User login
Arkansas cardiologist pays $900K to settle false claims allegations
in violation of the False Claims Act.
As part of the settlement, Dr. Tauth will enter into an integrity agreement with the U.S. Department of Health & Human Services, according to a news release from Henry Leventis, U.S. attorney for the Middle District of Tennessee.
“Health care fraud is a top priority of this office. We will aggressively pursue all those who are involved in fraud against government programs,” Mr. Leventis said.
Dr. Tauth formerly treated patients at National Park Medical Center (NPMC) in Hot Springs. The alleged false claims were submitted from September 2013 through August 2019.
The settlement with Dr. Tauth, aged 60, follows a November 2019 voluntary disclosure of the alleged false claims by Brentwood, Tenn.–based Lifepoint Health, which acquired NPMC and Hot Springs Cardiology Associates in November 2018.
NPMC and Hot Springs Cardiology entered into a settlement in October 2020 for the alleged violations and agreed to pay roughly $14.6 million, which includes over $9 million in restitution, according to the news release.
NPMC CEO Scott Smith said NPMC is “committed to maintaining high standards of integrity, legal compliance, and quality care for our patients. We regularly monitor our processes, procedures, and reporting and actively self-report concerns to regulators to ensure we are upholding these standards across our organization.”
“We are proud that our hospital took the appropriate steps to promptly self-report and finalize a settlement with the government for a swift resolution more than 2 years ago,” Mr. Smith said.
Dr. Tauth, however, maintains that the allegations made by NPMC are false.
“I am pleased to have reached a settlement agreement with the Department of Justice regarding allegations brought to them by my former employer, National Park Medical Center,” he said in a statement.
“The settlement agreement specifically states that it is not ‘an admission of liability’ by me, and I remain steadfast in my position that the allegations made by my former employer are false and without merit,” Dr. Tauth added.
He further stated that he has “chosen to enter into the settlement agreement because the legal process initiated by National Park’s allegations has been emotionally and financially damaging to me and my family in the extreme, and a settlement puts an end to the delays, uncertainties, inconveniences, and expenses of protracted litigation. Settlement is in the best interests of my family, my patients, and my medical practice.”
Dr. Tauth said he is “extremely grateful for the support I have received from my patients, medical staff, colleagues, friends, and family during this difficult time, and I look forward to providing high-quality cardiac care in the greater Hot Springs community for many years to come.”
A version of this article first appeared on Medscape.com.
in violation of the False Claims Act.
As part of the settlement, Dr. Tauth will enter into an integrity agreement with the U.S. Department of Health & Human Services, according to a news release from Henry Leventis, U.S. attorney for the Middle District of Tennessee.
“Health care fraud is a top priority of this office. We will aggressively pursue all those who are involved in fraud against government programs,” Mr. Leventis said.
Dr. Tauth formerly treated patients at National Park Medical Center (NPMC) in Hot Springs. The alleged false claims were submitted from September 2013 through August 2019.
The settlement with Dr. Tauth, aged 60, follows a November 2019 voluntary disclosure of the alleged false claims by Brentwood, Tenn.–based Lifepoint Health, which acquired NPMC and Hot Springs Cardiology Associates in November 2018.
NPMC and Hot Springs Cardiology entered into a settlement in October 2020 for the alleged violations and agreed to pay roughly $14.6 million, which includes over $9 million in restitution, according to the news release.
NPMC CEO Scott Smith said NPMC is “committed to maintaining high standards of integrity, legal compliance, and quality care for our patients. We regularly monitor our processes, procedures, and reporting and actively self-report concerns to regulators to ensure we are upholding these standards across our organization.”
“We are proud that our hospital took the appropriate steps to promptly self-report and finalize a settlement with the government for a swift resolution more than 2 years ago,” Mr. Smith said.
Dr. Tauth, however, maintains that the allegations made by NPMC are false.
“I am pleased to have reached a settlement agreement with the Department of Justice regarding allegations brought to them by my former employer, National Park Medical Center,” he said in a statement.
