User login
For MD-IQ use only
Are Children Born Through ART at Higher Risk for Cancer?
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The Value of Early Education
Early education is right up there with motherhood and apple pie as unarguable positive concepts. How could exposing young children to a school-like atmosphere not be a benefit, particularly in communities dominated by socioeconomic challenges? While there are some questions about the value of playing Mozart to infants, early education in the traditional sense continues to be viewed as a key strategy for providing young children a preschool foundation on which a successful academic career can be built. Several oft-cited randomized controlled trials have fueled both private and public interest and funding.
However, a recent commentary published in Science suggests that all programs are “not unequivocally positive and much more research is needed.” “Worrisome results in Tennessee,” “Success in Boston,” and “Largely null results for Headstart” are just a few of the article’s section titles and convey a sense of the inconsistency the investigators found as they reviewed early education systems around the country.
While there may be some politicians who may attempt to use the results of this investigation as a reason to cancel public funding of underperforming early education programs, the authors avoid this baby-and-the-bathwater conclusion. Instead, they urge more rigorous research “to understand how effective programs can be designed and implemented.”
The kind of re-thinking and brainstorming these investigators suggest takes time. While we’re waiting for this process to gain traction, this might be a good time to consider some of the benefits of early education that we don’t usually consider when our focus is on academic metrics.
A recent paper in Children’s Health Care by investigators at the Boston University Medical Center and School of Medicine considered the diet of children attending preschool. Looking at the dietary records of more than 300 children attending 30 childcare centers, the researchers found that the children’s diets before arrival at daycare was less healthy than while they were in daycare. “The hour after pickup appeared to be the least healthful” of any of the time periods surveyed. Of course, we will all conjure up images of what this chaotic post-daycare pickup may look like and cut the harried parents and grandparents some slack when it comes to nutritional choices. However, the bottom line is that for the group of children surveyed being in preschool or daycare protected them from a less healthy diet they were being provided outside of school hours.
Our recent experience with pandemic-related school closures provides more evidence that being in school was superior to any remote experience academically. School-age children and adolescents gained weight when school closures were the norm. Play patterns for children shifted from outdoor play to indoor play — often dominated by more sedentary video games. Both fatal and non-fatal gun-related injuries surged during the pandemic and, by far, the majority of these occur in the home and not at school.
Stepping back to look at this broader picture that includes diet, physical activity, and safety — not to mention the benefits of socialization — leads one to arrive at the unfortunate conclusion that Of course there will be those who point to the belief that schools are petri dishes putting children at greater risk for respiratory infections. On the other hand, we must accept that schools haven’t proved to be a major factor in the spread of COVID that many had feared.
The authors of the study in Science are certainly correct in recommending a more thorough investigation into the academic benefits of preschool education. However, we must keep in mind that preschool offers an environment that can be a positive influence on young children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Early education is right up there with motherhood and apple pie as unarguable positive concepts. How could exposing young children to a school-like atmosphere not be a benefit, particularly in communities dominated by socioeconomic challenges? While there are some questions about the value of playing Mozart to infants, early education in the traditional sense continues to be viewed as a key strategy for providing young children a preschool foundation on which a successful academic career can be built. Several oft-cited randomized controlled trials have fueled both private and public interest and funding.
However, a recent commentary published in Science suggests that all programs are “not unequivocally positive and much more research is needed.” “Worrisome results in Tennessee,” “Success in Boston,” and “Largely null results for Headstart” are just a few of the article’s section titles and convey a sense of the inconsistency the investigators found as they reviewed early education systems around the country.
While there may be some politicians who may attempt to use the results of this investigation as a reason to cancel public funding of underperforming early education programs, the authors avoid this baby-and-the-bathwater conclusion. Instead, they urge more rigorous research “to understand how effective programs can be designed and implemented.”
The kind of re-thinking and brainstorming these investigators suggest takes time. While we’re waiting for this process to gain traction, this might be a good time to consider some of the benefits of early education that we don’t usually consider when our focus is on academic metrics.
A recent paper in Children’s Health Care by investigators at the Boston University Medical Center and School of Medicine considered the diet of children attending preschool. Looking at the dietary records of more than 300 children attending 30 childcare centers, the researchers found that the children’s diets before arrival at daycare was less healthy than while they were in daycare. “The hour after pickup appeared to be the least healthful” of any of the time periods surveyed. Of course, we will all conjure up images of what this chaotic post-daycare pickup may look like and cut the harried parents and grandparents some slack when it comes to nutritional choices. However, the bottom line is that for the group of children surveyed being in preschool or daycare protected them from a less healthy diet they were being provided outside of school hours.
Our recent experience with pandemic-related school closures provides more evidence that being in school was superior to any remote experience academically. School-age children and adolescents gained weight when school closures were the norm. Play patterns for children shifted from outdoor play to indoor play — often dominated by more sedentary video games. Both fatal and non-fatal gun-related injuries surged during the pandemic and, by far, the majority of these occur in the home and not at school.
Stepping back to look at this broader picture that includes diet, physical activity, and safety — not to mention the benefits of socialization — leads one to arrive at the unfortunate conclusion that Of course there will be those who point to the belief that schools are petri dishes putting children at greater risk for respiratory infections. On the other hand, we must accept that schools haven’t proved to be a major factor in the spread of COVID that many had feared.
The authors of the study in Science are certainly correct in recommending a more thorough investigation into the academic benefits of preschool education. However, we must keep in mind that preschool offers an environment that can be a positive influence on young children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Early education is right up there with motherhood and apple pie as unarguable positive concepts. How could exposing young children to a school-like atmosphere not be a benefit, particularly in communities dominated by socioeconomic challenges? While there are some questions about the value of playing Mozart to infants, early education in the traditional sense continues to be viewed as a key strategy for providing young children a preschool foundation on which a successful academic career can be built. Several oft-cited randomized controlled trials have fueled both private and public interest and funding.
However, a recent commentary published in Science suggests that all programs are “not unequivocally positive and much more research is needed.” “Worrisome results in Tennessee,” “Success in Boston,” and “Largely null results for Headstart” are just a few of the article’s section titles and convey a sense of the inconsistency the investigators found as they reviewed early education systems around the country.
While there may be some politicians who may attempt to use the results of this investigation as a reason to cancel public funding of underperforming early education programs, the authors avoid this baby-and-the-bathwater conclusion. Instead, they urge more rigorous research “to understand how effective programs can be designed and implemented.”
The kind of re-thinking and brainstorming these investigators suggest takes time. While we’re waiting for this process to gain traction, this might be a good time to consider some of the benefits of early education that we don’t usually consider when our focus is on academic metrics.
A recent paper in Children’s Health Care by investigators at the Boston University Medical Center and School of Medicine considered the diet of children attending preschool. Looking at the dietary records of more than 300 children attending 30 childcare centers, the researchers found that the children’s diets before arrival at daycare was less healthy than while they were in daycare. “The hour after pickup appeared to be the least healthful” of any of the time periods surveyed. Of course, we will all conjure up images of what this chaotic post-daycare pickup may look like and cut the harried parents and grandparents some slack when it comes to nutritional choices. However, the bottom line is that for the group of children surveyed being in preschool or daycare protected them from a less healthy diet they were being provided outside of school hours.
Our recent experience with pandemic-related school closures provides more evidence that being in school was superior to any remote experience academically. School-age children and adolescents gained weight when school closures were the norm. Play patterns for children shifted from outdoor play to indoor play — often dominated by more sedentary video games. Both fatal and non-fatal gun-related injuries surged during the pandemic and, by far, the majority of these occur in the home and not at school.
Stepping back to look at this broader picture that includes diet, physical activity, and safety — not to mention the benefits of socialization — leads one to arrive at the unfortunate conclusion that Of course there will be those who point to the belief that schools are petri dishes putting children at greater risk for respiratory infections. On the other hand, we must accept that schools haven’t proved to be a major factor in the spread of COVID that many had feared.
The authors of the study in Science are certainly correct in recommending a more thorough investigation into the academic benefits of preschool education. However, we must keep in mind that preschool offers an environment that can be a positive influence on young children.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Prenatal Antibiotics May Increase Seborrheic Dermatitis Risk in Babies
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
, but this association was not as strong for childhood-onset SD.
The findings come from a large analysis of data from the United Kingdom that was presented during a late-breaking abstract session at the annual meeting of the Society for Investigative Dermatology.
SD is a common skin disease “that shares similarities with atopic dermatitis or atopic eczema as both are prevalent inflammatory skin diseases that can present with a chronic relapsing, remitting course,” the study’s corresponding author Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview. “Like atopic dermatitis, the pathophysiology of seborrheic dermatitis is thought to be complex and involves an interplay between genetics, immune dysregulation, and alterations in lipid composition and the skin microbiome, among others.”
In a previous study, she and colleagues showed that exposure to antibiotics both in utero and during the first 90 days of life increases the risk for atopic dermatitis (AD) in children, with risk being highest with exposure to penicillin even among children whose mothers did not have a history of AD.
For the current study, the researchers drew from a large electronic medical records database in the United Kingdom to perform a prospective cohort analysis of mother-child pairs that used proportional hazards models to examine the association between maternal in utero antibiotic exposure and SD in the child. The population included 1,023,140 children with linked maternal data who were followed for a mean of 10.2 years, which amounts to more than 10-million-person years of data. At baseline, the mean age of mothers was 28 years, 3% had SD, 14% had AD, and 51% of the children were male.
In unadjusted analyses, mothers with SD were more likely to receive an antibiotic during pregnancy than were those who did not have SD (odds ratio [OR], 1.42; 95% CI, 1.39-1.46). In addition, maternal in utero exposure to any antibiotic was associated with an increased risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) but less for childhood-onset SD (OR, 1.26; 95% CI, 1.20-1.32). “This effect changed little after adjustment and was still observed if mothers with SD and their babies were excluded,” the authors wrote in their poster abstract.
Any penicillin exposure during pregnancy increased the likelihood of a child having SD (OR, 1.54; 95% CI, 1.50-1.59), with the greater risk for infantile SD (OR, 1.70; 95% CI, 1.65-1.76) than for childhood-onset SD (OR, 1.25; 95% CI, 1.18-1.32). “The trimester of the in utero penicillin exposure did not seem to affect the association with SD,” the authors wrote. The risk was also increased with cephalosporin exposure but was less for sulfonamides and not for childhood-onset SD.
“We observed that antibiotic exposure in utero was primarily associated with an increased risk of infantile SD regardless of the mother’s history of SD, but this association was not as strong for childhood-onset SD,” Dr. Chiesa Fuxench said. “This would suggest that in utero exposure to antibiotics, particularly penicillin, may have its greatest effect on the colonization of skin microbiota in the newborn period leading to the development of infantile SD. Aside from seeking to improve our understanding of the pathophysiology of SD, our findings also suggest that infantile SD and childhood-onset SD may be separate entities with different risk factors, a hypothesis that needs to be further studied.”
She acknowledged certain limitations of the analysis, including the potential for unrecorded diagnoses of SD or misclassified cases in the database. For example, AD and psoriasis “may appear clinically like SD,” she said, although they performed sensitivity analysis excluding patients with these diagnoses and found similar results. In addition, there is the possibility that not all antibiotic exposures were captured in this database, and data on antibiotic exposure may be missing, she added.
Dr. Chiesa Fuxench disclosed that she received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, Vanda, and Incyte for work related to AD and from Menlo Therapeutics and Galderma for work related to prurigo nodularis. She has served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer and from Beiersdorf for work related to skin cancer and sun protection.
A version of this article appeared on Medscape.com .
FROM SID 2024
The Challenges of Delivering Allergen Immunotherapy in the Military Health System
Allergic rhinoconjunctivitis causes onerous symptoms of sneezing, rhinorrhea, postnasal drip, nasal congestion, and itchy, watery eyes. It is a common condition that affects 10% to 25% of the US population and up to 23% of military members with increased symptoms during deployments.1-3 Allergen immunotherapy (AIT), commonly known as allergy shots, is an effective treatment for allergic rhinoconjunctivitis, especially for patients whose symptoms are not controlled by allergy medications.4 Many military personnel who would like to receive AIT cannot continue with their immunotherapy because of frequent moves, deployments, and temporary duty assignments. This case report highlights the difficulty of managing AIT in the Military Health System.
Case Presentation
A 34-year-old active-duty US Air Force male surgeon with a medical history of allergic rhinoconjunctivitis was referred to the allergy clinic for evaluation and consideration of AIT. His symptoms included rhinorrhea, sneezing, nasal congestion, and itchy, watery eyes. The symptoms had been present for several years, occurring predominantly in the spring and fall, but also perennially when exposed to animals such as cats, dogs, and horses. The patient was raised on a ranch where he was exposed to these animals.
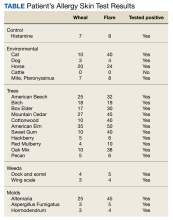
The patient had prior skin testing at the University of Nebraska Medical Center (UNMC) for aeroallergens and was positive for trees, grasses, weeds, molds, dust mites, cats, dogs, and horses. He received AIT at UNMC with great success for18 months. Regrettably, the patient discontinued AIT following a military move to Keesler Air Force Base in Mississippi. The patient’s examination was notable for injected conjunctiva, nasal mucosa edema, and a cobblestone throat. His symptoms were not alleviated with oral cetirizine and nasal fluticasone.
His skin testing was positive for trees, weeds, mold, cats, dogs, dust mites, and horsehair (Table). The risks and benefits of AIT were discussed with the patient, who elected to proceed with restarting AIT and received counseling on aeroallergen avoidance. The patient was unable to continue AIT at Keesler Medical Center because of a military deployment.
