User login
50-year-old man • foot pain • “purple” toe • history of smoking • Dx?
THE CASE
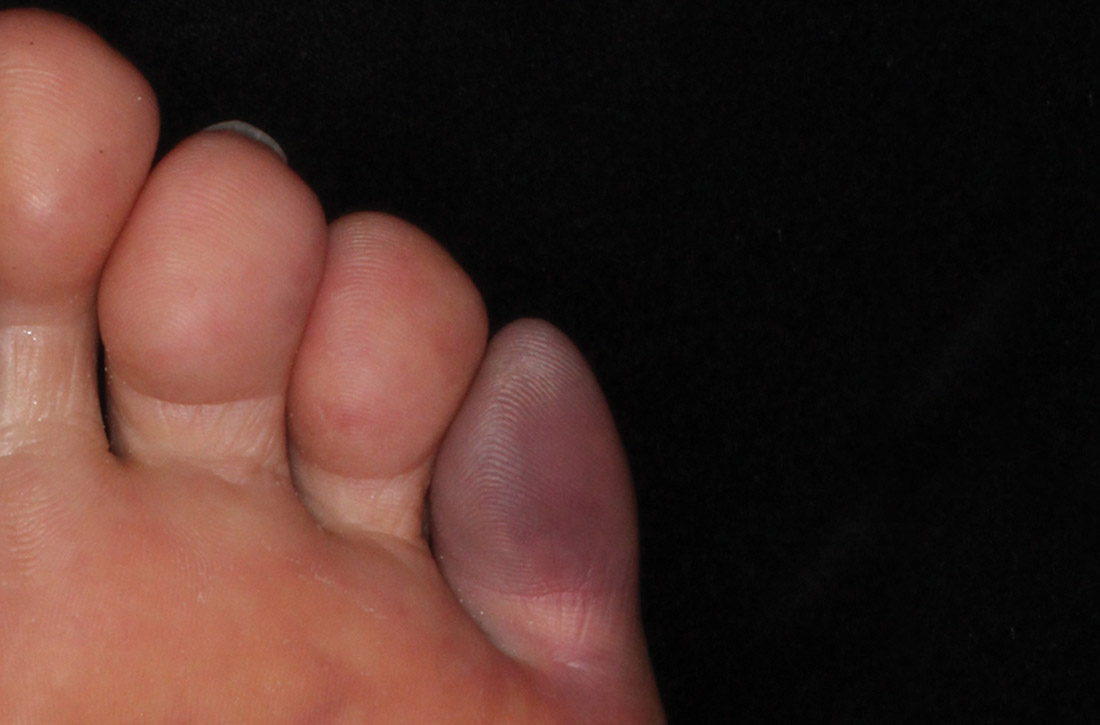
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; [email protected]
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.
THE CASE
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; [email protected]
THE CASE
A 50-year-old man presented to the primary care office for evaluation of foot pain. The day before, his left fifth toe had become exquisitely tender. He distinctly remembered that when he awoke, there was no discoloration or pain, but the toe later became “purple.” He denied any trauma. His medical record was notable for an extensive smoking history and a family history of early cardiovascular disease.
The patient appeared well but in obvious distress, secondary to the pain. His vital signs were unremarkable. His head, neck, lung, and cardiac exams revealed no abnormalities. Physical examination revealed a left fifth toe that was dusky purple and warm to the touch. Pain disproportionate to examination was noted on the anterior aspect of the toe, with limited range of motion. The patient walked with a compensated gait. Pulses were palpable on the posterior tibial (PT) and dorsalis pedis (DP) regions.
DIAGNOSIS
Based on our exam findings, we suspected a vascular injury and recommended an emergency consult by Podiatry, for which he was scheduled the following morning. The podiatric evaluation confirmed concern for a vascular injury and prompted a request for an emergent evaluation by Vascular Surgery.
The patient was seen emergently on Day 4 for a vascular surgery evaluation. Examination at that time showed a nearly absent femoral pulse on the left side and diminished and monophasic DP and PT pulses. His left foot demonstrated nonblanchable purpura that was clinically consistent with cholesterol embolization syndrome (CES).
We calculated the patient’s ankle-brachial index, and computed tomography angiography (CTA) was performed. While results were pending, the patient was started on aspirin 81 mg, clopidogrel 75 mg, and atorvastatin 40 mg, for a suspected slowly progressing iliac artery stenosis with a resulting acute atheroembolic event.
The CTA report showed a high-grade stenosis at the bifurcation of the left iliac artery, extending into both external and internal arteries. Of note, mild atherosclerotic disease without significant occlusion and runoff to the foot was observed into the tibial arteries. The stenosis extended into the profonda femoris artery, as well.
DISCUSSION
Atherosclerotic plaques are commonly encountered in patients with atherosclerotic disease; however, there are 2 varieties of emboli that arise from these plaques and one is often overlooked.1-4 The more common of these variants, thromboemboli, originates from an atherosclerotic plaque and can become lodged in a medium or large vessel as a single embolus.
Continue to: By contrast...
By contrast, atheroemboli (commonly known as cholesterol emboli or cholesterol crystal embolization) originate from atherosclerotic plaques in the aorta or another large artery,5 which are prone to embolize if the underlying plaque experiences stress. As the plaque erodes, cholesterol crystals break off and embolize distally. These smaller crystals flood into the circulation, allowing a shower of emboli over time to occlude the arterioles. As occlusion spreads through the arterioles, multiple organ systems are affected. (It was previously thought that procedure-associated cases were common, but a literature review has not borne this out.5)
The shower of emboli often triggers a systemic inflammatory response, causing nondescript abnormalities of laboratory inflammatory markers.6,7 Interestingly, hypereosinophilia is noted in about 80% of patients with CES.8
No disease-specific testing. A confounding factor in validating the diagnosis of CES is the lack of disease-specific testing. However, CES should be considered in a patient with acute kidney injury and hypereosinophilia. Making the diagnosis requires a high degree of clinical suspicion. Any organ can be affected, although the brain, kidneys, gastrointestinal tract, skin, and skeletal muscles of the lower extremities are most frequently involved.9 If left undiagnosed, the results can be devastating: slow and chronic injury to a variety of organ systems over time, which may not be recognized as a harbinger of an insidious underlying process causing end-organ damage.
Technically, definitive diagnosis can be made by biopsy of an affected organ. However, biopsy’s utility is limited due to potential for sampling error, accessibility (as noted, the location of the involved organ[s] may make biopsy nearly impossible without additional surgical risk9), and risk of poor healing to the biopsy site.10
Treatment is two-fold: supportive care for the affected end organ and prevention of subsequent embolic events. The latter entails aggressive risk factor reduction strategies, such as smoking cessation, statin therapy, blood pressure control, and blood sugar control. Warfarin is not recommended for treatment of CES due to the risk of further plaque rupture, hemorrhage, acute and chronic renal failure, and cholesterol microembolization to other organs.11,12
Continue to: Our patient
Our patient. After testing confirmed the diagnosis, the patient underwent an angioplasty. A stent was placed in his left iliac artery. He was continued on antiplatelet and statin therapy and was again counseled regarding smoking cessation.
THE TAKEAWAY
When patients present with symptoms suggestive of a vascular origin, consider CES. Although it can affect a multitude of organs, acute kidney injury and hypereosinophilia are the most common signs. Immediate intervention is required to save the affected organ; strategizing to reduce the risk for further embolic events is also key.
Prompt recognition of vascular emergencies, including those that are harbingers of atherosclerotic disease, is essential. As clinicians, it is imperative that we use all resources to address significant population health burdens. If CES is more prevalent than commonly thought, consideration should be given to increasing education about early detection and treatment of this disorder, including the reinforcement of primary prevention and aggressive treatment of risk factors for atherosclerotic cardiovascular disease.
CORRESPONDENCE
Meagan Vermeulen, MD, FAAFP, Department of Family Medicine, Rowan University School of Osteopathic Medicine, 42 East Laurel Road, Suite 2100A, Stratford, NJ 08084; [email protected]
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.
1. Tunick PA, Kronzon I. Atheromas of the thoracic aorta: clinical and therapeutic update. J Am Coll Cardiol. 2000;35:545-554.
2. Amarenco P, Duyckaerts C, Tzourio C, et al. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221-225.
3. Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474-1479.
4. Amarenco P, Cohen A, et al; French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334:1216-1221.
5. Ong HT, Elmsly WG, Friedlander DH. Cholesterol atheroembolism: an increasingly frequent complication of cardiac catheterisation. Med J Aust. 1991;154:412-414.
6. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631-641.
7. Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 2011;26:472-479.
8. Kasinath BS, Lewis EJ. Eosinophilia as a clue to the diagnosis of atheroembolic renal disease. Arch Intern Med. 1987;147:1384-1385.
9. Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 2013;15:315.
10. Jucgla A, Moreso F, Muniesa C, et al. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol. 2006;55:786-793.
11. Kim H, Zhen DB, Lieske JC, et al. Treatment of cholesterol embolization syndrome in the setting of an acute indication for anticoagulation therapy. J Med Cases. 2014;5:376-379.
12. Igarashi Y, Akimoto T, Kobayashi T, et al. Performing anticoagulation: a puzzling case of cholesterol embolization syndrome. Clin Med Insights Case Rep. 2017;10:1179547616684649. doi:10.1177/1179547616684649.
A guide to managing disorders of the ear pinna and canal
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
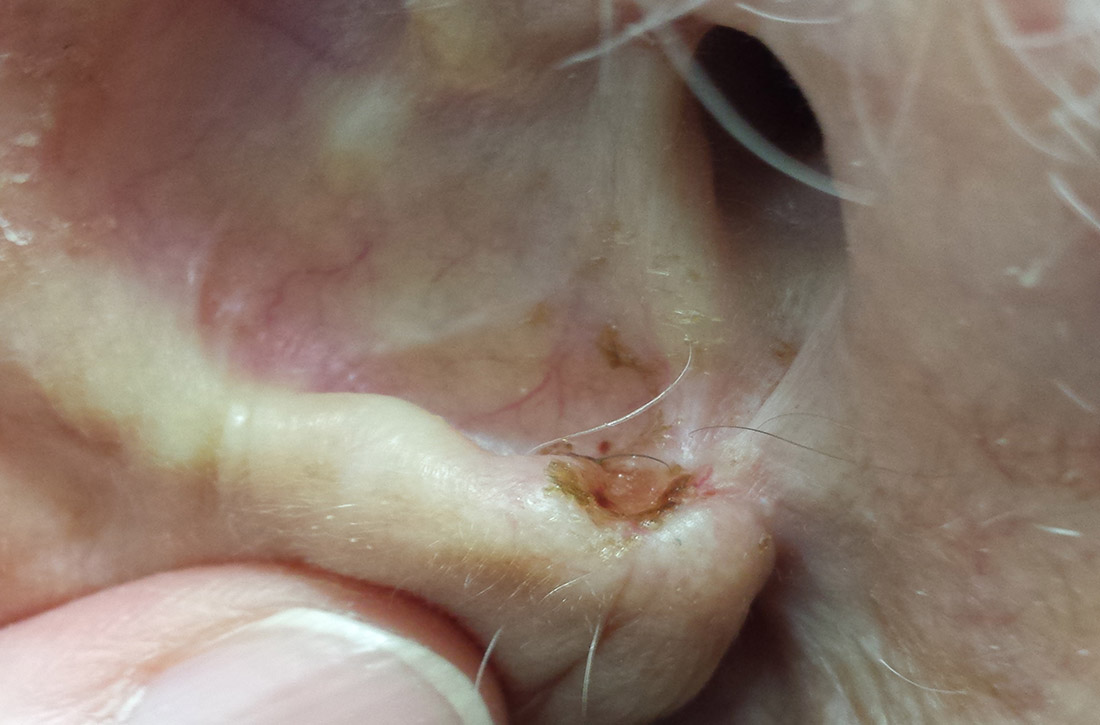
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
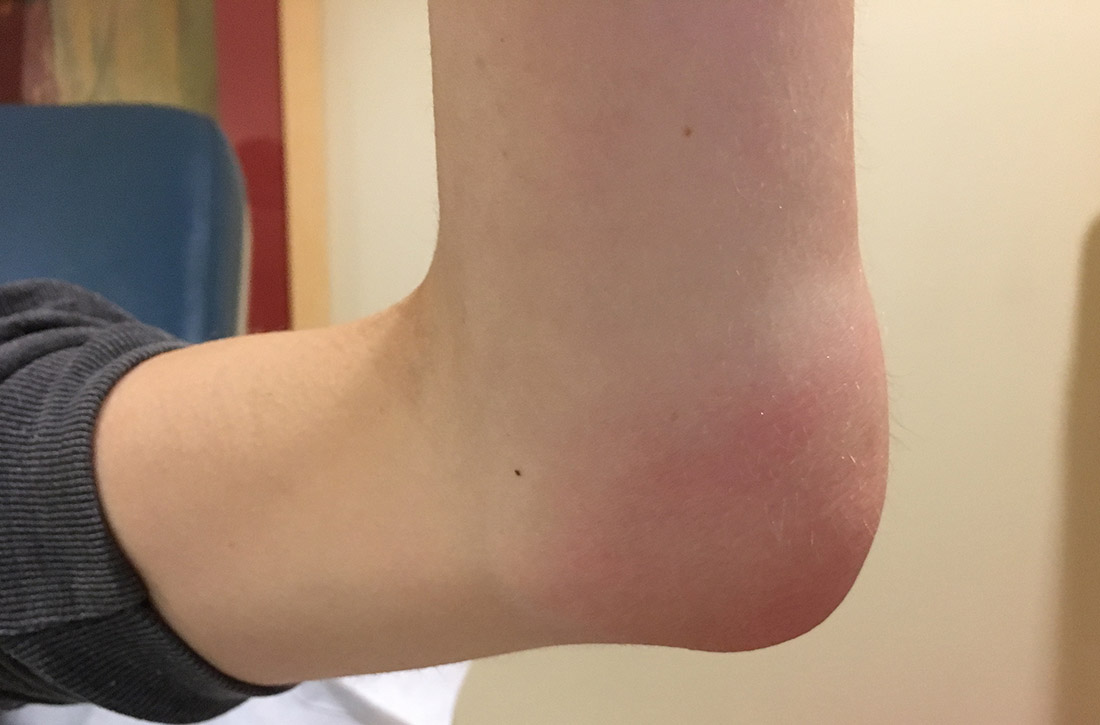
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
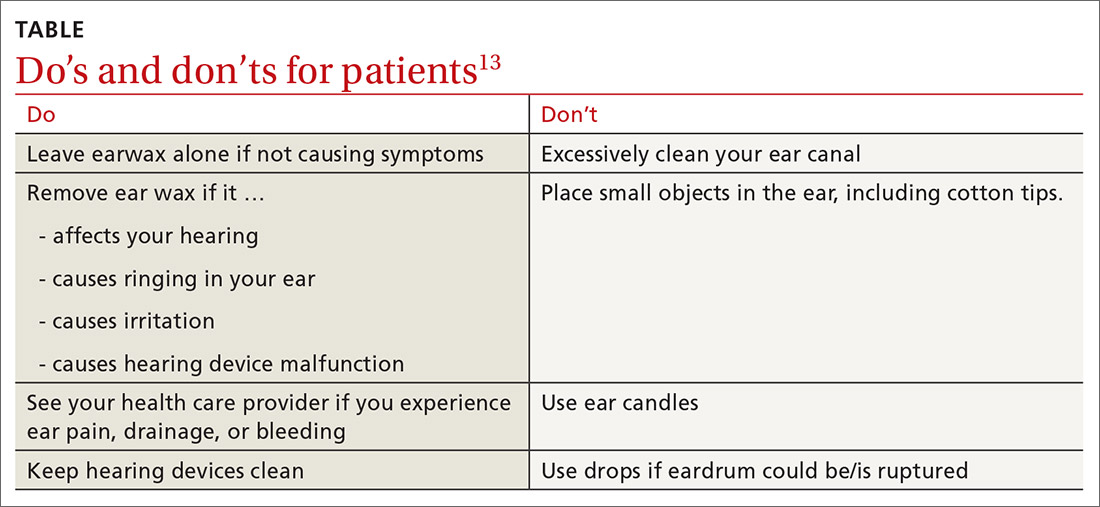
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13
How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
Which antibiotics are most useful for infection following ear piercing? When is it safe to attempt removal of a foreign body from the ear canal, and which cerumenolytic agent may be best for ear wax? This review covers common ailments of the outer ear, which are often readily diagnosed given a patient’s history and thorough physical examination. We also address more complicated matters such as deciding when to refer for treatment of suspected malignant otitis externa, and which lab markers to follow when managing it yourself.
A (very) brief review of ear anatomy
Understanding the unique embryology and intricate anatomy of the external ear informs our understanding of predictable infections, growths, and malformations.
