User login
Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.

The finding comes from the first long-term analysis of outcomes following endoscopic sleeve gastroplasty, a relatively new, minimally invasive weight-loss procedure that offers patients an alternative to bariatric surgery.
“Endoscopic sleeve gastrectomy is a 1-day outpatient procedure that uses a suturing device attached to an endoscope to create a series of sutures that cinch the stomach like an accordion down to roughly the size of a banana, and leaves no scars,” lead study author Reem Z. Sharaiha, MD, MSc, said during a media briefing in advance of the annual Digestive Disease Week®. “The procedure causes patients to eat less because they feel full faster. This results in weight loss.”
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
While previous studies have tracked ESG results for 1-2 years, her research team followed 203 patients who underwent the procedure between August 2013 and October 2018. “We felt that a longer-term study was needed to make sure weight loss was sustainable with this method of treatment, because research shows that if you keep weight loss for an extended period of time, you’re more likely to keep it off permanently, which is ultimately what we want for these patients,” said Dr. Sharaiha, who is an attending physician at New York–Presbyterian/Weill Cornell Medicine, New York.
At baseline, the mean age of the 203 patients was 46 years, 67% were female, and their mean body mass index was 39 kg/m2. Dr. Sharaiha and colleagues observed that maximum weight loss was generally achieved by 24 months after the procedure, after which patients tended to regain a small amount of their lost weight. For example, at 1 year, the mean weight loss was 18.1 kg, with a total body weight loss of 15.2% (P less than .0001 for both associations). At 2 years, the mean weight loss was 17.3 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 3 years, the mean weight loss was 20.8 kg, with a total body weight loss of 14.5% (P less than .0001 for both associations). At 5 years, the mean weight loss was 18.7 kg (P = .0003) and the total body weight loss was 14.5% (P = .0002).
Overall, patients gained an average 2.4 kg of weight after achieving their minimum weight after ESG until the end of follow-up. The researchers also found that failure to lose at least 10% of total body weight within the first 3 months after ESG decreased the chance of subsequent significant weight loss by 80%. Fewer than 1% of patients experienced complications, an improvement over surgical procedures.
“Our study showed very sustainable, significant weight loss for our patients between the 1 and 5 year mark,” Dr. Sharaiha said. “Out to 5 years, there was an average 15% total body weight loss. This is significant, because studies have shown that when people lose at least 10% of their body weight, they see improvement in blood pressure, diabetes, and heart outcomes, which are the comorbidities associated with obesity. We hope these findings will help persuade insurance companies that ESG is not experimental, but has value over patients’ lifespans.”
Dr. Sharaiha and colleagues plan to follow the current cohort for the next 10-20 years. “It’s important to show the value of these endoscopic procedures, so we’ll be looking at improvement in comorbidities such as diabetes, high blood pressure, and cholesterol,” she said. “We’re also part of a randomized study that’s currently under way looking at ESG in combination with diet and exercise.”
She reported having no financial disclosures.
FROM DDW 2019
Key clinical point: Endoscopic sleeve gastroplasty is an effective, minimally invasive weight-loss procedure that results in significant total body weight loss.
Major finding: Between 1 and 5 years after endoscopic sleeve gastroplasty, patients lost 15%-20% of their total body weight.
Study details: A retrospective study of prospectively collected data on 203 patients.
Disclosures: Dr. Sharaiha reported having no financial disclosures.
Maternal immunization protects against serious RSV infection in infancy
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
REPORTING FROM ESPID 2019
Are ObGyns knowledgeable about the risk factors for hepatitis C virus in pregnancy?
The American College of Obstetricians and Gynecologists (ACOG) recommends risk-based screening for hepatitis C virus (HCV) infection during pregnancy.1 However, the prevalence of HCV among pregnant women in the United States is on the rise. From 2009 to 2014, HCV infection present at delivery increased 89%.2 In addition, the risk of an HCV-infected mother transmitting the infection to her baby is about 4% to 7% per pregnancy.3 Currently, the Infectious Diseases Society of America and the American Association for the Study of Liver Diseases recommend universal HCV screening in pregnancy.4
Researchers at Tufts Medical Center in Boston, Massachusetts, a tertiary care center, presented survey findings on HCV screening among ObGyns at ACOG’s 2019 Annual Clinical and Scientific Meeting in Nashville, Tennessee.5 Katherine G. Koniares, MD, and colleagues sought to assess the opinions and clinical practices of ObGyns by emailing a 10-question electronic survey to providers. A total of 38 of 41 providers (93%) responded to the survey.
Survey results show lack of knowledge on risk factors
In response to the question, “Which pregnant patients do you believe should be screened for HCV,” 43.2% of providers stated “all pregnant women,” while 54.1% said “only pregnant women with risk factors for HCV.” A small percentage (2.7%) responded that they were not sure.
Providers also were asked which patients in their practice they screen for HCV. In response, 77.8% stated that they screen pregnant women for HCV based on risk factors, while 13.9% screen all pregnant patients for HCV; 8.3% do not screen for HCV.
When asked which risk factors providers use to screen patients for HCV, 42% to 85% said they screen for each indicated risk factor. Only 36% of providers, however, correctly identified all risk factors (for example, receiving blood products from donors who later tested positive for HCV; unexplained liver disease; and percutaneous/parenteral exposures in an unregulated setting, such as receiving tattoos outside a licensed parlor).
Further study needed on universal screening
The researchers assert that risk-based screening for HCV is not effective and that further research on universal HCV screening in pregnant patients is needed.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 86: Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
- Patrick SW, Bauer AM, Warren MD, et al. Hepatitis C virus infection among women giving birth—Tennessee and the United States, 2009-2014. MMWR Morbid Mortal Weekly Rep. 2017;66:470-473.
- Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011-2014. MMWR Morbid Mortal Weekly Rep. 2016;65:705-710.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). Recommendations for testing, management, and treating, hepatitis C. HCV testing and linkage to care. https://www.hcvguidelines.org/.