Discussion
There are several barriers to receiving AIT for active-duty patients with allergies. Due to previous skin test extracts, our patient had become desensitized to them. Though he had received aeroallergen immunotherapy with success for 18 months, the patients had to restart the build up phase of AIT due to a military-related move.
For patients to benefit from AIT, they must build up and maintain their immunotherapy injections for at least 3 to 5 years.4 The build-up period of immunotherapy lasts about 3 to 4 months. Patients typically receive weekly injections until they reach a maintenance immunotherapy dose of 0.5 mL of a 1:1 concentration ratio.4
Frequent deployments or temporary duty assignments are other barriers to AIT for active-duty patients. AIT is not usually given on deployments or temporary duty assignments unless the patient is located near a major military medical center. The US Air Force and Army operate allergy extender clinics at smaller bases and overseas locations to facilitate the maintenance of immunotherapy for military patients. Primary care physicians act as allergy extenders. These smaller allergy clinics are supervised by regional allergists at major military medical centers via telehealth and electronic/telephonic communication. These allergy clinics are not more widely available because there are not enough allergists and allergy medical technicians.
Allergen immunotherapy is not standardized, meaning civilian allergists use different aeroallergen immunotherapy formulations. While AIT is standardized in the US military through the Extract Laboratory Management System (ELMS), many active-duty patients are evaluated by civilian allergists in the TRICARE system who do not use ELMS, and when they move, AIT is not maintained.
Because up to 25% of active-duty personnel suffer from allergic rhinoconjunctivitis and AIT is not administered in many deployed settings, this issue could affect mission readiness and capabilities.3-6 These personnel may suffer from frequent and severe nasal and ocular allergy symptoms without being able to continue AIT. There is the potential for adverse effects on the military missions because of these impaired military personnel.5,6
Potential steps to improve the availability of allergen immunotherapy in the deployed setting include training deployed physicians, medical technicians, and other health care practitioners in administering and treating AIT so deployed personnel can receive therapy. Additionally, AIT should be standardized and ordered via the ELMS. Civilian allergists should be highly encouraged to use ELMS. This would create standardization of AIT for all active-duty allergy patients. The allergy extender system could be expanded to all military treatment facilities to provide easy access to allergen immunotherapy. The US Navy has the fewest allergists and allergy extenders, and would need to expand its network of allergy extenders to provide AIT at its health care facilities.
Conclusions
We present an active-duty servicemember with allergic rhinoconjunctivitis to trees, grasses, weeds, cats, dogs, dust mites, mold, and horses who had intermittent therapy that was interrupted by deployments. Our case highlights the difficulty of managing AIT in the military health system due to frequent moves, deployments, and temporary duty assignments. We also suggest steps that could help expand AIT for military personnel, including those deployed internationally.
1. Maciag MC, Phipatanakul W. Update on indoor allergens and their impact on pediatric asthma. Ann Allergy Asthma Immunol. 2022;128(6):652-658. doi:10.1016/j.anai.2022.02.009
2. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225-1232.
3. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172:1264–1269. doi:10.7205/milmed.172.12.1264
4. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. [published correction appears in J Allergy Clin Immunol. 2011 Mar;127(3):840]. J Allergy Clin Immunol. 2011;127(1 Suppl):S1-S55. doi:10.1016/j.jaci.2010.09.034
5. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. Increased allergic rhinitis rates among U.S. military personnel after deployment to the Persian Gulf. J Allergy Clin Immunol. 2008;121,S230. doi:10.1016/j.jaci.2007.12.909
6. Garshick E, Abraham JH, Baird CP, Ciminera P, et al. Respiratory ealth after military service in Southwest Asia and Afghanistan. An official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2019;16(8):e1-e16. doi:10.1513/AnnalsATS.201904-344WS
Allergic rhinoconjunctivitis causes onerous symptoms of sneezing, rhinorrhea, postnasal drip, nasal congestion, and itchy, watery eyes. It is a common condition that affects 10% to 25% of the US population and up to 23% of military members with increased symptoms during deployments.1-3 Allergen immunotherapy (AIT), commonly known as allergy shots, is an effective treatment for allergic rhinoconjunctivitis, especially for patients whose symptoms are not controlled by allergy medications.4 Many military personnel who would like to receive AIT cannot continue with their immunotherapy because of frequent moves, deployments, and temporary duty assignments. This case report highlights the difficulty of managing AIT in the Military Health System.
Case Presentation
A 34-year-old active-duty US Air Force male surgeon with a medical history of allergic rhinoconjunctivitis was referred to the allergy clinic for evaluation and consideration of AIT. His symptoms included rhinorrhea, sneezing, nasal congestion, and itchy, watery eyes. The symptoms had been present for several years, occurring predominantly in the spring and fall, but also perennially when exposed to animals such as cats, dogs, and horses. The patient was raised on a ranch where he was exposed to these animals.
The patient had prior skin testing at the University of Nebraska Medical Center (UNMC) for aeroallergens and was positive for trees, grasses, weeds, molds, dust mites, cats, dogs, and horses. He received AIT at UNMC with great success for18 months. Regrettably, the patient discontinued AIT following a military move to Keesler Air Force Base in Mississippi. The patient’s examination was notable for injected conjunctiva, nasal mucosa edema, and a cobblestone throat. His symptoms were not alleviated with oral cetirizine and nasal fluticasone.
His skin testing was positive for trees, weeds, mold, cats, dogs, dust mites, and horsehair (Table). The risks and benefits of AIT were discussed with the patient, who elected to proceed with restarting AIT and received counseling on aeroallergen avoidance. The patient was unable to continue AIT at Keesler Medical Center because of a military deployment.
Discussion
There are several barriers to receiving AIT for active-duty patients with allergies. Due to previous skin test extracts, our patient had become desensitized to them. Though he had received aeroallergen immunotherapy with success for 18 months, the patients had to restart the build up phase of AIT due to a military-related move.
For patients to benefit from AIT, they must build up and maintain their immunotherapy injections for at least 3 to 5 years.4 The build-up period of immunotherapy lasts about 3 to 4 months. Patients typically receive weekly injections until they reach a maintenance immunotherapy dose of 0.5 mL of a 1:1 concentration ratio.4
Frequent deployments or temporary duty assignments are other barriers to AIT for active-duty patients. AIT is not usually given on deployments or temporary duty assignments unless the patient is located near a major military medical center. The US Air Force and Army operate allergy extender clinics at smaller bases and overseas locations to facilitate the maintenance of immunotherapy for military patients. Primary care physicians act as allergy extenders. These smaller allergy clinics are supervised by regional allergists at major military medical centers via telehealth and electronic/telephonic communication. These allergy clinics are not more widely available because there are not enough allergists and allergy medical technicians.
Allergen immunotherapy is not standardized, meaning civilian allergists use different aeroallergen immunotherapy formulations. While AIT is standardized in the US military through the Extract Laboratory Management System (ELMS), many active-duty patients are evaluated by civilian allergists in the TRICARE system who do not use ELMS, and when they move, AIT is not maintained.
Because up to 25% of active-duty personnel suffer from allergic rhinoconjunctivitis and AIT is not administered in many deployed settings, this issue could affect mission readiness and capabilities.3-6 These personnel may suffer from frequent and severe nasal and ocular allergy symptoms without being able to continue AIT. There is the potential for adverse effects on the military missions because of these impaired military personnel.5,6
Potential steps to improve the availability of allergen immunotherapy in the deployed setting include training deployed physicians, medical technicians, and other health care practitioners in administering and treating AIT so deployed personnel can receive therapy. Additionally, AIT should be standardized and ordered via the ELMS. Civilian allergists should be highly encouraged to use ELMS. This would create standardization of AIT for all active-duty allergy patients. The allergy extender system could be expanded to all military treatment facilities to provide easy access to allergen immunotherapy. The US Navy has the fewest allergists and allergy extenders, and would need to expand its network of allergy extenders to provide AIT at its health care facilities.
Conclusions
We present an active-duty servicemember with allergic rhinoconjunctivitis to trees, grasses, weeds, cats, dogs, dust mites, mold, and horses who had intermittent therapy that was interrupted by deployments. Our case highlights the difficulty of managing AIT in the military health system due to frequent moves, deployments, and temporary duty assignments. We also suggest steps that could help expand AIT for military personnel, including those deployed internationally.
Allergic rhinoconjunctivitis causes onerous symptoms of sneezing, rhinorrhea, postnasal drip, nasal congestion, and itchy, watery eyes. It is a common condition that affects 10% to 25% of the US population and up to 23% of military members with increased symptoms during deployments.1-3 Allergen immunotherapy (AIT), commonly known as allergy shots, is an effective treatment for allergic rhinoconjunctivitis, especially for patients whose symptoms are not controlled by allergy medications.4 Many military personnel who would like to receive AIT cannot continue with their immunotherapy because of frequent moves, deployments, and temporary duty assignments. This case report highlights the difficulty of managing AIT in the Military Health System.
Case Presentation
A 34-year-old active-duty US Air Force male surgeon with a medical history of allergic rhinoconjunctivitis was referred to the allergy clinic for evaluation and consideration of AIT. His symptoms included rhinorrhea, sneezing, nasal congestion, and itchy, watery eyes. The symptoms had been present for several years, occurring predominantly in the spring and fall, but also perennially when exposed to animals such as cats, dogs, and horses. The patient was raised on a ranch where he was exposed to these animals.
The patient had prior skin testing at the University of Nebraska Medical Center (UNMC) for aeroallergens and was positive for trees, grasses, weeds, molds, dust mites, cats, dogs, and horses. He received AIT at UNMC with great success for18 months. Regrettably, the patient discontinued AIT following a military move to Keesler Air Force Base in Mississippi. The patient’s examination was notable for injected conjunctiva, nasal mucosa edema, and a cobblestone throat. His symptoms were not alleviated with oral cetirizine and nasal fluticasone.
His skin testing was positive for trees, weeds, mold, cats, dogs, dust mites, and horsehair (Table). The risks and benefits of AIT were discussed with the patient, who elected to proceed with restarting AIT and received counseling on aeroallergen avoidance. The patient was unable to continue AIT at Keesler Medical Center because of a military deployment.
Discussion
There are several barriers to receiving AIT for active-duty patients with allergies. Due to previous skin test extracts, our patient had become desensitized to them. Though he had received aeroallergen immunotherapy with success for 18 months, the patients had to restart the build up phase of AIT due to a military-related move.
For patients to benefit from AIT, they must build up and maintain their immunotherapy injections for at least 3 to 5 years.4 The build-up period of immunotherapy lasts about 3 to 4 months. Patients typically receive weekly injections until they reach a maintenance immunotherapy dose of 0.5 mL of a 1:1 concentration ratio.4
Frequent deployments or temporary duty assignments are other barriers to AIT for active-duty patients. AIT is not usually given on deployments or temporary duty assignments unless the patient is located near a major military medical center. The US Air Force and Army operate allergy extender clinics at smaller bases and overseas locations to facilitate the maintenance of immunotherapy for military patients. Primary care physicians act as allergy extenders. These smaller allergy clinics are supervised by regional allergists at major military medical centers via telehealth and electronic/telephonic communication. These allergy clinics are not more widely available because there are not enough allergists and allergy medical technicians.
Allergen immunotherapy is not standardized, meaning civilian allergists use different aeroallergen immunotherapy formulations. While AIT is standardized in the US military through the Extract Laboratory Management System (ELMS), many active-duty patients are evaluated by civilian allergists in the TRICARE system who do not use ELMS, and when they move, AIT is not maintained.
Because up to 25% of active-duty personnel suffer from allergic rhinoconjunctivitis and AIT is not administered in many deployed settings, this issue could affect mission readiness and capabilities.3-6 These personnel may suffer from frequent and severe nasal and ocular allergy symptoms without being able to continue AIT. There is the potential for adverse effects on the military missions because of these impaired military personnel.5,6
Potential steps to improve the availability of allergen immunotherapy in the deployed setting include training deployed physicians, medical technicians, and other health care practitioners in administering and treating AIT so deployed personnel can receive therapy. Additionally, AIT should be standardized and ordered via the ELMS. Civilian allergists should be highly encouraged to use ELMS. This would create standardization of AIT for all active-duty allergy patients. The allergy extender system could be expanded to all military treatment facilities to provide easy access to allergen immunotherapy. The US Navy has the fewest allergists and allergy extenders, and would need to expand its network of allergy extenders to provide AIT at its health care facilities.
Conclusions
We present an active-duty servicemember with allergic rhinoconjunctivitis to trees, grasses, weeds, cats, dogs, dust mites, mold, and horses who had intermittent therapy that was interrupted by deployments. Our case highlights the difficulty of managing AIT in the military health system due to frequent moves, deployments, and temporary duty assignments. We also suggest steps that could help expand AIT for military personnel, including those deployed internationally.
1. Maciag MC, Phipatanakul W. Update on indoor allergens and their impact on pediatric asthma. Ann Allergy Asthma Immunol. 2022;128(6):652-658. doi:10.1016/j.anai.2022.02.009
2. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225-1232.
3. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172:1264–1269. doi:10.7205/milmed.172.12.1264
4. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. [published correction appears in J Allergy Clin Immunol. 2011 Mar;127(3):840]. J Allergy Clin Immunol. 2011;127(1 Suppl):S1-S55. doi:10.1016/j.jaci.2010.09.034
5. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. Increased allergic rhinitis rates among U.S. military personnel after deployment to the Persian Gulf. J Allergy Clin Immunol. 2008;121,S230. doi:10.1016/j.jaci.2007.12.909
6. Garshick E, Abraham JH, Baird CP, Ciminera P, et al. Respiratory ealth after military service in Southwest Asia and Afghanistan. An official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2019;16(8):e1-e16. doi:10.1513/AnnalsATS.201904-344WS
1. Maciag MC, Phipatanakul W. Update on indoor allergens and their impact on pediatric asthma. Ann Allergy Asthma Immunol. 2022;128(6):652-658. doi:10.1016/j.anai.2022.02.009
2. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351(9111):1225-1232.
3. Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172:1264–1269. doi:10.7205/milmed.172.12.1264
4. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. [published correction appears in J Allergy Clin Immunol. 2011 Mar;127(3):840]. J Allergy Clin Immunol. 2011;127(1 Suppl):S1-S55. doi:10.1016/j.jaci.2010.09.034
5. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. Increased allergic rhinitis rates among U.S. military personnel after deployment to the Persian Gulf. J Allergy Clin Immunol. 2008;121,S230. doi:10.1016/j.jaci.2007.12.909
6. Garshick E, Abraham JH, Baird CP, Ciminera P, et al. Respiratory ealth after military service in Southwest Asia and Afghanistan. An official American Thoracic Society Workshop report. Ann Am Thorac Soc. 2019;16(8):e1-e16. doi:10.1513/AnnalsATS.201904-344WS
Bridging the Gap Between Inpatient and Outpatient Care
The Olin E. Teague Veterans’ Center (OETVC) in Temple, Texas, is a teaching hospital with 189 beds that provides patients access to medical, surgical, and specialty care. In 2022, 116,359 veterans received care at OETVC and 5393 inpatient admissions were noted. The inpatient ward consists of 3 teaching teams staffed by an attending physician, a second-year internal medicine resident, and 2 to 3 interns while hospitalists staff the 3 nonteaching teams. OETVC residents receive training on both routine and complex medical problems.
Each day, teaching teams discharge patients. With the complexity of discharges, there is always a risk of patients not following up with their primary care physicians, potential issues with filling medications, confusion about new medication regiments, and even potential postdischarge questions. In 1990, Holloway and colleagues evaluated potential risk factors for readmission among veterans. This study found that discharge from a geriatrics or intermediate care bed, chronic disease diagnosis, ≥ 2 procedures performed, increasing age, and distance from a veterans affairs medical center were risk factors.1
Several community hospital studies have evaluated readmission risk factors. One from 2000 noted that patients with more hospitalizations, lower mental health function, a diagnosis of chronic obstructive pulmonary disorder, and increased satisfaction with access to emergency care were associated with increased readmission in 90 days.2 Due to the readmission risks, OETVC decided to construct a program that would help these patients successfully transition from inpatient to outpatient care while establishing means to discuss their care with a physician for reassurance and guidance.
TRANSITION OF CARE PROGRAM
Transition of care programs have been implemented and evaluated in many institutions. A 2017 systematic review of transition of care programs supported the use of tailored discharge planning and postdischarge phone calls to reduce hospital readmission, noting that 6 studies demonstrated a statistically significant reduction in 30-day readmission rate.3 Another study found that pharmacy involvement in the transition of care reduced medication-related problems following discharge.4
Program Goals
The foundational goal of our program was to bridge the gap between inpatient and outpatient medicine. We hoped to improve patient adherence with their discharge regimens, improve access to primary care physicians, and improve discharge follow-up. Since hospitalization can be overwhelming, we hoped to capture potential barriers to medical care postdischarge when patients return home while decreasing hospital readmissions. Our second- and third-year resident physicians spend as much time as needed going through the patient’s course of illness throughout their hospitalization and treatment plans to ensure their understanding and potential success.
This program benefits residents by providing medical education and patient communication opportunities. Residents must review the patient’s clinical trajectory before calling them. In this process, residents develop an understanding of routine and complex illness scripts, or pathways of common illnesses. They also prepare for potential questions about the hospitalization, new medications, and follow-up care. Lastly, residents can focus on communication skills. Without the time pressures of returning to a busy rotation, the residents spend as much time discussing the hospital course and ensuring patient understanding as needed.
Program Description
At the beginning of each week, second- and third-year residents review the list of discharges from the 3 teaching teams. The list is generated by a medical service management analyst. The residents review patient records for inpatient services, laboratory results, medication changes, and proposed follow-up plans designed by the admission team prior to their phone call. The resident is also responsible for reviewing and reconciling discharge instructions and orders. Then, the resident calls the patient and reviews their hospitalization. If a patient does not answer, the resident leaves a voicemail that complies with the Health Insurance Portability and Accountability Act.
When patients answer the call, the resident follows a script (Appendix). Residents are encouraged to ask patients open-ended questions and address any new needs. They also discuss changes in symptoms, medications, functional status, and remind the patient about follow-up appointments. If imaging or specific orders were missed at discharge, the residents notify the chief resident, lead hospitalist, or deputy associate chief of staff for medical service. If additional laboratory tests need to be ordered, the resident devises a follow-up plan. If needed, specialty referrals can be placed. When residents feel there are multiple items that need to be addressed or if they notice any major concerns, they can recommend the patient present to the emergency department for evaluation. The chief resident, lead hospitalist, and deputy associate chair for medical service are available to assist with discussions about complex medical situations or new concerning symptoms. Residents document their encounters in the Computerized Patient Record System health record and any tests that need follow-up. This differs from the standard of care follow-up programs, which are conducted by primary care medicine nurses and do not fully discuss the hospitalization.
Implementation
This program was implemented as a 1-week elective for interested residents and part of the clinic rotation. The internal medicine medical service analyst pulls all discharges on Friday, which are then provided to the residents. The residents on rotation work through the discharges and find teaching team patients to follow up with and call.
Findings
Implementation of this program has yielded many benefits. The reminder of the importance of a primary care appointment has motivated patients to continue following up on an outpatient basis. Residents were also able to capture lapses in patient understanding. Residents could answer forgotten questions and help patients understand their admission pathology without time pressures. Residents have identified patients with hypoglycemia due to changed insulin regimens, set up specialist follow-up appointments, and provided additional education facilitating adherence. Additionally, several residents have expressed satisfaction with the ability to practice their communication skills. Others appreciated contributing to future patient successes.
While the focus on this article has been to share the program description, we have tabulated preliminary data. In January 2023, there were 239 internal medicine admissions; 158 admissions (66%) were teaching team patients, and 97 patients (61%) were called by a resident and spoken to regarding their care. There were 24 teaching team readmissions within 30 days, and 10 (42%) received a follow-up phone call. Eighty-one admitted patients were treated by nonteaching teams, 10 (12%) of whom were readmissions. Comparing 30-day readmission rates, 10 nonteaching team patients (12%), 10 teaching team patients (6.3%) who talk to a resident in the transition of care program were readmitted, and 24 teaching team patients who did not talk to a resident (10%) were readmitted.
DISCUSSION
The OETVC transition of care program was planned, formulated, and implemented without modeling after any other projects or institutions. This program aimed to utilize our residents as resources for patients.
Transition of care is defined as steps taken in a clinical encounter to assist with the coordination and continuity of patient care transferring between locations or levels of care.5 A 2018 study evaluating the utility of transition of care programs on adults aged ≥ 60 years found a reduction in rehospitalization rates, increased use of primary care services, and potential reduction in home health usage.6
In 2021, Johns Hopkins University School of Medicine implemented a program after polling residents and discovering their awareness of gaps in the transition of care.7 In 2002, pharmacists evaluated the impact of follow-up telephone calls to recently hospitalized patients. This group of pharmacists found that these calls were associated with increased patient satisfaction, resolution of medication-related problems and fewer emergency department returns.8
Our program differs from other transition of care programs in that resident physicians made the follow-up calls to patients. Residents could address all aspects of medical care, including new symptoms, new prescriptions, adverse events, and risk factors for readmission, or order new imaging and medications when appropriate. In the program, residents called all patients discharged after receiving care within their team. Calls were not based on risk assessments. The residents were able to speak with 61% of discharged patients. When readmission rates were compared between patients who received a resident follow-up phone call and those who did not, patients receiving the resident phone call were readmitted at a lower rate: 6.3% vs 10%, respectively.
While our data suggest a potential trend of decreased readmission, more follow-up over a longer period may be needed. We believe this program can benefit patients and our model can act as a template for other institutions interested in starting their own programs.
Challenges
Although our process is efficient, there have been some challenges. The discharge is created by the medical service management analyst and then sent to the chief resident, but there was concern that the list could be missed if either individual was unavailable. The chairperson for the department of medicine and their secretary are now involved in the process. To reduce unanswered telephone calls, residents use OETVC phones. Health Insurance Portability and Accountability Act-compliant voicemails providing a time for a follow-up call were implemented. As a result, veterans have answered their phones more regularly and are more aware of calls. Orders are generally placed by the chief resident, lead hospitalist, or chair of the medical service to ensure follow-up because residents are on rotation for 1 week at a time. Access to a physician also allows patients to discuss items unrelated to their hospitalization, introducing new symptoms, or situations requiring a resident to act with limited data.
CONCLUSIONS
The transition of care follow-up program described in this article may be beneficial for both internal medicine residents and patients. Second- and third-year residents are developing a better understanding of the trajectory of many illnesses and are given the opportunity to retrospectively analyze what they would do differently based on knowledge gained from their chart reviews. They are also given the opportunity to work on communication skills and explain courses of illnesses to patients in an easy-to-understand format without time constraints. Patients now have access to a physician following discharge to discuss any concerns with their hospitalization, condition, and follow-up. This program will continue to address barriers to care and adapt to improve the success of care transitions.
1. Holloway JJ, Medendorp SV, Bromberg J. Risk factors for early readmission among veterans. Health Serv Res. 1990;25(1 Pt 2):213-237.
2. Smith DM, Giobbie-Hurder A, Weinberger M, et al. Predicting non-elective hospital readmissions: a multi-site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions. J Clin Epidemiol. 2000;53(11):1113-1118. doi:10.1016/s0895-4356(00)00236-5
3. Kamermayer AK, Leasure AR, Anderson L. The Effectiveness of Transitions-of-Care Interventions in Reducing Hospital Readmissions and Mortality: A Systematic Review. Dimens Crit Care Nurs. 2017;36(6):311-316. doi:10.1097/DCC.0000000000000266
4. Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: A before-After prospective study. PLoS One. 2019;14(3):e0213593. Published 2019 Mar 12. doi:10.1371/journal.pone.0213593
5. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. doi:10.1046/j.1532-5415.2003.51185.x
6. Weeks LE, Macdonald M, Helwig M, Bishop A, Martin-Misener R, Iduye D. The impact of transitional care programs on health services utilization among community-dwelling older adults and their caregivers: a systematic review protocol of quantitative evidence. JBI Database System Rev Implement Rep. 2016;14(3):26-34. doi:10.11124/JBISRIR-2016-2568
7. Sheikh F, Gathecha E, Arbaje AI, Christmas C. Internal Medicine Residents’ Views About Care Transitions: Results of an Educational Intervention. J Med Educ Curric Dev. 2021;8:2382120520988590. Published 2021 Jan 20. doi:10.1177/2382120520988590
8. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. doi:10.1016/s0011-5029(02)90031-3
The Olin E. Teague Veterans’ Center (OETVC) in Temple, Texas, is a teaching hospital with 189 beds that provides patients access to medical, surgical, and specialty care. In 2022, 116,359 veterans received care at OETVC and 5393 inpatient admissions were noted. The inpatient ward consists of 3 teaching teams staffed by an attending physician, a second-year internal medicine resident, and 2 to 3 interns while hospitalists staff the 3 nonteaching teams. OETVC residents receive training on both routine and complex medical problems.
Each day, teaching teams discharge patients. With the complexity of discharges, there is always a risk of patients not following up with their primary care physicians, potential issues with filling medications, confusion about new medication regiments, and even potential postdischarge questions. In 1990, Holloway and colleagues evaluated potential risk factors for readmission among veterans. This study found that discharge from a geriatrics or intermediate care bed, chronic disease diagnosis, ≥ 2 procedures performed, increasing age, and distance from a veterans affairs medical center were risk factors.1
Several community hospital studies have evaluated readmission risk factors. One from 2000 noted that patients with more hospitalizations, lower mental health function, a diagnosis of chronic obstructive pulmonary disorder, and increased satisfaction with access to emergency care were associated with increased readmission in 90 days.2 Due to the readmission risks, OETVC decided to construct a program that would help these patients successfully transition from inpatient to outpatient care while establishing means to discuss their care with a physician for reassurance and guidance.
TRANSITION OF CARE PROGRAM
Transition of care programs have been implemented and evaluated in many institutions. A 2017 systematic review of transition of care programs supported the use of tailored discharge planning and postdischarge phone calls to reduce hospital readmission, noting that 6 studies demonstrated a statistically significant reduction in 30-day readmission rate.3 Another study found that pharmacy involvement in the transition of care reduced medication-related problems following discharge.4
Program Goals
The foundational goal of our program was to bridge the gap between inpatient and outpatient medicine. We hoped to improve patient adherence with their discharge regimens, improve access to primary care physicians, and improve discharge follow-up. Since hospitalization can be overwhelming, we hoped to capture potential barriers to medical care postdischarge when patients return home while decreasing hospital readmissions. Our second- and third-year resident physicians spend as much time as needed going through the patient’s course of illness throughout their hospitalization and treatment plans to ensure their understanding and potential success.
This program benefits residents by providing medical education and patient communication opportunities. Residents must review the patient’s clinical trajectory before calling them. In this process, residents develop an understanding of routine and complex illness scripts, or pathways of common illnesses. They also prepare for potential questions about the hospitalization, new medications, and follow-up care. Lastly, residents can focus on communication skills. Without the time pressures of returning to a busy rotation, the residents spend as much time discussing the hospital course and ensuring patient understanding as needed.
