User login
A Facility-Wide Plan to Increase Access to Medication for Opioid Use Disorder in Primary Care and General Mental Health Settings
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
In the United States, opioid use disorder (OUD) is a major public health challenge. In 2018 drug overdose deaths were 4 times higher than they were in 1999.1 This increase highlights a critical need to expand treatment access. Medication for opioid use disorder (MOUD), including methadone, naltrexone, and buprenorphine, improves outcomes for patients retained in care.2 Compared with the general population, veterans, particularly those with co-occurring posttraumatic stress disorder (PTSD) or depression, are more likely to receive higher dosages of opioid medications and experience opioid-related adverse outcomes (eg, overdose, OUD).3,4 As a risk reduction strategy, patients receiving potentially dangerous full-dose agonist opioid medication who are unable to taper to safer dosages may be eligible to transition to buprenorphine.5
Buprenorphine and naltrexone can be prescribed in office-based settings or in addiction, primary care, mental health, and pain clinics. Office-based opioid treatment with buprenorphine (OBOT-B) expands access to patients who are not reached by addiction treatment programs.6,7 This is particularly true in rural settings, where addiction care services are typically scarce.8 OBOT-B prevents relapse and maintains opioid-free days and may increase patient engagement by reducing stigma and providing treatment within an existing clinical care team.9 For many patients, OBOT-B results in good retention with just medical monitoring and minimal or no ancillary addiction counseling.10,11
Successful implementation of OBOT-B has occurred through a variety of care models in selected community health care settings.8,12,13 Historically in the Veterans Health Administration (VHA), MOUD has been prescribed in substance use disorder clinics by mental health practitioners. Currently, more than 44% of veterans with OUD are on MOUD.14
The VHA has invested significant resources to improve access to MOUD. In 2018, the Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative launched, with the aim to improve access within primary care, mental health, and pain clinics.15 SCOUTT emphasizes stepped-care treatment, with patients engaging in the step of care most appropriate to their needs. Step 0 is self-directed care/self-management, including mutual support groups; step-1 environments include office-based primary care, mental health, and pain clinics; and step-2 environments are specialty care settings. Through a series of remote webinars, an in-person national 2-day conference, and external facilitation, SCOUTT engaged 18 teams representing each Veterans Integrated Service Network (VISN) across the country to assist in implementing MOUD within 2 step-1 clinics. These teams have developed several models of providing step-1 care, including an interdisciplinary team-based primary care delivery model as well as a pharmacist care manager model.16, 17
US Department of Veterans Affairs (VA) Connecticut Health Care System (VACHS), which delivers care to approximately 58,000 veterans, was chosen to be a phase 1 SCOUTT site. Though all patients in VACHS have access to specialty care step-2 clinics, including methadone and buprenorphine programs, there remained many patients not yet on MOUD who could benefit from it. Baseline data (fiscal year [FY] 2018 4th quarter), obtained through electronic health record (EHR) database dashboards indicated that 710 (56%) patients with an OUD diagnosis were not receiving MOUD. International Classification of Disease, 10th Revision codes are the foundation for VA population management dashboards, and based their data on codes for opioid abuse and opioid dependence. These tools are limited by the accuracy of coding in EHRs. Additionally, 366 patients receiving long-term opioid prescriptions were identified as moderate, high, or very high risk for overdose or death based on an algorithm that considered prescribed medications, sociodemographics, and comorbid conditions, as characterized in the VA EHR (Stratification Tool for Opioid Risk Mitigation [STORM] report).18
This article describes the VACHSquality-improvement effort to extend OBOT-B into step-1 primary care and general mental health clinics. Our objectives are to (1) outline the process for initiating SCOUTT within VACHS; (2) examine barriers to implementation and the SCOUTT team response; (3) review VACHS patient and prescriber data at baseline and 1 year after implementation; and (4) explore future implementation strategies.
SCOUTT Team
A VACHS interdisciplinary team was formed and attended the national SCOUTT kickoff conference in 2018.15 Similar to other SCOUTT teams, the team consisted of VISN leadership (in primary care, mental health, and addiction care), pharmacists, and a team of health care practitioners (HCPs) from step-2 clinics (including 2 addiction psychiatrists, and an advanced practice registered nurse, a registered nurse specializing in addiction care), and a team of HCPs from prospective step-1 clinics (including a clinical psychologist and 2 primary care physicians). An external facilitator was provided from outside the VISN who met remotely with the team to assist in facilitation. Our team met monthly, with the goal to identify local barriers and facilitators to OBOT-B and implement interventions to enhance prescribing in step-1 primary care and general mental health clinics.
Implementation Steps
The team identified multiple barriers to dissemination of OBOT-B in target clinics (Table). The 3 main barriers were limited leadership engagement in promoting OBOT-B in target clinics, inadequate number of HCPs with active X-waivered prescribing status in the targeted clinics, and the need for standardized processes and tools to facilitate prescribing and follow-up.
To address leadership engagement, the SCOUTT team held quarterly presentations of SCOUTT goals and progress on target clinic leadership calls (usually 15 minutes) and arranged a 90-minute multidisciplinary leadership summit with key leadership representation from primary care, general mental health, specialty addiction care, nursing, and pharmacy. To enhance X-waivered prescribers in target clinics, the SCOUTT team sent quarterly emails with brief education points on MOUD and links to waiver trainings. At the time of implementation, in order to prescribe buprenorphine and meet qualifications to treat OUD, prescribers were required to complete specialized training as necessitated by the Drug Addiction Treatment Act of 2000. X-waivered status can now be obtained without requiring training
The SCOUTT team advocated for X-waivered status to be incentivized by performance pay for primary care practitioners and held quarterly case-based education sessions during preexisting allotted time. The onboarding process for new waivered prescribers to navigate from waiver training to active prescribing within the EHR was standardized via development of a standard operating procedure (SOP).
The SCOUTT team also assisted in the development of standardized processes and tools for prescribing in target clinics, including implementation of a standard operating procedure regarding prescribing (both initiation of buprenorphine, and maintenance) in target clinics. This procedure specifies that target clinic HCPs prescribe for patients requiring less intensive management, and who are appropriate for office-based treatment based on specific criteria (eAppendix
Templated progress notes were created for buprenorphine initiation and buprenorphine maintenance with links to recommended laboratory tests and urine toxicology test ordering, home induction guides, prescription drug monitoring database, naloxone prescribing, and pharmacy order sets. Communication with specialty HCPs was facilitated by development of e-consultation within the EHR and instant messaging options within the local intranet. In the SCOUTT team model, the prescriber independently completed assessment/follow-up without nursing or clinical pharmacy support.
