User login
April 2020
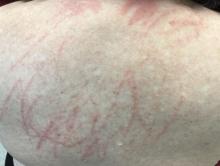
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Shiitake mushroom flagellate dermatitis
that resemble whiplash marks. The lesions may be extremely pruritic, and petechiae may be present in the streaks. The trunk is most commonly affected, although lesions can occur on the limbs. Mucosa is not affected. Sun exposure may exacerbate the condition. The dermatitis has been described in all ages and races, and males seem to be more affected than females.
Shiitake mushroom flagellate dermatitis typically occurs following the ingestion of raw or undercooked shiitake mushrooms (Lentinula edodes). The mushrooms contain a polysaccharide called lentinan. Ingestion of lentinan activates interleukin-1 (IL-1), resulting in vasodilation and the subsequent dermatitis that can occur within a few hours and up to 5 days post ingestion. Associated gastrointestinal symptoms, fever, and localized swelling have been reported. The rash will resolve spontaneously over a few days to weeks.
Flagellate erythema has been described with bleomycin treatment. Other reported associations include peplomycin (a bleomycin derivative) and docetaxel. The rash may appear following administration of bleomycin by any route and has been shown to be dose independent. Onset occurs anywhere from 1 day to several months after exposure. Over time, the erythema will develop into postinflammatory hyperpigmentation.
Dermatomyositis may present with flagellate erythema. Other symptoms include muscle weakness and an inflammatory myopathy. A heliotrope rash on the eyelids, Gottron’s papules on the hands, ragged cuticles with prominent vessels on nail folds may be seen. Blood work may reveal elevated antinuclear antibodies (ANA), anti–Mi-2 and anti–Jo-1. Adult-onset Still disease is characterized by fever, arthritis, and salmon-colored patches.
Our patient’s dermatitis resolved spontaneously without treatment.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Dermatology therapies evolve as disease knowledge and investment grow
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
For much of the past 50 years, many of the drugs used in dermatology have been adopted – and often adapted – from other specialties and used for dermatologic conditions.
“Almost every drug was more or less a hand-me-down” developed first for cancer or other diseases and found later, often serendipitously, to be useful for the skin, said William Eaglstein, MD, thinking back to the 1970s and recalling steroids, tetracyclines, methotrexate, and 5-flourouracil. “The perception always was that skin diseases weren’t serious, that the market was small.”
Much has changed. by dermatologist investigators, and “more and more companies are recognizing the importance of our diseases and the ability to get a return on investment,” said Dr. Eaglstein, past professor and chair of the departments of dermatology at the University of Miami and the University of Pittsburgh, who worked in industry after his academic career.
Psoriasis was a game changer, he and other dermatologists said in interviews. The tumor necrosis factor (TNF)–alpha blockers were first used for other indications, but their marked follow-on success in psoriasis “offered proof of concept clinically – showing that by targeting immune pathways in the skin we could achieve a clinical effect – and proof of concept commercially” that dermatology drugs are worth pursuing by pharmaceutical companies, said William Ju, MD, a cofounder and president of Advancing Innovation in Dermatology, a nonprofit organization that brings together stakeholders to develop novel dermatologic drugs and products.
This resulted in the approval of subsequent biologics, such as ustekinumab (Stelara) which inhibits the signaling of interleukin (IL)–12/IL-23, for psoriasis as their initial indication. Then, biologics targeting IL-17 followed this dermatology-first approach. “Researchers have continued further dissecting out the immunopathological pathways, and antibody drugs targeting IL-23p19 have been approved for psoriasis as the lead indication,” said Dr. Ju, a dermatologist who has worked in industry.
Seth Orlow, MD, PhD, who chairs the department of dermatology at NYU Langone Health, remembers the 1970s through the 1990s as the “era of topicals” developed for dermatologic conditions – topical antifungals, topical corticosteroids, and topical retinoids. The next decade was characterized by formulation tweaks and few novel treatments for dermatology, said Dr. Orlow, who is also professor of pediatric dermatology and director of the program in cutaneous biology at New York University.
Now, given the succession of psoriasis discoveries in the last decade, “large companies are interested in dermatology,” he said in an interview. “There’s an explosion of interest in atopic dermatitis. … and companies are dipping their toes in the water for alopecia areata and vitiligo. That’s amazing.”
Rare diseases like epidermolysis bullosa, ichthyosis, and basal cell nevus syndrome are getting attention as well, boosted by the Orphan Drug Act of 1983, in addition to increased research on disease pathways and growing appreciation of skin diseases. “There’s a lot under development, from small molecules to biologics to gene-based therapies,” Dr. Orlow commented.
The new frontier of atopic dermatitis
The approval in 2017 of dupilumab (Dupixent), a monoclonal antibody that inhibits the signaling of both IL-4 and IL-13) for moderate-severe atopic dermatitis (AD) in adults illustrates the new standing of dermatologic diseases in the field of drug development and commercialization. “Atopic dermatitis had always been the forgotten chronic disease in dermatology. … We’ve had no good treatments,” said Eric Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland. “Dupilumab coming to the forefront [as a dermatology-first indication] has changed the entire perspective of the field. … Everyone is now trying to find the next best drug.”
As with psoriasis, a targeted therapy for AD was made possible by the development in the 1990s of monoclonal antibody technology and the ensuing ability to create biologics that target specific molecules in the body – as well as bedside-to-bench research that homed in on the involvement of particular cytokines.
But there also is a “new understanding of the burden of the disease,” Dr. Simpson observed. In the last 5 years, he said, research funded by the National Eczema Association documented that AD “not only causes inflammation of the skin … but that it affects people at school and in the workplace, that people have multiple mental health comorbidities and skin infections, and that the disease profoundly affects the entire patient in ways that weren’t really recognized or appreciated.”
Having evolved in the footsteps of psoriasis, AD is at a higher starting point in terms of the safety and efficacy of its first biologic, sources said. On the other hand, AD is a much more complex and heterogeneous disease, and researchers are trying to determine which immune pathways and cytokines are most important – and in which populations.
“We’re at the beginning. We’re trying to figure out how to get 80% of patients clear or almost clear [as we can now with psoriasis biologics] rather than almost 40% [as in the dupilumab pivotal trials],” said Dr. Simpson, former cochair of the National Eczema Association’s scientific committee. Public data from ongoing phase 2 and 3 trials of other Th2 cytokine inhibitors suggest that 25%-45% of enrolled patients achieve high levels of clearance, he noted.
Emma Guttman-Yassky, MD, PhD, Sol and Clara Kest Professor and vice-chair for research in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said that AD’s heterogeneity involves “many factors, like ethnicity, age … and whether they have an atopic background such as asthma.”
Her research is showing, for instance, that AD in Asian and black patients is different than AD in European-American patients, and that the presence of comorbidities may well have treatment implications. She has also shown that children may have a different phenotype than adults, with greater activation of the Th17 axis that typifies psoriasis.
“For certain patients, we may need to target more than one pathway, or target a different pathway than the Th2 pathway. And treatment may be different in the setting of comorbidities,” said Dr. Guttman-Yassky, who is also director of the laboratory of inflammatory skin diseases at Mount Sinai. “We may think of one treatment – dupilumab, for example – for someone who has asthma and AD. But for patients who don’t have asthma and are Asian, for instance, or for children, we may need additional agents.”
Her research over the years on AD has taught her the importance of human studies over mouse model studies; it was in humans, she noted, that she and other investigators demonstrated “without doubt” that AD is an immune disease and not simply a barrier disease. The Th2 cytokine pathway appears to play the predominant role in AD, though “there still is a strong Th1 component,” she said.
“We’re in a better position to figure this out today [than in the past 20 or even 10 years],” said Dr. Guttman-Yassky, who recalls being told years ago that AD was a “dead end,” that it “would kill [her] career.” Given the evolution of science and the recognition of comorbidities and seriousness of dermatologic diseases, “the stars are aligned to get more [therapies] to these patients.”
Janus kinase (JAK) inhibitors are among these therapies. Three JAK inhibitors are in or have recently completed phase 3 studies for AD; two are currently approved for rheumatoid arthritis, and the other has been designed specifically for AD, Dr. Simpson pointed out. The drugs are oral small molecule drugs that block the JAK signaling pathways for certain proinflammatory cytokines.