The external ear is composed of the external auditory canal and auricle. The external auditory canal has a lateral (external) cartilaginous portion and a medial (internal) bony portion. The auricular structure is complex and formed by the helix, antihelix (crura; scaphoid fossa), tragus, antitragus, conchae, and lobule. The auricle is composed of elastic cartilage covered by skin. The lobule is composed of skin, adipose tissue, and connective tissue.
Embryologically, the auricle, auditory canal, and middle ear form from ectoderm of the first 2 branchial arches during early gestation. The auricle forms from the fusion of soft-tissue swellings (hillocks). Three hillocks arise from the first branchial arch and 3 from the second branchial arch during the fifth and sixth weeks of gestation. Tissues from the second branchial arch comprise the lobule, antihelix, and caudal helix. The cartilage of the tragus forms from the first branchial arch. The ear canal forms from an epithelial invagination of the first branchial arch that also occurs during the fifth week of gestation.1
Infections
Perichondritis
Inflammation or infection of the connective tissue layer surrounding the auricular cartilage (perichondrium) results in perichondritis. Further extension of infection can lead to an auricular abscess. Both of these conditions can have serious consequences.
What you’ll see. The most common risk factor for perichondritis is the popular practice of cosmetic transcartilaginous piercing.2 Piercing of the helix, scapha, or anti-helix (often referred to as “high” ear piercing) causes localized trauma that can strip the adjacent perichondrium, decrease blood supply, create cartilaginous microfractures, and lead to devascularization. Rates of infection as high as 35% have been reported with high-ear piercing.3
The most common microbes associated with perichondritis and pinna abscess formation are Pseudomonas and Staphylococcus species.2 P
Continue to: How to treat
How to treat. The cornerstone of treatment is early detection and antimicrobial coverage with antipseudomonal antibiotics. Ciprofloxacin is the oral antibiotic of choice because of its ability to penetrate the tissue.4 Other options include clindamycin and third- or fourth-generation cephalosporins. If the wound becomes abscessed, perform (or refer for) early surgical incision and drainage.5 A failure to promptly recognize perichondritis or to mistakenly prescribe non-antipseudomonal antibiotics contributes to increased rates of hospitalization.2 Cosmetic deformity is the most common complication of perichondritis. This may require reconstructive surgery.
Otitis externa
Acute otitis externa (AOE; “swimmer’s ear”) is cellulitis of the skin and subdermis of the external ear canal. It is most prevalent in warm, moist climates and almost always associated with acute bacterial infection, most commonly P aeruginosa or S aureus.6 There is also an increased association with poor water quality (containing higher bacterial loads). Anything breaching the integrity of the ear canal can potentially predispose to the development of AOE. This includes trauma from cleaning, cerumen removal, scratching due to allergic conditions, and placement of hearing-aid devices.6
What you’ll see. Suspect AOE when signs or symptoms of ear canal inflammation have appeared rapidly (generally within 2 days) over the past 3 weeks.7 Findings include otalgia, itching, fullness, tragal tenderness, ear canal edema, erythema with or without otorrhea, lymphadenitis, or cellulitis of the pinna or adjacent skin.7 AOE must be distinguished from other causes of otalgia and otorrhea, including dermatitis and viral infection.
How to treat. Topical therapy is recommended for the initial treatment of uncomplicated AOE, usually given over 7 days. Multiple topical preparations are available, such as ciprofloxacin 0.2%/hydrocortisone 1.0%; neomycin/polymyxin B/hydrocortisone; ofloxacin 0.3%; or acetic acid 2.0%.7 Avoid these agents, though, if you suspect tympanic membrane rupture. Quinolone drops are the only topical antimicrobials approved for middle ear use.7
Systemic antibiotics are not recommended for the initial treatment of AOE. Topical agents deliver a much higher concentration of medication than can be achieved systemically. Consider systemic antibiotics if there is extension outside the ear canal, a concern for necrotizing otitis externa (more on this in a bit), or the patient is immunodeficient.8
Continue to: Patient (or parent) education...
Patient (or parent) education is important to ensure proper medication administration. The patient should lie down with the affected ear facing up. After the canal is filled with drops, the patient should remain in this position for 3 to 5 minutes. Gently massaging the tragus can augment delivery. Patients should keep the ear canal as dry as possible and avoid inserting objects (eg, hearing aids, ear buds, cotton-tipped applicators) into the canal for the duration of treatment. The delivery of topical antibiotics can be enhanced by wick placement. Prescribe analgesics (typically nonsteroidal anti-inflammatory agents) based on severity of pain.7
Have patients abstain from water sports for 7 to 10 days. Showering is acceptable with minimal ear exposure to water; bathing is preferred when possible. If there is no clinical improvement in 48 to 72 hours, ask patients to return for re-evaluation.8 Prevention is essential for patients with a history of recurrent otitis externa. Acetic acid solutions create an acidic environment within the canal to help prevent recurrent AOE. Ear plugs and petroleum jelly–soaked cotton plugs prior to water exposure may also help prevent recurrent AOE.
Malignant otitis externa
Malignant, or necrotizing, otitis externa is an aggressive disease form of otitis externa that is most common in individuals with diabetes or other immunodeficiency disorders.9 Most cases are due to infection with P aeruginosa.10 Prior to the availability of effective antibiotics, mortality rates in patients with necrotizing otitis externa were as high as 50%.11
What you’ll see. Patients typically present with severe ear pain, otorrhea, conductive hearing loss, and a feeling of fullness in the external ear canal. Physical examination reveals purulent otorrhea and a swollen, tender ear canal. Exposed bone may be visible, most often on the floor of the canal. The tympanic membrane and middle ear are seldom involved on initial presentation.
The infection often originates at the junction of the bony and cartilaginous portion of the external canal, spreading through the fissures of Santorini to the skull base. If not aggressively treated, the infection spreads medially to the tympanomastoid suture causing intracranial complications—usually a facial nerve neuropathy.
Continue to: Given these clinical findings...
Given these clinical findings, promptly order laboratory studies and imaging to confirm the diagnosis. The erythrocyte sedimentation rate and C-reactive protein level are typically elevated, and either can be used as a marker to follow treatment. Computed tomography (CT) helps to determine the location and extent of disease and is recommended as the initial diagnostic imaging modality for patients with suspected malignant otitis externa.12
Magnetic resonance imaging helps define soft-tissue changes, dural enhancement, and involvement of medullary bone, making this the preferred modality to monitor therapeutic response.12 Technetium bone scanning can also be used for the initial diagnosis (particularly if CT findings are normal and clinical suspicion is high) and for follow-up with treatment.
How to treat. Management involves a team approach with otolaryngology, radiology, neurology, endocrinology, and infectious disease specialists. Long term (6-8 weeks) antipseudomonal antibiotic treatment is typical.
Let culture results guide the choice of antibiotic. Fluoroquinolone therapy, usually ciprofloxacin, is used most often.12 Surgical intervention may be required for local debridement and drainage of abscesses. Close follow-up is necessary due to reports of recurrence up to 1 year after treatment. If left untreated, necrotizing otitis externa can lead to osteomyelitis, meningitis, septic thrombosis, cerebral abscess, and death.11
Cerumen impaction
The relatively small diameter of the external auditory canal increases the risk for impaction of cerumen and foreign bodies. Cerumen impaction, in particular, is a common primary care complaint. Cerumen forms when glandular secretions from the outer two-thirds of the ear canal mix with exfoliated skin. It functions as a lubricant for the ear canal and as a barrier against infection, water accumulation, and foreign bodies.13
Continue to: What you'll see
What you’ll see. You may encounter cerumen impaction in an asymptomatic patient when it prevents visualization of the external auditory canal or tympanic membrane, or when a patient complains of conductive hearing loss, tinnitus, dizziness, ear pain, itching, and cough.13 It is found in 1 in 10 children and 1 in 20 adults.13 There is a higher incidence in patients who are elderly, are cognitively impaired, or wear hearing devices or ear plugs.13,14 Asymptomatic cerumen impaction should not be treated. A recent clinical guideline provides a useful “do and don’t” list for patient education (TABLE).13