Analysis
We examined changes in MOUD receipt and prescriber characteristics at baseline (FY 2018 4th quarter) and 1 year after implementation (FY 2019 4th quarter). Patient data were extracted from the VHA Corporate Data Warehouse (CDW), which contains data from all VHA EHRs. The VA STORM, is a CDW tool that automatically flags patients prescribed opioids who are at risk for overdose and suicide. Prescriber data were obtained from the Buprenorphine/X-Waivered Provider Report, a VA Academic Detailing Service database that provides details on HCP type, X-waivered status, and prescribing by location. χ2 analyses were conducted on before and after measures when total values were available.
Results
There was a 4% increase in patients with an OUD diagnosis receiving MOUD, from 552 (44%) to 582 (48%) (P = .04), over this time. The number of waivered prescribers increased from 67 to 131, the number of prescribers of buprenorphine in a 6-month span increased from 35 to 52, and the percentage of HCPs capable of prescribing within the EHR increased from 75% to 89% (P =.01).
Initially, addiction HCPs prescribed to about 68% of patients on buprenorphine, with target clinic HCPs prescribing to 24% (with the remaining coming from other specialty HCPs). On follow-up, addiction professionals prescribed to 63%, with target clinic clincians prescribing to 32%.
Interpretation
SCOUTT team interventions succeeded in increasing the number of patients receiving MOUD, a substantial increase in waivered HCPs, an increase in the number of waivered HCPs prescribing MOUD, and an increase in the proportion of patients receiving MOUD in step-1 target clinics. It is important to note that within the quality-improvement framework and goals of our SCOUTT team that the data were not collected as part of a research study but to assess impact of our interventions. Within this framework, it is not possible to directly attribute the increase in eligible patients receiving MOUD solely to SCOUTT team interventions, as other factors may have contributed, including improved awareness of HCPs.
Summary and Future Directions
Since implementation of SCOUTT in August 2018, VACHS has identified several barriers to buprenorphine prescribing in step-1 clinics and implemented strategies to overcome them. Describing our approach will hopefully inform other large health care systems (VA or non-VA) on changes required in order to scale up implementation of OBOT-B. The VACHS SCOUTT team was successful at enhancing a ready workforce in step-1 clinics, though noted a delay in changing prescribing practice and culture.
We recommend utilizing academic detailing to work with clinics and individual HCPs to identify and overcome barriers to prescribing. Also, we recommend implementation of a nursing or clinical pharmacy collaborative care model in target step-1 clinics (rather than the HCP-driven model). A collaborative care model reflects the patient aligned care team (PACT) principle of team-based efficient care, and PACT nurses or clinical pharmacists should be able to provide the minimal quarterly follow-up of clinically stable patients on MOUD within the step-1 clinics. Templated notes for assessment, initiation, and follow-up of patients on MOUD are now available from the SCOUTT national program and should be broadly implemented to facilitate adoption of the collaborative model in target clinics. In order to accomplish a full collaborative model, the VHA would need to enhance appropriate staffing to support this model, broaden access to telehealth, and expand incentives to teams/clinicians who prescribe in these settings.
Acknowledgments/Funding
This material is based upon work supported by the US Department of Veterans Affairs (VA), Office of Mental Health and Suicide Prevention, Veterans Health Administration; the VA Health Services Research and Development (HSR&D) Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (PEC) grants #19-001. Supporting organizations had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
1. Centers for Disease Control and Prevention. Understanding the epidemic. Updated March 17, 2021. Accessed September 17, 2021. https://www.cdc.gov/drugoverdose/epidemic/index.html
2. Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. Lancet. 2019;393(10182):1760-1772. doi:10.1016/S0140-6736(18)33078-2
3. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA. 2012 Jun 20;307(23):2489]. JAMA. 2012;307(9):940-947. doi:10.1001/jama.2012.234
4. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612. doi:10.1097/AJP.0000000000000011
5. US Department of Health and Human Services, Working Group on Patient-Centered Reduction or Discontinuation of Long-term Opioid Analgesics. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of Long-term opioid analgesics. Published October 2019. Accessed September 17, 2021. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
6. Sullivan LE, Chawarski M, O’Connor PG, Schottenfeld RS, Fiellin DA. The practice of office-based buprenorphine treatment of opioid dependence: is it associated with new patients entering into treatment?. Drug Alcohol Depend. 2005;79(1):113-116. doi:10.1016/j.drugalcdep.2004.12.008
7. LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts collaborative care model in community health centers. J Subst Abuse Treat. 2016;60:6-13. doi:10.1016/j.jsat.2015.06.010
8. Rubin R. Rural veterans less likely to get medication for opioid use disorder. JAMA. 2020;323(4):300. doi:10.1001/jama.2019.21856
9. Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011;57(3):281-289.
10. Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2-5 years. Am J Addict. 2008;17(2):116-120. doi:10.1080/10550490701860971
11. Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365-374. doi:10.1056/NEJMoa055255
12. Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: real-world substance abuse treatment outcomes. Drug Alcohol Depend. 2013;131(1-2):127-135. doi:10.1016/j.drugalcdep.2012.12.008
13. Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22(2):171-176. doi:10.1007/s11606-006-0023-1
14. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: Historical perspective, lessons learned, and next steps. Subst Abus. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
15. Gordon AJ, Drexler K, Hawkins EJ, et al. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst Abus. 2020;41(3):275-282. doi:10.1080/08897077.2020.1787299
16. Codell N, Kelley AT, Jones AL, et al. Aims, development, and early results of an interdisciplinary primary care initiative to address patient vulnerabilities. Am J Drug Alcohol Abuse. 2021;47(2):160-169. doi:10.1080/00952990.2020.1832507
17. DeRonne BM, Wong KR, Schultz E, Jones E, Krebs EE. Implementation of a pharmacist care manager model to expand availability of medications for opioid use disorder. Am J Health Syst Pharm. 2021;78(4):354-359. doi:10.1093/ajhp/zxaa405
18. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
19. US Department of Defense, US Department of Veterans Affairs, Opioid Therapy for Chronic Pain Work Group. VA/DoD clinical practice guideline for opioid therapy for chronic pain. Published February 2017. Accessed August 20, 2021. https://www.va.gov/HOMELESS/nchav/resources/docs/mental-health/substance-abuse/VA_DoD-CLINICAL-PRACTICE-GUIDELINE-FOR-OPIOID-THERAPY-FOR-CHRONIC-PAIN-508.pdf
Old wives’ tales, traditional medicine, and science
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].
