User login
Seven years of hot flashes common during, after menopause
For many women, vasomotor symptoms, including hot flashes and night sweats, last for more years and persist longer past the final menstrual period than previously thought, a large multiethnic, multiracial observational study has shown.
Of the 3,302 enrollees in the Study of Women’s Health Across the Nation (SWAN), 1,449 reported frequent (6 or more days in the previous 2 weeks) vasomotor symptoms (VMS), reported Nancy E. Avis, Ph.D., and her associates. This group experienced a median 7.4 years of VMS, with a median 4.5 years of symptoms after the final menstrual period (FMP) for the subset of 881 women who identified a definite FMP (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8063]).
The researchers also identified risk factors for more prolonged duration of VMS and longer persistence after FMP. Ethnicity was a significant factor in VMS variation (P <.001); African American women had the longest duration of VMS at 10.1 years, followed by Hispanic women (8.9 years), non-Hispanic white women (6.5 years), Chinese women (5.4 years), and Japanese women (4.8 years), said Dr. Avis of Wake Forest University, Winston-Salem, N.C., and her associates
African American women in the study also experienced the longest duration of VMS symptoms post-FMP. Depressive symptoms, anxiety, lower educational status, and higher perceived stress were among the other variables significantly associated with longer duration of VMS and longer persistence of symptoms after FMP.
Overall, the strongest single factor predicting both longer duration of VMS and longer symptom persistence after FMP was symptom onset occurring before menopause or during early perimenopause (P <.001).
Dr. Avis and her associates reported several limitations. For example, total VMS duration might have been underestimated. In addition, some women continued to report VMS beyond the 13-year follow-up period, “so longer follow-up is needed to better pinpoint the timing of cessation of VMS,” they noted.
Still, the findings can help clinicians “counsel patients about expectations regarding VMS and assist women in making treatment decisions,” the investigators wrote.
The research was supported by the National Institutes of Health, the Department of Health & Human Services, the National Institute on Aging, the National Institute of Nursing Research, and the Office of Research on Women’s Health. One author reported receiving grant support from Cephalon/Teva, serving as a consultant to Noven, and serving on an advisory board for Merck. None of the other study authors reported financial disclosures.
The study by Dr. Avis and her associates highlights the need to address the persistent and often troubling VMS symptoms that plague many women for years, often long after menopause. The study, which draws strength both from its large sample size and longitudinal design, makes clear that existing clinical guidelines have not recognized the full impact of VMS on women in midlife.
Many treatment strategies that focus on short-term symptom management might need to be revisited in light of this study’s findings. If symptom duration, for many, is longer than previously thought, then clinicians and patients will need to give careful consideration to longer-term risks and benefits for hormonal and nonhormonal treatment options.
Further, counseling regarding treatment initiation and options can now be individualized based on known risk factors. The wide range of treatment options and ongoing research in this area should contribute to improved outcomes for symptomatic women in midlife.
Gloria Richard-Davis, M.D., is affiliated with the department of ob.gyn. at the University of Arkansas Medical Sciences Center, Little Rock; JoAnn E. Manson, M.D., Dr.P.H., is affiliated with the division of preventive medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston. This commentary is drawn from the accompanying editorial (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8099]). The authors reported no conflicts of interest.
The study by Dr. Avis and her associates highlights the need to address the persistent and often troubling VMS symptoms that plague many women for years, often long after menopause. The study, which draws strength both from its large sample size and longitudinal design, makes clear that existing clinical guidelines have not recognized the full impact of VMS on women in midlife.
Many treatment strategies that focus on short-term symptom management might need to be revisited in light of this study’s findings. If symptom duration, for many, is longer than previously thought, then clinicians and patients will need to give careful consideration to longer-term risks and benefits for hormonal and nonhormonal treatment options.
Further, counseling regarding treatment initiation and options can now be individualized based on known risk factors. The wide range of treatment options and ongoing research in this area should contribute to improved outcomes for symptomatic women in midlife.
Gloria Richard-Davis, M.D., is affiliated with the department of ob.gyn. at the University of Arkansas Medical Sciences Center, Little Rock; JoAnn E. Manson, M.D., Dr.P.H., is affiliated with the division of preventive medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston. This commentary is drawn from the accompanying editorial (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8099]). The authors reported no conflicts of interest.
The study by Dr. Avis and her associates highlights the need to address the persistent and often troubling VMS symptoms that plague many women for years, often long after menopause. The study, which draws strength both from its large sample size and longitudinal design, makes clear that existing clinical guidelines have not recognized the full impact of VMS on women in midlife.
Many treatment strategies that focus on short-term symptom management might need to be revisited in light of this study’s findings. If symptom duration, for many, is longer than previously thought, then clinicians and patients will need to give careful consideration to longer-term risks and benefits for hormonal and nonhormonal treatment options.
Further, counseling regarding treatment initiation and options can now be individualized based on known risk factors. The wide range of treatment options and ongoing research in this area should contribute to improved outcomes for symptomatic women in midlife.
Gloria Richard-Davis, M.D., is affiliated with the department of ob.gyn. at the University of Arkansas Medical Sciences Center, Little Rock; JoAnn E. Manson, M.D., Dr.P.H., is affiliated with the division of preventive medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston. This commentary is drawn from the accompanying editorial (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8099]). The authors reported no conflicts of interest.
For many women, vasomotor symptoms, including hot flashes and night sweats, last for more years and persist longer past the final menstrual period than previously thought, a large multiethnic, multiracial observational study has shown.
Of the 3,302 enrollees in the Study of Women’s Health Across the Nation (SWAN), 1,449 reported frequent (6 or more days in the previous 2 weeks) vasomotor symptoms (VMS), reported Nancy E. Avis, Ph.D., and her associates. This group experienced a median 7.4 years of VMS, with a median 4.5 years of symptoms after the final menstrual period (FMP) for the subset of 881 women who identified a definite FMP (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8063]).
The researchers also identified risk factors for more prolonged duration of VMS and longer persistence after FMP. Ethnicity was a significant factor in VMS variation (P <.001); African American women had the longest duration of VMS at 10.1 years, followed by Hispanic women (8.9 years), non-Hispanic white women (6.5 years), Chinese women (5.4 years), and Japanese women (4.8 years), said Dr. Avis of Wake Forest University, Winston-Salem, N.C., and her associates
African American women in the study also experienced the longest duration of VMS symptoms post-FMP. Depressive symptoms, anxiety, lower educational status, and higher perceived stress were among the other variables significantly associated with longer duration of VMS and longer persistence of symptoms after FMP.
Overall, the strongest single factor predicting both longer duration of VMS and longer symptom persistence after FMP was symptom onset occurring before menopause or during early perimenopause (P <.001).
Dr. Avis and her associates reported several limitations. For example, total VMS duration might have been underestimated. In addition, some women continued to report VMS beyond the 13-year follow-up period, “so longer follow-up is needed to better pinpoint the timing of cessation of VMS,” they noted.
Still, the findings can help clinicians “counsel patients about expectations regarding VMS and assist women in making treatment decisions,” the investigators wrote.
The research was supported by the National Institutes of Health, the Department of Health & Human Services, the National Institute on Aging, the National Institute of Nursing Research, and the Office of Research on Women’s Health. One author reported receiving grant support from Cephalon/Teva, serving as a consultant to Noven, and serving on an advisory board for Merck. None of the other study authors reported financial disclosures.
For many women, vasomotor symptoms, including hot flashes and night sweats, last for more years and persist longer past the final menstrual period than previously thought, a large multiethnic, multiracial observational study has shown.
Of the 3,302 enrollees in the Study of Women’s Health Across the Nation (SWAN), 1,449 reported frequent (6 or more days in the previous 2 weeks) vasomotor symptoms (VMS), reported Nancy E. Avis, Ph.D., and her associates. This group experienced a median 7.4 years of VMS, with a median 4.5 years of symptoms after the final menstrual period (FMP) for the subset of 881 women who identified a definite FMP (JAMA Intern. Med. 2015 Feb. 16 [doi:10.1001/jamainternmed.2014.8063]).
The researchers also identified risk factors for more prolonged duration of VMS and longer persistence after FMP. Ethnicity was a significant factor in VMS variation (P <.001); African American women had the longest duration of VMS at 10.1 years, followed by Hispanic women (8.9 years), non-Hispanic white women (6.5 years), Chinese women (5.4 years), and Japanese women (4.8 years), said Dr. Avis of Wake Forest University, Winston-Salem, N.C., and her associates
African American women in the study also experienced the longest duration of VMS symptoms post-FMP. Depressive symptoms, anxiety, lower educational status, and higher perceived stress were among the other variables significantly associated with longer duration of VMS and longer persistence of symptoms after FMP.
Overall, the strongest single factor predicting both longer duration of VMS and longer symptom persistence after FMP was symptom onset occurring before menopause or during early perimenopause (P <.001).
Dr. Avis and her associates reported several limitations. For example, total VMS duration might have been underestimated. In addition, some women continued to report VMS beyond the 13-year follow-up period, “so longer follow-up is needed to better pinpoint the timing of cessation of VMS,” they noted.
Still, the findings can help clinicians “counsel patients about expectations regarding VMS and assist women in making treatment decisions,” the investigators wrote.
The research was supported by the National Institutes of Health, the Department of Health & Human Services, the National Institute on Aging, the National Institute of Nursing Research, and the Office of Research on Women’s Health. One author reported receiving grant support from Cephalon/Teva, serving as a consultant to Noven, and serving on an advisory board for Merck. None of the other study authors reported financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Vasomotor symptoms persist for more years and last longer past menopause than previously known.
Major finding: Women experiencing frequent vasomotor symptoms had a median 7.4 years of symptoms, with a median of 4.5 years of symptoms after the final menstrual period.
Data source: Multiracial/multiethnic observational study of 1,449 women in the menopausal transition experiencing frequent vasomotor symptoms.
Disclosures: The research was supported by the National Institutes of Health, the Department of Health & Human Services, the National Institute on Aging, the National Institute of Nursing Research, and the Office of Research on Women’s Health. One author reported receiving grant support from Cephalon/Teva, serving as a consultant to Noven, and serving on an advisory board for Merck. None of the other study authors reported financial disclosures.