How to treat. In asymptomatic patients, the presence of cerumen on examination is not an indication for removal. Based on current guidelines,13 impacted cerumen can safely be removed from the ear canal of symptomatic patients in several ways:
- Manual removal with cerumen loop/spoon or alligator forceps. This method decreases the risk for infection because it limits moisture exposure. However, it should be performed by a health care provider trained in its use because of the risk for trauma to the ear canal and tympanic membrane.
- Irrigation of the ear using tap water or a 50-50 solution of hydrogen peroxide and water. Irrigation can be achieved with a syringe or jet irrigator using a modified tip. This method also has a risk for trauma to the ear canal and tympanic membrane and should only be performed by appropriately trained health care professionals.
- Use of cerumenolytic agents to soften and thin earwax and promote natural extrusion. Several types of cerumenolytic drops (water-based and oil-based) are available and appear to be equally effective. Water-based solutions contain hydrogen peroxide, docusate sodium, acetic acid, and sodium bicarbonate. Oil-based drops may contain peanut, almond, or olive oils. A thorough allergic history should be performed to avoid using products in patients with nut allergies. In head-to-head laboratory comparisons, distilled water appears to be the best cerumenolytic.15
Foreign bodies
Foreign bodies in the external auditory canal (typically beads, cotton tips, and insects) are more common in children than adults.16
What you’ll see. Most foreign bodies are lodged in the bony part of the external auditory canal, and many patients try to remove the object before seeking medical care. Removal requires adequate visualization and skill.17 Although patients may be asymptomatic, most complain of pain, fullness, decreased hearing, or otorrhea.
How to treat. Directly visible objects can often be removed without referral. Suction, irrigation, forceps, probes, and fine hooks have been used. Insect removal can be facilitated by first flooding the canal with xylocaine, alcohol, or mineral oil. Acetone may be used to dissolve foreign bodies containing Styrofoam or to loosen glues. If the object is a button battery, avoid irrigation to prevent liquefaction tissue necrosis.
Continue to: Complications of foreign body removal...
Complications of foreign body removal include pain, otitis externa, otitis media, and trauma to the ear or tympanic membrane. The likelihood of successful removal of the object decreases and the risk for complications increases with each subsequent attempt.17 Consult an otolaryngologist if sedation or anesthesia is required, the foreign body is tightly wedged, there is trauma to the ear canal or tympanic membrane, the foreign body has a sharp edge (eg, glass or wire), or removal attempts have been unsuccessful.
Trauma
Sports injuries, motor vehicle accidents, bites, falls, and burns are the primary causes of trauma to the external ear.18
What you’ll see. Blunt auricular trauma predisposes to infection, necrosis, and scar contracture. One of the most common sequelae is cauliflower ear. Trauma is particularly common with contact sports such as boxing, wrestling, or mixed martial arts. The skin of the auricle attaches directly to the perichondrium. Following blunt or shearing trauma to the auricle, hematomas form within the space between the perichondrium and cartilage of the anterior ear.19
How to treat. Small hematomas can be managed by aspiration, while larger ones generally require open drainage.20 Newer treatments involving pressure dressings and the use of fibrin glue have been proposed.20 Recommend that athletes participating in contact sports wear appropriate protective headgear to prevent auricular hematoma and cauliflower ear.
Neoplasm
Roughly 5% of all skin cancers involve the ear, most frequently the pinna due to chronic sun exposure.21 The most frequently occurring malignancy of the external ear is basal cell carcinoma (BCC), which is responsible for 80% of all nonmelanoma skin cancers.22
Continue to: What you'll see
What you’ll see. BCC of the ear usually involves the preauricular area and the helix. The risk for BCC is related to exposure to ultraviolet radiation. BCC of the ear is more common in men and can be particularly aggressive, highlighting the importance of prevention and prompt recognition. BCC typically presents as a fleshy papule that is often translucent or “pearly’” and has overlying telangiectasia and a “rolled” border. Central ulceration can occur as well.
How to treat. Usual treatment of BCC is surgical excision. Prevention is critical and centers on sun avoidance or the use of appropriate sunscreens.
In addition to BCC, exposure of the external ear to sunlight and ultraviolet radiation predisposes patients to the development of squamous cell carcinoma (SCC) and melanoma. SCC has a variety of presentations including papules, plaques, and nodules. SCC has a higher metastatic potential than does BCC.
Keloid
Keloids are an abnormal healing response to soft-tissue injury: benign fibrocartilaginous growths that extend beyond the original wound.
What you’ll see. Keloids are more common in dark-skinned individuals and tend to result from burns, surgical incisions, infection, trauma, tattooing, injections, piercings, and arthropod bites. In some cases, they arise spontaneously. Keloids are more common in areas of increased skin tension (chest, shoulders, back), but may occur on the ears—most commonly after piercing or trauma. Keloids present clinically as slow-growing rubbery or firm nodules. The diagnosis is typically based on clinical appearance but can be confirmed by histopathology.
Continue to: How to treat
How to treat. Treatments vary and include observation, excision, intralesional injections, cryotherapy, enzyme therapy, silicone gel application, and irradiation.23 Recurrence is common; no therapy has been proven to be universally superior or preferred.
Congenital malformations
Atresia
Disruption of embryologic development (failed invagination of the external auditory canal) can lead to a stenotic or absent ear canal (aural atresia). Aural atresia is also often associated with fusion of the incus and malleus. This condition occurs predominantly in males. Unilateral atresia is more common than bilateral atresia, and the right ear is more often involved than the left.24
Microtia
Microtia is the incomplete development of the pinna leading to a small or deformed pinna. Microtia can be unilateral or bilateral. As with atresia, microtia more commonly affects males and, if unilateral, the right side is more often affected than the left. Microtia can occur in isolation but is often associated with genetic syndromes such as Treacher Collins syndrome and craniofacial microsomia (Goldenhar syndrome). When microtia is identified (typically at birth or early infancy), audiologic testing and a thorough physical examination for evidence of associated defects should be performed. Consult with an audiologist, clinical geneticist, or pediatric otolaryngologist.
Pre-auricular pits
Pre-auricular pits (sinuses) are tiny indentations anterior to the helix and superior to the tragus. While pre-auricular pits are more common on the right side, they are bilateral in 25% to 50% of cases.25 Pre-auricular pits occur in up to 1% of white children, 5% of black children, and 10% of Asian children.25 Children with this condition should undergo formal audiologic testing as their risk for hearing loss is higher compared with the general population.26
The branchio-oto-renal syndrome (associated with pre-auricular pits and hearing loss) also features structural defects of the ear, renal anomalies and/or nasolacrimal duct stenosis or fistulas. If this syndrome is suspected, renal ultrasound imaging is warranted. Other indications for renal ultrasound in patients with a pre-auricular pit are any dysmorphic feature, a family history of deafness, an auricular malformation, or a maternal history of gestational diabetes.27 Pre-auricular pits do not require surgery unless they drain chronically or become recurrently infected. Complete surgical excision is the treatment of choice in these cases.
CORRESPONDENCE
Mark Stephens, MD, 1850 Park Avenue, State College, PA 16801; [email protected]
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
1. Cox TC, Camci ED, Vora S, et al. The genetics of auricular development and malformation: new findings in model systems driving future directions for microtia research. Eur J Med Genet. 2014;57:394-401.
2. Sosin M, Weissler JM, Pulcrano M, et al. Transcartilaginous ear piercing and infectious complications: a systematic review and critical analysis of outcomes. Laryngoscope. 2015;125:1827-1834.
3. Stirn A. Body piercing: medical consequences and psychological motivations. Lancet. 2003;361:1205-1215.
4. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Larygol Oncol. 2013;127:505-508.
5. Mitchell S, Ditta K, Minhas S, et al. Pinna abscesses: can we manage them better? A case series and review of the literature. Eur Arch Otorhinolaryngol. 2015;272:3163-3167.
6. Stone KE. Otitis externa. Pediatr Rev. 2007;28:77-78.
7. Rosenfeld RM, Schwartz SR, Cannon CR, et al. Clinical practice guideline: acute otitis externa. Otolaryngol Head Neck Surg. 2014;150(1 suppl):S1-S24.
8. Prentice P. American Academy of Otolaryngology: Head and Neck Surgery Foundation clinical practice guideline on acute otitis externa. Arch Dis Child Educ Pract Ed. 2015;100:197.
9. Unadkat S, Kanzara T, Watters G. Necrotising otitis externa in the immunocompetent patient. J Laryngol Otol. 2018;132:71-74.
10. Carfrae MJ, Kesser BW. Malignant otitis externa. Otolarngol Clin N Am. 2008;41:537-549.
11. Chandler JR, Malignant otitis externa. Laryngoscope. 1968;78:1257-1294.
12. Hollis S, Evans K. Management of malignant (necrotising) otitis externa. J Laryngol Otol. 2011;125:1212-1217.
13. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156:S1-S29.
14. Guest JF, Greener MJ, Robinson AC, et al. Impacted cerumen: composition, production, epidemiology and management. QJM. 2004;97:477-488.
15. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127:1067-1070.
16. Awad AH, ElTaher M. ENT foreign bodies: an experience. Int Arch Otorhinolaryngol. 2018;22:146-151.
17. Heim SW, Maughan KL. Foreign bodies in the ear, nose, and throat. Am Fam Physician. 2007;76:1185-1189.
18. Sharma K, Goswami SC, Baruah DK. Auricular trauma and its management. Indian J Otolaryngol Head Neck Surg. 2006;58:232-234.
19. Haik J, Givol O, Kornhaber R, et al. Cauliflower ear–a minimally invasive treatment in a wrestling athlete: a case report. Int Med Case Rep J. 2018;11:5-7.
20. Ebrahimi A, Kazemi A, Rasouli HR, et al. Reconstructive surgery of auricular defects: an overview. Trauma Mon. 2015;20:e28202.
21. Warner E, Weston C, Barclay-Klingle N, et al. The swollen pinna. BMJ. 2017; 359; j5073.
22. Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
23. Ranjan SK, Ahmed A, Harsh V, et al. Giant bilateral keloids of the ear lobule: case report and brief review of the literature. J Family Med Prim Care. 2017;6:677-679.
24. Roland PS, Marple BF. Disorders of the external auditory canal. J Am Acad Audiol. 1997;8:367-378.
25. Scheinfeld NS, Silverberg NB, Weinberg JM, et al. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196.
26. Roth DA, Hildesheimer M, Bardestein S, et al. Preauricular skin tags and ear pits are associated with permanent hearing impairment in newborns. Pediatrics. 2008;122:e884-890.
27. Tan T, Constantinides H, Mitchell TE. The preauricular sinus: a review of its aetiology, clinical presentation and management. Int J Ped Otorhinolaryngol. 2005;69:1469-1474.
PRACTICE RECOMMENDATIONS
› Prescribe topical antibiotics for uncomplicated otitis externa, reserving systemic agents for infection extending outside the ear canal, necrotizing otitis externa, or patients who are immunodeficient. C
› Avoid clearing cerumen if a patient is asymptomatic and advise patients/parents on Do’s and Don’ts for ear wax accumulation. C
› Consider flooding the ear canal with xylocaine, alcohol, or mineral oil before attempting insect removal. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing amidst COVID-19 (and everything else that ails us)
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
This year, medical media has been dominated by reporting on the devastating COVID-19 pandemic. Many studies and analyses have shown that staying at home, social distancing, quarantining of close contacts, and wearing face masks and face shields are effective ways of preventing spread.
Although initially there were no known effective treatments for severe COVID-19 infection (other than oxygen and ventilator support), we now know that dexamethasone,1 remdesivir,2 and convalescent plasma3 are effective in lessening the severity of illness and perhaps preventing death. That said, we will continue to struggle with COVID-19 for the foreseeable future.
But other medical illnesses actually predominate in terms of morbidity and mortality, even during this pandemic. For example, although there has been an average of roughly 5600 COVID-19-related deaths per week for the past 4 months,4 there are, on average, more than 54,000 deaths per week in the United States from other causes.5 This means that we must continue to tend to the other health care needs of our patients even as we deal with COVID-19.
In that light, JFP continues to publish practical, evidence-based clinical reviews designed to keep family physicians and other primary health care clinicians up to date on a variety of topics. For instance, in this issue of JFP, we have articles on:
- Opioid prescribing. Although opioids have risks, they remain potent medications for relief from acute pain, as well as cancer-related pain and chronic pain not sufficiently treated with other medications. Mahvan et al provide expert advice on maximizing benefit and minimizing the risks of opioid prescribing.
- Secondary ischemic stroke prevention. For patients who have suffered a transient ischemic attack or minor stroke, a mainstay of prevention is antiplatelet therapy. Aspirin alone used to be the treatment of choice, but research has demonstrated the value of adding another antiplatelet agent. Helmer et al’s thorough review reminds us that the antiplatelet drug of choice, in addition to aspirin, is clopidogrel, which should be used only for the first 30 days after the event because of an increased bleeding risk.
- Combatting Clostridioides difficile infection. CDI has been a difficult condition to treat, especially in high-risk patients. Zukauckas et al provide a comprehensive review of diagnosis and management. Vancomycin is now the drug of choice, and fecal transplant is highly effective in preventing recurrent CDI.
This diverse range of timely, practical, evidence-based guidance—in addition to coverage of COVID-19 and other rapidly emerging medical news stories—can all be found on our Web site at www.mdedge.com/familymedicine. We remain committed to supplying you with all of the information you need to provide your patients with the very best care—no matter what brings them in to see you.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
1. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. Recovery: Randomised Evaluation of COVID-19 Therapy Web site. June 16, 2020. www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19. Accessed July 1, 2020.
2. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2007764.
3. Li L, Zhang W, Hu Y, et. al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial [published online ahead of print]. JAMA. doi:10.1001/jama.2020.10044.
4. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765.
5. Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;1-8.
Painful, swollen elbow
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.
Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.
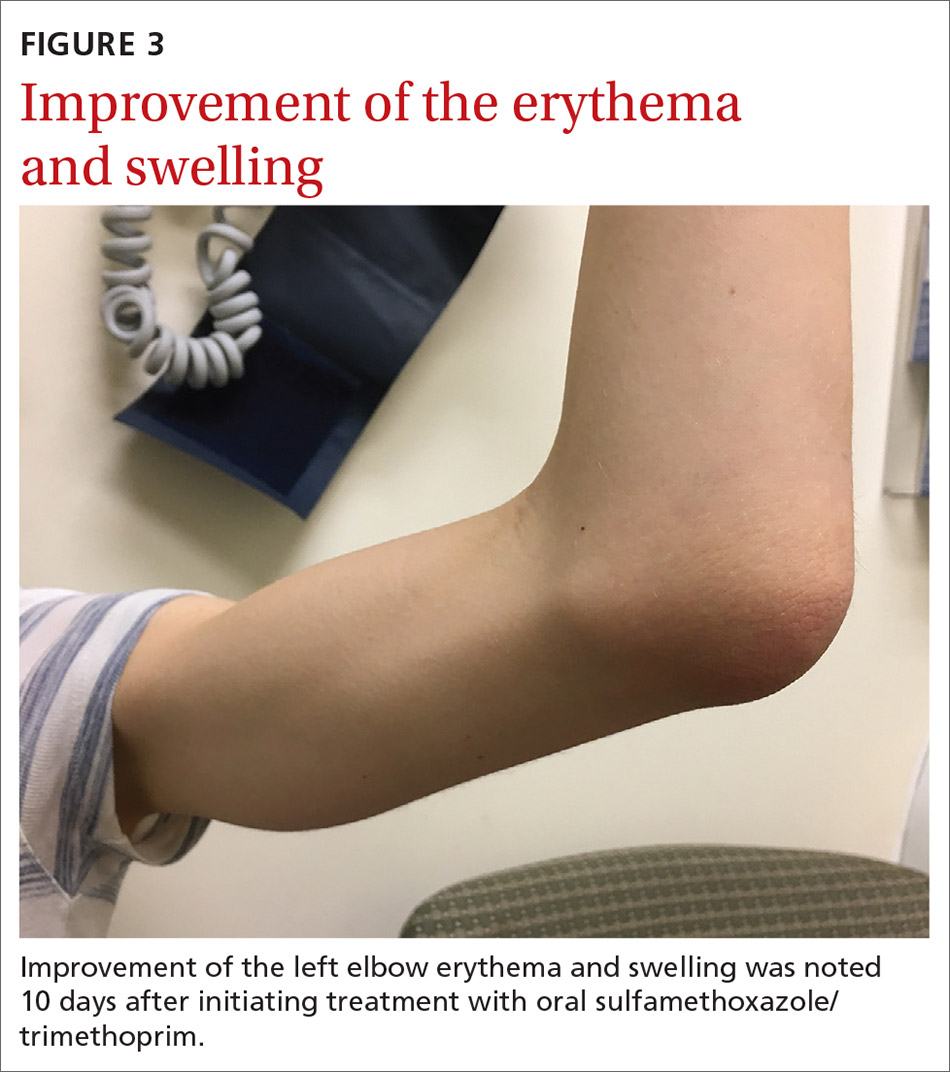
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. [email protected]
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.

Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.

CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. [email protected]
A 32-year-old woman presented to our clinic with left elbow swelling and pain of 6 days’ duration. She’d had a posterior interosseous nerve (PIN) injection (hydrodissection) at another facility 12 days earlier for refractory intersection syndrome.
During nerve hydrodissection, fluid is injected into the area surrounding the nerve in an effort to displace the muscles, tendons, and fascia and thus reduce friction on the nerve. This treatment, often completed with ultrasound guidance, is utilized by patients who want to obtain pain relief without undergoing surgery for nerve entrapment syndromes.
In this case, a combination of 1 mL (40 mg) of methylprednisolone acetate, 1 mL of lidocaine 2%, and 3 mL of normal saline was injected into the supinator muscle belly (proximal dorsal aspect of the forearm) under ultrasound guidance. Six days later, the patient began to experience elbow pain, redness, and swelling. The symptoms progressed within several hours and became so notable that she sought care at an urgent care facility the next morning. At this facility, she was told she had an infection and was prescribed oral levofloxacin 500 mg/d.
The patient presented to our clinic after 4 days of oral levofloxacin with no improvement of symptoms. She denied chills or fever and described her pain as moderate and radiating to her fingers. There was no history of trauma. The patient reported riding her bike more frequently, which had caused the original forearm pain that warranted the PIN injection. There were no other recent changes to activity. Her medical, social, and surgical histories were otherwise unremarkable.
Her vital signs were normal. Physical exam revealed an erythematous and warm left elbow (FIGURE 1). Her left elbow range of motion (extension and flexion) was mildly decreased due to the pain and swelling.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Iatrogenic septic olecranon bursitis
Aspiration of the patient’s olecranon bursa produced 3 mL of cloudy fluid (FIGURE 2). The patient’s painful, swollen, erythematous, warm elbow, cloudy aspirate, and history of preceding PIN hydrodissection were consistent with a diagnosis of septic olecranon bursitis.

Septic bursitis usually is caused by bacteria.1,2 Bursal infection can result from the spread of infection from nearby tissues or direct inoculation from skin trauma. It can also be iatrogenic and occur among healthy individuals.2,3 Injection anywhere close to the bursa can inoculate enough bacteria to progress to cellulitis first and then septic bursitis. Inflammatory conditions such as gout and rheumatoid arthritis also can cause acute and/or chronic superficial bursitis.1,2,4
Differentiating between septic and nonseptic bursitis can be challenging on history and physical exam alone, but specific signs and symptoms should warrant concern for infection.1,2,4,5 Fever is present in up to 75% of septic cases5; however, lack of fever does not rule out septic bursitis. Pain, erythema, warmth, and an overlying skin lesion also can indicate infection.4 Diagnostic imaging modalities may help distinguish different types of olecranon bursitis, but in most cases, they are not necessary.2
Other joint disorders factor into the differential
The differential diagnosis is broad and includes a variety of joint disorders in addition to septic (and nonseptic) bursitis.2,3
Septic arthritis is a deeper infection that involves the elbow joint and is considered an orthopedic emergency due to potential joint destruction.
Continue to: A simple joint effusion
A simple joint effusion also arises from the elbow joint, but this diagnosis becomes less likely when the joint aspirate appears cloudy. A simple joint effusion would not produce bacteria on gram stain and culture.
Crystalline inflammatory arthritis (gout, pseudogout) is due to intra-articular precipitation of crystals (uric acid crystals in gout, calcium pyrophosphate crystals in pseudogout).
Hematomas would produce gross blood or clot on joint aspiration.
Cellulitis is an infection of the superficial soft tissue (only) and thus, aspiration is not likely to yield fluid.
Diagnosis can be made with culture of fluid
Confirmation of septic olecranon bursitis is best attained by bursal needle aspiration and culture. Aspiration also can evaluate for other causes of elbow swelling. (If septic olecranon bursitis is suspected clinically, empiric antibiotics should be started while awaiting culture results.6) White blood cell counts from the aspirate also may be utilized but have a lower sensitivity and specificity for diagnosis.7
Continue to: In addition to aiding in diagnosis
In addition to aiding in diagnosis, bursal aspiration for a patient with septic bursitis can improve symptoms and reduce bacterial load.1-3,8 The use of a compressive bandage after aspiration may help reduce re-accumulation of the bursal fluid.1-3,8Staphylococcus aureus is responsible for the majority of septic olecranon bursitis cases.9-11
Tailoring the antibiotic regimen
There is wide variation in the treatment of septic olecranon bursitis due to the lack of strong evidence-based guidelines.1,2,8,11-13 When septic bursitis is strongly suspected (or confirmed) the patient should be started on an antibiotic regimen that covers S aureus.1,2 Once culture results and sensitivities return, the antibiotic regimen can be tailored appropriately.
In cases of mild-to-moderate septic olecranon bursitis in an immunocompetent host, the patient can be started on oral antibiotics and monitored closely as an outpatient.1-3,8 Patients with septic olecranon bursitis who meet the criteria for systemic inflammatory response syndrome or who are immunocompromised should be hospitalized and started on intravenous antibiotics.1-3 Recommended duration of antibiotic therapy varies but is usually about 10 to 14 days.1-3,8 In rare cases, surgical intervention with bursectomy may be necessary.1,2,14
Our patient was given a dose of ceftriaxone 250 mg intramuscularly and was started on oral sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily after aspiration of the bursa. Culture of the bursal fluid grew oxacillin-sensitive S aureus which was sensitive to a variety of antibiotics including levofloxacin and sulfamethoxazole/trimethoprim. Her symptoms gradually improved (FIGURE 3) and resolved after a 14-day course of oral sulfamethoxazole/trimethoprim.

CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, Department of Family Medicine & Orthopedics, AFW Clinic, 3055 Roslyn St, Denver, CO 80238; morteza. [email protected]
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
1. Baumbach SF, Lobo CM, Badyine I, et al. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134:359-370.
2. Khodaee M. Common superficial bursitis. Am Fam Physician. 2017;95:224-231.
3. Harris-Spinks C, Nabhan D, Khodaee M. Noniatrogenic septic olecranon bursitis: report of two cases and review of the literature. Curr Sports Med Rep. 2016;15:33-37.
4. Reilly D, Kamineni S. Olecranon bursitis. J Shoulder Elbow Surg. 2016;25:158-167.
5. Blackwell JR, Hay BA, Bolt AM, et al. Olecranon bursitis: a systematic overview. Shoulder Elbow. 2014;6:182-190.
6. Del Buono A, Franceschi F, Palumbo A, et al. Diagnosis and management of olecranon bursitis. Surgeon. 2012;10:297-300.
7. Stell IM, Gransden WR. Simple tests for septic bursitis: comparative study. BMJ. 1998;316:1877.
8. Abzug JM, Chen NC, Jacoby SM. Septic olecranon bursitis. J Hand Surg Am. 2012;37:1252-1253.
9. Cea-Pereiro JC, Garcia-Meijide J, Mera-Varela A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10-14.
10. Morrey BE. Bursitis. In: Morrey BE, Sanchez-Sotelo J, eds. The Elbow and its Disorders. 4th ed. Philadelphia, PA: Saunders Elsevier 2009:1164-1173.
11. Wingert NC, DeMaio M, Shenenberger DW. Septic olecranon bursitis, contact dermatitis, and pneumonitis in a gas turbine engine mechanic. J Shoulder Elbow Surg. 2012;21:E16-E20.
12. Baumbach SF, Michel M, Wyen H, et al. Current treatment concepts for olecranon and prepatellar bursitis in Austria. Z Orthop Unfall. 2013;151:149-155.
13. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: a systematic review. Arch Orthop Trauma Surg. 2014;134:1517-1536.
14. Ogilvie-Harris DJ, Gilbart M. Endoscopic bursal resection: the olecranon bursa and prepatellar bursa. Arthroscopy. 2000;16:249-253.
Tips and tools for safe opioid prescribing
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
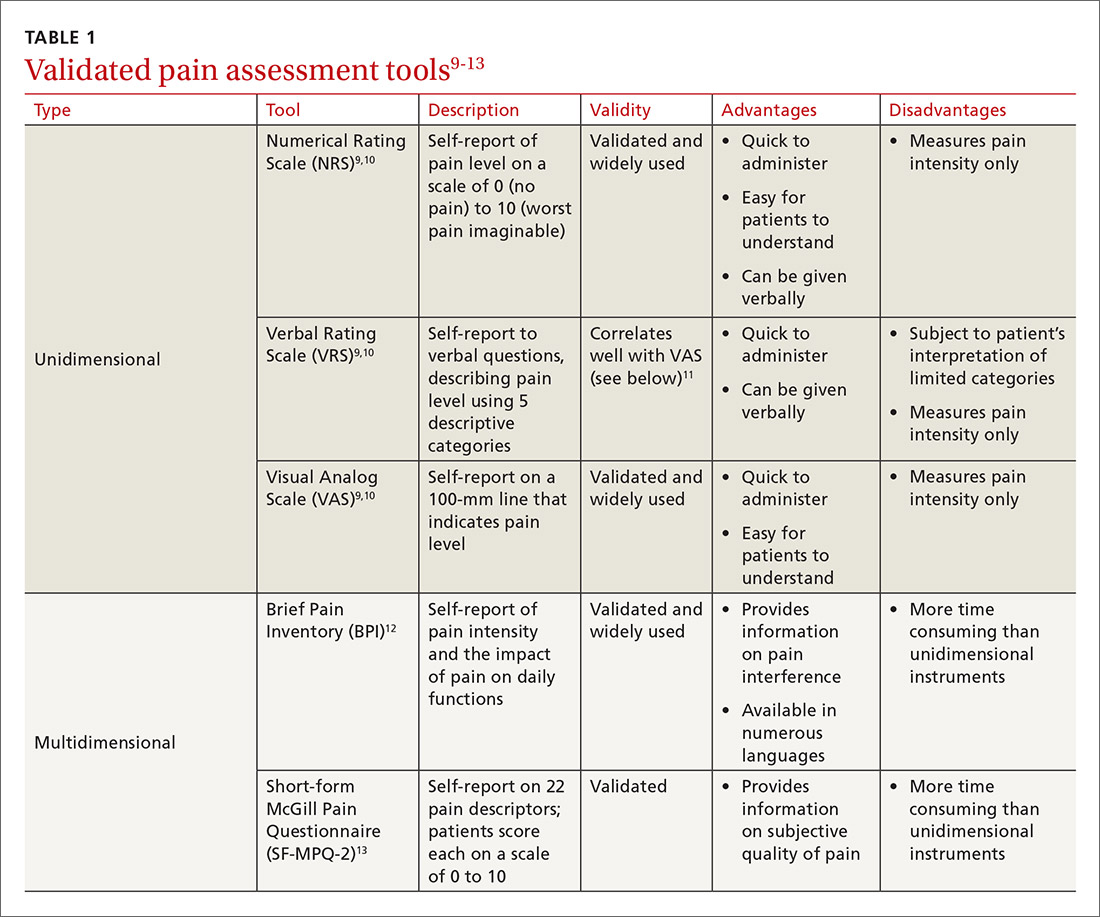
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).
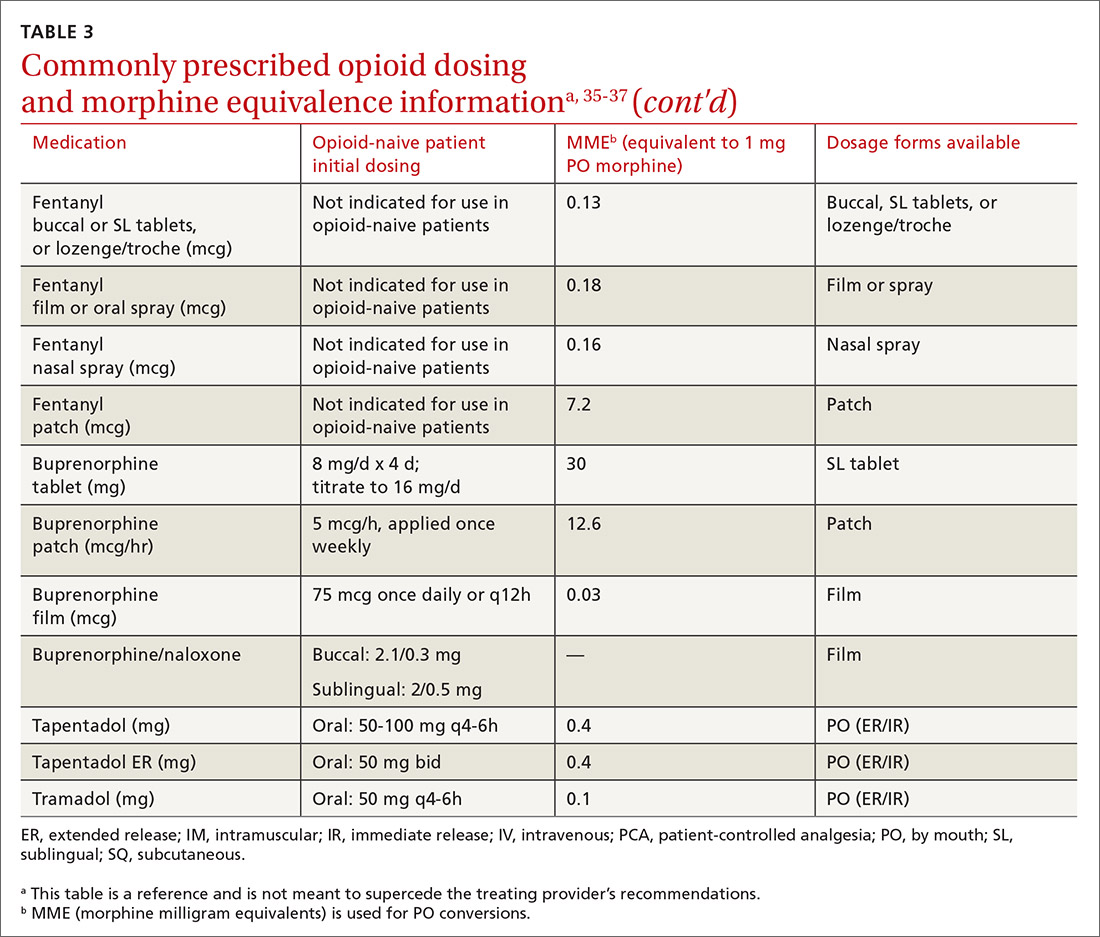
Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
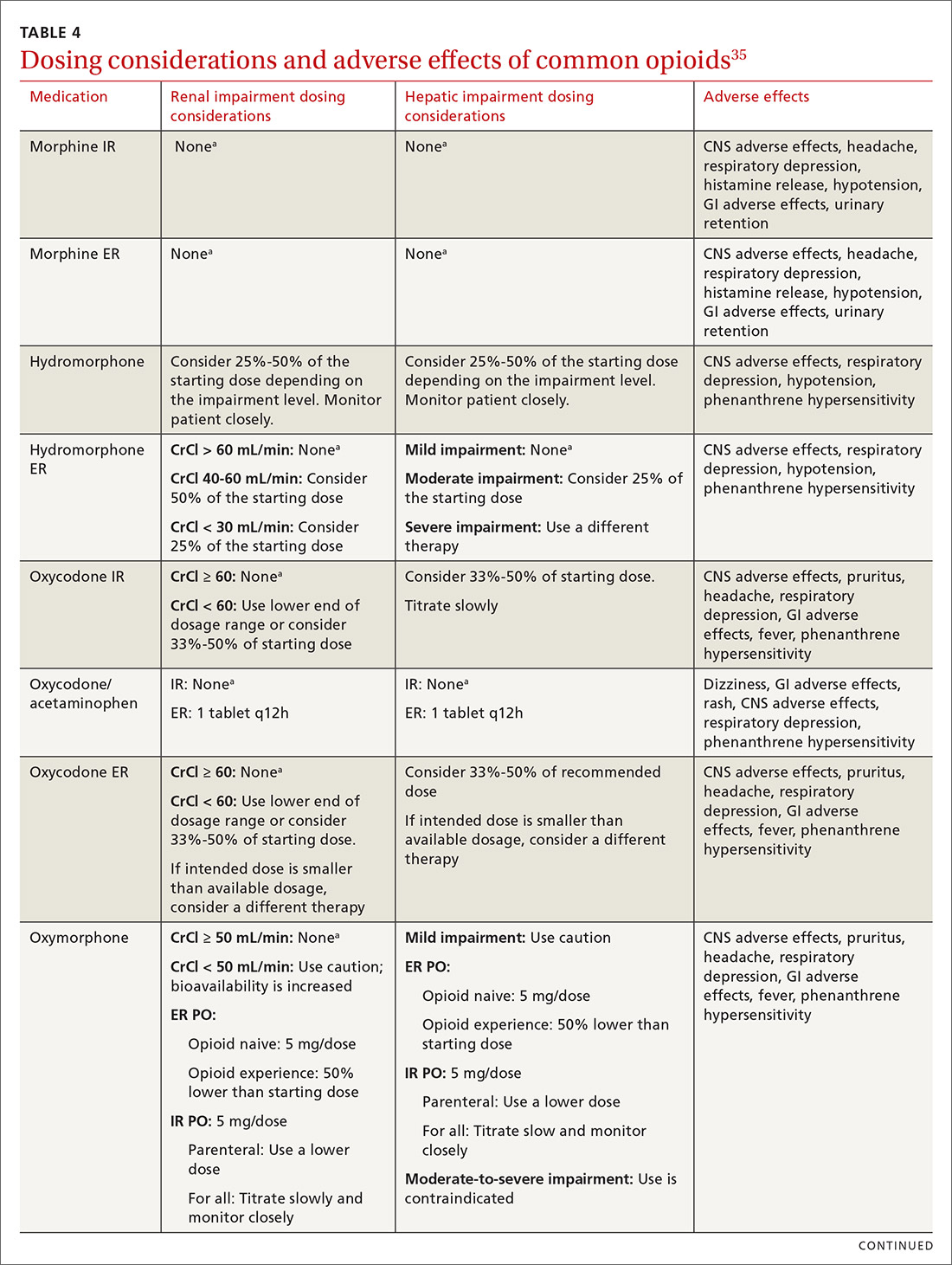
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
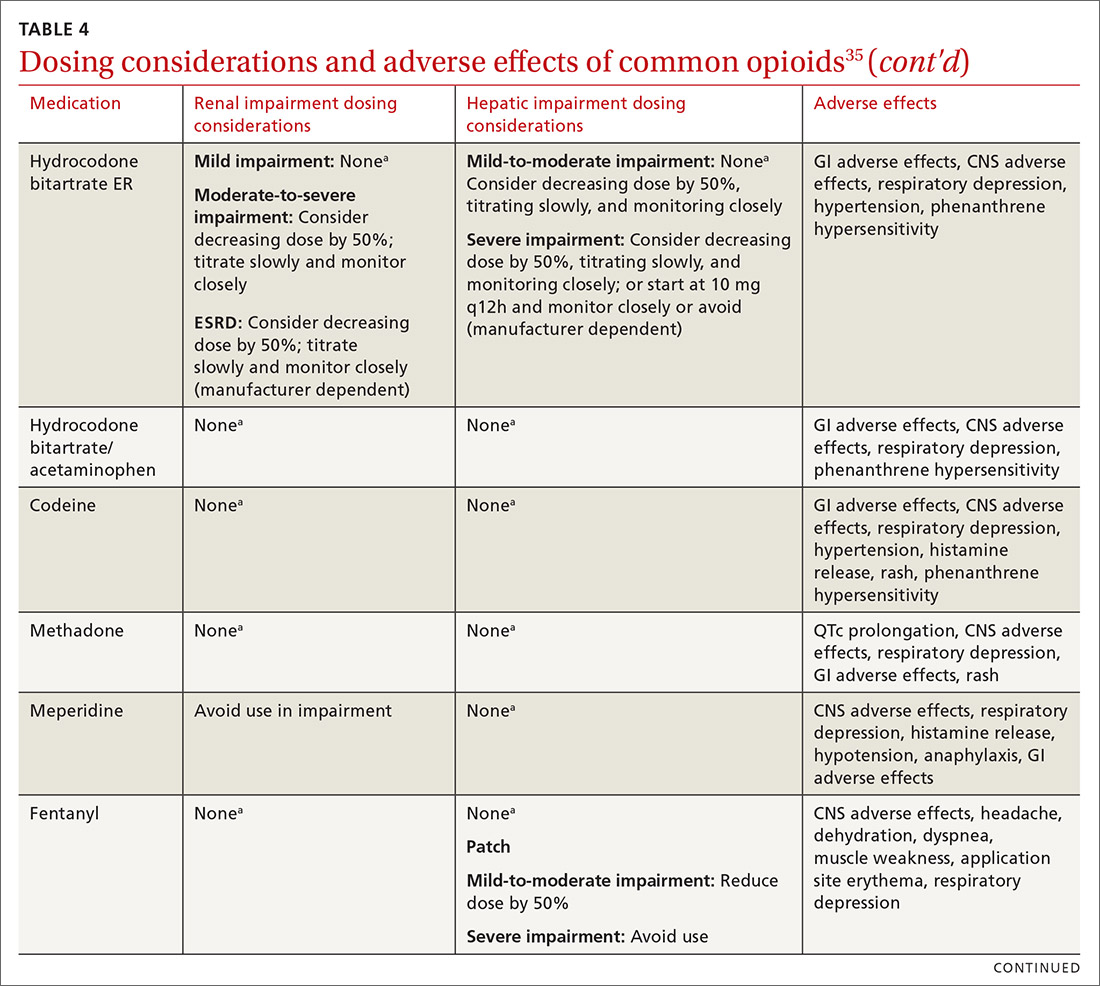
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
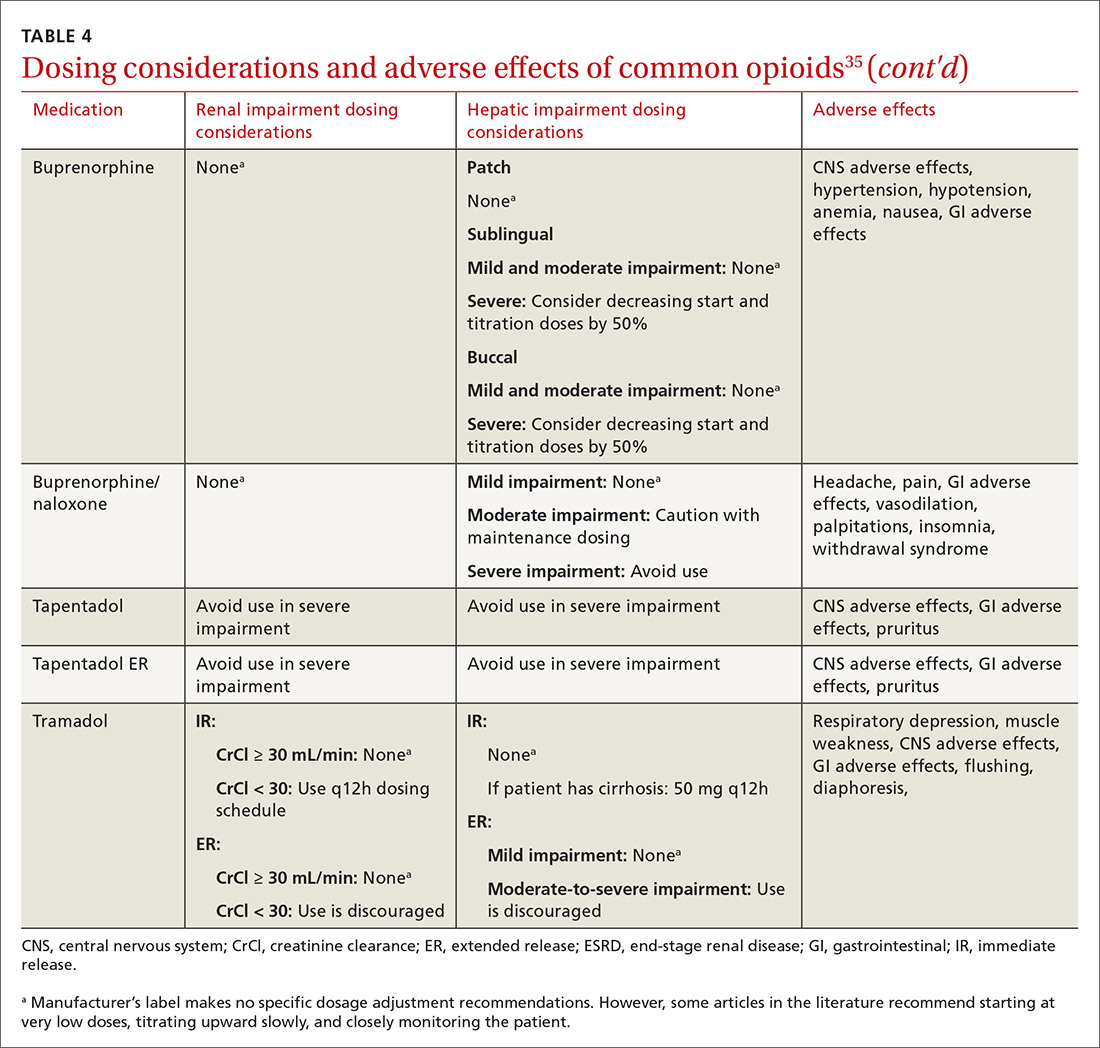
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
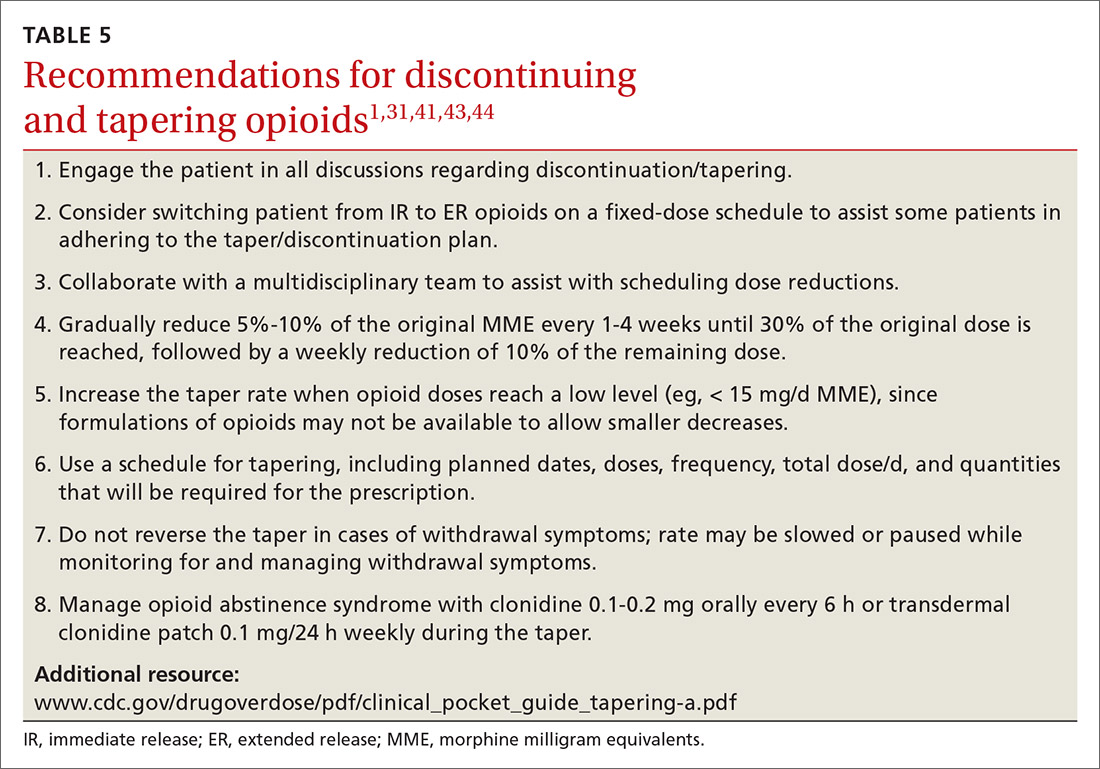
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16

Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28

CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.

Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31

Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44

General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1

The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16

Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30

Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,

At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28

CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.

Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31

Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44

General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.

CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
PRACTICE RECOMMENDATIONS
› Use a screening instrument such as the Opioid Risk Tool or the DIRE assessment to gauge a patient’s risk of opioid misuse and determine the frequency of monitoring. C
› Give as much priority to improving functional activity and minimizing adverse opioid effects as you do to relieving pain. C
› Prescribe an immediate-release, short-acting agent at first instead of a long-acting formulation; start with the lowest effective dosage and calculate total daily dose in terms of morphine milligram equivalents (MME). C
› Reduce the original MME dose by 5% to 10% every week when discontinuing an opioid. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Move over supplements, here come medical foods
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
As the Food and Drug Administration focuses on other issues, companies, both big and small, are looking to boost physician and consumer interest in their “medical foods” – products that fall somewhere between drugs and supplements and promise to mitigate symptoms, or even address underlying pathologies, of a range of diseases.
Manufacturers now market an array of medical foods, ranging from powders and capsules for Alzheimer disease to low-protein spaghetti for chronic kidney disease (CKD). The FDA has not been completely absent; it takes a narrow view of what medical conditions qualify for treatment with food products and has warned some manufacturers that their misbranded products are acting more like unapproved drugs.
By the FDA’s definition, medical food is limited to products that provide crucial therapy for patients with inborn errors of metabolism (IEM). An example is specialized baby formula for infants with phenylketonuria. Unlike supplements, medical foods are supposed to be used under the supervision of a physician. This has prompted some sales reps to turn up in the clinic, and most manufacturers have online approval forms for doctors to sign. Manufacturers, advisers, and regulators were interviewed for a closer look at this burgeoning industry.
The market
The global market for medical foods – about $18 billion in 2019 – is expected to grow steadily in the near future. It is drawing more interest, especially in Europe, where medical foods are more accepted by physicians and consumers, Meghan Donnelly, MS, RDN, said in an interview. She is a registered dietitian who conducts physician outreach in the United States for Flavis, a division of Dr. Schär. That company, based in northern Italy, started out targeting IEMs but now also sells gluten-free foods for celiac disease and low-protein foods for CKD.
It is still a niche market in the United States – and isn’t likely to ever approach the size of the supplement market, according to Marcus Charuvastra, the managing director of Targeted Medical Pharma, which markets Theramine capsules for pain management, among many other products. But it could still be a big win for a manufacturer if they get a small slice of a big market, such as for Alzheimer disease.
Defining medical food
According to an update of the Orphan Drug Act in 1988, a medical food is “a food which is formulated to be consumed or administered enterally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” The FDA issued regulations to accompany that law in 1993 but has since only issued a guidance document that is not legally binding.
Medical foods are not drugs and they are not supplements (the latter are intended only for healthy people). The FDA doesn’t require formal approval of a medical food, but, by law, the ingredients must be generally recognized as safe, and manufacturers must follow good manufacturing practices. However, the agency has taken a narrow view of what conditions require medical foods.
Policing medical foods hasn’t been a priority for the FDA, which is why there has been a proliferation of products that don’t meet the FDA’s view of the statutory definition of medical foods, according to Miriam Guggenheim, a food and drug law attorney in Washington, D.C. The FDA usually takes enforcement action when it sees a risk to the public’s health.
The agency’s stance has led to confusion – among manufacturers, physicians, consumers, and even regulators – making the market a kind of Wild West, according to Paul Hyman, a Washington, D.C.–based attorney who has represented medical food companies.
George A. Burdock, PhD, an Orlando-based regulatory consultant who has worked with medical food makers, believes the FDA will be forced to expand their narrow definition. He foresees a reconsideration of many medical food products in light of an October 2019 White House executive order prohibiting federal agencies from issuing guidance in lieu of rules.
Manufacturers and the FDA differ
One example of a product about which regulators and manufacturers differ is Theramine, which is described as “specially designed to supply the nervous system with the fuel it needs to meet the altered metabolic requirements of chronic pain and inflammatory disorders.”
It is not considered a medical food by the FDA, and the company has had numerous discussions with the agency about their diverging views, according to Mr. Charuvastra. “We’ve had our warning letters and we’ve had our sit downs, and we just had an inspection.”
Targeted Medical Pharma continues to market its products as medical foods but steers away from making any claims that they are like drugs, he said.
Confusion about medical foods has been exposed in the California Workers’ Compensation System by Leslie Wilson, PhD, and colleagues at the University of California, San Francisco. They found that physicians regularly wrote medical food prescriptions for non–FDA-approved uses and that the system reimbursed the majority of the products at a cost of $15.5 million from 2011 to 2013. More than half of these prescriptions were for Theramine.
Dr. Wilson reported that, for most products, no evidence supported effectiveness, and they were frequently mislabeled – for all 36 that were studied, submissions for reimbursement were made using a National Drug Code, an impossibility because medical foods are not drugs, and 14 were labeled “Rx only.”
Big-name companies joining in
The FDA does not keep a list of approved medical foods or manufacturers. Both small businesses and big food companies like Danone, Nestlé, and Abbott are players. Most products are sold online.