“The settlement agreement specifically states that it is not ‘an admission of liability’ by me, and I remain steadfast in my position that the allegations made by my former employer are false and without merit,” Dr. Tauth added.
He further stated that he has “chosen to enter into the settlement agreement because the legal process initiated by National Park’s allegations has been emotionally and financially damaging to me and my family in the extreme, and a settlement puts an end to the delays, uncertainties, inconveniences, and expenses of protracted litigation. Settlement is in the best interests of my family, my patients, and my medical practice.”
Dr. Tauth said he is “extremely grateful for the support I have received from my patients, medical staff, colleagues, friends, and family during this difficult time, and I look forward to providing high-quality cardiac care in the greater Hot Springs community for many years to come.”
A version of this article first appeared on Medscape.com.
in violation of the False Claims Act.
As part of the settlement, Dr. Tauth will enter into an integrity agreement with the U.S. Department of Health & Human Services, according to a news release from Henry Leventis, U.S. attorney for the Middle District of Tennessee.
“Health care fraud is a top priority of this office. We will aggressively pursue all those who are involved in fraud against government programs,” Mr. Leventis said.
Dr. Tauth formerly treated patients at National Park Medical Center (NPMC) in Hot Springs. The alleged false claims were submitted from September 2013 through August 2019.
The settlement with Dr. Tauth, aged 60, follows a November 2019 voluntary disclosure of the alleged false claims by Brentwood, Tenn.–based Lifepoint Health, which acquired NPMC and Hot Springs Cardiology Associates in November 2018.
NPMC and Hot Springs Cardiology entered into a settlement in October 2020 for the alleged violations and agreed to pay roughly $14.6 million, which includes over $9 million in restitution, according to the news release.
NPMC CEO Scott Smith said NPMC is “committed to maintaining high standards of integrity, legal compliance, and quality care for our patients. We regularly monitor our processes, procedures, and reporting and actively self-report concerns to regulators to ensure we are upholding these standards across our organization.”
“We are proud that our hospital took the appropriate steps to promptly self-report and finalize a settlement with the government for a swift resolution more than 2 years ago,” Mr. Smith said.
Dr. Tauth, however, maintains that the allegations made by NPMC are false.
“I am pleased to have reached a settlement agreement with the Department of Justice regarding allegations brought to them by my former employer, National Park Medical Center,” he said in a statement.
“The settlement agreement specifically states that it is not ‘an admission of liability’ by me, and I remain steadfast in my position that the allegations made by my former employer are false and without merit,” Dr. Tauth added.
He further stated that he has “chosen to enter into the settlement agreement because the legal process initiated by National Park’s allegations has been emotionally and financially damaging to me and my family in the extreme, and a settlement puts an end to the delays, uncertainties, inconveniences, and expenses of protracted litigation. Settlement is in the best interests of my family, my patients, and my medical practice.”
Dr. Tauth said he is “extremely grateful for the support I have received from my patients, medical staff, colleagues, friends, and family during this difficult time, and I look forward to providing high-quality cardiac care in the greater Hot Springs community for many years to come.”
A version of this article first appeared on Medscape.com.
Five thoughts on the Damar Hamlin collapse
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
The obvious first statement is that it’s neither wise nor appropriate to speculate on the specifics of Damar Hamlin’s cardiac event during a football game on Jan. 2 (including the possibility of commotio cordis) or his ongoing care. The public nature of his collapse induces intense curiosity but people with illness deserve privacy. Privacy in health care is in short supply. I disagree strongly with those who say his doctors ought to be giving public updates. That’s up to the family.
But there are important general concepts to consider about this incident. These include ...
Cardiac arrest can happen to anyone
People with structural heart disease or other chronic illnesses have a higher risk of arrhythmia, but the notion that athletes are immune from cardiac arrest is wrong. This sentence almost seems too obvious to write, but to this day, I hear clinicians express surprise that an athletic person has heart disease.
