User login
Suicidal behavior tied to increased all-cause mortality in MDD
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.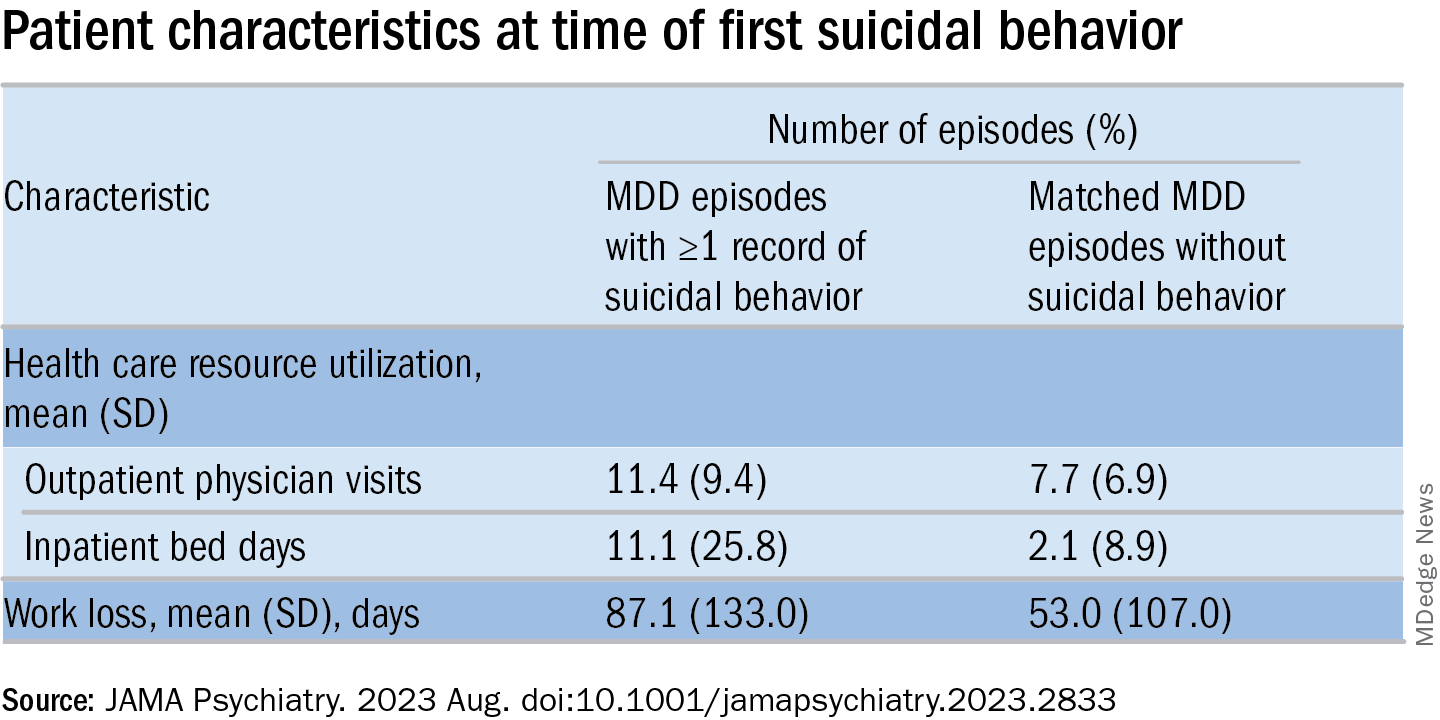
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Screen bipolar patients for eating disorders
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Risky drinking common in cancer survivors
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An analysis of more than 15,000 adults with a cancer diagnosis revealed that nearly 80% were current drinkers. Among current drinkers, 13% consumed a moderate amount of alcohol in a typical day, while close to 40% engaged in hazardous drinking.
The numbers are “staggering,” Yin Cao, ScD, MPH, of Washington University in St. Louis, said in an interview. “Most concerning is that those on cancer treatment are engaged in a similar level of risky drinking.”
The study was published online in JAMA Network Open.
Drinking alcohol can increase a person’s risk for a variety of cancers, including oral and pharyngeal cancer as well as esophageal, colorectal, liver, and female breast cancers.
Consuming alcohol is also associated with numerous risks among people diagnosed with cancer. In the short term, alcohol consumption can worsen postsurgical outcomes as well as impair cognition and amplify cardiotoxicity in patients undergoing chemotherapy. In the long term, drinking alcohol can elevate a person’s risk of recurrence, secondary tumors, and mortality.
The American Society of Clinical Oncology recently issued a statement reinforcing the need to prioritize alcohol consumption as a key modifiable behavioral factor in the cancer control research agenda.
The current American Cancer Society guidelines indicate that it’s best to avoid or, at least, minimize alcohol consumption. Men should limit their intake to no more than two drinks per day and women should have no more than one drink per day.
Despite this data and guidelines, alcohol drinking patterns among cancer survivors in the United States remain poorly understood.
To explore further, the researchers identified 15,199 adult cancer survivors enrolled in the National Institutes of Health’s All of Us Research Program.
Overall, 78% of the cohort – more than 11,800 individuals – were current drinkers. In a typical day, 24% engaged in binge drinking – consuming six or more drinks on a single occasion – and 38% engaged in hazardous drinking. Using the Alcohol Use Disorders Identification Test–Consumption, the researchers classified hazardous drinking as scores of 4 or higher in men and 3 or higher in women.
Drinking patterns looked similar in the subset of 1,839 patients undergoing cancer treatment. In this group, 76% were current drinkers. Among current drinkers, 12% exceeded moderate drinking levels, 23% reported binge drinking, and 38% engaged in hazardous drinking. In this group, men, Hispanics, people diagnosed with cancer before age 18, and smokers were more likely to engage in risky drinking behaviors.
“We know that many people who are diagnosed with cancer continue to drink alcohol, but this study provides much more detailed information about that,” said Farhad Islami, MD, PhD, senior scientific director for cancer disparity research at the American Cancer Society, Atlanta, who was not involved in the study.
Given the degree of drinking identified in this population, Dr. Cao highlighted the importance of talking to patients about alcohol.
“Our findings highlight an opportunity for enhanced support and intervention concerning risky drinking behaviors” in oncology, Dr. Cao said. “Given the societal norms surrounding alcohol and the general lack of awareness of alcohol’s short- and long-term impact on cancer outcomes, gently educating patients/survivors about potential risks while understanding the cultural and societal contexts of drinking can make a difference.”
Dr. Islami agreed that oncologists should talk to their patients about alcohol, “especially those going through active treatment because alcohol may affect the treatment or may be associated with more complications of the treatment.”
