User login
The latest migraine therapies – some you might not know about
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with my very good friend, Dr. Paul Williams. It’s time to talk about headaches. We did a great recent podcast on migraines, Headache Update: Making Migraines Less Painful with Dr. Kevin Weber. One of the quotes from that episode that stayed with me was when he said, “I tell my patients to think about migraine as an irritable old miser set in their ways, and your brain is set in its ways. It doesn’t like changes in routine. It doesn’t like lack of sleep, it doesn’t like being hungry, it doesn’t like being thirsty, and it doesn’t like changes in the weather.” That’s a reminder of the good, old-fashioned primary care tips for taking care of headache.
Paul N. Williams, MD: That’s right. Conservative supportive management goes by the wayside because we focus on the medications. But I thought that was a really nice way to start the episode.
Dr. Watto: I asked him about cervicogenic headaches, which I guess you have to diagnose by giving a cervical steroid injection and see if the patient feels better, but he said he doesn’t do this. This is expert opinion territory. He asks his patients with chronic headache about cervical neck pain, because if they have it, he goes after it with physical therapy, which can help with the headaches. I thought that was a great pearl that I hadn’t heard before.
Give the audience a pearl from this great episode.
Dr. Williams: We talked about foundational treatments. We reviewed some of the abortive therapies and over-the-counter products. Some patients do quite well with acetaminophen or NSAIDs. We also talked about triptans, which are the standard medicines that we all know about. You can use those in combination, by the way. Patients can take their triptan with the NSAID that works best for them. They don’t have to be used one at a time, trying one and then trying the other one if the first one doesn’t help. Dr. Weber gave us practical guides in terms of which triptans he favors. He mentioned rizatriptan and naratriptan, which is one that I had not used with any frequency. I’ve seen rizatriptan a fair amount and that one seems to be covered by most insurances. He favors those two triptans.
He also reminded us that even though there is theoretical concern for serotonin toxicity because these are serotonergic and you’ll see these scary pop-ups in your electronic health record, that concern is almost purely theoretical. It hasn’t been borne out. They are really safe medications to use. But do use caution if you have a patient with known cardiovascular disease or cerebrovascular disease. We spent a fair amount of time talking about chest pressure as a common side effect. We also talked about some of the newer agents.
Dr. Watto: I wanted to add something about the triptans. Part of the reason he favors rizatriptan and naratriptan is that they are newer. He thinks they tend to have fewer side effects. But he did mention sumatriptan because it comes in the most different formulations. If patients have severe nausea, there is a subcutaneous version of sumatriptan and also an intranasal version.
The new kids on the block are the CGRP receptor antagonists, and they are available for preventive and abortive therapy. The abortive therapies are probably what people will be seeing most often in primary care – ubrogepant and rimegepant. Patients can take ubrogepant for abortive therapy and then repeat it if necessary. That’s similar to what patients are used to with the triptans. Rimegepant is taken once daily for abortive therapy or every other day as a preventive agent. Those are two of the agents that you might see patients taking. I’ve certainly started to see them.
There are also a whole bunch of monoclonal antibodies that affect the CGRP pathway. Those are given either once a month by subcutaneous injection or once every 3 months, and one is an infusion. They are pretty safe, and the big appeal is that they can be used in patients with cardiovascular disease. He also said that he has some patients who take them because triptans can cause the medication overuse side effect, but the CGRP receptor antagonists don’t. It’s an option for some patients to take the CGRP receptor antagonists on certain days for abortive therapy and then they can take the triptans the rest of the month.
Dr. Weber said that in his practice, these new drugs have really been great, which I can imagine, if you’re a specialist, patients have exhausted many of the typical therapies we offer in primary care.
Paul, bring us home here. What else should we tell the audience about? In primary care, what can we offer these patients?
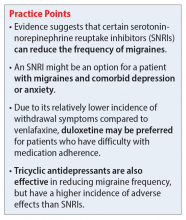
Dr. Williams: A lot of the stuff we can offer works, by the way. It’s exciting to have fancy new medications to use, but you don’t even necessarily need to get to that point. We have a lot of medications that we can use for migraine prophylaxis, such as the beta-blockers and antihypertensives. Candesartan was a new one to me, an angiotensin receptor blocker that apparently has good evidence for migraine prophylaxis and Dr. Weber swears by it. We talked about some of the antiseizure medications, such as topiramate, which is probably the one with the most comfort in primary care. Some older folks may be using valproic acid or the tricyclic antidepressants (amitriptyline and nortriptyline) because people with migraine often will have comorbid anxiety or trouble sleeping, so I find that can sometimes be an effective medication or if they have comorbid neuropathic pain.
Another one that was new to me was venlafaxine as migraine prophylaxis. It’s not something I’d heard about before this episode. Certainly, for someone with chronic pain or a mood disorder that’s comorbid with migraines, it may be worth a shot. So there are options that we can exhaust first, and we may actually be doing our specialist friends a favor by trying one or two of these in advance, because then by the time the patient gets to the neurologist, it makes the prior authorization process much easier for the newer, fancier-pants medications that we’re all very excited about.
Dr. Watto: Paul, we’ve teased this fantastic podcast episode filled with so much more great stuff, so people should check out Headache Update: Making Migraines Less Painful with Dr. Kevin Weber.
Until next time, this has been another episode of The Curbsiders, bringing you a little knowledge food for your brain hole.
The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with my very good friend, Dr. Paul Williams. It’s time to talk about headaches. We did a great recent podcast on migraines, Headache Update: Making Migraines Less Painful with Dr. Kevin Weber. One of the quotes from that episode that stayed with me was when he said, “I tell my patients to think about migraine as an irritable old miser set in their ways, and your brain is set in its ways. It doesn’t like changes in routine. It doesn’t like lack of sleep, it doesn’t like being hungry, it doesn’t like being thirsty, and it doesn’t like changes in the weather.” That’s a reminder of the good, old-fashioned primary care tips for taking care of headache.
Paul N. Williams, MD: That’s right. Conservative supportive management goes by the wayside because we focus on the medications. But I thought that was a really nice way to start the episode.
Dr. Watto: I asked him about cervicogenic headaches, which I guess you have to diagnose by giving a cervical steroid injection and see if the patient feels better, but he said he doesn’t do this. This is expert opinion territory. He asks his patients with chronic headache about cervical neck pain, because if they have it, he goes after it with physical therapy, which can help with the headaches. I thought that was a great pearl that I hadn’t heard before.
Give the audience a pearl from this great episode.
Dr. Williams: We talked about foundational treatments. We reviewed some of the abortive therapies and over-the-counter products. Some patients do quite well with acetaminophen or NSAIDs. We also talked about triptans, which are the standard medicines that we all know about. You can use those in combination, by the way. Patients can take their triptan with the NSAID that works best for them. They don’t have to be used one at a time, trying one and then trying the other one if the first one doesn’t help. Dr. Weber gave us practical guides in terms of which triptans he favors. He mentioned rizatriptan and naratriptan, which is one that I had not used with any frequency. I’ve seen rizatriptan a fair amount and that one seems to be covered by most insurances. He favors those two triptans.
He also reminded us that even though there is theoretical concern for serotonin toxicity because these are serotonergic and you’ll see these scary pop-ups in your electronic health record, that concern is almost purely theoretical. It hasn’t been borne out. They are really safe medications to use. But do use caution if you have a patient with known cardiovascular disease or cerebrovascular disease. We spent a fair amount of time talking about chest pressure as a common side effect. We also talked about some of the newer agents.
Dr. Watto: I wanted to add something about the triptans. Part of the reason he favors rizatriptan and naratriptan is that they are newer. He thinks they tend to have fewer side effects. But he did mention sumatriptan because it comes in the most different formulations. If patients have severe nausea, there is a subcutaneous version of sumatriptan and also an intranasal version.
The new kids on the block are the CGRP receptor antagonists, and they are available for preventive and abortive therapy. The abortive therapies are probably what people will be seeing most often in primary care – ubrogepant and rimegepant. Patients can take ubrogepant for abortive therapy and then repeat it if necessary. That’s similar to what patients are used to with the triptans. Rimegepant is taken once daily for abortive therapy or every other day as a preventive agent. Those are two of the agents that you might see patients taking. I’ve certainly started to see them.
