User login
For MD-IQ use only
What Effect Can a ‘Caring Message’ Intervention Have?
What Effect Can a ‘Caring Message’ Intervention Have?
Caring messages to veterans at risk for suicide come in many forms: cards, letters, phone calls, email, and text messages. Each message can have a major impact on the veteran’s mental health and their decision to use health care provided by the US Department of Veterans Affairs (VA). A recent study outlined ways to centralize that impact, ensuring the caring message reaches those who need it most.
The study examined the impact of the VA Veterans Crisis Line (VCL) caring letters intervention among veterans at increased psychiatric risk. It focused on veterans with ≥ 2 Veterans Health Administration (VHA) health service encounters within 24 months prior to VCL contact. The primary outcome was suicide-related events (SRE), including suicide attempts, intentional self-harm, and suicidal self-directed violence. Secondary outcomes included VHA health care use (all-cause inpatient and outpatient, mental health outpatient, mental health inpatient, and emergency department).
Of 186,514 VCL callers, 8.3% had a psychiatric hospitalization, 4.8% were flagged as high-risk by the REACH VET program, 6.2% had an SRE, and 12.9% met any of these criteria in the year prior to initial VCL contact. There was no association between caring letters and all-cause mortality or SRE, even though caring letters is one of the only interventions to demonstrate a reduction in suicide mortality as a randomized controlled trial.
While reducing suicide has not been the expected result, caring letters have consistently been associated with increased use of outpatient mental health services. The analysis found that veterans with and without indicators of elevated psychiatric risk were using services more. That, the researchers suggest, is more evidence that caring letters might prompt engagement with VHA care, even among veterans not identified as high risk.
Psychiatrist Jerome A. Motto, MD believed long-term supportive but nondemanding contact could reduce a suicidal person’s sense of isolation and enhance feelings of connectedness. His 1976 intervention established a plan to “exert a suicide prevention influence on high-risk persons who decline to enter the health care system.” In Motto’s 5-year follow-up study of 3,006 psychiatric inpatients, half of those who were not following their postdischarge treatment plan received calls or letters expressing interest in their well-being. Suicidal deaths were found to “diverge progressively,” leading Motto to claim the study showed “tentative evidence” that a high-risk population for suicide can be identified and that risk might be reduced through a systematic approach.
Despite those findings, the results of studies on repeated follow-up contact have been mixed. One review outlined how 5 studies showed a statistically significant reduction in suicidal behavior, 4 showed mixed results with trends toward a preventive effect, and 2 studies did not show a preventive effect.
In 2020, the VA launched an intervention for veterans who contacted the VCL. In the first 12 months, CLs were sent to > 100,000 veterans. In feedback interviews, participants described feeling appreciated, cared for, encouraged, and connected. They also said that the CLs helped them engage with community resources and made them more likely to seek VA care. Even veterans who were skeptical of the utility of the caring letters sometimes admitted keeping them.
Finding effective ways to prevent suicide among veterans has been a top priority for the VA. In 2021, then-US Surgeon General Jerome Adams issued a Call to Action that recommended using caring letters when gaps in care may exist, including following crisis line calls.
Caring messages to veterans at risk for suicide come in many forms: cards, letters, phone calls, email, and text messages. Each message can have a major impact on the veteran’s mental health and their decision to use health care provided by the US Department of Veterans Affairs (VA). A recent study outlined ways to centralize that impact, ensuring the caring message reaches those who need it most.
The study examined the impact of the VA Veterans Crisis Line (VCL) caring letters intervention among veterans at increased psychiatric risk. It focused on veterans with ≥ 2 Veterans Health Administration (VHA) health service encounters within 24 months prior to VCL contact. The primary outcome was suicide-related events (SRE), including suicide attempts, intentional self-harm, and suicidal self-directed violence. Secondary outcomes included VHA health care use (all-cause inpatient and outpatient, mental health outpatient, mental health inpatient, and emergency department).
Of 186,514 VCL callers, 8.3% had a psychiatric hospitalization, 4.8% were flagged as high-risk by the REACH VET program, 6.2% had an SRE, and 12.9% met any of these criteria in the year prior to initial VCL contact. There was no association between caring letters and all-cause mortality or SRE, even though caring letters is one of the only interventions to demonstrate a reduction in suicide mortality as a randomized controlled trial.
While reducing suicide has not been the expected result, caring letters have consistently been associated with increased use of outpatient mental health services. The analysis found that veterans with and without indicators of elevated psychiatric risk were using services more. That, the researchers suggest, is more evidence that caring letters might prompt engagement with VHA care, even among veterans not identified as high risk.
Psychiatrist Jerome A. Motto, MD believed long-term supportive but nondemanding contact could reduce a suicidal person’s sense of isolation and enhance feelings of connectedness. His 1976 intervention established a plan to “exert a suicide prevention influence on high-risk persons who decline to enter the health care system.” In Motto’s 5-year follow-up study of 3,006 psychiatric inpatients, half of those who were not following their postdischarge treatment plan received calls or letters expressing interest in their well-being. Suicidal deaths were found to “diverge progressively,” leading Motto to claim the study showed “tentative evidence” that a high-risk population for suicide can be identified and that risk might be reduced through a systematic approach.
Despite those findings, the results of studies on repeated follow-up contact have been mixed. One review outlined how 5 studies showed a statistically significant reduction in suicidal behavior, 4 showed mixed results with trends toward a preventive effect, and 2 studies did not show a preventive effect.
In 2020, the VA launched an intervention for veterans who contacted the VCL. In the first 12 months, CLs were sent to > 100,000 veterans. In feedback interviews, participants described feeling appreciated, cared for, encouraged, and connected. They also said that the CLs helped them engage with community resources and made them more likely to seek VA care. Even veterans who were skeptical of the utility of the caring letters sometimes admitted keeping them.
Finding effective ways to prevent suicide among veterans has been a top priority for the VA. In 2021, then-US Surgeon General Jerome Adams issued a Call to Action that recommended using caring letters when gaps in care may exist, including following crisis line calls.
Caring messages to veterans at risk for suicide come in many forms: cards, letters, phone calls, email, and text messages. Each message can have a major impact on the veteran’s mental health and their decision to use health care provided by the US Department of Veterans Affairs (VA). A recent study outlined ways to centralize that impact, ensuring the caring message reaches those who need it most.
The study examined the impact of the VA Veterans Crisis Line (VCL) caring letters intervention among veterans at increased psychiatric risk. It focused on veterans with ≥ 2 Veterans Health Administration (VHA) health service encounters within 24 months prior to VCL contact. The primary outcome was suicide-related events (SRE), including suicide attempts, intentional self-harm, and suicidal self-directed violence. Secondary outcomes included VHA health care use (all-cause inpatient and outpatient, mental health outpatient, mental health inpatient, and emergency department).
Of 186,514 VCL callers, 8.3% had a psychiatric hospitalization, 4.8% were flagged as high-risk by the REACH VET program, 6.2% had an SRE, and 12.9% met any of these criteria in the year prior to initial VCL contact. There was no association between caring letters and all-cause mortality or SRE, even though caring letters is one of the only interventions to demonstrate a reduction in suicide mortality as a randomized controlled trial.
While reducing suicide has not been the expected result, caring letters have consistently been associated with increased use of outpatient mental health services. The analysis found that veterans with and without indicators of elevated psychiatric risk were using services more. That, the researchers suggest, is more evidence that caring letters might prompt engagement with VHA care, even among veterans not identified as high risk.
Psychiatrist Jerome A. Motto, MD believed long-term supportive but nondemanding contact could reduce a suicidal person’s sense of isolation and enhance feelings of connectedness. His 1976 intervention established a plan to “exert a suicide prevention influence on high-risk persons who decline to enter the health care system.” In Motto’s 5-year follow-up study of 3,006 psychiatric inpatients, half of those who were not following their postdischarge treatment plan received calls or letters expressing interest in their well-being. Suicidal deaths were found to “diverge progressively,” leading Motto to claim the study showed “tentative evidence” that a high-risk population for suicide can be identified and that risk might be reduced through a systematic approach.
Despite those findings, the results of studies on repeated follow-up contact have been mixed. One review outlined how 5 studies showed a statistically significant reduction in suicidal behavior, 4 showed mixed results with trends toward a preventive effect, and 2 studies did not show a preventive effect.
In 2020, the VA launched an intervention for veterans who contacted the VCL. In the first 12 months, CLs were sent to > 100,000 veterans. In feedback interviews, participants described feeling appreciated, cared for, encouraged, and connected. They also said that the CLs helped them engage with community resources and made them more likely to seek VA care. Even veterans who were skeptical of the utility of the caring letters sometimes admitted keeping them.
Finding effective ways to prevent suicide among veterans has been a top priority for the VA. In 2021, then-US Surgeon General Jerome Adams issued a Call to Action that recommended using caring letters when gaps in care may exist, including following crisis line calls.
What Effect Can a ‘Caring Message’ Intervention Have?
What Effect Can a ‘Caring Message’ Intervention Have?
Military Service May Increase Risk for Early Menopause
Military Service May Increase Risk for Early Menopause
Traumatic and environmental exposures during military service may put women veterans at risk for early menopause, a recent longitudinal analysis of data from 668 women in the Gulf War Era Cohort Study found.
The study examined associations between possible early menopause (aged < 45 years) and participants’ Gulf War deployment, military environmental exposures (MEEs), Gulf War Illness, military sexual trauma (MST) and posttraumatic stress disorder (PTSD).
Of 384 Gulf War–deployed veterans, 63% reported MEEs and 26% reported MST during deployment. More than half (57%) of study participants (both Gulf War veterans and nondeployed veterans) met criteria for Gulf War Illness, and 23% met criteria for probable PTSD.
At follow-up, 15% of the women had possible early menopause—higher than population estimates for early menopause in the US, which range from 5% to 10%.
Gulf War deployment, Gulf War–related environmental exposures, and MST during deployment were not significantly associated with early menopause. However, both Gulf War Illness (odds ratio [OR], 1.83; 95% CI, 1.14 to 2.95) and probable PTSD (OR, 2.45; 95% CI, 1.54 to 3.90) were strongly associated with early menopause. Women with probable PTSD at baseline had more than double the odds of possible early menopause.
Previous research suggests that deployment, MEEs, and Gulf War Illness are broadly associated with adverse reproductive health conditions in women veterans. Exposure to persistent organic pollutants and combustion byproducts (eg, from industrial processes and burn pits) have been linked to ovarian dysfunction and oocyte destruction presumed to contribute to accelerated ovarian aging.
The average age for menopause in the US is 52 years. About 5% of women go through early menopause naturally. Early and premature (< 40 years) menopause may also result from a medical or surgical cause, such as a hysterectomy. Regardless the cause, early menopause can have a profound impact on a woman’s physical, emotional, and mental health. It is associated with premature mortality, poor bone health, sexual dysfunction, a 50% increased risk of cardiovascular disease, and 2-fold increased odds of depression.
“Sometimes we talk about menopause symptoms thinking that they're just sort of 1 brief point in time, but we're also talking about things that may affect women's health and functioning for a third or half of a lifespan,” Carolyn Gibson, PhD, MPH, said at the 2024 Spotlight on Women's Health Cyberseminar Series.
Gibson, a staff psychologist at the San Francisco Veterans Affairs (VA) Women’s Mental Health Program and lead author on the recent early-menopause study, pointed to some of the chronic physical health issues that might develop, such as cardiovascular disease, but also the psychological effects.
