User login
Neurologists need not discourage breastfeeding in women with MS
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
STOCKHOLM – Most neurologists are overly conservative when it comes to advising women with multiple sclerosis (MS) about breastfeeding, discouraging this broadly beneficial practice in favor of early resumption of treatment post pregnancy, Kerstin Hellwig, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“We should change our behavior, and I predict we will change it so that more women are breastfeeding while under MS medication within the next couple years. ,” said Dr. Hellwig, senior consultant and MS specialist in the department of neurology at St. Josef Hospital/Ruhr University in Bochum, Germany.
She was a coauthor of a groundbreaking 2012 meta-analysis that concluded that breastfeeding by MS patients is not harmful (J Neurol. 2012 Oct;259[10]:2246-8), a finding since confirmed in multiple additional studies.
“Women with MS who want to breastfeed should be supported in doing so,” Dr. Hellwig said.
In this regard, many neurologists are out of step with their colleagues in rheumatology and gastroenterology, who commonly endorse breastfeeding by their patients while on monoclonal antibodies for other autoimmune diseases, according to Dr. Hellwig.
It is important to recognize that most women of reproductive age with MS have milder forms of the disease, she said. They can safely breastfeed without being on any MS medications at all for the duration.
For women who want to breastfeed and have more-active disease where early treatment resumption is warranted, the key is to select a breastfeeding-compatible medication. The main determinant of whether a drug will enter the mother’s breast milk is the size of the drug molecule, with large molecules being unlikely to make their way into breast milk in anything approaching clinically meaningful amounts. The injectable first-line disease-modifying drugs are good options: For example, interferon-beta is a very large molecule which has been detected in breast milk at 0.0006% of the relative infant dose. That’s reassuring, Dr. Hellwig said, since anything less than a relative infant dose of 10% is generally considered to be safe for a baby. And while glatiramer acetate, another injectable, has not been tested, it is metabolized so rapidly that it is unlikely to be detectable in breast milk, according to Dr. Hellwig.
Monoclonal antibodies are also compatible with breastfeeding. Rituximab has been detected in breast milk at 1/240th of the maternal serum level, and natalizumab at less than 1/200th. These are large molecules with a low likelihood of infant absorption, since they are probably destroyed in the child’s gastrointestinal tract. Ocrelizumab has not been studied in breast milk, but it is an IgG1 monoclonal antibody, as is rituximab, and so should likewise pose “exceedingly low risk,” Dr. Hellwig said.
At last year’s ECTRIMS conference, she presented reassuring 1-year follow-up data on a cohort of infants breastfed by mothers with MS while on interferon-beta. “We do not see any growth disturbances, any severe infections, hospitalizations, excess antibiotic use, or postponed reaching of developmental milestones in babies being breastfed under the injectables,” she said.
Dr. Hellwig has served on scientific advisory board for Bayer, Biogen, Genzyme Sanofi, Teva, Roche, Novartis, and Merck. She has received speaker honoraria and research support from Bayer, Biogen, Merck, Novartis, SanofiGenzyme, and Teva, and has received support for congress participation from Bayer, Biogen, Genzyme, Teva, Roche, and Merck.
EXPERT ANALYSIS FROM ECTRIMS 2019
No decrease in preterm birth with n-3 fatty acid supplements
according to data published in the New England Journal of Medicine.
Maria Makrides, PhD, of the South Australian Health and Medical Research Institute, North Adelaide, and coauthors wrote there is evidence that n-3 long-chain polyunsaturated fatty acids play an essential role in labor initiation.
“Typical Western diets are relatively low in n-3 long-chain polyunsaturated fatty acids, which leads to a predominance of 2-series prostaglandin substrate in the fetoplacental unit and potentially confers a predisposition to preterm delivery,” they wrote, adding that epidemiologic studies have suggested associations between lower fish consumption in pregnancy and a higher rate of preterm delivery.
In a multicenter, double-blind trial, 5,517 women were randomized to either a daily fish oil supplement containing 900 mg of n-3 long-chain polyunsaturated fatty acids or vegetable oil capsules, from before 20 weeks’ gestation until 34 weeks’ gestation or delivery.
