User login
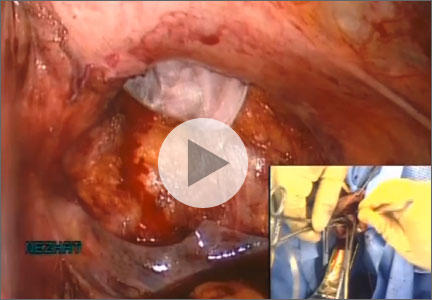
Laparoscopic myomectomy with enclosed transvaginal tissue extraction
Ceana Nezhat, MD, and Erica Dun, MD, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, Atlanta, Georgia, present this surgical case of a 41-year-old G0 with radiating lower abdominal pain and mennorhagia who desired removal of her symptomatic myomas. Preoperative transvaginal ultrasound revealed a 4-cm posterior pedunculated myoma and 5-cm fundal intramural myoma.
Ceana Nezhat, MD, and Erica Dun, MD, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, Atlanta, Georgia, present this surgical case of a 41-year-old G0 with radiating lower abdominal pain and mennorhagia who desired removal of her symptomatic myomas. Preoperative transvaginal ultrasound revealed a 4-cm posterior pedunculated myoma and 5-cm fundal intramural myoma.
Ceana Nezhat, MD, and Erica Dun, MD, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, Atlanta, Georgia, present this surgical case of a 41-year-old G0 with radiating lower abdominal pain and mennorhagia who desired removal of her symptomatic myomas. Preoperative transvaginal ultrasound revealed a 4-cm posterior pedunculated myoma and 5-cm fundal intramural myoma.
Early low-dose menopausal hormone therapy did not affect atherosclerosis
Two low-dose menopausal hormone regimens improved some cardiovascular parameters in healthy women but did not affect atherosclerosis progression, even when started early and continued for up to 4 years, investigators reported online July 28 in Annals of Internal Medicine.
Neither of the low-dose menopausal hormone therapy (MHT) regimens used in the study affected blood pressure, but both relieved hot flushes, added Dr. S. Mitchell Harman of the Phoenix Veterans Affairs Health Care System in Arizona and his associates.
Follow-up analyses of Women’s Health Initiative data had suggested that MHT might have cardiovascular benefits if started early enough or in younger women, the researchers noted.
To further explore the association, the Kronos Early Estrogen Prevention Study (KEEPS) enrolled 727 healthy women aged 42-58 years who were considered low risk for cardiovascular disease and were within 36 months of their last menses (Ann. Intern. Med. 2014 July 28 [doi: 10.7326/M14-0353]). The women were randomized in a double-blinded fashion to oral progesterone (200 mg for 12 days per month), plus either oral estrogen (conjugated equine estrogen, 0.45 mg per day) or transdermal estrogen (17-beta-estradiol, 50 mcg per day), or to a placebo, the researchers said.
Vascular ultrasonography at baseline and after up to 4 years of treatment revealed no effect of MHT on the progression of atherosclerosis, the researchers said. Average increases in carotid artery intima-media thickness were 0.0076 mm per year and were similar across treatment groups, as were increases in coronary artery calcium scores (17.4% for the oral estrogen group, 18.9% for the transdermal estrogen group, and 21.0% for the placebo group), they reported.
Neither hormone therapy regimen led to changes in blood pressure or interleukin-6 levels, although vasomotor symptoms (hot flushes) improved with both regimens, the investigators said. Lipoprotein cholesterol levels also improved in both treatment groups, and the oral estrogen group had improvements in C-reactive protein and sex hormone–binding globulin. The transdermal estrogen group had improvements in insulin sensitivity.
The study was inadequately powered to allow comparison of clinical events, and power for the coronary artery calcium score also was limited, the researchers noted. The results also might not be generalizable to women who have substantial cardiovascular risk factors, they added.
The Aurora Foundation funded the research, and Abbott Laboratories and Bayer Healthcare donated study medications. Dr. Harman reported no relevant conflicts of interest.
Two low-dose menopausal hormone regimens improved some cardiovascular parameters in healthy women but did not affect atherosclerosis progression, even when started early and continued for up to 4 years, investigators reported online July 28 in Annals of Internal Medicine.
Neither of the low-dose menopausal hormone therapy (MHT) regimens used in the study affected blood pressure, but both relieved hot flushes, added Dr. S. Mitchell Harman of the Phoenix Veterans Affairs Health Care System in Arizona and his associates.
Follow-up analyses of Women’s Health Initiative data had suggested that MHT might have cardiovascular benefits if started early enough or in younger women, the researchers noted.
To further explore the association, the Kronos Early Estrogen Prevention Study (KEEPS) enrolled 727 healthy women aged 42-58 years who were considered low risk for cardiovascular disease and were within 36 months of their last menses (Ann. Intern. Med. 2014 July 28 [doi: 10.7326/M14-0353]). The women were randomized in a double-blinded fashion to oral progesterone (200 mg for 12 days per month), plus either oral estrogen (conjugated equine estrogen, 0.45 mg per day) or transdermal estrogen (17-beta-estradiol, 50 mcg per day), or to a placebo, the researchers said.
Vascular ultrasonography at baseline and after up to 4 years of treatment revealed no effect of MHT on the progression of atherosclerosis, the researchers said. Average increases in carotid artery intima-media thickness were 0.0076 mm per year and were similar across treatment groups, as were increases in coronary artery calcium scores (17.4% for the oral estrogen group, 18.9% for the transdermal estrogen group, and 21.0% for the placebo group), they reported.
Neither hormone therapy regimen led to changes in blood pressure or interleukin-6 levels, although vasomotor symptoms (hot flushes) improved with both regimens, the investigators said. Lipoprotein cholesterol levels also improved in both treatment groups, and the oral estrogen group had improvements in C-reactive protein and sex hormone–binding globulin. The transdermal estrogen group had improvements in insulin sensitivity.
The study was inadequately powered to allow comparison of clinical events, and power for the coronary artery calcium score also was limited, the researchers noted. The results also might not be generalizable to women who have substantial cardiovascular risk factors, they added.
The Aurora Foundation funded the research, and Abbott Laboratories and Bayer Healthcare donated study medications. Dr. Harman reported no relevant conflicts of interest.
Two low-dose menopausal hormone regimens improved some cardiovascular parameters in healthy women but did not affect atherosclerosis progression, even when started early and continued for up to 4 years, investigators reported online July 28 in Annals of Internal Medicine.
Neither of the low-dose menopausal hormone therapy (MHT) regimens used in the study affected blood pressure, but both relieved hot flushes, added Dr. S. Mitchell Harman of the Phoenix Veterans Affairs Health Care System in Arizona and his associates.
Follow-up analyses of Women’s Health Initiative data had suggested that MHT might have cardiovascular benefits if started early enough or in younger women, the researchers noted.
To further explore the association, the Kronos Early Estrogen Prevention Study (KEEPS) enrolled 727 healthy women aged 42-58 years who were considered low risk for cardiovascular disease and were within 36 months of their last menses (Ann. Intern. Med. 2014 July 28 [doi: 10.7326/M14-0353]). The women were randomized in a double-blinded fashion to oral progesterone (200 mg for 12 days per month), plus either oral estrogen (conjugated equine estrogen, 0.45 mg per day) or transdermal estrogen (17-beta-estradiol, 50 mcg per day), or to a placebo, the researchers said.
Vascular ultrasonography at baseline and after up to 4 years of treatment revealed no effect of MHT on the progression of atherosclerosis, the researchers said. Average increases in carotid artery intima-media thickness were 0.0076 mm per year and were similar across treatment groups, as were increases in coronary artery calcium scores (17.4% for the oral estrogen group, 18.9% for the transdermal estrogen group, and 21.0% for the placebo group), they reported.
Neither hormone therapy regimen led to changes in blood pressure or interleukin-6 levels, although vasomotor symptoms (hot flushes) improved with both regimens, the investigators said. Lipoprotein cholesterol levels also improved in both treatment groups, and the oral estrogen group had improvements in C-reactive protein and sex hormone–binding globulin. The transdermal estrogen group had improvements in insulin sensitivity.
The study was inadequately powered to allow comparison of clinical events, and power for the coronary artery calcium score also was limited, the researchers noted. The results also might not be generalizable to women who have substantial cardiovascular risk factors, they added.
The Aurora Foundation funded the research, and Abbott Laboratories and Bayer Healthcare donated study medications. Dr. Harman reported no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Low-dose menopausal hormone therapy did not affect the progression of atherosclerosis, even when started early and continued for up to 4 years.
Major finding: Average increases in carotid artery intima-media thickness were 0.0076 mm per year and were similar across treatment groups, as were average changes in coronary artery calcium scores (17.4% for the oral estrogen group, 18.9% for the transdermal estrogen group, and 21.0% for the placebo group).
Data source: Multicenter, double-blind, placebo-controlled, randomized clinical trial of 727 healthy women within 36 months of their last menses. Women were randomized to oral conjugated equine estrogens (0.45 mg per day) and oral progesterone (200 mg for 12 days per month); the same dose of oral progesterone plus transdermal 17-beta-estradiol (50 mcg per day); or placebo.
Disclosures: The Aurora Foundation funded the research, and Abbott Laboratories and Bayer Healthcare donated study medications. Dr. Harman reported no relevant conflicts of interest.
New agent builds bone bigger, faster
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
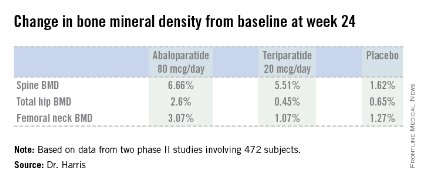
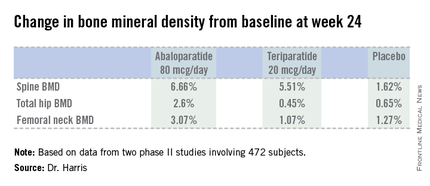
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
CHICAGO – Abaloparatide, a synthetic analog of human parathyroid hormone–related peptide, displayed jaw-dropping superiority to teriparatide in boosting bone mineral density at multiple anatomic sites in a head-to-head, placebo-controlled phase II study.
"Given the consistency of these increases in BMD [bone mineral density] seen in the phase II studies, abaloparatide may emerge as an important therapeutic agent in the treatment of postmenopausal osteoporosis," Dr. Alan G. Harris observed in presenting the results of two separate phase II abaloparatide studies at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.

"There is an unmet need for anabolic agents that preferentially increase bone formation as opposed to decreasing bone resorption. There is also a challenge we’re faced with in clinical practice: that is, the lack of early hip BMD increase with teriparatide," added Dr. Harris, chief medical officer at Radius Health of Cambridge, Mass., which is developing the agent.
The phase II data suggest abaloparatide at 80 mcg by once-daily subcutaneous injection meets both needs, he added.
Indeed, based upon the highly positive phase II work, a phase III, placebo- and teriparatide-controlled clinical trial with fracture endpoints is well underway. The 18-month trial involving more than 2,400 patients is due to be completed later this year.
Separately, Gary Hattersley, Ph.D., presented encouraging results from a 231-patient, 24-week, phase-II, dose-ranging study of abaloparatide delivered by transdermal patch.
"We look at this as being a strong proof-of-concept study demonstrating that this simple transdermal patch with only a 5-minute wear time is able to deliver meaningful amounts of abaloparatide through the skin without the need for a subcutaneous injection in order to achieve meaningful increases in BMD," said Dr. Hattersley, chief scientific officer at Radius Health.
"We recognize that there’s really a significant opportunity for an alternative to daily subcutaneous injection. This has the potential to improve both patient convenience as well as patient compliance," he added.
The increases in BMD with the patch – a 2.95% increase from baseline at the spine with the 150-mcg patch and a 1.49% rise in total hip BMD – were not as robust as in controls assigned to once-daily abaloparatide at 80 mcg, which is the optimal injectable dose also being used in the ongoing phase III trial. But Dr. Hattersley said he believes that higher-dose patches now under study will achieve substantially bigger increases in BMD.
