User login
Antimalarials in pregnancy and lactation
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
According to the World Health Organization, there were about 219 million cases of malaria and an estimated 660,000 deaths in 2010. Although huge, this was a 26% decrease from the rates in 2000. Six countries in Africa account for 47% of malaria cases: Cote d’Ivoire, Democratic Republic of the Congo, Mozambique, Nigeria, Uganda, and the United Republic of Tanzania. The second-most affected region in the world is Southeast Asia, which includes Myanmar, India, and Indonesia. In comparison, about 1,500 malaria cases and 5 deaths are reported annually in the United States, mostly from returned travelers.
if they will be traveling in any of the above regions. Malaria during pregnancy increases the risk for adverse pregnancy outcomes, including maternal anemia, prematurity, spontaneous abortion, and stillbirth.
As stated by the Centers for Disease Control and Prevention, no antimalarial agent is 100% protective. Therefore, whatever agent is used must be combined with personal protective measures such as wearing insect repellent, long sleeves, and long pants; sleeping in a mosquito-free setting; or using an insecticide-treated bed net.
There are nine antimalarial drugs available in the United States.
Atovaquone/Proguanil Hcl (Malarone and as generic)
This agent is good for last-minute travelers because the drug is started 1-2 days before traveling to areas where malaria transmission occurs. The combination can be classified as compatible in pregnancy. No reports in breastfeeding with atovaquone or the combination have been found. Proguanil is not available in the United States as a single agent.
Chloroquine (generic)
This is the drug of choice to prevent and treat sensitive malaria species during pregnancy. The drug crosses the placenta producing fetal concentrations that are similar to those in the mother. The drug appears to be low risk for embryo-fetal harm.
It is compatible in breastfeeding.
Dapsone (generic)
This agent does not appear to represent a major risk of harm to the fetus. Although it has been used in combination with pyrimethamine (an antiparasitic) or trimethoprim (an antibiotic) to prevent malaria, the efficacy of the combination has not been confirmed.
In breastfeeding, there is one case of mild hemolytic anemia in the mother and her breastfeeding infant that may have been caused by the drug.
Hydroxychloroquine (generic)
This agent is used for the treatment of malaria, systemic erythematosus, and rheumatoid arthritis. For antimalarial prophylaxis, 400 mg/week appears to be low risk for embryo-fetal harm. Doses used to treat malaria have been 200-400 mg/day.
Because very low concentrations of the drug have been found in breast milk, breastfeeding is probably compatible.
Mefloquine (generic)
This agent is a quinoline-methanol agent that does not appear to cause embryo-fetal harm based on a large number of pregnancy exposures.
There are no reports of its use while breastfeeding.
Primaquine (generic)
This agent is best avoided in pregnancy. There is no human pregnancy data, but it may cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiency (G6PD). Because the fetus is relatively G6PD deficient, it is best avoided in pregnancy regardless of the mother’s status.
There are no reports describing the use of the drug during lactation. Both the mother and baby should be tested for G6PD deficiency before the drug is used during breastfeeding.
Pyrimethamine (generic)
This agent has been used for the treatment or prophylaxis of malaria. Most studies have found this agent to be relatively safe and effective.
It is excreted into breast milk and has been effective in eliminating malaria parasites from breastfeeding infants.
Quinidine (generic)
Reports linking the use of this agent with congenital defects have not been found. Although the drug has data on its use as an antiarrhythmic, its published use to treat malaria is limited.
The drug is excreted into breast milk, but there are no reports of its during breastfeeding.
Quinine (generic)
This agent has a large amount of human pregnancy data (more than 1,000 exposures) that found no increased risk of birth defects. The drug has been replaced by newer agents but still may be used for chloroquine-resistant malaria.
The drug appears to be compatible during breastfeeding.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
Louisiana House passes 6-week abortion ban
about the time a heartbeat is usually detectable.
The legislation, which does not include an exception for rape or incest cases, passed by a 79-23 vote on May 29 in the Louisiana House. The bill does allow exceptions if the woman’s life is in danger, if the pregnancy poses risk of serious impairment to a woman’s body, of if the pregnancy is deemed medically futile. Democratic Gov. John Bel Edwards said he plans to sign the measure when it hits his desk.
“In 2015, I ran for governor as a pro-life candidate after serving as a pro-life legislator for 8 years,” Gov. Edwards said in a May 29 statement. “As governor, I have been true to my word and my beliefs on this issue. As I prepare to sign this bill, I call on the overwhelming bipartisan majority of legislators who voted for it to join me in continuing to build a better Louisiana that cares for the least among us and provides more opportunity for everyone.”
Six other states have enacted similar abortion bans: Alabama, Georgia, Kentucky, Mississippi, Missouri, and Ohio. While most of the laws bar abortions after a heartbeat is detected, Alabama’s measure prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
A number of lawsuits have been filed against the bans, including a May 24 legal challenge against Alabama’s law by Planned Parenthood Federation of America and the American Civil Liberties Union (ACLU), and a May 15 legal challenge against Ohio’s law by the ACLU and Planned Parenthood of Greater Ohio.
The U.S. Supreme Court on May 28 upheld part of an Indiana law that requires burial or cremation of fetal remains after an abortion, but the justices declined to address the measure’s prohibition on abortions sought because of race, sex, or disability of the fetus.
Court analysts say it’s only a matter of time before the Supreme Court takes up one of the abortion ban cases, most likely the legal challenge against Alabama’s law. Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Justice Brett M. Kavanaugh, and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe v. Wade, court watchers said.
about the time a heartbeat is usually detectable.
The legislation, which does not include an exception for rape or incest cases, passed by a 79-23 vote on May 29 in the Louisiana House. The bill does allow exceptions if the woman’s life is in danger, if the pregnancy poses risk of serious impairment to a woman’s body, of if the pregnancy is deemed medically futile. Democratic Gov. John Bel Edwards said he plans to sign the measure when it hits his desk.
“In 2015, I ran for governor as a pro-life candidate after serving as a pro-life legislator for 8 years,” Gov. Edwards said in a May 29 statement. “As governor, I have been true to my word and my beliefs on this issue. As I prepare to sign this bill, I call on the overwhelming bipartisan majority of legislators who voted for it to join me in continuing to build a better Louisiana that cares for the least among us and provides more opportunity for everyone.”
Six other states have enacted similar abortion bans: Alabama, Georgia, Kentucky, Mississippi, Missouri, and Ohio. While most of the laws bar abortions after a heartbeat is detected, Alabama’s measure prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
A number of lawsuits have been filed against the bans, including a May 24 legal challenge against Alabama’s law by Planned Parenthood Federation of America and the American Civil Liberties Union (ACLU), and a May 15 legal challenge against Ohio’s law by the ACLU and Planned Parenthood of Greater Ohio.
The U.S. Supreme Court on May 28 upheld part of an Indiana law that requires burial or cremation of fetal remains after an abortion, but the justices declined to address the measure’s prohibition on abortions sought because of race, sex, or disability of the fetus.
Court analysts say it’s only a matter of time before the Supreme Court takes up one of the abortion ban cases, most likely the legal challenge against Alabama’s law. Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Justice Brett M. Kavanaugh, and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe v. Wade, court watchers said.
about the time a heartbeat is usually detectable.
The legislation, which does not include an exception for rape or incest cases, passed by a 79-23 vote on May 29 in the Louisiana House. The bill does allow exceptions if the woman’s life is in danger, if the pregnancy poses risk of serious impairment to a woman’s body, of if the pregnancy is deemed medically futile. Democratic Gov. John Bel Edwards said he plans to sign the measure when it hits his desk.
“In 2015, I ran for governor as a pro-life candidate after serving as a pro-life legislator for 8 years,” Gov. Edwards said in a May 29 statement. “As governor, I have been true to my word and my beliefs on this issue. As I prepare to sign this bill, I call on the overwhelming bipartisan majority of legislators who voted for it to join me in continuing to build a better Louisiana that cares for the least among us and provides more opportunity for everyone.”
