User login
Guidance for PCI without on-site surgical backup updated
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
such as ambulatory surgery centers (ASCs) and office-based laboratories and which are best left to more traditional settings, such as hospitals with full cardiac support.
PCI has evolved quickly since SCAI issued its last update almost 9 years ago. The updated statement, published online in the Journal of the Society for Cardiovascular Angiography and Interventions, notes that the proportion of same-day PCI discharges has increased from 4.5% in 2009 to 28.6% in 2017.
The statement also notes that the Medicare facility fee for outpatient PCI in an ASC is about 40% less than the hospital fee: $6,111 versus $10,258 for the facility fee for 2022. The Centers for Medicare & Medicaid Services in 2020 extended coverage for PCIs in ASCs.
Rationale for update
Writing group chair Cindy Grines, MD, explained the rationale for updating the statement now. “The 2014 SCAI statement was very conservative, recommending only the simplest of cases be done without surgical backup,” Dr. Grines, chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta, said in an interview.
The statement drew on 12 global studies from 2015 to 2022 that evaluated more than 8 million PCIs at facilities with and without surgery on site. Dr. Grines noted those studies reported complication rates as low as 0.1% in PCI procedures in centers without surgical backup.
She also noted that the writing committee also received input that “by restricting the use of certain devices such as atherectomy, some patients who needed it as a bailout could be harmed.”
Another factor in prompting the statement update, Dr. Grines said: “Many hospitals have consolidated into heath systems, and these systems consolidated bypass surgery into one center. Therefore, centers with high volume, experienced operators, and excellent outcomes were now left with no surgery on site. It didn’t make sense to withdraw complex PCI from these centers who haven’t sent a patient for emergency bypass in several years.”
Statement guidance
The centerpiece of the update is an algorithm that covers the range of settings for PCI, from having a surgeon on site to ACS or office-based lab.
For example, indications for on-site surgical capability are PCI of the last remaining patent vessel or retrograde approach to epicardial chronic total occlusion (CTO), and when the patient is a candidate for surgery.
Indications for PCI in a hospital without on-site surgery but with percutaneous ventricular assist device or extracorporeal membrane oxygenation, calcium modification devices and high PCI volume are patients with decreased left ventricular ejection fraction, unprotected left main artery, CTO, or degenerated vein grafts.
For patients at high risk for transfusion, acute kidney injury or vascular complications, or who have high baseline respiratory risk, a hospital without on-site surgery but with respiratory care, blood bank, and vascular surgery services is indicated.
And for patients with none of the aforementioned characteristics or risks, ASC, office-based lab, or any hospital facility is acceptable.
The statement also provides guidance for operator experience. Those with less than 3 years’ experience, considered to have limited exposure to atherectomy devices and limited ST-segmented elevation MI (STEMI)/shock experience, should avoid doing PCIs in an ASC and performing atherectomy cases on their own, and have a colleague review case selection and assist in higher-risk cases. Experienced (3-10 years’ experience) and very experienced (more than 10 years’) should be able to perform in any setting and be competent with, if not highly experienced with, atherectomy and STEMI/shock.
Dr. Grines acknowledged the writing group didn’t want to set a specific operator volume requirement. “However, we recognize that lifetime operator experience is particularly important in more complex cases such as CTO, atherectomy, bifurcation stenoses, etc.,” she said. “In addition, performing these cases at a larger institution that has other operators that may assist in the event of a complication is very important. Specifically, we did not believe that recent fellow graduates with less than 3 years in practice or low-volume operators should attempt higher-risk cases in a no-SOS [surgeon-on-site] setting or perform cases in ASC or office-based labs where no colleagues are there to assist in case of a complication.”
In an interview, Gregory J. Dehmer, MD, professor of medicine at Virginia Tech University, Roanoke, reprised the theme of his accompanying editorial. “Things are evolving again, as Bob Dylan would say, ‘The Times They Are A-Changin’, so it’s very timely that the society in collaboration with other professional societies updated what are guidelines and rules of road if you’re going to do PCI in ASCs or office based laboratories,” said Dr. Dehmer, who chaired the writing committees of the 2007 and 2014 SCAI expert statements on PCI.
Having this statement is important for centers that don’t have on-site surgical backup, he said. “You couldn’t sustain a PCI operation at a rural hospital on just acute MIs alone. The key thing is that all of this built upon numerous studies both in the U.S. and abroad that showed the safety of doing elective cases – not only STEMIs, but elective PCI – at facilities without on-site surgery.”
CMS pushed the envelope when it decided to reimburse PCIs done in ASCs, Dr. Dehmer said. “That was not based on a lot of data. It was kind of a leap of faith. It’s logical that this should work, but in order for it to work and be safe for pats you have to follow the rules. That’s where SCAI stepped in at this point and said this is a whole new environment and we need to set some ground rules for physicians of who and who should not be having these procures in an office-based lab or an ambulatory surgery center.”
Dr. Grines and Dr. Dehmer have no relevant disclosures.
FROM THE JOURNAL OF SOCIETY FOR CARDIOVASCULAR ANGIOGRAPHY AND INTERVENTIONS
What happened to surgical mitral valve repair in the MitraClip era?
The overall case volume for surgical mitral valve (MV) repair of degenerative mitral regurgitation (DMR) hasn’t changed much nearly a decade into the age of transcatheter edge-to-edge repair (TEER). But, over the same period, there’s been a shift in the surgical–MV repair case mix at centers that have offered both the surgical option and TEER, a new study suggests.
Once TEER was introduced, those centers over time used the operative approach less in higher– and intermediate–surgical risk patients and more often in those deemed low risk for surgery. And that trend – at centers offering both approaches – paralleled improved risk-adjusted surgical repair short-term complications and 30-day and 5-year mortality.
The findings come from an analysis based on Society of Thoracic Surgeons and Medicare claims data collected from 2011 through 2018 at surgical–MV repair centers that also offered TEER for DMR after its 2013 approval. The transcatheter procedure, until only recently the exclusive domain of Abbott’s MitraClip in various incarnations, is officially indicated for patients judged too high risk for surgical MV repair.
A shift in surgical MV repair to predominantly lower-risk patients would be expected to improve outcomes. But the improvements seen in the current study seem to have a more complex explanation, Sreekanth Vemulapalli, MD, told this news organization.
The data seem to show TEER indication creep from higher-risk cases, for which there is clinical trial support, to intermediate-risk patients, that lack such evidence in favor of TEER. That seemed to push surgical repair toward even lower-risk cases. “I think that’s exactly right,” said Dr. Vemulapalli, Duke Clinical Research Institute, Durham, N.C.
Still, he observed, the analysis was adjusted for surgical risk, and “Even after that adjustment, it looked like surgical outcomes were getting better after the availability of transcatheter mitral repair techniques.”
That observation may be explained by an increasingly sharp, “more careful” process for selecting patients for surgical repair vs. TEER, said Dr. Vemulapalli, who is senior author of the study published in the Journal of the American College of Cardiology. Angela M. Lowenstern, MD, Vanderbilt University, Nashville, and Andrew M. Vekstein, MD, Duke Clinical Research Institute, were the lead authors.
Indeed, the report states, the analysis supports the view that “a systematic evaluation by a heart team able to direct patients towards either surgical or transcatheter approaches enhances both short-term and long-term surgical outcomes.”
“In a world where both surgical and transcatheter techniques are going to be available,” Dr. Vemulapalli said, “patient selection becomes very, very important.”
An accompanying editorial acknowledges the heart-team approach’s potential for improving the selection of patients for surgery and perhaps therefore outcomes. But it also cites issues with that interpretation of the data.
For example, the heart-team approach is not used in consistent ways across the United States. And “although the heart team is recommended in multiple guidelines for valvular heart therapies, there is little evidence for its efficacy, specifically regarding improving clinical outcomes,” write Matthew W. Sherwood, MD, MHS, and Wayne B. Batchelor, MD, MHS, Inova Heart and Vascular Institute, Falls Church, Va.
The editorialists highlight the study’s “significant downtrend in both high-risk and intermediate-risk surgical cases, with a concomitant increase in low-risk cases,” after introduction of TEER. That shift in case mix, they write, “would seem to be a more likely explanation for the modest improvement in outcomes for surgical MV repair.”
Also, importantly, the analysis didn’t include data on TEER procedures, only indirect evidence for TEER’s effect on surgical MV repair, the editorialists observe, and study authors acknowledge.
Still, the analysis looked at nearly 14,000 patients at 278 U.S. sites with surgical MV repair that launched TEER programs during the study period. They accounted for 6,806 surgical cases before and 7,153 surgical cases after the advent of TEER.
Their median annualized institutional surgical MV repair volume was 32 before and 29 after TEER availability (P = .06).