Dr. Harris presented data from two phase II studies on a total of 472 postmenopausal women with osteoporosis. In one, subjects were randomized to subcutaneous abaloparatide, teriparatide (Forteo) at its approved dose of 20 mcg by daily subcutaneous injection, or placebo. Although the primary endpoints in this study were assessed at 24 weeks, in an extension out to 48 weeks the increase in lumbar spine BMD over baseline was 12.9% with abaloparatide 80 mcg, 8.6% with teriparatide, and 0.7% with placebo.
In the other study, patients were randomized to abaloparatide or placebo. In this trial, patients on abaloparatide at 80 mcg showed a 5.8% increase over baseline in spine BMD at 24 weeks, along with a 2.74% increase in total hip BMD and a 2.76% increase in femoral neck BMD, as compared with a 0.44% increase in spine BMD with placebo and net BMD losses of less than 1% at each of the other two sites.
In both studies, side effects of abaloparatide were similar in type and incidence to placebo. Of note, the incidence of mild, transient hypercalcemia in abaloparatide-treated patients was half that of the teriparatide group.
Asked why the subcutaneous abaloparatide at 80 mcg is so much more effective at increasing BMD than teriparatide is at its approved dose, Dr. Harris replied, "They’re different peptides." In monkey studies, abaloparatide showed less increase in cortical bone porosity than in studies done using teriparatide. And in the head-to-head phase II study, the increase in bone turnover markers related to resorption was substantially greater with teriparatide. So abaloparatide’s greater BMD-building efficacy is because of greater selectivity for increased bone formation and less bone resorption relative to teriparatide, he suggested.
The abaloparatide patch utilizes proprietary technology developed by 3M. The dime-size patch contains 316 spearlike microprojections, each 500 mcm (micrometers) long. The tip of each microprojection is coated with abaloparatide. When the patch is applied to periumbilical skin, the microprojections penetrate the skin to a depth of about 250 mcm, putting the tip into the upper dermis. Patch application was painless and without side effects in the phase II study, according to Dr. Harris.
These phase II studies were funded by Radius Health.
AT ICE/ENDO 2014
Key clinical point: A novel anabolic agent being developed for the treatment of postmenopausal osteoporosis increased BMD faster and to a greater extent than did teriparatide.
Major finding: Total hip bone mineral density increased by 2.6% over baseline after 24 weeks of abaloparatide at 80 mcg daily, compared with 0.45% with teriparatide at 20 mcg daily and 0.65% with placebo.
Data source: This phase II randomized trial included 222 postmenopausal women with osteoporosis.
Disclosures: The study was sponsored by Radius Health. The presenter is the company’s chief medical officer.
Systemwide disparities seen in diagnosis, care of women with heart disease
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM WCC 2014
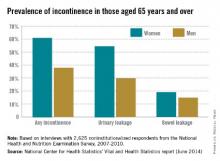
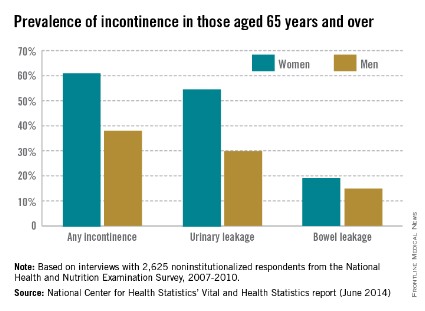
More than half of older women have experienced incontinence
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Among noninstitutionalized Americans aged 65 years and older, 61.2% of women and 38% of men experience at least occasional urinary or bowel incontinence, the National Center for Health Statistics reported.
Data from the 2007-2010 National Health and Nutrition Examination Survey (NHANES) show that 54.8% of women and 29.9% of men had urinary leakage at least a few times a month, while 19.2% of women and 14.9% of men had accidental bowel leakage of mucus, liquid stool, or solid stool at least one to three times a month, according to the NCHS (Vital Health Stat. 2014;3[36]).
Among women aged 65-74 years, 60.6% had some type of incontinence, compared with 61.9% of women aged 75 years and over. The difference was larger for men, however, with 34.1% of those aged 65-74 years reporting incontinence, compared with 42.4% of men aged 75 years and over, the report showed.
The NHANES data are based on in-home interviews with a nationally representative sample of 2,625 respondents.
Denosumab's benefits persist through 8 years
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
CHICAGO – Postmenopausal women on denosumab for 8 years straight showed a continued near-linear increase in bone mineral density at the lumbar spine as well as persistent reduction of bone turnover markers and a sustained low incidence of new vertebral and nonvertebral fractures in the ongoing FREEDOM open-label extension study.
Moreover, no new safety signals have emerged during 5 years of additional denosumab (Prolia) on top of an initial 3 years occurring in the pivotal, phase III, randomized, double-blind, placebo-controlled FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) trial.
Importantly, the overall risk of adverse events has not increased over time, and rates of malignancies, serious infections, eczema, hypocalcemia, and other adverse events in denosumab-treated patients remain comparable to rates seen in placebo-treated controls in the earlier double-blind phase, Dr. E. Michael Lewiecki reported at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The benefit/risk profile for denosumab remains favorable," declared Dr. Lewiecki, director of the New Mexico Clinical Research and Osteoporosis Center, Albuquerque.
The FREEDOM open-label extension is designed to assess the efficacy and safety of up to 10 years of denosumab therapy. Dr. Lewiecki presented the latest update, based on 8 years of treatment, or 5 years for patients in the original placebo arm who later elected to crossover to denosumab upon completing the 3-year double-blind phase. His report included 2,243 women on denosumab at 60 mg by subcutaneous injection every 6 months continuously for 8 years and another 2,207 on the drug for 5 years.
Five cases of osteonecrosis of the jaw have occurred in the long-term therapy group, as well as three cases among those who crossed over to the drug. In addition, there has been one atypical femoral fracture during 8 consecutive years on denosumab and one case in the crossover group.
Impressively, lumbar spine bone mineral density (BMD) has increased by 18.4% over baseline with 8 years of denosumab and by 13.7% with 5 years of active therapy.
"The near-linear slope of increase is unchanged over the course of the study. A similar slope is seen in the crossover group," the endocrinologist observed.
Total hip BMD increased by 8.3% with 8 years of denosumab and 4.9% with 5 years of therapy.
In the pivotal phase III FREEDOM trial, 3 years of denosumab reduced the risk of vertebral fractures by 68% compared to placebo, hip fractures by 40%, and nonvertebral fractures by 20% (N. Engl. J. Med. 2009;361:756-65).
The yearly incidence of new vertebral fractures was 0.7%-1.1% in denosumab-treated patients during years 1-3 and 1.1%-1.3% annually in open-label years 4-8. The annual incidence of new nonvertebral fractures in the denosumab group was 2.1%-2.6% in the first 3 years of double-blind therapy and has trended lower since then: 1.5% in year 4, 1.2% in year 5, 1.8% in year 6, 1.6% in year 7, and 0.7% in year 8.
"There’s a suggestion of a further reduction in the risk of nonvertebral fractures out to the 8-year time point. It’ll be fascinating to look at the data for years 9 and 10 to see if that’s just a statistical aberrance or that very low risk of nonvertebral fractures persists," Dr. Lewiecki commented.
Levels of the bone turnover markers serum C-terminal telopeptide of type 1 collagen and procollagen type 1 N-terminal propeptide quickly dropped upon initiation of denosumab and have remained low through 8 years.
Asked about the mechanism underlying the continued linear increase in lumbar spine BMD seen through 8 years of denosumab, in sharp contrast to the bisphosphonates, where BMD plateaus, Dr. Lewiecki confessed that "Many of us are scratching our heads about this and trying to come up with an explanation."
"I don’t know the reason why, but there are several hypotheses worth considering," he continued. "One is that the effects on cortical bone appear to be different with denosumab than with bisphosphonates. We see a reduction in cortical porosity and a larger improvement in bone mineral density at cortical skeletal sites. Secondly, there’s a possible parathyroid hormone effect that may play a role here: There is a larger and longer-lasting increase in parathyroid hormone with denosumab as compared with bisphosphonates. And finally, there’s animal data in monkeys showing ongoing bone modeling taking place with denosumab; it’s possible that may also play a role."
Denosumab is a fully human monoclonal antibody directed against the receptor activator of nuclear factor-kappa B (RANK) ligand.
The FREEDOM study is supported by Amgen. Dr. Lewiecki reported serving as a consultant to that company as well as to AgNovos Healthcare, Lilly, Merck, and Radius Health.
AT ICE/ENDO 2014
Key clinical point: An ongoing major study provides reassurance regarding the long-term safety and effectiveness of denosumab for postmenopausal osteoporosis.
Major finding: Eight years of denosumab was associated with sustained low fracture rates, steadily increasing bone mineral density, persistent reduction of bone turnover, and no increase in adverse events over time.
Data source: The FREEDOM trial open-label extension includes 4,450 women on denosumab for either 5 or 8 years and counting.
Disclosures: The study was sponsored by Amgen. The presenter serves as a consultant to the company.
Hormone therapy for menopausal vasomotor symptoms
Estrogen therapy is highly effective in the treatment of hot flashes among postmenopausal women. For postmenopausal women with a uterus, estrogen treatment for hot flashes is almost always combined with a progestin to reduce the risk of endometrial polyps, hyperplasia, and cancer. For instance, in the Postmenopausal Estrogen/Progestin Interventions Trial, 62% of the women with a uterus treated with conjugated equine estrogen (CEE) 0.625 mg daily without a progestin developed endometrial hyperplasia.1
In the United States, the most commonly prescribed progestin for hormone therapy has been medroxyprogesterone acetate (MPA; Provera). However, data from the Women’s Health Initiative (WHI) trials indicate that MPA, when combined with CEE, may have adverse health effects among postmenopausal women.
Let’s examine the WHI data
Among women 50 to 59 years of age with a uterus, the combination of CEE plus MPA was associated with a trend toward an increased risk of breast cancer, coronary heart disease, and myocardial infarction.2 In contrast, among women 50 to 59 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of invasive breast cancer, coronary heart disease, and myocardial infarction (TABLE 1).
Among women 50 to 79 years of age with a uterus, the combination of CEE plus MPA was associated with a significantly increased risk of breast cancer (hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.01–1.53; P = .04).2 In contrast, among women 50 to 79 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of breast cancer (HR, 0.79; 95% CI, 0.61–1.02, P = .07).2
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
When the analysis was limited to women consistently adherent to their CEE monotherapy, the estrogen treatment significantly decreased the risk of invasive breast cancer (HR, 0.67; 95% CI, 0.47–0.97; P = .03).3
The addition of MPA to CEE appears to reverse some of the health benefits of CEE monotherapy, although the biological mechanisms are unclear. This observation should prompt us to explore alternative and novel treatments of vasomotor symptoms that do not utilize MPA. Some options for MPA-free hormone therapy include:
- transdermal estradiol plus micronized progesterone
- CEE plus a levonorgestrel-releasing intrauterine system
- bazedoxifene plus CEE.
In addition, nonhormonal treatment of hot flashes is an option, with selective serotonin reuptake inhibitors (SSRIs).
Related article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence; January 2014)
MPA-free hormone therapy for hot flashes
Estrogen plus micronized progesterone
When using an estrogen plus progestin regimen to treat hot flashes, many experts favor a combination of low-dose transdermal estradiol and oral micronized progesterone (Prometrium). This combination is believed by some experts to result in a lower risk of venous thromboembolism, stroke, cardiovascular disease, and breast cancer than an estrogen-MPA combination.4–7
When prescribing transdermal estradiol plus oral micronized progesterone for a woman within 1 to 2 years of her last menses, a cyclic regimen can help reduce episodes of irregular, unscheduled uterine bleeding. I often use this cyclic regimen: transdermal estradiol 0.0375 mg plus cyclic oral micronized progesterone 200 mg prior to bedtime for calendar days 1 to 12.