Opioid prescribing mapped: Alabama highest, New York lowest
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.
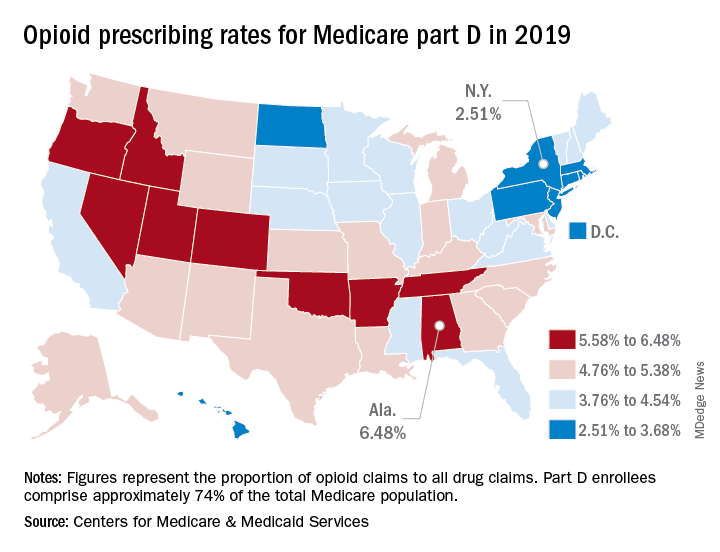
That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Scientists who unlocked secrets of pain sensation win nobel prize
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
for their discoveries of receptors for temperature and touch.
Their discoveries paved the way for new treatments for a wide range of disease conditions, including chronic pain.
“Our ability to sense heat, cold, and touch is essential for survival and underpins our interaction with the world around us,” the Nobel committee, in Stockholm, said in a news release.
“In our daily lives we take these sensations for granted, but how are nerve impulses initiated so that temperature and pressure can be perceived? This question has been solved by this year’s Nobel Prize laureates,” the committee added.
Science heats up
Dr. Julius and his collaborators used capsaicin, a pungent compound found in chili peppers that produces a burning sensation, to identify TRPV1, an ion channel activated by painful heat.
“The discovery of TRPV1 was a major breakthrough leading the way to the unravelling of additional temperature-sensing receptors,” the committee said.
Both Dr. Julius and Dr. Patapoutian used menthol to identify another receptor called TRPM8 that is activated by cold. Additional ion channels related to TRPV1 and TRPM8 were identified and found to be activated by a range of different temperatures.
The discoveries fueled other scientists to investigate the roles of these channels in thermal sensation.
“Julius’ discovery of TRPV1 was the breakthrough that allowed us to understand how differences in temperature can induce electrical signals in the nervous system,” the committee noted.
Science under pressure
As the mechanisms for temperature sensation began to unravel, Dr. Patapoutian and his collaborators used cultured pressure-sensitive cells to identify an ion channel activated by mechanical stimuli in the skin and internal organs. It was given the name Piezo1, after the Greek word for pressure.
Through its similarity to Piezo1, a second gene was discovered and named Piezo2. Sensory neurons were found to express high levels of Piezo2 and further studies firmly established that Piezo1 and Piezo2 are ion channels that are directly activated by the exertion of pressure on cell membranes.
“The groundbreaking discoveries of the TRPV1, TRPM8, and Piezo channels by this year’s Nobel Prize laureates have allowed us to understand how heat, cold, and mechanical force can initiate the nerve impulses that allow us to perceive and adapt to the world around us,” the Nobel committee said.
Dr. Julius and Dr. Patapoutian will receive a gold medal and share the $1.14 million prize money.
A version of this article first appeared on Medscape.com.
Opioid prescriptions following Mohs surgery dropped over the last decade
by 26.3% between 2009 and 2020, according to a cross-sectional analysis of national insurance claims data.
The findings suggest that dermatologic surgeons generally understood opioid prescription risks and public health warnings about the opioid epidemic, corresponding study author Surya A. Veerabagu said in an interview.
“The frequency of opioid prescriptions after Mohs surgery went up a little bit from 2009 to 2011, but then it subsequently decreased,” said Ms. Veerabagu, a 4th-year student at Tulane University, New Orleans. “It very much correlates with the overarching opioid trends of the time. From 2010 to 2015, research questioning the safety of opioids increased and in 2012, national prescriptions claims for opioids began to decrease. More media outlets voiced concerns over the growing opioid epidemic, as well.”
As she and her associates noted in their study, published online Sept. 22 in JAMA Dermatology, sales of opioids skyrocketed, increasing by 400% from 1999 to 2011, while prescription opioid–related deaths exceeded deaths caused by heroin and cocaine combined.
“In 2016, the U.S. Department of Health and Human Services declared the opioid epidemic a public health emergency, and the Centers for Disease Control and Prevention released guidelines to curtail unnecessary opioid prescriptions,” they wrote. “Unfortunately, overdose deaths involving prescription opioids continued to increase even after these measures.”
The researchers drew from Optum Clinformatics Data Mart (Optum CDM), a nationally representative insurance claims database, and limited the analysis to 358,012 adults who underwent Mohs surgery and obtained an opioid prescription within 2 days of surgery in the United States from Jan. 1, 2009, to June 1, 2020. They found that 34.6% of patients underwent Mohs surgery with opioid claims in 2009. This rose to a peak of 39.6% in 2011, then decreased annually to a rate of 11.7% in 2020.
The four opioids obtained most during the study period were hydrocodone (55%), codeine (16.3%), oxycodone (12%), and tramadol (11.6%). However, over time, the proportion of patients who obtained hydrocodone fell 21.7% from a peak of 67.1% in 2011 to 45.4% in 2020, while the proportion of patients who obtained tramadol – generally recognized as a safer option – increased 26.3% from a low of 1.6% in 2009 to 27.9% in 2020.
“The switch from very addictive opioids like hydrocodone and oxycodone to weaker opioids like tramadol was fascinating to see,” said Ms. Veerabagu, who conducted the study during her research fellowship in the department of dermatology at the University of Pennsylvania, Philadelphia. “I remember at first thinking I had coded the data wrong. I reviewed the results with the team to ensure it was correct. We noticed that propoxyphene prescriptions suddenly dropped to 0% in 2011.” She found that the FDA warning in 2010 and recall regarding the use of propoxyphene because of cardiotoxicity correlated with her data, which, “in addition to the thorough review, convinced me that my coding was correct.” Prior to 2011, propoxyphene constituted 28% of prescriptions in 2009 and 24% of prescriptions in 2010.