“Many people now know that smoking causes cancer, but unfortunately, many people do not know about the association of alcohol with cancer,” he said.
Outside of an awareness gap, there are numerous risk factors for substance abuse among cancer survivors, Marleen Meyers, MD, director of the cancer survivorship program at NYU Langone Perlmutter Cancer Center, New York, explained.
Alcohol can help some cancer survivors dull feelings of isolation, fear, stress, and poor pain management that may accompany their diagnosis and treatment, said Dr. Meyers, who was not involved in the research. That is why “it is important for patients to be honest with their providers and for providers to ask about substance use in a nonjudgmental way.”
In these conversations, oncologists should educate patients about the safety risks associated with alcohol intake during or after treatment and that there is no established “safe” amount of alcohol. Incorporating a mental health screening and questions about a family history of substance abuse can also help identify patients “most at risk so providers can be proactive,” she said.
The study was supported by a grant from the NIH. Dr. Cao, Dr. Islami, and Dr. Meyers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Mothers in medicine: What can we learn when worlds collide?
Across all industries, studies by the U.S. Department of Labor have shown that women, on average, earn 83.7 percent of what their male peers earn. While a lot has been written about the struggles women face in medicine, there have been decidedly fewer analyses that focus on women who choose to become mothers while working in medicine.
I’ve been privileged to work with medical students and residents for the last 8 years as the director of graduate and medical student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. Often, the women I see as patients speak about their struggles with the elusive goal of “having it all.” While both men and women in medicine have difficulty maintaining a work-life balance, I’ve learned, both personally and professionally, that many women face a unique set of challenges.
No matter what their professional status, our society often views a woman as the default parent. For example, the teacher often calls the mothers first. The camp nurse calls me first, not my husband, when our child scrapes a knee. After-school play dates are arranged by the mothers, not fathers.
But mothers also bring to medicine a wealth of unique experiences, ideas, and viewpoints. They learn firsthand how to foster affect regulation and frustration tolerance in their kids and become efficient at managing the constant, conflicting tug of war of demands.
Some may argue that, over time, women end up earning significantly less than their male counterparts because they leave the workforce while on maternity leave, ultimately delaying their upward career progression. It’s likely a much more complex problem. Many of my patients believe that, in our male-dominated society (and workforce), women are punished for being aggressive or stating bold opinions, while men are rewarded for the same actions. While a man may sound forceful and in charge, a women will likely be thought of as brusque and unappreciative.
Outside of work, many women may have more on their plate. A 2020 Gallup poll of more than 3,000 heterosexual couples found that women are responsible for the majority of household chores. Women continue to handle more of the emotional labor within their families, regardless of income, age, or professional status. This is sometimes called the “Mental Load’ or “Second Shift.” As our society continues to view women as the default parent for childcare, medical issues, and overarching social and emotional tasks vital to raising happy, healthy children, the struggle a female medical professional feels is palpable.
Raising kids requires a parent to consistently dole out control, predictability, and reassurance for a child to thrive. Good limit and boundary setting leads to healthy development from a young age.
Psychiatric patients (and perhaps all patients) also require control, predictability, and reassurance from their doctor. The lessons learned in being a good mother can be directly applied in patient care, and vice versa. The cross-pollination of this relationship continues to grow more powerful as a woman’s children grow and her career matures.
Pediatrician and psychoanalyst Donald Winnicott’s idea of a “good enough” mother cannot be a one-size-fits-all approach. Women who self-select into the world of medicine often hold themselves to a higher standard than “good enough.” Acknowledging that the demands from both home and work will fluctuate is key to achieving success both personally and professionally, and lessons from home can and should be utilized to become a more effective physician. The notion of having it all, and the definition of success, must evolve over time.
Dr. Maymind is director of medical and graduate student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. She has no relevant disclosures.
Across all industries, studies by the U.S. Department of Labor have shown that women, on average, earn 83.7 percent of what their male peers earn. While a lot has been written about the struggles women face in medicine, there have been decidedly fewer analyses that focus on women who choose to become mothers while working in medicine.
I’ve been privileged to work with medical students and residents for the last 8 years as the director of graduate and medical student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. Often, the women I see as patients speak about their struggles with the elusive goal of “having it all.” While both men and women in medicine have difficulty maintaining a work-life balance, I’ve learned, both personally and professionally, that many women face a unique set of challenges.
No matter what their professional status, our society often views a woman as the default parent. For example, the teacher often calls the mothers first. The camp nurse calls me first, not my husband, when our child scrapes a knee. After-school play dates are arranged by the mothers, not fathers.
But mothers also bring to medicine a wealth of unique experiences, ideas, and viewpoints. They learn firsthand how to foster affect regulation and frustration tolerance in their kids and become efficient at managing the constant, conflicting tug of war of demands.
Some may argue that, over time, women end up earning significantly less than their male counterparts because they leave the workforce while on maternity leave, ultimately delaying their upward career progression. It’s likely a much more complex problem. Many of my patients believe that, in our male-dominated society (and workforce), women are punished for being aggressive or stating bold opinions, while men are rewarded for the same actions. While a man may sound forceful and in charge, a women will likely be thought of as brusque and unappreciative.
Outside of work, many women may have more on their plate. A 2020 Gallup poll of more than 3,000 heterosexual couples found that women are responsible for the majority of household chores. Women continue to handle more of the emotional labor within their families, regardless of income, age, or professional status. This is sometimes called the “Mental Load’ or “Second Shift.” As our society continues to view women as the default parent for childcare, medical issues, and overarching social and emotional tasks vital to raising happy, healthy children, the struggle a female medical professional feels is palpable.
Raising kids requires a parent to consistently dole out control, predictability, and reassurance for a child to thrive. Good limit and boundary setting leads to healthy development from a young age.
Psychiatric patients (and perhaps all patients) also require control, predictability, and reassurance from their doctor. The lessons learned in being a good mother can be directly applied in patient care, and vice versa. The cross-pollination of this relationship continues to grow more powerful as a woman’s children grow and her career matures.
Pediatrician and psychoanalyst Donald Winnicott’s idea of a “good enough” mother cannot be a one-size-fits-all approach. Women who self-select into the world of medicine often hold themselves to a higher standard than “good enough.” Acknowledging that the demands from both home and work will fluctuate is key to achieving success both personally and professionally, and lessons from home can and should be utilized to become a more effective physician. The notion of having it all, and the definition of success, must evolve over time.
Dr. Maymind is director of medical and graduate student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. She has no relevant disclosures.