There are also a whole bunch of monoclonal antibodies that affect the CGRP pathway. Those are given either once a month by subcutaneous injection or once every 3 months, and one is an infusion. They are pretty safe, and the big appeal is that they can be used in patients with cardiovascular disease. He also said that he has some patients who take them because triptans can cause the medication overuse side effect, but the CGRP receptor antagonists don’t. It’s an option for some patients to take the CGRP receptor antagonists on certain days for abortive therapy and then they can take the triptans the rest of the month.
Dr. Weber said that in his practice, these new drugs have really been great, which I can imagine, if you’re a specialist, patients have exhausted many of the typical therapies we offer in primary care.
Paul, bring us home here. What else should we tell the audience about? In primary care, what can we offer these patients?
Dr. Williams: A lot of the stuff we can offer works, by the way. It’s exciting to have fancy new medications to use, but you don’t even necessarily need to get to that point. We have a lot of medications that we can use for migraine prophylaxis, such as the beta-blockers and antihypertensives. Candesartan was a new one to me, an angiotensin receptor blocker that apparently has good evidence for migraine prophylaxis and Dr. Weber swears by it. We talked about some of the antiseizure medications, such as topiramate, which is probably the one with the most comfort in primary care. Some older folks may be using valproic acid or the tricyclic antidepressants (amitriptyline and nortriptyline) because people with migraine often will have comorbid anxiety or trouble sleeping, so I find that can sometimes be an effective medication or if they have comorbid neuropathic pain.
Another one that was new to me was venlafaxine as migraine prophylaxis. It’s not something I’d heard about before this episode. Certainly, for someone with chronic pain or a mood disorder that’s comorbid with migraines, it may be worth a shot. So there are options that we can exhaust first, and we may actually be doing our specialist friends a favor by trying one or two of these in advance, because then by the time the patient gets to the neurologist, it makes the prior authorization process much easier for the newer, fancier-pants medications that we’re all very excited about.
Dr. Watto: Paul, we’ve teased this fantastic podcast episode filled with so much more great stuff, so people should check out Headache Update: Making Migraines Less Painful with Dr. Kevin Weber.
Until next time, this has been another episode of The Curbsiders, bringing you a little knowledge food for your brain hole.
The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Watto, here with my very good friend, Dr. Paul Williams. It’s time to talk about headaches. We did a great recent podcast on migraines, Headache Update: Making Migraines Less Painful with Dr. Kevin Weber. One of the quotes from that episode that stayed with me was when he said, “I tell my patients to think about migraine as an irritable old miser set in their ways, and your brain is set in its ways. It doesn’t like changes in routine. It doesn’t like lack of sleep, it doesn’t like being hungry, it doesn’t like being thirsty, and it doesn’t like changes in the weather.” That’s a reminder of the good, old-fashioned primary care tips for taking care of headache.
Paul N. Williams, MD: That’s right. Conservative supportive management goes by the wayside because we focus on the medications. But I thought that was a really nice way to start the episode.
Dr. Watto: I asked him about cervicogenic headaches, which I guess you have to diagnose by giving a cervical steroid injection and see if the patient feels better, but he said he doesn’t do this. This is expert opinion territory. He asks his patients with chronic headache about cervical neck pain, because if they have it, he goes after it with physical therapy, which can help with the headaches. I thought that was a great pearl that I hadn’t heard before.
Give the audience a pearl from this great episode.
Dr. Williams: We talked about foundational treatments. We reviewed some of the abortive therapies and over-the-counter products. Some patients do quite well with acetaminophen or NSAIDs. We also talked about triptans, which are the standard medicines that we all know about. You can use those in combination, by the way. Patients can take their triptan with the NSAID that works best for them. They don’t have to be used one at a time, trying one and then trying the other one if the first one doesn’t help. Dr. Weber gave us practical guides in terms of which triptans he favors. He mentioned rizatriptan and naratriptan, which is one that I had not used with any frequency. I’ve seen rizatriptan a fair amount and that one seems to be covered by most insurances. He favors those two triptans.
He also reminded us that even though there is theoretical concern for serotonin toxicity because these are serotonergic and you’ll see these scary pop-ups in your electronic health record, that concern is almost purely theoretical. It hasn’t been borne out. They are really safe medications to use. But do use caution if you have a patient with known cardiovascular disease or cerebrovascular disease. We spent a fair amount of time talking about chest pressure as a common side effect. We also talked about some of the newer agents.
Dr. Watto: I wanted to add something about the triptans. Part of the reason he favors rizatriptan and naratriptan is that they are newer. He thinks they tend to have fewer side effects. But he did mention sumatriptan because it comes in the most different formulations. If patients have severe nausea, there is a subcutaneous version of sumatriptan and also an intranasal version.
The new kids on the block are the CGRP receptor antagonists, and they are available for preventive and abortive therapy. The abortive therapies are probably what people will be seeing most often in primary care – ubrogepant and rimegepant. Patients can take ubrogepant for abortive therapy and then repeat it if necessary. That’s similar to what patients are used to with the triptans. Rimegepant is taken once daily for abortive therapy or every other day as a preventive agent. Those are two of the agents that you might see patients taking. I’ve certainly started to see them.
There are also a whole bunch of monoclonal antibodies that affect the CGRP pathway. Those are given either once a month by subcutaneous injection or once every 3 months, and one is an infusion. They are pretty safe, and the big appeal is that they can be used in patients with cardiovascular disease. He also said that he has some patients who take them because triptans can cause the medication overuse side effect, but the CGRP receptor antagonists don’t. It’s an option for some patients to take the CGRP receptor antagonists on certain days for abortive therapy and then they can take the triptans the rest of the month.
Dr. Weber said that in his practice, these new drugs have really been great, which I can imagine, if you’re a specialist, patients have exhausted many of the typical therapies we offer in primary care.
Paul, bring us home here. What else should we tell the audience about? In primary care, what can we offer these patients?
Dr. Williams: A lot of the stuff we can offer works, by the way. It’s exciting to have fancy new medications to use, but you don’t even necessarily need to get to that point. We have a lot of medications that we can use for migraine prophylaxis, such as the beta-blockers and antihypertensives. Candesartan was a new one to me, an angiotensin receptor blocker that apparently has good evidence for migraine prophylaxis and Dr. Weber swears by it. We talked about some of the antiseizure medications, such as topiramate, which is probably the one with the most comfort in primary care. Some older folks may be using valproic acid or the tricyclic antidepressants (amitriptyline and nortriptyline) because people with migraine often will have comorbid anxiety or trouble sleeping, so I find that can sometimes be an effective medication or if they have comorbid neuropathic pain.
Another one that was new to me was venlafaxine as migraine prophylaxis. It’s not something I’d heard about before this episode. Certainly, for someone with chronic pain or a mood disorder that’s comorbid with migraines, it may be worth a shot. So there are options that we can exhaust first, and we may actually be doing our specialist friends a favor by trying one or two of these in advance, because then by the time the patient gets to the neurologist, it makes the prior authorization process much easier for the newer, fancier-pants medications that we’re all very excited about.
Dr. Watto: Paul, we’ve teased this fantastic podcast episode filled with so much more great stuff, so people should check out Headache Update: Making Migraines Less Painful with Dr. Kevin Weber.
Until next time, this has been another episode of The Curbsiders, bringing you a little knowledge food for your brain hole.
The Curbsiders is an internal medicine podcast, in which three board-certified internists interview experts on clinically important topics. In a collaboration with Medscape, the Curbsiders share clinical pearls and practice-changing knowledge from selected podcasts.
A version of this article first appeared on Medscape.com.
Medications for Opioid Use Disorder Program in a VA Emergency Department
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
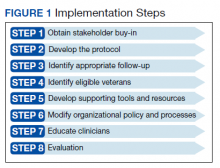
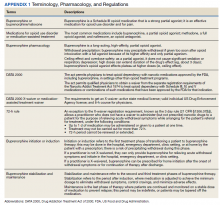
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
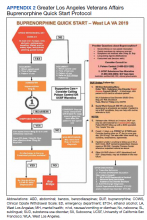
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
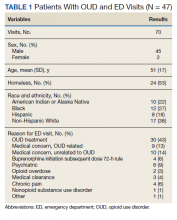
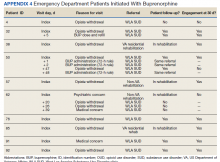
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
Soccer player with painful toe
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.