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard Medical School, Boston, said that the findings support recent opioid prescription recommendations following Mohs and other dermatologic procedures from professional societies including those from the American College of Mohs Surgery.
“More awareness has been raised in the past decade regarding the opioid epidemic and the rise of opioid abuse and deaths,” she said. “There has been increased scrutiny on procedures and prescribing of opioids post procedures.”
State-led efforts to lower the number of opioid prescriptions also play a role. For example, in 2016, Massachusetts launched the Massachusetts Prescription Awareness Tool (MassPAT), which imposes a 7-day limit on first-time prescriptions of opioids to patients and mandates that all prescribers check the prescription drug monitoring program before prescribing schedule II or III substances.
“The MassPAT system also gives you quarterly data on how your opioid prescriptions compare with those of your peers within your specialty and subspecialty,” Dr. Asgari said. “If you’re an outlier, I think that quickly leads you to change your prescribing patterns.”
Dr. Asgari noted that most opioids prescribed in the study by Ms. Veerabagu and colleagues were for cancers that arose on the head and neck. “There is still a perception among providers that cancers that arise in those anatomic sites can potentially cause more discomfort for the patient,” she said. “So, knowing more about the degree of pain among the head and neck cases would be an area of knowledge that would help provider behavior down the line.”
Ms. Veerabagu acknowledged certain limitations of the study, including the fact that unfilled prescriptions could not be accounted for, nor could opioids not taken or those obtained without a prescription. “We cannot survey patients in insurance claims database studies, so we have no way of knowing if everyone’s pain was adequately controlled from 2009 to 2020,” she said.
“The main takeaway message is to make sure doctors and patients share an open dialogue,” she added. “Informing patients of the major pros and cons of the appropriate postoperative pain management options available, including opioids’ addiction potential, is crucial. We hope our study adds to the larger continuing conversation of opioid usage within dermatology.”
The study’s senior author was Cerrene N. Giordano, MD, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia. Coauthor Jeremy R. Etzkorn, MD, is supported by a Dermatology Foundation Career Development Award in Dermatologic Surgery; coauthor Megan H. Noe, MD, MPH, reported receiving grants from Boehringer Ingelheim outside the submitted work. Another coauthor, Thuzar M. Shin, MD, PhD, reported receiving grants from Regeneron outside the submitted work. Dr. Asgari disclosed that she has received support from the Melanoma Research Alliance. She also contributes a chapter on skin cancer to UpToDate, for which she receives royalties.
by 26.3% between 2009 and 2020, according to a cross-sectional analysis of national insurance claims data.
The findings suggest that dermatologic surgeons generally understood opioid prescription risks and public health warnings about the opioid epidemic, corresponding study author Surya A. Veerabagu said in an interview.
“The frequency of opioid prescriptions after Mohs surgery went up a little bit from 2009 to 2011, but then it subsequently decreased,” said Ms. Veerabagu, a 4th-year student at Tulane University, New Orleans. “It very much correlates with the overarching opioid trends of the time. From 2010 to 2015, research questioning the safety of opioids increased and in 2012, national prescriptions claims for opioids began to decrease. More media outlets voiced concerns over the growing opioid epidemic, as well.”
As she and her associates noted in their study, published online Sept. 22 in JAMA Dermatology, sales of opioids skyrocketed, increasing by 400% from 1999 to 2011, while prescription opioid–related deaths exceeded deaths caused by heroin and cocaine combined.
“In 2016, the U.S. Department of Health and Human Services declared the opioid epidemic a public health emergency, and the Centers for Disease Control and Prevention released guidelines to curtail unnecessary opioid prescriptions,” they wrote. “Unfortunately, overdose deaths involving prescription opioids continued to increase even after these measures.”
The researchers drew from Optum Clinformatics Data Mart (Optum CDM), a nationally representative insurance claims database, and limited the analysis to 358,012 adults who underwent Mohs surgery and obtained an opioid prescription within 2 days of surgery in the United States from Jan. 1, 2009, to June 1, 2020. They found that 34.6% of patients underwent Mohs surgery with opioid claims in 2009. This rose to a peak of 39.6% in 2011, then decreased annually to a rate of 11.7% in 2020.
The four opioids obtained most during the study period were hydrocodone (55%), codeine (16.3%), oxycodone (12%), and tramadol (11.6%). However, over time, the proportion of patients who obtained hydrocodone fell 21.7% from a peak of 67.1% in 2011 to 45.4% in 2020, while the proportion of patients who obtained tramadol – generally recognized as a safer option – increased 26.3% from a low of 1.6% in 2009 to 27.9% in 2020.
“The switch from very addictive opioids like hydrocodone and oxycodone to weaker opioids like tramadol was fascinating to see,” said Ms. Veerabagu, who conducted the study during her research fellowship in the department of dermatology at the University of Pennsylvania, Philadelphia. “I remember at first thinking I had coded the data wrong. I reviewed the results with the team to ensure it was correct. We noticed that propoxyphene prescriptions suddenly dropped to 0% in 2011.” She found that the FDA warning in 2010 and recall regarding the use of propoxyphene because of cardiotoxicity correlated with her data, which, “in addition to the thorough review, convinced me that my coding was correct.” Prior to 2011, propoxyphene constituted 28% of prescriptions in 2009 and 24% of prescriptions in 2010.
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard Medical School, Boston, said that the findings support recent opioid prescription recommendations following Mohs and other dermatologic procedures from professional societies including those from the American College of Mohs Surgery.
“More awareness has been raised in the past decade regarding the opioid epidemic and the rise of opioid abuse and deaths,” she said. “There has been increased scrutiny on procedures and prescribing of opioids post procedures.”
State-led efforts to lower the number of opioid prescriptions also play a role. For example, in 2016, Massachusetts launched the Massachusetts Prescription Awareness Tool (MassPAT), which imposes a 7-day limit on first-time prescriptions of opioids to patients and mandates that all prescribers check the prescription drug monitoring program before prescribing schedule II or III substances.
“The MassPAT system also gives you quarterly data on how your opioid prescriptions compare with those of your peers within your specialty and subspecialty,” Dr. Asgari said. “If you’re an outlier, I think that quickly leads you to change your prescribing patterns.”
Dr. Asgari noted that most opioids prescribed in the study by Ms. Veerabagu and colleagues were for cancers that arose on the head and neck. “There is still a perception among providers that cancers that arise in those anatomic sites can potentially cause more discomfort for the patient,” she said. “So, knowing more about the degree of pain among the head and neck cases would be an area of knowledge that would help provider behavior down the line.”
