User login
Combo treatment eases nausea and vomiting of pregnancy
. While the benefit of either agent was clinically small for moderate to severe symptoms, the combination showed numerically larger and potentially more meaningful benefit, according to a team led by Xiao-Ke Wu, MD, PhD, of the department of obstetrics and gynecology at First Affiliated Hospital, Heilongjiang University of Chinese Medicine, and Heilongjiang Provincial Hospital in Harbin, China.
The treatments found small reductions in symptoms of less than one point to 1.6 points on an emesis scale. Nevertheless, Dr. Wu’s group wrote online June 19 in Annals of Internal Medicine that the finding “is especially significant because there is a pressing need to establish a pregnancy-safe treatment regimen and an integrative guideline for managing severe NVP.”
NVP affects as many as 85% of pregnant women, 80%-90% of whom have only mild symptoms, the authors noted. However, severe NVP and hyperemesis gravidarum, or HG, develop in about 10%. “Unfortunately, as many as 10% of wanted pregnancies with severe NVP or HG are terminated because of intolerable and untreatable symptoms and complications,” Dr. Wu told this news organization. And antiemetics may be underprescribed by general practitioners because of concerns about potential teratogenic effects, he said.
“Our findings suggest that either acupuncture or doxylamine-pyridoxine alone is a suitable for treating moderate to severe NVP, and a combination of both can be used to treat severe NVP and HG,” Dr. Wu said.
Commenting on the study but not involved in it, Catherine S. Stika, MD, a clinical professor of ob.gyn. at Northwestern University in Chicago, said the results suggest these two therapies are more suited to mild than severe symptoms. “But an RCT is important to do in order to support the use of these therapies since they’re not as widely accepted as they ought to be,” she said in an interview.
According to Dr. Stika, many pregnant women are reluctant to take drugs at all or participate in drug studies, “so the combination of nonpharmaceutical/pharmaceutical treatment might be a bit more appealing.” She noted that some women have such severe nausea they are literally starving and so weak they are bedridden or even hospitalized.
Both treatments have been recommended for some time, and the American College of Obstetricians and Gynecologists’ 2018 practice bulletin recommends acupuncture for mild nausea.
Design
The randomized, double-blind, placebo-controled 2x2 factorial trial was conducted at 13 tertiary-care hospitals in mainland China from June 2020 to February 2022. The researchers recruited 352 women in early pregnancy with moderate to severe NVP. The mean age of participants was about 29 years and the mean gestational age was about 9 weeks.
Participants were randomized into four 14-day treatment groups: active acupuncture for 30 minutes a day plus the antihistamine-vitamin B6 agent doxylamine-pyridoxine; sham acupuncture for 30 minutes daily plus doxylamine-pyridoxine; active acupuncture plus placebo; and sham acupuncture plus placebo.
The primary outcome was the reduction in Pregnancy-Unique Quantification of Emesis (PUQE) score at day 15 relative to baseline with a score of less than 6 indicating mild NVP, 6-12 indicating moderate NVP, and 13 or higher indicating severe NVP. Secondary outcomes ranged from quality of life and adverse events to maternal and perinatal complications. Acupuncture and combined treatment yielded larger though still small reductions in PUQE score, compared with control treatments. The mean differences were as follows: acupuncture, –.07; 95% confidence interval, 1.3-0.1); doxylamine-pyridoxine, –1.0: 95% CI, 1.6-0.4); combination of both, –1.6; 95% CI, 2.2-0.9). No significant interaction was detected between the interventions (P = .69).Compared with placebo treatments, pharmaceutical therapy resulted in more somnolence, while active acupuncture led to more frequent dyspnea, bruising, itching, and pain. A higher risk of babies born small for gestational age was observed in mothers who took doxylamine-pyridoxine versus placebo: odds ratio, 3.8; 95% CI, 1-14.1). Neither the placebo effects of the sham interventions nor the natural regression of symptoms experienced by many women were evaluated.
Suited to milder symptoms?
Dr. Stika called the study well-designed and well-written but cited several limitations, including the small cohort, the minor symptom improvement, and the lack of a comparator group receiving neither sham nor active treatment.
“Compared with sham combination treatments, the active combination arm was only about a point and a half better,” she said. “And would some women have got better over the 2 weeks anyway with no intervention at all? A large percentage of women with NVP do improve on their own.”
And in terms of acceptability to U.S. women, she cautioned, “The study cohort was entirely Chinese, and this is a population that already accepts acupuncture treatment.”
Countered Dr. Wu, “Medical care provided by licensed acupuncturists is approved in many countries. Certainly, it is ready to be prescribed by physicians when a pregnant patient is seeking NVP treatment.”
Dr. Stika stressed that these therapies are suited to milder NV, and would “barely take edge off severe symptoms,” for which a patient might have to “go up to a big gun like the antiemetic Zofran” (ondansetron). She is currently involved in a National Institutes of Health–funded clinical trial of the antidepressant mirtazapine (Remeron) for NVP.
Matthew Carroll, MD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, noted that doxylamine-pyridoxine is already an effective treatment for NVP, but in his experience it is often "not enough" to help patients deal with symptoms.
"Many patients are hesitant to take additional medications," he said. "If acupuncture can be safely done in pregnancy, then it seems a reasonable option as an adjuvant treatment for NVP. I think there is a cohort of pregnant people in the US who would be excited to try a complementary and nonpharmaceutical treatment option. Unfortunately, complementary therapies are rarely evaluated at a systems level for safety and so they are hard to recommend for obstetricians in the US," he added.
Dr. Carroll, who was not involved in the study. noted that "studies like this can help us counsel patients who may be seeking these treatments even if not approved or recommended by ACOG."
This study was funded by the National Key R&D Program of China and the Project of Heilongjiang Province “TouYan” Innovation Team. Support also came from the National Clinical Research Base of Chinese Medicine, the Heilongjiang Provincial Clinical Research Centre for Ovary Diseases, and the 2023 Capability Improvement Project for Evidence-based Assessment of Traditional Chinese Medicine.
Study coauthor Ben Willem J. Mol, MD, PhD, reported consulting fees from ObsEva and Merck and travel fees from Merck.
Dr. Stika and Dr. Carroll had no competing interests to disclose.
. While the benefit of either agent was clinically small for moderate to severe symptoms, the combination showed numerically larger and potentially more meaningful benefit, according to a team led by Xiao-Ke Wu, MD, PhD, of the department of obstetrics and gynecology at First Affiliated Hospital, Heilongjiang University of Chinese Medicine, and Heilongjiang Provincial Hospital in Harbin, China.
The treatments found small reductions in symptoms of less than one point to 1.6 points on an emesis scale. Nevertheless, Dr. Wu’s group wrote online June 19 in Annals of Internal Medicine that the finding “is especially significant because there is a pressing need to establish a pregnancy-safe treatment regimen and an integrative guideline for managing severe NVP.”
NVP affects as many as 85% of pregnant women, 80%-90% of whom have only mild symptoms, the authors noted. However, severe NVP and hyperemesis gravidarum, or HG, develop in about 10%. “Unfortunately, as many as 10% of wanted pregnancies with severe NVP or HG are terminated because of intolerable and untreatable symptoms and complications,” Dr. Wu told this news organization. And antiemetics may be underprescribed by general practitioners because of concerns about potential teratogenic effects, he said.
“Our findings suggest that either acupuncture or doxylamine-pyridoxine alone is a suitable for treating moderate to severe NVP, and a combination of both can be used to treat severe NVP and HG,” Dr. Wu said.
Commenting on the study but not involved in it, Catherine S. Stika, MD, a clinical professor of ob.gyn. at Northwestern University in Chicago, said the results suggest these two therapies are more suited to mild than severe symptoms. “But an RCT is important to do in order to support the use of these therapies since they’re not as widely accepted as they ought to be,” she said in an interview.
According to Dr. Stika, many pregnant women are reluctant to take drugs at all or participate in drug studies, “so the combination of nonpharmaceutical/pharmaceutical treatment might be a bit more appealing.” She noted that some women have such severe nausea they are literally starving and so weak they are bedridden or even hospitalized.
Both treatments have been recommended for some time, and the American College of Obstetricians and Gynecologists’ 2018 practice bulletin recommends acupuncture for mild nausea.
Design
The randomized, double-blind, placebo-controled 2x2 factorial trial was conducted at 13 tertiary-care hospitals in mainland China from June 2020 to February 2022. The researchers recruited 352 women in early pregnancy with moderate to severe NVP. The mean age of participants was about 29 years and the mean gestational age was about 9 weeks.
Participants were randomized into four 14-day treatment groups: active acupuncture for 30 minutes a day plus the antihistamine-vitamin B6 agent doxylamine-pyridoxine; sham acupuncture for 30 minutes daily plus doxylamine-pyridoxine; active acupuncture plus placebo; and sham acupuncture plus placebo.
The primary outcome was the reduction in Pregnancy-Unique Quantification of Emesis (PUQE) score at day 15 relative to baseline with a score of less than 6 indicating mild NVP, 6-12 indicating moderate NVP, and 13 or higher indicating severe NVP. Secondary outcomes ranged from quality of life and adverse events to maternal and perinatal complications. Acupuncture and combined treatment yielded larger though still small reductions in PUQE score, compared with control treatments. The mean differences were as follows: acupuncture, –.07; 95% confidence interval, 1.3-0.1); doxylamine-pyridoxine, –1.0: 95% CI, 1.6-0.4); combination of both, –1.6; 95% CI, 2.2-0.9). No significant interaction was detected between the interventions (P = .69).Compared with placebo treatments, pharmaceutical therapy resulted in more somnolence, while active acupuncture led to more frequent dyspnea, bruising, itching, and pain. A higher risk of babies born small for gestational age was observed in mothers who took doxylamine-pyridoxine versus placebo: odds ratio, 3.8; 95% CI, 1-14.1). Neither the placebo effects of the sham interventions nor the natural regression of symptoms experienced by many women were evaluated.
Suited to milder symptoms?
Dr. Stika called the study well-designed and well-written but cited several limitations, including the small cohort, the minor symptom improvement, and the lack of a comparator group receiving neither sham nor active treatment.