“It's just important,” she said during the Cyberseminar Series. “To think about the number of things that women in midlife tend to be juggling and managing, all of which may turn up the volume on symptom experience, effect of vulnerability to health and mental health challenges during this period.”
The findings of the study have clinical implications. Midlife women veterans (aged 45 to 64 years) are the largest group of women veterans enrolled in VA health care. Early menopause brings additional age-related care considerations. The authors advise prioritizing support for routine screening for menopause status and symptoms as well as gender-sensitive training, resources, and staffing to provide comprehensive, trauma-informed, evidence-based menopause care for women at any age.
Traumatic and environmental exposures during military service may put women veterans at risk for early menopause, a recent longitudinal analysis of data from 668 women in the Gulf War Era Cohort Study found.
The study examined associations between possible early menopause (aged < 45 years) and participants’ Gulf War deployment, military environmental exposures (MEEs), Gulf War Illness, military sexual trauma (MST) and posttraumatic stress disorder (PTSD).
Of 384 Gulf War–deployed veterans, 63% reported MEEs and 26% reported MST during deployment. More than half (57%) of study participants (both Gulf War veterans and nondeployed veterans) met criteria for Gulf War Illness, and 23% met criteria for probable PTSD.
At follow-up, 15% of the women had possible early menopause—higher than population estimates for early menopause in the US, which range from 5% to 10%.
Gulf War deployment, Gulf War–related environmental exposures, and MST during deployment were not significantly associated with early menopause. However, both Gulf War Illness (odds ratio [OR], 1.83; 95% CI, 1.14 to 2.95) and probable PTSD (OR, 2.45; 95% CI, 1.54 to 3.90) were strongly associated with early menopause. Women with probable PTSD at baseline had more than double the odds of possible early menopause.
Previous research suggests that deployment, MEEs, and Gulf War Illness are broadly associated with adverse reproductive health conditions in women veterans. Exposure to persistent organic pollutants and combustion byproducts (eg, from industrial processes and burn pits) have been linked to ovarian dysfunction and oocyte destruction presumed to contribute to accelerated ovarian aging.
The average age for menopause in the US is 52 years. About 5% of women go through early menopause naturally. Early and premature (< 40 years) menopause may also result from a medical or surgical cause, such as a hysterectomy. Regardless the cause, early menopause can have a profound impact on a woman’s physical, emotional, and mental health. It is associated with premature mortality, poor bone health, sexual dysfunction, a 50% increased risk of cardiovascular disease, and 2-fold increased odds of depression.
“Sometimes we talk about menopause symptoms thinking that they're just sort of 1 brief point in time, but we're also talking about things that may affect women's health and functioning for a third or half of a lifespan,” Carolyn Gibson, PhD, MPH, said at the 2024 Spotlight on Women's Health Cyberseminar Series.
Gibson, a staff psychologist at the San Francisco Veterans Affairs (VA) Women’s Mental Health Program and lead author on the recent early-menopause study, pointed to some of the chronic physical health issues that might develop, such as cardiovascular disease, but also the psychological effects.
“It's just important,” she said during the Cyberseminar Series. “To think about the number of things that women in midlife tend to be juggling and managing, all of which may turn up the volume on symptom experience, effect of vulnerability to health and mental health challenges during this period.”
The findings of the study have clinical implications. Midlife women veterans (aged 45 to 64 years) are the largest group of women veterans enrolled in VA health care. Early menopause brings additional age-related care considerations. The authors advise prioritizing support for routine screening for menopause status and symptoms as well as gender-sensitive training, resources, and staffing to provide comprehensive, trauma-informed, evidence-based menopause care for women at any age.
Traumatic and environmental exposures during military service may put women veterans at risk for early menopause, a recent longitudinal analysis of data from 668 women in the Gulf War Era Cohort Study found.
The study examined associations between possible early menopause (aged < 45 years) and participants’ Gulf War deployment, military environmental exposures (MEEs), Gulf War Illness, military sexual trauma (MST) and posttraumatic stress disorder (PTSD).
Of 384 Gulf War–deployed veterans, 63% reported MEEs and 26% reported MST during deployment. More than half (57%) of study participants (both Gulf War veterans and nondeployed veterans) met criteria for Gulf War Illness, and 23% met criteria for probable PTSD.
At follow-up, 15% of the women had possible early menopause—higher than population estimates for early menopause in the US, which range from 5% to 10%.
Gulf War deployment, Gulf War–related environmental exposures, and MST during deployment were not significantly associated with early menopause. However, both Gulf War Illness (odds ratio [OR], 1.83; 95% CI, 1.14 to 2.95) and probable PTSD (OR, 2.45; 95% CI, 1.54 to 3.90) were strongly associated with early menopause. Women with probable PTSD at baseline had more than double the odds of possible early menopause.
Previous research suggests that deployment, MEEs, and Gulf War Illness are broadly associated with adverse reproductive health conditions in women veterans. Exposure to persistent organic pollutants and combustion byproducts (eg, from industrial processes and burn pits) have been linked to ovarian dysfunction and oocyte destruction presumed to contribute to accelerated ovarian aging.
The average age for menopause in the US is 52 years. About 5% of women go through early menopause naturally. Early and premature (< 40 years) menopause may also result from a medical or surgical cause, such as a hysterectomy. Regardless the cause, early menopause can have a profound impact on a woman’s physical, emotional, and mental health. It is associated with premature mortality, poor bone health, sexual dysfunction, a 50% increased risk of cardiovascular disease, and 2-fold increased odds of depression.
“Sometimes we talk about menopause symptoms thinking that they're just sort of 1 brief point in time, but we're also talking about things that may affect women's health and functioning for a third or half of a lifespan,” Carolyn Gibson, PhD, MPH, said at the 2024 Spotlight on Women's Health Cyberseminar Series.
Gibson, a staff psychologist at the San Francisco Veterans Affairs (VA) Women’s Mental Health Program and lead author on the recent early-menopause study, pointed to some of the chronic physical health issues that might develop, such as cardiovascular disease, but also the psychological effects.
“It's just important,” she said during the Cyberseminar Series. “To think about the number of things that women in midlife tend to be juggling and managing, all of which may turn up the volume on symptom experience, effect of vulnerability to health and mental health challenges during this period.”
The findings of the study have clinical implications. Midlife women veterans (aged 45 to 64 years) are the largest group of women veterans enrolled in VA health care. Early menopause brings additional age-related care considerations. The authors advise prioritizing support for routine screening for menopause status and symptoms as well as gender-sensitive training, resources, and staffing to provide comprehensive, trauma-informed, evidence-based menopause care for women at any age.
Military Service May Increase Risk for Early Menopause
Military Service May Increase Risk for Early Menopause
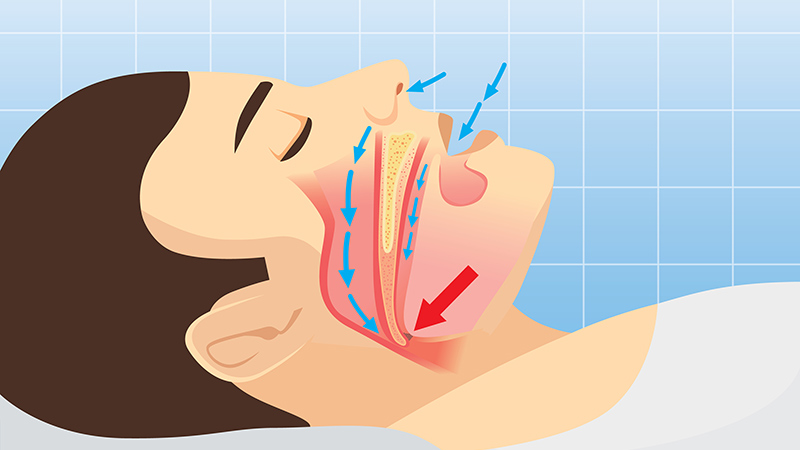
Stay Alert to Sleep Apnea Burden in the Military
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Obstructive sleep apnea (OSA) was associated with a significantly increased risk for adverse health outcomes and health care resource use among military personnel in the US, according to data from about 120,000 active-duty service members.
OSA and other clinical sleep disorders are common among military personnel, driven in part by demanding, nontraditional work schedules that can exacerbate sleep problems, but OSA’s impact in this population has not been well-studied, Emerson M. Wickwire, PhD, of the University of Maryland School of Medicine, Baltimore, and colleagues wrote in a new paper published in Chest.
In the current health economic climate of increasing costs and limited resources, the economic aspects of sleep disorders have never been more important, Wickwire said in an interview. The data in this study are the first to quantify the health and utilization burden of OSA in the US military and can support military decision-makers regarding allocation of scarce resources, he said.
To assess the burden of OSA in the military, they reviewed fully de-identified data from 59,203 active-duty military personnel with diagnoses of OSA and compared them with 59,203 active-duty military personnel without OSA. The participants ranged in age from 18 to 64 years; 7.4% were women and 64.5% were white individuals. Study outcomes included new diagnoses of physical and psychological health conditions, as well as health care resource use in the first year after the index date.
About one third of the participants were in the Army (38.7%), 25.6% were in the Air Force, 23.5% were in the Navy, 5.8% were in the Marines, 5.7% were in the Coast Guard, and 0.7% were in the Public Health Service.
Over the 1-year study period, military personnel with OSA diagnoses were significantly more likely to experience new physical and psychological adverse events than control individuals without OSA, based on proportional hazards models. The physical conditions with the greatest increased risk in the OSA group were traumatic brain injury and cardiovascular disease (which included acute myocardial infarction, atrial fibrillation, ischemic heart disease, and peripheral procedures), with hazard ratios (HRs) 3.27 and 2.32, respectively. The psychological conditions with the greatest increased risk in the OSA group vs control individuals were posttraumatic stress disorder (PTSD) and anxiety (HR, 4.41, and HR, 3.35, respectively).
Individuals with OSA also showed increased use of healthcare resources compared with control individuals without OSA, with an additional 170,511 outpatient visits, 66 inpatient visits, and 1,852 emergency department visits.
Don’t Discount OSA in Military Personnel
“From a clinical perspective, these findings underscore the importance of recognizing OSA as a critical risk factor for a wide array of physical and psychological health outcomes,” the researchers wrote in their discussion.
The results highlight the need for more clinical attention to patient screening, triage, and delivery of care, but efforts are limited by the documented shortage of sleep specialists in the military health system, they noted.
Key limitations of the study include the use of an administrative claims data source, which did not include clinical information such as disease severity or daytime symptoms, and the nonrandomized, observational study design, Wickwire told this news organization.
Looking ahead, the researchers at the University of Maryland School of Medicine and the Uniformed Services University, Bethesda, Maryland, are launching a new trial to assess the clinical effectiveness and cost-effectiveness of telehealth visits for military beneficiaries diagnosed with OSA as a way to manage the shortage of sleep specialists in the military health system, according to a press release from the University of Maryland.
“Although the association between poor sleep and traumatic stress is well-known, present results highlight striking associations between sleep apnea and posttraumatic stress disorder, traumatic brain injury, and musculoskeletal injuries, which are key outcomes from the military perspective,” Wickwire told this news organization.
“Our most important clinical recommendation is for healthcare providers to be on alert for signs and symptoms of OSA, including snoring, daytime sleepiness, and morning dry mouth,” said Wickwire. “Primary care and mental health providers should be especially attuned,” he added.