- Koniares KG, Fadlallah H, Kolettis DS, et al. A survey of hepatitis C virus (HCV) screening in pregnancy among ObGyns at a tertiary care center. Poster presented at: American College of Obstetricians and Gynecologists Annual Clinical and Scientific Meeting; May 3-6, 2019; Nashville, TN.
The American College of Obstetricians and Gynecologists (ACOG) recommends risk-based screening for hepatitis C virus (HCV) infection during pregnancy.1 However, the prevalence of HCV among pregnant women in the United States is on the rise. From 2009 to 2014, HCV infection present at delivery increased 89%.2 In addition, the risk of an HCV-infected mother transmitting the infection to her baby is about 4% to 7% per pregnancy.3 Currently, the Infectious Diseases Society of America and the American Association for the Study of Liver Diseases recommend universal HCV screening in pregnancy.4
Researchers at Tufts Medical Center in Boston, Massachusetts, a tertiary care center, presented survey findings on HCV screening among ObGyns at ACOG’s 2019 Annual Clinical and Scientific Meeting in Nashville, Tennessee.5 Katherine G. Koniares, MD, and colleagues sought to assess the opinions and clinical practices of ObGyns by emailing a 10-question electronic survey to providers. A total of 38 of 41 providers (93%) responded to the survey.
Survey results show lack of knowledge on risk factors
In response to the question, “Which pregnant patients do you believe should be screened for HCV,” 43.2% of providers stated “all pregnant women,” while 54.1% said “only pregnant women with risk factors for HCV.” A small percentage (2.7%) responded that they were not sure.
Providers also were asked which patients in their practice they screen for HCV. In response, 77.8% stated that they screen pregnant women for HCV based on risk factors, while 13.9% screen all pregnant patients for HCV; 8.3% do not screen for HCV.
When asked which risk factors providers use to screen patients for HCV, 42% to 85% said they screen for each indicated risk factor. Only 36% of providers, however, correctly identified all risk factors (for example, receiving blood products from donors who later tested positive for HCV; unexplained liver disease; and percutaneous/parenteral exposures in an unregulated setting, such as receiving tattoos outside a licensed parlor).
Further study needed on universal screening
The researchers assert that risk-based screening for HCV is not effective and that further research on universal HCV screening in pregnant patients is needed.
The American College of Obstetricians and Gynecologists (ACOG) recommends risk-based screening for hepatitis C virus (HCV) infection during pregnancy.1 However, the prevalence of HCV among pregnant women in the United States is on the rise. From 2009 to 2014, HCV infection present at delivery increased 89%.2 In addition, the risk of an HCV-infected mother transmitting the infection to her baby is about 4% to 7% per pregnancy.3 Currently, the Infectious Diseases Society of America and the American Association for the Study of Liver Diseases recommend universal HCV screening in pregnancy.4
Researchers at Tufts Medical Center in Boston, Massachusetts, a tertiary care center, presented survey findings on HCV screening among ObGyns at ACOG’s 2019 Annual Clinical and Scientific Meeting in Nashville, Tennessee.5 Katherine G. Koniares, MD, and colleagues sought to assess the opinions and clinical practices of ObGyns by emailing a 10-question electronic survey to providers. A total of 38 of 41 providers (93%) responded to the survey.
Survey results show lack of knowledge on risk factors
In response to the question, “Which pregnant patients do you believe should be screened for HCV,” 43.2% of providers stated “all pregnant women,” while 54.1% said “only pregnant women with risk factors for HCV.” A small percentage (2.7%) responded that they were not sure.
Providers also were asked which patients in their practice they screen for HCV. In response, 77.8% stated that they screen pregnant women for HCV based on risk factors, while 13.9% screen all pregnant patients for HCV; 8.3% do not screen for HCV.
When asked which risk factors providers use to screen patients for HCV, 42% to 85% said they screen for each indicated risk factor. Only 36% of providers, however, correctly identified all risk factors (for example, receiving blood products from donors who later tested positive for HCV; unexplained liver disease; and percutaneous/parenteral exposures in an unregulated setting, such as receiving tattoos outside a licensed parlor).
Further study needed on universal screening
The researchers assert that risk-based screening for HCV is not effective and that further research on universal HCV screening in pregnant patients is needed.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 86: Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
- Patrick SW, Bauer AM, Warren MD, et al. Hepatitis C virus infection among women giving birth—Tennessee and the United States, 2009-2014. MMWR Morbid Mortal Weekly Rep. 2017;66:470-473.
- Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011-2014. MMWR Morbid Mortal Weekly Rep. 2016;65:705-710.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). Recommendations for testing, management, and treating, hepatitis C. HCV testing and linkage to care. https://www.hcvguidelines.org/.
- Koniares KG, Fadlallah H, Kolettis DS, et al. A survey of hepatitis C virus (HCV) screening in pregnancy among ObGyns at a tertiary care center. Poster presented at: American College of Obstetricians and Gynecologists Annual Clinical and Scientific Meeting; May 3-6, 2019; Nashville, TN.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 86: Viral hepatitis in pregnancy. Obstet Gynecol. 2007;110:941-956.
- Patrick SW, Bauer AM, Warren MD, et al. Hepatitis C virus infection among women giving birth—Tennessee and the United States, 2009-2014. MMWR Morbid Mortal Weekly Rep. 2017;66:470-473.
- Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011-2014. MMWR Morbid Mortal Weekly Rep. 2016;65:705-710.
- American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA). Recommendations for testing, management, and treating, hepatitis C. HCV testing and linkage to care. https://www.hcvguidelines.org/.
- Koniares KG, Fadlallah H, Kolettis DS, et al. A survey of hepatitis C virus (HCV) screening in pregnancy among ObGyns at a tertiary care center. Poster presented at: American College of Obstetricians and Gynecologists Annual Clinical and Scientific Meeting; May 3-6, 2019; Nashville, TN.