Survival turns on rapid and effective intervention
In the old days of electrophysiology, we used to test implantable cardioverter-defibrillators during an implant procedure by inducing ventricular fibrillation (VF) and watching the device convert it. Thankfully, trials have shown that this is no longer necessary for most implants.
When you induce VF In the EP lab, you learn quickly that a) it causes loss of consciousness in a matter of seconds, b) rapid defibrillation restores consciousness, often without the patients knowing or remembering they passed out, and c) the failure of the shock to terminate VF results in deterioration in a matter of 1-2 minutes. Even 1 minute in VF feels so long.
Need is an appropriate word in VF treatment
Clinicians often use the verb need. As in, this patient needs this pill or this procedure. It’s rarely appropriate.
But in the case of treating VF, patients truly need rapid defibrillation. Survival of out-of-hospital cardiac arrest is low because there just aren’t enough automated external defibrillators (AEDs) or people trained to use them. A study of patients who had out-of-hospital cardiac arrest in Denmark found that 30-day survival almost doubled (28.8% vs. 16.4%), when the nearest AED was accessible.
Bystanders must act
The public messages are simple: If a person loses consciousness in front of you, and is not breathing normally, assume it is a cardiac arrest, call 911 to get professional help, and start hands-only chest compressions. Don’t spend time checking for a pulse or trying to wake the person. If this is not a cardiac arrest, they will soon tell you to stop compressing their chest. Seconds matter.
Chest compressions are important but what is really needed is defibrillation. A crucial step in CPR is to send someone to get an AED and get the pads attached. If this is a shockable rhythm, deliver the shock. Hamlin’s collapse emphasizes the importance of the AED; without it, his survival to the hospital would have been unlikely.
Widespread preparticipation screening of young athletes remains a bad idea
Whenever cardiac arrest occurs in an athlete, in such a public way, people think about prevention. Surely it is better to prevent such an event than react to it, goes the thinking. The argument against this idea has four prongs:
The incidence of cardiac disease in a young athlete is extremely low, which sets up a situation where most “positive” tests are false positive. A false positive screening ECG or echocardiogram can create harm in multiple ways. One is the risk from downstream procedures, but worse is the inappropriate disqualification from sport. Healthwise, few harms could be greater than creating long-term fear of exercise in someone.
There is also the problem of false-negative screening tests. An ECG may be normal in the setting of hypertrophic cardiomyopathy. And a normal echocardiogram does not exclude arrhythmogenic right ventricular cardiomyopathy or other genetic causes of cardiac arrest. In a 2018 study from a major sports cardiology center in London, 6 of the 8 sudden cardiac deaths in their series were in athletes who had no detectable abnormalities on screening.
Even when disease is found, it’s not clear that prohibiting participation in sports prevents sudden death. Many previous class III recommendations against participation in sport now carry class II – may be considered – designations.
Finally, screening for any disease loses value as treatments improve. Public education regarding rapid intervention with CPR and AED use is the best treatment option. A great example is the case of Christian Erikson, a Danish soccer player who suffered cardiac arrest during a match at the European Championships in 2021 and was rapidly defibrillated on the field. Therapy was so effective that he was conscious and able to wave to fans on his way out of the stadium. He has now returned to elite competition.
Proponents of screening might oppose my take by saying that National Football League players are intensely screened. But this is different from widespread screening of high school and college athletes. It might sound harsh to say, but professional teams have dualities of interests in the health of their athletes given the million-dollar contracts.
What’s more, professional teams can afford to hire expert cardiologists to perform the testing. This would likely reduce the rate of false-positive findings, compared with screening in the community setting. I often have young people referred to me because of asymptomatic bradycardia found during athletic screening – an obviously normal finding.
Conclusions
As long as there are sports, there will be athletes who suffer cardiac arrest.