“Compared with sham combination treatments, the active combination arm was only about a point and a half better,” she said. “And would some women have got better over the 2 weeks anyway with no intervention at all? A large percentage of women with NVP do improve on their own.”
And in terms of acceptability to U.S. women, she cautioned, “The study cohort was entirely Chinese, and this is a population that already accepts acupuncture treatment.”
Countered Dr. Wu, “Medical care provided by licensed acupuncturists is approved in many countries. Certainly, it is ready to be prescribed by physicians when a pregnant patient is seeking NVP treatment.”
Dr. Stika stressed that these therapies are suited to milder NV, and would “barely take edge off severe symptoms,” for which a patient might have to “go up to a big gun like the antiemetic Zofran” (ondansetron). She is currently involved in a National Institutes of Health–funded clinical trial of the antidepressant mirtazapine (Remeron) for NVP.
Matthew Carroll, MD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, noted that doxylamine-pyridoxine is already an effective treatment for NVP, but in his experience it is often "not enough" to help patients deal with symptoms.
"Many patients are hesitant to take additional medications," he said. "If acupuncture can be safely done in pregnancy, then it seems a reasonable option as an adjuvant treatment for NVP. I think there is a cohort of pregnant people in the US who would be excited to try a complementary and nonpharmaceutical treatment option. Unfortunately, complementary therapies are rarely evaluated at a systems level for safety and so they are hard to recommend for obstetricians in the US," he added.
Dr. Carroll, who was not involved in the study. noted that "studies like this can help us counsel patients who may be seeking these treatments even if not approved or recommended by ACOG."
This study was funded by the National Key R&D Program of China and the Project of Heilongjiang Province “TouYan” Innovation Team. Support also came from the National Clinical Research Base of Chinese Medicine, the Heilongjiang Provincial Clinical Research Centre for Ovary Diseases, and the 2023 Capability Improvement Project for Evidence-based Assessment of Traditional Chinese Medicine.
Study coauthor Ben Willem J. Mol, MD, PhD, reported consulting fees from ObsEva and Merck and travel fees from Merck.
Dr. Stika and Dr. Carroll had no competing interests to disclose.
. While the benefit of either agent was clinically small for moderate to severe symptoms, the combination showed numerically larger and potentially more meaningful benefit, according to a team led by Xiao-Ke Wu, MD, PhD, of the department of obstetrics and gynecology at First Affiliated Hospital, Heilongjiang University of Chinese Medicine, and Heilongjiang Provincial Hospital in Harbin, China.
The treatments found small reductions in symptoms of less than one point to 1.6 points on an emesis scale. Nevertheless, Dr. Wu’s group wrote online June 19 in Annals of Internal Medicine that the finding “is especially significant because there is a pressing need to establish a pregnancy-safe treatment regimen and an integrative guideline for managing severe NVP.”
NVP affects as many as 85% of pregnant women, 80%-90% of whom have only mild symptoms, the authors noted. However, severe NVP and hyperemesis gravidarum, or HG, develop in about 10%. “Unfortunately, as many as 10% of wanted pregnancies with severe NVP or HG are terminated because of intolerable and untreatable symptoms and complications,” Dr. Wu told this news organization. And antiemetics may be underprescribed by general practitioners because of concerns about potential teratogenic effects, he said.
“Our findings suggest that either acupuncture or doxylamine-pyridoxine alone is a suitable for treating moderate to severe NVP, and a combination of both can be used to treat severe NVP and HG,” Dr. Wu said.
Commenting on the study but not involved in it, Catherine S. Stika, MD, a clinical professor of ob.gyn. at Northwestern University in Chicago, said the results suggest these two therapies are more suited to mild than severe symptoms. “But an RCT is important to do in order to support the use of these therapies since they’re not as widely accepted as they ought to be,” she said in an interview.
According to Dr. Stika, many pregnant women are reluctant to take drugs at all or participate in drug studies, “so the combination of nonpharmaceutical/pharmaceutical treatment might be a bit more appealing.” She noted that some women have such severe nausea they are literally starving and so weak they are bedridden or even hospitalized.
Both treatments have been recommended for some time, and the American College of Obstetricians and Gynecologists’ 2018 practice bulletin recommends acupuncture for mild nausea.
Design
The randomized, double-blind, placebo-controled 2x2 factorial trial was conducted at 13 tertiary-care hospitals in mainland China from June 2020 to February 2022. The researchers recruited 352 women in early pregnancy with moderate to severe NVP. The mean age of participants was about 29 years and the mean gestational age was about 9 weeks.
Participants were randomized into four 14-day treatment groups: active acupuncture for 30 minutes a day plus the antihistamine-vitamin B6 agent doxylamine-pyridoxine; sham acupuncture for 30 minutes daily plus doxylamine-pyridoxine; active acupuncture plus placebo; and sham acupuncture plus placebo.
The primary outcome was the reduction in Pregnancy-Unique Quantification of Emesis (PUQE) score at day 15 relative to baseline with a score of less than 6 indicating mild NVP, 6-12 indicating moderate NVP, and 13 or higher indicating severe NVP. Secondary outcomes ranged from quality of life and adverse events to maternal and perinatal complications. Acupuncture and combined treatment yielded larger though still small reductions in PUQE score, compared with control treatments. The mean differences were as follows: acupuncture, –.07; 95% confidence interval, 1.3-0.1); doxylamine-pyridoxine, –1.0: 95% CI, 1.6-0.4); combination of both, –1.6; 95% CI, 2.2-0.9). No significant interaction was detected between the interventions (P = .69).Compared with placebo treatments, pharmaceutical therapy resulted in more somnolence, while active acupuncture led to more frequent dyspnea, bruising, itching, and pain. A higher risk of babies born small for gestational age was observed in mothers who took doxylamine-pyridoxine versus placebo: odds ratio, 3.8; 95% CI, 1-14.1). Neither the placebo effects of the sham interventions nor the natural regression of symptoms experienced by many women were evaluated.
Suited to milder symptoms?
Dr. Stika called the study well-designed and well-written but cited several limitations, including the small cohort, the minor symptom improvement, and the lack of a comparator group receiving neither sham nor active treatment.
“Compared with sham combination treatments, the active combination arm was only about a point and a half better,” she said. “And would some women have got better over the 2 weeks anyway with no intervention at all? A large percentage of women with NVP do improve on their own.”
And in terms of acceptability to U.S. women, she cautioned, “The study cohort was entirely Chinese, and this is a population that already accepts acupuncture treatment.”
Countered Dr. Wu, “Medical care provided by licensed acupuncturists is approved in many countries. Certainly, it is ready to be prescribed by physicians when a pregnant patient is seeking NVP treatment.”
Dr. Stika stressed that these therapies are suited to milder NV, and would “barely take edge off severe symptoms,” for which a patient might have to “go up to a big gun like the antiemetic Zofran” (ondansetron). She is currently involved in a National Institutes of Health–funded clinical trial of the antidepressant mirtazapine (Remeron) for NVP.
Matthew Carroll, MD, a professor of obstetrics and gynecology at Baylor College of Medicine and Texas Children’s Hospital in Houston, noted that doxylamine-pyridoxine is already an effective treatment for NVP, but in his experience it is often "not enough" to help patients deal with symptoms.
"Many patients are hesitant to take additional medications," he said. "If acupuncture can be safely done in pregnancy, then it seems a reasonable option as an adjuvant treatment for NVP. I think there is a cohort of pregnant people in the US who would be excited to try a complementary and nonpharmaceutical treatment option. Unfortunately, complementary therapies are rarely evaluated at a systems level for safety and so they are hard to recommend for obstetricians in the US," he added.
Dr. Carroll, who was not involved in the study. noted that "studies like this can help us counsel patients who may be seeking these treatments even if not approved or recommended by ACOG."
This study was funded by the National Key R&D Program of China and the Project of Heilongjiang Province “TouYan” Innovation Team. Support also came from the National Clinical Research Base of Chinese Medicine, the Heilongjiang Provincial Clinical Research Centre for Ovary Diseases, and the 2023 Capability Improvement Project for Evidence-based Assessment of Traditional Chinese Medicine.
Study coauthor Ben Willem J. Mol, MD, PhD, reported consulting fees from ObsEva and Merck and travel fees from Merck.
Dr. Stika and Dr. Carroll had no competing interests to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Did ob.gyn. residencies take a hit from abortion bans?
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
Emilee Gibson, MD, recently graduated from Southern Illinois University, Springfield, and starts her ob.gyn. residency at Vanderbilt University Medical Center in Nashville, Tenn., later this month. Abortion is permitted in Illinois but banned in Tennessee, a factor she weighed cautiously when she applied for residencies.
Dr. Gibson told this news organization that medical students, not just those interested in ob.gyn., are starting to think more about what it means to move to a state where it might be difficult to access abortion care. “Just from a personal standpoint, that’s a little scary.”
The Supreme Court’s decision to overturn Roe v. Wade abortion rights last June threatened to derail ob.gyns. in training from pursuing the specialty or locating in states that have banned or limited abortion.
, but some industry leaders, residents, and medical students say it may be too early to judge the full impact of the ruling because most students were already far along in their decision and application for a 2023 residency position.
At this point, some ob.gyn. students are planning careers on the basis of whether they have family ties in a particular state, whether limiting their search might hurt their potential to match in a competitive specialty, and whether their faith in the family planning and abortion training being offered by a program outweighs the drawbacks of being in a state with abortion bans or restrictions.
Lucy Brown, MD, a recent graduate of Indiana University, Indianapolis, said in an interview that she’d be “very nervous” about living and practicing in abortion-restricted Indiana if she were ready to start a family.
Dr. Brown said that she mostly limited applications in the recent Match to ob.gyn. residencies in states that protected abortion rights. Though she applied to a program in her home state of Kentucky, she noted that it – along with a program in Missouri – was very low on her rank list because of their abortion restrictions.
Ultimately, Dr. Brown matched at Johns Hopkins University, Baltimore, where she will receive abortion training and assist with abortions throughout her residency. Maryland’s abortion rights status was a big attraction, she said. “Abortion is integrated into every aspect of the education.”
