User login
Use of Fecal Immunochemical Testing in Acute Patient Care in a Safety Net Hospital System
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
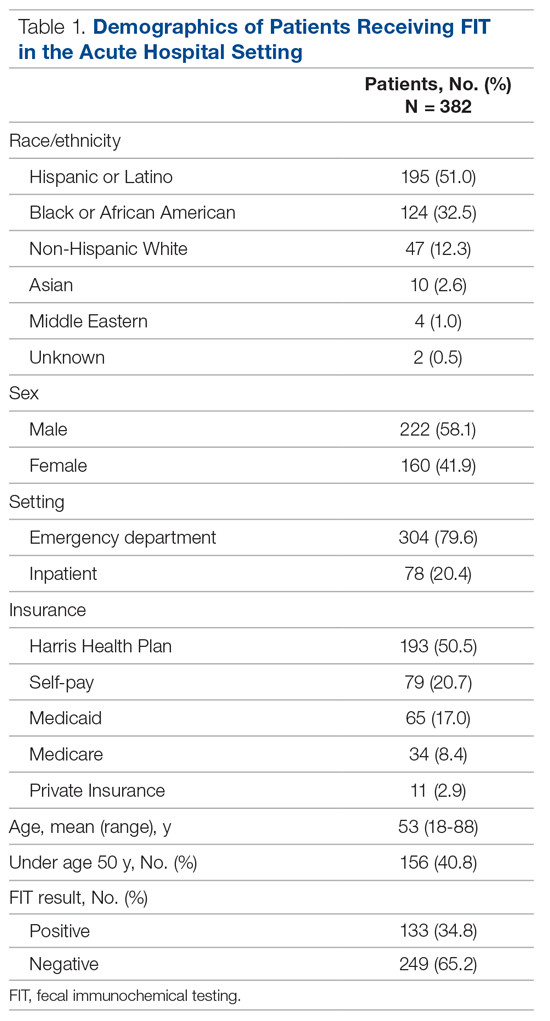
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.
Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).
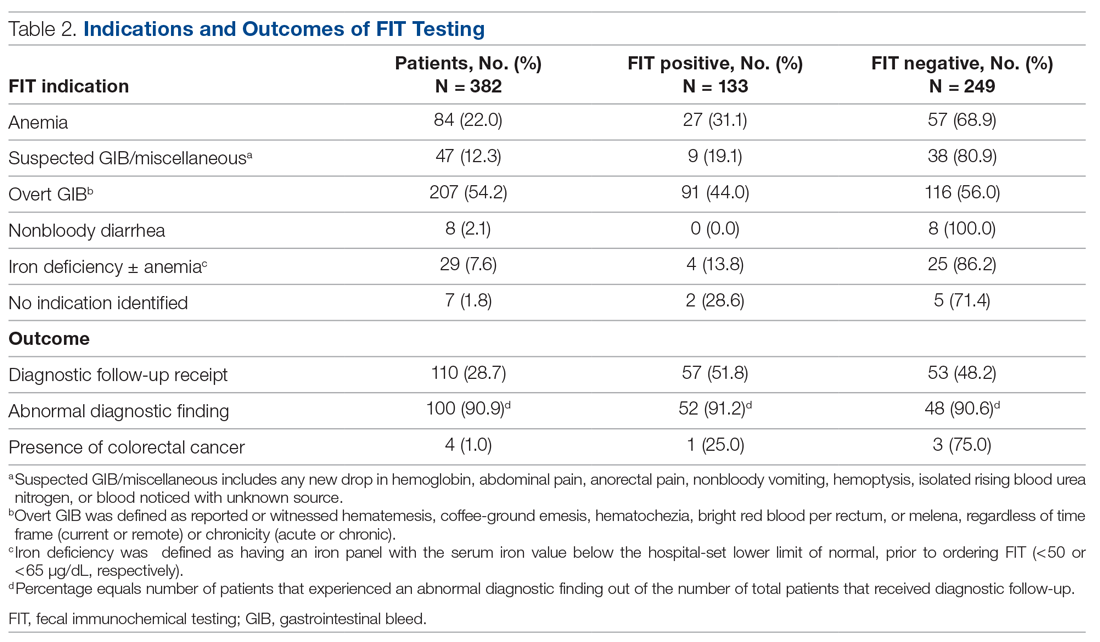
A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
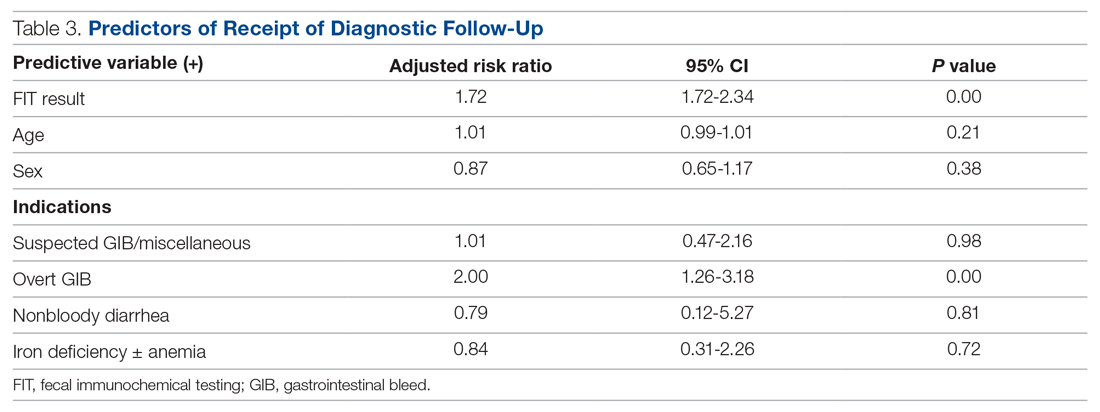
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).
Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
From Baylor College of Medicine, Houston, TX (Drs. Spezia-Lindner, Montealegre, Muldrew, and Suarez) and Harris Health System, Houston, TX (Shanna L. Harris, Maria Daheri, and Drs. Muldrew and Suarez).
Abstract
Objective: To characterize and analyze the prevalence, indications for, and outcomes of fecal immunochemical testing (FIT) in acute patient care within a safety net health care system’s emergency departments (EDs) and inpatient settings.
Design: Retrospective cohort study derived from administrative data.
Setting: A large, urban, safety net health care delivery system in Texas. The data gathered were from the health care system’s 2 primary hospitals and their associated EDs. This health care system utilizes FIT exclusively for fecal occult blood testing.
Participants: Adults ≥18 years who underwent FIT in the ED or inpatient setting between August 2016 and March 2017. Chart review abstractions were performed on a sample (n = 382) from the larger subset.
Measurements: Primary data points included total FITs performed in acute patient care during the study period, basic demographic data, FIT indications, FIT result, receipt of invasive diagnostic follow-up, and result of invasive diagnostic follow-up. Multivariable log-binomial regression was used to calculate risk ratios (RRs) to assess the association between FIT result and receipt of diagnostic follow-up. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result.
Results: During the 8-month study period, 2718 FITs were performed in the ED and inpatient setting, comprising 5.7% of system-wide FITs. Of the 382 patients included in the chart review who underwent acute care FIT, a majority had their test performed in the ED (304, 79.6%), 133 of which were positive (34.8%). The most common indication for FIT was evidence of overt gastrointestinal (GI) bleed (207, 54.2%), followed by anemia (84, 22.0%). While a positive FIT result was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72; P < 0.001), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00; P = 0.003). Of patients who underwent FIT and received diagnostic follow-up (n = 110), 48.2% were FIT negative. These patients were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86). Of the 382 patients in the study, 4 (1.0%) were subsequently diagnosed with colorectal cancer (CRC). Of those 4 patients, 1 (25%) was FIT positive.
Conclusion: FIT is being utilized in acute patient care outside of its established indication for CRC screening in asymptomatic, average-risk adults. Our study demonstrates that FIT is not useful in acute patient care.
Keywords: FOBT; FIT; fecal immunochemical testing; inpatient.
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States. It is estimated that in 2020, 147,950 individuals will be diagnosed with invasive CRC and 53,200 will die from it.1 While the overall incidence has been declining for decades, it is rising in young adults.2–4 Screening using direct visualization procedures (colonoscopy and sigmoidoscopy) and stool-based tests has been demonstrated to improve detection of precancerous and early cancerous lesions, thereby reducing CRC mortality.5 However, screening rates in the United States are suboptimal, with only 68.8% of adults aged 50 to 75 years screened according to guidelines in 2018.6Stool-based testing is a well-established and validated screening measure for CRC in asymptomatic individuals at average risk. Its widespread use in this population has been shown to cost-effectively screen for CRC among adults 50 years of age and older.5,7 Presently, the 2 most commonly used stool-based assays in the US health care system are guaiac-based tests (guaiac fecal occult blood test [gFOBT], Hemoccult) and
Despite the exclusive validation of FOBTs for use in CRC screening, studies have demonstrated that they are commonly used for a multitude of additional indications in emergency department (ED) and inpatient settings, most aimed at detecting or confirming GI blood loss. This may lead to inappropriate patient management, including the receipt of unnecessary follow-up procedures, which can incur significant costs to the patient and the health system.13-19 These costs may be particularly burdensome in safety net health systems (ie, those that offer access to care regardless of the patient’s ability to pay), which serve a large proportion of socioeconomically disadvantaged individuals in the United States.20,21 To our knowledge, no published study to date has specifically investigated the role of FIT in acute patient management.
This study characterizes the use of FIT in acute patient care within a large, urban, safety net health care system. Through a retrospective review of administrative data and patient charts, we evaluated FIT use prevalence, indications, and patient outcomes in the ED and inpatient settings.
Methods
Setting
This study was conducted in a large, urban, county-based integrated delivery system in Houston, Texas, that provides health care services to one of the largest uninsured and underinsured populations in the country.22 The health system includes 2 main hospitals and more than 20 ambulatory care clinics. Within its ambulatory care clinics, the health system implements a population-based screening strategy using stool-based testing. All adults aged 50 years or older who are due for FIT are identified through the health-maintenance module of the electronic medical record (EMR) and offered a take-home FIT. The health system utilizes FIT exclusively (OC-Light S FIT, Polymedco, Cortlandt Manor, NY); no guaiac-based assays are available.
Design and Data Collection
We began by using administrative records to determine the proportion of FITs conducted health system-wide that were ordered and completed in the acute care setting over the study period (August 2016-March 2017). Specifically, we used aggregate quality metric reports, which quantify the number of FITs conducted at each health system clinic and hospital each month, to calculate the proportion of FITs done in the ED and inpatient hospital setting.
We then conducted a retrospective cohort study of 382 adult patients who received FIT in the EDs and inpatient wards in both of the health system’s hospitals over the study period. All data were collected by retrospective chart review in Epic (Madison, WI) EMRs. Sampling was performed by selecting the medical record numbers corresponding to the first 50 completed FITs chronologically each month over the 8-month period, with a total of 400 charts reviewed.
Data collected included basic patient demographics, location of FIT ordering (ED vs inpatient), primary service ordering FIT, FIT indication, FIT result, and receipt and results of invasive diagnostic follow-up. Demographics collected included age, biological sex, race (self-selected), and insurance coverage.
FIT indication was determined based on resident or attending physician notes. The history of present illness, physical exam, and assessment and plan section of notes were reviewed by the lead author for a specific statement of indication for FIT or for evidence of clinical presentation for which FIT could reasonably be ordered. Indications were iteratively reviewed and collapsed into 6 different categories: anemia, iron deficiency with or without anemia, overt GIB, suspected GIB/miscellaneous, non-bloody diarrhea, and no indication identified. Overt GIB was defined as reported or witnessed hematemesis, coffee-ground emesis, hematochezia, bright red blood per rectum, or melena irrespective of time frame (current or remote) or chronicity (acute, subacute, or chronic). In cases where signs of overt bleed were not witnessed by medical professionals, determination of conditions such as melena or coffee-ground emesis were made based on health care providers’ assessment of patient history as documented in his or her notes. Suspected GIB/miscellaneous was defined with the following parameters: any new drop in hemoglobin, abdominal pain, anorectal pain, non-bloody vomiting, hemoptysis, isolated rising blood urea nitrogen, or patient noticing blood on self, clothing, or in the commode without an identified source. Patients who were anemic and found to have iron deficiency on recent lab studies (within 6 months) were reflexively categorized into iron deficiency with or without anemia as opposed to the “anemia” category, which was comprised of any anemia without recent iron studies or non-iron deficient anemia. FIT result was determined by test result entry in Epic, with results either reading positive or negative.
Diagnostic follow-up, for our purposes, was defined as receipt of an invasive procedure or surgery, including esophagogastroduodenoscopy (EGD), colonoscopy, flexible sigmoidoscopy, diagnostic and/or therapeutic abdominal surgical intervention, or any combination of these. Results of diagnostic follow-up were coded as normal or abnormal. A normal result was determined if all procedures performed were listed as normal or as “no pathological findings” on the operative or endoscopic report. Any reported pathologic findings on the operative/endoscopic report were coded as abnormal.
Statistical Analysis
Proportions were used to describe demographic characteristics of patients who received a FIT in acute hospital settings. Bivariable tables and Chi-square tests were used to compare indications and outcomes for FIT-positive and FIT-negative patients. The association between receipt of an invasive diagnostic follow-up (outcome) and the results of an inpatient FIT (predictor) was assessed using multivariable log-binomial regression to calculate risk ratios (RRs) and corresponding 95% confidence intervals. Log-binomial regression was used over logistic regression given that adjusted odds ratios generated by logistic regression often overestimate the association between the risk factor and the outcome when the outcome is common,23 as in the case of diagnostic follow-up. The model was adjusted for variables selected a priori, specifically, age, gender, and FIT indication. Chi-square analysis was used to compare the proportion of abnormal findings on diagnostic follow-up by FIT result (negative vs positive).
Results
During the 8-month study period, there were 2718 FITs ordered and completed in the acute care setting, compared to 44,662 FITs ordered and completed in the outpatient setting (5.7% performed during acute care).
Among the 400 charts reviewed, 7 were excluded from the analysis because they were duplicates from the same patient, and 11 were excluded due to insufficient information in the patient’s medical record, resulting in 382 patients included in the analysis. Patient demographic characteristics are described in Table 1. Patients were predominantly Hispanic/Latino or Black/African American (51.0% and 32.5%, respectively), a majority had insurance through the county health system (50.5%), and most were male (58.1%). The average age of those receiving FIT was 52 years (standard deviation, 14.8 years), with 40.8% being under the age of 50. For a majority of patients, FIT was ordered in the ED by emergency medicine providers (79.8%). The remaining FITs were ordered by providers in 12 different inpatient departments. Of the FITs ordered, 35.1% were positive.

Indications for ordering FIT are listed in Table 2. The largest proportion of FITs were ordered for overt signs of GIB (54.2%), followed by anemia (22.0%), suspected GIB/miscellaneous reasons (12.3%), iron deficiency with or without anemia (7.6%), and non-bloody diarrhea (2.1%). In 1.8% of cases, no indication for FIT was found in the EMR. No FITs were ordered for the indication of CRC detection. Of these indication categories, overt GIB yielded the highest percentage of FIT positive results (44.0%), and non-bloody diarrhea yielded the lowest (0%).