Results Not Surprising, but Research Gaps Remain
“The sleep health of active-duty military personnel is not only vital for optimal military performance but also impacts the health of Veterans after separation from the military,” said Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, Michigan, in an interview.
The current study identifies increased utilization of healthcare resources by active-duty personnel with sleep apnea, and outcomes were not surprising, said Shamim-Uzzaman, who is employed by the Veterans’ Health Administration, but was not involved in the current study.
The association between untreated OSA and medical and psychological comorbidities such as cardiovascular disease, diabetes, and mood disorders such as depression and anxiety is well-known, Shamim-Uzzaman said. “Patients with depression who also have sleep disturbances are at higher risk for suicide — the strength of this association is such that it led the Veterans’ Health Administration to mandate suicide screening for Veterans seen in its sleep clinics,” he added.
“We also know that untreated OSA is associated with excessive daytime sleepiness, slowed reaction times, and increased risk of motor vehicle accidents, all of which can contribute to sustaining injuries such as traumatic brain injury,” said Shamim-Uzzaman. “Emerging evidence also suggests that sleep disruption prior to exposure to trauma increases the risk of developing PTSD. Therefore, it is not surprising that patients with sleep apnea would have higher healthcare utilization for non-OSA conditions than those without sleep apnea,” he noted.
In clinical practice, the study underscores the importance of identifying and managing sleep health in military personnel, who frequently work nontraditional schedules with long, sustained shifts in grueling conditions not conducive to healthy sleep, Shamim-Uzzaman told this news organization. “Although the harsh work environments that our active-duty military endure come part and parcel with the job, clinicians caring for these individuals should ask specifically about their sleep and working schedules to optimize sleep as best as possible; this should include, but not be limited to, screening and testing for sleep disordered breathing and insomnia,” he said.
The current study has several limitations, including the inability to control for smoking or alcohol use, which are common in military personnel and associated with increased morbidity, said Shamim-Uzzaman. The study also did not assess the impact of other confounding factors, such as sleep duration and daytime sleepiness, that could impact the results, especially the association of OSA and traumatic brain injury, he noted. “More research is needed to assess the impact of these factors as well as the effect of treatment of OSA on comorbidities and healthcare utilization,” he said.
This study was supported by the Military Health Services Research Program.
Wickwire’s institution had received research funding from the American Academy of Sleep Medicine Foundation, Department of Defense, Merck, National Institutes of Health/National Institute on Aging, ResMed, the ResMed Foundation, and the SRS Foundation. Wickwire disclosed serving as a scientific consultant to Axsome Therapeutics, Dayzz, Eisai, EnsoData, Idorsia, Merck, Nox Health, Primasun, Purdue, and ResMed and is an equity shareholder in Well Tap.
Shamim-Uzzaman is an employee of the Veterans’ Health Administration.
A version of this article first appeared on Medscape.com.
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
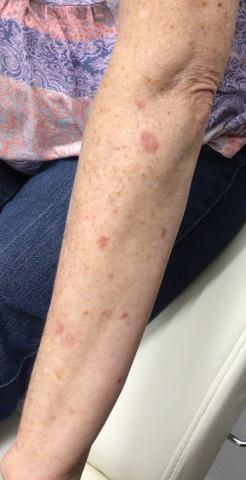
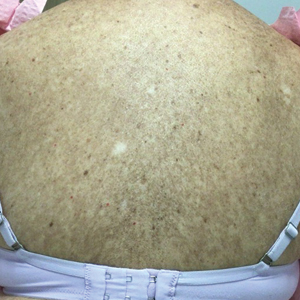
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
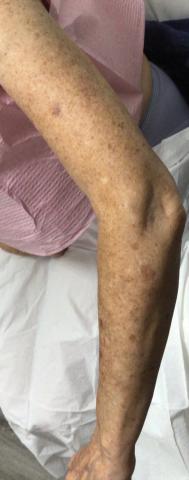
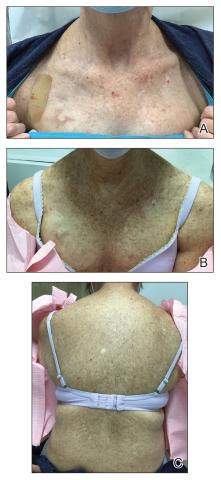
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
To the Editor:
Drug-induced hyperpigmentation is a common cause of an acquired increase in pigmentation. Belumosudil is an oral selective inhibitor of Rho-associated coiled-coil containing protein kinase (ROCK2) that is approved for the treatment of chronic graft-vs-host disease (GVHD). We describe a patient who developed diffuse skin bronzing 3 weeks after initiation of belumosudil treatment.
A 64-year-old fair-skinned woman presented to the dermatology clinic with bronzing of the skin and dystrophic nails 3 weeks after starting belumosudil for treatment of chronic GVHD. Six months prior to presentation, the patient had received a bone marrow transplant for chronic lymphoid leukemia. She presented to dermatology 6 months after the transplant with a new-onset rash that was suspicious for GVHD. Physical examination revealed pruritic pink papules diffusely scattered on the legs and forearms (Figure 1). The patient declined biopsy at that time and later followed up with oncology. The patient’s oncologist supported a diagnosis of GVHD, and the patient began treatment with belumosudil 200 mg/d which was intended to be taken until treatment failure due to progression of chronic GVHD.
Three weeks after starting belumosudil, the patient developed diffuse bronzing of the skin and brown, evenly colored patches scattered on the trunk, back, and upper and lower extremities on a background of the presumed GVHD rash (Figure 2). The hyperpigmentation was abrupt, starting on the chest and spreading to the abdomen, extremities, and back (Figure 3).
developed on the patient’s chest and back within 3 weeks of initiating treatment with belumosudil.
Again, the patient was offered biopsy for the new-onset pigmentation but declined. During this time, she had no notable sun exposure and primarily stayed indoors despite living in a region with a sunny semi-arid climate. Her medication and supplement list were reviewed and included acalabrutinib, a multivitamin, lutein, biotin, and a fish oil supplement. A compete blood cell count as well as ferritin, transferrin, cortisol, and adrenocorticotropic hormone levels were unremarkable.
The patient continued to take belumosudil for treatment of GVHD. The hyperpigmentation faded slightly by a 2-month follow-up visit but persisted and was stable. She has not tried other treatments for GVHD to manage the hyperpigmentation.
Conditions known to cause diffuse bronzing of the skin include Addison disease, hemochromatosis, Cushing disease, and medication adverse events. Our patient presented with an absence of systemic symptoms, normal laboratory results, and no clinical indicators suggesting alternate causes. Given that the onset of the hyperpigmentation was 3 weeks after she started a new medication, we hypothesized that the bronzing was an adverse effect of the belumosudil—though this correlation cannot be definitively proven by this case.
The most common offending agents for drug-induced skin hyperpigmentation are nonsteroidal anti- inflammatory drugs, antimalarials, amiodarone, cytotoxic drugs, and tetracyclines.1,2 Our patient’s medication list included the cytotoxic agent acalabrutinib, a Bruton tyrosine kinase inhibitor used for the treatment of non-Hodgkin lymphoma. It has been associated with dermatologic findings of ecchymosis, bruising, panniculitis, and cellulitis, but there are no known reports of hyperpigmentation.3 Our patient had been taking acalabrutinib for 6 months when the GVHD rash developed. At the time, she also was taking a multivitamin and lutein, biotin, and fish oil supplements, none of which have been associated with hyperpigmentation.
Polypharmacy adds a layer of difficulty in identifying the inciting cause of pigmentary change. In our case, symptoms began 3 weeks after the initiation of belumosudil. There were no cutaneous reactions observed in the ROCKstar study of belumosudil; the most common adverse events were upper respiratory tract infection, diarrhea, fatigue, nausea, increased liver enzymes, and dyspnea.4,5 Patients on belumosudil have developed aggressive cutaneous squamous cell carcinoma.6 However, a search of PubMed articles indexed for MEDLINE using the search terms acalabrutinib or belumosudil with hyperpigmentation or cutaneous reaction returned no reports of these medications causing hyperpigmentation or cutaneous deposits.
Treatment of drug-induced hyperpigmentation is difficult because discontinuation of the offending agent typically confirms diagnosis, but interruption of treatment is not always possible, as in our patient. The skin changes can fade over time, but effects typically are long lasting.
Dermatologists play a key role in the identification of drug-induced skin hyperpigmentation. After endocrine or metabolic causes of skin hyperpigmentation have been ruled out, a thorough review of the patient’s medication list should be done to assess for a drug-induced cause. Treatment is limited to sun avoidance, as interruption of treatment may not be possible, and lesions typically do fade over time. These chronic skin changes can have a psychosocial effect on patients and regular follow-up is recommended.
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
- Giménez García RM, Carrasco Molina S. Drug-induced hyperpigmentation: review and case series. J Am Board Fam Med. 2019;32:628-638. doi:10.3122/jabfm.2019.04.180212
- Dereure O. Drug-induced skin pigmentation. epidemiology, diagnosis and treatment. Am J Clin Dermatol. 2001;2:253-62. doi:10.2165/00128071-200102040-00006
- Sibaud V, Beylot-Barry M, Protin C, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am J Clin Dermatol. 2020; 21:799-812. doi:10.1007/s40257-020-00535-x
- Cutler C, Lee SJ, Arai S, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021;138:2278-2289. doi:10.1182/blood.2021012021
- Jagasia M, Lazaryan A, Bachier CR, et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graftversus- host disease. J Clin Oncol. 2021;39:1888-1898. doi:10.1200 /JCO.20.02754
- Lee GH, Guzman AK, Divito SJ, et al. Cutaneous squamous-cell carcinoma after treatment with ruxolitinib or belumosudil. N Engl J Med. 2023;389:188-190. doi:10.1056/NEJMc2304157
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
Atypical Skin Bronzing in Response to Belumosudil for Graft-vs-Host Disease
PRACTICE POINTS
- Drug-induced hyperpigmentation is a common cause of acquired hyperpigmentation and should be evaluated after metabolic or endocrine causes are ruled out.
- Belumosudil for chronic graft-vs-host disease can induce rapid-onset diffuse bronzing hyperpigmentation, even in the absence of other systemic or laboratory abnormalities.
- Treatment entails discontinuation of the offending agent and limitation of exacerbating factors such as sun exposure.
The Aftermath of Kennedy vs. Braidwood
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
In our June issue, I highlighted the potentially seismic clinical implications of the U.S. Supreme Court’s then-pending decision in the Kennedy vs. Braidwood Management, Inc., case. That ruling, recently released at the conclusion of the Court’s term, ultimately affirmed the Affordable Care Act’s mandate requiring insurers to cover certain preventive services, including colorectal cancer screening tests, without cost-sharing.
In doing so, however, the court determined that members of the U.S. Preventive Services Task Force (USPSTF), which recommends these services, are “inferior officers” appropriately appointed by the Secretary of Health and Human Services (HHS), rather than needing Senate confirmation. Thus, the decision reinforced the HHS Secretary’s authority to oversee and potentially influence USPSTF recommendations in the future. While the decision represented a victory in upholding a key provision of the ACA, it also signaled a potential threat to the scientific independence of the body charged with making those preventive care recommendations in a scientifically rigorous, unbiased manner.