By the numbers
For students applying to residencies this summer, evaluating the state legislative landscape is a little clearer than it was 1 year ago but is still evolving. As of June 1, 56 ob.gyn. residency programs and more than 1,100 medical residents are in states with the most restrictive bans in the country (19% of all programs), according to the Bixby Center for Reproductive Health at the University of California, San Francisco.
In terms of the latest abortion laws: 14 states banned abortion, 2 states banned abortion between 6 and 12 weeks, and 9 states banned abortion between 15 and 22 weeks, whereas abortion is legal in 25 states and Washington, according to a recent analysis by the Kaiser Family Foundation.
The impact on residencies? The Association of American Medical Colleges recently reported a 2% drop in the number of U.S. MD seniors who applied to residencies and a 5% decline in the number of seniors who applied to ob.gyn. residencies. In states where abortion was banned, the number of senior applicants to ob.gyn. programs dropped by more than 10%, according to AAMC’s Research and Action Institute.
“U.S. MD seniors appear, in general, more likely to avoid states where abortions are banned,” said Atul Grover, MD, PhD, executive director of the Research and Action Institute. “That’s a big difference between states where there are abortion bans and gestational limits and states with no bans or limits; it’s almost twice as large,” Dr. Grover said in an interview. “The question is: Was it a 1-year blip or something that will be the beginning of a trend?”
In a statement to this news organization, officials from the American College of Obstetricians and Gynecologists and the Association of Professors of Gynecology and Obstetrics said that they were aware of the AAMC data but needed to further evaluate the impact of the Dobbs ruling.
A survey released at ACOG’s annual meeting in May found that 58% of third- and fourth-year medical students were unlikely to apply to a residency program in a state with abortion restrictions. Conducted after the Dobbs ruling last year, the survey found that future physicians are choosing where to attend residency according to state abortion policies, indicating that access to abortion care is changing the landscape of medical practice.
“For personal as well as professional reasons, reproductive health care access is now a key factor in residency match decisions as a result of Dobbs,” lead author Ariana Traub, MPH, said. She studies at Emory University, Atlanta, where abortion is restricted.
“Many students, including myself, struggle when trying to decide whether to stay in restricted states where the need is greatest (highest maternal mortality, infant mortality, lower number of physicians), versus going to an unrestricted state” for more comprehensive training and care, Ms. Traub said. “Regardless of this decision, Dobbs and subsequent abortion laws are making students question what matters most and how they can provide the best care.”
In another recently published survey, University of Miami fourth-year student Morgan Levy, MD, MPH, and colleagues found that 77% of students would prefer to apply to a residency program in a state that preserves access to abortion. Ensuring access to those services for themselves or a family member was a key factor, according to the paper published in the Journal of General Internal Medicine.
For Dr. Levy, who recently graduated from a school in abortion-restricted Florida and will soon apply to ob.gyn. residencies, the Dobbs decision made her more committed to becoming an ob.gyn., an interest she’s had since college, she said.
“I do not intend to limit my search,” Dr. Levy said in an interview. “In the states where there are restrictions in place, it’s really important to make sure that people are getting good care,” she said.
Differing perspective
Though survey and anecdotal data show that students and residents expressing hesitation about states with bans or restrictive laws, it appears that most who applied to residency programs during the 2023 Match did not shy away from those states. Almost all the open ob.gyn. residency positions were filled, according to the National Resident Matching Program.
There was no change in how U.S. MD seniors applying for 2023 residency ranked programs on the basis of whether abortion was legal, limited, or banned in the state where a program was based, Donna Lamb, DHSc, MBA, BSN, president and CEO of the NRMP, said.
“We’re seeing what we’ve seen over the past 5 years, and that is a very high fill rate, a very high rate of preference for ob.gyn., and not a heck of a lot of change,” Dr. Lamb said, noting that ob.gyn. programs continue to be very competitive. “We have more applicants than we have positions available,” she said.
In the most recent Match, there were 2,100 applicants (more than half U.S. MD seniors) for about 1,500 slots, with 1,499 initial matches, according to NRMP data. The overall fill rate was 99.7% after the Supplemental Offer and Assistance Program and Electronic Residency Applications process, NRMP reported. The results are similar to what NRMP reported as its previous all-time high year for ob.gyn. placements.
There was a dip in applicants from 2022 to 2023, even though the slots available stayed the same, but it was not markedly different from the previous 5 years, Dr. Lamb said.
“While the Dobbs decision may, indeed, have impacted applicant and application numbers to residency programs, interventions such as signaling may also contribute to the decrease in numbers of applications submitted as well,” AnnaMarie Connolly, MD, ACOG chief of education and academic affairs, and Arthur Ollendorff, MD, APOG president, said in a statement to this news organization.
For the first time in 2022, Match Day applicants were required to “signal” interest in a particular program in an effort to reduce the number of applications and cost to medical students, they noted.
Personal view
When it was time for Dr. Gibson to apply for ob.gyn. residencies, she wondered: Where do you apply in this landscape? But she did not limit her applications: “If I don’t apply to Indiana, Missouri, Tennessee, Wisconsin, Iowa, I’m taking a lot of really great programs off the table.” She did not want to hurt her chances for a match in a competitive specialty, she said.
“Being in Tennessee is going to give me a very different, unique opportunity to hopefully do a lot of advocacy and lobbying and hopefully have my voice heard in maybe a different way than [in Illinois],” Dr. Gibson added.
Cassie Crifase, MPH, a fourth-year student at the University of Wisconsin–Madison applying to ob.gyn. residencies in next year’s Match, said in an interview that she’s concerned about the health risk of living in a state with abortion restrictions. Wisconsin is one of those.
“My list skews toward programs that are in abortion-protected states, but I also am applying to some programs that are in restricted states.” Those states would have to help her meet the Accreditation Council for Graduate Medical Education training requirements. And, she said, she’d want to know if she could still advocate for abortion access in the state.
Sereena Jivraj, a third-year medical student at Texas Christian University in Fort Worth, said that she won’t apply to programs in Alabama, Mississippi, Arkansas, and other nearby states with abortion restrictions. However, Texas is still on her list. “I’m from Texas, my family lives in Texas, and I go to school in Fort Worth, so I have made those connections,” Ms. Jivraj said.
Student advisers generally encourage ob.gyn. hopefuls to apply to 60-100 programs to ensure that they will match, Ms. Jivraj said. “How are you supposed to apply to 100 programs if many of them fall within states with high restrictions?”
What the future holds
Ms. Jivraj said that she’s concerned about what the future holds, especially if the law does not change in Texas. “I don’t want to go to work every day wondering if I’m going to go to jail for something that I say,” she said.
Dr. Crifase has similar fears. “I want to be able to provide the best care for my patients and that would require being able to do those procedures without having to have my first thought be: Is this legal?”
“Things feel very volatile and uncertain,” Pamela Merritt, executive director of the nonprofit Medical Students for Choice in Philadelphia, where abortion is permitted, said. “What we’re asking medical students to do right now is to envision a future in a profession, a lifetime of providing care, where the policies and procedures and standards of the profession are under attack by 26 state legislatures and the federal court system,” she said.
“I don’t think you’re going to see people as willing to take risk.” She added that if someone matches to a program and then has regrets, “You can’t easily jump from residency program to residency program.”
Dr. Levy believes that the impact of the Dobbs decision is “definitely going to be a more common question of applicants to their potential programs.”
Applicants undoubtedly are thinking about how abortion restrictions or bans might affect their own health or that of their partners or families, she said. In a 2022 survey, Dr. Levy and colleagues reported that abortion is not uncommon among physicians, with 11.5% of the 1,566 respondents who had been pregnant saying they had at least one therapeutic abortion.
Students are also considering the potential ramification of a ban on emergency contraception and laws that criminalize physicians’ provision of abortion care, Dr. Levy said. Another complicating factor is individuals’ family ties or roots in specific geographic areas, she said.
Prospective residents will also have a lot of questions about how they will receive family planning training, Dr. Levy commented. “If you’re somewhere that you can’t really provide full-spectrum reproductive health care, then the question will become: How is the program going to provide that training?”
A version of this article first appeared on Medscape.com.
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Therapeutic hypothermia to treat neonatal encephalopathy improves childhood outcomes
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7
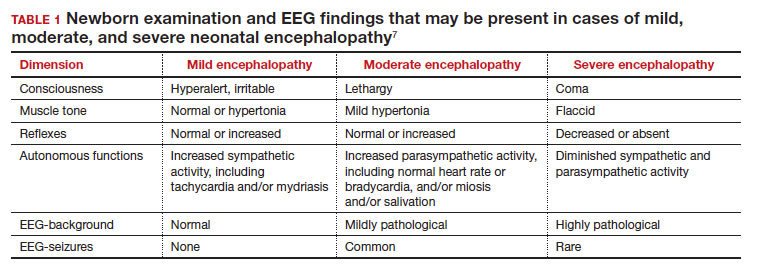
Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.
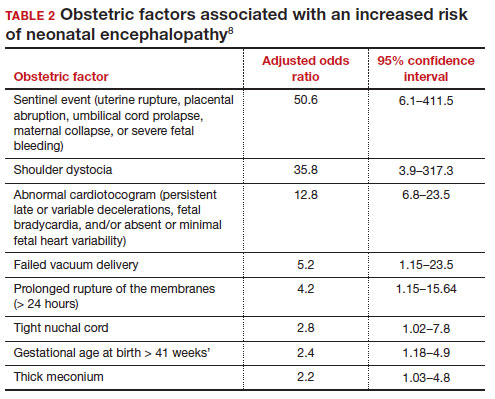
Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
Therapeutic hypothermia (TH) for moderate and severe neonatal encephalopathy has been shown to reduce the risk of newborn death, major neurodevelopmental disability, developmental delay, and cerebral palsy.1 It is estimated that 8 newborns with moderate or severe neonatal encephalopathy need to be treated with TH to prevent 1 case of cerebral palsy.1 The key elements of TH include:
- initiate hypothermia within 6 hoursof birth
- cool the newborn to a core temperature of 33.5˚ C to 34.5˚ C (92.3˚ F to 94.1˚ F) for 72 hours
- obtain brain ultrasonography to assess for intracranial hemorrhage
- obtain sequential MRI studies to assess brain structure and function
- initiate EEG monitoring for seizure activity.
