User login
Nonhormonal medication treatment of VMS
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
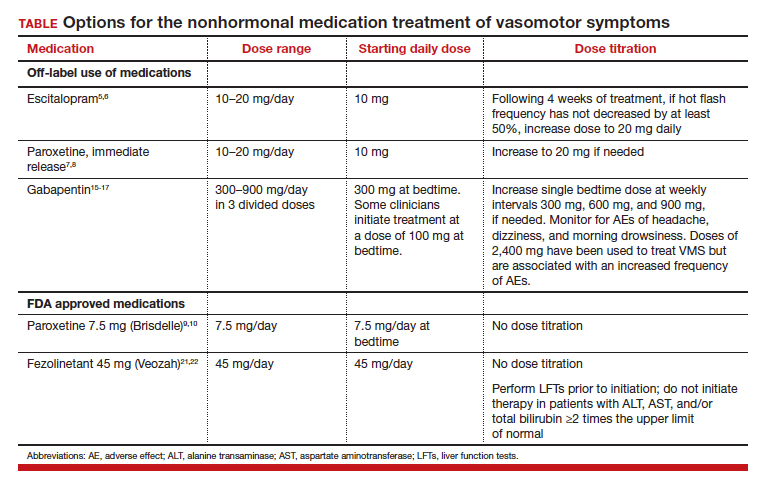
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.
Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
VMS, also known as hot flashes, night sweats, or cold sweats, occur for the majority of perimenopausal and menopausal women.1 In one study, the mean duration of clinically significant VMS was 5 years, and one-third of participants continued to have bothersome hot flashes 10 or more years after the onset of menopause.2 VMS may contribute to disrupted sleep patterns and depressed mood.3
All obstetrician-gynecologists know that estradiol and other estrogens are highly effective in the treatment of bothersome VMS. A meta-analysis reported that the frequency of VMS was reduced by 60% to 80% with oral estradiol (1 mg/day), transdermal estradiol(0.05 mg/day), and conjugated estrogen (0.625 mg).4 Breast tenderness and irregular uterine bleeding are common side effects of estrogen treatment of VMS. Estrogen treatment is contraindicated in patients with estrogen-responsive cancers, coronary heart disease, myocardial infarction, stroke, venous thromboembolism, and some cases of inherited thrombophilia. For these patients, an important option is the nonhormonal treatment of VMS, and several nonhormonal medications have been demonstrated to be effective therapy (TABLE 1). In this editorial I will review the medication treatment of VMS with escitalopram, paroxetine, gabapentin, and fezolinetant.

Escitalopram and paroxetine
Escitalopram and paroxetine have been shown to reduce VMS more than placebo in multiple clinical trials.5-10 In addition, escitalopram and paroxetine, at the doses tested, may be more effective for the treatment of VMS than sertraline, citalopram, or fluoxetine.11 In one trial assessing the efficacy of escitalopram to treat VMS, 205 patients with VMS were randomly assigned to 8 weeks of treatment with placebo or escitalopram.5 The initial escitalopram dose was 10 mg daily. At week 4:
- if VMS frequency was reduced by ≥ 50%, the patient remained on the 10-mg dose
- if VMS frequency was reduced by < 50%, the escitalopram dose was increased to 20 mg daily.
Following 8 weeks of treatment, the frequency of VMS decreased for patients in the placebo and escitalopram groups by 33% and 47%, respectively. Similar results have been reported in other studies.6
Paroxetine at a dose of 7.5 mg/day administered at bedtime is approved by the US Food and Drug Administration (FDA) for the treatment of VMS. In a pivotal study, 1,112 patients with VMS were randomly assigned to receive a placebo or paroxetine 7.5 mg at bedtime.9 In the 12-week study the reported decrease in mean weekly frequency of VMS for patients in the placebo and paroxetine groups were -37 and -44, respectively.9 Paroxetine 7.5 mg also reduced awakenings per night attributed to VMS and increased nighttime sleep duration.10
Depressed mood is prevalent among perimenopausal and postmenopausal patients.12 Prescribing escitalopram or paroxetine for VMS also may improve mood. Venlafaxine and desvenlafaxine are effective for the treatment of VMS;13,14 however, I seldom prescribe these medications for VMS because in my experience they are associated with more bothersome side effects, including dry mouth, decreased appetite, nausea, and insomnia than escitalopram or low-dose paroxetine.
Gabapentin
Numerous randomized clinical trials have reported that gabapentin is superior to placebo for the treatment of VMS.15 In one trial, 420 patients with breast cancer and VMS were randomly assigned to 8 weeks of treatment with placebo, gabapentin 300 mg/day (G300), or gabapentin 900 mg/day (G900) in 3 divided doses.16 Following 8 weeks of treatment, reduction in hot-flash severity score among patients receiving placebo, G300, or G900 was 15%, 31%, and 46%, respectively. Fatigue and somnolence were reported more frequently among patients taking gabapentin 900 mg/day. In a small trial, 60 patients with VMS were randomized to receive placebo, conjugated estrogen (0.2625 mg/day),or gabapentin (target dose of 2,400 mg/day in 3 divided doses).17 Following 12 weeks of treatment, the patient-reported decrease in VMS for those taking placebo, estrogen, or gabapentin was 54%, 72%, and 71%, respectively.
High-dose gabapentin treatment was associated with side effects of headache and dizziness more often than placebo or estrogen. Although gabapentin is not a treatment for insomnia, in my practice if a menopausal patient has prominent and bothersome symptoms of sleep disturbance and mild VMS symptoms, I will consider a trial of low-dose gabapentin. Some experts recommend initiating gabapentin at a dose of 100 mgdaily before bedtime to assess the effectiveness of a low dose that seldom causes significant side effects.

Fezolinetant
In a study of genetic variation associated with VMS, investigators discovered that nucleic acid variation in the neurokinin 3 (NK3) receptor was strongly associated with the prevalence of VMS, suggesting that this receptor is in the causal pathway to menopausal VMS.18 Additional research demonstrated that the kisspeptin/neurokinin B/dynorphin (KNDy) neurons, which are involved in the control of hypothalamic thermoregulation, are stimulated by neurokinin B, acting through the NK3 receptor, and suppressed by estradiol. A reduction in hypothalamic estrogen results in unopposed neurokinin B activity, which stimulates KNDy neurons, destabilizing the hypothalamic thermoregulatory center, causing vasodilation, which is perceived as hot flashes and sweating followed by chills.19
Fezolinetant is a high-affinity NK3 receptor antagonist that blocks the activity of neurokinin B, stabilizing the hypothalamic thermoregulatory center, thereby suppressing hot flashes. It is approved by the FDA for the treatment of moderate to severe VMS due to menopause using a fixed dose of 45 mg daily.20 In one clinical trial, 500 menopausal patients with bothersome VMS were randomly assigned to 12 weeks of treatment with placebo, fezolinetant 30 mg/day, or fezolinetant 45 mg/day. Following 12 weeks of treatment, the reported frequency rates of VMS among patients in the placebo, F30, and F45 groups were reduced by 43%, 61%, and 64%, respectively.21 In addition, following 12 weeks of treatment, the severity of VMS rates among patients in the placebo, F30, and F45 groups were reduced by 20%, 26%, and 32%, respectively.
Fezolinetant improved the quality of sleep and was associated with an improvement in patient-reported quality of life. Following 12 weeks of treatment, sleep quality among patients in the placebo, F30, and F45 groups was reported to be “much or moderately better” in 34%, 45%, and 54% of the patients, respectively.21 Similar results were reported in a companion study.22
Fezolinetant is contraindicated for patients with liver cirrhosis or severe renal impairment (estimated glomerular filtration rate of < 30 mL/min/1.73 m2). Before initiating treatment, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin (total and direct). Fezolinetant should not be prescribed if any of these tests are greater than twice the upper limit of normal. These tests should be repeated at 3, 6, and 9 months, and if the patient reports symptoms or signs of liver injury (nausea, vomiting, jaundice). Fezolinetant is metabolized by CYP1A2 and should not be prescribed to patients taking strong CYP1A2 inhibitors. The most common side effects associated with fezolinetant treatment are abdominal pain (4.3%), diarrhea (3.9%), insomnia (3.9%), back pain (3.0%), and hepatic transaminase elevation (2.3%). Fezolinetant has not been thoroughly evaluated in patients older than age 65. Following an oral dose of the medication, the median maximum concentration is reached in 1.5 hours, and the half-life is estimated to be 10 hours.20 Of all the medications discussed in this editorial, fezolinetant is the most expensive.
Effective VMS treatment improves overall health
Estrogen therapy is the gold standard treatment of VMS. However, many menopausal patients with bothersome VMS prefer not to take estrogen, and some have a medical condition that is a contraindication to estrogen treatment. The nonhormonal medication options for the treatment of VMS include escitalopram, paroxetine, gabapentin, and fezolinetant. Patients value the ability to choose the treatment they prefer, among all available hormonal and nonhormonal medication options. For mid-life women, effectively treating bothersome VMS is only one of many interventions that improves health. Optimal health is best achieved with23:
- high-quality diet
- daily physical activity
- appropriate body mass index
- nicotine avoidance
- a healthy sleep schedule
- normal blood pressure, lipid, and glucose levels.
Women who have a high-quality diet; daily physical activity; an appropriate body mass index; and normal blood pressure, cholesterol, and glucose levels are estimated to live 9 disease-free years longer than other women.24 ●
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopause transition: study of women’s health across the nation. Am J Pub Health. 2006;1226-1235.