Ms. Veerabagu acknowledged certain limitations of the study, including the fact that unfilled prescriptions could not be accounted for, nor could opioids not taken or those obtained without a prescription. “We cannot survey patients in insurance claims database studies, so we have no way of knowing if everyone’s pain was adequately controlled from 2009 to 2020,” she said.
“The main takeaway message is to make sure doctors and patients share an open dialogue,” she added. “Informing patients of the major pros and cons of the appropriate postoperative pain management options available, including opioids’ addiction potential, is crucial. We hope our study adds to the larger continuing conversation of opioid usage within dermatology.”
The study’s senior author was Cerrene N. Giordano, MD, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia. Coauthor Jeremy R. Etzkorn, MD, is supported by a Dermatology Foundation Career Development Award in Dermatologic Surgery; coauthor Megan H. Noe, MD, MPH, reported receiving grants from Boehringer Ingelheim outside the submitted work. Another coauthor, Thuzar M. Shin, MD, PhD, reported receiving grants from Regeneron outside the submitted work. Dr. Asgari disclosed that she has received support from the Melanoma Research Alliance. She also contributes a chapter on skin cancer to UpToDate, for which she receives royalties.
by 26.3% between 2009 and 2020, according to a cross-sectional analysis of national insurance claims data.
The findings suggest that dermatologic surgeons generally understood opioid prescription risks and public health warnings about the opioid epidemic, corresponding study author Surya A. Veerabagu said in an interview.
“The frequency of opioid prescriptions after Mohs surgery went up a little bit from 2009 to 2011, but then it subsequently decreased,” said Ms. Veerabagu, a 4th-year student at Tulane University, New Orleans. “It very much correlates with the overarching opioid trends of the time. From 2010 to 2015, research questioning the safety of opioids increased and in 2012, national prescriptions claims for opioids began to decrease. More media outlets voiced concerns over the growing opioid epidemic, as well.”
As she and her associates noted in their study, published online Sept. 22 in JAMA Dermatology, sales of opioids skyrocketed, increasing by 400% from 1999 to 2011, while prescription opioid–related deaths exceeded deaths caused by heroin and cocaine combined.
“In 2016, the U.S. Department of Health and Human Services declared the opioid epidemic a public health emergency, and the Centers for Disease Control and Prevention released guidelines to curtail unnecessary opioid prescriptions,” they wrote. “Unfortunately, overdose deaths involving prescription opioids continued to increase even after these measures.”
The researchers drew from Optum Clinformatics Data Mart (Optum CDM), a nationally representative insurance claims database, and limited the analysis to 358,012 adults who underwent Mohs surgery and obtained an opioid prescription within 2 days of surgery in the United States from Jan. 1, 2009, to June 1, 2020. They found that 34.6% of patients underwent Mohs surgery with opioid claims in 2009. This rose to a peak of 39.6% in 2011, then decreased annually to a rate of 11.7% in 2020.
The four opioids obtained most during the study period were hydrocodone (55%), codeine (16.3%), oxycodone (12%), and tramadol (11.6%). However, over time, the proportion of patients who obtained hydrocodone fell 21.7% from a peak of 67.1% in 2011 to 45.4% in 2020, while the proportion of patients who obtained tramadol – generally recognized as a safer option – increased 26.3% from a low of 1.6% in 2009 to 27.9% in 2020.
“The switch from very addictive opioids like hydrocodone and oxycodone to weaker opioids like tramadol was fascinating to see,” said Ms. Veerabagu, who conducted the study during her research fellowship in the department of dermatology at the University of Pennsylvania, Philadelphia. “I remember at first thinking I had coded the data wrong. I reviewed the results with the team to ensure it was correct. We noticed that propoxyphene prescriptions suddenly dropped to 0% in 2011.” She found that the FDA warning in 2010 and recall regarding the use of propoxyphene because of cardiotoxicity correlated with her data, which, “in addition to the thorough review, convinced me that my coding was correct.” Prior to 2011, propoxyphene constituted 28% of prescriptions in 2009 and 24% of prescriptions in 2010.
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard Medical School, Boston, said that the findings support recent opioid prescription recommendations following Mohs and other dermatologic procedures from professional societies including those from the American College of Mohs Surgery.
“More awareness has been raised in the past decade regarding the opioid epidemic and the rise of opioid abuse and deaths,” she said. “There has been increased scrutiny on procedures and prescribing of opioids post procedures.”
State-led efforts to lower the number of opioid prescriptions also play a role. For example, in 2016, Massachusetts launched the Massachusetts Prescription Awareness Tool (MassPAT), which imposes a 7-day limit on first-time prescriptions of opioids to patients and mandates that all prescribers check the prescription drug monitoring program before prescribing schedule II or III substances.
“The MassPAT system also gives you quarterly data on how your opioid prescriptions compare with those of your peers within your specialty and subspecialty,” Dr. Asgari said. “If you’re an outlier, I think that quickly leads you to change your prescribing patterns.”
Dr. Asgari noted that most opioids prescribed in the study by Ms. Veerabagu and colleagues were for cancers that arose on the head and neck. “There is still a perception among providers that cancers that arise in those anatomic sites can potentially cause more discomfort for the patient,” she said. “So, knowing more about the degree of pain among the head and neck cases would be an area of knowledge that would help provider behavior down the line.”
Ms. Veerabagu acknowledged certain limitations of the study, including the fact that unfilled prescriptions could not be accounted for, nor could opioids not taken or those obtained without a prescription. “We cannot survey patients in insurance claims database studies, so we have no way of knowing if everyone’s pain was adequately controlled from 2009 to 2020,” she said.
“The main takeaway message is to make sure doctors and patients share an open dialogue,” she added. “Informing patients of the major pros and cons of the appropriate postoperative pain management options available, including opioids’ addiction potential, is crucial. We hope our study adds to the larger continuing conversation of opioid usage within dermatology.”
The study’s senior author was Cerrene N. Giordano, MD, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia. Coauthor Jeremy R. Etzkorn, MD, is supported by a Dermatology Foundation Career Development Award in Dermatologic Surgery; coauthor Megan H. Noe, MD, MPH, reported receiving grants from Boehringer Ingelheim outside the submitted work. Another coauthor, Thuzar M. Shin, MD, PhD, reported receiving grants from Regeneron outside the submitted work. Dr. Asgari disclosed that she has received support from the Melanoma Research Alliance. She also contributes a chapter on skin cancer to UpToDate, for which she receives royalties.
