User login
With sexually transmitted infections off the charts, California pushes at-home tests
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
SACRAMENTO, CALIF. – California has become the first state to require health insurance plans to cover at-home tests for sexually transmitted infections such as HIV, chlamydia, and syphilis – which could help quell the STI epidemic that has raged nearly unchecked as public health departments have focused on COVID-19.
The rule, part of a broader law addressing the STI epidemic, took effect Jan. 1 for people with state-regulated private insurance plans and will kick in sometime later for the millions of low-income Californians enrolled in the state’s Medicaid program.
By making it easier and cheaper for Californians to self-administer tests in the privacy of their homes, the provision could bring better disease monitoring to rural and underserved parts of the state, reduce the stigma patients experience when seeking care, and give them more control over their health, say experts on infectious diseases.
“This is the first law of its kind, and I’d say it’s kind of cutting-edge,” said Stephanie Arnold Pang, senior director of policy and government relations for the National Coalition of STD Directors. “We want to bring down every single barrier for someone to get STI testing, and out-of-pocket cost is a huge factor.”
But being first has its downsides. Because the concept of insurance coverage for home STI tests is so new, the state’s Medicaid program, Medi-Cal, could not establish by Jan. 1 the billing codes it needs to start paying for tests. Federal regulators also haven’t approved the tests for home use, which could make labs reluctant to process them. And a state analysis predicts most in-network health care providers won’t start prescribing home tests for at least a year until they adjust their billing and other practices.
Nevertheless, the situation is urgent and requires action, said state Sen. Richard Pan (D-Sacramento), a pediatrician who wrote the law.
“We have children born in California with syphilis,” Dr. Pan said. “You’d think that went away in the Victorian era.”
Even before COVID, sexually transmitted infections hit all-time highs in the United States and California for 6 years in a row, according to 2019 data from the Centers for Disease Control and Prevention. Rates of congenital syphilis, which babies contract from their mothers, illustrate the severity of the STI epidemic: Cases were up 279% from 2015 to 2019 nationally and 232% in California. Of the 445 cases of congenital syphilis in California in 2019, 37 were stillbirths.
The pandemic only worsened the problem because health departments were overwhelmed responding to the COVID emergency, and stay-at-home orders kept people away from clinics.
In surveys of public health programs across the country since May 2020, the National Coalition of STD Directors found that most respondents – up to 78% in one survey – have diverted some of their STI workforces to test and monitor COVID. A report that accompanied the most recent survey found that some STIs were “completely unchecked” because of reductions in clinic hours, diversion of resources, shortages of testing kits and staff burnout.
Some at-home STI tests screen for a single disease but other kits can collect and send samples to check for a variety of infections. Depending on the test, patients collect a drop of blood with a lancet, or swab their mouth, vagina, anus, or penis.
Some tests require patients to send samples to a lab for analysis, while some oral HIV tests give results at home in a few minutes.
Ivan Beas, a 25-year-old graduate student at University of California, Los Angeles, was getting tested frequently as part of a 2-year research study. When clinics closed during the pandemic, researchers sent him a home kit.
The kit, which tests for HIV, hepatitis C, herpes, syphilis, chlamydia, gonorrhea, and trichomoniasis, was packaged discreetly and came with easy instructions. It took Mr. Beas about 10 minutes to prick his finger, swab his mouth and send the samples to the lab.
Mr. Beas wanted to continue screening himself every few months after the study ended, he said, but the kit he used retails for $289, which is out of reach for him.
The last time he went to a clinic in person, “I spent 2 hours waiting to even be seen by a doctor because of how busy they are,” he said. Until Medi-Cal begins covering home tests, he said, he will have to find time to get tested for free at a Planned Parenthood clinic.
“If insurance were to cover it, I’d definitely do it more,” he said.
Under California’s new law, plans regulated by the state must cover home STI tests when ordered by a health care provider.
Privately insured Californians can take advantage of the coverage immediately. How much they will owe out-of-pocket for the tests – if anything – depends on the type of plan they have, whether their provider is in-network, and whether they fall into a category the federal government has designated for free screening.
Medi-Cal patients almost never face out-of-pocket expenses, but they will have to wait for coverage because the Department of Health Care Services, which administers Medi-Cal, is working with the American Medical Association and the federal government to create billing codes. The reimbursement rates for those codes will then need federal approval.
The state doesn’t know how long that process will take, according to department spokesperson Anthony Cava.
The rule does not apply to the millions of Californians whose job-based health insurance plans are regulated by the federal government.
Other states and organizations have experimented with at-home STI tests. The public health departments in Alabama and the District of Columbia send free kits to residents who request them, but neither jurisdiction requires insurance coverage for them. The National Coalition of STD Directors is sending free kits to people through health departments in Philadelphia; Iowa; Virginia; Indiana; Puerto Rico; and Navajo County, Arizona. The list of recipients is expected to grow this month.
Iwantthekit.org, a project of Johns Hopkins University, has been sending free kits to Maryland residents since 2004, and to Alaskans since 2011. The program is funded by grants and works with local health departments.
Charlotte Gaydos, cofounder of the project, said that requests for test kits during the pandemic nearly tripled – and that she would expand to every state if she could bill insurance the way the California law mandates.
The tests fall into a murky regulatory area. While they have been approved by the Food and Drug Administration, none have been cleared for use at home. Patients are supposed to collect their own samples within the walls of a health facility, and some labs may not analyze samples collected at home.
Public health officials cited other potential challenges: Patients may not have the same access to counseling, treatment, or referrals to other services such as food banks that they would receive at clinics. And although patients are supposed to self-report the results of their tests to public health authorities, some people won’t follow through.
Vlad Carrillo, 31, experienced such trade-offs recently. Mr. Carrillo used to get tested at a San Francisco clinic, where they could get counseling and other services. But Carrillo lost their apartment during the pandemic and moved about 7 hours away to Bishop, the only incorporated city in rural Inyo County.
“Being away from the city, it took me a whole year to find a way to get tested,” Carrillo said.
Carrillo eventually got the kit through the mail, avoiding the stigma of going to the clinic in Bishop, which is “more focused on straight stuff,” like preventing pregnancy. Without the test, Carrillo couldn’t get PrEP, a medication to prevent HIV.
“Going without it for so long was really hard on me,” Carrillo said.