We can both hope for Hamlin’s full recovery and learn lessons to help reduce the rate of death from out-of-hospital cardiac arrest. This mostly involves education on how to help fellow humans and a public health commitment to access to AEDs.
John Mandrola, MD, practices cardiac electrophysiology in Louisville, Ky. and is a writer and podcaster for Medscape. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).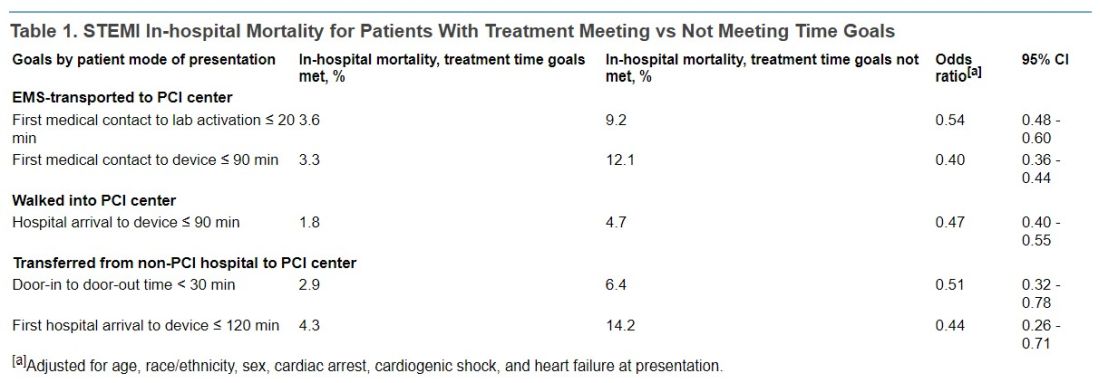
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Survival varies widely for cardiac arrests in U.S. cath labs
The chance of surviving a cardiac arrest varies widely across hospitals in the United States, even when the arrest occurs in the highly controlled setting of a cardiac catheterization lab, a new study indicates.
Among 4,787 patients who arrested in the cath lab at 231 hospitals in the Get With The Guidelines (GWTG) Resuscitation registry, only about one-third survived to discharge. The median risk-adjusted survival rate (RASR) for all hospitals was 36%.
When stratified by RASR tertiles, however, median survival rates were 20%, 36%, and 52% for hospitals in the lowest, middle, and highest tertiles.
The odds of survival differed by 71% in similar patients presenting at two randomly selected hospitals (median odds ratio, 1.71; 95% confidence interval, 1.52-1.87).
“The good news is that cardiac arrests in the cath lab are relatively infrequent, but the bad news is that they still occur and the outcomes are, in general, pretty dismal,” senior author Deepak L. Bhatt, MD, MPH, said in an interview. “So anything that we can do as hospitals [and] health care systems to improve the care of these patients could go a long way.”
He noted that data are sparse on cardiac arrests in the cath lab but that recent studies examining in-hospital arrests in the same registry report lower hospital-wide survival rates, between 17% and 24%.
Nevertheless, the current study included only those hospitals motivated and with the resources to participate in the American Heart Association’s voluntary GWTG Resuscitation registry between January 2003 and December 2017.
“It probably does provide the best case scenario of what’s going on and, if we included every hospital in the United States or the world, probably the outcomes would be substantially worse,” said Dr. Bhatt, who was recently named director of Mount Sinai Heart and the first Dr. Valentin Fuster Professor of Cardiovascular Medicine, New York.
The results were published in JACC Cardiovascular Interventions.
Hospital and patient factors
Possible explanations for the wide disparity in survival are the small number of cardiac arrests in the cath lab, the increasing complexity of cases, and the fact that patients are often very sick and may experience a problem during a procedure, or both, Dr. Bhatt suggested. Cath labs also vary in how they handle resuscitative efforts and access to advanced mechanical support devices, such as extracorporeal membrane oxygenation (ECMO).