Study finds link between intrahepatic cholestasis of pregnancy and risk of NAFLD
Patients with intrahepatic cholestasis of pregnancy (ICP) were nearly six times more likely to have a diagnosis of nonalcoholic fatty liver disease (NAFLD) than were controls, results from a retrospective, single-center study demonstrated.
“If this connection is confirmed with future studies, intrahepatic cholestasis of pregnancy may prove a novel model through which to investigate bile acid metabolism in patients with fatty liver disease,” one of the study authors, Tatyana Kushner, MD, MSCE, said during a media briefing in advance of the annual Digestive Disease Week. “This could have implications for future management of fatty liver disease. Additionally, these findings suggest that ICP patients should be seen by a liver specialist because they may go on to develop chronic liver disease or may already have already existing underlying liver disease.”
ICP is characterized by a build-up of bile acids during pregnancy and is associated with an increased risk of negative fetal outcomes and fetal death if left untreated, said Dr. Kushner, of the division of liver diseases at the Icahn School of Medicine at Mount Sinai, New York. The most notable symptom during pregnancy is severe pruritus. In what is believed to be the first study of its kind, Dr. Kushner and colleagues set out to evaluate the association between ICP and NAFLD and associated metabolic risk factors, including obesity, dyslipidemia, hypertension, and diabetes. Between January and December of 2017, they drew from the electronic medical records of a New York City health system to identify 149 pregnancies complicated by ICP and compared them to a control group of 200 pregnancies without an ICP diagnosis. The researchers used Pearson’s chi-square or Fisher’s exact test and Wilcoxon rank-sum tests to evaluate association of ICP with categorical variables and continuous variables, respectively, and unadjusted odds ratios to compare the ICP and control groups for clinically significant outcomes.
The median age of the study population was 30 years, their mean body mass index was 27.5 kg/m2, and there was a higher proportion of Hispanic women in the ICP group, compared with the control group (75% vs. 62%, respectively). Dr. Kushner and colleagues found that Hispanic women were nearly twice as likely to be diagnosed with ICP than non-Hispanic women (OR, 1.90; 95% confidence interval, 1.87-3.03). However, patients in both the ICP and control groups were similar for median age (OR, 1.02; 95% CI, 0.99-1.06), nulliparity (OR, 0.79; 95% CI, 0.48-1.30), and prevalence of hepatitis C (OR, 1.35; 95% CI, 0.08-21.67). The two groups were also similar for certain metabolic risk factors, including prevalence of obesity (OR, 1.01; 95% CI, 0.62-1.61), hypertension (OR, 0.69; 95% CI, 0.31-1.52), hemoglobin A1c greater than 5.5% (OR, 0.80; 95% CI, 0.34-1.9), and total cholesterol above 200 mg/dL (OR, 4.15; 95% CI, 0.83-20.84). Median bile acid levels were 30.6 micromoles (interquartile range, 11.6, 32.7) in the ICP group.
Compared with patients in the control group, those in the ICP group had higher median levels of alanine aminotransferase (ALT) (32 vs. 16 U/L; P less than .0001), alkaline phosphatase (181 vs. 128 U/L; P less than .0001), and total bilirubin (0.5 vs. 0.35 mg/dL; P less than .0001). ICP patients were also more likely than their counterparts to have ALT levels above 50 U/L (two times the upper limit of normal; OR, 3.22; 95% CI, 1.48-7.03), a history of biliary disease (OR, 3.29; 95% CI, 1.39-7.80), and to have evidence of steatosis on liver imaging (OR, 4.69; 95% CI, 1.68-13.12). When the researchers evaluated a diagnosis of NAFLD based on ICD-10 codes or evidence of steatosis on liver imaging, ICP patients were significantly more likely to have a diagnosis of NAFLD than controls (OR, 5.7; 95% CI, 2.08-15.65).
“We recommend additional research to look at differences in NAFLD progression in women who had NAFLD and were later diagnosed with ICP, compared to women with NAFLD who did not go on to develop ICP, because that may be a reflection of the role that bile acid metabolism plays in these particular patients,” Dr. Kushner said.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
The study’s primary author was Erica Monrose, MD. The researchers reported having no financial disclosures.
SOURCE: Monrose E et al. DDW 2019, Abstract Sa1562.
Patients with intrahepatic cholestasis of pregnancy (ICP) were nearly six times more likely to have a diagnosis of nonalcoholic fatty liver disease (NAFLD) than were controls, results from a retrospective, single-center study demonstrated.
“If this connection is confirmed with future studies, intrahepatic cholestasis of pregnancy may prove a novel model through which to investigate bile acid metabolism in patients with fatty liver disease,” one of the study authors, Tatyana Kushner, MD, MSCE, said during a media briefing in advance of the annual Digestive Disease Week. “This could have implications for future management of fatty liver disease. Additionally, these findings suggest that ICP patients should be seen by a liver specialist because they may go on to develop chronic liver disease or may already have already existing underlying liver disease.”
ICP is characterized by a build-up of bile acids during pregnancy and is associated with an increased risk of negative fetal outcomes and fetal death if left untreated, said Dr. Kushner, of the division of liver diseases at the Icahn School of Medicine at Mount Sinai, New York. The most notable symptom during pregnancy is severe pruritus. In what is believed to be the first study of its kind, Dr. Kushner and colleagues set out to evaluate the association between ICP and NAFLD and associated metabolic risk factors, including obesity, dyslipidemia, hypertension, and diabetes. Between January and December of 2017, they drew from the electronic medical records of a New York City health system to identify 149 pregnancies complicated by ICP and compared them to a control group of 200 pregnancies without an ICP diagnosis. The researchers used Pearson’s chi-square or Fisher’s exact test and Wilcoxon rank-sum tests to evaluate association of ICP with categorical variables and continuous variables, respectively, and unadjusted odds ratios to compare the ICP and control groups for clinically significant outcomes.
