User login
Soy isoflavones safe in postmenopausal women, data from 3-year trial showed
NATIONAL HARBOR, MD. – Soy isoflavones are safe for postmenopausal women to take long term, having no treatment effect on endometrial thickness, reproductive hormones, or thyroid function, the results of a 3-year study have shown.
Despite previous reports that soy isoflavones have deleterious effects, they still are often used by postmenopausal women as an alternative to hormone therapy, according to study author, D. Lee Alekel, Ph.D., program director of women’s health at the National Center for Complementary and Alternative Medicine at the National Institutes of Health.
“It behooves us to examine the safety-related outcomes and rates of any adverse events in women consuming over-the-counter soy isoflavone supplements, which could be 100 mg of isoflavones a day or more,” Dr. Alekel said at the annual meeting of the North American Menopause Society. “That’s five times the intake from typical Asian diets, so it’s quite a bit more.”
The findings are from the dual-site, double-blind, placebo-controlled Soy Isoflavones for Reducing Bone Loss (SIRBL) study of women randomly assigned to placebo, or either 80 mg or 120 mg of daily soy isoflavones. No treatment effects on endometrial thickness or circulating hormone concentrations, including thyroid function, were detected using transvaginal ultrasound in any of the study groups (Menopause 2014 [doi: 10.1097/GME.0000000000000280]).
All women in the study were between 48 and 65 years of age at the time of enrollment, had experienced natural onset of menopause, did not smoke, agreed to avoid soy-based foods for the duration of the study, and maintained a healthy weight. The investigators also took into account lactation duration, and excluded from the study any women using hormone therapy or chronic medication. All participants had to have an annual physical, mammogram, gynecologic exam, and breast exam performed by their own physicians in order to remain in the study.
After losing 12% to follow-up, 224 women remained in the intent-to-treat analysis, and 208 women who were compliant with their assigned treatment fully completed the study.
Assessments done at 6, 12, 18, 24, and 36 months showed declines in median endometrial thickness in all three treatment groups: from 1.5 to 1.1 mm at the Iowa site, and from 2.6 to 1.9 mm at the California site. Neither the 80 mg nor the 120 mg dose were found to have a treatment effect (P = .57 and P = 0.43, respectively).
Adverse event rates varied by site, but not treatment arms: a higher rate of upper respiratory tract infections was noted in women studied in Iowa, compared with women enrolled at the second site in southern California (P ≤ .0001). The investigators theorized this difference was due to Iowa having longer, harsher winters. The 80-mg arm had more genitourinary issues (P = .005), primarily urinary tract infections, than did the 120-mg group.
Previous studies also have suggested soy isoflavones have no adverse effect on the endometrium, regardless of the form or dose of isoflavone; however, one study cited by Dr. Alekel “did show that 150 mg of soy taken per day for up to 5 years increased endometrial hyperplasia about 4%, but there was no endometrial carcinoma detected” (Fertil. Steril. 2004 Jul;82:145-8).
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Soy isoflavones are safe for postmenopausal women to take long term, having no treatment effect on endometrial thickness, reproductive hormones, or thyroid function, the results of a 3-year study have shown.
Despite previous reports that soy isoflavones have deleterious effects, they still are often used by postmenopausal women as an alternative to hormone therapy, according to study author, D. Lee Alekel, Ph.D., program director of women’s health at the National Center for Complementary and Alternative Medicine at the National Institutes of Health.
“It behooves us to examine the safety-related outcomes and rates of any adverse events in women consuming over-the-counter soy isoflavone supplements, which could be 100 mg of isoflavones a day or more,” Dr. Alekel said at the annual meeting of the North American Menopause Society. “That’s five times the intake from typical Asian diets, so it’s quite a bit more.”
The findings are from the dual-site, double-blind, placebo-controlled Soy Isoflavones for Reducing Bone Loss (SIRBL) study of women randomly assigned to placebo, or either 80 mg or 120 mg of daily soy isoflavones. No treatment effects on endometrial thickness or circulating hormone concentrations, including thyroid function, were detected using transvaginal ultrasound in any of the study groups (Menopause 2014 [doi: 10.1097/GME.0000000000000280]).
All women in the study were between 48 and 65 years of age at the time of enrollment, had experienced natural onset of menopause, did not smoke, agreed to avoid soy-based foods for the duration of the study, and maintained a healthy weight. The investigators also took into account lactation duration, and excluded from the study any women using hormone therapy or chronic medication. All participants had to have an annual physical, mammogram, gynecologic exam, and breast exam performed by their own physicians in order to remain in the study.
After losing 12% to follow-up, 224 women remained in the intent-to-treat analysis, and 208 women who were compliant with their assigned treatment fully completed the study.
Assessments done at 6, 12, 18, 24, and 36 months showed declines in median endometrial thickness in all three treatment groups: from 1.5 to 1.1 mm at the Iowa site, and from 2.6 to 1.9 mm at the California site. Neither the 80 mg nor the 120 mg dose were found to have a treatment effect (P = .57 and P = 0.43, respectively).
Adverse event rates varied by site, but not treatment arms: a higher rate of upper respiratory tract infections was noted in women studied in Iowa, compared with women enrolled at the second site in southern California (P ≤ .0001). The investigators theorized this difference was due to Iowa having longer, harsher winters. The 80-mg arm had more genitourinary issues (P = .005), primarily urinary tract infections, than did the 120-mg group.
Previous studies also have suggested soy isoflavones have no adverse effect on the endometrium, regardless of the form or dose of isoflavone; however, one study cited by Dr. Alekel “did show that 150 mg of soy taken per day for up to 5 years increased endometrial hyperplasia about 4%, but there was no endometrial carcinoma detected” (Fertil. Steril. 2004 Jul;82:145-8).