“The JAK inhibitors are a real exciting story for dermatology,” he said. “Theoretically, by blocking more cytokines than biologics do, there could be some safety issues – that’s why we’re awaiting big phase 3 study results so we can figure out the risk-benefit balance and guide our patients as to which drug is best.”
Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center in Portland – a stand-alone dermatology clinical trial center founded in 1998 – likes to envision the evolution of drugs for dermatologic conditions as a funnel, with the most broad-acting drugs at the wide top of the funnel and the most targeted drugs at the bottom tip.
JAK inhibitors, he said, sit near the middle – more targeted and safer than cyclosporine and methotrexate, for instance, but not as targeted as the biologics now available for psoriasis and being developed for AD. “The oral medications that have been developed for psoriasis and those coming for AD are not quite as targeted to the disease,” he noted. “JAK inhibitors have great efficacy – it’s more a question of safety and being able to treat without causing collateral damage.”
Dr. Blauvelt expects the armamentarium of new drugs approved for AD to go from one (dupilumab) to seven within the next 2 years. This will include three new biologics and three new oral JAK inhibitors, he predicts. As the specialty sorts through and integrates these new drugs into practice, dermatologists will increasingly personalize treatment and will face the “nonscientific” challenge of the cost of new therapies and patient access to them, he noted.
In the meantime, said Dr. Simpson, recent drug discoveries have driven more non–pharmaceutical-funded translational research aimed at understanding the underlying biology of AD. The National Institutes of Health, for instance, “is interested in dupilumab and its impact on the skin barrier and skin defense mechanisms,” he said. “We’ll learn a lot more [in coming years].”
Spillover to other diseases
JAK inhibitors – some in oral and some in topical form – are showing efficacy in ongoing research for alopecia areata (AA) and vitiligo as well, Dr. Blauvelt said.
“We’re understanding more about the pathophysiology of these diseases, which historically have been tough diseases for dermatologists to treat,” he said. “The successes in alopecia areata and vitiligo are incredibly exciting actually – it’s very exciting to see hair and pigment coming back. And as we learn more, we should be able to develop [additional] drugs that are more disease targeted than the JAK inhibitors.”
Already, some of the biologics used to treat psoriasis have been studied in patients with hidradenitis suppurativa (HS), a disease in which painful lumps and sometimes tunnels form under the skin, with some success; adalimumab (Humira), a TNF-inhibitor, is now FDA approved for the treatment of moderate-severe HS, and studies are ongoing of IL-17 and IL-23 blockers for the disease.
“The pathophysiology [of HS] is very complex; it’s not nearly as straightforward as psoriasis, and there haven’t been any major breakthroughs yet,” Dr. Blauvelt said. “But the drugs seem to be working better than historical alternatives.”
Regarding AA, Dr. Guttman-Yassky, who is participating in a study of dupilumab for AA, recently found in a retrospective cross-sectional study that patients with the condition are more likely to have atopic comorbidities – asthma, allergic rhinitis, and AD, for instance. “The more comorbid conditions, the greater the risk of developing alopecia areata,” she said. “That could point to a potential pathogenic role of the Th2 axis in the disorder [challenging the traditional view of AA as a singularly Th1-centered disease.] The future will tell.”
Action on rare skin diseases
Both large and small companies have moved into the orphan drug space, investing in research and pursuing orphan drug indications for dermatologic conditions, because “it’s clear now in the marketplace that companies can develop effective drugs for rare disorders and be quite successful,” Dr. Orlow said.
According to a recent analysis, as a result of incentives for rare disease drug development contained in the Orphan Drug Act, 72 indications have been approved for rare skin disease, skin-related cancers, and hereditary disorders with prominent dermatologic manifestations since the law was passed in 1983 (J. Am. Acad. Dermatol. 2019;81[3]:867-77).
Epidermolysis bullosa (EB) is a good example, he and other sources said, of commercial interests merging with growing knowledge of disease pathogenesis as well as the tools needed to develop new treatments.
Research by dermatology scientists and others over the past 40 years, Dr. Ju explained, shed light on the molecular basis underlying the structure and function of the junction between the epidermis and dermis, including the pivotal role that type VII collagen plays in the normal adhesion of these two layers. Researchers then learned that, in EB, the family of genetic diseases characterized by skin fragility, “dystrophic types are caused by mutations in the gene encoding type VII collagen,” he said.
“Just as the advent of monoclonal antibodies allowed us to start attacking psoriasis and atopic dermatitis in unprecedented ways, the advent of gene therapy allows us to potentially address the fundamental molecular genetic defect of various types of EB,” Dr. Ju said.
While gene therapy is “still in its infancy,” companies have begun using the tools to address EB. One gene therapy in the pipeline – in phase 3 clinical trial testing – involves grafting back into patients with recessive dystrophic EB their skin cells that have been genetically modified to produce a correct (nonmutated) type VII collagen, he said.
Basal cell nevus syndrome, or Gorlin syndrome, a rare disease in which patients develop a multitude of basal cell carcinoma tumors, is another example of a “dermatology first” approach, Dr. Ju said. Research identified a genetic mutation that causes the hedgehog signaling pathway to be inappropriately activated in the disease, and a drug, vismodegib, was developed to inhibit this pathway. The drug was initially approved for patients with metastatic basal cell cancer and types of advanced basal cell cancer, and is now being tested in cancers affecting other organs, he said.
Basal cell cancer “is a huge market, but it was really unrecognized in the past,” Dr. Eaglstein said. “Seeing drugs come to market for basal cell cancer – this wouldn’t have happened [decades ago].”
Dr. Ju has worked in the pharmaceutical industry; all other sources in this story have worked with pharmaceutical manufacturers of treatments that are being developed or have been approved to treat dermatologic diseases mentioned in this story. In addition to Dr. Ju, Dr. Eaglstein and Dr. Orlow are cofounders of the Advancing Innovation in Dermatology group; Dr. Orlow is a member of the program committee for the organization’s dermatology summit conference.
Testing times for epidermolysis bullosa topical therapies
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
EXPERT ANALYSIS FROM EB 2020
Antifungal drug appears safe for pregnancy
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
Treatment with the according to results from a large registry study in Denmark.
Physicians have been reluctant to prescribe the drug during pregnancy because of the limited safety data. The drug has not been associated with any signs of fetal toxicity in animal studies, but only one study – in 54 pregnancies – has examined the issue in humans and did not identify an increased fetal risk, according to Niklas Worm Andersson, MD, of the department of clinical pharmacology, Copenhagen University Hospital at Bispebjerg and Frederiksberg, and coauthors.
The retrospective, nationwide cohort study analyzed exposure to oral and tropical terbinafine in a large pregnancy registry and found no increase in the risk of major malformations or spontaneous abortions in exposed versus unexposed pregnancies. The study was published in JAMA Dermatology.
Still, these results fell short of certainty, the authors noted. “Although our results may provide reassurance for pregnancies exposed to oral terbinafine by reporting no overall increased risk of major malformations, we cannot exclude a potential increased risk of a specific malformation,” they wrote.
“To our knowledge, this is by far the largest, most statistically rigorous study in the literature regarding this topic,” Jenny E. Murase, MD, of the department of dermatology at the University of California, San Francisco, and Mary Kathryn Abel, a medical student at UCSF, wrote in an accompanying editorial. They described the study as “a substantial contribution to the nearly absent literature regarding the use of terbinafine during pregnancy. Among the antifungal medications, it is possible that terbinafine is the safest one currently available for use in pregnancy, particularly of the oral formulations.”
However, since asymptomatic onychomycosis “is typically a cosmetic, rather than medical, concern, waiting until after pregnancy to initiate therapy is reasonable. ... It is important to acknowledge the uncertainty in this field and question the appropriateness of treating non–life-threatening diseases during pregnancy and lactation,” they wrote.
The Danish researchers drew from a registry of 1,650,649 pregnancies between 1997 and 2016, which included 891 pregnancies exposed to oral terbinafine, and 3,174 exposed to topical terbinafine. Matched outcome analyses compared the exposed pregnancies with up to 40,650 controls unexposed during pregnancy.
Propensity-matched comparisons showed no increased risk of major malformations for oral terbinafine exposure versus no exposure (odds ratio, 1.01; 95% confidence interval, 0.63-1.62) or topical exposure versus no exposure (OR, 1.08; 95% CI, 0.81-1.44). There was also no difference in oral versus topical exposure (OR, 1.18; 95% CI, 0.61-2.29).