During hypothermia the newborn is sedated, and oral feedings are reduced. During TH, important physiological goals are to maintain normal oxygenation, blood pressure, fluid balance, and glucose levels.1,2
TH: The basics
Most of the major published randomized clinical trials used the following inclusion criteria to initiate TH2:
- gestational age at birth of ≥ 35 weeks
- neonate is within 6 hours of birth
- an Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation at birth or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1
- moderate to severe encephalopathy or the presence of seizures
- absence of recognizable congenital abnormalities at birth.
However, in some institutions, expert neonatologists have developed more liberal criteria for the initiation of TH, to be considered on a case-by-case basis. These more inclusive criteria, which will result in more newborns being treated with TH, include3:
- gestational age at birth of ≥ 34 weeks
- neonate is within 12 hours of birth
- a sentinel event at birth or Apgar score ≤ 5 at 10 minutes of life or prolonged resuscitation or umbilical artery cord pH < 7.1 or neonatal blood gas within 60 minutes of life < 7.1 or postnatal cardiopulmonary failure
- moderate to severe encephalopathy or concern for the presence of seizures.
Birth at a gestational age ≤ 34 weeks is a contraindication to TH. Relative contraindications to initiation of TH include: birth weight < 1,750 g, severe congenital anomaly, major genetic disorders, known severe metabolic disorders, major intracranial hemorrhage, severe septicemia, and uncorrectable coagulopathy.3 Adverse outcomes of TH include thrombocytopenia, cardiac arrythmia, and fat necrosis.4
Diagnosing neonatal encephalopathy
Neonatal encephalopathy is a clinical diagnosis, defined as abnormal neurologic function in the first few days of life in an infant born at ≥ 35 weeks’ gestation. It is divided into 3 categories: mild (Stage 1), moderate (Stage 2), and severe (Stage 3).5,6 Institutions vary in the criteria used to differentiate mild from moderate neonatal encephalopathy, the two most frequent forms of encephalopathy. Newborns with mild encephalopathy are not routinely treated with TH because TH has not been shown to be helpful in this setting. Institutions with liberal criteria for diagnosing moderate encephalopathy will initiate TH in more cases. Involvement of a pediatric neurologist in the diagnosis of moderate encephalopathy may help confirm the diagnosis made by the primary neonatologist and provide an independent, second opinion about whether the newborn should be diagnosed with mild or moderate encephalopathy, a clinically important distinction. Physical examination and EEG findings associated with cases of mild, moderate, and severe encephalopathy are presented in TABLE 1.7

Continue: Obstetric factors that may be associated with neonatal encephalopathy...
Obstetric factors that may be associated with neonatal encephalopathy
In a retrospective case-control study that included 405 newborns at ≥ 35 weeks’ gestational age with neonatal encephalopathy thought to be due to hypoxia, 8 obstetric factors were identified as being associated with an increased risk of neonatal encephalopathy, including (TABLE 2)8:
1. an obstetric sentinel event (uterine rupture, placental abruption, umbilical cord prolapse, maternal collapse, or severe fetal bleeding)
2. shoulder dystocia
3. abnormal cardiotocogram (persistent late or variable decelerations, fetal bradycardia, and/or absent or minimal fetal heart variability)
4. failed vacuum delivery
5. prolonged rupture of the membranes (> 24 hours)
6. tight nuchal cord
7. gestational age at birth > 41 weeks
8. thick meconium.

Similar findings have been reported by other investigators analyzing the obstetric risk factors for neonatal encephalopathy.7,9
Genetic causes of neonatal seizures and neonatal encephalopathy
Many neonatologists practice with the belief that for a newborn with encephalopathy in the setting of a sentinel labor event, a low Apgar score at 5 minutes, an umbilical cord artery pH < 7.00, and/or an elevated lactate level, the diagnosis of hypoxic ischemic encephalopathy is warranted. However, there are many causes of neonatal encephalopathy not related to intrapartum events. For example, neonatal encephalopathy and seizures may be caused by infectious, vascular, metabolic, medications, or congenital problems.10
There are genetic disorders that can be associated with both neonatal seizures and encephalopathy, suggesting that in some cases the primary cause of the encephalopathy is a genetic problem, not management of labor. Mutations in the potassium channel and sodium channel genes are well recognized causes of neonatal seizures.11,12 Cerebral palsy, a childhood outcome that may follow neonatal encephalopathy, also has numerous etiologies, including genetic causes. Among 1,345 children with cerebral palsy referred for exome sequencing, investigators reported that a genetic abnormality was identified in 33% of the cases.13 Mutations in 86 genes were identified in multiple children. Similar results have been reported in other cohorts.14-16 Maintaining an open mind about the causes of a case of neonatal encephalopathy and not jumping to a conclusion before completing an evaluation is an optimal approach.
Parent’s evolving emotional and intellectual reaction to the initiation of TH
Initiation of TH for a newborn with encephalopathy catalyzes parents to wonder, “How did my baby develop an encephalopathy?”, “Did my obstetrician’s management of labor and delivery contribute to the outcome?” and “What is the prognosis for my baby?” These are difficult questions with high emotional valence for both patients and clinicians. Obstetricians and neonatologists should collaborate to provide consistent responses to these questions.
The presence of a low umbilical cord artery pH and high lactate in combination with a low Apgar score at 5 minutes may lead the neonatologist to diagnose hypoxic-ischemic encephalopathy in the medical record. The diagnosis of brain hypoxia and ischemia in a newborn may be interpreted by parents as meaning that labor events caused or contributed to the encephalopathy. During the 72 hours of TH, the newborn is sedated and separated from the parents, causing additional emotional stress and uncertainty. When a baby is transferred from a community hospital to a neonatal intensive care unit (NICU) at a tertiary center, the parents may be geographically separated from their baby during a critical period of time, adding to their anxiety. At some point during the care process most newborns treated with TH will have an EEG, brain ultrasound, and brain magnetic resonance imaging (MRI). These data will be discussed with the parent(s) and may cause confusion and additional stress.
The optimal approach to communicating with parents whose newborn is treated with TH continues to evolve. Best practices may include17-20:
- in-person, regular multidisciplinary family meetings with the parents, including neonatologists, obstetricians, social service specialists and mental health experts when possible
- providing emotional support to parents, recognizing the psychological trauma of the clinical events
- encouraging parents to have physical contact with the newborn during TH
- elevating the role of the parents in the care process by having them participate in care events such as diapering the newborn
- ensuring that clinicians do not blame other clinicians for the clinical outcome
- communicating the results and interpretation of advanced physiological monitoring and imaging studies, with an emphasis on clarity, recognizing the limitations of the studies
- providing educational materials for parents about TH, early intervention programs, and support resources.
Coordinated and consistent communication with the parents is often difficult to facilitate due to many factors, including the unique perspectives and vocabularies of clinicians from different specialties and the difficulty of coordinating communications with all those involved over multiple shifts and sites of care. In terms of vocabulary, neonatologists are comfortable with making a diagnosis of hypoxic-ischemic encephalopathy in a newborn, but obstetricians would prefer that neonatologists use the more generic diagnosis of encephalopathy, holding judgment on the cause until additional data are available. In terms of coordinating communication over multiple shifts and sites of care, interactions between an obstetrician and their patient typically occurs in the postpartum unit, while interactions between neonatologists and parents occur in the NICU.
Parents of a baby with neonatal encephalopathy undergoing TH may have numerous traumatic experiences during the care process. For weeks or months after birth, they may recall or dream about the absence of sounds from their newborn at birth, the resuscitation events including chest compressions and intubation, the shivering of the baby during TH, and the jarring pivot from the expectation of holding and bonding with a healthy newborn to the reality of a sick newborn requiring intensive care. Obstetricians are also traumatized by these events and support from peers and mental health experts may help them recognize, explore, and adapt to the trauma. Neonatologists believe that TH can help improve the childhood outcomes of newborns with encephalopathy, a goal endorsed by all clinicians and family members. ●
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
- Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischemic encephalopathy. Cochrane Database Syst Rev. 2013;CD003311.
- Committee on Fetus and Newborn; Papile E, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146-1150.
- Academic Medical Center Patient Safety Organization. Therapeutic hypothermia in neonates. Recommendations of the neonatal encephalopathy task force. 2016. https://www.rmf.harvard. edu/-/media/Files/_Global/KC/PDFs/Guide lines/crico_neonates.pdf. Accessed May 25, 2023.
- Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2017;74:51-61.
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696-705.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischemic encephalopathy in predicting neurodevelopmental outcome. Acta Pediatr. 1997;86:757-761.
- Lundgren C, Brudin L, Wanby AS, et al. Ante- and intrapartum risk factors for neonatal hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:1595-1601.
- Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, et al. Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2013;132:e952-e959.
- Lorain P, Bower A, Gottardi E, et al. Risk factors for hypoxic-ischemic encephalopathy in cases of severe acidosis: a case-control study. Acta Obstet Gynecol Scand. 2022;101:471-478.
- Russ JB, Simmons R, Glass HC. Neonatal encephalopathy: beyond hypoxic-ischemic encephalopathy. Neo Reviews. 2021;22:e148-e162.
- Allen NM, Mannion M, Conroy J, et al. The variable phenotypes of KCNQ-related epilepsy. Epilepsia. 2014;55:e99-e105.
- Zibro J, Shellhaas RA. Neonatal seizures: diagnosis, etiologies and management. Semin Neurol. 2020;40:246-256.
- Moreno-De-Luca A, Millan F, Peacreta DR, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475.
- Srivastava S, Lewis SA, Cohen JS, et al. Molecular diagnostic yield of exome sequencing and chromosomal microarray in cerebral palsy. A systematic review and meta-analysis. JAMA Neurology. 2022;79:1287-1295.
- Gonzalez-Mantilla PJ, Hu Y, Myers SM, et al. Diagnostic yield of exome sequencing in cerebral palsy and implications for genetic testing guidelines. A systematic review and meta-analysis. JAMA Pediatr. Epub March 6, 2023.
