User login
Cannabis use: Messages remain mixed across diagnoses
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
FROM PSYCHOPHARMACOLOGY UPDATE
Good news, bad news for buprenorphine in opioid use disorder
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misuse of buprenorphine in the United States by patients with opioid use disorder (OUD) dropped sharply between 2015 and 2019, new research shows.
Analyses of data from the National Survey on Drug Use and Health also showed that about 50% of the patients with OUD were not receiving substance use treatment – and that some may be misusing buprenorphine in an effort to self-treat their addiction.
Interestingly, there was no association between buprenorphine misuse and income among those with OUD or with race, ethnicity, or insurance status regardless of OUD status, which bucks commonly held perceptions of those with the disorder.
Overall, the findings “underscore the need to pursue actions that expand access to buprenorphine-based OUD treatment, to develop strategies to monitor and reduce buprenorphine misuse, and to address associated conditions,” the investigators, led by Beth Han, MD, PhD, National Institute on Drug Abuse (NIDA), write.
The study was published online October 15 in JAMA Network Open.
Opioid deaths
Centers for Disease Control and Prevention data Of those deaths, 69,710 involved opioids.
Buprenorphine, a medication approved by the U.S. Food and Drug Administration to treat OUD, has been shown to reduce opioid cravings and withdrawal symptoms and lower overdose risk.
The new survey included responses from 214,505 adults. Of these, 51.7% were women, 45.5% were age 50 years or older, and 63.9% were non-Hispanic White.
Responses were collected between 2015-2019 as part of an annual survey administered annually by the Substance Abuse and Mental Health Services Administration.
Misuse was defined as any use outside the prescribed amount, frequency, duration, or indication.
In 2019, hydrocodone, oxycodone, codeine, and tramadol were the most misused prescription opioid products. An estimated 2.4 million adults used buprenorphine, with 1.7 million reporting no misuse in the past 12 months.
While buprenorphine misuse was stable between 2015 and 2019 among individuals without OUD, misuse declined significantly among those with OUD – from 20.5% in 2015 to 15.9% in 2019 (P = .04).
A different picture of misuse
The demographic data reveals a picture of buprenorphine misuse that researchers note is quite different from common perceptions about people with substance use.
Those with OUD who misused buprenorphine were more likely to be non-Hispanic White (82.9% vs. 73.6%, respectively) and less likely to live in large metropolitan areas (47.7% vs. 58.1%).
Among participants with OUD, buprenorphine misuse was significantly associated with age, especially in those between 24 and 34 years (adjusted odds ratio [aOR], 2.9; 95% confidence interval, 1.4-5.8) and between 35 and 49 years (aOR, 2.3; 95% CI, 1.2-4.5).
It was also significantly associated with living in nonmetropolitan areas (aOR, 1.8; 95% CI, 1.0-3.0) and having past-year polysubstance use and use disorders (aOR, 3.9; 95% CI, 1.3-11.2); but negatively associated with past-year treatment for illicit drug use–only treatment (aOR, 0.4; 95% CI, 0.3-0.7).
There was no significant association between buprenorphine misuse and income in participants with OUD or with race, ethnicity, or insurance status, regardless of OUD status.
“Perceptions that persons of racial and ethnic minority groups and people living in poverty are more likely to misuse their medication are incorrect,” the researchers write.
“Nevertheless, these factors have been found to be important factors associated with opioid harms and receipt of buprenorphine treatment,” they add.
Between 2015 and 2017, the largest increase in opioid-related drug overdose deaths was among Black people aged 25 to 34, and the largest increase involving synthetic opioids was among Hispanic individuals aged 45 to 54. At the same time, White people were more likely to receive buprenorphine treatment for OUD.
‘Don’t exaggerate concerns’
Among survey participants with OUD, 57% of those who had misused buprenorphine in the past year had received no substance use treatment. Among those with OUD who had not misused the drug in the past year, 49% had received no treatment for their addiction.
The most common reason for buprenorphine misuse cited by those with OUD was “because I am hooked” (27.3%), which researchers said suggests people may be taking buprenorphine without a prescription to self-treat their OUD.
The investigators note that although buprenorphine is inexpensive and effective, clinicians currently must receive a federal waiver to prescribe it to more than 30 patients at a time.
Concern over potential misuse may be one reason some clinicians have been reluctant to complete the training process. However, the study results showed misuse rates of other opioids, including oxycodone and hydrocodone, were higher than those reported for buprenorphine.
“Many other prescription opioids are misused at much higher rates,” co-investigator Wilson Compton, MD, MPE, deputy director of NIDA, told this news organization.
“While there are concerns about all of them, we want to make sure that people don’t exaggerate the concerns – and understanding that oxycodone and hydrocodone are so much more frequently misused is important,” added Dr. Compton.
Symptom of inadequate access?
Commenting on the research, Bobby Mukkamala, MD, chair of the American Medical Association Board of Trustees, said individuals who misuse buprenorphine “commonly do so to alleviate uncontrolled pain or symptoms of withdrawal.”
“So-called misuse of buprenorphine is a symptom of inadequate access to physicians to treat opioid use disorder,” said Dr. Mukkamala, who also chairs the AMA Substance Use and Pain Care Task Force.
A 2020 study from the U.S. Department of Health & Human Services showed 40% of U.S. counties have no clinicians with a federal waiver permitting them to prescribe buprenorphine in an office setting.
In April, the HHS released new practice guidelines that allow certain practitioners licensed under state law who have a valid Drug Enforcement Administration registration to treat up to 30 patients with buprenorphine without having to complete requirements related to training, counseling, and other ancillary services known as an “X-waiver.”