This story was produced by Kaiser Health News (KHN), which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Write an exercise Rx to improve patients' cardiorespiratory fitness
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
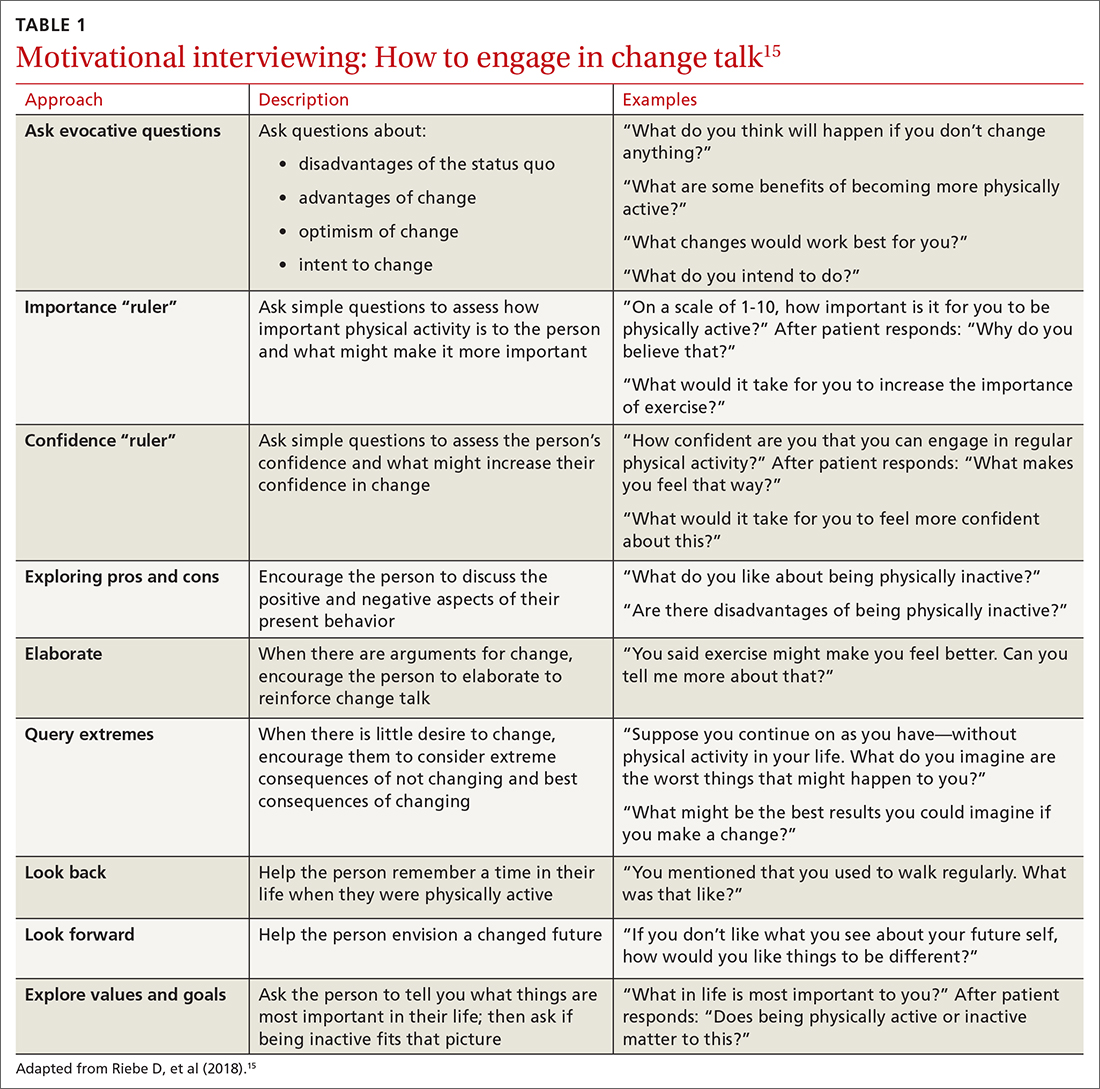
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
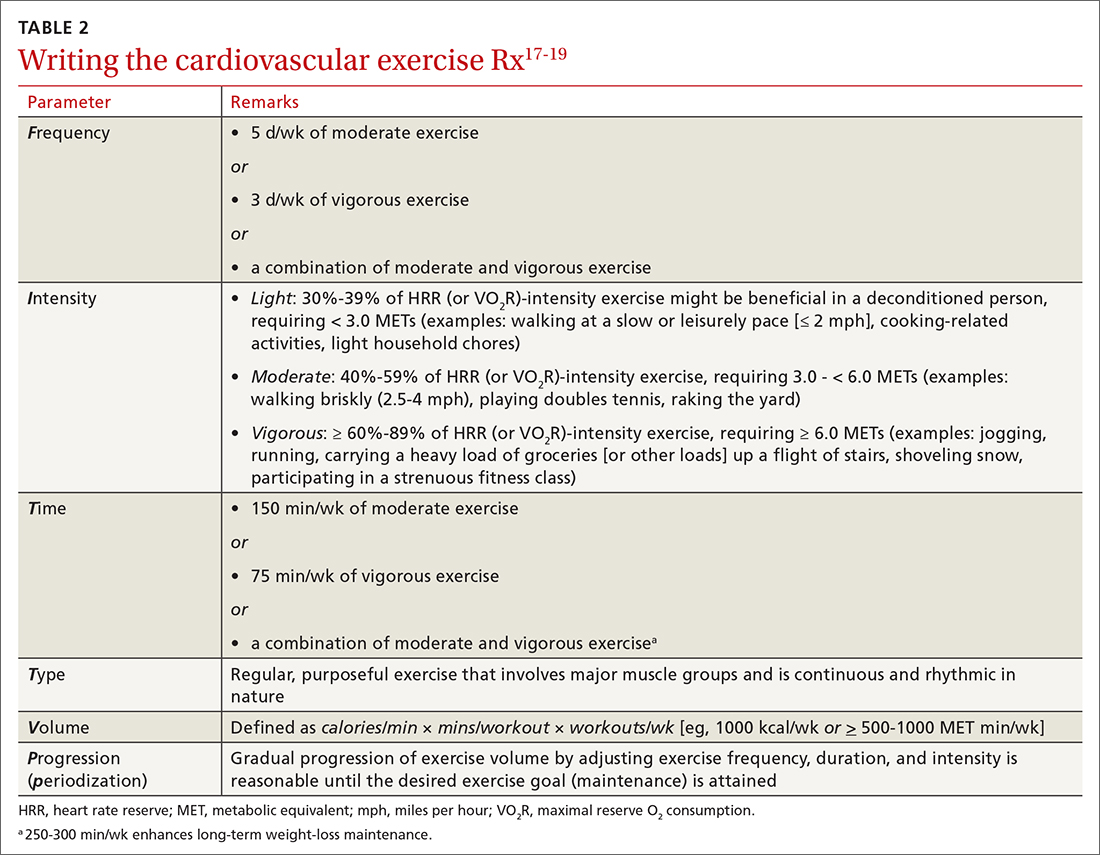
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.
SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
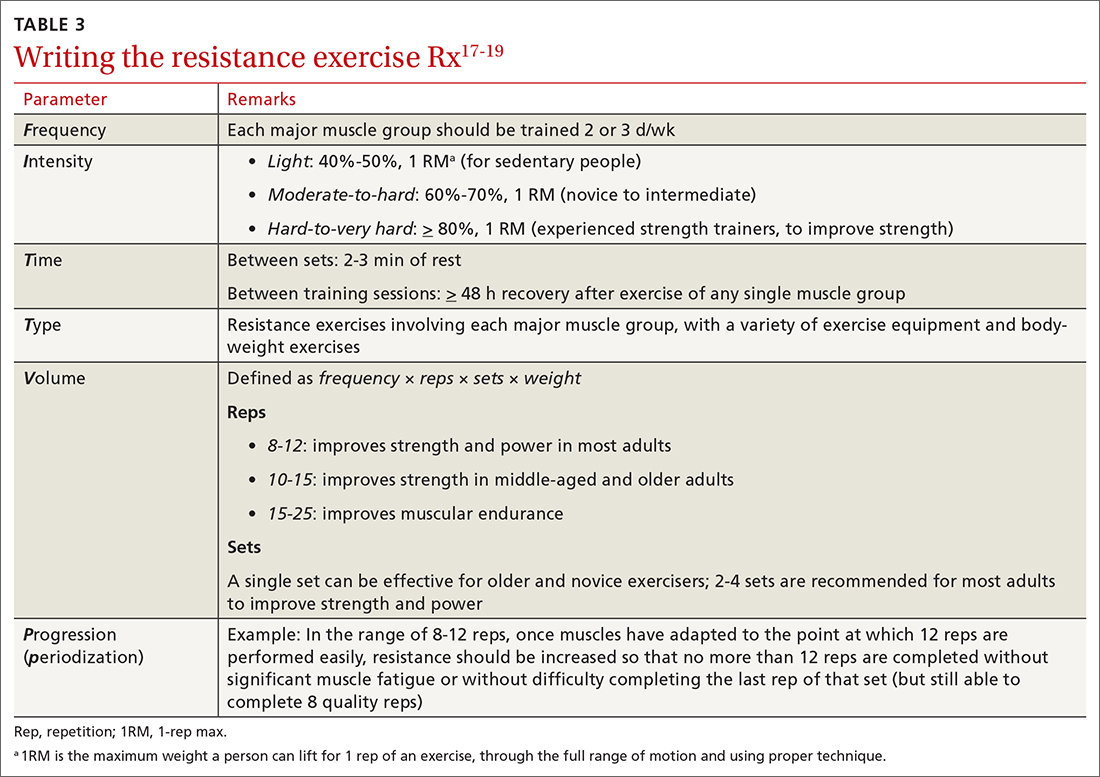
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
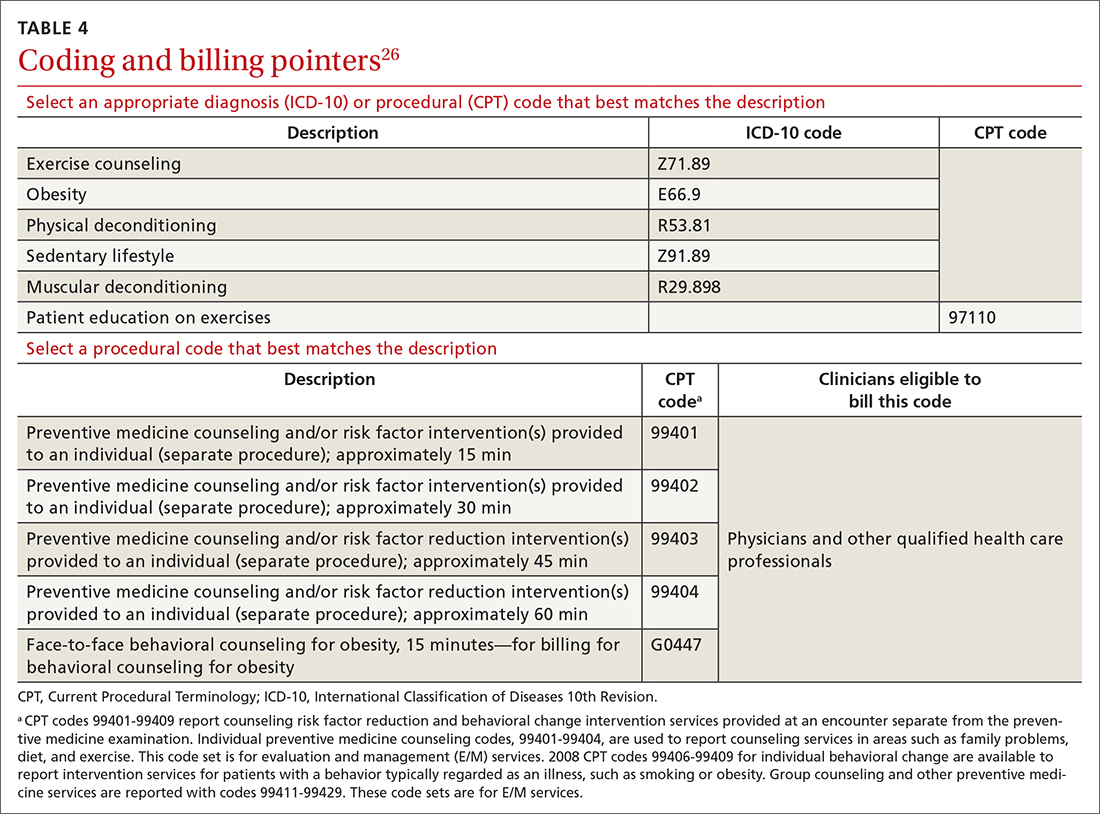
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.
SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.
SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
PRACTICE RECOMMENDATIONS
› Encourage children and adolescents (6 to 17 years of age) to engage in 60 min of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening endeavors on most, if not all, days of the week. A
› Encourage adults to perform approximately 150 to 300 min of moderate or 75 to 150 min of vigorous physical activity (or an equivalent combination) per week, along with moderate-intensity muscle-strengthening activities on ≥ 2 days per week. A
› Counsel patients that even a small (eg, 1-2 metabolic equivalents) increase in cardiorespiratory fitness is associated with a 10% to 30% lower rate of adverse events. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Could Viagra help prevent Alzheimer’s?
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
published in the journal Nature Aging.
Patients who used the drug sildenafil, the generic name for Viagra, were 69% less likely to develop the disease than were nonusers.
“Sildenafil, which has been shown to significantly improve cognition and memory in preclinical models, presented as the best drug candidate,” Feixiong Cheng, PhD, the lead study author in the Cleveland Clinic’s Genomic Medicine Institute, said in a statement.
“Notably, we found that sildenafil use reduced the likelihood of Alzheimer’s in individuals with coronary artery disease, hypertension, and type 2 diabetes, all of which are comorbidities significantly associated with risk of the disease, as well as in those without,” he said.
Alzheimer’s, which is the most common form of age-related dementia, affects hundreds of millions of people worldwide. The disease is expected to affect nearly 14 million Americans by 2050. There is no approved treatment for it.
Dr. Cheng and colleagues at the Cleveland Clinic used a large gene-mapping network to analyze whether more than 1,600 Food and Drug Administration–approved drugs could work against Alzheimer’s. They gave higher scores to drugs that target both amyloid and tau proteins in the brain, which are two hallmarks of the disease. Sildenafil appeared at the top of the list.
Then the researchers used a database of health insurance claims for more than 7 million people in the U.S. to understand the relationship between sildenafil and Alzheimer’s disease outcomes. They compared sildenafil users to nonusers and found that those who used the drug were 69% less likely to have the neurodegenerative disease, even after 6 years of follow-up.
After that, the research team came up with a lab model that showed the sildenafil increased brain cell growth and targeted tau proteins. The lab model could indicate how the drug influences disease-related brain changes.
But Dr. Cheng cautioned against drawing strong conclusions. The study doesn’t demonstrate a causal relationship between sildenafil and Alzheimer’s disease. Researchers will need to conduct clinical trials with a placebo control to see how well the drug works.
Other researchers said the findings offer a new avenue for research but don’t yet provide solid answers.
“Being able to repurpose a drug already licensed for health conditions could help speed up the drug discovery process and bring about life-changing dementia treatments sooner,” Susan Kohlhaas, PhD, director of research at Alzheimer’s Research UK, told the Science Media Centre.
“Importantly, this research doesn’t prove that sildenafil is responsible for reducing dementia risk, or that it slows or stops the disease,” she continued. “If you want to discuss any treatments you are receiving, the first port of call is to speak to your doctor.”
And doctors won’t likely recommend it as a treatment just yet either.
“While these data are interesting scientifically, based on this study, I would not rush out to start taking sildenafil as a prevention for Alzheimer’s disease,” Tara Spires-Jones, PhD, deputy director of the Centre for Discovery Brain Sciences at the University of Edinburgh, told the Science Media Centre.
A version of this article first appeared on WebMD.com.
FROM NATURE AGING
Association Between Physiotherapy Outcome Measures and the Functional Independence Measure: A Retrospective Analysis
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
From Illawarra Shoalhaven Local Health District, New South Wales, Australia (Maren Jones, Dr. Hewitt, Philippa King, Rhiannon Thorn, Edward Davidson, and Tiana-Lee Elphick), and Illawarra Health and Medical Research Institute, University of Wollongong, Wollongong, New South Wales, Australia (Dr. Hewitt)
Objective: To assess the association between change scores in the Functional Independence Measure (FIM) with evaluative measures used in physiotherapy to objectively show that use of the FIM in isolation is limited.