“It’s not available in every cath lab and, even in hospitals that have it, they may not have a given ECMO circuit available at the exact time the patient’s having a cardiac arrest,” he said. “That’s one example of something that can make, in my opinion, a big difference in whether a patient lives or dies if they’re having a cardiac arrest but may not always be easily deployed.”
When the investigators looked specifically at hospital-level factors, only yearly volume of cardiac arrests in the cath lab was significantly associated with risk-adjusted survival (P < .01), whereas hospital size, rural or urban setting, teaching status, and geographic location were not.
In multivariate adjusted analyses, factors associated with survival to discharge included age (OR, 0.78), Black race (OR, 0.68), respiratory insufficiency (OR, 0.75), and initial cardiac arrest rhythm (OR, 3.32).
The median hospital RASR was 27% higher for ventricular tachycardia or ventricular fibrillation arrests than for arrests with a nonshockable rhythm of asystole and pulseless electrical activity (55% vs. 28%).
Notably, hospitals in the lowest tertile of risk-adjusted survival rates had a higher prevalence of non-White patients, renal and respiratory comorbidity, and arrest with nonshockable rhythm.
“We want to make sure as we’re contemplating whether to resuscitate a patient or how aggressively to resuscitate, that we aren’t letting any of our own biases, whether they have to do with race or potentially sex and gender, interfere with more objective assessments of whether the patient can in fact be saved or not,” Dr. Bhatt said.
Reached for comment, Srihari S. Naidu, MD, who chaired the writing group for the Society for Cardiovascular Angiography and Interventions’ (SCAI) consensus statement on cardiogenic shock and co-authored its document on best practices in the cardiac cath lab, said the findings show that survival in the cath lab is higher than that seen in-hospital. “Still, there’s a lot of room for improvement,” he said.
He was particularly struck by the variability in survival. “Underprivileged individuals, so those who are non-White populations and have respiratory and renal problems, they seem to have a worse survival and that makes sense – patients with comorbidities – but it feeds into the issue of, ‘Are we treating our population similarly in terms of their baseline race and ethnicity as a gap in care?’ ”
Better survival at hospitals with high volumes likely reflects more experience in handling these events, a rapid response and personnel to help with resuscitation, and overall better critical care and cath lab environment, said Dr. Naidu, director of the cardiac cath lab at Westchester Medical Center and professor of medicine at New York Medical College, both in Valhalla, N.Y.
“So that leads into two things,” he said. “One is that probably we should be working on having all high-risk patients go to centers of excellence. So, for example, [for] patients in shock, patients with STEMI, regionalization of care to the high-volume cath labs that are experienced in cardiac arrest and critical care management may be a way to go.”
“Second, if experience counts, can that experience be simulated through drills and simulations in the cath lab?” Dr. Naidu said. “Should all cath labs have drills where we have a cardiac arrest patient, and how would we respond to that? Who’s going to do the compressions? Where’s the mechanical support device? What are the things we need to have a seamless cardiac arrest protocol for arrests during the cath lab?”
Dr. Bhatt and colleagues acknowledge that despite adjustment for many key variables, the study lacked procedural details that may affect survival and information related to resuscitation efforts.
“We really do need to focus more research efforts, potentially more in the way of quality-improvement efforts, to try and help patients get these sorts of patients who are in dire straits to the cath lab but hopefully also through the hospital discharge and back home,” Dr. Bhatt said.
In an editorial accompanying the study, Matthew L. Tomey, MD, Icahn School of Medicine at Mount Sinai, New York, writes that the “findings and limitations of this study together sound a call to action.”
He also signaled the need for more research and for registries and reporting instruments to capture variables particular to in-laboratory cardiac arrest and resuscitation in the cardiac cath lab. “A necessary first step is the development of consensus data elements for supplemental reporting in cases of ILCA,” such as indication for cath lab presentation, timing of arrest relative to procedure, and cause of arrest.