The move was welcomed by many in the field, but Dr. Mukkamala said the agency did not go far enough.
“The AMA supports removing the federal X-waiver requirement to help destigmatize the provision of buprenorphine as well as remove the many administrative barriers that come with the federal requirement,” he said.
The study was funded by the National Institute on Drug Abuse. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
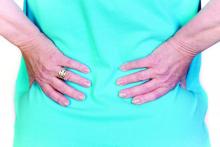
Is exercise therapy effective treatment for low back pain?
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
EVIDENCE SUMMARY
General exercise offers benefit …at least for chronic LBP
A 2017 systematic review of 4 systematic reviews and 50 RCTs (122 total trials) evaluated general exercise vs usual care for acute (< 4 weeks), subacute (4 to 12 weeks), or chronic (≥ 12 weeks) LBP with or without radiculopathy in adults.1 Exercise was not consistently associated with decreased pain in acute or subacute LBP. For chronic LBP, 3 RCTs (n = 200) associated exercise with decreased pain (weighted mean difference [WMD] = –9.2 on a 0-100 point visual acuity scale; 95% CI, –16.0 to –2.4) and improved function (WMD = –12.4 on the Oswestry Disability Index; 95% CI, –23.0 to –1.7) at short-term follow-up (≤ 3 months). This effect was found to decrease at long-term (≥ 1 year) follow-up (WMD for pain = –4.9; 95% CI, –10.5 to 0.6 and WMD for function = –3.2; 95% CI, 6.0 to –0.4). In a meta-analysis of 10 studies (n = 1992) included in this systematic review, exercise was associated with a lower likelihood of work disability (odds ratio, 0.66; CI, 0.48 to 0.92) at 12 months.1
Yoga, Pilates, and motor control exercise: Your results may vary
Several reviews have explored the effects of specific exercise modalities on LBP. A 2017 meta-analysis of 9 RCTs in the United States, United Kingdom, and India of nonpregnant adults (≥ 18 years old) with chronic LBP (N = 810) found that yoga (any tradition of yoga with a physical component) vs no exercise demonstrated a statistically, but not clinically, significant decrease in pain at 3 to 4 months (mean difference [MD] = –4.6 on a 0-100 point scale; 95% CI, –7.0 to –2.1), 6 months (MD = –7.8; 95% CI, –13.4 to –2.3), and 12 months (MD = –5.4; 95% CI, –14.5 to –3.7). Clinically significant pain benefit was considered a change of 15 or more points.2
A 2015 meta-analysis of RCTs (10 trials; N = 510) comparing the effects of Pilates (a form of body conditioning involving isometric contractions and core exercises focusing on stability) vs minimal intervention on chronic (> 12 weeks) LBP in nonpregnant adults (≥ 16 years old) found low-quality evidence for decreased pain at short-term follow-up (≤ 3 months; MD = –14.1 on a 0-100 point scale; 95% CI, –18.9 to –9.2). There was moderate-quality evidence for decreased pain at intermediate follow-up (3-12 months; MD = –10.5; 95% CI, –18.5 to –2.6).3
A 2016 systematic review evaluated motor control exercise (MCE; a form of exercise that focuses on trunk muscle control and coordination) in adults (≥ 16 years old) with chronic LBP (≥ 12 weeks). There was low- to moderate-quality evidence that, compared to minimal intervention, MCE decreases pain at short-term (≤ 6 months; 4 RCTs; MD = –10.0 on a 0-100 point scale; 95% CI, –15.7 to –4.4), intermediate (6-12 months; 4 RCTs; MD = –12.6; 95% CI, –20.5 to –4.7), and long-term follow-up (> 12 months; 3 RCTs; MD = –13.0; 95% CI, –18.5 to –7.4). When comparing MCE to general exercise, there were no clinically significant differences in pain or disability at intermediate and long-term follow-up.4Common limitations included heterogeneity of intervention methodology, inability to blind results, inability to assess cointerventions, and in some cases, small sample sizes of trials.
Recommendations from others
The 2017 American College of Physicians (ACP) clinical practice guideline on noninvasive treatments for LBP does not recommend exercise therapy in acute or subacute LBP; recommended therapies include superficial heat, massage, acupuncture, or spinal manipulation.5 The ACP recommends general exercise, yoga, tai chi, or MCE for chronic LBP, in addition to multidisciplinary rehabilitation, acupuncture, mindfulness-based stress reduction, progressive relaxation, biofeedback, laser therapy, operant therapy, cognitive behavioral therapy, or spinal manipulation.
The 2017 US Department of Veterans Affairs and US Department of Defense clinical practice guideline on treatment of LBP notes insufficient evidence for benefit of clinician-guided exercise therapy in acute LBP.6 For chronic LBP, clinician-directed exercise, yoga, tai chi, or Pilates is recommended.
Editor’s takeaway
Convincing evidence demonstrates that exercise modestly improves chronic LBP—but only modestly (4% to 15%), and not in acute LBP. This small magnitude of effect may disappoint expectations, but exercise remains among our better interventions for this common chronic problem. Few—if any—interventions have proven better, and exercise has beneficial side effects, a low cost, and widespread availability.