The median age of the study population was 30 years, their mean body mass index was 27.5 kg/m2, and there was a higher proportion of Hispanic women in the ICP group, compared with the control group (75% vs. 62%, respectively). Dr. Kushner and colleagues found that Hispanic women were nearly twice as likely to be diagnosed with ICP than non-Hispanic women (OR, 1.90; 95% confidence interval, 1.87-3.03). However, patients in both the ICP and control groups were similar for median age (OR, 1.02; 95% CI, 0.99-1.06), nulliparity (OR, 0.79; 95% CI, 0.48-1.30), and prevalence of hepatitis C (OR, 1.35; 95% CI, 0.08-21.67). The two groups were also similar for certain metabolic risk factors, including prevalence of obesity (OR, 1.01; 95% CI, 0.62-1.61), hypertension (OR, 0.69; 95% CI, 0.31-1.52), hemoglobin A1c greater than 5.5% (OR, 0.80; 95% CI, 0.34-1.9), and total cholesterol above 200 mg/dL (OR, 4.15; 95% CI, 0.83-20.84). Median bile acid levels were 30.6 micromoles (interquartile range, 11.6, 32.7) in the ICP group.
Compared with patients in the control group, those in the ICP group had higher median levels of alanine aminotransferase (ALT) (32 vs. 16 U/L; P less than .0001), alkaline phosphatase (181 vs. 128 U/L; P less than .0001), and total bilirubin (0.5 vs. 0.35 mg/dL; P less than .0001). ICP patients were also more likely than their counterparts to have ALT levels above 50 U/L (two times the upper limit of normal; OR, 3.22; 95% CI, 1.48-7.03), a history of biliary disease (OR, 3.29; 95% CI, 1.39-7.80), and to have evidence of steatosis on liver imaging (OR, 4.69; 95% CI, 1.68-13.12). When the researchers evaluated a diagnosis of NAFLD based on ICD-10 codes or evidence of steatosis on liver imaging, ICP patients were significantly more likely to have a diagnosis of NAFLD than controls (OR, 5.7; 95% CI, 2.08-15.65).
“We recommend additional research to look at differences in NAFLD progression in women who had NAFLD and were later diagnosed with ICP, compared to women with NAFLD who did not go on to develop ICP, because that may be a reflection of the role that bile acid metabolism plays in these particular patients,” Dr. Kushner said.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
The study’s primary author was Erica Monrose, MD. The researchers reported having no financial disclosures.
SOURCE: Monrose E et al. DDW 2019, Abstract Sa1562.
Patients with intrahepatic cholestasis of pregnancy (ICP) were nearly six times more likely to have a diagnosis of nonalcoholic fatty liver disease (NAFLD) than were controls, results from a retrospective, single-center study demonstrated.
“If this connection is confirmed with future studies, intrahepatic cholestasis of pregnancy may prove a novel model through which to investigate bile acid metabolism in patients with fatty liver disease,” one of the study authors, Tatyana Kushner, MD, MSCE, said during a media briefing in advance of the annual Digestive Disease Week. “This could have implications for future management of fatty liver disease. Additionally, these findings suggest that ICP patients should be seen by a liver specialist because they may go on to develop chronic liver disease or may already have already existing underlying liver disease.”
ICP is characterized by a build-up of bile acids during pregnancy and is associated with an increased risk of negative fetal outcomes and fetal death if left untreated, said Dr. Kushner, of the division of liver diseases at the Icahn School of Medicine at Mount Sinai, New York. The most notable symptom during pregnancy is severe pruritus. In what is believed to be the first study of its kind, Dr. Kushner and colleagues set out to evaluate the association between ICP and NAFLD and associated metabolic risk factors, including obesity, dyslipidemia, hypertension, and diabetes. Between January and December of 2017, they drew from the electronic medical records of a New York City health system to identify 149 pregnancies complicated by ICP and compared them to a control group of 200 pregnancies without an ICP diagnosis. The researchers used Pearson’s chi-square or Fisher’s exact test and Wilcoxon rank-sum tests to evaluate association of ICP with categorical variables and continuous variables, respectively, and unadjusted odds ratios to compare the ICP and control groups for clinically significant outcomes.
The median age of the study population was 30 years, their mean body mass index was 27.5 kg/m2, and there was a higher proportion of Hispanic women in the ICP group, compared with the control group (75% vs. 62%, respectively). Dr. Kushner and colleagues found that Hispanic women were nearly twice as likely to be diagnosed with ICP than non-Hispanic women (OR, 1.90; 95% confidence interval, 1.87-3.03). However, patients in both the ICP and control groups were similar for median age (OR, 1.02; 95% CI, 0.99-1.06), nulliparity (OR, 0.79; 95% CI, 0.48-1.30), and prevalence of hepatitis C (OR, 1.35; 95% CI, 0.08-21.67). The two groups were also similar for certain metabolic risk factors, including prevalence of obesity (OR, 1.01; 95% CI, 0.62-1.61), hypertension (OR, 0.69; 95% CI, 0.31-1.52), hemoglobin A1c greater than 5.5% (OR, 0.80; 95% CI, 0.34-1.9), and total cholesterol above 200 mg/dL (OR, 4.15; 95% CI, 0.83-20.84). Median bile acid levels were 30.6 micromoles (interquartile range, 11.6, 32.7) in the ICP group.
Compared with patients in the control group, those in the ICP group had higher median levels of alanine aminotransferase (ALT) (32 vs. 16 U/L; P less than .0001), alkaline phosphatase (181 vs. 128 U/L; P less than .0001), and total bilirubin (0.5 vs. 0.35 mg/dL; P less than .0001). ICP patients were also more likely than their counterparts to have ALT levels above 50 U/L (two times the upper limit of normal; OR, 3.22; 95% CI, 1.48-7.03), a history of biliary disease (OR, 3.29; 95% CI, 1.39-7.80), and to have evidence of steatosis on liver imaging (OR, 4.69; 95% CI, 1.68-13.12). When the researchers evaluated a diagnosis of NAFLD based on ICD-10 codes or evidence of steatosis on liver imaging, ICP patients were significantly more likely to have a diagnosis of NAFLD than controls (OR, 5.7; 95% CI, 2.08-15.65).
“We recommend additional research to look at differences in NAFLD progression in women who had NAFLD and were later diagnosed with ICP, compared to women with NAFLD who did not go on to develop ICP, because that may be a reflection of the role that bile acid metabolism plays in these particular patients,” Dr. Kushner said.
Digestive Disease Week is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE), and the Society for Surgery of the Alimentary Tract (SSAT).
The study’s primary author was Erica Monrose, MD. The researchers reported having no financial disclosures.
SOURCE: Monrose E et al. DDW 2019, Abstract Sa1562.
REPORTING FROM DDW 2019
C-sections play role in 300% higher severe maternal morbidity in twin pregnancies
according to findings from the prospective EPIMOMS study.
The population-based incidence of severe acute maternal morbidity occurring between 22 weeks’ of gestation and 42 days post partum in the 2012-2013 French multicenter study was 6.2% among 3,202 twin pregnancies and 1.3% among 179,107 singleton pregnancies, Hugo Madar, MD, MPH, of Bordeaux University Hospital, France, and colleagues reported on behalf of the EPIMOMS (Epidémiologie de la Morbidité Maternelle Sévère) study group.
For the current analysis – a population-based, cohort-nested, case-control analysis of study data – the investigators compared 2,500 case patients (8% had twin pregnancies) and 3,650 controls (2% had twin pregnancies) who did not experience severe acute maternal morbidity during that time period (odds ratio, 4.7). After accounting for confounding factors, the increased risk among women with twin versus singleton pregnancies persisted (OR, 4.2) during both the antepartum (OR, 4.1) and intrapartum/postpartum (OR, 4.2) periods.
The majority of events (77%) occurred during the latter periods, and the two most common underlying causal conditions were severe obstetric hemorrhage (66%) and severe hypertensive complications (20%); however, the increased risk in twin pregnancies was apparent, regardless of the underlying cause.
The cesarean delivery rates for twin versus singleton pregnancies were 72% and 34%, respectively, in the case group, and 58% and 18%, respectively, in the control group. A path analysis taking potential indication bias into account showed that 21% of the total risk of intrapartum or postpartum severe acute maternal morbidity risk associated with twin pregnancy was mediated by cesarean delivery, Dr. Madar and associates noted, explaining that, “in other words, if twin pregnancies had the same probability of cesarean delivery as singleton pregnancies, the association found between twin pregnancy and intrapartum or postpartum severe acute maternal morbidity would be reduced by one-fifth.”
This provides further support for limiting the use of cesarean for twin deliveries to cases with clear medical indications, as increasing the rate of vaginal deliveries may decrease the rate of severe acute maternal morbidity, they concluded.
EPIMOMS was supported by the National Research Agency and the Ile de France Regional Health Agency. Dr. Madar received a training grant from the Aquitaine Regional Health Agency. The authors reported having no other relevant financial disclosures.
SOURCE: Madar H et al. Obstet Gynecol. 2019;133:1141-50.
Twin pregnancies are known to be associated with increased risk of maternal morbidity, so the findings of this “very well-designed” study by Madar et al. are “not strikingly different than what we know,” according to Ozhan M. Turan, MD, PhD.
These data alone will do little to change practice, but paired with an increased focus on training with respect to vaginal twin delivery – including in cases of breech presentation of the second baby – they could lead to improved maternal outcomes, he explained, adding that “breech extraction can be very fast and safe in skilled hands.”
Except for the lack of information in the study about whether the twins were monozygotic or dizygotic, the study is sound, and the data may prove useful for counseling patients about the risks and benefits of vaginal versus cesarean delivery and for promoting improved training of residents, maternal-fetal medicine fellows, and junior obstetricians in vaginal twin delivery techniques, he said.
Dr. Turan is director of the division of maternal and fetal medicine and of fetal therapy & complex obstetric surgery at the University of Maryland, Baltimore County. He reported having no relevant financial disclosures.
Twin pregnancies are known to be associated with increased risk of maternal morbidity, so the findings of this “very well-designed” study by Madar et al. are “not strikingly different than what we know,” according to Ozhan M. Turan, MD, PhD.
These data alone will do little to change practice, but paired with an increased focus on training with respect to vaginal twin delivery – including in cases of breech presentation of the second baby – they could lead to improved maternal outcomes, he explained, adding that “breech extraction can be very fast and safe in skilled hands.”
Except for the lack of information in the study about whether the twins were monozygotic or dizygotic, the study is sound, and the data may prove useful for counseling patients about the risks and benefits of vaginal versus cesarean delivery and for promoting improved training of residents, maternal-fetal medicine fellows, and junior obstetricians in vaginal twin delivery techniques, he said.
Dr. Turan is director of the division of maternal and fetal medicine and of fetal therapy & complex obstetric surgery at the University of Maryland, Baltimore County. He reported having no relevant financial disclosures.
Twin pregnancies are known to be associated with increased risk of maternal morbidity, so the findings of this “very well-designed” study by Madar et al. are “not strikingly different than what we know,” according to Ozhan M. Turan, MD, PhD.
These data alone will do little to change practice, but paired with an increased focus on training with respect to vaginal twin delivery – including in cases of breech presentation of the second baby – they could lead to improved maternal outcomes, he explained, adding that “breech extraction can be very fast and safe in skilled hands.”
Except for the lack of information in the study about whether the twins were monozygotic or dizygotic, the study is sound, and the data may prove useful for counseling patients about the risks and benefits of vaginal versus cesarean delivery and for promoting improved training of residents, maternal-fetal medicine fellows, and junior obstetricians in vaginal twin delivery techniques, he said.
Dr. Turan is director of the division of maternal and fetal medicine and of fetal therapy & complex obstetric surgery at the University of Maryland, Baltimore County. He reported having no relevant financial disclosures.
according to findings from the prospective EPIMOMS study.
The population-based incidence of severe acute maternal morbidity occurring between 22 weeks’ of gestation and 42 days post partum in the 2012-2013 French multicenter study was 6.2% among 3,202 twin pregnancies and 1.3% among 179,107 singleton pregnancies, Hugo Madar, MD, MPH, of Bordeaux University Hospital, France, and colleagues reported on behalf of the EPIMOMS (Epidémiologie de la Morbidité Maternelle Sévère) study group.
For the current analysis – a population-based, cohort-nested, case-control analysis of study data – the investigators compared 2,500 case patients (8% had twin pregnancies) and 3,650 controls (2% had twin pregnancies) who did not experience severe acute maternal morbidity during that time period (odds ratio, 4.7). After accounting for confounding factors, the increased risk among women with twin versus singleton pregnancies persisted (OR, 4.2) during both the antepartum (OR, 4.1) and intrapartum/postpartum (OR, 4.2) periods.
The majority of events (77%) occurred during the latter periods, and the two most common underlying causal conditions were severe obstetric hemorrhage (66%) and severe hypertensive complications (20%); however, the increased risk in twin pregnancies was apparent, regardless of the underlying cause.
The cesarean delivery rates for twin versus singleton pregnancies were 72% and 34%, respectively, in the case group, and 58% and 18%, respectively, in the control group. A path analysis taking potential indication bias into account showed that 21% of the total risk of intrapartum or postpartum severe acute maternal morbidity risk associated with twin pregnancy was mediated by cesarean delivery, Dr. Madar and associates noted, explaining that, “in other words, if twin pregnancies had the same probability of cesarean delivery as singleton pregnancies, the association found between twin pregnancy and intrapartum or postpartum severe acute maternal morbidity would be reduced by one-fifth.”
This provides further support for limiting the use of cesarean for twin deliveries to cases with clear medical indications, as increasing the rate of vaginal deliveries may decrease the rate of severe acute maternal morbidity, they concluded.
EPIMOMS was supported by the National Research Agency and the Ile de France Regional Health Agency. Dr. Madar received a training grant from the Aquitaine Regional Health Agency. The authors reported having no other relevant financial disclosures.
SOURCE: Madar H et al. Obstet Gynecol. 2019;133:1141-50.
according to findings from the prospective EPIMOMS study.
The population-based incidence of severe acute maternal morbidity occurring between 22 weeks’ of gestation and 42 days post partum in the 2012-2013 French multicenter study was 6.2% among 3,202 twin pregnancies and 1.3% among 179,107 singleton pregnancies, Hugo Madar, MD, MPH, of Bordeaux University Hospital, France, and colleagues reported on behalf of the EPIMOMS (Epidémiologie de la Morbidité Maternelle Sévère) study group.
For the current analysis – a population-based, cohort-nested, case-control analysis of study data – the investigators compared 2,500 case patients (8% had twin pregnancies) and 3,650 controls (2% had twin pregnancies) who did not experience severe acute maternal morbidity during that time period (odds ratio, 4.7). After accounting for confounding factors, the increased risk among women with twin versus singleton pregnancies persisted (OR, 4.2) during both the antepartum (OR, 4.1) and intrapartum/postpartum (OR, 4.2) periods.
The majority of events (77%) occurred during the latter periods, and the two most common underlying causal conditions were severe obstetric hemorrhage (66%) and severe hypertensive complications (20%); however, the increased risk in twin pregnancies was apparent, regardless of the underlying cause.
The cesarean delivery rates for twin versus singleton pregnancies were 72% and 34%, respectively, in the case group, and 58% and 18%, respectively, in the control group. A path analysis taking potential indication bias into account showed that 21% of the total risk of intrapartum or postpartum severe acute maternal morbidity risk associated with twin pregnancy was mediated by cesarean delivery, Dr. Madar and associates noted, explaining that, “in other words, if twin pregnancies had the same probability of cesarean delivery as singleton pregnancies, the association found between twin pregnancy and intrapartum or postpartum severe acute maternal morbidity would be reduced by one-fifth.”
This provides further support for limiting the use of cesarean for twin deliveries to cases with clear medical indications, as increasing the rate of vaginal deliveries may decrease the rate of severe acute maternal morbidity, they concluded.
EPIMOMS was supported by the National Research Agency and the Ile de France Regional Health Agency. Dr. Madar received a training grant from the Aquitaine Regional Health Agency. The authors reported having no other relevant financial disclosures.
SOURCE: Madar H et al. Obstet Gynecol. 2019;133:1141-50.
FROM OBSTETRICS & GYNECOLOGY
Magnetic beads functionalized with VEGF could treat preeclampsia
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
A method of apheresis using vascular endothelial growth factor functionalized magnetic beads reduced levels of the soluble form of the vascular endothelial growth factor 1 in blood from women with preeclampsia, according to recent research published in the journal Hypertension.
The approach both reduces levels of the soluble form of the vascular endothelial growth factor 1 (sFlt-1) and releases placental growth factor (PlGF), which could help restore endothelial function in women with preeclampsia. The researchers said they chose sFlt-1 as a target because of “mounting evidence of its involvement in the pathogenesis of preeclampsia.” sFlt-1 has been suspected of inhibiting angiogenic signaling through “direct sequestration of angiogenic ligands” vascular endothelial growth factor (VEGF) and PlGF as well as “dominant-negative heterodimerization with surface VEGFRs.”
“During normal pregnancy, massive amounts of PlGF are produced by the placenta, reaching concentrations of free PlGF around 400 pg/mL, whereas during preeclampsia, free PlGF is extremely low due to the release of sFlt-1 into the maternal circulation,” the researchers said.
Using VEGF-functionalized magnetic beads, the researchers performed static and dynamic experiments using phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia. Under static conditions, there was a decrease of 33% for sFlt-1 and an increase of 27% for PlGF, while in dynamic conditions, there was a 40% decrease in sFlt-1 and a twofold increase in freed PlGF. When tested with plasma from women with preeclampsia, the ratio of sFlt-1/PlGF decreased by 63%, and VEGF release was associated with apheresis.
“This was a proof of concept study and our approach aims to restore physiologic levels of angiogenic factors,” Vassilis Tsatsaris, MD, PhD, of Cochin Hospital, Paris, said in a press release. “The reduction of sFlt-1 and the release of angiogenic factors is very significant and promising.”
Dr. Tsatsaris and his colleagues noted their next steps are to optimize the process of reducing sFlt-1 and examining how the approach works in an animal model.
“During normal pregnancy, circulating free VEGF levels are very low, almost undetectable with noncompetitive [enzyme-linked immunosorbent assay] ELISA. Whether these extremely low levels of VEGF have a physiological role during pregnancy is not known,” they wrote.
This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors report no relevant conflicts of interest.
SOURCE: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
FROM HYPERTENSION
Key clinical point: Use of magnetic beads functionalized with vascular endothelial growth factor (VEGF) reduced the soluble form of endothelial growth factor 1 (sFlt-1) in the blood of women with preeclampsia.
Major finding: sFlt-1 was reduced by 40% under dynamic conditions, and there was a twofold increase in the amount of freed placental growth factor.
Study details: A proof-of-concept study using VEGF-functionalized magnetic beads and phosphate buffered saline (PBS), conditioned media, and plasma from women with preeclampsia.
Disclosures: This study was funded by Agence Nationale pour la recherche, Institut Pierre Gilles de Gennes, and the PremUP Foundation. One author reported receiving a grant from the Ecole Normale Supérieure and a second author reported receiving a grant from the Fondation pour la Recherche Médicale. The other authors reported no relevant conflicts of interest.
Source: Trapiella-Alfonso L et al. Hypertension. 2019. doi: 10.1161/HYPERTENSIONAHA.118.12380.
N.Y. hospitals report near-universal CMV screening when newborns fail hearing tests
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
BALTIMORE – Over the past 2 years, Northwell Health, a large medical system in the metropolitan New York area, increased cytomegalovirus screening for infants who fail hearing tests from 6.6% to 95% at five of its birth hospitals, according to a presentation at the Pediatric Academic Societies annual meeting.
Three cases of congenital cytomegalovirus (CMV) have been picked up so far. The plan is to roll the program out to all 10 of the system’s birth hospitals, where over 40,000 children are born each year.
“We feel very satisfied and proud” of the progress that’s been made at Northwell in such a short time, said Alia Chauhan, MD, a Northwell pediatrician who presented the findings.
Northwell launched its “Hearing Plus” program in 2017 to catch the infection before infants leave the hospital. Several other health systems around the country have launched similar programs, and a handful of states – including New York – now require CMV screening for infants who fail mandated hearing tests.
The issue is gaining traction because hearing loss is often the only sign of congenital CMV, so it’s a bellwether for infection. Screening children with hearing loss is an easy way to pick it up early, so steps can be taken to prevent problems down the road. As it is, congenital CMV is the leading nongenetic cause of hearing loss in infants, accounting for at least 10% of cases.
The Northwell program kicked off with an education campaign to build consensus among pediatricians, hospitalists, and nurses. A flyer was made about CMV screening for moms whose infants fail hearing tests, printed in both English and Spanish.
Initially, the program used urine PCR [polymerase chain reaction] to screen for CMV, but waiting for infants to produce a sample often delayed discharge, so a switch was soon made to saliva swab PCRs, which take seconds, with urine PCR held in reserve to confirm positive swabs.
To streamline the process, a standing order was added to the electronic records system so nurses could order saliva PCRs without having to get physician approval. “I think [that] was one of the biggest things that’s helped us,” Dr. Chauhan said.
Children who test positive must have urine confirmation within 21 days of birth; most are long gone from the hospital by then and have to be called back in. “We haven’t lost anyone to follow-up, but it can be stressful trying to get someone to come back,” she said.
Six of 449 infants have screened positive on saliva – three were false positives with negative urine screens. Of the three confirmed cases, two infants later turned out to have normal hearing on repeat testing and were otherwise asymptomatic.
These days, Dr. Chauhan said, if children have a positive saliva PCR but later turn out to have normal hearing, and are otherwise free of symptoms with no CMV risk factors, “we are not confirming with urine.”
Dr. Chauhan did not have any disclosures. No funding source was mentioned.
SOURCE: Chauhan A et al. PAS 2019. Abstract 306
REPORTING FROM PAS 2019
Key clinical point: A metropolitan N.Y. health system provides a model for how to implement cytomegalovirus screening for infants who fail hearing tests.
Major finding: .
Study details: Pre-post quality improvement project.
Disclosures: The lead investigator had no disclosures. No funding source was mentioned.
Source: Chauhan A et al. PAS 2019. Abstract 306.
Managing 2nd trimester loss: Shared decision making, honor patient preference
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
NASHVILLE, TENN. – according to Sara W. Prager, MD.
Information transfer between the physician and patient, as opposed to a provider-driven or patient-driven decision-making process, better ensures that “the best possible decision” will be reached, Dr. Prager, director of the family planning division and family planning fellowship at the University of Washington in Seattle, said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Engaging the patient in the process – actively involving and supporting her in health care and treatment decision-making activities – is critically important, especially when dealing with pregnancy loss, which involves an acute sense of powerlessness, she said. Patient engagement is essential for respecting her autonomy, enhancing her agency, improving health status, reducing decisional conflict, and improving overall satisfaction.
Shared decision making requires a discussion about how the two approaches compare, particularly with respect to specific complications associated with each, Dr. Prager said, noting that discussion of values also should be encouraged.
Although surgical management is used more often, both approaches are safe and effective, and in the absence of clear contraindications in settings where both medication and a practitioner skilled in dilatation and evacuation are available, patient preference should honored, she said.