Short-term hormone replacement therapy upped ovarian cancer risk
Current, short-term use of hormone replacement therapy led to a 43% increase in risk of serous and endometrioid ovarian cancers, according to a large meta-analysis reported online in the Lancet.
Risk fell after women stopped hormone replacement therapy (HRT), but “remained appreciable” for the next few years, wrote the Collaborative Group on Epidemiological Studies of Ovarian Cancer. And long-term users had an estimated 25% increase in ovarian cancer risk, even a full decade after stopping HRT (relative risk, 1.25; 95% confidence interval, 1.07 to 1.46; P = .005), the group reported.
The findings “strongly suggest a causal relationship – i.e., that among otherwise similar women, use of hormone therapy increases the probability of developing the two most common types of ovarian cancer,” the group wrote (Lancet 2015 Feb. 13 [doi: 10.1016/ S0140-6736(14)61687-1]).
Guidelines on HRT from the United States and Europe do not mention ovarian cancer, and those from the United Kingdom describe only risk with long-term use. The findings are “directly relevant to medical advice, personal choices, and the current efforts to revise ... guidelines,” the investigators wrote.
The meta-analysis included 52 observational cohort studies, 17 of which were prospective. In all, 12,110 postmenopausal women in the prospective studies developed ovarian cancer, and 55% had used HRT. Women who had been on HRT for less than 5 years were 1.43 times more likely to develop ovarian cancer than were nonusers (RR, 1.43; 95% CI, 1.29 to 1.46; P < .0001). Thus, HRT might cause one extra case of ovarian cancer per 1,000 users, and one extra death from ovarian cancer per 1,700 users, the group wrote.
Risk of ovarian cancer was similar regardless of age and type of HRT use, the investigators said.
The Medical Research Council and Cancer Research UK funded the analysis. The authors declared no relevant financial conflicts of interest.
The findings support adding ovarian cancer to the list of adverse effects associated with hormone therapy use. However, compared with cardiovascular diseases and breast cancer, ovarian cancer is far less common, suggesting that overall risk assessment of hormone therapy will not be strongly affected by these results.
The present study did not evaluate dose, and median year of diagnosis was 2001 — slightly before the widespread change in use patterns occurred with publication of the Women’s Health Initiative findings.
It is still not clear whether the current recommendation to use hormone therapy for the shortest duration possible is appropriate for women who are concerned about an increased risk of ovarian cancer. The current report underlines the importance and limitations of observational data for rare and long-term outcomes, especially for the complex associations between regimen, dose, duration, route of administration, and timing of hormone therapy use with ovarian, breast, and endometrial cancers.
Dr. Nicolas Wentzensen and Dr. Britton Trabert are investigators with the division of cancer epidemiology & genetics at the National Cancer Institute, Bethesda, Md. These comments are taken from their accompanying editorial (Lancet 2015 Feb. 13 [doi:10.1016/ S0140-6736(14)62458-2]). They had no competing interests.
The findings support adding ovarian cancer to the list of adverse effects associated with hormone therapy use. However, compared with cardiovascular diseases and breast cancer, ovarian cancer is far less common, suggesting that overall risk assessment of hormone therapy will not be strongly affected by these results.
The present study did not evaluate dose, and median year of diagnosis was 2001 — slightly before the widespread change in use patterns occurred with publication of the Women’s Health Initiative findings.
It is still not clear whether the current recommendation to use hormone therapy for the shortest duration possible is appropriate for women who are concerned about an increased risk of ovarian cancer. The current report underlines the importance and limitations of observational data for rare and long-term outcomes, especially for the complex associations between regimen, dose, duration, route of administration, and timing of hormone therapy use with ovarian, breast, and endometrial cancers.
Dr. Nicolas Wentzensen and Dr. Britton Trabert are investigators with the division of cancer epidemiology & genetics at the National Cancer Institute, Bethesda, Md. These comments are taken from their accompanying editorial (Lancet 2015 Feb. 13 [doi:10.1016/ S0140-6736(14)62458-2]). They had no competing interests.
The findings support adding ovarian cancer to the list of adverse effects associated with hormone therapy use. However, compared with cardiovascular diseases and breast cancer, ovarian cancer is far less common, suggesting that overall risk assessment of hormone therapy will not be strongly affected by these results.
The present study did not evaluate dose, and median year of diagnosis was 2001 — slightly before the widespread change in use patterns occurred with publication of the Women’s Health Initiative findings.
It is still not clear whether the current recommendation to use hormone therapy for the shortest duration possible is appropriate for women who are concerned about an increased risk of ovarian cancer. The current report underlines the importance and limitations of observational data for rare and long-term outcomes, especially for the complex associations between regimen, dose, duration, route of administration, and timing of hormone therapy use with ovarian, breast, and endometrial cancers.
Dr. Nicolas Wentzensen and Dr. Britton Trabert are investigators with the division of cancer epidemiology & genetics at the National Cancer Institute, Bethesda, Md. These comments are taken from their accompanying editorial (Lancet 2015 Feb. 13 [doi:10.1016/ S0140-6736(14)62458-2]). They had no competing interests.
Current, short-term use of hormone replacement therapy led to a 43% increase in risk of serous and endometrioid ovarian cancers, according to a large meta-analysis reported online in the Lancet.
Risk fell after women stopped hormone replacement therapy (HRT), but “remained appreciable” for the next few years, wrote the Collaborative Group on Epidemiological Studies of Ovarian Cancer. And long-term users had an estimated 25% increase in ovarian cancer risk, even a full decade after stopping HRT (relative risk, 1.25; 95% confidence interval, 1.07 to 1.46; P = .005), the group reported.
The findings “strongly suggest a causal relationship – i.e., that among otherwise similar women, use of hormone therapy increases the probability of developing the two most common types of ovarian cancer,” the group wrote (Lancet 2015 Feb. 13 [doi: 10.1016/ S0140-6736(14)61687-1]).
Guidelines on HRT from the United States and Europe do not mention ovarian cancer, and those from the United Kingdom describe only risk with long-term use. The findings are “directly relevant to medical advice, personal choices, and the current efforts to revise ... guidelines,” the investigators wrote.
The meta-analysis included 52 observational cohort studies, 17 of which were prospective. In all, 12,110 postmenopausal women in the prospective studies developed ovarian cancer, and 55% had used HRT. Women who had been on HRT for less than 5 years were 1.43 times more likely to develop ovarian cancer than were nonusers (RR, 1.43; 95% CI, 1.29 to 1.46; P < .0001). Thus, HRT might cause one extra case of ovarian cancer per 1,000 users, and one extra death from ovarian cancer per 1,700 users, the group wrote.
Risk of ovarian cancer was similar regardless of age and type of HRT use, the investigators said.
The Medical Research Council and Cancer Research UK funded the analysis. The authors declared no relevant financial conflicts of interest.
Current, short-term use of hormone replacement therapy led to a 43% increase in risk of serous and endometrioid ovarian cancers, according to a large meta-analysis reported online in the Lancet.
Risk fell after women stopped hormone replacement therapy (HRT), but “remained appreciable” for the next few years, wrote the Collaborative Group on Epidemiological Studies of Ovarian Cancer. And long-term users had an estimated 25% increase in ovarian cancer risk, even a full decade after stopping HRT (relative risk, 1.25; 95% confidence interval, 1.07 to 1.46; P = .005), the group reported.
The findings “strongly suggest a causal relationship – i.e., that among otherwise similar women, use of hormone therapy increases the probability of developing the two most common types of ovarian cancer,” the group wrote (Lancet 2015 Feb. 13 [doi: 10.1016/ S0140-6736(14)61687-1]).
Guidelines on HRT from the United States and Europe do not mention ovarian cancer, and those from the United Kingdom describe only risk with long-term use. The findings are “directly relevant to medical advice, personal choices, and the current efforts to revise ... guidelines,” the investigators wrote.
The meta-analysis included 52 observational cohort studies, 17 of which were prospective. In all, 12,110 postmenopausal women in the prospective studies developed ovarian cancer, and 55% had used HRT. Women who had been on HRT for less than 5 years were 1.43 times more likely to develop ovarian cancer than were nonusers (RR, 1.43; 95% CI, 1.29 to 1.46; P < .0001). Thus, HRT might cause one extra case of ovarian cancer per 1,000 users, and one extra death from ovarian cancer per 1,700 users, the group wrote.
Risk of ovarian cancer was similar regardless of age and type of HRT use, the investigators said.
The Medical Research Council and Cancer Research UK funded the analysis. The authors declared no relevant financial conflicts of interest.
Key clinical point: Hormone replacement therapy (HRT) was linked to an increased risk of ovarian cancer.
Major finding: Current use of HRT for less than 5 years was associated with a 43% increase in ovarian cancer risk (P < .0001).
Data source: Meta-analysis of data from 17 prospective studies.
Disclosures: The Medical Research Council and Cancer Research UK funded the study. The authors declared no relevant financial conflicts of interest.
Unnecessary hysterectomies still significant, Michigan data indicate
Almost one in five hysterectomies for benign indications were unnecessary, based on 2013 data from 52 Michigan hospitals.
Uterine pathology reports did not match or support the indication for surgery in 18% of the 3,397 hysterectomies reviewed from the Michigan Surgical Quality Collaborative, a state-wide program to improve surgical care. In women under 40 years old, pathology did not support surgery in 38% of hysterectomies for benign indications.
Endometriosis and chronic pain were the most common reasons for unnecessary uterus removal; pathology was unsupportive of surgery in about 40% of those cases. Pathology also was unsupportive in about 14% of women with fibroid or acute uterine bleeding (AUB) and in about 20% of the remaining cases, which were mostly indicated for a blend of bleeding, pain, and other problems (Am. J. Obstet. Gynecol. 2014 Dec 23 [doi:http://dx.doi.org/10.1016/j.ajog.2014.11.031]).
