User login
Two questions can help establish a diagnosis of hidradenitis suppurativa
According to Iltefat H. Hamzavi, MD,
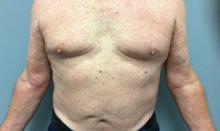
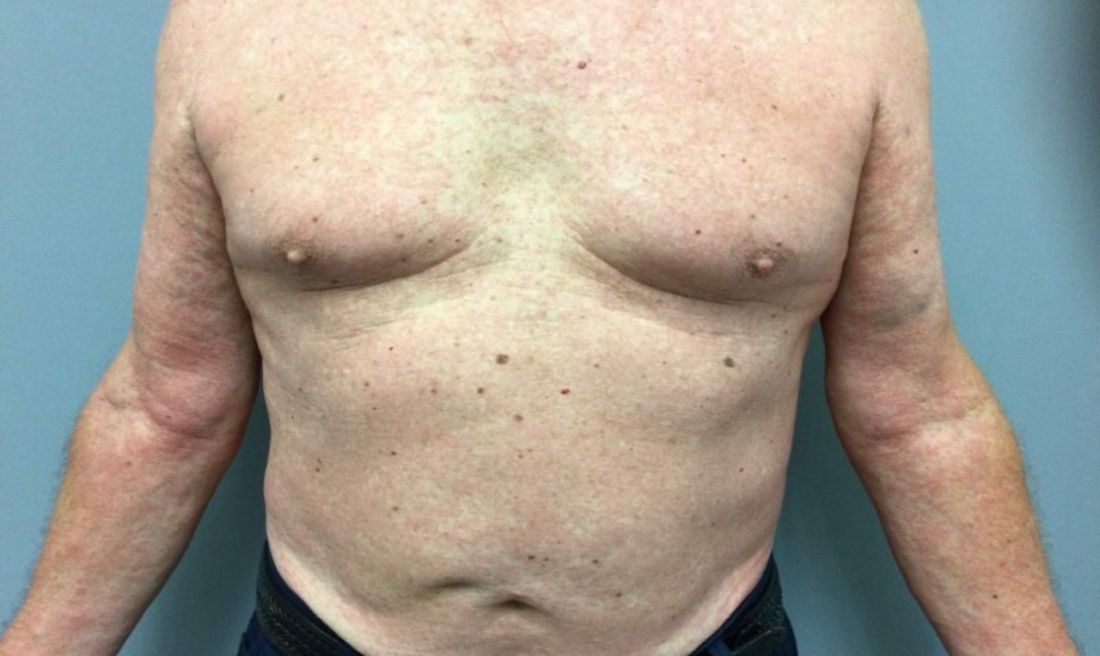
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Certain opioids hold promise for treating itch
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
A 73-year-old White male presented with 2 days of a very pruritic rash
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
What is the diagnosis?
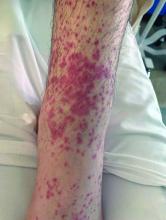
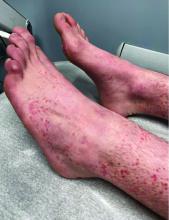
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Numerous morphologies of skin rashes have been described in the setting of COVID-19, including pernio, livedoid rash, exanthem, and vasculitis. This classic constellation of symptoms (palpable purpura on buttocks/legs, abdominal pain, arthralgia, hematuria) is highly consistent with Henoch-Schonlein purpura (HSP). There are now multiple case reports of COVID-19–associated HSP.
HSP is the most common type of childhood systemic vasculitis. It is mediated by immunoglobulin A (IgA) immune complex deposition and has been associated with respiratory tract infections, streptococcal species, parainfluenza virus, and human parvovirus B19, medications, vaccinations, and malignancies. HSP is usually a self-limiting disease, with a course over 4-6 weeks, and can affect multiple organs, including the skin, gastrointestinal tract, joints, and the kidneys. The diagnostic criteria include palpable purpura in the presence of one or more of the following: diffuse abdominal pain, arthritis or arthralgia, any biopsy showing predominant IgA deposition, and renal involvement in the form of hematuria or proteinuria. Renal disease is variable and is the most significant indicator of long-term prognosis. This teenager was treated with oral corticosteroids because of the severe periarticular edema and responded rapidly. His subsequent urine analyses normalized.
What is on the differential?
Multisystem inflammatory syndrome in children (MIS-C) is a rare, potentially fatal, complication of COVID-19 infection that causes inflammation of multiple organs, including the heart, lungs, kidneys, brain, skin, eyes, or the gastrointestinal tract. It commonly affects children around ages 8-9 years. Initial symptoms include fever, rash, red eyes, diarrhea, and vomiting that appear 2-6 weeks post COVID-19 infection. Like HSP, MIS-C can present with edema of the extremities, worsening hand/foot pain, and hematuria; however, the absence of both fever and the pattern of system involvement seen with MIS-C and classic findings in this patient are more consistent with HSP.
Reactive infectious mucocutaneous eruption (RIME) was recently coined to encompass both infection-associated Stevens-Johnson eruptions including Mycoplasma pneumoniae-induced rash and mucositis (MIRM) and mucocutaneous eruptions caused by nonmycoplasma pathogens (including Chlamydia pneumoniae, human parainfluenza virus 2, rhinovirus, adenovirus, enterovirus, human metapneumovirus, influenza B virus, and COVID-19). It is usually seen in male children and adolescents. Prodromal symptoms include cough, fever, and malaise and they precede the prominent feature of mucositis. Our patient’s lack of mucosal involvement is not consistent with RIME.
Perniosis (chilblains) is characterized by localized edematous patches of erythema or cyanosis on exposed extremities, that may be associated with cold exposure. Lesions are usually symmetric and self-limiting, and symptoms can include numbness, tingling, pruritus, burning, or pain. Pernio-like skin lesions have been seen during the COVID-19 pandemic, though many patients have negative testing for infection by PCR and serology. Pernio may also be seen with autoimmune diseases or malignancy.
Meningococcemia is a rare disease caused by infection with gram-negative diplococci bacteria Neisseria meningitidis and spreads through saliva or respiratory secretions. Its clinical presentation can vary widely, from transient fever to fulminant disease. It is characterized by upper respiratory tract infection, fever, and petechial lesions associated with thrombocytopenia and coagulopathy.
Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital. Dr. Eichenfield and Ms. Laborada have no relevant financial disclosures.
References
AlGhoozi DA, AlKhayyat HM. BMJ Case Reports CP 2021;14:e239910.
Jacobi M et al. Pediatr Infect Dis J. 2021;40(2):e93-4.
Paller A, Mancini AJ. Hurwitz clinical pediatric dermatology: A textbook of skin disorders of childhood and adolescence. 4th ed. Philadelphia (PA): Elsevier Saunders; 2011.
Radia T et al. Paediatr Respir Rev. 2021;38:51-7.
Ramien ML. Clin Exp Dermatol. 2021;46(3):420-9.
Cannabinoids being studied for a variety of dermatologic conditions
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
.
“When you walk into places like CVS or Walgreens, you see lots of displays for CBD creams and oils,” Todd S. Anhalt, MD, said during the annual meeting of the Pacific Dermatologic Association. “The problem is, we don’t know what’s in them or who made them or how good they are. That’s going to be a problem for a while.”
According to Dr. Anhalt, clinical professor emeritus of dermatology at Stanford (Calif.) University, there are about 140 active cannabinoid compounds in cannabis, but the most important ones are THC and cannabidiol (CBD). There are three types of cannabinoids, based on where the cannabidiol is produced: endocannabinoids, which are produced in the human body; phytocannabinoids, which are derived from plants such as marijuana and hemp; and synthetic cannabinoids, which are derived in labs.
Dr. Anhalt described the endocannabinoid system as a conserved network of molecular signaling made of several components: signaling molecules (endocannabinoids), endocannabinoid receptors (CB-1 and CB-2), enzymes, and transporters. There is also overlap between cannabinoids and terpenes, which are responsible for flavor and aroma in plants and marijuana and can enhance the effects of CBD.
“For the most part, CB-1 receptors are in the central nervous system and CB-2 [receptors] are mostly in the periphery,” including the skin and digestive system, said Dr. Anhalt, who practices at the California Skin Institute in Los Altos, Calif. “This is interesting because one of the main conditions I recommend cannabidiol for is in patients with peripheral neuropathy, despite the fact they may be on all sorts of medications such as Neurontin and Lyrica or tricyclic antidepressants. Sometimes they don’t get much relief from those. I have had many patients tell me that they have had reduction of pain and increased functionality using the CBD creams.” CB-2 receptors, he noted, are located in keratinocytes, sensory receptors, sweat glands, fibroblasts, Langerhans cells, melanocytes, and sebaceous glands.
Recent research shows that the endocannabinoid system is involved in modulation of the CNS and in immune function, particularly skin homeostasis and barrier function. “We know that barrier function can be affected by the generation of oxidative species,” he said. “The stress that it causes can decrease barrier function and lead to cytokine release and itch. CBDs have been shown to enter cells, target and upregulate genes with decreased oxidation and inflammation, and protect membrane integrity in skin cells. Therefore, this might be helpful in atopic dermatitis.” Other potential uses in dermatology include wound healing, acne, hair growth modulation, skin and hair pigmentation, skin infections, psoriasis, and cutaneous malignancies, as well as neuropathic pain.
Evidence is strongest for neuropathic pain, he said, which is mediated by CB-1 receptors peripherally, followed by itch and atopic dermatitis. The authors of a 2017 systematic review concluded that “low-strength” evidence exists to suggest that cannabis alleviates neuropathic pain, with insufficient evidence for other types of pain.
Topical CBD comes in various forms: oils (usually hemp oil), creams, and lotions, Dr. Anhalt said. “I advise patients to apply it 2-4 times per day depending on how anxious or uncomfortable they are. It takes my patients 10 days to 2 weeks before they notice anything at all.”
For atopic dermatitis, it could be useful “not to use it instead of a moisturizer, but as a moisturizer,” Dr. Anhalt advised. “You can have a patient get big jars of CBD creams and lotions. They may have to try a few before they find one that they really like, but you can replace all of the other moisturizers that you’re using right now in patients who have a lot of itch.”
As for CBD’s effect on peripheral neuropathy, the medical literature is lacking, but some studies show low to moderate evidence of efficacy. For example, a Cochrane Review found that a 30% or greater pain reduction was achieved by 39% of patients who used cannabis-based treatments, vs. 33% of those on placebo.
“I would not suggest CBD as a first-line drug unless it’s very mild peripheral neuropathy, but for patients who are on gabapentin who are better but not better enough, this is an excellent adjunct,” Dr. Anhalt said. “It’s worth trying. It’s not too expensive and it’s really safe.”
The application of topical CBD to treat cutaneous malignancies has not yet shown evidence of significant efficacy, while using CBDs for acne holds promise. “The endogenous cannabinoid system is involved in the production of lipids,” he said. “Cannabinoids have an antilipogenic activity, so they decrease sebum production. CBD could help patients with mild acne who are reluctant to use other types of medications. For this and other potential dermatologic applications, lots more studies need to be done.”
Dr. Anhalt reported having no financial disclosures.