In the United States, Danone’s Nutricia division sells formulas and low-protein foods for IEMs. They also sell Ketocal, a powder or ready-to-drink liquid that is pitched as a balanced medical food to simplify and optimize the ketogenic diet for children with intractable epilepsy. Yet the FDA does not include epilepsy among the conditions that medical foods can treat.
Nestlé sells traditional medical foods for IEMs and also markets a range of what it calls nutritional therapies for such conditions as irritable bowel syndrome and dysphagia.
Nestlé is a minority shareholder in Axona, a product originally developed by Accera (Cerecin as of 2018). Jacquelyn Campo, senior director of global communications at Nestlé Health Sciences, said that the company is not actively involved in the operations management of Cerecin. However, on its website, Nestlé touts Axona, which is only available in the United States, as a “medical food” that “is intended for the clinical dietary management of mild to moderate Alzheimer disease.” The Axona site claims that the main ingredient, caprylic triglyceride, is broken down into ketones that provide fuel to treat cerebral hypometabolism, a precursor to Alzheimer disease. In a 2009 study, daily dosing of a preliminary formulation was associated with improved cognitive performance compared with placebo in patients with mild to moderate Alzheimer disease.
In 2013, the FDA warned Accera that it was misbranding Axona as a medical food and that the therapeutic claims the company was making would make the product an unapproved drug. Ms. Campo said Nestlé is aware of the agency’s warning, but added, “to our knowledge, Cerecin provided answers to the issues raised by the FDA.”
With the goal of getting drug approval, Accera went on to test a tweaked formulation in a 400-patient randomized, placebo-controlled trial called NOURISH AD that ultimately failed. Nevertheless, Axona is still marketed as a medical food. It costs about $100 for a month’s supply.
Repeated requests for comment from Cerecin were not answered. Danielle Schor, an FDA spokesperson, said the agency will not discuss the status of individual products.
More disputes and insurance coverage
Mary Ann DeMarco, executive director of sales and marketing for the Scottsdale, Ariz.–based medical food maker Primus Pharmaceuticals, said the company believes its products fit within the FDA’s medical foods rubric.
These include Fosteum Plus capsules, which it markets “for the clinical dietary management of the metabolic processes of osteopenia and osteoporosis.” The capsules contain a combination of genistein, zinc, calcium, phosphate, vitamin K2, and vitamin D. As proof of effectiveness, the company cites clinical data on some of the ingredients – not the product itself.
Primus has run afoul of the FDA before when it similarly positioned another product, called Limbrel, as a medical food for osteoarthritis. From 2007 to 2017, the FDA received 194 adverse event reports associated with Limbrel, including reports of drug-induced liver injury, pancreatitis, and hypersensitivity pneumonitis. In December 2017, the agency urged Primus to recall Limbrel, a move that it said was “necessary to protect the public health and welfare.” Primus withdrew the product but laid out a defense of Limbrel on a devoted website.
The FDA would not comment any further, said Ms. Schor. Ms. DeMarco said that Primus is working with the FDA to bring Limbrel back to market.
A lack of insurance coverage – even for approved medical foods for IEMs – has frustrated advocates, parents, and manufacturers. They are putting their weight behind the Medical Nutrition Equity Act, which would mandate public and private payer coverage of medical foods for IEMs and digestive conditions such as Crohn disease. That 2019 House bill has 56 cosponsors; there is no Senate companion bill.
“If you can get reimbursement, it really makes the market,” for Primus and the other manufacturers, Mr. Hyman said.
Primus Pharmaceuticals has launched its own campaign, Cover My Medical Foods, to enlist consumers and others to the cause.
Partnering with advocates
Although its low-protein breads, pastas, and baking products are not considered medical foods by the FDA, Dr. Schär is marketing them as such in the United States. They are trying to make a mark in CKD, according to Ms. Donnelly. She added that Dr. Schär has been successful in Europe, where nutrition therapy is more integrated in the health care system.
In 2019, Flavis and the National Kidney Foundation joined forces to raise awareness of nutritional interventions and to build enthusiasm for the Flavis products. The partnership has now ended, mostly because Flavis could no longer afford it, according to Ms. Donnelly.
“Information on diet and nutrition is the most requested subject matter from the NKF,” said Anthony Gucciardo, senior vice president of strategic partnerships at the foundation. The partnership “has never been necessarily about promoting their products per se; it’s promoting a healthy diet and really a diet specific for CKD.”
The NKF developed cobranded materials on low-protein foods for physicians and a teaching tool they could use with patients. Consumers could access nutrition information and a discount on Flavis products on a dedicated webpage. The foundation didn’t describe the low-protein products as medical foods, said Mr. Gucciardo, even if Flavis promoted them as such.
In patients with CKD, dietary management can help prevent the progression to end-stage renal disease. Although Medicare covers medical nutrition therapy – in which patients receive personalized assessments and dietary advice – uptake is abysmally low, according to a 2018 study.
Dr. Burdock thinks low-protein foods for CKD do meet the FDA’s criteria for a medical food but that the agency might not necessarily agree with him. The FDA would not comment.
Physician beware
When it comes to medical foods, the FDA has often looked the other way because the ingredients may already have been proven safe and the danger to an individual or to the public’s health is relatively low, according to Dr. Burdock and Mr. Hyman.
However, if the agency “feels that a medical food will prevent people from seeking medical care or there is potential to defraud the public, it is justified in taking action against the company,” said Dr. Burdock.
According to Dr. Wilson, the pharmacist who reported on the inappropriate medical food prescriptions in the California system, the FDA could help by creating a list of approved medical foods. Physicians should take time to learn about the difference between medical foods and supplements, she said, adding that they should also not hesitate to “question the veracity of the claims for them.”
Ms. Guggenheim believed doctors need to know that, for the most part, these are not FDA-approved products. She emphasized the importance of evaluating the products and looking at the data of their impact on a disease or condition.
“Many of these companies strongly believe that the products work and help people, so clinicians need to be very data driven,” she said.
A version of this article originally appeared on Medscape.com.
New insight into neurobehavioral effects of legalized cannabis
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Researchers have published one of the first studies to characterize the association between the consumption of legal cannabis and subsequent pharmacologic and neurobehavioral outcomes, with somewhat surprising results.
The study showed that, although cannabis consumption did not affect most short-term neurobehavioral measures, it delayed recall memory and impaired balance.
The investigation also showed that users of much more potent cannabis concentrates actually demonstrated similar or lower levels of subjective drug intoxication and short-term impairment than did their counterparts who used lower-potency forms of the cannabis flower.
“It does not appear that the potency being used matters that much,” senior investigator Kent E. Hutchison, PhD, said in an interview. “People seem to be titrating to a certain level of intoxication or a certain level of feeling high. And for some people that requires a lot of drug, and for other people not as much.”
added Dr. Hutchison, a professor of psychology and neuroscience at the University of Colorado Boulder.
The study was published online June 10 in JAMA Psychiatry.
Widespread availability, little research
Recreational cannabis is now legal in 11 states and the District of Columbia, while medical cannabis is legal in 33. However, despite growing popularity of cannabis, there is little research on its potential health and biobehavioral risks, largely because of federal restrictions on cannabis research.
Cannabis users typically consume various forms of the cannabis flower, which can boast concentrations of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (THC) of up to 30%. However, use of concentrated forms of cannabis – which are made by extracting plant cannabinoids into a different form – is increasing.
Such formulations can boast THC concentrations as high as 90%. Nevertheless, data regarding the relative risks of these higher-strength products are limited.
Previous research has shown a variety of negative short-term and long-term neurobehavioral effects associated with cannabis use, including harmful cognitive and motor effects. Extended exposure to THC may also negatively affect brain regions that are associated with the control of coordinated movement, and create brain-activation deficits in motor control regions that persist well beyond the effects of short-term intoxication.
Despite such findings, Dr. Hutchison said the existing literature on the subject does not yield a real-world view of current cannabis use because it tends to focus on low-THC products that are increasingly less common in today’s legal market.
Given such shortcomings, the investigators wanted to address persistent questions surrounding the neurobehavioral effects of legal cannabis flower products (16% or 24% THC) and cannabis concentrate products (70% or 90% THC). In doing so, they examined three primary topics:
- The association between short-term use of these products and THC plasma levels; subjective intoxication; and mood, cognitive performance, and balance
- Differences in such associations between users of cannabis flower and concentrate products
- Potential variations in these associations by THC potency
High- versus low-potency varieties
The study included 133 individuals (aged 21-70 years), who were designated as either cannabis flower users or cannabis concentrate users. Participants had all used cannabis at least four times in the previous month with no adverse reaction and were not receiving treatment for a psychotic disorder or bipolar disorder.
Participants were randomly assigned to consume either higher-potency or lower-potency products that had been purchased from a local dispensary. Flower users were randomized to purchase 3 g of either a 16% THC or 24% THC product, while concentrate users were randomized to purchase 1g of either 70% THC or 90% THC.
Participants completed a series of four assessments, one at baseline and three others at a mobile laboratory. The mobile laboratory assessments occurred before, immediately after, and one hour after participants had all consumed their cannabis ad libitum.
Of the original cohort of 133 participants, 55 flower cannabis users (mean age, 28.8 years; 46% women) and 66 concentrate cannabis users (mean age, 28.3 years; 45% women) complied with the study’s instructions and had complete data.
The study’s primary outcome measures included plasma cannabinoids, subjective drug intoxication and mood, and neurobehavioral outcomes such as attention, memory, inhibitory control, and balance.
Mixed results
With respect to cannabis concentrations, results showed that users of concentrate exhibited higher levels of both plasma THC and the active metabolite of THC (11-hydroxy-delta9-THC) across all points than did their counterparts who used cannabis flower products.
Specifically, mean plasma THC levels were 1,016 ± 1,380 mcg/ml in concentrate users and 455±503 mcg/mL in flower users after ad libitum cannabis consumption. Nevertheless, self-reported levels of intoxication were no different between users of cannabis flower or concentrate products.
Although results also showed that most neurobehavioral measures were not altered by short-term cannabis consumption, there were some notable exceptions. There was a negative linear effect with delayed verbal recall errors, suggesting poorer performance after cannabis use (F1, 203 = 32.31; P < .001).
On the other hand, investigators found a positive linear effect with inhibitory control and working memory, which actually suggests better performance after cannabis use. This finding, the researchers note, may be the result of a practice effect. Cannabis flower users performed better across all inhibitory control assessments.
The researchers also tested participants’ balance with their eyes open and closed. In the eyes-open condition, they found a trend toward impaired balance after cannabis use, though this normalized within an hour. When subjects closed their eyes, however, researchers observed a significant short-term increase in sway after cannabis use, which fell back to pre-use levels one hour after use (F1, 203 = 18.88; P < .001).
Of note, outcomes did not differ between groups according to the type of cannabis product consumed or its relative potency.
The study yielded several surprising findings, beginning with self-reported intoxication levels, which were not statistically significant between different cannabis flower and concentrate users, despite significantly different plasma THC levels between the two groups.
Dr. Hutchison explained that this may be the result of greater THC tolerance among concentrate users, THC saturation of cannabinoid receptors, or interindividual differences among users with respect to cannabis metabolism or sensitivity.
“I thought for sure that high-potency users would be much more compromised,” he said. “I guess it just goes to show we have a lot to learn about how these things work.”
Additionally, there were virtually no significant changes in acute performance after cannabis use, with the exception of delayed verbal recall. In fact, the most marked change observed in the study was the effect of cannabis on balance immediately after drug use, though these changes seemed to abate within an hour.
Nevertheless, the study highlights several potential public health implications of cannabis consumption, Dr. Hutchison added. “What happens when people with high blood concentrations decide to quit?” he asked. “Do they have trouble quitting? Do they have withdrawal symptoms?”
The long-term effects of cannabis use is another important question that still needs to be answered, he added.
Finally, Dr. Hutchison noted that, although the study showed little difference between users of cannabis flower and concentrates, study participants were all experienced users.
“There is certainly the potential for harm when a naive person uses cannabis concentrate,” he said. “Suddenly they have way more THC than they thought they were going to get, and that’s where a lot of people get into trouble with cannabis.”
Pitfalls and hurdles
In an accompanying editorial, Margaret Haney, PhD, of Columbia University Irving Medical Center, New York, explained that cannabis’ awkward position as simultaneously legal and illegal, medical and recreational, has hampered researchers’ ability to study its effects as comprehensively as they would otherwise like.
“With a federally illegal drug legalized in individual states, scientists constrained, and federal agencies somewhat silent, clinicians have none of the data that guide their decisions for other medications (eg, which indication, product, cannabinoid ratio, dose, or route of administration; what risks for individual patients [eg, pregnant, adolescent, psychiatric?]),” Dr. Haney wrote.
These pitfalls are compounded by the significant regulatory hurdles.
“The FDA is appropriately cautious about what it allows scientists to test in patients, and none of the products available in dispensaries or online have undergone the safety and manufacturing procedures needed for FDA approval,” she continued. “How then to conduct the studies so needed?”
Yet as Haney noted, giving cannabinoid researchers a Schedule I exemption may help address many of the barriers facing these scientists. Such a move, she said, would increase the number of randomized controlled trials being performed, “and thereby begin to breach the divide between the use of these products and empirical evidence.”
Dr. Hutchison has disclosed no relevant financial relationships. Dr. Haney disclosed funding from the US National Institute on Drug Abuse and from the Thompson Family Foundation Initiative. The study was funded by the NIH and Colorado Department of Public Health and Environment.
A version of this article originally appeared on Medscape.com.
Access to Pain Care From Compensation Clinics: A Relational Coordination Perspective
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
Chronic pain is common in veterans, and early engagement in pain treatment is recommended to forestall consequences of untreated pain, including depression, disability, and substance use disorders. The Veterans Health Administration (VHA) employs a stepped care model of pain treatment, with the majority of pain care based in primary care (step 1), and an array of specialty/multimodal treatment options made available at each step in the model for patients with more complex problems, or those who do not respond to more conservative interventions.1
Recognizing the need for comprehensive pain care, the US Congress passed the Comprehensive Addiction and Recovery Act, 21 USC §1521 (2016), which included provisions for VHA facilities to offer multimodal pain treatment and to report the availability of pain care options at each step in the stepped care model.2, With the passage of the Veterans Access, Choice, and Accountability Act of 2014, 38 USC §101 (2014) and now the MISSION Act of 2018, 38 USC §703 (2018) veterans whose VHA facilities are too distant, who require care unavailable at that facility, or who have to wait too long to receive care are eligible for treatment at either VHA or non-VHA facilities.3 These laws allocate the same pool of funds to both VHA and community care and thus create an incentive to engage veterans in care within the VHA network so the funds are not spent out of network.4
An opportunity to connect veterans with VHA care arises at specialized VHA Compensation and Pension (C&P) clinics during examinations that determine whether a veteran’s health conditions were caused or exacerbated by their military service. Veterans file claims with the US Department of Veterans Affairs (VA) Veterans Benefits Administration (VBA), which sends the patient to either a VHA facility or private practitioners for these examinations. Although the number of examinations conducted each year is not available, there were 274,528 veterans newly awarded compensation in fiscal year 2018, and a substantial number of the total of 4,743,108 veterans with C&P awards had reevaluation examinations for at least 1 of their conditions during that year.5 Based largely on the compensation examination results, military service records, and medical records, veterans are granted a service-connected rating for conditions deemed related to military service. A service-connection rating between 0% and 100% is assigned by the VBA, with higher ratings indicating more impairment and, consequently, more financial compensation. Service-connection ratings also are used to decide which veterans are in the highest priority groups for receipt of VHA health care services and are exempt from copayments.
Although traditionally thought of as a forensic evaluation with no clinical purpose, the C&P examination process affords many opportunities to explain VHA care to veterans in distress who file claims.6 A randomized clinical trials (RCT) involving veterans with mental health claims and a second RCT including veterans with musculoskeletal claims each found that veterans use more VHA services if offered outreach at the time of the C&P examination.7,8 In addition to clinical benefits, outreach around the time of C&P examinations also might mitigate the well documented adversarial aspects of the service-connection claims process.6,9,10 Currently, such outreach is not part of routine VHA procedures. Ironically, it is the VBA and not VHA that contacts veterans who are awarded service-connection with information about their eligibility for VHA care based on their award.
Connecting veterans to pain treatment can involve clarifying eligibility for VHA care for veterans in whom eligibility is unknown, involving primary care providers (PCPs) who are the fulcrum of VHA pain care referrals, and motivating veterans to seek specific pain treatment modalities. Connecting veterans to treatment at the time of their compensation examinations also likely involves bidirectional cooperation between the specialized C&P clinics where veterans are examined and the clinics that provide treatment.