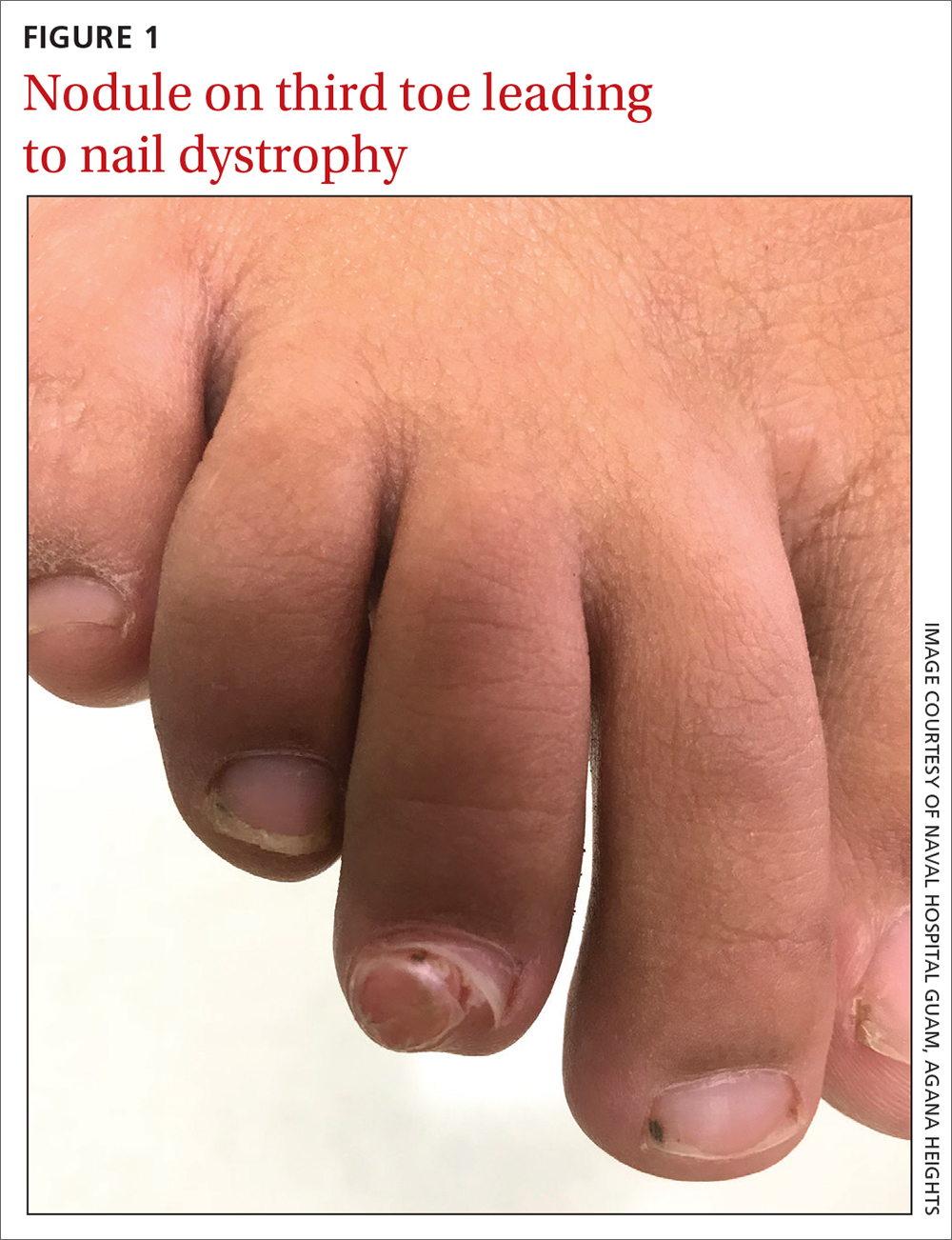
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3
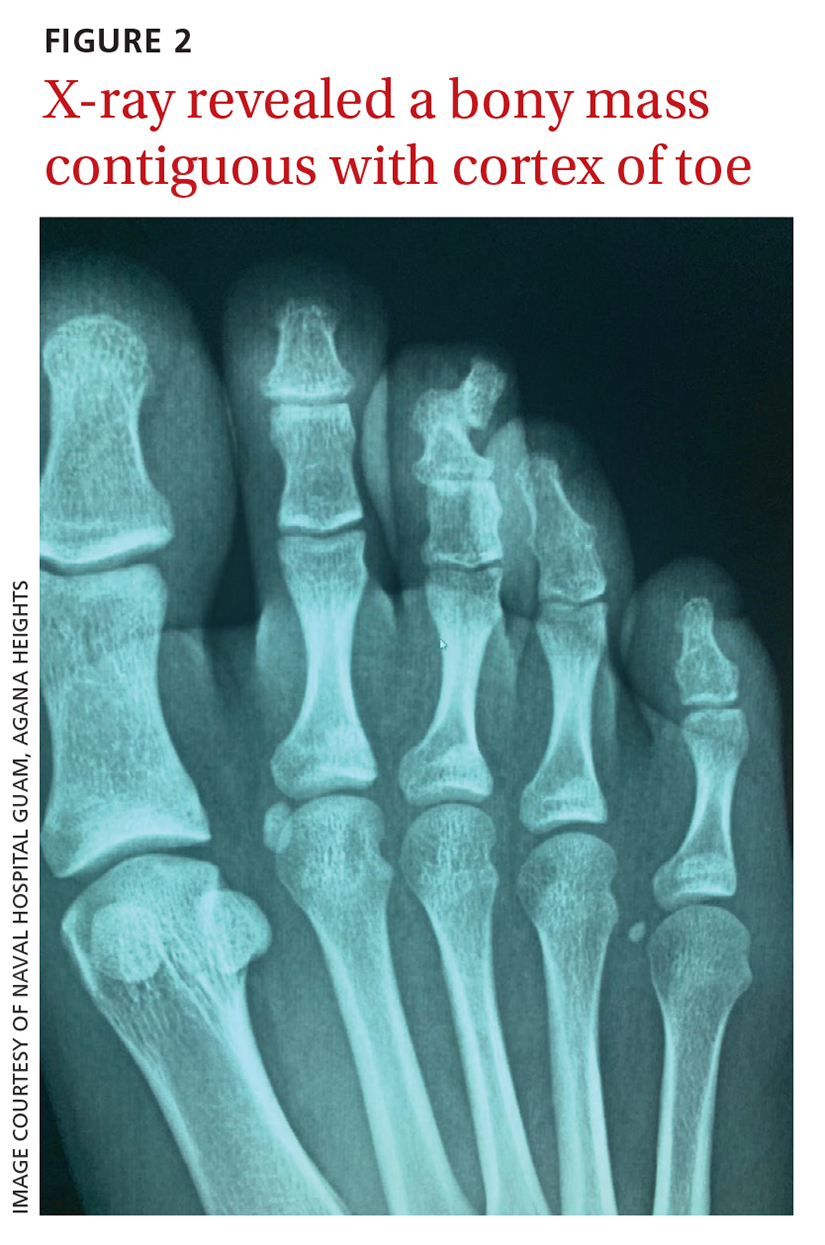
Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3

Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3

Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
Using SNRIs to prevent migraines in patients with depression
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.