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
1. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-506. doi: 10.7326/M16-2459
2. Wieland LS, Skoetz N, Pilkington K, et al. Yoga treatment for chronic non-specific low back pain (review). Cochrane Database Syst Rev. 2017;1:CD010671. doi: 10.1002/14651858.CD010671.pub2
3. Yamato TP, Maher CG, Saragiotto BT, et al. Pilates for low back pain. Cochrane Database Syst Rev. 2015;7:CD010265. doi: 10.1002/14651858.CD010265.pub2
4. Saragiotto BT, Maher CG, Yamato TP, et. al. Motor control exercise for chronic non‐specific low‐back pain. Cochrane Database Syst Rev. 2016;1:CD012004. doi: 10.1002/14651858.CD012004
5. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530. doi: 10.7326/M16-2367
6. Pangarkar SS, Kang DG, Sandbrink F, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34:2620-2629. doi: 10.1007/s11606-019-05086-4
EVIDENCE-BASED ANSWER:
Yes, it is somewhat effective. Exercise therapy—including general exercise, yoga, Pilates, and motor control exercise—has been shown to modestly decrease pain in chronic low back pain (LBP); levels of benefit in short- (≤ 3 months) and long- (≥ 1 year) term follow-up range from 4% to 15% improvement (strength of recommendation [SOR] A, based on a systematic review of randomized controlled trials [RCTs]).
Exercise therapy may improve function and decrease work disability in subacute and chronic LBP, respectively (SOR A, based on a meta-analysis of RCTs). Exercise therapy has not been associated with improvement in acute LBP (SOR A, based on a meta-analysis of RCTs).
DIY nerve stimulation effective in episodic migraine
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
results from a phase 3 study show.
This is great news for headache patients who want to explore nondrug treatment options, said study investigator Deena E. Kuruvilla, MD, neurologist and headache specialist at the Westport Headache Institute, Connecticut.
She added that such devices “aren’t always part of the conversation when we’re discussing preventive and acute treatments with our patients. Making this a regular part of the conversation might be helpful to patients.”
The findings were presented at ANA 2021: 146th Annual Meeting of the American Neurological Association (ANA), which was held online.
A key therapeutic target
The randomized, double-blind trial compared E-TNS with sham stimulation for the acute treatment of migraine.
The E-TNS device (Verum Cefaly Abortive Program) stimulates the supraorbital nerve in the forehead. “This nerve is a branch of the trigeminal nerve, which is thought to be the key player in migraine pathophysiology,” Dr. Kuruvilla noted.
The device has been cleared by the U.S. Food and Drug Administration for acute and preventive treatment of migraine.
During a run-in period before randomization, patients were asked to keep a detailed headache diary and to become comfortable using the trial device to treat an acute migraine attack at home.
The study enrolled 538 adult patients at 10 centers. The patients were aged 18 to 65 years, and they had been having episodic migraines, with or without aura, for at least a year. The participants had to have received a migraine diagnosis before age 50, and they had to be experiencing an attack of migraine 2 to 8 days per month.
The patients used the device only for a migraine of at least moderate intensity that was accompanied by at least one migraine-associated symptom, such as photophobia, phonophobia, or nausea. They were asked not to take rescue medication prior to or during a therapy session.
Study participants applied either neurostimulation or sham stimulation for a continuous 2-hour period within 4 hours of a migraine attack over the 2-month study period.
The two primary endpoints were pain freedom and freedom from the most bothersome migraine-associated symptoms at 2 hours.
Compared to sham treatment, active stimulation was more effective in achieving pain freedom (P = .043) and freedom from the most bothersome migraine-associated symptom (P = .001) at 2 hours.
“So the study did meet both primary endpoints with statistical significance,” said Dr. Kuruvilla.
The five secondary endpoints included pain relief at 2 hours; absence of all migraine-associated symptoms at 2 hours; use of rescue medication within 24 hours; sustained pain freedom at 24 hours; and sustained pain relief at 24 hours.
All but one of these endpoints reached statistical significance, showing superiority for the active intervention. The only exception was in regard to use of rescue medication.
The most common adverse event (AE) was forehead paresthesia, discomfort, or burning, which was more common in the active-treatment group than in the sham-treatment group (P = .009). There were four cases of nausea or vomiting in the active-treatment group and none in the sham-treatment group. There were no serious AEs.
Available over the counter
Both moderators of the headache poster tour that featured this study – Justin C. McArthur, MBBS, from Johns Hopkins University, Baltimore, and Steven Galetta, MD, from NYU Grossman School of Medicine – praised the presentation.
Dr. Galetta questioned whether patients were receiving preventive therapies. Dr. Kuruvilla said that the patients were allowed to enter the trial while taking preventive therapies, including antiepileptic treatments, blood pressure medications, and antidepressants, but that they had to be receiving stable doses.
The investigators didn’t distinguish between participants who were taking preventive therapies and those who weren’t, she said. “The aim was really to look at acute treatment for migraine,” and patients taking such medication “had been stable on their regimen for a pretty prolonged period of time.”
Dr. McArthur asked about the origin of the nausea some patients experienced.
It was difficult to determine whether the nausea was an aspect of an individual patient’s migraine attack or was an effect of the stimulation, said Dr. Kuruvilla. She noted that some patients found the vibrating sensation from the device uncomfortable and that nausea could be associated with pain at the site.
The device costs $300 to $400 (U.S.) and is available over the counter.
Dr. Kuruvilla is a consultant for Cefaly, Neurolief, Theranica, Now What Media, and Kx Advisors. She is on the speakers bureau for AbbVie/Allergan, Amgen/Novartis, Lilly, the American Headache Society, Biohaven, and CME meeting, and she is on an advisory board at AbbVie/Allergan, Lilly, Theranica, and Amgen/Novartis. She is editor and associate editor of Healthline and is an author for WebMD/Medscape, Healthline.