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause. 2014;21:924-932.
- Hatcher KM, Smith RL, Chiang C, et al. Nocturnal hot flashes, but not serum hormone concentrations as a predictor of insomnia in menopausal women: results from the Midlife Women’s Health Study. J Women’s Health. 2023;32:94-101.
- Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610.
- Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267-227.
- Carpenter JS, Guthrie KA, Larson JC, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertil Steril. 2012;97:1399-1404.e1.
- Slaton RM, Champion MN, Palmore KB. A review of paroxetine for the treatment of vasomotor symptoms. J Pharm Pract. 2015;28:266-274.
- Stearns V, Slack R, Greep N, et al. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919-6930.
- Simon JA, Portman DJ, Kaunitz AM, et al. Lowdose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027-1035.
- Pinkerton JV, Joffe H, Kazempour K, et al. Lowdose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50-58.
- Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and metaanalysis of randomized trials. J Gen Intern Med. 2014;29:204-213.
- Freeman EW. Depression in the menopause transition: risks in the changing hormone milieu as observed in the general population. Womens Midlife Health. 2015;1:2.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059-2063.
- Sun Z, Hao Y, Zhang M. Efficacy and safety of desvenlafaxine treatment for hot flashes associated with menopause: a meta-analysis of randomized controlled trials. Gynecol Obstet Invest. 2013;75:255-262.
- Toulis KA, Tzellos T, Kouvelas D, et al. Gabapentin for the treatment of hot flashes in women with natural or tamoxifen-induced menopause: a systematic review and meta-analysis. Clin Ther. 2009;31:221-235.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebocontrolled trial. Lancet. 2005;366:818-824.
- Reddy SY, Warner H, Guttuso T Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41-48.
- Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women’s Health Initiative Study. Menopause. 2017;24:252.
- Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neurendocrinol. 2013;34:211-227.
- Veozah (package insert). Astellas Pharma; Northbrook, Illinois. May 2023.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-severe vasomotor symptoms associated with menopause: a Phase 3 RCT. J Clin Endocrinol Metab. 2023;108:1981-1997.
- Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet. 2023;401:1091-1102.
- Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18-43.
- Wang X, Ma H, Li X, et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in U.K. adults. JAMA Int Med. 2023;183:340-349.
Hormone therapy less effective in menopausal women with obesity
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
AT THE MENOPAUSE SOCIETY ANNUAL MEETING
False-positive Pap smear may indicate genitourinary syndrome
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a poster presented at The Menopause Society 2023 annual meeting.
METHODOLOGY:
- Starting in 2010, researchers in Florida and Antigua saw an increase in the number of perimenopausal women with no history of cervical abnormalities and low risk for sexually transmitted infections (STIs) presenting with abnormal Pap smears at their clinics.
- They studied 1,500 women aged 30-70 from several clinics. The women had low risk for STIs, a maximum of two sexual partners, and the presence of cervical dysplasia over a period of 12 years.
TAKEAWAY:
- Nearly all (96.7%) of the women who received local estrogen treatment had a normal Pap smear following therapy.
- A high number of patients who initially presented with cervical dysplasia underwent interventions such as colposcopies, biopsies, LEEP excisions, cryotherapy, cone biopsies, and hysterectomies because of cervical atrophy.
- The researchers concluded that local estrogen treatment could save patients money spent on treatments for cervical atrophy.
- Some women who underwent cone biopsies and hysterectomies and did not receive local estrogen still had vaginal dysplasia.
IN PRACTICE:
“In this study, we report an early sign of genitourinary syndrome of menopause: false positive cervical dysplasia caused by cervicovaginal atrophy resulting from decreased estrogen levels during perimenopause,” say the investigators. “We also demonstrate how the use of local estrogen therapy can prevent a significant number of interventions and procedures, resulting in significant cost savings. This is particularly relevant as the number of Pap smears conducted in this population represents 50%-60% of all Pap smears performed on women.”
SOURCE:
The data were presented at The Menopause Society 2023 annual meeting. The study was led by Alberto Dominguez-Bali, MD, from the Miami Center for Obstetrics, Gynecology and Human Sexuality.
LIMITATIONS:
The study authors report no limitations.
DISCLOSURES:
The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY ANNUAL MEETING
CBT effectively treats sexual concerns in menopausal women
PHILADELPHIA – . Four CBT sessions specifically focused on sexual concerns resulted in decreased sexual distress and concern, reduced depressive and menopausal symptoms, and increased sexual desire and functioning, as well as improved body image and relationship satisfaction.
An estimated 68%-87% of perimenopausal and postmenopausal women report sexual concerns, Sheryl Green, PhD, CPsych, an associate professor of psychiatry and behavioral neurosciences at McMaster University and a psychologist at St. Joseph’s Healthcare’s Women’s Health Concerns Clinic, both in Hamilton, Ont., told attendees at the meeting.
“Sexual concerns over the menopausal transition are not just physical, but they’re also psychological and emotional,” Dr. Green said. “Three common challenges include decreased sexual desire, a reduction in physical arousal and ability to achieve an orgasm, and sexual pain and discomfort during intercourse.”
The reasons for these concerns are multifactorial, she said. Decreased sexual desire can stem from stress, medical problems, their relationship with their partner, or other causes. A woman’s difficulty with reduced physical arousal or ability to have an orgasm can result from changes in hormone levels and vaginal changes, such as vaginal atrophy, which can also contribute to the sexual pain or discomfort reported by 17%-45% of postmenopausal women.
Two pharmacologic treatments exist for sexual concerns: oral flibanserin (Addyi) and injectable bremelanotide (Vyleesi). But many women may be unable or unwilling to take medication for their concerns. Previous research from Lori Brotto has found cognitive behavioral therapy and mindfulness interventions to effectively improve sexual functioning in women treated for gynecologic cancer and in women without a history of cancer.
“Sexual function needs to be understood from a bio-psychosocial model, looking at the biologic factors, the psychological factors, the sociocultural factors, and the interpersonal factors,” Sheryl Kingsberg, PhD, a professor of psychiatry and reproductive biology at Case Western Reserve University and a psychologist at University Hospitals in Cleveland, said in an interview.
“They can all overlap, and the clinician can ask a few pointed questions that help identify what the source of the problem is,” said Dr. Kingsberg, who was not involved in this study. She noted that the International Society for the Study of Women’s Sexual Health has an algorithm that can help in determining the source of the problems.
“Sometimes it’s going to be a biologic condition for which pharmacologic options are nice, but even if it is primarily pharmacologic, psychotherapy is always useful,” Dr. Kingsberg said. “Once the problem is there, even if it’s biologically based, then you have all the things in terms of the cognitive distortion, anxiety,” and other issues that a cognitive behavioral approach can help address. “And access is now much wider because of telehealth,” she added.
‘Psychology of menopause’
The study led by Dr. Green focused on peri- and postmenopausal women, with an average age of 50, who were experiencing primary sexual concerns based on a score of at least 26 on the Female Sexual Function Index (FSFI). Among the 20 women recruited for the study, 6 had already been prescribed hormone therapy for sexual concerns.
All reported decreased sexual desire, 17 reported decreased sexual arousal, 14 had body image dissatisfaction related to sexual concerns, and 6 reported urogenital problems. Nine of the women were in full remission from major depressive disorder, one had post-traumatic stress syndrome, and one had subclinical generalized anxiety disorder.
After spending 4 weeks on a wait list as self-control group for the study, the 15 women who completed the trial underwent four individual CBT sessions focusing on sexual concerns. The first session focused on psychoeducation and thought monitoring, and the second focused on cognitive distortions, cognitive strategies, and unhelpful beliefs or expectations related to sexual concerns. The third session looked at the role of problematic behaviors and behavioral experiments, and the fourth focused on continuation of strategies, long-term goals, and maintaining gains.
The participants completed eight measures at baseline, after the 4 weeks on the wait list, and after the four CBT sessions to assess the following:
- Sexual satisfaction, distress, and desire, using the FSFI, the Female Sexual Distress Scale-Revised (FSDS-R), and the Female Sexual Desire Questionnaire (FSDQ).
- Menopause symptoms, using the Greene Climacteric Scale (GCS).
- Body image, using the Dresden Body Image Questionnaire (DBIQ).
- Relationship satisfaction, using the Couples Satisfaction Index (CSI).
- Depression, using the Beck Depression Inventory-II (BDI-II).
- Anxiety, using the Hamilton Anxiety Rating Scale (HAM-A).
The women did not experience any significant changes while on the wait list except a slight decrease on the FSDQ concern subscale. Following the CBT sessions, however, the women experienced a significant decrease in sexual distress and concern as well as an increase in sexual dyadic desire and sexual functioning (P = .003 for FSFI, P = .002 for FSDS-R, and P = .003 for FSDQ).
Participants also experienced a decrease in depression (P < .0001) and menopausal symptoms (P = .001) and an increase in body-image satisfaction (P = .018) and relationship satisfaction (P = .0011) after the CBT sessions. The researchers assessed participants’ satisfaction with the Client Satisfaction Questionnaire after the CBT sessions and reported some of the qualitative findings.