Almost half of the women had no documentation in their charts that alternatives to hysterectomy were tried or even considered. Hormonal management, operative hysteroscopy, endometrial ablation, levonorgestrel intrauterine devices (IUDs), and other approaches were documented in 68% of women under 40 years old, but documentation was less likely in women over 40 years old. Alternatives approaches were more likely in women with larger uteri and in women with endometriosis, but were, overall, “underutilized,” Dr. Daniel Morgan, an associate professor of obstetrics and gynecology at the University of Michigan in Ann Arbor, and his fellow researchers concluded.
Parity, body mass index, insurance, and common medical comorbidities did not seem to influence the use of alternatives in the study.
The researchers noted that checklists for preoperative appropriateness have been shown in previous studies to reduce the rate of benign hysterectomies, and increase the likelihood that pathology will support the reason for the operation.
The checklist approach “could help standardize treatment and ensure appropriate uterine-sparing management has been offered. The use of electronic medical records systems could potentially facilitate this type of standardization with relative ease,” the researchers wrote.
Also, the levonorgestrel IUD, “a highly effective, cost-saving intervention for women with acute uterine bleeding and pelvic pain, was considered [in] only 12%” of the Michigan cases. Increasing its use is another “important area for quality improvement and cost savings,” they added.
“We are now in the process of developing institution-specific reports ... on use of alternatives prior to hysterectomy and rates of negative pathology. It is our goal that each institution in the Collaborative will see their data and act on it accordingly. We hope that it will lead to more use (or at least consideration) of alternatives to hysterectomy and lower rates of negative pathology.”
The Michigan Surgical Quality Collaborative is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network. Dr. Morgan reported no conflicts of interest.
Almost one in five hysterectomies for benign indications were unnecessary, based on 2013 data from 52 Michigan hospitals.
Uterine pathology reports did not match or support the indication for surgery in 18% of the 3,397 hysterectomies reviewed from the Michigan Surgical Quality Collaborative, a state-wide program to improve surgical care. In women under 40 years old, pathology did not support surgery in 38% of hysterectomies for benign indications.
Endometriosis and chronic pain were the most common reasons for unnecessary uterus removal; pathology was unsupportive of surgery in about 40% of those cases. Pathology also was unsupportive in about 14% of women with fibroid or acute uterine bleeding (AUB) and in about 20% of the remaining cases, which were mostly indicated for a blend of bleeding, pain, and other problems (Am. J. Obstet. Gynecol. 2014 Dec 23 [doi:http://dx.doi.org/10.1016/j.ajog.2014.11.031]).
Almost half of the women had no documentation in their charts that alternatives to hysterectomy were tried or even considered. Hormonal management, operative hysteroscopy, endometrial ablation, levonorgestrel intrauterine devices (IUDs), and other approaches were documented in 68% of women under 40 years old, but documentation was less likely in women over 40 years old. Alternatives approaches were more likely in women with larger uteri and in women with endometriosis, but were, overall, “underutilized,” Dr. Daniel Morgan, an associate professor of obstetrics and gynecology at the University of Michigan in Ann Arbor, and his fellow researchers concluded.
Parity, body mass index, insurance, and common medical comorbidities did not seem to influence the use of alternatives in the study.
The researchers noted that checklists for preoperative appropriateness have been shown in previous studies to reduce the rate of benign hysterectomies, and increase the likelihood that pathology will support the reason for the operation.
The checklist approach “could help standardize treatment and ensure appropriate uterine-sparing management has been offered. The use of electronic medical records systems could potentially facilitate this type of standardization with relative ease,” the researchers wrote.
Also, the levonorgestrel IUD, “a highly effective, cost-saving intervention for women with acute uterine bleeding and pelvic pain, was considered [in] only 12%” of the Michigan cases. Increasing its use is another “important area for quality improvement and cost savings,” they added.
“We are now in the process of developing institution-specific reports ... on use of alternatives prior to hysterectomy and rates of negative pathology. It is our goal that each institution in the Collaborative will see their data and act on it accordingly. We hope that it will lead to more use (or at least consideration) of alternatives to hysterectomy and lower rates of negative pathology.”
The Michigan Surgical Quality Collaborative is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network. Dr. Morgan reported no conflicts of interest.
Almost one in five hysterectomies for benign indications were unnecessary, based on 2013 data from 52 Michigan hospitals.
Uterine pathology reports did not match or support the indication for surgery in 18% of the 3,397 hysterectomies reviewed from the Michigan Surgical Quality Collaborative, a state-wide program to improve surgical care. In women under 40 years old, pathology did not support surgery in 38% of hysterectomies for benign indications.
Endometriosis and chronic pain were the most common reasons for unnecessary uterus removal; pathology was unsupportive of surgery in about 40% of those cases. Pathology also was unsupportive in about 14% of women with fibroid or acute uterine bleeding (AUB) and in about 20% of the remaining cases, which were mostly indicated for a blend of bleeding, pain, and other problems (Am. J. Obstet. Gynecol. 2014 Dec 23 [doi:http://dx.doi.org/10.1016/j.ajog.2014.11.031]).
Almost half of the women had no documentation in their charts that alternatives to hysterectomy were tried or even considered. Hormonal management, operative hysteroscopy, endometrial ablation, levonorgestrel intrauterine devices (IUDs), and other approaches were documented in 68% of women under 40 years old, but documentation was less likely in women over 40 years old. Alternatives approaches were more likely in women with larger uteri and in women with endometriosis, but were, overall, “underutilized,” Dr. Daniel Morgan, an associate professor of obstetrics and gynecology at the University of Michigan in Ann Arbor, and his fellow researchers concluded.
Parity, body mass index, insurance, and common medical comorbidities did not seem to influence the use of alternatives in the study.
The researchers noted that checklists for preoperative appropriateness have been shown in previous studies to reduce the rate of benign hysterectomies, and increase the likelihood that pathology will support the reason for the operation.
The checklist approach “could help standardize treatment and ensure appropriate uterine-sparing management has been offered. The use of electronic medical records systems could potentially facilitate this type of standardization with relative ease,” the researchers wrote.
Also, the levonorgestrel IUD, “a highly effective, cost-saving intervention for women with acute uterine bleeding and pelvic pain, was considered [in] only 12%” of the Michigan cases. Increasing its use is another “important area for quality improvement and cost savings,” they added.
“We are now in the process of developing institution-specific reports ... on use of alternatives prior to hysterectomy and rates of negative pathology. It is our goal that each institution in the Collaborative will see their data and act on it accordingly. We hope that it will lead to more use (or at least consideration) of alternatives to hysterectomy and lower rates of negative pathology.”
The Michigan Surgical Quality Collaborative is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network. Dr. Morgan reported no conflicts of interest.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Key clinical point: Take the time to try levonorgestrel IUDs and other alternatives before removing a woman’s uterus.
Major finding: About 40% of hysterectomies for benign indications in women younger than 40 years old were not supported by post-surgical pathology.
Data source: Chart review of 3,397 hysterectomies at 52 Michigan hospitals
Disclosures:The Michigan Surgical Quality Collaborative is funded by Blue Cross and Blue Shield of Michigan/Blue Care Network. The lead investigator has no financial conflicts of interest.
Hot flashes linked to increased hip fracture risk
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Hot flashes are associated with a significant increase in the risk of hip fracture, regardless of age, body mass index, and other confounders such as smoking, according to analysis of data from the Women’s Health Study.
The prospective, observational study among 4,867 women aged 50-79 years found a 78% increase in the risk of hip fracture among women with moderate to severe menopausal vasomotor symptoms at baseline, compared with women with no symptoms.
Vasomotor symptom severity was also inversely associated with bone mineral density (BMD) at both the femoral neck and the spine. Compared with women who had no vasomotor symptoms, those with moderate or severe symptoms had 0.015 g/cm2 lower femoral neck BMD and 0.016 g/cm2 lower lumbar spine BMD, according to an analysis published on Dec. 18 in the Journal of Clinical Endocrinology and Metabolism (2014 [doi:10.1210/jc.2014-3062]).
“Despite being younger and heavier than asymptomatic women, characteristics associated with higher BMD, women with moderate/severe [vasomotor symptoms] had a higher risk of hip fractures that was also independent of other established risk factors for fractures,” wrote Dr. Carolyn J. Crandall of the University of California, Los Angeles, and her colleagues.
The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Key clinical point: The severity of vasomotor menopause symptoms is associated with risk of hip fracture.
Major finding: Women with moderate to severe vasomotor symptoms have a 78% increase in their risk of hip fracture.
Data source: A prospective, observational study among 4,867 women aged 50-79 years.
Disclosures: The study was funded by the National Institutes of Health. Two of the study authors reported consulting and other financial relationships with drug and device companies.
Hormone therapy ‘timing hypothesis’ gains ground in ELITE
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.

|
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.

|
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.

|
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Hormone therapy cut atherosclerotic progression, compared with placebo, in women 6 or fewer years out from menopause start, but not in those 10 or more years out.
Major finding: In women up to 6 years out from menopause, hormone therapy was linked with 40% less atherosclerotic progression than with placebo.
Data source: ELITE,a single-center, randomized study with 643 postmenopausal women.
Disclosures: Dr. Hodis had no disclosures.
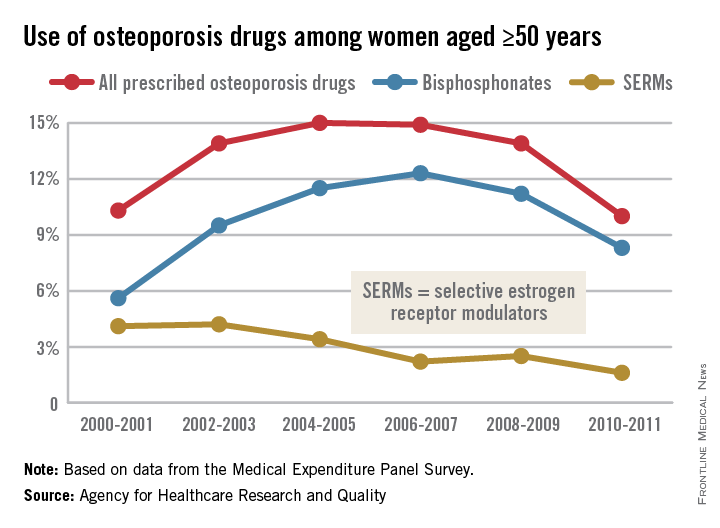
Osteoporosis medication use down in older women
Even as the number of women aged 50 years and older continues to rise, the percentage who are taking osteoporosis medications has fallen by one-third since 2004-2005, the Agency for Healthcare Research and Quality reported.