A total of 110 patients (28.7%) underwent FIT and received invasive diagnostic follow-up. Of these 110 patients, 57 (51.8%) underwent EGD (2 of whom had further surgical intervention), 21 (19.1%) underwent colonoscopy (1 of whom had further surgical intervention), 25 (22.7%) underwent dual EGD and colonoscopy, 1 (0.9%) underwent flexible sigmoidoscopy, and 6 (5.5%) directly underwent abdominal surgical intervention. There was a significantly higher rate of diagnostic follow-up for FIT-positive vs FIT-negative patients (42.9% vs 21.3%; P < 0.001). However, of the 110 patients who underwent subsequent diagnostic follow-up, 48.2% were FIT negative. FIT-negative patients who received diagnostic follow-up were just as likely to have an abnormal finding as FIT-positive patients (90.6% vs 91.2%; P = 0.86).
Of the 382 patients in the study, 4 were diagnosed with CRC through diagnostic follow-up (1.0%). Of those 4 patients, 1 was FIT positive.
The results of the multivariable analyses to evaluate predictors of diagnostic colonoscopy are described in Table 3. Variables in the final model were FITresult, age, and FIT indication. After adjusting for other variables in the model, receipt of diagnostic follow-up was significantly associated with having a positive FIT (adjusted RR, 1.72; P < 0.001) and an overt GIB as an indication (adjusted RR, 2.00; P < 0.01).

Discussion
During the time frame of our study, 5.7% of all FITs ordered within our health system were ordered in the acute patient care setting at our hospitals. The most common indication was overt GIB, which was the indication for 54.2% of patients. Of note, none of the FITs ordered in the acute patient care setting were ordered for CRC screening. These findings support the evidence in the literature that stool-based screening tests, including FIT, are commonly used in US health care systems for diagnostic purposes and risk stratification in acute patient care to detect GIBs.13-18
Our data suggest that FIT was not a clinically useful test in determining a patient’s need for diagnostic follow-up. While having a positive FIT was significantly associated with obtaining a diagnostic exam in multivariate analysis (RR, 1.72), having signs of overt GI bleeding was a stronger predictor of diagnostic follow-up (RR, 2.00). This salient finding is evidence that a thorough clinical history and physical exam may more strongly predict whether a patient will undergo endoscopy or other follow-up than a FIT result. These findings support other studies in the literature that have called into question the utility of FOBTs in these acute settings.13-19 Under such circumstances, FOBTs have been shown to rarely influence patient management and thus represent an unnecessary expense.13–17 Additionally, in some cases, FOBT use in these settings may negatively affect patient outcomes. Such adverse effects include delaying treatment until results are returned or obfuscating indicated management with the results (eg, a patient with indications for colonoscopy not being referred due to a negative FOBT).13,14,17
We found that, for patients who subsequently went on to have diagnostic follow-up (most commonly endoscopy), there was no difference in the likelihood of FIT-positive and FIT-negative patients to have an abnormality discovered (91.2% vs 90.6%; P = 0.86). This analysis demonstrates no post-hoc support for FIT positivity as a predictor of presence of pathology in patients who were discriminately selected for diagnostic follow-up on clinical grounds by gastroenterologists and surgeons. It does, however, further support that clinical judgment about the need for diagnostic follow-up—irrespective of FIT result—has a very high yield for discovery of pathology in the acute setting.
There are multiple reasons why FOBTs, and specifically FIT, contribute little in management decisions for patients with suspected GI blood loss. Use of FIT raises concern for both false-negatives and false-positives when used outside of its indication. Regarding false- negatives, FIT is an unreliable test for detection of blood loss from the upper GI tract. As FITs utilize antibodies to detect the presence of globin, a byproduct of red blood cell breakdown, it is expected that FIT would fail to detect many cases of upper GI bleeding, as globin is broken down in the upper GI tract.24 This fact is part of what has made FIT a more effective CRC screening test than its guaiac-based counterparts—it has greater specificity for lower GI tract blood loss compared to tests relying on detection of heme.8 While guaiac-based assays like Hemoccult have also been shown to be poor tests in acute patient care, they may more frequently, though still unreliably, detect blood of upper GI origin. We believe that part of the ongoing use of FIT in patients with a suspected upper GIB may be from lack of understanding among providers on the mechanistic difference between gFOBTs and FITs, even though gFOBTs also yield highly unreliable results.
FIT does not have the same risk of false-positive results that guaiac-based tests have, which can yield positive results with extra-intestinal blood ingestion, aspirin, or alcohol use; insignificant GI bleeding; and consumption of peroxidase-containing foods.13,17,25 However, from a clinical standpoint, there are several scenarios of insignificant bleeding that would yield a positive FIT result, such as hemorrhoids, which are common in the US population.26,27 Additionally, in the ED, where most FITs were performed in our study, it is possible that samples for FITs are being obtained via digital rectal exam (DRE) given patients’ acuity of medical conditions and time constraints. However, FIT has been validated when using a formed stool sample. Obtaining FIT via DRE may lead to microtrauma to the rectum, which could hypothetically yield a positive FIT.
Strengths of this study include its use of in-depth chart data on a large number of FIT-positive patients, which allowed us to discern indications, outcomes, and other clinical data that may have influenced clinical decision-making. Additionally, whereas other studies that address FOBT use in acute patient care have focused on guaiac-based assays, our findings regarding the lack of utility of FIT are novel and have particular relevance as FITs continue to grow in popularity. Nonetheless, there are certain limitations future research should seek to address. In this study, the diagnostic follow-up result was coded by presence or absence of pathologic findings but did not qualify findings by severity or attempt to determine whether the pathology noted on diagnostic follow-up was the definitive source of the suspected GI bleed. These variables could help determine whether there was a difference in severity of bleeding between FIT-positive and FIT-negative patients and could potentially be studied with a prospective research design. Our own study was not designed to address the question of whether FIT result informs patient management decisions. To answer this directly, interviews would have to be conducted with those making the follow-up decision (ie, endoscopists and surgeons). Additionally, this study was not adequately powered to make determinations on the efficacy of FIT in the acute care setting for detection of CRC. As mentioned, only 1 of the 4 patients (25%) who went on to be diagnosed with CRC on follow-up was initially FIT-positive. This would require further investigation.
Conclusion
FIT is being utilized for diagnostic purposes in the acute care of symptomatic patients, which is a misuse of an established screening test for CRC. While our study was not designed to answer whether and how often a FIT result informs subsequent patient management, our results indicate that FIT is an ineffective diagnostic and risk-stratification tool when used in the acute care setting. Our findings add to existing evidence that indicates FOBTs should not be used in acute patient care.
Taken as a whole, the results of our study add to a growing body of evidence demonstrating no role for FOBTs, and specifically FIT, in acute patient care. In light of this evidence, some health care systems have already demonstrated success with system-wide disinvestment from the test in acute patient care settings, with one group publishing about their disinvestment process.28 After completion of our study, our preliminary data were presented to leadership from the internal medicine, emergency medicine, and laboratory divisions within our health care delivery system to galvanize complete disinvestment of FIT from acute care at our hospitals, a policy that was put into effect in July 2019.
Corresponding author: Nathaniel J. Spezia-Lindner, MD, Baylor College of Medicine, 7200 Cambridge St, BCM 903, Ste A10.197, Houston, TX 77030; [email protected].
Financial disclosures: None.
Funding: Cancer Prevention and Research Institute of Texas, CPRIT (PP170094, PDs: ML Jibaja-Weiss and JR Montealegre).
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 10.1CA Cancer 10.1J Clin. 2020;70(1):7-30.
2. Howlader NN, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. National Cancer Institute; 2017:1-2.
3. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. 10.1J Natl Cancer Inst. 2017;109(8):djw322.
4. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. 10.25JAMA Surg. 2015;150(1):17-22.
5. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. 10.25JAMA. 2016;315(23):2576-2594.
6. Centers for Disease Control and Prevention (CDC). Use of colorectal cancer screening tests. Behavioral Risk Factor Surveillance System. October 22, 2019. Accessed February 10, 2021. https://www.cdc.gov/cancer/colorectal/statistics/use-screening-tests-BRFSS.htm
7. Hewitson P, Glasziou PP, Irwig L, et al. Screening for colorectal cancer using the fecal occult blood test, Hemoccult. 10.25Cochrane Database Syst Rev. 2007;2007(1):CD001216.
8. Bujanda L, Lanas Á, Quintero E, et al. Effect of aspirin and antiplatelet drugs on the outcome of the fecal immunochemical test. 10.25Mayo Clin Proc. 2013;88(7):683-689.
9. Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. 10.25J Natl Cancer Inst. 2007;99(19):1462-1470.
10. Dancourt V, Lejeune C, Lepage C, et al. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. 10.25Eur J Cancer. 2008;44(15):2254-2258.
11. Hol L, Wilschut JA, van Ballegooijen M, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. 10.25Br J Cancer. 2009;100(7):1103-1110.
12. Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128(10):2415-2424.
13. Friedman A, Chan A, Chin LC, et al. Use and abuse of faecal occult blood tests in an acute hospital inpatient setting. Intern Med J. 2010;40(2):107-111.
14. Narula N, Ulic D, Al-Dabbagh R, et al. Fecal occult blood testing as a diagnostic test in symptomatic patients is not useful: a retrospective chart review. Can J Gastroenterol Hepatol. 2014;28(8):421-426.
15. Ip S, Sokoro AA, Kaita L, et al. Use of fecal occult blood testing in hospitalized patients: results of an audit. Can J Gastroenterol Hepatol. 2014;28(9):489-494.
16. Mosadeghi S, Ren H, Catungal J, et al. Utilization of fecal occult blood test in the acute hospital setting and its impact on clinical management and outcomes. J Postgrad Med. 2016;62(2):91-95.
17. van Rijn AF, Stroobants AK, Deutekom M, et al. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24(11):1266-1269.
18. Sharma VK, Komanduri S, Nayyar S, et al. An audit of the utility of in-patient fecal occult blood testing. Am J Gastroenterol. 2001;96(4):1256-1260.
19. Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183(13):1474-1481.
20. Chokshi DA, Chang JE, Wilson RM. Health reform and the changing safety net in the United States. N Engl J Med. 2016;375(18):1790-1796.
21. Nguyen OK, Makam AN, Halm EA. National use of safety net clinics for primary care among adults with non-Medicaid insurance in the United States. PLoS One. 2016;11(3):e0151610.
22. United States Census Bureau. American Community Survey. Selected Economic Characteristics. 2019. Accessed February 20, 2021. https://data.census.gov/cedsci/table?q=ACSDP1Y2019.DP03%20Texas&g=0400000US48&tid=ACSDP1Y2019.DP03&hidePreview=true
23. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943.
24. Rockey DC. Occult gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34(4):699-718.
25. Macrae FA, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82(5 pt 1):891-898.
26. Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology. 1990;98(2):380-386.
27. Fleming JL, Ahlquist DA, McGill DB, et al. Influence of aspirin and ethanol on fecal blood levels as determined by using the HemoQuant assay. Mayo Clin Proc. 1987;62(3):159-163.
28. Gupta A, Tang Z, Agrawal D. Eliminating in-hospital fecal occult blood testing: our experience with disinvestment. Am J Med. 2018;131(7):760-763.
Implementing the AMI READMITS Risk Assessment Score to Increase Referrals Among Patients With Type I Myocardial Infarction
From The Johns Hopkins Hospital, Baltimore, MD (Dr. Muganlinskaya and Dr. Skojec, retired); The George Washington University, Washington, DC (Dr. Posey); and Johns Hopkins University, Baltimore, MD (Dr. Resar).
Abstract
Objective: Assessing the risk characteristics of patients with acute myocardial infarction (MI) can help providers make appropriate referral decisions. This quality improvement project sought to improve timely, appropriate referrals among patients with type I MI by adding a risk assessment, the AMI READMITS score, to the existing referral protocol.
Methods: Patients’ chart data were analyzed to assess changes in referrals and timely follow-up appointments from pre-intervention to intervention. A survey assessed providers’ satisfaction with the new referral protocol.
Results: Among 57 patients (n = 29 preintervention; n = 28 intervention), documented referrals increased significantly from 66% to 89% (χ2 = 4.571, df = 1, P = 0.033); and timely appointments increased by 10%, which was not significant (χ2 = 3.550, df = 2, P = 0.169). Most providers agreed that the new protocol was easy to use, useful in making referral decisions, and improved the referral process. All agreed the risk score should be incorporated into electronic clinical notes. Provider opinions related to implementing the risk score in clinical practice were mixed. Qualitative feedback suggests this was due to limited validation of the AMI READMITS score in reducing readmissions.
Conclusions: Our risk-based referral protocol helped to increase appropriate referrals among patients with type I MI. Provider adoption may be enhanced by incorporating the protocol into electronic clinical notes. Research to further validate the accuracy of the AMI READMITS score in predicting readmissions may support adoption of the protocol in clinical practice.
Keywords: quality improvement; type I myocardial infarction; referral process; readmission risk; risk assessment; chart review.
Early follow-up after discharge is an important strategy to reduce the risk of unplanned hospital readmissions among patients with various conditions.1-3 While patient confounding factors, such as chronic health problems, environment, socioeconomic status, and literacy, make it difficult to avoid all unplanned readmissions, early follow-up may help providers identify and appropriately manage some health-related issues, and as such is a pivotal element of a readmission prevention strategy.4 There is evidence that patients with non-ST elevation myocardial infarction (NSTEMI) who have an outpatient appointment with a physician within 7 days after discharge have a lower risk of 30-day readmission.5
Our hospital’s postmyocardial infarction clinic was created to prevent unplanned readmissions within 30 days after discharge among patients with type I myocardial infarction (MI). Since inception, the number of referrals has been much lower than expected. In 2018, the total number of patients discharged from the hospital with type I MI and any troponin I level above 0.40 ng/mL was 313. Most of these patients were discharged from the hospital’s cardiac units; however, only 91 referrals were made. To increase referrals, the cardiology nurse practitioners (NPs) developed a post-MI referral protocol (Figure 1). However, this protocol was not consistently used and referrals to the clinic remained low.
Evidence-based risk assessment tools have the potential to increase effective patient management. For example, cardiology providers at the hospital utilize various scores, such as CHA2DS2-VASc6 and the Society of Thoracic Surgery risk score,7 to plan patient management. Among the scores used to predict unplanned readmissions for MI patients, the most promising is the AMI READMITS score.8 Unlike other nonspecific prediction models, the AMI READMITS score was developed based on variables extracted from the electronic health records (EHRs) of patients who were hospitalized for MI and readmitted within 30 days after discharge. Recognizing the potential to increase referrals by integrating an MI-specific risk assessment, this quality improvement study modified the existing referral protocol to include the patients’ AMI READMITS score and recommendations for follow-up.
Currently, there are no clear recommendations on how soon after discharge patients with MI should undergo follow-up. As research data vary, we selected 7 days follow-up for patients from high risk groups based on the “See you in 7” initiative for patients with heart failure (HF) and MI,9,10 as well as evidence that patients with NSTEMI have a lower risk of 30-day readmission if they have follow-up within 7 days after discharge5; and we selected 14 days follow-up for patients from low-risk groups based on evidence that postdischarge follow-up within 14 days reduces risk of 30-day readmission in patients with acute myocardial infarction (AMI) and/or acutely decompensated HF.11
Methods
This project was designed to answer the following question: For adult patients with type I MI, does implementation of a readmission risk assessment referral protocol increase the percentage of referrals and appointments scheduled within a recommended time? Anticipated outcomes included: (1) increased referrals to a cardiologist or the post-MI clinic; (2) increased scheduled follow-up appointments within 7 to 14 days; (3) provider satisfaction with the usability and usefulness of the new protocol; and (4) consistent provider adoption of the new risk assessment referral protocol.
To evaluate the degree to which these outcomes were achieved, we reviewed patient charts for 2 months prior and 2 months during implementation of the new referral protocol. As shown in Figure 2, the new protocol added the following process steps to the existing protocol: calculation of the AMI READMITS score, recommendations for follow-up based on patients’ risk score, and guidance to refer patients to the post-MI clinic if patients did not have an appointment with a cardiologist within 7 to 14 days after discharge. Patients’ risk assessment scores were obtained from forms completed by clinicians during the intervention. Clinician’s perceptions related to the usability and usefulness of the new protocol and feedback related to its long-term adoption were assessed using a descriptive survey.
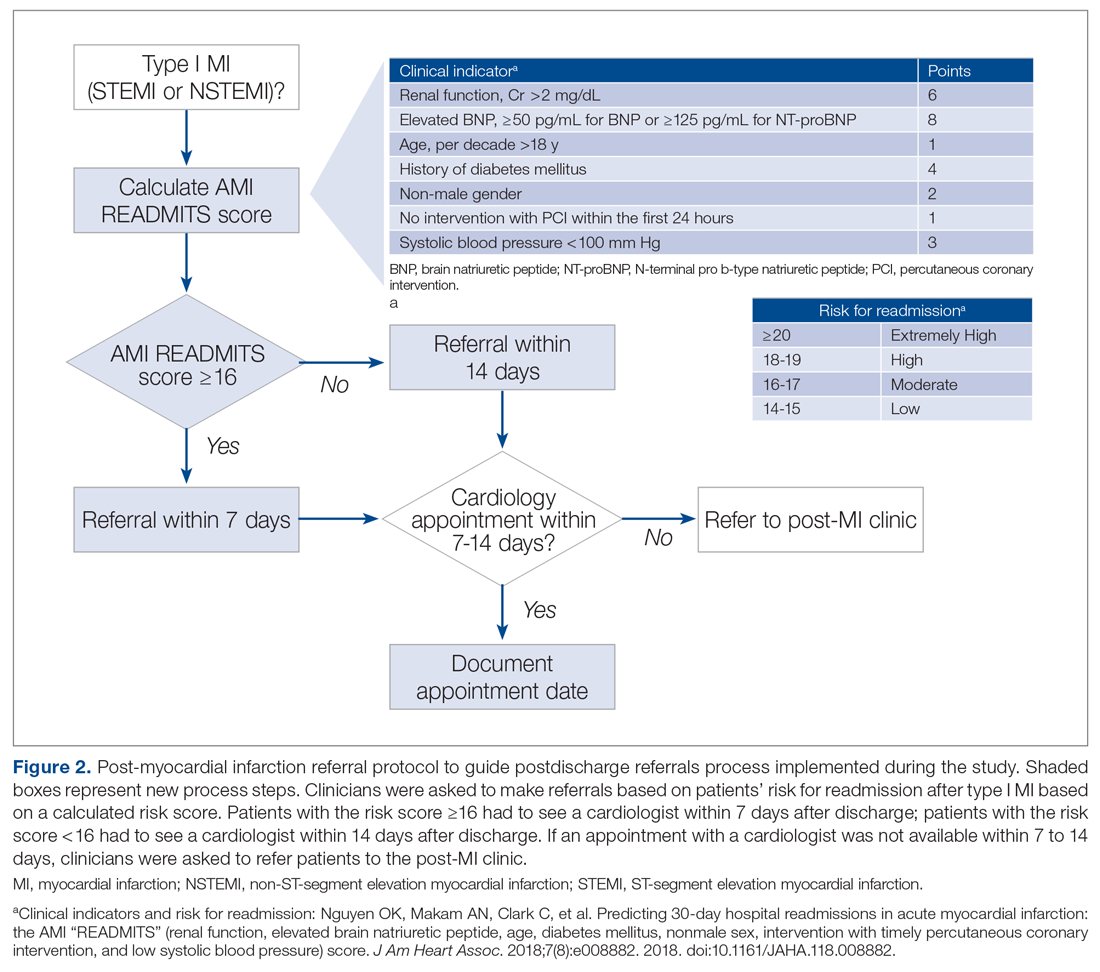
The institutional review board classified this project as a quality improvement project. To avoid potential loss of patient privacy, no identifiable data were collected, a unique identifier unrelated to patients’ records was generated for each patient, and data were saved on a password-protected cardiology office computer.
Population
The project population included all adult patients (≥ 18 years old) with type I MI who were admitted or transferred to the hospital, had a percutaneous coronary intervention (PCI), or were managed without PCI and discharged from the hospital’s cardiac care unit (CCU) and progressive cardiac care unit (PCCU). The criteria for type I MI included the “detection of a rise and/or fall of cardiac troponin with at least 1 value above the 99th percentile and with at least 1 of the following: symptoms of acute myocardial ischemia; new ischemic electrocardiographic (ECG) changes; development of new pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; identification of a coronary thrombus by angiography including intracoronary imaging or by autopsy.”12 The study excluded patients with type I MI who were referred for coronary bypass surgery.
Intervention
The revised risk assessment protocol was implemented within the CCU and PCCU. The lead investigator met with each provider to discuss the role of the post-MI clinic, current referral rates, the purpose of the project, and the new referral process to be completed during the project for each patient discharged with type I MI. Cardiology NPs, fellows, and residents were asked to use the risk-assessment form to calculate patients’ risk for readmission, and refer patients to the post-MI clinic if an appointment with a cardiologist was not available within 7 to 14 days after discharge. Every week during the intervention phase, the investigator sent reminder emails to ensure form completion. Providers were asked to calculate and write the score, the discharge and referral dates, where referrals were made (a cardiologist or the post-MI clinic), date of appointment, and reason for not scheduling an appointment or not referring on the risk assessment form, and to drop the completed forms in specific labeled boxes located at the CCU and PCCU work stations. The investigator collected the completed forms weekly. When the number of discharged patients did not match the number of completed forms, the investigator followed up with discharging providers to understand why.
Data and Data Collection
Data to determine whether the use of the new protocol increased discharge referrals among patients with type I MI within the recommended timeframes were collected by electronic chart review. Data included discharging unit, patients’ age, gender, admission and discharge date, diagnosis, referral to a cardiologist and the post-MI clinic, and appointment date. Clinical data needed to calculate the AMI READMITS score was also collected: PCI within 24 hours, serum creatinine, systolic blood pressure (SBP), brain natriuretic peptide (BNP), and diabetes status.
Data to assess provider satisfaction with the usability and usefulness of the new protocol were gathered through an online survey. The survey included 1 question related to the providers’ role, 1 question asking whether they used the risk assessment for each patient, and 5 Likert-items assessing the ease of usage. An additional open-ended question asked providers to share feedback related to integrating the AMI READMITS risk assessment score to the post-MI referral protocol long term.
To evaluate how consistently providers utilized the new referral protocol when discharging patients with type I MI, the number of completed forms was compared with the number of those patients who were discharged.
Statistical Analysis
Descriptive statistics were used to summarize patient demographics and to calculate the frequency of referrals before and during the intervention. Chi-square statistics were calculated to determine whether the change in percentage of referrals and timely referrals was significant. Descriptive statistics were used to determine the level of provider satisfaction related to each survey item. A content analysis method was used to synthesize themes from the open-ended question asking clinicians to share their feedback related to the new protocol.
Results
Fifty-seven patients met the study inclusion criteria: 29 patients during the preintervention phase and 28 patients during the intervention phase. There were 35 male (61.4%) and 22 female (38.6%) patients. Twenty-five patients (43.9%) were from age groups 41 through 60 years and 61 through 80 years, respectively, representing the majority of included patients. Seven patients (12.3%) were from the 81 years and older age group. There were no patients in the age group 18 through 40 years. Based on the AMI READMITS score calculation, 57.9% (n = 33) patients were from a low-risk group (includes extremely low and low risk for readmission) and 42.1% (n = 24) were from a high-risk group (includes moderate, high, and extremely high risk for readmission).
Provider adoption of the new protocol during the intervention was high. Referral forms were completed for 82% (n = 23) of the 28 patients during the intervention. Analysis findings showed a statistically significant increase in documented referrals after implementing the new referral protocol. During the preintervention phase, 66% (n = 19) of patients with type I MI were referred to see a cardiologist or an NP at a post-MI clinic and there was no documented referral for 34% (n = 10) of patients. During the intervention phase, 89% (n = 25) of patients were referred and there was no documented referral for 11% (n = 3) of patients. Chi-square results indicated that the increase in referrals was significant (χ2 = 4.571, df = 1, P = 0.033).
Data analysis examined whether patient referrals fell within the recommended timeframe of 7 days for the high-risk group (included moderate-to-extremely high risk) and 14 days for the low-risk group (included low-to-extremely low risk). During the preintervention phase, 31% (n = 9) of patient referrals were scheduled as recommended; 28% (n = 8) of patient referrals were scheduled but delayed; and there was no referral date documented for 41% (n = 12) of patients. During the intervention phase, referrals scheduled as recommended increased to 53% (n = 15); 25% (n = 7) of referrals were scheduled but delayed; and there was no referral date documented for 21.4% (n = 6) of patients. The change in appointments scheduled as recommended was not significant (χ2 = 3.550, df = 2, P = 0.169).
Surveys were emailed to 25 cardiology fellows and 3 cardiology NPs who participated in this study. Eighteen of the 28 clinicians (15 cardiology fellows and 3 cardiology NPs) responded for a response rate of 64%. One of several residents who rotated through the CCU and PCCU during the intervention also completed the survey, for a total of 19 participants. When asked if the protocol was easy to use, 79% agreed or strongly agreed. Eighteen of the 19 participants (95%) agreed or strongly agreed that the protocol was useful in making referral decisions. Sixty-eight percent agreed or strongly agreed that the AMI READMITS risk assessment score improves referral process. All participants agreed or strongly agreed that there should be an option to incorporate the AMI READMITS risk assessment score into electronic clinical notes. When asked whether the AMI READMITS risk score should be implemented in clinical practice, responses were mixed (Figure 3). A common theme among the 4 participants who responded with comments was the need for additional data to validate the usefulness of the AMI READMITS to reduce readmissions. In addition, 1 participant commented that “manual calculation [of the risk score] is not ideal.”
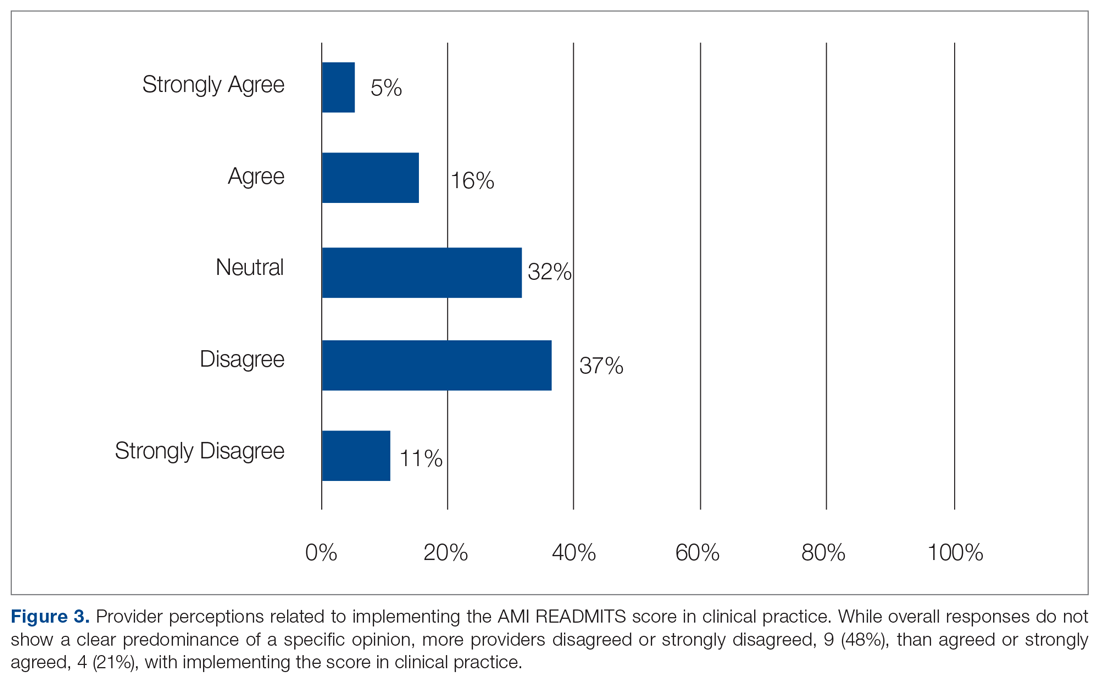
Discussion
This project demonstrated that implementing an evidence-based referral protocol integrating the AMI-READMITS score can increase timely postdischarge referrals among patients with type I MI. The percentage of appropriately scheduled appointments increased during the intervention phase; however, a relatively high number of appointments were scheduled outside of the recommended timeframe, similar to preintervention. Thus, while the new protocol increased referrals and provider documentation of these referrals, it appears that challenges in scheduling timely referral appointments remained. This project did not examine the reasons for delayed appointments.
The survey findings indicated that providers were generally satisfied with the usability and usefulness of the new risk assessment protocol. A large majority agreed or strongly agreed that it was easy to use and useful in making referral decisions, and most agreed or strongly agreed that it improves the referral process. Mixed opinions regarding implementing the AMI READMITS score in clinical practice, combined with qualitative findings, suggest that a lack of external validation of the AMI READMITS presents a barrier to its long-term adoption. All providers who participated in the survey agreed or strongly agreed that the risk assessment should be incorporated into electronic clinical notes. We have begun the process of working with the EHR vendor to automate the AMI risk-assessment within the referral work-flow, which will provide an opportunity for a follow-up quality improvement study.
This quality improvement project has several limitations. First, it implemented a small change in 2 inpatient units at 1 hospital using a simple pre- posttest design. Therefore, the findings are not generalizable to other settings. Prior to the intervention, some referrals may have been made without documentation. While the authors were able to trace undocumented referrals for patients who were referred to the post-MI clinic or to a cardiologist affiliated with the hospital, some patients may have been referred to cardiologists who were not affiliated with the hospital. Another limitation was that the self-created provider survey used was not tested in other clinical settings; thus, it cannot be determined whether the sensitivity and specificity of the survey questions are high. In addition, the clinical providers who participated in the study knew the study team, which may have influenced their behavior during the study period. Furthermore, the identified improvement in clinicians’ referral practices may not be sustainable due to the complexity and effort required to manually calculate the risk score. This limitation could be eliminated by integrating the risk score calculation into the EHR.
Conclusion
Early follow-up after discharge plays an important role in supporting patients’ self-management of some risk factors (ie, diet, weight, and smoking) and identifying gaps in postdischarge care which may lead to readmission. This project provides evidence that integrating the AMI READMITS risk assessment score into the referral process can help to guide discharge decision-making and increase timely, appropriate referrals for patients with MI. Integration of a specific risk assessment, such as the AMI READMITS, within the post-MI referral protocol may help clinicians make more efficient, educated referral decisions. Future studies should explore more specifically how and why the new protocol impacts clinicians’ decision-making and behavior related to post-MI referrals. In addition, future studies should investigate challenges associated with scheduling postdischarge appointments. It will be important to investigate how integration of the new protocol within the EHR may increase efficiency, consistency, and provider satisfaction with the new referral process. Additional research investigating the effects of the AMI READMITS score on readmissions reduction will be important to promote long-term adoption of the improved referral protocol in clinical practice.
Acknowledgments: The authors thank Shelly Conaway, ANP-BC, MSN, Angela Street, ANP-BC, MSN, Andrew Geis, ACNP-BC, MSN, Richard P. Jones II, MD, Eunice Young, MD, Joy Rothwell, MSN, RN-BC, Allison Olazo, MBA, MSN, RN-BC, Elizabeth Heck, RN-BC, and Matthew Trojanowski, MHA, MS, RRT, CSSBB for their support of this study.
Corresponding author: Nailya Muganlinskaya, DNP, MPH, ACNP-BC, MSN, The Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21287; [email protected].
Financial disclosures: None.
1. Why it is important to improve care transitions? Society of Hospital Medicine. Accessed June 15, 2020. https://www.hospitalmedicine.org/clinical-topics/care-transitions/
2. Tong L, Arnold T, Yang J, et al. The association between outpatient follow-up visits and all-cause non-elective 30-day readmissions: a retrospective observational cohort study. PloS One. 2018;13(7):e0200691.
3. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-22.
4. Health Research & Educational Trust. Preventable Readmissions Change Package. American Hospital Association. Updated December 2015. Accessed June 10, 2020. https://www.aha.org/sites/default/files/hiin/HRETHEN_ChangePackage_Readmissions.pd
5. Tung Y-C, Chang G-M, Chang H-Y, Yu T-H. Relationship between early physician follow-up and 30-day readmission after acute myocardial infarction and heart failure. Plos One. 2017;12(1):e0170061.
6. Kaplan RM, Koehler J, Zieger PD, et al. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639-46.
7. Balan P, Zhao Y, Johnson S, et al. The Society of Thoracic Surgery Risk Score as a predictor of 30-day mortality in transcatheter vs surgical aortic valve replacement: a single-center experience and its implications for the development of a TAVR risk-prediction model. J Invasive Cardiol. 2017;29(3):109-14.
8. Smith LN, Makam AN, Darden D, et al. Acute myocardial infarction readmission risk prediction models: A systematic review of model performance. Circ Cardiovasc Qual Outcomes9.9. 2018;11(1):e003885.
9. Baker H, Oliver-McNeil S, Deng L, Hummel SL. See you in 7: regional hospital collaboration and outcomes in Medicare heart failure patients. JACC Heart Fail. 2015;3(10):765-73.
10. Batten A, Jaeger C, Griffen D, et al. See you in 7: improving acute myocardial infarction follow-up care. BMJ Open Qual. 2018;7(2):e000296.
11. Lee DW, Armistead L, Coleman H, et al. Abstract 15387: Post-discharge follow-up within 14 days reduces 30-day hospital readmission rates in patients with acute myocardial infarction and/or acutely decompensated heart failure. Circulation. 2018;134 (1):A 15387.
12. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Circulation. 2018;138 (20):e:618-51.
From The Johns Hopkins Hospital, Baltimore, MD (Dr. Muganlinskaya and Dr. Skojec, retired); The George Washington University, Washington, DC (Dr. Posey); and Johns Hopkins University, Baltimore, MD (Dr. Resar).
Abstract
Objective: Assessing the risk characteristics of patients with acute myocardial infarction (MI) can help providers make appropriate referral decisions. This quality improvement project sought to improve timely, appropriate referrals among patients with type I MI by adding a risk assessment, the AMI READMITS score, to the existing referral protocol.
Methods: Patients’ chart data were analyzed to assess changes in referrals and timely follow-up appointments from pre-intervention to intervention. A survey assessed providers’ satisfaction with the new referral protocol.
Results: Among 57 patients (n = 29 preintervention; n = 28 intervention), documented referrals increased significantly from 66% to 89% (χ2 = 4.571, df = 1, P = 0.033); and timely appointments increased by 10%, which was not significant (χ2 = 3.550, df = 2, P = 0.169). Most providers agreed that the new protocol was easy to use, useful in making referral decisions, and improved the referral process. All agreed the risk score should be incorporated into electronic clinical notes. Provider opinions related to implementing the risk score in clinical practice were mixed. Qualitative feedback suggests this was due to limited validation of the AMI READMITS score in reducing readmissions.
Conclusions: Our risk-based referral protocol helped to increase appropriate referrals among patients with type I MI. Provider adoption may be enhanced by incorporating the protocol into electronic clinical notes. Research to further validate the accuracy of the AMI READMITS score in predicting readmissions may support adoption of the protocol in clinical practice.
Keywords: quality improvement; type I myocardial infarction; referral process; readmission risk; risk assessment; chart review.
Early follow-up after discharge is an important strategy to reduce the risk of unplanned hospital readmissions among patients with various conditions.1-3 While patient confounding factors, such as chronic health problems, environment, socioeconomic status, and literacy, make it difficult to avoid all unplanned readmissions, early follow-up may help providers identify and appropriately manage some health-related issues, and as such is a pivotal element of a readmission prevention strategy.4 There is evidence that patients with non-ST elevation myocardial infarction (NSTEMI) who have an outpatient appointment with a physician within 7 days after discharge have a lower risk of 30-day readmission.5
Our hospital’s postmyocardial infarction clinic was created to prevent unplanned readmissions within 30 days after discharge among patients with type I myocardial infarction (MI). Since inception, the number of referrals has been much lower than expected. In 2018, the total number of patients discharged from the hospital with type I MI and any troponin I level above 0.40 ng/mL was 313. Most of these patients were discharged from the hospital’s cardiac units; however, only 91 referrals were made. To increase referrals, the cardiology nurse practitioners (NPs) developed a post-MI referral protocol (Figure 1). However, this protocol was not consistently used and referrals to the clinic remained low.

Evidence-based risk assessment tools have the potential to increase effective patient management. For example, cardiology providers at the hospital utilize various scores, such as CHA2DS2-VASc6 and the Society of Thoracic Surgery risk score,7 to plan patient management. Among the scores used to predict unplanned readmissions for MI patients, the most promising is the AMI READMITS score.8 Unlike other nonspecific prediction models, the AMI READMITS score was developed based on variables extracted from the electronic health records (EHRs) of patients who were hospitalized for MI and readmitted within 30 days after discharge. Recognizing the potential to increase referrals by integrating an MI-specific risk assessment, this quality improvement study modified the existing referral protocol to include the patients’ AMI READMITS score and recommendations for follow-up.
Currently, there are no clear recommendations on how soon after discharge patients with MI should undergo follow-up. As research data vary, we selected 7 days follow-up for patients from high risk groups based on the “See you in 7” initiative for patients with heart failure (HF) and MI,9,10 as well as evidence that patients with NSTEMI have a lower risk of 30-day readmission if they have follow-up within 7 days after discharge5; and we selected 14 days follow-up for patients from low-risk groups based on evidence that postdischarge follow-up within 14 days reduces risk of 30-day readmission in patients with acute myocardial infarction (AMI) and/or acutely decompensated HF.11
Methods
This project was designed to answer the following question: For adult patients with type I MI, does implementation of a readmission risk assessment referral protocol increase the percentage of referrals and appointments scheduled within a recommended time? Anticipated outcomes included: (1) increased referrals to a cardiologist or the post-MI clinic; (2) increased scheduled follow-up appointments within 7 to 14 days; (3) provider satisfaction with the usability and usefulness of the new protocol; and (4) consistent provider adoption of the new risk assessment referral protocol.
To evaluate the degree to which these outcomes were achieved, we reviewed patient charts for 2 months prior and 2 months during implementation of the new referral protocol. As shown in Figure 2, the new protocol added the following process steps to the existing protocol: calculation of the AMI READMITS score, recommendations for follow-up based on patients’ risk score, and guidance to refer patients to the post-MI clinic if patients did not have an appointment with a cardiologist within 7 to 14 days after discharge. Patients’ risk assessment scores were obtained from forms completed by clinicians during the intervention. Clinician’s perceptions related to the usability and usefulness of the new protocol and feedback related to its long-term adoption were assessed using a descriptive survey.

The institutional review board classified this project as a quality improvement project. To avoid potential loss of patient privacy, no identifiable data were collected, a unique identifier unrelated to patients’ records was generated for each patient, and data were saved on a password-protected cardiology office computer.
Population
The project population included all adult patients (≥ 18 years old) with type I MI who were admitted or transferred to the hospital, had a percutaneous coronary intervention (PCI), or were managed without PCI and discharged from the hospital’s cardiac care unit (CCU) and progressive cardiac care unit (PCCU). The criteria for type I MI included the “detection of a rise and/or fall of cardiac troponin with at least 1 value above the 99th percentile and with at least 1 of the following: symptoms of acute myocardial ischemia; new ischemic electrocardiographic (ECG) changes; development of new pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; identification of a coronary thrombus by angiography including intracoronary imaging or by autopsy.”12 The study excluded patients with type I MI who were referred for coronary bypass surgery.
Intervention
The revised risk assessment protocol was implemented within the CCU and PCCU. The lead investigator met with each provider to discuss the role of the post-MI clinic, current referral rates, the purpose of the project, and the new referral process to be completed during the project for each patient discharged with type I MI. Cardiology NPs, fellows, and residents were asked to use the risk-assessment form to calculate patients’ risk for readmission, and refer patients to the post-MI clinic if an appointment with a cardiologist was not available within 7 to 14 days after discharge. Every week during the intervention phase, the investigator sent reminder emails to ensure form completion. Providers were asked to calculate and write the score, the discharge and referral dates, where referrals were made (a cardiologist or the post-MI clinic), date of appointment, and reason for not scheduling an appointment or not referring on the risk assessment form, and to drop the completed forms in specific labeled boxes located at the CCU and PCCU work stations. The investigator collected the completed forms weekly. When the number of discharged patients did not match the number of completed forms, the investigator followed up with discharging providers to understand why.
Data and Data Collection
Data to determine whether the use of the new protocol increased discharge referrals among patients with type I MI within the recommended timeframes were collected by electronic chart review. Data included discharging unit, patients’ age, gender, admission and discharge date, diagnosis, referral to a cardiologist and the post-MI clinic, and appointment date. Clinical data needed to calculate the AMI READMITS score was also collected: PCI within 24 hours, serum creatinine, systolic blood pressure (SBP), brain natriuretic peptide (BNP), and diabetes status.
Data to assess provider satisfaction with the usability and usefulness of the new protocol were gathered through an online survey. The survey included 1 question related to the providers’ role, 1 question asking whether they used the risk assessment for each patient, and 5 Likert-items assessing the ease of usage. An additional open-ended question asked providers to share feedback related to integrating the AMI READMITS risk assessment score to the post-MI referral protocol long term.
To evaluate how consistently providers utilized the new referral protocol when discharging patients with type I MI, the number of completed forms was compared with the number of those patients who were discharged.
Statistical Analysis
Descriptive statistics were used to summarize patient demographics and to calculate the frequency of referrals before and during the intervention. Chi-square statistics were calculated to determine whether the change in percentage of referrals and timely referrals was significant. Descriptive statistics were used to determine the level of provider satisfaction related to each survey item. A content analysis method was used to synthesize themes from the open-ended question asking clinicians to share their feedback related to the new protocol.
Results
Fifty-seven patients met the study inclusion criteria: 29 patients during the preintervention phase and 28 patients during the intervention phase. There were 35 male (61.4%) and 22 female (38.6%) patients. Twenty-five patients (43.9%) were from age groups 41 through 60 years and 61 through 80 years, respectively, representing the majority of included patients. Seven patients (12.3%) were from the 81 years and older age group. There were no patients in the age group 18 through 40 years. Based on the AMI READMITS score calculation, 57.9% (n = 33) patients were from a low-risk group (includes extremely low and low risk for readmission) and 42.1% (n = 24) were from a high-risk group (includes moderate, high, and extremely high risk for readmission).
Provider adoption of the new protocol during the intervention was high. Referral forms were completed for 82% (n = 23) of the 28 patients during the intervention. Analysis findings showed a statistically significant increase in documented referrals after implementing the new referral protocol. During the preintervention phase, 66% (n = 19) of patients with type I MI were referred to see a cardiologist or an NP at a post-MI clinic and there was no documented referral for 34% (n = 10) of patients. During the intervention phase, 89% (n = 25) of patients were referred and there was no documented referral for 11% (n = 3) of patients. Chi-square results indicated that the increase in referrals was significant (χ2 = 4.571, df = 1, P = 0.033).
Data analysis examined whether patient referrals fell within the recommended timeframe of 7 days for the high-risk group (included moderate-to-extremely high risk) and 14 days for the low-risk group (included low-to-extremely low risk). During the preintervention phase, 31% (n = 9) of patient referrals were scheduled as recommended; 28% (n = 8) of patient referrals were scheduled but delayed; and there was no referral date documented for 41% (n = 12) of patients. During the intervention phase, referrals scheduled as recommended increased to 53% (n = 15); 25% (n = 7) of referrals were scheduled but delayed; and there was no referral date documented for 21.4% (n = 6) of patients. The change in appointments scheduled as recommended was not significant (χ2 = 3.550, df = 2, P = 0.169).
Surveys were emailed to 25 cardiology fellows and 3 cardiology NPs who participated in this study. Eighteen of the 28 clinicians (15 cardiology fellows and 3 cardiology NPs) responded for a response rate of 64%. One of several residents who rotated through the CCU and PCCU during the intervention also completed the survey, for a total of 19 participants. When asked if the protocol was easy to use, 79% agreed or strongly agreed. Eighteen of the 19 participants (95%) agreed or strongly agreed that the protocol was useful in making referral decisions. Sixty-eight percent agreed or strongly agreed that the AMI READMITS risk assessment score improves referral process. All participants agreed or strongly agreed that there should be an option to incorporate the AMI READMITS risk assessment score into electronic clinical notes. When asked whether the AMI READMITS risk score should be implemented in clinical practice, responses were mixed (Figure 3). A common theme among the 4 participants who responded with comments was the need for additional data to validate the usefulness of the AMI READMITS to reduce readmissions. In addition, 1 participant commented that “manual calculation [of the risk score] is not ideal.”

Discussion
This project demonstrated that implementing an evidence-based referral protocol integrating the AMI-READMITS score can increase timely postdischarge referrals among patients with type I MI. The percentage of appropriately scheduled appointments increased during the intervention phase; however, a relatively high number of appointments were scheduled outside of the recommended timeframe, similar to preintervention. Thus, while the new protocol increased referrals and provider documentation of these referrals, it appears that challenges in scheduling timely referral appointments remained. This project did not examine the reasons for delayed appointments.
The survey findings indicated that providers were generally satisfied with the usability and usefulness of the new risk assessment protocol. A large majority agreed or strongly agreed that it was easy to use and useful in making referral decisions, and most agreed or strongly agreed that it improves the referral process. Mixed opinions regarding implementing the AMI READMITS score in clinical practice, combined with qualitative findings, suggest that a lack of external validation of the AMI READMITS presents a barrier to its long-term adoption. All providers who participated in the survey agreed or strongly agreed that the risk assessment should be incorporated into electronic clinical notes. We have begun the process of working with the EHR vendor to automate the AMI risk-assessment within the referral work-flow, which will provide an opportunity for a follow-up quality improvement study.
This quality improvement project has several limitations. First, it implemented a small change in 2 inpatient units at 1 hospital using a simple pre- posttest design. Therefore, the findings are not generalizable to other settings. Prior to the intervention, some referrals may have been made without documentation. While the authors were able to trace undocumented referrals for patients who were referred to the post-MI clinic or to a cardiologist affiliated with the hospital, some patients may have been referred to cardiologists who were not affiliated with the hospital. Another limitation was that the self-created provider survey used was not tested in other clinical settings; thus, it cannot be determined whether the sensitivity and specificity of the survey questions are high. In addition, the clinical providers who participated in the study knew the study team, which may have influenced their behavior during the study period. Furthermore, the identified improvement in clinicians’ referral practices may not be sustainable due to the complexity and effort required to manually calculate the risk score. This limitation could be eliminated by integrating the risk score calculation into the EHR.
Conclusion
Early follow-up after discharge plays an important role in supporting patients’ self-management of some risk factors (ie, diet, weight, and smoking) and identifying gaps in postdischarge care which may lead to readmission. This project provides evidence that integrating the AMI READMITS risk assessment score into the referral process can help to guide discharge decision-making and increase timely, appropriate referrals for patients with MI. Integration of a specific risk assessment, such as the AMI READMITS, within the post-MI referral protocol may help clinicians make more efficient, educated referral decisions. Future studies should explore more specifically how and why the new protocol impacts clinicians’ decision-making and behavior related to post-MI referrals. In addition, future studies should investigate challenges associated with scheduling postdischarge appointments. It will be important to investigate how integration of the new protocol within the EHR may increase efficiency, consistency, and provider satisfaction with the new referral process. Additional research investigating the effects of the AMI READMITS score on readmissions reduction will be important to promote long-term adoption of the improved referral protocol in clinical practice.
Acknowledgments: The authors thank Shelly Conaway, ANP-BC, MSN, Angela Street, ANP-BC, MSN, Andrew Geis, ACNP-BC, MSN, Richard P. Jones II, MD, Eunice Young, MD, Joy Rothwell, MSN, RN-BC, Allison Olazo, MBA, MSN, RN-BC, Elizabeth Heck, RN-BC, and Matthew Trojanowski, MHA, MS, RRT, CSSBB for their support of this study.
Corresponding author: Nailya Muganlinskaya, DNP, MPH, ACNP-BC, MSN, The Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21287; [email protected].
Financial disclosures: None.
From The Johns Hopkins Hospital, Baltimore, MD (Dr. Muganlinskaya and Dr. Skojec, retired); The George Washington University, Washington, DC (Dr. Posey); and Johns Hopkins University, Baltimore, MD (Dr. Resar).
Abstract
Objective: Assessing the risk characteristics of patients with acute myocardial infarction (MI) can help providers make appropriate referral decisions. This quality improvement project sought to improve timely, appropriate referrals among patients with type I MI by adding a risk assessment, the AMI READMITS score, to the existing referral protocol.
Methods: Patients’ chart data were analyzed to assess changes in referrals and timely follow-up appointments from pre-intervention to intervention. A survey assessed providers’ satisfaction with the new referral protocol.
Results: Among 57 patients (n = 29 preintervention; n = 28 intervention), documented referrals increased significantly from 66% to 89% (χ2 = 4.571, df = 1, P = 0.033); and timely appointments increased by 10%, which was not significant (χ2 = 3.550, df = 2, P = 0.169). Most providers agreed that the new protocol was easy to use, useful in making referral decisions, and improved the referral process. All agreed the risk score should be incorporated into electronic clinical notes. Provider opinions related to implementing the risk score in clinical practice were mixed. Qualitative feedback suggests this was due to limited validation of the AMI READMITS score in reducing readmissions.
Conclusions: Our risk-based referral protocol helped to increase appropriate referrals among patients with type I MI. Provider adoption may be enhanced by incorporating the protocol into electronic clinical notes. Research to further validate the accuracy of the AMI READMITS score in predicting readmissions may support adoption of the protocol in clinical practice.
Keywords: quality improvement; type I myocardial infarction; referral process; readmission risk; risk assessment; chart review.
Early follow-up after discharge is an important strategy to reduce the risk of unplanned hospital readmissions among patients with various conditions.1-3 While patient confounding factors, such as chronic health problems, environment, socioeconomic status, and literacy, make it difficult to avoid all unplanned readmissions, early follow-up may help providers identify and appropriately manage some health-related issues, and as such is a pivotal element of a readmission prevention strategy.4 There is evidence that patients with non-ST elevation myocardial infarction (NSTEMI) who have an outpatient appointment with a physician within 7 days after discharge have a lower risk of 30-day readmission.5
Our hospital’s postmyocardial infarction clinic was created to prevent unplanned readmissions within 30 days after discharge among patients with type I myocardial infarction (MI). Since inception, the number of referrals has been much lower than expected. In 2018, the total number of patients discharged from the hospital with type I MI and any troponin I level above 0.40 ng/mL was 313. Most of these patients were discharged from the hospital’s cardiac units; however, only 91 referrals were made. To increase referrals, the cardiology nurse practitioners (NPs) developed a post-MI referral protocol (Figure 1). However, this protocol was not consistently used and referrals to the clinic remained low.

Evidence-based risk assessment tools have the potential to increase effective patient management. For example, cardiology providers at the hospital utilize various scores, such as CHA2DS2-VASc6 and the Society of Thoracic Surgery risk score,7 to plan patient management. Among the scores used to predict unplanned readmissions for MI patients, the most promising is the AMI READMITS score.8 Unlike other nonspecific prediction models, the AMI READMITS score was developed based on variables extracted from the electronic health records (EHRs) of patients who were hospitalized for MI and readmitted within 30 days after discharge. Recognizing the potential to increase referrals by integrating an MI-specific risk assessment, this quality improvement study modified the existing referral protocol to include the patients’ AMI READMITS score and recommendations for follow-up.
Currently, there are no clear recommendations on how soon after discharge patients with MI should undergo follow-up. As research data vary, we selected 7 days follow-up for patients from high risk groups based on the “See you in 7” initiative for patients with heart failure (HF) and MI,9,10 as well as evidence that patients with NSTEMI have a lower risk of 30-day readmission if they have follow-up within 7 days after discharge5; and we selected 14 days follow-up for patients from low-risk groups based on evidence that postdischarge follow-up within 14 days reduces risk of 30-day readmission in patients with acute myocardial infarction (AMI) and/or acutely decompensated HF.11
Methods
This project was designed to answer the following question: For adult patients with type I MI, does implementation of a readmission risk assessment referral protocol increase the percentage of referrals and appointments scheduled within a recommended time? Anticipated outcomes included: (1) increased referrals to a cardiologist or the post-MI clinic; (2) increased scheduled follow-up appointments within 7 to 14 days; (3) provider satisfaction with the usability and usefulness of the new protocol; and (4) consistent provider adoption of the new risk assessment referral protocol.
To evaluate the degree to which these outcomes were achieved, we reviewed patient charts for 2 months prior and 2 months during implementation of the new referral protocol. As shown in Figure 2, the new protocol added the following process steps to the existing protocol: calculation of the AMI READMITS score, recommendations for follow-up based on patients’ risk score, and guidance to refer patients to the post-MI clinic if patients did not have an appointment with a cardiologist within 7 to 14 days after discharge. Patients’ risk assessment scores were obtained from forms completed by clinicians during the intervention. Clinician’s perceptions related to the usability and usefulness of the new protocol and feedback related to its long-term adoption were assessed using a descriptive survey.

The institutional review board classified this project as a quality improvement project. To avoid potential loss of patient privacy, no identifiable data were collected, a unique identifier unrelated to patients’ records was generated for each patient, and data were saved on a password-protected cardiology office computer.
Population
The project population included all adult patients (≥ 18 years old) with type I MI who were admitted or transferred to the hospital, had a percutaneous coronary intervention (PCI), or were managed without PCI and discharged from the hospital’s cardiac care unit (CCU) and progressive cardiac care unit (PCCU). The criteria for type I MI included the “detection of a rise and/or fall of cardiac troponin with at least 1 value above the 99th percentile and with at least 1 of the following: symptoms of acute myocardial ischemia; new ischemic electrocardiographic (ECG) changes; development of new pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with an ischemic etiology; identification of a coronary thrombus by angiography including intracoronary imaging or by autopsy.”12 The study excluded patients with type I MI who were referred for coronary bypass surgery.
Intervention
The revised risk assessment protocol was implemented within the CCU and PCCU. The lead investigator met with each provider to discuss the role of the post-MI clinic, current referral rates, the purpose of the project, and the new referral process to be completed during the project for each patient discharged with type I MI. Cardiology NPs, fellows, and residents were asked to use the risk-assessment form to calculate patients’ risk for readmission, and refer patients to the post-MI clinic if an appointment with a cardiologist was not available within 7 to 14 days after discharge. Every week during the intervention phase, the investigator sent reminder emails to ensure form completion. Providers were asked to calculate and write the score, the discharge and referral dates, where referrals were made (a cardiologist or the post-MI clinic), date of appointment, and reason for not scheduling an appointment or not referring on the risk assessment form, and to drop the completed forms in specific labeled boxes located at the CCU and PCCU work stations. The investigator collected the completed forms weekly. When the number of discharged patients did not match the number of completed forms, the investigator followed up with discharging providers to understand why.
Data and Data Collection
Data to determine whether the use of the new protocol increased discharge referrals among patients with type I MI within the recommended timeframes were collected by electronic chart review. Data included discharging unit, patients’ age, gender, admission and discharge date, diagnosis, referral to a cardiologist and the post-MI clinic, and appointment date. Clinical data needed to calculate the AMI READMITS score was also collected: PCI within 24 hours, serum creatinine, systolic blood pressure (SBP), brain natriuretic peptide (BNP), and diabetes status.
Data to assess provider satisfaction with the usability and usefulness of the new protocol were gathered through an online survey. The survey included 1 question related to the providers’ role, 1 question asking whether they used the risk assessment for each patient, and 5 Likert-items assessing the ease of usage. An additional open-ended question asked providers to share feedback related to integrating the AMI READMITS risk assessment score to the post-MI referral protocol long term.
To evaluate how consistently providers utilized the new referral protocol when discharging patients with type I MI, the number of completed forms was compared with the number of those patients who were discharged.
Statistical Analysis
Descriptive statistics were used to summarize patient demographics and to calculate the frequency of referrals before and during the intervention. Chi-square statistics were calculated to determine whether the change in percentage of referrals and timely referrals was significant. Descriptive statistics were used to determine the level of provider satisfaction related to each survey item. A content analysis method was used to synthesize themes from the open-ended question asking clinicians to share their feedback related to the new protocol.
Results
Fifty-seven patients met the study inclusion criteria: 29 patients during the preintervention phase and 28 patients during the intervention phase. There were 35 male (61.4%) and 22 female (38.6%) patients. Twenty-five patients (43.9%) were from age groups 41 through 60 years and 61 through 80 years, respectively, representing the majority of included patients. Seven patients (12.3%) were from the 81 years and older age group. There were no patients in the age group 18 through 40 years. Based on the AMI READMITS score calculation, 57.9% (n = 33) patients were from a low-risk group (includes extremely low and low risk for readmission) and 42.1% (n = 24) were from a high-risk group (includes moderate, high, and extremely high risk for readmission).
Provider adoption of the new protocol during the intervention was high. Referral forms were completed for 82% (n = 23) of the 28 patients during the intervention. Analysis findings showed a statistically significant increase in documented referrals after implementing the new referral protocol. During the preintervention phase, 66% (n = 19) of patients with type I MI were referred to see a cardiologist or an NP at a post-MI clinic and there was no documented referral for 34% (n = 10) of patients. During the intervention phase, 89% (n = 25) of patients were referred and there was no documented referral for 11% (n = 3) of patients. Chi-square results indicated that the increase in referrals was significant (χ2 = 4.571, df = 1, P = 0.033).
Data analysis examined whether patient referrals fell within the recommended timeframe of 7 days for the high-risk group (included moderate-to-extremely high risk) and 14 days for the low-risk group (included low-to-extremely low risk). During the preintervention phase, 31% (n = 9) of patient referrals were scheduled as recommended; 28% (n = 8) of patient referrals were scheduled but delayed; and there was no referral date documented for 41% (n = 12) of patients. During the intervention phase, referrals scheduled as recommended increased to 53% (n = 15); 25% (n = 7) of referrals were scheduled but delayed; and there was no referral date documented for 21.4% (n = 6) of patients. The change in appointments scheduled as recommended was not significant (χ2 = 3.550, df = 2, P = 0.169).
Surveys were emailed to 25 cardiology fellows and 3 cardiology NPs who participated in this study. Eighteen of the 28 clinicians (15 cardiology fellows and 3 cardiology NPs) responded for a response rate of 64%. One of several residents who rotated through the CCU and PCCU during the intervention also completed the survey, for a total of 19 participants. When asked if the protocol was easy to use, 79% agreed or strongly agreed. Eighteen of the 19 participants (95%) agreed or strongly agreed that the protocol was useful in making referral decisions. Sixty-eight percent agreed or strongly agreed that the AMI READMITS risk assessment score improves referral process. All participants agreed or strongly agreed that there should be an option to incorporate the AMI READMITS risk assessment score into electronic clinical notes. When asked whether the AMI READMITS risk score should be implemented in clinical practice, responses were mixed (Figure 3). A common theme among the 4 participants who responded with comments was the need for additional data to validate the usefulness of the AMI READMITS to reduce readmissions. In addition, 1 participant commented that “manual calculation [of the risk score] is not ideal.”

Discussion
This project demonstrated that implementing an evidence-based referral protocol integrating the AMI-READMITS score can increase timely postdischarge referrals among patients with type I MI. The percentage of appropriately scheduled appointments increased during the intervention phase; however, a relatively high number of appointments were scheduled outside of the recommended timeframe, similar to preintervention. Thus, while the new protocol increased referrals and provider documentation of these referrals, it appears that challenges in scheduling timely referral appointments remained. This project did not examine the reasons for delayed appointments.
The survey findings indicated that providers were generally satisfied with the usability and usefulness of the new risk assessment protocol. A large majority agreed or strongly agreed that it was easy to use and useful in making referral decisions, and most agreed or strongly agreed that it improves the referral process. Mixed opinions regarding implementing the AMI READMITS score in clinical practice, combined with qualitative findings, suggest that a lack of external validation of the AMI READMITS presents a barrier to its long-term adoption. All providers who participated in the survey agreed or strongly agreed that the risk assessment should be incorporated into electronic clinical notes. We have begun the process of working with the EHR vendor to automate the AMI risk-assessment within the referral work-flow, which will provide an opportunity for a follow-up quality improvement study.
This quality improvement project has several limitations. First, it implemented a small change in 2 inpatient units at 1 hospital using a simple pre- posttest design. Therefore, the findings are not generalizable to other settings. Prior to the intervention, some referrals may have been made without documentation. While the authors were able to trace undocumented referrals for patients who were referred to the post-MI clinic or to a cardiologist affiliated with the hospital, some patients may have been referred to cardiologists who were not affiliated with the hospital. Another limitation was that the self-created provider survey used was not tested in other clinical settings; thus, it cannot be determined whether the sensitivity and specificity of the survey questions are high. In addition, the clinical providers who participated in the study knew the study team, which may have influenced their behavior during the study period. Furthermore, the identified improvement in clinicians’ referral practices may not be sustainable due to the complexity and effort required to manually calculate the risk score. This limitation could be eliminated by integrating the risk score calculation into the EHR.
Conclusion
Early follow-up after discharge plays an important role in supporting patients’ self-management of some risk factors (ie, diet, weight, and smoking) and identifying gaps in postdischarge care which may lead to readmission. This project provides evidence that integrating the AMI READMITS risk assessment score into the referral process can help to guide discharge decision-making and increase timely, appropriate referrals for patients with MI. Integration of a specific risk assessment, such as the AMI READMITS, within the post-MI referral protocol may help clinicians make more efficient, educated referral decisions. Future studies should explore more specifically how and why the new protocol impacts clinicians’ decision-making and behavior related to post-MI referrals. In addition, future studies should investigate challenges associated with scheduling postdischarge appointments. It will be important to investigate how integration of the new protocol within the EHR may increase efficiency, consistency, and provider satisfaction with the new referral process. Additional research investigating the effects of the AMI READMITS score on readmissions reduction will be important to promote long-term adoption of the improved referral protocol in clinical practice.
Acknowledgments: The authors thank Shelly Conaway, ANP-BC, MSN, Angela Street, ANP-BC, MSN, Andrew Geis, ACNP-BC, MSN, Richard P. Jones II, MD, Eunice Young, MD, Joy Rothwell, MSN, RN-BC, Allison Olazo, MBA, MSN, RN-BC, Elizabeth Heck, RN-BC, and Matthew Trojanowski, MHA, MS, RRT, CSSBB for their support of this study.
Corresponding author: Nailya Muganlinskaya, DNP, MPH, ACNP-BC, MSN, The Johns Hopkins Hospital, 1800 Orleans St, Baltimore, MD 21287; [email protected].
Financial disclosures: None.
1. Why it is important to improve care transitions? Society of Hospital Medicine. Accessed June 15, 2020. https://www.hospitalmedicine.org/clinical-topics/care-transitions/
2. Tong L, Arnold T, Yang J, et al. The association between outpatient follow-up visits and all-cause non-elective 30-day readmissions: a retrospective observational cohort study. PloS One. 2018;13(7):e0200691.
3. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-22.
4. Health Research & Educational Trust. Preventable Readmissions Change Package. American Hospital Association. Updated December 2015. Accessed June 10, 2020. https://www.aha.org/sites/default/files/hiin/HRETHEN_ChangePackage_Readmissions.pd
5. Tung Y-C, Chang G-M, Chang H-Y, Yu T-H. Relationship between early physician follow-up and 30-day readmission after acute myocardial infarction and heart failure. Plos One. 2017;12(1):e0170061.
6. Kaplan RM, Koehler J, Zieger PD, et al. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639-46.
7. Balan P, Zhao Y, Johnson S, et al. The Society of Thoracic Surgery Risk Score as a predictor of 30-day mortality in transcatheter vs surgical aortic valve replacement: a single-center experience and its implications for the development of a TAVR risk-prediction model. J Invasive Cardiol. 2017;29(3):109-14.
8. Smith LN, Makam AN, Darden D, et al. Acute myocardial infarction readmission risk prediction models: A systematic review of model performance. Circ Cardiovasc Qual Outcomes9.9. 2018;11(1):e003885.
9. Baker H, Oliver-McNeil S, Deng L, Hummel SL. See you in 7: regional hospital collaboration and outcomes in Medicare heart failure patients. JACC Heart Fail. 2015;3(10):765-73.
10. Batten A, Jaeger C, Griffen D, et al. See you in 7: improving acute myocardial infarction follow-up care. BMJ Open Qual. 2018;7(2):e000296.
11. Lee DW, Armistead L, Coleman H, et al. Abstract 15387: Post-discharge follow-up within 14 days reduces 30-day hospital readmission rates in patients with acute myocardial infarction and/or acutely decompensated heart failure. Circulation. 2018;134 (1):A 15387.
12. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Circulation. 2018;138 (20):e:618-51.
1. Why it is important to improve care transitions? Society of Hospital Medicine. Accessed June 15, 2020. https://www.hospitalmedicine.org/clinical-topics/care-transitions/
2. Tong L, Arnold T, Yang J, et al. The association between outpatient follow-up visits and all-cause non-elective 30-day readmissions: a retrospective observational cohort study. PloS One. 2018;13(7):e0200691.
3. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-22.
4. Health Research & Educational Trust. Preventable Readmissions Change Package. American Hospital Association. Updated December 2015. Accessed June 10, 2020. https://www.aha.org/sites/default/files/hiin/HRETHEN_ChangePackage_Readmissions.pd
5. Tung Y-C, Chang G-M, Chang H-Y, Yu T-H. Relationship between early physician follow-up and 30-day readmission after acute myocardial infarction and heart failure. Plos One. 2017;12(1):e0170061.
6. Kaplan RM, Koehler J, Zieger PD, et al. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140(20):1639-46.
7. Balan P, Zhao Y, Johnson S, et al. The Society of Thoracic Surgery Risk Score as a predictor of 30-day mortality in transcatheter vs surgical aortic valve replacement: a single-center experience and its implications for the development of a TAVR risk-prediction model. J Invasive Cardiol. 2017;29(3):109-14.
8. Smith LN, Makam AN, Darden D, et al. Acute myocardial infarction readmission risk prediction models: A systematic review of model performance. Circ Cardiovasc Qual Outcomes9.9. 2018;11(1):e003885.
9. Baker H, Oliver-McNeil S, Deng L, Hummel SL. See you in 7: regional hospital collaboration and outcomes in Medicare heart failure patients. JACC Heart Fail. 2015;3(10):765-73.
10. Batten A, Jaeger C, Griffen D, et al. See you in 7: improving acute myocardial infarction follow-up care. BMJ Open Qual. 2018;7(2):e000296.
11. Lee DW, Armistead L, Coleman H, et al. Abstract 15387: Post-discharge follow-up within 14 days reduces 30-day hospital readmission rates in patients with acute myocardial infarction and/or acutely decompensated heart failure. Circulation. 2018;134 (1):A 15387.
12. Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction. Circulation. 2018;138 (20):e:618-51.
PCOS equivalent in men: No ovaries required
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
FROM ENDO 2021
Is the WHO’s HPV vaccination target within reach?
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
FROM PREVENTIVE MEDICINE
Self-management techniques help relieve lower urinary tract symptoms
The researchers reviewed the literature and analyzed eight randomized controlled trials enrolling a total of 1,006 men, who were experiencing lower urinary tract symptoms, according to the paper published in the Annals of Family Medicine. The self-management techniques practiced by patients as part of the trials included adjusting the timing of when patients drank fluids, reducing or eliminating caffeine and alcohol, adjusting the schedules of or replacing medications for other conditions, adjusting patients’ habits for urinating, and performing pelvic floor exercises for better performance of muscles controlling urination.
“Self-management interventions for lower urinary tract symptoms should be considered as a cheap and safe alternative to drug interventions with unfavorable safety profiles,” said study author Loai Albarqouni, MD, MSc, PhD, a post-doctoral fellow at Bond University in Australia.
Self-management yielded better results than usual care
Some of the symptoms experienced by participants in the trials included increased frequency of urination, urgency of urination, urination hesitancy, and dribbling. The researchers excluded research involving men with LUTS attributed to infections, those with prostate cancer, men who had undergone prostate surgery, and men with neurologic conditions.
Self-management techniques, which frequently included watchful waiting, significantly reduced symptom severity, compared with usual care in two of the trials, which included a total of 350 participants. Symptom severity was measured using the International Prostate Symptom Score (IPSS), with a mean difference of 7.44 points in favor of self-management (95% confidence interval, –8.82 to –6.06). A drop of 3 points on the IPSS scale is considered clinically meaningful.
The researchers found no difference in symptom severity at 6-12 weeks between self-management and drug therapy in their analysis of four trials that compared these approaches. Self-management resulted in better results in terms of waking at night because of the need to urinate, but there was no difference in the number of times urinating per day.
In two of the studies, investigators examined a combined self-management and drug therapy approach, compared with drug therapy by itself. In one of these studies, which included 133 participants, using the combination of treatments resulted in significantly lower symptom severity, compared with using drug therapy alone at 6 weeks, on the IPSS, with a mean difference of 2.30 (95% CI, –4.11 to –0.49).
One study involving men with involuntary loss of urine immediately after urination compared utilizing counseling, pelvic floor exercises, and urethral milking to work urine through the urethra. Pelvic floor exercise was the most effective at reducing urine loss.
Study author Dr. Albarqouni said better tools for physician education could help with implementing these strategies more effectively.
Analysis draws more attention to self-management approaches for men
Outside experts said that, while self-management approaches for these symptoms have long been recognized for women, this analysis draws more attention to the growing use of self-management approaches for men. They noted that hurdles, such as time constraints and physician education on proper technique, remain.
“Evidence suggests that the regular use of nondrug interventions is suboptimal for various reasons, including the inadequate reporting of the details of the interventions in the literature,” Dr. Albarqouni said.
Camille Vaughan, MD, MS, assistant professor of medicine at Emory University, where she has researched lower urinary tract symptoms, said advising patients on self-care is common in her practice, but should be more widely adopted in primary care.
Many patients don’t want to add to drugs that are often already a long list of medications, for fear of side effects and interactions, she said.
“If there are behavioral-based approaches that are appropriate, they’re often really interested in those strategies,” she said.
Barriers include the time it takes to teach patients these strategies and the confidence of the physicians themselves to instruct patients correctly, Dr. Vaughan said. Some physicians might be interested in the self-management approach for their patients, but “may not feel like they have all of the information at hand to share with patients,” she added.
“I think there are several decades of work showing the benefit of these types of strategies in women,” she said. “It’s relatively recent for men.” The analysis is a useful summary, she said.
“I think this should be really encouraging for providers and patients alike, because it’s highlighting the benefits of behavior and lifestyle-based strategies. A lot of these issues are going to impact men as they age,” she added.
High-quality data on self-management techniques have been limited
Scott Bauer, MD, MS, assistant professor of medicine at the University of California, San Francisco, and general internist at the San Francisco VA Medical Center, said he often prescribes self-management but has often had to review primary data from smaller trials and adapt that information to his own practice.
“I have felt like, for a long time, there’s been a lack of high-quality data and good synthesis of that data to really guide what I should specifically be recommending,” he said. “I’m very happy to see efforts to try to synthesize the data in a more comprehensive way and maybe work toward guidelines that can be applied more easily in clinical care.” It shows, he said, that “there is a decent amount of signal that should really be taken seriously both in a clinical context and for future research studies.”
Dr. Bauer noted that there is still a need to identify which patients are best suited for which approaches.
“We are very poor at diagnosing the specific etiology of LUTS – we don’t have great diagnostic tests or even phenotyping, and so that leaves clinicians with a very heterogeneous group of patients who all have the same syndrome of symptoms,” he explained. “But we don’t have much to guide us in terms of identifying who would benefit most from self-management overall, who would benefit from specific self-management techniques, and who would benefit from medication to target very specific mechanisms.”
Dr. Vaughan reported receiving funding from the Department of Veterans Affairs and National institutes of Health for research related to urinary symptom management, and that her spouse is an employee of Kimberly-Clark, which makes adult care products. Dr. Albarqouni and Dr. Bauer reported no relevant financial disclosures.
The researchers reviewed the literature and analyzed eight randomized controlled trials enrolling a total of 1,006 men, who were experiencing lower urinary tract symptoms, according to the paper published in the Annals of Family Medicine. The self-management techniques practiced by patients as part of the trials included adjusting the timing of when patients drank fluids, reducing or eliminating caffeine and alcohol, adjusting the schedules of or replacing medications for other conditions, adjusting patients’ habits for urinating, and performing pelvic floor exercises for better performance of muscles controlling urination.
“Self-management interventions for lower urinary tract symptoms should be considered as a cheap and safe alternative to drug interventions with unfavorable safety profiles,” said study author Loai Albarqouni, MD, MSc, PhD, a post-doctoral fellow at Bond University in Australia.
Self-management yielded better results than usual care
Some of the symptoms experienced by participants in the trials included increased frequency of urination, urgency of urination, urination hesitancy, and dribbling. The researchers excluded research involving men with LUTS attributed to infections, those with prostate cancer, men who had undergone prostate surgery, and men with neurologic conditions.
Self-management techniques, which frequently included watchful waiting, significantly reduced symptom severity, compared with usual care in two of the trials, which included a total of 350 participants. Symptom severity was measured using the International Prostate Symptom Score (IPSS), with a mean difference of 7.44 points in favor of self-management (95% confidence interval, –8.82 to –6.06). A drop of 3 points on the IPSS scale is considered clinically meaningful.
The researchers found no difference in symptom severity at 6-12 weeks between self-management and drug therapy in their analysis of four trials that compared these approaches. Self-management resulted in better results in terms of waking at night because of the need to urinate, but there was no difference in the number of times urinating per day.
In two of the studies, investigators examined a combined self-management and drug therapy approach, compared with drug therapy by itself. In one of these studies, which included 133 participants, using the combination of treatments resulted in significantly lower symptom severity, compared with using drug therapy alone at 6 weeks, on the IPSS, with a mean difference of 2.30 (95% CI, –4.11 to –0.49).
One study involving men with involuntary loss of urine immediately after urination compared utilizing counseling, pelvic floor exercises, and urethral milking to work urine through the urethra. Pelvic floor exercise was the most effective at reducing urine loss.
Study author Dr. Albarqouni said better tools for physician education could help with implementing these strategies more effectively.
Analysis draws more attention to self-management approaches for men
Outside experts said that, while self-management approaches for these symptoms have long been recognized for women, this analysis draws more attention to the growing use of self-management approaches for men. They noted that hurdles, such as time constraints and physician education on proper technique, remain.
“Evidence suggests that the regular use of nondrug interventions is suboptimal for various reasons, including the inadequate reporting of the details of the interventions in the literature,” Dr. Albarqouni said.
Camille Vaughan, MD, MS, assistant professor of medicine at Emory University, where she has researched lower urinary tract symptoms, said advising patients on self-care is common in her practice, but should be more widely adopted in primary care.
Many patients don’t want to add to drugs that are often already a long list of medications, for fear of side effects and interactions, she said.
“If there are behavioral-based approaches that are appropriate, they’re often really interested in those strategies,” she said.
Barriers include the time it takes to teach patients these strategies and the confidence of the physicians themselves to instruct patients correctly, Dr. Vaughan said. Some physicians might be interested in the self-management approach for their patients, but “may not feel like they have all of the information at hand to share with patients,” she added.
“I think there are several decades of work showing the benefit of these types of strategies in women,” she said. “It’s relatively recent for men.” The analysis is a useful summary, she said.
“I think this should be really encouraging for providers and patients alike, because it’s highlighting the benefits of behavior and lifestyle-based strategies. A lot of these issues are going to impact men as they age,” she added.
High-quality data on self-management techniques have been limited
Scott Bauer, MD, MS, assistant professor of medicine at the University of California, San Francisco, and general internist at the San Francisco VA Medical Center, said he often prescribes self-management but has often had to review primary data from smaller trials and adapt that information to his own practice.