Across all industries, studies by the U.S. Department of Labor have shown that women, on average, earn 83.7 percent of what their male peers earn. While a lot has been written about the struggles women face in medicine, there have been decidedly fewer analyses that focus on women who choose to become mothers while working in medicine.
I’ve been privileged to work with medical students and residents for the last 8 years as the director of graduate and medical student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. Often, the women I see as patients speak about their struggles with the elusive goal of “having it all.” While both men and women in medicine have difficulty maintaining a work-life balance, I’ve learned, both personally and professionally, that many women face a unique set of challenges.
No matter what their professional status, our society often views a woman as the default parent. For example, the teacher often calls the mothers first. The camp nurse calls me first, not my husband, when our child scrapes a knee. After-school play dates are arranged by the mothers, not fathers.
But mothers also bring to medicine a wealth of unique experiences, ideas, and viewpoints. They learn firsthand how to foster affect regulation and frustration tolerance in their kids and become efficient at managing the constant, conflicting tug of war of demands.
Some may argue that, over time, women end up earning significantly less than their male counterparts because they leave the workforce while on maternity leave, ultimately delaying their upward career progression. It’s likely a much more complex problem. Many of my patients believe that, in our male-dominated society (and workforce), women are punished for being aggressive or stating bold opinions, while men are rewarded for the same actions. While a man may sound forceful and in charge, a women will likely be thought of as brusque and unappreciative.
Outside of work, many women may have more on their plate. A 2020 Gallup poll of more than 3,000 heterosexual couples found that women are responsible for the majority of household chores. Women continue to handle more of the emotional labor within their families, regardless of income, age, or professional status. This is sometimes called the “Mental Load’ or “Second Shift.” As our society continues to view women as the default parent for childcare, medical issues, and overarching social and emotional tasks vital to raising happy, healthy children, the struggle a female medical professional feels is palpable.
Raising kids requires a parent to consistently dole out control, predictability, and reassurance for a child to thrive. Good limit and boundary setting leads to healthy development from a young age.
Psychiatric patients (and perhaps all patients) also require control, predictability, and reassurance from their doctor. The lessons learned in being a good mother can be directly applied in patient care, and vice versa. The cross-pollination of this relationship continues to grow more powerful as a woman’s children grow and her career matures.
Pediatrician and psychoanalyst Donald Winnicott’s idea of a “good enough” mother cannot be a one-size-fits-all approach. Women who self-select into the world of medicine often hold themselves to a higher standard than “good enough.” Acknowledging that the demands from both home and work will fluctuate is key to achieving success both personally and professionally, and lessons from home can and should be utilized to become a more effective physician. The notion of having it all, and the definition of success, must evolve over time.
Dr. Maymind is director of medical and graduate student mental health at Rowan-Virtua School of Osteopathic Medicine in Mt. Laurel, N.J. She has no relevant disclosures.
Sleep disturbance may predict increased risk of suicidal thoughts
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
Suicide remains the second leading cause of death in young adults, but factors that may predict increased suicide risk have not been characterized, wrote Rebecca C. Cox, PhD, of the University of Colorado Boulder, and colleagues.
“Sleep disturbance is a promising modifiable risk factor for acute changes in suicide risk,” they noted. “Previous research has found multiple aspects of sleep disturbance are linked to elevated SI, including insomnia symptoms, both short and long sleep duration, nocturnal wakefulness, and nightmares.”
However, data on the impact of nightly sleep disturbance on suicide risk are limited, the researchers said. They hypothesized that use of ecological momentary assessment (EMA) to assess daily variability in sleep might offer more insight into the relationship between various components of sleep disturbance and changes in suicide risk.
In a study published in Psychiatry Research , the investigators recruited 102 young adults aged 18-35 years who had a history of suicidal behavior; 74.5% were female, 64.7% were White. Participants completed seven semi-random surveys per day for between wake and sleep schedules over 21 days. Each survey asked participants to report on whether they had experienced suicidal ideation (SI) since the last survey. The researchers examined within-person and between-person sleep variables including bedtime, sleep onset latency, sleep onset, number of awakenings, wake after sleep onset, sleep duration, sleep timing, sleep quality, and nightmares.
Overall, nightmares had a significant, positive effect on passive SI at both within- and between-person levels, but no significant effect on active SI. Sleep latency showed a significant, positive effect on passive and active SI at the between-person level, meaning that “participants who took longer to fall asleep on average were more likely to experience passive and active SI during the sampling period,” the researchers noted.
In addition, days following nights of more time awake between sleep onset and offset were days with increased likelihood of passive and active SI. Similarly, days following nights of worse sleep quality than normally reported for an individual were days with increased likelihood of passive and active SI. Sleep timing and duration had no significant effects on SI at the within- or between-person level.
“Notably, tests of reverse models found no relation between daily passive or active SI and any component of the subsequent night’s sleep, suggesting a unidirectional relation between sleep disturbance and subsequent SI,” the researchers wrote in their discussion. If future research replicates the study findings, the results could support the inclusion of sleep difficulties on standard risk assessments as a way to identify risk for SI and initiate prevention approaches, they said.
The findings were limited by several factors including the potential for unmeasured variables impacting the associations between sleep and SI, the researchers noted. Other limitations included the lack of data on more severe levels of SI such as planning and intent, and on suicidal behaviors such as preparatory behaviors, aborted attempts, and actual attempts. The findings also may not generalize to other age groups such as children, adolescents, or older adults, they said.
More research is needed to determine which sleep disturbance components are acute risk factors for which suicide-related outcomes, the researchers said. However, the study is the first to provide evidence for daily sleep disturbances as a near-term predictor of SI in young adults, they concluded.
The study was supported in part by the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
The three pillars of perinatal care: Babies, parents, dyadic relationships
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Perinatal depression (PND) is the most common obstetric complication in the United States. Even when screening results are positive, mothers often do not receive further evaluation, and even when PND is diagnosed, mothers do not receive evidence-based treatments.
Meta-analytic estimates show that pregnant women suffer from PND at rates from 6.5% to 12.9% across pregnancy to 3-months post partum.1 Women from low-income families and adolescent mothers are at highest risk, where rates are double and triple respectively.
Fathers also suffer from PND, with a prevalence rate from 2% to 25%, increasing to 50% when the mother experiences PND.
The American Academy of Pediatrics issued a Policy Statement (January 2019) about the need to recognize and manage PND. They recommended that pediatric medical homes establish a system to implement the screening of mothers at the 1-, 2-, 4-, and 6-month well-child visits, to use community resources for the treatment and referral of the mother with depression, and to provide support for the maternal-child relationship.2
The American Academy of Pediatrics also recommends advocacy for workforce development for mental health professionals who care for young children and mother-infant dyads, and for promotion of evidence-based interventions focused on healthy attachment and parent-child relationships.