Program Description
At the beginning of each week, second- and third-year residents review the list of discharges from the 3 teaching teams. The list is generated by a medical service management analyst. The residents review patient records for inpatient services, laboratory results, medication changes, and proposed follow-up plans designed by the admission team prior to their phone call. The resident is also responsible for reviewing and reconciling discharge instructions and orders. Then, the resident calls the patient and reviews their hospitalization. If a patient does not answer, the resident leaves a voicemail that complies with the Health Insurance Portability and Accountability Act.
When patients answer the call, the resident follows a script (Appendix). Residents are encouraged to ask patients open-ended questions and address any new needs. They also discuss changes in symptoms, medications, functional status, and remind the patient about follow-up appointments. If imaging or specific orders were missed at discharge, the residents notify the chief resident, lead hospitalist, or deputy associate chief of staff for medical service. If additional laboratory tests need to be ordered, the resident devises a follow-up plan. If needed, specialty referrals can be placed. When residents feel there are multiple items that need to be addressed or if they notice any major concerns, they can recommend the patient present to the emergency department for evaluation. The chief resident, lead hospitalist, and deputy associate chair for medical service are available to assist with discussions about complex medical situations or new concerning symptoms. Residents document their encounters in the Computerized Patient Record System health record and any tests that need follow-up. This differs from the standard of care follow-up programs, which are conducted by primary care medicine nurses and do not fully discuss the hospitalization.
Implementation
This program was implemented as a 1-week elective for interested residents and part of the clinic rotation. The internal medicine medical service analyst pulls all discharges on Friday, which are then provided to the residents. The residents on rotation work through the discharges and find teaching team patients to follow up with and call.
Findings
Implementation of this program has yielded many benefits. The reminder of the importance of a primary care appointment has motivated patients to continue following up on an outpatient basis. Residents were also able to capture lapses in patient understanding. Residents could answer forgotten questions and help patients understand their admission pathology without time pressures. Residents have identified patients with hypoglycemia due to changed insulin regimens, set up specialist follow-up appointments, and provided additional education facilitating adherence. Additionally, several residents have expressed satisfaction with the ability to practice their communication skills. Others appreciated contributing to future patient successes.
While the focus on this article has been to share the program description, we have tabulated preliminary data. In January 2023, there were 239 internal medicine admissions; 158 admissions (66%) were teaching team patients, and 97 patients (61%) were called by a resident and spoken to regarding their care. There were 24 teaching team readmissions within 30 days, and 10 (42%) received a follow-up phone call. Eighty-one admitted patients were treated by nonteaching teams, 10 (12%) of whom were readmissions. Comparing 30-day readmission rates, 10 nonteaching team patients (12%), 10 teaching team patients (6.3%) who talk to a resident in the transition of care program were readmitted, and 24 teaching team patients who did not talk to a resident (10%) were readmitted.
DISCUSSION
The OETVC transition of care program was planned, formulated, and implemented without modeling after any other projects or institutions. This program aimed to utilize our residents as resources for patients.
Transition of care is defined as steps taken in a clinical encounter to assist with the coordination and continuity of patient care transferring between locations or levels of care.5 A 2018 study evaluating the utility of transition of care programs on adults aged ≥ 60 years found a reduction in rehospitalization rates, increased use of primary care services, and potential reduction in home health usage.6
In 2021, Johns Hopkins University School of Medicine implemented a program after polling residents and discovering their awareness of gaps in the transition of care.7 In 2002, pharmacists evaluated the impact of follow-up telephone calls to recently hospitalized patients. This group of pharmacists found that these calls were associated with increased patient satisfaction, resolution of medication-related problems and fewer emergency department returns.8
Our program differs from other transition of care programs in that resident physicians made the follow-up calls to patients. Residents could address all aspects of medical care, including new symptoms, new prescriptions, adverse events, and risk factors for readmission, or order new imaging and medications when appropriate. In the program, residents called all patients discharged after receiving care within their team. Calls were not based on risk assessments. The residents were able to speak with 61% of discharged patients. When readmission rates were compared between patients who received a resident follow-up phone call and those who did not, patients receiving the resident phone call were readmitted at a lower rate: 6.3% vs 10%, respectively.
While our data suggest a potential trend of decreased readmission, more follow-up over a longer period may be needed. We believe this program can benefit patients and our model can act as a template for other institutions interested in starting their own programs.
Challenges
Although our process is efficient, there have been some challenges. The discharge is created by the medical service management analyst and then sent to the chief resident, but there was concern that the list could be missed if either individual was unavailable. The chairperson for the department of medicine and their secretary are now involved in the process. To reduce unanswered telephone calls, residents use OETVC phones. Health Insurance Portability and Accountability Act-compliant voicemails providing a time for a follow-up call were implemented. As a result, veterans have answered their phones more regularly and are more aware of calls. Orders are generally placed by the chief resident, lead hospitalist, or chair of the medical service to ensure follow-up because residents are on rotation for 1 week at a time. Access to a physician also allows patients to discuss items unrelated to their hospitalization, introducing new symptoms, or situations requiring a resident to act with limited data.
CONCLUSIONS
The transition of care follow-up program described in this article may be beneficial for both internal medicine residents and patients. Second- and third-year residents are developing a better understanding of the trajectory of many illnesses and are given the opportunity to retrospectively analyze what they would do differently based on knowledge gained from their chart reviews. They are also given the opportunity to work on communication skills and explain courses of illnesses to patients in an easy-to-understand format without time constraints. Patients now have access to a physician following discharge to discuss any concerns with their hospitalization, condition, and follow-up. This program will continue to address barriers to care and adapt to improve the success of care transitions.
The Olin E. Teague Veterans’ Center (OETVC) in Temple, Texas, is a teaching hospital with 189 beds that provides patients access to medical, surgical, and specialty care. In 2022, 116,359 veterans received care at OETVC and 5393 inpatient admissions were noted. The inpatient ward consists of 3 teaching teams staffed by an attending physician, a second-year internal medicine resident, and 2 to 3 interns while hospitalists staff the 3 nonteaching teams. OETVC residents receive training on both routine and complex medical problems.
Each day, teaching teams discharge patients. With the complexity of discharges, there is always a risk of patients not following up with their primary care physicians, potential issues with filling medications, confusion about new medication regiments, and even potential postdischarge questions. In 1990, Holloway and colleagues evaluated potential risk factors for readmission among veterans. This study found that discharge from a geriatrics or intermediate care bed, chronic disease diagnosis, ≥ 2 procedures performed, increasing age, and distance from a veterans affairs medical center were risk factors.1
Several community hospital studies have evaluated readmission risk factors. One from 2000 noted that patients with more hospitalizations, lower mental health function, a diagnosis of chronic obstructive pulmonary disorder, and increased satisfaction with access to emergency care were associated with increased readmission in 90 days.2 Due to the readmission risks, OETVC decided to construct a program that would help these patients successfully transition from inpatient to outpatient care while establishing means to discuss their care with a physician for reassurance and guidance.
TRANSITION OF CARE PROGRAM
Transition of care programs have been implemented and evaluated in many institutions. A 2017 systematic review of transition of care programs supported the use of tailored discharge planning and postdischarge phone calls to reduce hospital readmission, noting that 6 studies demonstrated a statistically significant reduction in 30-day readmission rate.3 Another study found that pharmacy involvement in the transition of care reduced medication-related problems following discharge.4
Program Goals
The foundational goal of our program was to bridge the gap between inpatient and outpatient medicine. We hoped to improve patient adherence with their discharge regimens, improve access to primary care physicians, and improve discharge follow-up. Since hospitalization can be overwhelming, we hoped to capture potential barriers to medical care postdischarge when patients return home while decreasing hospital readmissions. Our second- and third-year resident physicians spend as much time as needed going through the patient’s course of illness throughout their hospitalization and treatment plans to ensure their understanding and potential success.
This program benefits residents by providing medical education and patient communication opportunities. Residents must review the patient’s clinical trajectory before calling them. In this process, residents develop an understanding of routine and complex illness scripts, or pathways of common illnesses. They also prepare for potential questions about the hospitalization, new medications, and follow-up care. Lastly, residents can focus on communication skills. Without the time pressures of returning to a busy rotation, the residents spend as much time discussing the hospital course and ensuring patient understanding as needed.
Program Description
At the beginning of each week, second- and third-year residents review the list of discharges from the 3 teaching teams. The list is generated by a medical service management analyst. The residents review patient records for inpatient services, laboratory results, medication changes, and proposed follow-up plans designed by the admission team prior to their phone call. The resident is also responsible for reviewing and reconciling discharge instructions and orders. Then, the resident calls the patient and reviews their hospitalization. If a patient does not answer, the resident leaves a voicemail that complies with the Health Insurance Portability and Accountability Act.
When patients answer the call, the resident follows a script (Appendix). Residents are encouraged to ask patients open-ended questions and address any new needs. They also discuss changes in symptoms, medications, functional status, and remind the patient about follow-up appointments. If imaging or specific orders were missed at discharge, the residents notify the chief resident, lead hospitalist, or deputy associate chief of staff for medical service. If additional laboratory tests need to be ordered, the resident devises a follow-up plan. If needed, specialty referrals can be placed. When residents feel there are multiple items that need to be addressed or if they notice any major concerns, they can recommend the patient present to the emergency department for evaluation. The chief resident, lead hospitalist, and deputy associate chair for medical service are available to assist with discussions about complex medical situations or new concerning symptoms. Residents document their encounters in the Computerized Patient Record System health record and any tests that need follow-up. This differs from the standard of care follow-up programs, which are conducted by primary care medicine nurses and do not fully discuss the hospitalization.
Implementation
This program was implemented as a 1-week elective for interested residents and part of the clinic rotation. The internal medicine medical service analyst pulls all discharges on Friday, which are then provided to the residents. The residents on rotation work through the discharges and find teaching team patients to follow up with and call.
Findings
Implementation of this program has yielded many benefits. The reminder of the importance of a primary care appointment has motivated patients to continue following up on an outpatient basis. Residents were also able to capture lapses in patient understanding. Residents could answer forgotten questions and help patients understand their admission pathology without time pressures. Residents have identified patients with hypoglycemia due to changed insulin regimens, set up specialist follow-up appointments, and provided additional education facilitating adherence. Additionally, several residents have expressed satisfaction with the ability to practice their communication skills. Others appreciated contributing to future patient successes.
While the focus on this article has been to share the program description, we have tabulated preliminary data. In January 2023, there were 239 internal medicine admissions; 158 admissions (66%) were teaching team patients, and 97 patients (61%) were called by a resident and spoken to regarding their care. There were 24 teaching team readmissions within 30 days, and 10 (42%) received a follow-up phone call. Eighty-one admitted patients were treated by nonteaching teams, 10 (12%) of whom were readmissions. Comparing 30-day readmission rates, 10 nonteaching team patients (12%), 10 teaching team patients (6.3%) who talk to a resident in the transition of care program were readmitted, and 24 teaching team patients who did not talk to a resident (10%) were readmitted.
DISCUSSION
The OETVC transition of care program was planned, formulated, and implemented without modeling after any other projects or institutions. This program aimed to utilize our residents as resources for patients.
Transition of care is defined as steps taken in a clinical encounter to assist with the coordination and continuity of patient care transferring between locations or levels of care.5 A 2018 study evaluating the utility of transition of care programs on adults aged ≥ 60 years found a reduction in rehospitalization rates, increased use of primary care services, and potential reduction in home health usage.6
In 2021, Johns Hopkins University School of Medicine implemented a program after polling residents and discovering their awareness of gaps in the transition of care.7 In 2002, pharmacists evaluated the impact of follow-up telephone calls to recently hospitalized patients. This group of pharmacists found that these calls were associated with increased patient satisfaction, resolution of medication-related problems and fewer emergency department returns.8
Our program differs from other transition of care programs in that resident physicians made the follow-up calls to patients. Residents could address all aspects of medical care, including new symptoms, new prescriptions, adverse events, and risk factors for readmission, or order new imaging and medications when appropriate. In the program, residents called all patients discharged after receiving care within their team. Calls were not based on risk assessments. The residents were able to speak with 61% of discharged patients. When readmission rates were compared between patients who received a resident follow-up phone call and those who did not, patients receiving the resident phone call were readmitted at a lower rate: 6.3% vs 10%, respectively.
While our data suggest a potential trend of decreased readmission, more follow-up over a longer period may be needed. We believe this program can benefit patients and our model can act as a template for other institutions interested in starting their own programs.
Challenges
Although our process is efficient, there have been some challenges. The discharge is created by the medical service management analyst and then sent to the chief resident, but there was concern that the list could be missed if either individual was unavailable. The chairperson for the department of medicine and their secretary are now involved in the process. To reduce unanswered telephone calls, residents use OETVC phones. Health Insurance Portability and Accountability Act-compliant voicemails providing a time for a follow-up call were implemented. As a result, veterans have answered their phones more regularly and are more aware of calls. Orders are generally placed by the chief resident, lead hospitalist, or chair of the medical service to ensure follow-up because residents are on rotation for 1 week at a time. Access to a physician also allows patients to discuss items unrelated to their hospitalization, introducing new symptoms, or situations requiring a resident to act with limited data.
CONCLUSIONS
The transition of care follow-up program described in this article may be beneficial for both internal medicine residents and patients. Second- and third-year residents are developing a better understanding of the trajectory of many illnesses and are given the opportunity to retrospectively analyze what they would do differently based on knowledge gained from their chart reviews. They are also given the opportunity to work on communication skills and explain courses of illnesses to patients in an easy-to-understand format without time constraints. Patients now have access to a physician following discharge to discuss any concerns with their hospitalization, condition, and follow-up. This program will continue to address barriers to care and adapt to improve the success of care transitions.
1. Holloway JJ, Medendorp SV, Bromberg J. Risk factors for early readmission among veterans. Health Serv Res. 1990;25(1 Pt 2):213-237.