- van Eyk C, MacLennon SC, MacLennan AH. All patients with cerebral palsy diagnosis merit genomic sequencing. JAMA Pediatr. Epub March 6, 2023.
- Craig AK, James C, Bainter J, et al. Parental perceptions of neonatal therapeutic hypothermia; emotional and healing experiences. J Matern Fetal Neonatal Med. 2020;33:2889-2896. doi: 10.1080/14767058.2018.1563592.
- Sagaser A, Pilon B, Goeller A, et al. Parent experience of hypoxic-ischemic encephalopathy and hypothermia: a call for trauma informed care. Am J Perinatol. Epub March 4, 2022.
- Cascio A, Ferrand A, Racine E, et al. Discussing brain magnetic resonance imaging results for neonates with hypoxic-ischemic encephalopathy treated with hypothermia: a challenge for clinicians and parents. E Neurological Sci. 2022;29:100424.
- Thyagarajan B, Baral V, Gunda R, et al. Parental perceptions of hypothermia treatment for neonatal hypoxic-ischaemic encephalopathy. J Matern Fetal Neonatal Med. 2018;31:2527-2533.
Can cffDNA technology be used to determine the underlying cause of pregnancy loss to better inform future pregnancy planning?
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
Hartwig TJ, Ambye L, Gruhn JR, et al. Cell-free fetal DNA for genetic evaluation in Copenhagen Pregnancy Loss Study (COPL): a prospective cohort study. Lancet. 2023;401:762-771. https://doi.org/10.1016/S0140-6736(22)02610-1.
Expert Commentary
A devastating outcome for women, pregnancy loss is directly proportional to maternal age, estimated to occur in approximately 15% of clinically recognized pregnancies and 30% of preclinical pregnancies.1 Approximately 80% of pregnancy losses occur in the first trimester.2 The frequency of clinically recognized early pregnancy loss for women aged 20–30 years is 9% to 17%, and these rates increase sharply, from 20% at age 35 years to 40% at age 40 years, and 80% at age 45 years. Recurrent pregnancy loss (RPL), defined as the spontaneous loss of 2 or more clinically recognized pregnancies, affects less than 5% of women.3 Genetic testing using chromosomal microarray analysis (CMA) has identified aneuploidy in about 55% of cases of miscarriage.4
Following ASRM guidelines for the evaluation of RPL, which consists of analyzing parental chromosomal abnormalities, congenital and acquired uterine anomalies, endocrine imbalances, and autoimmune factors (including antiphospholipid syndrome), no explainable cause is determined in 50% of cases.3 Recently, it has been shown that more than 90% of patients with RPL will have a probable or definitive cause identified when CMA testing on miscarriage tissue with the ASRM evaluation guidelines.5
Details of the study
In this prospective cohort study from Denmark, the authors analyzed maternal serum for cell-free fetal DNA (cffDNA) to determine the ploidy status of the pregnancy loss. One thousand women older than age 18 were included (those who demonstrated an ultrasound-confirmed intrauterine pregnancy loss prior to 22 weeks’ gestation). Maternal blood was obtained while pregnancy tissue was in situ or within 24 hours of passage of products of conception (POC), then analyzed by genome-wide sequencing of cffDNA.
For the first 333 recruited women (validation phase), direct sequencing of the POC was performed for sensitivity and specificity. Following the elimination of inconclusive samples, 302 of the 333 cases demonstrated a sensitivity of 85% and specificity of 93%. In the subsequent evaluation of 667 women, researchers analyzed maternal serum from the gestational age of fetuses ranging from 35 days to 149 days.
Results. In total, nearly 90% of cases yielded conclusive results, with 50% euploid, 46% aneuploid, and 4% multiple aneuploidies. Earlier gestational ages (less than 7 weeks) had a no-call rate (ie, inconclusive) of approximately 50% (only based on 16 patients), with results typically obtained in maternal serum following passage of POC; in pregnancies at gestational ages past 7 weeks, the no-call rate was about 10%. In general, the longer the time after the pregnancy tissue passed, the higher likelihood of a no-call result.
Applying the technology of single-nucleotide polymorphism (SNP)-based CMA can improve identification of fetal and/or maternal sources as causes of pregnancy loss with accuracy, but it does require collection of POC. Of note, samples were deficient in this study, the authors cite, in one-third of the cases. Given this limitation of collection, the authors argue for use of the noninvasive method of cffDNA, obtained from maternal serum.
Study strengths and weaknesses
Several weaknesses of this study are highlighted. Of the validation cohort, one-third of pregnancy tissue could not be analyzed due to insufficient collection. Only 73% of cases allowed for DNA isolation from fetal tissue or chorionic villi; in 27% of cases samples were labeled “unknown tissue.” In those cases classified as unknown, 70% were further determined to be maternal. When all female and monosomy cases were excluded in an effort to assuredly reduce the risk of contamination with maternal DNA, sensitivity of the cffDNA testing process declined to 78%. Another limitation was the required short window for maternal blood sampling (within 24 hours) and its impact on the no-call rate.
The authors note an association with later-life morbidity in patients with a history of pregnancy loss and RPL (including cardiovascular disease, type 2 diabetes, and mental health disorders), thereby arguing for cffDNA-based testing versus no causal testing; however, no treatment has been proven to be effective at reducing pregnancy loss. ●
The best management course for unexplained RPL is uncertain. Despite its use for a euploid miscarriage or parental chromosomal structural rearrangement, in vitro fertilization with preimplantation genetic testing remains an unproven modality.6,7 Given that approximately 70% of human conceptions never achieve viability, and 50% fail spontaneously before being detected,8 the authors’ findings demonstrate peripheral maternal blood can provide a reasonably high sensitivity and specificity for fetal ploidy status when compared with direct sequencing of pregnancy tissue. As fetal aneuploidy offers a higher percentage of subsequent successful pregnancy outcomes, cffDNA may offer reassurance, or direct further testing, following a pregnancy loss. As an application of their results, evaluation may be deferred for an aneuploid miscarriage.
—MARK P. TROLICE, MD, MBA
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
- Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391-400. doi: 10.1055/s-0028-1087105.
- Wang X, Chen C , Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79:577-584.
- Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2012;98: 1103-1111.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/tacg.s320778.
- Popescu F, Jaslow FC, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33:579-587. https://doi.org/10.1093/humrep/dey021.
- Dahdouh EM, Balayla J, Garcia-Velasco JA, et al. PGT-A for recurrent pregnancy loss: evidence is growing but the issue is not resolved. Hum Reprod. 2021;36:2805-2806. https://doi.org/10.1093/humrep/deab194.
- Iews M, Tan J, Taskin O, et al. Does preimplantation genetic diagnosis improve reproductive outcome in couples with recurrent pregnancy loss owing to structural chromosomal rearrangement? A systematic review. Reproductive Bio Medicine Online. 2018;36:677-685. https://doi.org/10.1016 /j.rbmo.2018.03.005.
- Papas RS, Kutteh WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. 2021;14:321-329. https://doi.org/10.2147/TACG.S320778.
How has cannabis legalization affected pregnant mothers?
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A population-based study shows that the rate of cannabis-related acute care use during pregnancy increased from 11 per 100,000 pregnancies before legalization to 20 per 100,000 pregnancies afterward: an increase of 82%. Absolute increases were small, however.
“Our findings are consistent with studies highlighting that cannabis use during pregnancy has been increasing in North America, and this study suggests that cannabis legalization might contribute to and accelerate such trends,” study author Daniel Myran, MD, MPH, a public health and preventive medicine physician at the University of Ottawa in Ontario, said in an interview.
The study was published online in the Canadian Medical Association Journal.
Risks for newborns
In a 2019 study, 7% of U.S. women reported using cannabis during pregnancy during 2016-2017, which was double the rate of 3.4% for 2002-2003.
Dr. Myran and colleagues hypothesized that legalizing nonmedical cannabis has affected the drug’s use during pregnancy in Ontario. “We also hypothesized that hospital care for cannabis use would be associated with adverse neonatal outcomes, even after adjusting for other important risk factors that may differ between people with and without cannabis use,” he said.
The researchers’ repeated cross-sectional analysis evaluated changes in the number of pregnant people who received acute care from January 2015 to July 2021 among all patients who were eligible for Ontario’s public health coverage. The final study cohort included 691,242 pregnant patients, of whom 533 had at least one pregnancy with cannabis-related acute care visits. These mothers had a mean age of 24 years vs. 30 for their counterparts with no such visits.
Using segmented regression, the researchers compared changes in the quarterly rate of pregnant people with acute care related to cannabis use (the primary outcome) with those of acute care for mental health conditions or for noncannabis substance use (the control conditions).
“Severe morning sickness was a major risk factor for care in the emergency department or hospital for cannabis use,” said Dr. Myran. “Prior work has found that people who use cannabis during pregnancy often state that it was used to manage challenging symptoms of pregnancy such as morning sickness.”
Most acute care events (72.2%) were emergency department visits. The most common reasons for acute care were harmful cannabis use (57.6%), followed by cannabis dependence or withdrawal (21.5%), and acute cannabis intoxication (12.8%).
Compared with pregnancies without acute care, those with acute care related to cannabis had higher rates of adverse neonatal outcomes such as birth before 37 weeks’ gestational age (16.9% vs. 7.2%), birth weight at or below the bottom fifth percentile after adjustment for gestational age (12.1% vs. 4.4%), and neonatal intensive care unit admission in the first 28 days of life (31.5% vs. 13%).
An adjusted analysis found that patients younger than 35 years and those living in rural settings or the lowest-income neighborhoods had higher odds of acute cannabis-related care during pregnancy. Patients who received acute care for any substance use or schizophrenia before pregnancy or who accessed outpatient mental health services before pregnancy had higher risk for cannabis-related acute care during pregnancy. Mothers receiving acute care for cannabis also had higher risk for acute care for hyperemesis gravidarum during pregnancy (30.9%).
The rate of acute care for other types of substance use such as alcohol and opioids did not change after cannabis legalization, and acute care for mental health conditions such as anxiety and depression during pregnancy declined by 14%, Dr. Myran noted.