Six other states have enacted similar abortion bans: Alabama, Georgia, Kentucky, Mississippi, Missouri, and Ohio. While most of the laws bar abortions after a heartbeat is detected, Alabama’s measure prohibits abortion at every pregnancy stage and penalizes physicians with a Class A felony for performing an abortion and a Class C felony for attempting to perform an abortion. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
A number of lawsuits have been filed against the bans, including a May 24 legal challenge against Alabama’s law by Planned Parenthood Federation of America and the American Civil Liberties Union (ACLU), and a May 15 legal challenge against Ohio’s law by the ACLU and Planned Parenthood of Greater Ohio.
The U.S. Supreme Court on May 28 upheld part of an Indiana law that requires burial or cremation of fetal remains after an abortion, but the justices declined to address the measure’s prohibition on abortions sought because of race, sex, or disability of the fetus.
Court analysts say it’s only a matter of time before the Supreme Court takes up one of the abortion ban cases, most likely the legal challenge against Alabama’s law. Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Justice Brett M. Kavanaugh, and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe v. Wade, court watchers said.
FIGO outlines global standards for preeclampsia screening
as a one-step procedure, according to new recommendations from The International Federation of Gynecology and Obstetrics (FIGO).
FIGO “encourages all countries and its member associations to adopt and promote strategies to ensure [universal screening],” Liona C. Poon, MD, of Prince of Wales Hospital, the Chinese University of Hong Kong, Shatin, and colleagues wrote in a guide published in the International Journal of Gynecology & Obstetrics.
“The best combined test is one that includes maternal risk factors, measurements of mean arterial pressure (MAP), serum placental growth factor (PLGF), and uterine artery pulsatility index (UTPI),” the authors said, noting that the baseline screening test plus a combination of maternal risk factors with MAP is an alternative when PLGF and/or UTPI can’t be measured.
The FIGO recommendations are the culmination of an initiative on PE, which involved a group of international experts convened to discuss and evaluate current knowledge on PE and to “develop a document to frame the issues and suggest key actions to address the health burden posed by PE.” Among the group’s objectives are raising awareness of the links between PE and poor outcomes, and demanding a “clearly defined global health agenda” to address the issue because preeclampsia affects 2%-5% of all pregnant women and is a leading cause of maternal and perinatal morbidity and mortality.
The recommendations represent a consensus document that provides guidance for the first trimester screening and prevention of preterm PE.
“Based on high‐quality evidence, the document outlines current global standards for the first‐trimester screening and prevention of preterm PE, which is in line with FIGO good clinical practice advice on first trimester screening and prevention of preeclampsia in singleton pregnancy,” the authors said, explaining that “[it] provides both the best and the most pragmatic recommendations according to the level of acceptability, feasibility, and ease of implementation that have the potential to produce the most significant impact in different resource settings” (Int J Gynecol Obstet. 2019;145[Suppl. 1]:1-33).
Specific suggestions are made based on region and resources, and research priorities are outlined to “bridge the current knowledge and evidence gap.”
In addition to universal first trimester screening for PE, the guide stresses a need for improved public health focus, and contingent screening approaches in areas with limited resources (including routine screening for preterm PE by maternal factors and MAP in most cases, with PLGF and UTPI measurement reserved for higher-risk women). It also recommends that women at high risk should receive prophylactic measures such as aspirin therapy beginning at 11–14+6 weeks of gestation at a dose of about 150 mg to be taken every night until 36 weeks of gestation, when delivery occurs, or when PE is diagnosed.
Mary E. D’Alton, MD, a maternal-fetal medicine specialist who is chair of the department of obstetrics and gynecology and the Willard C. Rappleye Professor of Obstetrics & Gynecology at Columbia University, New York, was asked to comment on Dr. Sibai’s concerns about the guidelines. “I would simply say that ACOG and SMFM are the organizations in the United States [that] provide educational guidelines about practice in the United States.” Dr. D’Alton assisted Dr. Poon and her associates on the guidelines as an expert on preeclampsia.
Dr. Poon, given a chance to comment on the concern that the FIGO guidelines diverged from those of ACOG and SMFM, responded in an interview, “FIGO, being the global voice for women’s health, likes to ensure that our recommendations are resource appropriate. The objective of these guidelines is to provide a best practice approach, and also offers other pragmatic options for lower resource settings to ensure that preeclampsia testing can be implemented globally. We urge the broader membership of FIGO to adapt these guidelines to their local contexts.”*
She also emphasized that “PerkinElmer’s sponsorship was an unrestricted grant. The company had no involvement in writing the guideline.”*
This work was funded by an unrestricted grant from PerkinElmer, which markets an assay used for first trimester preeclampsia screening. Dr. Poon and her associates reported having no conflicts of interest.
[email protected]
*This article was updated 5/31/2019.
The recommendations regarding screening and management of first trimester preeclampsia as issued by FIGO are largely inconsistent with those from the American College of Obstetricians and Gynecologists (ACOG) and from the Society for Maternal-Fetal Medicine (SMFM), according to Baha M. Sibai, MD.
The FIGO recommendation for first trimester screening and use of low-dose aspirin at 150 mg daily starting at 13 weeks also contradicts the ACOG and SMFM recommendations, he said, noting that a 2019 ACOG practice bulletin on preeclampsia recommends 81 mg of aspirin daily initiated between 12 and 28 weeks of gestation (optimally before 16 weeks of gestation) and continuing until delivery; this is for women with any of the high risk factors for preeclampsia and for women with more than one of the moderate risk factors. Under that recommendation, more women would be eligible based on clinical risk factors. The FIGO approach is not cost effective and will miss many cases of preeclampsia, compared with the ACOG recommendations, he said.
It also should be noted that the FIGO document is funded by a grant from PerkinElmer, which markets a test for PE, he said, stressing that there are “no data suggesting that this test is valid in U.S. pregnancies.”
Dr. Sibai is a maternal-fetal medicine specialist with UT Physicians Maternal-Fetal Medicine Center–Texas Medical Center, and a professor in the department of obstetrics, gynecology, and reproductive sciences at the University of Texas Health Science Center, Houston. He was asked to comment on the article by Poon et al. He said he had no relevant financial disclosures.
The recommendations regarding screening and management of first trimester preeclampsia as issued by FIGO are largely inconsistent with those from the American College of Obstetricians and Gynecologists (ACOG) and from the Society for Maternal-Fetal Medicine (SMFM), according to Baha M. Sibai, MD.
The FIGO recommendation for first trimester screening and use of low-dose aspirin at 150 mg daily starting at 13 weeks also contradicts the ACOG and SMFM recommendations, he said, noting that a 2019 ACOG practice bulletin on preeclampsia recommends 81 mg of aspirin daily initiated between 12 and 28 weeks of gestation (optimally before 16 weeks of gestation) and continuing until delivery; this is for women with any of the high risk factors for preeclampsia and for women with more than one of the moderate risk factors. Under that recommendation, more women would be eligible based on clinical risk factors. The FIGO approach is not cost effective and will miss many cases of preeclampsia, compared with the ACOG recommendations, he said.
It also should be noted that the FIGO document is funded by a grant from PerkinElmer, which markets a test for PE, he said, stressing that there are “no data suggesting that this test is valid in U.S. pregnancies.”
Dr. Sibai is a maternal-fetal medicine specialist with UT Physicians Maternal-Fetal Medicine Center–Texas Medical Center, and a professor in the department of obstetrics, gynecology, and reproductive sciences at the University of Texas Health Science Center, Houston. He was asked to comment on the article by Poon et al. He said he had no relevant financial disclosures.