Among the 5,486 pregnancies included in the final analysis, there were no differences between the intervention and control groups in the primary outcome of early preterm delivery, which occurred in 2.2% of pregnancies in the n-3 fatty acid group and 2% of the control group (P = 0.5).
The study also saw no significant differences between the two groups in other outcomes such as the rates of preterm delivery, preterm spontaneous labor, postterm induction, or gestational age at delivery. Similarly, there were no apparent effects of supplementation on maternal and neonatal outcomes including low birth weight, admission to neonatal intensive care, gestational diabetes, postpartum hemorrhage, or preeclampsia.
The analysis did suggest a greater incidence of infants born very large for gestational age – with a birth weight above the 97th percentile – among women in the fatty acid supplement group, but this did not correspond to an increased rate of interventions such as cesarean section or postterm induction.
The authors commented that their finding of more very-large-for-gestational-age babies added to the debate about whether n-3 long-chain polyunsaturated fatty acid supplementation did have a direct impact on fetal growth, although they also noted that it could be a chance finding.
There were also no significant differences between the two groups in serious adverse events, including miscarriage.
The authors noted that the baseline level of n-3 long-chain polyunsaturated fatty acids in the women enrolled in trial may have been higher than in previous studies.
The study was supported by the Australian National Health and Medical Research Council and the Thyne Reid Foundation, with in-kind support from Croda UK and Efamol/Wassen UK. Two authors declared advisory board fees from private industry, and one also declared a patent relating to fatty acids in research. No other conflicts of interest were declared.
SOURCE: Makrides M et al. N Engl J Med. 2019;381:1035-45.
according to data published in the New England Journal of Medicine.
Maria Makrides, PhD, of the South Australian Health and Medical Research Institute, North Adelaide, and coauthors wrote there is evidence that n-3 long-chain polyunsaturated fatty acids play an essential role in labor initiation.
“Typical Western diets are relatively low in n-3 long-chain polyunsaturated fatty acids, which leads to a predominance of 2-series prostaglandin substrate in the fetoplacental unit and potentially confers a predisposition to preterm delivery,” they wrote, adding that epidemiologic studies have suggested associations between lower fish consumption in pregnancy and a higher rate of preterm delivery.
In a multicenter, double-blind trial, 5,517 women were randomized to either a daily fish oil supplement containing 900 mg of n-3 long-chain polyunsaturated fatty acids or vegetable oil capsules, from before 20 weeks’ gestation until 34 weeks’ gestation or delivery.
Among the 5,486 pregnancies included in the final analysis, there were no differences between the intervention and control groups in the primary outcome of early preterm delivery, which occurred in 2.2% of pregnancies in the n-3 fatty acid group and 2% of the control group (P = 0.5).
The study also saw no significant differences between the two groups in other outcomes such as the rates of preterm delivery, preterm spontaneous labor, postterm induction, or gestational age at delivery. Similarly, there were no apparent effects of supplementation on maternal and neonatal outcomes including low birth weight, admission to neonatal intensive care, gestational diabetes, postpartum hemorrhage, or preeclampsia.
The analysis did suggest a greater incidence of infants born very large for gestational age – with a birth weight above the 97th percentile – among women in the fatty acid supplement group, but this did not correspond to an increased rate of interventions such as cesarean section or postterm induction.
The authors commented that their finding of more very-large-for-gestational-age babies added to the debate about whether n-3 long-chain polyunsaturated fatty acid supplementation did have a direct impact on fetal growth, although they also noted that it could be a chance finding.
There were also no significant differences between the two groups in serious adverse events, including miscarriage.
The authors noted that the baseline level of n-3 long-chain polyunsaturated fatty acids in the women enrolled in trial may have been higher than in previous studies.
The study was supported by the Australian National Health and Medical Research Council and the Thyne Reid Foundation, with in-kind support from Croda UK and Efamol/Wassen UK. Two authors declared advisory board fees from private industry, and one also declared a patent relating to fatty acids in research. No other conflicts of interest were declared.
SOURCE: Makrides M et al. N Engl J Med. 2019;381:1035-45.
according to data published in the New England Journal of Medicine.
Maria Makrides, PhD, of the South Australian Health and Medical Research Institute, North Adelaide, and coauthors wrote there is evidence that n-3 long-chain polyunsaturated fatty acids play an essential role in labor initiation.