“The treatment program was able to assist me with recognizing that some of my sexual concerns were normal, emotional as well as physical and hormonal, and provided me the ability to delve more deeply into the psychology of menopause and how to work through symptoms and concerns in more manageable pieces,” one participant wrote. Another found helpful the “homework exercises of recognizing a thought/feeling/emotion surrounding how I feel about myself/body and working through. More positive thought pattern/restructuring a response the most helpful.”
The main complaint about the program was that it was too short, with women wanting more sessions to help continue their progress.
Not an ‘either-or’ approach
Dr. Kingsberg said ISSWSH has a variety of sexual medicine practitioners, including providers who can provide CBT for sexual concerns, and the American Association of Sexuality Educators, Counselors and Therapists has a referral directory.
“Keeping in mind the bio-psychosocial model, sometimes psychotherapy is going to be a really effective treatment for sexual concerns,” Dr. Kingsberg said. “Sometimes the pharmacologic option is going to be a really effective treatment for some concerns, and sometimes the combination is going to have a really nice treatment effect. So it’s not a one-size-fits-all, and it doesn’t have to be an either-or.”
The sexual concerns of women still do not get adequately addressed in medical schools and residencies, Dr. Kingsberg said, which is distinctly different from how male sexual concerns are addressed in health care.
“Erectile dysfunction is kind of in the norm, and women are still a little hesitant to bring up their sexual concerns,” Dr. Kingsberg said. “They don’t know if it’s appropriate and they’re hoping that their clinician will ask.”
One way clinicians can do that is with a global question for all their patients: “Most of my patients have sexual questions or concerns; what concerns do you have?”
“They don’t have to go through a checklist of 10 things,” Dr. Kingsberg said. If the patient does not bring anything up, providers can then ask a single follow up question: “Do you have any concerns with desire, arousal, orgasm, or pain?” That question, Dr. Kingsberg said, covers the four main areas of concern.
The study was funded by the Canadian Institute of Health Research. Dr. Green reported no disclosures. Dr. Kingsberg has consulted for or served on the advisory board for Alloy, Astellas, Bayer, Dare Bioscience, Freya, Reunion Neuroscience, Materna Medical, Madorra, Palatin, Pfizer, ReJoy, Sprout, Strategic Science Technologies, and MsMedicine.
PHILADELPHIA – . Four CBT sessions specifically focused on sexual concerns resulted in decreased sexual distress and concern, reduced depressive and menopausal symptoms, and increased sexual desire and functioning, as well as improved body image and relationship satisfaction.
An estimated 68%-87% of perimenopausal and postmenopausal women report sexual concerns, Sheryl Green, PhD, CPsych, an associate professor of psychiatry and behavioral neurosciences at McMaster University and a psychologist at St. Joseph’s Healthcare’s Women’s Health Concerns Clinic, both in Hamilton, Ont., told attendees at the meeting.
“Sexual concerns over the menopausal transition are not just physical, but they’re also psychological and emotional,” Dr. Green said. “Three common challenges include decreased sexual desire, a reduction in physical arousal and ability to achieve an orgasm, and sexual pain and discomfort during intercourse.”
The reasons for these concerns are multifactorial, she said. Decreased sexual desire can stem from stress, medical problems, their relationship with their partner, or other causes. A woman’s difficulty with reduced physical arousal or ability to have an orgasm can result from changes in hormone levels and vaginal changes, such as vaginal atrophy, which can also contribute to the sexual pain or discomfort reported by 17%-45% of postmenopausal women.
Two pharmacologic treatments exist for sexual concerns: oral flibanserin (Addyi) and injectable bremelanotide (Vyleesi). But many women may be unable or unwilling to take medication for their concerns. Previous research from Lori Brotto has found cognitive behavioral therapy and mindfulness interventions to effectively improve sexual functioning in women treated for gynecologic cancer and in women without a history of cancer.
“Sexual function needs to be understood from a bio-psychosocial model, looking at the biologic factors, the psychological factors, the sociocultural factors, and the interpersonal factors,” Sheryl Kingsberg, PhD, a professor of psychiatry and reproductive biology at Case Western Reserve University and a psychologist at University Hospitals in Cleveland, said in an interview.
“They can all overlap, and the clinician can ask a few pointed questions that help identify what the source of the problem is,” said Dr. Kingsberg, who was not involved in this study. She noted that the International Society for the Study of Women’s Sexual Health has an algorithm that can help in determining the source of the problems.
“Sometimes it’s going to be a biologic condition for which pharmacologic options are nice, but even if it is primarily pharmacologic, psychotherapy is always useful,” Dr. Kingsberg said. “Once the problem is there, even if it’s biologically based, then you have all the things in terms of the cognitive distortion, anxiety,” and other issues that a cognitive behavioral approach can help address. “And access is now much wider because of telehealth,” she added.
‘Psychology of menopause’
The study led by Dr. Green focused on peri- and postmenopausal women, with an average age of 50, who were experiencing primary sexual concerns based on a score of at least 26 on the Female Sexual Function Index (FSFI). Among the 20 women recruited for the study, 6 had already been prescribed hormone therapy for sexual concerns.
All reported decreased sexual desire, 17 reported decreased sexual arousal, 14 had body image dissatisfaction related to sexual concerns, and 6 reported urogenital problems. Nine of the women were in full remission from major depressive disorder, one had post-traumatic stress syndrome, and one had subclinical generalized anxiety disorder.
After spending 4 weeks on a wait list as self-control group for the study, the 15 women who completed the trial underwent four individual CBT sessions focusing on sexual concerns. The first session focused on psychoeducation and thought monitoring, and the second focused on cognitive distortions, cognitive strategies, and unhelpful beliefs or expectations related to sexual concerns. The third session looked at the role of problematic behaviors and behavioral experiments, and the fourth focused on continuation of strategies, long-term goals, and maintaining gains.
The participants completed eight measures at baseline, after the 4 weeks on the wait list, and after the four CBT sessions to assess the following:
- Sexual satisfaction, distress, and desire, using the FSFI, the Female Sexual Distress Scale-Revised (FSDS-R), and the Female Sexual Desire Questionnaire (FSDQ).
- Menopause symptoms, using the Greene Climacteric Scale (GCS).
- Body image, using the Dresden Body Image Questionnaire (DBIQ).
- Relationship satisfaction, using the Couples Satisfaction Index (CSI).
- Depression, using the Beck Depression Inventory-II (BDI-II).
- Anxiety, using the Hamilton Anxiety Rating Scale (HAM-A).
The women did not experience any significant changes while on the wait list except a slight decrease on the FSDQ concern subscale. Following the CBT sessions, however, the women experienced a significant decrease in sexual distress and concern as well as an increase in sexual dyadic desire and sexual functioning (P = .003 for FSFI, P = .002 for FSDS-R, and P = .003 for FSDQ).
Participants also experienced a decrease in depression (P < .0001) and menopausal symptoms (P = .001) and an increase in body-image satisfaction (P = .018) and relationship satisfaction (P = .0011) after the CBT sessions. The researchers assessed participants’ satisfaction with the Client Satisfaction Questionnaire after the CBT sessions and reported some of the qualitative findings.
“The treatment program was able to assist me with recognizing that some of my sexual concerns were normal, emotional as well as physical and hormonal, and provided me the ability to delve more deeply into the psychology of menopause and how to work through symptoms and concerns in more manageable pieces,” one participant wrote. Another found helpful the “homework exercises of recognizing a thought/feeling/emotion surrounding how I feel about myself/body and working through. More positive thought pattern/restructuring a response the most helpful.”
The main complaint about the program was that it was too short, with women wanting more sessions to help continue their progress.
Not an ‘either-or’ approach
Dr. Kingsberg said ISSWSH has a variety of sexual medicine practitioners, including providers who can provide CBT for sexual concerns, and the American Association of Sexuality Educators, Counselors and Therapists has a referral directory.
“Keeping in mind the bio-psychosocial model, sometimes psychotherapy is going to be a really effective treatment for sexual concerns,” Dr. Kingsberg said. “Sometimes the pharmacologic option is going to be a really effective treatment for some concerns, and sometimes the combination is going to have a really nice treatment effect. So it’s not a one-size-fits-all, and it doesn’t have to be an either-or.”
The sexual concerns of women still do not get adequately addressed in medical schools and residencies, Dr. Kingsberg said, which is distinctly different from how male sexual concerns are addressed in health care.
“Erectile dysfunction is kind of in the norm, and women are still a little hesitant to bring up their sexual concerns,” Dr. Kingsberg said. “They don’t know if it’s appropriate and they’re hoping that their clinician will ask.”
One way clinicians can do that is with a global question for all their patients: “Most of my patients have sexual questions or concerns; what concerns do you have?”
“They don’t have to go through a checklist of 10 things,” Dr. Kingsberg said. If the patient does not bring anything up, providers can then ask a single follow up question: “Do you have any concerns with desire, arousal, orgasm, or pain?” That question, Dr. Kingsberg said, covers the four main areas of concern.
The study was funded by the Canadian Institute of Health Research. Dr. Green reported no disclosures. Dr. Kingsberg has consulted for or served on the advisory board for Alloy, Astellas, Bayer, Dare Bioscience, Freya, Reunion Neuroscience, Materna Medical, Madorra, Palatin, Pfizer, ReJoy, Sprout, Strategic Science Technologies, and MsMedicine.
PHILADELPHIA – . Four CBT sessions specifically focused on sexual concerns resulted in decreased sexual distress and concern, reduced depressive and menopausal symptoms, and increased sexual desire and functioning, as well as improved body image and relationship satisfaction.