Relational coordination is a theoretical framework that can describe the horizontal relationships between different teams within the same medical facility. Relational coordination theorizes that communication between workgroups is related synergistically to the quality of relationships between workgroups. Relational coordination is better between workgroups that share goals and often have high levels of relational coordination, which is thought to be especially important when activities are ambiguous, require cooperation, and are conducted under time pressure.11 High relational coordination also has been associated with high staff job satisfaction, high satisfaction with delivered services, and adherence to treatment guidelines.12-14 An observational cohort study suggested that relational coordination can be improved by targeted interventions that bring workgroups together and facilitate intercommunication.15
To better understand referral and engagement for pain treatment at compensation examinations, VA staff from primary care, mental health, pain management, and C&P teams at the 8 VHA medical centers in New England were invited to complete a validated relational coordination survey.11,16 A subset of invited staff participated in a semistructured interview about pain treatment referral practices within their medical centers.
Assessments were conducted as part of a mixed methods formative evaluation involving quantitative and qualitative methods for a clinical trial at the 8 VHA medical centers in New England. The trial is testing an intervention in which veterans presenting for service-connection examinations for musculoskeletal conditions receive brief counseling to engage them in nonopioid pain treatments. The VHA Central Institutional Review Board approved this formative evaluation and the clinical trial has begun (ClinicalTrials.gov NCT04062214).
Potential interviewees were involved in referrals to and provision of nonpharmacologic pain treatment and were identified by site investigators in the randomized trial. Identified interviewees were clinical and administrative staff belonging to VHA Primary Care, Pain Management, and Compensation and Pension clinics. A total of 83 staff were identified.
Semistructured Interviews
A subset of the 83 staff were invited to participate in a semistructured interview because their position impacted coordination of pain care at their facilities or they worked in C&P. Staff at a site were interviewed until no new themes emerged from additional interviews, and each of the 8 sites was represented. Interviews were conducted between June and August 2018. Standardized scripts describing the study and inviting participation in a semistructured interview were e-mailed to VA staff. At the time of the interview the study purpose was restated and consent for audiotaping was obtained. The interviews followed a guide designed to assess a relational coordination framework among various workgroups. The data in this manuscript were elicited by specific prompts concerning: (1) How veterans learn about pain care when they come through C&P; and (2) How staff in C&P communicate with treatment providers about veterans who have chronic pain. Each interview lasted about 30 minutes.
Relational Coordination Survey
All identified staff were invited to participate in a relational coordination survey. The survey was administered through VA REDCap. Survey invitations were e-mailed from REDCap to VA staff and included a description of the study and assurances of the confidentiality of data collected. Surveys took < 10 minutes to complete. To begin, respondents identified their primary workgroup (C&P, primary care, pain management, or administrative leadership or staff), secondary workgroup (if they were in > 1), and site. Respondents provided no other identifying information and were assured their responses would be confidential.
The survey consisted of 7 questions regarding beliefs about the quality of communication and interactions among workgroup members in obtaining a shared goal.11 The shared goal in the survey used in this study was providing pain care services for veterans with musculoskeletal conditions. Using a 5-point Likert scale, the 7 questions concerned frequency, timeliness, and accuracy of communication; response to problems providing pain services; sharing goals; and knowledge and respect for respondent’s job function. Higher scores indicated better relational coordination among members of a workgroup. Using the survey’s 7 items, composite mean relational coordination scores were calculated for each of the 4 primary workgroups. To account for the possibility that a member rated their own workgroups, 2 scores were created for each workgroup; one included members of the workgroup and another excluded them.
Data Analysis
The audio-recorded semistructured interviews were transcribed and entered into Atlas.ti qualitative data analysis software. To identify cross-cutting themes, a semistructured telephone interview guide was developed by the qualitative study team that emphasized interrelationships between different clinical teams. The transcripts were then analyzed using the grounded theory approach, a systematic methodology to reduce themes from collected qualitative data. Two research staff read each transcript twice; first to familiarize themselves with the text and then, using open coding, to identify important concepts that emerged from the language and assign codes to segments of text. To ensure accuracy, researchers included suitable contextual information in the coding. Using the constant comparative method, research staff then met to examine the themes that emerged in the interviews, discuss and coalesce coding discrepancies, and compare perspectives.17
The composite score (mean of the 7 items and 95% CI) of the survey responses was analyzed to identify significant differences in coordination across the 4 workgroups. Analysis of variance (ANOVA) was used to examine each relational coordination score by respondents’ workgroup. Post hoc analyses examined relational coordination survey differences among the 4 respondent groups.
Results
Thirty-nine survey respondents participated in the semistructured interviews. C&P examiners expressed varying degrees of comfort with their role in extending access to pain care for veterans. Some of the examiners strongly believed that their role was purely forensic, and going beyond this forensic role to refer or recommend treatment to veterans would be a violation of their role to conduct a forensic examination. “We don’t have an ongoing therapeutic relationship with any of the patients,” a C&P examiner explained: “We see them once; they’re out the door. It’s forensic. We’re investigating the person as a claimant, we’re investigating it and using our tools to go and review information from 30, 40 years ago.”
Other examiners had a less strict approach for working with veterans in C&P, even though examiners are asked not to provide advice or therapy. One C&P examiner noted that because he “can’t watch people in pain,” during the examination this doctor recommends that patients go to the office that determines whether they are eligible for benefits and choose a PCP. Another C&P examiner concurred with this approach. “I certainly spend a little time with the veteran talking to them about their personal life, who they are, what they do, what they’ve done, what they’re going to do to kind of break the ice between us,” the second examiner explained. “At the end, I will make some suggestions to them. I’m comfortable doing that. I don’t know that everybody is.”
Many of the VHA providers we interviewed had little knowledge of the C&P process or whether C&P examiners had any role or responsibilities in referring veterans for pain care. Most VHA providers could not name any C&P examiners at their facility and were generally unfamiliar with the content of C&P examinations. One provider bluntly said, “I’ve never communicated with anyone in comp and pen [C&P].”
Another PCP also expressed concerns with referrals, suggesting that C&P and primary care “are totally separate and should remain separate,” the PCP explained. “My concern with getting referral from comp and pen is that is it then they’re seeking all sorts of treatment that they wouldn’t necessarily need or ask for otherwise.”
Conversely a different PCP had a positive outlook on how C&P examiners might help ease the transition into the VHA for veterans with pain, especially for newly discharged veterans. “Having comp and pen address these issues is really going to be helpful. I think it could be significant that the topic is introduced early on.”
Relational Coordination Survey
Relational coordination surveys were sent to 83 participants of whom 66 responded. Respondents were from
The relational coordination composite scores were lowest for C&P. This finding remained whether C&P staff surveys were included or removed from the C&P responses. As demonstrated by the 95% CI, when team members’ surveys were included, C&P scores (95% CI, 2.01-2.42) were significantly lower than the primary care (95% CI, 3.34-3.64) and pain management (95% CI, 3.61-3.96) groups. All the relational coordination composite scores were slightly lower when staff who described their own workgroup were removed (ie, respondents rated their own workgroups as having higher relational coordination than others did). Using the composite scores excluding same workgroup members, the composite scores of the C&P remained significantly lower than all 3 other workgroups (Table). Means values for each individual item in the C&P group were significantly less than all other group means for each item except for the question on responses to problems providing pain services (data not shown). On this item only, the mean C&P rating was > 3 (3.19), but this was still lower than the means of the primary care and pain management workgroups.
Further analyses were undertaken to understand the importance of stakeholders’ ratings of their own workgroup compared with ratings by others of that workgroup. A 1-way ANOVA of workgroup was conducted and displayed significant workgroup differences between member and nonmember relational coordination ratings on 3 of the 4 workgroup’s scores C&P (F = 5.75, 3, 62 df; P < .01) primary care (F = 4.30, 3, 62 df; P < .008) and pain management (F = 8.22, 3, 62 df; P < .001). Post hoc contrasts between the different workgroups doing the rating revealed: (1) significant differences in the assessment of the C&P workgroup between the C&P workgroup and both the primary care (P < .01) and pain management groups (P < .001) with C&P rating their own workgroup significantly higher; (2) a significant difference in the scoring of the primary care workgroup with the primary care group rating themselves significantly higher than the C&P group; and (3) significant differences in the scoring of the pain management workgroup with both pain management and primary care groups rating the pain management group significantly higher than the C&P group. The results were not substantially changed by removing the 18 respondents who identified themselves as being part of > 1 workgroup .
Discussion
Mixed methods revealed disparate viewpoints about the role of C&P in referring veterans to pain care services. Overall, C&P teams coordinated less with other workgroups than the other groups coordinated with each other, and the C&P clinics took only limited steps to engage veterans in VHA treatment. The relational coordination results appeared to be valid. The mean scores were near the middle of the relational coordination rating scale, with standard deviations indicating a range of responses. The lower relational coordination scores of the C&P group remained after removing stakeholders who were rating their own workgroup. Further support for the validity of the relational coordination survey results is that they were consistent with the reports of C&P clinic isolation in the semistructured interviews.
The interview data suggest that one reason the C&P teams had low relational coordination scores is that VA staff interpret the emphasis on evaluative rather than therapeutic examinations to preclude other attempts to engage veterans into VHA treatment, even though such treatment engagement is permitted within existing guidelines. VBA referrals for examinations say nothing, either way, about engaging veterans in VHA care. The relational coordination results suggest that an intervention that might increase treatment referrals from the C&P clinics would be to explain the (existing) policy allowing for outreach around the time of compensation examinations to VHA staff so this goal is clearly agreed-upon. Another approach to facilitating treatment engagement at the C&P examination is to use other interventions that have been associated with better relational coordination such as intergroup meetings, horizontal integration more generally, and an atmosphere is which people from different backgrounds feel empowered to speak frankly to each other.15,18,19 An important linkage to forge is between C&P teams and the administrative workgroups responsible for verifying a veteran’s eligibility for VHA care and enrolling eligible veterans in VHA treatment. Having C&P clinicians who are familiar with the eligibility and treatment engagement processes would facilitate providing that information to veterans, without compromising the evaluative format of the compensation examination.
An interesting ancillary finding is that relational coordination ratings by members of 3 of the 4 workgroups were higher than ratings by other staff of that workgroup. A possible explanation for this finding is that workgroup members are more aware of the relational coordination efforts made by their own workgroup than those by other workgroups, and therefore rate their own workgroup higher. This also might be part of a broader self-aggrandizement heuristic that has been described in multiple domains.20 Staff may apply this heuristic in reporting that their staff engage in more relational coordination, reflecting the social desirability of being cooperative.
There are simple facility-level interventions that would facilitate veterans access to care such as conducting C&P examinations for potentially treatment-eligible veterans at VHA facilities (vs conducted outside VHA) and having access to materials that explain the treatment options to veterans when they check in for their compensation examinations. The approach to C&P-based treatment engagement that was successfully employed in 2 clinical trials involved having counselors not connected with the C&P clinic contact veterans around the time of their compensation examination to explain VA treatment options and motivate veterans to pursue treatment.8,9 This independent counselor approach is being evaluated in a larger study.
Limitations
These data are from a small number of VA staff evaluating veterans in a single region of the US. They do not show causation, and it is possible that relational coordination is not necessary for referrals from C&P clinics. Relational coordination might not be necessary when referral processes can be simply routinized with little need for communication.11 However, other analyses in these clinics have found that pain treatment referrals in fact are not routinized, with substantial variability within and across institutions. Another possibility is that features that have been associated with less relational coordination, such as male gender and medical specialist guild, were disproportionately present in C&P clinics compared to the other clinics.21
Conclusions
There have been public calls to improve the evaluation of service-connection claims such that this process includes approaches to engage veterans in treatment.22 Referring veterans to treatment when they come for C&P examinations will likely involve improving relational coordination between the C&P service and other parts of VHA. Nationwide, sites that integrate C&P more fully may have valuable lessons to impart about the benefits of such integration. An important step towards better relational coordination will be clarifying that engaging veterans in VHA care around the time of their C&P examinations is a facility-wide goal.
Acknowledgments
The authors thank Brian Linde and Efia James for their perspectives on C&P procedures. This work was supported by the Veterans Integrated Service Network 1 Mental Illness Research Education and Clinical Center (MIRECC) and National Institute of Health, National Center for Complementary and Integrative Health Project # 5UG3AT009758-02. (MIR, SM mPIs).
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
1. US Department Veterans Affairs, Veterans Health Administration. VHA Directive 2009-053: pain management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2019. Accessed June 18, 2020.
2. Rosenberger PH, Phillip EJ, Lee A, Kerns RD. The VHA’s national pain management strategy: implementing the stepped care model. Fed Pract. 2011;28(8):39-42.
3. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: A Qualitative Examination of Rapid Policy Implementation in the Department of Veterans Affairs. Med Care. 2017;55 Suppl 7 Suppl 1:S71-S75. doi:10.1097/MLR.0000000000000667
4. Rieselbach RE, Epperly T, Nycz G, Shin P. Community health centers could provide better outsourced primary care for veterans. J Gen Intern Med. 2019;34(1):150-153. doi:10.1007/s11606-018-4691-4
5. US Department of Veterans Affairs, Veterans Benefit Administration. VBA annual benefits report fiscal year 2018. https://www.benefits.va.gov/REPORTS/abr/docs/2018-abr.pdf. Updated March 29, 2019. Accessed June 17, 2020.
6. Rosen MI. Compensation examinations for PTSD-an opportunity for treatment? J Rehabil Res Dev. 2010;47(5):xv-xxii. doi:10.1682/jrrd.2010.04.0075
7. Rosen MI, Ablondi K, Black AC, et al. Work outcomes after benefits counseling among veterans applying for service connection for a psychiatric condition. Psychiatr Serv. 2014;65(12):1426-1432. doi:10.1176/appi.ps.201300478
8. Rosen MI, Becker WC, Black AC, Martino S, Edens EL, Kerns RD. Brief counseling for veterans with musculoskeletal disorder, risky substance use, and service connection claims. Pain Med. 2019;20(3):528-542. doi:10.1093/pm/pny071
9. Meshberg-Cohen S, DeViva JC, Rosen MI. Counseling veterans applying for service connection status for mental health conditions. Psychiatr Serv. 2017;68(4):396-399. doi:10.1176/appi.ps.201500533
10. Sayer NA, Spoont M, Nelson DB. Post-traumatic stress disorder claims from the viewpoint of veterans service officers. Mil Med. 2005;170(10):867-870. doi:10.7205/milmed.170.10.867
11. Gittell JH. Coordinating mechanisms in care provider groups: relational coordination as a mediator and input uncertainty as a moderator of performance effects. Manage Sci. 2002;48(11):1408-1426. doi: 10.1287/mnsc.48.11.1408.268
12. Havens DS, Gittell JH, Vasey J. Impact of relational coordination on nurse job satisfaction, work engagement and burnout: achieving the quadruple aim. J Nurs Adm. 2018;48(3):132-140. doi:10.1097/NNA.0000000000000587
13. Gittell JH, Logan C, Cronenwett J, et al. Impact of relational coordination on staff and patient outcomes in outpatient surgical clinics. Health Care Manage Rev. 2020;45(1):12-20. doi:10.1097/HMR.0000000000000192
14. Cramm JM, Nieboer AP. Relational coordination promotes quality of chronic care delivery in Dutch disease-management programs. Health Care Manage Rev. 2012;37(4):301-309. doi:10.1097/HMR.0b013e3182355ea4
15. Abu-Rish Blakeney E, Lavallee DC, Baik D, Pambianco S, O’Brien KD, Zierler BK. Purposeful interprofessional team intervention improves relational coordination among advanced heart failure care teams. J Interprof Care. 2019;33(5):481-489. doi:10.1080/13561820.2018.1560248
16. Valentine MA, Nembhard IM, Edmondson AC. Measuring teamwork in health care settings: a review of survey instruments. Med Care. 2015;53(4):e16-e30. doi:10.1097/MLR.0b013e31827feef6
17. Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL. Transaction Publishers; 2009.
18. Gittell JH. How interdependent parties build relational coordination to achieve their desired outcomes. Negot J. 2015;31(4):387-391. doi: 10.1111/nejo.12114
19. Solberg MT, Hansen TW, Bjørk IT. The need for predictability in coordination of ventilator treatment of newborn infants--a qualitative study. Intensive Crit Care Nurs. 2015;31(4):205-212. doi:10.1016/j.iccn.2014.12.003
20. Taylor SE, Brown JD. Illusion and well-being: a social psychological perspective on mental health. Psychol Bull. 1988;103(2):193-210.
21. Hartgerink JM, Cramm JM, Bakker TJ, van Eijsden AM, Mackenbach JP, Nieboer AP. The importance of multidisciplinary teamwork and team climate for relational coordination among teams delivering care to older patients. J Adv Nurs. 2014;70(4):791-799. doi:10.1111/jan.12233
22. Bilmes L. soldiers returning from iraq and afghanistan: the long-term costs of providing veterans medical care and disability benefits RWP07-001. https://research.hks.harvard.edu/publications/getFile.aspx?Id=237. Published January 2007. Accessed June 18, 2020.
Use of nonopioid pain meds is on the rise
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
FROM CPDD 2020