As anticipated, the HHS Secretary responded to the Supreme Court’s ruling by abruptly canceling the USPSTF’s scheduled July meeting. This decision, coupled with his recent disbanding of the entire 17-member Advisory Committee on Immunization Practices — the group responsible for shaping evidence-based vaccine policy — has raised serious concerns across the healthcare field. On July 9th, AGA joined a coalition of 104 health organizations in submitting a letter to the Chair and Ranking Members of the Senate Committee on Health, Education, Labor and Pensions and the House Committee on Energy and Commerce, urging them to protect the integrity of the USPSTF.
The fight to protect science-based health policy is far from over — effective advocacy necessitates that clinicians use their professional platforms to push back against the politicization of science – not only for the integrity of the medical profession, but for the health and future of the patients we serve.
Megan A. Adams, MD, JD, MSc
Editor in Chief
At-Home Alzheimer’s Testing Is Here: Are Physicians Ready?
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
Given the opportunity, 90% of Americans say they would take a blood biomarker test for Alzheimer’s disease (AD) — even in the absence of symptoms. Notably, 80% say they wouldn’t wait for a physician to order a test, they’d request one themselves.
The findings, from a recent nationwide survey by the Alzheimer’s Association, suggest a growing desire to predict the risk for or show evidence of AD and related dementias with a simple blood test. For consumers with the inclination and the money, that desire can now become reality.
Once limited to research settings or only available via a physician’s order, blood-based diagnostics for specific biomarkers — primarily pTau-217 and beta-amyloid 42/20 — are now offered by at least four companies in the US. Several others sell blood-based “dementia” panels without those biomarkers and screens for apolipoprotein (APOE) genes, including APOE4, a variant that confers a higher risk for AD.
The companies promote testing to all comers, not just those with a family history or concerns about cognitive symptoms. Test prices range from hundreds to thousands of dollars, depending on whether they are included in a company membership, often designed to encourage repeat testing. Blood draws are conducted at home or at certified labs. Buyers don’t need a prescription or to consult with a physician after receiving results.
Knowing results of such tests could be empowering and may encourage people to prepare for their illness, Jessica Mozersky, PhD, assistant professor of medicine at the Bioethics Research Center at Washington University in St. Louis, told this news organization. A direct-to-consumer (DTC) test also eliminates potential physician-created barriers to testing, she added.
But there are also potential harms.
Based on results, individuals may interpret everyday forgetfulness — like misplacing keys — as a sign that dementia is inevitable. This can lead them to change life plans, rethink the way they spend their time, or begin viewing their future negatively. “It creates unnecessary worry and anxiety,” Mozersky said.
The growing availability of DTC tests — heralded by some experts and discouraged by others — comes as AD and dementia specialists continue to debate whether AD diagnostic and staging criteria should be based only on biomarkers or on criteria that includes both pathology and symptomology.
For many, it raises a fundamental concern: If experts haven’t reached a consensus on blood-based AD biomarker testing, how can consumers be expected to interpret at-home test results?
Growing Demand
In 2024, the number of people living with AD passed 7 million. A recent report from the Alzheimer’s Association estimates that number will nearly double by 2060.
The demand for testing also appears to be rising. Similar to the findings in the Alzheimer’s Association’s survey, a small observational study published last year showed that 90% of patients who received a cerebrospinal fluid AD biomarker test ordered by a physician said the decision to get the test was “easy.” For 82%, getting results was positive because it allowed them to plan ahead and to adopt or continue healthy behaviors such as exercise and cognitive activities.
Until now, blood biomarker tests for AD have primarily been available only through a doctor. The tests measure beta-amyloid 42/20 and pTau-217, both of which are strong biomarkers of AD. Some other blood-based biomarkers under investigation include neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP).
As reported by Medscape Medical News, the FDA approved the first blood-based AD diagnostic test in May. The Lumipulse G pTau 217/Beta-Amyloid 1-42 is for the early detection of amyloid plaques associated with AD in adults aged 55 years or older who show signs and symptoms of the disease. But it is only available by prescription.
Quest Diagnostics tested the DTC market in 2023, promoting a consumer-initiated test for beta-amyloid 42/40 that had previously been available only through physicians. It was not well-received by clinicians and ethicists. The company withdrew it later that year but continues to sell beta-amyloid 42/20 and pTau-217 tests through physicians, as does its competitor Labcorp.
Today, at least a handful of companies in the US market AD biomarkers directly to the public: Apollo Health, BetterBrain, Function Health, Neurogen Biomarking, and True Health Labs. None of the companies have disclosed ties to pharmaceutical or device companies or test developers.
What Can Consumers Get?
Some companies direct customers to a lab for blood sample collection, whereas others send a technician to customers’ homes. The extent of biomarker testing and posttest consultation also vary by company.
Apollo Health customers can order a “BrainScan” for $799, which includes screens for pTau-217, GFAP, and NfL. Buyers get a detailed report that explains each test, the result (in nanograms per liter) and optimal range (ng/L) and potential next steps. A pTau-217 result in the normal range, for instance, would come with a recommendation for repeat testing every 2 years. If someone receives an abnormal result, they are contacted by a health coach who can make a physician referral.
At Function Health, members pay $499 a year to have access to hundreds of tests and a written summary of results by a clinician. All of its “Brain Health” tests, including “Beta-Amyloid 42/40 Ratio,” pTau-217, APOE, MTHFR, DNA, and NfL, are available for an additional undisclosed charge.
BetterBrain has a $399 membership that covers an initial 75-minute consultation with cognitive tests, a “personalized brain health plan,” and a blood test that is a basic panel without AD biomarkers. A $499 membership includes all of that plus an APOE test. A pTau-217 test is available for an additional undisclosed fee.
At Neurogen Biomarking, which started in January, a consumer orders an at-home test kit, and a phlebotomist comes to their home for a blood draw. The consumer then fills out an online cognitive assessment. Test results are reviewed by a board-certified neurologist and discussed with the consumer via a virtual visit. If the person is at low-risk, they are given some educational material. Those at higher risk are referred to Neurogen’s “team of specialty-trained neurologists” for continuing care. Testing costs were not provided by the company.
Consumers can order “Beta-Amyloid 42/40” for $749 and pTau-217 for $229 directly through True Health Labs. No consultations or services are offered.
DTC Testing Raises Alarms
It’s unclear where DTC tests fall in terms of regulation. The FDA does not usually review at-home tests for low-risk medical purposes but will generally do so for diagnostics that are for higher-risk conditions “to determine the validity of test claims,” according to the agency’s website.
Consumers, however, don’t usually have easy access to information on biomarker tests’ sensitivity, specificity, or other characteristics that would be used by clinicians or regulatory authorities to assess a test’s validity.
The lack of regulation of consumer-initiated AD testing is one issue cited by critics of at-home tests, including the Alzheimer’s Association.
“None of these tests have been scientifically proven to be accurate,” the association noted in a statement, adding that “the tests can have false positive results, meaning that individuals can have results saying they have dementia when in fact they do not.”
“For these and other reasons, the Alzheimer’s Association believes that home screening tests cannot and should not be used as a substitute for a thorough examination by a skilled physician. The whole process of assessment and diagnosis should be carried out within the context of an ongoing relationship with a responsible and qualified healthcare professional,” the statement said.
The association also said that biomarker tests should not be ordered — even by physicians — for asymptomatic individuals.
The American Academy of Neurology (AAN) does not have a position on DTC tests for AD biomarkers, a spokesperson told this news organization. In a 2021 paper on ethical considerations for diagnosis and care, an AAN committee said that biomarker testing could be clinically useful for some symptomatic patients, but testing asymptomatic individuals is “recommended solely in a research setting” because of potential harms “and the absence of interventions capable of favorably altering the natural history of the disease.”
Eric Topol, MD, chair of the Department of Translational Medicine at Scripps Research in La Jolla, California, is bullish on the potential for blood-based biomarker tests. In a blog post, he called the pTau-217 biomarker “one of the most exciting advances in neurology for decades, giving us a new opportunity to accurately predict and potentially prevent (or at least substantially delay) mild cognitive impairment and Alzheimer’s.”
But, wrote Topol, who is the former editor in chief of MedscapeMedical News, “I don’t think these biomarkers are going to be useful in people at low risk.” He wrote that testing should not be used by people who are “cognitively intact” or to tell someone they have pre-AD. “More work needs to be done to determine whether lowering one’s pTau-217 will alter the brain plaque progression and be seen as a disease-modifier,” wrote Topol.
The Risks of Knowing
Some people don’t want to know their biomarker status. In a study in May in JAMA Network Open, Mozersky and colleagues reported that while 81% of a group of cognitively normal participants in a longitudinal study of dementia said they wanted to see results, only 60% ultimately opted to get results after testing. Participants said they did not want to know because they didn’t want to become a burden on their family or that they felt fine; others had concerns about whether the tests were accurate.
That low number “surprised us,” said Mozersky. “Our study certainly suggests that when you’re really faced with knowing, that your answer is more likely to possibly be no,” she added.
DTC companies tell buyers that results could motivate them to change their lifestyle to reduce their future risk for AD and dementia. But some participants in Mozersky’s study said they didn’t want to know their status because there were no preventive treatments. Test results weren’t seen as “actionable,” she said.
Some studies have shown a degree of fatalism in individuals after receiving a test result, whether it’s positive or negative.
A group of Israeli researchers studied responses of people given PET scans to detect beta-amyloid. Before testing, all participants said they were motivated to adopt lifestyle changes to fight dementia. However, after testing, both those who had elevated beta-amyloid and those who did not reported a much lower desire to change their lifestyle. Those with normal scans probably felt relieved, wrote the researchers. The group with abnormal scans was too small to fully understand their reaction, they wrote.
Concerns about insurance coverage might also deter potential test-takers. Overall, 44% of those responding to the Alzheimer’s Association survey said they were worried that insurers might not cover healthcare costs in the future if they had received a positive test earlier. Respondents also worried about test accuracy, the cost of testing, and whether a positive test might lead to a prohibition on some activities, like driving.
What About the Doctors?
The DTC companies promise buyers that results will be private and won’t be shared with insurers — or with clinicians. And that raises another issue for many who are concerned about the lack of a physician intermediary with at-home testing.
“You remove the opportunity for clinicians to both review the result and figure out how to interpret it before it’s communicated to the patient,” Jalayne J. Arias, JD, a bioethicist and associate professor of Health Policy and Behavioral Sciences at Georgia State University, Atlanta, told this news organization.
Many in the field have been “thinking really carefully about how do we provide guidance to clinicians about biomarker testing,” she said. “Those issues are just heightened when we put it into a direct-to-consumer model,” Arias said.
Arias — who with colleagues published an analysis of potential insurance issues with biomarker tests in JAMA Neurology — said that prohibitions against discrimination based on preexisting conditions means that most likely, health insurers could not use testing data to deny coverage or increase premiums.
But, she said, “there are some question marks around the discrimination risks.” This is especially true for people seeking long-term care, disability or life insurance, she added.
If a test result is not documented in a medical record, it’s not clear whether the individual has an obligation to disclose the result to an insurer, said Arias.
Given all the unanswered questions about how results should be interpreted, to whom the results should be disclosed, and when and how to have discussions with patients, “it’s hard for me to imagine that we’re quite ready for a direct-to-consumer” test, Arias said.
Mozersky noted that Washington University has a financial stake in C2N Diagnostics, which makes the PrecivityAD — biomarker tests for AD. Arias reported having no conflicts of interest.