“Physicians who care for pregnant people should consider increasing screening for cannabis use during pregnancy,” said Dr. Myran. “In addition, repeated nonstigmatizing screening and counseling may be indicated for higher-risk groups identified in the study, including pregnancies with severe morning sickness.”
The U.S. perspective
Commenting on the study, M. Camille Hoffman, MD, MSc, a maternal-fetal medicine specialist at the University of Colorado in Aurora, said that the findings likely indicate that legalization has made cannabis users less reluctant to come forward for urgent care. “They cannot really claim that this is equivalent to more use, just that more people are willing to present,” she said. Dr. Hoffman was not involved in the study.
The Canadian results do not align perfectly with what is seen in the United States. “It does suggest that there may be more cannabinoid hyperemesis being coded as hyperemesis gravidarum, which is a pregnancy-specific condition vs. a cannabis-dependence-related one,” said Dr. Hoffman.
Literature in the United States often includes tobacco use as a covariate, she added. “This study does not appear to do that,” she said. “Rather, it uses any substance use. Because of this, it is difficult to really know the contribution of cannabis to the adverse pregnancy outcomes vs. the combination of tobacco and cannabis.”
Finally, she pointed out, the proportion of those presenting for acute care for substance use in the 2 years before conception was 22% for acute care visits for cannabis vs 1% for no acute care visits. “This suggests to me that this was a highly vulnerable group before the legalization of cannabis as well. The overall absolute difference is nine in total per 100,000 – hardly enough to draw any real conclusions. Again, maybe those nine were simply more willing to come forth with concerns with cannabis being legal.”
There is no known safe level of cannabis consumption, and its use by pregnant women has been linked to later neurodevelopmental issues in their offspring. A 2022 U.S. study suggested that cannabis exposure in the womb may leave children later in life at risk for autism, psychiatric disorders, and problematic substance abuse, particularly as they enter peak periods of vulnerability in late adolescence.
As to the impact of legalization in certain U.S. states, a 2022 study found that women perceived legalization to mean greater access to cannabis, increased acceptance of use, and greater trust in cannabis retailers. In line with Dr. Hoffman’s view, this study suggested that legalization made pregnant women more willing to discuss cannabis use during pregnancy honestly with their care providers.
In the United States, prenatal cannabis use is still included in definitions of child abuse or neglect and can lead to termination of parental rights, even in states with full legalization.
“These findings highlight the need for ongoing monitoring of markers of cannabis use during pregnancy after legalization,” said Dr. Myran. He also called for effective policies in regions with legal cannabis, such as increased warning labels on cannabis products.
This study was supported by the Canadian Institutes of Health Research and the University of Ottawa site of ICES, which is funded by an annual grant from the Ontario Ministry of Health and Ministry of Long-Term Care. Dr. Myran reports a speaker fee from McMaster University. Dr. Hoffman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMAJ
Fibroid characteristics can help us anticipate postpartum hemorrhage
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Fibroids, or leiomyomas, are noncancerous monoclonal tumors of the smooth muscle layer of the uterus. Fibroids occur more frequently in Black patients and their prevalence increases with age. The hormonally responsive nature of fibroids, frequently leading to growth with estrogen and progesterone exposure, makes them of particular concern during pregnancy.
Although most patients with fibroids do not have pregnancy complications directly attributable to their fibroids, prior studies have reported several associations, including painful degeneration, early pregnancy loss, preterm birth, placental abruption, malpresentation, and postpartum hemorrhage. Fibroids may predispose to uterine atony and hemorrhage by disrupting or impairing the synchronization and coordination of uterine contractions. Within the current body of literature, it remains less certain whether certain fibroid characteristics are associated with increased hemorrhage risk.
Prior studies evaluating the association between specific fibroid characteristics and postpartum hemorrhage have yielded inconsistent findings. In our study, we evaluated whether certain fibroid characteristics are associated with hemorrhage requiring blood transfusion. Specifically, our goal was to determine whether larger or more numerous fibroids increase the risk of transfusion.
This was a retrospective cohort study spanning 2019-2022. A total of 4,421 patients were included in this study. Fibroid characteristics were collected, including size, number, and location. Fibroid size was classified as small (< 5 cm), medium (5-10 cm), and large (> 10 cm).
In terms of number of fibroids, there was no significant increase in transfusions when comparing one fibroid to multiple fibroids. When assessing fibroid size, however, we did observe a significant incremental increase in rate of transfusions with increasing fibroid size. In terms of fibroid location, patients with fibroids in the lower uterine segment or cervix were about 1.5 times more likely to have hemorrhage requiring transfusion, compared with those without a fibroid in that location.
This study allows practitioners to better risk-stratify patients from the practical perspective of postpartum hemorrhage requiring transfusions. In pregnant patients with fibroids, the specific fibroid characteristics can help us better anticipate clinically significant postpartum hemorrhage. In such patients, it is important to document specific fibroid characteristics, especially the largest fibroid diameter and fibroid location in the lower uterine segment or cervix. This emphasizes the importance of careful sonographic evaluation and consistent documentation of fibroids in pregnant patients.
Our study helps guide more informed counseling and risk stratification in this population, with increasing risk according to fibroid size and location. Patients with high-risk features, that is, medium or large fibroids and those with fibroids located in the lower uterine segment or cervix, should thus receive counseling about their increased risk of hemorrhage. As providers, we can help ameliorate this risk by optimizing hemoglobin levels of those at increased risk prior to delivery, and by ensuring availability of appropriate resources at the time of delivery.
Dr. Yaghoubian is a maternal-fetal medicine fellow at North Shore University Hospital/Long Island Jewish Medical Center in Manhasset, N.Y., and will be joining the faculty at the same institution. Email Dr. Yaghoubian at [email protected].
Intervention reduces severe postpartum hemorrhage by 60% in developing nations
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
Postpartum hemorrhage (PPH) is the leading cause of maternal deaths worldwide, particularly in the least developed and developing countries. Of the 14 million female patients affected each year, approximately 70,000 cases result in death. However, according to a new study conducted by the World Health Organization and the University of Birmingham (England), a simple and affordable strategy may reduce the occurrence of severe cases during vaginal delivery.
These cases are defined as entailing blood loss greater than or equal to 1,000 mL in the 24 hours following delivery. This intervention also substantially reduced the need for blood transfusions, which are often costly and difficult to obtain.
In this trial, 80 secondary-level hospitals in Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Researchers identified that, among hospitals and patients with data, a primary outcome event occurred in 1.6% of the patients in the intervention group, compared with 4.3% of those in the usual-care group. In addition, PPH was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group. The treatment bundle was used in 91.2% and 19.4%, respectively.
The E-MOTIVE intervention, which is intended for use by health care professionals, consists of three elements:
- A strategy for early detection of PPH, which allows triggering of the “first response” treatment bundle
- A first response bundle called MOTIVE, which is based on WHO guidelines and consists of uterine massage, oxytocic drugs, tranexamic acid, IV fluids, and examination of the genital tract and escalation
- An implementation strategy that focuses on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with trigger line, and MOTIVE emergency trolley or carry case
During a WHO press conference, study author Arri Coomarasamy, MD, said, “This new approach to treating postpartum hemorrhage could radically improve women’s chances of surviving childbirth globally, helping them get the treatment they need when they need it.”
Dr. Coomarasamy, who is also co-director of the WHO Collaborating Centre on Global Women’s Health at the University of Birmingham, added, “Time is of the essence when responding to postpartum bleeding, so interventions that eliminate delays in diagnosis or treatment should be game-changers for maternal health.”
PPH a ‘preventable’ problem
In Brazil, maternal mortality is still one of the most significant challenges in public health. In recent years, the COVID-19 pandemic exacerbated the difficulties and weaknesses in the health care system for pregnant women and new mothers.
The maternal mortality ratio (MMR) is the number of maternal deaths per 100,000 live births from any cause related to or aggravated by pregnancy or its management (excluding accidental or incidental causes) during pregnancy and childbirth or within 42 days of termination of pregnancy. In 2021, the MMR was 113. This figure was almost double the 55.3 maternal deaths per 100,000 live births reported in 2019, which was before the pandemic. Preliminary data from the Brazilian Ministry of Health collected by the Brazilian Obstetric Observatory (OOBr) indicate that the MMR in 2022 decreased to 50.6 maternal deaths per 100,000 live births. However, these numbers could increase, because the maternal mortality committees are still reviewing cases.
Rossana Pulcineli Vieira Francisco, MD, PhD, a professor of obstetrics and gynecology at the University of São Paulo School of Medicine and obstetrics coordinator of the OOBr, affirmed that although these numbers have dropped, they are still much higher than the targets set by health authorities. Brazil is a participant of the UN agreement that aims to reduce MMR to a maximum of 30 maternal deaths per 100,000 live births per year by 2030. “We still have a long way to go to reach this goal within the next 7 years,” Dr. Francisco warned.
She compared rates in Brazil with those of more developed regions. According to the data, the mean MMR in Europe is 13 maternal deaths per 100,000 live births. “Portugal was shocked when maternal deaths surpassed 20 [maternal deaths per 100,000 live births in 2020] amidst the COVID-19 pandemic. The ratio in Brazil, even before the pandemic, was 55,” she said.
“Maternal mortality and infant mortality ratios are powerful indicators of the quality of the health care system,” added the OOBr coordinator, who asserted that investing in primary and prenatal care is essential. Dr. Francisco also pointed out the preventable nature of maternal mortality in Brazil. “The three main causes of direct maternal mortality in Brazil are high blood pressure, postpartum hemorrhage, and infection, particularly in the postpartum period. These issues are all considered preventable.”
Although it is difficult to prevent preeclampsia, hospital care and maternity care measures can significantly reduce the number of deaths caused by this condition. “For high blood pressure, what we most miss is having specialized prenatal care for at-risk women when the problem is diagnosed during pregnancy.”
Regarding PPH, Dr. Francisco calls attention to the importance of training teams to treat the problem. “In Brazil, the lack of training [for professionals] is still a serious problem.”