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Soy isoflavones are safe for postmenopausal women to take long term, having no treatment effect on endometrial thickness, reproductive hormones, or thyroid function, the results of a 3-year study have shown.
Despite previous reports that soy isoflavones have deleterious effects, they still are often used by postmenopausal women as an alternative to hormone therapy, according to study author, D. Lee Alekel, Ph.D., program director of women’s health at the National Center for Complementary and Alternative Medicine at the National Institutes of Health.
“It behooves us to examine the safety-related outcomes and rates of any adverse events in women consuming over-the-counter soy isoflavone supplements, which could be 100 mg of isoflavones a day or more,” Dr. Alekel said at the annual meeting of the North American Menopause Society. “That’s five times the intake from typical Asian diets, so it’s quite a bit more.”
The findings are from the dual-site, double-blind, placebo-controlled Soy Isoflavones for Reducing Bone Loss (SIRBL) study of women randomly assigned to placebo, or either 80 mg or 120 mg of daily soy isoflavones. No treatment effects on endometrial thickness or circulating hormone concentrations, including thyroid function, were detected using transvaginal ultrasound in any of the study groups (Menopause 2014 [doi: 10.1097/GME.0000000000000280]).
All women in the study were between 48 and 65 years of age at the time of enrollment, had experienced natural onset of menopause, did not smoke, agreed to avoid soy-based foods for the duration of the study, and maintained a healthy weight. The investigators also took into account lactation duration, and excluded from the study any women using hormone therapy or chronic medication. All participants had to have an annual physical, mammogram, gynecologic exam, and breast exam performed by their own physicians in order to remain in the study.
After losing 12% to follow-up, 224 women remained in the intent-to-treat analysis, and 208 women who were compliant with their assigned treatment fully completed the study.
Assessments done at 6, 12, 18, 24, and 36 months showed declines in median endometrial thickness in all three treatment groups: from 1.5 to 1.1 mm at the Iowa site, and from 2.6 to 1.9 mm at the California site. Neither the 80 mg nor the 120 mg dose were found to have a treatment effect (P = .57 and P = 0.43, respectively).
Adverse event rates varied by site, but not treatment arms: a higher rate of upper respiratory tract infections was noted in women studied in Iowa, compared with women enrolled at the second site in southern California (P ≤ .0001). The investigators theorized this difference was due to Iowa having longer, harsher winters. The 80-mg arm had more genitourinary issues (P = .005), primarily urinary tract infections, than did the 120-mg group.
Previous studies also have suggested soy isoflavones have no adverse effect on the endometrium, regardless of the form or dose of isoflavone; however, one study cited by Dr. Alekel “did show that 150 mg of soy taken per day for up to 5 years increased endometrial hyperplasia about 4%, but there was no endometrial carcinoma detected” (Fertil. Steril. 2004 Jul;82:145-8).
On Twitter @whitneymcknight
AT THE NAMS 2014 ANNUAL MEETING
Key clinical point: Soy isoflavones taken long term can be an effective nonpharmacogenic treatment of postmenopausal symptoms.
Major finding: Neither 80 mg (P = 0.57) nor 120 mg dose (P = 0.43) taken daily for 3 years had an effect on endometrial thickness.
Data source: Double-blind, random, controlled, multisite study of 208 postmenopausal women given placebo, 80 mg daily, or 120 mg daily of soy isoflavones for 36 months.
Disclosures: Dr. Alekel did not have any relevant disclosures.
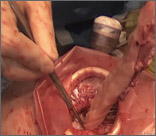
The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique
As a result of recent concerns regarding the use of power morcellation, clinicians have been faced with the need to develop alternative techniques for contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.
The following video represents a refined and reproducible approach that incorporates a containment bag (Anchor Medical) and a self-retaining retractor (Applied Medical) in order to meet the following objectives:
- tissue extraction without the need for power morcellation
- specimen containment to avoid intraperitoneal spillage
- ability to continue to offer minimally invasive surgical options to patients through a safe and standardized approach to tissue extraction.
The example case is real-time, contained, intact removal of an 8-cm, 130-g fibroid.

I hope you enjoy the featured opening session on best tissue extraction standards at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver and stop by to visit me at the OBG Management booth.
— Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As a result of recent concerns regarding the use of power morcellation, clinicians have been faced with the need to develop alternative techniques for contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.
The following video represents a refined and reproducible approach that incorporates a containment bag (Anchor Medical) and a self-retaining retractor (Applied Medical) in order to meet the following objectives:
- tissue extraction without the need for power morcellation
- specimen containment to avoid intraperitoneal spillage
- ability to continue to offer minimally invasive surgical options to patients through a safe and standardized approach to tissue extraction.
The example case is real-time, contained, intact removal of an 8-cm, 130-g fibroid.

I hope you enjoy the featured opening session on best tissue extraction standards at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver and stop by to visit me at the OBG Management booth.
— Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
As a result of recent concerns regarding the use of power morcellation, clinicians have been faced with the need to develop alternative techniques for contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.
The following video represents a refined and reproducible approach that incorporates a containment bag (Anchor Medical) and a self-retaining retractor (Applied Medical) in order to meet the following objectives:
- tissue extraction without the need for power morcellation
- specimen containment to avoid intraperitoneal spillage
- ability to continue to offer minimally invasive surgical options to patients through a safe and standardized approach to tissue extraction.
The example case is real-time, contained, intact removal of an 8-cm, 130-g fibroid.

I hope you enjoy the featured opening session on best tissue extraction standards at the AAGL Global Congress on Minimally Invasive Gynecology in Vancouver and stop by to visit me at the OBG Management booth.