Design: Retrospective observational study.
Setting: Five rehabilitation inpatient wards from 1 public local health district in NSW Australia.
Participants: Patient data over a 5-year time frame (2015 to 2019) were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups (Australasian Rehabilitation Outcome Centre classification) were identified for inclusion in this study: Reconditioning (n = 742, mean age 76.88 years); Orthopedic Fracture (n = 585, mean age 77.46 years); and Orthopedic Replacement (n = 377, mean age 73.84 years).
Measurements: The difference between the admission and discharge scores were calculated for each measure. Kruskal-Wallis and χ2 tests were used to analyze the data.
Results: Pearson correlation (r) coefficients between FIM Motor change to the de Morton’s Mobility Index (DEMMI) change was r = 0.396, FIM Motor change to the Timed Up and Go (TUG) change was r = -0.217, and the FIM Motor change to the Ten Meter Walk Test (10MWT) change was .194.
Conclusion: The FIM Motor change scores showed a weak positive association to the DEMMI change and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, functional mobility, and dynamic balance.
Keywords: physiotherapy; rehabilitation; clinical outcome measures.
Patients receive interdisciplinary inpatient rehabilitation treatment after they have sustained a lower limb fracture, a lower limb joint replacement, or have generalized deconditioning (muscle wasting and disuse atrophy) following hospitalization for surgery or illness. The degree of a patient’s impairment or loss of functional capacity, as well as their ability to manage at home safely, is assessed using standardized outcome measures during their recovery and rehabilitation.1,2
Physiotherapists routinely use validated outcome measures to assess patient progress and to measure goal attainment through assessment of functional independence, dynamic balance performance, and ambulatory ability. These objective assessments provide clinicians with information about the effectiveness of the rehabilitation program, as well as the patient’s ability to manage in their home environment, to determine the need for assistive devices, level of caregiver support, future level of autonomy, and strategies for falls prevention.3-7
There is a view among service providers that rehabilitation decisions can be based on a singular measure of function known as the Functional Independence Measure (FIM). This is an understandable position because not only is the FIM an internationally recognized, valid, and reliable tool, but, as a singular measure, it also means measurement consistency across rehabilitation sites is more likely. However, rehabilitation is complex, and it is risky to base decisions on a single measure, which might not capture the results of rehabilitation treatment ingredients on individual patient targets.8,9
The patient’s progress is objectively assessed using functional outcome measures such as the FIM. Other measures used typically in our service include the de Morton’s Mobility Index (DEMMI), Timed Up and Go (TUG), and the Ten Meter Walk Test (10MWT), which measure patient mobility, balance during directional changes, and walking ability, respectively. Additional measures include patient progression to a less supportive level of assistance (ie, number of persons required to assist or level of supervision) or the selection of a walking aid (eg, forearm support frame, crutches). This progression—or lack thereof—assists in decision-making regarding the individual’s future once they are discharged from rehabilitation. Such considerations would include the need to modify the home environment, selection of assistive devices, community access (walking indoors, outdoors, and shopping), personal care needs, and age-appropriate care facility recommendations (ie, level of care). The use of outcome measures also indicates the need for further referrals to other care providers upon discharge from the rehabilitation facility.
There is widespread support in the literature for the use of the FIM, DEMMI, TUG, and 10MWT in rehabilitation population groups. For example, DEMMI has been validated in hip fracture patients during rehabilitation,10 as well as among older people hospitalized for medical illness.11-13 It has also been shown to be a predictor of discharge destination for patients living with frailty in geriatric rehabilitation settings,14 and to have moderate predictive validity for functional independence after 4 weeks of rehabilitation.15 Similarly, TUG has been validated for use among hospitalized and community-dwelling individuals,16-18 and for patients after joint arthroplasty19,20 or hip fracture.21 It has also been shown to be an indicator of fall risk,22-24 as well as a predictor of fracture incidence.25 Furthermore, TUG has been identified as an indicator of a patient’s ability to walk in the community without the need for a walking device.26 It has also been shown to be an early identifier of patients in need of rehabilitation.27 Normative values for TUG have been reported, and the association with gait time established.28
Gait speed has been shown to predict adverse outcomes in community-dwelling older people.29 In fact, the 10MWT has been established as a powerful tool to benchmark rehabilitation recovery after a medical event.30 Results of the test relate to overall quality of walking, health status, morbidity, and the rate of mortality.31-33 Meaningful improvement, minimum detectable change (0.19-0.34 m/s), and responsiveness in common physical performance in older adults has been reported.26,34,36
Structural and functional impairment has been used to define rehabilitation classes by the Australasian Rehabilitation Outcome Centre (AROC) in the Australian National Sub-Acute and Non-Acute Patient Classification (AN-SNAP) Version 4.37-43 Variables used for grouping are age, care type, function, and impairment for rehabilitation. FIM was developed in order to assess patients’ outcomes after inpatient multidisciplinary care, and is an internationally accepted measure of functioning.44 It is a holistic outcome measure, which can be used to determine the patient’s level of disability and burden of care, and is widely used in both public and private inpatient rehabilitation settings. Each patient classification is reported separately within the case mix structure.45 Inpatient rehabilitation centers are evaluated and compared by the AROC,46 with an emphasis on length of stay and the FIM change. The most successful centers demonstrate shorter length of stay and greater FIM improvement. Although the FIM is a valuable measure, it does not provide a complete picture of the individual patient’s rehabilitation gain: ie, the specific attributes of patients’ mobility, walking ability, or balance during directional changes.
A large-scale analysis of the association between the holistic disability measure of the FIM and the more mobility- and ambulation-focused physiotherapy outcomes has not been documented.
The well-documented DEMMI accumulates points for the patient’s mobility in a similar fashion to the FIM, but with more mobility detail. These 2 outcome measures allow for the full range of patients, from the very dependent up to and including the independently ambulant patients. The DEMMI may show a positive relationship to the FIM, yet the association is unknown. The association of the TUG to the 10MWT has been established28; however, their relationship to the FIM is unknown.
Current practice in the participating public health inpatient rehabilitation wards is to use the DEMMI, TUG, 10MWT, and FIM to ensure physiotherapy and allow the wider multidisciplinary team to more effectively evaluate patient mobility outcomes. The 3 most frequent patient groups identified within the current patient population are expected to present clinical differences and will be analyzed for comparison. If an association is found between the outcome measures in question, clinical efficiency could be improved.
The aim of the current study is to assess the association between change scores in the FIM with evaluative measures of outcomes typically used in physiotherapy to objectively show that use of the FIM in isolation is limited in our population of patients.
Methods
Study design and setting
This retrospective descriptive observational study complied with the STROBE-RECORD guidance and checklist (available at mdedge.com/jcomjournal) and analyzed the routinely collected data from rehabilitation patients who were admitted to 5 different rehabilitation wards in 4 different public hospitals from 1 regional local health district (20-24 beds per ward) from 2015 to 2019. As this study conducted secondary analyses using existing de-identified data from a public health facility and did not involve interaction with any human subjects, ethical approval was not required.46 Approval to conduct this study was granted by the health district’s institutional review committee, as per the National Statement on Ethical Conduct in Human Research 2015.
Participants
Patient data over a 5-year time frame were reviewed (N = 2378). The patient data from the 3 most prevalent impairment groups were identified for inclusion in this study: reconditioning, orthopedic fracture, and orthopedic replacement. (See Table 1 for the specific AN-SNAP impairment groups used in this study.)