With respect to spontaneous abortions, there was no significant association with oral terbinafine (hazard ratio, 1.06; 95% CI, 0.86-1.32) or topical terbinafine (HR, 1.04; 95% CI, 0.88-1.21), compared with unexposed pregnancies, or oral over topical terbinafine-exposed pregnancies (HR, 1.19; 95% CI, 0.84-1.70).
The study is limited by the fact that it was conducted in a Danish population, and the data relied on filled prescriptions for determining exposure, which did not account for adherence. Residual confounding is possible because of the retrospective nature of the study, the authors pointed out.
No source of funding was disclosed. One of the authors has received grants and personal fees from Novartis. Dr. Murase has received fees from Sanofi Genzyme, Dermira, UCB, Regeneron, Ferndale, and UpToDate.
SOURCES: Andersson NW et al. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2020.0142; Murase JE, Abel MK. JAMA Dermatol. 2020 Mar 4. doi: 10.1001/jamadermatol.2019.5036.
FROM JAMA DERMATOLOGY
Nemolizumab tames itching in prurigo nodularis patients in phase 2 study
compared with patients treated with placebo, according to data from a phase 2 trial of 70 patients.
Prurigo nodularis (PN) is a chronic skin condition distinguished by hyperkeratotic red nodules and extreme pruritus, which contributes to crusting and excoriation of the lesions. Lesions can range in size from a few millimeters to 3 centimeters in diameter, and number from a handful to hundreds.
“The pathogenesis of prurigo nodularis is not well understood, but it has been considered to include a form of neuronal sensitization of itch-processing neurons and the development of an itch–scratch cycle,” wrote Sonja Ständer, MD, of the department of dermatology and Center for Chronic Pruritus, University Hospital Münster, Germany, and coauthors. “Interleukin-31, when bound to its receptor complex, has been shown to be a mediator of pruritus, especially in atopic dermatitis and prurigo nodularis,” they noted.
In a study published in the New England Journal of Medicine, the researchers randomized 70 adults with PN to nemolizumab, a monoclonal antibody targeting the interleukin-31 receptor, or a placebo. Patients received 0.5 mg/kg of the drug or placebo subcutaneously at baseline and again at 4 and 8 weeks; safety data were assessed through 18 weeks.
At week 4, the primary outcome of average peak pruritus score based on the numerical rating scale (PP-NRS) decreased by 4.5 points in the nemolizumab group, compared with 1.7 points in the placebo group (reductions of 53% and 20%, respectively). At baseline, weekly peak scores on the PP-NRS were 8.4 for both groups.
In addition, 24% of the nemolizumab patients reported 75% or more healed lesions at week 4, compared with 11% of the placebo group, and at the week 18 final follow-up visit, “the least-squares mean change in the lesion count was −13.3 in the nemolizumab group and − 7.5 in the placebo group,” the researchers said.
Adverse events were similar in both nemolizumab and placebo groups (68% and 67%, respectively) with the most common being gastrointestinal symptoms and musculoskeletal pain. Four nemolizumab patients and three placebo patients experienced serious adverse events. The serious adverse events in the nemolizumab patients included one case each of psoriasiform rash, clavicular fracture, fibromyalgia, and bladder lithiasis. Two patients in each group discontinued the study because of adverse events.
The study findings were limited by the small sample size and short duration, the researchers noted. However, the Food and Drug Administration granted nemolizumab a Breakthrough Therapy Designation in 2019, according to Galderma, and the company is planning a phase 3 trial of nemolizumab in adults with PN to begin later in 2020.
Galderma sponsored and designed the study. Lead author Dr. Ständer disclosed ties with Galderma, Almirall, Beiersdorf, Bellus Health, Cara Therapeutics, Celgene, LEO Pharma, Sienna Biopharmaceuticals, and Vanda Pharmaceuticals. Several coauthors were Galderma employees.
SOURCE: Ständer S et al. N Engl J Med. 2020 Feb 20;382(8):706-16.
compared with patients treated with placebo, according to data from a phase 2 trial of 70 patients.
Prurigo nodularis (PN) is a chronic skin condition distinguished by hyperkeratotic red nodules and extreme pruritus, which contributes to crusting and excoriation of the lesions. Lesions can range in size from a few millimeters to 3 centimeters in diameter, and number from a handful to hundreds.
“The pathogenesis of prurigo nodularis is not well understood, but it has been considered to include a form of neuronal sensitization of itch-processing neurons and the development of an itch–scratch cycle,” wrote Sonja Ständer, MD, of the department of dermatology and Center for Chronic Pruritus, University Hospital Münster, Germany, and coauthors. “Interleukin-31, when bound to its receptor complex, has been shown to be a mediator of pruritus, especially in atopic dermatitis and prurigo nodularis,” they noted.
In a study published in the New England Journal of Medicine, the researchers randomized 70 adults with PN to nemolizumab, a monoclonal antibody targeting the interleukin-31 receptor, or a placebo. Patients received 0.5 mg/kg of the drug or placebo subcutaneously at baseline and again at 4 and 8 weeks; safety data were assessed through 18 weeks.
At week 4, the primary outcome of average peak pruritus score based on the numerical rating scale (PP-NRS) decreased by 4.5 points in the nemolizumab group, compared with 1.7 points in the placebo group (reductions of 53% and 20%, respectively). At baseline, weekly peak scores on the PP-NRS were 8.4 for both groups.
In addition, 24% of the nemolizumab patients reported 75% or more healed lesions at week 4, compared with 11% of the placebo group, and at the week 18 final follow-up visit, “the least-squares mean change in the lesion count was −13.3 in the nemolizumab group and − 7.5 in the placebo group,” the researchers said.
Adverse events were similar in both nemolizumab and placebo groups (68% and 67%, respectively) with the most common being gastrointestinal symptoms and musculoskeletal pain. Four nemolizumab patients and three placebo patients experienced serious adverse events. The serious adverse events in the nemolizumab patients included one case each of psoriasiform rash, clavicular fracture, fibromyalgia, and bladder lithiasis. Two patients in each group discontinued the study because of adverse events.
The study findings were limited by the small sample size and short duration, the researchers noted. However, the Food and Drug Administration granted nemolizumab a Breakthrough Therapy Designation in 2019, according to Galderma, and the company is planning a phase 3 trial of nemolizumab in adults with PN to begin later in 2020.
Galderma sponsored and designed the study. Lead author Dr. Ständer disclosed ties with Galderma, Almirall, Beiersdorf, Bellus Health, Cara Therapeutics, Celgene, LEO Pharma, Sienna Biopharmaceuticals, and Vanda Pharmaceuticals. Several coauthors were Galderma employees.
SOURCE: Ständer S et al. N Engl J Med. 2020 Feb 20;382(8):706-16.
compared with patients treated with placebo, according to data from a phase 2 trial of 70 patients.
Prurigo nodularis (PN) is a chronic skin condition distinguished by hyperkeratotic red nodules and extreme pruritus, which contributes to crusting and excoriation of the lesions. Lesions can range in size from a few millimeters to 3 centimeters in diameter, and number from a handful to hundreds.
“The pathogenesis of prurigo nodularis is not well understood, but it has been considered to include a form of neuronal sensitization of itch-processing neurons and the development of an itch–scratch cycle,” wrote Sonja Ständer, MD, of the department of dermatology and Center for Chronic Pruritus, University Hospital Münster, Germany, and coauthors. “Interleukin-31, when bound to its receptor complex, has been shown to be a mediator of pruritus, especially in atopic dermatitis and prurigo nodularis,” they noted.
In a study published in the New England Journal of Medicine, the researchers randomized 70 adults with PN to nemolizumab, a monoclonal antibody targeting the interleukin-31 receptor, or a placebo. Patients received 0.5 mg/kg of the drug or placebo subcutaneously at baseline and again at 4 and 8 weeks; safety data were assessed through 18 weeks.
At week 4, the primary outcome of average peak pruritus score based on the numerical rating scale (PP-NRS) decreased by 4.5 points in the nemolizumab group, compared with 1.7 points in the placebo group (reductions of 53% and 20%, respectively). At baseline, weekly peak scores on the PP-NRS were 8.4 for both groups.