“I have felt like, for a long time, there’s been a lack of high-quality data and good synthesis of that data to really guide what I should specifically be recommending,” he said. “I’m very happy to see efforts to try to synthesize the data in a more comprehensive way and maybe work toward guidelines that can be applied more easily in clinical care.” It shows, he said, that “there is a decent amount of signal that should really be taken seriously both in a clinical context and for future research studies.”
Dr. Bauer noted that there is still a need to identify which patients are best suited for which approaches.
“We are very poor at diagnosing the specific etiology of LUTS – we don’t have great diagnostic tests or even phenotyping, and so that leaves clinicians with a very heterogeneous group of patients who all have the same syndrome of symptoms,” he explained. “But we don’t have much to guide us in terms of identifying who would benefit most from self-management overall, who would benefit from specific self-management techniques, and who would benefit from medication to target very specific mechanisms.”
Dr. Vaughan reported receiving funding from the Department of Veterans Affairs and National institutes of Health for research related to urinary symptom management, and that her spouse is an employee of Kimberly-Clark, which makes adult care products. Dr. Albarqouni and Dr. Bauer reported no relevant financial disclosures.
The researchers reviewed the literature and analyzed eight randomized controlled trials enrolling a total of 1,006 men, who were experiencing lower urinary tract symptoms, according to the paper published in the Annals of Family Medicine. The self-management techniques practiced by patients as part of the trials included adjusting the timing of when patients drank fluids, reducing or eliminating caffeine and alcohol, adjusting the schedules of or replacing medications for other conditions, adjusting patients’ habits for urinating, and performing pelvic floor exercises for better performance of muscles controlling urination.
“Self-management interventions for lower urinary tract symptoms should be considered as a cheap and safe alternative to drug interventions with unfavorable safety profiles,” said study author Loai Albarqouni, MD, MSc, PhD, a post-doctoral fellow at Bond University in Australia.
Self-management yielded better results than usual care
Some of the symptoms experienced by participants in the trials included increased frequency of urination, urgency of urination, urination hesitancy, and dribbling. The researchers excluded research involving men with LUTS attributed to infections, those with prostate cancer, men who had undergone prostate surgery, and men with neurologic conditions.
Self-management techniques, which frequently included watchful waiting, significantly reduced symptom severity, compared with usual care in two of the trials, which included a total of 350 participants. Symptom severity was measured using the International Prostate Symptom Score (IPSS), with a mean difference of 7.44 points in favor of self-management (95% confidence interval, –8.82 to –6.06). A drop of 3 points on the IPSS scale is considered clinically meaningful.
The researchers found no difference in symptom severity at 6-12 weeks between self-management and drug therapy in their analysis of four trials that compared these approaches. Self-management resulted in better results in terms of waking at night because of the need to urinate, but there was no difference in the number of times urinating per day.
In two of the studies, investigators examined a combined self-management and drug therapy approach, compared with drug therapy by itself. In one of these studies, which included 133 participants, using the combination of treatments resulted in significantly lower symptom severity, compared with using drug therapy alone at 6 weeks, on the IPSS, with a mean difference of 2.30 (95% CI, –4.11 to –0.49).
One study involving men with involuntary loss of urine immediately after urination compared utilizing counseling, pelvic floor exercises, and urethral milking to work urine through the urethra. Pelvic floor exercise was the most effective at reducing urine loss.
Study author Dr. Albarqouni said better tools for physician education could help with implementing these strategies more effectively.
Analysis draws more attention to self-management approaches for men
Outside experts said that, while self-management approaches for these symptoms have long been recognized for women, this analysis draws more attention to the growing use of self-management approaches for men. They noted that hurdles, such as time constraints and physician education on proper technique, remain.
“Evidence suggests that the regular use of nondrug interventions is suboptimal for various reasons, including the inadequate reporting of the details of the interventions in the literature,” Dr. Albarqouni said.
Camille Vaughan, MD, MS, assistant professor of medicine at Emory University, where she has researched lower urinary tract symptoms, said advising patients on self-care is common in her practice, but should be more widely adopted in primary care.
Many patients don’t want to add to drugs that are often already a long list of medications, for fear of side effects and interactions, she said.
“If there are behavioral-based approaches that are appropriate, they’re often really interested in those strategies,” she said.
Barriers include the time it takes to teach patients these strategies and the confidence of the physicians themselves to instruct patients correctly, Dr. Vaughan said. Some physicians might be interested in the self-management approach for their patients, but “may not feel like they have all of the information at hand to share with patients,” she added.
“I think there are several decades of work showing the benefit of these types of strategies in women,” she said. “It’s relatively recent for men.” The analysis is a useful summary, she said.
“I think this should be really encouraging for providers and patients alike, because it’s highlighting the benefits of behavior and lifestyle-based strategies. A lot of these issues are going to impact men as they age,” she added.
High-quality data on self-management techniques have been limited
Scott Bauer, MD, MS, assistant professor of medicine at the University of California, San Francisco, and general internist at the San Francisco VA Medical Center, said he often prescribes self-management but has often had to review primary data from smaller trials and adapt that information to his own practice.
“I have felt like, for a long time, there’s been a lack of high-quality data and good synthesis of that data to really guide what I should specifically be recommending,” he said. “I’m very happy to see efforts to try to synthesize the data in a more comprehensive way and maybe work toward guidelines that can be applied more easily in clinical care.” It shows, he said, that “there is a decent amount of signal that should really be taken seriously both in a clinical context and for future research studies.”
Dr. Bauer noted that there is still a need to identify which patients are best suited for which approaches.
“We are very poor at diagnosing the specific etiology of LUTS – we don’t have great diagnostic tests or even phenotyping, and so that leaves clinicians with a very heterogeneous group of patients who all have the same syndrome of symptoms,” he explained. “But we don’t have much to guide us in terms of identifying who would benefit most from self-management overall, who would benefit from specific self-management techniques, and who would benefit from medication to target very specific mechanisms.”
Dr. Vaughan reported receiving funding from the Department of Veterans Affairs and National institutes of Health for research related to urinary symptom management, and that her spouse is an employee of Kimberly-Clark, which makes adult care products. Dr. Albarqouni and Dr. Bauer reported no relevant financial disclosures.
Testosterone decline after steroid abuse revealed with new biomarker
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).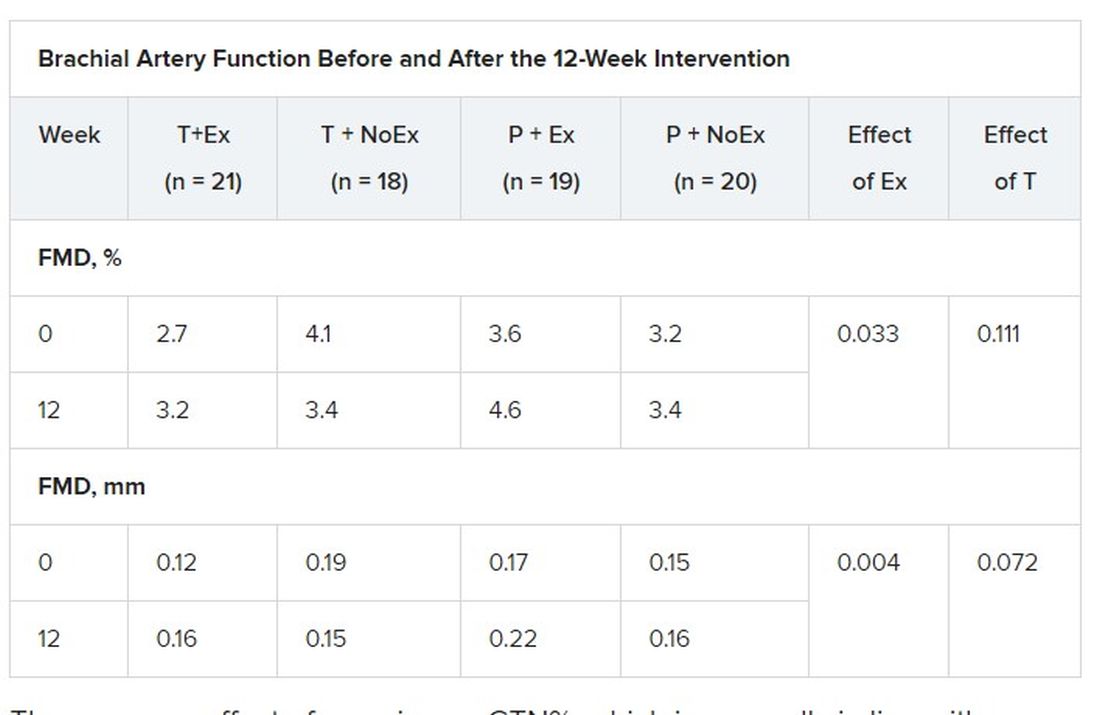
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Surveillance after testicular cancer: New approaches slash radiation exposure
Two new approaches to surveillance imaging after treatment of stage I testicular seminoma sharply reduce or eliminate radiation exposure relative to the standard approach, without substantially compromising relapse detection, the phase 3 TRISST study suggests.
Results were reported at the 2021 Genitourinary Cancers Symposium (Abstract 374).
“Stage I seminoma has a survival that approaches 100%. Over recent years, CT surveillance has become an international standard of care and has largely replaced the use of adjuvant treatment,” said investigator Robert A. Huddart, MRCP, FRCR, PhD, of The Royal Marsden NHS Foundation Trust, London.
“A typical surveillance protocol, however, consists of multiple CT scans taken over a period of a few years and results in quite a high diagnostic radiation dose, which has raised questions about the long-term risk of second malignancies related to this program,” he noted. “At the moment, there is no evidence base to inform how frequently imaging should be undertaken and the type of imaging that should be used.”
Results of TRISST showed that, with a median 6-year follow-up during which men were monitored with various surveillance protocols, 1.5% experienced a relapse that was advanced (stage IIC or higher) at detection.
The incidence of relapse was 0.3% with the standard schedule of seven abdominal surveillance scans and a statistically noninferior 2.8% with three more widely spaced scans. Also, compared with the standard CT scans, which yielded an incidence of 2.6%, MRI scans were noninferior, yielding an incidence of 0.6%.
“The three-scan schedule was noninferior to seven scans in our protocol, and in fact, with the three-scan schedule, we would use over 1,000 fewer scans at a cost of perhaps having four relapses that could have been avoided,” Dr. Huddart pointed out. “We can conclude that MRI is noninferior to CT and should be recommended to avoid irradiation. This study will provide an evidence base for making the transition to MRI, which is important. The MRI scan is more complex – it takes longer and is more resource heavy. So we do need to supply the evidence that it is the right thing to do for patients.”
Need for expertise in interpreting MRI scans is a valid concern, he acknowledged. “There is a degree of specialization in the UK for testis cancer management, and clearly, you had to be specialist to take part in the study. So I can’t say it is your everyday radiologist, but the data would suggest we actually saw less errors in terms of pickup with the MRI scan than with the CT scan,” he said. “You do need to have a level of expertise, but it doesn’t require super-specialist expertise. I suppose that will be a learning lesson for all of us, to learn better MRI interpretation if we are using MRI.”
Trial details
The 669 men randomized in TRISST (NCT00589537), a multicenter trial with a factorial and noninferiority design, had undergone orchiectomy for stage I seminoma and did not have any adjuvant therapy planned.
They were randomized once on number of surveillance scans: seven scans (at 6, 12, 18, 24, 36, 48, and 60 months) vs. three scans (at 6, 18, and 36 months). And they were randomized again on scan modality: CT versus MRI. All groups had similar follow-up otherwise, consisting of periodic chest radiographs, tumor marker tests, and clinical assessments.
The primary outcome, 6-year incidence of advanced relapse defined as stage IIC or higher (i.e., measuring 5 cm or greater) by Royal Marsden Hospital criteria, was chosen because, when the study began, this was the dividing point between using local therapy and using systemic multiagent chemotherapy to treat a relapse, Dr. Huddart explained.
Among men remaining on surveillance, compliance was good, with 94% of all scans attended and 79% performed on time, he reported.
Overall, 12% of the randomized population experienced a relapse of any stage during follow-up, with nearly all relapses occurring within the first 3 years.
The 6-year incidence of advanced relapse was just 1.5% in the entire trial population, lower than the 5.7% expected in trial planning, according to Dr. Huddart.
In intention-to-treat analyses, the incidence was 2.8% with the three-scan schedule and 0.3% with the seven-scan schedule, with the difference of 2.6% and the bounds of the 90% confidence interval falling within the predefined noninferiority margin of 5.7%.
Using three scans instead of seven scans increased the proportion of patients with relapse who had advanced stage from 3% to 20%. Four of the nine advanced relapses occurring with the three-scan schedule could possibly have been detected earlier with the seven-scan schedule.
The 6-year incidence of advanced relapse was 0.6% with MRI scans and 2.6% with CT scans. The difference of –1.9% and the bounds of the 90% confidence interval fell within the noninferiority margin. Use of MRI instead of CT reduced the proportion of patients with relapse who had advanced stage from 20% to 5%.
For both the scan frequency comparison and the scan modality comparison, findings were essentially the same in per protocol analyses and in analyses that instead looked at relapses measuring 3 cm or greater, according to Dr. Huddart.
Fully 89% of patients with advanced relapses were treated with chemotherapy only, and 56% of all patients with advanced relapse had a response to their treatment.
Most patients experiencing a relapse of any stage, 93%, were alive and free of disease at their most recent follow-up, Dr. Huddart reported. Overall survival for the trial population was 99% and similar across surveillance groups, with no deaths due to testicular cancer.
Risk-tailored surveillance
“Noninferiority trials are much more challenging than equivalence or superiority trials,” observed invited discussant Pilar Laguna, MD, PhD, of Istanbul Medipol University, Turkey.
She expressed some reservations about TRISST results, including the much lower than expected incidence of advanced relapse, which may have affected comparisons, and problematic compliance, as about one-quarter of patients stopped surveillance before relapse or withdrew from the trial before 6 years of follow-up.
Recurrence after treatment of stage I seminoma is largely driven by the risk factors of tumor size exceeding 4 cm and presence of rete testis invasion, and 54% of TRISST patients did not have either of these factors, Dr. Laguna noted.
“While a more relaxed schedule may well suit those patients at low risk, more intense schedules will be appropriate for patients with risk factors,” she maintained. “I am pretty sure that the TRISST trial will impact future guideline recommendations, although still I think that one approach may not fit all.”
TRISST was funded by Cancer Research UK and the MRC Clinical Trials Unit. Dr. Huddart disclosed relationships with Janssen Oncology, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme, Nektar, and Roche. Dr. Laguna disclosed that she is chair of the EAU Testicular Cancer Guidelines Panel.
Two new approaches to surveillance imaging after treatment of stage I testicular seminoma sharply reduce or eliminate radiation exposure relative to the standard approach, without substantially compromising relapse detection, the phase 3 TRISST study suggests.
Results were reported at the 2021 Genitourinary Cancers Symposium (Abstract 374).
“Stage I seminoma has a survival that approaches 100%. Over recent years, CT surveillance has become an international standard of care and has largely replaced the use of adjuvant treatment,” said investigator Robert A. Huddart, MRCP, FRCR, PhD, of The Royal Marsden NHS Foundation Trust, London.
“A typical surveillance protocol, however, consists of multiple CT scans taken over a period of a few years and results in quite a high diagnostic radiation dose, which has raised questions about the long-term risk of second malignancies related to this program,” he noted. “At the moment, there is no evidence base to inform how frequently imaging should be undertaken and the type of imaging that should be used.”