A version of this article first appeared on Medscape.com.
FROM ANA
FDA approves combo pill for severe, acute pain
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
enough to require an opioid analgesic and for which alternative treatments fail to provide adequate pain relief.
Celecoxib is a nonsteroidal anti-inflammatory drug and tramadol is an opioid agonist. Seglentis contains 56 mg of celecoxib and 44 mg of tramadol.
“The unique co-crystal formulation of Seglentis provides effective pain relief via a multimodal approach,” Craig A. Sponseller, MD, chief medical officer of Kowa Pharmaceuticals America, said in a news release.
Esteve Pharmaceuticals has entered into an agreement with Kowa Pharmaceuticals America to commercialize the pain medicine in the United States, with a launch planned for early 2022.
“Seglentis uses four different and complementary mechanisms of analgesia and offers healthcare providers an important option to treat acute pain in adults that is severe enough to require opioid treatment and for which alternative treatments are inadequate,” Dr. Sponseller said.
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, the FDA will require a Risk Evaluation and Mitigation Strategy (REMS) for Seglentis.
The label states that the drug should be initiated as two tablets every 12 hours as needed and should be prescribed for the shortest duration consistent with individual patient treatment goals.
Patients should be monitored for respiratory depression, especially within the first 24 to 72 hours of initiating therapy with Seglentis.
Prescribers should discuss naloxone (Narcan) with patients and consider prescribing the opioid antagonist naloxone based on the patient’s risk factors for overdose.
Full prescribing information is available online.
A version of this article was first published on Medscape.com.
Tramadol linked to higher risk of mortality, compared with codeine
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
Tramadol is increasingly used to manage chronic noncancer pain, but as compared with opioids, it appears to be linked to a higher risk for adverse outcomes, according to new data.
Among a cohort of patients who received a prescription for either tramadol or codeine for orthopedic-related pain, tramadol was significantly associated with a higher risk of mortality, cardiovascular events, and fractures.
However, there was no significant difference in the risk of falls, delirium, constipation, opioid abuse/dependence, or sleep disorders between the two drugs.
“However, this is a retrospective cohort study, and despite it providing information that would otherwise be impossible to gather – such as from randomized controlled trials – clinicians should not solely base their decision on this study,” cautioned lead author Carlen Reyes, MD, PhD, of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol), Barcelona.
Dr. Reyes noted that the intake of tramadol and codeine was analyzed using the number of “packages” that were dispensed, as an approximation of the real intake. “Logically we could think that the more packages dispensed of one drug, the more dose the patient is taking, but this is not always true given the availability of different doses commercialized of tramadol and different doses prescribed,” she said. “Given that we did not account for the real dose prescribed, we can only suspect an increased risk of these outcomes and reinforce the need for further prospective studies with more specific dose-response analysis comparing tramadol and codeine.”
The paper was published Oct. 19 in JAMA.
Tramadol has been considered to be a relatively safe opioid and was even strongly recommended by the American Academy of Orthopaedic Surgeons for patients experiencing symptomatic knee osteoarthritis. The authors point out that studies looking at opioid use from 2019 to 2020 show that tramadol was the most prescribed opioid in England, the Netherlands, and Spain.
In the United States, the age-adjusted rate of drug overdose deaths from synthetic opioids rose from 1.0 per 100 000 in 2013 to 11.4 in 2019. Most of these deaths were attributable to fentanyl but some were also related to tramadol.
But despite its wide use in managing chronic noncancer pain, results of recent studies suggest adverse outcomes as compared with other agents. Last year, one study found that older patients who received tramadol had a significant increase in the risk of hip fracture vs. those using NSAIDs or codeine. Another study, also published in 2020, showed that patients with osteoarthritis who were treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs.
In the current paper, Dr. Reyes and colleagues evaluated the association of tramadol with mortality and other adverse clinical outcomes in outpatient settings, compared with codeine.
They conducted a retrospective, population-based, propensity score–matched cohort study using a primary care database that routinely collects medical records and pharmacy dispensations for more than 80% of the population of Catalonia, Spain. The cohort included people 18 years or older who had been prescribed tramadol or codeine from 2007 to 2017 and were followed up to Dec. 31, 2017.
After propensity score matching, the final analysis included 368,960 participants: 184,480 in the tramadol arm and 184,480 in the codeine arm.
The mean age of patients was 52.7 years in the tramadol arm and 53.5 years in the codeine arm, and the prevalence of cancer was 3.2% and 3.3%, respectively. The most common diagnoses in this cohort were back pain (47.5% vs. 48.5%), neck/shoulder pain (28.6% vs. 29.5%), and osteoarthritis (15.3% vs. 15.5%). The most commonly used drugs were ibuprofen (34.4% vs. 34.3%) and paracetamol/acetaminophen (37.1% vs. 36.8%)
Higher risk of adverse outcomes
As compared with codeine, tramadol use was significantly associated with a higher risk of mortality (13.00 vs. 5.61 per 1,000 person-years; hazard ratio, 2.31; 95% confidence interval, 2.08-2.56); absolute rate differences (7.37 per 1,000 person-years; 95% CI, 6.09-8.78), cardiovascular events (10.03 vs. 8.67 per 1,000 person-years; HR, 1.15; 95% CI, 1.05-1.27; ARD, 1.36 per 1,000 person-years; 95% CI, 0.45-2.36), and fractures (12.26 vs. 8.13 per 1,000 person-years; HR, 1.50; 95% CI, 1.37-1.65; ARD, 4.10 per 1,000 person-years; 95% CI, 3.02-5.29).