In this video interview, Dr. Prager further explains her position. “Using evidence-based medicine to have a shared decision-making process ... is extremely helpful for patients to feel like they have some control in this out-of-control situation where they’re experiencing a pregnancy loss.”
She also discussed how the use of mifepristone plus misoprostol for medical management of second-trimester loss has the potential to improve access.
“This is medication that, because of stigma surrounding abortion, is not always available ... so actually using it for non–abortion-related activities can be a way to help reduce that stigma around the medication itself, and get it into clinical sites, because it really does meaningfully improve management in the second trimester, as well as in the first trimester.”
In fact, the combination can cut nearly in half the amount of time it takes from the start of an induction until the end of the induction process, she said.
Dr. Prager also discussed surgical training resources and how to advocate for patient access to family planning experts who have the appropriate training.
Dr. Prager said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Is routine induction of labor in a healthy pregnancy at 39 weeks reasonable?
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
NASHVILLE, TENN. – Aaron B. Caughey, MD, PhD, discussed this in a video at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
In a healthy pregnancy, with no medical indications for induction of labor, 39-40 weeks’ gestation is a time when there is a relatively low risk of stillbirth, although the risk is not zero, Dr. Caughey explained. The same is true for neonatal death. This gestational age is a time when there is a low risk for respiratory complications and a low risk for meconium.
“This might be a nice time to have a baby,” said Dr. Caughey, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. “The trade-off is intervention. Don’t you increase the risk of C-sections?”
Actually, numerous retrospective studies have shown that there is either no difference or a decreased rate of C-sections with induction of labor at 39-40 weeks’ gestation, compared with expectant management.
These findings led to a prospective, randomized study by William A. Grobman, MD, and associates for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network called the ARRIVE trial (N Engl J Med. 2018;379:513-23). In that trial, the investigators randomized 3,062 women to induction of labor and 3,044 to expectant management. A significantly lower percentage of women in the induction of labor group underwent C-section than did women randomized to expectant management: 19% vs. 22% (relative risk, 0.84; P less than .001) – that is, 16% fewer C-sections. Also, 36% fewer women in the induction of labor group experienced preeclampsia. No significant differences were found between the two groups in terms of neonatal outcomes.
However, this is just one study, Dr. Caughey noted. What does is mean for a local community hospital? What does it mean for a busy private obstetrics practice?
Watch this video for his answer.
REPORTING FROM ACOG 2019
Most pregnancy-related deaths are preventable
according to the Centers for Disease Control and Prevention.
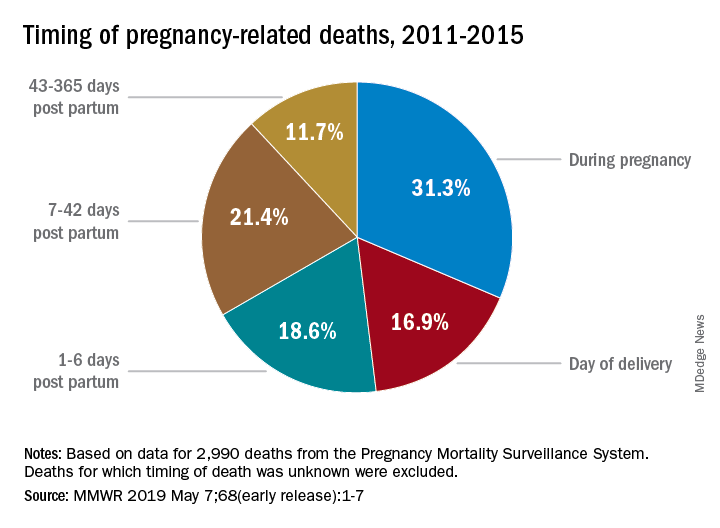
Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”
according to the Centers for Disease Control and Prevention.

Deaths from pregnancy-related complications can occur “up to a year after delivery,” the CDC emphasized in a report released May 7. Indeed, 31% of pregnancy-related deaths happen during pregnancy, 36% happen at delivery or in the week after, and 33% happen 1 week to 1 year post partum. Yet detailed data from 13 state maternal mortality review committees (MMRCs) showed that 60% of such deaths are preventable.
There were 17 pregnancy-related deaths per 100,000 live births during 2011-2015, based on another data source: 3,410 pregnancy-related deaths (an average of 682 deaths per year) in the CDC’s Pregnancy Mortality Surveillance System. That pregnancy-related mortality ratio varied by race/ethnicity, Emily E. Peterson, MD, and the other CDC investigators reported in Morbidity and Mortality Weekly Report: Hispanic (11 deaths per 100,000), white (13), Asian/Pacific Islander (14), American Indian/Alaska Native (33), and black (43).
One aspect of the disparity was addressed by Wanda Barfield, MD, MPH, director of the CDC’s division of reproductive health and assistant surgeon general in the U.S. Public Health Service. “Recent studies have shown that racial and ethnic minority women deliver at different and lower-quality hospitals than white women and that these hospitals disproportionately care for black women at delivery,” she said at a CDC telebriefing.
Analysis of the timing of 2,990 deaths from the Pregnancy Mortality Surveillance System showed that almost a third (31.3%) occurred during pregnancy and 16.9% occurred on the day of delivery. Dr. Peterson and her CDC associates noted that more than half of pregnancy-related deaths, however, took place later: 1-6 days post partum (18.6%), 7-42 days (21.4%), and 43-365 days (11.7%).
The data on preventability were collected by the state MMRCs and included 232 deaths that occurred during 2013-2017. The MMRCs considered deaths preventable if they could be “averted by one or more reasonable changes to patient, community, provider, health facility, and/or system factors.”
“Our new analysis underscores the need for access to quality services, risk awareness, and early diagnosis, but it also highlights opportunities for preventing future pregnancy-related deaths,” Dr. Barfield said. “By identifying and promptly responding to warning signs not just during pregnancy, but even up to a year after delivery, we can save lives.”