— Dr. Arnold Advincula, AAGL 2014 Scientific Program Chair
Share your thoughts on this video! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
VIDEO: SERMs move beyond osteoporosis, breast cancer prevention
HONOLULU– Uses for selective estrogen receptor modulators have grown beyond prevention of breast cancer or osteoporosis to treatment of postmenopausal symptoms.
Dr. Cynthia Stuenkel spoke about selective estrogen receptor modifiers (SERMs) and menopause in a keynote address at the annual meeting of the American Society for Reproductive Medicine.
In a video interview, Dr. Stuenkel talks about two of the newer SERM options – ospemifene for dyspareunia and a combination of the SERM bazedoxifene and conjugated equine estrogens that’s available outside of the United States to treat vasomotor symptoms or for prevention of bone loss.
These new tools expand clinical options – but, as with any new therapy – longer and larger studies of the newer agents are needed to more carefully assess long-term safety, said Dr. Stuenkel of the University of California, San Diego.
She reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
HONOLULU– Uses for selective estrogen receptor modulators have grown beyond prevention of breast cancer or osteoporosis to treatment of postmenopausal symptoms.
Dr. Cynthia Stuenkel spoke about selective estrogen receptor modifiers (SERMs) and menopause in a keynote address at the annual meeting of the American Society for Reproductive Medicine.
In a video interview, Dr. Stuenkel talks about two of the newer SERM options – ospemifene for dyspareunia and a combination of the SERM bazedoxifene and conjugated equine estrogens that’s available outside of the United States to treat vasomotor symptoms or for prevention of bone loss.
These new tools expand clinical options – but, as with any new therapy – longer and larger studies of the newer agents are needed to more carefully assess long-term safety, said Dr. Stuenkel of the University of California, San Diego.
She reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
HONOLULU– Uses for selective estrogen receptor modulators have grown beyond prevention of breast cancer or osteoporosis to treatment of postmenopausal symptoms.
Dr. Cynthia Stuenkel spoke about selective estrogen receptor modifiers (SERMs) and menopause in a keynote address at the annual meeting of the American Society for Reproductive Medicine.
In a video interview, Dr. Stuenkel talks about two of the newer SERM options – ospemifene for dyspareunia and a combination of the SERM bazedoxifene and conjugated equine estrogens that’s available outside of the United States to treat vasomotor symptoms or for prevention of bone loss.
These new tools expand clinical options – but, as with any new therapy – longer and larger studies of the newer agents are needed to more carefully assess long-term safety, said Dr. Stuenkel of the University of California, San Diego.
She reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
EXPERT ANALYSIS FROM 2014 ASRM
Ovarian aging may be tip-off for cardiovascular risk
HONOLULU – Women with greater ovarian reserve had lower cardiovascular risk in a prospective study of 1,092 healthy, ovulating women aged 25-45 years, 250 of whom were followed for 3-5 years.
Measures of ovarian aging at baseline were inversely correlated with measures of systemic cellular aging. At the follow-up assessment, women who’d had lower ovarian reserve at baseline were more likely to have higher Framingham Heart Study assessment scores predicting increased risk for cardiovascular events.
“The ovary may be particularly sensitive to cellular aging” and could provide a relatively simple, objective way to identify long-term cardiovascular risk earlier in some women, Dr. Marcelle I. Cedars said at the annual meeting of the American Society for Reproductive Medicine.
She and her associates tested the women for anti-Müllerian hormone levels (AMH) and antral follicle counts (AFC) as markers of ovarian reserve. They assessed cellular aging by examining telomere length and mitochondrial DNA copy numbers in circulating white blood cells as biomarkers of cellular senescence. Shorter telomere length has been associated with worse cardiovascular risk in previous studies, she noted. The current study also measured lipid levels, fasting glucose, insulin levels, body mass index, waist circumference, and blood pressure.
Women with higher AFC and AMH at baseline had healthier lipid profiles at follow-up 3-5 years later, reported Dr. Cedars, director of the division of reproductive endocrinology and of the Center for Reproductive Health at the University of California, San Francisco. The upper 10th percentile of AFC levels were associated with significantly longer telomeres and significantly lower numbers of mitochondrial DNA copies, compared with the lowest 10th percentile of AFC.
Women with higher AFC or AMH at baseline had significantly lower Framingham risk scores at follow-up, compared with women with low AFC or average AFC, she said. The Framingham scores at follow-up showed a 10-year risk of MI of 2.1% in women in the low AFC tertiles, 1.3% in women in middle AFC tertiles, and 1.1% in women in high AFC tertiles.
The difference in cardiovascular risk level was statistically significant between the high and low tertiles for AFC and between the middle and low tertiles for AFC. For AMH levels, the difference in cardiovascular risk was significant between high and low tertiles, between high and middle tertiles, and between middle and low tertiles.
The ovary’s sensitivity to oxidative stress and telomere shortening plus the need for functional mitochondrial DNA in the oocyte may underlie the process that makes ovarian age a potential window onto cardiovascular risk, the investigators hypothesized.
Previous studies have shown that women with cardiovascular disease tend to be diagnosed later and generally to have a worse prognosis, compared with men, Dr. Cedars said. Using ovarian “age” as a window onto cardiovascular risk might help close that gap, she suggested.
Dr. Cedars reported financial associations with Nora Therapeutics, Ferring Pharmaceuticals, and the founders of Telome Health, a company that measures telomere length.
On Twitter @sherryboschert
This study supports the systemic view of reproductive health in the context of one’s overall health. Ovarian aging is something that can be measured fairly easily and it could become a useful way to identify women at particular risk for cardiovascular disease. Because women are typically diagnosed later in the course of their cardiovascular disease and have a worse prognosis than men, new ways to earlier identify women at particular risk could help improve outcomes for them.