A subgroup and sensitivity analysis showed that the increased mortality risk associated with tramadol was significantly higher in younger persons vs. older ones (HR, 3.14; 95% CI, 1.82-5.41 vs. 2.39; 95% CI, 2.20-2.60]; P < .001 for interaction). In addition, women had a significantly greater risk of cardiovascular events versus men (HR, 1.32; 95% CI, 1.19-1.46] vs. 1.03; 95% CI, 0.9-1.13]; P < .001 for interaction).
Potential for confounding
Weighing in on the data, Daniel Solomon, MD, MPH, chief of clinical sciences, division of rheumatology, Brigham and Women’s Hospital, and professor of medicine, Harvard Medical School, Boston, noted that because it is extremely unlikely that anyone will ever conduct a large, head-to-head safety trial comparing different opioids, the results of this paper are important to consider.
“However, as the authors appropriately caution, this type of analysis is limited by the strong potential for residual confounding,” he said. “In other words, even though the authors used state-of-the-art methods to limit imbalances between the patients initiating tramadol versus codeine, there is strong reason to believe that imbalances that may account for the differences in adverse events exist.”
For example, he noted that if one looks at the distribution of comorbid conditions in the before-matching group, tramadol initiators demonstrate a higher frequency of chronic kidney disease, diabetes, and overall chronic comorbid diseases. “This suggests to me that prescribers apply selection criteria when choosing who to prescribe which opioid,” Dr. Solomon explained.
“While the authors’ use of propensity score matching limits confounding, it only can improve balance for measured confounders,” he said. “Other factors not measured in this type of data set – blood pressure, pain, physical activity, tobacco use, body mass index – may still demonstrate imbalances even after matching.”
But after these limitations are taken into consideration, the results remain concerning, Dr. Solomon emphasized, particularly the all-cause mortality excess of tramadol versus codeine users. “This study did not include cause of death, which would help the reader understand why users of tramadol were dying more frequently,” he added. “It also might help in understanding whether this is a true biologic effect or residual confounding.”
Perceived safety
In an accompanying editorial, Howard S. Kim, MD, MS, and colleagues from Northwestern University, Chicago, write that the greatest risk of tramadol may involve the perception that it is “inherently safer than other opioids.”
“In actuality, the mechanisms of action and variable metabolism of tramadol in a given population create considerable therapeutic uncertainty and introduce additional risk exposure,” they say, as demonstrated in the current study.
Therefore, when clinicians determine that an opioid is needed for pain relief, it may be a better option to select a pure opioid agonist that has a more predictable therapeutic effect and known adverse effect profile, such as morphine or hydrocodone. “This would allow clinicians and patients to more properly weigh the risks and benefits of initiating opioid therapy through shared decision-making and prompt the level of counseling on safe use, storage, and disposal practices that all opioids deserve,” write the editorialists.
The study was funded by the Fundació Institut Universitari per a la recerca a l’Atenció Primària de Salut Jordi Gol i Gurina. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre. Dr. Reyes has disclosed no relevant financial relationships. Dr. Solomon disclosed salary support from research contracts to his hospital from Amgen, AbbVie, Moderna, the Rheumatology Research Foundation, and National Institutes of Health; and royalties from UpToDate. Dr. Kim reported unrelated grant support from the Agency for Healthcare Research and Quality.
A version of this article first appeared on Medscape.com.
FDA OKs new high-dose naloxone product for opioid overdose
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.
ZIMHI from Adamis Pharmaceuticals is administered using a single-dose, prefilled syringe that delivers 5 mg of naloxone hydrochloride solution through intramuscular or subcutaneous injection.
Naloxone is an opioid antagonist that works by blocking or reversing the effects of the opioid, including extreme drowsiness, slowed breathing, or loss of consciousness.
Opioid-related overdose deaths — driven partly by prescription drug overdoses — remain a leading cause of death in the United States.
ZIMHI “provides an additional option in the treatment of opioid overdoses,” the FDA said in a statement announcing approval.
In a statement from Adamis Pharmaceuticals, Jeffrey Galinkin, MD, an anesthesiologist and former member of the FDA advisory committee for analgesics and addiction products, said he is “pleased to see this much-needed, high-dose naloxone product will become part of the treatment tool kit as a countermeasure to the continued surge in fentanyl related deaths.”
“The higher intramuscular doses of naloxone in ZIMHI should result in more rapid and higher levels of naloxone in the systemic circulation, which in turn, should result in more successful resuscitations,” Dr. Galinkin said.
Last spring the FDA approved a higher-dose naloxone hydrochloride nasal spray (Kloxxado) for the emergency treatment of opioid overdose.
Kloxxado delivers 8 mg of naloxone into the nasal cavity, which is twice as much as the 4 mg of naloxone contained in Narcan nasal spray.
The FDA approved ZIMHI (and Kloxxado) through the 505(b)(2) regulatory pathway, which allows the agency to refer to previous findings of safety and efficacy for an already-approved product, as well as to review findings from further studies of the product.
The company plans to launch ZIMHI in the first quarter of 2022.
A version of this article first appeared on Medscape.com.
New antimigraine drugs linked with less risk for adverse events
“[T]he lack of cardiovascular risks of these new classes of migraine-specific treatments may provide alternative treatment options for individuals for whom currently available acute treatments have failed or for those with cardiovascular contraindications,” write lead author Chun-Pai Yang, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and colleagues, in the paper, published online in JAMA Network Open.