Family research
There is a bidirectional association between family relational stress and PND. Lack of family support is both a predictor and a consequence of perinatal depression. Frequent arguments, conflict because one or both partners did not want the pregnancy, division of labor, poor support following stressful life events, lack of partner availability, and low intimacy are associated with increased perinatal depressive symptoms.
Gender role stress is also included as a risk factor. For example, men may fear performance failure related to work and sex, and women may fear disruption in the couple relationship due to the introduction of a child.
When depressed and nondepressed women at 2 months post delivery were compared, the women with depressive symptoms perceived that their partners did not share similar interests, provided little companionship, expressed disinterest in infant care, did not provide a feeling of connection, did not encourage them to get assistance to cope with difficulties, and expressed disagreement in infant care.3
A high-quality intimate relationship is protective for many illnesses and PND is no exception.4
Assessment
Despite the availability of effective treatments, perinatal mental health utilization rates are strikingly low. There are limited providers and a general lack of awareness of the need for this care. The stigma for assessing and treating PND is high because the perception is that pregnancy is supposed to be a joyous time and with time, PND will pass.
The first step is a timely and accurate assessment of the mother, which should, if possible, include the father and other family support people. The preferred standard for women is the Edinburgh Postnatal Depression Scale (EPDS), a checklist of 10 items (listed below) with a maximum score of 30, and any score over 10 warrants further assessment.5 This scale is used worldwide in obstetric clinics and has been used to identify PND in fathers.
- I have been able to laugh and see the funny side of things.
- I have looked forward with enjoyment to things.
- I have blamed myself unnecessarily when things went wrong.
- I have been anxious or worried for no good reason.
- I have felt scared or panicky for no good reason.
- Things have been getting to me.
- I have been so unhappy that I have had difficulty sleeping.
- I have felt sad or miserable.
- I have been so unhappy that I have been crying.
- The thought of harming myself has occurred to me.
A new ultrabrief tool with only four questions is the Brief Multidimensional Assessment Scale (BMAS), which measures the ability to get things done, emotional support in important relationships, quality of life, and sense of purpose in life. It demonstrates concurrent validity with other measures and discriminates between nonclinical participants and participants from most clinical contexts.6
For those interested in assessing family health, an easy-to-use assessment tool is the 12-item Family Assessment Device (FAD).7
Family therapy interventions
A systematic review and meta-analysis of the current evidence on the usefulness of family therapy interventions in the prevention and treatment of PND identified seven studies.
In these studies, there were statistically significant reductions in depressive symptoms at postintervention in intervention group mothers. Intervention intensity and level of family involvement moderated the impacts of intervention on maternal depression, and there was a trend in improved family functioning in intervention group couples.8
Evidence-based interventions are usually psychoeducational or cognitive-behavioral family therapy models where focused interventions target the following three areas:
- Communication skills related to expectations (including those that pertain to gender roles and the transition to parenthood) and emotional support.
- Conflict management.
- Problem-solving skills related to shared responsibility in infant care and household activities.
Intensive day program for mothers and babies
There is a growing awareness of the effectiveness of specialized mother-baby day hospital programs for women with psychiatric distress during the peripartum period.9
The Women & Infants’ Hospital (WIH) in Providence, R.I., established a mother-baby postpartum depression day program in 2000, adjacent to the obstetrical hospital, the ninth largest obstetrical service in the United States. The day program is integrated with the hospital’s obstetric medicine team and referrals are also accepted from the perinatal practices in the surrounding community. The treatment day includes group, individual, and milieu treatment, as well as consultation with psychiatrists, nutritionists, social workers, lactation specialists and others.
The primary theoretical model utilized by the program is interpersonal psychotherapy (IPT), with essential elements of the program incorporating cognitive behavioral therapy (CBT), and experiential strategies (for instance, mindfulness, breathing, progressive muscle relaxation) to improve self-care and relaxation skills. Patient satisfaction surveys collected from 800 women, (54% identified as White) treated at the program between 2007 and 2012 found that women were highly satisfied with the treatment received, noting that the inclusion of the baby in their treatment is a highly valued aspect of care.
A similar program in Minnesota reported that 328 women who consented to participation in research had significant improvements (P < .001) in self-report scales assessing depression, anxiety, and maternal functioning, improving mental health and parenting functioning.10
Lastly, a recent study out of Brussels, on the benefit of a mother-baby day program analyzed patient data from 2015 and 2020. This clinical population of 92 patients (43% identifying as North African) was comparable to the population of the inpatient mother-baby units in terms of psychosocial fragility except that the parents entering the day program had less severe illnesses, more anxiety disorder, and less postpartum psychosis. In the day program, all the babies improved in terms of symptoms and relationships, except for those with significant developmental difficulties.
The dyadic relationship was measured using “levels of adaptation of the parent–child relationship” scale which has four general levels of adjustment, from well-adjusted to troubled or dangerous relationship. Unlike programs in the United States, this program takes children up to 2.5 years old and the assessment period is up to 8 weeks.11
Prevention of mental illness is best achieved by reducing the known determinants of illness. For PND, the research is clear, so why not start at the earliest possible stage, when we know that change is possible? Pushing health care systems to change is not easy, but as the research accumulates and the positive results grow, our arguments become stronger.
Dr. Heru is a psychiatrist in Aurora, Colo. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Gavin NI et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005 Nov;106(5 Pt 1):1071-83. doi: 10.1097/01.AOG.0000183597.31630.db.
2. Rafferty J et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019 Jan;143(1):e20183260. doi: 10.1542/peds.2018-3260.
3. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
4. Kumar SA et al. Promoting resilience to depression among couples during pregnancy: The protective functions of intimate relationship satisfaction and self-compassion. Family Process. 2022 May;62(1):387-405. doi: 10.1111/famp.12788.
5. Cox JL et al. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987 Jun;150:782-6. doi: 10.1192/bjp.150.6.782.
6. Keitner GI et al. The Brief Multidimensional Assessment Scale (BMAS): A broad measure of patient well-being. Am J Psychother. 2023 Feb 1;76(2):75-81. doi: 10.1176/appi.psychotherapy.20220032.
7. Boterhoven de Haan KL et al. Reliability and validity of a short version of the general functioning subscale of the McMaster Family Assessment Device. Fam Process. 2015 Mar;54(1):116-23. doi: 10.1111/famp.12113.
8. Cluxton-Keller F, Bruce ML. Clinical effectiveness of family therapeutic interventions in the prevention and treatment of perinatal depression: A systematic review and meta-analysis. PLoS One. 2018 Jun 14;13(6):e0198730. doi: 10.1371/journal.pone.0198730.