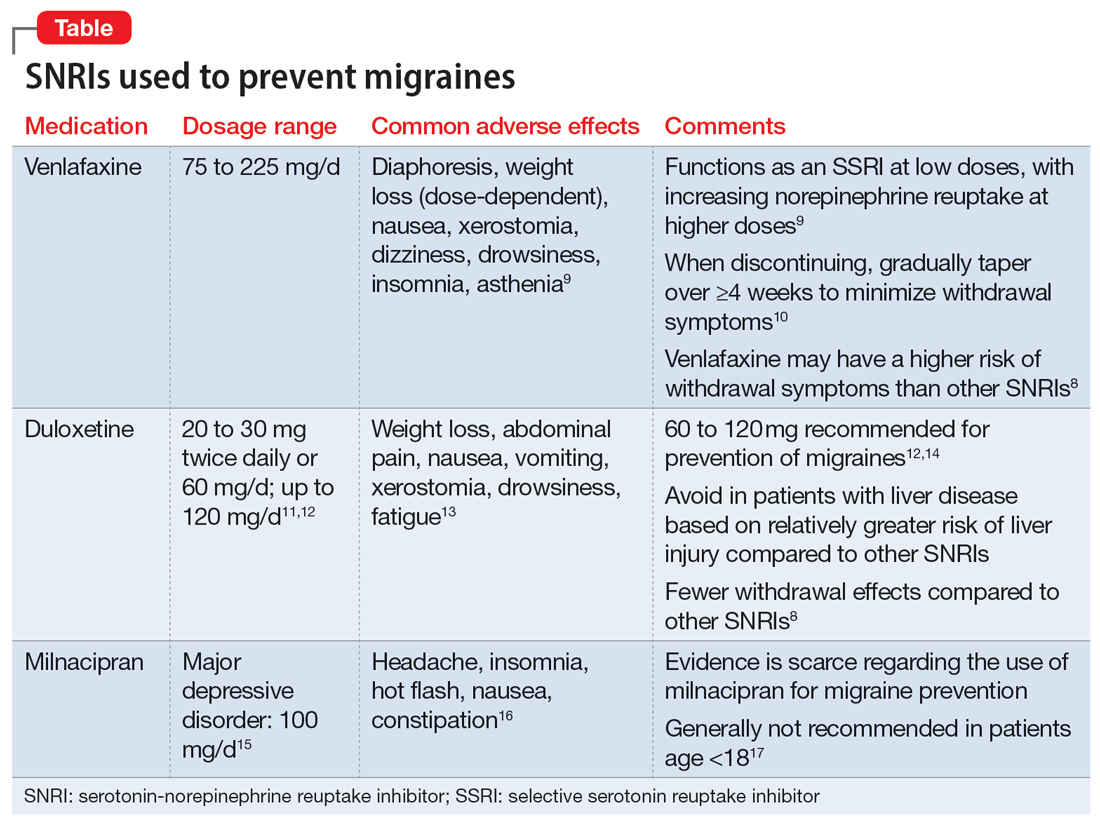
Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
Ms. D, age 45, has major depressive disorder (MDD), generalized anxiety disorder (GAD), migraines, and hypertension. At a follow-up visit, she says she has been under a lot of stress at work in the past several months and feels her antidepressant is not working well for her depression or anxiety. Ms. D notes that lately she has had more frequent migraines, occurring approximately 4 times per month during the past 3 months. She describes a severe throbbing frontal pain that occurs primarily on the left side of her head, but sometimes on the right side. Ms. D says she experiences nausea, vomiting, and photophobia during these migraine episodes. The migraines last up to 12 hours, but often resolve with sumatriptan 50 mg as needed.
Ms. D takes fluoxetine 60 mg/d for depression and anxiety, lisinopril 20 mg/d for hypertension, as well as a women’s multivitamin and vitamin D3 daily. She has not tried other antidepressants and misses doses of her medications about once every other week. Her blood pressure is 125/80 mm Hg; heart rate is 80 beats per minute; and temperature is 37° C. Ms. D’s treatment team is considering switching her to a medication that can act as preventative therapy for migraines while also treating her depression and anxiety.
Migraine is a chronic, disabling neurovascular disorder that affects approximately 15% of the United States population.1 It is the second-leading disabling condition worldwide and may negatively affect social, family, personal, academic, and occupational domains.2 Migraine is often characterized by throbbing pain, is frequently unilateral, and may last 24 to 72 hours.3 It may occur with or without aura and can be associated with nausea, vomiting, or sensitivity to light.3 Episodic migraines occur <15 days a month, while chronic migraines occur ≥15 days a month.4
Many psychiatric, neurologic, vascular, and cardiac comorbidities are more prevalent in individuals who experience migraine headaches compared to the general population. Common psychiatric comorbidities found in patients with migraines are depression, bipolar disorder, GAD, panic disorder, and posttraumatic stress disorder5; MDD is the most common.4 A person who experiences migraine headaches is 2 to 4 times more likely to develop MDD than one who does not experience migraine headaches.4
First-line treatments for preventing migraine including divalproex, topiramate, metoprolol, propranolol, and timolol.6 However, for some patients with migraines and comorbid depression or anxiety, an antidepressant may be an option. This article briefly reviews the evidence for using antidepressants that have been studied for their ability to decrease migraine frequency.
Antidepressants that can prevent migraine
Tricyclic antidepressants (TCAs) are second- or third-line options for migraine prevention.6 While TCAs have proven to be effective for preventing migraines, many patients are unable to tolerate their adverse effects (ie, anticholinergic effects, sedation).7 TCAs may be more appealing for younger patients, who may be less bothered by anticholinergic burden, or those who have difficulty sleeping.
Serotonin-norepinephrine reuptake inhibitors (SNRIs). There has been growing interest in understanding the potential utility of SNRIs as a preventative treatment for migraines. Research has found that SNRIs are as effective as TCAs for preventing migraines and also more tolerable in terms of adverse effects.7 SNRIs such as venlafaxine and duloxetine are currently prescribed off-label to prevent migraines despite a lack of FDA approval for this indication.8
Continue to: Understanding the safety and efficacy...
Understanding the safety and efficacy of SNRIs as preventative treatment for episodic migraines is useful, particularly for patients with comorbid depression. The Table8-17 details clinical information related to SNRI use.

Duloxetine has demonstrated efficacy in preventing migraines in patients with comorbid depression.8 In a 2019 study, Kisler et al14 found that duloxetine 60 mg/d for 7 weeks was more effective for migraine prophylaxis than placebo as measured by the percentage of self-estimated migraine improvement by each patient compared to pretreatment levels (duloxetine: 52.3% ± 30.4%; placebo: 26.0% ± 27.3%; P = .001).
Venlafaxine has also demonstrated efficacy for preventing migraines in patients with comorbid depression.8 One study demonstrated a significant decrease in headaches per month with the use of venlafaxine 150 mg/d compared to placebo.18 Adelman et al19 found a reduction in migraine headaches per month (16.1 to 11.1, P < .0001) in patients who took venlafaxine for an average of 6 months with a mean dose of 150 mg/d. In a study of patients who did not have a mood disorder, Tarlaci20 found that venlafaxine reduced migraine headache independent of its antidepressant action.
Though milnacipran has not been studied as extensively as other SNRIs, evidence suggests it reduces the incidence of headaches and migraines, especially among episodic migraine patients. Although it has an equipotent effect on both serotonin and norepinephrine (NE) reuptake, milnacipran has a greater NE effect compared to other SNRIs approved for treating mood disorders. A prospective, single-arm study by Engel et al21 found a significant (P < .005) reduction from baseline in all headache and migraine days per month with the use of milnacipran 100 mg/d over the course of 3 months. The number of headache days per month was reduced by 4.2 compared to baseline. This same study reported improved functionality and reduced use of acute and symptomatic medications overall due to the decrease in headaches and migraines.21
In addition to demonstrating that certain SNRIs can effectively prevent migraine, some evidence suggests certain patients may benefit from the opportunity to decrease pill burden by using a single medication to treat both depression and migraine.22 Duloxetine may be preferred for patients who struggle with adherence (such as Ms. D) due to its relatively lower incidence of withdrawal symptoms compared to venlafaxine.8
CASE CONTINUED
Ms. D’s psychiatrist concludes she would be an appropriate candidate for treatment with an SNRI due to her history of MDD and chronic migraines. Because Ms. D expresses some difficulty remembering to take her medications, the psychiatrist recommends duloxetine because it is less likely to produce withdrawal symptoms compared to venlafaxine. To decrease pill burden, fluoxetine 60 mg is stopped with no taper due to its long half-life, and duloxetine is started at 30 mg/d, with a planned increase to 60 mg/d after 1 to 2 weeks as tolerated to target both mood and migraine prophylaxis. Duloxetine will not interact with Ms. D’s current medication regimen, including lisinopril, women’s multivitamin, or vitamin D3. The psychiatrist discusses the importance of medication adherence to improve her conditions effectively and safely. Ms. D’s heart rate and blood pressure will continue to be monitored.