FROM JAMA DERMATOLOGY
FDA okays new oral CGRP antagonist for migraine prevention
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
‘Alarming’ increase in fake pills laced with fentanyl, methamphetamine, DEA warns
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
The U.S. Drug Enforcement Administration has issued a public safety alert over an “alarming” increase in fake prescription pills laced with the synthetic opioid fentanyl or the stimulant methamphetamine.
“The United States is facing an unprecedented crisis of overdose deaths fueled by illegally manufactured fentanyl and methamphetamine,” DEA Administrator Anne Milgram said in the alert.
“Counterfeit pills that contain these dangerous and extremely addictive drugs are more lethal and more accessible than ever before. DEA is focusing resources on taking down the violent drug traffickers causing the greatest harm and posing the greatest threat to the safety and health of Americans,” Ms. Milgram said.
Criminal drug networks are mass-producing fake fentanyl- and methamphetamine-laced pills and deceptively marketing them as legitimate prescription pills, the DEA warns.
such as oxycodone (Oxycontin, Percocet), hydrocodone (Vicodin), and alprazolam (Xanax); or stimulants like amphetamines (Adderall).
The agency has seized fake pills in every U.S. state. More than 9.5 million fake pills have been seized so far this year – more than the last 2 years combined.
The number of seized counterfeit pills with fentanyl has jumped nearly 430% since 2019. DEA lab tests reveal that two out of every five pills with fentanyl contain a potentially lethal dose.
These deadly pills are widely accessible and often sold on social media and e-commerce platforms – making them available to anyone with a smartphone, including minors, the DEA warns.
More than 93,000 people died of a drug overdose in the United States last year, according to federal statistics, and fentanyl is the primary driver of this alarming increase in overdose deaths, the DEA says.
The agency has launched a “One Pill Can Kill” public awareness campaign to educate the public of the dangers of counterfeit pills purchased outside of a licensed pharmacy. These pills are “illegal, dangerous, and potentially lethal,” the DEA warns.
This alert does not apply to legitimate pharmaceutical medications prescribed by doctors and dispensed by licensed pharmacists, the DEA says.
“The legitimate prescription supply chain is not impacted. Anyone filling a prescription at a licensed pharmacy can be confident that the medications they receive are safe when taken as directed by a medical professional,” the agency says.
A version of this article first appeared on Medscape.com.
Consensus statement warns against acetaminophen use during pregnancy
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Pregnant women should use paracetamol/acetaminophen only with a medical indication and at the lowest effective dose for the shortest possible time, according to an international consensus statement published online Sept. 23 in Nature Reviews Endocrinology.
With global rates of use high and risks considered negligible, the expert panel of 13 U.S. and European authors call for focused research into how this analgesic and febrifuge may impair fetal development and lead to adverse outcomes in children. They outline several precautionary measures to be taken in the meantime.
According to first author and epidemiologist Ann Z. Bauer, ScD, a postdoctoral research fellow at the University of Massachusetts in Lowell, and colleagues, this drug is used by an estimated 65% of pregnant women in the United States, and more than 50% worldwide. It is currently the active ingredient in more than 600 prescription and nonprescription medications, including Tylenol, which historically has been deemed safe in all trimesters of pregnancy.
But a growing body of experimental and epidemiological evidence suggests prenatal exposure to paracetamol (N-acetyl-p-aminophenol, or APAP) might alter fetal development and elevate the risks of neurodevelopmental, reproductive and urogenital disorders in both sexes. Exposure in utero has been linked, for example, to potential behavioral problems in children.
The new recommendations are based on a review of experimental animal and cell-based research as well as human epidemiological data published from January 1995 to October 2020. The authors include clinicians, epidemiologists, and scientists specializing in toxicology, endocrinology, reproductive medicine and neurodevelopment.
Recommendations
Although the new guidance does not differ markedly from current advice, the authors believe stronger communication and greater awareness of risks are needed. In addition to restricting use of this medication to low doses for short periods when medically necessary, expectant mothers should receive counseling before conception or early in pregnancy. If uncertain about its use, they should consult their physicians or pharmacists.
In other recommendations, the panel said:
- The 2015 FDA Drug Safety Communication recommendations should be updated based on evaluation of all available scientific evidence.
- The European Medicines Agency Pharmacovigilance Risk Assessment Committee should review the most recent epidemiologic and experimental research and issue an updated Drug Safety Communication.
- Obstetric and gynecological associations should update their guidance after reviewing all available research.
- The Acetaminophen Awareness Coalition (“Know Your Dose” Campaign) should add standardized warnings and specifically advise pregnant women to forgo APAP unless it’s medically indicated.
- All sales of APAP-containing medications should be accompanied by recommendations specifically for use in pregnancy. This information should include warning labels on packaging, and if possible, APAP should be sold only in pharmacies (as in France).
Mechanism of action
APAP is an endocrine disruptor (Neuroscientist. 2020 Sep 11. doi: 10.1177/1073858420952046). “Chemicals that disrupt the endocrine system are concerning because they can interfere with the activity of endogenous hormones that are essential for healthy neurological, urogenital, and reproductive development,” researchers wrote.
“The precise mechanism is not clear but its toxicity is thought to be due mainly to hormone disruption,” Dr. Bauer said in an interview.
Moreover, APAP readily crosses the placenta and blood–brain barrier, and changes in APAP metabolism during pregnancy might make women and their fetuses more vulnerable to its toxic effects. For instance, the molar dose fraction of APAP converted to the oxidative metabolite N-acetyl-p-benzoquinone imine increases during pregnancy. In addition to its hepatotoxicity, this poisonous byproduct is thought to be a genotoxin that increases DNA cleavage by acting on the enzyme topoisomerase II.
Asked for her perspective on the statement, Kjersti Aagaard, MD, PhD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, called the expert panel’s statement thoughtful and comprehensive, but she urged caution in interpreting the role of acetaminophen.
The challenge in linking any commonly used medication to adverse effects and congenital defects, she said, is “teasing out an association from causation. Given the commonality of the use of acetaminophen with the relative rarity of the outcomes, it is clear that not all cases of exposure result in adverse outcomes.”
As for judicious use, she said, one would be to reduce a high fever, which can cause miscarriage, neural tube defects, and potential heart disease in adulthood. Acetaminophen is the drug of choice in this case since nonsteroidal anti-inflammatory drugs such as ibuprofen are not recommended owing to their known risks to the fetal heart.