When using transdermal estradiol plus oral micronized progesterone in a woman more than 2 years from her last menses, a continuous regimen is often prescribed. I often use this continuous regimen: transdermal estradiol 0.0375 mg plus continuous oral micronized progesterone 100 mg daily prior to bedtime.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Estrogen plus a levonorgestrel-releasing intrauterine systemThe levonorgestrel intrauterine system (LNG-IUS; 20 µg daily; Mirena) is frequently used in Europe to protect the endometrium against the adverse effects of estrogen therapy in postmenopausal women. In a meta-analysis of five clinical trials involving postmenopausal women, the LNG-IUS provided excellent protection against endometrial hyperplasia, compared with MPA.8
One caution about using the LNG-IUS system with estrogen in postmenopausal women is that an observational study of all women with breast cancer in Finland from 1995 through 2007 reported a significantly increased risk of breast cancer among postmenopausal women using an LNG-IUS compared with women who did not use hormones or used only estrogen because they had a hysterectomy (TABLE 2).9 This study was not a randomized clinical trial and patients at higher baseline risk for breast cancer, including women with a high body mass index, may have been preferentially treated with an LNG-IUS. More information is needed to better understand the relationship between the LNG-IUS and breast cancer in postmenopausal women.
Related article: What we’ve learned from 2 decades’ experience with the LNG-IUS. Q&A with Oskari Heikinheimo, MD, PhD (February 2011)
Progestin-free hormone treatment, bazedoxifene plus CEE
The main reason for adding a progestin to estrogen therapy for vasomotor symptoms in postmenopausal women with a uterus is to prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer. A major innovation in hormone therapy is the discovery that third-generation selective estrogen receptor modulators (SERMs), such as bazedoxifene (BZA), can prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer but do not interfere with the efficacy of estrogen in the treatment of vasomotor symptoms.
BZA is an estrogen agonist in bone and an estrogen antagonist in the endometrium.10–12 The combination of BZA (20 mg daily) plus CEE (0.45 mg daily) (Duavee) is approved for the treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis.13–15 Over 24 months of therapy, various doses of BZA plus CEE reduced reported daily hot flashes by 52% to 86%.16 In the same study, placebo treatment was associated with a 17% reduction in hot flashes.16
The main adverse effect of BZA/CEE is an increased risk of deep venous thrombosis. Therefore, BZA/CEE is contraindicated in women with a known thrombophilia or a personal history of hormone-induced deep venous thrombosis. The effect of BZA/CEE on the risk of developing invasive breast cancer is not known; over 52 weeks of therapy it did not increase breast density on mammogram.17,18
BZA/CEE is a remarkable advance in hormone therapy. It is progestin-free, uses estrogen to treat vasomotor symptoms, and uses BZA to protect the endometrium against estrogen-induced hyperplasia.
Related article: New option for treating menopausal vasomotor symptoms receives FDA approval. (News for your Practice; October 2013)
Nonhormone treatment of vasomotor symptoms Paroxetine mesylateFor postmenopausal women with vasomotor symptoms who cannot take estrogen, SSRIs are modestly effective in reducing moderate to severe hot flashes. The US Food and Drug Administration recently approved paroxetine mesylate (Brisdelle) for the treatment of postmenopausal vasomotor symptoms. The approved dose is 7.5 mg daily taken at bedtime.
Data supporting the efficacy of paroxetine mesylate are available from two studies involving 1,184 menopausal women with vasomotor symptoms randomly assigned to receive paroxetine 7.5 mg daily or placebo for 12 weeks of treatment.19-22 In one of the two clinical trials, women treated with paroxetine mesylate 7.5 mg daily had 5.6 fewer moderate to severe hot flashes daily after 12 weeks of treatment compared with 3.9 fewer hot flashes with placebo (median treatment difference, 1.7; P<.001).21
Paroxetine can block the metabolism of tamoxifen to its highly potent metabolite, endoxifen. Consequently, paroxetine may reduce the effectiveness of tamoxifen treatment for breast cancer and should be used with caution in postmenopausal women with breast cancer being treated with tamoxifen.
Related article: Paroxetine mesylate 7.5 mg found to be a safe alternative to hormone therapy for menopausal women with hot flashes. (News for your Practice; June 2014)
Escitalopram
Gynecologists are familiar with the use of venlafaxine, desvenlafaxine, clonidine, citalopram, sertraline, and fluoxetine for the treatment of postmenopausal hot flashes. Recently, escitalopram (Lexapro) at doses of 10 to 20 mg daily has been shown to be more effective than placebo in the treatment of hot flashes and sleep disturbances in postmenopausal women.23,24 In one trial of escitalopram 10 to 20 mg daily versus placebo in 205 postmenopausal women averaging 9.8 hot flashes daily at baseline, escitalopram and placebo reduced mean daily hot flashes by 4.6 and 3.2, respectively (P<.001), after 8 weeks of treatment.
In a meta-analysis of SSRIs for the treatment of hot flashes, data from a mixed-treatment comparison analysis indicated that the rank order from most to least effective therapy for hot flashes was: escitalopram > paroxetine > sertraline > citalopram > fluoxetine.25 Venlafaxine and desvenlafaxine, two serotonin and norepinephrine reuptake inhibitors that are effective in the treatment of hot flashes, were not included in the mixed-treatment comparison.
Use of alternatives to MPA could mean fewer health risks for women on a wide scale
Substantial data indicate that MPA is not an optimal progestin to combine with estrogen for hormone therapy. Currently, many health insurance plans and Medicare use pharmacy management formularies that prioritize dispensing MPA for postmenopausal hormone therapy. Dispensing an alternative to MPA, such as micronized progesterone, often requires the patient to make a significant copayment.
Hopefully, health insurance companies, Medicare, and their affiliated pharmacy management administrators will soon stop their current policy of using financial incentives to favor dispensing MPA when hormone therapy is prescribed because alternatives to MPA appear to be associated with fewer health risks for postmenopausal women.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–375.
2. Manson JE, Chlebowski RT, Stefnick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
3. Stefanick ML, Anderson GL, Margolis KL, et al; WHI Investigators. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657.
4. Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? [published online head of print January 6, 2014]. Menopause. PMID: 24398406.
5. Manson JE. Current recommendations: What is the clinician to do? Fertil Steril. 2014;101(4):916–921.
6. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study [published correction appears in Breast Cancer Res Treat. 2008;107(2):307–308]. Breast Cancer Res Treat. 2008;107(1):103–111.
7. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519.
8. Somboonpom W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: Systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
9. Lyytinen HK, Dyba T, Ylikorkala O, Pukkala EI. A case-control study on hormone therapy as a risk factor for breast cancer in Finland: Intrauterine system carries a risk as well. Int J Cancer. 2010;126(2):483–489.
10. Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143C:207–222.
11. Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause. 2013;20(7):777–784.
12. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: A randomized trial. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
13. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
14. Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt). 2014;23(1):18–28.
15. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: A randomized controlled trial. Obstet Gynecol. 2013;121(5):959–968.
16. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
17. Harvey JA, Holm MK, Ranganath R, Guse PA, Trott EA, Helzner E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause. 2009;16(6):1193–1196.
18. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20(2):138–145.
19. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: Two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
20. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
21. Orleans RJ, Li L, Kim MJ, et al. FDA approval of paroxetine for menopausal hot flashes. N Engl J Med. 2014;370(19):1777–1779.
22. Paroxetine (Brisdelle) for hot flashes. Med Lett Drugs Ther. 2013;55(1428):85–86.
23. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in health menopausal women. A randomized controlled trial. JAMA. 2011;305(3):267–274.
24. Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: A randomized controlled trial. Menopause. 2012;19(8):848–855.
25. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: A systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204–213.
Estrogen therapy is highly effective in the treatment of hot flashes among postmenopausal women. For postmenopausal women with a uterus, estrogen treatment for hot flashes is almost always combined with a progestin to reduce the risk of endometrial polyps, hyperplasia, and cancer. For instance, in the Postmenopausal Estrogen/Progestin Interventions Trial, 62% of the women with a uterus treated with conjugated equine estrogen (CEE) 0.625 mg daily without a progestin developed endometrial hyperplasia.1
In the United States, the most commonly prescribed progestin for hormone therapy has been medroxyprogesterone acetate (MPA; Provera). However, data from the Women’s Health Initiative (WHI) trials indicate that MPA, when combined with CEE, may have adverse health effects among postmenopausal women.
Let’s examine the WHI data
Among women 50 to 59 years of age with a uterus, the combination of CEE plus MPA was associated with a trend toward an increased risk of breast cancer, coronary heart disease, and myocardial infarction.2 In contrast, among women 50 to 59 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of invasive breast cancer, coronary heart disease, and myocardial infarction (TABLE 1).
Among women 50 to 79 years of age with a uterus, the combination of CEE plus MPA was associated with a significantly increased risk of breast cancer (hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.01–1.53; P = .04).2 In contrast, among women 50 to 79 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of breast cancer (HR, 0.79; 95% CI, 0.61–1.02, P = .07).2
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
When the analysis was limited to women consistently adherent to their CEE monotherapy, the estrogen treatment significantly decreased the risk of invasive breast cancer (HR, 0.67; 95% CI, 0.47–0.97; P = .03).3
The addition of MPA to CEE appears to reverse some of the health benefits of CEE monotherapy, although the biological mechanisms are unclear. This observation should prompt us to explore alternative and novel treatments of vasomotor symptoms that do not utilize MPA. Some options for MPA-free hormone therapy include:
- transdermal estradiol plus micronized progesterone
- CEE plus a levonorgestrel-releasing intrauterine system
- bazedoxifene plus CEE.
In addition, nonhormonal treatment of hot flashes is an option, with selective serotonin reuptake inhibitors (SSRIs).
Related article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence; January 2014)
MPA-free hormone therapy for hot flashes
Estrogen plus micronized progesterone
When using an estrogen plus progestin regimen to treat hot flashes, many experts favor a combination of low-dose transdermal estradiol and oral micronized progesterone (Prometrium). This combination is believed by some experts to result in a lower risk of venous thromboembolism, stroke, cardiovascular disease, and breast cancer than an estrogen-MPA combination.4–7
When prescribing transdermal estradiol plus oral micronized progesterone for a woman within 1 to 2 years of her last menses, a cyclic regimen can help reduce episodes of irregular, unscheduled uterine bleeding. I often use this cyclic regimen: transdermal estradiol 0.0375 mg plus cyclic oral micronized progesterone 200 mg prior to bedtime for calendar days 1 to 12.
When using transdermal estradiol plus oral micronized progesterone in a woman more than 2 years from her last menses, a continuous regimen is often prescribed. I often use this continuous regimen: transdermal estradiol 0.0375 mg plus continuous oral micronized progesterone 100 mg daily prior to bedtime.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Estrogen plus a levonorgestrel-releasing intrauterine systemThe levonorgestrel intrauterine system (LNG-IUS; 20 µg daily; Mirena) is frequently used in Europe to protect the endometrium against the adverse effects of estrogen therapy in postmenopausal women. In a meta-analysis of five clinical trials involving postmenopausal women, the LNG-IUS provided excellent protection against endometrial hyperplasia, compared with MPA.8
One caution about using the LNG-IUS system with estrogen in postmenopausal women is that an observational study of all women with breast cancer in Finland from 1995 through 2007 reported a significantly increased risk of breast cancer among postmenopausal women using an LNG-IUS compared with women who did not use hormones or used only estrogen because they had a hysterectomy (TABLE 2).9 This study was not a randomized clinical trial and patients at higher baseline risk for breast cancer, including women with a high body mass index, may have been preferentially treated with an LNG-IUS. More information is needed to better understand the relationship between the LNG-IUS and breast cancer in postmenopausal women.
Related article: What we’ve learned from 2 decades’ experience with the LNG-IUS. Q&A with Oskari Heikinheimo, MD, PhD (February 2011)
Progestin-free hormone treatment, bazedoxifene plus CEE
The main reason for adding a progestin to estrogen therapy for vasomotor symptoms in postmenopausal women with a uterus is to prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer. A major innovation in hormone therapy is the discovery that third-generation selective estrogen receptor modulators (SERMs), such as bazedoxifene (BZA), can prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer but do not interfere with the efficacy of estrogen in the treatment of vasomotor symptoms.