“Typical Western diets are relatively low in n-3 long-chain polyunsaturated fatty acids, which leads to a predominance of 2-series prostaglandin substrate in the fetoplacental unit and potentially confers a predisposition to preterm delivery,” they wrote, adding that epidemiologic studies have suggested associations between lower fish consumption in pregnancy and a higher rate of preterm delivery.
In a multicenter, double-blind trial, 5,517 women were randomized to either a daily fish oil supplement containing 900 mg of n-3 long-chain polyunsaturated fatty acids or vegetable oil capsules, from before 20 weeks’ gestation until 34 weeks’ gestation or delivery.
Among the 5,486 pregnancies included in the final analysis, there were no differences between the intervention and control groups in the primary outcome of early preterm delivery, which occurred in 2.2% of pregnancies in the n-3 fatty acid group and 2% of the control group (P = 0.5).
The study also saw no significant differences between the two groups in other outcomes such as the rates of preterm delivery, preterm spontaneous labor, postterm induction, or gestational age at delivery. Similarly, there were no apparent effects of supplementation on maternal and neonatal outcomes including low birth weight, admission to neonatal intensive care, gestational diabetes, postpartum hemorrhage, or preeclampsia.
The analysis did suggest a greater incidence of infants born very large for gestational age – with a birth weight above the 97th percentile – among women in the fatty acid supplement group, but this did not correspond to an increased rate of interventions such as cesarean section or postterm induction.
The authors commented that their finding of more very-large-for-gestational-age babies added to the debate about whether n-3 long-chain polyunsaturated fatty acid supplementation did have a direct impact on fetal growth, although they also noted that it could be a chance finding.
There were also no significant differences between the two groups in serious adverse events, including miscarriage.
The authors noted that the baseline level of n-3 long-chain polyunsaturated fatty acids in the women enrolled in trial may have been higher than in previous studies.
The study was supported by the Australian National Health and Medical Research Council and the Thyne Reid Foundation, with in-kind support from Croda UK and Efamol/Wassen UK. Two authors declared advisory board fees from private industry, and one also declared a patent relating to fatty acids in research. No other conflicts of interest were declared.
SOURCE: Makrides M et al. N Engl J Med. 2019;381:1035-45.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: No decrease in preterm birth was seen with n-3 fatty acid supplementation during pregnancy, compared with controls.
Major finding: Early preterm delivery occurred in 2.2% of pregnancies in the n-3 fatty acid group and 2% of the control group (P = 0.5).
Study details: A multicenter, double-blind trial in 5,517 women.
Disclosures: The study was supported by the Australian National Health and Medical Research Council and the Thyne Reid Foundation, with in-kind support from Croda UK and Efamol/Wassen UK. Two authors declared advisory board fees from private industry, and one also declared a patent relating to fatty acids in research. No other conflicts of interest were declared.
Source: Makrides M et al. N Engl J Med. 2019;381:1035-45.