In addition, 24% of the nemolizumab patients reported 75% or more healed lesions at week 4, compared with 11% of the placebo group, and at the week 18 final follow-up visit, “the least-squares mean change in the lesion count was −13.3 in the nemolizumab group and − 7.5 in the placebo group,” the researchers said.
Adverse events were similar in both nemolizumab and placebo groups (68% and 67%, respectively) with the most common being gastrointestinal symptoms and musculoskeletal pain. Four nemolizumab patients and three placebo patients experienced serious adverse events. The serious adverse events in the nemolizumab patients included one case each of psoriasiform rash, clavicular fracture, fibromyalgia, and bladder lithiasis. Two patients in each group discontinued the study because of adverse events.
The study findings were limited by the small sample size and short duration, the researchers noted. However, the Food and Drug Administration granted nemolizumab a Breakthrough Therapy Designation in 2019, according to Galderma, and the company is planning a phase 3 trial of nemolizumab in adults with PN to begin later in 2020.
Galderma sponsored and designed the study. Lead author Dr. Ständer disclosed ties with Galderma, Almirall, Beiersdorf, Bellus Health, Cara Therapeutics, Celgene, LEO Pharma, Sienna Biopharmaceuticals, and Vanda Pharmaceuticals. Several coauthors were Galderma employees.
SOURCE: Ständer S et al. N Engl J Med. 2020 Feb 20;382(8):706-16.
FROM NEW ENGLAND JOURNAL OF MEDICINE
SCC survival remains poor in epidermolysis bullosa
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
REPORTING FROM EB 2020
Prioritize oral health in children with DEB
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
LONDON – , pediatric dentist Susanne Krämer told attendees at the first EB World Congress.
While it may not be the first thing on the minds of families coming to terms with their children having a chronic and potentially debilitating skin disease, it is important to consider oral health early to ensure healthy dentition and mouth function, both of which will affect the ability to eat and thus nutrition.
When there are a lot of other health issues, “dentistry is not a priority,” Dr. Krämer acknowledged in an interview at the meeting, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Something as simple as brushing teeth can be very distressing for parents of a child with EB, she observed, especially if there is dysphagia and toothpaste may be getting into the airways accidentally.
Oral health was one of the topics that patients with EB and their families said would be good to have some guidance on when they were surveyed by DEBRA International. This led the charity to develop its first clinical practice guideline in 2012. Dr. Krämer was the lead author of the guidelines, which are about to be updated and republished.
The “Oral Health for Patients with Epidermolysis Bullosa – Best Clinical Practice Guidelines” (Int J Paediatr Dent. 2012;22 Suppl 1:1-35) are in the final stages of being revised, said Dr. Krämer, who is head of the department of pediatric dentistry at the University of Chile in Santiago. Although there is not much new evidence since the guidelines were first published, “we do have a lot of new technologies within dentistry that can aid the care of EB,” she said.
An important addition to the upcoming 2020 guidelines is a chapter on the patient-clinician partnership. This was added because “you can have fantastic technologies, but if you don’t have a confident relationship with the family and the patient, you won’t be able to proceed.” Dr. Krämer explained: “Patients with EB are so fragile and so afraid of being hurt that they won’t open their mouth unless there is a confidence with the clinician and they trust [him or her]; once they trust, they [will] open the mouth and you can work.”
Dr. Krämer noted that timing of the first dental appointment will depend on the referral pathway for every country and then every service. In her specialist practice the aim is to see newly diagnosed babies before the age of 3 months. “Lots of people would argue they don’t have teeth, but I need to educate the families on several aspects of oral health from early on.”
Older patients with EB may be more aware of the importance of a healthy mouth from a functional point of view and the need to eat and swallow normally, Dr. Krämer said, adding that the “social aspects of having a healthy smile are very important as well.”
Oral care in EB has come a long way since the 1970s when teeth extraction was recommended as the primary dental treatment option. “If you refer to literature in the 90s, that said we can actually restore the teeth in the patients with EB, and what we are now saying is that we have to prevent oral disease,” Dr. Krämer said.
Can oral disease be prevented completely? Yes, she said, but only in a few patients. “We still have decay in a lot of our patients, but far less than what we have had before. It will depend on the compliance of the family and the patient,” Dr. Krämer noted.
Compliance also is a factor in improving mouth function after surgery, which may be done to prevent the tongue from fusing to the bottom of the mouth and to relieve or prevent microstomia, which limits mouth opening.
“We are doing a lot of surgeries to release the fibrotic scars ... we have done it in both children and adults, but there have been better results in adults, because they are able to comply with the course of exercises” after surgery, Dr. Krämer said.
Results of an as-yet unpublished randomized controlled trial of postoperative mouth exercises demonstrate that patients who did the exercises, which involved using a device to stretch the mouth three times a day for 3 months, saw improvements in mouth opening. Once they stopped doing the exercises, however, these improvements faded. Considering the time spent on dressing changes and other exercises, this is perhaps understandable, she acknowledged.
Prevention, education, continual follow-up, and early referral are key to good oral health, Dr. Krämer emphasized. “If there is patient-clinician partnership confidence, they can have regular checkups with dental cleaning, with a fluoride varnish, different preventive strategies so they do not need to get to the point where they need general anesthesia or extractions.” Extractions still will be done, she added, but more for orthodontic reasons, because the teeth do not fit in the mouth. “That is our ideal world, that is where we want to go.”
REPORTING FROM EB 2020
Target plantar keratoderma when managing ‘mild’ EBS
LONDON – Hardened feet are a major determinant of the clinical course of epidermolysis bullosa simplex (EBS), according to research presented by a German team of investigators at the EB World Congress.
In a study of 157 individuals with EBS, 75.8% had plantar keratoderma, a condition associated with a vicious circle of pain, reduced mobility, subsequent weight gain, and further foot problems.
“EBS has severe impacts on various aspects of everyday life,” Antonia Reimer, MD, and associates at the University of Freiburg, Germany, reported in a poster presentation. “Plantar involvement and [plantar keratoderma] are serious complications of all EBS subtypes, correlating with excessive weight gain, pain, local infections, and limited mobility.”
The researchers suggested that “targeting [plantar keratoderma] should be a priority in EBS therapy and research.”
In their retrospective cohort study, clinical and molecular data were retrieved from patient records, and major determinants of the clinical course of EBS investigated. As such, the researchers looked at how weight changes affected EBS, the effect of hardening skin on the feet, pain, mobility, and working life.
“EB simplex is generally regarded as the ‘mildest’ EB type,” Dr. Reimer and colleagues wrote, “however, individuals with EBS report a high disease burden and frequent pain.” The team found that just under 30% of patients (n = 46) experienced frequent pain, particularly those with localized and severe EBS. Of the patients experiencing pain, the majority (75.2%) had plantar keratoderma. Furthermore, those with blisters underneath the hardened skin reported having the most painful lesions.
Palmoplantar hyperhidrosis was present in slightly more than 40% of cases, and was especially common in individuals with localized EBS, Dr. Reimer and colleagues found. They also found that bacterial and fungal infections occurred in 14% and 7% of patients, respectively, and this correlated significantly with diffuse plantar keratoderma.
A third of patients experience mobility problems, and 8.2% required a wheelchair; 16.4% “were in occupational disability,” the team reported.
“Hyperkeratosis is important because it isn’t just about treating the hyperkeratosis, it’s also looking at the mechanical balance of the foot,” Tariq Khan, PhD, said during an unrelated oral presentation. Dr. Khan, a consultant podiatrist specializing in EB at Great Ormond Street Hospital NHS Foundation Trust in London, discussed how to best manage the feet of people with EB.
“Podiatry technology and how we treat can often be detrimental to an EB patient,” Dr. Khan cautioned at the meeting, which was organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
For example, “certain devices, certain types of material, will add more friction and pressure and cause more blistering,” he added, making treatment challenging.
Having worked with the EB community for the past 22 years, he noted that he had seen how podiatry practices had been refined to deal with this patient population. Dr. Khan is one of several experts behind EB podiatry guidelines issued by DEBRA International last year (Br J Dermatol. 2019 Aug 9. doi: 10.1111/bjd.18381) and has run the charity’s first practical EB podiatry skills course to educate more podiatrists on the intricacies of managing EB feet.