In 2004-2005, 15% of all women aged 50 years and over were using some form of prescribed osteoporosis drug, but that number had dropped to 10% by 2010-2011, according to data from the Medical Expenditure Panel Survey. The total number of women aged 50 and older increased from 47.8 million to 55.2 million over that same time period.
In 2010-2011, 8.3% of all women aged at least 50 years were using bisphosphonates, down from a high of 12.3% in 2006-2007 but up from 5.6% in 2000-2001. Use of the next most popular form of osteoporosis medication, the selective estrogen receptor modulators (SERMS), declined from 4.2% of all older women in 2002-2003 to 1.6% in 2010-2011, the AHRQ said.
Data for denosumab (Prolia), a fully human monoclonal antibody drug approved for the treatment of osteoporosis, were not available in the MEPS during the analysis period.
Even as the number of women aged 50 years and older continues to rise, the percentage who are taking osteoporosis medications has fallen by one-third since 2004-2005, the Agency for Healthcare Research and Quality reported.
In 2004-2005, 15% of all women aged 50 years and over were using some form of prescribed osteoporosis drug, but that number had dropped to 10% by 2010-2011, according to data from the Medical Expenditure Panel Survey. The total number of women aged 50 and older increased from 47.8 million to 55.2 million over that same time period.
In 2010-2011, 8.3% of all women aged at least 50 years were using bisphosphonates, down from a high of 12.3% in 2006-2007 but up from 5.6% in 2000-2001. Use of the next most popular form of osteoporosis medication, the selective estrogen receptor modulators (SERMS), declined from 4.2% of all older women in 2002-2003 to 1.6% in 2010-2011, the AHRQ said.
Data for denosumab (Prolia), a fully human monoclonal antibody drug approved for the treatment of osteoporosis, were not available in the MEPS during the analysis period.
Even as the number of women aged 50 years and older continues to rise, the percentage who are taking osteoporosis medications has fallen by one-third since 2004-2005, the Agency for Healthcare Research and Quality reported.
In 2004-2005, 15% of all women aged 50 years and over were using some form of prescribed osteoporosis drug, but that number had dropped to 10% by 2010-2011, according to data from the Medical Expenditure Panel Survey. The total number of women aged 50 and older increased from 47.8 million to 55.2 million over that same time period.
In 2010-2011, 8.3% of all women aged at least 50 years were using bisphosphonates, down from a high of 12.3% in 2006-2007 but up from 5.6% in 2000-2001. Use of the next most popular form of osteoporosis medication, the selective estrogen receptor modulators (SERMS), declined from 4.2% of all older women in 2002-2003 to 1.6% in 2010-2011, the AHRQ said.
Data for denosumab (Prolia), a fully human monoclonal antibody drug approved for the treatment of osteoporosis, were not available in the MEPS during the analysis period.
New use for old DXA scanners?
NATIONAL HARBOR, MD. – If you’re one of the many physicians with an X-ray absorptiometry machine collecting dust in the nether regions of your clinic, recent findings from a small pilot study might interest you.
Dr. Steven R. Goldstein said he found that roughly 20% of postmenopausal women whose body mass index (BMI) measurement indicated they had a normal weight actually had body fat measurements greater than the 75th percentile when their body fat was assessed using a X-ray absorptiometry (DXA) scanner.
Similarly, about a fifth of postmenopausal women in the study whose BMI indicated they were in the overweight range actually had DXA scanner readings indicating their percentage of body fat placed them below the 25th percentile.
“When you have 20% in each group, that’s not a tiny number,” Dr. Goldstein said in an interview at this year’s annual meeting of the North American Menopause Society. “BMI or weight alone does not always predict metabolic health.”
BMI, a measurement first created for use by insurance actuaries by Belgian mathematician Adolphe Quetelet in the mid-1800s, has come under scrutiny across the specialties in recent years, particularly since 1998 when the Centers for Disease Control and Prevention aligned with the World Health Organization determined that the criteria for being overweight was a BMI of 25 kg/m2 instead of 27.8 kg/m2.
“This instantly rendered 29 million previously healthy Americans as now being overweight,” Dr. Goldstein, a professor of obstetrics and gynecology at New York (N.Y.) University Langone Medical Center, told the audience.
Additionally, as one audience member noted during the question and answer period of the presentation, many members of nonAnglo populations, such as those from Mexico and Central America, are strong, healthy endomorphs, even though their BMIs would indicate otherwise.
“Most people don’t think about the fact that BMI is 170 years old and that with it, a 25-year-old man who weighs 150 pounds and is 5 foot 8 inches, has the same BMI as a 60-year-old woman who also weighs 150 pounds,” Dr. Goldstein said in an interview. “And yet, it’s used almost as another vital sign.”
Instead, Dr. Goldstein said that data from DXA scanners could be used by insurance companies to make more accurate decisions about obesity-related medical procedures, such as bariatric surgery. Studies have shown that some adults considered normal weight have cardiovascular abnormalities, and some considered obese are metabolically healthy (Arch. Intern. Med. 2008;168:1617-24).
“Using BMI, some people are being denied bariatric surgery when they need it, but there may be other people who are having the surgery who don’t actually need it,” Dr. Goldstein said.
The scanners also have met with some darker times, especially as reimbursements for the technology primarily used to determine bone mineral density in an aging population have fluctuated over the years, leaving many physicians with thousands of dollars in equipment they no longer use for fear they will not be paid for their time.
DXA scanners use two different energy levels that are absorbed differently by bone and lean and fat tissues, then uses the differences between them to determine the amount of lean and fat tissue across the entire body.
Dr. Goldstein and his colleagues analyzed DXA data taken from 50 postmenopausal women who visited his clinic for a routine bone mass DXA scan. The scanner software was used to calculate the women’s body fat percentile according to age and sex. Women who were between the 25th and 75th percentile were considered to have “normal” body fat, whereas below the 25th percentile or above the 75th percentile was considered abnormally lean or heavy, respectively. The measurements then were compared with the women’s BMI measurements.
Just over 18% of the cohort had normal BMI readings but placed above the 75th percentile for body fat when measured by the scanner. Conversely, 22% of women in the lowest percentile for body fat per their DXA scan also had normal BMI weight. Twenty-three percent of women considered overweight according to their BMI fell into the normal range for DXA body fat results. All of the women in the study considered obese by BMI were in the 100th percentile using the DXA scanner.
“DXA determination of body fat percentile seems like a reasonable and probable surrogate for metabolic health that is more accurate than BMI,” Dr. Goldstein said.
“We have the equipment, it’s just a matter of updating the software,” he said.
Dr. Goldstein said he had no relevant disclosures.
On Twitter @whitneymcknight
Body mass index is the best screening tool we have to identify and stage the disease of obesity. Like other diseases, there are additional prognostic factors that influence disease severity. Additional information regarding fat composition can indicate more risk in addition to BMI. For example, more visceral or abdominal fat could imply greater cardiac risk particularly in Asian populations. BMI is the fifth vital sign, and any BMI >30 warrants discussion regarding treatment. Additional studies like DXA scans can elaborate additional risk that can be mitigated through safe and effective treatments for obesity like bariatric surgery.
John Morton, M.D., is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He was asked to comment on this study.
Body mass index is the best screening tool we have to identify and stage the disease of obesity. Like other diseases, there are additional prognostic factors that influence disease severity. Additional information regarding fat composition can indicate more risk in addition to BMI. For example, more visceral or abdominal fat could imply greater cardiac risk particularly in Asian populations. BMI is the fifth vital sign, and any BMI >30 warrants discussion regarding treatment. Additional studies like DXA scans can elaborate additional risk that can be mitigated through safe and effective treatments for obesity like bariatric surgery.
John Morton, M.D., is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He was asked to comment on this study.
Body mass index is the best screening tool we have to identify and stage the disease of obesity. Like other diseases, there are additional prognostic factors that influence disease severity. Additional information regarding fat composition can indicate more risk in addition to BMI. For example, more visceral or abdominal fat could imply greater cardiac risk particularly in Asian populations. BMI is the fifth vital sign, and any BMI >30 warrants discussion regarding treatment. Additional studies like DXA scans can elaborate additional risk that can be mitigated through safe and effective treatments for obesity like bariatric surgery.
John Morton, M.D., is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He was asked to comment on this study.
NATIONAL HARBOR, MD. – If you’re one of the many physicians with an X-ray absorptiometry machine collecting dust in the nether regions of your clinic, recent findings from a small pilot study might interest you.
Dr. Steven R. Goldstein said he found that roughly 20% of postmenopausal women whose body mass index (BMI) measurement indicated they had a normal weight actually had body fat measurements greater than the 75th percentile when their body fat was assessed using a X-ray absorptiometry (DXA) scanner.
Similarly, about a fifth of postmenopausal women in the study whose BMI indicated they were in the overweight range actually had DXA scanner readings indicating their percentage of body fat placed them below the 25th percentile.
“When you have 20% in each group, that’s not a tiny number,” Dr. Goldstein said in an interview at this year’s annual meeting of the North American Menopause Society. “BMI or weight alone does not always predict metabolic health.”
BMI, a measurement first created for use by insurance actuaries by Belgian mathematician Adolphe Quetelet in the mid-1800s, has come under scrutiny across the specialties in recent years, particularly since 1998 when the Centers for Disease Control and Prevention aligned with the World Health Organization determined that the criteria for being overweight was a BMI of 25 kg/m2 instead of 27.8 kg/m2.
“This instantly rendered 29 million previously healthy Americans as now being overweight,” Dr. Goldstein, a professor of obstetrics and gynecology at New York (N.Y.) University Langone Medical Center, told the audience.
Additionally, as one audience member noted during the question and answer period of the presentation, many members of nonAnglo populations, such as those from Mexico and Central America, are strong, healthy endomorphs, even though their BMIs would indicate otherwise.