FROM PDA 2021
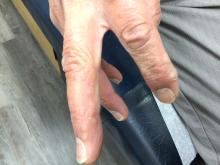
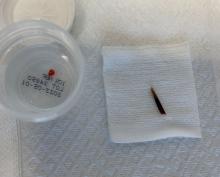
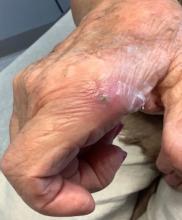
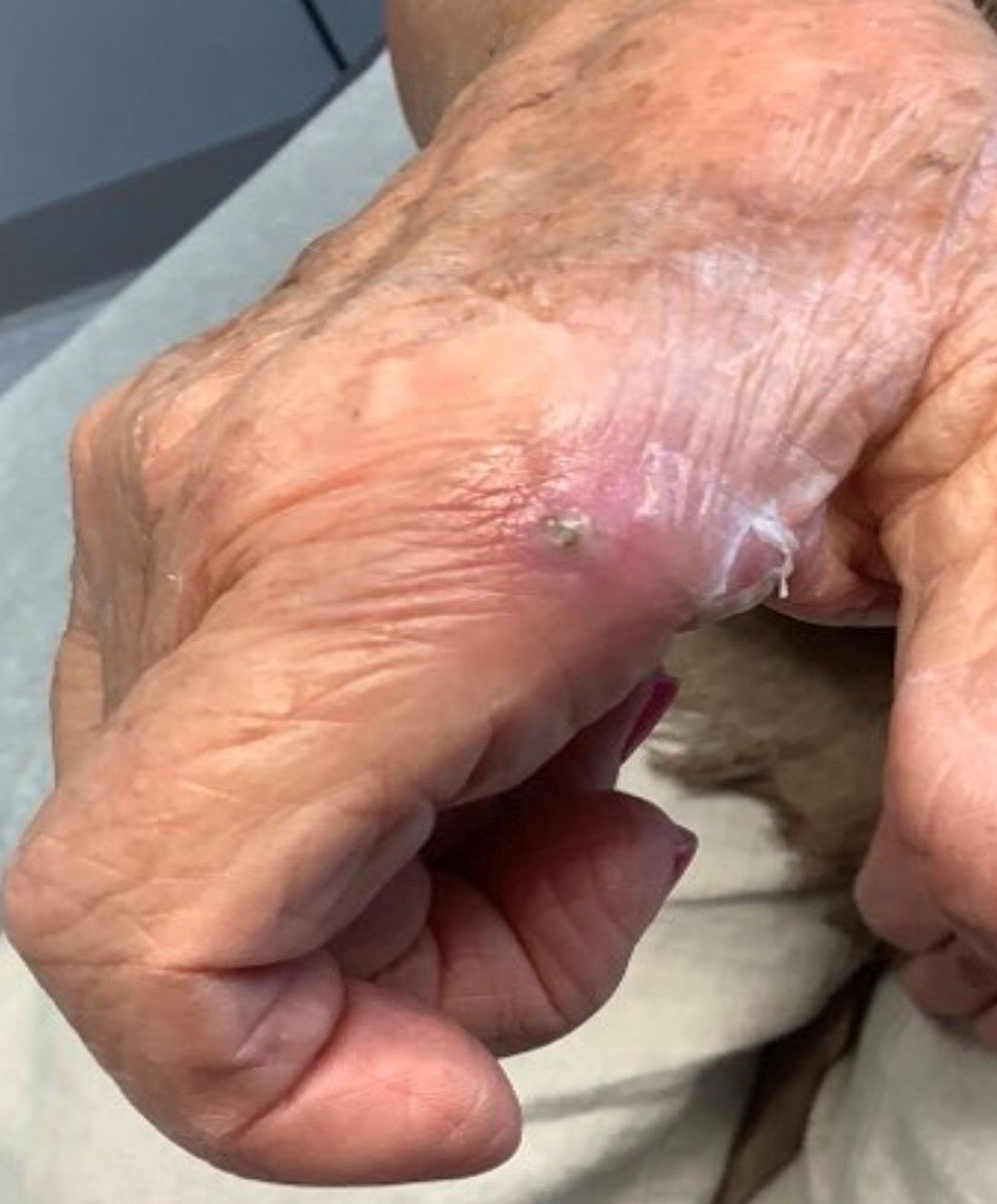
A 70-year-old man presents with firm papules on his hand and fingers
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
, although women are more often affected than men. GA most commonly appears in the first 3 decades of life. Although the etiology is not known, GA may represent a delayed hypersensitivity reaction. A link between GA and diabetes mellitus, autoimmune thyroiditis, dyslipidemia, and rarely, malignancy may exist.