Related Resources
- Leo RJ, Khalid K. Antidepressants for chronic pain. Current Psychiatry. 2019;18(2):8-16,21-22.
- Williams AM, Knox ED. When to prescribe antidepressants to treat comorbid depression and pain disorders. Current Psychiatry. 2017;16(1):55-58.
Drug Brand Names
Divalproex • Depakote
Duloxetine • Cymbalta
Fluoxetine • Prozac
Lisinopril • Zestril, Prinivil
Milnacipran • Savella
Sumatriptan • Imitrex
Topiramate • Topamax
Venlafaxine • Effexor
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
1. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
2. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi:10.1016/S1474-4422(18)30322-3
3. Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N Engl J Med. 2002;346(4):257-270. doi:10.1056/NEJMra010917
4. Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504-515. doi:10.1080/09540261.2017.1326882
5. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi:10.1016/j.ncl.2019.06.001
6. Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17-24.
7. Xu XM, Liu Y, Dong MX, et al. Tricyclic antidepressants for preventing migraine in adults. Medicine (Baltimore). 2017;96(22):e6989. doi:10.1097/MD.0000000000006989
8. Burch R. Antidepressants for preventive treatment of migraine. Curr Treat Options Neurol. 2019;21(4):18. doi:10.1007/s11940-019-0557-2
9. Venlafaxine. Lexicomp. 2021. http://online.lexi.com/
10. Ogle NR, Akkerman SR. Guidance for the discontinuation or switching of antidepressant therapies in adults. J Pharm Pract. 2013;26(4):389-396. doi:10.1177/0897190012467210
11. Duloxetine [package insert]. Indianapolis, IN: Eli Lilly and Company; 2004.
12. Young WB, Bradley KC, Anjum MW, et al. Duloxetine prophylaxis for episodic migraine in persons without depression: a prospective study. Headache. 2013;53(9):1430-1437.
13. Duloxetine. Lexicomp. 2021. http://online.lexi.com/
14. Kisler LB, Weissman-Fogel I, Coghill RC, et al. Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain. 2019;35(9):753-765.
15. Mansuy L. Antidepressant therapy with milnacipran and venlafaxine. Neuropsychiatr Dis Treat. 2010;6 (Suppl I):17-22.
16. Milnacipran. Lexicomp. 2021. http://online.lexi.com/
17. Milnacipran. MedlinePlus. Updated January 22, 2022. Accessed August 19, 2022. https://medlineplus.gov/druginfo/meds/a609016.html
18. Ozyalcin SN, Talu GK, Kiziltan E, et al. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache. 2005;45(2):144-152. doi:10.1111/j.1526-4610.2005.05029.x
19. Adelman LC, Adelman JU, Von Seggern R, et al. Venlafaxine extended release (XR) for the prophylaxis of migraine and tension-type headache: a retrospective study in a clinical setting. Headache. 2000;40(7):572-580. doi:10.1046/j.1526-4610.2000.00089.x
20. Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clin Neuropharmacol. 2009;32(5):254-258. doi:10.1097/WNF.0b013e3181a8c84f
21. Engel ER, Kudrow D, Rapoport AM. A prospective, open-label study of milnacipran in the prevention of headache in patients with episodic or chronic migraine. Neurol Sci. 2014;35(3):429-435. doi:10.1007/s10072-013-1536-0
22. Baumgartner A, Drame K, Geutjens S, et al. Does the polypill improve patient adherence compared to its individual formulations? A systematic review. Pharmaceutics. 2020;12(2):190.
Postop analgesia in Saudi Arabia and the United States: A resident’s perspective
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
Could a vaccine (and more) fix the fentanyl crisis?
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
Postpartum sexual enjoyment: Does mode of delivery matter?
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
Children with sickle cell anemia not getting treatments, screening
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
Fewer than half of children aged 2-16 years with sickle cell anemia are receiving recommended annual screening for stroke, a common complication of the disease, according to a new Vital Signs report from the Centers for Disease Control and Prevention.
Many of these children also are not receiving the recommended medication, hydroxyurea, which can reduce pain and acute chest syndrome and improve anemia and quality of life, according to the report released Sept. 20.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD), which is a red blood cell disorder that primarily affects Black and African American people in the United States. It is associated with severe complications such as stroke, vison damage, frequent infections, and delayed growth, and a reduction in lifespan of more than 20 years.
SCD affects approximately 100,000 Americans and SCA accounts for about 75% of those cases.
Physician remembers her patients’ pain
In a briefing to reporters in advance of the report’s release, Debra Houry, MD, MPH, the CDC’s acting principal deputy director, recalled “long, tough nights with these young sickle cell warriors” in her career as an emergency department physician.
“[S]eeing children and teens suffering from the severe pain that often accompanies sickle cell anemia was heartbreaking,” she said.
She asked health care providers to confront racism as they build better systems for ensuring optimal treatment for children and adolescents with SCA.
“Health care providers can educate themselves, their colleagues, and their institutions about the specialized needs of people with sickle cell anemia, including how racism inhibits optimal care,” Dr. Houry said.
She said people with SCA report difficulty accessing care and when they do, they often report feeling stigmatized.
Lead author of the report, Laura Schieve, PhD, an epidemiologist with CDC’s National Center on Birth Defects and Developmental Disabilities, and colleagues looked at data from more than 3,300 children with SCA who were continuously enrolled in Medicaid during 2019. The data came from the IBM MarketScan Multi-State Medicaid Database.
Key recommendations issued in 2014
In 2014, the National Heart, Lung, and Blood Institute (NHLBI) issued two key recommendations to prevent or reduce complications in children and adolescents with SCA.
One was annual screening of children and adolescents aged 2-16 years with transcranial Doppler (TCD) ultrasound to identify those at risk for stroke. The second was offering hydroxyurea therapy, which keeps red blood cells from sickling and blocking small blood vessels, to children and adolescents who were at least 9 months old to reduce pain and the risk for several life-threatening complications.
The researchers, however, found that in 2019, only 47% and 38% of children and adolescents aged 2-9 and 10-16 years, respectively, had TCD screening and 38% and 53% of children and adolescents aged 2-9 years and 10-16 years, respectively, used hydroxyurea.
“These complications are preventable – not inevitable. We must do more to help lessen the pain and complications associated with this disease by increasing the number of children who are screened for stroke and using the medication that can help reduce painful episodes,” said Karen Remley, MD, MPH, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a press release.
Bridging the gap
Providers, parents, health systems, and governmental agencies all have roles in bringing evidence-based recommended care to young SCA patients, Dr. Houry noted.
Community organizations can also help connect families with resources and tools to increase understanding.
Dr. Schieve pointed to access barriers in that families may have trouble traveling to specialized centers where the TCD screening is given. In addition, appointments for the screening may be limited.
Children taking hydroxyurea must be monitored for the proper dosage of medication, she explained, and that can be logistically challenging as well.
Providers report they often don’t get timely information back from TCD screening programs to keep up with which children need their annual screening.
Overall, the nation lacks providers with expertise in SCD and that can lead to symptoms being dismissed, Dr. Schieve said.
Hematologists and others have a role in advocating for patients with governmental entities to raise awareness of this issue, she added.
It’s also important that electronic health records give prompts and provide information so that all providers who care for a child can track screening and medication for the condition, Dr. Schieve and Dr. Houry said.
New funding for sickle cell data collection
Recent funding to the CDC Sickle Cell Data Collection Program may help more people get appropriate care, Dr. Houry said.
The program is currently active in 11 states and collects data from people all over the United States with SCD to study trends and treatment access for those with the disease.
The data help drive decisions such as where new sickle cell clinics are needed.
“We will expand to more states serving more people affected by this disease,” Dr. Houry said.
The authors declared no relevant financial relationships.
FROM THE MMWR
‘Cracking’ technology shows promise for reducing environmental inhaled nitrous oxide impacts during labor
New evidence indicates that the use of “cracking” technology can significantly reduce the ambient levels of inhaled nitrous oxide (N2O) during labor, especially when women are coached on how best to use it.
The findings, from a quality improvement study conducted by anesthetists and midwives in the United Kingdom, appear to have implications for minimizing staff exposures and for lowering N2O’s environmental effect overall. The potent greenhouse gas has a carbon footprint that is 265 times larger than carbon dioxide.