According to her, investments in rapid response systems are also needed. “As the baby needs nutrients and oxygen, the uterus becomes full of blood vessels at the end of pregnancy. As a result, a PPH leads to significant blood loss. In Brazil, some hospitals don’t even have blood bags. And in some cases, there may not be enough time to get a blood bag from somewhere else.”
Dr. Francisco also points out that, although it may not be feasible for all of Brazil’s health care units to have blood banks, integrated structures could be created to facilitate access to blood in case of emergency.
A grant from the Bill & Melinda Gates Foundation supported the E-MOTIVE project.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Prenatal sleep problems, depression linked to poorer outcomes
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Together, the two studies suggest that commonly overlooked experiences in the prenatal period can have negative effects down the line if clinicians aren’t asking patients about them and addressing the issue.
”I think the national conversation around mental health in general will hopefully carry us forward to better supporting the patients who are coming in with preexisting conditions,” lead author Minnie Jang, a 4th-year medical student at Johns Hopkins University, Baltimore, said in an interview.
Most of the attention on mood disorders of pregnancy focus on the postpartum period, but preexisting or new-onset depression during pregnancy deserves more attention, Ms. Jang told attendees. ACOG recommends that clinicians screen all patients at least once during the perinatal period, but that could be anywhere from early pregnancy to the postpartum period. Ms. Jang would like to see recommendations addressing both early pregnancy and the postpartum period.
“I think there’s this framing that postpartum depression is a distinct entity from other mental health conditions whereas it’s really part of a continuum,” Ms. Jang said in an interview.
She retrospectively analyzed the medical records of all pregnant women who completed the Edinburgh Postnatal Depression Scale (EPDS) during their first or second trimesters between 2002 and 2021 at Johns Hopkins Hospital. Among the 718 women who were screened in early pregnancy, 44.6% were Black or African American, 39.7% were white, and 15.7% were of a different race. Nearly all (94%) were not Hispanic/Latino.
Most (59%) were partnered, employed (68%), and had private insurance (58%). Only 7% used tobacco while 11% used alcohol and 6% used illicit drugs.
Twelve percent of the patients scored positive for depression, with a score of at least 10 or an affirmative answer to question 10 regarding self-harm. These women tended to be younger (P = .034), with an median age of 28 at their first visit versus 31 for those who screened negative, and were more likely to be publicly insured (P = .013) and without a partner (P = .005).
Patients who screened positive were more likely to have a history of substance use or history of a previous psychiatric diagnosis (P < .0001 for both). In addition, more patients who screened positive (49%) than those who screened negative (26%) had fetal complications (P < .001).
”There are some interesting subgroups of patients who are screening positive for depressive symptoms early on in pregnancy,” Ms. Jang said. Some come into pregnancy with preexisting mental health conditions while others have situational depressive symptoms, such as the subgroup referred to social work who had diagnosed fetal complications, she said. “Then there’s a whole other group of patients who are developing new symptoms during pregnancy.”
Patients who screened positive tended to start prenatal care later, at a median 12.3 weeks gestational age, than patients who screened negative, at a median 10.7 weeks gestational age (P = .002), the analysis found.
The number of routine prenatal care visits did not significantly differ between those who screened positive and those who screened negative, but patients with positive depression screens were almost half as likely to complete glucose tolerance testing (odds ratio, 0.6) or group B streptococcus testing (OR, 0.56) after adjusting for insurance status, gravidity, and gestational age at the patient’s first visit.
The researchers also identified a significant positive association between higher EPDS scores and the number of labor and delivery triage visits (P = .006). There were no significant differences in the rates of Tdap vaccination or screening for sexually transmitted infections between the two groups.
Poor sleep linked to later depression
The other study was prospective, using data from the PATCH Prenatal Care and Maternal and Child Health Outcomes study, which initially “compared health outcomes and satisfaction with prenatal care between patients receiving Centering Pregnancy group prenatal care and patients receiving traditional prenatal care,” the authors explained. This secondary analysis looked at sleep problems and postpartum depression.
“We don’t routinely ask patients about sleep or screen patients for sleeping issues,” lead author Carolyn Sinow, MD, a 4th-year resident at Kaiser Permanente Santa Clara (Calif.) Medical Center, said in an interview. “I think that we need to take sleep complaints more seriously overall, especially in early pregnancy.” While sleep problems in the third trimester often have more to do with discomforts from pregnancy itself, better sleep “in the first and second trimester is something we can really target with good sleep hygiene,” she added.
The 336 pregnant participants were recruited from Health Connect as long as they had a singleton pregnancy, were receiving prenatal care from Kaiser Permanente Northern California, and completed baseline questionnaires about their sleep and depression and anxiety symptoms during their first trimester between August 2020 and April 2021. Those with clinical depression or a high-risk pregnancy were excluded. The participants then completed the questionnaires again between 4 and 8 weeks post partum.
After adjusting for baseline depression and potential confounders, patients with poor sleep quality, indicated by a score greater than 5 on the Pittsburgh Sleep Quality Index (PSQI), were 12% more likely to develop postpartum depression, indicated by a score on the Patient Health Questionnaire depression scale (PHQ-8) of 10 or greater (relative risk, 1.12; 95% confidence interval, 1.01-1.25).
The two aspects of sleep that specifically correlated with postpartum depression were sleep quality and sleep latency, or taking a long time to fall asleep. Those reporting poor sleep quality were twice as likely to develop postpartum depression (relative risk, 2.18; 95% CI, 1.22-3.91), and those who took a while to fall asleep were 52% more likely to develop postpartum depression (RR, 1.52; 95% CI, 1.06-2.17).
Though the study also found prenatal sleep problems correlated with higher postpartum anxiety scores on the General Anxiety Disorder scale (GAD-7), the results were not statistically significant.
Kathleen Morrell, MD, MPH, an ob.gyn. in New York, was not involved in the study and said she was surprised it wasn’t something that had been studied much before because it makes sense.
“I always like it when studies confirm what we think should make sense, so it’s nice to see it,” Dr. Morrell said in an interview. “I think anytime you put something out, research it, and define it with numbers for doctors, that sometimes allows us to [realize], ‘Oh, that’s probably something we should be paying more attention to, especially if we have available treatments for it,’” she added.
“The clinical takeaway is that we really need to be screening for sleep pattern disruptions early in pregnancy, because even though it makes logical sense, it might not be something on our radar to think about,” Dr. Morrell said. “If people aren’t sleeping, well, their mental health is negatively affected.”
The most promising therapy for sleep issues currently is cognitive-behavioral therapy, which can accessed through various apps, Dr. Sinow said in an interview. “There are also safe interventions, such as melatonin and Unisom, that are totally safe in pregnancy that we can use to target sleep in early pregnancy.”
Dr. Morrell added that vitamin B6, often taken for nausea and vomiting during pregnancy, can also sometimes help people sleep and is safe during pregnancy.
“We know that postpartum depression does not necessarily only have a negative effect on the mother, but also has a negative effect on the infant and the family dynamic as well,” Dr. Morrell said. “So, we should be looking and screening for it so that we can offer people potential treatment because we know it can have long-term effects.”
Ms. Jang and Dr. Sinow did not have any disclosures. Dr. Morrell has done training for Nexplanon. Neither study noted external funding.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Together, the two studies suggest that commonly overlooked experiences in the prenatal period can have negative effects down the line if clinicians aren’t asking patients about them and addressing the issue.
”I think the national conversation around mental health in general will hopefully carry us forward to better supporting the patients who are coming in with preexisting conditions,” lead author Minnie Jang, a 4th-year medical student at Johns Hopkins University, Baltimore, said in an interview.
Most of the attention on mood disorders of pregnancy focus on the postpartum period, but preexisting or new-onset depression during pregnancy deserves more attention, Ms. Jang told attendees. ACOG recommends that clinicians screen all patients at least once during the perinatal period, but that could be anywhere from early pregnancy to the postpartum period. Ms. Jang would like to see recommendations addressing both early pregnancy and the postpartum period.
“I think there’s this framing that postpartum depression is a distinct entity from other mental health conditions whereas it’s really part of a continuum,” Ms. Jang said in an interview.
She retrospectively analyzed the medical records of all pregnant women who completed the Edinburgh Postnatal Depression Scale (EPDS) during their first or second trimesters between 2002 and 2021 at Johns Hopkins Hospital. Among the 718 women who were screened in early pregnancy, 44.6% were Black or African American, 39.7% were white, and 15.7% were of a different race. Nearly all (94%) were not Hispanic/Latino.
Most (59%) were partnered, employed (68%), and had private insurance (58%). Only 7% used tobacco while 11% used alcohol and 6% used illicit drugs.
Twelve percent of the patients scored positive for depression, with a score of at least 10 or an affirmative answer to question 10 regarding self-harm. These women tended to be younger (P = .034), with an median age of 28 at their first visit versus 31 for those who screened negative, and were more likely to be publicly insured (P = .013) and without a partner (P = .005).
Patients who screened positive were more likely to have a history of substance use or history of a previous psychiatric diagnosis (P < .0001 for both). In addition, more patients who screened positive (49%) than those who screened negative (26%) had fetal complications (P < .001).
”There are some interesting subgroups of patients who are screening positive for depressive symptoms early on in pregnancy,” Ms. Jang said. Some come into pregnancy with preexisting mental health conditions while others have situational depressive symptoms, such as the subgroup referred to social work who had diagnosed fetal complications, she said. “Then there’s a whole other group of patients who are developing new symptoms during pregnancy.”
Patients who screened positive tended to start prenatal care later, at a median 12.3 weeks gestational age, than patients who screened negative, at a median 10.7 weeks gestational age (P = .002), the analysis found.
The number of routine prenatal care visits did not significantly differ between those who screened positive and those who screened negative, but patients with positive depression screens were almost half as likely to complete glucose tolerance testing (odds ratio, 0.6) or group B streptococcus testing (OR, 0.56) after adjusting for insurance status, gravidity, and gestational age at the patient’s first visit.
The researchers also identified a significant positive association between higher EPDS scores and the number of labor and delivery triage visits (P = .006). There were no significant differences in the rates of Tdap vaccination or screening for sexually transmitted infections between the two groups.