The recommendations regarding screening and management of first trimester preeclampsia as issued by FIGO are largely inconsistent with those from the American College of Obstetricians and Gynecologists (ACOG) and from the Society for Maternal-Fetal Medicine (SMFM), according to Baha M. Sibai, MD.
The FIGO recommendation for first trimester screening and use of low-dose aspirin at 150 mg daily starting at 13 weeks also contradicts the ACOG and SMFM recommendations, he said, noting that a 2019 ACOG practice bulletin on preeclampsia recommends 81 mg of aspirin daily initiated between 12 and 28 weeks of gestation (optimally before 16 weeks of gestation) and continuing until delivery; this is for women with any of the high risk factors for preeclampsia and for women with more than one of the moderate risk factors. Under that recommendation, more women would be eligible based on clinical risk factors. The FIGO approach is not cost effective and will miss many cases of preeclampsia, compared with the ACOG recommendations, he said.
It also should be noted that the FIGO document is funded by a grant from PerkinElmer, which markets a test for PE, he said, stressing that there are “no data suggesting that this test is valid in U.S. pregnancies.”
Dr. Sibai is a maternal-fetal medicine specialist with UT Physicians Maternal-Fetal Medicine Center–Texas Medical Center, and a professor in the department of obstetrics, gynecology, and reproductive sciences at the University of Texas Health Science Center, Houston. He was asked to comment on the article by Poon et al. He said he had no relevant financial disclosures.
as a one-step procedure, according to new recommendations from The International Federation of Gynecology and Obstetrics (FIGO).
FIGO “encourages all countries and its member associations to adopt and promote strategies to ensure [universal screening],” Liona C. Poon, MD, of Prince of Wales Hospital, the Chinese University of Hong Kong, Shatin, and colleagues wrote in a guide published in the International Journal of Gynecology & Obstetrics.
“The best combined test is one that includes maternal risk factors, measurements of mean arterial pressure (MAP), serum placental growth factor (PLGF), and uterine artery pulsatility index (UTPI),” the authors said, noting that the baseline screening test plus a combination of maternal risk factors with MAP is an alternative when PLGF and/or UTPI can’t be measured.
The FIGO recommendations are the culmination of an initiative on PE, which involved a group of international experts convened to discuss and evaluate current knowledge on PE and to “develop a document to frame the issues and suggest key actions to address the health burden posed by PE.” Among the group’s objectives are raising awareness of the links between PE and poor outcomes, and demanding a “clearly defined global health agenda” to address the issue because preeclampsia affects 2%-5% of all pregnant women and is a leading cause of maternal and perinatal morbidity and mortality.
The recommendations represent a consensus document that provides guidance for the first trimester screening and prevention of preterm PE.
“Based on high‐quality evidence, the document outlines current global standards for the first‐trimester screening and prevention of preterm PE, which is in line with FIGO good clinical practice advice on first trimester screening and prevention of preeclampsia in singleton pregnancy,” the authors said, explaining that “[it] provides both the best and the most pragmatic recommendations according to the level of acceptability, feasibility, and ease of implementation that have the potential to produce the most significant impact in different resource settings” (Int J Gynecol Obstet. 2019;145[Suppl. 1]:1-33).
Specific suggestions are made based on region and resources, and research priorities are outlined to “bridge the current knowledge and evidence gap.”
In addition to universal first trimester screening for PE, the guide stresses a need for improved public health focus, and contingent screening approaches in areas with limited resources (including routine screening for preterm PE by maternal factors and MAP in most cases, with PLGF and UTPI measurement reserved for higher-risk women). It also recommends that women at high risk should receive prophylactic measures such as aspirin therapy beginning at 11–14+6 weeks of gestation at a dose of about 150 mg to be taken every night until 36 weeks of gestation, when delivery occurs, or when PE is diagnosed.
Mary E. D’Alton, MD, a maternal-fetal medicine specialist who is chair of the department of obstetrics and gynecology and the Willard C. Rappleye Professor of Obstetrics & Gynecology at Columbia University, New York, was asked to comment on Dr. Sibai’s concerns about the guidelines. “I would simply say that ACOG and SMFM are the organizations in the United States [that] provide educational guidelines about practice in the United States.” Dr. D’Alton assisted Dr. Poon and her associates on the guidelines as an expert on preeclampsia.
Dr. Poon, given a chance to comment on the concern that the FIGO guidelines diverged from those of ACOG and SMFM, responded in an interview, “FIGO, being the global voice for women’s health, likes to ensure that our recommendations are resource appropriate. The objective of these guidelines is to provide a best practice approach, and also offers other pragmatic options for lower resource settings to ensure that preeclampsia testing can be implemented globally. We urge the broader membership of FIGO to adapt these guidelines to their local contexts.”*
She also emphasized that “PerkinElmer’s sponsorship was an unrestricted grant. The company had no involvement in writing the guideline.”*
This work was funded by an unrestricted grant from PerkinElmer, which markets an assay used for first trimester preeclampsia screening. Dr. Poon and her associates reported having no conflicts of interest.
[email protected]
*This article was updated 5/31/2019.
as a one-step procedure, according to new recommendations from The International Federation of Gynecology and Obstetrics (FIGO).
FIGO “encourages all countries and its member associations to adopt and promote strategies to ensure [universal screening],” Liona C. Poon, MD, of Prince of Wales Hospital, the Chinese University of Hong Kong, Shatin, and colleagues wrote in a guide published in the International Journal of Gynecology & Obstetrics.
“The best combined test is one that includes maternal risk factors, measurements of mean arterial pressure (MAP), serum placental growth factor (PLGF), and uterine artery pulsatility index (UTPI),” the authors said, noting that the baseline screening test plus a combination of maternal risk factors with MAP is an alternative when PLGF and/or UTPI can’t be measured.
The FIGO recommendations are the culmination of an initiative on PE, which involved a group of international experts convened to discuss and evaluate current knowledge on PE and to “develop a document to frame the issues and suggest key actions to address the health burden posed by PE.” Among the group’s objectives are raising awareness of the links between PE and poor outcomes, and demanding a “clearly defined global health agenda” to address the issue because preeclampsia affects 2%-5% of all pregnant women and is a leading cause of maternal and perinatal morbidity and mortality.
The recommendations represent a consensus document that provides guidance for the first trimester screening and prevention of preterm PE.
“Based on high‐quality evidence, the document outlines current global standards for the first‐trimester screening and prevention of preterm PE, which is in line with FIGO good clinical practice advice on first trimester screening and prevention of preeclampsia in singleton pregnancy,” the authors said, explaining that “[it] provides both the best and the most pragmatic recommendations according to the level of acceptability, feasibility, and ease of implementation that have the potential to produce the most significant impact in different resource settings” (Int J Gynecol Obstet. 2019;145[Suppl. 1]:1-33).
Specific suggestions are made based on region and resources, and research priorities are outlined to “bridge the current knowledge and evidence gap.”
In addition to universal first trimester screening for PE, the guide stresses a need for improved public health focus, and contingent screening approaches in areas with limited resources (including routine screening for preterm PE by maternal factors and MAP in most cases, with PLGF and UTPI measurement reserved for higher-risk women). It also recommends that women at high risk should receive prophylactic measures such as aspirin therapy beginning at 11–14+6 weeks of gestation at a dose of about 150 mg to be taken every night until 36 weeks of gestation, when delivery occurs, or when PE is diagnosed.
Mary E. D’Alton, MD, a maternal-fetal medicine specialist who is chair of the department of obstetrics and gynecology and the Willard C. Rappleye Professor of Obstetrics & Gynecology at Columbia University, New York, was asked to comment on Dr. Sibai’s concerns about the guidelines. “I would simply say that ACOG and SMFM are the organizations in the United States [that] provide educational guidelines about practice in the United States.” Dr. D’Alton assisted Dr. Poon and her associates on the guidelines as an expert on preeclampsia.