Use of genetic testing for congenital heart defect management
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Genetic assessment for CHD: Case-specific, stepwise
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.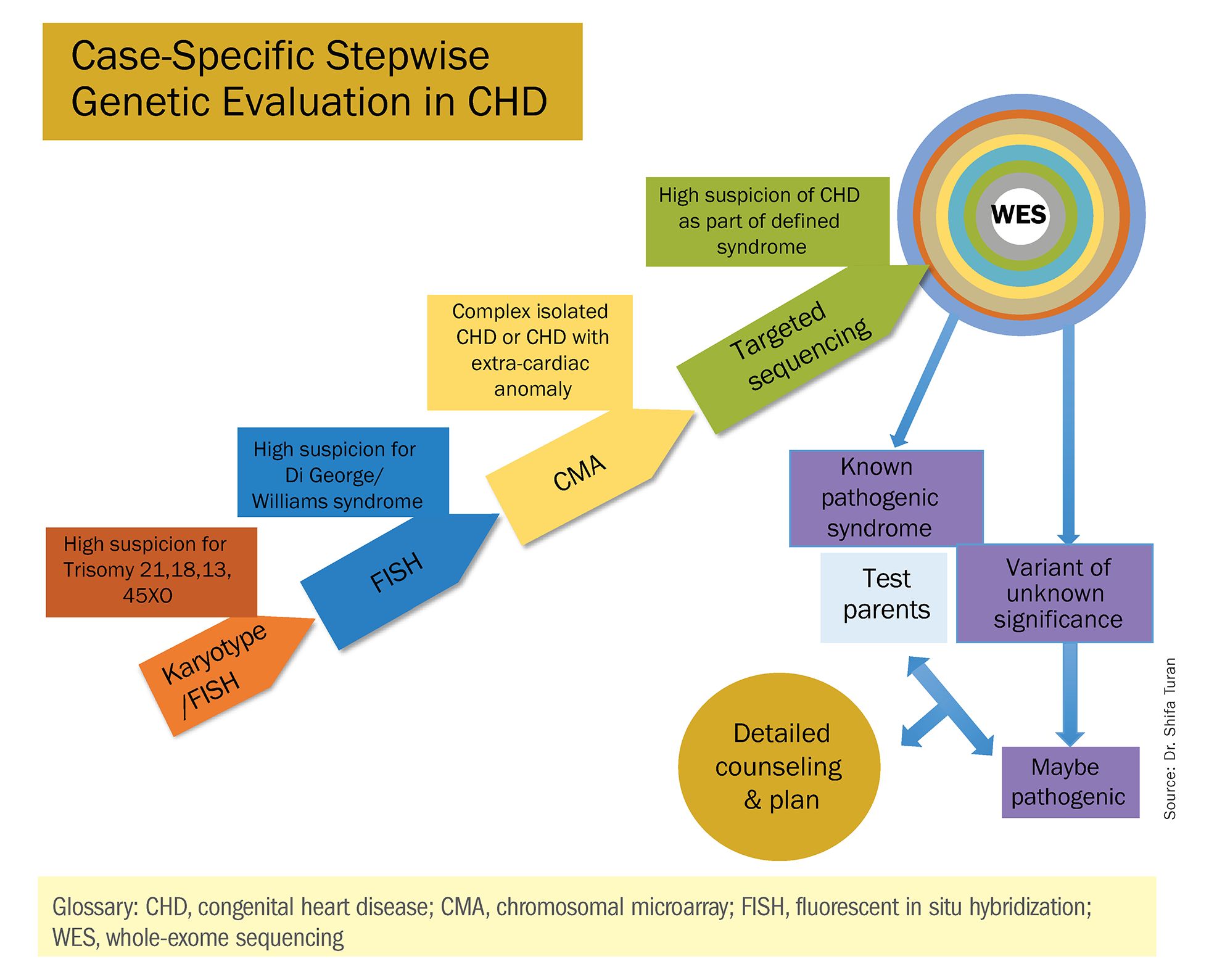
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.
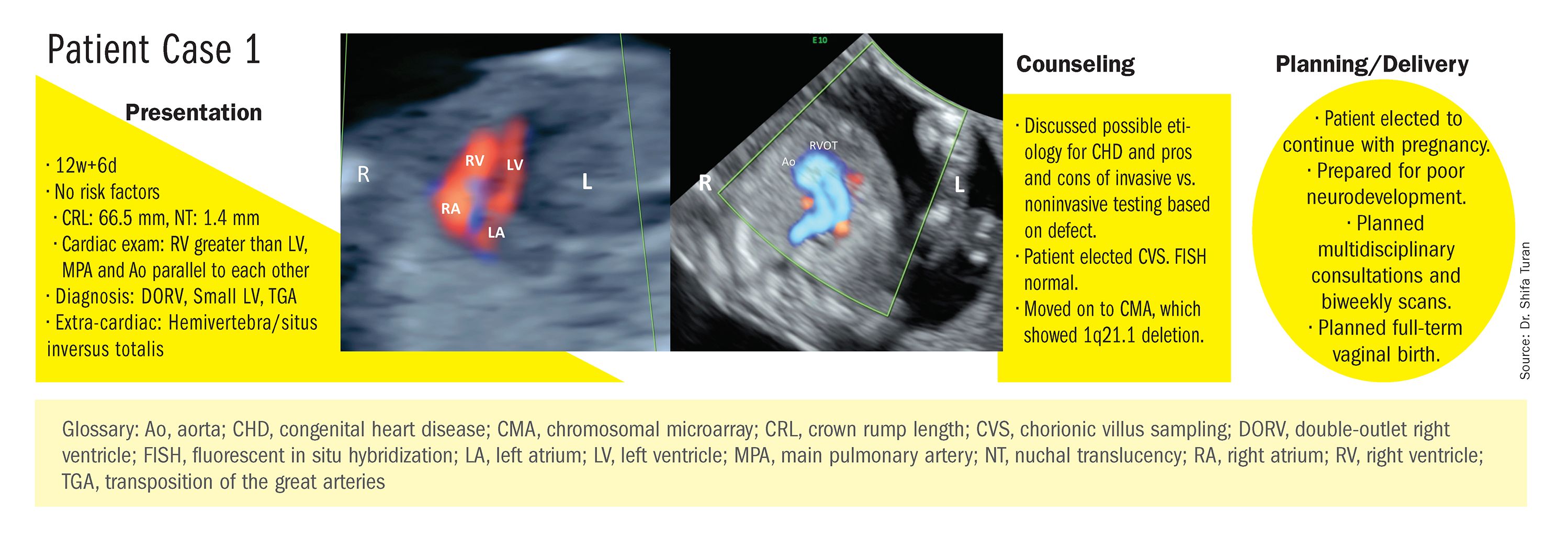
At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.
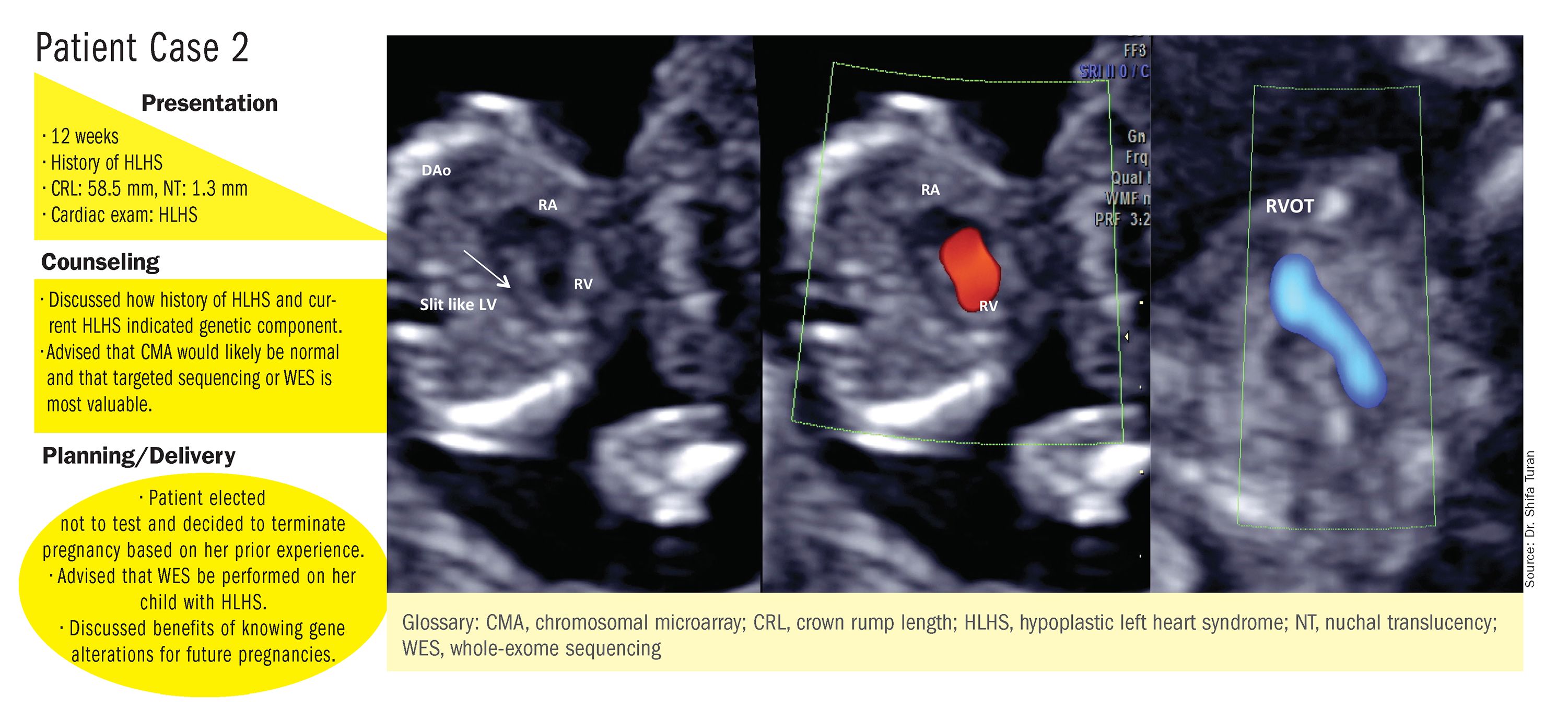
These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Zulresso: Hope and lingering questions
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Cannabis use before and during pregnancy is on the rise


Oral drug for postpartum depression aces phase 3 trial
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
REPORTING FROM THE ECNP 2019
Chronic hypertension in pregnancy increased 13-fold since 1970
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
FROM HYPERTENSION
Chlamydia trachomatis is associated with adverse reproductive health outcomes
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
FROM CLINICAL INFECTIOUS DISEASES
Targeting US maternal mortality: ACOG’s recent strides and future action
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.
Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.

Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
Real progress was achieved in 2018 in the effort to reduce the US maternal mortality rate, the highest of any developed nation and where women of color are 3 to 4 times more likely than others to die of childbirth-related causes. Importantly, the United States is the only nation other than Afghanistan and Sudan where the rate is rising.1
In May 2019, the Centers for Disease Control and Prevention (CDC) published a Vital Signs document focused on preventable maternal deaths.2 It affirmed that about 60% of the 700 pregnancy-related deaths that occur annually in the United States are preventable, and it provided important information on when and why these deaths occur.
Among the CDC findings, about:
- one-third of deaths (31%) occurred during pregnancy (before delivery)
- one-third (36%) occurred at delivery or in the week after
- one-third (33%) occurred 1 week to 1 year postpartum.
In addition, the CDC highlighted that:
- Heart disease and stroke caused more than 1 in 3 deaths (34%). Infections and severe bleeding were other leading causes of death.
- Black and American Indian/Alaska Native women were about 3 times as likely to die from a pregnancy-related cause as white women.
The American College of Obstetricians and Gynecologists (ACOG), under the leadership of President Lisa Hollier, MD, MPH (2018–2019), fully embraced the challenge and responsibility of meaningfully improving health care for every mom. In this article, I review some of the critical steps taken in 2018 and preview ACOG’s continued commitment for 2019 and beyond.

Efforts succeed: Bills are now laws of the land
ACOG and our partner organizations, including the Society for Maternal-Fetal Medicine and the March of Dimes, have long recognized the value of state-based maternal mortality review committees (MMRCs) in slowing and reversing the rate of maternal mortality. An MMRC brings together local experts to examine the causes of maternal deaths—not to find fault, but to find ways to prevent future deaths. With the right framework and support, MMRCs already are providing us with data and driving policy recommendations.
Supporting MMRCs in all states. With this in mind, ACOG helped pass and push to enactment HR 1318, the Preventing Maternal Deaths Act of 2018 (Public Law No. 115-344), a bipartisan bill designed to help develop and provide support for MMRCs in every state. The bill was introduced in the US House of Representatives by Rep. Jaime Herrera Beutler (R-WA) and Rep. Diana DeGette (D-CO) and in the US Senate by Sen. Heidi Heitkamp (D-ND) and Sen. Shelley Moore Capito (R-WV). ACOG Fellow and US Rep. Michael Burgess, MD (R-TX), also was instrumental in the bill’s success. The CDC is actively working toward implementation of this law, and grantees are expected to be announced by the end of September.
Continue to: In addition, ACOG worked with Congress...
In addition, ACOG worked with Congress to secure $50 million in federal funding to reduce maternal mortality, allocated thusly:
- $12 million to support state MMRCs
- $3 million to support the Alliance for Innovation on Maternal Health
- $23 million for State Maternal Health Innovation Program grants
- $12 million to address maternal mortality in the Healthy Start program.