The risk-adjusted odds ratio for 30-day mortality after vs. before TEER became an option was 0.73 (95% confidence interval, 0.54-0.99). The corresponding hazard ratio for mortality at 5 years was 0.75 (95% CI, 0.66-0.86).
Other risk-adjusted surgical outcomes improved once TEER became available, including MV adverse outcomes (OR, 0.71; 95% CI, 0.58-0.86; P < .001), operative mortality (OR, 0.73; 95% CI, 0.54-0.99; P = .041), and major morbidity (OR, 0.85; 95% CI, 0.73-0.98; P = .026)
Despite the data’s suggestion of TEER indication creep from solely high–surgical risk patients to those at intermediate risk, Dr. Vemulapalli said, “I don’t think that people should be doing transcatheter mitral repair in intermediate- or low-risk patients as a general rule.” Although, he added, “there will always be certain exceptions, depending on the patient’s specific situation.”
Dr. Vemulapalli pointed to several ongoing trials comparing TEER vs. surgical MR repair in patients with DMR at intermediate surgical risk, including REPAIR MR and PRIMARY.
The study was supported by the National Institutes of Health and Abbott. Dr. Vemulapalli discloses receiving grants or contracts from the American College of Cardiology, the Society of Thoracic Surgeons, Cytokinetics, Abbott Vascular, the National Institutes of Health, and Boston Scientific; and consulting or serving on an advisory board for Janssen, the American College of Physicians, HeartFlow, and Edwards LifeSciences. Dr. Sherwood discloses receiving honoraria or consulting fees from Medtronic and Boston Scientific. Dr. Batchelor discloses receiving consulting fees from Medtronic, Boston Scientific, Edwards Lifesciences, and Abbott.
A version of this article first appeared on Medscape.com.
The overall case volume for surgical mitral valve (MV) repair of degenerative mitral regurgitation (DMR) hasn’t changed much nearly a decade into the age of transcatheter edge-to-edge repair (TEER). But, over the same period, there’s been a shift in the surgical–MV repair case mix at centers that have offered both the surgical option and TEER, a new study suggests.
Once TEER was introduced, those centers over time used the operative approach less in higher– and intermediate–surgical risk patients and more often in those deemed low risk for surgery. And that trend – at centers offering both approaches – paralleled improved risk-adjusted surgical repair short-term complications and 30-day and 5-year mortality.
The findings come from an analysis based on Society of Thoracic Surgeons and Medicare claims data collected from 2011 through 2018 at surgical–MV repair centers that also offered TEER for DMR after its 2013 approval. The transcatheter procedure, until only recently the exclusive domain of Abbott’s MitraClip in various incarnations, is officially indicated for patients judged too high risk for surgical MV repair.
A shift in surgical MV repair to predominantly lower-risk patients would be expected to improve outcomes. But the improvements seen in the current study seem to have a more complex explanation, Sreekanth Vemulapalli, MD, told this news organization.
The data seem to show TEER indication creep from higher-risk cases, for which there is clinical trial support, to intermediate-risk patients, that lack such evidence in favor of TEER. That seemed to push surgical repair toward even lower-risk cases. “I think that’s exactly right,” said Dr. Vemulapalli, Duke Clinical Research Institute, Durham, N.C.
Still, he observed, the analysis was adjusted for surgical risk, and “Even after that adjustment, it looked like surgical outcomes were getting better after the availability of transcatheter mitral repair techniques.”
That observation may be explained by an increasingly sharp, “more careful” process for selecting patients for surgical repair vs. TEER, said Dr. Vemulapalli, who is senior author of the study published in the Journal of the American College of Cardiology. Angela M. Lowenstern, MD, Vanderbilt University, Nashville, and Andrew M. Vekstein, MD, Duke Clinical Research Institute, were the lead authors.
Indeed, the report states, the analysis supports the view that “a systematic evaluation by a heart team able to direct patients towards either surgical or transcatheter approaches enhances both short-term and long-term surgical outcomes.”
“In a world where both surgical and transcatheter techniques are going to be available,” Dr. Vemulapalli said, “patient selection becomes very, very important.”
An accompanying editorial acknowledges the heart-team approach’s potential for improving the selection of patients for surgery and perhaps therefore outcomes. But it also cites issues with that interpretation of the data.
For example, the heart-team approach is not used in consistent ways across the United States. And “although the heart team is recommended in multiple guidelines for valvular heart therapies, there is little evidence for its efficacy, specifically regarding improving clinical outcomes,” write Matthew W. Sherwood, MD, MHS, and Wayne B. Batchelor, MD, MHS, Inova Heart and Vascular Institute, Falls Church, Va.
The editorialists highlight the study’s “significant downtrend in both high-risk and intermediate-risk surgical cases, with a concomitant increase in low-risk cases,” after introduction of TEER. That shift in case mix, they write, “would seem to be a more likely explanation for the modest improvement in outcomes for surgical MV repair.”
Also, importantly, the analysis didn’t include data on TEER procedures, only indirect evidence for TEER’s effect on surgical MV repair, the editorialists observe, and study authors acknowledge.
Still, the analysis looked at nearly 14,000 patients at 278 U.S. sites with surgical MV repair that launched TEER programs during the study period. They accounted for 6,806 surgical cases before and 7,153 surgical cases after the advent of TEER.
Their median annualized institutional surgical MV repair volume was 32 before and 29 after TEER availability (P = .06).
The risk-adjusted odds ratio for 30-day mortality after vs. before TEER became an option was 0.73 (95% confidence interval, 0.54-0.99). The corresponding hazard ratio for mortality at 5 years was 0.75 (95% CI, 0.66-0.86).
Other risk-adjusted surgical outcomes improved once TEER became available, including MV adverse outcomes (OR, 0.71; 95% CI, 0.58-0.86; P < .001), operative mortality (OR, 0.73; 95% CI, 0.54-0.99; P = .041), and major morbidity (OR, 0.85; 95% CI, 0.73-0.98; P = .026)
Despite the data’s suggestion of TEER indication creep from solely high–surgical risk patients to those at intermediate risk, Dr. Vemulapalli said, “I don’t think that people should be doing transcatheter mitral repair in intermediate- or low-risk patients as a general rule.” Although, he added, “there will always be certain exceptions, depending on the patient’s specific situation.”
Dr. Vemulapalli pointed to several ongoing trials comparing TEER vs. surgical MR repair in patients with DMR at intermediate surgical risk, including REPAIR MR and PRIMARY.
The study was supported by the National Institutes of Health and Abbott. Dr. Vemulapalli discloses receiving grants or contracts from the American College of Cardiology, the Society of Thoracic Surgeons, Cytokinetics, Abbott Vascular, the National Institutes of Health, and Boston Scientific; and consulting or serving on an advisory board for Janssen, the American College of Physicians, HeartFlow, and Edwards LifeSciences. Dr. Sherwood discloses receiving honoraria or consulting fees from Medtronic and Boston Scientific. Dr. Batchelor discloses receiving consulting fees from Medtronic, Boston Scientific, Edwards Lifesciences, and Abbott.
A version of this article first appeared on Medscape.com.
The overall case volume for surgical mitral valve (MV) repair of degenerative mitral regurgitation (DMR) hasn’t changed much nearly a decade into the age of transcatheter edge-to-edge repair (TEER). But, over the same period, there’s been a shift in the surgical–MV repair case mix at centers that have offered both the surgical option and TEER, a new study suggests.
Once TEER was introduced, those centers over time used the operative approach less in higher– and intermediate–surgical risk patients and more often in those deemed low risk for surgery. And that trend – at centers offering both approaches – paralleled improved risk-adjusted surgical repair short-term complications and 30-day and 5-year mortality.
The findings come from an analysis based on Society of Thoracic Surgeons and Medicare claims data collected from 2011 through 2018 at surgical–MV repair centers that also offered TEER for DMR after its 2013 approval. The transcatheter procedure, until only recently the exclusive domain of Abbott’s MitraClip in various incarnations, is officially indicated for patients judged too high risk for surgical MV repair.
A shift in surgical MV repair to predominantly lower-risk patients would be expected to improve outcomes. But the improvements seen in the current study seem to have a more complex explanation, Sreekanth Vemulapalli, MD, told this news organization.
The data seem to show TEER indication creep from higher-risk cases, for which there is clinical trial support, to intermediate-risk patients, that lack such evidence in favor of TEER. That seemed to push surgical repair toward even lower-risk cases. “I think that’s exactly right,” said Dr. Vemulapalli, Duke Clinical Research Institute, Durham, N.C.
Still, he observed, the analysis was adjusted for surgical risk, and “Even after that adjustment, it looked like surgical outcomes were getting better after the availability of transcatheter mitral repair techniques.”
That observation may be explained by an increasingly sharp, “more careful” process for selecting patients for surgical repair vs. TEER, said Dr. Vemulapalli, who is senior author of the study published in the Journal of the American College of Cardiology. Angela M. Lowenstern, MD, Vanderbilt University, Nashville, and Andrew M. Vekstein, MD, Duke Clinical Research Institute, were the lead authors.
Indeed, the report states, the analysis supports the view that “a systematic evaluation by a heart team able to direct patients towards either surgical or transcatheter approaches enhances both short-term and long-term surgical outcomes.”
“In a world where both surgical and transcatheter techniques are going to be available,” Dr. Vemulapalli said, “patient selection becomes very, very important.”
An accompanying editorial acknowledges the heart-team approach’s potential for improving the selection of patients for surgery and perhaps therefore outcomes. But it also cites issues with that interpretation of the data.
For example, the heart-team approach is not used in consistent ways across the United States. And “although the heart team is recommended in multiple guidelines for valvular heart therapies, there is little evidence for its efficacy, specifically regarding improving clinical outcomes,” write Matthew W. Sherwood, MD, MHS, and Wayne B. Batchelor, MD, MHS, Inova Heart and Vascular Institute, Falls Church, Va.
The editorialists highlight the study’s “significant downtrend in both high-risk and intermediate-risk surgical cases, with a concomitant increase in low-risk cases,” after introduction of TEER. That shift in case mix, they write, “would seem to be a more likely explanation for the modest improvement in outcomes for surgical MV repair.”
Also, importantly, the analysis didn’t include data on TEER procedures, only indirect evidence for TEER’s effect on surgical MV repair, the editorialists observe, and study authors acknowledge.
Still, the analysis looked at nearly 14,000 patients at 278 U.S. sites with surgical MV repair that launched TEER programs during the study period. They accounted for 6,806 surgical cases before and 7,153 surgical cases after the advent of TEER.
Their median annualized institutional surgical MV repair volume was 32 before and 29 after TEER availability (P = .06).
The risk-adjusted odds ratio for 30-day mortality after vs. before TEER became an option was 0.73 (95% confidence interval, 0.54-0.99). The corresponding hazard ratio for mortality at 5 years was 0.75 (95% CI, 0.66-0.86).
Other risk-adjusted surgical outcomes improved once TEER became available, including MV adverse outcomes (OR, 0.71; 95% CI, 0.58-0.86; P < .001), operative mortality (OR, 0.73; 95% CI, 0.54-0.99; P = .041), and major morbidity (OR, 0.85; 95% CI, 0.73-0.98; P = .026)
Despite the data’s suggestion of TEER indication creep from solely high–surgical risk patients to those at intermediate risk, Dr. Vemulapalli said, “I don’t think that people should be doing transcatheter mitral repair in intermediate- or low-risk patients as a general rule.” Although, he added, “there will always be certain exceptions, depending on the patient’s specific situation.”
Dr. Vemulapalli pointed to several ongoing trials comparing TEER vs. surgical MR repair in patients with DMR at intermediate surgical risk, including REPAIR MR and PRIMARY.
The study was supported by the National Institutes of Health and Abbott. Dr. Vemulapalli discloses receiving grants or contracts from the American College of Cardiology, the Society of Thoracic Surgeons, Cytokinetics, Abbott Vascular, the National Institutes of Health, and Boston Scientific; and consulting or serving on an advisory board for Janssen, the American College of Physicians, HeartFlow, and Edwards LifeSciences. Dr. Sherwood discloses receiving honoraria or consulting fees from Medtronic and Boston Scientific. Dr. Batchelor discloses receiving consulting fees from Medtronic, Boston Scientific, Edwards Lifesciences, and Abbott.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
Legacy ICDs exposed to MRI still shock, pace as needed
Functions like sensing and pacing in implantable cardioverter defibrillators (ICDs) tend to resist interference from the energy fields generated by MRI, as long as device programming is properly adjusted before the scan.
That applies even to patients with older “legacy” devices implanted before the 2015 advent of MRI-conditional ICDs despite, in practice, prevalent but misguided resistance to obtaining MRI scans in such cases.
Less is known whether such non–MRI-conditional devices, once exposed to MRI, will then reliably deliver antiarrhythmic shocks or antitachycardia pacing (ATP) when needed.
A new cohort study has tried to fill in some of that knowledge gap. It showed no evidence of an excess risk for death or ICD failure to deliver therapy within about 2 years of clinically indicated MRI scans in 629 patients with non–MRI-conditional devices.
The findings, published online in the Annals of Internal Medicine, come with caveats. For example, they’re based on the experience of one, albeit major, center and on MRIs that were for varied indications using 1.5-tesla equipment only.
Despite such safety evidence for appropriately adjusted non–MRI-conditional ICDs, many patients with the devices don›t receive clinically indicated MRI scans due to “perceived risk” that the ICDs won’t then reliably deliver appropriate therapy, observe the authors, led by Joshua Ra, MD, University of California, San Francisco.
Any such risks are “largely theoretical,” but may still explain “why some institutions are shying away from offering MRI exams” to patients with non–MRI-conditional ICDs, Dr. Ra told this news organization.
Many such hospitals refer such patients to more experienced centers, creating “significant logistical barriers in terms of patient access to these MRIs,” he said. “That seems to still be prevalent, unfortunately.”
The current findings “provide another layer of reassurance” that MRI scans in patients with non–MRI-conditional ICDs don’t impair a device’s ability to deliver shocks or ATP, Dr. Ra said.
The cohort consisted of 629 patients with non–MRI-conditional ICDs who underwent 813 clinically indicated MRI exams from 2003 to early 2015 at Johns Hopkins University, Baltimore.
Scans performed within 4 weeks of device implantation were excluded because, the report notes, that’s when spontaneous lead dislodgements or changes to device parameters are most likely to occur. Also excluded were patients with permanent epicardial leads, abandoned leads, or subcutaneous ICD lead systems, the report states.
Still, Dr. Ra said, the cohort is fairly representative of “the modern patient population” of non–MRI-conditional ICD recipients.
A total of 4,177 arrhythmia episodes were documented during a median 2.2 years between scans and last device interrogation prior to pulse-generator change-out or lead exchange.
Of note, Dr. Ra observed, the arrhythmias were confirmed in only 85% of the cohort. Most of the remainder were referral patients who were lost to follow-up whose devices were unavailable for interrogation.
Device therapy terminated “nearly all” documented spontaneous arrhythmias in that 85% of patients, the report states. They included 757 episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF), including 130 that were shocked and the remainder that were managed with ATP. There were also 105 supraventricular tachycardias, all successfully terminated with shocks.
There were no cases of VT or VF detection delay from undersensing or instances of syncope because of “abnormalities” in device detection of arrhythmias, the report states.
Of the 210 known deaths, which occurred a median 1.7 years after the scan, about half were noncardiac and more than a third were cardiac but nonarrhythmic.
Ten patients died from arrhythmia-related cardiac causes, representing 5% of deaths; but 7% of deaths were of undetermined cause.
“No direct relationship of deaths attributable to prior MRI exposure was found or reported,” the report states.
The researchers informally compared outcomes between older and more recently implanted non–MRI-conditional ICDs, the latter presumably with more modern design features. Their data, based on device interrogations, Dr. Ra said, “seem to suggest there were no differences.”
The study was supported by Johns Hopkins University and the National Institutes of Health. Author disclosures are available at apconline.org.
A version of this article first appeared on Medscape.com.
Functions like sensing and pacing in implantable cardioverter defibrillators (ICDs) tend to resist interference from the energy fields generated by MRI, as long as device programming is properly adjusted before the scan.
That applies even to patients with older “legacy” devices implanted before the 2015 advent of MRI-conditional ICDs despite, in practice, prevalent but misguided resistance to obtaining MRI scans in such cases.
Less is known whether such non–MRI-conditional devices, once exposed to MRI, will then reliably deliver antiarrhythmic shocks or antitachycardia pacing (ATP) when needed.
A new cohort study has tried to fill in some of that knowledge gap. It showed no evidence of an excess risk for death or ICD failure to deliver therapy within about 2 years of clinically indicated MRI scans in 629 patients with non–MRI-conditional devices.
The findings, published online in the Annals of Internal Medicine, come with caveats. For example, they’re based on the experience of one, albeit major, center and on MRIs that were for varied indications using 1.5-tesla equipment only.
Despite such safety evidence for appropriately adjusted non–MRI-conditional ICDs, many patients with the devices don›t receive clinically indicated MRI scans due to “perceived risk” that the ICDs won’t then reliably deliver appropriate therapy, observe the authors, led by Joshua Ra, MD, University of California, San Francisco.
Any such risks are “largely theoretical,” but may still explain “why some institutions are shying away from offering MRI exams” to patients with non–MRI-conditional ICDs, Dr. Ra told this news organization.
Many such hospitals refer such patients to more experienced centers, creating “significant logistical barriers in terms of patient access to these MRIs,” he said. “That seems to still be prevalent, unfortunately.”
The current findings “provide another layer of reassurance” that MRI scans in patients with non–MRI-conditional ICDs don’t impair a device’s ability to deliver shocks or ATP, Dr. Ra said.
The cohort consisted of 629 patients with non–MRI-conditional ICDs who underwent 813 clinically indicated MRI exams from 2003 to early 2015 at Johns Hopkins University, Baltimore.
Scans performed within 4 weeks of device implantation were excluded because, the report notes, that’s when spontaneous lead dislodgements or changes to device parameters are most likely to occur. Also excluded were patients with permanent epicardial leads, abandoned leads, or subcutaneous ICD lead systems, the report states.
Still, Dr. Ra said, the cohort is fairly representative of “the modern patient population” of non–MRI-conditional ICD recipients.
A total of 4,177 arrhythmia episodes were documented during a median 2.2 years between scans and last device interrogation prior to pulse-generator change-out or lead exchange.
Of note, Dr. Ra observed, the arrhythmias were confirmed in only 85% of the cohort. Most of the remainder were referral patients who were lost to follow-up whose devices were unavailable for interrogation.
Device therapy terminated “nearly all” documented spontaneous arrhythmias in that 85% of patients, the report states. They included 757 episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF), including 130 that were shocked and the remainder that were managed with ATP. There were also 105 supraventricular tachycardias, all successfully terminated with shocks.
There were no cases of VT or VF detection delay from undersensing or instances of syncope because of “abnormalities” in device detection of arrhythmias, the report states.
Of the 210 known deaths, which occurred a median 1.7 years after the scan, about half were noncardiac and more than a third were cardiac but nonarrhythmic.
Ten patients died from arrhythmia-related cardiac causes, representing 5% of deaths; but 7% of deaths were of undetermined cause.
“No direct relationship of deaths attributable to prior MRI exposure was found or reported,” the report states.
The researchers informally compared outcomes between older and more recently implanted non–MRI-conditional ICDs, the latter presumably with more modern design features. Their data, based on device interrogations, Dr. Ra said, “seem to suggest there were no differences.”
The study was supported by Johns Hopkins University and the National Institutes of Health. Author disclosures are available at apconline.org.
A version of this article first appeared on Medscape.com.
Functions like sensing and pacing in implantable cardioverter defibrillators (ICDs) tend to resist interference from the energy fields generated by MRI, as long as device programming is properly adjusted before the scan.
That applies even to patients with older “legacy” devices implanted before the 2015 advent of MRI-conditional ICDs despite, in practice, prevalent but misguided resistance to obtaining MRI scans in such cases.
Less is known whether such non–MRI-conditional devices, once exposed to MRI, will then reliably deliver antiarrhythmic shocks or antitachycardia pacing (ATP) when needed.
A new cohort study has tried to fill in some of that knowledge gap. It showed no evidence of an excess risk for death or ICD failure to deliver therapy within about 2 years of clinically indicated MRI scans in 629 patients with non–MRI-conditional devices.
The findings, published online in the Annals of Internal Medicine, come with caveats. For example, they’re based on the experience of one, albeit major, center and on MRIs that were for varied indications using 1.5-tesla equipment only.
Despite such safety evidence for appropriately adjusted non–MRI-conditional ICDs, many patients with the devices don›t receive clinically indicated MRI scans due to “perceived risk” that the ICDs won’t then reliably deliver appropriate therapy, observe the authors, led by Joshua Ra, MD, University of California, San Francisco.
Any such risks are “largely theoretical,” but may still explain “why some institutions are shying away from offering MRI exams” to patients with non–MRI-conditional ICDs, Dr. Ra told this news organization.
Many such hospitals refer such patients to more experienced centers, creating “significant logistical barriers in terms of patient access to these MRIs,” he said. “That seems to still be prevalent, unfortunately.”
The current findings “provide another layer of reassurance” that MRI scans in patients with non–MRI-conditional ICDs don’t impair a device’s ability to deliver shocks or ATP, Dr. Ra said.
The cohort consisted of 629 patients with non–MRI-conditional ICDs who underwent 813 clinically indicated MRI exams from 2003 to early 2015 at Johns Hopkins University, Baltimore.
Scans performed within 4 weeks of device implantation were excluded because, the report notes, that’s when spontaneous lead dislodgements or changes to device parameters are most likely to occur. Also excluded were patients with permanent epicardial leads, abandoned leads, or subcutaneous ICD lead systems, the report states.
Still, Dr. Ra said, the cohort is fairly representative of “the modern patient population” of non–MRI-conditional ICD recipients.
A total of 4,177 arrhythmia episodes were documented during a median 2.2 years between scans and last device interrogation prior to pulse-generator change-out or lead exchange.
Of note, Dr. Ra observed, the arrhythmias were confirmed in only 85% of the cohort. Most of the remainder were referral patients who were lost to follow-up whose devices were unavailable for interrogation.
Device therapy terminated “nearly all” documented spontaneous arrhythmias in that 85% of patients, the report states. They included 757 episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF), including 130 that were shocked and the remainder that were managed with ATP. There were also 105 supraventricular tachycardias, all successfully terminated with shocks.
There were no cases of VT or VF detection delay from undersensing or instances of syncope because of “abnormalities” in device detection of arrhythmias, the report states.
Of the 210 known deaths, which occurred a median 1.7 years after the scan, about half were noncardiac and more than a third were cardiac but nonarrhythmic.
Ten patients died from arrhythmia-related cardiac causes, representing 5% of deaths; but 7% of deaths were of undetermined cause.
“No direct relationship of deaths attributable to prior MRI exposure was found or reported,” the report states.
The researchers informally compared outcomes between older and more recently implanted non–MRI-conditional ICDs, the latter presumably with more modern design features. Their data, based on device interrogations, Dr. Ra said, “seem to suggest there were no differences.”
The study was supported by Johns Hopkins University and the National Institutes of Health. Author disclosures are available at apconline.org.
A version of this article first appeared on Medscape.com.
CV deaths jumped in 2020, reflecting pandemic toll
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
Cardiovascular-related deaths increased dramatically in 2020, marking the largest single-year increase since 2015 and surpassing the previous record from 2003, according to the American Heart Association’s 2023 Statistical Update.
During the first year of the COVID-19 pandemic, the largest increases in cardiovascular disease (CVD) deaths were seen among Asian, Black, and Hispanic people.
“We thought we had been improving as a country with respect to CVD deaths over the past few decades,” Connie Tsao, MD, chair of the AHA Statistical Update writing committee, told this news organization.
Since 2020, however, those trends have changed. Dr. Tsao, a staff cardiologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, both in Boston, noted the firsthand experience that many clinicians had in seeing the shift.
“We observed this sharp rise in age-adjusted CVD deaths, which corresponds to the COVID-19 pandemic,” she said. “Those of us health care providers knew from the overfull hospitals and ICUs that clearly COVID took a toll, particularly in those with cardiovascular risk factors.”
The AHA Statistical Update was published online in the journal Circulation.
Data on deaths
Each year, the American Heart Association and National Institutes of Health report the latest statistics related to heart disease, stroke, and cardiovascular risk factors. The 2023 update includes additional information about pandemic-related data.
Overall, the number of people who died from cardiovascular disease increased during the first year of the pandemic, rising from 876,613 in 2019 to 928,741 in 2020. This topped the previous high of 910,000 in 2003.
In addition, the age-adjusted mortality rate increased for the first time in several years, Dr. Tsao said, by a “fairly substantial” 4.6%. The age-adjusted mortality rate incorporates the variability in the aging population from year to year, accounting for higher death rates among older people.
“Even though our total number of deaths has been slowly increasing over the past decade, we have seen a decline each year in our age-adjusted rates – until 2020,” she said. “I think that is very indicative of what has been going on within our country – and the world – in light of people of all ages being impacted by the COVID-19 pandemic, especially before vaccines were available to slow the spread.”
The largest increases in CVD-related deaths occurred among Asian, Black, and Hispanic people, who were most heavily affected during the first year of the pandemic.
“People from communities of color were among those most highly impacted, especially early on, often due to a disproportionate burden of cardiovascular risk factors, such as hypertension and obesity,” Michelle Albert, MD, MPH, president of AHA and a professor of medicine at the University of California, San Francisco, said in a statement.
Dr. Albert, who is also the director of UCSF’s Center for the Study of Adversity and Cardiovascular Disease, does research on health equity and noted the disparities seen in the 2020 numbers. “Additionally, there are socioeconomic considerations, as well as the ongoing impact of structural racism on multiple factors, including limiting the ability to access quality health care,” she said.
Additional considerations
In a special commentary, the Statistical Update writing committee pointed to the need to track data for other underrepresented communities, including LGBTQ people and those living in rural or urban areas. The authors outlined several ways to better understand the effects of identity and social determinants of health, as well as strategies to reduce cardiovascular-related disparities.
“This year’s writing group made a concerted effort to gather information on specific social factors related to health risk and outcomes, including sexual orientation, gender identity, urbanization, and socioeconomic position,” Dr. Tsao said. “However, the data are lacking because these communities are grossly underrepresented in clinical and epidemiological research.”
For the next several years, the AHA Statistical Update will likely include more insights about the effects of the COVID-19 pandemic, as well as ongoing disparities.
“For sure, we will be continuing to see the effects of the pandemic for years to come,” Dr. Tsao said. “Recognition of the disparities in outcomes among vulnerable groups should be a call to action among health care providers and researchers, administration, and policy leaders to investigate the reasons and make changes to reverse these trends.”
The statistical update was prepared by a volunteer writing group on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
STS, new president apologize for predecessor’s speech amid Twitter backlash
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
The Society of Thoracic Surgeons (STS) and its newly installed president have posted an apology for a speech delivered by its outgoing president that appeared, in part, to disparage affirmative action as a means to promote diversity, equity, and inclusion in the field.
The speech, entitled “Three Score & More,” presented Jan. 22 at the STS 58th annual meeting in San Diego by John H. Calhoon, MD, University of Texas Health Science Center at San Antonio, unleashed a cascade of tweets, some circumspect but many expressing outrage and dismay.
Many of the tweets were from individuals who acknowledged not hearing the speech but who had seen at least one accompanying slide which, by then, had been widely circulated on the platform. It contained phrases such as “Affirmative Action is not equal opportunity” and “Defining people by color, gender, religion only tends to ingrain bias and discrimination,” all under the heading of “Virtuous Ideals.”
Reactions on Twitter included comments such as “This is bad beyond description” and a description of the slide’s content as “the blueprint & thought process for those actively maintaining Whiteness & the Patriarchy in medicine.”
Following an early onslaught of such tweets, the STS and new president Thomas E. MacGillivray, MD, MedStar Health, Washington, issued a statement disowning at least the controversial parts of Dr. Calhoon’s presentation, stating they were “inconsistent with STS’s core values of diversity, equity, and inclusion.”
The post continues, “The STS apologizes for these remarks. We know these comments were hurtful and we regret the pain they have caused to so many valued colleagues.” It then states, “Diversity, equity, and inclusion are central principles of our Society, and what we strive for in our profession and our practice. STS is committed to learning from this experience and taking action to reinforce our commitment to these values.”
“I believe that either the slide and/or my remarks were misinterpreted by some. I don’t want to hurt anybody. I’m profoundly sorry and apologize,” Dr. Calhoon said in an interview.
“I’m proud of my own group’s record on diversity and using equity and inclusion to get there,” he said. “We’re committed to it. We’ve had a wonderfully diverse group. I tried to highlight that in my remarks.”
About the Twitter response to the slide in question, Dr. Calhoon said, “I have no idea how they were thinking.” He added, “I can only comment that I’m really proud of our record and, for that matter, the STS’s record on diversity, equity, and inclusion.”
A version of this article first appeared on Medscape.com.
Damar Hamlin’s cardiac arrest: Key lessons
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Jan. 9, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert D. Glatter, medical adviser for Medscape Emergency Medicine. Today, we have Dr. Paul E. Pepe, an emergency medicine physician based in Florida and a highly recognized expert in emergency medical services (EMS), critical care, sports and event medicine, and resuscitation. Also joining us is Dr. Michael S. (“Mick”) Malloy, an emergency medicine physician based in Ireland, also an expert in prehospital care, resuscitation, and sports and event medicine. Welcome, gentlemen.
Dr. Pepe: Thanks for having us here.
Dr. Glatter: the Buffalo Bills safety who went down suffering a cardiac arrest in front of millions of people. Although we don’t know the exact cause of the events that transpired, the goal of our discussion is to guide our audience through a systematic approach to evaluation and management of an athlete suffering blunt force chest and neck trauma, and then suffering a cardiac arrest. We do know, obviously, that Damar was successfully resuscitated, thanks to the medical staff and trainers.
Almost 50 years ago, Chuck Hughes, a Detroit Lions receiver, went down and died with just a minute to go in the game and, unfortunately, didn’t survive.
Paul, can you tell me your impressions after viewing the replay of the events that evening? What were the most likely causes of this syncopal event and the subsequent cardiac arrest?
Dr. Pepe: We don’t know anything specifically. It’s being kept private about what the events were. It’s a little bit complicated in a sense that he basically had an extended resuscitation in the hospital. My experience has been that most people that have ventricular fibrillation, from whatever cause, will most likely be waking up on the field if you get to them. I’ve had personal experience with that.
More importantly than when it starts, when someone goes down on the field, both Dr. Malloy and I take a broader view. We don’t get tunnel vision and think, “Oh, it was a traumatic event,” or “It was cardiac event,” and we just have our minds open. There are many things that could make you stop breathing on the field. It could be a neck or a severe head injury, and then any kind of other internal injury that occurs.
When I saw in the video that Damar Hamlin stood up, that made it a less likely to be a spinal injury. He seemed to be physically functioning, and then he suddenly collapsed. That went along with something that looks like a ventricular fibrillation or ventricular tachycardia type of event and made me think right away that it was commotio cordis. I’m not a Latin scholar, but commotio is like commotion. A literal translation might be an agitation of the heart. I was thinking that he probably got hit somewhere in the middle of the chest at the right moment where the heart is resetting in that repolarization phase, like an R-on-T phenomenon, and then caused this sudden ventricular dysrhythmia.
Most people associate it to that because we have a couple of dozen cases a year of people getting hockey pucks or a baseball hitting their chest, which is very common with adolescents. On the other hand, you can’t get it from a blunt injury like this, and it was too early for it to be, say, a direct cardiac contusion, unless there was a direct injury there. It just happened so quickly.
In Europe, they’ve had a large amount of experience with this same kind of problem before, even just from a direct shoulder hit, for example. Mick Malloy is the dean of the faculty of sports and exercise medicine at the Royal College of Surgeons in Ireland and has vast experience, and now he is the person overseeing the procedures for this. Mick, have you had those kinds of experiences as well?
Dr. Molloy: Yes. It’s something that has occurred over recent decades and has been more recognized. I note that in professional sports, it’s a very different thing because you’ve got such huge teams and teams trained to respond very quickly. And that’s the most important thing in this scenario – having a team that is well functioning as a high-class emergency response team ready to get out on to that field very quickly after the person collapses, getting the automated external defibrillator (AED) on, and then recognizing whether there needs to be a shock given or not. The machine will tell you all that.
In our scenario, we run courses called CARES (Care of the Athlete Resuscitation and Emergencies in Sport) to make sure that our team physicians and team physiotherapists and trainers are all speaking as one when an emergency arises.
I don’t worry so much about the professional sport. It’s more with the amateur sports and the kids sports that I get a bit more concerned because there isn’t the same level of medical care there. Having everybody trained in basic life support would be very important to reduce unnecessary deaths from these types of conditions.
As Paul mentioned, there is a very specific cardiac cause in some of these circumstances, where you get hit just at the wrong time and that hit occurs at a particular electrical point in time. It causes this ventricular fibrillation, and the only real treatment there is the defibrillator as quickly as possible.
Dr. Glatter: What you’re saying ultimately is an important part about rapid defibrillation, and at first, cardiopulmonary resuscitation (CPR). People are concerned about whether they should begin CPR. We’re talking about out-of-hospital cardiac arrest that is outside of a football stadium, for example. Some people are obsessed with taking a person’s pulse, and that’s been a point of contention. If someone is unconscious and not breathing, we should start CPR. Wouldn›t you agree? They will wake up quickly if you begin chest compressions if they’re not necessary.
Dr. Pepe: I tell people, just do it. You’re right, people will wake up and feel it if they don’t need it.
Getting back to Mick’s point of having things ready to go, for example, 8 years ago, we had a professional player on the bench who suddenly collapsed right there in front of the entire audience. We immediately did CPR, and we got the AED on. We shocked him and he was ready, willing, and able to get back on the bench again. It turns out he had underlying coronary artery disease, but we got him back right away.
I did an initial study where we placed an AED in a public place at the Chicago O’Hare Airport to see if the public would use these. Most cardiac arrests occur at home, of course, but in public places, that was a good place to try it. We had almost 10 cases the first year. What was fascinating was that we had almost no survivors over the previous decade, even though there were paramedics at the airport. When we put these out there, we had nine people go down that first year, and six people who had never operated an AED or seen one before knew to get one and use it. Every one of those people survived neurologically intact, and almost every person was waking up before traditional responders got there. That’s how effective this is, but you need to know where the AED is.
Dr. Glatter: How to turn it on, where it is, and how to operate it.