Methods
The new study compared the outcomes for acute migraine management using the ditan, lasmiditan (a 5-hydroxytryptamine [5HT]1F–receptor agonist), and the two gepants, rimegepant, and ubrogepant (calcitonin gene–related peptide [CGRP] antagonists), with standard triptan (selective 5-HT1B/1D–receptor agonist) therapy.
The researchers evaluated 64 double-blind randomized clinical trials which included 46,442 patients, the majority of whom (74%-87%) were women with an age range of 36-43 years.
The primary outcome evaluated was the odds ratio for freedom from pain at 2 hours after a single dose and secondary outcomes were the OR for pain relief at 2 hours following a dose, as well as any adverse events.
Results
Dr. Yang and colleagues found that virtually all medications with widespread clinical use, regardless of class, were associated with higher ORs for pain freedom when compared with placebo.
Compared to ditan and gepant agents, however, triptans were associated with significantly higher ORs for pain freedom. The odds ratio ranges were 1.72-3.40 for lasmiditan, 1.58-3.13 for rimegepant, and 1.54-3.05 for ubrogepant.
With respect to pain relief at 2 hours, while all medications were more effective than placebo, triptans were associated with higher ORs when compared with the other drug classes: lasmiditan (range: OR, 1.46; 95% confidence interval, 1.09-1.96 to OR, 3.31; 95% CI, 2.41-4.55), rimegepant (range: OR, 1.33; 95% CI, 1.01-1.76 to OR, 3.01; 95% CI, 2.33-3.88), and ubrogepant (range: OR, 1.38; 95% CI, 1.02-1.88 to OR, 3.13; 95% CI, 2.35-4.15)
When assessing tolerability, the researchers found that overall, triptans were associated with the higher ORs for any adverse events (AE) with a trend of dose-response relationship. Lasmiditan (in the ditan class) was associated with the highest risk for AEs among all treatments. Most of the AEs were mild to moderate and included chest pain, tightness, heaviness, and pressure.
Dr. Yang and colleagues note that, “although these two new classes of antimigraine drugs may not be as efficacious as triptans, these novel abortive agents without cardiovascular risks might offer an alternative to current specific migraine treatments for patients at risk of cardiovascular disease.”
Balancing efficacy and tolerability
“When choosing an acute medication for a patient there is always a balance between efficacy and tolerability,” headache specialist and associate director of North Shore Headache and Spine Lauren Natbony, MD, said in an interview.
“A medication can only be effective if a patient is able to tolerate it and will actually use it,” Dr. Natbony said.
With respect to the current review, Dr. Natbony pointed out, “response to acute therapy can differ between migraine attacks and may be based on variables not controlled for, such as how early in an attack the medication was taken, associated symptoms such as nausea that may make oral medications less efficacious, etc.”
The authors acknowledge that the focus on short-term responses and AEs after a single dose is a limitation of the study. They also pointed out what they considered to be a strength of the study, which was its network meta-analysis design. According to the authors, this design allowed for “multiple direct and indirect comparisons, ranking the efficacy and safety of individual pharmacologic interventions and providing more precise estimates than those of RCTs and traditional meta-analysis.”
Funding for this study was provided through grants from the Ministry of Science and Technology, Taiwan; the Brain Research Center; and National Yang Ming Chiao Tung University.
Dr. Yang has received personal fees and grants from various pharmaceutical companies. He has also received grants from the Taiwan Ministry of Technology and Science, the Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. The other authors and Dr. Natbony disclosed no relevant financial relationships.
“[T]he lack of cardiovascular risks of these new classes of migraine-specific treatments may provide alternative treatment options for individuals for whom currently available acute treatments have failed or for those with cardiovascular contraindications,” write lead author Chun-Pai Yang, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and colleagues, in the paper, published online in JAMA Network Open.
Methods
The new study compared the outcomes for acute migraine management using the ditan, lasmiditan (a 5-hydroxytryptamine [5HT]1F–receptor agonist), and the two gepants, rimegepant, and ubrogepant (calcitonin gene–related peptide [CGRP] antagonists), with standard triptan (selective 5-HT1B/1D–receptor agonist) therapy.
The researchers evaluated 64 double-blind randomized clinical trials which included 46,442 patients, the majority of whom (74%-87%) were women with an age range of 36-43 years.
The primary outcome evaluated was the odds ratio for freedom from pain at 2 hours after a single dose and secondary outcomes were the OR for pain relief at 2 hours following a dose, as well as any adverse events.
Results
Dr. Yang and colleagues found that virtually all medications with widespread clinical use, regardless of class, were associated with higher ORs for pain freedom when compared with placebo.
Compared to ditan and gepant agents, however, triptans were associated with significantly higher ORs for pain freedom. The odds ratio ranges were 1.72-3.40 for lasmiditan, 1.58-3.13 for rimegepant, and 1.54-3.05 for ubrogepant.
With respect to pain relief at 2 hours, while all medications were more effective than placebo, triptans were associated with higher ORs when compared with the other drug classes: lasmiditan (range: OR, 1.46; 95% confidence interval, 1.09-1.96 to OR, 3.31; 95% CI, 2.41-4.55), rimegepant (range: OR, 1.33; 95% CI, 1.01-1.76 to OR, 3.01; 95% CI, 2.33-3.88), and ubrogepant (range: OR, 1.38; 95% CI, 1.02-1.88 to OR, 3.13; 95% CI, 2.35-4.15)
When assessing tolerability, the researchers found that overall, triptans were associated with the higher ORs for any adverse events (AE) with a trend of dose-response relationship. Lasmiditan (in the ditan class) was associated with the highest risk for AEs among all treatments. Most of the AEs were mild to moderate and included chest pain, tightness, heaviness, and pressure.