Dr. Aagaard emphasized that while acetaminophen use is temporally associated with learning and behavioral problems, and urogenital disorders at birth in male infants such as like hypospadias, so is exposure to multiple environmental chemicals and pollutants, as well as climate change. “It would be a real mistake with real life implications if we associated any congenital disease or disorder with a commonly used medication with known benefits if the true causal link lies elsewhere.”
She said the precautionary statements fall into the time-honored therapeutic principle of first do no harm. “However, the call for research action must be undertaken earnestly and sincerely.”
According to Dr. Bauer, the statement’s essential take-home message is that “physicians should educate themselves and educate women about what we’re learning about the risks of acetaminophen in pregnancy.” Risk can be minimized by using the lowest effective dose for the shortest time and only when medically indicated. “Pregnant women should speak to their physicians about acetaminophen. It’s about empowerment and making smart decisions,” she said.
This study received no specific funding. Coauthor Dr. R.T. Mitchell is supported by a UK Research Institute fellowship.
Recognizing and treating trigger finger
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.
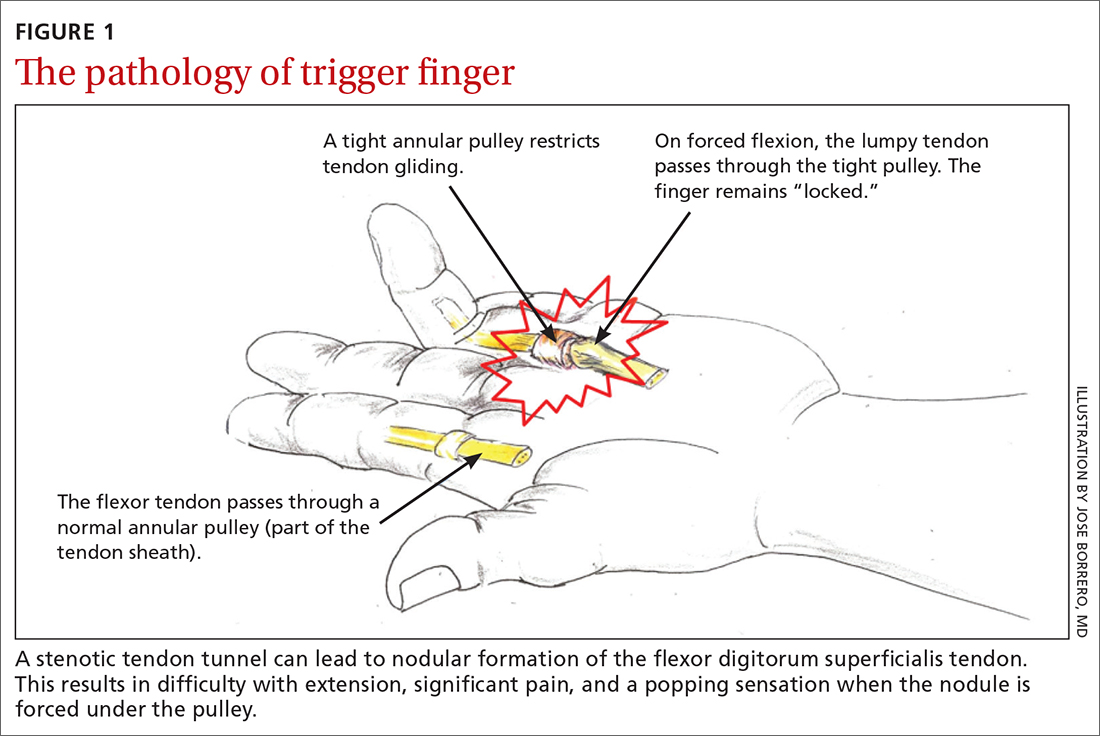
What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).
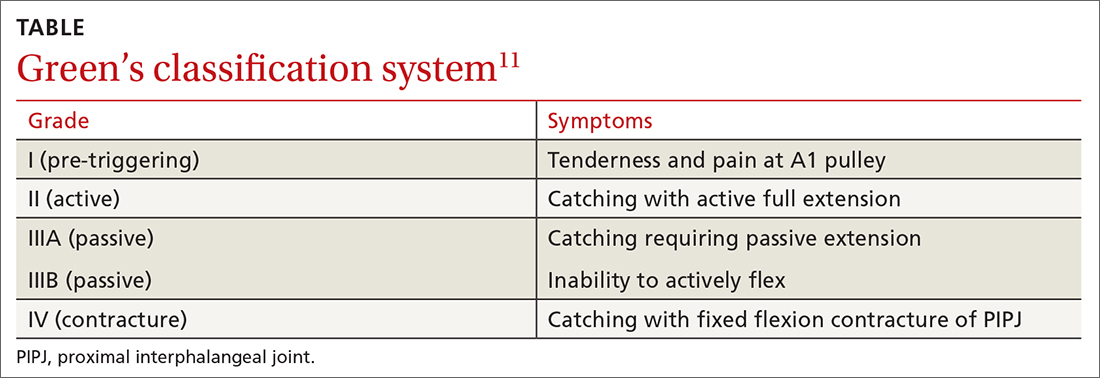
Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20
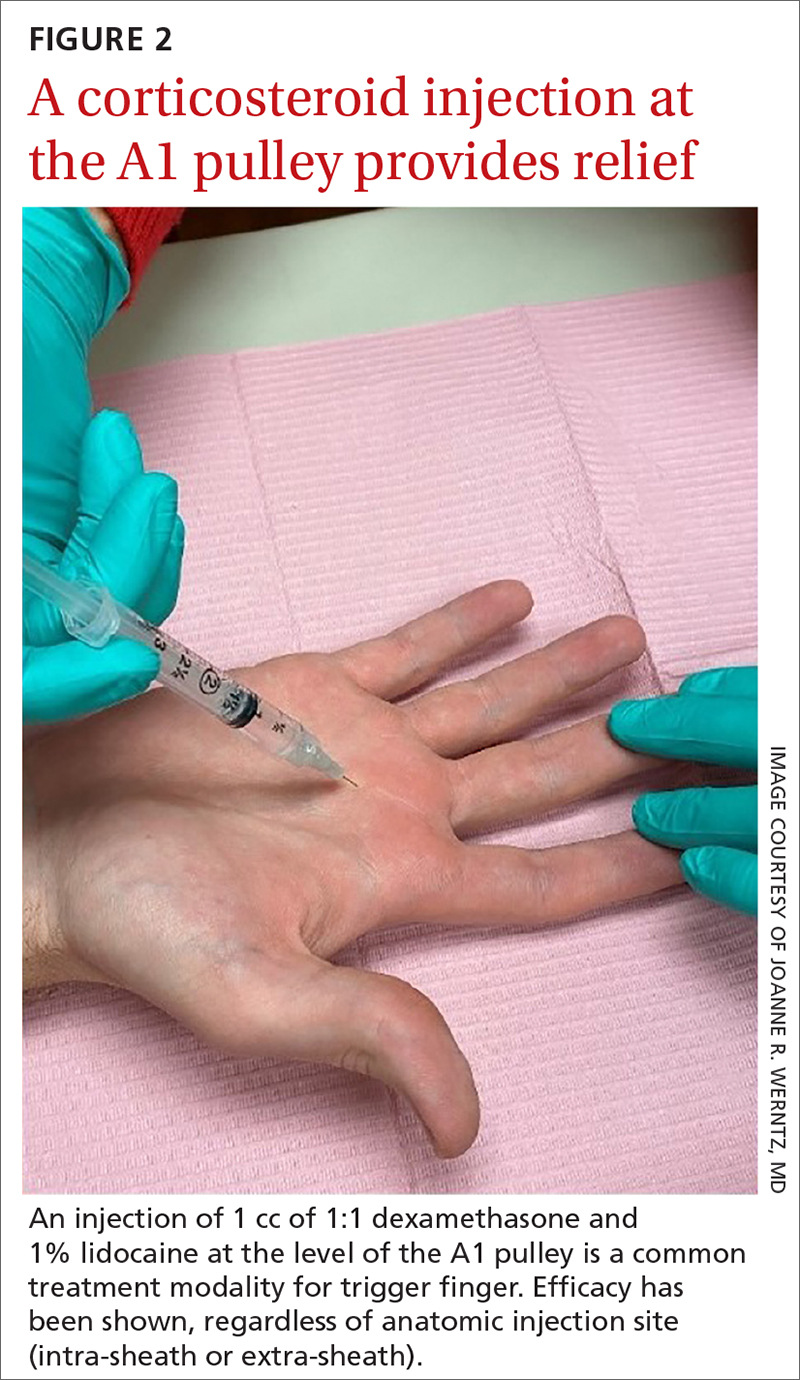
A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.
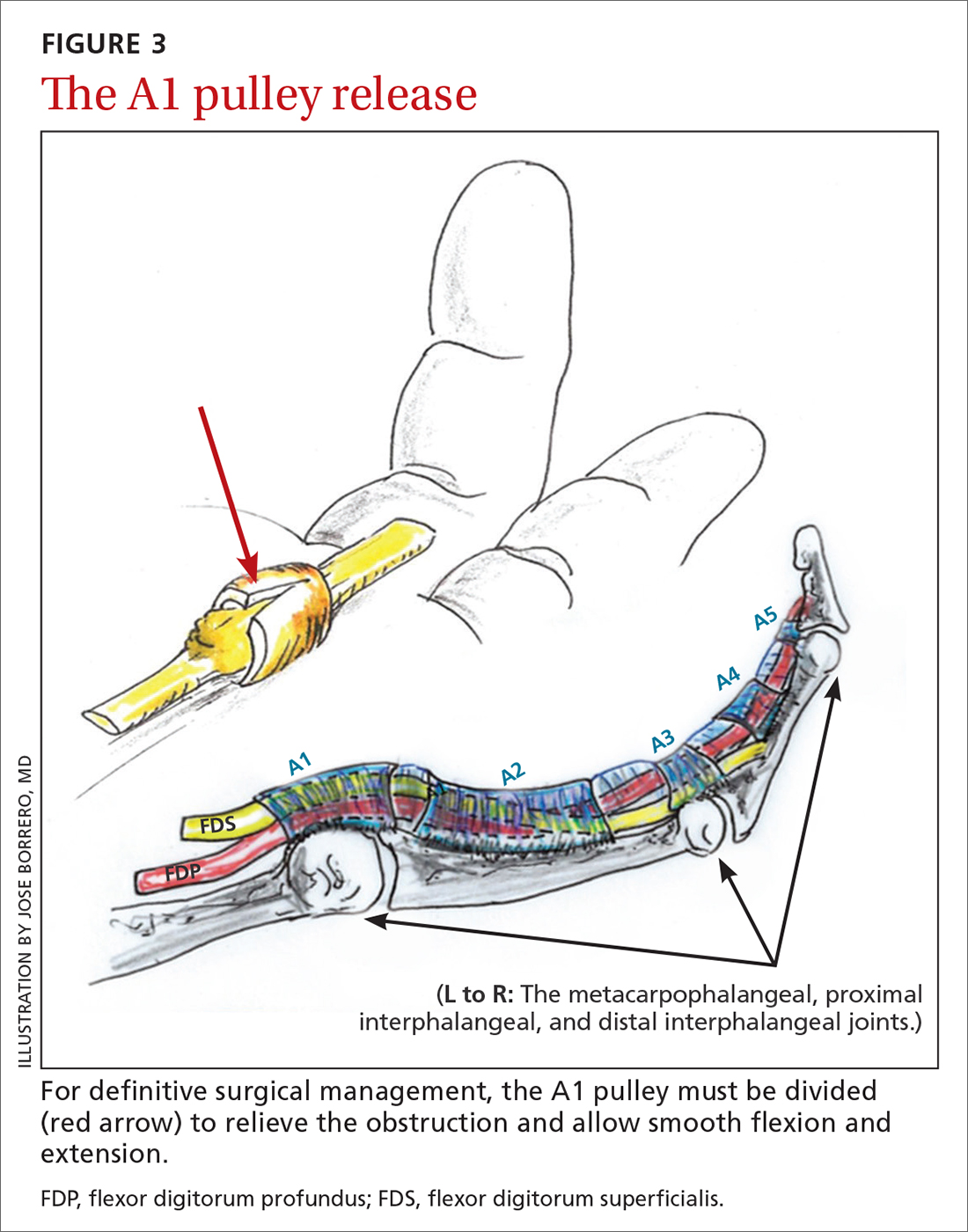
Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
PRACTICE RECOMMENDATIONS
› Recommend splinting as a first-line conservative treatment for trigger finger if there is not a fixed contracture. B
› Prescribe corticosteroids, which may completely resolve trigger finger in the majority of patients without diabetes. A
› Refer patients for surgical release if they do not respond to conservative management. The surgical success rate is as high as 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