An estimated 68%-87% of perimenopausal and postmenopausal women report sexual concerns, Sheryl Green, PhD, CPsych, an associate professor of psychiatry and behavioral neurosciences at McMaster University and a psychologist at St. Joseph’s Healthcare’s Women’s Health Concerns Clinic, both in Hamilton, Ont., told attendees at the meeting.
“Sexual concerns over the menopausal transition are not just physical, but they’re also psychological and emotional,” Dr. Green said. “Three common challenges include decreased sexual desire, a reduction in physical arousal and ability to achieve an orgasm, and sexual pain and discomfort during intercourse.”
The reasons for these concerns are multifactorial, she said. Decreased sexual desire can stem from stress, medical problems, their relationship with their partner, or other causes. A woman’s difficulty with reduced physical arousal or ability to have an orgasm can result from changes in hormone levels and vaginal changes, such as vaginal atrophy, which can also contribute to the sexual pain or discomfort reported by 17%-45% of postmenopausal women.
Two pharmacologic treatments exist for sexual concerns: oral flibanserin (Addyi) and injectable bremelanotide (Vyleesi). But many women may be unable or unwilling to take medication for their concerns. Previous research from Lori Brotto has found cognitive behavioral therapy and mindfulness interventions to effectively improve sexual functioning in women treated for gynecologic cancer and in women without a history of cancer.
“Sexual function needs to be understood from a bio-psychosocial model, looking at the biologic factors, the psychological factors, the sociocultural factors, and the interpersonal factors,” Sheryl Kingsberg, PhD, a professor of psychiatry and reproductive biology at Case Western Reserve University and a psychologist at University Hospitals in Cleveland, said in an interview.
“They can all overlap, and the clinician can ask a few pointed questions that help identify what the source of the problem is,” said Dr. Kingsberg, who was not involved in this study. She noted that the International Society for the Study of Women’s Sexual Health has an algorithm that can help in determining the source of the problems.
“Sometimes it’s going to be a biologic condition for which pharmacologic options are nice, but even if it is primarily pharmacologic, psychotherapy is always useful,” Dr. Kingsberg said. “Once the problem is there, even if it’s biologically based, then you have all the things in terms of the cognitive distortion, anxiety,” and other issues that a cognitive behavioral approach can help address. “And access is now much wider because of telehealth,” she added.
‘Psychology of menopause’
The study led by Dr. Green focused on peri- and postmenopausal women, with an average age of 50, who were experiencing primary sexual concerns based on a score of at least 26 on the Female Sexual Function Index (FSFI). Among the 20 women recruited for the study, 6 had already been prescribed hormone therapy for sexual concerns.
All reported decreased sexual desire, 17 reported decreased sexual arousal, 14 had body image dissatisfaction related to sexual concerns, and 6 reported urogenital problems. Nine of the women were in full remission from major depressive disorder, one had post-traumatic stress syndrome, and one had subclinical generalized anxiety disorder.
After spending 4 weeks on a wait list as self-control group for the study, the 15 women who completed the trial underwent four individual CBT sessions focusing on sexual concerns. The first session focused on psychoeducation and thought monitoring, and the second focused on cognitive distortions, cognitive strategies, and unhelpful beliefs or expectations related to sexual concerns. The third session looked at the role of problematic behaviors and behavioral experiments, and the fourth focused on continuation of strategies, long-term goals, and maintaining gains.
The participants completed eight measures at baseline, after the 4 weeks on the wait list, and after the four CBT sessions to assess the following:
- Sexual satisfaction, distress, and desire, using the FSFI, the Female Sexual Distress Scale-Revised (FSDS-R), and the Female Sexual Desire Questionnaire (FSDQ).
- Menopause symptoms, using the Greene Climacteric Scale (GCS).
- Body image, using the Dresden Body Image Questionnaire (DBIQ).
- Relationship satisfaction, using the Couples Satisfaction Index (CSI).
- Depression, using the Beck Depression Inventory-II (BDI-II).
- Anxiety, using the Hamilton Anxiety Rating Scale (HAM-A).
The women did not experience any significant changes while on the wait list except a slight decrease on the FSDQ concern subscale. Following the CBT sessions, however, the women experienced a significant decrease in sexual distress and concern as well as an increase in sexual dyadic desire and sexual functioning (P = .003 for FSFI, P = .002 for FSDS-R, and P = .003 for FSDQ).
Participants also experienced a decrease in depression (P < .0001) and menopausal symptoms (P = .001) and an increase in body-image satisfaction (P = .018) and relationship satisfaction (P = .0011) after the CBT sessions. The researchers assessed participants’ satisfaction with the Client Satisfaction Questionnaire after the CBT sessions and reported some of the qualitative findings.
“The treatment program was able to assist me with recognizing that some of my sexual concerns were normal, emotional as well as physical and hormonal, and provided me the ability to delve more deeply into the psychology of menopause and how to work through symptoms and concerns in more manageable pieces,” one participant wrote. Another found helpful the “homework exercises of recognizing a thought/feeling/emotion surrounding how I feel about myself/body and working through. More positive thought pattern/restructuring a response the most helpful.”
The main complaint about the program was that it was too short, with women wanting more sessions to help continue their progress.
Not an ‘either-or’ approach
Dr. Kingsberg said ISSWSH has a variety of sexual medicine practitioners, including providers who can provide CBT for sexual concerns, and the American Association of Sexuality Educators, Counselors and Therapists has a referral directory.
“Keeping in mind the bio-psychosocial model, sometimes psychotherapy is going to be a really effective treatment for sexual concerns,” Dr. Kingsberg said. “Sometimes the pharmacologic option is going to be a really effective treatment for some concerns, and sometimes the combination is going to have a really nice treatment effect. So it’s not a one-size-fits-all, and it doesn’t have to be an either-or.”
The sexual concerns of women still do not get adequately addressed in medical schools and residencies, Dr. Kingsberg said, which is distinctly different from how male sexual concerns are addressed in health care.
“Erectile dysfunction is kind of in the norm, and women are still a little hesitant to bring up their sexual concerns,” Dr. Kingsberg said. “They don’t know if it’s appropriate and they’re hoping that their clinician will ask.”
One way clinicians can do that is with a global question for all their patients: “Most of my patients have sexual questions or concerns; what concerns do you have?”
“They don’t have to go through a checklist of 10 things,” Dr. Kingsberg said. If the patient does not bring anything up, providers can then ask a single follow up question: “Do you have any concerns with desire, arousal, orgasm, or pain?” That question, Dr. Kingsberg said, covers the four main areas of concern.
The study was funded by the Canadian Institute of Health Research. Dr. Green reported no disclosures. Dr. Kingsberg has consulted for or served on the advisory board for Alloy, Astellas, Bayer, Dare Bioscience, Freya, Reunion Neuroscience, Materna Medical, Madorra, Palatin, Pfizer, ReJoy, Sprout, Strategic Science Technologies, and MsMedicine.
AT NAMS 2023
Hyaluronic acid suppository improves menopause symptoms
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
Menopausal hormone therapy less prescribed for Black women
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
PHILADELPHIA – , according to a review of published studies presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
“Gaps in treatment can be used to inform health care providers about menopausal HT prescribing disparities, with the goal of improving equitable and advanced patient care among disadvantaged populations,” wrote Danette Conklin, PhD, an assistant professor of psychiatry and reproductive biology at Case Western Reserve University, Cleveland, and a psychologist at University Hospitals Cleveland Medical Center; Sally MacPhedran, MD, an associate professor of reproductive biology at Case Western Reserve University and an ob.gyn at MetroHealth Medical Center, also in Cleveland; and their colleagues.
The researchers combed through PubMed, CINAHL, Cochrane Library, Web of Science and PsychInfo databases to identify all studies conducted in the United States since 1940 that contained data on patient demographics and prescribing patterns for hormone therapy to treat menopausal symptoms. In addition to excluding men, children, teens, trans men, and women who had contraindications for HT, the investigators excluded randomized clinical trials so that prescribing patterns would not be based on protocols or RCT participatory criteria.
The researchers identified 20 studies, ranging from 1973 through 2015, including 9 national studies and the others across different U.S. regions. They then analyzed differences in HT prescribing according to age, race/ethnicity, education, income, insurance type, body mass index, and mental health, including alcohol or substance use.
Seven of the studies assessed HT use based on patient surveys, seven used medical or medication records showing an HT prescription, two studies used insurance claims to show an HT prescription, and one study surveyed patients about whether they received an HT prescription. Another four studies used surveys that asked patients whether they received HT counseling but did not indicate if the patients received a prescription.
Half of the studies showed racial disparities in HT prescribing. In all of them, Black women used or were prescribed or counseled on using HT less than white, Hispanic, or Asian women. White women had greater use, prescribing, or counseling than all other races/ethnicities except one study in which Hispanic women were prescribed vaginal estrogen more often than white women.
Six of the studies showed education disparities in which menopausal women with lower education levels used less HT or were prescribed or counseled on HT less than women with higher education.
Complex reasons
Monica Christmas, MD, an associate professor of obstetrics and gynecology at the University of Chicago and director of the Menopause Program and the Center for Women’s Integrated Health, said the study’s findings were not surprising, but the reasons for the racial disparities are likely complex.
Implicit bias in providers is likely one contributing factor, with some providers not thinking of offering HT to certain patients or not expecting the patients to be interested in it. Providers may also hesitate to prescribe HT to patients with more comorbidities because of concerns about HT risks, so if Black patients have more comorbidities, that could play a role in how many are offered or counseled on HT, she said.
“Probably the biggest take home is that it is important to be asking all of our patients about their symptoms and being proactive about talking about it,” Dr. Christmas said in an interview.
At the same time, in her anecdotal experience at a previous institution, Dr. Christmas noticed that her Black patients were less receptive to using hormone therapy than her White patients even though her Black patients tended to exhibit or report greater or more severe symptoms. But there’s been a “paradigm shift” more recently, Dr. Christmas said. With awareness about menopause growing in the media and particularly on social media, and with greater awareness about racial disparities in menopausal symptoms and care – including that shown in Dr. Christmas’s work in the SWAN Study – Dr. Christmas has had more Black patients asking about HT and other treatments for their menopausal symptoms more recently.
“Just 10 years ago, I was trying to talk to people about hormones, and I’ve been giving them to people that need them for a long time, and I couldn’t,” Dr. Christmas said. “Now people are coming in, saying ‘no one’s ever talked to me about it’ or ‘I deserve this.’ It shows you the persuasion that social media and the Internet have on our thinking too, and I think that’s going to be interesting to look at, to see how that impacts people’s perception about wanting treatment.”
Dr. Conklin agreed that reasons for the disparities likely involve a combination of factors, including providers’ assumptions about different racial groups’ knowledge and receptiveness toward different treatments. One of the studies in their review also reported provider barriers to prescribing HT, which included lack of time, lack of adequate knowledge, and concern about risks to patients’ health.
“Medical providers tend to have less time with their patients compared to PhDs, and that time factor really makes a big difference in terms of what the focus is going to be in that [short] appointment,” Dr. Conklin said in an interview. “Perhaps from a provider point of view, they are prioritizing what they think is more important to their patient and not really listening deeply to what their patient is saying.”
Educating clinicians
Potentially supporting that possibility, Dr. Conklin and Dr. MacPhedran also had a poster at the conference that looked at prescribing of HT in both Black and White women with a diagnosis of depression, anxiety, or bipolar disorder.
“In a population with a high percentage of Black patients known to have more menopause symptoms, the data demonstrated a surprisingly low rate of documented menopause symptoms (11%) compared to prior reports of up to 80%,” the researchers reported. “This low rate may be related to patient reporting, physician inquiry, or physician documentation of menopause symptoms.” They further found that White women with menopause symptoms and one of those psychiatric diagnosis were 40% more likely to receive an HT prescription for menopausal symptoms than Black women with the same diagnoses and symptoms.
Dr. Conklin emphasized the importance of providers not overlooking women who have mental health disorders when it comes to treating menopausal symptoms, particularly since mental health conditions and menopausal symptoms can exacerbate each other.
“Their depression could worsen irritability, and anxiety can worsen during the transition, and it could be overlooked or thought of as another [psychiatric] episode,” Dr. Conklin said. Providers may need to “dig a little deeper,” especially if patients are reporting having hot flashes or brain fog.
A key way to help overcome the racial disparities – whether they result from systemic issues, implicit bias or assumptions, or patients’ own reticence – is education, Dr. Conklin said. She recommended that providers have educational material about menopause and treatments for menopausal symptoms in the waiting room and then ask patients about their symptoms and invite patients to ask questions.
Dr. MacPhedran added that education for clinicians is key as well.
“Now is a great time – menopause is hot, menopause is interesting, and it’s getting a little bit of a push in terms of research dollars,” Dr. MacPhedran said. “That will trickle down to more emphasis in medical education, whether that’s nurse practitioners, physicians, PAs, or midwives. Everybody needs more education on menopause so they can be more comfortable asking and answering these questions.”
Dr. Conklin said she would like to see expanded education on menopause for medical residents and in health psychology curricula as well.
Among the 13 studies that found disparities in prescribing patterns by age, seven studies showed that older women used or were prescribed or counseled on HT more often than younger women. Four studies found the opposite, with older women less likely to use or be prescribed or counseled about HT. One study had mixed results, and one study had expected prescribing patterns.
Five studies found income disparities and five studies found disparities by medical conditions in terms of HT use, prescribing, or counseling. Other disparities identified in smaller numbers of studies (four or fewer) included natural versus surgical menopause, insurance coverage, body mass index, geographic region, smoking and alcohol use.
The two biggest limitations of the research were its heterogeneity and the small number of studies included, which points to how scarce research on racial disparities in HT use really are, Dr. Conklin said.
The research did not use any external funding. The authors had no industry disclosures. Dr. Christmas has done an educational video for FertilityIQ.
AT NAMS 2023
This symptom signals UTI in 83% of cases
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Dyspareunia is a major indicator of urinary tract infections, being present in 83% of cases.
METHODOLOGY:
- Dyspareunia is a common symptom of UTIs, especially in premenopausal women, but is rarely inquired about during patient evaluations, according to researchers from Florida Atlantic University.
- In 2010, the researchers found that among 3,000 of their female Latinx patients aged 17-72 years in South Florida, 80% of those with UTIs reported experiencing pain during sexual intercourse.
- Since then, they have studied an additional 2,500 patients from the same population.
TAKEAWAY:
- Among all 5,500 patients, 83% of those who had UTIs experienced dyspareunia.
- Eighty percent of women of reproductive age with dyspareunia had an undiagnosed UTI.
- During the perimenopausal and postmenopausal years, dyspareunia was more often associated with genitourinary syndrome than UTIs.
- Ninety-four percent of women with UTI-associated dyspareunia responded positively to antibiotics.
IN PRACTICE:
“We have found that this symptom is extremely important as part of the symptomatology of UTI [and is] frequently found along with the classical symptoms,” the researchers reported. “Why has something so clear, so frequently present, never been described? The answer is simple: Physicians and patients do not talk about sex, despite dyspareunia being more a clinical symptom than a sexual one. Medical schools and residency programs in all areas, especially in obstetrics and gynecology, urology, and psychiatry, have been neglecting the education of physicians-in-training in this important aspect of human health. In conclusion, this is [proof] of how medicine has sometimes been influenced by religion, culture, and social norms far away from science.”
SOURCE:
The data were presented at the 2023 meeting of the Menopause Society. The study was led by Alberto Dominguez-Bali, MD, from Florida Atlantic University, Boca Raton, Fla.
LIMITATIONS:
The study authors reported no limitations.
DISCLOSURES:
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormone replacement therapy for postmenopausal osteoporosis
The actress Sally Field recently described her struggles with postmenopausal osteoporosis – she was given the diagnosis when she was 60 years old despite being physically active and engaging in activities such as biking, hiking, and yoga. As a slim, White woman in her sixth decade of life, she certainly had several risk factors for osteoporosis.
Osteoporosis, a condition associated with weak bones and an increased risk for fracture, is common in women after menopause. It’s defined as a bone mineral density (BMD) T-score of less than or equal to –2.5 on dual-energy x-ray absorptiometry (DXA) scan, occurrence of a spine or hip fracture regardless of BMD, or a BMD T-score between –1 and –2.5, along with a history of certain kinds of fractures or increased fracture risk based on the Fracture Risk Assessment Tool (FRAX).
The National Health and Nutrition Examination Survey from 2013 to 2014 reported that 16.5 % of women aged 50 years or older in the U.S. have osteoporosis (vs. only 5% of men of a similar age), with an increasing prevalence with increasing age. For example, the risk for osteoporosis of the hip increases from about 7% in women 50-59 years of age to about 35% in those aged 80 years or older. The risk for postmenopausal osteoporosis is reported to be highest in Asian women (40%), followed by Hispanic (20.5%), non-Hispanic White (17%), and non-Hispanic Black women (8.2%).
Why increased fracture risk in postmenopausal women?
The primary cause of postmenopausal osteoporosis is the cessation of estrogen production by the ovaries around the menopausal transition. Estrogen is very important for bone health. It reduces bone loss by reducing levels of receptor activator of NF-kappa B ligand (RANKL) and sclerostin, and it probably also increases bone formation through its effects on sclerostin.
Around menopause, the decrease in estrogen levels results in an increase in RANKL and sclerostin, with a consequent increase in bone loss at a pace that exceeds the rate of bone formation, thereby leading to osteoporosis.
Many factors further increase the risk for osteoporosis and fracture in postmenopausal women. These include a sedentary lifestyle, lower body weight, family history of osteoporosis, smoking, and certain medications and diseases. Medications that adversely affect bone health at this age include (but are not limited to) glucocorticoids such as hydrocortisone, prednisone, and dexamethasone; letrozole; excess thyroid hormone; certain drugs used to treat cancer; immunosuppressive drugs; certain antiseizure medications; proton pump inhibitors (such as omeprazole); sodium-glucose cotransporter 2 inhibitors and certain other drugs used to treat type 2 diabetes; and selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors (used to treat anxiety and depression).
Diseases associated with increased osteoporosis risk include certain genetic conditions affecting bone, a history of early ovarian insufficiency, hyperthyroidism, high levels of cortisol, diabetes, hyperparathyroidism, eating disorders, obesity, calcium and vitamin D deficiency, excess urinary excretion of calcium, malabsorption and certain gastrointestinal surgeries, chronic kidney disease, rheumatoid arthritis, certain types of cancer, and frailty.