BZA is an estrogen agonist in bone and an estrogen antagonist in the endometrium.10–12 The combination of BZA (20 mg daily) plus CEE (0.45 mg daily) (Duavee) is approved for the treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis.13–15 Over 24 months of therapy, various doses of BZA plus CEE reduced reported daily hot flashes by 52% to 86%.16 In the same study, placebo treatment was associated with a 17% reduction in hot flashes.16
The main adverse effect of BZA/CEE is an increased risk of deep venous thrombosis. Therefore, BZA/CEE is contraindicated in women with a known thrombophilia or a personal history of hormone-induced deep venous thrombosis. The effect of BZA/CEE on the risk of developing invasive breast cancer is not known; over 52 weeks of therapy it did not increase breast density on mammogram.17,18
BZA/CEE is a remarkable advance in hormone therapy. It is progestin-free, uses estrogen to treat vasomotor symptoms, and uses BZA to protect the endometrium against estrogen-induced hyperplasia.
Related article: New option for treating menopausal vasomotor symptoms receives FDA approval. (News for your Practice; October 2013)
Nonhormone treatment of vasomotor symptoms Paroxetine mesylateFor postmenopausal women with vasomotor symptoms who cannot take estrogen, SSRIs are modestly effective in reducing moderate to severe hot flashes. The US Food and Drug Administration recently approved paroxetine mesylate (Brisdelle) for the treatment of postmenopausal vasomotor symptoms. The approved dose is 7.5 mg daily taken at bedtime.
Data supporting the efficacy of paroxetine mesylate are available from two studies involving 1,184 menopausal women with vasomotor symptoms randomly assigned to receive paroxetine 7.5 mg daily or placebo for 12 weeks of treatment.19-22 In one of the two clinical trials, women treated with paroxetine mesylate 7.5 mg daily had 5.6 fewer moderate to severe hot flashes daily after 12 weeks of treatment compared with 3.9 fewer hot flashes with placebo (median treatment difference, 1.7; P<.001).21
Paroxetine can block the metabolism of tamoxifen to its highly potent metabolite, endoxifen. Consequently, paroxetine may reduce the effectiveness of tamoxifen treatment for breast cancer and should be used with caution in postmenopausal women with breast cancer being treated with tamoxifen.
Related article: Paroxetine mesylate 7.5 mg found to be a safe alternative to hormone therapy for menopausal women with hot flashes. (News for your Practice; June 2014)
Escitalopram
Gynecologists are familiar with the use of venlafaxine, desvenlafaxine, clonidine, citalopram, sertraline, and fluoxetine for the treatment of postmenopausal hot flashes. Recently, escitalopram (Lexapro) at doses of 10 to 20 mg daily has been shown to be more effective than placebo in the treatment of hot flashes and sleep disturbances in postmenopausal women.23,24 In one trial of escitalopram 10 to 20 mg daily versus placebo in 205 postmenopausal women averaging 9.8 hot flashes daily at baseline, escitalopram and placebo reduced mean daily hot flashes by 4.6 and 3.2, respectively (P<.001), after 8 weeks of treatment.
In a meta-analysis of SSRIs for the treatment of hot flashes, data from a mixed-treatment comparison analysis indicated that the rank order from most to least effective therapy for hot flashes was: escitalopram > paroxetine > sertraline > citalopram > fluoxetine.25 Venlafaxine and desvenlafaxine, two serotonin and norepinephrine reuptake inhibitors that are effective in the treatment of hot flashes, were not included in the mixed-treatment comparison.
Use of alternatives to MPA could mean fewer health risks for women on a wide scale
Substantial data indicate that MPA is not an optimal progestin to combine with estrogen for hormone therapy. Currently, many health insurance plans and Medicare use pharmacy management formularies that prioritize dispensing MPA for postmenopausal hormone therapy. Dispensing an alternative to MPA, such as micronized progesterone, often requires the patient to make a significant copayment.
Hopefully, health insurance companies, Medicare, and their affiliated pharmacy management administrators will soon stop their current policy of using financial incentives to favor dispensing MPA when hormone therapy is prescribed because alternatives to MPA appear to be associated with fewer health risks for postmenopausal women.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Estrogen therapy is highly effective in the treatment of hot flashes among postmenopausal women. For postmenopausal women with a uterus, estrogen treatment for hot flashes is almost always combined with a progestin to reduce the risk of endometrial polyps, hyperplasia, and cancer. For instance, in the Postmenopausal Estrogen/Progestin Interventions Trial, 62% of the women with a uterus treated with conjugated equine estrogen (CEE) 0.625 mg daily without a progestin developed endometrial hyperplasia.1
In the United States, the most commonly prescribed progestin for hormone therapy has been medroxyprogesterone acetate (MPA; Provera). However, data from the Women’s Health Initiative (WHI) trials indicate that MPA, when combined with CEE, may have adverse health effects among postmenopausal women.
Let’s examine the WHI data
Among women 50 to 59 years of age with a uterus, the combination of CEE plus MPA was associated with a trend toward an increased risk of breast cancer, coronary heart disease, and myocardial infarction.2 In contrast, among women 50 to 59 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of invasive breast cancer, coronary heart disease, and myocardial infarction (TABLE 1).
Among women 50 to 79 years of age with a uterus, the combination of CEE plus MPA was associated with a significantly increased risk of breast cancer (hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.01–1.53; P = .04).2 In contrast, among women 50 to 79 years of age without a uterus, CEE monotherapy was associated with a trend toward a decreased risk of breast cancer (HR, 0.79; 95% CI, 0.61–1.02, P = .07).2
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
When the analysis was limited to women consistently adherent to their CEE monotherapy, the estrogen treatment significantly decreased the risk of invasive breast cancer (HR, 0.67; 95% CI, 0.47–0.97; P = .03).3
The addition of MPA to CEE appears to reverse some of the health benefits of CEE monotherapy, although the biological mechanisms are unclear. This observation should prompt us to explore alternative and novel treatments of vasomotor symptoms that do not utilize MPA. Some options for MPA-free hormone therapy include:
- transdermal estradiol plus micronized progesterone
- CEE plus a levonorgestrel-releasing intrauterine system
- bazedoxifene plus CEE.
In addition, nonhormonal treatment of hot flashes is an option, with selective serotonin reuptake inhibitors (SSRIs).
Related article: Is one oral estrogen formulation safer than another for menopausal women? Andrew M. Kaunitz, MD (Examining the Evidence; January 2014)
MPA-free hormone therapy for hot flashes
Estrogen plus micronized progesterone
When using an estrogen plus progestin regimen to treat hot flashes, many experts favor a combination of low-dose transdermal estradiol and oral micronized progesterone (Prometrium). This combination is believed by some experts to result in a lower risk of venous thromboembolism, stroke, cardiovascular disease, and breast cancer than an estrogen-MPA combination.4–7
When prescribing transdermal estradiol plus oral micronized progesterone for a woman within 1 to 2 years of her last menses, a cyclic regimen can help reduce episodes of irregular, unscheduled uterine bleeding. I often use this cyclic regimen: transdermal estradiol 0.0375 mg plus cyclic oral micronized progesterone 200 mg prior to bedtime for calendar days 1 to 12.
When using transdermal estradiol plus oral micronized progesterone in a woman more than 2 years from her last menses, a continuous regimen is often prescribed. I often use this continuous regimen: transdermal estradiol 0.0375 mg plus continuous oral micronized progesterone 100 mg daily prior to bedtime.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Estrogen plus a levonorgestrel-releasing intrauterine systemThe levonorgestrel intrauterine system (LNG-IUS; 20 µg daily; Mirena) is frequently used in Europe to protect the endometrium against the adverse effects of estrogen therapy in postmenopausal women. In a meta-analysis of five clinical trials involving postmenopausal women, the LNG-IUS provided excellent protection against endometrial hyperplasia, compared with MPA.8
One caution about using the LNG-IUS system with estrogen in postmenopausal women is that an observational study of all women with breast cancer in Finland from 1995 through 2007 reported a significantly increased risk of breast cancer among postmenopausal women using an LNG-IUS compared with women who did not use hormones or used only estrogen because they had a hysterectomy (TABLE 2).9 This study was not a randomized clinical trial and patients at higher baseline risk for breast cancer, including women with a high body mass index, may have been preferentially treated with an LNG-IUS. More information is needed to better understand the relationship between the LNG-IUS and breast cancer in postmenopausal women.
Related article: What we’ve learned from 2 decades’ experience with the LNG-IUS. Q&A with Oskari Heikinheimo, MD, PhD (February 2011)
Progestin-free hormone treatment, bazedoxifene plus CEE
The main reason for adding a progestin to estrogen therapy for vasomotor symptoms in postmenopausal women with a uterus is to prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer. A major innovation in hormone therapy is the discovery that third-generation selective estrogen receptor modulators (SERMs), such as bazedoxifene (BZA), can prevent estrogen-induced development of endometrial polyps, hyperplasia, and cancer but do not interfere with the efficacy of estrogen in the treatment of vasomotor symptoms.
BZA is an estrogen agonist in bone and an estrogen antagonist in the endometrium.10–12 The combination of BZA (20 mg daily) plus CEE (0.45 mg daily) (Duavee) is approved for the treatment of moderate to severe vasomotor symptoms and prevention of osteoporosis.13–15 Over 24 months of therapy, various doses of BZA plus CEE reduced reported daily hot flashes by 52% to 86%.16 In the same study, placebo treatment was associated with a 17% reduction in hot flashes.16
The main adverse effect of BZA/CEE is an increased risk of deep venous thrombosis. Therefore, BZA/CEE is contraindicated in women with a known thrombophilia or a personal history of hormone-induced deep venous thrombosis. The effect of BZA/CEE on the risk of developing invasive breast cancer is not known; over 52 weeks of therapy it did not increase breast density on mammogram.17,18
BZA/CEE is a remarkable advance in hormone therapy. It is progestin-free, uses estrogen to treat vasomotor symptoms, and uses BZA to protect the endometrium against estrogen-induced hyperplasia.
Related article: New option for treating menopausal vasomotor symptoms receives FDA approval. (News for your Practice; October 2013)
Nonhormone treatment of vasomotor symptoms Paroxetine mesylateFor postmenopausal women with vasomotor symptoms who cannot take estrogen, SSRIs are modestly effective in reducing moderate to severe hot flashes. The US Food and Drug Administration recently approved paroxetine mesylate (Brisdelle) for the treatment of postmenopausal vasomotor symptoms. The approved dose is 7.5 mg daily taken at bedtime.
Data supporting the efficacy of paroxetine mesylate are available from two studies involving 1,184 menopausal women with vasomotor symptoms randomly assigned to receive paroxetine 7.5 mg daily or placebo for 12 weeks of treatment.19-22 In one of the two clinical trials, women treated with paroxetine mesylate 7.5 mg daily had 5.6 fewer moderate to severe hot flashes daily after 12 weeks of treatment compared with 3.9 fewer hot flashes with placebo (median treatment difference, 1.7; P<.001).21
Paroxetine can block the metabolism of tamoxifen to its highly potent metabolite, endoxifen. Consequently, paroxetine may reduce the effectiveness of tamoxifen treatment for breast cancer and should be used with caution in postmenopausal women with breast cancer being treated with tamoxifen.
Related article: Paroxetine mesylate 7.5 mg found to be a safe alternative to hormone therapy for menopausal women with hot flashes. (News for your Practice; June 2014)
Escitalopram
Gynecologists are familiar with the use of venlafaxine, desvenlafaxine, clonidine, citalopram, sertraline, and fluoxetine for the treatment of postmenopausal hot flashes. Recently, escitalopram (Lexapro) at doses of 10 to 20 mg daily has been shown to be more effective than placebo in the treatment of hot flashes and sleep disturbances in postmenopausal women.23,24 In one trial of escitalopram 10 to 20 mg daily versus placebo in 205 postmenopausal women averaging 9.8 hot flashes daily at baseline, escitalopram and placebo reduced mean daily hot flashes by 4.6 and 3.2, respectively (P<.001), after 8 weeks of treatment.