“Most people don’t think about the fact that BMI is 170 years old and that with it, a 25-year-old man who weighs 150 pounds and is 5 foot 8 inches, has the same BMI as a 60-year-old woman who also weighs 150 pounds,” Dr. Goldstein said in an interview. “And yet, it’s used almost as another vital sign.”
Instead, Dr. Goldstein said that data from DXA scanners could be used by insurance companies to make more accurate decisions about obesity-related medical procedures, such as bariatric surgery. Studies have shown that some adults considered normal weight have cardiovascular abnormalities, and some considered obese are metabolically healthy (Arch. Intern. Med. 2008;168:1617-24).
“Using BMI, some people are being denied bariatric surgery when they need it, but there may be other people who are having the surgery who don’t actually need it,” Dr. Goldstein said.
The scanners also have met with some darker times, especially as reimbursements for the technology primarily used to determine bone mineral density in an aging population have fluctuated over the years, leaving many physicians with thousands of dollars in equipment they no longer use for fear they will not be paid for their time.
DXA scanners use two different energy levels that are absorbed differently by bone and lean and fat tissues, then uses the differences between them to determine the amount of lean and fat tissue across the entire body.
Dr. Goldstein and his colleagues analyzed DXA data taken from 50 postmenopausal women who visited his clinic for a routine bone mass DXA scan. The scanner software was used to calculate the women’s body fat percentile according to age and sex. Women who were between the 25th and 75th percentile were considered to have “normal” body fat, whereas below the 25th percentile or above the 75th percentile was considered abnormally lean or heavy, respectively. The measurements then were compared with the women’s BMI measurements.
Just over 18% of the cohort had normal BMI readings but placed above the 75th percentile for body fat when measured by the scanner. Conversely, 22% of women in the lowest percentile for body fat per their DXA scan also had normal BMI weight. Twenty-three percent of women considered overweight according to their BMI fell into the normal range for DXA body fat results. All of the women in the study considered obese by BMI were in the 100th percentile using the DXA scanner.
“DXA determination of body fat percentile seems like a reasonable and probable surrogate for metabolic health that is more accurate than BMI,” Dr. Goldstein said.
“We have the equipment, it’s just a matter of updating the software,” he said.
Dr. Goldstein said he had no relevant disclosures.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – If you’re one of the many physicians with an X-ray absorptiometry machine collecting dust in the nether regions of your clinic, recent findings from a small pilot study might interest you.
Dr. Steven R. Goldstein said he found that roughly 20% of postmenopausal women whose body mass index (BMI) measurement indicated they had a normal weight actually had body fat measurements greater than the 75th percentile when their body fat was assessed using a X-ray absorptiometry (DXA) scanner.
Similarly, about a fifth of postmenopausal women in the study whose BMI indicated they were in the overweight range actually had DXA scanner readings indicating their percentage of body fat placed them below the 25th percentile.
“When you have 20% in each group, that’s not a tiny number,” Dr. Goldstein said in an interview at this year’s annual meeting of the North American Menopause Society. “BMI or weight alone does not always predict metabolic health.”
BMI, a measurement first created for use by insurance actuaries by Belgian mathematician Adolphe Quetelet in the mid-1800s, has come under scrutiny across the specialties in recent years, particularly since 1998 when the Centers for Disease Control and Prevention aligned with the World Health Organization determined that the criteria for being overweight was a BMI of 25 kg/m2 instead of 27.8 kg/m2.
“This instantly rendered 29 million previously healthy Americans as now being overweight,” Dr. Goldstein, a professor of obstetrics and gynecology at New York (N.Y.) University Langone Medical Center, told the audience.
Additionally, as one audience member noted during the question and answer period of the presentation, many members of nonAnglo populations, such as those from Mexico and Central America, are strong, healthy endomorphs, even though their BMIs would indicate otherwise.
“Most people don’t think about the fact that BMI is 170 years old and that with it, a 25-year-old man who weighs 150 pounds and is 5 foot 8 inches, has the same BMI as a 60-year-old woman who also weighs 150 pounds,” Dr. Goldstein said in an interview. “And yet, it’s used almost as another vital sign.”
Instead, Dr. Goldstein said that data from DXA scanners could be used by insurance companies to make more accurate decisions about obesity-related medical procedures, such as bariatric surgery. Studies have shown that some adults considered normal weight have cardiovascular abnormalities, and some considered obese are metabolically healthy (Arch. Intern. Med. 2008;168:1617-24).
“Using BMI, some people are being denied bariatric surgery when they need it, but there may be other people who are having the surgery who don’t actually need it,” Dr. Goldstein said.
The scanners also have met with some darker times, especially as reimbursements for the technology primarily used to determine bone mineral density in an aging population have fluctuated over the years, leaving many physicians with thousands of dollars in equipment they no longer use for fear they will not be paid for their time.
DXA scanners use two different energy levels that are absorbed differently by bone and lean and fat tissues, then uses the differences between them to determine the amount of lean and fat tissue across the entire body.
Dr. Goldstein and his colleagues analyzed DXA data taken from 50 postmenopausal women who visited his clinic for a routine bone mass DXA scan. The scanner software was used to calculate the women’s body fat percentile according to age and sex. Women who were between the 25th and 75th percentile were considered to have “normal” body fat, whereas below the 25th percentile or above the 75th percentile was considered abnormally lean or heavy, respectively. The measurements then were compared with the women’s BMI measurements.
Just over 18% of the cohort had normal BMI readings but placed above the 75th percentile for body fat when measured by the scanner. Conversely, 22% of women in the lowest percentile for body fat per their DXA scan also had normal BMI weight. Twenty-three percent of women considered overweight according to their BMI fell into the normal range for DXA body fat results. All of the women in the study considered obese by BMI were in the 100th percentile using the DXA scanner.
“DXA determination of body fat percentile seems like a reasonable and probable surrogate for metabolic health that is more accurate than BMI,” Dr. Goldstein said.
“We have the equipment, it’s just a matter of updating the software,” he said.
Dr. Goldstein said he had no relevant disclosures.
On Twitter @whitneymcknight
AT THE 2014 NAMS MEETING
Key clinical point: Dual energy X-ray absorptiometry (DXA) data may be better than BMI for assessing metabolic health in postmenopausal women, bariatric surgery candidates.
Major finding: Approximately 20% of postmenopausal women were possibly misdiagnosed as metabolically healthy when BMI was only measurement used.
Data source: Pilot study of 50 consecutive postmenopausal women given DXA scan at a single site.
Disclosures: Dr. Goldstein said he had no relevant disclosures.
2014 Update on osteoporosis
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Ospemifene’s effect more comprehensive than its FDA indication
NATIONAL HARBOR, MD. – Ospemifene, a treatment for dyspareunia in postmenopausal women, also improved other symptoms of vaginal vulvar atrophy, according to a secondary analysis of two pivotal phase III trials presented here.
Marketed as Osphena (Shionogi), ospemifene is an oral estrogen agonist/antagonist approved in 2013 by the U.S. Food and Drug Administration for use in postmenopausal women with dyspareunia.
Had the drug been approved prior to FDA guidance added in 2013, however, ospemifene’s indications might have been more inclusive.
“A lot of people will look at this indication and [think], ‘Why would you just improve dyspareunia when you’re actually working with the estrogen receptor?’ ” said Dr. Ginger Constantine, a researcher at EndoRheum in Malvern, Pa., and a presenter at this year’s annual meeting of the North American Menopause Society, whose secondary analysis of the studies indicated the drug also eases vaginal dryness, itching, irritation, and painful urination in postmenopausal women.
“One would think that you would also be improving dryness ... and that is what you do see,” she said. However, when the patient-reported most bothersome symptom experienced at baseline is considered, different endpoints are achieved, resulting in packaging inserts that don’t necessarily reflect the full scope of what the product can successfully be used to treat, according to Dr. Constantine.
The estradiol acetate vaginal ring, Femring (Actavis), which was FDA-approved in 2002, has a more general indication, based on several combined study endpoints of relief from moderate to severe menopausal symptoms, including vulvar and vaginal atrophy.
Current guidance is more specific, calling for improvement in the patient-reported most bothersome symptom at baseline, as rated on a scale of 0-3, with 2 being moderate and 3 being severe. Dr. Constantine said this has led to controversy because women often have more than one symptom. “A patient might score a 3 for vaginal dryness and a 2 for dyspareunia, but she may not be so worried about her vaginal dryness,” Dr. Constantine said. “It’s her dyspareunia that’s more problematic [for her].”
Factoring in only the most bothersome symptom could impair investigators’ ability to detect statistical significance, and the result of predicating a product’s indication on the most bothersome symptom as part of a study’s construct has the potential to confuse both the clinician and the patient. “Most [postmenopausal] women have vaginal dryness, and now we’re asking them to tell us what ‘really’ bothers them, and they have to flip a coin,” Dr. Constantine said in response to an audience member’s question about whether the FDA’s labeling was too restrictive.
For the analysis, Dr. Constantine and her associates reviewed data from a 12-week, double-blind, pivotal phase III study of 826 postmenopausal women randomly assigned to either 30 mg of daily oral ospemifene, 60 mg daily of the study drug, or placebo.
Although 544 women across the study reported more than one symptom at baseline, the study’s endpoint was improvement in the most bothersome symptom. At baseline, a third of all participants reported experiencing vaginal dryness; 43% reported dyspareunia; and nearly a fifth reported vaginal itching and irritation. However, the No. 1 most bothersome symptom was dyspareunia, with nearly half, 45%, of all women reporting it as their chief complaint.
By the study’s end, the severity scores for the 120 women who’d reported dyspareunia as their most bothersome symptom were significantly improved from baseline (P = .023).
When dryness was assessed either with or without the consideration of a most bothersome symptom, then significant improvements were found (P = .001 and P = .021, respectively).
In a second pivotal, double-blind, phase III study reviewed by Dr. Constantine and her coinvestigators, 919 postmenopausal women were randomly assigned to 60 mg of daily oral ospemifene or placebo for 12 weeks, and were stratified according to the most bothersome symptom of either dyspareunia or dryness.
When compared with placebo, an improvement in dryness as the most bothersome symptom was not statistically significant (P = .0803), although an improvement from baseline in moderate to severe dryness was (P < .001).
Because the endpoints in both studies were improvement in the most bothersome symptom, the treatment was given the indication for dyspareunia only. As to why the statistical significance for dryness was diminished, Dr. Constantine said that because the women studied were allowed to use lubricants, which meant “the dryness really did get better,” it could have interfered with the results.
Compared with placebo, dyspareunia was significantly improved both as a moderate to severe symptom at baseline, and as the most bothersome symptom (P = .0003 and P < .0001, respectively).
Other symptoms such as irritation, itching, and painful urination also were improved, but did not achieve statistical significance in either study.
Given the data seen in this context, Dr. Constantine concluded that ospemifene’s limited indication was predicated more on study constructs than on failure to provide relief from other moderate to severe symptoms.
Dr. Constantine is a paid consultant and board member for Shionogi, the maker of Osphena.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Ospemifene, a treatment for dyspareunia in postmenopausal women, also improved other symptoms of vaginal vulvar atrophy, according to a secondary analysis of two pivotal phase III trials presented here.
Marketed as Osphena (Shionogi), ospemifene is an oral estrogen agonist/antagonist approved in 2013 by the U.S. Food and Drug Administration for use in postmenopausal women with dyspareunia.
Had the drug been approved prior to FDA guidance added in 2013, however, ospemifene’s indications might have been more inclusive.
“A lot of people will look at this indication and [think], ‘Why would you just improve dyspareunia when you’re actually working with the estrogen receptor?’ ” said Dr. Ginger Constantine, a researcher at EndoRheum in Malvern, Pa., and a presenter at this year’s annual meeting of the North American Menopause Society, whose secondary analysis of the studies indicated the drug also eases vaginal dryness, itching, irritation, and painful urination in postmenopausal women.
“One would think that you would also be improving dryness ... and that is what you do see,” she said. However, when the patient-reported most bothersome symptom experienced at baseline is considered, different endpoints are achieved, resulting in packaging inserts that don’t necessarily reflect the full scope of what the product can successfully be used to treat, according to Dr. Constantine.
The estradiol acetate vaginal ring, Femring (Actavis), which was FDA-approved in 2002, has a more general indication, based on several combined study endpoints of relief from moderate to severe menopausal symptoms, including vulvar and vaginal atrophy.
Current guidance is more specific, calling for improvement in the patient-reported most bothersome symptom at baseline, as rated on a scale of 0-3, with 2 being moderate and 3 being severe. Dr. Constantine said this has led to controversy because women often have more than one symptom. “A patient might score a 3 for vaginal dryness and a 2 for dyspareunia, but she may not be so worried about her vaginal dryness,” Dr. Constantine said. “It’s her dyspareunia that’s more problematic [for her].”
Factoring in only the most bothersome symptom could impair investigators’ ability to detect statistical significance, and the result of predicating a product’s indication on the most bothersome symptom as part of a study’s construct has the potential to confuse both the clinician and the patient. “Most [postmenopausal] women have vaginal dryness, and now we’re asking them to tell us what ‘really’ bothers them, and they have to flip a coin,” Dr. Constantine said in response to an audience member’s question about whether the FDA’s labeling was too restrictive.
For the analysis, Dr. Constantine and her associates reviewed data from a 12-week, double-blind, pivotal phase III study of 826 postmenopausal women randomly assigned to either 30 mg of daily oral ospemifene, 60 mg daily of the study drug, or placebo.
Although 544 women across the study reported more than one symptom at baseline, the study’s endpoint was improvement in the most bothersome symptom. At baseline, a third of all participants reported experiencing vaginal dryness; 43% reported dyspareunia; and nearly a fifth reported vaginal itching and irritation. However, the No. 1 most bothersome symptom was dyspareunia, with nearly half, 45%, of all women reporting it as their chief complaint.
By the study’s end, the severity scores for the 120 women who’d reported dyspareunia as their most bothersome symptom were significantly improved from baseline (P = .023).
When dryness was assessed either with or without the consideration of a most bothersome symptom, then significant improvements were found (P = .001 and P = .021, respectively).
In a second pivotal, double-blind, phase III study reviewed by Dr. Constantine and her coinvestigators, 919 postmenopausal women were randomly assigned to 60 mg of daily oral ospemifene or placebo for 12 weeks, and were stratified according to the most bothersome symptom of either dyspareunia or dryness.
When compared with placebo, an improvement in dryness as the most bothersome symptom was not statistically significant (P = .0803), although an improvement from baseline in moderate to severe dryness was (P < .001).
Because the endpoints in both studies were improvement in the most bothersome symptom, the treatment was given the indication for dyspareunia only. As to why the statistical significance for dryness was diminished, Dr. Constantine said that because the women studied were allowed to use lubricants, which meant “the dryness really did get better,” it could have interfered with the results.
Compared with placebo, dyspareunia was significantly improved both as a moderate to severe symptom at baseline, and as the most bothersome symptom (P = .0003 and P < .0001, respectively).
Other symptoms such as irritation, itching, and painful urination also were improved, but did not achieve statistical significance in either study.
Given the data seen in this context, Dr. Constantine concluded that ospemifene’s limited indication was predicated more on study constructs than on failure to provide relief from other moderate to severe symptoms.
Dr. Constantine is a paid consultant and board member for Shionogi, the maker of Osphena.
On Twitter @whitneymcknight
NATIONAL HARBOR, MD. – Ospemifene, a treatment for dyspareunia in postmenopausal women, also improved other symptoms of vaginal vulvar atrophy, according to a secondary analysis of two pivotal phase III trials presented here.
Marketed as Osphena (Shionogi), ospemifene is an oral estrogen agonist/antagonist approved in 2013 by the U.S. Food and Drug Administration for use in postmenopausal women with dyspareunia.
Had the drug been approved prior to FDA guidance added in 2013, however, ospemifene’s indications might have been more inclusive.
“A lot of people will look at this indication and [think], ‘Why would you just improve dyspareunia when you’re actually working with the estrogen receptor?’ ” said Dr. Ginger Constantine, a researcher at EndoRheum in Malvern, Pa., and a presenter at this year’s annual meeting of the North American Menopause Society, whose secondary analysis of the studies indicated the drug also eases vaginal dryness, itching, irritation, and painful urination in postmenopausal women.
“One would think that you would also be improving dryness ... and that is what you do see,” she said. However, when the patient-reported most bothersome symptom experienced at baseline is considered, different endpoints are achieved, resulting in packaging inserts that don’t necessarily reflect the full scope of what the product can successfully be used to treat, according to Dr. Constantine.
The estradiol acetate vaginal ring, Femring (Actavis), which was FDA-approved in 2002, has a more general indication, based on several combined study endpoints of relief from moderate to severe menopausal symptoms, including vulvar and vaginal atrophy.
Current guidance is more specific, calling for improvement in the patient-reported most bothersome symptom at baseline, as rated on a scale of 0-3, with 2 being moderate and 3 being severe. Dr. Constantine said this has led to controversy because women often have more than one symptom. “A patient might score a 3 for vaginal dryness and a 2 for dyspareunia, but she may not be so worried about her vaginal dryness,” Dr. Constantine said. “It’s her dyspareunia that’s more problematic [for her].”
Factoring in only the most bothersome symptom could impair investigators’ ability to detect statistical significance, and the result of predicating a product’s indication on the most bothersome symptom as part of a study’s construct has the potential to confuse both the clinician and the patient. “Most [postmenopausal] women have vaginal dryness, and now we’re asking them to tell us what ‘really’ bothers them, and they have to flip a coin,” Dr. Constantine said in response to an audience member’s question about whether the FDA’s labeling was too restrictive.
For the analysis, Dr. Constantine and her associates reviewed data from a 12-week, double-blind, pivotal phase III study of 826 postmenopausal women randomly assigned to either 30 mg of daily oral ospemifene, 60 mg daily of the study drug, or placebo.
Although 544 women across the study reported more than one symptom at baseline, the study’s endpoint was improvement in the most bothersome symptom. At baseline, a third of all participants reported experiencing vaginal dryness; 43% reported dyspareunia; and nearly a fifth reported vaginal itching and irritation. However, the No. 1 most bothersome symptom was dyspareunia, with nearly half, 45%, of all women reporting it as their chief complaint.
By the study’s end, the severity scores for the 120 women who’d reported dyspareunia as their most bothersome symptom were significantly improved from baseline (P = .023).
When dryness was assessed either with or without the consideration of a most bothersome symptom, then significant improvements were found (P = .001 and P = .021, respectively).
In a second pivotal, double-blind, phase III study reviewed by Dr. Constantine and her coinvestigators, 919 postmenopausal women were randomly assigned to 60 mg of daily oral ospemifene or placebo for 12 weeks, and were stratified according to the most bothersome symptom of either dyspareunia or dryness.
When compared with placebo, an improvement in dryness as the most bothersome symptom was not statistically significant (P = .0803), although an improvement from baseline in moderate to severe dryness was (P < .001).
Because the endpoints in both studies were improvement in the most bothersome symptom, the treatment was given the indication for dyspareunia only. As to why the statistical significance for dryness was diminished, Dr. Constantine said that because the women studied were allowed to use lubricants, which meant “the dryness really did get better,” it could have interfered with the results.
Compared with placebo, dyspareunia was significantly improved both as a moderate to severe symptom at baseline, and as the most bothersome symptom (P = .0003 and P < .0001, respectively).
Other symptoms such as irritation, itching, and painful urination also were improved, but did not achieve statistical significance in either study.
Given the data seen in this context, Dr. Constantine concluded that ospemifene’s limited indication was predicated more on study constructs than on failure to provide relief from other moderate to severe symptoms.
Dr. Constantine is a paid consultant and board member for Shionogi, the maker of Osphena.
On Twitter @whitneymcknight
AT THE NAMS 2014 ANNUAL MEETING
Key clinical point: Ospemifene could be an effective treatment for a range of moderate to severe menopausal symptoms.
Major finding: Ospemifine 60 mg daily, FDA-approved for dyspareunia, also yielded statistically significant improvement in vaginal dryness.
Data source: Secondary analysis of two randomly controlled, double-blind, pivotal phase III trials of 826 women treated with either 30 mg or 60 mg of daily oral ospemifene or placebo, and 919 women treated with either 60 mg ospemifene daily or placebo, stratified at randomization by most bothersome symptom at baseline.
Disclosures: Dr. Constantine is a paid consultant and board member for Shionogi, maker of Osphena.
Try testosterone only in women with hypoactive sexual desire
Testosterone supplementation is not recommended for women, unless they have a clinical diagnosis of hypoactive sexual desire disorder, a joint task force led by the Endocrine Society has determined.
While a number of studies have shown that testosterone supplementation helps improve sexual desire, cognition, and other outcomes, most are either too small, too short, or confounded by other factors to provide irrefutable evidence of the hormone’s benefit for any indication other than hypoactive sexual desire disorder (HSDD), Dr. Margaret E. Wierman and her colleagues wrote in the Journal of Clinical Endocrinology and Metabolism (J. Clin. Endocrinol. Metab. 2014;99:3489-510).
A trial of testosterone is only appropriate for postmenopausal women with a diagnosed HSDD, wrote the task force, which also noted that libido and response improvements don’t necessarily correlate with testosterone serum levels. If there’s no clinical response after a 6-month trial, the hormone should be discontinued.
The hormone-health connection is clearly important to women, and many have already done Internet research on the topic, said Dr. Margery Gass, executive director of the North American Menopause Society. A Google query for “hormones to control aging” turned up 9.5 million results, she said.
Although NAMS was not involved in creating the new clinical guideline, the society supports it, she said in an interview.
“The Endocrine Society has published an excellent set of guidelines on the use of androgen therapy in women. These guidelines are in harmony with NAMS’ comments on the use of testosterone and dehydroepiandrosterones,” included in the group’s clinical practice guideline for the care of midlife women (Menopause 2014;21:1038-62). That paper also recommends a trial of testosterone therapy “in carefully selected postmenopausal women with female sexual interest/arousal disorder and no other etiology for their sexual problems.”
Dr. Gass continued, “I encourage clinicians to use both publications to help women understand the misinformation about testosterone and other androgens that they are seeing on the Internet.”
Dr. Wierman, chief of endocrinology at the University of Colorado, Aurora, led the task force, composed of representatives from the American Society for Reproductive Medicine, the American Congress of Obstetricians and Gynecologists, the European Society of Endocrinology, and the International Menopause Society. Together, they reviewed the extant literature and, in addition, commissioned meta-analyses to examine the issue of androgen supplementation in premenopausal and postmenopausal women – either healthy or with specific conditions. Evidence was graded as level 1 (strong) or level 2 (weak).
The group considered androgen supplementation for a number of indications: infertility; sexual dysfunction; cognition; cardiovascular, metabolic, and bone health; and general well-being. It found little strong data to support the practice in any of these except HSDD.
As a basic tenet, the task force agreed that androgen testing for most women is inaccurate, and thus virtually incapable of providing useful diagnostic information. Testosterone levels vary among women and within their different hormonal life stages, and there are no validated reference ranges.
Sexual function
A controversial issue, women’s sexual health remains somewhat of a mystery, the team noted – a complex web of physical, emotional, and psychological factors. Evidence derived from testosterone therapy in postmenopausal women shows that the hormone may influence all aspects of sexual response by improving desire, subjective arousal, and vaginal blood flow, and increasing frequency of orgasm.
However, decreasing sexuality is a common phenomenon for women, and is not dependent on physiologic testosterone levels. While response to testosterone therapy didn’t always correlate with plasma levels, most trials found that a daily dose of 300 mcg provided optimal benefit. One study found a more than 115% increase in reported orgasms, compared with a 38% increase in the placebo group, and another found 2.12 more satisfactory sexual events per month, compared with 0.73 per month with placebo.
One trial of a testosterone gel was negative, but was only available as an abstract. A large randomized trial of transdermal testosterone gel that recruited 3,565 naturally and surgically postmenopausal women, and accrued several thousand women-years of data, has yet to be published.
For postmenopausal women with properly diagnosed HSDD, the task force recommended a 3- to 6-month trial of testosterone for those who request therapy and in whom it is not contraindicated.
Testosterone levels should be measured at baseline and after 3-6 weeks of initial treatment to assess patient overuse. In cases of ongoing testosterone therapy, levels should be reviewed every 6 months to monitor for excessive use and signs of androgen excess.
Other indications
The task force noted insufficient evidence for the following indications:
• Infertility. One meta-analysis reported an overall 11% increase in live births; another found no benefit. The individual studies were marred by methodological issues, the team said.
• Bone. Several small studies showed increased bone density in some locations after testosterone therapy. However, many women received concomitant estrogens, so the studies were confounded, the team noted.
• Cognition. Several studies have hinted that testosterone may be protective against Alzheimer’s and other dementias in women, possibly by mediating the accumulation of neurotoxic amyloid beta brain plaques. Women taking the hormone scored better on cognitive tests, including verbal memory, as well as spatial and mathematical reasoning. “In addition to having neuroprotective effects, testosterone has positive effects on endothelial function and acts as a vasodilator, providing another potential pathway through which testosterone may confer neuroprotection,” the investigators said. Again, however, they felt that the body and quality of evidence were not enough to justify a recommendation.
• Cardiovascular. Higher endogenous testosterone in women has been associated with better endothelial function and lower carotid intima-medial thickness; exogenous testosterone has not been adequately studied. However, testosterone supplementation does not seem to negatively affect lipids or increase other cardiovascular risk markers.
• Body composition. Several large studies of women with testosterone-alone and testosterone-estradiol therapy found increased lean muscle mass and lower body fat mass. These results were seen in healthy women as well as those with Turner syndrome, anorexia nervosa, and HIV infections. “Thus,” the investigators concluded, “testosterone increases lean body mass and may decrease fat mass in women, but effects are less dramatic than in men.”
• Mood. Testosterone therapy resulted in improved mood and lower depression scores in several studies, but subjects in some were not screened for comorbidities, including sexual dysfunction.
• Side effects. The potential for masculinizing effects – acne, hirsutism, deepening of the voice, and androgenic alopecia – exists. They are dose dependent, uncommon, and short-lived once supraphysiologic levels are decreased. Findings on breast cancer risk are contradictory; the team said more study is necessary.
The task force commissioned a testosterone therapy meta-analysis of published randomized trials of testosterone-alone or in addition to hormone replacement therapy. “Across all trials, testosterone use was associated with a statistically significant improvement in satisfaction, pleasure, orgasm, and libido. The quality of evidence was moderate to high for pleasure and orgasm outcomes, and moderate for satisfaction and libido outcomes,” with minimal effect on lipids and none on hair growth or loss.
“However,” the task force wrote, “data on adverse effects were not extensive, particularly for long-term use,” with a follow-up of 6 weeks to 2 years.
Dr. Wierman had no financial disclosures. However, several coauthors had numerous disclosures, including financial ties with pharmaceutical companies.
On Twitter @alz_gal
Testosterone supplementation is not recommended for women, unless they have a clinical diagnosis of hypoactive sexual desire disorder, a joint task force led by the Endocrine Society has determined.
While a number of studies have shown that testosterone supplementation helps improve sexual desire, cognition, and other outcomes, most are either too small, too short, or confounded by other factors to provide irrefutable evidence of the hormone’s benefit for any indication other than hypoactive sexual desire disorder (HSDD), Dr. Margaret E. Wierman and her colleagues wrote in the Journal of Clinical Endocrinology and Metabolism (J. Clin. Endocrinol. Metab. 2014;99:3489-510).
A trial of testosterone is only appropriate for postmenopausal women with a diagnosed HSDD, wrote the task force, which also noted that libido and response improvements don’t necessarily correlate with testosterone serum levels. If there’s no clinical response after a 6-month trial, the hormone should be discontinued.
The hormone-health connection is clearly important to women, and many have already done Internet research on the topic, said Dr. Margery Gass, executive director of the North American Menopause Society. A Google query for “hormones to control aging” turned up 9.5 million results, she said.
Although NAMS was not involved in creating the new clinical guideline, the society supports it, she said in an interview.
“The Endocrine Society has published an excellent set of guidelines on the use of androgen therapy in women. These guidelines are in harmony with NAMS’ comments on the use of testosterone and dehydroepiandrosterones,” included in the group’s clinical practice guideline for the care of midlife women (Menopause 2014;21:1038-62). That paper also recommends a trial of testosterone therapy “in carefully selected postmenopausal women with female sexual interest/arousal disorder and no other etiology for their sexual problems.”
Dr. Gass continued, “I encourage clinicians to use both publications to help women understand the misinformation about testosterone and other androgens that they are seeing on the Internet.”
Dr. Wierman, chief of endocrinology at the University of Colorado, Aurora, led the task force, composed of representatives from the American Society for Reproductive Medicine, the American Congress of Obstetricians and Gynecologists, the European Society of Endocrinology, and the International Menopause Society. Together, they reviewed the extant literature and, in addition, commissioned meta-analyses to examine the issue of androgen supplementation in premenopausal and postmenopausal women – either healthy or with specific conditions. Evidence was graded as level 1 (strong) or level 2 (weak).
The group considered androgen supplementation for a number of indications: infertility; sexual dysfunction; cognition; cardiovascular, metabolic, and bone health; and general well-being. It found little strong data to support the practice in any of these except HSDD.
As a basic tenet, the task force agreed that androgen testing for most women is inaccurate, and thus virtually incapable of providing useful diagnostic information. Testosterone levels vary among women and within their different hormonal life stages, and there are no validated reference ranges.
Sexual function
A controversial issue, women’s sexual health remains somewhat of a mystery, the team noted – a complex web of physical, emotional, and psychological factors. Evidence derived from testosterone therapy in postmenopausal women shows that the hormone may influence all aspects of sexual response by improving desire, subjective arousal, and vaginal blood flow, and increasing frequency of orgasm.