GA is most commonly localized, presenting as an asymptomatic, erythematous, annular plaque with a firm border and central clearing localized to the wrists, ankles, and dorsal hands or feet. This form is the type most often seen in children. Generalized GA is far less common and presents later in life as multiple asymptomatic or pruritic papules and plaques on the trunk and extremities. Less common variants include subcutaneous GA, patch GA, atypical GA, and perforating GA. Perforating GA occurs on the dorsal hands and presents as (umbilicated) papules, and seems consistent with this patient’s clinical presentation. Histologically, transepidermal elimination of collagen is typically seen in perforating GA.1
Histology in this patient’s biopsy revealed a granulomatous dermatitis consistent with granuloma annulare. A palisaded arrangement of histiocytic cells surrounding altered collagen with increased dermal mucin was seen. There was associated perivascular mononuclear inflammatory infiltrates. The overlying epidermis was unremarkable.
Granuloma annulare often spontaneously resolves without sequelae. In some cases, atrophy may result. Lesions may also recur. Localized GA is often treated with high-potency topical corticosteroids or intralesional corticosteroids. For generalized GA, topical or intralesional corticosteroids may be used for select lesions. Topical calcineurin inhibitors, light therapy, cryotherapy, imiquimod, hydroxychloroquine, isotretinoin, and dapsone have also been reported in the literature as possible treatments.
This case and photo were provided by Dr. Berke, of Three Rivers Dermatology, Pittsburgh, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1 Alves J, Barreiros H, Bartolo E. Healthcare (Basel). 2014 Sep 4;2(3):338-45.
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
3. “Andrews’ Diseases of the Skin,” 13th ed. James W et al. Philadelphia: Saunders Elsevier, 2006.
Oteseconazole promising for recurrent yeast infections
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ketosis, including ketogenic diets, implicated in prurigo pigmentosa
, according to a dermatologist, who reviewed skin conditions common to patients of Asian descent at the Skin of Color Update 2021.
“Ketogenic diets are gaining popularity globally for weight loss. After 2-4 weeks [on a strict ketogenic diet], some patients start to notice very pruritic papules on their trunk, the so-called keto rash,” reported Hye Jin Chung, MD, director of the Asian Skin Clinic, Beth Israel Deaconess Medical Center, Boston. “Keto rash is actually prurigo pigmentosa.”
The exact pathogenesis of prurigo pigmentosa, a highly pruritic macular and papular rash with gross reticular pigmentation, is unclear, but Dr. Chung reported that the strong link with ketosis might explain why more cases are now being encountered outside of east Asia. Ketosis or conditions associated with a high risk for ketosis, such as anorexia nervosa, diabetes mellitus, or recent bariatric surgery, have been linked to prurigo pigmentosa in all skin types and ethnicities.
“I tell my residents that this is a disease you will never forget after your first case,” she said.
The differential diagnosis includes contact dermatitis and other inflammatory disorders, but Dr. Chung said that the reticular pattern of the lesions is a relatively unique feature. Confluent and reticulated papillomatosis (CARP) shares a pattern of reticulated lesions, but Dr. Chung said it lacks the inflammatory erythematous papules and the severe pruritus common to prurigo pigmentosa.
Histologically, the pattern evolves. It begins as a perivascular infiltration dominated by neutrophils and eosinophils with hyperkeratosis, acanthosis, and spongiosis. Over time, Dr. Chung said that the histologic picture shows an increasing degree of dyskeratosis as keratinocytes die.
Prurigo pigmentosa was first described 50 years ago by Masaji Nagashima, MD, who published a report on eight patients in Japan with a pruriginous truncal dermatosis featuring symmetrical pigmentation. Most subsequent reports were also from Japan or other east Asian countries, but it has since spread.
This global spread was captured in a recently published review of 115 published studies and case reports from 24 countries. In this review, the proportion of studies from Europe (36.5%) approached that of those from east Asia (38.2%), even if 76% of the patients for whom race was reported were of Asian ethnicity.
Of the 369 patients evaluated in these studies and case reports, 72.1% were female. The mean age was 25.6 years. In the studies originating outside of Asia, prurigo pigmentosa was reported in a spectrum of skin types and ethnicities, including Whites, Blacks, and Hispanics. The lowest reported incidence has been in the latter two groups, but the authors of the review speculated that this condition is likely being underdiagnosed in non-Asian individuals.
Dr. Chung agreed, and she cautioned that the consequences typically result in a significant delay for achieving disease control. In recounting a recent case of prurigo pigmentosa at her center, she said that the 59-year-old Asian patient had been initiated on topical steroids and oral antihistamines by her primary care physician before she was referred. This is a common and reasonable strategy for a highly pruritic rash potentially caused by contact dermatitis, but it is ineffective for this disorder.
“Prurigo pigmentosa requires anti-inflammatory agents,” she explained. She said that doxycycline and minocycline are the treatments of choice, but noted that there are also reports of efficacy with dapsone, macrolide antibiotics, and isotretinoin.
In her most recent case, she initiated the patient on 100 mg of doxycycline twice daily. There was significant improvement within 2 weeks, and the rash resolved within a month with no relapse in follow-up that now exceeds 12 months, Dr. Chung said.
According to Dr. Chung, Asian-Americans are the most rapidly growing ethnic group in the United States, making it increasingly important to be familiar with conditions common or unique to Asian skin, but prurigo pigmentosa is no longer confined to those of Asian descent. She encouraged clinicians to recognize this disorder to reduce the common delays to effective treatment.
The senior author of the recently published review of studies, Jensen Yeung, MD, of the department of dermatology, University of Toronto, agreed. He, too, believes that dermatologists need to increase their awareness of the signs and symptoms of prurigo pigmentosa – and not just in Asian patients or patients of Asian descent.
“This diagnosis is often missed,” he contended in an interview. “This condition has become more common in the past 5 years in my clinical experience.” He added that the increasing incidence might not just be related to better diagnostic accuracy, although the most significant of other possible explanations “is not yet well understood.”
Dr. Chung reports that she has no relevant financial relationships to disclose. Dr. Yeung reports financial relationships with more than 25 pharmaceutical companies, some of which produce treatments employed in the control of prurigo pigmentosa.
, according to a dermatologist, who reviewed skin conditions common to patients of Asian descent at the Skin of Color Update 2021.
“Ketogenic diets are gaining popularity globally for weight loss. After 2-4 weeks [on a strict ketogenic diet], some patients start to notice very pruritic papules on their trunk, the so-called keto rash,” reported Hye Jin Chung, MD, director of the Asian Skin Clinic, Beth Israel Deaconess Medical Center, Boston. “Keto rash is actually prurigo pigmentosa.”
The exact pathogenesis of prurigo pigmentosa, a highly pruritic macular and papular rash with gross reticular pigmentation, is unclear, but Dr. Chung reported that the strong link with ketosis might explain why more cases are now being encountered outside of east Asia. Ketosis or conditions associated with a high risk for ketosis, such as anorexia nervosa, diabetes mellitus, or recent bariatric surgery, have been linked to prurigo pigmentosa in all skin types and ethnicities.
“I tell my residents that this is a disease you will never forget after your first case,” she said.
The differential diagnosis includes contact dermatitis and other inflammatory disorders, but Dr. Chung said that the reticular pattern of the lesions is a relatively unique feature. Confluent and reticulated papillomatosis (CARP) shares a pattern of reticulated lesions, but Dr. Chung said it lacks the inflammatory erythematous papules and the severe pruritus common to prurigo pigmentosa.
Histologically, the pattern evolves. It begins as a perivascular infiltration dominated by neutrophils and eosinophils with hyperkeratosis, acanthosis, and spongiosis. Over time, Dr. Chung said that the histologic picture shows an increasing degree of dyskeratosis as keratinocytes die.
Prurigo pigmentosa was first described 50 years ago by Masaji Nagashima, MD, who published a report on eight patients in Japan with a pruriginous truncal dermatosis featuring symmetrical pigmentation. Most subsequent reports were also from Japan or other east Asian countries, but it has since spread.
This global spread was captured in a recently published review of 115 published studies and case reports from 24 countries. In this review, the proportion of studies from Europe (36.5%) approached that of those from east Asia (38.2%), even if 76% of the patients for whom race was reported were of Asian ethnicity.
Of the 369 patients evaluated in these studies and case reports, 72.1% were female. The mean age was 25.6 years. In the studies originating outside of Asia, prurigo pigmentosa was reported in a spectrum of skin types and ethnicities, including Whites, Blacks, and Hispanics. The lowest reported incidence has been in the latter two groups, but the authors of the review speculated that this condition is likely being underdiagnosed in non-Asian individuals.
Dr. Chung agreed, and she cautioned that the consequences typically result in a significant delay for achieving disease control. In recounting a recent case of prurigo pigmentosa at her center, she said that the 59-year-old Asian patient had been initiated on topical steroids and oral antihistamines by her primary care physician before she was referred. This is a common and reasonable strategy for a highly pruritic rash potentially caused by contact dermatitis, but it is ineffective for this disorder.
“Prurigo pigmentosa requires anti-inflammatory agents,” she explained. She said that doxycycline and minocycline are the treatments of choice, but noted that there are also reports of efficacy with dapsone, macrolide antibiotics, and isotretinoin.
In her most recent case, she initiated the patient on 100 mg of doxycycline twice daily. There was significant improvement within 2 weeks, and the rash resolved within a month with no relapse in follow-up that now exceeds 12 months, Dr. Chung said.
According to Dr. Chung, Asian-Americans are the most rapidly growing ethnic group in the United States, making it increasingly important to be familiar with conditions common or unique to Asian skin, but prurigo pigmentosa is no longer confined to those of Asian descent. She encouraged clinicians to recognize this disorder to reduce the common delays to effective treatment.
The senior author of the recently published review of studies, Jensen Yeung, MD, of the department of dermatology, University of Toronto, agreed. He, too, believes that dermatologists need to increase their awareness of the signs and symptoms of prurigo pigmentosa – and not just in Asian patients or patients of Asian descent.
“This diagnosis is often missed,” he contended in an interview. “This condition has become more common in the past 5 years in my clinical experience.” He added that the increasing incidence might not just be related to better diagnostic accuracy, although the most significant of other possible explanations “is not yet well understood.”
Dr. Chung reports that she has no relevant financial relationships to disclose. Dr. Yeung reports financial relationships with more than 25 pharmaceutical companies, some of which produce treatments employed in the control of prurigo pigmentosa.
, according to a dermatologist, who reviewed skin conditions common to patients of Asian descent at the Skin of Color Update 2021.
“Ketogenic diets are gaining popularity globally for weight loss. After 2-4 weeks [on a strict ketogenic diet], some patients start to notice very pruritic papules on their trunk, the so-called keto rash,” reported Hye Jin Chung, MD, director of the Asian Skin Clinic, Beth Israel Deaconess Medical Center, Boston. “Keto rash is actually prurigo pigmentosa.”