In a meta-analysis of SSRIs for the treatment of hot flashes, data from a mixed-treatment comparison analysis indicated that the rank order from most to least effective therapy for hot flashes was: escitalopram > paroxetine > sertraline > citalopram > fluoxetine.25 Venlafaxine and desvenlafaxine, two serotonin and norepinephrine reuptake inhibitors that are effective in the treatment of hot flashes, were not included in the mixed-treatment comparison.
Use of alternatives to MPA could mean fewer health risks for women on a wide scale
Substantial data indicate that MPA is not an optimal progestin to combine with estrogen for hormone therapy. Currently, many health insurance plans and Medicare use pharmacy management formularies that prioritize dispensing MPA for postmenopausal hormone therapy. Dispensing an alternative to MPA, such as micronized progesterone, often requires the patient to make a significant copayment.
Hopefully, health insurance companies, Medicare, and their affiliated pharmacy management administrators will soon stop their current policy of using financial incentives to favor dispensing MPA when hormone therapy is prescribed because alternatives to MPA appear to be associated with fewer health risks for postmenopausal women.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–375.
2. Manson JE, Chlebowski RT, Stefnick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
3. Stefanick ML, Anderson GL, Margolis KL, et al; WHI Investigators. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657.
4. Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? [published online head of print January 6, 2014]. Menopause. PMID: 24398406.
5. Manson JE. Current recommendations: What is the clinician to do? Fertil Steril. 2014;101(4):916–921.
6. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study [published correction appears in Breast Cancer Res Treat. 2008;107(2):307–308]. Breast Cancer Res Treat. 2008;107(1):103–111.
7. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519.
8. Somboonpom W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: Systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
9. Lyytinen HK, Dyba T, Ylikorkala O, Pukkala EI. A case-control study on hormone therapy as a risk factor for breast cancer in Finland: Intrauterine system carries a risk as well. Int J Cancer. 2010;126(2):483–489.
10. Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143C:207–222.
11. Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause. 2013;20(7):777–784.
12. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: A randomized trial. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
13. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
14. Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt). 2014;23(1):18–28.
15. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: A randomized controlled trial. Obstet Gynecol. 2013;121(5):959–968.
16. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
17. Harvey JA, Holm MK, Ranganath R, Guse PA, Trott EA, Helzner E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause. 2009;16(6):1193–1196.
18. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20(2):138–145.
19. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: Two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
20. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
21. Orleans RJ, Li L, Kim MJ, et al. FDA approval of paroxetine for menopausal hot flashes. N Engl J Med. 2014;370(19):1777–1779.
22. Paroxetine (Brisdelle) for hot flashes. Med Lett Drugs Ther. 2013;55(1428):85–86.
23. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in health menopausal women. A randomized controlled trial. JAMA. 2011;305(3):267–274.
24. Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: A randomized controlled trial. Menopause. 2012;19(8):848–855.
25. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: A systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204–213.
1. The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275(5):370–375.
2. Manson JE, Chlebowski RT, Stefnick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
3. Stefanick ML, Anderson GL, Margolis KL, et al; WHI Investigators. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA. 2006;295(14):1647–1657.
4. Simon JA. What if the Women’s Health Initiative had used transdermal estradiol and oral progesterone instead? [published online head of print January 6, 2014]. Menopause. PMID: 24398406.
5. Manson JE. Current recommendations: What is the clinician to do? Fertil Steril. 2014;101(4):916–921.
6. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study [published correction appears in Breast Cancer Res Treat. 2008;107(2):307–308]. Breast Cancer Res Treat. 2008;107(1):103–111.
7. Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ. 2010;340:c2519.
8. Somboonpom W, Panna S, Temtanakitpaisan T, Kaewrudee S, Soontrapa S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: Systematic review and meta-analysis. Menopause. 2011;18(10):1060–1066.
9. Lyytinen HK, Dyba T, Ylikorkala O, Pukkala EI. A case-control study on hormone therapy as a risk factor for breast cancer in Finland: Intrauterine system carries a risk as well. Int J Cancer. 2010;126(2):483–489.
10. Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143C:207–222.
11. Ethun KF, Wood CE, Cline JM, Register TC, Appt SE, Clarkson TB. Endometrial profile of bazedoxifene acetate alone and in combination with conjugated equine estrogens in a primate model. Menopause. 2013;20(7):777–784.
12. Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: A randomized trial. J Clin Endocrinol Metab. 2014;99(2):E189–E198.
13. Pinkerton JV, Pickar JH, Racketa J, Mirkin S. Bazedoxifene/conjugated estrogens for menopausal symptom treatment and osteoporosis prevention. Climacteric. 2012;15(5):411–418.
14. Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt). 2014;23(1):18–28.
15. Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: A randomized controlled trial. Obstet Gynecol. 2013;121(5):959–968.
16. Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92(3):1025–1038.
17. Harvey JA, Holm MK, Ranganath R, Guse PA, Trott EA, Helzner E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause. 2009;16(6):1193–1196.
18. Harvey JA, Pinkerton JV, Baracat EC, Shi H, Chines AA, Mirkin S. Breast density changes in a randomized controlled trial evaluating bazedoxifene/conjugated estrogens. Menopause. 2013;20(2):138–145.
19. Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7.5 mg for menopausal vasomotor symptoms: Two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
20. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate-to-severe vasomotor symptoms. Obstet Gynecol. 2014;123(suppl 1):132S–133S.
21. Orleans RJ, Li L, Kim MJ, et al. FDA approval of paroxetine for menopausal hot flashes. N Engl J Med. 2014;370(19):1777–1779.
22. Paroxetine (Brisdelle) for hot flashes. Med Lett Drugs Ther. 2013;55(1428):85–86.
23. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in health menopausal women. A randomized controlled trial. JAMA. 2011;305(3):267–274.
24. Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: A randomized controlled trial. Menopause. 2012;19(8):848–855.
25. Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: A systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29(1):204–213.
Enigmatic inconsistencies between WHI data and conclusions; Exaggerated or intentionally fabricated data?
Enigmatic inconsistencies between WHI data and conclusions
The issue of the inconsistencies between the Women’s Health Initiative (WHI) data and conclusions is enigmatic because the integrity and judgment of researchers has in the past and should remain above reproach.
For those of us in the private sector of obstetrics and gynecology, the feeling that there must be information that intentionally or unintentionally has been omitted from our view remains the most comfortable and convenient explanation for the discrepancies.
The vindication of observational studies predating WHI by the reanalysis of WHI data seems to be continually suppressed in the literature, and the unreasoning exclusion of the critical issue of timing in the initiation and continued administration of estrogen therapy (ET) and hormone therapy (HT) is inexplicable. One is repeatedly tempted to consider some underlying agenda.
Elimination of sampling by Wyeth, and now exclusion from drug formularies with the attendant exorbitant increases in cost have, in addition to the absence of a defense by researchers or manufacturers, discouraged continuing use of this valuable medication, even among those in whom the safety and benefits of Premarin and Prempro have been established over years of experience and scores of studies.
It is much like the children’s story, “The Emperor’s New Clothes.” It seems incredible that so many knowledgeable authorities seem unable to recognize a 60% reduction in coronary artery plaque in recently menopausal women (ages 50–59 years) after 5.2 years of HT, which underscores the importance of patient selection and timing of administration.1 Wyeth’s explanation, when I inquired, was that this involved “off-label” use, although the data are from reanalysis of WHI data. I would think that a 60% reduction of arterial plaque deserves front-page coverage.
When articles about the discontinuation of WHI began to appear in 2001, stated reasons included the overwhelming predominance of new breast cancer cases in estrogen-administered subjects, but no one seems to appreciate the 47% decrease in breast cancer mortality discovered in the reanalysis, due to the chronologically earlier appearance of disease at earlier clinical stages. In my practice, we are finding in situ disease in HT and ET patients after 4 to 5 years of use.
Consequently, I am appreciative of Dr. Thacker’s mention of the Sarrel data2 and her expansion into the “so often” overlooked issues. I think that it’s overdue—integrity must be restored to the interpretation of NIH’s $780,000,000 expenditure of taxpayer dollars. After all, WHI was to be the statistically unimpeachable clarification of estrogen and hormone replacement.
Glenn N. Hayashi, MD
Honolulu, Hawaii
Exaggerated or intentionally fabricated data?
Thank you for publishing Dr. Holly Thacker’s commentary regarding the travesty that was and is WHI. I enthusiastically support her admonition to “look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.”
My only disagreement is Dr. Thacker’s choice of the word “exaggerated” when describing the harms linked to HT. I would have chosen instead the words “intentionally fabricated.” How? By taking data out of context, by releasing data selectively, by withholding data—all for the purpose of achieving and then protecting their frighteningly negative and destructive initial conclusions.
I wish it were the case that an independent commission might right these wrongs. Unfortunately, that cannot happen in today’s intellectual context. The fundamental error that made WHI’s multitude of errors possible was the notion that we can dispense with the difficult work of considering “the totality of the data” by placing our faith in “statistical significance” derived from a single “randomized controlled trial.” That fundamental error is too deeply entrenched, too highly remunerative, and too propitiously useful to those seeking a world concordant with their fantasies.
Reality demands that we account for every fact and will in time put an end to this deadly conceit.
Geoffrey C. Kincaid, MD
Knoxville, Tennessee
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
1. Manson JE, Allison MA, Rossouw JE, et al; WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602.
2. Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
Enigmatic inconsistencies between WHI data and conclusions
The issue of the inconsistencies between the Women’s Health Initiative (WHI) data and conclusions is enigmatic because the integrity and judgment of researchers has in the past and should remain above reproach.
For those of us in the private sector of obstetrics and gynecology, the feeling that there must be information that intentionally or unintentionally has been omitted from our view remains the most comfortable and convenient explanation for the discrepancies.
The vindication of observational studies predating WHI by the reanalysis of WHI data seems to be continually suppressed in the literature, and the unreasoning exclusion of the critical issue of timing in the initiation and continued administration of estrogen therapy (ET) and hormone therapy (HT) is inexplicable. One is repeatedly tempted to consider some underlying agenda.
Elimination of sampling by Wyeth, and now exclusion from drug formularies with the attendant exorbitant increases in cost have, in addition to the absence of a defense by researchers or manufacturers, discouraged continuing use of this valuable medication, even among those in whom the safety and benefits of Premarin and Prempro have been established over years of experience and scores of studies.
It is much like the children’s story, “The Emperor’s New Clothes.” It seems incredible that so many knowledgeable authorities seem unable to recognize a 60% reduction in coronary artery plaque in recently menopausal women (ages 50–59 years) after 5.2 years of HT, which underscores the importance of patient selection and timing of administration.1 Wyeth’s explanation, when I inquired, was that this involved “off-label” use, although the data are from reanalysis of WHI data. I would think that a 60% reduction of arterial plaque deserves front-page coverage.
When articles about the discontinuation of WHI began to appear in 2001, stated reasons included the overwhelming predominance of new breast cancer cases in estrogen-administered subjects, but no one seems to appreciate the 47% decrease in breast cancer mortality discovered in the reanalysis, due to the chronologically earlier appearance of disease at earlier clinical stages. In my practice, we are finding in situ disease in HT and ET patients after 4 to 5 years of use.
Consequently, I am appreciative of Dr. Thacker’s mention of the Sarrel data2 and her expansion into the “so often” overlooked issues. I think that it’s overdue—integrity must be restored to the interpretation of NIH’s $780,000,000 expenditure of taxpayer dollars. After all, WHI was to be the statistically unimpeachable clarification of estrogen and hormone replacement.
Glenn N. Hayashi, MD
Honolulu, Hawaii
Exaggerated or intentionally fabricated data?
Thank you for publishing Dr. Holly Thacker’s commentary regarding the travesty that was and is WHI. I enthusiastically support her admonition to “look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.”