However, decreasing sexuality is a common phenomenon for women, and is not dependent on physiologic testosterone levels. While response to testosterone therapy didn’t always correlate with plasma levels, most trials found that a daily dose of 300 mcg provided optimal benefit. One study found a more than 115% increase in reported orgasms, compared with a 38% increase in the placebo group, and another found 2.12 more satisfactory sexual events per month, compared with 0.73 per month with placebo.
One trial of a testosterone gel was negative, but was only available as an abstract. A large randomized trial of transdermal testosterone gel that recruited 3,565 naturally and surgically postmenopausal women, and accrued several thousand women-years of data, has yet to be published.
For postmenopausal women with properly diagnosed HSDD, the task force recommended a 3- to 6-month trial of testosterone for those who request therapy and in whom it is not contraindicated.
Testosterone levels should be measured at baseline and after 3-6 weeks of initial treatment to assess patient overuse. In cases of ongoing testosterone therapy, levels should be reviewed every 6 months to monitor for excessive use and signs of androgen excess.
Other indications
The task force noted insufficient evidence for the following indications:
• Infertility. One meta-analysis reported an overall 11% increase in live births; another found no benefit. The individual studies were marred by methodological issues, the team said.
• Bone. Several small studies showed increased bone density in some locations after testosterone therapy. However, many women received concomitant estrogens, so the studies were confounded, the team noted.
• Cognition. Several studies have hinted that testosterone may be protective against Alzheimer’s and other dementias in women, possibly by mediating the accumulation of neurotoxic amyloid beta brain plaques. Women taking the hormone scored better on cognitive tests, including verbal memory, as well as spatial and mathematical reasoning. “In addition to having neuroprotective effects, testosterone has positive effects on endothelial function and acts as a vasodilator, providing another potential pathway through which testosterone may confer neuroprotection,” the investigators said. Again, however, they felt that the body and quality of evidence were not enough to justify a recommendation.
• Cardiovascular. Higher endogenous testosterone in women has been associated with better endothelial function and lower carotid intima-medial thickness; exogenous testosterone has not been adequately studied. However, testosterone supplementation does not seem to negatively affect lipids or increase other cardiovascular risk markers.
• Body composition. Several large studies of women with testosterone-alone and testosterone-estradiol therapy found increased lean muscle mass and lower body fat mass. These results were seen in healthy women as well as those with Turner syndrome, anorexia nervosa, and HIV infections. “Thus,” the investigators concluded, “testosterone increases lean body mass and may decrease fat mass in women, but effects are less dramatic than in men.”
• Mood. Testosterone therapy resulted in improved mood and lower depression scores in several studies, but subjects in some were not screened for comorbidities, including sexual dysfunction.
• Side effects. The potential for masculinizing effects – acne, hirsutism, deepening of the voice, and androgenic alopecia – exists. They are dose dependent, uncommon, and short-lived once supraphysiologic levels are decreased. Findings on breast cancer risk are contradictory; the team said more study is necessary.
The task force commissioned a testosterone therapy meta-analysis of published randomized trials of testosterone-alone or in addition to hormone replacement therapy. “Across all trials, testosterone use was associated with a statistically significant improvement in satisfaction, pleasure, orgasm, and libido. The quality of evidence was moderate to high for pleasure and orgasm outcomes, and moderate for satisfaction and libido outcomes,” with minimal effect on lipids and none on hair growth or loss.
“However,” the task force wrote, “data on adverse effects were not extensive, particularly for long-term use,” with a follow-up of 6 weeks to 2 years.
Dr. Wierman had no financial disclosures. However, several coauthors had numerous disclosures, including financial ties with pharmaceutical companies.
On Twitter @alz_gal
Testosterone supplementation is not recommended for women, unless they have a clinical diagnosis of hypoactive sexual desire disorder, a joint task force led by the Endocrine Society has determined.
While a number of studies have shown that testosterone supplementation helps improve sexual desire, cognition, and other outcomes, most are either too small, too short, or confounded by other factors to provide irrefutable evidence of the hormone’s benefit for any indication other than hypoactive sexual desire disorder (HSDD), Dr. Margaret E. Wierman and her colleagues wrote in the Journal of Clinical Endocrinology and Metabolism (J. Clin. Endocrinol. Metab. 2014;99:3489-510).
A trial of testosterone is only appropriate for postmenopausal women with a diagnosed HSDD, wrote the task force, which also noted that libido and response improvements don’t necessarily correlate with testosterone serum levels. If there’s no clinical response after a 6-month trial, the hormone should be discontinued.
The hormone-health connection is clearly important to women, and many have already done Internet research on the topic, said Dr. Margery Gass, executive director of the North American Menopause Society. A Google query for “hormones to control aging” turned up 9.5 million results, she said.
Although NAMS was not involved in creating the new clinical guideline, the society supports it, she said in an interview.
“The Endocrine Society has published an excellent set of guidelines on the use of androgen therapy in women. These guidelines are in harmony with NAMS’ comments on the use of testosterone and dehydroepiandrosterones,” included in the group’s clinical practice guideline for the care of midlife women (Menopause 2014;21:1038-62). That paper also recommends a trial of testosterone therapy “in carefully selected postmenopausal women with female sexual interest/arousal disorder and no other etiology for their sexual problems.”
Dr. Gass continued, “I encourage clinicians to use both publications to help women understand the misinformation about testosterone and other androgens that they are seeing on the Internet.”
Dr. Wierman, chief of endocrinology at the University of Colorado, Aurora, led the task force, composed of representatives from the American Society for Reproductive Medicine, the American Congress of Obstetricians and Gynecologists, the European Society of Endocrinology, and the International Menopause Society. Together, they reviewed the extant literature and, in addition, commissioned meta-analyses to examine the issue of androgen supplementation in premenopausal and postmenopausal women – either healthy or with specific conditions. Evidence was graded as level 1 (strong) or level 2 (weak).
The group considered androgen supplementation for a number of indications: infertility; sexual dysfunction; cognition; cardiovascular, metabolic, and bone health; and general well-being. It found little strong data to support the practice in any of these except HSDD.
As a basic tenet, the task force agreed that androgen testing for most women is inaccurate, and thus virtually incapable of providing useful diagnostic information. Testosterone levels vary among women and within their different hormonal life stages, and there are no validated reference ranges.
Sexual function
A controversial issue, women’s sexual health remains somewhat of a mystery, the team noted – a complex web of physical, emotional, and psychological factors. Evidence derived from testosterone therapy in postmenopausal women shows that the hormone may influence all aspects of sexual response by improving desire, subjective arousal, and vaginal blood flow, and increasing frequency of orgasm.
However, decreasing sexuality is a common phenomenon for women, and is not dependent on physiologic testosterone levels. While response to testosterone therapy didn’t always correlate with plasma levels, most trials found that a daily dose of 300 mcg provided optimal benefit. One study found a more than 115% increase in reported orgasms, compared with a 38% increase in the placebo group, and another found 2.12 more satisfactory sexual events per month, compared with 0.73 per month with placebo.
One trial of a testosterone gel was negative, but was only available as an abstract. A large randomized trial of transdermal testosterone gel that recruited 3,565 naturally and surgically postmenopausal women, and accrued several thousand women-years of data, has yet to be published.
For postmenopausal women with properly diagnosed HSDD, the task force recommended a 3- to 6-month trial of testosterone for those who request therapy and in whom it is not contraindicated.
Testosterone levels should be measured at baseline and after 3-6 weeks of initial treatment to assess patient overuse. In cases of ongoing testosterone therapy, levels should be reviewed every 6 months to monitor for excessive use and signs of androgen excess.
Other indications
The task force noted insufficient evidence for the following indications:
• Infertility. One meta-analysis reported an overall 11% increase in live births; another found no benefit. The individual studies were marred by methodological issues, the team said.
• Bone. Several small studies showed increased bone density in some locations after testosterone therapy. However, many women received concomitant estrogens, so the studies were confounded, the team noted.
• Cognition. Several studies have hinted that testosterone may be protective against Alzheimer’s and other dementias in women, possibly by mediating the accumulation of neurotoxic amyloid beta brain plaques. Women taking the hormone scored better on cognitive tests, including verbal memory, as well as spatial and mathematical reasoning. “In addition to having neuroprotective effects, testosterone has positive effects on endothelial function and acts as a vasodilator, providing another potential pathway through which testosterone may confer neuroprotection,” the investigators said. Again, however, they felt that the body and quality of evidence were not enough to justify a recommendation.
• Cardiovascular. Higher endogenous testosterone in women has been associated with better endothelial function and lower carotid intima-medial thickness; exogenous testosterone has not been adequately studied. However, testosterone supplementation does not seem to negatively affect lipids or increase other cardiovascular risk markers.
• Body composition. Several large studies of women with testosterone-alone and testosterone-estradiol therapy found increased lean muscle mass and lower body fat mass. These results were seen in healthy women as well as those with Turner syndrome, anorexia nervosa, and HIV infections. “Thus,” the investigators concluded, “testosterone increases lean body mass and may decrease fat mass in women, but effects are less dramatic than in men.”
• Mood. Testosterone therapy resulted in improved mood and lower depression scores in several studies, but subjects in some were not screened for comorbidities, including sexual dysfunction.
• Side effects. The potential for masculinizing effects – acne, hirsutism, deepening of the voice, and androgenic alopecia – exists. They are dose dependent, uncommon, and short-lived once supraphysiologic levels are decreased. Findings on breast cancer risk are contradictory; the team said more study is necessary.
The task force commissioned a testosterone therapy meta-analysis of published randomized trials of testosterone-alone or in addition to hormone replacement therapy. “Across all trials, testosterone use was associated with a statistically significant improvement in satisfaction, pleasure, orgasm, and libido. The quality of evidence was moderate to high for pleasure and orgasm outcomes, and moderate for satisfaction and libido outcomes,” with minimal effect on lipids and none on hair growth or loss.
“However,” the task force wrote, “data on adverse effects were not extensive, particularly for long-term use,” with a follow-up of 6 weeks to 2 years.
Dr. Wierman had no financial disclosures. However, several coauthors had numerous disclosures, including financial ties with pharmaceutical companies.
On Twitter @alz_gal