During his talk, Dr. Kahn mentioned several innovations that came about by working with external companies, such as the production of special cotton socks containing silver fibers to help reduce the symptom of hot feet, and development of a cooling insole that helped draw moisture and odor away from the foot while providing comfort to the wearer.
One of the main problems for those with EB is finding comfortable footwear that doesn’t aggravate their symptoms, Dr. Khan emphasized.
According to the EB podiatry guidelines, footwear needs to be supportive, and “its primary focus should be aimed at minimizing blistering by reducing friction.” If blisters are already present, the guidelines note that dressings and topical antiseptics or antibiotics might be used until the blisters heal. “Therefore, suitable shoes or footwear are essential to accommodate dressings and not lead to further trauma to the damaged area. Footwear that is adjustable may be beneficial in these circumstances.”
What constitutes appropriate footwear is open to debate and was the topic of a separate poster presentation at meeting. Mark O’Sullivan, EB team podiatrist at Solihull and Birmingham Women’s and Children’s NHS Foundation Trust, and associates looked at whether wearing rocker bottom footwear could ease the formation of blisters in patients with EBS.
The team studied nine patients who reported regular plantar blistering. An in-shoe measurement system was devised to measure patients’ plantar pressure while they were wearing their existing footwear and then again when they were wearing new footwear with a rocker bottom. Participants completed questionnaires about the development of blisters on their feet, their activity levels, and pain.
The rocker bottom footwear reduced the peak plantar pressure by 30.5% and the total plantar pressure by 31.8%, compared with regular footwear. A shift in the average pressure under the foot was seen, moving from the heels of the feet to the midfoot area, while remaining similar in the front foot area.
“Patient feedback has been mixed,” Mr. O’Sullivan said when presenting the poster. “Patients state that blisters have often reduced in the heels and forefoot, but new blisters have developed in the midfoot.” As a result, some study participants chose to alternate wearing the rocker bottom footwear with their normal shoes, to even out the places where blisters might form.
Although the jury is still out on the benefit of rocker bottom footwear, one thing that might help those with EBS who develop regular foot blisters may be to keep their weight in check. In a separate poster presentation given by Lynn Hubbard, a specialist EB dietitian in the department of nutrition and dietetics at St. Thomas’ Hospital in London, it was shown that almost a third of patients with EB simplex were obese, compared with 26% of adults in the general U.K. population.
“People with EBS are known to have hyperkeratosis and foot-blistering,” Ms. Hubbard observed in the poster. This can lead to reduce mobility and pain, which “may in turn have an impact on body weight, and an increased BMI [body mass index] may further affect mobility.”
Data were collected on 90 patients who attended a U.K. EBS clinic over an 11-month period. While 45.5% of patients had a normal weight, the majority was overweight (21.1%), obese (21.1%), or morbidly obese (10%).
Fifteen patients completed questionnaires about their mobility, and almost all felt that their weight had an adverse effect on their feet, as did EBS. Several also noted problems with their EBS, in the skin folds around the bra, waist, and sock lines.
“We now plan to begin a pilot study to establish a supportive weight management program for people with EBS and evaluate both weight loss and impact on mobility,” Ms. Hubbard reported.
No conflicts of interest were declared by any of the speakers.
SOURCES: EB 2020. Reimer A et al. Poster 26; Khan T. oral presentation; O’Sullivan M et al. Poster 93; Hubbard L. Poster 19.
LONDON – Hardened feet are a major determinant of the clinical course of epidermolysis bullosa simplex (EBS), according to research presented by a German team of investigators at the EB World Congress.
In a study of 157 individuals with EBS, 75.8% had plantar keratoderma, a condition associated with a vicious circle of pain, reduced mobility, subsequent weight gain, and further foot problems.
“EBS has severe impacts on various aspects of everyday life,” Antonia Reimer, MD, and associates at the University of Freiburg, Germany, reported in a poster presentation. “Plantar involvement and [plantar keratoderma] are serious complications of all EBS subtypes, correlating with excessive weight gain, pain, local infections, and limited mobility.”
The researchers suggested that “targeting [plantar keratoderma] should be a priority in EBS therapy and research.”
In their retrospective cohort study, clinical and molecular data were retrieved from patient records, and major determinants of the clinical course of EBS investigated. As such, the researchers looked at how weight changes affected EBS, the effect of hardening skin on the feet, pain, mobility, and working life.
“EB simplex is generally regarded as the ‘mildest’ EB type,” Dr. Reimer and colleagues wrote, “however, individuals with EBS report a high disease burden and frequent pain.” The team found that just under 30% of patients (n = 46) experienced frequent pain, particularly those with localized and severe EBS. Of the patients experiencing pain, the majority (75.2%) had plantar keratoderma. Furthermore, those with blisters underneath the hardened skin reported having the most painful lesions.
Palmoplantar hyperhidrosis was present in slightly more than 40% of cases, and was especially common in individuals with localized EBS, Dr. Reimer and colleagues found. They also found that bacterial and fungal infections occurred in 14% and 7% of patients, respectively, and this correlated significantly with diffuse plantar keratoderma.
A third of patients experience mobility problems, and 8.2% required a wheelchair; 16.4% “were in occupational disability,” the team reported.
“Hyperkeratosis is important because it isn’t just about treating the hyperkeratosis, it’s also looking at the mechanical balance of the foot,” Tariq Khan, PhD, said during an unrelated oral presentation. Dr. Khan, a consultant podiatrist specializing in EB at Great Ormond Street Hospital NHS Foundation Trust in London, discussed how to best manage the feet of people with EB.
“Podiatry technology and how we treat can often be detrimental to an EB patient,” Dr. Khan cautioned at the meeting, which was organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
For example, “certain devices, certain types of material, will add more friction and pressure and cause more blistering,” he added, making treatment challenging.
Having worked with the EB community for the past 22 years, he noted that he had seen how podiatry practices had been refined to deal with this patient population. Dr. Khan is one of several experts behind EB podiatry guidelines issued by DEBRA International last year (Br J Dermatol. 2019 Aug 9. doi: 10.1111/bjd.18381) and has run the charity’s first practical EB podiatry skills course to educate more podiatrists on the intricacies of managing EB feet.
During his talk, Dr. Kahn mentioned several innovations that came about by working with external companies, such as the production of special cotton socks containing silver fibers to help reduce the symptom of hot feet, and development of a cooling insole that helped draw moisture and odor away from the foot while providing comfort to the wearer.
One of the main problems for those with EB is finding comfortable footwear that doesn’t aggravate their symptoms, Dr. Khan emphasized.
According to the EB podiatry guidelines, footwear needs to be supportive, and “its primary focus should be aimed at minimizing blistering by reducing friction.” If blisters are already present, the guidelines note that dressings and topical antiseptics or antibiotics might be used until the blisters heal. “Therefore, suitable shoes or footwear are essential to accommodate dressings and not lead to further trauma to the damaged area. Footwear that is adjustable may be beneficial in these circumstances.”
What constitutes appropriate footwear is open to debate and was the topic of a separate poster presentation at meeting. Mark O’Sullivan, EB team podiatrist at Solihull and Birmingham Women’s and Children’s NHS Foundation Trust, and associates looked at whether wearing rocker bottom footwear could ease the formation of blisters in patients with EBS.
The team studied nine patients who reported regular plantar blistering. An in-shoe measurement system was devised to measure patients’ plantar pressure while they were wearing their existing footwear and then again when they were wearing new footwear with a rocker bottom. Participants completed questionnaires about the development of blisters on their feet, their activity levels, and pain.
The rocker bottom footwear reduced the peak plantar pressure by 30.5% and the total plantar pressure by 31.8%, compared with regular footwear. A shift in the average pressure under the foot was seen, moving from the heels of the feet to the midfoot area, while remaining similar in the front foot area.
“Patient feedback has been mixed,” Mr. O’Sullivan said when presenting the poster. “Patients state that blisters have often reduced in the heels and forefoot, but new blisters have developed in the midfoot.” As a result, some study participants chose to alternate wearing the rocker bottom footwear with their normal shoes, to even out the places where blisters might form.
Although the jury is still out on the benefit of rocker bottom footwear, one thing that might help those with EBS who develop regular foot blisters may be to keep their weight in check. In a separate poster presentation given by Lynn Hubbard, a specialist EB dietitian in the department of nutrition and dietetics at St. Thomas’ Hospital in London, it was shown that almost a third of patients with EB simplex were obese, compared with 26% of adults in the general U.K. population.