My only disagreement is Dr. Thacker’s choice of the word “exaggerated” when describing the harms linked to HT. I would have chosen instead the words “intentionally fabricated.” How? By taking data out of context, by releasing data selectively, by withholding data—all for the purpose of achieving and then protecting their frighteningly negative and destructive initial conclusions.
I wish it were the case that an independent commission might right these wrongs. Unfortunately, that cannot happen in today’s intellectual context. The fundamental error that made WHI’s multitude of errors possible was the notion that we can dispense with the difficult work of considering “the totality of the data” by placing our faith in “statistical significance” derived from a single “randomized controlled trial.” That fundamental error is too deeply entrenched, too highly remunerative, and too propitiously useful to those seeking a world concordant with their fantasies.
Reality demands that we account for every fact and will in time put an end to this deadly conceit.
Geoffrey C. Kincaid, MD
Knoxville, Tennessee
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
Enigmatic inconsistencies between WHI data and conclusions
The issue of the inconsistencies between the Women’s Health Initiative (WHI) data and conclusions is enigmatic because the integrity and judgment of researchers has in the past and should remain above reproach.
For those of us in the private sector of obstetrics and gynecology, the feeling that there must be information that intentionally or unintentionally has been omitted from our view remains the most comfortable and convenient explanation for the discrepancies.
The vindication of observational studies predating WHI by the reanalysis of WHI data seems to be continually suppressed in the literature, and the unreasoning exclusion of the critical issue of timing in the initiation and continued administration of estrogen therapy (ET) and hormone therapy (HT) is inexplicable. One is repeatedly tempted to consider some underlying agenda.
Elimination of sampling by Wyeth, and now exclusion from drug formularies with the attendant exorbitant increases in cost have, in addition to the absence of a defense by researchers or manufacturers, discouraged continuing use of this valuable medication, even among those in whom the safety and benefits of Premarin and Prempro have been established over years of experience and scores of studies.
It is much like the children’s story, “The Emperor’s New Clothes.” It seems incredible that so many knowledgeable authorities seem unable to recognize a 60% reduction in coronary artery plaque in recently menopausal women (ages 50–59 years) after 5.2 years of HT, which underscores the importance of patient selection and timing of administration.1 Wyeth’s explanation, when I inquired, was that this involved “off-label” use, although the data are from reanalysis of WHI data. I would think that a 60% reduction of arterial plaque deserves front-page coverage.
When articles about the discontinuation of WHI began to appear in 2001, stated reasons included the overwhelming predominance of new breast cancer cases in estrogen-administered subjects, but no one seems to appreciate the 47% decrease in breast cancer mortality discovered in the reanalysis, due to the chronologically earlier appearance of disease at earlier clinical stages. In my practice, we are finding in situ disease in HT and ET patients after 4 to 5 years of use.
Consequently, I am appreciative of Dr. Thacker’s mention of the Sarrel data2 and her expansion into the “so often” overlooked issues. I think that it’s overdue—integrity must be restored to the interpretation of NIH’s $780,000,000 expenditure of taxpayer dollars. After all, WHI was to be the statistically unimpeachable clarification of estrogen and hormone replacement.
Glenn N. Hayashi, MD
Honolulu, Hawaii
Exaggerated or intentionally fabricated data?
Thank you for publishing Dr. Holly Thacker’s commentary regarding the travesty that was and is WHI. I enthusiastically support her admonition to “look at the totality of the data on menopausal HT, evaluate our patients individually, treat those who are truly hormonally deficient and suffering, and counsel them that many of the harms linked to HT have been exaggerated.”
My only disagreement is Dr. Thacker’s choice of the word “exaggerated” when describing the harms linked to HT. I would have chosen instead the words “intentionally fabricated.” How? By taking data out of context, by releasing data selectively, by withholding data—all for the purpose of achieving and then protecting their frighteningly negative and destructive initial conclusions.
I wish it were the case that an independent commission might right these wrongs. Unfortunately, that cannot happen in today’s intellectual context. The fundamental error that made WHI’s multitude of errors possible was the notion that we can dispense with the difficult work of considering “the totality of the data” by placing our faith in “statistical significance” derived from a single “randomized controlled trial.” That fundamental error is too deeply entrenched, too highly remunerative, and too propitiously useful to those seeking a world concordant with their fantasies.
Reality demands that we account for every fact and will in time put an end to this deadly conceit.
Geoffrey C. Kincaid, MD
Knoxville, Tennessee
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
1. Manson JE, Allison MA, Rossouw JE, et al; WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602.
2. Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
1. Manson JE, Allison MA, Rossouw JE, et al; WHI and WHI-CACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–2602.
2. Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59. Am J Pub Health. 2013;103(9):1583–1588.
Paroxetine mesylate 7.5 mg found to be a safe alternative to hormone therapy for menopausal women with hot flashes
The US Food and Drug Administration (FDA) recently approved paroxetine mesylate 7.5 mg (Brisdelle) for the treatment of moderate to severe menopausal vasomotor symptoms (VMS). Paroxetine, formerly known as low-dose mesylate salt of paroxetine (LDMP), is a nonhormonal agent, which makes it an alternative hot flash therapy for menopausal women who cannot or do not want to use hormones. Paroxetine mesylate (Pexeva, Brisdelle) and paroxetine hydrochloride (Paxil, and generics) are two salts of the same active compound (paroxetine). They may have somewhat different metabolism.
The efficacy and safety of paroxetine mesylate, a selective serotonin-reuptake inhibitor (SSRI), were evaluated individually in three Phase 2 or 3 multicenter, double-blind, randomized, placebo-controlled trials, published by James Simon, MD, from George Washington University School of Medicine, and colleagues,1 and Joffe and colleagues.2 Most treatment-emergent adverse events (TEAEs) in the individual studies were mild or moderate in severity, with minimal acute discontinuation symptoms reported on treatment cessation.
In a study3 presented April 29, at the 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG) in Chicago, Illinois, Simon and colleagues further reported on the overall tolerability and safety profile of paroxetine mesylate 7.5 mg using pooled data from the three randomized trials. In their post-hoc analyses, they specifically examined the emergence of adverse events linked to the use of SSRIs when prescribed for psychiatric disorders at therapeutically higher doses than 7.5 mg. The adverse events focused on included weight gain, decreased libido, and sleep disturbance, as well as suicidality, abnormal bleeding, and bone fracture.
Study details. A total of 1,276 postmenopausal women (approximately 70% white) aged 40 years or older (median age, 54 years) with moderate to severe VMS (7−8 hot flashes/day; 50−60 hot flashes/wk) received either paroxetine mesylate or placebo at bedtime for 8 (Phase 2), 12 (Phase 3), or 24 (Phase 3) weeks. The study was sponsored by Noven Therapeutics, LLC.
Treatment-emergent adverse events and discontinuation
About half (50.4%) of the 635 women in the paroxetine group and 47.0% of the 641 women in the placebo group reported at least one TEAE. Most commonly reported TEAEs in the paroxetine group (reported in ≥2% of patients and with a twofold or higher frequency than in the placebo group) were nausea, fatigue, and dizziness.
TEAEs that were determined to be related to the study drug were reported in 19.5% in the paroxetine group and in 17.6% in the placebo group. These most frequent TEAEs were fatigue, nausea, dizziness, and diarrhea.
Severe AEs were reported in 3.9% and 3.6% of women in the paroxetine and placebo groups, respectively, although the investigator determined that less than 1% were related to paroxetine treatment.
TEAEs that led to discontinuation occurred in 4.7% of paroxetine-treated women and in 3.7% of placebo-treated women, although the incidence of study drug interruptions from TEAEs was similar (0.9%) between treatments. The most frequent adverse reactions leading to discontinuation in the paroxetine arm were abdominal pain (0.3%), attention disturbances (0.3%), headache (0.3%), and suicidal ideation (0.3%).
Of the most common AEs, nausea occurred mainly within the first 4 weeks of treatment; fatigue occurred primarily within the first week of treatment and decreased in frequency with continued therapy. Incidences and types of AEs that began after 12 weeks were similar to those that began during the first 12 weeks of treatment.
AEs related to SSRIs not found to be problematic
No differences were found between groups with regard to TEAEs related to weight, libido, or sleep. No clinically meaningful changes in laboratory values, vital signs, or ECGs were observed with either group. No clinically important findings on abnormal bleeding, bone fracture, or suicidality were evident in the paroxetine arm.
In the Phase 3 studies:
- One suicide attempt was reported in the paroxetine group in the 24-week study, but was determined by investigators to be unrelated to treatment
- Incidence rates of gastrointestinal and other bleeding events were similar between groups
- Five bone fractures were reported: One in the paroxetine group and four among three participants in the placebo group.
One death occurred in the 12-week Phase 3 study due to acute respiratory failure with evidence of hypertension-mediated pulmonary edema and hypertensive cardiovascular disease. The investigator did not consider the death to be related to the study drug.
Study conclusion
The authors concluded that paroxetine 7.5 mg had favorable tolerability in menopausal women with moderate to severe VMS.
“Paroxetine 7.5 mg offers a nonhormonal treatment option for women who seek treatment for moderate to severe hot flashes associated with menopause,” said Dr. Simon.3
Tell us what you think! Send your Letter to the Editor: [email protected]
1. Simon JA, et al. Low dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
2. Joffe H et al. Low-dose mesylate salt of paroxetine (LDMP) in treatment of vasomotor symptoms (VMS) in menopause. Presented at: 2012 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists; May 7, 2012; San Diego, CA. Poster 43.
3. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate to severe vasomotor symptoms. Poster presented at: 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG); April 26–30, 2014; Chicago, IL.
The US Food and Drug Administration (FDA) recently approved paroxetine mesylate 7.5 mg (Brisdelle) for the treatment of moderate to severe menopausal vasomotor symptoms (VMS). Paroxetine, formerly known as low-dose mesylate salt of paroxetine (LDMP), is a nonhormonal agent, which makes it an alternative hot flash therapy for menopausal women who cannot or do not want to use hormones. Paroxetine mesylate (Pexeva, Brisdelle) and paroxetine hydrochloride (Paxil, and generics) are two salts of the same active compound (paroxetine). They may have somewhat different metabolism.
The efficacy and safety of paroxetine mesylate, a selective serotonin-reuptake inhibitor (SSRI), were evaluated individually in three Phase 2 or 3 multicenter, double-blind, randomized, placebo-controlled trials, published by James Simon, MD, from George Washington University School of Medicine, and colleagues,1 and Joffe and colleagues.2 Most treatment-emergent adverse events (TEAEs) in the individual studies were mild or moderate in severity, with minimal acute discontinuation symptoms reported on treatment cessation.
In a study3 presented April 29, at the 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG) in Chicago, Illinois, Simon and colleagues further reported on the overall tolerability and safety profile of paroxetine mesylate 7.5 mg using pooled data from the three randomized trials. In their post-hoc analyses, they specifically examined the emergence of adverse events linked to the use of SSRIs when prescribed for psychiatric disorders at therapeutically higher doses than 7.5 mg. The adverse events focused on included weight gain, decreased libido, and sleep disturbance, as well as suicidality, abnormal bleeding, and bone fracture.
Study details. A total of 1,276 postmenopausal women (approximately 70% white) aged 40 years or older (median age, 54 years) with moderate to severe VMS (7−8 hot flashes/day; 50−60 hot flashes/wk) received either paroxetine mesylate or placebo at bedtime for 8 (Phase 2), 12 (Phase 3), or 24 (Phase 3) weeks. The study was sponsored by Noven Therapeutics, LLC.
Treatment-emergent adverse events and discontinuation
About half (50.4%) of the 635 women in the paroxetine group and 47.0% of the 641 women in the placebo group reported at least one TEAE. Most commonly reported TEAEs in the paroxetine group (reported in ≥2% of patients and with a twofold or higher frequency than in the placebo group) were nausea, fatigue, and dizziness.
TEAEs that were determined to be related to the study drug were reported in 19.5% in the paroxetine group and in 17.6% in the placebo group. These most frequent TEAEs were fatigue, nausea, dizziness, and diarrhea.
Severe AEs were reported in 3.9% and 3.6% of women in the paroxetine and placebo groups, respectively, although the investigator determined that less than 1% were related to paroxetine treatment.
TEAEs that led to discontinuation occurred in 4.7% of paroxetine-treated women and in 3.7% of placebo-treated women, although the incidence of study drug interruptions from TEAEs was similar (0.9%) between treatments. The most frequent adverse reactions leading to discontinuation in the paroxetine arm were abdominal pain (0.3%), attention disturbances (0.3%), headache (0.3%), and suicidal ideation (0.3%).
Of the most common AEs, nausea occurred mainly within the first 4 weeks of treatment; fatigue occurred primarily within the first week of treatment and decreased in frequency with continued therapy. Incidences and types of AEs that began after 12 weeks were similar to those that began during the first 12 weeks of treatment.
AEs related to SSRIs not found to be problematic
No differences were found between groups with regard to TEAEs related to weight, libido, or sleep. No clinically meaningful changes in laboratory values, vital signs, or ECGs were observed with either group. No clinically important findings on abnormal bleeding, bone fracture, or suicidality were evident in the paroxetine arm.
In the Phase 3 studies:
- One suicide attempt was reported in the paroxetine group in the 24-week study, but was determined by investigators to be unrelated to treatment
- Incidence rates of gastrointestinal and other bleeding events were similar between groups
- Five bone fractures were reported: One in the paroxetine group and four among three participants in the placebo group.
One death occurred in the 12-week Phase 3 study due to acute respiratory failure with evidence of hypertension-mediated pulmonary edema and hypertensive cardiovascular disease. The investigator did not consider the death to be related to the study drug.
Study conclusion
The authors concluded that paroxetine 7.5 mg had favorable tolerability in menopausal women with moderate to severe VMS.
“Paroxetine 7.5 mg offers a nonhormonal treatment option for women who seek treatment for moderate to severe hot flashes associated with menopause,” said Dr. Simon.3
Tell us what you think! Send your Letter to the Editor: [email protected]
The US Food and Drug Administration (FDA) recently approved paroxetine mesylate 7.5 mg (Brisdelle) for the treatment of moderate to severe menopausal vasomotor symptoms (VMS). Paroxetine, formerly known as low-dose mesylate salt of paroxetine (LDMP), is a nonhormonal agent, which makes it an alternative hot flash therapy for menopausal women who cannot or do not want to use hormones. Paroxetine mesylate (Pexeva, Brisdelle) and paroxetine hydrochloride (Paxil, and generics) are two salts of the same active compound (paroxetine). They may have somewhat different metabolism.
The efficacy and safety of paroxetine mesylate, a selective serotonin-reuptake inhibitor (SSRI), were evaluated individually in three Phase 2 or 3 multicenter, double-blind, randomized, placebo-controlled trials, published by James Simon, MD, from George Washington University School of Medicine, and colleagues,1 and Joffe and colleagues.2 Most treatment-emergent adverse events (TEAEs) in the individual studies were mild or moderate in severity, with minimal acute discontinuation symptoms reported on treatment cessation.
In a study3 presented April 29, at the 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG) in Chicago, Illinois, Simon and colleagues further reported on the overall tolerability and safety profile of paroxetine mesylate 7.5 mg using pooled data from the three randomized trials. In their post-hoc analyses, they specifically examined the emergence of adverse events linked to the use of SSRIs when prescribed for psychiatric disorders at therapeutically higher doses than 7.5 mg. The adverse events focused on included weight gain, decreased libido, and sleep disturbance, as well as suicidality, abnormal bleeding, and bone fracture.
Study details. A total of 1,276 postmenopausal women (approximately 70% white) aged 40 years or older (median age, 54 years) with moderate to severe VMS (7−8 hot flashes/day; 50−60 hot flashes/wk) received either paroxetine mesylate or placebo at bedtime for 8 (Phase 2), 12 (Phase 3), or 24 (Phase 3) weeks. The study was sponsored by Noven Therapeutics, LLC.
Treatment-emergent adverse events and discontinuation
About half (50.4%) of the 635 women in the paroxetine group and 47.0% of the 641 women in the placebo group reported at least one TEAE. Most commonly reported TEAEs in the paroxetine group (reported in ≥2% of patients and with a twofold or higher frequency than in the placebo group) were nausea, fatigue, and dizziness.
TEAEs that were determined to be related to the study drug were reported in 19.5% in the paroxetine group and in 17.6% in the placebo group. These most frequent TEAEs were fatigue, nausea, dizziness, and diarrhea.
Severe AEs were reported in 3.9% and 3.6% of women in the paroxetine and placebo groups, respectively, although the investigator determined that less than 1% were related to paroxetine treatment.
TEAEs that led to discontinuation occurred in 4.7% of paroxetine-treated women and in 3.7% of placebo-treated women, although the incidence of study drug interruptions from TEAEs was similar (0.9%) between treatments. The most frequent adverse reactions leading to discontinuation in the paroxetine arm were abdominal pain (0.3%), attention disturbances (0.3%), headache (0.3%), and suicidal ideation (0.3%).
Of the most common AEs, nausea occurred mainly within the first 4 weeks of treatment; fatigue occurred primarily within the first week of treatment and decreased in frequency with continued therapy. Incidences and types of AEs that began after 12 weeks were similar to those that began during the first 12 weeks of treatment.
AEs related to SSRIs not found to be problematic
No differences were found between groups with regard to TEAEs related to weight, libido, or sleep. No clinically meaningful changes in laboratory values, vital signs, or ECGs were observed with either group. No clinically important findings on abnormal bleeding, bone fracture, or suicidality were evident in the paroxetine arm.
In the Phase 3 studies:
- One suicide attempt was reported in the paroxetine group in the 24-week study, but was determined by investigators to be unrelated to treatment
- Incidence rates of gastrointestinal and other bleeding events were similar between groups
- Five bone fractures were reported: One in the paroxetine group and four among three participants in the placebo group.
One death occurred in the 12-week Phase 3 study due to acute respiratory failure with evidence of hypertension-mediated pulmonary edema and hypertensive cardiovascular disease. The investigator did not consider the death to be related to the study drug.
Study conclusion
The authors concluded that paroxetine 7.5 mg had favorable tolerability in menopausal women with moderate to severe VMS.
“Paroxetine 7.5 mg offers a nonhormonal treatment option for women who seek treatment for moderate to severe hot flashes associated with menopause,” said Dr. Simon.3
Tell us what you think! Send your Letter to the Editor: [email protected]
1. Simon JA, et al. Low dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
2. Joffe H et al. Low-dose mesylate salt of paroxetine (LDMP) in treatment of vasomotor symptoms (VMS) in menopause. Presented at: 2012 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists; May 7, 2012; San Diego, CA. Poster 43.
3. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate to severe vasomotor symptoms. Poster presented at: 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG); April 26–30, 2014; Chicago, IL.
1. Simon JA, et al. Low dose paroxetine 7.5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20(10):1027–1035.
2. Joffe H et al. Low-dose mesylate salt of paroxetine (LDMP) in treatment of vasomotor symptoms (VMS) in menopause. Presented at: 2012 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists; May 7, 2012; San Diego, CA. Poster 43.
3. Simon JA, Portman DJ, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Safety profile of paroxetine 7.5 mg in women with moderate to severe vasomotor symptoms. Poster presented at: 2014 Annual Clinical Meeting of The American College of Obstetricians and Gynecologists (ACOG); April 26–30, 2014; Chicago, IL.
Chronic vulvar irritation, itching, and pain. What is the diagnosis?
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Chronic irritation, itching, and pain are only rarely due to infection. These symptoms are more likely to be caused by dermatoses, vaginal abnormalities, and pain syndromes that may be difficult to diagnose. Careful evaluation should include a wet mount and culture to eliminate infection as a cause so that the correct diagnosis can be ascertained and treated.
In Part 2 of this two-part series, we focus on five cases of vulvar dermatologic disruptions:
- atrophic vagina
- irritant and allergic contact dermatitis
- complex vulvar aphthosis
- desquamative inflammatory vaginitis
- inverse psoriasis.
CASE 1. INTROITAL BURNING AND A FEAR OF BREAST CANCER
A 56-year-old woman visits your office for management of recent-onset introital burning during sexual activity. She reports that her commercial lubricant causes irritation. Topical and oral antifungal therapies have not been beneficial. She has a strong family history of breast cancer.
On examination, she exhibits small, smooth labia minora and experiences pain when a cotton swab is pressed against the vestibule. The vagina is also smooth, with scant secretions. Microscopically, these secretions are almost acellular, with no increase in white blood cells and no clue cells, yeast forms, or lactobacilli. The pH is greater than 6.5, and most epithelial cells are parabasal (FIGURE 1).
You prescribe topical estradiol cream for vaginal use three nights per week, but when the patient returns 1 month later, her condition is unchanged. She explains that she never used the cream after reading the package insert, which reports a risk of breast cancer.
Diagnosis: Atrophic vagina (not atrophic vaginitis, as there is no increase in white blood cells).
Treatment: Re-estrogenization should relieve her symptoms.
Several options for local estrogen replacement are available. Creams include estradiol (Estrace) and conjugated equine estrogen (Premarin), the latter of which is arguably slightly more irritating. These are prescribed at a starting dose of 1 g in the vagina three nights per week. After several weeks, they can be titrated to the lowest frequency that controls symptoms.
The risk of vaginal candidiasis is fairly high during the first 2 or 3 weeks of re-estrogenization, so patients should be warned of this possibility. Also consider prophylactic weekly fluconazole or an azole suppository two or three times a week for the first few weeks. Estradiol tablets (Vagifem) inserted in the vagina are effective, less messy, and more expensive, as is the estradiol ring (Estring), which is inserted and changed quarterly.
It is not unusual for a woman to avoid use of topical estrogen out of fear, or to use insufficient amounts only on the vulva, or to use it for only 1 or 2 weeks.1
Women should be scheduled for a return visit to ensure they have been using the estrogen, their wet mount has normalized, and discomfort has cleared.
Related article: Your menopausal patient's breast biopsy reveals atypical hyperplasia. JoAnn V. Pinkerton, MD (Cases in Menopause; May 2013)
When a woman is reluctant to use local estrogen
We counsel women that small doses of vaginal estrogen used for limited periods of time are unlikely to influence their breast cancer risk and are the most effective treatment for symptoms of atrophy. Usually, this explanation is sufficient to reassure a woman that topical estrogen is safe. Otherwise, use of commercial personal lubricants (silicone-based lubricants are well tolerated) and moisturizers such as Replens and RePhresh can be comforting.
The topical anesthetics lidocaine 2% jelly or lidocaine 5% ointment (which sometimes burns) can minimize pain with sexual activity for those requiring more than lubrication.
Ospemifene (Osphena) is used by some clinicians in this situation, but this medication is labeled as a risk for all of the same contraindications as systemic estrogen, and it is much more expensive than topical estrogen. Ospemifene is an estrogen agonist/antagonist. Although it is the only oral medication indicated for the treatment of menopause-related dyspareunia, the long-term effects on breast cancer risk are unknown. Also, it has an agonist effect on the endometrium and, again, the long-term risk is unknown.
Related article: New treatment option for vulvar and vaginal atrophy. Andrew M. Kaunitz, MD (News for your Practice; May 2013)
Fluconazole use is contraindicated with ospemifene, as is the use of any estrogen products.
CASE 2. RECALCITRANT ITCHING, BURNING, AND REDNESS
A 25-year-old woman reports anogenital itching, burning, and redness, which have been present for 3 months. She says she developed a yeast infection after antibiotic therapy for a dental infection; the yeast infection was treated with terconazole. She reports an allergic reaction to the terconazole, with immediate severe burning, redness, and swelling. The clobetasol cream she was given to use twice daily also caused burning, so she discontinued it. Her symptoms improved when she tried cool soaks and applied topical benzocaine gel as a local anesthetic. However,
2 weeks later, she experienced increasing redness, itching, and burning. Although the benzocaine relieved these symptoms, it required almost continual reapplication for comfort.
A physical examination of the vulva reveals generalized, poorly demarcated redness, edema, and superficial erosions (FIGURE 2).
Diagnosis: Irritant contact dermatitis (as opposed to allergic contact dermatitis) associated with the use of terconazole and clobetasol. This was followed by allergic contact dermatitis in association with benzocaine.
Treatment: Withdrawal of benzocaine, with reinitiation of cool soaks and a switch to clobetasol ointment rather than cream. Nighttime sedation allows the patient to sleep through the itching and gradually allows her skin to heal.
Contact dermatitis is a fairly common cause of vulvar irritation, with two main types:
- Irritant contact dermatitis—The most common form, it occurs in any individual exposed to an irritating substance in sufficient quantity or frequency. Irritant contact dermatitis is characterized mostly by sensations of rawness or burning and generally is caused by urine, feces, perspiration, friction, alcohols in topical creams, overwashing, and use of harsh soaps.
- Allergic contact dermatitis—This form is characterized by itching, although secondary pain and burning from scratching and blistering can occur as well. Common allergens in the genital area include benzocaine, diphenhydramine (Benadryl), neomycin in triple antibiotic ointment (Neosporin), and latex. Allergic contact dermatitis occurs after 1 or 2 weeks of initial exposure or 1 or 2 days after re-exposure.
The diagnosis of an irritant or allergic contact dermatitis can be based on a history of incontinence, application of high-risk substances, or inappropriate washing. Management generally involves discontinuation of all panty liners and topical agents except for water, with a topical steroid ointment used twice a day and pure petroleum jelly used as often as necessary for comfort. Nighttime sedation to allow a reprieve from rubbing and scratching may be helpful, and narcotic pain medications may be useful for the first 1 to 2 weeks of treatment.
Women who fail to respond to treatment should be referred for patch testing by a
dermatologist.
Related article: Vulvar pain syndromes: Making the correct diagnosis. Neal M. Lonky, MD, MPH; Libby Edwards, MD; Jennifer Gunter, MD; Hope K. Haefner, MD (Roundtable, part 1 of 3; September 2011)
CASE 3. TEENAGER WITH VULVAR PAIN AND SORES
A woman brings her 13-year-old daughter to your office for treatment of sudden-onset vulvar pain and sores. The child developed a sore throat and low-grade fever 3 days earlier, with vulvar pain and vulvar dysuria the next day. The pediatrician diagnosed a herpes simplex virus infection and prescribed oral acyclovir, but the girl’s condition has not improved, and the mother believes her daughter’s claims of sexual abstinence.
The girl is otherwise healthy, aside from a history of trivial oral canker sores without arthritis, headaches, abdominal pain, eye pain, or vision changes.
Physical examination of the vulva reveals soft, painful, well-demarcated ulcers with a white fibrin base (FIGURE 3).
Diagnosis: Complex aphthosis. Further testing is unnecessary.
Treatment: Prednisone 40 mg/day plus hydrocodone in usual doses of 5/325, one or two tablets every 4 to 6 hours, as needed; topical petroleum jelly (especially before urination); and sitz baths. When the patient returns 1 week later, she is much improved.
Aphthae are believed to be of hyperimmune origin, often precipitated by a viral syndrome. They are most common in girls aged 9 to 18 years. Vulvar aphthae are triggered by various viral infections, including Epstein-Barr.2 The offending virus is not located in the ulcer proper, however, but is identified serologically.
Aphthae are uncommon and under-recognized on the vulva, and genital aphthae are usually much larger than oral aphthae. Most patients initially are mistakenly evaluated and treated for sexually transmitted disease, but the large, well-demarcated, painful, nonindurated deep nature of the ulcer is pathognomonic for an aphthous ulcer.
The presence of oral and genital aphthae does not constitute a diagnosis of Behçet disease, an often-devastating systemic inflammatory condition occurring almost exclusively in men in the Middle and Far East. The diagnosis of Behçet disease requires the identification of objective inflammatory disease of the eyes, joints, gastrointestinal tract, or neurologic system. True Behçet disease is incredibly uncommon in the United States. When it is diagnosed in Western countries, it takes an attenuated form, most often occurring in women who experience multisystem discomfort rather than identifiable inflammatory disease. End-organ damage is uncommon. Evaluation for Behçet disease in women with vulvar aphthae generally is not indicated, although a directed review of systems is reasonable. The rare patient who experiences frequent recurrence and symptoms of systemic disease should be referred to an ophthalmologist and other relevant specialists to evaluate for inflammatory disease.
The treatment of vulvar aphthae consists of systemic corticosteroids such as prednisone 40 mg/day for smaller individuals and 60 mg/day for larger women, with follow-up to ensure a good response. Often, the prednisone can be discontinued when pain relents rather than continued through complete healing. Reassurance, without discussing Behçet disease, is paramount, as is pain control. The heavy application of petroleum jelly can decrease pain and prevent urine from touching the ulcer.
Some patients experience recurrent ulcers. A second prescription of prednisone can be provided for immediate reinstitution with onset of symptoms. However, frequent recurrences may require ongoing suppressive medication, with dapsone being the usual first choice. Colchicine often is used, and thalidomide and tumor necrosis factor-a blockers (adalimumab, etanercept, and infliximab) also are extremely beneficial.3,4
CASE 4. INCREASED NEUTROPHILS AND NO LACTOBACILLI
A 36-year-old woman visits your office reporting introital itching, vulvar dysuria, and superficial dyspareunia that have lasted 6 months. She has tried over-the-counter antifungal therapy, with only slight improvement while using the cream. Her health is normal otherwise, lacking pain syndromes or abnormalities suggestive of pelvic floor dysfunction. She experienced comfortable sexual activity until 6 months ago.
The only abnormalities apparent on physical examination are redness of the vestibule, medial labia minora, and vaginal walls, with edema of the surrounding skin and no oral lesions (FIGURE 4A). Copious vaginal secretions are visible at the introitus. A wet mount shows a marked increase in neutrophils with scattered parabasal cells (FIGURE 4B). There are no clue cells, lactobacilli, or yeast forms. The patient’s pH level is greater than 6.5. Routine and fungal cultures and molecular studies for chlamydia, trichomonas, and gonorrhea are returned as normal.
Diagnosis: Desquamative inflammatory vaginitis.
Treatment: Clindamycin vaginal cream, 1/2 to 1 full applicator nightly, with a weekly oral fluconazole tablet (200 mg is more easily covered by insurance) to prevent secondary candidiasis. You schedule a follow-up visit in 1 month.
Desquamative inflammatory vaginitis (DIV) is described as noninfectious inflammatory vaginitis in a setting of normal estrogen and absence of skin disease of the mucous membranes of the vagina. The condition is characterized by an increase in white blood cells and parabasal cells, and absent lactobacilli, with relatively high vaginal pH. DIV is thought to represent an inflammatory dermatosis of the vaginal epithelium.5 Although some clinicians believe that DIV is actually lichen planus, the latter exhibits erosions as well as redness, nearly always affects the mouth and the vulva, and produces remarkable scarring. DIV does not erode, affect any other skin surfaces, or scar.
Other rare skin diseases that produce erosions and scarring also can be ruled out by the presence of erosions, absence of oral disease, and absence of other mucosal involvement. These diseases include cicatricial pemphigoid, pemphigus vulgaris, Stevens-Johnson syndrome, and toxic epidermal necrolysis. Infectious diseases characterized by inflammation are excluded by culture or molecular studies, and atrophic vaginitis and retained foreign bodies (especially retained tampons) can produce a similar picture.
The vulvar itching and irritation that occur with DIV most likely represent an irritant contact dermatitis, with vaginal secretions serving as the irritant.
How to treat DIV
The management of DIV consists of either topical clindamycin cream (theoretically for its anti-inflammatory rather than antimicrobial properties) or intravaginal corticosteroids, especially hydrocortisone acetate.6 Hydrocortisone can be tried at the low commercially available dose of 25-mg rectal suppositories, which should be inserted into the vagina nightly, or it can be compounded at 100 or 200 mg, if needed. If the condition is recalcitrant, combination therapy can be used.
When signs and symptoms abate, the frequency of use can be decreased, or hydrocortisone can be discontinued and restarted again with any recurrence of discomfort. Many clinicians also prescribe weekly fluconazole to prevent intercurrent candidiasis.
Related article: Successful treatment of chronic vaginitis. Robert L. Barbieri, MD (Editorial, July 2013)
CASE 5. PLAQUES ON VULVA AND IN SKIN FOLDS
A 43-year-old woman reports a recalcitrant yeast infection of the vulva, with itching and irritation. She is overweight and diabetic, with mild stress incontinence.
Physical examination reveals a fairly well-demarcated plaque of redness of the vulva and labiocrural folds, with satellite red papules and peripheral peeling (FIGURE 5). An examination of other skin surfaces reveals similar plaques in the gluteal cleft, umbilicus, and axillae as well as under the breasts. A fungal preparation of the vagina and skin is negative. You obtain a fungal culture and prescribe topical and oral antifungal therapy and see the patient again 1 week later. Her condition is unchanged.
Diagnosis: You make a presumptive diagnosis of inverse psoriasis and do a confirmatory punch biopsy.
Treatment: Clobetasol ointment applied to the skin folds, along with continuation of the topical miconazole cream. A week later, the patient’s condition is remarkably improved, and her biopsy shows psoriasiform dermatitis. You reduce the potency of her corticosteroid, switching to desonide cream sparingly applied daily.
Psoriasis is a common skin disease of immunologic origin. The skin is classically red and thick, with heavy white scale produced by rapid turnover of epithelium. However, there are several morphologic types of psoriasis, and anogenital psoriasis is most often of the inverse pattern. Inverse psoriasis preferentially affects skin folds and is frequently mistaken for (and often initially superinfected with) candidiasis. Scale is thin and unapparent, and there often is a shiny, glazed appearance to the skin. Tiny satellite lesions often are visible as well. A skin biopsy of inverse psoriasis often is not diagnostic, showing only nonspecific psoriasiform dermatitis; this does not disprove psoriasis.
Psoriasis is a systemic condition and is associated with metabolic syndrome, carrying an increased risk of overweight, hypertension, diabetes, and cardiovascular disease. Management of these conditions is very important in the treatment of the patient overall.
Unlike lichen planus and lichen sclerosus, scarring is rare with psoriasis, and squamous cell carcinoma generally is unassociated.7,8
Anogenital psoriasis is treated with topical corticosteroids and, when needed, topical vitamin D preparations. Generally, inverse psoriasis is controlled with low-potency topical corticosteroids, with management of secondary infection and irritants. Otherwise, ultraviolet light is a time-honored therapy for psoriasis but not practical for skin folds. It also is difficult for many patients to manage with a busy life. Systemic therapy, including methotrexate and oral retinoids are often used, as are newer biologic agents such as etanercept, adalimumab, infliximab, and ustekinumab.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
- Kingsberg SA, Krychman ML. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis.
J Sex Med. 2013;10(6):1567–1574. - Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: A manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19(3):195–204.
- O’Neill ID. Efficacy of tumour necrosis factor-a antagonists in aphthous ulceration: Review of published individual patient data. J Eur Acad Dermatol Venereol. 2012;26(2):231–235.
- Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Ortego-Centeno N. Recalcitrant, recurrent aphthous stomatitis successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2009;23(2):206.
- Stockdale CK. Clinical spectrum of desquamative inflammatory vaginitis. Curr Infect Dis Rep. 2010;12(6):479–483.
- Sobel JD, Reichman O, Misra D, Yoo W. Prognosis and treatment of desquamative inflammatory vaginitis. Obstet Gynecol. 2011;117(4):850–855.
- Albert S, Neill S, Derrick EK, Calonje E. Psoriasis associated with vulvar scarring. Clin Exp Dermatol. 2004;29(4):354–356.
- Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden. J Invest Dermatol. 2001;117(6):1531–1537.
Read Part 1: Chronic vulvar symptoms and dermatologic disruptions: How to make the correct diagnosis (May 2014)