“Our results indicate that cracking technology can reduce ambient nitrous oxide levels in the obstetric setting, with potential for reductions in environmental impacts and occupational exposure,” reported Annie Pinder, MBChB, a fellow in sustainable anesthesia at North West School of Anaesthesia, Manchester, England, and colleagues in Anaesthesia.
Proportionally, the United Kingdom is one of the largest users of inhaled N2O during labor, often for first-line pain control. A 2017 survey by the Care Quality Commission estimated that 77% of women in labor used inhaled N2O for pain, and that it didn’t preclude them from using other types of analgesia, including opioids, epidurals, and nonpharmacologic approaches.
Previous research has established the effectiveness of cracking, which uses a catalyst to convert N2O into nitrogen and oxygen. However, little is known about the effectiveness of scavenging devices that minimize waste N2O in a real-world setting, the authors said.
For the study, median ambient N2O levels were recorded for 36 women during the final 30 minutes of uncomplicated labor. Ambient N2O levels were initially recorded in 12 patients without use of three N2O scavenging devices, and then in three groups of eight patients using either a mouthpiece, a facemask with an air-filled cushion, or a low-profile facemask. Women were also coached on how to use the devices, and given feedback.
“Given that a similar magnitude of reduction in nitrous oxide levels was seen with mouthpieces and low-profile face masks, we suggest that pregnant women should be offered the option of either device when cracking is used,” the study authors wrote.
Staff feedback was generally positive, but some found use of the technology cumbersome. Sufficient staff engagement is the key to successful implementation, the researchers pointed out.
The results showed that when women consistently exhaled into the mouthpiece, median ambient N2O levels were 71% lower compared with levels recorded prior to use of the scavenging device. When women exhaled into a lightweight face mask with a flexible seal, median ambient N2O levels were 81% lower compared with baseline.
These data are consistent with the United Kingdom’s goal of achieving a net zero carbon footprint for the National Health Service by 2040, the researchers said. The study findings are also in keeping with predictions that cracking technology could reduce greenhouse gas emissions associated with N2O by an estimated 75%.
“Although cracking may make nitrous oxide ‘greener,’ it does not make it ‘green,’ ” noted Dr. Pinder and coauthor Cliff Shelton, MBChB, in an interview. Dr. Shelton is a senior clinical lecturer in anesthesia at Lancaster (England) University and a consultant anesthetist at Wythenshawe Hospital, Manchester.
Even with the use of cracking technology, the occupational effect of inhaled N2O is likely to remain higher than for other, more effective forms of anesthesia, such as epidurals and remifentanil (Ultiva), Dr. Pinder and Dr. Shelton said. Furthermore, ambient N2O levels are not a direct measure of the proportion of nitrous oxide cracked, “so there is scope for further work to more precisely understand the ‘carbon footprint’ impacts,” they pointed out.
Inhaled N20 is widely used for labor pain in the Scandinavian countries, as well as in Canada, Australia, and New Zealand. It’s also making a comeback in the United States, facilitated by the Food and Drug Administration’s (FDA) approval of a portable N2O delivery system in 2012.
The system, which delivers a mixture of 50% nitrous oxide and 50% oxygen, has offered a new option for laboring mothers, said Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, and coauthors in a 2014 report.
“Nitrous oxide works really well as an adjunct to other analgesia,” said Laura Goetzl, MD, MPH, professor of obstetrics, gynecology, and reproductive sciences at University of Texas at Houston Health Science Center. Women in labor really like having the option of inhaled N2O to manage pain, she said in an interview. “The more options that we have to offer, the better for women.”
“Not only does nitrous oxide help with perception of pain, it’s also highly effective for reducing patient anxiety,” Dr. Goetzl explained. “If a patient is waiting for an epidural, the use of nitrous oxide can be particularly helpful.”
Dr. Shelton reported that he is executive editor of Anaesthesia Reports. Dr. Pinder and the remaining coauthors disclosed having no conflicts of interest. Dr. Goetzl reported that she is on the medical advisory board of Mirvie.
This story was updated on Sept. 27, 2022.
New evidence indicates that the use of “cracking” technology can significantly reduce the ambient levels of inhaled nitrous oxide (N2O) during labor, especially when women are coached on how best to use it.
The findings, from a quality improvement study conducted by anesthetists and midwives in the United Kingdom, appear to have implications for minimizing staff exposures and for lowering N2O’s environmental effect overall. The potent greenhouse gas has a carbon footprint that is 265 times larger than carbon dioxide.
“Our results indicate that cracking technology can reduce ambient nitrous oxide levels in the obstetric setting, with potential for reductions in environmental impacts and occupational exposure,” reported Annie Pinder, MBChB, a fellow in sustainable anesthesia at North West School of Anaesthesia, Manchester, England, and colleagues in Anaesthesia.
Proportionally, the United Kingdom is one of the largest users of inhaled N2O during labor, often for first-line pain control. A 2017 survey by the Care Quality Commission estimated that 77% of women in labor used inhaled N2O for pain, and that it didn’t preclude them from using other types of analgesia, including opioids, epidurals, and nonpharmacologic approaches.
Previous research has established the effectiveness of cracking, which uses a catalyst to convert N2O into nitrogen and oxygen. However, little is known about the effectiveness of scavenging devices that minimize waste N2O in a real-world setting, the authors said.
For the study, median ambient N2O levels were recorded for 36 women during the final 30 minutes of uncomplicated labor. Ambient N2O levels were initially recorded in 12 patients without use of three N2O scavenging devices, and then in three groups of eight patients using either a mouthpiece, a facemask with an air-filled cushion, or a low-profile facemask. Women were also coached on how to use the devices, and given feedback.
“Given that a similar magnitude of reduction in nitrous oxide levels was seen with mouthpieces and low-profile face masks, we suggest that pregnant women should be offered the option of either device when cracking is used,” the study authors wrote.
Staff feedback was generally positive, but some found use of the technology cumbersome. Sufficient staff engagement is the key to successful implementation, the researchers pointed out.
The results showed that when women consistently exhaled into the mouthpiece, median ambient N2O levels were 71% lower compared with levels recorded prior to use of the scavenging device. When women exhaled into a lightweight face mask with a flexible seal, median ambient N2O levels were 81% lower compared with baseline.
These data are consistent with the United Kingdom’s goal of achieving a net zero carbon footprint for the National Health Service by 2040, the researchers said. The study findings are also in keeping with predictions that cracking technology could reduce greenhouse gas emissions associated with N2O by an estimated 75%.
“Although cracking may make nitrous oxide ‘greener,’ it does not make it ‘green,’ ” noted Dr. Pinder and coauthor Cliff Shelton, MBChB, in an interview. Dr. Shelton is a senior clinical lecturer in anesthesia at Lancaster (England) University and a consultant anesthetist at Wythenshawe Hospital, Manchester.
Even with the use of cracking technology, the occupational effect of inhaled N2O is likely to remain higher than for other, more effective forms of anesthesia, such as epidurals and remifentanil (Ultiva), Dr. Pinder and Dr. Shelton said. Furthermore, ambient N2O levels are not a direct measure of the proportion of nitrous oxide cracked, “so there is scope for further work to more precisely understand the ‘carbon footprint’ impacts,” they pointed out.
Inhaled N20 is widely used for labor pain in the Scandinavian countries, as well as in Canada, Australia, and New Zealand. It’s also making a comeback in the United States, facilitated by the Food and Drug Administration’s (FDA) approval of a portable N2O delivery system in 2012.
The system, which delivers a mixture of 50% nitrous oxide and 50% oxygen, has offered a new option for laboring mothers, said Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, and coauthors in a 2014 report.
“Nitrous oxide works really well as an adjunct to other analgesia,” said Laura Goetzl, MD, MPH, professor of obstetrics, gynecology, and reproductive sciences at University of Texas at Houston Health Science Center. Women in labor really like having the option of inhaled N2O to manage pain, she said in an interview. “The more options that we have to offer, the better for women.”
“Not only does nitrous oxide help with perception of pain, it’s also highly effective for reducing patient anxiety,” Dr. Goetzl explained. “If a patient is waiting for an epidural, the use of nitrous oxide can be particularly helpful.”
Dr. Shelton reported that he is executive editor of Anaesthesia Reports. Dr. Pinder and the remaining coauthors disclosed having no conflicts of interest. Dr. Goetzl reported that she is on the medical advisory board of Mirvie.