Dr. Poon, given a chance to comment on the concern that the FIGO guidelines diverged from those of ACOG and SMFM, responded in an interview, “FIGO, being the global voice for women’s health, likes to ensure that our recommendations are resource appropriate. The objective of these guidelines is to provide a best practice approach, and also offers other pragmatic options for lower resource settings to ensure that preeclampsia testing can be implemented globally. We urge the broader membership of FIGO to adapt these guidelines to their local contexts.”*
She also emphasized that “PerkinElmer’s sponsorship was an unrestricted grant. The company had no involvement in writing the guideline.”*
This work was funded by an unrestricted grant from PerkinElmer, which markets an assay used for first trimester preeclampsia screening. Dr. Poon and her associates reported having no conflicts of interest.
[email protected]
*This article was updated 5/31/2019.
FROM THE INTERNATIONAL JOURNAL OF GYNECOLOGY & OBSTETRICS
Maternal mortality: Critical next steps in addressing the crisis
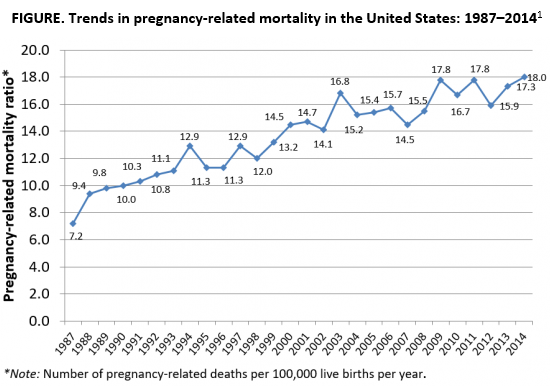
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1
A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
As the rest of the industrialized world has seen a decline in maternal mortality, the United States has seen a substantial rise over the last 30 years (FIGURE).1 It is estimated that more than 60% of these pregnancy-related deaths are preventable. Additionally, substantial disparities exist, with African-American women 3 to 4 times more likely to die of pregnancy-related complications than white women.1

A good first step
The Preventing Maternal Deaths Act was passed by the 115th Congress and signed into law December 2018 in an effort to support and expand maternal mortality review committees (MMRCs) on a state level while allowing the Centers for Disease Control and Prevention (CDC) to further study disparities within maternal mortality. Although these efforts are a good first step to help reduce maternal mortality, more needs to be done to quell this growing epidemic.
We must now improve care access
One strategy to aid in decreasing maternal morbidity and mortality is to improve affordable access to medical care. Medicaid is the largest single payer of maternity care in the United States, covering 42.6% of births. Currently, in many states, Medicaid coverage only lasts until a woman is 60 days postpartum.2 Although 31 states, including the District of Columbia, have adopted Medicaid expansion programs to allow women to extend coverage beyond those 60 days, offering these programs is not a federal law. In the 19 remaining states with no extension options, the vast majority of women will lose their Medicaid coverage just after they are 2 months postpartum and will have no alternative health insurance coverage.2
Why does this coverage cutoff matter? Pregnancy-related deaths are defined as up to 12 months postpartum. A report reviewing 9 MMRCs found that 38% of pregnancy-related deaths occurred while a woman was pregnant, 45% of deaths occurred within 42 days of delivery, and 18% from 43 days to 1 year after delivery.3 Additionally, nearly half of women with Medicaid do not come to their 6-week postpartum visit (for a variety of reasons), missing a critical opportunity to address health concerns.2 Of the deaths that occurred in this later postpartum period, leading causes were cardiomyopathy (32%), mental health conditions (16%), and embolism (11%).3 Prevention and management of these conditions require regular follow-up with an ObGyn, as well as potentially from subspecialists in cardiology, psychiatry, hematology, and other subspecialties. Women not having access to affordable health care during the critical postpartum period greatly increases their risk of death or severe morbidity.
An important next step beyond the Preventing Maternal Deaths Act is to extend Medicaid coverage to 12 months postpartum for all women everywhere. MMRCs have concluded that extending coverage would ensure that “medical and behavioral health conditions [could be] managed and treated before becoming progressively severe.”3 This would presumably help decrease the risk of pregnancy-related death and address worsening morbidity. Additionally, the postpartum period is a well-established time of increased stress and can be an overwhelming and emotional time for many new mothers, especially for those with limited resources for childcare, transportation, stable housing, etc.6 Providing and ensuring ongoing medical care would substantially improve the lives and health of women and the health of their families.
We, as a country, need to make changes
Every step of the way, a woman faces challenges to safely and affordably access health care. Providing access to insurance coverage for 12 months postpartum can help to decrease our country’s rising maternal mortality and morbidity rates.
Take action
Congresswoman Robin Kelly (D-IL) and Senator Dick Durbin (D-IL) have introduced the MOMMA Act (H.R. 1897/S. 916) to help address the rising maternal mortality rate.
This Act would:
- Expand Medicaid coverage to 1 year postpartum.
- Work with the CDC to uniformly collect data to accurately assess maternal mortality and morbidity.
- Ensure the sharing of best practices of care across hospital systems.
- Focus on culturally-competent care to address implicit bias among health care workers.
- Support and expand the Alliance for Innovation on Maternal Health (AIM)—a data-driven initiative to implement safety protocols in hospitals across the country.
To call or contact your representative to co-sponsor this bill, click here. To review if your Congressperson is a co-sponsor, click here. To review if your Senator is a co-sponsor, click here.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
- The Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System, Trends in Pregnancy-Related Deaths. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pregnancy-mortality-surveillance-system.htm. Accessed May 29, 2019.
- Stuebe A, Moore JE, Mittal P, et al. Extending medicaid coverage for postpartum moms. May 6, 2019. https://www.healthaffairs.org/do/10.1377/hblog20190501.254675/full/. Accessed May 29, 2019.
- Building U.S. Capacity to Review and Prevent Maternal Deaths. Report from nine maternal mortality review committees. 2018. Color/Word_R17_G85_B204http://reviewtoaction.org/Report_from_Nine_MMRCs. Accessed May 29, 2019.
- MacDorman MF, Declercq E, Cabral H, et al. Recent increases in the U.S. maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447-455.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2016. Natl Vital Stat Rep. 2018;67:1-55.
- Vestal C. For addicted women, the year after childbirth is the deadliest. August 14, 2018. https://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2018/08/14/for-addicted-women-the-year-after-childbirth-is-the-deadliest. Accessed May 29, 2019.
What closing Missouri’s last abortion clinic will mean for neighboring states
ST. LOUIS – As the last abortion clinic in Missouri warned that it will have to stop providing the procedure as soon as May 31, abortion providers in surrounding states said they are anticipating an uptick of even more Missouri patients.
At Hope Clinic in Granite City, Ill., just 10 minutes from downtown St. Louis, Deputy Director Alison Dreith said on May 28 that her clinic was preparing for more patients as news about Missouri spread.
“We’re really scrambling today about the need for increased staff and how fast can we hire and train,” Dreith said.
And at a Trust Women clinic in Wichita, Kan., that already has to fly in doctors, the staff didn’t know what it would mean for their overloaded patient schedule.
“God forbid we see that people can’t get services in Missouri,” said Julie Burkhart, Trust Women founder and CEO. “What is that going to mean on our limited physician days?”
If St. Louis’ Planned Parenthood clinic is unable to offer abortions, the group said, Missouri would be the only state in the country to not have an operating abortion clinic. Five other states – Kentucky, Mississippi, North Dakota, South Dakota and West Virginia – reportedly have only one abortion clinic. And 90% of U.S. counties didn’t have an abortion clinic as of 2014, according to the Guttmacher Institute, a reproductive rights research and advocacy group.
For some, this echoes back to the days before abortion was legalized nationwide in 1973 with the Supreme Court’s Roe v. Wade decision, when patients who could afford to travel would go to more liberal states like California or New York where abortion was legal.
But providers in Kansas and Illinois say this influx from Missouri isn’t new. About half of their clients already come from the Show Me State. To the south, in neighboring Arkansas, where a 72-hour waiting period will go into effect in July, the vast majority of its patients still live within the state.
Over the past 10 years, four Missouri abortion clinics have closed because of increased regulations, including a mandatory 72-hour waiting period after receiving counseling on abortion, thus requiring two trips to a facility; requirements that physicians have hospital admitting privileges within 15 minutes of their clinics; and a rule requiring two-parent notification for minors and one-parent notarized consent. All those limits left one clinic in downtown St. Louis to serve the whole state.
Now Planned Parenthood, which operates that final abortion clinic, said on May 28 that it will be forced to end its abortion services altogether by May 31 if the state suspends its license. The closure is not related to new anti-abortion laws that Missouri Gov. Mike Parson, a Republican, signed on May 24 to ban most abortions after 8 weeks of pregnancy. The new laws don’t take effect until August.
Already the number of patients in Missouri seeking an abortion at the clinic from April 2018 until this April had dropped by 50% compared with the same period the previous year. Planned Parenthood spokesman Jesse Lawder attributes two-thirds of the decrease to the clinic’s refusal to do pelvic exams for abortions performed through medication – recently required by the state – thus forcing all such abortions to be performed out of state.
For Dreith, while she expects the Missouri numbers to continue to grow at her Illinois clinic across the Mississippi River, it’s not the only state sending patients her way.
“Patients were literally coming to us from the last remaining clinics in Kentucky ... so that they wouldn’t get past 24 weeks,” Dreith said. “We don’t want these patients in surrounding states traveling [to] New York [or] California like they once had to.”
That’s how it was prior to the Roe v. Wade ruling, according to Mary Ziegler, a professor at Florida State University College of Law who is writing her third book on the history of the legal battle around abortion access. She anticipates the pattern of privilege will repeat itself.
“You would still expect women with resources to be able to travel as far as they needed,” she said. “And you would expect women without resources to not be able to travel. ... The more the court retreats from protecting abortion rights, the more stark those differences will become.”
For Dreith, the historical comparison to the pre-Roe era rings true, albeit with improved medical practices.
There are safer, easier, and more effective ways to perform abortions now than the “horror stories that we saw pre-Roe,” said Dreith. “But I think the travel will be one of the huge throwbacks and the scariest part will be the criminalization.”
States such as Missouri could feel pressure to start arresting women who perform their own abortions with pills at home or travel out of state, Ziegler said. But, she said, “punishing women isn’t something that’s thought to be very popular.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
ST. LOUIS – As the last abortion clinic in Missouri warned that it will have to stop providing the procedure as soon as May 31, abortion providers in surrounding states said they are anticipating an uptick of even more Missouri patients.
At Hope Clinic in Granite City, Ill., just 10 minutes from downtown St. Louis, Deputy Director Alison Dreith said on May 28 that her clinic was preparing for more patients as news about Missouri spread.
“We’re really scrambling today about the need for increased staff and how fast can we hire and train,” Dreith said.
And at a Trust Women clinic in Wichita, Kan., that already has to fly in doctors, the staff didn’t know what it would mean for their overloaded patient schedule.
“God forbid we see that people can’t get services in Missouri,” said Julie Burkhart, Trust Women founder and CEO. “What is that going to mean on our limited physician days?”
If St. Louis’ Planned Parenthood clinic is unable to offer abortions, the group said, Missouri would be the only state in the country to not have an operating abortion clinic. Five other states – Kentucky, Mississippi, North Dakota, South Dakota and West Virginia – reportedly have only one abortion clinic. And 90% of U.S. counties didn’t have an abortion clinic as of 2014, according to the Guttmacher Institute, a reproductive rights research and advocacy group.
For some, this echoes back to the days before abortion was legalized nationwide in 1973 with the Supreme Court’s Roe v. Wade decision, when patients who could afford to travel would go to more liberal states like California or New York where abortion was legal.
But providers in Kansas and Illinois say this influx from Missouri isn’t new. About half of their clients already come from the Show Me State. To the south, in neighboring Arkansas, where a 72-hour waiting period will go into effect in July, the vast majority of its patients still live within the state.
Over the past 10 years, four Missouri abortion clinics have closed because of increased regulations, including a mandatory 72-hour waiting period after receiving counseling on abortion, thus requiring two trips to a facility; requirements that physicians have hospital admitting privileges within 15 minutes of their clinics; and a rule requiring two-parent notification for minors and one-parent notarized consent. All those limits left one clinic in downtown St. Louis to serve the whole state.
Now Planned Parenthood, which operates that final abortion clinic, said on May 28 that it will be forced to end its abortion services altogether by May 31 if the state suspends its license. The closure is not related to new anti-abortion laws that Missouri Gov. Mike Parson, a Republican, signed on May 24 to ban most abortions after 8 weeks of pregnancy. The new laws don’t take effect until August.
Already the number of patients in Missouri seeking an abortion at the clinic from April 2018 until this April had dropped by 50% compared with the same period the previous year. Planned Parenthood spokesman Jesse Lawder attributes two-thirds of the decrease to the clinic’s refusal to do pelvic exams for abortions performed through medication – recently required by the state – thus forcing all such abortions to be performed out of state.
For Dreith, while she expects the Missouri numbers to continue to grow at her Illinois clinic across the Mississippi River, it’s not the only state sending patients her way.
“Patients were literally coming to us from the last remaining clinics in Kentucky ... so that they wouldn’t get past 24 weeks,” Dreith said. “We don’t want these patients in surrounding states traveling [to] New York [or] California like they once had to.”
That’s how it was prior to the Roe v. Wade ruling, according to Mary Ziegler, a professor at Florida State University College of Law who is writing her third book on the history of the legal battle around abortion access. She anticipates the pattern of privilege will repeat itself.
“You would still expect women with resources to be able to travel as far as they needed,” she said. “And you would expect women without resources to not be able to travel. ... The more the court retreats from protecting abortion rights, the more stark those differences will become.”
For Dreith, the historical comparison to the pre-Roe era rings true, albeit with improved medical practices.
There are safer, easier, and more effective ways to perform abortions now than the “horror stories that we saw pre-Roe,” said Dreith. “But I think the travel will be one of the huge throwbacks and the scariest part will be the criminalization.”
States such as Missouri could feel pressure to start arresting women who perform their own abortions with pills at home or travel out of state, Ziegler said. But, she said, “punishing women isn’t something that’s thought to be very popular.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
ST. LOUIS – As the last abortion clinic in Missouri warned that it will have to stop providing the procedure as soon as May 31, abortion providers in surrounding states said they are anticipating an uptick of even more Missouri patients.
At Hope Clinic in Granite City, Ill., just 10 minutes from downtown St. Louis, Deputy Director Alison Dreith said on May 28 that her clinic was preparing for more patients as news about Missouri spread.
“We’re really scrambling today about the need for increased staff and how fast can we hire and train,” Dreith said.
And at a Trust Women clinic in Wichita, Kan., that already has to fly in doctors, the staff didn’t know what it would mean for their overloaded patient schedule.
“God forbid we see that people can’t get services in Missouri,” said Julie Burkhart, Trust Women founder and CEO. “What is that going to mean on our limited physician days?”
If St. Louis’ Planned Parenthood clinic is unable to offer abortions, the group said, Missouri would be the only state in the country to not have an operating abortion clinic. Five other states – Kentucky, Mississippi, North Dakota, South Dakota and West Virginia – reportedly have only one abortion clinic. And 90% of U.S. counties didn’t have an abortion clinic as of 2014, according to the Guttmacher Institute, a reproductive rights research and advocacy group.
For some, this echoes back to the days before abortion was legalized nationwide in 1973 with the Supreme Court’s Roe v. Wade decision, when patients who could afford to travel would go to more liberal states like California or New York where abortion was legal.
But providers in Kansas and Illinois say this influx from Missouri isn’t new. About half of their clients already come from the Show Me State. To the south, in neighboring Arkansas, where a 72-hour waiting period will go into effect in July, the vast majority of its patients still live within the state.
Over the past 10 years, four Missouri abortion clinics have closed because of increased regulations, including a mandatory 72-hour waiting period after receiving counseling on abortion, thus requiring two trips to a facility; requirements that physicians have hospital admitting privileges within 15 minutes of their clinics; and a rule requiring two-parent notification for minors and one-parent notarized consent. All those limits left one clinic in downtown St. Louis to serve the whole state.
Now Planned Parenthood, which operates that final abortion clinic, said on May 28 that it will be forced to end its abortion services altogether by May 31 if the state suspends its license. The closure is not related to new anti-abortion laws that Missouri Gov. Mike Parson, a Republican, signed on May 24 to ban most abortions after 8 weeks of pregnancy. The new laws don’t take effect until August.
Already the number of patients in Missouri seeking an abortion at the clinic from April 2018 until this April had dropped by 50% compared with the same period the previous year. Planned Parenthood spokesman Jesse Lawder attributes two-thirds of the decrease to the clinic’s refusal to do pelvic exams for abortions performed through medication – recently required by the state – thus forcing all such abortions to be performed out of state.
For Dreith, while she expects the Missouri numbers to continue to grow at her Illinois clinic across the Mississippi River, it’s not the only state sending patients her way.
“Patients were literally coming to us from the last remaining clinics in Kentucky ... so that they wouldn’t get past 24 weeks,” Dreith said. “We don’t want these patients in surrounding states traveling [to] New York [or] California like they once had to.”
That’s how it was prior to the Roe v. Wade ruling, according to Mary Ziegler, a professor at Florida State University College of Law who is writing her third book on the history of the legal battle around abortion access. She anticipates the pattern of privilege will repeat itself.
“You would still expect women with resources to be able to travel as far as they needed,” she said. “And you would expect women without resources to not be able to travel. ... The more the court retreats from protecting abortion rights, the more stark those differences will become.”
For Dreith, the historical comparison to the pre-Roe era rings true, albeit with improved medical practices.
There are safer, easier, and more effective ways to perform abortions now than the “horror stories that we saw pre-Roe,” said Dreith. “But I think the travel will be one of the huge throwbacks and the scariest part will be the criminalization.”
States such as Missouri could feel pressure to start arresting women who perform their own abortions with pills at home or travel out of state, Ziegler said. But, she said, “punishing women isn’t something that’s thought to be very popular.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Syncope during pregnancy increases risk for poor outcomes
a retrospective population-based cohort study finds. Risks appeared highest with first-trimester syncope.
“There are very limited data on the frequency of fainting during pregnancy,” Padma Kaul, Ph.D., senior study author and professor of medicine at the University of Alberta, Edmonton, said in a statement. “In our study, fainting during pregnancy occurred in about 1%, or 10 per 1,000 pregnancies, but appears to be increasing by 5% each year.”
“Fainting during pregnancy has previously been thought to follow a relatively benign course,” Dr. Kaul said. “The findings of our study suggest that timing of fainting during pregnancy may be important. When the faint happens early during pregnancy or multiple times during pregnancy, it may be associated with both short- and long-term health issues for the baby and the mother.”
First authors Safia Chatur, MD, of the University of Calgary (Alta.) and Sunjidatul Islam, MBBS, of the Canadian Vigour Centre, Edmonton, Alta., and associates analyzed 481,930 pregnancies occurring during 2005-2014 in the province.
Study results, reported in the Journal of the American Heart Association, showed that syncope occurred in almost 1% of pregnancies (9.7 episodes per 1,000 pregnancies) overall. Incidence increased by 5% per year during the study period.
Syncope episodes were distributed across the first trimester (32%), second trimester (44%), and third trimester (24%). Eight percent of pregnancies had more than one episode.
Compared with unaffected peers, women who experienced syncope were younger (age younger than 25 years, 35% vs. 21%; P less than .001) and more often primiparous (52% vs. 42%; P less than .001).
The rate of preterm birth was 18%, 16%, and 14% in pregnancies with an initial syncope episode during the first, second, and third trimester, respectively, compared with 15% in pregnancies without syncope (P less than .01 across groups).
With a median follow-up of about 5 years, compared with peers of syncope-free pregnancies, children of pregnancies complicated by syncope had a higher incidence of congenital anomalies (3.1% vs. 2.6%; P = .023). Incidence was highest in pregnancies with multiple episodes of syncope (5% vs. 3%; P less than .01).
In adjusted analyses that accounted for multiple pregnancies in individual women, relative to counterparts with no syncope during pregnancy, women who experienced syncope during the first trimester had higher odds of giving birth preterm (odds ratio, 1.3; P = .001) and of having an infant small for gestational age (OR, 1.2; P = .04) or with congenital anomalies (OR, 1.4; P = .036). Women with multiple syncope episodes versus none were twice as likely to have offspring with congenital anomalies (OR, 2.0; P = .003).
Relative to peers who did not experience syncope in pregnancy, women who did had higher incidences of cardiac arrhythmias (0.8% vs. 0.2%; P less than .01) and syncope episodes (1.4% vs. 0.2%; P less than .01) in the first year after delivery.
“Our data suggest that syncope during pregnancy may not be a benign occurrence,” Dr. Chatur and associates said. “More detailed clinical data are needed to identify potential causes for the observed increase in syncope during pregnancy in our study.“Whether women who experience syncope during pregnancy may benefit from closer monitoring during the obstetric and postpartum periods requires further study,” they concluded.
The investigators disclosed no relevant conflicts of interest. This study was funded by a grant from the Cardiac Arrhythmia Network of Canada.
SOURCE: Chatur S et al. J Am Heart Assoc. 2019;8:e011608.
a retrospective population-based cohort study finds. Risks appeared highest with first-trimester syncope.
“There are very limited data on the frequency of fainting during pregnancy,” Padma Kaul, Ph.D., senior study author and professor of medicine at the University of Alberta, Edmonton, said in a statement. “In our study, fainting during pregnancy occurred in about 1%, or 10 per 1,000 pregnancies, but appears to be increasing by 5% each year.”
“Fainting during pregnancy has previously been thought to follow a relatively benign course,” Dr. Kaul said. “The findings of our study suggest that timing of fainting during pregnancy may be important. When the faint happens early during pregnancy or multiple times during pregnancy, it may be associated with both short- and long-term health issues for the baby and the mother.”
First authors Safia Chatur, MD, of the University of Calgary (Alta.) and Sunjidatul Islam, MBBS, of the Canadian Vigour Centre, Edmonton, Alta., and associates analyzed 481,930 pregnancies occurring during 2005-2014 in the province.
Study results, reported in the Journal of the American Heart Association, showed that syncope occurred in almost 1% of pregnancies (9.7 episodes per 1,000 pregnancies) overall. Incidence increased by 5% per year during the study period.
Syncope episodes were distributed across the first trimester (32%), second trimester (44%), and third trimester (24%). Eight percent of pregnancies had more than one episode.
Compared with unaffected peers, women who experienced syncope were younger (age younger than 25 years, 35% vs. 21%; P less than .001) and more often primiparous (52% vs. 42%; P less than .001).
The rate of preterm birth was 18%, 16%, and 14% in pregnancies with an initial syncope episode during the first, second, and third trimester, respectively, compared with 15% in pregnancies without syncope (P less than .01 across groups).
With a median follow-up of about 5 years, compared with peers of syncope-free pregnancies, children of pregnancies complicated by syncope had a higher incidence of congenital anomalies (3.1% vs. 2.6%; P = .023). Incidence was highest in pregnancies with multiple episodes of syncope (5% vs. 3%; P less than .01).