2. Smith DM, Giobbie-Hurder A, Weinberger M, et al. Predicting non-elective hospital readmissions: a multi-site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions. J Clin Epidemiol. 2000;53(11):1113-1118. doi:10.1016/s0895-4356(00)00236-5
3. Kamermayer AK, Leasure AR, Anderson L. The Effectiveness of Transitions-of-Care Interventions in Reducing Hospital Readmissions and Mortality: A Systematic Review. Dimens Crit Care Nurs. 2017;36(6):311-316. doi:10.1097/DCC.0000000000000266
4. Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: A before-After prospective study. PLoS One. 2019;14(3):e0213593. Published 2019 Mar 12. doi:10.1371/journal.pone.0213593
5. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. doi:10.1046/j.1532-5415.2003.51185.x
6. Weeks LE, Macdonald M, Helwig M, Bishop A, Martin-Misener R, Iduye D. The impact of transitional care programs on health services utilization among community-dwelling older adults and their caregivers: a systematic review protocol of quantitative evidence. JBI Database System Rev Implement Rep. 2016;14(3):26-34. doi:10.11124/JBISRIR-2016-2568
7. Sheikh F, Gathecha E, Arbaje AI, Christmas C. Internal Medicine Residents’ Views About Care Transitions: Results of an Educational Intervention. J Med Educ Curric Dev. 2021;8:2382120520988590. Published 2021 Jan 20. doi:10.1177/2382120520988590
8. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. doi:10.1016/s0011-5029(02)90031-3
1. Holloway JJ, Medendorp SV, Bromberg J. Risk factors for early readmission among veterans. Health Serv Res. 1990;25(1 Pt 2):213-237.
2. Smith DM, Giobbie-Hurder A, Weinberger M, et al. Predicting non-elective hospital readmissions: a multi-site study. Department of Veterans Affairs Cooperative Study Group on Primary Care and Readmissions. J Clin Epidemiol. 2000;53(11):1113-1118. doi:10.1016/s0895-4356(00)00236-5
3. Kamermayer AK, Leasure AR, Anderson L. The Effectiveness of Transitions-of-Care Interventions in Reducing Hospital Readmissions and Mortality: A Systematic Review. Dimens Crit Care Nurs. 2017;36(6):311-316. doi:10.1097/DCC.0000000000000266
4. Daliri S, Hugtenburg JG, Ter Riet G, et al. The effect of a pharmacy-led transitional care program on medication-related problems post-discharge: A before-After prospective study. PLoS One. 2019;14(3):e0213593. Published 2019 Mar 12. doi:10.1371/journal.pone.0213593
5. Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549-555. doi:10.1046/j.1532-5415.2003.51185.x
6. Weeks LE, Macdonald M, Helwig M, Bishop A, Martin-Misener R, Iduye D. The impact of transitional care programs on health services utilization among community-dwelling older adults and their caregivers: a systematic review protocol of quantitative evidence. JBI Database System Rev Implement Rep. 2016;14(3):26-34. doi:10.11124/JBISRIR-2016-2568
7. Sheikh F, Gathecha E, Arbaje AI, Christmas C. Internal Medicine Residents’ Views About Care Transitions: Results of an Educational Intervention. J Med Educ Curric Dev. 2021;8:2382120520988590. Published 2021 Jan 20. doi:10.1177/2382120520988590
8. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239-248. doi:10.1016/s0011-5029(02)90031-3
The ASCO Annual Meeting Starts This Week
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
From its origins in 1964, ASCO’s annual event has grown to become the world’s largest clinical oncology meeting, drawing attendees from across the globe.
More than 7000 abstracts were submitted for this year’s meeting a new record — and over 5000 were selected for presentation.
This year’s chair of the Annual Meeting Education Committee, Thomas William LeBlanc, MD, told us he has been attending the meeting since his training days more than a decade ago.
The event is “just incredibly empowering and energizing,” Dr. LeBlanc said, with opportunities to catch up with old colleagues and meet new ones, learn how far oncology has come and where it’s headed, and hear clinical pearls to take back the clinic.
This year’s theme, selected by ASCO President Lynn M. Schuchter, MD, is “The Art and Science of Cancer Care: From Comfort to Cure.”
Dr. LeBlanc, a blood cancer specialist at Duke University, Durham, North Carolina, said the theme has been woven throughout the abstract and educational sessions. Most sessions will have at least one presentation related to how we support people — not only “when we cure them but also when we can’t cure them,” he said.
Topics will include patient well-being, comfort measures, and survivorship. And for the first time the plenary session will include a palliative care abstract that addresses whether or not palliative care can be delivered effectively through telemedicine. The session is on Sunday, June 2.
Other potentially practice changing plenary abstracts tackle immunotherapy combinations for resectable melanoma, perioperative chemotherapy vs neoadjuvant chemoradiation for esophageal cancer, and osimertinib after definitive chemoradiotherapy for unresectable non–small cell lung cancer.
ASCO is piloting a slightly different format for research presentations this year. Instead of starting with context and background, speakers have been asked to present study results upfront as well as repeat them at the end of the talk. The reason behind the tweak is that engagement and retention tend to be better when results are presented upfront, instead of just at the end of a talk.
A popular session — ASCO Voices — has also been given a more central position in the conference: Friday, May 31. In this session, speakers will give short presentations about their personal experiences as providers, researchers, or patients.
ASCO Voices is a relatively recent addition to the meeting that has grown and gotten better. The talks are usually “very powerful narratives” that remind clinicians about “the importance of what they’re doing each day,” Dr. LeBlanc said.
Snippets of the talks will be played while people wait for sessions to begin at the meeting, so attendees who miss the Friday talks can still hear them.
In terms of educational sessions, Dr. LeBlanc highlighted two that might be of general interest to practicing oncologists: A joint ASCO/American Association for Cancer Research session entitled “Drugging the ‘Undruggable’ Target: Successes, Challenges, and the Road Ahead,” on Sunday morning and “Common Sense Oncology: Equity, Value, and Outcomes That Matter” on Monday morning.
As a blood cancer specialist, he said he is particularly interested in the topline results from the ASC4FIRST trial of asciminib, a newer kinase inhibitor, in newly diagnosed chronic myeloid leukemia, presented on Friday.
As in past years, this news organization will be on hand providing coverage with a dedicated team of reporters, editors, and videographers. Stop by our exhibit hall booth — number 26030 — to learn about the tools we offer to support your practice.
A version of this article appeared on Medscape.com .
ASTRO Releases New EBRT Guideline for Symptomatic Bone Mets
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
The guideline was needed to update previous recommendations and incorporate new high-quality evidence for the management of symptomatic bone metastases, Sara Alcorn, MD, PhD, of the University of Minnesota, Minneapolis, and colleagues wrote in Practical Radiation Oncology.
The focus was on the efficacy of EBRT in reducing pain, improving skeletal function, and enhancing quality of life, they wrote in the clinical practice guideline.
In developing their recommendations, the ASTRO task force reviewed evidence from 53 randomized controlled trials (RCTs) and 31 nonrandomized studies, and considered clinical experience.
Indications for Palliative Radiation
EBRT is strongly recommended for reducing pain from osseous metastasis and improving ambulatory status, sphincter function, and reducing pain in patients with spinal metastases causing compression of the spinal cord or cauda equina.
For patients with symptomatic bone metastases and an anticipated life expectancy of at least 4 weeks, EBRT is conditionally recommended to improve quality of life.
Implementation of other Treatments Alongside Palliative Radiation
Instead of RT alone, surgery with postoperative RT is conditionally recommended for patients with compression of the spinal cord or cauda equina.
Postoperative RT is strongly recommended for patients who have undergone surgery for non-spine bone metastases or spine metastases without involving spinal cord or cauda equina compression.
For patients with spinal bone metastases compressing the spinal cord or cauda equina, combining RT with dexamethasone is strongly recommended over RT alone.
Techniques, Dose-Fractionation, and Dose-Constraints for Initial Palliative Radiation
For patients with symptomatic bone metastases undergoing conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 3000 cGy in 10 fractions.
For patients with spinal bone metastases causing compression of the spinal cord or cauda equina who are not candidates for initial surgical decompression and are treated with conventional palliative RT, strongly recommended doses are 800 cGy in 1 fraction, 1600 cGy in 2 fractions, 2000 cGy in 5 fractions, or 3000 cGy in 10 fractions.
When selecting dose-fractionation, consider patient and disease factors such as prognosis and radiosensitivity, the authors wrote.
Highly conformal planning and delivery techniques, such as intensity-modulated radiation therapy, are conditionally recommended for patients with spinal bone metastases compressing the spinal cord or cauda equina who are receiving dose-escalated palliative RT.
The strongly recommended stereotactic body radiotherapy (SBRT) doses for patients with symptomatic bone metastases are 1200 to 1600 cGy in 1 fraction for non-spine metastases and 2400 cGy in 2 fractions for spine metastases. Other established SBRT dose and fractionation regimens with similar biologically effective doses may be considered based on patient tumor characteristics, normal tissue factors, and physician experience.
For patients with symptomatic bone metastases who have an ECOG PS of 0-2, are not undergoing surgical intervention, and have no neurological symptoms, SBRT is conditionally recommended over conventional palliative RT. Other factors to consider include life expectancy, tumor radiosensitivity, and metastatic disease burden, the guideline says.
Techniques, Dose-Fractionation, and Dose-Constraints for Palliative Reirradiation
For patients with spinal bone metastases requiring reirradiation to the same site, the strongly recommended conventional palliative RT regimens are 800 cGy in 1 fraction, 2000 cGy in 5 fractions, 2400 cGy in 6 fractions, or 2000 cGy in 8 fractions. When determining the RT dose-fractionation, consider the prior RT dose, time interval, and total spinal cord tolerance, the guideline says.
Treatment with SBRT is conditionally recommended for patients with spinal bone metastases needing reirradiation at the same site. When determining if SBRT is appropriate, consider patient factors such as urgency of treatment, prognosis, and radio-resistance. In addition, consider the prior RT dose, time interval, and total spinal cord tolerance when determining the RT dose-fractionation, the authors say.
The strongly recommended options for patients with symptomatic non-spine bone metastases needing reirradiation at the same site are single-fraction RT (800 cGy in 1 fraction) or multifraction conventional palliative RT (2000 cGy in 5 fractions or 2400 cGy in 6 fractions).
Impact of Techniques and Dose-fractionation on Quality of Life and Toxicity
For patients with bone metastases undergoing palliative radiation, it is strongly recommended to use a shared decision-making approach to determine the dose, fractionation, and supportive measures to optimize quality of life.
“Based on published data, the ASTRO task force’s recommendations inform best clinical practices on palliative RT for symptomatic bone metastases,” the guideline panelists said.
Limitations
While the guideline provides comprehensive recommendations, the panelists underscored the importance of individualized treatment approaches. Future research is needed to address gaps in evidence, particularly regarding advanced RT techniques and reirradiation strategies.
Guideline development was funded by ASTRO, with the systematic evidence review funded by the Patient-Centered Outcomes Research Institute. The panelists disclosed relationships with AstraZeneca, Elekta, Teladoc, and others.
FROM PRACTICAL RADIATION ONCOLOGY
Obesity and Cancer: Untangling a Complex Web
According to the Centers for Disease Control and Prevention (CDC), over 684,000 Americans are diagnosed with an “obesity-associated” cancer each year.
The incidence of many of these cancers has been rising in recent years, particularly among younger people — a trend that sits in contrast with the overall decline in cancers with no established relationship to excess weight, such as lung and skin cancers.
Is obesity the new smoking? Not exactly.
While about 42% of cancers — including common ones such as colorectal and postmenopausal breast cancers — are considered obesity-related, only about 8% of incident cancers are attributed to excess body weight. People often develop those diseases regardless of weight.
Although plenty of evidence points to excess body fat as a cancer risk factor, it’s unclear at what point excess weight has an effect. Is gaining weight later in life, for instance, better or worse for cancer risk than being overweight or obese from a young age?
There’s another glaring knowledge gap: Does losing weight at some point in adulthood change the picture? In other words, how many of those 684,000 diagnoses might have been prevented if people shed excess pounds?
When it comes to weight and cancer risk, “there’s a lot we don’t know,” said Jennifer W. Bea, PhD, associate professor, health promotion sciences, University of Arizona, Tucson.
A Consistent but Complicated Relationship
Given the growing incidence of obesity — which currently affects about 42% of US adults and 20% of children and teenagers — it’s no surprise that many studies have delved into the potential effects of excess weight on cancer rates.
Although virtually all the evidence comes from large cohort studies, leaving the cause-effect question open, certain associations keep showing up.
“What we know is that, consistently, a higher body mass index [BMI] — particularly in the obese category — leads to a higher risk of multiple cancers,” said Jeffrey A. Meyerhardt, MD, MPH, codirector, Colon and Rectal Cancer Center, Dana-Farber Cancer Institute, Boston.
In a widely cited report published in The New England Journal of Medicine in 2016, the International Agency for Research on Cancer (IARC) analyzed over 1000 epidemiologic studies on body fat and cancer. The agency pointed to over a dozen cancers, including some of the most common and deadly, linked to excess body weight.
That list includes esophageal adenocarcinoma and endometrial cancer — associated with the highest risk — along with kidney, liver, stomach (gastric cardia), pancreatic, colorectal, postmenopausal breast, gallbladder, ovarian, and thyroid cancers, plus multiple myeloma and meningioma. There’s also “limited” evidence linking excess weight to additional cancer types, including aggressive prostate cancer and certain head and neck cancers.
At the same time, Dr. Meyerhardt said, many of those same cancers are also associated with issues that lead to, or coexist with, overweight and obesity, including poor diet, lack of exercise, and metabolic conditions such as diabetes.