9. Battle CL, Howard MM. A mother-baby psychiatric day hospital: History, rationale, and why perinatal mental health is important for obstetric medicine. Obstet Med. 2014 Jun;7(2):66-70. doi: 10.1177/1753495X13514402.
10. Kim HG et al. Keeping Parent, Child, and Relationship in Mind: Clinical Effectiveness of a Trauma-informed, Multigenerational, Attachment-Based, Mother-Baby Partial Hospital Program in an Urban Safety Net Hospital. Matern Child Health J. 2021 Nov;25(11):1776-86. doi: 10.1007/s10995-021-03221-4.
11. Moureau A et al. A 5 years’ experience of a parent-baby day unit: impact on baby’s development. Front Psychiatry. 2023 June 15;14. doi: 10.3389/fpsyt.2023.1121894.
Child assault tied to triple the risk for mental illness within 1 year
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
The greatest risk was found in the first year following the assault, increasing to three times the risk of being diagnosed with mental illness, compared with children not assaulted. Mood and anxiety disorders were the most common diagnoses.
“From a clinical and policy perspective, our study highlights that there is a critical opportunity for health care clinicians to support children in the first year following physical assault,” Natasha Saunders, MD, MSc, of the Hospital for Sick Children, Toronto, and colleagues wrote. “There is a need to develop and implement targeted mental illness prevention, screening, and treatment programs for assaulted children.”
The findings were published online in JAMA Network Open.
While it has been well established that children exposed to assault have an increased risk for subsequent mental illness, Dr. Saunders and coinvestigators noted that using an age-matched, population-based cohort study would enable them to obtain detailed information on the patterns and timing of subsequent psychiatric diagnoses.
To that end, the researchers used several medical databases in Ontario to find 5,487 children (infants to age 13 years) who required an ED visit or hospitalization for a physical assault in Ontario between 2006 and 2014.
These children were matched on a 1:4 basis with 21,948 children not exposed to physical assault. The children were followed until their 18th birthday or until the study ended in March 2019.
The researchers found that more than a third of the children (39%) who were exposed to assault received a mental health diagnosis, according to health records, compared with 23% of unexposed children.
Mood and anxiety disorders were the most common diagnoses among children exposed to assault (16.2% vs. 10.6%, respectively); followed by select childhood behavior disorders, such as ADHD, oppositional defiant disorder, or conduct disorder (9.9% vs. 5.2%); and substance use disorders (2.4% vs. 0.4%).
Triple risk of mental illness in first year
The researchers found that the children exposed to assault were nearly twice as likely to be diagnosed with a mental illness over a median follow-up of 7 years, compared with those not exposed to assault (adjusted hazard ratio, 1.96; 95% confidence interval, 1.85,2.08).
In the year following the assault, children exposed to assault bore three times the risk of being diagnosed with a mental illness, compared with unexposed children (aHR, 3.08; 95% CI, 2.68,3.54).
In addition, the children who had been assaulted were more likely to be diagnosed in an acute care setting than those who were not assaulted (14% vs. 2.8%).
The children who had been assaulted were an average age of 7 years and were more often boys (55% vs. 45%). Children who were assaulted were also more likely to have mothers with mental illness (35% vs. 19%).
The investigators noted that the study likely underestimated the number of children exposed to assault, as many do not end up in the ED.
In addition to highlighting the need for medical personnel to support children in the first year following assault, the investigators wrote that “our results also advocate for accessible mental health care outside of the acute setting and for care that addresses the social and health needs of mothers, who themselves have high social and health risks.”
This study received funding from the National Foundation to End Child Abuse and Neglect and the Ontario Ministry of Health and the Ministry of Long-Term Care. Dr. Saunders reported receiving personal fees from The BMJ Group, Archives of Diseases in Childhood outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Telehealth visit helps reconnect adolescents lost to follow-up
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
A telehealth primary care visit more than doubled the well-visit show rate for a cohort of hard-to-reach adolescents, results of a small pilot study show.
Brian P. Jenssen, MD, MSHP, department of pediatrics, University of Pennsylvania, Philadelphia, led the pilot study and the project team, which included physicians, researchers, and experts in innovation, quality improvement, and data analytics.
Findings were published online in Annals of Family Medicine.
Keeping adolescents in consistent primary care can be challenging for many reasons. The study authors note, “Only 50% of adolescents have had a health supervision visit in the past year, missing a critical opportunity for clinicians to influence health, development, screening, and counseling.”
Interest high in hard-to-reach group
This study included a particularly hard-to-reach group of 18-year-old patients at an urban primary care clinic who were lost to follow-up and had Medicaid insurance. They had not completed a well visit in more than 2 years and had a history of no-show visits.
Interest in the pilot program was high. The authors write: “We contacted patients (or their caregivers) to gauge interest in a virtual well visit with a goal to fill five telehealth slots in one evening block with one clinician. Due to high patient interest and demand, we expanded to 15 slots over three evenings, filling the slots after contacting just 24 patients.”
Professional organizations have recommended a telehealth/in-person hybrid care model to meet hard-to-reach adolescents “wherever they are,” the authors note, but the concept has not been well studied.
Under the hybrid model, the first visit is through telehealth and in-person follow-up is scheduled as needed.
Navigators contacted patients to remind them of the appointment, and helped activate the patient portal and complete previsit screening questions for depression and other health risks.
Telehealth visits were billed as preventive visits and in-person follow-up visits as no-charge nurse visits, and these payments were supported by Medicaid.
Sharp increase in show rate
In the pilot study, of the 15 patients scheduled for the telehealth visit, 11 connected virtually (73% show rate). Of those, nine needed in-person follow-up, and five completed the follow-up.
Before the intervention, the average well-visit show rate for this patient group was 33%.
Clinicians counseled all the patients about substance use and safe sex. One patient screened positive for depression and was then connected to services. Two patients were started on birth control.
During the in-person follow-up, all patients received vaccinations (influenza, meningococcal, and/or COVID-19) and were screened for sexually transmitted infection. Eight patients completed the satisfaction survey and all said they liked the convenience of the telehealth visit.
Telehealth may reduce barriers for teens
Anthony Cheng, MD, a family medicine physician at Oregon Health & Science University in Portland, who was not part of the study, said he found the hybrid model promising.
One reason is that telehealth eliminates the need for transportation to medical appointments, which can be a barrier for adolescents.
Among the top causes of death for young people are mental health issues and addressing those, Dr. Cheng noted, is well-suited to a telehealth visit.