In adjusted analyses that accounted for multiple pregnancies in individual women, relative to counterparts with no syncope during pregnancy, women who experienced syncope during the first trimester had higher odds of giving birth preterm (odds ratio, 1.3; P = .001) and of having an infant small for gestational age (OR, 1.2; P = .04) or with congenital anomalies (OR, 1.4; P = .036). Women with multiple syncope episodes versus none were twice as likely to have offspring with congenital anomalies (OR, 2.0; P = .003).
Relative to peers who did not experience syncope in pregnancy, women who did had higher incidences of cardiac arrhythmias (0.8% vs. 0.2%; P less than .01) and syncope episodes (1.4% vs. 0.2%; P less than .01) in the first year after delivery.
“Our data suggest that syncope during pregnancy may not be a benign occurrence,” Dr. Chatur and associates said. “More detailed clinical data are needed to identify potential causes for the observed increase in syncope during pregnancy in our study.“Whether women who experience syncope during pregnancy may benefit from closer monitoring during the obstetric and postpartum periods requires further study,” they concluded.
The investigators disclosed no relevant conflicts of interest. This study was funded by a grant from the Cardiac Arrhythmia Network of Canada.
SOURCE: Chatur S et al. J Am Heart Assoc. 2019;8:e011608.
a retrospective population-based cohort study finds. Risks appeared highest with first-trimester syncope.
“There are very limited data on the frequency of fainting during pregnancy,” Padma Kaul, Ph.D., senior study author and professor of medicine at the University of Alberta, Edmonton, said in a statement. “In our study, fainting during pregnancy occurred in about 1%, or 10 per 1,000 pregnancies, but appears to be increasing by 5% each year.”
“Fainting during pregnancy has previously been thought to follow a relatively benign course,” Dr. Kaul said. “The findings of our study suggest that timing of fainting during pregnancy may be important. When the faint happens early during pregnancy or multiple times during pregnancy, it may be associated with both short- and long-term health issues for the baby and the mother.”
First authors Safia Chatur, MD, of the University of Calgary (Alta.) and Sunjidatul Islam, MBBS, of the Canadian Vigour Centre, Edmonton, Alta., and associates analyzed 481,930 pregnancies occurring during 2005-2014 in the province.
Study results, reported in the Journal of the American Heart Association, showed that syncope occurred in almost 1% of pregnancies (9.7 episodes per 1,000 pregnancies) overall. Incidence increased by 5% per year during the study period.
Syncope episodes were distributed across the first trimester (32%), second trimester (44%), and third trimester (24%). Eight percent of pregnancies had more than one episode.
Compared with unaffected peers, women who experienced syncope were younger (age younger than 25 years, 35% vs. 21%; P less than .001) and more often primiparous (52% vs. 42%; P less than .001).
The rate of preterm birth was 18%, 16%, and 14% in pregnancies with an initial syncope episode during the first, second, and third trimester, respectively, compared with 15% in pregnancies without syncope (P less than .01 across groups).
With a median follow-up of about 5 years, compared with peers of syncope-free pregnancies, children of pregnancies complicated by syncope had a higher incidence of congenital anomalies (3.1% vs. 2.6%; P = .023). Incidence was highest in pregnancies with multiple episodes of syncope (5% vs. 3%; P less than .01).
In adjusted analyses that accounted for multiple pregnancies in individual women, relative to counterparts with no syncope during pregnancy, women who experienced syncope during the first trimester had higher odds of giving birth preterm (odds ratio, 1.3; P = .001) and of having an infant small for gestational age (OR, 1.2; P = .04) or with congenital anomalies (OR, 1.4; P = .036). Women with multiple syncope episodes versus none were twice as likely to have offspring with congenital anomalies (OR, 2.0; P = .003).
Relative to peers who did not experience syncope in pregnancy, women who did had higher incidences of cardiac arrhythmias (0.8% vs. 0.2%; P less than .01) and syncope episodes (1.4% vs. 0.2%; P less than .01) in the first year after delivery.
“Our data suggest that syncope during pregnancy may not be a benign occurrence,” Dr. Chatur and associates said. “More detailed clinical data are needed to identify potential causes for the observed increase in syncope during pregnancy in our study.“Whether women who experience syncope during pregnancy may benefit from closer monitoring during the obstetric and postpartum periods requires further study,” they concluded.
The investigators disclosed no relevant conflicts of interest. This study was funded by a grant from the Cardiac Arrhythmia Network of Canada.
SOURCE: Chatur S et al. J Am Heart Assoc. 2019;8:e011608.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Rapid urine test could aid in preeclampsia diagnosis
NASHVILLE, TENN. – according to findings from a study of urine samples from a prospective cohort of women referred to a labor and delivery triage center to rule out the condition.
The study involved the evaluation of 349 frozen urine samples from the cohort, which included 89 preeclampsia cases (26%) as diagnosed by expert adjudication based on 2013 American College of Obstetricians and Gynecologists guidelines. Visual scoring by a blinded user at 3 minutes after application of urine to the test device for 329 available samples showed 84% test sensitivity, 77% test specificity, and 93% negative predictive value, compared with the adjudicated diagnoses, Wendy L. Davis reported during an e-poster session at ACOG’s annual clinical and scientific meeting.
Of the women in the study cohort, 52% were multiparous, 91% were overweight or obese, 38% were early preterm, 31% were late preterm, and 31% were at term. Cases were described by at least one referring physician as “particularly challenging and ambiguous,” said Ms. Davis, founder and CEO of GestVision in Groton, Conn., and colleagues.
The findings, which suggest that this rapid test holds promise as an aid in the diagnosis of preeclampsia, are important as preeclampsia-associated morbidity and mortality most often occur because of a delay or misdiagnosis, they explained, also noting that diagnostic criteria for preeclampsia are “inadequate even in best care situations.”
The test – an in vitro diagnostic device known as the GestAssured Test Kit – is being developed by GestVision based on data showing that aberrant protein misfolding and aggregation is a pathogenic feature of preeclampsia. That data initially led to development of the Congo Red Dot test as a laboratory batch test, followed by development and validation of a point-of-care version of the test to allow for better integration in clinical work flow.
The GestAssured Test Kit currently in development for commercial use is based on that technology, and the current data suggest that it has slightly higher sensitivity, slightly lower specificity, and slightly higher negative predictive value than the Congo Red Dot test.
“The GestAssured test was developed specifically for preeclampsia and has a high negative predictive value, suggesting that this device, in conjunction with ACOG task force guidelines for hypertension in pregnancy, can assist in ruling out disease in patients suspected of preeclampsia,” the investigators wrote.
“[It is] particularly useful in a triage setting where you have a complex collection of patients coming in,” Ms. Davis said during the poster presentation. “And it warrants ongoing U.S. multicenter clinical studies.”
This study was funded by GestVision and Saving Lives at Birth. Ms. Davis is an employee and shareholder of GestVision and is named as an inventor or coinventor on patents licensed for commercialization to the company.
NASHVILLE, TENN. – according to findings from a study of urine samples from a prospective cohort of women referred to a labor and delivery triage center to rule out the condition.
The study involved the evaluation of 349 frozen urine samples from the cohort, which included 89 preeclampsia cases (26%) as diagnosed by expert adjudication based on 2013 American College of Obstetricians and Gynecologists guidelines. Visual scoring by a blinded user at 3 minutes after application of urine to the test device for 329 available samples showed 84% test sensitivity, 77% test specificity, and 93% negative predictive value, compared with the adjudicated diagnoses, Wendy L. Davis reported during an e-poster session at ACOG’s annual clinical and scientific meeting.
Of the women in the study cohort, 52% were multiparous, 91% were overweight or obese, 38% were early preterm, 31% were late preterm, and 31% were at term. Cases were described by at least one referring physician as “particularly challenging and ambiguous,” said Ms. Davis, founder and CEO of GestVision in Groton, Conn., and colleagues.
The findings, which suggest that this rapid test holds promise as an aid in the diagnosis of preeclampsia, are important as preeclampsia-associated morbidity and mortality most often occur because of a delay or misdiagnosis, they explained, also noting that diagnostic criteria for preeclampsia are “inadequate even in best care situations.”
The test – an in vitro diagnostic device known as the GestAssured Test Kit – is being developed by GestVision based on data showing that aberrant protein misfolding and aggregation is a pathogenic feature of preeclampsia. That data initially led to development of the Congo Red Dot test as a laboratory batch test, followed by development and validation of a point-of-care version of the test to allow for better integration in clinical work flow.
The GestAssured Test Kit currently in development for commercial use is based on that technology, and the current data suggest that it has slightly higher sensitivity, slightly lower specificity, and slightly higher negative predictive value than the Congo Red Dot test.
“The GestAssured test was developed specifically for preeclampsia and has a high negative predictive value, suggesting that this device, in conjunction with ACOG task force guidelines for hypertension in pregnancy, can assist in ruling out disease in patients suspected of preeclampsia,” the investigators wrote.
“[It is] particularly useful in a triage setting where you have a complex collection of patients coming in,” Ms. Davis said during the poster presentation. “And it warrants ongoing U.S. multicenter clinical studies.”
This study was funded by GestVision and Saving Lives at Birth. Ms. Davis is an employee and shareholder of GestVision and is named as an inventor or coinventor on patents licensed for commercialization to the company.
NASHVILLE, TENN. – according to findings from a study of urine samples from a prospective cohort of women referred to a labor and delivery triage center to rule out the condition.
The study involved the evaluation of 349 frozen urine samples from the cohort, which included 89 preeclampsia cases (26%) as diagnosed by expert adjudication based on 2013 American College of Obstetricians and Gynecologists guidelines. Visual scoring by a blinded user at 3 minutes after application of urine to the test device for 329 available samples showed 84% test sensitivity, 77% test specificity, and 93% negative predictive value, compared with the adjudicated diagnoses, Wendy L. Davis reported during an e-poster session at ACOG’s annual clinical and scientific meeting.
Of the women in the study cohort, 52% were multiparous, 91% were overweight or obese, 38% were early preterm, 31% were late preterm, and 31% were at term. Cases were described by at least one referring physician as “particularly challenging and ambiguous,” said Ms. Davis, founder and CEO of GestVision in Groton, Conn., and colleagues.
The findings, which suggest that this rapid test holds promise as an aid in the diagnosis of preeclampsia, are important as preeclampsia-associated morbidity and mortality most often occur because of a delay or misdiagnosis, they explained, also noting that diagnostic criteria for preeclampsia are “inadequate even in best care situations.”
The test – an in vitro diagnostic device known as the GestAssured Test Kit – is being developed by GestVision based on data showing that aberrant protein misfolding and aggregation is a pathogenic feature of preeclampsia. That data initially led to development of the Congo Red Dot test as a laboratory batch test, followed by development and validation of a point-of-care version of the test to allow for better integration in clinical work flow.
The GestAssured Test Kit currently in development for commercial use is based on that technology, and the current data suggest that it has slightly higher sensitivity, slightly lower specificity, and slightly higher negative predictive value than the Congo Red Dot test.
“The GestAssured test was developed specifically for preeclampsia and has a high negative predictive value, suggesting that this device, in conjunction with ACOG task force guidelines for hypertension in pregnancy, can assist in ruling out disease in patients suspected of preeclampsia,” the investigators wrote.
“[It is] particularly useful in a triage setting where you have a complex collection of patients coming in,” Ms. Davis said during the poster presentation. “And it warrants ongoing U.S. multicenter clinical studies.”
This study was funded by GestVision and Saving Lives at Birth. Ms. Davis is an employee and shareholder of GestVision and is named as an inventor or coinventor on patents licensed for commercialization to the company.
REPORTING FROM ACOG 2019
Active psoriatic arthritis, ankylosing spondylitis linked to increase in adverse pregnancy outcomes
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
Women with psoriatic arthritis and ankylosing spondylitis generally have favorable pregnancy outcomes, but high disease activity during pregnancy could increase the risk of adverse labor and delivery outcomes, according to 2004-2018 data from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project.
Corticosteroid use further increased risk for preterm delivery among women with ankylosing spondylitis.
While more research is needed, these findings suggest that better obstetric outcomes might be achieved via better disease control and minimal use of corticosteroids, according to Chelsey J. F. Smith, MD, of the University of California, San Diego, and colleagues.
“Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions,” Dr. Smith and coauthors said in a report on the study in Arthritis Care and Research.
Many women affected by psoriatic arthritis and ankylosing spondylitis are of child-bearing age and consider planning a family, according to the researchers. Data on pregnancy outcomes are lacking, they said, “often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.”
The study from Dr. Smith and coinvestigators comprised 963 women who enrolled in the OTIS prospective cohort study within 20 weeks of gestation and delivered at least one live-born infant. Of that cohort, 129 had ankylosing spondylitis, 117 had psoriatic arthritis, and the remaining 717 served as a control group.
Psoriatic arthritis conferred an 81% increased risk for moderate preterm delivery at 32-36 weeks gestation, compared with healthier women, 13.7% and 7.7% respectively. Risk was increased among women with psoriatic arthritis for preterm labor, 16.2% and 8.4% (adjusted risk ratio, 2.05, 95% confidence interval, 1.21-3.48), caesarean delivery, 48.7% and 26.2% (aRR, 1.63, 95% CI, 1.26-2.12), and oligohydramnios, 25% and 11% (aRR, 3.79, 95% CI, 1.34-10.74). Women with psoriatic arthritis were 2 years older on average and their average body mass index was 27 kg/m2 vs. 24.5 kg/m2 in the control group.
In women with ankylosing spondylitis, risk of infant hospitalization in the neonatal intensive care unit was increased by 67%, 17.2% vs. 11.9% in the control group.
Active disease measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3) was linked to increased risk of adverse obstetric outcomes in some cases, the investigators said.
For example, risk of preterm delivery was increased in women with psoriatic arthritis who had active disease at 32 weeks as measured by HAQ (27 women) and RAPID3 (28 women) scores, while in ankylosing spondylitis, active disease measured at intake by RAPID3 (46 women) was associated with increased risk of caesarean delivery.
Medication use in women with psoriatic arthritis was not associated with increased preterm delivery risk. However, women with ankylosing spondylitis who used corticosteroids in the second trimester had an increased risk of preterm delivery.
The rate of corticosteroid use was “surprisingly high” at 38% among the women with ankylosing spondylitis, Dr. Smith and coinvestigators said.
“The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of ankylosing spondylitis, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis,” they said in their report.
Dr. Smith and coauthors reported no conflicts of interest. The OTIS Collaborative Research Group has received research funding from AbbVie, Amgen, Bristol-Myers Squibb, Janssen, Pfizer, and others.
SOURCE: Smith CJF et al. Arthritis Care Res. 2019 May 10. doi: 10.1002/acr.23924.
FROM ARTHRITIS CARE & RESEARCH
Better screening needed to reduce pregnancy-related overdose, death
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Drug-induced death is the leading cause of pregnancy-associated death in Utah, primarily occurring in the late postpartum period.
Major finding: Despite known history, pregnant women were not systematically screened for drug use or treated for mental health disorders or drug misuse.
Study details: A retrospective cohort study of 136 pregnancy-associated deaths, of which 26% were drug induced.
Disclosures: Dr. Smid is supported by the Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
Source: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
Benzodiazepines nearly double the odds of spontaneous abortion
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
FROM JAMA PSYCHIATRY