Dr. Pepe: That was the point: These rescuers saved lives in the first year, and it was tremendous. Two points I make about it are that one, you need to know where it is, and two, just go turn it on. It gives you the instructions to follow through; just be in the Nike mode, because it basically won’t hurt a person. It’s rare that there’s ever been any complication of that. The machine algorithms are so good.
Dr. Glatter: Mick, I want to turn to you about the European experience. Specifically in Denmark, we know that there’s a large public health initiative to have AEDs accessible. There have been studies showing that when the public is engaged, especially with studies looking at an app when access is available, survivability doubled in the past 10 years from having access to AEDs. What’s your experience in Ireland in terms of public access to defibrillators?
Dr. Molloy: We’ve got two different streams here. There was a big push to have more AEDs at all sports venues. That was great, but some of the sporting clubs put them inside the locked door. I said that there’s no point to that because nobody can access it. You need to have an external building and you need to leave it open. If somebody needs to use it, they need to know how to get it, open it, and get away, and not get in through a locked door to get access to a defibrillator. We have AEDs now in most stadiums and even in small rural areas, where you might have only 200 people turn up for a game.
From another public access side, if you dial in – in our scenario, it’s 112, not 911 –we have Community First Responder groups. In the rural areas, you have local people who’ve been trained in basic life support and community first response who have AEDs. They’ll have periods of the day where they come home from work as a teacher, a nurse, a policeman, or a fireman, and they turn on an app on their phone and say, “I’m available for the next 5 hours.” If there’s a cardiac arrest rung in within 5 miles of their community, they will drive directly there with the AED that they have. We’ve had numerous saves from that in the country because it could take 40 minutes to get an EMS vehicle there, and obviously, time is crucial in these scenarios. Our dispatchers will talk people through CPR, and then the community responders arrive with the AED. It has been a fantastic initiative.
Dr. Pepe: In many places, people have apps on their phones where they’re locked into the system, and it will go off and tell them there is something nearby and even GPS them into it, and it’s been fantastic.
The two points I want to make to responding to what we just heard Dean Malloy say is one, we always have a designated spot to have these in various places. If I’m at City Hall, we always have them near the red elevators on every floor and down at security. In all the public high schools, we always have one right below the clock where everybody can see it. We set it up in a very standardized form that anybody and everybody will know where it is at the time an event happens.
The other point he made about having the response teams is fantastic. I live in a large high rise and there are two complexes with many people here, and many are older, so there’s going to be a higher risk for having an event. In fact, we’ve just had one recently. The concept we developed here was a community emergency response team, where we sometimes have doctors, nurses, and paramedics who live here be on call and be responsible, or you could try to find an AED. More importantly, we made sure everybody here knew where they were and where to get them. We’ve got most of the people trained, and we’re doing more training in what actions to take during these periods of time when such events happen.
Dr. Glatter: Yes, it’s critical. I wanted to point out that we’ve looked at the use of drones, especially here in the United States. There have been some pilot studies looking at their utility in the setting of out-of-hospital cardiac arrest. I want to get both of your thoughts on this and the feasibility of this.
Dr. Molloy: In a rural area, it’s a fantastic idea. You’re going to get something there as the crow flies very quickly. You probably have to look at exactly in, say, a rural area like Ireland of 32,000 square kilometers, how many you›ll have to put, what kind of distances they can realistically cover, and make sure the batteries are charged. Certainly, that’s a very good initiative because with the AEDs, you can’t do anything wrong. You can’t give a shock unless a shock needs to be given. The machine directs you what to do, so somebody who has had no training can pick one of these out of the box and start to work with it quickly and confidently that they can’t do anything wrong.
It’s a great idea. It would be a little expensive potentially at the moment in getting the drones and having that volume of drones around. In the U.S., you have completely different air traffic than we have, and in cities, you have more helicopters flying around. We certainly wouldn’t have that in our cities because that could cause a challenge if you’ve got drones flying around as well. It’s about making it safe that nothing else can go wrong from a drone in somebody else’s flight path.
Dr. Pepe: In my experience, the earlier the intervention, the better the results. There is a limit here in terms of the drones if they just can’t get there soon enough. Having said that, we are so fortunate in the city of Seattle to have most citizens knowing CPR, and we’d get that person resuscitated because they were doing such a good job with the CPR up front.
That’s why you’re going to see the Buffalo Bills player survive neurologically intact – because he did get immediate treatment right then and there. In the future, we may even have some better devices that will actually even restore normal blood flow right then and there while you’re still in cardiac arrest. There are limitations in every case. But on the other hand, it’s exciting and it paid off in this case recently.
Dr. Molloy: Just a point of interest coming from this small little country over here. The first portable defibrillator was developed in Belfast, Ireland, in the back of a cardiac response car. Despite us being a tiny little country, we do have some advances ahead of the United States.
Dr. Pepe: That was a breakthrough. Dr. Frank Pantridge and John Geddes did this great work and that caught the imagination of everybody here. At first, they were just going out to give people oxygen and sedate them for their chest pain. It turned out that their defibrillators are what made the difference as they went out there. Absolutely, I have to acknowledge the folks in Ireland for giving us this. Many of the EMS systems got started because of the article they published in The Lancet back in 1967.
Dr. Glatter: I wanted to briefly talk about screening of the athletes at the high school/college level, but also at the professional level. Obviously, there are issues, including the risk for false-positives in terms of low incidence, but there are also false negatives, as the case with Christian Eriksen, who had a cardiac arrest in 2021 and who has been through extensive testing. We can debate the validity of such testing, but I wanted to get both of your takes on the utility of screening in such a population.
Dr. Molloy: That’s a very emotive subject. False-positives are difficult because you’re now saying to somebody that they can’t compete in your sport at a decent level. The difficult part is telling somebody that this is the end of their career.
The false-negative is a little bit more difficult. I don’t know Christian Eriksen and I’m not involved in his team in any way, but that is a one-point examination, and you’re dependent on the scale of the process interpreting the ECG, which is again only a couple of seconds and that particular arrhythmia may not have shown up on that.
Also, athletes, by nature of what they’re doing, are operating at 99% of efficiency on a frequent basis. They are at the peak of their physiologic fitness, and it does make them a little bit more prone to picking up viral illnesses from time to time. They may get a small viral myopericarditis, which causes a new arrhythmia that nobody knew about. They had the screening 2 or 3 years ago, and they now developed a new problem because of what they do, which just may not show up.
I was actually surprised that the gentleman came through it very well, which is fantastic. He wasn’t allowed to play football in the country where he was employed, and he has now moved to another country and is playing football with a defibrillator inserted. I don’t know what the rules are in American football where you can play with implantable defibrillators. I’m not so sure it’s a great idea to do that.
Dr. Pepe: One thing that we should bring up is that there are athletes with underlying cardiomyopathies or hypertrophies and things like that, but that was unlikely in this case. It’s possible, but it’s unlikely, because it would have manifested itself before. In terms of screening, I’ve met some very smart medical doctors who have run those tests, and they have been very encouraged even at the high school levels to have screenings done, whether it’s electrocardiography, echocardiography, and so on. I have to reiterate what Dr Malloy just said in that it may have its downsides as well. If you can pick up real obvious cases, I think that may be of value.
Dr. Glatter: I want to conclude and get some pearls and takeaways from each of you regarding the events that transpired and what our audience can really hold onto.
Dr. Molloy: Look at Formula One in the past 50 years. In Formula One, in the beginning it was a 2-minute job to change a tire. Now, they have this down where they’re measuring in fractions of a second and criticizing each other if one guy is 2.6 seconds and the other guy is 2.9 seconds. For me, that’s phenomenal. It takes me 25 minutes to change a tire.
We’ve looked at that from a resuscitation perspective, and we now do pit crew resuscitation before our events. We’ve planned our team and know who’s going to be occupying what role. After the events at the UEFA championships, we had a new rule brought in by UEFA where they handed me a new document saying, “This is what we would like you to do for resuscitation.” It was a three-man triangle, and I said, “No, we’re not going to do that here.” And they said, “Why, you have to; it’s our rule.”
I said, “No, our rule in Ireland is we have a six-person triangle. We’re not downing our standards because of what you have internationally. You’re covering games in some very low-resource environments, I know that. We have a particular standard here that we’re sticking to. We have a six-person group. We know what we’re all doing; we come very quickly to those downed players and get involved and we’ve had good outcomes, so we’re not going to change the standards.”
That’s the thing: You need to practice these things. The players don’t go out on the weekend and do a move for the very first time without practicing it hundreds of times. We need to look at it the same way as the medical team who are looking after that group of players and the crowd because we also look after the crowd.
A particular challenge in some of our stadiums is that the upper decks are so steep, and it’s very hard to get a patient onto a trolley and do CPR as you’re bringing them down to a zone to get them flat. We’ve had to come up with some innovative techniques to try and do that and accommodate that using some of the mechanical CPR devices. That’s the result you’ll only get from having practiced these events and trying to extricate patients. We want to check response times, so you have to practice your response team activity very frequently.