This story was updated on Sept. 27, 2022.
New evidence indicates that the use of “cracking” technology can significantly reduce the ambient levels of inhaled nitrous oxide (N2O) during labor, especially when women are coached on how best to use it.
The findings, from a quality improvement study conducted by anesthetists and midwives in the United Kingdom, appear to have implications for minimizing staff exposures and for lowering N2O’s environmental effect overall. The potent greenhouse gas has a carbon footprint that is 265 times larger than carbon dioxide.
“Our results indicate that cracking technology can reduce ambient nitrous oxide levels in the obstetric setting, with potential for reductions in environmental impacts and occupational exposure,” reported Annie Pinder, MBChB, a fellow in sustainable anesthesia at North West School of Anaesthesia, Manchester, England, and colleagues in Anaesthesia.
Proportionally, the United Kingdom is one of the largest users of inhaled N2O during labor, often for first-line pain control. A 2017 survey by the Care Quality Commission estimated that 77% of women in labor used inhaled N2O for pain, and that it didn’t preclude them from using other types of analgesia, including opioids, epidurals, and nonpharmacologic approaches.
Previous research has established the effectiveness of cracking, which uses a catalyst to convert N2O into nitrogen and oxygen. However, little is known about the effectiveness of scavenging devices that minimize waste N2O in a real-world setting, the authors said.
For the study, median ambient N2O levels were recorded for 36 women during the final 30 minutes of uncomplicated labor. Ambient N2O levels were initially recorded in 12 patients without use of three N2O scavenging devices, and then in three groups of eight patients using either a mouthpiece, a facemask with an air-filled cushion, or a low-profile facemask. Women were also coached on how to use the devices, and given feedback.
“Given that a similar magnitude of reduction in nitrous oxide levels was seen with mouthpieces and low-profile face masks, we suggest that pregnant women should be offered the option of either device when cracking is used,” the study authors wrote.
Staff feedback was generally positive, but some found use of the technology cumbersome. Sufficient staff engagement is the key to successful implementation, the researchers pointed out.
The results showed that when women consistently exhaled into the mouthpiece, median ambient N2O levels were 71% lower compared with levels recorded prior to use of the scavenging device. When women exhaled into a lightweight face mask with a flexible seal, median ambient N2O levels were 81% lower compared with baseline.
These data are consistent with the United Kingdom’s goal of achieving a net zero carbon footprint for the National Health Service by 2040, the researchers said. The study findings are also in keeping with predictions that cracking technology could reduce greenhouse gas emissions associated with N2O by an estimated 75%.
“Although cracking may make nitrous oxide ‘greener,’ it does not make it ‘green,’ ” noted Dr. Pinder and coauthor Cliff Shelton, MBChB, in an interview. Dr. Shelton is a senior clinical lecturer in anesthesia at Lancaster (England) University and a consultant anesthetist at Wythenshawe Hospital, Manchester.
Even with the use of cracking technology, the occupational effect of inhaled N2O is likely to remain higher than for other, more effective forms of anesthesia, such as epidurals and remifentanil (Ultiva), Dr. Pinder and Dr. Shelton said. Furthermore, ambient N2O levels are not a direct measure of the proportion of nitrous oxide cracked, “so there is scope for further work to more precisely understand the ‘carbon footprint’ impacts,” they pointed out.
Inhaled N20 is widely used for labor pain in the Scandinavian countries, as well as in Canada, Australia, and New Zealand. It’s also making a comeback in the United States, facilitated by the Food and Drug Administration’s (FDA) approval of a portable N2O delivery system in 2012.
The system, which delivers a mixture of 50% nitrous oxide and 50% oxygen, has offered a new option for laboring mothers, said Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, and coauthors in a 2014 report.
“Nitrous oxide works really well as an adjunct to other analgesia,” said Laura Goetzl, MD, MPH, professor of obstetrics, gynecology, and reproductive sciences at University of Texas at Houston Health Science Center. Women in labor really like having the option of inhaled N2O to manage pain, she said in an interview. “The more options that we have to offer, the better for women.”
“Not only does nitrous oxide help with perception of pain, it’s also highly effective for reducing patient anxiety,” Dr. Goetzl explained. “If a patient is waiting for an epidural, the use of nitrous oxide can be particularly helpful.”
Dr. Shelton reported that he is executive editor of Anaesthesia Reports. Dr. Pinder and the remaining coauthors disclosed having no conflicts of interest. Dr. Goetzl reported that she is on the medical advisory board of Mirvie.
This story was updated on Sept. 27, 2022.
FROM ANAESTHESIA
Online yoga program improves physical function in OA
Although pain did not significantly improve in the yoga group, participants only completed about two-thirds of the recommended sessions, suggesting that more benefit may be possible with greater adherence, wrote lead author Kim L. Bennell, PhD, of the University of Melbourne, and colleagues in the Annals of Internal Medicine.
“To date, an online yoga program specifically for people with knee osteoarthritis has not been investigated,” the investigators said. “The need for such evidence-based packaged online exercise programs is highlighted in the 2020 U.S. National Public Health Agenda for Osteoarthritis.”
Methods and results
The trial involved 212 adults aged 45 years or older with symptomatic knee osteoarthritis. All patients had access to online educational materials about managing osteoarthritis.
Half of the participants were randomized into the 12-week online yoga program. This self-directed, unsupervised course consisted of 12 prerecorded 30-minute instructional yoga sessions, each with a unique sequence of poses to be completed three times in one week before moving on to the next class the following week. After 12 weeks, these participants could choose to continue doing yoga via the online program for 12 additional weeks, if desired.
The primary outcomes were knee pain and physical function, gauged by a 10-point numerical rating scale and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), respectively. Adherence was defined as completion of at least 2 yoga sessions within the preceding week.
At the 12-week mark, the yoga group did not show any significant improvement in knee pain (–0.6; 95% confidence interval, –1.2 to 0.1), but they did achieve a mean 4-point reduction in WOMAC, suggesting significant improvement in knee function (–4.0; 95% CI, –6.8 to –1.3). Of note, however, this improvement was not enough to meet the threshold for minimal clinically important difference. At 24 weeks, the yoga group no longer showed significant improvement in knee function versus baseline.
“I don’t think a longer program would necessarily reduce knee pain, as benefits from a whole range of different types of exercise for knee osteoarthritis generally can show benefits within 8 weeks,” Dr. Bennell said in an interview.
Still, she noted that the average outcome in the trial may not represent what is possible if a patient commits to a regular yoga routine.
“I think it relates more to adherence [than duration], and I think benefits for knee pain would have been seen if a greater number of people had fully adhered to the program three times a week,” she said.
At 12 weeks, 68.8% of those in the yoga group were adherent, while just 28.4% were still adherent at week 24 after the optional extension period.
“As this was a self-directed program, adherence might be expected to be less than that of a supervised program,” Dr. Bennell noted.
Referring to unpublished data, Dr. Bennell said a sensitivity analysis showed that participants in the yoga group who completed yoga at least twice a week did show greater improvements in function and pain than those who did yoga less than twice per week.
“So it does suggest that adherence is important, as we might expect,” she said.
Another tool in the OA toolbox
Nick Trasolini, MD, of Wake Forest University School of Medicine, Winston-Salem, N.C., described the benefits in the trial as “modest” and noted that the improvement in function did not meet the threshold for minimal clinically important difference.
“Nevertheless,” he said in a written comment, “the [yoga] program was safe and associated with high participant satisfaction [mean satisfaction, 8 out of 10]. While this may not be the ‘silver bullet,’ it is another tool that we can offer to sufficiently motivated patients seeking non-operative solutions for knee osteoarthritis.”
Unfortunately, these tools remain “fraught with challenges,” Dr. Trasolini added.
“While multiple injection options are available (including corticosteroid, hyaluronic acid viscosupplementation, and biologic injections), the benefits of these injections can be short-lived,” he said. “This is frustrating to patients and physicians alike. Physical therapy is beneficial for knee osteoarthritis when deconditioning has led to decreased knee, hip, and core stability. However, physical therapy can be time consuming, painful, and cost prohibitive.”
In the present study, participants in the yoga group were somewhat willing (mean willingness, 5 out of 10) to pay for their 12-week yoga program. They reported that they would pay approximately $80 U.S. dollars for chance to do it all again.