“People with EBS are known to have hyperkeratosis and foot-blistering,” Ms. Hubbard observed in the poster. This can lead to reduce mobility and pain, which “may in turn have an impact on body weight, and an increased BMI [body mass index] may further affect mobility.”
Data were collected on 90 patients who attended a U.K. EBS clinic over an 11-month period. While 45.5% of patients had a normal weight, the majority was overweight (21.1%), obese (21.1%), or morbidly obese (10%).
Fifteen patients completed questionnaires about their mobility, and almost all felt that their weight had an adverse effect on their feet, as did EBS. Several also noted problems with their EBS, in the skin folds around the bra, waist, and sock lines.
“We now plan to begin a pilot study to establish a supportive weight management program for people with EBS and evaluate both weight loss and impact on mobility,” Ms. Hubbard reported.
No conflicts of interest were declared by any of the speakers.
SOURCES: EB 2020. Reimer A et al. Poster 26; Khan T. oral presentation; O’Sullivan M et al. Poster 93; Hubbard L. Poster 19.
LONDON – Hardened feet are a major determinant of the clinical course of epidermolysis bullosa simplex (EBS), according to research presented by a German team of investigators at the EB World Congress.
In a study of 157 individuals with EBS, 75.8% had plantar keratoderma, a condition associated with a vicious circle of pain, reduced mobility, subsequent weight gain, and further foot problems.
“EBS has severe impacts on various aspects of everyday life,” Antonia Reimer, MD, and associates at the University of Freiburg, Germany, reported in a poster presentation. “Plantar involvement and [plantar keratoderma] are serious complications of all EBS subtypes, correlating with excessive weight gain, pain, local infections, and limited mobility.”
The researchers suggested that “targeting [plantar keratoderma] should be a priority in EBS therapy and research.”
In their retrospective cohort study, clinical and molecular data were retrieved from patient records, and major determinants of the clinical course of EBS investigated. As such, the researchers looked at how weight changes affected EBS, the effect of hardening skin on the feet, pain, mobility, and working life.
“EB simplex is generally regarded as the ‘mildest’ EB type,” Dr. Reimer and colleagues wrote, “however, individuals with EBS report a high disease burden and frequent pain.” The team found that just under 30% of patients (n = 46) experienced frequent pain, particularly those with localized and severe EBS. Of the patients experiencing pain, the majority (75.2%) had plantar keratoderma. Furthermore, those with blisters underneath the hardened skin reported having the most painful lesions.
Palmoplantar hyperhidrosis was present in slightly more than 40% of cases, and was especially common in individuals with localized EBS, Dr. Reimer and colleagues found. They also found that bacterial and fungal infections occurred in 14% and 7% of patients, respectively, and this correlated significantly with diffuse plantar keratoderma.
A third of patients experience mobility problems, and 8.2% required a wheelchair; 16.4% “were in occupational disability,” the team reported.
“Hyperkeratosis is important because it isn’t just about treating the hyperkeratosis, it’s also looking at the mechanical balance of the foot,” Tariq Khan, PhD, said during an unrelated oral presentation. Dr. Khan, a consultant podiatrist specializing in EB at Great Ormond Street Hospital NHS Foundation Trust in London, discussed how to best manage the feet of people with EB.
“Podiatry technology and how we treat can often be detrimental to an EB patient,” Dr. Khan cautioned at the meeting, which was organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
For example, “certain devices, certain types of material, will add more friction and pressure and cause more blistering,” he added, making treatment challenging.
Having worked with the EB community for the past 22 years, he noted that he had seen how podiatry practices had been refined to deal with this patient population. Dr. Khan is one of several experts behind EB podiatry guidelines issued by DEBRA International last year (Br J Dermatol. 2019 Aug 9. doi: 10.1111/bjd.18381) and has run the charity’s first practical EB podiatry skills course to educate more podiatrists on the intricacies of managing EB feet.
During his talk, Dr. Kahn mentioned several innovations that came about by working with external companies, such as the production of special cotton socks containing silver fibers to help reduce the symptom of hot feet, and development of a cooling insole that helped draw moisture and odor away from the foot while providing comfort to the wearer.
One of the main problems for those with EB is finding comfortable footwear that doesn’t aggravate their symptoms, Dr. Khan emphasized.
According to the EB podiatry guidelines, footwear needs to be supportive, and “its primary focus should be aimed at minimizing blistering by reducing friction.” If blisters are already present, the guidelines note that dressings and topical antiseptics or antibiotics might be used until the blisters heal. “Therefore, suitable shoes or footwear are essential to accommodate dressings and not lead to further trauma to the damaged area. Footwear that is adjustable may be beneficial in these circumstances.”
What constitutes appropriate footwear is open to debate and was the topic of a separate poster presentation at meeting. Mark O’Sullivan, EB team podiatrist at Solihull and Birmingham Women’s and Children’s NHS Foundation Trust, and associates looked at whether wearing rocker bottom footwear could ease the formation of blisters in patients with EBS.
The team studied nine patients who reported regular plantar blistering. An in-shoe measurement system was devised to measure patients’ plantar pressure while they were wearing their existing footwear and then again when they were wearing new footwear with a rocker bottom. Participants completed questionnaires about the development of blisters on their feet, their activity levels, and pain.
The rocker bottom footwear reduced the peak plantar pressure by 30.5% and the total plantar pressure by 31.8%, compared with regular footwear. A shift in the average pressure under the foot was seen, moving from the heels of the feet to the midfoot area, while remaining similar in the front foot area.
“Patient feedback has been mixed,” Mr. O’Sullivan said when presenting the poster. “Patients state that blisters have often reduced in the heels and forefoot, but new blisters have developed in the midfoot.” As a result, some study participants chose to alternate wearing the rocker bottom footwear with their normal shoes, to even out the places where blisters might form.
Although the jury is still out on the benefit of rocker bottom footwear, one thing that might help those with EBS who develop regular foot blisters may be to keep their weight in check. In a separate poster presentation given by Lynn Hubbard, a specialist EB dietitian in the department of nutrition and dietetics at St. Thomas’ Hospital in London, it was shown that almost a third of patients with EB simplex were obese, compared with 26% of adults in the general U.K. population.
“People with EBS are known to have hyperkeratosis and foot-blistering,” Ms. Hubbard observed in the poster. This can lead to reduce mobility and pain, which “may in turn have an impact on body weight, and an increased BMI [body mass index] may further affect mobility.”
Data were collected on 90 patients who attended a U.K. EBS clinic over an 11-month period. While 45.5% of patients had a normal weight, the majority was overweight (21.1%), obese (21.1%), or morbidly obese (10%).
Fifteen patients completed questionnaires about their mobility, and almost all felt that their weight had an adverse effect on their feet, as did EBS. Several also noted problems with their EBS, in the skin folds around the bra, waist, and sock lines.
“We now plan to begin a pilot study to establish a supportive weight management program for people with EBS and evaluate both weight loss and impact on mobility,” Ms. Hubbard reported.
No conflicts of interest were declared by any of the speakers.
SOURCES: EB 2020. Reimer A et al. Poster 26; Khan T. oral presentation; O’Sullivan M et al. Poster 93; Hubbard L. Poster 19.
REPORTING FROM EB 2020
Hand deformity happens early in children with dystrophic epidermolysis bullosa
London – A predictable course of hand contracture was seen in a U.K. study of children with recessive dystrophic epidermolysis bullosa (RDEB), with all children experiencing moderate or severe hand deformity by the age of 12 years.
This stark finding, reported at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), highlighted the importance of intervening early with surgical methods that aim to prevent the pseudosyndactyly, or “mitten” hand deformity, which is an unfortunate characteristic of the genetic skin condition.
The investigative team, from the plastic and reconstructive surgery department at Great Ormond Street Hospital for Children NHS Trust, London, presented data from a retrospective case review of 24 children who attended their specialist pediatric EB center between 2010 and 2019. Of these, seven children had surgery to release hand contractures.
A total of 250 hand assessments were made via the novel Assessment of the Component Hand Contractures in Epidermolysis Bullosa (ACE). The assessment provides a hand deformity grade (HDG) – none, mild, moderate, and severe –based on the typical contractures that are seen in RDEB, such as between the fingers (web space contractures), finger flexion contractures, and thumb adduction contractures.