Furthermore, older age, low bone density, a previous history of fracture, a family history of hip fracture, smoking, and excessive alcohol intake increase the risk for an osteoporotic fracture in a postmenopausal woman.
Bone density assessment using DXA is recommended in postmenopausal women who are at increased risk for low bone density and fracture. Monitoring of bone density is typically initiated about 5 years after the menopausal transition but should be considered earlier in those at high risk for osteoporosis. Women who are aged 70 or older, and those who have had significant height loss, should also get radiography of the spine to look for vertebral fractures.
Optimal nutrition is important for all postmenopausal women. Weight extremes are to be avoided. Although the use of calcium and vitamin D supplementation in postmenopausal women is still debated, the Institute of Medicine recommends that women 51-70 years of age take 1,000-1,200 mg of calcium and 400-600 IU of vitamin D daily, and that those older than 70 years take 1,000-1,200 mg of calcium and 400-800 IU of vitamin D daily.
Women with low vitamin D levels often require higher doses of vitamin D. It’s very important to avoid smoking and excessive alcohol consumption. Optimizing protein intake and exercises that improve muscle strength and improve balance can reduce the risk for falls, a key contributor to osteoporotic fractures.
Estrogen to prevent fracture risk
Because estrogen deficiency is a key cause of postmenopausal osteoporosis, estrogen replacement therapy has been used to prevent this condition, particularly early in the menopausal transition (51-60 years). Different formulations of estrogen given via oral or transdermal routes have been demonstrated to prevent osteoporosis; transdermal estrogen is often preferred because of a lower risk for blood clots and stroke. Women who have an intact uterus should also receive a progestin preparation either daily or cyclically, because estrogen alone can increase the risk for uterine cancer in the long run. Estrogen replacement has been associated with a 34% reduction in vertebral, hip, and total fractures in women of this age group.
Sally Field did receive hormone replacement therapy, which was helpful for her bones. However, as typically happens, her bone density dropped again when she discontinued hormone replacement. She also had low vitamin D levels, but vitamin D supplementation was not helpful. She received other medical intervention, with recovery back to good bone health.
Raloxifene is a medication that acts on the estrogen receptor, with beneficial effects on bone, and is approved for prevention and treatment of postmenopausal osteoporosis.
Medications that reduce bone loss (antiresorptive drugs), such as bisphosphonates and denosumab, and those that increase bone formation (osteoanabolic drugs), such as teriparatide, abaloparatide, and romosozumab, are used alone or in combination in women whose osteoporosis doesn’t respond to lifestyle and preventive strategies. The osteoanabolic drugs are typically reserved for women at very high risk for fractures, such as those with a BMD T-score ≤ less than or equal to –3, older women with recent fractures, and those with other risk factors. Treatment is typically lifelong.
(such as fractures of the spine and hip). It’s important to recognize those at greatest risk for this condition; implement bone health monitoring in a timely fashion; and ensure optimal nutrition, calcium and vitamin D supplementation, and exercises that optimize muscle strength and balance. Hormone replacement therapy is a consideration in many women. Some women will require antiresorptive or osteoanabolic drugs to manage this condition. With optimal treatment, older women can live long and productive lives.
Dr. Misra is Chief, Division of Pediatric Endocrinology, Mass General for Children; Associate Director, Harvard Catalyst Translation and Clinical Research Center; Director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; Professor, department of pediatrics, Harvard Medical School, Boston. She has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Sanofi; Ipsen.
A version of this article first appeared on Medscape.com.
The actress Sally Field recently described her struggles with postmenopausal osteoporosis – she was given the diagnosis when she was 60 years old despite being physically active and engaging in activities such as biking, hiking, and yoga. As a slim, White woman in her sixth decade of life, she certainly had several risk factors for osteoporosis.
Osteoporosis, a condition associated with weak bones and an increased risk for fracture, is common in women after menopause. It’s defined as a bone mineral density (BMD) T-score of less than or equal to –2.5 on dual-energy x-ray absorptiometry (DXA) scan, occurrence of a spine or hip fracture regardless of BMD, or a BMD T-score between –1 and –2.5, along with a history of certain kinds of fractures or increased fracture risk based on the Fracture Risk Assessment Tool (FRAX).
The National Health and Nutrition Examination Survey from 2013 to 2014 reported that 16.5 % of women aged 50 years or older in the U.S. have osteoporosis (vs. only 5% of men of a similar age), with an increasing prevalence with increasing age. For example, the risk for osteoporosis of the hip increases from about 7% in women 50-59 years of age to about 35% in those aged 80 years or older. The risk for postmenopausal osteoporosis is reported to be highest in Asian women (40%), followed by Hispanic (20.5%), non-Hispanic White (17%), and non-Hispanic Black women (8.2%).
Why increased fracture risk in postmenopausal women?
The primary cause of postmenopausal osteoporosis is the cessation of estrogen production by the ovaries around the menopausal transition. Estrogen is very important for bone health. It reduces bone loss by reducing levels of receptor activator of NF-kappa B ligand (RANKL) and sclerostin, and it probably also increases bone formation through its effects on sclerostin.
Around menopause, the decrease in estrogen levels results in an increase in RANKL and sclerostin, with a consequent increase in bone loss at a pace that exceeds the rate of bone formation, thereby leading to osteoporosis.
Many factors further increase the risk for osteoporosis and fracture in postmenopausal women. These include a sedentary lifestyle, lower body weight, family history of osteoporosis, smoking, and certain medications and diseases. Medications that adversely affect bone health at this age include (but are not limited to) glucocorticoids such as hydrocortisone, prednisone, and dexamethasone; letrozole; excess thyroid hormone; certain drugs used to treat cancer; immunosuppressive drugs; certain antiseizure medications; proton pump inhibitors (such as omeprazole); sodium-glucose cotransporter 2 inhibitors and certain other drugs used to treat type 2 diabetes; and selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors (used to treat anxiety and depression).
Diseases associated with increased osteoporosis risk include certain genetic conditions affecting bone, a history of early ovarian insufficiency, hyperthyroidism, high levels of cortisol, diabetes, hyperparathyroidism, eating disorders, obesity, calcium and vitamin D deficiency, excess urinary excretion of calcium, malabsorption and certain gastrointestinal surgeries, chronic kidney disease, rheumatoid arthritis, certain types of cancer, and frailty.
Furthermore, older age, low bone density, a previous history of fracture, a family history of hip fracture, smoking, and excessive alcohol intake increase the risk for an osteoporotic fracture in a postmenopausal woman.
Bone density assessment using DXA is recommended in postmenopausal women who are at increased risk for low bone density and fracture. Monitoring of bone density is typically initiated about 5 years after the menopausal transition but should be considered earlier in those at high risk for osteoporosis. Women who are aged 70 or older, and those who have had significant height loss, should also get radiography of the spine to look for vertebral fractures.
Optimal nutrition is important for all postmenopausal women. Weight extremes are to be avoided. Although the use of calcium and vitamin D supplementation in postmenopausal women is still debated, the Institute of Medicine recommends that women 51-70 years of age take 1,000-1,200 mg of calcium and 400-600 IU of vitamin D daily, and that those older than 70 years take 1,000-1,200 mg of calcium and 400-800 IU of vitamin D daily.
Women with low vitamin D levels often require higher doses of vitamin D. It’s very important to avoid smoking and excessive alcohol consumption. Optimizing protein intake and exercises that improve muscle strength and improve balance can reduce the risk for falls, a key contributor to osteoporotic fractures.
Estrogen to prevent fracture risk
Because estrogen deficiency is a key cause of postmenopausal osteoporosis, estrogen replacement therapy has been used to prevent this condition, particularly early in the menopausal transition (51-60 years). Different formulations of estrogen given via oral or transdermal routes have been demonstrated to prevent osteoporosis; transdermal estrogen is often preferred because of a lower risk for blood clots and stroke. Women who have an intact uterus should also receive a progestin preparation either daily or cyclically, because estrogen alone can increase the risk for uterine cancer in the long run. Estrogen replacement has been associated with a 34% reduction in vertebral, hip, and total fractures in women of this age group.
Sally Field did receive hormone replacement therapy, which was helpful for her bones. However, as typically happens, her bone density dropped again when she discontinued hormone replacement. She also had low vitamin D levels, but vitamin D supplementation was not helpful. She received other medical intervention, with recovery back to good bone health.
Raloxifene is a medication that acts on the estrogen receptor, with beneficial effects on bone, and is approved for prevention and treatment of postmenopausal osteoporosis.
Medications that reduce bone loss (antiresorptive drugs), such as bisphosphonates and denosumab, and those that increase bone formation (osteoanabolic drugs), such as teriparatide, abaloparatide, and romosozumab, are used alone or in combination in women whose osteoporosis doesn’t respond to lifestyle and preventive strategies. The osteoanabolic drugs are typically reserved for women at very high risk for fractures, such as those with a BMD T-score ≤ less than or equal to –3, older women with recent fractures, and those with other risk factors. Treatment is typically lifelong.
(such as fractures of the spine and hip). It’s important to recognize those at greatest risk for this condition; implement bone health monitoring in a timely fashion; and ensure optimal nutrition, calcium and vitamin D supplementation, and exercises that optimize muscle strength and balance. Hormone replacement therapy is a consideration in many women. Some women will require antiresorptive or osteoanabolic drugs to manage this condition. With optimal treatment, older women can live long and productive lives.