“There’s so much we can do if we can establish a relationship and maintain a relationship with our patients as young adults,” he said. “People do better when they have a regular source of care.”
He added that adolescents also have grown up communicating via screens so it’s often more comfortable for them to communicate with health care providers this way.
Dr. Cheng said adopting such a model may be difficult for providers reluctant to switch from the practice model with which they are most comfortable.
“We prefer to do things we have the most confidence in,” he said. “It does take an investment to train staff and build your own clinical comfort. If that experience wasn’t good over the past 3 years, you may be anxious to get back to your normal way of doing business.”
The authors and Dr. Cheng have no relevant financial relationships to disclose.
FROM ANNALS OF INTERNAL MEDICINE
How much pain is in the mind? This doctor thinks the answer is, most
More than 3 decades ago, John E. Sarno, MD, published Healing Back Pain, a popular book that garnered something of a cult following. Looking at his own practice, Dr. Sarno, a rehabilitation medicine specialist in New York, saw that most of his patients with chronic pain did not have evidence of acute injury or degenerative disk disease. Their persistent pain appeared to be independent of any structural damage to the spine. Dr. Sarno attributed the pain to what he called tension myoneural syndrome (TMS), or the body’s reaction to suppressed stress and emotional turmoil. Resolving that psychological conflict, Dr. Sarno believed, would lead to an improvement in pain.
Dr. Sarno’s theory has met skepticism from the mainstream community, but glowing testimonies from patients who say they benefited from his strategies fill the Internet. Dr. Sarno wrote several books on his ideas before his death in 2017. But he published only one peer-reviewed study, a 2003 review in the Archives of Physical Medicine and Rehabilitation coauthored by Ira Rashbaum, MD.
The interview has been edited for length and clarity.
What is your theory of back pain?
Dr. Rashbaum: My null hypothesis is that back pain is not due to psychological issues, so as to not be a biased doctor, I try to accept the null hypothesis or reject the null hypothesis. In most cases chronic back pain is not due to structural etiology. My sense is it’s a mind-body issue – the avoidance of feeling strong emotions like anger, rage, sadness, fear, shame, and guilt. Patients can embrace psychoeducational programs and if they don’t get better, we work with a psychotherapist or a licensed mental health counselor to help work through the patient’s feelings. That’s my experience over a number of years.
How do you determine if a patient has back pain from a mind-body issue or another cause?
Dr. Rashbaum: I do a very careful medical history, including a physical examination and review of any diagnostic studies they’ve undergone. In most situations, there’s not really a medical cause of the back pain. For instance, a lot of asymptomatic individuals have all sorts of horrible findings on medical imaging like CTs and MRIs, and the reverse is also true – many people with negative findings on imaging tests experience significant pain. My job as a diagnostician is to see how much of this is really a mind-body problem or something that stems from structural pathology.
How well do your patients react to being told that their back pain is, in a way, “in their head?”
Dr. Rashbaum: I have a skewed population. I’m sort of like a guru in mind-body back pain, so the people who come to me are already thinking along those lines. I ask: “What’s going on in your life?” Maybe there are job issues, marital issues, health issues, and I’d say that it’s certainly possible that stress can be causing this back pain.
Sometimes when I see a patient referred from another physician, I’m a bit hesitant to ask about what’s going on in their life. Even earlier today, I’d seen a patient with back pain and I had a sense that they were not really going to be open to a mind-body approach. So I said, do physical therapy.
What do you recommend primary care clinicians do with patients with back pain?
Dr. Rashbaum: You have to do a proper neurologic examination and musculoskeletal examination. It’s a tough situation because doctors in primary care have limited time to take care of patients. It’s difficult to have a deeper dive just to kind of see what’s going on in their life. But you can recommend useful agents like acetaminophen and muscle relaxants, which are sometimes okay.
What sorts of things do you tell patients to say to themselves when they’re experiencing pain?
Dr. Rashbaum: If the pain is severe, I recommend they take medication – over-the-counter analgesics or a muscle relaxant, if they have them – and take a warm shower or bath. I prefer acetaminophen up to three times per day, if that’s okay with the patient’s primary care physician, over NSAIDs because most pain is noninflammatory in nature. Once the pain is more manageable, patients should journal about what’s going on in their lives and/or meditate, and try to feel any strong emotions, such as anger, sadness, or fear.
What do you say to clinicians who are dismissive of the notion that chronic pain may stem from emotional repression, and that addressing the latter can resolve the former – particularly those who point to a lack of peer-reviewed data for such a link?
Dr. Rashbaum: I would tell them they could be looking harder for that evidence. For example, in a patient page from JAMA from April 24, 2013, on low back pain, often the cause of back pain is unknown. There are data in spine surgical journals that patients with psychological issues do worse with spine surgery. And in 2016 JAMA published a study from Cherkin and colleagues, which found that, among adults with chronic low back pain, treatment with mindfulness-based stress reduction or cognitive behavioral therapy resulted in greater improvement in back pain and functional limitations at 26 weeks, compared with usual care.
My feeling is that these psychosocial interventions are easy to try, relatively inexpensive, noninvasive, and, in my experience, often can lead to marked improvements. I believe that, for the vast majority of people with chronic pain, it makes much more sense to start by addressing mind-body issues than turning to that approach as a last resort.
Dr. Rashbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 decades ago, John E. Sarno, MD, published Healing Back Pain, a popular book that garnered something of a cult following. Looking at his own practice, Dr. Sarno, a rehabilitation medicine specialist in New York, saw that most of his patients with chronic pain did not have evidence of acute injury or degenerative disk disease. Their persistent pain appeared to be independent of any structural damage to the spine. Dr. Sarno attributed the pain to what he called tension myoneural syndrome (TMS), or the body’s reaction to suppressed stress and emotional turmoil. Resolving that psychological conflict, Dr. Sarno believed, would lead to an improvement in pain.
Dr. Sarno’s theory has met skepticism from the mainstream community, but glowing testimonies from patients who say they benefited from his strategies fill the Internet. Dr. Sarno wrote several books on his ideas before his death in 2017. But he published only one peer-reviewed study, a 2003 review in the Archives of Physical Medicine and Rehabilitation coauthored by Ira Rashbaum, MD.
The interview has been edited for length and clarity.
What is your theory of back pain?
Dr. Rashbaum: My null hypothesis is that back pain is not due to psychological issues, so as to not be a biased doctor, I try to accept the null hypothesis or reject the null hypothesis. In most cases chronic back pain is not due to structural etiology. My sense is it’s a mind-body issue – the avoidance of feeling strong emotions like anger, rage, sadness, fear, shame, and guilt. Patients can embrace psychoeducational programs and if they don’t get better, we work with a psychotherapist or a licensed mental health counselor to help work through the patient’s feelings. That’s my experience over a number of years.