As these federal congressional initiatives worked their way into law, the states actively supported MMRCs as well. As of this writing, only 3 states—North Dakota, South Dakota, and Wyoming—have not yet developed an MMRC.3
Filling the gaps in ObGyn care. Another key ACOG-sponsored bill signed into law will help bring more ObGyns into shortage areas. Sponsored by Rep. Burgess, Rep. Anna Eshoo (D-CA), and Rep. Lucille Roybal-Allard (D-CA) and by Sen. Tammy Baldwin (D-WI) and Sen. Lisa Murkowski (R-AK), the Improving Access to Maternity Care Act (Public Law No. 115-320) requires the Department of Health and Human Services to identify maternity health professional target areas for use by the National Health Service Corps to bring ObGyns to where they are most needed.
Following up on that new law, ACOG currently is working closely with the American Academy of Family Physicians (AAFP) and the National Rural Health Association (NRHA) on the unique challenges women in rural areas face in accessing maternity and other women’s health care services. In June, Dr. Hollier represented ACOG at the Rural Maternal Health forum, which was convened by the Centers for Medicare and Medicaid and sponsored by ACOG, AAFP, and NRHA.4 We are pursuing policies designed to increase the number of ObGyns and other physicians who choose to train in rural areas and increase the clinical use of telehealth to help connect rural physicians and patients with subspecialists in urban areas.
Projects in the works
Congress is ready to do more. Already, 5 ACOG-supported bills have been introduced, including bills that extend women’s Medicaid coverage to 12 months postpartum (consistent with coverage for babies), support state perinatal quality collaboratives, and more. This interest is augmented by the work of the recently formed congressional Black Maternal Health Caucus, focused on reducing racial disparities in health care. In July, ACOG joined 12 members of Congress in a caucus summit to partner with these important congressional allies.
ACOG is expanding support for these legislative efforts through our work with another important ally, the American Medical Association (AMA). ACOG’s delegation to the 2019 Annual Meeting of the AMA House of Delegates in June scored important policy wins, including AMA support for Medicaid coverage for women 12 months postpartum and improving access to care in rural communities.
There is momentum on Capitol Hill to take action on these important issues, and ACOG’s priority is to ensure that any legislative package complements the important work many ObGyns are already doing to improve maternal health outcomes. ACOG has an important seat at the table and will continue to advocate each and every day for your practices and your patients as Congress deliberates legislative action.
Continue to: Your voice matters...
Your voice matters
Encourage your representatives in the House and the Senate to support ACOG-endorsed legislation and be sure they know the importance of ensuring access to women’s health care in your community. Get involved in advocacy; start by visiting the ACOG advocacy web page (www.acog.org/advocacy). Also note that members of Congress are back in their home states during seasonal breaks and many hold town halls and constituent meetings. The health of moms and babies is always an important issue, and you are the expert.
ACOG’s commitment to ensuring healthy moms and babies, and ensuring that our members can continue providing high-quality care, runs through everything we do.
Acknowledgments
The author thanks ACOG former Vice President for Health Policy Barbara Levy, MD, ACOG Senior Director Jeanne Mahoney, and ACOG Federal Affairs Director Rachel Tetlow for their helpful review and comments.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.
- Council on Patient Safety in Women's Health Care. Alliance for Innovation on Maternal Health Program. https://safehealthcareforeverywoman.org/aim-program/. Accessed August 19, 2019.
- Centers for Disease Control and Prevention. Vital signs: pregnancy-related deaths. https://www.cdc.gov/vitalsigns/maternal-deaths/index.html. Accessed August 19, 2019.
- American College of Obstetricians and Gynecologists. State Maternal Mortality Review Committees, PQCs, and AIM. https://www.acog.org/-/media/Departments/Government-Relations-and-Outreach/MMRC_AIM-State-Fact-Sheet_Mar-2019.pdf. Accessed August 19, 2019.
- Centers for Medicare and Medicaid Services. A conversation on maternal health care in rural communities: charting a path to improved access, quality and outcomes. June 12, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/equity-initiatives/rural-health/rural-maternal-health.html. Accessed August 19, 2019.