Dr. Pepe: There are two points made by Mick that I want to react to. One, the pit crew approach is critical in so many ways. We do the same thing in what we call the medical first attack, where we knew who the A, B, and C person would be. When we took it out to the NBA trainers, I recommended for them to have a similar approach so that if an event does happen right in the middle of prime time, they are coordinated.
The second point is that we do mass-gathering medicine. It’s not just the sportspeople on the field or the entertainers that we’re looking after; it is the people in the stands. We will see a cardiac arrest once a month. If you think about it, you might see a cardiac arrest occur in any community on a regular basis. Now you’ve got 100,000 people in one stadium, and something is bound to go wrong over those 3 or 4 hours where they are there and may have a critical emergency. Preparation for all of that is really important as well.
The final point is that on a day-to-day basis, most cardiac arrests do occur in the home. Granted, 80% of them are nonshockable cases, but the people who are more apt to survive are going to be the ones who have an electrical event. In fact, when we looked at our data years ago, we found that, of the cases of people with ventricular fibrillation that we resuscitated, half didn’t even have heart damage. Their enzymes were normal. It was a pure electrical event, and they were more resuscitable. They may have an underlying problem, but we can fix that once we get them back.
Everybody needs to know how to do bystander CPR, and second, we must make sure we have AEDs strategically placed, as I alluded to before. We also go out to other parts of the community and give them advice. All those things must be put in place, but more importantly, just get the training and make the training simple. It’s really a “just do it” philosophy, but make it simple.
For example, when I teach a course, I can do it in 15 minutes, and people retain it because I keep reiterating things like, “Okay, there’s one thing you need to know about choking: Pop the cork.” You give them a physiologic image of what’s happening. Everybody says, “I remember you saying to just do it, pop the cork.”
With AEDs, know where it is – that’s why we should have it in standardized places. Go get it, turn it on, and then follow the instructions. Also, the most important thing is making sure you’re doing quality compressions; and there are videos that can help you with that, as well as classes that you can take that will get you through it.
Dr. Glatter: Absolutely. The public still has the misconception that you need to do mouth-to-mouth resuscitation. The message has not permeated through society that you don’t need to do mouth-to-mouth. Hands-only CPR is the gold standard now.
Dr. Pepe: If people have a reversible cause like ventricular fibrillation, often they’re already gasping, which is better than a delivered breath, by the way. Most important, then, are the compressions to make sure you have oxygen going up to the brain, because you’re still theoretically loaded with oxygen in your bloodstream if you had a sudden cardiac arrest from a ventricular fibrillation.
Your points are well taken, and we found that we had better outcomes when we just gave instructions to do compressions only, and that became the standard. Mick, you’ve had some experiences with that as well.
Dr. Molloy: If we’re going to have a long-term benefit from all this, we have to start doing this in elementary school and teaching kids basic life support and some basic health messaging.
I remember trying to get this across to a teacher one day and the teacher saying, “But why would we teach young kids to resuscitate each other?” I said, “I think you forget that the only 60-year-old person in the room is you. You train them, and we train them. They’re the ones who are going to respond and keep you alive. That’s the way you should be looking at this.” That completely changed the mindset of whether we should be doing this for the kids or not.
Dr. Pepe: In fact, what we find is that that’s exactly who gets saved. I had case after case where the kids at the school had learned CPR and saved the teachers or the administrator at the high school or elementary school. It’s a fantastic point that you bring up, Dr. Malloy.
Dr. Glatter: One other brief thing we can interject here is that the team was excellent on field in that they evaluated Damar Hamlin in a primary survey sense of ABCs (i.e., airway, breathing, and circulation) for things like a tension pneumothorax. In the sense in which he was hit, there are reversible causes. Making sure he didn’t have a tension pneumothorax that caused the arrest, in my mind, was critical.
Dr. Pepe: We do the same thing on a day-to-day basis with a car wreck, because it could be that the person had ventricular fibrillation and then had the wreck. It’s not always trauma. That’s a fantastic point that you’re making. That’s exactly what I think happened, and that’s what we do.
Dr. Glatter: Well, thank you, gentlemen. This was an informative and helpful discussion for our audience. I appreciate your time and expertise.
Dr. Glatter, is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Pepe is a professor of internal medicine, surgery, pediatrics, public health, and emergency medicine at University of Texas Health Science Center in Houston. He’s also a global coordinator of the U.S. Metropolitan Municipalities EMS Medical Directors (“Eagles”) Coalition.
Dr. Molloy works clinically as a consultant in emergency medicine in Wexford General Hospital, part of the Ireland East Hospital Group (IEHG). Internationally, he is a member of the Disaster Medicine Section of the European Society of Emergency Medicine (EUSEM) and has been appointed by the Irish Medical Organization (IMO) as one of two Irish delegates to serve on the European Board and Section of Emergency Medicine of the European Union of Medical Specialists (UEMS), having served for a number of years on its predecessor, the Multidisciplinary Joint Committee on Emergency Medicine.
A version of this article first appeared on Medscape.com.
Simulation-based training effective for transesophageal echo
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Simulation-based teaching of transesophageal echocardiography (TEE) improved cardiology fellows’ knowledge, skills, and comfort with the procedure, compared with traditional training, a new study shows.
“TEE learning may be hampered by the lack of availability of teachers and equipment and by the need for esophageal intubation, which is semi-invasive,” Augustin Coisne, MD, PhD, of the Cardiovascular Research Foundation in New York, said in an interview. “In this setting, simulation emerges as a key educational tool, but we were lacking evidence supporting simulation-based educational programs.”
Fellows in the simulation group achieved higher theoretical test scores and practical test scores after the training than did those in the traditional group.
Furthermore, Dr. Coisne said, “the results of the subgroup analyses were surprising and unexpected. The effect of the simulation-based training was greater among fellows at the beginning of fellowship – i.e., 2 years or less of training – in both theoretical and practical tests and in women [versus men] for the theoretical test.”
Their results, from the randomized SIMULATOR study, were published online in JAMA Cardiology.
More ready, more confident
The researchers randomly assigned 324 cardiology fellows (mean age, 26.4 years; about 30% women) inexperienced in TEE from 42 French university centers to TEE training with or without simulation support. Both groups participated in traditional didactic training using e-learning with an online course that is compulsory for all cardiology fellows in France.
The simulation group also participated in two 2-hour teaching sessions using a TEE simulator.
Each fellow completed a theoretical and a practical test prior to training to assess their baseline TEE level and again 3 months after the end of the training program. A TEE simulator (U/S Mentor Simulator; 3D Systems Simbionix) was used for all tests, and 24 certified echocardiography teachers served as both trainers and raters.
The theoretical tests included 20 online video-based questions to evaluate recognition of standard TEE views, normal anatomy, and some pathological cases. Fellows had 90 seconds to choose the best answer for each question from five multiple-choice options.
For the practical tests, fellows had 3 minutes to familiarize themselves with the handling of the simulator, without specific training and before the probe introduction.
They were asked to show 10 basic views on the simulator and had a maximum of 1 minute for each view.
The coprimary outcomes were the scores in the final theoretical and practical tests. TEE duration and the fellows’ self-assessment of their proficiency were also evaluated.
At baseline, the theoretical and practical test scores were similar between the groups (33.0 for the simulator group vs. 32.5 for the traditional group, and 44.2 vs. 46.1, respectively).
After training, the fellows in the simulation group had higher theoretical and practical test scores than those in the traditional group (47.2% vs. 38.3% and 74.5% vs. 59.0%, respectively).
Score changes were consistently higher when the pretraining scores were lower, an association that was stronger in the simulation group.
Dr. Coisne noted that subgroup analyses showed that the effectiveness of the simulation training was greater when performed at the beginning of the fellowship. On the theoretical test, the point increase was 11.9 for the simulation group versus 4.25 points for the traditional training group; for the practical test, the increases were 24.0 points versus 10.1 points.
After training, it took significantly less time for the simulation group to complete a TEE than it did the traditional group (8.3 vs. 9.4 minutes).
Furthermore, simulation group fellows reported that they felt more ready (mean score, 3.0 vs. 1.7) and more confident (mean score, 3.3 vs. 2.4) about performing a TEE alone after training.
“The simulation approach is definitively scalable to every institution,” Dr. Coisne said. “However, a medico-economic analysis should be interesting because the cost of the simulator and its maintenance might be a limitation to spread simulation-based teaching. The possibility for smaller hospitals to pool their financial input to share a TEE simulator could be considered to increase its cost-effectiveness.”
Real-world outcomes required
Commenting on the study, S. Justin Szawlewicz, MD, chair of cardiovascular medicine at Deborah Heart and Lung Center in Brown Mills, N.J., pointed out that the authors indicated that the number of TEEs performed by the trainees was not collected.