The study was supported by grants from the National Health and Medical Research Council Program and the Centres of Research Excellence. The investigators disclosed additional relationships with Pfizer, Lilly, TLCBio, and others. Dr. Trasolini disclosed no relevant conflicts of interest.
Although pain did not significantly improve in the yoga group, participants only completed about two-thirds of the recommended sessions, suggesting that more benefit may be possible with greater adherence, wrote lead author Kim L. Bennell, PhD, of the University of Melbourne, and colleagues in the Annals of Internal Medicine.
“To date, an online yoga program specifically for people with knee osteoarthritis has not been investigated,” the investigators said. “The need for such evidence-based packaged online exercise programs is highlighted in the 2020 U.S. National Public Health Agenda for Osteoarthritis.”
Methods and results
The trial involved 212 adults aged 45 years or older with symptomatic knee osteoarthritis. All patients had access to online educational materials about managing osteoarthritis.
Half of the participants were randomized into the 12-week online yoga program. This self-directed, unsupervised course consisted of 12 prerecorded 30-minute instructional yoga sessions, each with a unique sequence of poses to be completed three times in one week before moving on to the next class the following week. After 12 weeks, these participants could choose to continue doing yoga via the online program for 12 additional weeks, if desired.
The primary outcomes were knee pain and physical function, gauged by a 10-point numerical rating scale and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), respectively. Adherence was defined as completion of at least 2 yoga sessions within the preceding week.
At the 12-week mark, the yoga group did not show any significant improvement in knee pain (–0.6; 95% confidence interval, –1.2 to 0.1), but they did achieve a mean 4-point reduction in WOMAC, suggesting significant improvement in knee function (–4.0; 95% CI, –6.8 to –1.3). Of note, however, this improvement was not enough to meet the threshold for minimal clinically important difference. At 24 weeks, the yoga group no longer showed significant improvement in knee function versus baseline.
“I don’t think a longer program would necessarily reduce knee pain, as benefits from a whole range of different types of exercise for knee osteoarthritis generally can show benefits within 8 weeks,” Dr. Bennell said in an interview.
Still, she noted that the average outcome in the trial may not represent what is possible if a patient commits to a regular yoga routine.
“I think it relates more to adherence [than duration], and I think benefits for knee pain would have been seen if a greater number of people had fully adhered to the program three times a week,” she said.
At 12 weeks, 68.8% of those in the yoga group were adherent, while just 28.4% were still adherent at week 24 after the optional extension period.
“As this was a self-directed program, adherence might be expected to be less than that of a supervised program,” Dr. Bennell noted.
Referring to unpublished data, Dr. Bennell said a sensitivity analysis showed that participants in the yoga group who completed yoga at least twice a week did show greater improvements in function and pain than those who did yoga less than twice per week.
“So it does suggest that adherence is important, as we might expect,” she said.
Another tool in the OA toolbox
Nick Trasolini, MD, of Wake Forest University School of Medicine, Winston-Salem, N.C., described the benefits in the trial as “modest” and noted that the improvement in function did not meet the threshold for minimal clinically important difference.
“Nevertheless,” he said in a written comment, “the [yoga] program was safe and associated with high participant satisfaction [mean satisfaction, 8 out of 10]. While this may not be the ‘silver bullet,’ it is another tool that we can offer to sufficiently motivated patients seeking non-operative solutions for knee osteoarthritis.”
Unfortunately, these tools remain “fraught with challenges,” Dr. Trasolini added.
“While multiple injection options are available (including corticosteroid, hyaluronic acid viscosupplementation, and biologic injections), the benefits of these injections can be short-lived,” he said. “This is frustrating to patients and physicians alike. Physical therapy is beneficial for knee osteoarthritis when deconditioning has led to decreased knee, hip, and core stability. However, physical therapy can be time consuming, painful, and cost prohibitive.”
In the present study, participants in the yoga group were somewhat willing (mean willingness, 5 out of 10) to pay for their 12-week yoga program. They reported that they would pay approximately $80 U.S. dollars for chance to do it all again.
The study was supported by grants from the National Health and Medical Research Council Program and the Centres of Research Excellence. The investigators disclosed additional relationships with Pfizer, Lilly, TLCBio, and others. Dr. Trasolini disclosed no relevant conflicts of interest.
Although pain did not significantly improve in the yoga group, participants only completed about two-thirds of the recommended sessions, suggesting that more benefit may be possible with greater adherence, wrote lead author Kim L. Bennell, PhD, of the University of Melbourne, and colleagues in the Annals of Internal Medicine.
“To date, an online yoga program specifically for people with knee osteoarthritis has not been investigated,” the investigators said. “The need for such evidence-based packaged online exercise programs is highlighted in the 2020 U.S. National Public Health Agenda for Osteoarthritis.”
Methods and results
The trial involved 212 adults aged 45 years or older with symptomatic knee osteoarthritis. All patients had access to online educational materials about managing osteoarthritis.
Half of the participants were randomized into the 12-week online yoga program. This self-directed, unsupervised course consisted of 12 prerecorded 30-minute instructional yoga sessions, each with a unique sequence of poses to be completed three times in one week before moving on to the next class the following week. After 12 weeks, these participants could choose to continue doing yoga via the online program for 12 additional weeks, if desired.
The primary outcomes were knee pain and physical function, gauged by a 10-point numerical rating scale and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), respectively. Adherence was defined as completion of at least 2 yoga sessions within the preceding week.
At the 12-week mark, the yoga group did not show any significant improvement in knee pain (–0.6; 95% confidence interval, –1.2 to 0.1), but they did achieve a mean 4-point reduction in WOMAC, suggesting significant improvement in knee function (–4.0; 95% CI, –6.8 to –1.3). Of note, however, this improvement was not enough to meet the threshold for minimal clinically important difference. At 24 weeks, the yoga group no longer showed significant improvement in knee function versus baseline.
“I don’t think a longer program would necessarily reduce knee pain, as benefits from a whole range of different types of exercise for knee osteoarthritis generally can show benefits within 8 weeks,” Dr. Bennell said in an interview.
Still, she noted that the average outcome in the trial may not represent what is possible if a patient commits to a regular yoga routine.
“I think it relates more to adherence [than duration], and I think benefits for knee pain would have been seen if a greater number of people had fully adhered to the program three times a week,” she said.
At 12 weeks, 68.8% of those in the yoga group were adherent, while just 28.4% were still adherent at week 24 after the optional extension period.
“As this was a self-directed program, adherence might be expected to be less than that of a supervised program,” Dr. Bennell noted.
Referring to unpublished data, Dr. Bennell said a sensitivity analysis showed that participants in the yoga group who completed yoga at least twice a week did show greater improvements in function and pain than those who did yoga less than twice per week.
“So it does suggest that adherence is important, as we might expect,” she said.
Another tool in the OA toolbox
Nick Trasolini, MD, of Wake Forest University School of Medicine, Winston-Salem, N.C., described the benefits in the trial as “modest” and noted that the improvement in function did not meet the threshold for minimal clinically important difference.
“Nevertheless,” he said in a written comment, “the [yoga] program was safe and associated with high participant satisfaction [mean satisfaction, 8 out of 10]. While this may not be the ‘silver bullet,’ it is another tool that we can offer to sufficiently motivated patients seeking non-operative solutions for knee osteoarthritis.”
Unfortunately, these tools remain “fraught with challenges,” Dr. Trasolini added.
“While multiple injection options are available (including corticosteroid, hyaluronic acid viscosupplementation, and biologic injections), the benefits of these injections can be short-lived,” he said. “This is frustrating to patients and physicians alike. Physical therapy is beneficial for knee osteoarthritis when deconditioning has led to decreased knee, hip, and core stability. However, physical therapy can be time consuming, painful, and cost prohibitive.”
In the present study, participants in the yoga group were somewhat willing (mean willingness, 5 out of 10) to pay for their 12-week yoga program. They reported that they would pay approximately $80 U.S. dollars for chance to do it all again.
The study was supported by grants from the National Health and Medical Research Council Program and the Centres of Research Excellence. The investigators disclosed additional relationships with Pfizer, Lilly, TLCBio, and others. Dr. Trasolini disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE