User login
What is the clinical and economic return on taxpayers’ $260M investment in the WHI?
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: [email protected]
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: [email protected]
The WHI estrogen plus progestin (EPT) clinical trial, a $260 million venture, is among the most expensive projects ever undertaken by the National Institutes of Health. Following 2002 publication of its initial findings, use of EPT and estrogen alone (ET) hormone therapy (HT) among US women plummeted. Investigators, including WHI leadership, estimated the clinical and economic impact of this trial from a payer perspective.
Details of the study
For the years 2003 to 2012, the authors used a disease-simulation model to evaluate the effect of the WHI EPT trial on women aged 50 to 79 with an intact uterus (women who were combined-HT [cHT], or EPT, eligible). They compared outcomes between a “WHI scenario,” in which the prevalence of cHT use was based on actual WHI findings, with a “no-WHI” scenario, in which pre-WHI trends in cHT use (from 1998 to 2002) were linearly extrapolated.
The simulation model predicted that 9.5 million women used cHT in the no-WHI scenario, 4.3 million more than actually used cHT in the WHI scenario. The authors estimated that, compared with the no-WHI scenario, 126,000, 76,000, and 80,000 fewer respective cases of breast cancer, cardiovascular disease (CVD), and venous thromboembolism occurred and that 263,000 and 15,000 more respective cases of fractures and colorectal cancer occurred among women as a result of the WHI.
Related article: When should a menopausal woman discontinue hormone therapy? Andrew M. Kaunitz, MD (Cases in Menopause; February 2014)
Regarding economic outcomes, the authors estimated that the WHI resulted in $35.2 billion in direct medical expenditure savings—principally from fewer prescriptions for EPT and associated office visits ($26.2 billion), but also from decreased breast cancer incidence ($4.5 billion) and decreased CVD incidence ($2.2 billion), among other savings, which offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Related article: In the latest report from the WHI, the data contradict the conclusions. Holly Thacker, MD (Commentary; March 2014)
In addition, the investigators reported a tremendous gain in quality of life years (145,000) in the WHI versus the no-WHI scenario, attributing the difference to the greater health-related quality-of-life effect associated with decreased breast cancer and CVD incidence in the WHI scenario.
What this evidence means for practice
At first glance, the clinical and economic benefits of the WHI EPT trial appear enormous. However, the authors surprisingly failed to take into consideration relevant issues well known to women’s health clinicians: lower use of systemic HT (both in women with an intact uterus and those posthysterectomy) has resulted in many more women suffering from bothersome vasomotor and sleep-related menopausal symptoms, with resultant impairment of quality of life.
In addition, the authors did not account for the major reduction in use of ET after the 2002 WHI findings in women who have had a hysterectomy; given that ET reduces the incidence of breast cancer and cardiovascular disease, declines in ET use have resulted in increased morbidity and mortality from these conditions.1
Finally, as the profound declines in use of systemic HT have not been accompanied by a substantive increase in the use of vaginal estrogen, we have an epidemic of symptomatic vulvovaginal atrophy, with attendant sexual dysfunction and impaired quality of life.
Andrew M. Kaunitz, MD
TELL US WHAT YOU THINK!
Share your thoughts on this article. Send your Letter to the Editor: [email protected]
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
Reference
- Sarrel PM, Njike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50 to 59 years. Am J Public Health. 2013;103(9):1583–1588.
Hemorrhagic ovarian cysts: One entity with many appearances
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
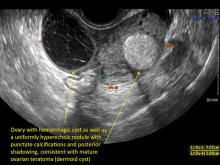
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
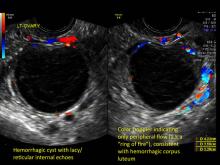
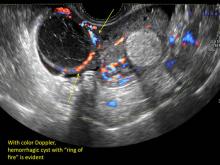
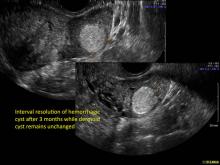
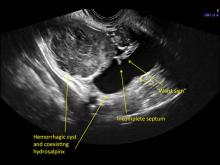
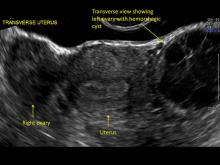
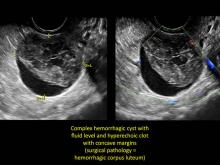
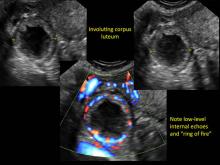
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
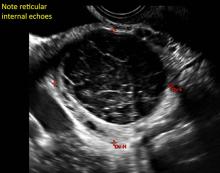
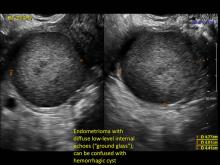
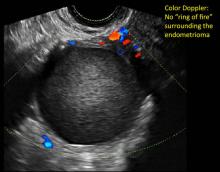
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
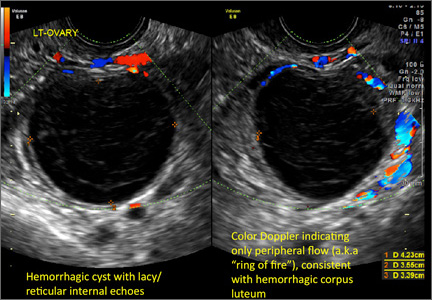
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
FOREWARD
Steven R. Goldstein, MD, CCD, NCMP
Professor, Department of Obstetrics and Gynecology, New York University School of Medicine; Director, Gynecologic Ultrasound; and Co-Director, Bone Densitometry, New York University Medical Center, New York
This is the inaugural offering in a new series, titled Images in Gyn Ultrasound. It is interesting and important that Dr. Michelle Stalnaker and Dr. Andrew Kaunitz have chosen hemorrhagic ovarian cysts as their debut topic.
Realize that since the vaginal probe was introduced in the 1980s, our entire specialty has had to undergo a learning curve--just as individuals will have a learning curve. In the early days of transvaginal ultrasound, an imager often provided a differential for such masses, along the lines of “compatible with hemorrhagic cyst, endometrioma, dermoid…cannot rule out neoplasia.” Today, however, with better understanding, and especially with the addition of color flow Doppler, very often a definitive diagnosis can be made.
These “hemorrhagic cysts” are nothing more than bleeding into a corpus luteum at the time of ovulation−the more blood that collects before tamponade or clot stops its accumulation, the larger the “cyst” can become. As the cyst goes through a “maturation” process and undergoes clot retraction and clot lysis, the variable internal echo patterns presented in the following images are possible, but there will ALWAYS only be peripheral blood flow as evidenced by the morphologic appearance of the vascular distribution. See video.
Study these images carefully as they are very representative of the many faces of the hemorrhagic corpus luteum.
Hemorrhagic ovarian cysts: One entity with many appearances
Michelle L. Stalnaker, MD
Assistant Professor and Associate Program Director, Obstetrics and Gynecology Residency, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville
Andrew M. Kaunitz, MD
University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology at the University of Florida College of Medicine–Jacksonville. Dr. Kaunitz is a member of the OBG Management Board of Editors.
Hemorrhagic cysts are normal in ovulatory women, usually resolving within 8 weeks. They can be quite variable in appearance, however, and can be confused with ovarian endometriomae. Presenting characteristics can include:
- reticular (lacy, cobweb, fishnet) internal echoes due to fibrin strands
- a solid-appearing area with concave margins
- on Color Doppler: circumferential peripheral vascular flow (“ring of fire”), with no internal flow
Management. With respect to hemorrhagic cysts, the Society of Radiologists in Ultrasound 2010 Consensus Conference Statement indicates:
- For premenopausal women:
- No follow-up imaging needed unless there’s an uncertain diagnosis or if the cyst is larger than 5 cm
- Cyst size > 5 cm; short-interval follow-up ultrasound is indicated (6-12 weeks)
- For recently menopausal women:
- Follow-up ultrasound in 6 to 12 weeks to ensure resolution of the initial findings
- For later postmenopausal women:
- Cyst possibly neoplastic; consider surgical removal
Women’s Health Initiative study netted $37 billion in savings
The massive Women’s Health Initiative estrogen plus progestin clinical trial netted more than $37 billion in savings in the 10 years after it was published, largely by curtailing postmenopausal women’s use of combined hormone therapy, which in turn prevented 126,000 cases of breast cancer and 76,000 cardiovascular events, according to a report published online May 5 in Annals of Internal Medicine.
"The net health yield for women in the United States was approximately 145,000 more quality-of-life-years than would have occurred in the absence of the trial," said Joshua A. Roth, Ph.D., of the public health sciences division, Fred Hutchinson Cancer Research Center and Group Health Research Institute, Seattle, and his associates.
So even though the 2002 trial was one of the most expensive publicly funded studies ever – costing the National Institutes of Health an estimated $260 million in 2012 U.S. dollars – it yielded clinical and economic returns of approximately $140 for every dollar invested in it, they noted.
"Our findings suggest that large public research investments can yield considerable clinical and economic value when targeted to address research questions with great clinical relevance and public health effect," the investigators wrote.
One of the primary debates regarding public funding of research concerns its overall "returns" to society. To estimate the returns of the WHI-EP trial, Dr. Roth and his colleagues developed several mathematical models so they could simulate the 10-year health outcomes of American women aged 50-79 years if the study had never taken place – that is, if it had never been reported that combined hormone therapy (HT) raised the risks of cardiovascular disease, venous thromboembolism, and breast cancer (albeit reducing the risks of fracture and colon cancer).
Publication of those results led to an immediate 50% decrease in the use of combined HT and a continuing decline of 5%-10% per year thereafter, the researchers said.
They compared disease incidence, survival rates, and direct medical expenditures between a "WHI scenario" and a "no WHI scenario" to calculate the net economic and clinical returns of the trial through the year 2012.
Approximately 39.1 million women were eligible for combined HT during the study period. An estimated 5.2 million used combined HT in the WHI scenario, but 9.5 million would have used it if there had never been a WHI, given the usage trends during the years preceding the trial.
Relative to the no-WHI scenario, there were 126,000 fewer cases of breast cancer, 76,000 fewer cases of cardiovascular disease (CVD), and 80,000 fewer cases of venous thromboembolism in the WHI scenario. On the other side of the scale, there also were 263,000 more osteoporotic fractures and 15,000 more cases of colorectal cancer.
"Compared with the no-WHI scenario, the WHI scenario resulted in $35.2 billion in direct medical expenditure savings. Most of the savings came from fewer combined HT users and associated office visits ($26.2 billion), decreased breast cancer incidence ($4.5 billion), and decreased CVD incidence ($2.2 billion), offsetting increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion)," Dr. Roth and his associates said (Ann. Intern. Med. 2014;160:594-602).
The WHI scenario, compared with the no-WHI scenario, yielded 145,000 QALYs (quality-adjusted life-years), mainly because of the improved quality of life of women who avoided breast cancer and CVD. This greatly offset the reductions in QALYs that would have been due to increased fractures in the no-WHI scenario.
The net economic return of the WHI was calculated to be $37.1 billion. Savings from reduced use of combined HT drove the early economic value of the trial, and later value was driven by a combination of combined HT expenditure savings and QALY gains.
"This level of value was robust across plausible uncertainty ranges, and remained greater than $20 billion in all simulations that we evaluated," they wrote.
"Our analysis of the economic return from the WHI-EP trial suggests that, in certain circumstances, public investments in large prospective trials with high clinical and public health relevance could provide a similarly large positive rate of return in the long term," the investigators added.
The massive Women’s Health Initiative estrogen plus progestin clinical trial netted more than $37 billion in savings in the 10 years after it was published, largely by curtailing postmenopausal women’s use of combined hormone therapy, which in turn prevented 126,000 cases of breast cancer and 76,000 cardiovascular events, according to a report published online May 5 in Annals of Internal Medicine.
"The net health yield for women in the United States was approximately 145,000 more quality-of-life-years than would have occurred in the absence of the trial," said Joshua A. Roth, Ph.D., of the public health sciences division, Fred Hutchinson Cancer Research Center and Group Health Research Institute, Seattle, and his associates.
So even though the 2002 trial was one of the most expensive publicly funded studies ever – costing the National Institutes of Health an estimated $260 million in 2012 U.S. dollars – it yielded clinical and economic returns of approximately $140 for every dollar invested in it, they noted.
"Our findings suggest that large public research investments can yield considerable clinical and economic value when targeted to address research questions with great clinical relevance and public health effect," the investigators wrote.
One of the primary debates regarding public funding of research concerns its overall "returns" to society. To estimate the returns of the WHI-EP trial, Dr. Roth and his colleagues developed several mathematical models so they could simulate the 10-year health outcomes of American women aged 50-79 years if the study had never taken place – that is, if it had never been reported that combined hormone therapy (HT) raised the risks of cardiovascular disease, venous thromboembolism, and breast cancer (albeit reducing the risks of fracture and colon cancer).
Publication of those results led to an immediate 50% decrease in the use of combined HT and a continuing decline of 5%-10% per year thereafter, the researchers said.
They compared disease incidence, survival rates, and direct medical expenditures between a "WHI scenario" and a "no WHI scenario" to calculate the net economic and clinical returns of the trial through the year 2012.
Approximately 39.1 million women were eligible for combined HT during the study period. An estimated 5.2 million used combined HT in the WHI scenario, but 9.5 million would have used it if there had never been a WHI, given the usage trends during the years preceding the trial.
Relative to the no-WHI scenario, there were 126,000 fewer cases of breast cancer, 76,000 fewer cases of cardiovascular disease (CVD), and 80,000 fewer cases of venous thromboembolism in the WHI scenario. On the other side of the scale, there also were 263,000 more osteoporotic fractures and 15,000 more cases of colorectal cancer.
"Compared with the no-WHI scenario, the WHI scenario resulted in $35.2 billion in direct medical expenditure savings. Most of the savings came from fewer combined HT users and associated office visits ($26.2 billion), decreased breast cancer incidence ($4.5 billion), and decreased CVD incidence ($2.2 billion), offsetting increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion)," Dr. Roth and his associates said (Ann. Intern. Med. 2014;160:594-602).
The WHI scenario, compared with the no-WHI scenario, yielded 145,000 QALYs (quality-adjusted life-years), mainly because of the improved quality of life of women who avoided breast cancer and CVD. This greatly offset the reductions in QALYs that would have been due to increased fractures in the no-WHI scenario.
The net economic return of the WHI was calculated to be $37.1 billion. Savings from reduced use of combined HT drove the early economic value of the trial, and later value was driven by a combination of combined HT expenditure savings and QALY gains.
"This level of value was robust across plausible uncertainty ranges, and remained greater than $20 billion in all simulations that we evaluated," they wrote.
"Our analysis of the economic return from the WHI-EP trial suggests that, in certain circumstances, public investments in large prospective trials with high clinical and public health relevance could provide a similarly large positive rate of return in the long term," the investigators added.
The massive Women’s Health Initiative estrogen plus progestin clinical trial netted more than $37 billion in savings in the 10 years after it was published, largely by curtailing postmenopausal women’s use of combined hormone therapy, which in turn prevented 126,000 cases of breast cancer and 76,000 cardiovascular events, according to a report published online May 5 in Annals of Internal Medicine.
"The net health yield for women in the United States was approximately 145,000 more quality-of-life-years than would have occurred in the absence of the trial," said Joshua A. Roth, Ph.D., of the public health sciences division, Fred Hutchinson Cancer Research Center and Group Health Research Institute, Seattle, and his associates.
So even though the 2002 trial was one of the most expensive publicly funded studies ever – costing the National Institutes of Health an estimated $260 million in 2012 U.S. dollars – it yielded clinical and economic returns of approximately $140 for every dollar invested in it, they noted.
"Our findings suggest that large public research investments can yield considerable clinical and economic value when targeted to address research questions with great clinical relevance and public health effect," the investigators wrote.
One of the primary debates regarding public funding of research concerns its overall "returns" to society. To estimate the returns of the WHI-EP trial, Dr. Roth and his colleagues developed several mathematical models so they could simulate the 10-year health outcomes of American women aged 50-79 years if the study had never taken place – that is, if it had never been reported that combined hormone therapy (HT) raised the risks of cardiovascular disease, venous thromboembolism, and breast cancer (albeit reducing the risks of fracture and colon cancer).
Publication of those results led to an immediate 50% decrease in the use of combined HT and a continuing decline of 5%-10% per year thereafter, the researchers said.
They compared disease incidence, survival rates, and direct medical expenditures between a "WHI scenario" and a "no WHI scenario" to calculate the net economic and clinical returns of the trial through the year 2012.
Approximately 39.1 million women were eligible for combined HT during the study period. An estimated 5.2 million used combined HT in the WHI scenario, but 9.5 million would have used it if there had never been a WHI, given the usage trends during the years preceding the trial.
Relative to the no-WHI scenario, there were 126,000 fewer cases of breast cancer, 76,000 fewer cases of cardiovascular disease (CVD), and 80,000 fewer cases of venous thromboembolism in the WHI scenario. On the other side of the scale, there also were 263,000 more osteoporotic fractures and 15,000 more cases of colorectal cancer.
"Compared with the no-WHI scenario, the WHI scenario resulted in $35.2 billion in direct medical expenditure savings. Most of the savings came from fewer combined HT users and associated office visits ($26.2 billion), decreased breast cancer incidence ($4.5 billion), and decreased CVD incidence ($2.2 billion), offsetting increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion)," Dr. Roth and his associates said (Ann. Intern. Med. 2014;160:594-602).
The WHI scenario, compared with the no-WHI scenario, yielded 145,000 QALYs (quality-adjusted life-years), mainly because of the improved quality of life of women who avoided breast cancer and CVD. This greatly offset the reductions in QALYs that would have been due to increased fractures in the no-WHI scenario.
The net economic return of the WHI was calculated to be $37.1 billion. Savings from reduced use of combined HT drove the early economic value of the trial, and later value was driven by a combination of combined HT expenditure savings and QALY gains.
"This level of value was robust across plausible uncertainty ranges, and remained greater than $20 billion in all simulations that we evaluated," they wrote.
"Our analysis of the economic return from the WHI-EP trial suggests that, in certain circumstances, public investments in large prospective trials with high clinical and public health relevance could provide a similarly large positive rate of return in the long term," the investigators added.
FROM ANNALS OF INTERNAL MEDICINE
Major Finding: The WHI resulted in $35.2 billion in direct medical expenditure savings, most of which came from fewer combined HT users and associated office visits ($26.2 billion), decreased breast cancer incidence ($4.5 billion), and decreased CVD incidence ($2.2 billion); this offset increases in expenditures for greater fracture incidence ($4.8 billion) and colorectal cancer ($1.0 billion).
Data Source: An analysis of mathematical models that estimated clinical and economic outcomes if the WHI-EP had not been performed in 2002, the risk/benefit profile of combined HT had not been reported, and women had continued to use combined HT through 2012.
Disclosures: This study was supported in part by the National Institute on Aging; the WHI was funded by the National Heart, Lung, and Blood Institute. Dr. Roth and her associates reported no financial conflicts of interest.
Yearly monitoring does not predict fractures after bisphosphonate cessation
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.
Chronic vulvar symptoms and dermatologic disruptions: How to make the correct diagnosis
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
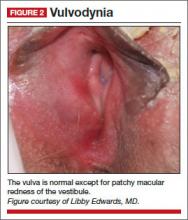
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
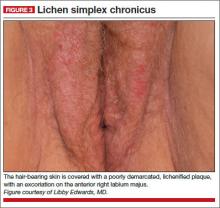
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
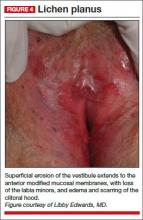
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
Nearly one in every six women will experience chronic vulvar symptoms at some point, from ongoing itching to sensations of rawness, burning, or dyspareunia. Regrettably, clinicians generally are taught only a few possible causes for these symptoms, primarily infections such as yeast, bacterial vaginosis, herpes simplex virus, or anogenital warts. However, infections rarely produce chronic symptoms that do not respond, at least temporarily, to therapy.
In this two-part series, we focus on a total of 10 cases of vulvar symptoms, zeroing in on diagnosis and treatment. In this first part, we describe five patient scenarios illustrating the diagnosis and treatment of:
- lichen sclerosus
- vulvodynia
- lichen simplex chronicus
- lichen planus
- hidradenitis suppurativa.
In many chronic cases, more than one entity is the cause
Specific skin diseases, sensations of rawness from various external and internal irritants, neuropathy, and psychological issues are all much more common causes of chronic vulvar symptoms than infection. Moreover, most women with chronic vulvar symptoms have more than one entity producing their discomfort.
Very often, the cause of a patient’s symptoms is not clear at the first visit, with nonspecific redness or even normal skin seen on examination. Pathognomonic skin findings can be obscured by irritant contact dermatitis caused by unnecessary medications or overwashing, atrophic vaginitis, and/or rubbing and scratching. In such cases, obvious abnormalities must be eliminated and the patient reevaluated to definitively discover and treat the cause of the symptoms.
CASE 1. ANOGENITAL ITCHING AND DYSPAREUNIA
A 62-year-old woman schedules a visit to address her anogenital itching. She reports pain with scratching and has developed introital dyspareunia. On physical examination, you find a well-demarcated white plaque of thickened, crinkled skin (FIGURE 1). A wet mount shows parabasal cells and no lactobacilli.
Diagnosis: Lichen sclerosus and atrophic vagina.
Treatment: Halobetasol ointment, an ultra-potent topical corticosteroid, once or twice daily; along with estradiol cream (0.5 g intravaginally) 3 times a week.
Lichen sclerosus is a skin disease found most often on the vulva of postmenopausal women, although it also can affect prepubertal children and reproductive-age women. Lichen sclerosus is multifactorial in pathogenesis, including prominent autoimmune factors, local environmental factors, and genetic predisposition.1
Although there is no cure for lichen sclerosus, the symptoms and clinical abnormalities usually can be well managed with ultra-potent topical corticosteroids. However, scarring and architectural changes are not reversible. Moreover, poorly controlled lichen sclerosus exhibits malignant transformation on anogenital skin in about 3% of affected patients.
The standard of care is application of an ultra-potent topical corticosteroid ointment once or twice daily until the skin texture normalizes again. The most common of such corticosteroids are clobetasol, halobetasol, and betamethasone dipropionate in an augmented vehicle (betamethasone dipropionate in the usual vehicle is only a medium-high medication in terms of potency.) One of us (L.E.) finds that some women experience irritation with generic clobetasol.
The ointment form of the selected corticosteroid is preferred, as creams are irritating to the vulva in most women because they contain more alcohols and preservatives than ointments do. The amount to be used is very small—far smaller than the pea-sized amount often suggested. By using this smaller amount, we avoid spread to the surrounding hair-bearing skin, which is at greater risk for steroid dermatitis and atrophy than the modified mucous membranes.
Related video: Lichen sclerosis: My approach to treatment Michael Baggish, MD
Even asymptomatic lichen sclerosus can progress
Most vulvologists agree that when the skin normalizes (not when symptoms subside), it is best to either decrease the frequency of application of the ultra-potent corticosteroid to two or three times a week, or to continue daily use with a lower-potency corticosteroid such as triamcinolone ointment 0.1%. Discontinuation of therapy usually results in recurrence.2
Treatment should not be based solely on symptoms, as asymptomatic lichen sclerosus can progress and cause permanent scarring and an increased risk for squamous cell carcinoma.
Although no studies have shown a decreased risk for squamous cell carcinoma with ongoing use of a corticosteroid, vulvologists have observed that malignant transformation occurs uniformly in the setting of poorly controlled lichen sclerosus. Immune dysregulation and inflammation may play an important role, so careful management to minimize inflammation may help prevent a malignancy.3
Secondary treatment choices
Secondary choices for lichen sclerosus include the topical calcineurin inhibitors tacrolimus (Protopic) and pimecrolimus (Elidel) but not testosterone, which has been shown to be ineffective. Tacrolimus and pimecrolimus are useful but often burn upon application, and they are “black-boxed” for cutaneous squamous cell carcinoma and lymphoma. Therefore, although squamous cell carcinoma associated with their use is extraordinarily uncommon, patients should be advised of these risks, particularly because lichen sclerosus already exhibits this association.
Most postmenopausal women with lichen sclerosus also exhibit hypothyroidism, so they should be monitored for this. However, thyroid function testing in 18 children showed no evidence of hypothyroidism in that age group (L.E. unpublished data).
Estrogen replacement may be advised
Postmenopausal women who have prominent introital lichen sclerosus or dyspareunia should receive estrogen replacement of some type so that there is only one cause, rather than two, for their dyspareunia, thinning, fragility, and inelasticity.
Women with well-controlled lichen sclerosus should be followed twice a year to ensure that their disease remains suppressed with ongoing therapy, and to evaluate for active disease, adverse effects of therapy, and the appearance of dysplasia or squamous cell carcinoma.
Women with lichen sclerosus occasionally experience discomfort after their clinical skin disease has cleared. These women now have developed vulvodynia triggered by their lichen sclerosus.
Related series: Vulvar Pain Syndromes—A 3-part roundtable
Part 1. Making the correct diagnosis (September 2011)
Part 2. A bounty of treatments—but not all of them proven (October 2011)
Part 3. Causes and treatment of vestibulodynia (November 2011)
CASE 2. IS IT REALLY CHRONIC YEAST INFECTION?
A 36-year-old woman consults you about her history of chronic yeast infection that manifests as introital burning, discharge, and dyspareunia. She is otherwise healthy, except for irritable bowel syndrome and fibromyalgia.
Physical examination reveals a mild patchy redness of the vestibule and surrounding modified mucous membranes (FIGURE 2). Gentle probing with a cotton swab triggers exquisite pain in the vestibule, with slight extension to the labia minora. A wet mount shows no evidence of increased white blood cells, parabasal cells, clue cells, or yeast forms. Lactobacilli are abundant.
Diagnosis: Vulvodynia, with a nearly vestibulodynia pattern.
Treatment: Venlafaxine and pelvic floor physical therapy.
Vulvodynia is a genital pain syndrome defined as sensations of chronic burning, irritation, rawness, and soreness in the absence of objective disease and infection that could explain the discomfort. Vulvodynia occurs in approximately 7% to 8% of women.4
Vulvodynia generally is believed to be a multifactorial symptom, occurring as a result of pelvic floor dysfunction and neuropathic pain,5,6 with anxiety/depression issues exacerbating symptoms. Some recent studies have shown the presence of biochemical mediators of inflammation in the absence of clinical and histologic inflammation.7 Discomfort often is worsened by infections or the application of common irritants (creams, panty liners, soaps, some topical anesthetics). Estrogen deficiency is another common exacerbating factor.
Women tend to exhibit other pain syndromes such as chronic headaches, fibromyalgia, temperomandibular disorder, or premenstrual syndrome, as well as prominent anxiety, depression, sleep disorder, and so on.
Almost uniformly present are symptoms of pelvic floor dysfunction, such as constipation, irritable bowel syndrome, and interstitial cystitis or urinary symptoms in the absence of a urinary tract infection. These women also are frequently unusually intolerant of medications.
Classifying vulvodynia
There are two primary patterns of vulvodynia. The first and most common is vestibulodynia, formerly called vulvar vestibulitis. The term vestibulitis was eliminated to reflect the absence of clinical and histologic inflammation. Vestibulodynia refers to pain that is always limited to the vestibule. Generalized vulvodynia, however, extends beyond the vestibule, is migratory, or does not include the vestibule.
Several vulvologists have found that many patients exhibit features of both types of vulvodynia, and these patterns probably exist on a spectrum. The difference is probably unimportant in clinical practice, except that vestibulodynia can be treated with vestibulectomy.
How we manage vulvodynia
We focus on pelvic floor physical therapy and on the provision of medication for neuropathic pain, which is initiated at very small doses and gradually increased to active doses.8 The medications used and the ultimate doses often required include:
- amitriptyline or desipramine 150 mg
- gabapentin 600 to 1,200 mg three times daily
- venlafaxine XR 150 mg daily
- pregabalin 150 mg twice a day
- duloxetine 60 mg a day.
Compounded amitriptyline 2% with baclofen 2% cream applied three times daily is beneficial for many patients, and topical lidocaine jelly 2% or ointment 5% (which often burns) can help provide immediate temporary relief.
Most patients require sex therapy and counseling for maximal improvement. Women with vestibulodynia in whom these therapies fail are good candidates for vestibulectomy if their pain is strictly limited to the vestibule. Fortunately, most women do not require this aggressive therapy.
Related article: Successful treatment of chronic vaginitis Robert L. Barbieri, MD (Editorial; July 2013)
CASE 3. SEVERE ITCHING DISRUPTS SLEEP
A 34-year-old patient reports excruciating itching, with disruption of daily activities and sleep. She has been treated for candidiasis on multiple occasions, but in your office her wet mount and confirmatory culture are negative. Physical examination reveals a pink, lichenified plaque with excoriation (FIGURE 3).
Diagnosis: Lichen simplex chronicus.
Treatment: Ultra-potent corticosteroid ointment applied very sparingly twice daily and covered with petroleum jelly. You also order nighttime sedation with amitriptyline to break the itch-scratch cycle. When the patient’s itching resolves and her skin clears, you taper her off the corticosteroid, warning her that recurrence is likely, and instruct her to restart the medication immediately should itching recur.
Lichen simplex chronicus (formerly called squamous hyperplasia or hyperplastic dystrophy, and also known as eczema, neurodermatitis, or localized atopic dermatitis) occurs when irritation from any cause produces itching in a predisposed person. The subsequent scratching and rubbing both produce the rash and exacerbate the irritation that drives the itching, even after the original cause is gone. The rubbing and scratching perpetuate the irritation and itching, producing the “itch-scratch” cycle.
The appearance of lichen simplex chronicus is produced by rubbing (where the skin thickens and lichenifies) or scratching (where the skin becomes red with linear erosions, called excoriations, caused by fingernails).
The initial trigger for lichen simplex chronicus often is an infection—often yeast—but overwashing, stress, sweat, heat, urine, irritating lubricants, and use of panty liners also may precipitate the itching. At the office visit, the original infection or other cause of irritation often is no longer present, and only lichen simplex chronicus can be identified.
How to treat lichen simplex chronicus
Management of lichen simplex chronicus requires very sparing application of an ultra-potent topical corticosteroid (clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle ointment) twice daily, with the ointment covered with petroleum jelly. Care also must be taken to avoid irritants.
In addition, nighttime sedation helps to interrupt the itch-scratch cycle by preventing rubbing during sleep.
When the skin appears normal and itching has resolved, taper the medication down or off, warning the patient that recurrence is common with any future irritation.
Restart therapy immediately upon recurrence to prevent lichenification and chronic problems.
Second-line medications include calcineurin inhibitors (tacrolimus or pimecrolimus). Although these agents do not contribute to atrophy, they are less effective than topical corticosteroids,9 cost more, and can cause burning upon application.
Unlike lichen sclerosus, lichen simplex chronicus does not always recur upon cessation of treatment, and there is no need for concern about an increased risk of malignancy or significant scarring.
Related article: New treatment option for vulvar and vaginal atrophy Andrew M. Kaunitz, MD (News for your Practice; May 2013)
CASE 4. ORAL AND VULVAR INVOLVEMENT
A 73-year-old patient seeks your help in alleviating longstanding introital itching and rawness, with dyspareunia. She has tried topical estradiol cream intravaginally three times weekly in combination with weekly fluconazole, to no avail.
Physical examination reveals deep red patches and erosions of the vestibule, with complete resorption of the labia minora (FIGURE 4). Patchy redness of the vagina is apparent as well, so you examine the patient’s mouth and find deep redness of the gingivae and erosions of the buccal mucosae, with surrounding white, lacy papules. A wet mount shows a marked increase in lymphocytes and parabasal cells, with a pH of more than 7.
Diagnosis: After correlating the vulvar and oral findings, you make a diagnosis of lichen planus.
Treatment: You initiate halobetasol ointment twice daily, to be applied to the vulva. You also continue vaginal estradiol cream but add hydrocortisone acetate 200 mg compounded vaginal suppositories nightly, as well as clobetasol gel to be applied to oral lesions three times a day. You follow the patient closely for secondary yeast of the mouth and vagina.
Erosive multimucosal lichen planus is a disease of cell-mediated immunity that overwhelmingly affects menopausal women. The most common surfaces involved are the mouth, vagina, rectal mucosa, and vulva; usually, at least two surfaces are affected. The esophagus, extra-auditory canals, nasal mucosa, and eyes also can be involved. Dry, extragenital skin usually is not affected in the setting of erosive vulvovaginal lichen planus.
Vulvar lichen planus most often is controlled with ultra-potent topical corticosteroids (again, clobetasol, halobetasol, or betamethasone dipropionate in an augmented vehicle), but other mucosal surfaces often are more difficult to manage. Although there is no definitive cure for this condition, careful local care, estrogen replacement, and suppression of oral and vulvovaginal candidiasis usually provide relief.
Calcineurin inhibitors (tacrolimus, pimecrolimus) sometimes are useful in patients who improve only partially after treatment with a topical corticosteroid, provided burning with application is tolerable.10 Systemic immunosuppressants such as hydroxychloroquine, methotrexate, mycophenolate mofetil, azathioprine, cyclosporine, cyclophosphamide, and tumor necrosis factor (TNF) alpha blockers (etanercept, adalimumab, infliximab), as well as oral retinoids, can be added for more recalcitrant disease.11
How to manage disease that affects the vagina
When the vagina is involved in lichen planus, treatment is important to prevent scarring, as well as rawness and pain from irritant contact dermatitis caused by purulent vaginal secretions. Occasionally, a 25-mg hydrocortisone acetate rectal suppository inserted into the vagina nightly improves vaginal lichen planus, but sometimes more potent suppositories, such as doses of 100 to 200 mg, may be compounded. Dilators should be inserted daily to prevent vaginal synechiae.
Oral involvement requires targeted treatment
The mouth is almost always involved in lichen planus. If a dermatologist is not involved in patient care, a prescription for dexamethasone/nystatin elixir (50:50) (5 mL swish, hold, and spit four times daily) can improve oral symptoms remarkably. Alternatively, clobetasol gel applied to affected areas of the mouth three or four times daily can be helpful. Secondary yeast of the vagina and mouth are common with the use of topical corticosteroids.
Careful clinical follow-up is advised
Like uncontrolled lichen sclerosus, erosive lichen planus of the vulva produces scarring and sometimes eventuates into squamous cell carcinoma. Therefore, careful clinical surveillance is warranted. And therapy must be continued to prevent recurrence of lichen planus (as it must be for lichen sclerosus), scarring, and to decrease the risk of squamous cell carcinoma. And like lichen sclerosus, lichen planus sometimes triggers vulvodynia.
CASE 5. MULTIPLE BOILS IN THE GROIN
A 31-year-old morbidly obese African American woman comes to your office with continually evolving boils in the groin. A culture shows Bacterioides spp, Escherichia coli, and Peptococcus spp. In the past, multiple courses of various antibiotics have provided only modest relief.
Physical examination reveals fluctuant nodules, scars, and draining sinus tracts of the hair-bearing vulva and crural crease (FIGURE 5). The axillae are clear.
Diagnosis: Hidradenitis suppurativa.
Treatment: The patient begins taking minocycline 100 mg twice daily. Because she is a smoker, you refer her to an aggressive primary care provider for smoking cessation and weight loss management.
Three months later, the patient is developing only about two nodules a month, managed by early intralesional injections of triamcinolone acetonide.
Hidradenitis suppurativa is sometimes called inverse acne because the underlying pathogenesis is similar to cystic acne. Follicular plugging with keratin debris occurs, with additional keratin, sebaceous material, and normal skin bacteria trapped below the occlusion and distending the follicle. As the follicle wall stretches, thins, and allows for leakage of keratin debris into surrounding dermis, a brisk foreign-body response produces a noninfectious abscess.
Hidradenitis suppurativa affects more than 2% of the population.12 It appears only in areas of the body that contain apocrine glands and in individuals who have double- or triple-outlet follicles that predispose them to follicular occlusion. Therefore, this disease has a genetic component.
Other risk factors include male sex, African genetic background, obesity, and smoking. The prevalence of metabolic syndrome is significantly higher in individuals with hidradenitis suppurativa than in the general population.13
Recommended management
Treatments include:
- chronic antibiotics with nonspecific anti-inflammatory activity (tetracyclines, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole)
- intralesional injection of corticosteroids for early nodules (which often aborts their development)
- TNF alpha blockers (etanercept, adalimumab, infliximab)14–16
- surgical removal of affected skin—the definitive therapy.
Note, however, that anogenital hidradenitis often is too extensive for surgery to be practical. In patients who have localized hidradenitis, primary excision is an excellent early therapy, provided the patient is advised that recurrence may occur in apocrine-containing nearby skin. Aggressive curettage of the roof of the cysts has been performed by some clinicians with good response.
Don’t overlook adjuvant approaches
Smoking cessation and weight loss often are useful.
Other therapies backed by anecdotal evidence include oral contraceptives or spironolactone for their anti-androgen effect, as well as metformin, a more recently studied agent.
Local care with antibacterial soaps and topical antibiotics may be useful for some women.
MORE CASES TO COME
In Part 2 of this series, which will appear in the June 2014 issue of OBG Management, we will discuss the following cases:
- atrophic vagina and atrophic vaginitis
- contact dermatitis
- vulvar aphthae
- desquamative inflammatory vaginitis
- psoriasis.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to: [email protected] Please include the city and state in which you practice.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
- Doulaveri G, Armira K, Kouris A, et al. Genital vulvar lichen sclerosus in monozygotic twin women: A case report and review of the literature. Case Rep Dermatol. 2013;5(3):321–325.
- Virgili A, Minghetti S, Borghi A, Corazza M. Proactive maintenance therapy with a topical corticosteroid for vulvar lichen sclerosus: Preliminary results of a randomized study. Br J Dermatol. 2013;168(6):1316–1324.
- Brodrick B, Belkin ZR, Goldstein AT. Influence of treatments on prognosis for vulvar lichen sclerosus: Facts and controversies. Clin Dermatol. 2013;31(6):780–786.
- Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: Population-based estimates from two geographic regions. Am J Obstet Gynecol. 2014;210(1):40.e1–e8.
- Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. J Sex Med. 2014;11(3):776–785.
- Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589.
- Omoigui S. The biochemical origin of pain: the origin of all pain is inflammation and the inflammatory response. Part 2 of 3: Inflammatory profile of pain syndromes. Med Hypotheses. 2007;69(6):1169–1178.
- Haefner HK, Collins ME, Davis GD, et al. The vulvodynia guideline. J Low Genit Tract Dis. 2005;9(1):40–51.
- Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13(2):113–123.
- Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. 2012;16(4):221–229.
- Mirowski GW, Goddard A. Treatment of vulvovaginal lichen planus. Dermatol Clin. 2010;28(4):717–725.
- Vinding GR, Miller IM, Zarchi K, et al. The prevalence of inverse recurrent suppuration: A population-based study of possible hidradenitis suppurativa [published online ahead of print December 16, 2013]. Br J Dermatol. doi:10.1111/bjd.12787.
- Gold DA, Reeder VJ, Mahan MG, Hamzavi IH. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J Am Acad Dermatol. 2014;70(4):699–703.
- Scheinfeld N. Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19(4):1.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa:
A parallel randomized trial. Ann Intern Med. 2012;157(12):846–855. - Chinniah N, Cains GD. Moderate to severe hidradenitis suppurativa treated with biological therapies [published online ahead of print January 23, 2014]. Australas J Dermatol. doi:10.1111/ajd.12136.
Read Part 2: Chronic vulvar irritation, itching, and pain. What is the diagnosis? (June 2014)
Ospemifene found to have minimal effects on the endometrium at 52 weeks
Ospemifene was FDA-approved in 2013 to treat moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA) due to menopause. This nonestrogenenic drug has tissue selective agonist/antagonist effects—a selective estrogen-receptor modulator (SERM). Tamoxifen, a first-generation SERM, increases the risk of endometrial cancer. However, the second-generation SERM raloxifene is not associated with this increased risk. In preclinical studies and clinical trials, ospemifene has been shown to exert positive effects on the vaginal epithelium and minimal effects on the endometrium.
Steven R. Goldstein, MD, from New York University School of Medicine, and colleagues set out to determine the endometrial safety of ospemifene in six Phase 2/3 clinical trials of postemenopausal women with up to 52 weeks of exposure to ospemifene 60 mg/day versus placebo.
Endometrial safety of the study drug was assessed in a total of 1,349 women with an intact uterus (851 in the ospemifene group vs 543 in the placebo group).
Results
Endometrial biopsies obtained at 52 weeks revealed a rate of endometrial hyperplasia of 0.3%.
Of 342 biopsied women, “there was a single case of a woman with simple hyperplasia,” says Dr. Goldstein. “She was 52 years old, had become menopausal at age 49 and had been taking hormone therapy for about 2 years before entering the trial. After 4 months of ospemifene, she had an episode of bleeding and was diagnosed with proliferative endometrium. The study drug was stopped with a plan to follow up in 3 months; 89 days later she had another episode of bleeding and was diagnosed with simple hyperplasia. She was treated with a single course of progestogen, the hyperplasia resolved, and then she was noted to have a benign polyp.”
No complex hyperplasias or carcinomas were found.
Ospemifene participants with histologic findings other than inactive, atrophic, or insufficient was 3.5% at 52 weeks, and this finding was similar to baseline endometrial biopsy results for placebo (4.0%).
The incidence of active and disordered type endometrial proliferation was less than 1% of participants treated with ospemifene. The vaginal bleeding incidence was similar in the treatment and placebo groups.
“This data tells me that this drug is clearly acting like its cousin raloxifene in the uterus, with virtually no active proliferation and no true hyperplasia. The FDA guidance for any of these products is less than 1% hyperplasia in 1 year, and there was a single case out of 342 biopsies, says Dr. Goldstein.
MORE NEWS and HIGHLIGHTS from ACOG's 2014 ANNUAL CLINICAL MEETING
Survey: Most average-risk pregnant women preferred NIPT to invasive testing
Adding infertility assessment and treatment to your practice
Delivery notes after shoulder dystocia often lack critical elements
Reference
Goldstein SR, Archer DF, Simon JS, Constantine GD. Endometrial safety of ospemifene and the ability of transvaginal ultrasound to detect small changes in endometrial thickness. Poster presented at the American Congress of Obstetricians and Gynecologists (ACOG) Annual Clinical Meeting. Chicago, IL; April 28, 2014.
Ospemifene was FDA-approved in 2013 to treat moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA) due to menopause. This nonestrogenenic drug has tissue selective agonist/antagonist effects—a selective estrogen-receptor modulator (SERM). Tamoxifen, a first-generation SERM, increases the risk of endometrial cancer. However, the second-generation SERM raloxifene is not associated with this increased risk. In preclinical studies and clinical trials, ospemifene has been shown to exert positive effects on the vaginal epithelium and minimal effects on the endometrium.
Steven R. Goldstein, MD, from New York University School of Medicine, and colleagues set out to determine the endometrial safety of ospemifene in six Phase 2/3 clinical trials of postemenopausal women with up to 52 weeks of exposure to ospemifene 60 mg/day versus placebo.
Endometrial safety of the study drug was assessed in a total of 1,349 women with an intact uterus (851 in the ospemifene group vs 543 in the placebo group).
Results
Endometrial biopsies obtained at 52 weeks revealed a rate of endometrial hyperplasia of 0.3%.
Of 342 biopsied women, “there was a single case of a woman with simple hyperplasia,” says Dr. Goldstein. “She was 52 years old, had become menopausal at age 49 and had been taking hormone therapy for about 2 years before entering the trial. After 4 months of ospemifene, she had an episode of bleeding and was diagnosed with proliferative endometrium. The study drug was stopped with a plan to follow up in 3 months; 89 days later she had another episode of bleeding and was diagnosed with simple hyperplasia. She was treated with a single course of progestogen, the hyperplasia resolved, and then she was noted to have a benign polyp.”
No complex hyperplasias or carcinomas were found.
Ospemifene participants with histologic findings other than inactive, atrophic, or insufficient was 3.5% at 52 weeks, and this finding was similar to baseline endometrial biopsy results for placebo (4.0%).
The incidence of active and disordered type endometrial proliferation was less than 1% of participants treated with ospemifene. The vaginal bleeding incidence was similar in the treatment and placebo groups.
“This data tells me that this drug is clearly acting like its cousin raloxifene in the uterus, with virtually no active proliferation and no true hyperplasia. The FDA guidance for any of these products is less than 1% hyperplasia in 1 year, and there was a single case out of 342 biopsies, says Dr. Goldstein.
MORE NEWS and HIGHLIGHTS from ACOG's 2014 ANNUAL CLINICAL MEETING
Survey: Most average-risk pregnant women preferred NIPT to invasive testing
Adding infertility assessment and treatment to your practice
Delivery notes after shoulder dystocia often lack critical elements
Ospemifene was FDA-approved in 2013 to treat moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA) due to menopause. This nonestrogenenic drug has tissue selective agonist/antagonist effects—a selective estrogen-receptor modulator (SERM). Tamoxifen, a first-generation SERM, increases the risk of endometrial cancer. However, the second-generation SERM raloxifene is not associated with this increased risk. In preclinical studies and clinical trials, ospemifene has been shown to exert positive effects on the vaginal epithelium and minimal effects on the endometrium.
Steven R. Goldstein, MD, from New York University School of Medicine, and colleagues set out to determine the endometrial safety of ospemifene in six Phase 2/3 clinical trials of postemenopausal women with up to 52 weeks of exposure to ospemifene 60 mg/day versus placebo.
Endometrial safety of the study drug was assessed in a total of 1,349 women with an intact uterus (851 in the ospemifene group vs 543 in the placebo group).
Results
Endometrial biopsies obtained at 52 weeks revealed a rate of endometrial hyperplasia of 0.3%.
Of 342 biopsied women, “there was a single case of a woman with simple hyperplasia,” says Dr. Goldstein. “She was 52 years old, had become menopausal at age 49 and had been taking hormone therapy for about 2 years before entering the trial. After 4 months of ospemifene, she had an episode of bleeding and was diagnosed with proliferative endometrium. The study drug was stopped with a plan to follow up in 3 months; 89 days later she had another episode of bleeding and was diagnosed with simple hyperplasia. She was treated with a single course of progestogen, the hyperplasia resolved, and then she was noted to have a benign polyp.”
No complex hyperplasias or carcinomas were found.
Ospemifene participants with histologic findings other than inactive, atrophic, or insufficient was 3.5% at 52 weeks, and this finding was similar to baseline endometrial biopsy results for placebo (4.0%).
The incidence of active and disordered type endometrial proliferation was less than 1% of participants treated with ospemifene. The vaginal bleeding incidence was similar in the treatment and placebo groups.
“This data tells me that this drug is clearly acting like its cousin raloxifene in the uterus, with virtually no active proliferation and no true hyperplasia. The FDA guidance for any of these products is less than 1% hyperplasia in 1 year, and there was a single case out of 342 biopsies, says Dr. Goldstein.
MORE NEWS and HIGHLIGHTS from ACOG's 2014 ANNUAL CLINICAL MEETING
Survey: Most average-risk pregnant women preferred NIPT to invasive testing
Adding infertility assessment and treatment to your practice
Delivery notes after shoulder dystocia often lack critical elements
Reference
Goldstein SR, Archer DF, Simon JS, Constantine GD. Endometrial safety of ospemifene and the ability of transvaginal ultrasound to detect small changes in endometrial thickness. Poster presented at the American Congress of Obstetricians and Gynecologists (ACOG) Annual Clinical Meeting. Chicago, IL; April 28, 2014.
Reference
Goldstein SR, Archer DF, Simon JS, Constantine GD. Endometrial safety of ospemifene and the ability of transvaginal ultrasound to detect small changes in endometrial thickness. Poster presented at the American Congress of Obstetricians and Gynecologists (ACOG) Annual Clinical Meeting. Chicago, IL; April 28, 2014.
Why it's important to open the sexual health dialogue
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
In this 5-minute audiocast, Dr. Krychman offers key takeaways from his seminar, "Sexuality in the Elder Woman," at the 62nd Annual Clinical Meeting of the American College of Obstetricians and Gynecologists.
Topical lidocaine reduces menopausal dyspareunia
Applying topical liquid lidocaine to the vulvar vestibule prior to penetration allows for comfortable intercourse in breast cancer survivors with severe menopausal dyspareunia, according to findings from a randomized, controlled study involving 46 women.
During a double-blind phase of the study, patients randomized to apply 4% aqueous lidocaine had less intercourse pain than those who applied saline (median pain scores of 1.0 and 5.3 out of 10, respectively), according to Dr. Martha F. Goetsch of Oregon Health and Science University, Portland, who will report the finding at the annual meeting of the American Congress of Obstetricians and Gynecologists.
During an open-label phase of the study in which all patients were allowed to apply lidocaine, 37 of 41 (90%) reported comfortable penetration, and sexual distress scores decreased from a median of 30.5 to a median of 14. Additionally, 17 of 20 women (85%) who were abstaining from intercourse because of the discomfort had resumed penetrative intimacy, said Dr. Goetsch, whose abstract was awarded first prize among oral presentations by ACOG.
Patients included in the study were estrogen-deficient breast cancer survivors with severe penetrative dyspareunia not associated with pelvic muscle or organ pain. All had severe vulvovaginal atrophy. During the 1-month blinded phase of the study, the women applied either the lidocaine or the saline to the vulvar vestibule for 3 minutes prior to penetration. Effects of twice-weekly tampon insertion or intercourse were documented in a diary. No partners complained of numbness resulting from the lidocaine.
The findings are notable, because breast cancer survivors number in the millions in the United States alone.
"They often suffer from severe dyspareunia and are urged to refrain from using estrogen, which is the therapy most effective for dyspareunia in menopause," Dr. Goetsch said in an interview.
Furthermore, prior research has focused primarily on vaginal atrophy as the cause of dyspareunia in postmenopausal women.
"This study showed that pain could be prevented even though atrophy was unchanged," she said, noting that this suggests that perhaps atrophy is the wrong therapeutic focus.
"Success came with therapy to the vestibule, not the vagina," she said.
Dr. Goetsch reported having no disclosures.
Applying topical liquid lidocaine to the vulvar vestibule prior to penetration allows for comfortable intercourse in breast cancer survivors with severe menopausal dyspareunia, according to findings from a randomized, controlled study involving 46 women.
During a double-blind phase of the study, patients randomized to apply 4% aqueous lidocaine had less intercourse pain than those who applied saline (median pain scores of 1.0 and 5.3 out of 10, respectively), according to Dr. Martha F. Goetsch of Oregon Health and Science University, Portland, who will report the finding at the annual meeting of the American Congress of Obstetricians and Gynecologists.
During an open-label phase of the study in which all patients were allowed to apply lidocaine, 37 of 41 (90%) reported comfortable penetration, and sexual distress scores decreased from a median of 30.5 to a median of 14. Additionally, 17 of 20 women (85%) who were abstaining from intercourse because of the discomfort had resumed penetrative intimacy, said Dr. Goetsch, whose abstract was awarded first prize among oral presentations by ACOG.
Patients included in the study were estrogen-deficient breast cancer survivors with severe penetrative dyspareunia not associated with pelvic muscle or organ pain. All had severe vulvovaginal atrophy. During the 1-month blinded phase of the study, the women applied either the lidocaine or the saline to the vulvar vestibule for 3 minutes prior to penetration. Effects of twice-weekly tampon insertion or intercourse were documented in a diary. No partners complained of numbness resulting from the lidocaine.
The findings are notable, because breast cancer survivors number in the millions in the United States alone.
"They often suffer from severe dyspareunia and are urged to refrain from using estrogen, which is the therapy most effective for dyspareunia in menopause," Dr. Goetsch said in an interview.
Furthermore, prior research has focused primarily on vaginal atrophy as the cause of dyspareunia in postmenopausal women.
"This study showed that pain could be prevented even though atrophy was unchanged," she said, noting that this suggests that perhaps atrophy is the wrong therapeutic focus.
"Success came with therapy to the vestibule, not the vagina," she said.
Dr. Goetsch reported having no disclosures.
Applying topical liquid lidocaine to the vulvar vestibule prior to penetration allows for comfortable intercourse in breast cancer survivors with severe menopausal dyspareunia, according to findings from a randomized, controlled study involving 46 women.
During a double-blind phase of the study, patients randomized to apply 4% aqueous lidocaine had less intercourse pain than those who applied saline (median pain scores of 1.0 and 5.3 out of 10, respectively), according to Dr. Martha F. Goetsch of Oregon Health and Science University, Portland, who will report the finding at the annual meeting of the American Congress of Obstetricians and Gynecologists.
During an open-label phase of the study in which all patients were allowed to apply lidocaine, 37 of 41 (90%) reported comfortable penetration, and sexual distress scores decreased from a median of 30.5 to a median of 14. Additionally, 17 of 20 women (85%) who were abstaining from intercourse because of the discomfort had resumed penetrative intimacy, said Dr. Goetsch, whose abstract was awarded first prize among oral presentations by ACOG.
Patients included in the study were estrogen-deficient breast cancer survivors with severe penetrative dyspareunia not associated with pelvic muscle or organ pain. All had severe vulvovaginal atrophy. During the 1-month blinded phase of the study, the women applied either the lidocaine or the saline to the vulvar vestibule for 3 minutes prior to penetration. Effects of twice-weekly tampon insertion or intercourse were documented in a diary. No partners complained of numbness resulting from the lidocaine.
The findings are notable, because breast cancer survivors number in the millions in the United States alone.
"They often suffer from severe dyspareunia and are urged to refrain from using estrogen, which is the therapy most effective for dyspareunia in menopause," Dr. Goetsch said in an interview.
Furthermore, prior research has focused primarily on vaginal atrophy as the cause of dyspareunia in postmenopausal women.
"This study showed that pain could be prevented even though atrophy was unchanged," she said, noting that this suggests that perhaps atrophy is the wrong therapeutic focus.
"Success came with therapy to the vestibule, not the vagina," she said.
Dr. Goetsch reported having no disclosures.
FROM THE ACOG ANNUAL CLINICAL MEETING
Key clinical point: Consider lidocaine as an option for women with severe menopausal dyspareunia for whom estrogen therapy is not recommended.
Major finding: 37 of 41 patients (90%) reported comfortable penetration during intercourse.
Data source: A randomized, controlled, double-blind study of 46 women.
Disclosures: Dr. Goetsch reported having no disclosures.
Menopause doesn’t drive severe asthma
MADRID – Menopause is blamed for many things, but it’s unlikely to be the reason for the increased risk of severe asthma or worse quality of life in elderly asthmatic women, a study suggests.
"The increased unadjusted asthma severity and need for health care utilization in postmenopausal women are more likely due to other factors like age and other comorbidities rather than menopause per se," Dr. Joe Zein said at the world congress of the American College of Chest Physicians.
The investigators used a propensity score matching method to analyze the effect of menopause on asthma severity, quality of life, and health care utilization in 166 menopausal and 538 premenopausal women enrolled in the Severe Asthma Research program from 2002 to 2011. Subsequent multivariate logistic regression analyses were used to adjust for the covariates of age at enrollment, hypertension, gastroesophageal reflux disease (GERD), and hormone therapy, which was used in only 35 menopausal women.
Compared with premenopausal women, menopausal women were older and reported less atopy and more comorbidities, such as higher body mass index, diabetes mellitus, hypertension, GERD, obstructive sleep apnea, sinusitis, and nasal polyps, said Dr. Zein, a pulmonologist at Cleveland Clinic.
Menopausal women also had lower lung function and higher neutrophil percentage in both induced sputum and bronchoalveolar lavage fluid.
Severe asthma was present in 31% (167/538) of premenopausal and 72% (119/166) of menopausal women.
In unadjusted analysis, the risk of severe asthma was almost sixfold higher in menopausal women (odds ratio, 5.62; 95% confidence interval 3.83-8.26), but dropped dramatically in the adjusted analysis (OR, 1.46), he said.
Menopausal women also had lower average scores than did premenopausal women (4.06 vs. 4.56) on the 7-point Asthma Quality of Life Questionnaire, with 7 being "not impaired at all" and 1 being "severely impaired." The mean difference between groups pointed to worse quality of life among menopausal women in unadjusted analysis (–0.5), but again this faded after multivariate adjustment (0.31; 95% C.I. –0.30 to 0.93).
Similar trends were observed for health care utilization including emergency department visits (unadjusted OR, 1.33; adjusted OR, 1.15) and hospitalization (unadjusted OR, 2.93; adjusted OR, 0.70), Dr. Zein said.
Finally, an analysis stratified by menopausal status that looked at the association between enrollment age and the probability of severe asthma, suggested a rise in severe asthma among premenopausal women and those in early menopause, followed by a steady decline around age 55 years. Two possible hypotheses are that insulin resistance is higher during the period around menopause and thus may worsen asthma and that estrogen levels initially rise during early menopause before declining and also may increase asthma severity, Dr. Zein said.
"We don’t know exactly, but I think we should not look at menopause as one entity."
Several studies have tried to tease out the effects of menopause and aging on asthma severity, with conflicting results.
A recent study reported that menopausal women in their fifties and sixties are more than twice as likely to be hospitalized for asthma as men the same age (Ann. Allergy Asthma Immunol. 2013;111:176-81).
The Harvard Nurses Health Study, however, found that postmenopausal women who never used hormone therapy had a significantly lower age-adjusted risk of asthma than premenopausal women (Am. J. Respir. Crit. Care Med. 1995;152:1183-8).
The role of estrogen in asthma remains controversial, Dr. Zein observed. The incidence of asthma is twice as high among boys during childhood, but this switches during puberty when girls have a higher incidence of asthma as well as asthma-related hospitalizations and health care utilization, he noted.
Dr. Zein reported no financial disclosures; a coauthor reported grant monies from the National Institutes of Health.
MADRID – Menopause is blamed for many things, but it’s unlikely to be the reason for the increased risk of severe asthma or worse quality of life in elderly asthmatic women, a study suggests.
"The increased unadjusted asthma severity and need for health care utilization in postmenopausal women are more likely due to other factors like age and other comorbidities rather than menopause per se," Dr. Joe Zein said at the world congress of the American College of Chest Physicians.
The investigators used a propensity score matching method to analyze the effect of menopause on asthma severity, quality of life, and health care utilization in 166 menopausal and 538 premenopausal women enrolled in the Severe Asthma Research program from 2002 to 2011. Subsequent multivariate logistic regression analyses were used to adjust for the covariates of age at enrollment, hypertension, gastroesophageal reflux disease (GERD), and hormone therapy, which was used in only 35 menopausal women.
Compared with premenopausal women, menopausal women were older and reported less atopy and more comorbidities, such as higher body mass index, diabetes mellitus, hypertension, GERD, obstructive sleep apnea, sinusitis, and nasal polyps, said Dr. Zein, a pulmonologist at Cleveland Clinic.
Menopausal women also had lower lung function and higher neutrophil percentage in both induced sputum and bronchoalveolar lavage fluid.
Severe asthma was present in 31% (167/538) of premenopausal and 72% (119/166) of menopausal women.
In unadjusted analysis, the risk of severe asthma was almost sixfold higher in menopausal women (odds ratio, 5.62; 95% confidence interval 3.83-8.26), but dropped dramatically in the adjusted analysis (OR, 1.46), he said.
Menopausal women also had lower average scores than did premenopausal women (4.06 vs. 4.56) on the 7-point Asthma Quality of Life Questionnaire, with 7 being "not impaired at all" and 1 being "severely impaired." The mean difference between groups pointed to worse quality of life among menopausal women in unadjusted analysis (–0.5), but again this faded after multivariate adjustment (0.31; 95% C.I. –0.30 to 0.93).
Similar trends were observed for health care utilization including emergency department visits (unadjusted OR, 1.33; adjusted OR, 1.15) and hospitalization (unadjusted OR, 2.93; adjusted OR, 0.70), Dr. Zein said.
Finally, an analysis stratified by menopausal status that looked at the association between enrollment age and the probability of severe asthma, suggested a rise in severe asthma among premenopausal women and those in early menopause, followed by a steady decline around age 55 years. Two possible hypotheses are that insulin resistance is higher during the period around menopause and thus may worsen asthma and that estrogen levels initially rise during early menopause before declining and also may increase asthma severity, Dr. Zein said.
"We don’t know exactly, but I think we should not look at menopause as one entity."
Several studies have tried to tease out the effects of menopause and aging on asthma severity, with conflicting results.
A recent study reported that menopausal women in their fifties and sixties are more than twice as likely to be hospitalized for asthma as men the same age (Ann. Allergy Asthma Immunol. 2013;111:176-81).
The Harvard Nurses Health Study, however, found that postmenopausal women who never used hormone therapy had a significantly lower age-adjusted risk of asthma than premenopausal women (Am. J. Respir. Crit. Care Med. 1995;152:1183-8).
The role of estrogen in asthma remains controversial, Dr. Zein observed. The incidence of asthma is twice as high among boys during childhood, but this switches during puberty when girls have a higher incidence of asthma as well as asthma-related hospitalizations and health care utilization, he noted.
Dr. Zein reported no financial disclosures; a coauthor reported grant monies from the National Institutes of Health.
MADRID – Menopause is blamed for many things, but it’s unlikely to be the reason for the increased risk of severe asthma or worse quality of life in elderly asthmatic women, a study suggests.
"The increased unadjusted asthma severity and need for health care utilization in postmenopausal women are more likely due to other factors like age and other comorbidities rather than menopause per se," Dr. Joe Zein said at the world congress of the American College of Chest Physicians.
The investigators used a propensity score matching method to analyze the effect of menopause on asthma severity, quality of life, and health care utilization in 166 menopausal and 538 premenopausal women enrolled in the Severe Asthma Research program from 2002 to 2011. Subsequent multivariate logistic regression analyses were used to adjust for the covariates of age at enrollment, hypertension, gastroesophageal reflux disease (GERD), and hormone therapy, which was used in only 35 menopausal women.
Compared with premenopausal women, menopausal women were older and reported less atopy and more comorbidities, such as higher body mass index, diabetes mellitus, hypertension, GERD, obstructive sleep apnea, sinusitis, and nasal polyps, said Dr. Zein, a pulmonologist at Cleveland Clinic.
Menopausal women also had lower lung function and higher neutrophil percentage in both induced sputum and bronchoalveolar lavage fluid.
Severe asthma was present in 31% (167/538) of premenopausal and 72% (119/166) of menopausal women.
In unadjusted analysis, the risk of severe asthma was almost sixfold higher in menopausal women (odds ratio, 5.62; 95% confidence interval 3.83-8.26), but dropped dramatically in the adjusted analysis (OR, 1.46), he said.
Menopausal women also had lower average scores than did premenopausal women (4.06 vs. 4.56) on the 7-point Asthma Quality of Life Questionnaire, with 7 being "not impaired at all" and 1 being "severely impaired." The mean difference between groups pointed to worse quality of life among menopausal women in unadjusted analysis (–0.5), but again this faded after multivariate adjustment (0.31; 95% C.I. –0.30 to 0.93).
Similar trends were observed for health care utilization including emergency department visits (unadjusted OR, 1.33; adjusted OR, 1.15) and hospitalization (unadjusted OR, 2.93; adjusted OR, 0.70), Dr. Zein said.
Finally, an analysis stratified by menopausal status that looked at the association between enrollment age and the probability of severe asthma, suggested a rise in severe asthma among premenopausal women and those in early menopause, followed by a steady decline around age 55 years. Two possible hypotheses are that insulin resistance is higher during the period around menopause and thus may worsen asthma and that estrogen levels initially rise during early menopause before declining and also may increase asthma severity, Dr. Zein said.
"We don’t know exactly, but I think we should not look at menopause as one entity."
Several studies have tried to tease out the effects of menopause and aging on asthma severity, with conflicting results.
A recent study reported that menopausal women in their fifties and sixties are more than twice as likely to be hospitalized for asthma as men the same age (Ann. Allergy Asthma Immunol. 2013;111:176-81).
The Harvard Nurses Health Study, however, found that postmenopausal women who never used hormone therapy had a significantly lower age-adjusted risk of asthma than premenopausal women (Am. J. Respir. Crit. Care Med. 1995;152:1183-8).
The role of estrogen in asthma remains controversial, Dr. Zein observed. The incidence of asthma is twice as high among boys during childhood, but this switches during puberty when girls have a higher incidence of asthma as well as asthma-related hospitalizations and health care utilization, he noted.
Dr. Zein reported no financial disclosures; a coauthor reported grant monies from the National Institutes of Health.
AT CHEST WORLD CONGRESS 2014
Major finding: The odds ratio for severe asthma was 5.62 for menopausal vs. premenopausal women in unadjusted analysis, but 1.46 after adjustment.
Data source: A retrospective analysis of 166 menopausal and 538 premenopausal asthmatic women.
Disclosures: Dr. Zein reported no financial disclosures; a coauthor reported grant monies from the National Institutes of Health.
It’s time to put to rest the calcium supplement controversy
SAN DIEGO – Lingering concern over increased risk cardiovascular events and mortality associated with calcium supplementation do not outweigh the bone benefits of calcium supplements, Connie Weaver, Ph.D., said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
In her opinion, the best randomized, controlled trial is from the Women’s Health Initiative, which found that calcium supplementation significantly reduced the risk of hip fracture and breast cancer, said Dr. Weaver, distinguished professor and head of the department of nutrition science at Purdue University, West Lafayette, Ind. In addition, there were no significant associations observed in the risk of myocardial infarction, heart disease, cardiovascular diseases, or death (Osteoporos. Int. 2013;24:567-80).
Other studies showing that calcium supplementation increased the risk for cardiovascular events were flawed in a number of ways, she said.
Questions about the value of calcium supplementation began to surface following publication of a meta-analysis of 15 trials that found a 30% increased in the incidence of myocardial infarction and smaller, nonsignificant increased in the risk of stroke and mortality (BMJ 2010;341:c3691). According to Dr. Weaver, who is also director of the Indiana Clinical and Translational Science Institute, this and other studies on the topic are limited by the fact that they are secondary analyses of studies designed to investigate bone. "So, if myocardial infarction or any other outcome measures are not part of your original plan, you may not be taking the measurements with the same care," she explained. "If you didn’t adjudicate, your self-reported information may turn out to be gastrointestinal discomfort, and the patient might confuse indigestion with myocardial infarction. That’s the kind of trouble you can get into with secondary analysis."
Other limitations of studies on this topic include a lack of baseline cardiovascular data and lack of standards for cardiovascular event ascertainment. "Self-reported data are very problematic," she maintained." In addition, no dose-response relationship was examined in many of these analyses, there was low compliance in intent to treat analyses, but the main thing for me was there was no underlying mechanism that was demonstrated."
Even so, in 2013 the U. S. Preventive Services Task Force weighed the existing evidence and recommended against daily supplements of less than 400 IU vitamin D3 and less than 1,000 mg of calcium for the prevention of fractures in postmenopausal women (Ann. Intern. Med. 2013;159:824-34). Further, they concluded that the current evidence is insufficient to recommend greater than 400 IU vitamin D3 and greater than 1,000 mg calcium for the prevention of fractures in postmenopausal women, premenopausal women, and men. "The controversy is unlikely to be resolved by epidemiological studies or secondary analysis of randomized controlled trials," Dr. Weaver said.
The mechanism by which calcium may lessen cardiovascular health remains elusive. Some speculate that a spike in circulating calcium following excessive calcium intake exacerbates coronary artery calcification and leads to subsequent cardiovascular dysfunction. "There is no research to show that this happens, but this is the speculated mechanism," Dr. Weaver said. However, in 2013 investigators published a study that demonstrated that an increase in serum calcium following a 1,000-mg oral dose of calcium citrate did not cause harmful changes in cardiovascular function (J. Bone Miner. Res. 2013; 28:412-8).
Data from one animal study may lend support to the safety of calcium supplements. Dr. Weaver led an interdisciplinary team to study soft tissue calcification in 24 Ossabaw miniature swine. Previous studies of these pigs have demonstrated that when fed an excess calorie atherogenic diet, they develop metabolic syndrome and progress to coronary atherosclerosis.
Dr. Weaver and her associates randomized the pigs into to three dietary intake groups: control, high dairy (nonfat milk) or high supplement (calcium carbonate). After a little more than 5 months on their allocated diet, the pigs underwent jugular injection of the tracer 41Ca, which has a long half-life and can be monitored sensitively at low levels expected in tissue. The researchers collected blood and urine samples, conducted intravascular ultrasound imaging and PET-CT to assess coronary artery disease, and performed an in vitro wire myography on the proximal 1.5 cm of left anterior descending artery. This procedure "examines functional responses and vascular reactivity of arteries and is a sensitive and early indicator of compromised cell function," Dr. Weaver explained.
Intravascular ultrasound detected no significant differences in plaque wall coverage between the three dietary groups and PET-CT found that stroke volume and ejection fraction did not differ among the groups. In vitro myography also demonstrated no differences between the three groups, even when smooth muscle of the left anterior descending artery was exposed to bradykinin and sodium nitroprusside. Histology also demonstrated no differences between the three groups.
"We hope our case study puts to rest these concerns about calcium supplements so we cover our bones as well as not worry about cardiovascular effects," Dr. Weaver concluded. "Adequate intake of calcium should be below the tolerable upper limit and should be encouraged for bone health. Most dietary guidance committees recommend foods as the first choice, but supplements should be used to fill dietary shortcomings. So if the dietary guidelines recommend 3 cups/day of low-fat dairy product equivalents to get enough calcium, the addition of a 300-mg calcium supplement is recommended for every one serving missed."
"Bone health is a lifelong concern," she said. "Peak skeletal mass is achieved by ages 20-30 and the adult skeleton is remodeled and replaced every 10 years."
Milk contains enough calcium to meet dietary recommendations, but milk consumption is decreasing among Americans in all age groups, especially in children, she said. "This decline could set them up to grow lower levels of bone mass. Over life, that could position them to be in a fracture zone earlier. So we have to be concerned: How do we get these minerals needed if use of the main dietary source – milk – is declining? The controversy of calcium supplements comes to the forefront, because that is one alternative to milk. There are not that many foods that are concentrated in well-absorbed calcium."
Dr. Weaver disclosed that she serves on the boards of the National Osteoporosis Foundation, the International Life Sciences Institute, Showalter, and Pharmavite. She has also received grant support from the National Institutes of Health, the Dairy Research Institute, Nestle, and Tate & Lyle.
SAN DIEGO – Lingering concern over increased risk cardiovascular events and mortality associated with calcium supplementation do not outweigh the bone benefits of calcium supplements, Connie Weaver, Ph.D., said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
In her opinion, the best randomized, controlled trial is from the Women’s Health Initiative, which found that calcium supplementation significantly reduced the risk of hip fracture and breast cancer, said Dr. Weaver, distinguished professor and head of the department of nutrition science at Purdue University, West Lafayette, Ind. In addition, there were no significant associations observed in the risk of myocardial infarction, heart disease, cardiovascular diseases, or death (Osteoporos. Int. 2013;24:567-80).
Other studies showing that calcium supplementation increased the risk for cardiovascular events were flawed in a number of ways, she said.
Questions about the value of calcium supplementation began to surface following publication of a meta-analysis of 15 trials that found a 30% increased in the incidence of myocardial infarction and smaller, nonsignificant increased in the risk of stroke and mortality (BMJ 2010;341:c3691). According to Dr. Weaver, who is also director of the Indiana Clinical and Translational Science Institute, this and other studies on the topic are limited by the fact that they are secondary analyses of studies designed to investigate bone. "So, if myocardial infarction or any other outcome measures are not part of your original plan, you may not be taking the measurements with the same care," she explained. "If you didn’t adjudicate, your self-reported information may turn out to be gastrointestinal discomfort, and the patient might confuse indigestion with myocardial infarction. That’s the kind of trouble you can get into with secondary analysis."
Other limitations of studies on this topic include a lack of baseline cardiovascular data and lack of standards for cardiovascular event ascertainment. "Self-reported data are very problematic," she maintained." In addition, no dose-response relationship was examined in many of these analyses, there was low compliance in intent to treat analyses, but the main thing for me was there was no underlying mechanism that was demonstrated."
Even so, in 2013 the U. S. Preventive Services Task Force weighed the existing evidence and recommended against daily supplements of less than 400 IU vitamin D3 and less than 1,000 mg of calcium for the prevention of fractures in postmenopausal women (Ann. Intern. Med. 2013;159:824-34). Further, they concluded that the current evidence is insufficient to recommend greater than 400 IU vitamin D3 and greater than 1,000 mg calcium for the prevention of fractures in postmenopausal women, premenopausal women, and men. "The controversy is unlikely to be resolved by epidemiological studies or secondary analysis of randomized controlled trials," Dr. Weaver said.
The mechanism by which calcium may lessen cardiovascular health remains elusive. Some speculate that a spike in circulating calcium following excessive calcium intake exacerbates coronary artery calcification and leads to subsequent cardiovascular dysfunction. "There is no research to show that this happens, but this is the speculated mechanism," Dr. Weaver said. However, in 2013 investigators published a study that demonstrated that an increase in serum calcium following a 1,000-mg oral dose of calcium citrate did not cause harmful changes in cardiovascular function (J. Bone Miner. Res. 2013; 28:412-8).
Data from one animal study may lend support to the safety of calcium supplements. Dr. Weaver led an interdisciplinary team to study soft tissue calcification in 24 Ossabaw miniature swine. Previous studies of these pigs have demonstrated that when fed an excess calorie atherogenic diet, they develop metabolic syndrome and progress to coronary atherosclerosis.
Dr. Weaver and her associates randomized the pigs into to three dietary intake groups: control, high dairy (nonfat milk) or high supplement (calcium carbonate). After a little more than 5 months on their allocated diet, the pigs underwent jugular injection of the tracer 41Ca, which has a long half-life and can be monitored sensitively at low levels expected in tissue. The researchers collected blood and urine samples, conducted intravascular ultrasound imaging and PET-CT to assess coronary artery disease, and performed an in vitro wire myography on the proximal 1.5 cm of left anterior descending artery. This procedure "examines functional responses and vascular reactivity of arteries and is a sensitive and early indicator of compromised cell function," Dr. Weaver explained.
Intravascular ultrasound detected no significant differences in plaque wall coverage between the three dietary groups and PET-CT found that stroke volume and ejection fraction did not differ among the groups. In vitro myography also demonstrated no differences between the three groups, even when smooth muscle of the left anterior descending artery was exposed to bradykinin and sodium nitroprusside. Histology also demonstrated no differences between the three groups.
"We hope our case study puts to rest these concerns about calcium supplements so we cover our bones as well as not worry about cardiovascular effects," Dr. Weaver concluded. "Adequate intake of calcium should be below the tolerable upper limit and should be encouraged for bone health. Most dietary guidance committees recommend foods as the first choice, but supplements should be used to fill dietary shortcomings. So if the dietary guidelines recommend 3 cups/day of low-fat dairy product equivalents to get enough calcium, the addition of a 300-mg calcium supplement is recommended for every one serving missed."
"Bone health is a lifelong concern," she said. "Peak skeletal mass is achieved by ages 20-30 and the adult skeleton is remodeled and replaced every 10 years."
Milk contains enough calcium to meet dietary recommendations, but milk consumption is decreasing among Americans in all age groups, especially in children, she said. "This decline could set them up to grow lower levels of bone mass. Over life, that could position them to be in a fracture zone earlier. So we have to be concerned: How do we get these minerals needed if use of the main dietary source – milk – is declining? The controversy of calcium supplements comes to the forefront, because that is one alternative to milk. There are not that many foods that are concentrated in well-absorbed calcium."
Dr. Weaver disclosed that she serves on the boards of the National Osteoporosis Foundation, the International Life Sciences Institute, Showalter, and Pharmavite. She has also received grant support from the National Institutes of Health, the Dairy Research Institute, Nestle, and Tate & Lyle.
SAN DIEGO – Lingering concern over increased risk cardiovascular events and mortality associated with calcium supplementation do not outweigh the bone benefits of calcium supplements, Connie Weaver, Ph.D., said at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
In her opinion, the best randomized, controlled trial is from the Women’s Health Initiative, which found that calcium supplementation significantly reduced the risk of hip fracture and breast cancer, said Dr. Weaver, distinguished professor and head of the department of nutrition science at Purdue University, West Lafayette, Ind. In addition, there were no significant associations observed in the risk of myocardial infarction, heart disease, cardiovascular diseases, or death (Osteoporos. Int. 2013;24:567-80).
Other studies showing that calcium supplementation increased the risk for cardiovascular events were flawed in a number of ways, she said.
Questions about the value of calcium supplementation began to surface following publication of a meta-analysis of 15 trials that found a 30% increased in the incidence of myocardial infarction and smaller, nonsignificant increased in the risk of stroke and mortality (BMJ 2010;341:c3691). According to Dr. Weaver, who is also director of the Indiana Clinical and Translational Science Institute, this and other studies on the topic are limited by the fact that they are secondary analyses of studies designed to investigate bone. "So, if myocardial infarction or any other outcome measures are not part of your original plan, you may not be taking the measurements with the same care," she explained. "If you didn’t adjudicate, your self-reported information may turn out to be gastrointestinal discomfort, and the patient might confuse indigestion with myocardial infarction. That’s the kind of trouble you can get into with secondary analysis."
Other limitations of studies on this topic include a lack of baseline cardiovascular data and lack of standards for cardiovascular event ascertainment. "Self-reported data are very problematic," she maintained." In addition, no dose-response relationship was examined in many of these analyses, there was low compliance in intent to treat analyses, but the main thing for me was there was no underlying mechanism that was demonstrated."
Even so, in 2013 the U. S. Preventive Services Task Force weighed the existing evidence and recommended against daily supplements of less than 400 IU vitamin D3 and less than 1,000 mg of calcium for the prevention of fractures in postmenopausal women (Ann. Intern. Med. 2013;159:824-34). Further, they concluded that the current evidence is insufficient to recommend greater than 400 IU vitamin D3 and greater than 1,000 mg calcium for the prevention of fractures in postmenopausal women, premenopausal women, and men. "The controversy is unlikely to be resolved by epidemiological studies or secondary analysis of randomized controlled trials," Dr. Weaver said.
The mechanism by which calcium may lessen cardiovascular health remains elusive. Some speculate that a spike in circulating calcium following excessive calcium intake exacerbates coronary artery calcification and leads to subsequent cardiovascular dysfunction. "There is no research to show that this happens, but this is the speculated mechanism," Dr. Weaver said. However, in 2013 investigators published a study that demonstrated that an increase in serum calcium following a 1,000-mg oral dose of calcium citrate did not cause harmful changes in cardiovascular function (J. Bone Miner. Res. 2013; 28:412-8).
Data from one animal study may lend support to the safety of calcium supplements. Dr. Weaver led an interdisciplinary team to study soft tissue calcification in 24 Ossabaw miniature swine. Previous studies of these pigs have demonstrated that when fed an excess calorie atherogenic diet, they develop metabolic syndrome and progress to coronary atherosclerosis.
Dr. Weaver and her associates randomized the pigs into to three dietary intake groups: control, high dairy (nonfat milk) or high supplement (calcium carbonate). After a little more than 5 months on their allocated diet, the pigs underwent jugular injection of the tracer 41Ca, which has a long half-life and can be monitored sensitively at low levels expected in tissue. The researchers collected blood and urine samples, conducted intravascular ultrasound imaging and PET-CT to assess coronary artery disease, and performed an in vitro wire myography on the proximal 1.5 cm of left anterior descending artery. This procedure "examines functional responses and vascular reactivity of arteries and is a sensitive and early indicator of compromised cell function," Dr. Weaver explained.
Intravascular ultrasound detected no significant differences in plaque wall coverage between the three dietary groups and PET-CT found that stroke volume and ejection fraction did not differ among the groups. In vitro myography also demonstrated no differences between the three groups, even when smooth muscle of the left anterior descending artery was exposed to bradykinin and sodium nitroprusside. Histology also demonstrated no differences between the three groups.
"We hope our case study puts to rest these concerns about calcium supplements so we cover our bones as well as not worry about cardiovascular effects," Dr. Weaver concluded. "Adequate intake of calcium should be below the tolerable upper limit and should be encouraged for bone health. Most dietary guidance committees recommend foods as the first choice, but supplements should be used to fill dietary shortcomings. So if the dietary guidelines recommend 3 cups/day of low-fat dairy product equivalents to get enough calcium, the addition of a 300-mg calcium supplement is recommended for every one serving missed."
"Bone health is a lifelong concern," she said. "Peak skeletal mass is achieved by ages 20-30 and the adult skeleton is remodeled and replaced every 10 years."
Milk contains enough calcium to meet dietary recommendations, but milk consumption is decreasing among Americans in all age groups, especially in children, she said. "This decline could set them up to grow lower levels of bone mass. Over life, that could position them to be in a fracture zone earlier. So we have to be concerned: How do we get these minerals needed if use of the main dietary source – milk – is declining? The controversy of calcium supplements comes to the forefront, because that is one alternative to milk. There are not that many foods that are concentrated in well-absorbed calcium."
Dr. Weaver disclosed that she serves on the boards of the National Osteoporosis Foundation, the International Life Sciences Institute, Showalter, and Pharmavite. She has also received grant support from the National Institutes of Health, the Dairy Research Institute, Nestle, and Tate & Lyle.
EXPERT ANALYSIS FROM THE NATURAL SUPPLEMENTS UPDATE