“This would be useful information to determine if those who received simulator training sought out and performed more TEEs, and also to determine if cardiology trainees in France perform a similar number of TEEs as cardiology trainees in the United States.”
In addition, he said, “the 4 hours of simulator training in TEE is extra education and experience that the standard trainees didn’t get. Would 4 extra hours of standard training didactics also improve trainees’ scores?”
Noting that the fellows’ ability to perform TEE in real patients was not assessed, Dr. Szawlewicz said, “a study could be designed that evaluated TEE images from real patients to see if trainees receiving simulator training performed better, more comprehensive and efficient TEEs than standard training.”
Nevertheless, he concluded, “Four hours of simulator training appears to improve TEE knowledge and skills. This is something we would consider at our institution.”
Like Dr. Szawlewicz, Michael Spooner, MD, MBA, of Mercy One North Iowa Heart Center in Mason City, and Kathryn Bertlacher, MD, of the University of Pittsburgh Medical Center, noted in a related editorial, “data are not provided about change in the learner’s behavior or performance on an actual TEE after the course, nor are there data about clinical outcomes such as patient safety or completeness of subsequent TEEs.
“This limitation, which is a limitation of most of the existing TEE simulation literature, is a high bar to cross,” they concluded. “Reaching this bar will require studies such as this to provide foundational understanding.”
Twin-Medical provided the TEE simulators. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
By the numbers: Cardiology slow to add women, IMGs join more quickly
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.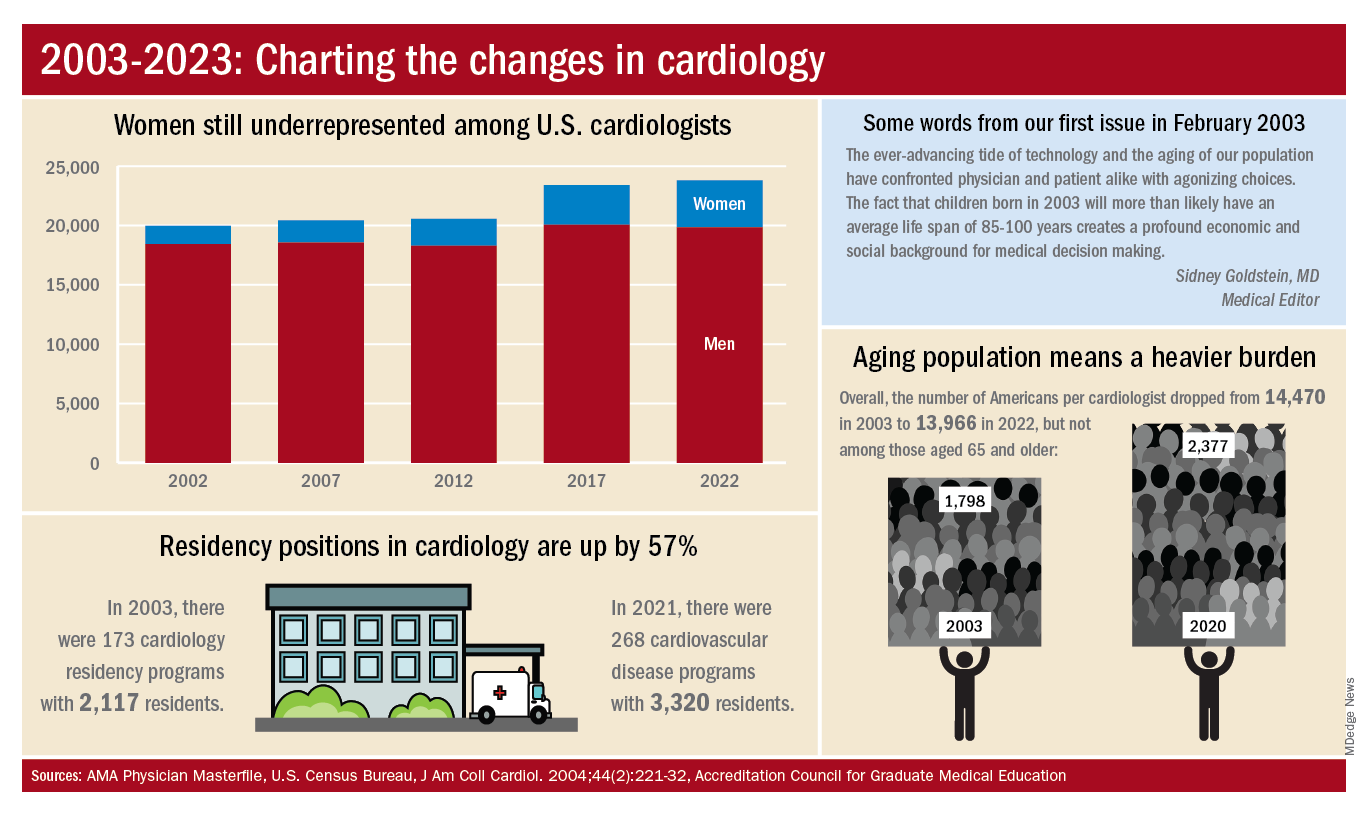
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
Despite Mark Twain’s assertion that “there are three kinds of lies: lies, damned lies, and statistics,” we’re going to dive into 20 years’ worth of data and, hopefully, come up with a few statistics that shed some light on the specialty’s workforce since Cardiology News published its first issue in February 2003.
We start with a major issue over these last 20 years: The participation of women in the specialty.
Back in July of 2002, just a few months before the first issue of Cardiology News was published, W. Bruce Fye, MD, then-president of the American College of Cardiology, wrote, “We need to do more to attract female medical graduates to our specialty because they represent almost one-half of the new doctors trained in this country. Cardiology needs to take full advantage of this large talent pool”
Data from the American Medical Association confirm that assertion: Of the nearly 20,000 postgraduate cardiologists in practice that year, only 7.8% were women. And that was at a time when more than 42% of medical school graduates were women, Dr. Fye noted, while also pointing out that “only 10% of cardiology trainees are female, and just 6% of ACC fellows are women.”
The gap between men and women has closed somewhat in the last 20 years, but the specialty continues to lag behind the profession as a whole. Women represented 16.7% of cardiologists in 2022, versus 37% of physicians overall, AMA data show. In 2019, for the first time, the majority of U.S. medical school students (50.5%) were women, according to the Association of American Medical Colleges.
A look at residency numbers from the Accreditation Council for Graduate Medical Education shows that continued slow improvement in the number of women can be expected, as 25.5% of cardiovascular disease residents were women during the 2021-2022 academic year. Only 2 of the 19 other internal medicine subspecialties were lower, and they happened to be interventional cardiology (20.1%) and clinical cardiac electrophysiology (14.5%).
When men are added to the mix, cardiovascular disease had a total of 3,320 active residents training in 268 programs in 2021-2022, making it the largest of the IM subspecialties in both respects. The resident total is up 57% since 2003, when it came in at 2,117, while programs have increased 55% from the 173 that were operating 2 decades ago. During the year in the middle (2011-2012), there were 2,521 residents in 187 programs, so a larger share of the growth has occurred in the last 10 years, the ACGME data indicate.
The shortage of cardiologists that Dr. Fye and others wrote about 20 years ago has not gone away. A 2018 report from health consulting firm PYA noted the increase in obesity and the low number of medical school graduates choosing the specialty. “Older and fewer physicians specializing in cardiology, coupled with the aging of baby boomers and gravitation toward practice in urban areas, will continue to exacerbate shortages in physician services in the specialty of cardiology, especially in rural areas, over the next decade,” PYA principal Lyle Oelrich wrote.
A little math appears to back up the claims of a cardiologist shortage. Based on census figures for the U.S. population in 2003, there were 14,470 Americans for each of the cardiologists reported by the AMA. That figure dropped to 13,966 by 2022, which seems like an improvement, but it comes with a caveat. The number of Americans aged 65 years and older increased from 1,798 to 2,377 per cardiologist as of 2020, the latest year for which population data were available by age.
One source of growth in the cardiology workforce has been perhaps its most significant minority: international medical graduates. Even by 2004, IMGs represented a much larger segment of all cardiologists (30.0%) than did women (9.3%), based on AMA data. To put it another way, there were more IMGs specializing in cardiovascular disease (6,615) in 2004 than there were women (3,963) in 2022.
The latest data on cardiology training programs – overall numbers were not available – put IMGs at 39.2% for the 2019-2020 academic year. The 2022 fellowship match provides a slightly smaller proportion of IMGs (37.4%) filling cardiovascular disease positions, according to the National Resident Matching Program.
After PCI, 1-month beats 12-month DAPT in high-risk patients
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
FROM JACC ASIA