Dr. Misra is Chief, Division of Pediatric Endocrinology, Mass General for Children; Associate Director, Harvard Catalyst Translation and Clinical Research Center; Director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; Professor, department of pediatrics, Harvard Medical School, Boston. She has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Sanofi; Ipsen.
A version of this article first appeared on Medscape.com.
The actress Sally Field recently described her struggles with postmenopausal osteoporosis – she was given the diagnosis when she was 60 years old despite being physically active and engaging in activities such as biking, hiking, and yoga. As a slim, White woman in her sixth decade of life, she certainly had several risk factors for osteoporosis.
Osteoporosis, a condition associated with weak bones and an increased risk for fracture, is common in women after menopause. It’s defined as a bone mineral density (BMD) T-score of less than or equal to –2.5 on dual-energy x-ray absorptiometry (DXA) scan, occurrence of a spine or hip fracture regardless of BMD, or a BMD T-score between –1 and –2.5, along with a history of certain kinds of fractures or increased fracture risk based on the Fracture Risk Assessment Tool (FRAX).
The National Health and Nutrition Examination Survey from 2013 to 2014 reported that 16.5 % of women aged 50 years or older in the U.S. have osteoporosis (vs. only 5% of men of a similar age), with an increasing prevalence with increasing age. For example, the risk for osteoporosis of the hip increases from about 7% in women 50-59 years of age to about 35% in those aged 80 years or older. The risk for postmenopausal osteoporosis is reported to be highest in Asian women (40%), followed by Hispanic (20.5%), non-Hispanic White (17%), and non-Hispanic Black women (8.2%).
Why increased fracture risk in postmenopausal women?
The primary cause of postmenopausal osteoporosis is the cessation of estrogen production by the ovaries around the menopausal transition. Estrogen is very important for bone health. It reduces bone loss by reducing levels of receptor activator of NF-kappa B ligand (RANKL) and sclerostin, and it probably also increases bone formation through its effects on sclerostin.
Around menopause, the decrease in estrogen levels results in an increase in RANKL and sclerostin, with a consequent increase in bone loss at a pace that exceeds the rate of bone formation, thereby leading to osteoporosis.
Many factors further increase the risk for osteoporosis and fracture in postmenopausal women. These include a sedentary lifestyle, lower body weight, family history of osteoporosis, smoking, and certain medications and diseases. Medications that adversely affect bone health at this age include (but are not limited to) glucocorticoids such as hydrocortisone, prednisone, and dexamethasone; letrozole; excess thyroid hormone; certain drugs used to treat cancer; immunosuppressive drugs; certain antiseizure medications; proton pump inhibitors (such as omeprazole); sodium-glucose cotransporter 2 inhibitors and certain other drugs used to treat type 2 diabetes; and selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors (used to treat anxiety and depression).
Diseases associated with increased osteoporosis risk include certain genetic conditions affecting bone, a history of early ovarian insufficiency, hyperthyroidism, high levels of cortisol, diabetes, hyperparathyroidism, eating disorders, obesity, calcium and vitamin D deficiency, excess urinary excretion of calcium, malabsorption and certain gastrointestinal surgeries, chronic kidney disease, rheumatoid arthritis, certain types of cancer, and frailty.
Furthermore, older age, low bone density, a previous history of fracture, a family history of hip fracture, smoking, and excessive alcohol intake increase the risk for an osteoporotic fracture in a postmenopausal woman.
Bone density assessment using DXA is recommended in postmenopausal women who are at increased risk for low bone density and fracture. Monitoring of bone density is typically initiated about 5 years after the menopausal transition but should be considered earlier in those at high risk for osteoporosis. Women who are aged 70 or older, and those who have had significant height loss, should also get radiography of the spine to look for vertebral fractures.
Optimal nutrition is important for all postmenopausal women. Weight extremes are to be avoided. Although the use of calcium and vitamin D supplementation in postmenopausal women is still debated, the Institute of Medicine recommends that women 51-70 years of age take 1,000-1,200 mg of calcium and 400-600 IU of vitamin D daily, and that those older than 70 years take 1,000-1,200 mg of calcium and 400-800 IU of vitamin D daily.
Women with low vitamin D levels often require higher doses of vitamin D. It’s very important to avoid smoking and excessive alcohol consumption. Optimizing protein intake and exercises that improve muscle strength and improve balance can reduce the risk for falls, a key contributor to osteoporotic fractures.
Estrogen to prevent fracture risk
Because estrogen deficiency is a key cause of postmenopausal osteoporosis, estrogen replacement therapy has been used to prevent this condition, particularly early in the menopausal transition (51-60 years). Different formulations of estrogen given via oral or transdermal routes have been demonstrated to prevent osteoporosis; transdermal estrogen is often preferred because of a lower risk for blood clots and stroke. Women who have an intact uterus should also receive a progestin preparation either daily or cyclically, because estrogen alone can increase the risk for uterine cancer in the long run. Estrogen replacement has been associated with a 34% reduction in vertebral, hip, and total fractures in women of this age group.
Sally Field did receive hormone replacement therapy, which was helpful for her bones. However, as typically happens, her bone density dropped again when she discontinued hormone replacement. She also had low vitamin D levels, but vitamin D supplementation was not helpful. She received other medical intervention, with recovery back to good bone health.
Raloxifene is a medication that acts on the estrogen receptor, with beneficial effects on bone, and is approved for prevention and treatment of postmenopausal osteoporosis.
Medications that reduce bone loss (antiresorptive drugs), such as bisphosphonates and denosumab, and those that increase bone formation (osteoanabolic drugs), such as teriparatide, abaloparatide, and romosozumab, are used alone or in combination in women whose osteoporosis doesn’t respond to lifestyle and preventive strategies. The osteoanabolic drugs are typically reserved for women at very high risk for fractures, such as those with a BMD T-score ≤ less than or equal to –3, older women with recent fractures, and those with other risk factors. Treatment is typically lifelong.
(such as fractures of the spine and hip). It’s important to recognize those at greatest risk for this condition; implement bone health monitoring in a timely fashion; and ensure optimal nutrition, calcium and vitamin D supplementation, and exercises that optimize muscle strength and balance. Hormone replacement therapy is a consideration in many women. Some women will require antiresorptive or osteoanabolic drugs to manage this condition. With optimal treatment, older women can live long and productive lives.
Dr. Misra is Chief, Division of Pediatric Endocrinology, Mass General for Children; Associate Director, Harvard Catalyst Translation and Clinical Research Center; Director, Pediatric Endocrine-Sports Endocrine-Neuroendocrine Lab, Mass General Hospital; Professor, department of pediatrics, Harvard Medical School, Boston. She has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Sanofi; Ipsen.
A version of this article first appeared on Medscape.com.
Premenstrual disorders may be preview of early menopause
Women with premenstrual disorders may be more likely go through menopause before they are 45 years old, a new study suggests.
Women with premenstrual disorders, or PMDs, were also more likely to have moderate or severe night sweats or hot flashes during menopause, the researchers found.
Published in JAMA Network Open, the new findings stem from data from more than 3,600 nurses who contributed their health information to a database between 1991 and 2017. Women with PMDs were more than twice as likely as women without PMDs to have early menopause.
Most women have menopause between the ages of 45 and 55 years old, according to the World Health Organization.
There are numerous PMDs, including the well-known premenstrual syndrome, which is considered a mild disorder affecting up to 30% of women that causes symptoms like crankiness and bloating. A less common PMD is premenstrual dysphoric disorder, which can severely impact a woman’s life through psychological, gastrointestinal, skin, and neurological problems.
Previous research has linked PMDs during the reproductive years and postmenopausal issues like hot flashes and night sweats to increased risks of health problems like high blood pressure, heart conditions, and diabetes.
“It is important to identify women at risk for early menopause because of its link with poorer heart, brain, and bone health,” Stephanie Faubion, MD, MBA, a doctor at the Mayo Clinic and medical director of the North American Menopause Society, told CNN. Dr. Faubion was not involved in the study.
That said, it’s important to note that the study was observational – meaning researchers can’t say for certain that PMDs will cause early menopause. Rather, the study shows there may be a correlation between the two, Donghao Lu, MD, an associate professor in the department of medical epidemiology and biostatistics at the Karolinska Institute, told CNN.
A version of this article first appeared on Medscape.com.
Women with premenstrual disorders may be more likely go through menopause before they are 45 years old, a new study suggests.
Women with premenstrual disorders, or PMDs, were also more likely to have moderate or severe night sweats or hot flashes during menopause, the researchers found.
Published in JAMA Network Open, the new findings stem from data from more than 3,600 nurses who contributed their health information to a database between 1991 and 2017. Women with PMDs were more than twice as likely as women without PMDs to have early menopause.
Most women have menopause between the ages of 45 and 55 years old, according to the World Health Organization.
There are numerous PMDs, including the well-known premenstrual syndrome, which is considered a mild disorder affecting up to 30% of women that causes symptoms like crankiness and bloating. A less common PMD is premenstrual dysphoric disorder, which can severely impact a woman’s life through psychological, gastrointestinal, skin, and neurological problems.
Previous research has linked PMDs during the reproductive years and postmenopausal issues like hot flashes and night sweats to increased risks of health problems like high blood pressure, heart conditions, and diabetes.
“It is important to identify women at risk for early menopause because of its link with poorer heart, brain, and bone health,” Stephanie Faubion, MD, MBA, a doctor at the Mayo Clinic and medical director of the North American Menopause Society, told CNN. Dr. Faubion was not involved in the study.
That said, it’s important to note that the study was observational – meaning researchers can’t say for certain that PMDs will cause early menopause. Rather, the study shows there may be a correlation between the two, Donghao Lu, MD, an associate professor in the department of medical epidemiology and biostatistics at the Karolinska Institute, told CNN.
A version of this article first appeared on Medscape.com.
Women with premenstrual disorders may be more likely go through menopause before they are 45 years old, a new study suggests.
Women with premenstrual disorders, or PMDs, were also more likely to have moderate or severe night sweats or hot flashes during menopause, the researchers found.
Published in JAMA Network Open, the new findings stem from data from more than 3,600 nurses who contributed their health information to a database between 1991 and 2017. Women with PMDs were more than twice as likely as women without PMDs to have early menopause.
Most women have menopause between the ages of 45 and 55 years old, according to the World Health Organization.
There are numerous PMDs, including the well-known premenstrual syndrome, which is considered a mild disorder affecting up to 30% of women that causes symptoms like crankiness and bloating. A less common PMD is premenstrual dysphoric disorder, which can severely impact a woman’s life through psychological, gastrointestinal, skin, and neurological problems.
Previous research has linked PMDs during the reproductive years and postmenopausal issues like hot flashes and night sweats to increased risks of health problems like high blood pressure, heart conditions, and diabetes.
“It is important to identify women at risk for early menopause because of its link with poorer heart, brain, and bone health,” Stephanie Faubion, MD, MBA, a doctor at the Mayo Clinic and medical director of the North American Menopause Society, told CNN. Dr. Faubion was not involved in the study.
That said, it’s important to note that the study was observational – meaning researchers can’t say for certain that PMDs will cause early menopause. Rather, the study shows there may be a correlation between the two, Donghao Lu, MD, an associate professor in the department of medical epidemiology and biostatistics at the Karolinska Institute, told CNN.
A version of this article first appeared on Medscape.com.
PCPs facing increased patient demand for knowledgeable menopause care
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.
In 2017, a survey of 20 U.S. residency programs in family medicine, internal medicine, and ob.gyn. showed that only 6.8% of residents felt they were being adequately prepared to manage menopausal patients effectively, including how to use hormone therapy (HT).
Of the 177 residents who responded to the survey, 102 (56%) were in either family medicine or internal medicine.
“My guess is that there has been no substantial evolution in medical training to this day,” said lead survey study author Juliana Kling, MD, MPH, professor of medicine, chair of women’s health internal medicine, and dean, Mayo Clinic Alix School of Medicine, Scottsdale, Ariz.
The survey showed that overall 98% of residents thought it was important to know about menopause. However, 34% said they wouldn’t recommend HT in a severely symptomatic woman with no contraindications, and 60% said they wouldn’t recommend HT until at least the natural age of menopause in a prematurely menopausal woman. Some even recommended against it.
“Hormone therapy is effective, and for most healthy women younger than 60, the benefits are going to outweigh the risks,” said Dr. Kling. “We need to be comfortable, even in internal medicine, with prescribing hormones for the right women.”
The researchers concluded that “residual ambivalence about [hormone therapy] on the part of educators” may have played a role in curriculums that didn’t acknowledge the clinical relevance of menopause or include current evidence on the use of HT. Physicians should be taught to recognize menopausal symptoms, know the risks and benefits of HT and the alternatives, and how to select suitable candidates, they said.
Up to 80% of women in the United States are affected by menopausal vasomotor symptoms, but only one in four receive treatment, Dr. Kling pointed out. “Women will spend about a third of their lives after menopause, so being prepared to manage the consequences of menopause, such as bone health, vaginal dryness and painful intercourse, and increased cardiovascular disease risk, is critically important to all of us caring for women,” she emphasized. “These aren’t just ‘bothersome symptoms.’ ”
It is estimated that by 2060, there will be 90 million postmenopausal women in the United States. “Given the number of women who will experience symptoms of menopause and the considerable associated burden to their health and to the health care system, it is important to invest in educating future clinicians to provide evidence-based, comprehensive menopause management,” said Dr. Kling and coauthors in a February 2023 review of menopause treatments.
HT is the standard for the treatment of hot flashes and night sweats, and is highly effective for the prevention of bone loss and managing genitourinary syndrome of menopause. Among the alternatives to HT, the nonhormonal pharmacologic fezolinetant (Veozah) was approved by the U.S. Food and Drug Administration last May.
Following the early negative reports from the Women’s Health Initiative study of HT in 2002 and 2004, however, steep declines in HT prescription rates were seen among internists and family medicine practitioners. By 2009, only 18% of all HT prescriptions were written by primary care providers, and today, many remain wary about prescribing HT, despite evidence of its clinical value and safety.
“I think there’s a whole generation of family physicians who were taught that [hormone therapy] is dangerous and still feel very uncomfortable about using it to treat menopausal symptoms,” said Santina J.G. Wheat, MD, MPH, associate professor of family and community medicine at Northwestern University, Chicago. “These are the physicians educating the next generation of physicians,” said Dr. Wheat, who is program director for the McGaw Northwestern Family Medicine Residency Erie Humboldt Park.
Heather Hirsch, MD, an internist who specializes in menopause medicine in Columbus, Ohio, estimates that there are 300 internists among the 1,000 or so health care providers currently certified in menopause medicine through The Menopause Society (formerly the North American Menopause Society or NAMS). With 63 million women in the United States between the ages of 34 and 65, “that adds up to one doctor for several million patients,” she pointed out.
“In my opinion, the impact on menopausal care is profound,” said Jennifer T. Allen, MD, associate professor of obstetrics and gynecology, and director of menopause and midlife health at the Medical College of Georgia, Augusta. “If a physician was not exposed to menopause medicine in medical school or residency and does not choose to learn about menopause after training, then the opportunity to fully care for perimenopausal and postmenopausal women is extinguished.”
Not everyone agrees. “There’s no question that women’s health in general and menopausal issues specifically are a critical part of health care that is typically covered in most family medicine curriculums,” said Neil S. Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College in Philadelphia. “In family medicine, we really do attend to women’s health – particularly women’s health around menopause – as an important part of resident physician training,” emphasized Dr. Skolnik who is also and also associate director of the family medicine residency program at Abington Jefferson Health in Jenkintown, Penn.
"Family physicians are in a unique position to offer female patients effective care at perimenopause and beyond," added Karen L. Smith, MD, a family physician from Raeford, N.C., who is a board member of the American Academy of Family Physicians.*
Even so, many primary care physicians remain unsure about the use of HT, according to William E. Golden, MD, an internist and geriatrician, and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock.
“On the whole area of hot flashes and vasomotor instability, I think we’re in a state of significant flux and confusion,” Dr. Golden said in an interview. “For a long time, a lot of doctors told patients, ‘It’s okay, you’ll age out of it.’ Then the data started showing that the vasomotor symptoms continued for years so physicians began to reevaluate how to manage them. Now, the pendulum has swung back to giving estrogen.”
Many family physicians have been left to their own devices to figure out how to manage menopausal patients, said Dr. Wheat. “When there are significant changes to clinical management – or in the case of HT, a real reversal in how menopausal symptoms are managed – getting information out to physicians can be challenging.”
Meanwhile, patient demand for answers to their questions about menopause and the use of HT is changing the conversation, where it’s taking place, and with whom.
Some media-savvy doctors have taken to TikTok, where a lot of women started educating themselves about menopause during the pandemic. Dr. Hirsch is one of them. She uses the social media platform to talk about menopause and FDA-approved HT, but warned that for every clinician who is certified in menopause medicine “there are five more selling snake oil.”
Mainstream media has also jumped on the menopause bandwagon. The New York Times was one of the first, declaring that “menopause is having a moment.” On Feb. 1, the newspaper stormed the gates of the medical establishment with an article asking why more doctors weren’t offering HT to women experiencing hot flashes, sleeplessness, and pain during sex. The headline: “Women have been misled about menopause.”
On April 5, “The Menopause Talk” was posted to Oprah Daily, along with a menopause curriculum to give viewers “the tools to stay firmly in the driver’s seat as you navigate perimenopause and then menopause.” Popular topics included how to get your sex life back, premature menopause survival, and ways to work with insurers so that treatment is affordable.
“There’s been a sea-change in the culture that’s being driven by patient demand,” said Dr. Kling. “The conversation, colloquially, in the media, and with our patients, is evolving. Menopause is no longer such a taboo topic, and our patients are really demanding that we have answers for them. Clinicians are recognizing that they need better training in menopause and seeking that out.”
Last June, “Transforming Women’s Health” – the Mayo Clinic’s annual CME program held in partnership with The Menopause Society – had record physician attendance. “We’re going to make sure that our trainees are learning the up-to-date recommendations, not the ones from 20 years ago when the initial WHI reports made everyone fearful of hormones,” said Dr. Kling.
Dr. Kling disclosed that she is a medical editor for Everyday Health, and has a relationship with Evolve Medical Education. Dr. Skolnik reported relationships with numerous pharmaceutical companies. He is an MDedge Family Medicine board member. Dr. Golden is an MDedge Internal Medicine board member, and Dr. Wheat is an MDedge Family Medicine board member. Dr. Allen reported having no potential conflicts of interest.
* This story was updated on Sept 18, 2023. The quotation is attributable to Dr. Smith, not Dr. Skolnik.