Dr. Rebecca Z. Sokol is president of the American Society for Reproductive Medicine. She gave these comments in a statement released by the society, which said that she has no financial disclosures.
This study supports the systemic view of reproductive health in the context of one’s overall health. Ovarian aging is something that can be measured fairly easily and it could become a useful way to identify women at particular risk for cardiovascular disease. Because women are typically diagnosed later in the course of their cardiovascular disease and have a worse prognosis than men, new ways to earlier identify women at particular risk could help improve outcomes for them.
Dr. Rebecca Z. Sokol is president of the American Society for Reproductive Medicine. She gave these comments in a statement released by the society, which said that she has no financial disclosures.
This study supports the systemic view of reproductive health in the context of one’s overall health. Ovarian aging is something that can be measured fairly easily and it could become a useful way to identify women at particular risk for cardiovascular disease. Because women are typically diagnosed later in the course of their cardiovascular disease and have a worse prognosis than men, new ways to earlier identify women at particular risk could help improve outcomes for them.
Dr. Rebecca Z. Sokol is president of the American Society for Reproductive Medicine. She gave these comments in a statement released by the society, which said that she has no financial disclosures.
HONOLULU – Women with greater ovarian reserve had lower cardiovascular risk in a prospective study of 1,092 healthy, ovulating women aged 25-45 years, 250 of whom were followed for 3-5 years.
Measures of ovarian aging at baseline were inversely correlated with measures of systemic cellular aging. At the follow-up assessment, women who’d had lower ovarian reserve at baseline were more likely to have higher Framingham Heart Study assessment scores predicting increased risk for cardiovascular events.
“The ovary may be particularly sensitive to cellular aging” and could provide a relatively simple, objective way to identify long-term cardiovascular risk earlier in some women, Dr. Marcelle I. Cedars said at the annual meeting of the American Society for Reproductive Medicine.
She and her associates tested the women for anti-Müllerian hormone levels (AMH) and antral follicle counts (AFC) as markers of ovarian reserve. They assessed cellular aging by examining telomere length and mitochondrial DNA copy numbers in circulating white blood cells as biomarkers of cellular senescence. Shorter telomere length has been associated with worse cardiovascular risk in previous studies, she noted. The current study also measured lipid levels, fasting glucose, insulin levels, body mass index, waist circumference, and blood pressure.
Women with higher AFC and AMH at baseline had healthier lipid profiles at follow-up 3-5 years later, reported Dr. Cedars, director of the division of reproductive endocrinology and of the Center for Reproductive Health at the University of California, San Francisco. The upper 10th percentile of AFC levels were associated with significantly longer telomeres and significantly lower numbers of mitochondrial DNA copies, compared with the lowest 10th percentile of AFC.
Women with higher AFC or AMH at baseline had significantly lower Framingham risk scores at follow-up, compared with women with low AFC or average AFC, she said. The Framingham scores at follow-up showed a 10-year risk of MI of 2.1% in women in the low AFC tertiles, 1.3% in women in middle AFC tertiles, and 1.1% in women in high AFC tertiles.
The difference in cardiovascular risk level was statistically significant between the high and low tertiles for AFC and between the middle and low tertiles for AFC. For AMH levels, the difference in cardiovascular risk was significant between high and low tertiles, between high and middle tertiles, and between middle and low tertiles.
The ovary’s sensitivity to oxidative stress and telomere shortening plus the need for functional mitochondrial DNA in the oocyte may underlie the process that makes ovarian age a potential window onto cardiovascular risk, the investigators hypothesized.
Previous studies have shown that women with cardiovascular disease tend to be diagnosed later and generally to have a worse prognosis, compared with men, Dr. Cedars said. Using ovarian “age” as a window onto cardiovascular risk might help close that gap, she suggested.
Dr. Cedars reported financial associations with Nora Therapeutics, Ferring Pharmaceuticals, and the founders of Telome Health, a company that measures telomere length.
On Twitter @sherryboschert
HONOLULU – Women with greater ovarian reserve had lower cardiovascular risk in a prospective study of 1,092 healthy, ovulating women aged 25-45 years, 250 of whom were followed for 3-5 years.
Measures of ovarian aging at baseline were inversely correlated with measures of systemic cellular aging. At the follow-up assessment, women who’d had lower ovarian reserve at baseline were more likely to have higher Framingham Heart Study assessment scores predicting increased risk for cardiovascular events.
“The ovary may be particularly sensitive to cellular aging” and could provide a relatively simple, objective way to identify long-term cardiovascular risk earlier in some women, Dr. Marcelle I. Cedars said at the annual meeting of the American Society for Reproductive Medicine.
She and her associates tested the women for anti-Müllerian hormone levels (AMH) and antral follicle counts (AFC) as markers of ovarian reserve. They assessed cellular aging by examining telomere length and mitochondrial DNA copy numbers in circulating white blood cells as biomarkers of cellular senescence. Shorter telomere length has been associated with worse cardiovascular risk in previous studies, she noted. The current study also measured lipid levels, fasting glucose, insulin levels, body mass index, waist circumference, and blood pressure.
Women with higher AFC and AMH at baseline had healthier lipid profiles at follow-up 3-5 years later, reported Dr. Cedars, director of the division of reproductive endocrinology and of the Center for Reproductive Health at the University of California, San Francisco. The upper 10th percentile of AFC levels were associated with significantly longer telomeres and significantly lower numbers of mitochondrial DNA copies, compared with the lowest 10th percentile of AFC.
Women with higher AFC or AMH at baseline had significantly lower Framingham risk scores at follow-up, compared with women with low AFC or average AFC, she said. The Framingham scores at follow-up showed a 10-year risk of MI of 2.1% in women in the low AFC tertiles, 1.3% in women in middle AFC tertiles, and 1.1% in women in high AFC tertiles.
The difference in cardiovascular risk level was statistically significant between the high and low tertiles for AFC and between the middle and low tertiles for AFC. For AMH levels, the difference in cardiovascular risk was significant between high and low tertiles, between high and middle tertiles, and between middle and low tertiles.
The ovary’s sensitivity to oxidative stress and telomere shortening plus the need for functional mitochondrial DNA in the oocyte may underlie the process that makes ovarian age a potential window onto cardiovascular risk, the investigators hypothesized.
Previous studies have shown that women with cardiovascular disease tend to be diagnosed later and generally to have a worse prognosis, compared with men, Dr. Cedars said. Using ovarian “age” as a window onto cardiovascular risk might help close that gap, she suggested.
Dr. Cedars reported financial associations with Nora Therapeutics, Ferring Pharmaceuticals, and the founders of Telome Health, a company that measures telomere length.
On Twitter @sherryboschert
AT THE ASRM ANNUAL MEETING
Key clinical point: “Older” ovaries were associated with increased cardiovascular risk.
Major finding: The 10-year risk of MI was 2.1% in women in the lowest tertiles of AFC, 1.3% in middle tertiles, and 1.1% in high tertiles.
Data source: Prospective measures of ovarian reserve in 1,092 healthy women and cardiovascular risk assessments on 250 at 3-5 years.
Disclosures: Dr. Cedars reported financial associations with Nora Therapeutics, Ferring Pharmaceuticals, and the founders of Telome Health, a company that measures telomere length.
Purified pollen extract for hot flashes did not inhibit tamoxifen’s efficacy
NATIONAL HARBOR, MD. – Relizen, a nonhormonal purified pollen extract with demonstrated efficacy in treating vasomotor symptoms of menopause, had negligible effects on the CYP2D6 enzyme at five times the recommended daily dose, according to a poster presentation.
Because tamoxifen is metabolized into endoxifen, the active metabolite in the CYP2D6 enzyme, the findings could mean women with severe hot flashes who are taking tamoxifen now have a viable treatment option that won’t increase their risk of death from breast cancer.
“Tamoxifen creates some of the worst hot flashes we will ever see,” Dr. Steven R. Goldstein, professor of obstetrics and gynecology at New York University, said in an interview at this year’s annual meeting of the North American Menopause Society. Even so, because systemic estrogen is contraindicated in women using either tamoxifen or raloxifene for the treatment or chemoprevention of breast cancer, and questions remain about the safety of phytoestrogens, many of these women forgo the commonly prescribed estrogen-based treatments for menopausal symptoms.
Instead, some physicians will prescribe off-label low doses of antidepressants to treat vasomotor symptoms in this patient population. However, a 2010 population-based cohort study of 2,430 Canadian women taking tamoxifen concurrently with an SSRI showed that the risk of death from breast cancer increased 24%-91% in those taking paroxetine in particular, depending on the increasing overlap of tamoxifen treatment and antidepressant use (BMJ 2010;340:c693 [doi:10.1136/bmj.c693]). Last year, the Food and Drug Administration approved the vasomotor symptoms of menopause as an indication for the SSRI paroxetine (Paxil, Brisdelle), but required Noven, the drug’s manufacturer, to state paroxetine’s countering effect on tamoxifen’s efficacy.
Until now, according to Dr. Goldstein, fears over estrogen and SSRI’s potential ill-effects have meant that many women have chosen to “tough it out” when it comes to hot flashes, night sweats, and other symptoms.
The importance of his study, he said, is that because Relizen (Sérélyspharma, France), a powdered, pollen cytoplasmic extract harvested in southern Sweden, doesn’t affect the CYP enzyme system, it’s safe to give to tamoxifen patients. “It is the only nonpharmacologic product that I have ever been aware of that has a double-blind, randomized, placebo-controlled, parallel study showing that it reduces vasomotor symptoms and improves quality of life in menopausal women,” Dr. Goldstein said.
In an in vitro study of Relizen’s effect on the isoenzyme CYP2D6 in pooled human liver microsomes, Dr. Goldstein and his colleagues tested the supplement against quinidine, a known CYP2D6 inhibitor. The end point was the conversion of bufuralol to 1-OH-bufuralol in the CYP enzyme system. Six concentrations of each compound were tested, with all reactions being performed in triplicate. The Relizen concentrations ranged from 1.65 mcg/mL to 400 mcg/mL, five times the Relizen recommended daily dose of 80 mcg/mL. The quinidine concentrations ranged from 2.06 nM to 500 nM.
The study compound’s inhibition of CYP2D6 was negligible, ranging from –6.53% to 10.67%, compared with quinidine’s CYP2D6 linear dose-related increase from –7.07% at 2.06 nM to 84.05% at 500 nM.
Relizen’s apparent clinical utility in women at high risk of death from breast cancer is the “low-hanging fruit,” Dr. Goldstein said. “The tamoxifen patient has no other place to go. But if a woman is 51 years old and she comes to me and says she has hot flashes, now I have multiple options. I can give her hormones, I can give her 7.5 mg of paroxetine, or I can give her Relizen. This is just another arrow in my quiver.”
In the placebo-controlled study of Relizen, 65% of the active treatment group of 32 peri- and postmenopausal women reported decreased vasomotor symptoms by the end of the 12 week trial, compared with 38% of the placebo group (Climacteric 2005; 8:162-70).
“I know [Relizen] is effective, and it’s tested against placebo,” Dr. Goldstein said. “But is it as strong as estrogen? I have no clue. A head-to-head trial is not going to happen.”
Relizen has been widely available in Europe under a variety of names, including Femal, since 1999, and has had no reported adverse effects, according to the manufacturer. JDS Pharmaceuticals (Noven) began distributing the supplement in the United States earlier this year.
Dr. Goldstein is a paid consultant for JDS Pharmaceuticals, a division of Noven. The study was sponsored by the supplement manufacturer, Sérélyspharma(France). JDS Pharmaceuticals (Noven) is the licensed distributor of Relizen in the United States.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Relizen, a nonhormonal purified pollen extract with demonstrated efficacy in treating vasomotor symptoms of menopause, had negligible effects on the CYP2D6 enzyme at five times the recommended daily dose, according to a poster presentation.
Because tamoxifen is metabolized into endoxifen, the active metabolite in the CYP2D6 enzyme, the findings could mean women with severe hot flashes who are taking tamoxifen now have a viable treatment option that won’t increase their risk of death from breast cancer.
“Tamoxifen creates some of the worst hot flashes we will ever see,” Dr. Steven R. Goldstein, professor of obstetrics and gynecology at New York University, said in an interview at this year’s annual meeting of the North American Menopause Society. Even so, because systemic estrogen is contraindicated in women using either tamoxifen or raloxifene for the treatment or chemoprevention of breast cancer, and questions remain about the safety of phytoestrogens, many of these women forgo the commonly prescribed estrogen-based treatments for menopausal symptoms.
Instead, some physicians will prescribe off-label low doses of antidepressants to treat vasomotor symptoms in this patient population. However, a 2010 population-based cohort study of 2,430 Canadian women taking tamoxifen concurrently with an SSRI showed that the risk of death from breast cancer increased 24%-91% in those taking paroxetine in particular, depending on the increasing overlap of tamoxifen treatment and antidepressant use (BMJ 2010;340:c693 [doi:10.1136/bmj.c693]). Last year, the Food and Drug Administration approved the vasomotor symptoms of menopause as an indication for the SSRI paroxetine (Paxil, Brisdelle), but required Noven, the drug’s manufacturer, to state paroxetine’s countering effect on tamoxifen’s efficacy.
Until now, according to Dr. Goldstein, fears over estrogen and SSRI’s potential ill-effects have meant that many women have chosen to “tough it out” when it comes to hot flashes, night sweats, and other symptoms.
The importance of his study, he said, is that because Relizen (Sérélyspharma, France), a powdered, pollen cytoplasmic extract harvested in southern Sweden, doesn’t affect the CYP enzyme system, it’s safe to give to tamoxifen patients. “It is the only nonpharmacologic product that I have ever been aware of that has a double-blind, randomized, placebo-controlled, parallel study showing that it reduces vasomotor symptoms and improves quality of life in menopausal women,” Dr. Goldstein said.
In an in vitro study of Relizen’s effect on the isoenzyme CYP2D6 in pooled human liver microsomes, Dr. Goldstein and his colleagues tested the supplement against quinidine, a known CYP2D6 inhibitor. The end point was the conversion of bufuralol to 1-OH-bufuralol in the CYP enzyme system. Six concentrations of each compound were tested, with all reactions being performed in triplicate. The Relizen concentrations ranged from 1.65 mcg/mL to 400 mcg/mL, five times the Relizen recommended daily dose of 80 mcg/mL. The quinidine concentrations ranged from 2.06 nM to 500 nM.
The study compound’s inhibition of CYP2D6 was negligible, ranging from –6.53% to 10.67%, compared with quinidine’s CYP2D6 linear dose-related increase from –7.07% at 2.06 nM to 84.05% at 500 nM.
Relizen’s apparent clinical utility in women at high risk of death from breast cancer is the “low-hanging fruit,” Dr. Goldstein said. “The tamoxifen patient has no other place to go. But if a woman is 51 years old and she comes to me and says she has hot flashes, now I have multiple options. I can give her hormones, I can give her 7.5 mg of paroxetine, or I can give her Relizen. This is just another arrow in my quiver.”
In the placebo-controlled study of Relizen, 65% of the active treatment group of 32 peri- and postmenopausal women reported decreased vasomotor symptoms by the end of the 12 week trial, compared with 38% of the placebo group (Climacteric 2005; 8:162-70).
“I know [Relizen] is effective, and it’s tested against placebo,” Dr. Goldstein said. “But is it as strong as estrogen? I have no clue. A head-to-head trial is not going to happen.”
Relizen has been widely available in Europe under a variety of names, including Femal, since 1999, and has had no reported adverse effects, according to the manufacturer. JDS Pharmaceuticals (Noven) began distributing the supplement in the United States earlier this year.
Dr. Goldstein is a paid consultant for JDS Pharmaceuticals, a division of Noven. The study was sponsored by the supplement manufacturer, Sérélyspharma(France). JDS Pharmaceuticals (Noven) is the licensed distributor of Relizen in the United States.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Relizen, a nonhormonal purified pollen extract with demonstrated efficacy in treating vasomotor symptoms of menopause, had negligible effects on the CYP2D6 enzyme at five times the recommended daily dose, according to a poster presentation.
Because tamoxifen is metabolized into endoxifen, the active metabolite in the CYP2D6 enzyme, the findings could mean women with severe hot flashes who are taking tamoxifen now have a viable treatment option that won’t increase their risk of death from breast cancer.