Dr. Yang and colleagues note that, “although these two new classes of antimigraine drugs may not be as efficacious as triptans, these novel abortive agents without cardiovascular risks might offer an alternative to current specific migraine treatments for patients at risk of cardiovascular disease.”
Balancing efficacy and tolerability
“When choosing an acute medication for a patient there is always a balance between efficacy and tolerability,” headache specialist and associate director of North Shore Headache and Spine Lauren Natbony, MD, said in an interview.
“A medication can only be effective if a patient is able to tolerate it and will actually use it,” Dr. Natbony said.
With respect to the current review, Dr. Natbony pointed out, “response to acute therapy can differ between migraine attacks and may be based on variables not controlled for, such as how early in an attack the medication was taken, associated symptoms such as nausea that may make oral medications less efficacious, etc.”
The authors acknowledge that the focus on short-term responses and AEs after a single dose is a limitation of the study. They also pointed out what they considered to be a strength of the study, which was its network meta-analysis design. According to the authors, this design allowed for “multiple direct and indirect comparisons, ranking the efficacy and safety of individual pharmacologic interventions and providing more precise estimates than those of RCTs and traditional meta-analysis.”
Funding for this study was provided through grants from the Ministry of Science and Technology, Taiwan; the Brain Research Center; and National Yang Ming Chiao Tung University.
Dr. Yang has received personal fees and grants from various pharmaceutical companies. He has also received grants from the Taiwan Ministry of Technology and Science, the Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. The other authors and Dr. Natbony disclosed no relevant financial relationships.
“[T]he lack of cardiovascular risks of these new classes of migraine-specific treatments may provide alternative treatment options for individuals for whom currently available acute treatments have failed or for those with cardiovascular contraindications,” write lead author Chun-Pai Yang, MD, PhD, of Taichung (Taiwan) Veterans General Hospital and colleagues, in the paper, published online in JAMA Network Open.
Methods
The new study compared the outcomes for acute migraine management using the ditan, lasmiditan (a 5-hydroxytryptamine [5HT]1F–receptor agonist), and the two gepants, rimegepant, and ubrogepant (calcitonin gene–related peptide [CGRP] antagonists), with standard triptan (selective 5-HT1B/1D–receptor agonist) therapy.
The researchers evaluated 64 double-blind randomized clinical trials which included 46,442 patients, the majority of whom (74%-87%) were women with an age range of 36-43 years.
The primary outcome evaluated was the odds ratio for freedom from pain at 2 hours after a single dose and secondary outcomes were the OR for pain relief at 2 hours following a dose, as well as any adverse events.
Results
Dr. Yang and colleagues found that virtually all medications with widespread clinical use, regardless of class, were associated with higher ORs for pain freedom when compared with placebo.
Compared to ditan and gepant agents, however, triptans were associated with significantly higher ORs for pain freedom. The odds ratio ranges were 1.72-3.40 for lasmiditan, 1.58-3.13 for rimegepant, and 1.54-3.05 for ubrogepant.
With respect to pain relief at 2 hours, while all medications were more effective than placebo, triptans were associated with higher ORs when compared with the other drug classes: lasmiditan (range: OR, 1.46; 95% confidence interval, 1.09-1.96 to OR, 3.31; 95% CI, 2.41-4.55), rimegepant (range: OR, 1.33; 95% CI, 1.01-1.76 to OR, 3.01; 95% CI, 2.33-3.88), and ubrogepant (range: OR, 1.38; 95% CI, 1.02-1.88 to OR, 3.13; 95% CI, 2.35-4.15)
When assessing tolerability, the researchers found that overall, triptans were associated with the higher ORs for any adverse events (AE) with a trend of dose-response relationship. Lasmiditan (in the ditan class) was associated with the highest risk for AEs among all treatments. Most of the AEs were mild to moderate and included chest pain, tightness, heaviness, and pressure.
Dr. Yang and colleagues note that, “although these two new classes of antimigraine drugs may not be as efficacious as triptans, these novel abortive agents without cardiovascular risks might offer an alternative to current specific migraine treatments for patients at risk of cardiovascular disease.”
Balancing efficacy and tolerability
“When choosing an acute medication for a patient there is always a balance between efficacy and tolerability,” headache specialist and associate director of North Shore Headache and Spine Lauren Natbony, MD, said in an interview.
“A medication can only be effective if a patient is able to tolerate it and will actually use it,” Dr. Natbony said.
With respect to the current review, Dr. Natbony pointed out, “response to acute therapy can differ between migraine attacks and may be based on variables not controlled for, such as how early in an attack the medication was taken, associated symptoms such as nausea that may make oral medications less efficacious, etc.”
The authors acknowledge that the focus on short-term responses and AEs after a single dose is a limitation of the study. They also pointed out what they considered to be a strength of the study, which was its network meta-analysis design. According to the authors, this design allowed for “multiple direct and indirect comparisons, ranking the efficacy and safety of individual pharmacologic interventions and providing more precise estimates than those of RCTs and traditional meta-analysis.”
Funding for this study was provided through grants from the Ministry of Science and Technology, Taiwan; the Brain Research Center; and National Yang Ming Chiao Tung University.
Dr. Yang has received personal fees and grants from various pharmaceutical companies. He has also received grants from the Taiwan Ministry of Technology and Science, the Brain Research Center, National Yang Ming Chiao Tung University, and Taipei Veterans General Hospital outside the submitted work. The other authors and Dr. Natbony disclosed no relevant financial relationships.