The exact pathogenesis of prurigo pigmentosa, a highly pruritic macular and papular rash with gross reticular pigmentation, is unclear, but Dr. Chung reported that the strong link with ketosis might explain why more cases are now being encountered outside of east Asia. Ketosis or conditions associated with a high risk for ketosis, such as anorexia nervosa, diabetes mellitus, or recent bariatric surgery, have been linked to prurigo pigmentosa in all skin types and ethnicities.
“I tell my residents that this is a disease you will never forget after your first case,” she said.
The differential diagnosis includes contact dermatitis and other inflammatory disorders, but Dr. Chung said that the reticular pattern of the lesions is a relatively unique feature. Confluent and reticulated papillomatosis (CARP) shares a pattern of reticulated lesions, but Dr. Chung said it lacks the inflammatory erythematous papules and the severe pruritus common to prurigo pigmentosa.
Histologically, the pattern evolves. It begins as a perivascular infiltration dominated by neutrophils and eosinophils with hyperkeratosis, acanthosis, and spongiosis. Over time, Dr. Chung said that the histologic picture shows an increasing degree of dyskeratosis as keratinocytes die.
Prurigo pigmentosa was first described 50 years ago by Masaji Nagashima, MD, who published a report on eight patients in Japan with a pruriginous truncal dermatosis featuring symmetrical pigmentation. Most subsequent reports were also from Japan or other east Asian countries, but it has since spread.
This global spread was captured in a recently published review of 115 published studies and case reports from 24 countries. In this review, the proportion of studies from Europe (36.5%) approached that of those from east Asia (38.2%), even if 76% of the patients for whom race was reported were of Asian ethnicity.
Of the 369 patients evaluated in these studies and case reports, 72.1% were female. The mean age was 25.6 years. In the studies originating outside of Asia, prurigo pigmentosa was reported in a spectrum of skin types and ethnicities, including Whites, Blacks, and Hispanics. The lowest reported incidence has been in the latter two groups, but the authors of the review speculated that this condition is likely being underdiagnosed in non-Asian individuals.
Dr. Chung agreed, and she cautioned that the consequences typically result in a significant delay for achieving disease control. In recounting a recent case of prurigo pigmentosa at her center, she said that the 59-year-old Asian patient had been initiated on topical steroids and oral antihistamines by her primary care physician before she was referred. This is a common and reasonable strategy for a highly pruritic rash potentially caused by contact dermatitis, but it is ineffective for this disorder.
“Prurigo pigmentosa requires anti-inflammatory agents,” she explained. She said that doxycycline and minocycline are the treatments of choice, but noted that there are also reports of efficacy with dapsone, macrolide antibiotics, and isotretinoin.
In her most recent case, she initiated the patient on 100 mg of doxycycline twice daily. There was significant improvement within 2 weeks, and the rash resolved within a month with no relapse in follow-up that now exceeds 12 months, Dr. Chung said.
According to Dr. Chung, Asian-Americans are the most rapidly growing ethnic group in the United States, making it increasingly important to be familiar with conditions common or unique to Asian skin, but prurigo pigmentosa is no longer confined to those of Asian descent. She encouraged clinicians to recognize this disorder to reduce the common delays to effective treatment.
The senior author of the recently published review of studies, Jensen Yeung, MD, of the department of dermatology, University of Toronto, agreed. He, too, believes that dermatologists need to increase their awareness of the signs and symptoms of prurigo pigmentosa – and not just in Asian patients or patients of Asian descent.
“This diagnosis is often missed,” he contended in an interview. “This condition has become more common in the past 5 years in my clinical experience.” He added that the increasing incidence might not just be related to better diagnostic accuracy, although the most significant of other possible explanations “is not yet well understood.”
Dr. Chung reports that she has no relevant financial relationships to disclose. Dr. Yeung reports financial relationships with more than 25 pharmaceutical companies, some of which produce treatments employed in the control of prurigo pigmentosa.
FROM SOC 2021
An 80-year-old female developed a painful purulent nodule a day after gardening
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
Rare hematologic malignancy may first present to a dermatologist
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
FROM PDA 2021