“Tamoxifen creates some of the worst hot flashes we will ever see,” Dr. Steven R. Goldstein, professor of obstetrics and gynecology at New York University, said in an interview at this year’s annual meeting of the North American Menopause Society. Even so, because systemic estrogen is contraindicated in women using either tamoxifen or raloxifene for the treatment or chemoprevention of breast cancer, and questions remain about the safety of phytoestrogens, many of these women forgo the commonly prescribed estrogen-based treatments for menopausal symptoms.
Instead, some physicians will prescribe off-label low doses of antidepressants to treat vasomotor symptoms in this patient population. However, a 2010 population-based cohort study of 2,430 Canadian women taking tamoxifen concurrently with an SSRI showed that the risk of death from breast cancer increased 24%-91% in those taking paroxetine in particular, depending on the increasing overlap of tamoxifen treatment and antidepressant use (BMJ 2010;340:c693 [doi:10.1136/bmj.c693]). Last year, the Food and Drug Administration approved the vasomotor symptoms of menopause as an indication for the SSRI paroxetine (Paxil, Brisdelle), but required Noven, the drug’s manufacturer, to state paroxetine’s countering effect on tamoxifen’s efficacy.
Until now, according to Dr. Goldstein, fears over estrogen and SSRI’s potential ill-effects have meant that many women have chosen to “tough it out” when it comes to hot flashes, night sweats, and other symptoms.
The importance of his study, he said, is that because Relizen (Sérélyspharma, France), a powdered, pollen cytoplasmic extract harvested in southern Sweden, doesn’t affect the CYP enzyme system, it’s safe to give to tamoxifen patients. “It is the only nonpharmacologic product that I have ever been aware of that has a double-blind, randomized, placebo-controlled, parallel study showing that it reduces vasomotor symptoms and improves quality of life in menopausal women,” Dr. Goldstein said.
In an in vitro study of Relizen’s effect on the isoenzyme CYP2D6 in pooled human liver microsomes, Dr. Goldstein and his colleagues tested the supplement against quinidine, a known CYP2D6 inhibitor. The end point was the conversion of bufuralol to 1-OH-bufuralol in the CYP enzyme system. Six concentrations of each compound were tested, with all reactions being performed in triplicate. The Relizen concentrations ranged from 1.65 mcg/mL to 400 mcg/mL, five times the Relizen recommended daily dose of 80 mcg/mL. The quinidine concentrations ranged from 2.06 nM to 500 nM.
The study compound’s inhibition of CYP2D6 was negligible, ranging from –6.53% to 10.67%, compared with quinidine’s CYP2D6 linear dose-related increase from –7.07% at 2.06 nM to 84.05% at 500 nM.
Relizen’s apparent clinical utility in women at high risk of death from breast cancer is the “low-hanging fruit,” Dr. Goldstein said. “The tamoxifen patient has no other place to go. But if a woman is 51 years old and she comes to me and says she has hot flashes, now I have multiple options. I can give her hormones, I can give her 7.5 mg of paroxetine, or I can give her Relizen. This is just another arrow in my quiver.”
In the placebo-controlled study of Relizen, 65% of the active treatment group of 32 peri- and postmenopausal women reported decreased vasomotor symptoms by the end of the 12 week trial, compared with 38% of the placebo group (Climacteric 2005; 8:162-70).
“I know [Relizen] is effective, and it’s tested against placebo,” Dr. Goldstein said. “But is it as strong as estrogen? I have no clue. A head-to-head trial is not going to happen.”
Relizen has been widely available in Europe under a variety of names, including Femal, since 1999, and has had no reported adverse effects, according to the manufacturer. JDS Pharmaceuticals (Noven) began distributing the supplement in the United States earlier this year.
Dr. Goldstein is a paid consultant for JDS Pharmaceuticals, a division of Noven. The study was sponsored by the supplement manufacturer, Sérélyspharma(France). JDS Pharmaceuticals (Noven) is the licensed distributor of Relizen in the United States.
On Twitter @whitneymcknight
Key clinical point: When SSRIs are contraindicated for treatment of vasomotor symptoms, such as in women taking tamoxifen, Relizen was found to be a safe, effective alternative.
Major finding: Relizen’s inhibition of the CYP2D6 enzyme, key to tamoxifen’s efficacy, was found negligible at five times the daily recommended dose.
Data source: In vitro study using pooled human liver microsomes exposed to either Relizen or known CYP2D6 inhibitor, quinidine.
Disclosures: Dr. Goldstein is a paid consultant for JDS Pharmaceuticals, a division of Noven. The study was sponsored by the supplement manufacturer, Sérélyspharma, (France). JDS Pharmaceuticals (Noven) is the licensed distributor of Relizen in the United States.
Female sexual dysfunction: Is pharmacologic treatment a future reality or just wishful thinking?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Novel and other therapies for vaginal dryness
NATIONAL HARBOR, MD. – Menopausal women who experience vaginal dryness often find the resultant pain of sexual intercourse so prohibitive that, according to Dr. JoAnne Pinkerton, often these women go months or years without intimate relations.
There are many treatment options that can benefit patients with the condition, explained Dr. Pinkerton, medical director of the Midlife Health Center at the University of Virginia, Charlottesville, but physicians aren’t always aware of what’s available.
In a video interview at annual meeting of the North American Menopause Society, Dr. Pinkerton discussed the range of therapies physicians can offer menopausal patients with vaginal dryness.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Menopausal women who experience vaginal dryness often find the resultant pain of sexual intercourse so prohibitive that, according to Dr. JoAnne Pinkerton, often these women go months or years without intimate relations.
There are many treatment options that can benefit patients with the condition, explained Dr. Pinkerton, medical director of the Midlife Health Center at the University of Virginia, Charlottesville, but physicians aren’t always aware of what’s available.
In a video interview at annual meeting of the North American Menopause Society, Dr. Pinkerton discussed the range of therapies physicians can offer menopausal patients with vaginal dryness.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Menopausal women who experience vaginal dryness often find the resultant pain of sexual intercourse so prohibitive that, according to Dr. JoAnne Pinkerton, often these women go months or years without intimate relations.
There are many treatment options that can benefit patients with the condition, explained Dr. Pinkerton, medical director of the Midlife Health Center at the University of Virginia, Charlottesville, but physicians aren’t always aware of what’s available.
In a video interview at annual meeting of the North American Menopause Society, Dr. Pinkerton discussed the range of therapies physicians can offer menopausal patients with vaginal dryness.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE NAMS 2014 ANNUAL MEETING
VIDEO: Causes of low libido in postmenopausal women are complex, don’t always require pharmacologic treatment
NATIONAL HARBOR, MD. – Menopause is often accompanied by complex changes in sexual health and function, and factors contributing to low libido and other sexual problems in postmenopausal women may include a combination of hormonal and psychological components, according to experts at the annual meeting of the North American Menopause Society (NAMS).
In this roundtable discussion, Dr. Jan L. Shifren of Harvard Medical School, Boston; NAMS executive director Dr. Margery Gass of Cleveland Clinic; and psychologist Sheryl A. Kingsberg, Ph.D., of the Case Western Reserve University, Cleveland, discuss the physiological, psychological, and relationship factors that affect sexual function and desire in postmenopausal women.
They also talk about treatments such as psychotherapy, low-dose estrogen therapy, and testosterone therapy that may be used to address postmenopausal sexual problems, and how to determine when psychological intervention alone is adequate, and when pharmacologic intervention may be needed.
But the most important thing, they stressed, was to ask women if they are having any changes in sexual health. This is not a topic most women are likely to bring up themselves.
Dr. Kingsberg disclosed relationships with Apricus Biosciences, Emotional Brain, Metagenics, Novo Nordisk, Palatin Technologies, Pfizer, Shionogi, Sprout Pharmaceuticals, Strategic Science and Technologies, and Teva Pharmaceuticals. Dr. Gass and Dr. Shifren did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Menopause is often accompanied by complex changes in sexual health and function, and factors contributing to low libido and other sexual problems in postmenopausal women may include a combination of hormonal and psychological components, according to experts at the annual meeting of the North American Menopause Society (NAMS).
In this roundtable discussion, Dr. Jan L. Shifren of Harvard Medical School, Boston; NAMS executive director Dr. Margery Gass of Cleveland Clinic; and psychologist Sheryl A. Kingsberg, Ph.D., of the Case Western Reserve University, Cleveland, discuss the physiological, psychological, and relationship factors that affect sexual function and desire in postmenopausal women.
They also talk about treatments such as psychotherapy, low-dose estrogen therapy, and testosterone therapy that may be used to address postmenopausal sexual problems, and how to determine when psychological intervention alone is adequate, and when pharmacologic intervention may be needed.
But the most important thing, they stressed, was to ask women if they are having any changes in sexual health. This is not a topic most women are likely to bring up themselves.
Dr. Kingsberg disclosed relationships with Apricus Biosciences, Emotional Brain, Metagenics, Novo Nordisk, Palatin Technologies, Pfizer, Shionogi, Sprout Pharmaceuticals, Strategic Science and Technologies, and Teva Pharmaceuticals. Dr. Gass and Dr. Shifren did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Menopause is often accompanied by complex changes in sexual health and function, and factors contributing to low libido and other sexual problems in postmenopausal women may include a combination of hormonal and psychological components, according to experts at the annual meeting of the North American Menopause Society (NAMS).
In this roundtable discussion, Dr. Jan L. Shifren of Harvard Medical School, Boston; NAMS executive director Dr. Margery Gass of Cleveland Clinic; and psychologist Sheryl A. Kingsberg, Ph.D., of the Case Western Reserve University, Cleveland, discuss the physiological, psychological, and relationship factors that affect sexual function and desire in postmenopausal women.
They also talk about treatments such as psychotherapy, low-dose estrogen therapy, and testosterone therapy that may be used to address postmenopausal sexual problems, and how to determine when psychological intervention alone is adequate, and when pharmacologic intervention may be needed.
But the most important thing, they stressed, was to ask women if they are having any changes in sexual health. This is not a topic most women are likely to bring up themselves.
Dr. Kingsberg disclosed relationships with Apricus Biosciences, Emotional Brain, Metagenics, Novo Nordisk, Palatin Technologies, Pfizer, Shionogi, Sprout Pharmaceuticals, Strategic Science and Technologies, and Teva Pharmaceuticals. Dr. Gass and Dr. Shifren did not report any relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
FROM THE NAMS ANNUAL MEETING
MenoPro: An app from NAMS for you and your menopausal patient
AUDIO: Conjugated estrogen, bazedoxifene combo offers menopause treatment option
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Treating menopause symptoms in women who still have a uterus can be difficult, but alternatives to estrogen are available.
For many women, the use of estrogen therapies is contraindicated – and even if it is prescribed, many women are noncompliant because of fears of increased risk of breast or uterine cancer, explained Dr. JoAnn Pinkerton of the University of Virginia, Charlottesville.
However, with the U.S. Food and Drug Administration’s recent approval of Duavee, a combination of conjugated estrogen and bazedoxifene, Dr. Pinkerton noted, women who don’t want to take estrogen have another option for quelling their menopause symptoms.
In an interview at the annual meeting of the North American Menopause Society, Dr. Pinkerton analyzes the benefits of combination therapy.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE NAMS ANNUAL MEETING