A version of this article first appeared on Medscape.com.
First New PTSD Drug in Two Decades On the Horizon?
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
The Psychopharmacologic Drugs Advisory Committee of the FDA is set to meet on July 18 to consider a supplemental new drug application for brexpiprazole (Rexulti, Otsuka Pharmaceutical Co., Ltd.), in combination with sertraline, for the treatment of adults with posttraumatic stress disorder (PTSD).
If approved, it would be the first new treatment for PTSD in more than 20 years.
“It is my hope that the FDA does approve this treatment for two related reasons — the data look positive and compelling, and there’s a tremendous unmet need in PTSD,” Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the Mood Disorders Psychopharmacology Unit, University of Toronto, Toronto, Ontario, Canada, told this news organization.
What’s in the Treatment Toolbox Now?
PTSD is a “common, severe, and nonremitting condition,” McIntyre noted. According to the National Center for PTSD, the condition affects roughly 13 million adults in the US in any given year. This represents about 5% of the adult population.
PTSD can develop following exposure to traumatic events such as combat, assault, disasters, or severe accidents. Core symptoms of PTSD include intrusive memories and flashbacks, avoidance behaviors, negative alterations in mood and cognition, and hyperarousal.
Currently, the selective serotonin reuptake inhibitors (SSRI), sertraline and paroxetine, are the only FDA-approved medications for PTSD, and while these medications can be effective, many patients fail to achieve remission or discontinue treatment due to adverse effects or lack of response.
Other medications used off-label to treat PTSD — including prazosin, mirtazapine, atypical antipsychotics, and mood stabilizers — have shown variable efficacy.
There has not been a new FDA-approved drug for PTSD in over two decades, underscoring the need for better therapeutic options, particularly for patients who do not fully respond to SSRI alone.
Why Brexpiprazole Plus Sertraline?
Brexpiprazole is an atypical antipsychotic currently approved as adjunctive treatment of major depressive disorder (MDD) in adults; treatment of schizophrenia in adults and adolescents aged 13 years or older; and treatment of agitation associated with Alzheimer’s dementia.
The combination of brexpiprazole and sertraline could address the limitations of SSRI alone by working synergistically to treat PTSD.
Sertraline increases serotonin levels in the brain to improve mood and reduce anxiety. Brexpiprazole has a complex mechanism of action involving multiple neurotransmitter systems, including but not limited to serotonin and dopamine.
Together, they may target different aspects of PTSD, potentially leading to a more comprehensive reduction in symptoms.
What Do the Phase 3 Data Show?
In a pivotal, double-blind, randomized controlled, phase 3 trial, brexpiprazole plus sertraline provided significantly greater relief of PTSD symptoms than sertraline plus placebo.
The results were published late last year in JAMA Psychiatry and reported by this news organization at that time.
The trial enrolled 416 adults (mean age, 37 years; 75% women) aged 18-65 years with a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of PTSD and symptoms for at least 6 months prior to screening.
At baseline, participants had a mean Clinician Administered PTSD Scale (CAPS-5) for DSM-5 total score of 38.4, indicating moderate to high severity PTSD. The average time from the index traumatic event was 4 years, and three fourths had no prior exposure to PTSD prescription medications.
Participants underwent a 1-week placebo run-in period followed by randomization to daily oral brexpiprazole 2-3 mg plus sertraline 150 mg or daily sertraline 150 mg plus placebo for 11 weeks.
At week 10, brexpiprazole plus sertraline demonstrated statistically significant greater improvement in the CAPS-5 total score (primary outcome) than sertraline plus placebo (mean change, -19.2 points vs -13.6 points; P < .001).
Brexpiprazole plus sertraline also led to statistically significant greater improvement on all key secondary and other efficacy endpoints, both clinician-reported and patient-reported, including measures of anxiety, depression, intrusive symptoms, hyperarousal, and overall functioning.
Combining an atypical antipsychotic with an antidepressant for PTSD “builds on what we’ve been doing in depression,” Elspeth Ritchie, MD, chair of Psychiatry, MedStar Washington Hospital Center, Washington, DC, noted in an interview with this news organization.
“We have found that a combination of a low-dose antipsychotic and an antidepressant is helpful for depression, so it makes sense that it will be helpful for PTSD. However, this has been mostly based on clinical decisions, without a heavy research background. Good science is always helpful to support those clinical decisions,” Ritchie told this news organization.
What About Safety?
In the phase 3 trial, brexpiprazole plus sertraline had a safety profile consistent with that of brexpiprazole in approved indications. The rate of discontinuation due to adverse events was low (3.9% for brexpiprazole plus sertraline vs 10.2% for sertraline plus placebo), indicating that most participants tolerated the brexpiprazole and sertraline combination treatment, the study team said.
In both treatment groups, the only treatment-emergent adverse event (TEAE) with incidence greater than 10% was nausea, a known adverse effect of sertraline treatment.
Weight gain was greater in participants receiving the combination. At the last visit, a weight gain of 7% or greater from week 1 was experienced by 8% of participants taking brexpiprazole with the sertraline group and 5% of those taking the sertraline plus placebo. Previous analyses in schizophrenia and MDD show that brexpiprazole is associated with moderate weight gain (+3 to 4 kg over 1 year).
The incidence of sedating TEAEs (a concern with some antipsychotics) was generally low, although fatigue (7% vs 4%) and somnolence (5% vs 3%) were more common with brexpiprazole plus sertraline than with sertraline alone.
There were no clinically meaningful between-group differences in changes in laboratory test parameters, vital signs, or ECG and participant-reported TEAEs related to suicidality.
Potential Concerns
As with any new drug application, several questions and issues are likely to be raised by the advisory committee. They could include whether the clinical benefit is substantial enough to warrant approval and how the observed effect sizes compare to existing approved therapies and evidence-based psychotherapies.
McIntyre told this news organization what’s particularly noteworthy is that the magnitude of the improvement in PTSD symptoms with brexpiprazole plus sertraline is greater than with sertraline alone. “That’s a very important statement. And this high level of efficacy was consistent on the secondary outcome measures, and the overall tolerability and safety seemed very acceptable,” he said.
What’s equally important, said McIntyre, is that most people with PTSD have depression and anxiety, and the brexpiprazole plus sertraline combination was more helpful than sertraline alone on the measures of anxiety and depression. “This is really important, especially in light of the fact that this medication [brexpiprazole] is already approved for adults living with major depressive disorder and inadequate response to antidepressants,” McIntyre said.
McIntyre added he suspects some questions the committee may have could relate to the extent to which it’s the case that brexpiprazole is effective in PTSD regardless of the antidepressant that is prescribed with it.
“There also will be the inevitable questions about the absence of long-term data which I think will need to be addressed given how chronic and relapse prone this condition is,” McIntyre said.
The committee may ask how trauma and PTSD will be screened in primary care and how outcomes related to this therapy will be evaluated in everyday clinical practice, McIntyre said.
Overall, McIntyre said brexpiprazole plus sertraline in PTSD is a “very positive” development for the field.
“PTSD is a terrible condition. It’s so darn common, and we just don’t have enough treatments for it. The data look good for my perspective. My fingers are crossed for the patients with PTSD and their families,” said McIntyre.
Ritchie reported having no relevant disclosures. McIntyre received speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and atai Life Sciences. McIntyre is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
State-Mandated ‘Gold Card’ Programs to Ease Prior Authorization Burdens Offer Little Relief, Experts Say
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
“Gold card” programs were supposed to make it easier for frustrated physicians to deal with insurers’ burdensome prior authorization demands.
The idea: Insurers would reward doctors whose past prior authorization requests were typically approved by exempting them from red tape in the future.
At least 10 states have required insurers to establish gold card programs amid mounting concerns nationwide that overuse of prior authorization jeopardizes patient health. Last month, leading insurers joined with the White House in a voluntary pledge to reduce their use of the practice, which they contend is necessary to control costs and minimize unnecessary care.
But Texas’ experience with gold card programs may signal the limits of that approach.
Only 3% of Clinicians Qualified
The Lone Star State was an early adopter, passing a 2021 law enabling health providers with a high prior authorization success rate to earn a “gold card” exemption from insurers.
But statewide, only 3% of providers met that bar, according to a testimony provided by the Texas Department of Insurance earlier this year.
“I think it’s safe to say that the impact of this law on prior authorizations for our physicians is underwhelming,” said Ezequiel “Zeke” Silva III, MD, a San Antonio-based interventional radiologist who chairs the Texas Medical Association’s Council on Legislation. “We would have hoped for a greater percentage of our physicians to have been granted the ‘gold card’ status.”
At least nine other states have enacted gold card laws, according to the National Conference of State Legislatures (NCSL).
Care Delayed and Denied
Physicians maintain that excessive prior authorization paperwork impedes timely patient care, with clinicians and staffers devoting 13 hours weekly to documentation, according to a 2024 American Medical Association survey.
Insurers view the review as a guardrail against unnecessary care driving up costs. Studies show that restricting prior authorization could boost premiums by 5.6%-16.7%, a Texas Association of Health Plans official testified during the legislative session.
In June, Texas Gov. Greg Abbott signed a revised version of the state’s “gold card” law — part of an emerging national attempt to streamline the prior review process. Cigna, Humana, UnitedHealthcare, and other large insurers have voluntarily committed to reducing the scope of claims involved, according to the America’s Health Insurance Plans trade group.
Meanwhile, federal officials have finalized requirements that direct some insurers, including Medicaid and Medicare Advantage programs, to speed up responses to prior authorization requests, among other measures. Some of those requirements begin in 2026.
Gold Card Designs
As in other states, Texas’ “gold card” legislation applies only to state-regulated insurers, which comprise about one fifth of the state’s market. Under HB 3812, which takes effect on September 1, insurers will evaluate health providers based on a year of prior authorization requests rather than 6 months under the 2021 law.
To be evaluated, providers must have submitted at least five requests for a specific health service during that period. To achieve “gold card” status, insurers must approve at least 90% of requests, the same threshold as set by the 2021 law. But the new law stipulates that insurers review a broader pool of requests, including those made directly to the health plan as well as any related affiliates, according to the Texas Department of Insurance.
The new law continues to limit exemptions only to “top-performing physicians” who repeatedly provide cost-effective care, said Blake Hutson, director of public affairs at the Texas Association of Health Plans. “Even with the change to 1 year, and the bill also adds in a broader array of claims that will be looked at, you still have to meet 90%.”
A key addition requires insurers to release an annual report detailing how many exemptions they have granted or denied, making decisions more transparent to the public, Silva said. “Not just what’s being approved and what’s not being approved, but to potentially evaluate for trends that presently we just have no ability to evaluate,” he said.
Gold card laws vary from state to state, and some exclude prescription drugs, according to an NCSL legislative summary. Other states with gold card programs include Arkansas, Colorado, Illinois, Louisiana, Michigan, New Mexico, Vermont, West Virginia, and Wyoming.
In Illinois, legislation passed last year targeted hospital services for Medicaid patients, as denial rates were routinely higher in that population, said Dave Gross, senior vice president of Government Relations and Communications at the Illinois Health and Hospital Association, Naperville, Illinois. “We’re not seeing this problem in the commercial space,” he noted.
Real-World Implications
To some degree, the “gold card” concept makes intuitive sense, recognizing physicians who have a track record of getting their medical care requests approved, said Ravi Gupta, MD, an assistant professor of medicine at Johns Hopkins University School of Medicine, Baltimore, who has studied prior authorization patterns.
But Gupta raised equity concerns. Physicians in large medical groups and hospital systems will have access to staff and other resources to better navigate the prior approval process than those in smaller private practices.
Plus, he added, there’s the potential that physicians who achieve exemptions may become “more indiscriminate” about the services that they recommend.
Insurers’ stated aim is to reduce unnecessary and low-value medical care through prior authorization gatekeeping, Gupta said. But a study he helped conduct, assessing policies across five Medicare Advantage insurers, found a significant lack of consensus on what treatments should be included. Treatments comprising only 12% of Medicare spending would have required prior authorization by all five insurers. Most of that consensus, he wrote, “was devoted to a small number of costly services.”
The administrative burdens affect patients as well. Two thirds of patients with cancer in one 2023 study become personally involved, including calling the insurer or appealing a denial. The patients also reported less trust in insurers and the health system overall, which could have worrisome downstream effects, Fumiko Chino, MD, the study’s lead author and an assistant professor of radiation oncology at Houston’s MD Anderson Cancer Center, said.
“If you don’t trust healthcare,” she said, “why on earth would you get a vaccine or get cancer screening or get your blood pressure checked?”
More Than X Percent?
Gupta views the leading health insurers’ pledge as encouraging in concept — but he notes that they are voluntary commitments without any accountability.
In the interim, gold carding remains no more than a workaround, he said.
“Gold cards aren’t really fixing that [prior authorization] problem,” he said. “They’re just rewarding certain clinicians who can demonstrate that they have been able to get through the prior authorization process successfully for X amount of time before they’re rewarded with a gold card.”
In Illinois, regulators are still hashing out gold card rules, including whether the required 90% approval threshold will be based on a specific hospital service or a broader pool of services, Gross said. The hospital association also will closely watch whether Illinois’ experience begins to mirror that in Texas, he said.
“We have some of the best hospitals in the country here in Chicago,” he said. “If we end up with a 3% approval rating of gold cards, we’re going to have to go back to the legislature.”
A version of this article first appeared on Medscape.com.
Dementia Risk May Follow a Geographic Pattern
TOPLINE:
Dementia incidence varied significantly by US region in a new study, with the Southeast showing a 25% higher risk and the Northwest and Rocky Mountains each showing a 23% higher risk compared to the Mid-Atlantic. Investigators said the findings highlight the need for a geographically tailored approach to address dementia risk factors and diagnostic services.
METHODOLOGY:
- Researchers conducted a cohort study using data from the US Veterans Health Administration for more than 1.2 million older adults without dementia (mean age, 73.9 years; 98%% men) from 1999 to 2021. The average follow-up was 12.6 years.
- Ten geographical regions across the US were defined using the CDC National Center for Chronic Disease Prevention and Health Promotion definition.
- The diagnosis of dementia was made using International Classification of Diseases, Ninth and Tenth Revision codes from inpatient and outpatient visits.
TAKEAWAY:
- Dementia incidence rates per 1000 person-years were lowest in the Mid-Atlantic (11.2; 95% CI, 11.1-11.4) and highest in the Southeast (14.0; 95% CI, 13.8-14.2).
- After adjusting for demographics, compared with the Mid-Atlantic region, dementia incidence was highest in the Southeast (rate ratio [RR], 1.25), followed by the Northwest and Rocky Mountains (RR for both, 1.23), South (RR, 1.18), Southwest (RR, 1.13), and Midwest and South Atlantic (RR for both, 1.12). The Great Lakes and Northeast regions had < a 10% difference in incidence.
- Results remained consistent after adjusting for rurality and cardiovascular comorbidities, and after accounting for competing risk for death.
IN PRACTICE:
“This study provides valuable insights into the regional variation in dementia incidence among US veterans in that we observed more than 20% greater incidence in several regions compared with the Mid-Atlantic region,” the investigators wrote.
“By identifying areas with the highest incidence rates, resources can be better allocated and targeted interventions designed to mitigate the impact of dementia on vulnerable populations,” they added.
SOURCE:
This study was led by Christina S. Dintica, PhD, University of California, San Francisco. It was published online on June 9 in JAMA Neurology.
LIMITATIONS:
This study population was limited to US veterans, limiting the generalizability of the findings. Education level was defined using educational attainment rates in the participants’ zip codes rather than individual data. Additionally, because residential history was limited to a single location per participant, migration patterns could not be tracked.
DISCLOSURES:
This study was supported by grants from the Alzheimer’s Association, the National Institute on Aging, and the Department of Defense. One author reported serving on data and safety monitoring boards for studies sponsored by the National Institutes of Health, as well as holding advisory board membership and receiving personal fees from industry. Full details are listed in the original article. The other four investigators reported no relevant financial conflicts.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dementia incidence varied significantly by US region in a new study, with the Southeast showing a 25% higher risk and the Northwest and Rocky Mountains each showing a 23% higher risk compared to the Mid-Atlantic. Investigators said the findings highlight the need for a geographically tailored approach to address dementia risk factors and diagnostic services.
METHODOLOGY:
- Researchers conducted a cohort study using data from the US Veterans Health Administration for more than 1.2 million older adults without dementia (mean age, 73.9 years; 98%% men) from 1999 to 2021. The average follow-up was 12.6 years.
- Ten geographical regions across the US were defined using the CDC National Center for Chronic Disease Prevention and Health Promotion definition.
- The diagnosis of dementia was made using International Classification of Diseases, Ninth and Tenth Revision codes from inpatient and outpatient visits.
TAKEAWAY:
- Dementia incidence rates per 1000 person-years were lowest in the Mid-Atlantic (11.2; 95% CI, 11.1-11.4) and highest in the Southeast (14.0; 95% CI, 13.8-14.2).
- After adjusting for demographics, compared with the Mid-Atlantic region, dementia incidence was highest in the Southeast (rate ratio [RR], 1.25), followed by the Northwest and Rocky Mountains (RR for both, 1.23), South (RR, 1.18), Southwest (RR, 1.13), and Midwest and South Atlantic (RR for both, 1.12). The Great Lakes and Northeast regions had < a 10% difference in incidence.
- Results remained consistent after adjusting for rurality and cardiovascular comorbidities, and after accounting for competing risk for death.
IN PRACTICE:
“This study provides valuable insights into the regional variation in dementia incidence among US veterans in that we observed more than 20% greater incidence in several regions compared with the Mid-Atlantic region,” the investigators wrote.
“By identifying areas with the highest incidence rates, resources can be better allocated and targeted interventions designed to mitigate the impact of dementia on vulnerable populations,” they added.
SOURCE:
This study was led by Christina S. Dintica, PhD, University of California, San Francisco. It was published online on June 9 in JAMA Neurology.
LIMITATIONS:
This study population was limited to US veterans, limiting the generalizability of the findings. Education level was defined using educational attainment rates in the participants’ zip codes rather than individual data. Additionally, because residential history was limited to a single location per participant, migration patterns could not be tracked.
DISCLOSURES:
This study was supported by grants from the Alzheimer’s Association, the National Institute on Aging, and the Department of Defense. One author reported serving on data and safety monitoring boards for studies sponsored by the National Institutes of Health, as well as holding advisory board membership and receiving personal fees from industry. Full details are listed in the original article. The other four investigators reported no relevant financial conflicts.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dementia incidence varied significantly by US region in a new study, with the Southeast showing a 25% higher risk and the Northwest and Rocky Mountains each showing a 23% higher risk compared to the Mid-Atlantic. Investigators said the findings highlight the need for a geographically tailored approach to address dementia risk factors and diagnostic services.
METHODOLOGY:
- Researchers conducted a cohort study using data from the US Veterans Health Administration for more than 1.2 million older adults without dementia (mean age, 73.9 years; 98%% men) from 1999 to 2021. The average follow-up was 12.6 years.
- Ten geographical regions across the US were defined using the CDC National Center for Chronic Disease Prevention and Health Promotion definition.
- The diagnosis of dementia was made using International Classification of Diseases, Ninth and Tenth Revision codes from inpatient and outpatient visits.
TAKEAWAY:
- Dementia incidence rates per 1000 person-years were lowest in the Mid-Atlantic (11.2; 95% CI, 11.1-11.4) and highest in the Southeast (14.0; 95% CI, 13.8-14.2).
- After adjusting for demographics, compared with the Mid-Atlantic region, dementia incidence was highest in the Southeast (rate ratio [RR], 1.25), followed by the Northwest and Rocky Mountains (RR for both, 1.23), South (RR, 1.18), Southwest (RR, 1.13), and Midwest and South Atlantic (RR for both, 1.12). The Great Lakes and Northeast regions had < a 10% difference in incidence.
- Results remained consistent after adjusting for rurality and cardiovascular comorbidities, and after accounting for competing risk for death.
IN PRACTICE:
“This study provides valuable insights into the regional variation in dementia incidence among US veterans in that we observed more than 20% greater incidence in several regions compared with the Mid-Atlantic region,” the investigators wrote.
“By identifying areas with the highest incidence rates, resources can be better allocated and targeted interventions designed to mitigate the impact of dementia on vulnerable populations,” they added.
SOURCE:
This study was led by Christina S. Dintica, PhD, University of California, San Francisco. It was published online on June 9 in JAMA Neurology.
LIMITATIONS:
This study population was limited to US veterans, limiting the generalizability of the findings. Education level was defined using educational attainment rates in the participants’ zip codes rather than individual data. Additionally, because residential history was limited to a single location per participant, migration patterns could not be tracked.
DISCLOSURES:
This study was supported by grants from the Alzheimer’s Association, the National Institute on Aging, and the Department of Defense. One author reported serving on data and safety monitoring boards for studies sponsored by the National Institutes of Health, as well as holding advisory board membership and receiving personal fees from industry. Full details are listed in the original article. The other four investigators reported no relevant financial conflicts.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).
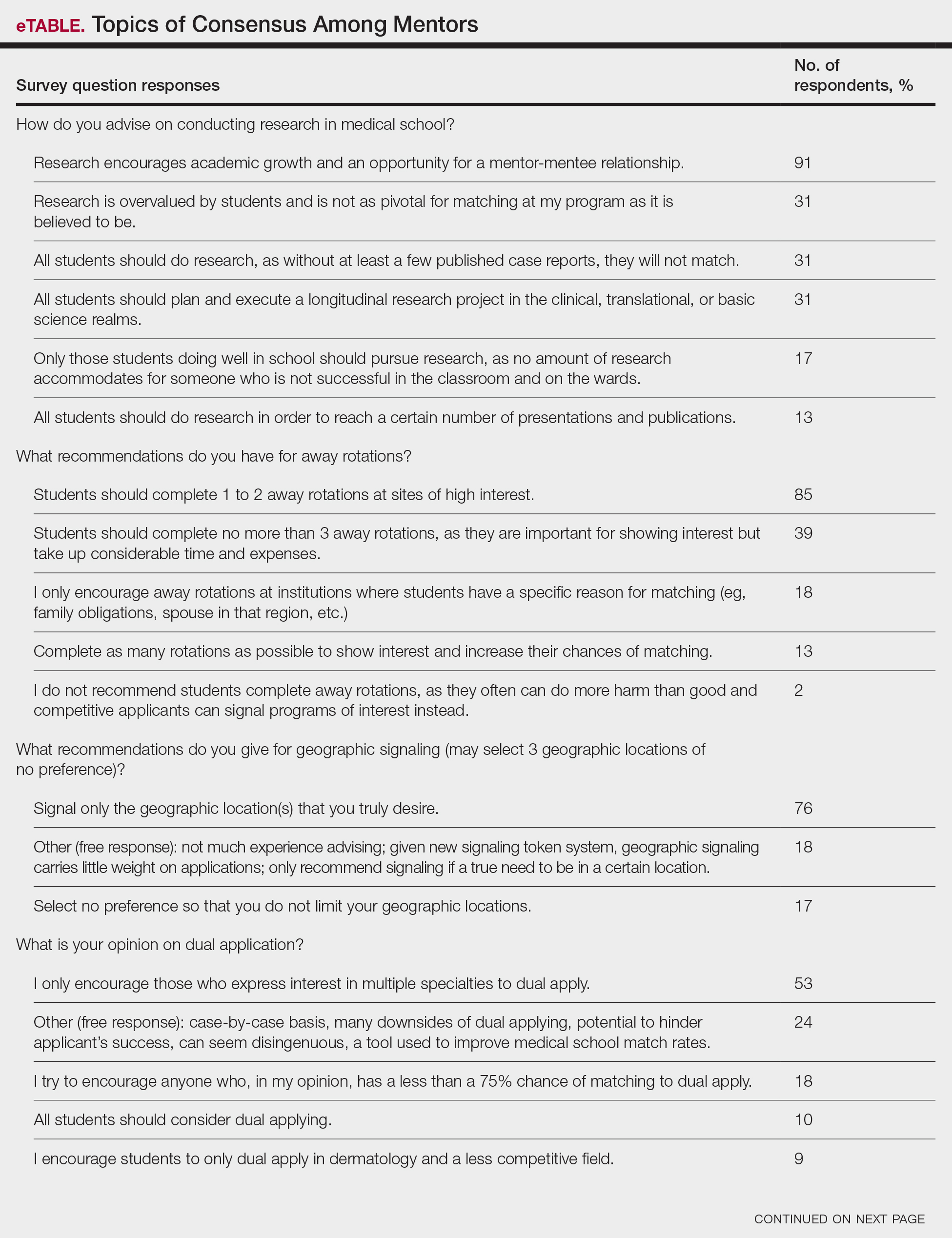

Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.
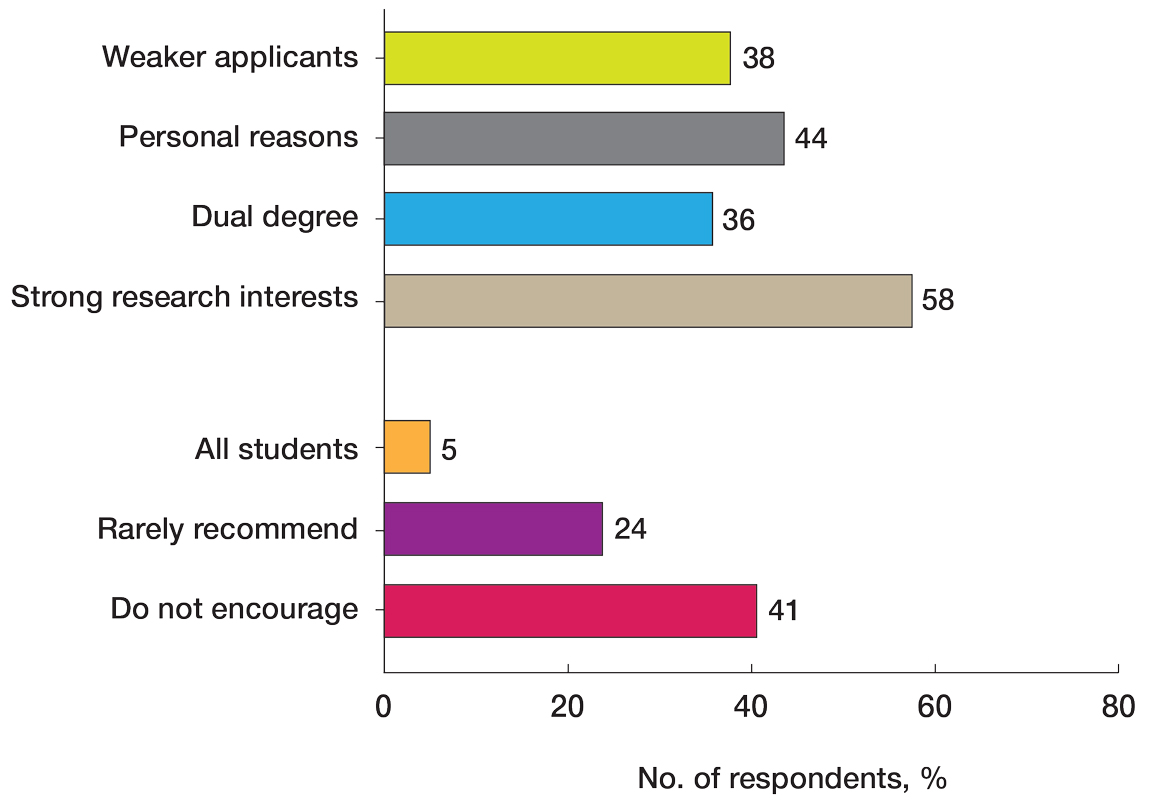
Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).
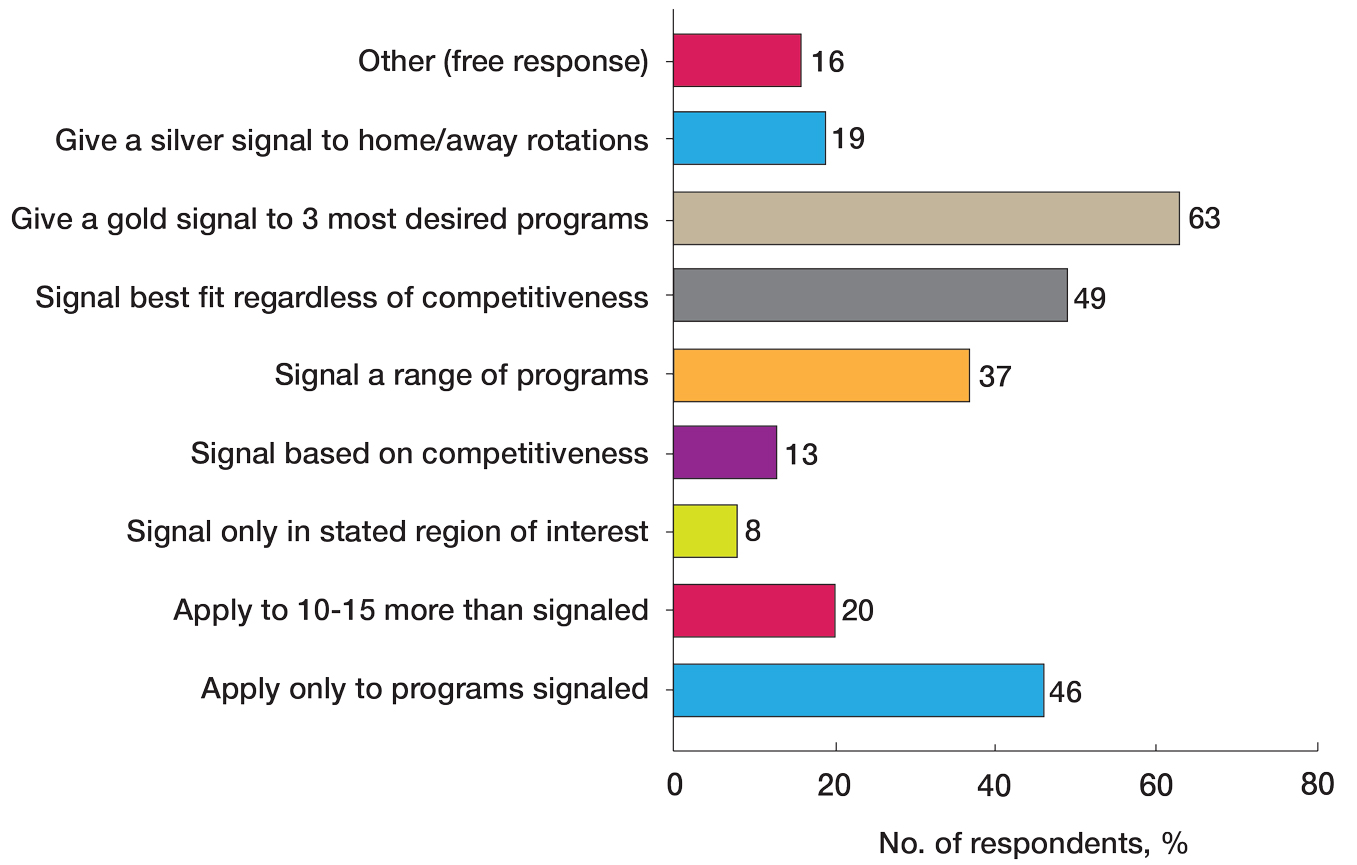
Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).


Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.

Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).

Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
While strong relationships with mentors and advisers are critical to navigating the competitive dermatology match process, the advice medical students receive from different individuals can be contradictory. Unaccredited information online—particularly on social media—as well as data reported by applicants can add to potential confusion.1 Published research has elicited comments and observations from successfully matched medical students about highly discussed topics such as presentations and publications, letters of recommendation, away rotations, and interviews.2,3 However, there currently are no published data about advice that dermatology mentors actually offer medical students. In this study, we aimed to investigate this gap in the current literature and examine the advice dermatology faculty, program directors, and other mentors at institutions accredited by the Accreditation Council for Graduate Medical Education across the United States give to medical students applying to dermatology residency.
Methods
A 14-question Johns Hopkins Qualtrics survey was sent via the Association of Professors of Dermatology (APD) listserve in June 2024 soliciting responses from members who consider themselves to be mentors to dermatology applicants across the United States. The survey included multiple-choice questions with the option to select multiple answers and a space for open-ended responses. The questions first gathered information on the respondents, including the capacity in which the mentors advised medical students (eg, program director, department chair, clinical faculty). Mentors were asked for the number of years they had been advising mentees and if they were advising students with a home dermatology program. In addition, mentors were asked what advice they give their mentees about aspects of the application process, including gap years, dual applications, research involvement, couples matching, program signaling, away rotations, internship year, letters of recommendation, geographic signaling, interviewing advice, and volunteering during medical school.
On August 18, 2024, survey results from 115 respondents were aggregated. The responses for each question were quantitatively assessed to determine whether there was consensus on specific advice offered. The open-ended responses also were qualitatively assessed to determine the most common responses.
Results
The respondents included program directors (30% [35/115]), clinical faculty (22% [25/115]), department chairs (18% [21/115]), assistant program directors (15% [17/115]), medical school clerkship directors (8% [9/115]), primary mentors (ie, faculty who did not fall into any of the aforementioned categories but still advised medical students interested in dermatology)(5% [6/115]), division chiefs (1% [1/115]), and deans (1% [1/115]). Respondents had been advising students for a median of 10 years (range, 1-40 years [25th percentile, 5.00 years; 75th percentile, 13.75 years]). The majority (90% [103/115]) of mentors surveyed were advising students with a home dermatology program.
Areas of Consensus
In some areas, there was broad consensus among the advice offered by the mentors that were surveyed (eTable).


Research During Medical School—More than 91% (105/115) of the respondents recommended research to encourage academic growth and indicated that the most important reason for conducting research during medical school is to foster mentor-mentee relationships; however, more than one-third of respondents believed research is overvalued by students and research productivity is not as critical for matching as they perceive it to be. When these responses were categorized by respondent positions, 29% (15/52) of program or assistant directors indicated agreement with the statement that research is overvalued.
Away Rotations—There also was a consensus about the importance of away rotations, with 85% (98/115) of respondents advising students to complete 1 to 2 away rotations at sites of high interest, and 13% (15/115) suggesting that students complete as many away rotations as possible. It is worth noting, however, that the official APD Residency Program Directors Section’s statement on away rotations recommends no more than 2 away rotations (or no more than 3 for students with no home program).4
Reapplication Advice—Additionally, in a situation where students do not match into a dermatology residency program, the vast majority (71% [82/115]) of respondents advised students to rank competitive intern years to foster connections and improve the chance of matching on the second attempt.
Volunteering During Medical School—Seventy-seven percent (89/115) of mentors encouraged students to engage in volunteerism and advocacy during medical school to create a well-rounded application, and 69% (79/115) of mentors encouraged students to display leadership in their volunteer efforts.
Areas Without Consensus
Letters of Recommendation—Most respondents recommended submitting letters of recommendation only from dermatology professionals (55% [63/115]), with the remainder recommending students request a letter from anyone who could provide a strong recommendation regardless of specialty mix (42% [48/115]).
Dermatologic Subspecialties—For students interested in dermatologic subspecialties, 73% (84/115) of mentors advised that students be honest during interviews but keep an open mind that interests during residencies may change. Forty-three percent (49/115) of respondents encouraged students to promote a subspecialty interest during their interview only if they can demonstrate effort within that subspecialty on their application.
Couples Matching—Most respondents approach couples matching on a case-by-case basis and assess individual priorities when they do advise on this topic. Respondents often advise applicants to identify a few cities/regions and focus strongly on the programs within those regions to avoid spreading themselves too thin; however, one-third (38/115) of respondents indicated that they do not personally offer advice regarding the couples match.
Areas With Diverse Opinions
Gap Years—Nearly one-quarter (24% [28/115]) of mentors reported that they rarely recommend students take a year off and only support those who are adamant about doing so, or that they never support taking a gap year at all. A slight majority (58% [67/115]) recommend a gap year for students strongly interested in dermatologic research, and 38% (44/115) recommend a gap year for students with weaker applications (Figure 1). We received many open-ended responses to this question, with mentors frequently indicating that they advise students to take a gap year on a case-by-case basis, with 44% (51/115) of commenters recommending that students only take paid gap-year research positions.

Program Signaling—The dermatology residency application process implemented a system of preference signaling tokens (PSTs) starting with the 2021-2022 cycle. Not quite half (46% [53/115]) of respondents recommend students apply only to places that they signaled, while 20% (23/115) advise responding to 10 to 15 additional programs. Very few (8% [9/115]) advise students to signal only in their stated region of interest. Approximately half (49% [56/115]) of mentors recommend students only signal based on the programs they feel would be the best fit for them without regard for perceived competitiveness—which aligns with the APD Residency Program Directors Section’s recommendation4—while 37% (43/115) recommend students distribute their signals to a wide range of programs. Sixty-three percent (72/115) of respondents recommend gold signaling to the student’s 3 most desired programs regardless of home and away rotation considerations, while 19% (22/115) recommend students give silver signals to their home and away rotation programs, as a rotation is already a signal of a strong desire to be there (Figure 2).

Dual Application—Fifty-three percent (61/115) of mentors recommended dual applying only for those truly interested in multiple specialties. Eighteen percent (21/115) of respondents advised dual applying for those with less than a 75% chance of matching. Twenty-five percent (29/115) of respondents free-wrote comments about approaching dual applying on a case-by-case basis, with many discussing the downsides of dual application and raising concerns that dual applications can hinder applicants’ success, can seem disingenuous, and seem to be a tool used to improve medical school match rates without benefit for the student.
We also stratified the data to compare overall responses from the total cohort with those from only program and assistant program directors. Across the 14 questions, responses from program and assistant program directors alone were similar to the overall cohort results
Comment
This study evaluated nationwide data on mentorship advising in dermatology, detailing mentors’ advice regarding research, gap years, dual applications, away rotations, intern year, couples matching, program signaling, and volunteering during medical school. Based on our results, most respondents agree on the importance of research during medical school, the utility of away rotations, and the value of volunteering during medical school. Similarly, respondents agreed on the importance of having strong letters of recommendation; while some advised asking only dermatology faculty to write letters, others did not have a specialty preference for the letter writers. Respondents also had varying views about sharing interest in subspecialties during residency interviews. Many of the respondents do not provide recommendations regarding geographic signaling and couples matching, expressing that these are parts of an application that are important to approach on a case-by-case basis. Lastly, respondents had diverse opinions regarding the utility of gap years, whether to encourage or discourage dual applications, and how to advise regarding program signaling.
Our results also showed that one-third of respondents believed that research is not as important as it is perceived to be by dermatology applicants. While engaging in research during medical school was almost unanimously encouraged to foster mentor-mentee relationships, respondents expressed that the number of research experiences and publications was not critical. This is an important topic of discussion, as taking a dedicated year away from medical school to complete a research fellowship is becoming a trend among dermatology applicants.5 There has been discussion both on unofficial online platforms as well as in the published literature regarding the pressure for medical students interested in dermatology to publish, which may result in a gap year for research.6 The literature on the utility of a gap year in match rates is sparse, with one study showing no difference in match rates among Mayo Clinic dermatology residents who took research years vs those who did not.7 However, this contrasts with match rates at top dermatology residency programs where 41% of applicants who took a gap year matched vs 19% who did not.7,8 These conflicting data are reflected in our study results, with respondents expressing different opinions on the utility of gap years.
There also are important equity concerns regarding the role of research years in the dermatology residency match process. Dermatology is one of the least racially diverse specialties, although there have been efforts to increase representation among residents and attending physicians.9-11 Research years can be important contributors to this lack of representation, as these often are unpaid and can discourage economically disadvantaged students from applying.9-11 Additionally, applicants may not have the flexibility to defer future salary for a year to match into dermatology; therefore, mentors should offer multiple options to individual applicants instead of solely encouraging gap years, given the conflicting feelings regarding their productivity.
Another topic of disagreement was dual application. Approximately one-third of respondents said they encourage either all students or those with less than a 75% chance of matching to dual apply, while about half only encourage students who are truly interested in multiple specialties to do so. Additionally, a large subset of respondents said they do not encourage dual applications due to concerns that they make applicants a worse candidate for each specialty and overall have negative effects on matching. Twenty-five percent of respondents opted to leave an open-ended response to this question: some offered the perspective that, if applicants feel a need to dual apply due to a weaker application, they do not advise the applicant to apply to dermatology. Many open ended responses underscored that the respondent does not encourage dual applications because they are inherently more time consuming, could hinder the applicant’s success, can seem disingenuous, and are a tool used to improve medical school match rates without being beneficial for the student. Some respondents also favored reapplying to dermatology the following year instead of dual applying. Finally, a subset of mentors indicated that they approach dual applications on a case-by-case basis, and others reported they do not have much experience advising on this topic. Currently, there are no known data in the literature on the efficacy and utility of dual applications in the dermatology match process; therefore, our study provides valuable insight for applicants interested in the impacts of the dual application. Overall, students should approach this option with mentors on an individual basis but ultimately should be aware of the concerns and mixed perceptions of the dual application process.
With regard to program signaling, previous research has shown that PSTs have a large impact on the chance of being granted an interview.12 In our study, we provide a comprehensive overview of advising regarding these signals. While mentors often responded that they did not have much experience advising in this domain—and it is too soon to tell the impact of this program signaling—many offered differing opinions. Many said they recommend that students give a gold signal to their 3 most desired programs regardless of home and away rotations and perceived competitiveness, which follows the guidelines issued by the APD; however, 19% recommend only giving silver signals to home and away rotation programs, as participation in those programs is considered a sufficient signal of interest. Additionally, about half of mentors recommended that students only apply where they signal, whereas 20% recommended applying to 10 to 15 programs beyond those signaled. Future studies should investigate the impact of PSTs on interview invitations once sufficient application cycles have occurred.
Study Limitations
This study was conducted via email to the APD listserve. The total number of faculty on this listserve is unknown; therefore, we do not know the total response rate of the survey. Additionally, we surveyed mentors in this listserve, who therefore receive more emails and overall correspondence about the dermatology match and may be more involved in these conversations. The mentors who responded to our survey may have a different approach and response to our various survey questions than a given mentor across the United States who did not respond to this survey. A final limitation of our study is that the survey responses a mentor gives may not fully match the advice that they give their students privately.
Conclusion
Our survey of dermatology mentors across the United States provides valuable insight into how mentors advise for a strong dermatology residency application. Mentors agreed on the importance of research during medical school, away rotations, strong letters of recommendation, and volunteerism and advocacy to promote a strong residency application. Important topics of disagreement include the decision for dermatology applicants to take a dedicated gap year in medical school, how to use tokens/signals effectively, and the dual application process. Our findings also underscore important application components that applicants and mentors should approach on an individual basis. Future studies should investigate the impact of signals/tokens on the match process as well as the utility of gap years and dual applications, working to standardize the advice applicants receive.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the match. Dermatol Online J. 2020;26:13030 /qt4604h1w4.
- Kolli SS, Feldman SR, Huang WW. The dermatology residency application process. Dermatol Online J. 2021;26:13030/qt4k1570vj.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202. doi:10.1001/archdermatol.2010.303
- Association of Professors of Dermatology Residency Program Directors Section Information Regarding the 2023-2024 Application Cycle. Published 2023. Accessed June 1, 2024. https://students-residents.aamc.org/media/12386/download
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:4.
- Wang JV, Keller M. Pressure to publish for residency applicants in dermatology. Dermatol Online J. 2016;22:13030/qt56x1t7ww.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230. doi:10.1111/ijd.15964
- Yeh C, Desai AD, Wassef C, et al. The importance of mentorship during research gap years for the dermatology residency match. Int J Dermatol. 2023;62:E209-E210. doi:10.1111/ijd.16084
- Zheng DX, Gallo Marin B, Mulligan KM, et al. Inequity concerns surrounding research years and the dermatology residency match. Int J Dermatol. 2022;61:E247-E248. doi:10.1111/ijd.16179
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatology residency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Cordova A, et al. Challenging the status quo: increasing diversity in dermatology. J Am Acad Dermatol. 2020;83:E421. doi:10.1016/j.jaad.2020.04.185
- Dirr MA, Brownstone N, Zakria D, et al. Dermatology match preference signaling tokens: impact and implications. Dermatol Surg. 2022;48:1367-1368. doi:10.1097/DSS.0000000000003645
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
A Nationwide Survey of Dermatology Faculty and Mentors on Their Advice for the Dermatology Match Process
PRACTICE POINTS
- Dermatology mentors should abide by Association of Professors of Dermatology guidelines when advising regarding signals and away rotations.
- Mentors agree with the utility of research during medical school, completing away rotations, and volunteering during medical school.
- There are differing opinions regarding the utility of a research year, program signaling, couples matching, and dual applying.