It’s a complicated web, and it’s likely, Dr. Meyerhardt said, that high BMI both directly affects cancer risk and is part of a “causal pathway” of other factors that do.
Regarding direct effects, preclinical research has pointed to multiple ways in which excess body fat could contribute to cancer, said Karen M. Basen-Engquist, PhD, MPH, professor, Division of Cancer Prevention and Population Services, The University of Texas MD Anderson Cancer Center, Houston.
One broad mechanism to help explain the obesity-cancer link is chronic systemic inflammation because excess fat tissue can raise levels of substances in the body, such as tumor necrosis factor alpha and interleukin 6, which fuel inflammation. Excess fat also contributes to hyperinsulinemia — too much insulin in the blood — which can help promote the growth and spread of tumor cells.
But the underlying reasons also appear to vary by cancer type, Dr. Basen-Engquist said. With hormonally driven cancer types, such as breast and endometrial, excess body fat may alter hormone levels in ways that spur tumor growth. Extra fat tissue may, for example, convert androgens into estrogens, which could help feed estrogen-dependent tumors.
That, Dr. Basen-Engquist noted, could be why excess weight is associated with postmenopausal, not premenopausal, breast cancer: Before menopause, body fat is a relatively minor contributor to estrogen levels but becomes more important after menopause.
How Big Is the Effect?
While more than a dozen cancers have been consistently linked to excess weight, the strength of those associations varies considerably.
Endometrial and esophageal cancers are two that stand out. In the 2016 IARC analysis, people with severe obesity had a seven-times greater risk for endometrial cancer and 4.8-times greater risk for esophageal adenocarcinoma vs people with a normal BMI.
With other cancers, the risk increases for those with severe obesity compared with a normal BMI were far more modest: 10% for ovarian cancer, 30% for colorectal cancer, and 80% for kidney and stomach cancers, for example. For postmenopausal breast cancer, every five-unit increase in BMI was associated with a 10% relative risk increase.
A 2018 study from the American Cancer Society, which attempted to estimate the proportion of cancers in the United States attributable to modifiable risk factors — including alcohol consumption, ultraviolet rays exposure, and physical inactivity — found that smoking accounted for the highest proportion of cancer cases by a wide margin (19%), but excess weight came in second (7.8%).
Again, weight appeared to play a bigger role in certain cancers than others: An estimated 60% of endometrial cancers were linked to excess weight, as were roughly one third of esophageal, kidney, and liver cancers. At the other end of the spectrum, just over 11% of breast, 5% of colorectal, and 4% of ovarian cancers were attributable to excess weight.
Even at the lower end, those rates could make a big difference on the population level, especially for groups with higher rates of obesity.
CDC data show that obesity-related cancers are rising among women younger than 50 years, most rapidly among Hispanic women, and some less common obesity-related cancers, such as stomach, thyroid and pancreatic, are also rising among Black individuals and Hispanic Americans.
Obesity may be one reason for growing cancer disparities, said Leah Ferrucci, PhD, MPH, assistant professor, epidemiology, Yale School of Public Health, New Haven, Connecticut. But, she added, the evidence is limited because Black individuals and Hispanic Americans are understudied.
When Do Extra Pounds Matter?
When it comes to cancer risk, at what point in life does excess weight, or weight gain, matter? Is the standard weight gain in middle age, for instance, as hazardous as being overweight or obese from a young age?
Some evidence suggests there’s no “safe” time for putting on excess pounds.
A recent meta-analysis concluded that weight gain at any point after age 18 years is associated with incremental increases in the risk for postmenopausal breast cancer. A 2023 study in JAMA Network Open found a similar pattern with colorectal and other gastrointestinal cancers: People who had sustained overweight or obesity from age 20 years through middle age faced an increased risk of developing those cancers after age 55 years.
The timing of weight gain didn’t seem to matter either. The same elevated risk held among people who were normal weight in their younger years but became overweight after age 55 years.
Those studies focused on later-onset disease. But, in recent years, experts have tracked a troubling rise in early-onset cancers — those diagnosed before age 50 years — particularly gastrointestinal cancers.
An obvious question, Dr. Meyerhardt said, is whether the growing prevalence of obesity among young people is partly to blame.
There’s some data to support that, he said. An analysis from the Nurses’ Health Study II found that women with obesity had double the risk for early-onset colorectal cancer as those with a normal BMI. And every 5-kg increase in weight after age 18 years was associated with a 9% increase in colorectal cancer risk.
But while obesity trends probably partly explain the rise in early-onset cancers, there is likely more to the story, Dr. Meyerhardt said.
“I think all of us who see an increasing number of patients under 50 with colorectal cancer know there’s a fair number who do not fit that [high BMI] profile,” he said. “There’s a fair number over 50 who don’t either.”
Does Weight Loss Help?
With all the evidence pointing to high BMI as a cancer risk factor, a logical conclusion is that weight loss should reduce that excess risk. However, Dr. Bea said, there’s actually little data to support that, and what exists comes from observational studies.
Some research has focused on people who had substantial weight loss after bariatric surgery, with encouraging results. A study published in JAMA found that among 5053 people who underwent bariatric surgery, 2.9% developed an obesity-related cancer over 10 years compared with 4.9% in the nonsurgery group.
Most people, however, aim for less dramatic weight loss, with the help of diet and exercise or sometimes medication. Some evidence shows that a modest degree of weight loss may lower the risks for postmenopausal breast and endometrial cancers.
A 2020 pooled analysis found, for instance, that among women aged ≥ 50 years, those who lost as little as 2.0-4.5 kg, or 4.4-10.0 pounds, and kept it off for 10 years had a lower risk for breast cancer than women whose weight remained stable. And losing more weight — 9 kg, or about 20 pounds, or more — was even better for lowering cancer risk.
But other research suggests the opposite. A recent analysis found that people who lost weight within the past 2 years through diet and exercise had a higher risk for a range of cancers compared with those who did not lose weight. Overall, though, the increased risk was quite low.
Whatever the research does, or doesn’t, show about weight and cancer risk, Dr. Basen-Engquist said, it’s important that risk factors, obesity and otherwise, aren’t “used as blame tools.”
“With obesity, behavior certainly plays into it,” she said. “But there are so many influences on our behavior that are socially determined.”
Both Dr. Basen-Engquist and Dr. Meyerhardt said it’s important for clinicians to consider the individual in front of them and for everyone to set realistic expectations.
People with obesity should not feel they have to become thin to be healthier, and no one has to leap from being sedentary to exercising several hours a week.
“We don’t want patients to feel that if they don’t get to a stated goal in a guideline, it’s all for naught,” Dr. Meyerhardt said.
A version of this article appeared on Medscape.com.
According to the Centers for Disease Control and Prevention (CDC), over 684,000 Americans are diagnosed with an “obesity-associated” cancer each year.
The incidence of many of these cancers has been rising in recent years, particularly among younger people — a trend that sits in contrast with the overall decline in cancers with no established relationship to excess weight, such as lung and skin cancers.
Is obesity the new smoking? Not exactly.
While about 42% of cancers — including common ones such as colorectal and postmenopausal breast cancers — are considered obesity-related, only about 8% of incident cancers are attributed to excess body weight. People often develop those diseases regardless of weight.
Although plenty of evidence points to excess body fat as a cancer risk factor, it’s unclear at what point excess weight has an effect. Is gaining weight later in life, for instance, better or worse for cancer risk than being overweight or obese from a young age?
There’s another glaring knowledge gap: Does losing weight at some point in adulthood change the picture? In other words, how many of those 684,000 diagnoses might have been prevented if people shed excess pounds?
When it comes to weight and cancer risk, “there’s a lot we don’t know,” said Jennifer W. Bea, PhD, associate professor, health promotion sciences, University of Arizona, Tucson.
A Consistent but Complicated Relationship
Given the growing incidence of obesity — which currently affects about 42% of US adults and 20% of children and teenagers — it’s no surprise that many studies have delved into the potential effects of excess weight on cancer rates.
Although virtually all the evidence comes from large cohort studies, leaving the cause-effect question open, certain associations keep showing up.
“What we know is that, consistently, a higher body mass index [BMI] — particularly in the obese category — leads to a higher risk of multiple cancers,” said Jeffrey A. Meyerhardt, MD, MPH, codirector, Colon and Rectal Cancer Center, Dana-Farber Cancer Institute, Boston.
In a widely cited report published in The New England Journal of Medicine in 2016, the International Agency for Research on Cancer (IARC) analyzed over 1000 epidemiologic studies on body fat and cancer. The agency pointed to over a dozen cancers, including some of the most common and deadly, linked to excess body weight.
That list includes esophageal adenocarcinoma and endometrial cancer — associated with the highest risk — along with kidney, liver, stomach (gastric cardia), pancreatic, colorectal, postmenopausal breast, gallbladder, ovarian, and thyroid cancers, plus multiple myeloma and meningioma. There’s also “limited” evidence linking excess weight to additional cancer types, including aggressive prostate cancer and certain head and neck cancers.
At the same time, Dr. Meyerhardt said, many of those same cancers are also associated with issues that lead to, or coexist with, overweight and obesity, including poor diet, lack of exercise, and metabolic conditions such as diabetes.
It’s a complicated web, and it’s likely, Dr. Meyerhardt said, that high BMI both directly affects cancer risk and is part of a “causal pathway” of other factors that do.
Regarding direct effects, preclinical research has pointed to multiple ways in which excess body fat could contribute to cancer, said Karen M. Basen-Engquist, PhD, MPH, professor, Division of Cancer Prevention and Population Services, The University of Texas MD Anderson Cancer Center, Houston.
One broad mechanism to help explain the obesity-cancer link is chronic systemic inflammation because excess fat tissue can raise levels of substances in the body, such as tumor necrosis factor alpha and interleukin 6, which fuel inflammation. Excess fat also contributes to hyperinsulinemia — too much insulin in the blood — which can help promote the growth and spread of tumor cells.
But the underlying reasons also appear to vary by cancer type, Dr. Basen-Engquist said. With hormonally driven cancer types, such as breast and endometrial, excess body fat may alter hormone levels in ways that spur tumor growth. Extra fat tissue may, for example, convert androgens into estrogens, which could help feed estrogen-dependent tumors.
That, Dr. Basen-Engquist noted, could be why excess weight is associated with postmenopausal, not premenopausal, breast cancer: Before menopause, body fat is a relatively minor contributor to estrogen levels but becomes more important after menopause.
How Big Is the Effect?
While more than a dozen cancers have been consistently linked to excess weight, the strength of those associations varies considerably.
Endometrial and esophageal cancers are two that stand out. In the 2016 IARC analysis, people with severe obesity had a seven-times greater risk for endometrial cancer and 4.8-times greater risk for esophageal adenocarcinoma vs people with a normal BMI.
With other cancers, the risk increases for those with severe obesity compared with a normal BMI were far more modest: 10% for ovarian cancer, 30% for colorectal cancer, and 80% for kidney and stomach cancers, for example. For postmenopausal breast cancer, every five-unit increase in BMI was associated with a 10% relative risk increase.
A 2018 study from the American Cancer Society, which attempted to estimate the proportion of cancers in the United States attributable to modifiable risk factors — including alcohol consumption, ultraviolet rays exposure, and physical inactivity — found that smoking accounted for the highest proportion of cancer cases by a wide margin (19%), but excess weight came in second (7.8%).
Again, weight appeared to play a bigger role in certain cancers than others: An estimated 60% of endometrial cancers were linked to excess weight, as were roughly one third of esophageal, kidney, and liver cancers. At the other end of the spectrum, just over 11% of breast, 5% of colorectal, and 4% of ovarian cancers were attributable to excess weight.
Even at the lower end, those rates could make a big difference on the population level, especially for groups with higher rates of obesity.
CDC data show that obesity-related cancers are rising among women younger than 50 years, most rapidly among Hispanic women, and some less common obesity-related cancers, such as stomach, thyroid and pancreatic, are also rising among Black individuals and Hispanic Americans.
Obesity may be one reason for growing cancer disparities, said Leah Ferrucci, PhD, MPH, assistant professor, epidemiology, Yale School of Public Health, New Haven, Connecticut. But, she added, the evidence is limited because Black individuals and Hispanic Americans are understudied.
When Do Extra Pounds Matter?
When it comes to cancer risk, at what point in life does excess weight, or weight gain, matter? Is the standard weight gain in middle age, for instance, as hazardous as being overweight or obese from a young age?
Some evidence suggests there’s no “safe” time for putting on excess pounds.
A recent meta-analysis concluded that weight gain at any point after age 18 years is associated with incremental increases in the risk for postmenopausal breast cancer. A 2023 study in JAMA Network Open found a similar pattern with colorectal and other gastrointestinal cancers: People who had sustained overweight or obesity from age 20 years through middle age faced an increased risk of developing those cancers after age 55 years.
The timing of weight gain didn’t seem to matter either. The same elevated risk held among people who were normal weight in their younger years but became overweight after age 55 years.
Those studies focused on later-onset disease. But, in recent years, experts have tracked a troubling rise in early-onset cancers — those diagnosed before age 50 years — particularly gastrointestinal cancers.
An obvious question, Dr. Meyerhardt said, is whether the growing prevalence of obesity among young people is partly to blame.
There’s some data to support that, he said. An analysis from the Nurses’ Health Study II found that women with obesity had double the risk for early-onset colorectal cancer as those with a normal BMI. And every 5-kg increase in weight after age 18 years was associated with a 9% increase in colorectal cancer risk.
But while obesity trends probably partly explain the rise in early-onset cancers, there is likely more to the story, Dr. Meyerhardt said.
“I think all of us who see an increasing number of patients under 50 with colorectal cancer know there’s a fair number who do not fit that [high BMI] profile,” he said. “There’s a fair number over 50 who don’t either.”
Does Weight Loss Help?
With all the evidence pointing to high BMI as a cancer risk factor, a logical conclusion is that weight loss should reduce that excess risk. However, Dr. Bea said, there’s actually little data to support that, and what exists comes from observational studies.
Some research has focused on people who had substantial weight loss after bariatric surgery, with encouraging results. A study published in JAMA found that among 5053 people who underwent bariatric surgery, 2.9% developed an obesity-related cancer over 10 years compared with 4.9% in the nonsurgery group.
Most people, however, aim for less dramatic weight loss, with the help of diet and exercise or sometimes medication. Some evidence shows that a modest degree of weight loss may lower the risks for postmenopausal breast and endometrial cancers.
A 2020 pooled analysis found, for instance, that among women aged ≥ 50 years, those who lost as little as 2.0-4.5 kg, or 4.4-10.0 pounds, and kept it off for 10 years had a lower risk for breast cancer than women whose weight remained stable. And losing more weight — 9 kg, or about 20 pounds, or more — was even better for lowering cancer risk.
But other research suggests the opposite. A recent analysis found that people who lost weight within the past 2 years through diet and exercise had a higher risk for a range of cancers compared with those who did not lose weight. Overall, though, the increased risk was quite low.
Whatever the research does, or doesn’t, show about weight and cancer risk, Dr. Basen-Engquist said, it’s important that risk factors, obesity and otherwise, aren’t “used as blame tools.”
“With obesity, behavior certainly plays into it,” she said. “But there are so many influences on our behavior that are socially determined.”
Both Dr. Basen-Engquist and Dr. Meyerhardt said it’s important for clinicians to consider the individual in front of them and for everyone to set realistic expectations.
People with obesity should not feel they have to become thin to be healthier, and no one has to leap from being sedentary to exercising several hours a week.
“We don’t want patients to feel that if they don’t get to a stated goal in a guideline, it’s all for naught,” Dr. Meyerhardt said.
A version of this article appeared on Medscape.com.
According to the Centers for Disease Control and Prevention (CDC), over 684,000 Americans are diagnosed with an “obesity-associated” cancer each year.
The incidence of many of these cancers has been rising in recent years, particularly among younger people — a trend that sits in contrast with the overall decline in cancers with no established relationship to excess weight, such as lung and skin cancers.
Is obesity the new smoking? Not exactly.
While about 42% of cancers — including common ones such as colorectal and postmenopausal breast cancers — are considered obesity-related, only about 8% of incident cancers are attributed to excess body weight. People often develop those diseases regardless of weight.
Although plenty of evidence points to excess body fat as a cancer risk factor, it’s unclear at what point excess weight has an effect. Is gaining weight later in life, for instance, better or worse for cancer risk than being overweight or obese from a young age?
There’s another glaring knowledge gap: Does losing weight at some point in adulthood change the picture? In other words, how many of those 684,000 diagnoses might have been prevented if people shed excess pounds?
When it comes to weight and cancer risk, “there’s a lot we don’t know,” said Jennifer W. Bea, PhD, associate professor, health promotion sciences, University of Arizona, Tucson.
A Consistent but Complicated Relationship
Given the growing incidence of obesity — which currently affects about 42% of US adults and 20% of children and teenagers — it’s no surprise that many studies have delved into the potential effects of excess weight on cancer rates.
Although virtually all the evidence comes from large cohort studies, leaving the cause-effect question open, certain associations keep showing up.
“What we know is that, consistently, a higher body mass index [BMI] — particularly in the obese category — leads to a higher risk of multiple cancers,” said Jeffrey A. Meyerhardt, MD, MPH, codirector, Colon and Rectal Cancer Center, Dana-Farber Cancer Institute, Boston.
In a widely cited report published in The New England Journal of Medicine in 2016, the International Agency for Research on Cancer (IARC) analyzed over 1000 epidemiologic studies on body fat and cancer. The agency pointed to over a dozen cancers, including some of the most common and deadly, linked to excess body weight.
That list includes esophageal adenocarcinoma and endometrial cancer — associated with the highest risk — along with kidney, liver, stomach (gastric cardia), pancreatic, colorectal, postmenopausal breast, gallbladder, ovarian, and thyroid cancers, plus multiple myeloma and meningioma. There’s also “limited” evidence linking excess weight to additional cancer types, including aggressive prostate cancer and certain head and neck cancers.
At the same time, Dr. Meyerhardt said, many of those same cancers are also associated with issues that lead to, or coexist with, overweight and obesity, including poor diet, lack of exercise, and metabolic conditions such as diabetes.
It’s a complicated web, and it’s likely, Dr. Meyerhardt said, that high BMI both directly affects cancer risk and is part of a “causal pathway” of other factors that do.
Regarding direct effects, preclinical research has pointed to multiple ways in which excess body fat could contribute to cancer, said Karen M. Basen-Engquist, PhD, MPH, professor, Division of Cancer Prevention and Population Services, The University of Texas MD Anderson Cancer Center, Houston.
One broad mechanism to help explain the obesity-cancer link is chronic systemic inflammation because excess fat tissue can raise levels of substances in the body, such as tumor necrosis factor alpha and interleukin 6, which fuel inflammation. Excess fat also contributes to hyperinsulinemia — too much insulin in the blood — which can help promote the growth and spread of tumor cells.
But the underlying reasons also appear to vary by cancer type, Dr. Basen-Engquist said. With hormonally driven cancer types, such as breast and endometrial, excess body fat may alter hormone levels in ways that spur tumor growth. Extra fat tissue may, for example, convert androgens into estrogens, which could help feed estrogen-dependent tumors.
That, Dr. Basen-Engquist noted, could be why excess weight is associated with postmenopausal, not premenopausal, breast cancer: Before menopause, body fat is a relatively minor contributor to estrogen levels but becomes more important after menopause.
How Big Is the Effect?
While more than a dozen cancers have been consistently linked to excess weight, the strength of those associations varies considerably.
Endometrial and esophageal cancers are two that stand out. In the 2016 IARC analysis, people with severe obesity had a seven-times greater risk for endometrial cancer and 4.8-times greater risk for esophageal adenocarcinoma vs people with a normal BMI.
With other cancers, the risk increases for those with severe obesity compared with a normal BMI were far more modest: 10% for ovarian cancer, 30% for colorectal cancer, and 80% for kidney and stomach cancers, for example. For postmenopausal breast cancer, every five-unit increase in BMI was associated with a 10% relative risk increase.
A 2018 study from the American Cancer Society, which attempted to estimate the proportion of cancers in the United States attributable to modifiable risk factors — including alcohol consumption, ultraviolet rays exposure, and physical inactivity — found that smoking accounted for the highest proportion of cancer cases by a wide margin (19%), but excess weight came in second (7.8%).
Again, weight appeared to play a bigger role in certain cancers than others: An estimated 60% of endometrial cancers were linked to excess weight, as were roughly one third of esophageal, kidney, and liver cancers. At the other end of the spectrum, just over 11% of breast, 5% of colorectal, and 4% of ovarian cancers were attributable to excess weight.
Even at the lower end, those rates could make a big difference on the population level, especially for groups with higher rates of obesity.
CDC data show that obesity-related cancers are rising among women younger than 50 years, most rapidly among Hispanic women, and some less common obesity-related cancers, such as stomach, thyroid and pancreatic, are also rising among Black individuals and Hispanic Americans.
Obesity may be one reason for growing cancer disparities, said Leah Ferrucci, PhD, MPH, assistant professor, epidemiology, Yale School of Public Health, New Haven, Connecticut. But, she added, the evidence is limited because Black individuals and Hispanic Americans are understudied.
When Do Extra Pounds Matter?
When it comes to cancer risk, at what point in life does excess weight, or weight gain, matter? Is the standard weight gain in middle age, for instance, as hazardous as being overweight or obese from a young age?
Some evidence suggests there’s no “safe” time for putting on excess pounds.
A recent meta-analysis concluded that weight gain at any point after age 18 years is associated with incremental increases in the risk for postmenopausal breast cancer. A 2023 study in JAMA Network Open found a similar pattern with colorectal and other gastrointestinal cancers: People who had sustained overweight or obesity from age 20 years through middle age faced an increased risk of developing those cancers after age 55 years.
The timing of weight gain didn’t seem to matter either. The same elevated risk held among people who were normal weight in their younger years but became overweight after age 55 years.
Those studies focused on later-onset disease. But, in recent years, experts have tracked a troubling rise in early-onset cancers — those diagnosed before age 50 years — particularly gastrointestinal cancers.
An obvious question, Dr. Meyerhardt said, is whether the growing prevalence of obesity among young people is partly to blame.
There’s some data to support that, he said. An analysis from the Nurses’ Health Study II found that women with obesity had double the risk for early-onset colorectal cancer as those with a normal BMI. And every 5-kg increase in weight after age 18 years was associated with a 9% increase in colorectal cancer risk.
But while obesity trends probably partly explain the rise in early-onset cancers, there is likely more to the story, Dr. Meyerhardt said.
“I think all of us who see an increasing number of patients under 50 with colorectal cancer know there’s a fair number who do not fit that [high BMI] profile,” he said. “There’s a fair number over 50 who don’t either.”
Does Weight Loss Help?
With all the evidence pointing to high BMI as a cancer risk factor, a logical conclusion is that weight loss should reduce that excess risk. However, Dr. Bea said, there’s actually little data to support that, and what exists comes from observational studies.
Some research has focused on people who had substantial weight loss after bariatric surgery, with encouraging results. A study published in JAMA found that among 5053 people who underwent bariatric surgery, 2.9% developed an obesity-related cancer over 10 years compared with 4.9% in the nonsurgery group.
Most people, however, aim for less dramatic weight loss, with the help of diet and exercise or sometimes medication. Some evidence shows that a modest degree of weight loss may lower the risks for postmenopausal breast and endometrial cancers.
A 2020 pooled analysis found, for instance, that among women aged ≥ 50 years, those who lost as little as 2.0-4.5 kg, or 4.4-10.0 pounds, and kept it off for 10 years had a lower risk for breast cancer than women whose weight remained stable. And losing more weight — 9 kg, or about 20 pounds, or more — was even better for lowering cancer risk.
But other research suggests the opposite. A recent analysis found that people who lost weight within the past 2 years through diet and exercise had a higher risk for a range of cancers compared with those who did not lose weight. Overall, though, the increased risk was quite low.
Whatever the research does, or doesn’t, show about weight and cancer risk, Dr. Basen-Engquist said, it’s important that risk factors, obesity and otherwise, aren’t “used as blame tools.”
“With obesity, behavior certainly plays into it,” she said. “But there are so many influences on our behavior that are socially determined.”
Both Dr. Basen-Engquist and Dr. Meyerhardt said it’s important for clinicians to consider the individual in front of them and for everyone to set realistic expectations.
People with obesity should not feel they have to become thin to be healthier, and no one has to leap from being sedentary to exercising several hours a week.
“We don’t want patients to feel that if they don’t get to a stated goal in a guideline, it’s all for naught,” Dr. Meyerhardt said.
A version of this article appeared on Medscape.com.
Most women can conceive after breast cancer treatment
The findings, presented May 23 in advance of the annual meeting of the American Society of Clinical Oncology (ASCO) represent the most comprehensive look to date at fertility outcomes following treatment for women diagnosed with breast cancer before age 40 (Abstract 1518).
Kimia Sorouri, MD, a research fellow at the Dana-Farber Cancer Center in Boston, Massachusetts, and her colleagues, looked at data from the Young Women’s Breast Cancer study, a multicenter longitudinal cohort study, for 1213 U.S. and Canadian women (74% non-Hispanic white) who were diagnosed with stages 0-III breast cancer between 2006 and 2016. None of the included patients had metastatic disease, prior hysterectomy, or prior oophorectomy at diagnosis.
During a median 11 years of follow up, 197 of the women reported attempting pregnancy. Of these, 73% reported becoming pregnant, and 65% delivered a live infant a median 4 years after cancer diagnosis. The median age at diagnosis was 32 years, and 28% opted for egg or embryo freezing to preserve fertility. Importantly, 68% received chemotherapy, which can impair fertility, with only a small percentage undergoing ovarian suppression during chemotherapy treatment.
Key predictors of pregnancy or live birth in this study were “financial comfort,” a self-reported measure defined as having money left over to spend after bills are paid (odds ratio [OR], 2.04; 95% CI 1.01-4.12; P = .047); younger age at the time of diagnosis; and undergoing fertility preservation interventions at diagnosis (OR, 2.78; 95% CI 1.29-6.00; P = .009). Chemotherapy and other treatment factors were not seen to be associated with pregnancy or birth outcomes.
“Current research that informs our understanding of the impact of breast cancer treatment on pregnancy and live birth rates is fairly limited,” Dr. Sorouri said during an online press conference announcing the findings. Quality data on fertility outcomes has been limited to studies in certain subgroups, such as women with estrogen receptor–positive breast cancers, she noted, while other studies “have short-term follow-up and critically lack prospective assessment of attempt at conception.”
The new findings show, Dr. Sorouri said, “that in this modern cohort with a heightened awareness of fertility, access to fertility preservation can help to mitigate a portion of the damage from chemotherapy and other agents. Importantly, this highlights the need for increased accessibility of fertility preservation services for women newly diagnosed with breast cancer who are interested in a future pregnancy.”
Commenting on Dr. Sorouri and colleagues’ findings, Julie Gralow, MD, a breast cancer researcher and ASCO’s chief medical officer, stressed that, while younger age at diagnosis and financial comfort were two factors outside the scope of clinical oncology practice, “we can impact fertility preservation prior to treatment.”