Using the ACE tool, “we found four significant time points regarding hand contracture development,” Catherine Miller, one of the team’s occupational therapists, said during a poster presentation. At birth, none of the children had any signs of hand deformity, but by 2 years of age half had mild hand contracture. By age 6, all children had some form of hand deformity, Ms. Miller said, and by age 12 all had moderate to severe hand deformity, “so adding to the data that hand deformities really are inevitable.”
Other findings were that the thumb and finger web spaces were the first to contract, Ms. Miller said. “So they tend to develop earlier and progress relatively slowly.” By contrast the finger flexion contractures occurred later on, “but progress more relatively rapidly,” she observed.
“Our data are limited as not every child is included at every age, and out tool has not yet been validated,” Ms. Miller and team acknowledged in the poster. “We assume that hand contractures do not improve, and therefore have included operated hands (mean age 6 years) at their last preoperative HDG in order to represent older children and more advanced hand deformities.”
In an interview, Ms. Miller noted that families have a lot going on when their newborn is diagnosed with RDEB, so introducing the idea that there will be substantial hand deformities in the future “is a difficult conversation. We have to take that gently.”
There are nonsurgical approaches to keeping the hands open, such as “encouraging them to open their hands in play, daily stretches; we can make splints with a silicon substance and other thermoplastic materials,” Ms. Miller said.
Hand surgery is a ‘blunt tool’
“The primary problem, of course, is the dermal fibrosis that we see that creates scarring and secondary problems,” said Gill Smith, a plastic surgery consultant who works with Ms. Miller at the hospital.
“In an ideal world, you would bandage up [the children] so that they could never injure their hands, but then they couldn’t use them, they couldn’t grow properly, and they could not develop,” Ms. Smith said in an oral presentation about hand surgery in children with RDEB. “You do not want them to get to the secondary stage, because the secondary stage is a real problem – you get all these impairments of hand function – pseudosyndactyly, finger contraction, and first web contracture, and ending up in a ‘mitten’ hand.”
Surgery is a very “crude” and “blunt tool,” Ms. Smith emphasized. Prevention is key, and perhaps in the future gene therapy, mesenchymal stem cells, and the like will mean that there is less need for hand surgery, she intimated. Until then, there are some things that can be done surgically – such as wrapping the hands, using gloves to protect the skin, stretching out the web spaces of the palm, and using splints. “All of these things we are trying to improve all the time, and come up with new ideas.”
The question is when to intervene? Ms. Smith said that in any other type of hand surgery, particularly in children where growth and function might be affected, the aim would be to “go in early.” In children with RDEB, however, the timing is not so clear: “Should we be going in early, before secondary joint changes, before we get secondary tendon shortening?” Perhaps this would result in less complex surgery, she suggested, but “it is a really huge deal for families and for children. For the moment we are still only really doing it when there [are] quite significant functional difficulties.”
When it comes to the type of surgery done to release the hands, “everyone has variants on the release technique,” but none are known to be better than any other, Ms. Smith said. Surgical release deals with consequences of dermal fibrosis but also creates more fibrosis, she cautioned.
Effects of hand surgery do not last long
How long the surgery’s effect will last is “what everyone wants to know, and I don’t think anyone has found a really good answer. It is variable, but unfortunately it’s a lot shorter than we’d like,” said Ms. Smith.
Indeed, data in another poster presentation by Ms. Smith and colleagues showed that the situation can be ‘back to square one’ within just a couple of years. Of the seven patients who had surgery at a mean 7 years of age (range 6-10 years), “most had returned to their original total score by 2 years post surgery,” the team wrote. All children “were initially happy with both appearance and function after surgery” they added; however, “happiness gradually decreased with time as they lost function and their scores increased with recurrence of contracture.”
The team noted that “sometimes after surgery a different component of the hand contracture worsened but function was preserved.”
While the ACE tool used by the team has not yet been validated, they believe it to be “a systematic tool with a structured method of administration.” As such it can help with informed decision making, they believe, and it could be used with functional measures to see how hand contractures might be impacting hand function and quality of life.
The ACE tool can be downloaded for free from the GOSH website.
SOURCE: Jessop N et al. EB 2020. Posters 42 and 43; Smith G et al. Poster 63.
London – A predictable course of hand contracture was seen in a U.K. study of children with recessive dystrophic epidermolysis bullosa (RDEB), with all children experiencing moderate or severe hand deformity by the age of 12 years.
This stark finding, reported at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), highlighted the importance of intervening early with surgical methods that aim to prevent the pseudosyndactyly, or “mitten” hand deformity, which is an unfortunate characteristic of the genetic skin condition.
The investigative team, from the plastic and reconstructive surgery department at Great Ormond Street Hospital for Children NHS Trust, London, presented data from a retrospective case review of 24 children who attended their specialist pediatric EB center between 2010 and 2019. Of these, seven children had surgery to release hand contractures.
A total of 250 hand assessments were made via the novel Assessment of the Component Hand Contractures in Epidermolysis Bullosa (ACE). The assessment provides a hand deformity grade (HDG) – none, mild, moderate, and severe –based on the typical contractures that are seen in RDEB, such as between the fingers (web space contractures), finger flexion contractures, and thumb adduction contractures.
Using the ACE tool, “we found four significant time points regarding hand contracture development,” Catherine Miller, one of the team’s occupational therapists, said during a poster presentation. At birth, none of the children had any signs of hand deformity, but by 2 years of age half had mild hand contracture. By age 6, all children had some form of hand deformity, Ms. Miller said, and by age 12 all had moderate to severe hand deformity, “so adding to the data that hand deformities really are inevitable.”
Other findings were that the thumb and finger web spaces were the first to contract, Ms. Miller said. “So they tend to develop earlier and progress relatively slowly.” By contrast the finger flexion contractures occurred later on, “but progress more relatively rapidly,” she observed.
“Our data are limited as not every child is included at every age, and out tool has not yet been validated,” Ms. Miller and team acknowledged in the poster. “We assume that hand contractures do not improve, and therefore have included operated hands (mean age 6 years) at their last preoperative HDG in order to represent older children and more advanced hand deformities.”
In an interview, Ms. Miller noted that families have a lot going on when their newborn is diagnosed with RDEB, so introducing the idea that there will be substantial hand deformities in the future “is a difficult conversation. We have to take that gently.”
There are nonsurgical approaches to keeping the hands open, such as “encouraging them to open their hands in play, daily stretches; we can make splints with a silicon substance and other thermoplastic materials,” Ms. Miller said.
Hand surgery is a ‘blunt tool’
“The primary problem, of course, is the dermal fibrosis that we see that creates scarring and secondary problems,” said Gill Smith, a plastic surgery consultant who works with Ms. Miller at the hospital.
“In an ideal world, you would bandage up [the children] so that they could never injure their hands, but then they couldn’t use them, they couldn’t grow properly, and they could not develop,” Ms. Smith said in an oral presentation about hand surgery in children with RDEB. “You do not want them to get to the secondary stage, because the secondary stage is a real problem – you get all these impairments of hand function – pseudosyndactyly, finger contraction, and first web contracture, and ending up in a ‘mitten’ hand.”
Surgery is a very “crude” and “blunt tool,” Ms. Smith emphasized. Prevention is key, and perhaps in the future gene therapy, mesenchymal stem cells, and the like will mean that there is less need for hand surgery, she intimated. Until then, there are some things that can be done surgically – such as wrapping the hands, using gloves to protect the skin, stretching out the web spaces of the palm, and using splints. “All of these things we are trying to improve all the time, and come up with new ideas.”
The question is when to intervene? Ms. Smith said that in any other type of hand surgery, particularly in children where growth and function might be affected, the aim would be to “go in early.” In children with RDEB, however, the timing is not so clear: “Should we be going in early, before secondary joint changes, before we get secondary tendon shortening?” Perhaps this would result in less complex surgery, she suggested, but “it is a really huge deal for families and for children. For the moment we are still only really doing it when there [are] quite significant functional difficulties.”
When it comes to the type of surgery done to release the hands, “everyone has variants on the release technique,” but none are known to be better than any other, Ms. Smith said. Surgical release deals with consequences of dermal fibrosis but also creates more fibrosis, she cautioned.