FROM JAMA NETWORK OPEN
Lumbar epidural steroid jab lowers bone formation in older women
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among postmenopausal women who received an epidural steroid injection (ESI) in the lumbar spine to treat back and leg pain arising from a compressed nerve in the spine, levels of bone formation biomarkers were decreased. The decrease in levels persisted more than 12 weeks, results from a new study show.
In addition, serum cortisol levels decreased by 50% at week 1 after the ESI, indicating systemic absorption of the steroid.
“The extent and duration of these effects suggest that patients who receive multiple [ESIs in the lumbar spine] may be at particular risk for harmful skeletal consequences,” Shannon Clare reported in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research.
Further studies are needed of the relationship between these short-term changes in bone turnover and bone loss and the risk for fracture among the burgeoning population treated with ESIs, added Ms. Clare, of the Hospital for Special Surgery, New York.
The researchers examined changes in serum levels of bone formation and resorption markers and other analytes in 24 women who received a lumbar ESI for radicular back pain and in 8 other women from the hospital population who served as control persons.
Among the women who received ESI, 1 week after the injection, serum levels of two bone formation biomarkers – total procollagen type 1 N-terminal peptide (P1NP) and osteocalcin – were about 27% lower than at baseline. The suppression persisted beyond 12 weeks.
Serum levels of the bone resorption biomarker C-terminal telopeptide of type I collagen (CTX) did not differ significantly after ESI.
“Our results are notable because we found that the duration of suppression of bone formation extended beyond 12 weeks, a far longer duration than seen previously with intra-articular injections” of glucocorticoids, said Ms. Clare and senior author Emily M. Stein, MD, director of research for the Metabolic Bone Service and an endocrinologist at the Hospital for Special Surgery and is associate professor of medicine at Weill Cornell Medicine, both in New York.
The findings suggest that patients should not receive multiple doses within a 12-week period, they told this news organization in a joint email response.
Women are not typically screened for osteopenia or osteoporosis before ESI. However, “our results suggest that physicians should consider screening women for osteoporosis who receive ESI, particularly those who are treated with multiple doses,” said Ms. Clare and Dr. Stein. “Steroid exposure should be minimized as much as possible by having patients space injections as far as they can tolerate.”
Systemic absorption, negative impact on bone turnover markers
“The hypothesis that [ESIs] interfere with the vertebral osseous microenvironment and increase the risk of vertebral fractures has been supported with evidence in the literature,” Mohamad Bydon, MD, professor of neurosurgery, orthopedic surgery, and health services research at the Mayo Clinic, Rochester, Minn., said in an interview.
Prior studies have demonstrated a decrease in bone mineral density (BMD) and an increase in vertebral fractures following ESI, added Dr. Bydon, senior author of a 2018 review of the effect of ESI on BMD and vertebral fracture risk that was published in Pain Medicine. He was not involved with the current study.
“The article by Clare et al. provides evidence on the systemic absorption of glucocorticoids by demonstrating a drop in serum cortisol following ESI,” he noted. “The measurement of bone metabolism biomarkers offers molecular confirmation of clinical and radiological observations of previous studies” showing that ESI affects the vertebrae.
More than 9 million ESIs each year
Each year, more than 9 million ESIs are administered to patients in the United States to relieve radicular back and leg pain that may be caused by a herniated disc or spinal stenosis (a gradual narrowing of the open spaces in the spinal column, which is common in older adults), the researchers explained.
Some patients experience sufficient pain relief with ESIs. Others may not be eligible for surgery and may receive multiple ESIs annually for many years because they provide pain relief.
It is well established that oral and intravenous glucocorticoids profoundly suppress bone formation and transiently increase bone resorption, causing substantial bone loss and increased fracture risk within 3 months of administration, Ms. Clare explained in the session.
Long-term use of high-dose inhaled glucocorticoids has been associated with bone loss and fractures. However, the effect of ESIs on bone has been less well studied.
The researchers hypothesized that ESIs are systemically absorbed and cause suppression of bone formation without a compensatory decrease in bone resorption.
They enrolled 24 patients who had undergone lumbar ESIs and 8 control patients. The mean age of the patients in the two groups was 63 years and 68 years, respectively. Most patients were White (88% and 100%, respectively). The mean body mass index was 27 kg/m2 and 28 kg/m2, respectively. On average, the patients had entered menopause 12 and 16 years earlier, respectively.
In the group that received steroid injections, almost two-thirds (15 patients, 63%) received triamcinolone. The rest received dexamethasone (six patients, 25%) or betamethasone (three patients, 12%) at doses that were equivalent to 80 mg triamcinolone.
The patients’ baseline serum levels of 25-hydroxy vitamin D, parathyroid hormone, cortisol, P1NP, osteocalcin, and CTX were within the reference ranges and were similar in the two groups.
The researchers also determined serum levels of cortisol (to assess suppression of endogenous glucocorticoids), osteocalcin, P1NP, and CTX in the patients and control persons at 1, 4, 12, 26, and 52 weeks after patients had received the ESI.
The researchers acknowledged that the small sample is a study limitation. In addition, the first serum samples were taken 1 week after the injection, and so any earlier changes in analyte levels were not captured. The patients also received different types of steroids, although the doses were similar when converted to triamcinolone equivalents.
The study was supported by a Spine Service grant from the Hospital for Special Surgery. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Retraining the brain may eliminate chronic back pain
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.