How do you determine if a patient has back pain from a mind-body issue or another cause?
Dr. Rashbaum: I do a very careful medical history, including a physical examination and review of any diagnostic studies they’ve undergone. In most situations, there’s not really a medical cause of the back pain. For instance, a lot of asymptomatic individuals have all sorts of horrible findings on medical imaging like CTs and MRIs, and the reverse is also true – many people with negative findings on imaging tests experience significant pain. My job as a diagnostician is to see how much of this is really a mind-body problem or something that stems from structural pathology.
How well do your patients react to being told that their back pain is, in a way, “in their head?”
Dr. Rashbaum: I have a skewed population. I’m sort of like a guru in mind-body back pain, so the people who come to me are already thinking along those lines. I ask: “What’s going on in your life?” Maybe there are job issues, marital issues, health issues, and I’d say that it’s certainly possible that stress can be causing this back pain.
Sometimes when I see a patient referred from another physician, I’m a bit hesitant to ask about what’s going on in their life. Even earlier today, I’d seen a patient with back pain and I had a sense that they were not really going to be open to a mind-body approach. So I said, do physical therapy.
What do you recommend primary care clinicians do with patients with back pain?
Dr. Rashbaum: You have to do a proper neurologic examination and musculoskeletal examination. It’s a tough situation because doctors in primary care have limited time to take care of patients. It’s difficult to have a deeper dive just to kind of see what’s going on in their life. But you can recommend useful agents like acetaminophen and muscle relaxants, which are sometimes okay.
What sorts of things do you tell patients to say to themselves when they’re experiencing pain?
Dr. Rashbaum: If the pain is severe, I recommend they take medication – over-the-counter analgesics or a muscle relaxant, if they have them – and take a warm shower or bath. I prefer acetaminophen up to three times per day, if that’s okay with the patient’s primary care physician, over NSAIDs because most pain is noninflammatory in nature. Once the pain is more manageable, patients should journal about what’s going on in their lives and/or meditate, and try to feel any strong emotions, such as anger, sadness, or fear.
What do you say to clinicians who are dismissive of the notion that chronic pain may stem from emotional repression, and that addressing the latter can resolve the former – particularly those who point to a lack of peer-reviewed data for such a link?
Dr. Rashbaum: I would tell them they could be looking harder for that evidence. For example, in a patient page from JAMA from April 24, 2013, on low back pain, often the cause of back pain is unknown. There are data in spine surgical journals that patients with psychological issues do worse with spine surgery. And in 2016 JAMA published a study from Cherkin and colleagues, which found that, among adults with chronic low back pain, treatment with mindfulness-based stress reduction or cognitive behavioral therapy resulted in greater improvement in back pain and functional limitations at 26 weeks, compared with usual care.
My feeling is that these psychosocial interventions are easy to try, relatively inexpensive, noninvasive, and, in my experience, often can lead to marked improvements. I believe that, for the vast majority of people with chronic pain, it makes much more sense to start by addressing mind-body issues than turning to that approach as a last resort.
Dr. Rashbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 decades ago, John E. Sarno, MD, published Healing Back Pain, a popular book that garnered something of a cult following. Looking at his own practice, Dr. Sarno, a rehabilitation medicine specialist in New York, saw that most of his patients with chronic pain did not have evidence of acute injury or degenerative disk disease. Their persistent pain appeared to be independent of any structural damage to the spine. Dr. Sarno attributed the pain to what he called tension myoneural syndrome (TMS), or the body’s reaction to suppressed stress and emotional turmoil. Resolving that psychological conflict, Dr. Sarno believed, would lead to an improvement in pain.
Dr. Sarno’s theory has met skepticism from the mainstream community, but glowing testimonies from patients who say they benefited from his strategies fill the Internet. Dr. Sarno wrote several books on his ideas before his death in 2017. But he published only one peer-reviewed study, a 2003 review in the Archives of Physical Medicine and Rehabilitation coauthored by Ira Rashbaum, MD.
The interview has been edited for length and clarity.
What is your theory of back pain?
Dr. Rashbaum: My null hypothesis is that back pain is not due to psychological issues, so as to not be a biased doctor, I try to accept the null hypothesis or reject the null hypothesis. In most cases chronic back pain is not due to structural etiology. My sense is it’s a mind-body issue – the avoidance of feeling strong emotions like anger, rage, sadness, fear, shame, and guilt. Patients can embrace psychoeducational programs and if they don’t get better, we work with a psychotherapist or a licensed mental health counselor to help work through the patient’s feelings. That’s my experience over a number of years.
How do you determine if a patient has back pain from a mind-body issue or another cause?
Dr. Rashbaum: I do a very careful medical history, including a physical examination and review of any diagnostic studies they’ve undergone. In most situations, there’s not really a medical cause of the back pain. For instance, a lot of asymptomatic individuals have all sorts of horrible findings on medical imaging like CTs and MRIs, and the reverse is also true – many people with negative findings on imaging tests experience significant pain. My job as a diagnostician is to see how much of this is really a mind-body problem or something that stems from structural pathology.
How well do your patients react to being told that their back pain is, in a way, “in their head?”
Dr. Rashbaum: I have a skewed population. I’m sort of like a guru in mind-body back pain, so the people who come to me are already thinking along those lines. I ask: “What’s going on in your life?” Maybe there are job issues, marital issues, health issues, and I’d say that it’s certainly possible that stress can be causing this back pain.
Sometimes when I see a patient referred from another physician, I’m a bit hesitant to ask about what’s going on in their life. Even earlier today, I’d seen a patient with back pain and I had a sense that they were not really going to be open to a mind-body approach. So I said, do physical therapy.
What do you recommend primary care clinicians do with patients with back pain?
Dr. Rashbaum: You have to do a proper neurologic examination and musculoskeletal examination. It’s a tough situation because doctors in primary care have limited time to take care of patients. It’s difficult to have a deeper dive just to kind of see what’s going on in their life. But you can recommend useful agents like acetaminophen and muscle relaxants, which are sometimes okay.
What sorts of things do you tell patients to say to themselves when they’re experiencing pain?
Dr. Rashbaum: If the pain is severe, I recommend they take medication – over-the-counter analgesics or a muscle relaxant, if they have them – and take a warm shower or bath. I prefer acetaminophen up to three times per day, if that’s okay with the patient’s primary care physician, over NSAIDs because most pain is noninflammatory in nature. Once the pain is more manageable, patients should journal about what’s going on in their lives and/or meditate, and try to feel any strong emotions, such as anger, sadness, or fear.