Patient data from the less-frequent impairment groups were excluded (n = 673, 28.19%), including stroke (n = 343), brain dysfunction (n = 45), amputation of limb (n = 45), spinal cord dysfunction (n = 36), neurological dysfunction (n = 34), cardiac (n = 24), and others (n = 25) who may have benefitted from other outcome measures due to their medical condition. Ten patient data sets were excluded for missing discharge outcome measure data, from when the patient became ill and returned to acute services or was discharged at short notice. To be included in the study, both the admission and discharge scores from the FIM and the admission and discharge scores from at least 1 of the physiotherapy outcome measures were required for each patient (n = 1704, 71.39%): Reconditioning (n = 742), Orthopedic Fracture (n = 585), and Orthopedic Replacement (n = 377). Information regarding the type of walking aid and the amount of assistance required for safe ambulation was also recorded. These items were included in the study’s descriptive analysis. Only 1.7% of these descriptors were missing.
Outcome measures
DEMMI tasks of bed mobility, sitting balance, transfers, walking, and balance were scored with an assigned value according to the patient’s performance. This was then tallied and the results scaled, to provide an overall score out of 100 available points. The total score from admission and discharge was then compared. Improvement (change) was identified by the increase in scores.
The TUG assesses a patient’s dynamic balance performance.47 The number of seconds it took the patient to complete the procedure was recorded at admission and discharge. Improvement (change) was identified by the reduction in time taken at discharge from the admission score.
The 10MWT measures the unidirectional walking speed of a person over 10 meters and is recorded in seconds and reported in meters per second. Improvement (change) was identified by the reduction in the time taken to increase walking speed.
Concurrent to the physiotherapy measures were the FIM scores, recorded by the accredited nursing staff from each rehabilitation ward. Improvement is demonstrated by the accumulation of points on the ordinal scale of the FIM Total, including mobility, dressing, bladder and bowel care, cognition, and social interaction, and is represented as a score between 18 and 126. The FIM Motor category is reported as a score between 13 and 91.
The 2 data sets were matched by unique identifier and admission dates, then de-identified for analysis.
Statistical analysis
Patient demographic information was analyzed using descriptive statistics (mean, SD, frequencies, percentages) for each impairment group (orthopedic fracture, orthopedic replacement, reconditioning). Differences in continuous demographic variables for each impairment group were assessed using Kruskal-Wallis tests and χ2 tests for categorical variables. Functional outcome scores were compared at admission, discharge, and change between the impairment groups. Association of the functional outcome change scores was determined with the Pearson correlation coefficient (r) between the FIM and the DEMMI, TUG, and 10MWT. Graphs were plotted for each of these (Figure available online at mdedge.com/jcomjournal). A strong, moderate, and weak association was described as > 0.6, > 0.4, and > 0.2, respectively.46 Statistical significance was set at P < .05. Analyses were conducted using Stata (StataCorp LLC, USA).
Results
The patient descriptive data (site from which data were collected, admission length of stay, age at admission, discharge destination, walk aid improvement, and walk assistance improvement) from the 3 impairment groups are reported in Table 2. The functional outcomes for DEMMI, TUG, 10MWT, FIM Motor, FIM Total at admission, discharge, and the change scores are presented in Table 3.
Orthopedic fracture patients had the greatest improvement in their functional outcomes, with a DEMMI improvement of 18 points, TUG score change of 23.49 seconds (s), 10MWT change of 0.30 meters/second (m/s), FIM Motor change of 20.62, and a FIM Total change of 21.9 points. The outcome measures exceeded the minimum detectable change as reported in the literature for DEMMI (8.8 points48), TUG (2.08 s26), walking speed 0.19 m/s26, and FIM Motor (14.6 points49).
Association of functional outcomes (change scores)
There was a significant weak positive correlation between DEMMI change score and both the FIM Motor (r = 0.396) and FIM Total change scores (r = 0.373). When viewing the specific items within the FIM Motor labelled FIM Walk change, FIM MobilityBedChair change, and FIM stairs change, r values were 0.100, 0.379, and 0.126, respectively. In addition, there was a weak negative correlation between TUG change scores and both FIM Motor (r = -0.217) and FIM Total change scores (r = -0.207). There was a very weak positive correlation between 10MWT (m/s) change scores and both FIM Motor (r = 0.194) and FIM Total change scores (r = 0.187) (Table 4, Figure). There was a moderate correlation between 10MWT change (s) and TUG change (s) (r = 0.72, P < .001).
Discussion
The purpose of this study was to ascertain the association between the DEMMI, TUG, 10MWT, and FIM measures using retrospective data collected from 5 public hospital inpatient rehabilitation wards. The results of this retrospective analysis demonstrate that a variety of objective outcome measures are required for the multidisciplinary team to accurately measure a patient’s functional improvement during their inpatient rehabilitation stay. No single outcome measure in this study fully reported all mobility attributes, and we note the risk of basing decisions on a single measure evaluating rehabilitation outcomes. Although the internationally used FIM has a strong place in rehabilitation reporting and benchmarking, it does not predict change nor provide a proxy for the patient’s whole-body motor control as they extend their mobility, dynamic balance, and ambulatory ability. Multiple objective outcome measures should therefore be required to evaluate the patient’s progress and functional performance toward discharge planning.
The FIM is a measure of disability or care needs, incorporating cognitive, social, and physical components of disability. It is a valid, holistic measure of an individual’s functional ability at a given time. Rehabilitation sites internationally utilize this assessment tool to evaluate a patient’s progress and the efficacy of intervention. The strength of this measure is its widespread use and the inclusion of the personal activities of daily living to provide an overall evaluation encompassing all aspects of a person’s ability to function independently. However, as our study results suggest, patient improvement measured by the FIM Motor components were not correlated to other widely used physiotherapy measures of ambulation and balance, such as the 10MWT or TUG. This is perhaps largely because the FIM Motor components only consider the level of assistance (eg, physical assistance, assistive device, independence) and do not consider assessment of balance and gait ability as assessed in the 10MWT and TUG. The 10MWT and TUG provide assessment of velocity and dynamic balance during walking, which have been shown to predict an individual’s risk of falling.22,23 This is a pertinent issue in the rehabilitation and geriatric population.29 Furthermore, the use of the FIM as a benchmarking tool to compare facility efficiency may not provide a complete assessment of all outcomes achieved on the inpatient rehabilitation ward, such as reduced falls risk or improved ambulatory ability and balance.
Of the objective measures evaluated in our paper, the DEMMI assessment has the most similar components to those of the FIM Motor. It includes evaluating independence with bed mobility, standing up, and ambulation. In addition, the DEMMI includes assessment of both static and dynamic balance. As a result of these commonalities, there was only a weak positive correlation between the change in DEMMI and the change in FIM Motor and FIM Total. However, this correlation is not statistically significant. Therefore, the FIM is not recommended as a replacement of the DEMMI, nor can one be used to predict the other.
It has previously been confirmed that there is a significant positive correlation between the 10MWT and the TUG.27 This retrospective analysis has also supported these findings. This is possibly due to the similarity in the assessments, as they both incorporate ambulation ability and dynamic movement.
Each of the 4 outcome measures assess different yet vital aspects of an individual’s functional mobility and ambulation ability during their subacute rehabilitation journey. The diversity of patient age, functional impairment, and mobility level needs a range of outcomes to provide baselines, targets, and goal attainment for discharge home.