Results of TRISST showed that, with a median 6-year follow-up during which men were monitored with various surveillance protocols, 1.5% experienced a relapse that was advanced (stage IIC or higher) at detection.
The incidence of relapse was 0.3% with the standard schedule of seven abdominal surveillance scans and a statistically noninferior 2.8% with three more widely spaced scans. Also, compared with the standard CT scans, which yielded an incidence of 2.6%, MRI scans were noninferior, yielding an incidence of 0.6%.
“The three-scan schedule was noninferior to seven scans in our protocol, and in fact, with the three-scan schedule, we would use over 1,000 fewer scans at a cost of perhaps having four relapses that could have been avoided,” Dr. Huddart pointed out. “We can conclude that MRI is noninferior to CT and should be recommended to avoid irradiation. This study will provide an evidence base for making the transition to MRI, which is important. The MRI scan is more complex – it takes longer and is more resource heavy. So we do need to supply the evidence that it is the right thing to do for patients.”
Need for expertise in interpreting MRI scans is a valid concern, he acknowledged. “There is a degree of specialization in the UK for testis cancer management, and clearly, you had to be specialist to take part in the study. So I can’t say it is your everyday radiologist, but the data would suggest we actually saw less errors in terms of pickup with the MRI scan than with the CT scan,” he said. “You do need to have a level of expertise, but it doesn’t require super-specialist expertise. I suppose that will be a learning lesson for all of us, to learn better MRI interpretation if we are using MRI.”
Trial details
The 669 men randomized in TRISST (NCT00589537), a multicenter trial with a factorial and noninferiority design, had undergone orchiectomy for stage I seminoma and did not have any adjuvant therapy planned.
They were randomized once on number of surveillance scans: seven scans (at 6, 12, 18, 24, 36, 48, and 60 months) vs. three scans (at 6, 18, and 36 months). And they were randomized again on scan modality: CT versus MRI. All groups had similar follow-up otherwise, consisting of periodic chest radiographs, tumor marker tests, and clinical assessments.
The primary outcome, 6-year incidence of advanced relapse defined as stage IIC or higher (i.e., measuring 5 cm or greater) by Royal Marsden Hospital criteria, was chosen because, when the study began, this was the dividing point between using local therapy and using systemic multiagent chemotherapy to treat a relapse, Dr. Huddart explained.
Among men remaining on surveillance, compliance was good, with 94% of all scans attended and 79% performed on time, he reported.
Overall, 12% of the randomized population experienced a relapse of any stage during follow-up, with nearly all relapses occurring within the first 3 years.
The 6-year incidence of advanced relapse was just 1.5% in the entire trial population, lower than the 5.7% expected in trial planning, according to Dr. Huddart.
In intention-to-treat analyses, the incidence was 2.8% with the three-scan schedule and 0.3% with the seven-scan schedule, with the difference of 2.6% and the bounds of the 90% confidence interval falling within the predefined noninferiority margin of 5.7%.
Using three scans instead of seven scans increased the proportion of patients with relapse who had advanced stage from 3% to 20%. Four of the nine advanced relapses occurring with the three-scan schedule could possibly have been detected earlier with the seven-scan schedule.
The 6-year incidence of advanced relapse was 0.6% with MRI scans and 2.6% with CT scans. The difference of –1.9% and the bounds of the 90% confidence interval fell within the noninferiority margin. Use of MRI instead of CT reduced the proportion of patients with relapse who had advanced stage from 20% to 5%.
For both the scan frequency comparison and the scan modality comparison, findings were essentially the same in per protocol analyses and in analyses that instead looked at relapses measuring 3 cm or greater, according to Dr. Huddart.
Fully 89% of patients with advanced relapses were treated with chemotherapy only, and 56% of all patients with advanced relapse had a response to their treatment.
Most patients experiencing a relapse of any stage, 93%, were alive and free of disease at their most recent follow-up, Dr. Huddart reported. Overall survival for the trial population was 99% and similar across surveillance groups, with no deaths due to testicular cancer.
Risk-tailored surveillance
“Noninferiority trials are much more challenging than equivalence or superiority trials,” observed invited discussant Pilar Laguna, MD, PhD, of Istanbul Medipol University, Turkey.
She expressed some reservations about TRISST results, including the much lower than expected incidence of advanced relapse, which may have affected comparisons, and problematic compliance, as about one-quarter of patients stopped surveillance before relapse or withdrew from the trial before 6 years of follow-up.
Recurrence after treatment of stage I seminoma is largely driven by the risk factors of tumor size exceeding 4 cm and presence of rete testis invasion, and 54% of TRISST patients did not have either of these factors, Dr. Laguna noted.
“While a more relaxed schedule may well suit those patients at low risk, more intense schedules will be appropriate for patients with risk factors,” she maintained. “I am pretty sure that the TRISST trial will impact future guideline recommendations, although still I think that one approach may not fit all.”
TRISST was funded by Cancer Research UK and the MRC Clinical Trials Unit. Dr. Huddart disclosed relationships with Janssen Oncology, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme, Nektar, and Roche. Dr. Laguna disclosed that she is chair of the EAU Testicular Cancer Guidelines Panel.
Two new approaches to surveillance imaging after treatment of stage I testicular seminoma sharply reduce or eliminate radiation exposure relative to the standard approach, without substantially compromising relapse detection, the phase 3 TRISST study suggests.
Results were reported at the 2021 Genitourinary Cancers Symposium (Abstract 374).
“Stage I seminoma has a survival that approaches 100%. Over recent years, CT surveillance has become an international standard of care and has largely replaced the use of adjuvant treatment,” said investigator Robert A. Huddart, MRCP, FRCR, PhD, of The Royal Marsden NHS Foundation Trust, London.
“A typical surveillance protocol, however, consists of multiple CT scans taken over a period of a few years and results in quite a high diagnostic radiation dose, which has raised questions about the long-term risk of second malignancies related to this program,” he noted. “At the moment, there is no evidence base to inform how frequently imaging should be undertaken and the type of imaging that should be used.”
Results of TRISST showed that, with a median 6-year follow-up during which men were monitored with various surveillance protocols, 1.5% experienced a relapse that was advanced (stage IIC or higher) at detection.
The incidence of relapse was 0.3% with the standard schedule of seven abdominal surveillance scans and a statistically noninferior 2.8% with three more widely spaced scans. Also, compared with the standard CT scans, which yielded an incidence of 2.6%, MRI scans were noninferior, yielding an incidence of 0.6%.
“The three-scan schedule was noninferior to seven scans in our protocol, and in fact, with the three-scan schedule, we would use over 1,000 fewer scans at a cost of perhaps having four relapses that could have been avoided,” Dr. Huddart pointed out. “We can conclude that MRI is noninferior to CT and should be recommended to avoid irradiation. This study will provide an evidence base for making the transition to MRI, which is important. The MRI scan is more complex – it takes longer and is more resource heavy. So we do need to supply the evidence that it is the right thing to do for patients.”
Need for expertise in interpreting MRI scans is a valid concern, he acknowledged. “There is a degree of specialization in the UK for testis cancer management, and clearly, you had to be specialist to take part in the study. So I can’t say it is your everyday radiologist, but the data would suggest we actually saw less errors in terms of pickup with the MRI scan than with the CT scan,” he said. “You do need to have a level of expertise, but it doesn’t require super-specialist expertise. I suppose that will be a learning lesson for all of us, to learn better MRI interpretation if we are using MRI.”
Trial details
The 669 men randomized in TRISST (NCT00589537), a multicenter trial with a factorial and noninferiority design, had undergone orchiectomy for stage I seminoma and did not have any adjuvant therapy planned.
They were randomized once on number of surveillance scans: seven scans (at 6, 12, 18, 24, 36, 48, and 60 months) vs. three scans (at 6, 18, and 36 months). And they were randomized again on scan modality: CT versus MRI. All groups had similar follow-up otherwise, consisting of periodic chest radiographs, tumor marker tests, and clinical assessments.
The primary outcome, 6-year incidence of advanced relapse defined as stage IIC or higher (i.e., measuring 5 cm or greater) by Royal Marsden Hospital criteria, was chosen because, when the study began, this was the dividing point between using local therapy and using systemic multiagent chemotherapy to treat a relapse, Dr. Huddart explained.
Among men remaining on surveillance, compliance was good, with 94% of all scans attended and 79% performed on time, he reported.
Overall, 12% of the randomized population experienced a relapse of any stage during follow-up, with nearly all relapses occurring within the first 3 years.
The 6-year incidence of advanced relapse was just 1.5% in the entire trial population, lower than the 5.7% expected in trial planning, according to Dr. Huddart.
In intention-to-treat analyses, the incidence was 2.8% with the three-scan schedule and 0.3% with the seven-scan schedule, with the difference of 2.6% and the bounds of the 90% confidence interval falling within the predefined noninferiority margin of 5.7%.
Using three scans instead of seven scans increased the proportion of patients with relapse who had advanced stage from 3% to 20%. Four of the nine advanced relapses occurring with the three-scan schedule could possibly have been detected earlier with the seven-scan schedule.
The 6-year incidence of advanced relapse was 0.6% with MRI scans and 2.6% with CT scans. The difference of –1.9% and the bounds of the 90% confidence interval fell within the noninferiority margin. Use of MRI instead of CT reduced the proportion of patients with relapse who had advanced stage from 20% to 5%.
For both the scan frequency comparison and the scan modality comparison, findings were essentially the same in per protocol analyses and in analyses that instead looked at relapses measuring 3 cm or greater, according to Dr. Huddart.
Fully 89% of patients with advanced relapses were treated with chemotherapy only, and 56% of all patients with advanced relapse had a response to their treatment.
Most patients experiencing a relapse of any stage, 93%, were alive and free of disease at their most recent follow-up, Dr. Huddart reported. Overall survival for the trial population was 99% and similar across surveillance groups, with no deaths due to testicular cancer.
Risk-tailored surveillance
“Noninferiority trials are much more challenging than equivalence or superiority trials,” observed invited discussant Pilar Laguna, MD, PhD, of Istanbul Medipol University, Turkey.
She expressed some reservations about TRISST results, including the much lower than expected incidence of advanced relapse, which may have affected comparisons, and problematic compliance, as about one-quarter of patients stopped surveillance before relapse or withdrew from the trial before 6 years of follow-up.
Recurrence after treatment of stage I seminoma is largely driven by the risk factors of tumor size exceeding 4 cm and presence of rete testis invasion, and 54% of TRISST patients did not have either of these factors, Dr. Laguna noted.
“While a more relaxed schedule may well suit those patients at low risk, more intense schedules will be appropriate for patients with risk factors,” she maintained. “I am pretty sure that the TRISST trial will impact future guideline recommendations, although still I think that one approach may not fit all.”
TRISST was funded by Cancer Research UK and the MRC Clinical Trials Unit. Dr. Huddart disclosed relationships with Janssen Oncology, Bayer, Bristol-Myers Squibb, Merck Sharp & Dohme, Nektar, and Roche. Dr. Laguna disclosed that she is chair of the EAU Testicular Cancer Guidelines Panel.
FROM GUCS 2021
Large study finds trans men on testosterone at risk for blood clots
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Over 10% of transgender men (females transitioning to male) who take testosterone develop high hematocrit levels that could put them at greater risk for a thrombotic event, and the largest increase in levels occurs in the first year after starting therapy, a new Dutch study indicates.
Erythrocytosis, defined as a hematocrit greater than 0.50 L/L, is a potentially serious side effect of testosterone therapy, say Milou Cecilia Madsen, MD, and colleagues in their article published online Feb. 18, 2021, in the Journal of Clinical Endocrinology & Metabolism.
When hematocrit was measured twice, 11.1% of the cohort of 1073 trans men had levels in excess of 0.50 L/L over a 20-year follow-up.
“Erythrocytosis is common in transgender men treated with testosterone, especially in those who smoke, have [a] high BMI [body mass index], and [who] use testosterone injections,” Dr. Madsen, of the VU University Medical Center Amsterdam, said in a statement from the Endocrine Society.
“A reasonable first step in the care of transgender men with high red blood cells while on testosterone is to advise them to quit smoking, switch injectable testosterone to gel, and, if BMI is high, to lose weight,” she added.
First large study of testosterone in trans men with 20-year follow-up
Transgender men often undergo testosterone therapy as part of gender-affirming treatment.
Secondary erythrocytosis, a condition where the body makes too many red blood cells, is a common side effect of testosterone therapy that can increase the risk of thrombolic events, heart attack, and stroke, Dr. Madsen and colleagues explained.
This is the first study of a large cohort of trans men taking testosterone therapy followed for up to 20 years. Because of the large sample size, statistical analysis with many determinants could be performed. And because of the long follow-up, a clear time relation between initiation of testosterone therapy and hematocrit could be studied, they noted.
Participants were part of the Amsterdam Cohort of Gender Dysphoria study, a large cohort of individuals seen at the Center of Expertise on Gender Dysphoria at Amsterdam University Medical Center between 1972 and 2015.
Laboratory measurements taken between 2004 and 2018 were available for analysis. Trans men visited the center every 3-6 months during their first year of testosterone therapy and were then monitored every year or every other year.
Long-acting undecanoate injection was associated with the highest risk of a hematocrit level greater than 0.50 L/L, and the risk of erythrocytosis in those who took long-acting intramuscular injections was about threefold higher, compared with testosterone gel (adjusted odds ratio, 3.1).
In contrast, short-acting ester injections and oral administration of testosterone had a similar risk for erythrocytosis, as did testosterone gel.
Other determinants of elevated hematocrit included smoking, medical history of a number of comorbid conditions, and older age on initiation of testosterone.
In contrast, “higher testosterone levels per se were not associated with an increased odds of hematocrit greater than 0.50 L/L”, the authors noted.
Current advice for trans men based on old guidance for hypogonadism
The authors said that current advice for trans men is based on recommendations for testosterone-treated hypogonadal cis men (those assigned male at birth) from 2008, which advises a hematocrit greater than 0.50 L/L has a moderate to high risk of adverse outcome. For levels greater than 0.54 L/L, cessation of testosterone therapy, a dose reduction, or therapeutic phlebotomy to reduce the risk of adverse events is advised. For levels 0.50-0.54 L/L, no clear advice is given.
But questions remain as to whether these guidelines are applicable to trans men because the duration of testosterone therapy is much longer in trans men and hormone treatment often cannot be discontinued without causing distress.
Meanwhile, hematology guidelines indicate an upper limit for hematocrit for cis females of 0.48 L/L.
“It could be argued that the upper limit for cis females should be applied, as trans men are born with female genetics,” the authors said. “This is a subject for further research.”
Duration of testosterone therapy impacts risk of erythrocytosis
In the study, the researchers found that longer duration of testosterone therapy increased the risk of developing hematocrit levels greater than 0.50 L/L. For example, after 1 year, the cumulative incidence of erythrocytosis was 8%; after 10 years, it was 38%; and after 14 years, it was 50%.
Until more specific guidance is developed for trans men, if hematocrit levels rise to 0.50-0.54 L/L, the researchers suggested taking “reasonable” steps to prevent a further increase:
- Consider switching patients who use injectable testosterone to transdermal products.
- Advise patients with a BMI greater than 25 kg/m2 to lose weight to attain a BMI of 18.5-25.
- Advise patients to stop smoking.
- Pursue treatment optimization for chronic lung disease or sleep apnea.
The study had no external funding. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.