What do you say to clinicians who are dismissive of the notion that chronic pain may stem from emotional repression, and that addressing the latter can resolve the former – particularly those who point to a lack of peer-reviewed data for such a link?
Dr. Rashbaum: I would tell them they could be looking harder for that evidence. For example, in a patient page from JAMA from April 24, 2013, on low back pain, often the cause of back pain is unknown. There are data in spine surgical journals that patients with psychological issues do worse with spine surgery. And in 2016 JAMA published a study from Cherkin and colleagues, which found that, among adults with chronic low back pain, treatment with mindfulness-based stress reduction or cognitive behavioral therapy resulted in greater improvement in back pain and functional limitations at 26 weeks, compared with usual care.
My feeling is that these psychosocial interventions are easy to try, relatively inexpensive, noninvasive, and, in my experience, often can lead to marked improvements. I believe that, for the vast majority of people with chronic pain, it makes much more sense to start by addressing mind-body issues than turning to that approach as a last resort.
Dr. Rashbaum reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nutritional psychiatry: Does it exist?
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.
Matt was diagnosed with ADHD combined type when he was 6 years old. Given his age, the family was reluctant to try medications, but after a couple years of parenting classes and reward charts, the parents requested a stimulant. He had significant improvement in focus and impulsivity but also reduced appetite. Now at age 13, irritability and depressive symptoms have been increasing for 9 months. Skeptical of adding another medication, his parents ask whether nutrition might be an alternative tool to treat his symptoms?
What a healthy diet even consists of seems to be a moving target over decades and years. Trendy research, supplements, and dietary approaches proliferate alongside appealing theories of action. In the end, weighing which intervention is effective for which disorder and at what cost becomes murky.
Yet several fundamental principles seem clear and consistent over time and across studies.
Starting early
There is reliable evidence that in the perinatal environment, nutrition sets the stage for many aspects of healthy development. These effects are likely mediated variously through the hypothalamic-pituitary-adrenal axis, the trillions of gut bacteria that make up the microbiome, gene-environment interactions, and more. Maternal malnutrition and stress prenatally puts infants at risk for not only poor birth outcomes but also psychiatric challenges throughout childhood, such as ADHD, anxiety, depression, and autism.1
Intervening in the perinatal period has long-term benefits. A first step includes assessing food security, beginning with consistent access to nutritious food. It is important to inquire about the role of food and nutrition in the family’s history and culture, as well as identifying resources to support access to affordable nutrition. This can be paired with parenting interventions, such as family meals without screens. This may require scaffolding positive conversations in high-conflict family settings (see The Family Dinner Project).
Healthy diets promote mental health
If food security is achieved, what is next? Clinicians can inquire about the who, what, where, when, and why of nutrition to learn about a family’s eating habits.2 While randomized controlled data is very limited, both cross-sectional and longitudinal studies show that healthy diets in youth correlate with mental health – more healthy foods reducing internalizing and externalizing disorders, and more typical Western diets increasing the risk. On average, dietary interventions include higher levels of fruits and vegetables, fish, and nuts, and lower levels of processed foods.2 There is not evidence that restrictive diets or fasting is appropriate or safe for youth. Additionally, involving children in getting, growing, or preparing food with gradually increasing autonomy fosters self-confidence and skill development.
In those struggling with restrictive eating disorders, food is medicine – helping those with restrictive diets to develop more balanced and adequate intake for metabolic needs. Outside of diagnosable eating disorders, weight or body mass index is less of a goal or marker when it comes to mental health. Instead, look for participation in enjoyable activities, opportunities to move and rest, and a body image that supports self-care and self-confidence (see the National Institutes of Health’s We Can! Program). Creating dissonance with cultural ideals of appearance centered on thinness can prevent future eating disorders.3
Nutraceutical options
Outside of eating disorders, specific foods and plants with health or medicinal properties – variously called nutraceuticals, phytoceuticals, or micronutrients – have emerging evidence in mental health. A 2022 expert academic consensus panel reviewed the literature to create clinical guidelines in this area.4 For major depression, adding omega-3 fatty acids to standard antidepressant treatment or standalone St. John’s wort have adequate evidence to recommend, while adjunctive probiotics, zinc, saffron, and curcumin have sufficient though less robust evidence. S-adenosyl methionine, vitamin D, and methyfolate showed only weak evidence for depression, while vitamin C, magnesium, creatine, N-acetylcysteine, folate, and monotherapy omega-3s do not have sufficient evidence to be recommended. For ADHD there was weak support for vitamin D, but no clear evidence for omega-3s, zinc, gingko, or acetyl L-carnitine. For anxiety, there is moderate evidence for ashwagandha and lavender in adults. A child psychiatry review suggests also trying chamomile for generalized anxiety based on the evidence in young adults, and underscores some data for N-acetylcysteine for OCD in particular.5
Many of these nutraceuticals exhibit small or moderate effects in a limited number of trials, with generally much less data for youth, compared with adults. While the same could be said for many on- and off-label uses of psychiatric medications for kids, clinicians would be wise to consider these highly specific nutritional interventions as items on the menu of treatment options rather than stand-alone treatments.
Revisitng the case study
Reflecting on Matt’s care, his pediatrician first assessed his dietary patterns, noting late-night eating and caffeine use with minimal hydration or fiber across the day. Recommendations for keeping fruit and vegetable snacks easily accessible as well as carrying a water flask are well received. They also discuss adding omega-3 fatty acids and probiotics with his morning stimulant while he awaits a referral for cognitive-behavioral therapy in order to address his depressive symptoms and minimize medication needs.
Beyond addressing food security and balanced family meals, specific interventions may be appropriate as initial treatment adjuncts for mild and some moderate mental illness. For more intense moderate to severe illness, nutritional psychiatry may be considered in combination with treatments with stronger evidence. At a community level, clinicians can help advocate for universal school meal programs to address food security, and so-called salad bar interventions to increase fruit/vegetable uptake among school-age children.
Dr. Rosenfeld is associate professor of psychiatry and pediatrics at University of Vermont and the Vermont Center for Children, Youth, and Families, both in Burlington. He has no disclosures.
References
1 Vohr BR et al. Pediatrics. 2017;139:S38-49.
2. Hosker DK et al. Child Adol Psychiatr Clin N Am. 2019;28(2):171-93.
3. Stice E et al. Int J Eat Disord. 2013;46(5):478-85.
4. Sarris J et al. World J Biol Psychiatry. 2022;23(6):424-55.
5. Simkin DR et al. Child Adolesc Psychiatric Clin N Am. 2023;32:193-216.