Consistent with the AROC AN-SNAP reporting of Length of Stay and FIM change separated into the weighted impairment groups, the data analysis of this study demonstrated significant differences between the Reconditioning, Orthopedic Fracture, and Orthopedic Replacement patient data. Tables 2 and 3 describe the differences between the groups. The fracture population in this study improved the most across each outcome measure. In contrast, the reconditioning population showed the least improvement. This may be expected due to the pathophysiological differences between the groups. Furthermore, for the elderly who sustain fractures because of a fall, rehabilitation will be required to address not only the presenting injury but also the premorbid falls risk factors which may include polypharmacy or impaired balance.
Any conclusions drawn from the findings of this study need to take into consideration that it has focused on patients from 1 local health district and therefore may not be generalizable to a wider national or international context. As this study was a retrospective study, controlling for data collection quality, measurement bias due to nonblinding and missing data is a limitation. However, clinicians regularly completed these outcome assessments and recorded this information as part of their standard care practices within this health district. There may have been slight differences in definitions of practice between the 5 rehabilitation sites. To ensure reliability, each individual site’s protocols for the FIM, DEMMI, TUG, and 10MWT were reviewed and confirmed to be consistent.
It is important, too, to consider the ceiling effect for the FIM scores. For patients requiring a walking aid well after discharge, the highest level of independence from the walking aid will not be achieved. It is acknowledged that the floor effect of the 10MWT and TUG may also influence the outcomes of this study. In addition, data were not collected on preadmission functional measures to enable further evaluation of the population groups. The proportion of variance in change from admission to discharge for TUG and 10MWT to FIM was less than 5%, so the correlation interpretation from this type of scaling is limited. Further research into outcome measures for inpatient rehabilitation in respect to variables such as patient age, length of stay, discharge destination, and efficacy of intervention is warranted.
Conclusion
The FIM Motor change scores showed a weak positive association to the DEMMI change, and no association to the TUG and 10MWT change, demonstrating that the outcome measures do not measure the same attributes. Thorough reporting of clinical outcomes is much more meaningful to assess and guide the physiotherapy component of rehabilitation. To review rehabilitation effectiveness from a management perspective, it is recommended that all measures are reviewed to assess the burden of care, mobility, functional capacity, and dynamic balance.
Acknowledgements: The authors thank Anne Smith, MSHLM, BAppSc, Head of the Physiotherapy Department, and the physiotherapists and allied health assistants who have contributed to the collection of this valuable data over several years. They also thank Lina Baytieh, MS, BS, from Research Central, Illawarra Shoalhaven Local Health District, for her assistance with the analysis.
Corresponding author: Maren Jones, MPH, BS, Physiotherapy Department, Port Kembla Hospital, Illawarra Shoalhaven Local Health District, Warrawong, New South Wales, 2505 Australia; [email protected].
Financial disclosures: None.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
1. Centers for Disease Control and Prevention. Disability and health overview. Impairments, activity limitations and participation restrictions. September 16, 2020. https://www.cdc.gov/ncbddd/disabilityandhealth/disability.html
2. The Royal Australasian College of Physicians. Australasian Faculty of Rehabilitation Medicine. Standards for the Provision of Inpatient Adult Rehabilitation Medicine Services in Public and Private Hospitals. February 2019:7-9. https://www.racp.edu.au/docs/default-source/advocacy-library/afrm-standards-for-the-provision-of-inpatient-adult-rehabilitation-medicine-services-in-public-and-private-hospitals.pdf?sfvrsn=4690171a_4
3. NSW Agency for Clinical Innovation. NSW rehabilitation model of care. June 1, 2015. https://aci.health.nsw.gov.au/resources/rehabilitation/rehabilitation-model-of-care/rehabilitation-moc
4. The State of Queensland (Queensland Health). Clinical task instructions. June 22, 2021. https://www.health.qld.gov.au/ahwac/html/clintaskinstructions
5. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148-157. doi:10.1111/j.1532-5415.2010.03234.x
6. Suwannarat P, Kaewsanmung S, Thaweewannakij T, Amatachaya S. The use of functional performance tests by primary health-care providers to determine walking ability with and without a walking device in community-dwelling elderly. Physiother Theory Pract. 2021;37(1):64-72. doi:10.1080/09593985.2019.1606372
7. Lee K-J, Um S-H, Kim Y-H. Postoperative rehabilitation after hip fracture: a literature review. Hip Pelvis. 2020;32(3):125-131. doi:10.5371/hp.2020.32.3.125
8. Wade DT, Smeets RJEM, Verbunt JA. Research in rehabilitation medicine: methodological challenges. J Clin Epidemiol. 2010;63(7):699-704. doi:10.1016/j.clinepi.2009.07.010
9. Wade DT. Outcome measures for clinical rehabilitation trials: impairment, function, quality of life, or value? Am J Phys Med Rehabil. 2003;82(suppl 10):S26-S31. doi:10.1097/01.PHM.0000086996.89383.A1
10. de Morton NA, Harding KE, Taylor NF, Harrison G. Validity of the de Morton NA Mobility Index (DEMMI) for measuring the mobility of patients with hip fracture during rehabilitation. Disabil Rehabil. 2013;35(4):325-333. doi:10.3109/09638288.2012.705220
11. Trøstrup J, Andersen H, Kam CAM, et al. Assessment of mobility in older people hospitalized for medical illness using the de Morton Mobility Index and cumulated ambulation score—validity and minimal clinical important difference. J Geriatr Phys Ther. 2019;42(3):153-160. doi:10.1519/JPT.0000000000000170
12. Gazzoti A, Meyer U, Freystaetter G, et al. Physical performance among patients aged 70+ in acute care: a preliminary comparison between the Short Physical Performance Battery and the De Morton Mobility Index with regard to sensitivity to change and prediction of discharge destination. Aging Clin Exp Res. 2020;32(4):579-586. doi:10.1007/s40520-019-1249-9
13. Tavares LS, Moreno NA, de Aquino BG, et al. Reliability, validity, interpretability and responsiveness of the DEMMI mobility index for Brazilian older hospitalized patients. PLoS One. 2020;15(3):e0230047. doi:10.1371/journal.pone.0230047
14. Braun T, Schulz R-J, Reinke J. Reliability and validity of the German translation of the de Morton Mobility Index performed by physiotherapists in patients admitted to a sub-acute inpatient geriatric rehabilitation hospital. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0035-y
15. Søndergaard K, Petersen LE, Pedersen MK, et al. The responsiveness and predictive validity of the de Morton Mobility Index in geriatric rehabilitation. Disabil Rehabil. 2020 Jun 12. [Epub ahead of print] doi:10.1080/09638288.2020.1771438
16. de Morton NA, Brusco NK, Wood L, et al. The de Morton Mobility Index (DEMMI) provides a valid method for measuring and monitoring the mobility of patients making the transition from hospital to the community: an observational study. J Physiother. 2011;57(2):109-116. doi:10.1016/S1836-9553(11)70021-2
17. Caronni A, Sterpi I, Antoniotti P, et al. Criterion validity of the instrumented Timed Up and Go test: a partial least square regression study. Gait Posture. 2018;61(3):287-293. doi:10.1016/j.gaitpost.2018.01.015
18. Kristensen MT, Bloch ML, Jonsson LR, Jakobsen TL. Interrater reliability of the standardized Timed Up and Go Test when used in hospitalized and community-dwelling individuals. Physiother Res Int. 2019;24(2):e1769. doi:10.1002/pri.1769
19. Yuksel E, Kalkan S, Cekmece S, et al. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32(2):426-430. doi:10.1016/j.arth.2016.07.031
20. Yuksel E, Unver B, Kalkan S, Karatosun V. Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int. 2021;31(1):43-49. doi:10.1177/1120700019888614
21. Faleide AGH, Bogen BE, Magnussen LH. Intra-session test-retest reliability of the Timed “Up & Go” Test when performed by patients with hip fractures. Eur J Physiother. 2015;17(2):89-97. doi:10.3109/21679169.2015.1043579
22. Barry E, Galvin R, Keogh C, et al. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi:10.1186/1471-2318-14-14
23. Kojima G, Masud T, Kendrick D, et al. Does the timed up and go test predict future falls among British community-dwelling older people? Prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15:38. doi:10.1186/s12877-015-0039-7
24. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896-903.
25. Jeong SM, Shin DW, Han K, et al. Timed Up-and-Go test is a useful predictor of fracture incidence. Bone. 2019;127:474-481. doi:10.1016/j.bone.2019.07.018
26. Donaghue OA, Savva GM, Börsch-Supan A, Kenny RA. Reliability, measurement error and minimum detectable change in reliability measurement error and minimum detectable change in mobility measures: a cohort study of community dwelling adults aged 50 years and over in Ireland. BMJ Open. 2019;9(11):e030475. doi:10/1136.bmjopen-2019-030475
27. Freter SH, Fruchter N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96-101. doi:10.1191/026921500675545616
28. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. 2017;8(1):9-13. doi:10.1177/2150131916659282
29. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people: an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10)881-889. doi:10.1007/s12603-009-0246-z
30. Unver B, Baris RH, Yusel E, et al. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017;39(25):2572-2576. doi:10.1080/09638288.2016.1236153
31. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):46-49.
32. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. doi:10.1001/jama.2010.1923
33. Bohannon R. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19. doi:10.1093/ageing/26.1.15
34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance in older adults. J Am Geriatr Soc. 2006;54(5):743-749. doi:10.1111/j.1532-5415.2006.00701.x
35. Hollman J, Beckman B, Brandt R, et al. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther. 2008;31(2):53-56. doi:10.1519/00139143-200831020-00003
36. Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97(3):182-189. doi:10.1016/j.physio.2010.12.004
37. Granger CV, Hamilton BB, Keith RA, et al. Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
38. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The Functional Independence Measure: a new tool for rehabilitation. In: Eisenberg MG, Grzesiak RC, eds. Advances in Clinical Rehabilitation. Springer-Verlag; 1987:6-18.
39. Linacre JM, Heinemann AW, Wright BD, et al. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75(2):127-132.
40. Coster WJ, Haley SM, Jette AM. Measuring patient-reported outcomes after discharge from inpatient rehabilitation settings. J Rehabil Med. 2006;38(4):237-242. doi:10.1080/16501970600609774
41. Street L. Frequently asked questions about FIM. Journal of the Australasian Rehabilitation Nurses Association. 2014;17(1):21-22. https://ro.uow.edu.au/ahsri/296/
42. Green JP, Gordon R, Blanchard MB, et al. Development of the Australian National Subacute and Non-acute Patient (AN-SNAP) Classification. Version 4 Final Report. Australian Health Services Research Institute, University of Wollongong, 2015. https://ro.uow.edu.au/ahsri/760
43. Australasian Rehabilitation Outcomes Centre. University of Wollongong, Australia. https://www.uow.edu.au/ahsri/aroc/
44. Green J, Gordon R, Kobel C, et al; Centre for Health Service Development. The Australian National Subacute and Non-acute Patient Classification. AN-SNAP V4 User Manual. May 2015. https://documents.uow.edu.au/content/groups/public/@web/@chsd/@aroc/documents/doc/uow194637.pdf
45. Alexander TL, Simmonds FD, Capelle JT, Green LJ. Anywhere Hospital AROC Impairment Specific Report on Reconditioning (Inpatient–Pathway 3), July 2018–June 2019. Australasian Rehabilitation Outcomes Centre, Australian Health Services Research Institute, University of Wollongong; 2019. ro.uow.edu.au/ahsri/1110
46. Evans JD. Straightforward Statistics for the Behavioural Sciences. Brooks/Cole Publishing; 1996.
47. Lee SP, Dufek J, Hickman R, Schuerman S. Influence of procedural factors on the reliability and performance of the timed up-and-go test in older adults. Int J Gerontol. 2016;10(1):37-42. doi:10.1016/j.ijge.2015
48. New PW, Scroggie GD, Williams CM. The validity, reliability, responsiveness and minimal clinically important difference of the de Morton Mobility Index in rehabilitation. Disabil Rehabil. 2017;39(10):1039-1043. doi:10.10801/09638288.2016.1179800
49. Nakaguchi T, Ishimoto Y, Akazawa N. Functional Independence Measure for patients with locomotor disorders in convalescent rehabilitation wards. Clinically significant minimum difference in exercise score gain. Physiotherapy Science. 2018;33(2):235-240.
Shock, disbelief as NCCN changes prostate cancer guidance
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
For over a decade, the influential National Comprehensive Cancer Network (NCCN) has been recommending that men with low-risk prostate cancer be offered active surveillance as the lone “preferred” initial treatment option.
But the NCCN has now reversed this long-standing recommendation in the latest revision of its prostate cancer guideline.
The organization now recommends that low-risk disease be managed with either active surveillance or radiation therapy or surgery, with equal weight given to all three of these initial options.
The complaints were voiced in unusually blunt and strong language for physicians.
“This is a terrible step back that impacts every urologist,” commented John Griffith, MD, of Hartford Healthcare, who practices in New Britain, Conn.
Dr. Griffith explained that he prints out the NCCN guidance with “every patient newly diagnosed” and that the preferred designation is a “huge help” in reassuring them about not treating low-risk disease initially.
In a Twitter thread, Benjamin Davies, MD, of the University of Pittsburgh, facetiously wondered if a time warp was at play: “To suggest for a millisecond that active surveillance isn’t the preferred method for low-risk men is bizarre thinking ... Is this 1980?”
“I’m baffled,” said Brian Chapin, MD, of MD Anderson Cancer Center, Houston, in another Twitter thread.
“This is ludicrous,” said Andrew Vickers, PhD, of Memorial Sloan Kettering Cancer Center in New York City in a tweet.
Alexander Kutikov, MD, of Fox Chase Cancer Center in Philadelphia, commented on Twitter that the change “seems off the rails…a bit stunned by this.”
Matthew Cooperberg, MD, of the University of California San Francisco, and Minhaj Siddiqui, MD, of the University of Maryland in Baltimore both called the move a “step backward.”
Many others also expressed disappointment in the NCCN, whose guidelines are hugely influential because of the role they play clinically as well as with payors and the legal system.
“A huge setback & frankly a disgrace for @NCCN and its processes,” commented Fox Chase’s Dr. Kutikov.
Stacy Loeb, MD, of NYU Langone Health in New York City, suggested the new guidance may stunt use of active surveillance in the United States. She tweeted: “The updated NCCN guideline certainly won’t help the lagging and heterogenous uptake of active surveillance in the U.S. We should be carefully expanding the pool for active surveillance, not narrowing it.”
The purpose of active surveillance is to avoid adverse events from treatment, which can be life-changing as they include incontinence and erectile dysfunction.
The rationale is that many men with low-risk prostate cancer may not need treatment for their disease, as the disease may be slow-growing and may never threaten their life. With active surveillance, men are instead monitored with blood tests, scans, and biopsies to watch for worsening disease, and treated only when there are signs of disease progression.
This active surveillance approach has grown in acceptance among American patients since 2010.
The concern now is that the change in guidance from the NCCN will lead to a reduction in active surveillance, and an increase in initial treatment with surgery and radiotherapy for low-risk disease, which is considered by many to be “overtreatment’ of this disease and may not be medically necessary.
For example, UCSF’s Dr. Cooperberg said he feared that the changed guidance “will be used by urologists and radiation oncologists to justify overtreatment of low-risk disease.”
Dr. Kutikov agreed but described that possibility differently, citing the risk of lawsuits. He observed that without the NCCN’s “medico-legal buffer” of active surveillance as the preferred initial treatment, there are “further incentives” for overtreatment.
The new NCCN guidance also conflicts with the American Urological Association’s guidelines and dissolves what was once a mostly united front from the two major organizations on active surveillance and low-risk disease.
The AUA Guideline reads: “Clinicians should recommend active surveillance as the preferable care option for most low-risk localized prostate cancer patients (Moderate Recommendation; Evidence Level: Grade B)
Patients protest change in wording
Not surprisingly, the revised NCCN guidance was criticized by multiple patient advocacy groups, including Active Surveillance Patients International (ASPI), which wrote a letter to the NCCN protesting the change.
In that letter, the ASPI writes that active surveillance is now chosen as the initial approach for low-risk prostate cancer in about 90% of cases in some European nations, and in about 50% of cases in the United States. It also warns that eliminating the word “preferred” from the NCCN guidelines represents a retreat, and “will have repercussions far beyond what we may first conceive.”
“Active surveillance should be the preferred choice to preserve quality of life for men with low-risk cancer,” the advocacy group states. “The PIVOT trials indicate for low-risk disease there is basically no advantage to intervention. Why would one risk the side effects if they knew that?”
Why now?
The NCCN’s move to alter its low-risk prostate cancer guidance is especially striking because, 11 years ago, the NCCN broke new ground in recommending active surveillance as the sole initial treatment option for low-risk men. (It was also the first guidelines group to recommend the same for very low-risk men.)
So why the change now? This news organization requested, but did not receive, comment from the NCCN and its chair of the prostate cancer panel, Edward Schaeffer, MD, of Northwestern University in Chicago.
However, on Twitter, Dr. Schaeffer hinted at what had turned the tables for the NCCN panel – the risk that, over time, some men with low-risk disease who are on active surveillance are reclassified on biopsy as having a higher risk.
He highlighted a 2020 study on that very subject from the University of California, San Francisco, published in the Journal of Urology. Those authors concluded that: “Given the heterogeneity of the disease, some tumors characterized as low risk may merit early treatment while others may be followed much less intensely over some time interval.”
Dr. Schaeffer tweeted: “I think this nicely sums up the low-risk space ...”
Experts reacting to Dr. Schaeffer’s tweet were not swayed.
Looking at additional measures such as genomic scores and PSA density, as advocated by Dr. Schaeffer via the posted 2020 study, is good for assessing individual risk, “but still, active surveillance is the preferred option for low risk,” said MD Anderson’s Dr. Chapin.
UCSF’s Dr. Cooperberg, who was a co-author on that 2020 Journal of Urology paper, commented that the university’s urology department had “spent the past quarter century arguing active surveillance is ‘preferred’ for almost all low risk [disease]!”
“Many on active surveillance need treatment someday, but that does not justify immediate overtreatment,” he concluded.
A version of this article first appeared on Medscape.com.
Antiepileptic medications linked to increased priapism risk
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several antiepileptic drugs (AEDs) are associated with an increased risk for priapism, new research suggests.
After analyzing U.S. adverse event reporting data, investigators found that among nearly 200 cases of priapism, a persistent, often painful erection unrelated to sexual interest or stimulation that lasts more than 4 hours, eight AEDs were associated with a positive “safety signal” for priapism.
These included valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine. Of these, valpromide had the largest association.
“Based on our results, we would recommend to clinicians to be cautious about the possibility of encountering priapism” in patients receiving the eight AEDs identified, lead researcher Ana Pejcic, PhD, department of pharmacology and toxicology, University of Kragujevac, Serbia, told meeting attendees.
If clinicians encounter such cases, they should be “reported to the regulatory authorities,” Dr. Pejcic added.
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
Noteworthy limitations
Dr. Pejcic told this news organization that the safety signal with AEDs “does not directly mean that a medicine has caused the reported adverse event” because an illness or other drug taken by the patient could be responsible instead.
She also noted that the U.S. Food and Drug Administration’s Adverse Event Reporting System relies on “spontaneous reports of adverse events,” which have multiple limitations.
These limitations include that the FDA “does not require that a causal relationship between a drug and event be proven, and reports do not always have enough information to properly evaluate an event.”
Nevertheless, Dr. Pejcic added that if a causal relationship was to be shown, the underlying mechanism could be linked to the pharmacological properties of the individual antiepileptic, such as altered alpha-1 adrenergic receptor expression or increased dopamine release.
Still, that would require “further evaluation in larger pharmacoepidemiological studies, with adjustment for potential confounding variables,” she said.
Replication needed
Priapism has recently been observed in case reports in association with the use of some AEDs. In addition, use of the drugs has been associated with hypo- and hypersexuality, as well as erectile and ejaculatory dysfunction.
Because the relationship between priapism and AED use “has not been well characterized,” the researchers mined data from the FDA’s Adverse Event Reporting System.
They examined entries from the first quarter of 2004 and the third quarter of 2020, focusing on 47 AEDs from the N03A subgroup of the Anatomical Therapeutic Chemical Classification System.
The researchers identified 8,122,037 cases for data analysis, of which 1,936 involved priapism as an adverse event. In total, 16 antiepileptic medications had at least one case of an adverse event involving priapism.
A positive safety signal was defined as a Proportional Reporting Ratio (PRR) of at least two, a chi-squared of at least four, or three or more cases. The signal was detected for valpromide, brivaracetam, valproic acid, topiramate, oxcarbazepine, clonazepam, levetiracetam, and carbamazepine.
The largest association with priapism was with valpromide, at a PRR of 61.79. That was followed by PRR of 9.61 for brivaracetam, 7.28 for valproic acid, and 3.23 for topiramate.
“Considering that the proportionality analysis we applied in our study is used for hypothesis generation, our results will need to confirm in large cohorts and case-control studies,” said Dr. Pejcic.
New and important hypothesis?
Commenting on the study, Daniel Goldenholz, MD, PhD, instructor in the Division of Epilepsy, Beth Israel Deaconess Medical Center, Boston, said priapism is not something that practicing epileptologists are instructed “to look for.”
He noted that “the idea of looking for a hidden signal in a massive database like this is very appealing” because it could reveal patterns that were previously undetected.
However, the event rate in the study suggests priapism, which “in the right context would be considered a medical emergency, [is] relatively uncommon,” said Dr. Goldenholz, who was not involved with the research.
He noted that medications that could cause priapism, “such as antidepressants, blood pressure meds, and anticoagulants,” are commonly used by many people – including those with epilepsy.
It is consequently possible that “the finding from this study can be explained by comorbid medical problems,” Dr. Goldenholz said. This is particularly likely because many of the AEDs in question “have been on the market for decades,” he added.
“If a seemingly dangerous symptom would be happening as a result of one of these medications, ,” he said.
Still, Dr. Goldenholz noted that it is “possible that these authors have a new and important hypothesis which must now be tested: Does priapism occur in patients with antiseizure medications when other causes are already ruled out?”
The investigators and Dr. Goldenholz have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
The male biological clock – How to tell the time
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
Vitamin supplementation in healthy patients: What does the evidence support?
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96
B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96
B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96
B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
GRADE DEFINITIONS
For an explanation of USPSTF grade definitions, see www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
Customized brain stimulation: New hope for severe depression
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
USPSTF update: Screen young asymptomatic women for chlamydia and gonorrhea
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
But evidence for screening men remains insufficient, task force says
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
FROM JAMA