She called it “critical” that every patient be informed of the impact of a breast cancer diagnosis and treatment on future fertility, and that all young patients interested in future fertility be offered fertility preservation prior to beginning treatment.
Ann Partridge, MD, of Dana-Farber, said in an interview that the findings reflected a decades’ long change in approach. “Twenty years ago when we first started this cohort, people would tell women ‘you can’t get pregnant. It’s too dangerous. You won’t be able to.’ And some indeed aren’t able to, but the majority who are attempting are succeeding, especially if they preserve their eggs or embryos. So even if chemo puts you into menopause or made you subfertile, if you’ve preserved eggs or embryos, we now can mitigate that distressing effect that many cancer patients have suffered from historically. That’s the good news here.”
Nonetheless, Dr. Partridge, an oncologist and the last author of the study, noted, the results reflected success only for women actively attempting pregnancy. “Remember, we’re not including the people who didn’t attempt. There may be some who went into menopause who never banked eggs or embryos, and may never have tried because they went to a doctor who told them they’re not fertile.” Further, she said, not all insurances cover in vitro fertilization for women who have had breast cancer.
The fact that financial comfort was correlated with reproductive success, Dr. Partridge said, speaks to broader issues about access. “It may not be all about insurers. It may be to have the ability, to have the time, the education and the wherewithal to do this right — and about being with doctors who talk about it.”
Dr. Sorouri and colleagues’ study was sponsored by the Breast Cancer Research Foundation and Susan G. Komen. Several co-authors disclosed receiving speaking and/or consulting fees from pharmaceutical companies, and one reported being an employee of GlaxoSmithKline. Dr. Sorouri reported no industry funding, while Dr. Partridge reported research funding from Novartis.
The findings, presented May 23 in advance of the annual meeting of the American Society of Clinical Oncology (ASCO) represent the most comprehensive look to date at fertility outcomes following treatment for women diagnosed with breast cancer before age 40 (Abstract 1518).
Kimia Sorouri, MD, a research fellow at the Dana-Farber Cancer Center in Boston, Massachusetts, and her colleagues, looked at data from the Young Women’s Breast Cancer study, a multicenter longitudinal cohort study, for 1213 U.S. and Canadian women (74% non-Hispanic white) who were diagnosed with stages 0-III breast cancer between 2006 and 2016. None of the included patients had metastatic disease, prior hysterectomy, or prior oophorectomy at diagnosis.
During a median 11 years of follow up, 197 of the women reported attempting pregnancy. Of these, 73% reported becoming pregnant, and 65% delivered a live infant a median 4 years after cancer diagnosis. The median age at diagnosis was 32 years, and 28% opted for egg or embryo freezing to preserve fertility. Importantly, 68% received chemotherapy, which can impair fertility, with only a small percentage undergoing ovarian suppression during chemotherapy treatment.
Key predictors of pregnancy or live birth in this study were “financial comfort,” a self-reported measure defined as having money left over to spend after bills are paid (odds ratio [OR], 2.04; 95% CI 1.01-4.12; P = .047); younger age at the time of diagnosis; and undergoing fertility preservation interventions at diagnosis (OR, 2.78; 95% CI 1.29-6.00; P = .009). Chemotherapy and other treatment factors were not seen to be associated with pregnancy or birth outcomes.
“Current research that informs our understanding of the impact of breast cancer treatment on pregnancy and live birth rates is fairly limited,” Dr. Sorouri said during an online press conference announcing the findings. Quality data on fertility outcomes has been limited to studies in certain subgroups, such as women with estrogen receptor–positive breast cancers, she noted, while other studies “have short-term follow-up and critically lack prospective assessment of attempt at conception.”
The new findings show, Dr. Sorouri said, “that in this modern cohort with a heightened awareness of fertility, access to fertility preservation can help to mitigate a portion of the damage from chemotherapy and other agents. Importantly, this highlights the need for increased accessibility of fertility preservation services for women newly diagnosed with breast cancer who are interested in a future pregnancy.”
Commenting on Dr. Sorouri and colleagues’ findings, Julie Gralow, MD, a breast cancer researcher and ASCO’s chief medical officer, stressed that, while younger age at diagnosis and financial comfort were two factors outside the scope of clinical oncology practice, “we can impact fertility preservation prior to treatment.”
She called it “critical” that every patient be informed of the impact of a breast cancer diagnosis and treatment on future fertility, and that all young patients interested in future fertility be offered fertility preservation prior to beginning treatment.
Ann Partridge, MD, of Dana-Farber, said in an interview that the findings reflected a decades’ long change in approach. “Twenty years ago when we first started this cohort, people would tell women ‘you can’t get pregnant. It’s too dangerous. You won’t be able to.’ And some indeed aren’t able to, but the majority who are attempting are succeeding, especially if they preserve their eggs or embryos. So even if chemo puts you into menopause or made you subfertile, if you’ve preserved eggs or embryos, we now can mitigate that distressing effect that many cancer patients have suffered from historically. That’s the good news here.”
Nonetheless, Dr. Partridge, an oncologist and the last author of the study, noted, the results reflected success only for women actively attempting pregnancy. “Remember, we’re not including the people who didn’t attempt. There may be some who went into menopause who never banked eggs or embryos, and may never have tried because they went to a doctor who told them they’re not fertile.” Further, she said, not all insurances cover in vitro fertilization for women who have had breast cancer.
The fact that financial comfort was correlated with reproductive success, Dr. Partridge said, speaks to broader issues about access. “It may not be all about insurers. It may be to have the ability, to have the time, the education and the wherewithal to do this right — and about being with doctors who talk about it.”
Dr. Sorouri and colleagues’ study was sponsored by the Breast Cancer Research Foundation and Susan G. Komen. Several co-authors disclosed receiving speaking and/or consulting fees from pharmaceutical companies, and one reported being an employee of GlaxoSmithKline. Dr. Sorouri reported no industry funding, while Dr. Partridge reported research funding from Novartis.
The findings, presented May 23 in advance of the annual meeting of the American Society of Clinical Oncology (ASCO) represent the most comprehensive look to date at fertility outcomes following treatment for women diagnosed with breast cancer before age 40 (Abstract 1518).
Kimia Sorouri, MD, a research fellow at the Dana-Farber Cancer Center in Boston, Massachusetts, and her colleagues, looked at data from the Young Women’s Breast Cancer study, a multicenter longitudinal cohort study, for 1213 U.S. and Canadian women (74% non-Hispanic white) who were diagnosed with stages 0-III breast cancer between 2006 and 2016. None of the included patients had metastatic disease, prior hysterectomy, or prior oophorectomy at diagnosis.
During a median 11 years of follow up, 197 of the women reported attempting pregnancy. Of these, 73% reported becoming pregnant, and 65% delivered a live infant a median 4 years after cancer diagnosis. The median age at diagnosis was 32 years, and 28% opted for egg or embryo freezing to preserve fertility. Importantly, 68% received chemotherapy, which can impair fertility, with only a small percentage undergoing ovarian suppression during chemotherapy treatment.
Key predictors of pregnancy or live birth in this study were “financial comfort,” a self-reported measure defined as having money left over to spend after bills are paid (odds ratio [OR], 2.04; 95% CI 1.01-4.12; P = .047); younger age at the time of diagnosis; and undergoing fertility preservation interventions at diagnosis (OR, 2.78; 95% CI 1.29-6.00; P = .009). Chemotherapy and other treatment factors were not seen to be associated with pregnancy or birth outcomes.
“Current research that informs our understanding of the impact of breast cancer treatment on pregnancy and live birth rates is fairly limited,” Dr. Sorouri said during an online press conference announcing the findings. Quality data on fertility outcomes has been limited to studies in certain subgroups, such as women with estrogen receptor–positive breast cancers, she noted, while other studies “have short-term follow-up and critically lack prospective assessment of attempt at conception.”
The new findings show, Dr. Sorouri said, “that in this modern cohort with a heightened awareness of fertility, access to fertility preservation can help to mitigate a portion of the damage from chemotherapy and other agents. Importantly, this highlights the need for increased accessibility of fertility preservation services for women newly diagnosed with breast cancer who are interested in a future pregnancy.”
Commenting on Dr. Sorouri and colleagues’ findings, Julie Gralow, MD, a breast cancer researcher and ASCO’s chief medical officer, stressed that, while younger age at diagnosis and financial comfort were two factors outside the scope of clinical oncology practice, “we can impact fertility preservation prior to treatment.”
She called it “critical” that every patient be informed of the impact of a breast cancer diagnosis and treatment on future fertility, and that all young patients interested in future fertility be offered fertility preservation prior to beginning treatment.
Ann Partridge, MD, of Dana-Farber, said in an interview that the findings reflected a decades’ long change in approach. “Twenty years ago when we first started this cohort, people would tell women ‘you can’t get pregnant. It’s too dangerous. You won’t be able to.’ And some indeed aren’t able to, but the majority who are attempting are succeeding, especially if they preserve their eggs or embryos. So even if chemo puts you into menopause or made you subfertile, if you’ve preserved eggs or embryos, we now can mitigate that distressing effect that many cancer patients have suffered from historically. That’s the good news here.”
Nonetheless, Dr. Partridge, an oncologist and the last author of the study, noted, the results reflected success only for women actively attempting pregnancy. “Remember, we’re not including the people who didn’t attempt. There may be some who went into menopause who never banked eggs or embryos, and may never have tried because they went to a doctor who told them they’re not fertile.” Further, she said, not all insurances cover in vitro fertilization for women who have had breast cancer.
The fact that financial comfort was correlated with reproductive success, Dr. Partridge said, speaks to broader issues about access. “It may not be all about insurers. It may be to have the ability, to have the time, the education and the wherewithal to do this right — and about being with doctors who talk about it.”
Dr. Sorouri and colleagues’ study was sponsored by the Breast Cancer Research Foundation and Susan G. Komen. Several co-authors disclosed receiving speaking and/or consulting fees from pharmaceutical companies, and one reported being an employee of GlaxoSmithKline. Dr. Sorouri reported no industry funding, while Dr. Partridge reported research funding from Novartis.
FROM ASCO 2024
Study Finds Injuries, Stress Levels Increased Among Mohs Surgeons
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
PHOENIX — In addition, most surgeons did not feel prepared to manage or prevent these symptoms.
“Our study highlights the need to implement ergonomic training and emotion-focused coping skills, as part of fellowship training and the continuing medical education curriculum, to alleviate and prevent emotional burnout,” said lead author Eduardo A. Michelen-Gómez, MD, a dermatology resident at the University of Puerto Rico School of Medicine, San Juano. “This interaction also must be designed to cater to generation and sex specific needs.”
Dr. Michelen-Gómez presented the findings at the annual meeting of the American College of Mohs Surgery.
Mohs is a demanding procedure that involves repetitive motion, strict attention to detail, and high practice efficiency, all of which must be balanced with the need to prioritize patient safety and well-being. “All of these factors predispose Mohs surgeons to be at an increased risk of physical and emotional stress,” he said.
Despite these concerns, however, the literature is limited concerning work-associated stressors among Mohs surgeons. To further explore this issue, Dr. Michelen-Gómez and colleagues conducted a survey study of ACMS members to investigate not only the prevalence of emotional and physical stressors associated with being a Mohs surgeon but also what specific actions physicians were taking to prevent and/or treat these stressors.
They designed a 21-question cross-sectional electronic survey that was sent to all active ACMS members in 2023. Outcomes evaluated were gender, years of practice, concern for and prevalence of occupational musculoskeletal disorders, emotional stress and burnout, and surgeon’s knowledge and training to manage these symptoms. A total of 473 Mohs surgeons responded.
High Prevalence of Injury and Burnout
“Almost 90% of respondents reported moderate to severe concern for occupational musculoskeletal injuries,” said Dr. Michelen-Gómez. “The prevalence of these injuries was 68%, with neck injuries being the most common complaint. Of the entire cohort, 67% have adopted ergonomic practices patterns.”
Female surgeons had a higher prevalence of musculoskeletal injuries than men, and there was no correlation between years of practice and prevalence of these injuries.
Their results also showed that 70% of respondents reported experiencing psychological and emotional stress or burnout associated with being a Mohs surgeon. The cause of emotional stress differed between men and women. “In males, the most common cause was patient care–related anxiety, while in females, it was finding an adequate work-life balance,” he said.
Surgeons with fewer years of experience were more likely to experience emotional stress (P = .01), and female surgeons had a higher prevalence of burnout and musculoskeletal disorders (71.0% and 71.4%, respectively) than male surgeons (67.7% and 65.2%, respectively).
To prevent or manage musculoskeletal injury, respondents reported using interventions such as physical therapy, yoga/stretching/Pilates, massage therapy, cupping, and using a physical trainer. Specific actions for preventing or managing emotional stress and burnout included engaging with a therapist, working with a life coach, practicing meditation or mindfulness, journaling, relying on religion or spirituality, and exercise.
However, among those who reported musculoskeletal disorders or emotional stress, only 40.56% and 46.67%, respectively, felt they had sufficient knowledge and the resources to manage them appropriately.
“In addition, we found a positive correlation between the development of psychological stress and physical issues,” said Dr. Michelen-Gómez. Future studies can include determining the most effective methods to address the emotional and physical stressors of practicing Mohs Surgery.”
Asked to comment on the study findings, Jesse M. Lewin, MD, chief of Mohs micrographic and dermatologic surgery and vice chair of surgical operations at the Icahn School of Medicine at Mount Sinai, New York City, said that the real-world take-home messages from this study are twofold.
“It is important to focus on physician wellness and prevention of burnout and physical injury to protect our physician workforce, and two, we should equip physicians-in-training with tools to protect their physical and emotional health,” he said.
Dr. Michelen-Gómez and Dr. Lewin, who was not involved with the study, had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACMS 2024