Effects of hand surgery do not last long
How long the surgery’s effect will last is “what everyone wants to know, and I don’t think anyone has found a really good answer. It is variable, but unfortunately it’s a lot shorter than we’d like,” said Ms. Smith.
Indeed, data in another poster presentation by Ms. Smith and colleagues showed that the situation can be ‘back to square one’ within just a couple of years. Of the seven patients who had surgery at a mean 7 years of age (range 6-10 years), “most had returned to their original total score by 2 years post surgery,” the team wrote. All children “were initially happy with both appearance and function after surgery” they added; however, “happiness gradually decreased with time as they lost function and their scores increased with recurrence of contracture.”
The team noted that “sometimes after surgery a different component of the hand contracture worsened but function was preserved.”
While the ACE tool used by the team has not yet been validated, they believe it to be “a systematic tool with a structured method of administration.” As such it can help with informed decision making, they believe, and it could be used with functional measures to see how hand contractures might be impacting hand function and quality of life.
The ACE tool can be downloaded for free from the GOSH website.
SOURCE: Jessop N et al. EB 2020. Posters 42 and 43; Smith G et al. Poster 63.
London – A predictable course of hand contracture was seen in a U.K. study of children with recessive dystrophic epidermolysis bullosa (RDEB), with all children experiencing moderate or severe hand deformity by the age of 12 years.
This stark finding, reported at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA), highlighted the importance of intervening early with surgical methods that aim to prevent the pseudosyndactyly, or “mitten” hand deformity, which is an unfortunate characteristic of the genetic skin condition.
The investigative team, from the plastic and reconstructive surgery department at Great Ormond Street Hospital for Children NHS Trust, London, presented data from a retrospective case review of 24 children who attended their specialist pediatric EB center between 2010 and 2019. Of these, seven children had surgery to release hand contractures.
A total of 250 hand assessments were made via the novel Assessment of the Component Hand Contractures in Epidermolysis Bullosa (ACE). The assessment provides a hand deformity grade (HDG) – none, mild, moderate, and severe –based on the typical contractures that are seen in RDEB, such as between the fingers (web space contractures), finger flexion contractures, and thumb adduction contractures.
Using the ACE tool, “we found four significant time points regarding hand contracture development,” Catherine Miller, one of the team’s occupational therapists, said during a poster presentation. At birth, none of the children had any signs of hand deformity, but by 2 years of age half had mild hand contracture. By age 6, all children had some form of hand deformity, Ms. Miller said, and by age 12 all had moderate to severe hand deformity, “so adding to the data that hand deformities really are inevitable.”
Other findings were that the thumb and finger web spaces were the first to contract, Ms. Miller said. “So they tend to develop earlier and progress relatively slowly.” By contrast the finger flexion contractures occurred later on, “but progress more relatively rapidly,” she observed.
“Our data are limited as not every child is included at every age, and out tool has not yet been validated,” Ms. Miller and team acknowledged in the poster. “We assume that hand contractures do not improve, and therefore have included operated hands (mean age 6 years) at their last preoperative HDG in order to represent older children and more advanced hand deformities.”
In an interview, Ms. Miller noted that families have a lot going on when their newborn is diagnosed with RDEB, so introducing the idea that there will be substantial hand deformities in the future “is a difficult conversation. We have to take that gently.”
There are nonsurgical approaches to keeping the hands open, such as “encouraging them to open their hands in play, daily stretches; we can make splints with a silicon substance and other thermoplastic materials,” Ms. Miller said.
Hand surgery is a ‘blunt tool’
“The primary problem, of course, is the dermal fibrosis that we see that creates scarring and secondary problems,” said Gill Smith, a plastic surgery consultant who works with Ms. Miller at the hospital.
“In an ideal world, you would bandage up [the children] so that they could never injure their hands, but then they couldn’t use them, they couldn’t grow properly, and they could not develop,” Ms. Smith said in an oral presentation about hand surgery in children with RDEB. “You do not want them to get to the secondary stage, because the secondary stage is a real problem – you get all these impairments of hand function – pseudosyndactyly, finger contraction, and first web contracture, and ending up in a ‘mitten’ hand.”
Surgery is a very “crude” and “blunt tool,” Ms. Smith emphasized. Prevention is key, and perhaps in the future gene therapy, mesenchymal stem cells, and the like will mean that there is less need for hand surgery, she intimated. Until then, there are some things that can be done surgically – such as wrapping the hands, using gloves to protect the skin, stretching out the web spaces of the palm, and using splints. “All of these things we are trying to improve all the time, and come up with new ideas.”
The question is when to intervene? Ms. Smith said that in any other type of hand surgery, particularly in children where growth and function might be affected, the aim would be to “go in early.” In children with RDEB, however, the timing is not so clear: “Should we be going in early, before secondary joint changes, before we get secondary tendon shortening?” Perhaps this would result in less complex surgery, she suggested, but “it is a really huge deal for families and for children. For the moment we are still only really doing it when there [are] quite significant functional difficulties.”
When it comes to the type of surgery done to release the hands, “everyone has variants on the release technique,” but none are known to be better than any other, Ms. Smith said. Surgical release deals with consequences of dermal fibrosis but also creates more fibrosis, she cautioned.
Effects of hand surgery do not last long
How long the surgery’s effect will last is “what everyone wants to know, and I don’t think anyone has found a really good answer. It is variable, but unfortunately it’s a lot shorter than we’d like,” said Ms. Smith.
Indeed, data in another poster presentation by Ms. Smith and colleagues showed that the situation can be ‘back to square one’ within just a couple of years. Of the seven patients who had surgery at a mean 7 years of age (range 6-10 years), “most had returned to their original total score by 2 years post surgery,” the team wrote. All children “were initially happy with both appearance and function after surgery” they added; however, “happiness gradually decreased with time as they lost function and their scores increased with recurrence of contracture.”
The team noted that “sometimes after surgery a different component of the hand contracture worsened but function was preserved.”
While the ACE tool used by the team has not yet been validated, they believe it to be “a systematic tool with a structured method of administration.” As such it can help with informed decision making, they believe, and it could be used with functional measures to see how hand contractures might be impacting hand function and quality of life.
The ACE tool can be downloaded for free from the GOSH website.
SOURCE: Jessop N et al. EB 2020. Posters 42 and 43; Smith G et al. Poster 63.
REPORTING FROM EB 2020
Hyperhidrosis treatment options include glycopyrrolate
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
LAHAINA, HAWAII – Hyperhidrosis affects nearly 5% of the U.S. population, and in a survey of U.S. teenagers, about 17% reported excessive sweating, Jashin Wu, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
In an interview with MDedge reporter Bruce Jancin, Dr. Wu, founder of the Dermatology Research and Education Foundation, Irvine, Calif., discussed the off-label use of oral agents to treat hyperhidrosis. Dr. Wu said he is a fan of oral glycopyrrolate in particular, which he tends to use even earlier than suggested in the International Hyperhidrosis Society guidelines.
Glycopyrrolate is available in 1 mg and 2 mg tablets; Dr. Wu starts patients at a dose of 1 mg twice a day, escalating by 1 mg per week until the “desired effects occur” or the patient has problems tolerating treatment because of side effects.
Other oral options include oxybutynin and propranolol. Sofpironium bromide, an analog of glycopyrrolate, is in the pipeline, he said.
During the interview, Dr. Wu discussed mydriasis, an adverse effect associated with both topical and systemic anticholinergic treatment. In the two pivotal phase 3 randomized trials of prescription glycopyrronium cloth (Qbrexza) for axillary hyperhidrosis, the incidence of mydriasis was 6.8% in 463 patients on active treatment for 4 weeks. Three-quarters of cases were unilateral. The mydriasis resolved without permanent treatment discontinuation in 27 of the 31 patients (J Am Acad Dermatol. 2019 Jan;80[1]:128-138.e2).
“The most important point is that patients need to be educated that they need to wash their hands very well after they apply it to the affected areas” to prevent accidental medication contact with the eyes, he advised.
Alarm bells can go off when a patient with anticholinergic therapy–induced mydriasis presents to an ED without mentioning their treatment status, Dr. Wu observed.
Dr. Wu had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
To listen to the interview, click the play button below.
REPORTING FROM THE HAWAII DERMATOLOGY SEMINAR