Poor sleep linked to later depression
The other study was prospective, using data from the PATCH Prenatal Care and Maternal and Child Health Outcomes study, which initially “compared health outcomes and satisfaction with prenatal care between patients receiving Centering Pregnancy group prenatal care and patients receiving traditional prenatal care,” the authors explained. This secondary analysis looked at sleep problems and postpartum depression.
“We don’t routinely ask patients about sleep or screen patients for sleeping issues,” lead author Carolyn Sinow, MD, a 4th-year resident at Kaiser Permanente Santa Clara (Calif.) Medical Center, said in an interview. “I think that we need to take sleep complaints more seriously overall, especially in early pregnancy.” While sleep problems in the third trimester often have more to do with discomforts from pregnancy itself, better sleep “in the first and second trimester is something we can really target with good sleep hygiene,” she added.
The 336 pregnant participants were recruited from Health Connect as long as they had a singleton pregnancy, were receiving prenatal care from Kaiser Permanente Northern California, and completed baseline questionnaires about their sleep and depression and anxiety symptoms during their first trimester between August 2020 and April 2021. Those with clinical depression or a high-risk pregnancy were excluded. The participants then completed the questionnaires again between 4 and 8 weeks post partum.
After adjusting for baseline depression and potential confounders, patients with poor sleep quality, indicated by a score greater than 5 on the Pittsburgh Sleep Quality Index (PSQI), were 12% more likely to develop postpartum depression, indicated by a score on the Patient Health Questionnaire depression scale (PHQ-8) of 10 or greater (relative risk, 1.12; 95% confidence interval, 1.01-1.25).
The two aspects of sleep that specifically correlated with postpartum depression were sleep quality and sleep latency, or taking a long time to fall asleep. Those reporting poor sleep quality were twice as likely to develop postpartum depression (relative risk, 2.18; 95% CI, 1.22-3.91), and those who took a while to fall asleep were 52% more likely to develop postpartum depression (RR, 1.52; 95% CI, 1.06-2.17).
Though the study also found prenatal sleep problems correlated with higher postpartum anxiety scores on the General Anxiety Disorder scale (GAD-7), the results were not statistically significant.
Kathleen Morrell, MD, MPH, an ob.gyn. in New York, was not involved in the study and said she was surprised it wasn’t something that had been studied much before because it makes sense.
“I always like it when studies confirm what we think should make sense, so it’s nice to see it,” Dr. Morrell said in an interview. “I think anytime you put something out, research it, and define it with numbers for doctors, that sometimes allows us to [realize], ‘Oh, that’s probably something we should be paying more attention to, especially if we have available treatments for it,’” she added.
“The clinical takeaway is that we really need to be screening for sleep pattern disruptions early in pregnancy, because even though it makes logical sense, it might not be something on our radar to think about,” Dr. Morrell said. “If people aren’t sleeping, well, their mental health is negatively affected.”
The most promising therapy for sleep issues currently is cognitive-behavioral therapy, which can accessed through various apps, Dr. Sinow said in an interview. “There are also safe interventions, such as melatonin and Unisom, that are totally safe in pregnancy that we can use to target sleep in early pregnancy.”
Dr. Morrell added that vitamin B6, often taken for nausea and vomiting during pregnancy, can also sometimes help people sleep and is safe during pregnancy.
“We know that postpartum depression does not necessarily only have a negative effect on the mother, but also has a negative effect on the infant and the family dynamic as well,” Dr. Morrell said. “So, we should be looking and screening for it so that we can offer people potential treatment because we know it can have long-term effects.”
Ms. Jang and Dr. Sinow did not have any disclosures. Dr. Morrell has done training for Nexplanon. Neither study noted external funding.
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Together, the two studies suggest that commonly overlooked experiences in the prenatal period can have negative effects down the line if clinicians aren’t asking patients about them and addressing the issue.
”I think the national conversation around mental health in general will hopefully carry us forward to better supporting the patients who are coming in with preexisting conditions,” lead author Minnie Jang, a 4th-year medical student at Johns Hopkins University, Baltimore, said in an interview.
Most of the attention on mood disorders of pregnancy focus on the postpartum period, but preexisting or new-onset depression during pregnancy deserves more attention, Ms. Jang told attendees. ACOG recommends that clinicians screen all patients at least once during the perinatal period, but that could be anywhere from early pregnancy to the postpartum period. Ms. Jang would like to see recommendations addressing both early pregnancy and the postpartum period.
“I think there’s this framing that postpartum depression is a distinct entity from other mental health conditions whereas it’s really part of a continuum,” Ms. Jang said in an interview.
She retrospectively analyzed the medical records of all pregnant women who completed the Edinburgh Postnatal Depression Scale (EPDS) during their first or second trimesters between 2002 and 2021 at Johns Hopkins Hospital. Among the 718 women who were screened in early pregnancy, 44.6% were Black or African American, 39.7% were white, and 15.7% were of a different race. Nearly all (94%) were not Hispanic/Latino.
Most (59%) were partnered, employed (68%), and had private insurance (58%). Only 7% used tobacco while 11% used alcohol and 6% used illicit drugs.
Twelve percent of the patients scored positive for depression, with a score of at least 10 or an affirmative answer to question 10 regarding self-harm. These women tended to be younger (P = .034), with an median age of 28 at their first visit versus 31 for those who screened negative, and were more likely to be publicly insured (P = .013) and without a partner (P = .005).
Patients who screened positive were more likely to have a history of substance use or history of a previous psychiatric diagnosis (P < .0001 for both). In addition, more patients who screened positive (49%) than those who screened negative (26%) had fetal complications (P < .001).
”There are some interesting subgroups of patients who are screening positive for depressive symptoms early on in pregnancy,” Ms. Jang said. Some come into pregnancy with preexisting mental health conditions while others have situational depressive symptoms, such as the subgroup referred to social work who had diagnosed fetal complications, she said. “Then there’s a whole other group of patients who are developing new symptoms during pregnancy.”
Patients who screened positive tended to start prenatal care later, at a median 12.3 weeks gestational age, than patients who screened negative, at a median 10.7 weeks gestational age (P = .002), the analysis found.
The number of routine prenatal care visits did not significantly differ between those who screened positive and those who screened negative, but patients with positive depression screens were almost half as likely to complete glucose tolerance testing (odds ratio, 0.6) or group B streptococcus testing (OR, 0.56) after adjusting for insurance status, gravidity, and gestational age at the patient’s first visit.
The researchers also identified a significant positive association between higher EPDS scores and the number of labor and delivery triage visits (P = .006). There were no significant differences in the rates of Tdap vaccination or screening for sexually transmitted infections between the two groups.
Poor sleep linked to later depression
The other study was prospective, using data from the PATCH Prenatal Care and Maternal and Child Health Outcomes study, which initially “compared health outcomes and satisfaction with prenatal care between patients receiving Centering Pregnancy group prenatal care and patients receiving traditional prenatal care,” the authors explained. This secondary analysis looked at sleep problems and postpartum depression.
“We don’t routinely ask patients about sleep or screen patients for sleeping issues,” lead author Carolyn Sinow, MD, a 4th-year resident at Kaiser Permanente Santa Clara (Calif.) Medical Center, said in an interview. “I think that we need to take sleep complaints more seriously overall, especially in early pregnancy.” While sleep problems in the third trimester often have more to do with discomforts from pregnancy itself, better sleep “in the first and second trimester is something we can really target with good sleep hygiene,” she added.
The 336 pregnant participants were recruited from Health Connect as long as they had a singleton pregnancy, were receiving prenatal care from Kaiser Permanente Northern California, and completed baseline questionnaires about their sleep and depression and anxiety symptoms during their first trimester between August 2020 and April 2021. Those with clinical depression or a high-risk pregnancy were excluded. The participants then completed the questionnaires again between 4 and 8 weeks post partum.
After adjusting for baseline depression and potential confounders, patients with poor sleep quality, indicated by a score greater than 5 on the Pittsburgh Sleep Quality Index (PSQI), were 12% more likely to develop postpartum depression, indicated by a score on the Patient Health Questionnaire depression scale (PHQ-8) of 10 or greater (relative risk, 1.12; 95% confidence interval, 1.01-1.25).
The two aspects of sleep that specifically correlated with postpartum depression were sleep quality and sleep latency, or taking a long time to fall asleep. Those reporting poor sleep quality were twice as likely to develop postpartum depression (relative risk, 2.18; 95% CI, 1.22-3.91), and those who took a while to fall asleep were 52% more likely to develop postpartum depression (RR, 1.52; 95% CI, 1.06-2.17).
Though the study also found prenatal sleep problems correlated with higher postpartum anxiety scores on the General Anxiety Disorder scale (GAD-7), the results were not statistically significant.
Kathleen Morrell, MD, MPH, an ob.gyn. in New York, was not involved in the study and said she was surprised it wasn’t something that had been studied much before because it makes sense.
“I always like it when studies confirm what we think should make sense, so it’s nice to see it,” Dr. Morrell said in an interview. “I think anytime you put something out, research it, and define it with numbers for doctors, that sometimes allows us to [realize], ‘Oh, that’s probably something we should be paying more attention to, especially if we have available treatments for it,’” she added.
“The clinical takeaway is that we really need to be screening for sleep pattern disruptions early in pregnancy, because even though it makes logical sense, it might not be something on our radar to think about,” Dr. Morrell said. “If people aren’t sleeping, well, their mental health is negatively affected.”
The most promising therapy for sleep issues currently is cognitive-behavioral therapy, which can accessed through various apps, Dr. Sinow said in an interview. “There are also safe interventions, such as melatonin and Unisom, that are totally safe in pregnancy that we can use to target sleep in early pregnancy.”
Dr. Morrell added that vitamin B6, often taken for nausea and vomiting during pregnancy, can also sometimes help people sleep and is safe during pregnancy.
“We know that postpartum depression does not necessarily only have a negative effect on the mother, but also has a negative effect on the infant and the family dynamic as well,” Dr. Morrell said. “So, we should be looking and screening for it so that we can offer people potential treatment because we know it can have long-term effects.”
Ms. Jang and Dr. Sinow did not have any disclosures. Dr. Morrell has done training for Nexplanon. Neither study noted external funding.
AT ACOG 2023