Dr. Bhatt reported numerous relationships with industry. Dr. Naidu and Dr. Tomey report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The chance of surviving a cardiac arrest varies widely across hospitals in the United States, even when the arrest occurs in the highly controlled setting of a cardiac catheterization lab, a new study indicates.
Among 4,787 patients who arrested in the cath lab at 231 hospitals in the Get With The Guidelines (GWTG) Resuscitation registry, only about one-third survived to discharge. The median risk-adjusted survival rate (RASR) for all hospitals was 36%.
When stratified by RASR tertiles, however, median survival rates were 20%, 36%, and 52% for hospitals in the lowest, middle, and highest tertiles.
The odds of survival differed by 71% in similar patients presenting at two randomly selected hospitals (median odds ratio, 1.71; 95% confidence interval, 1.52-1.87).
“The good news is that cardiac arrests in the cath lab are relatively infrequent, but the bad news is that they still occur and the outcomes are, in general, pretty dismal,” senior author Deepak L. Bhatt, MD, MPH, said in an interview. “So anything that we can do as hospitals [and] health care systems to improve the care of these patients could go a long way.”
He noted that data are sparse on cardiac arrests in the cath lab but that recent studies examining in-hospital arrests in the same registry report lower hospital-wide survival rates, between 17% and 24%.
Nevertheless, the current study included only those hospitals motivated and with the resources to participate in the American Heart Association’s voluntary GWTG Resuscitation registry between January 2003 and December 2017.
“It probably does provide the best case scenario of what’s going on and, if we included every hospital in the United States or the world, probably the outcomes would be substantially worse,” said Dr. Bhatt, who was recently named director of Mount Sinai Heart and the first Dr. Valentin Fuster Professor of Cardiovascular Medicine, New York.
The results were published in JACC Cardiovascular Interventions.
Hospital and patient factors
Possible explanations for the wide disparity in survival are the small number of cardiac arrests in the cath lab, the increasing complexity of cases, and the fact that patients are often very sick and may experience a problem during a procedure, or both, Dr. Bhatt suggested. Cath labs also vary in how they handle resuscitative efforts and access to advanced mechanical support devices, such as extracorporeal membrane oxygenation (ECMO).
“It’s not available in every cath lab and, even in hospitals that have it, they may not have a given ECMO circuit available at the exact time the patient’s having a cardiac arrest,” he said. “That’s one example of something that can make, in my opinion, a big difference in whether a patient lives or dies if they’re having a cardiac arrest but may not always be easily deployed.”
When the investigators looked specifically at hospital-level factors, only yearly volume of cardiac arrests in the cath lab was significantly associated with risk-adjusted survival (P < .01), whereas hospital size, rural or urban setting, teaching status, and geographic location were not.
In multivariate adjusted analyses, factors associated with survival to discharge included age (OR, 0.78), Black race (OR, 0.68), respiratory insufficiency (OR, 0.75), and initial cardiac arrest rhythm (OR, 3.32).
The median hospital RASR was 27% higher for ventricular tachycardia or ventricular fibrillation arrests than for arrests with a nonshockable rhythm of asystole and pulseless electrical activity (55% vs. 28%).
Notably, hospitals in the lowest tertile of risk-adjusted survival rates had a higher prevalence of non-White patients, renal and respiratory comorbidity, and arrest with nonshockable rhythm.
“We want to make sure as we’re contemplating whether to resuscitate a patient or how aggressively to resuscitate, that we aren’t letting any of our own biases, whether they have to do with race or potentially sex and gender, interfere with more objective assessments of whether the patient can in fact be saved or not,” Dr. Bhatt said.
Reached for comment, Srihari S. Naidu, MD, who chaired the writing group for the Society for Cardiovascular Angiography and Interventions’ (SCAI) consensus statement on cardiogenic shock and co-authored its document on best practices in the cardiac cath lab, said the findings show that survival in the cath lab is higher than that seen in-hospital. “Still, there’s a lot of room for improvement,” he said.
He was particularly struck by the variability in survival. “Underprivileged individuals, so those who are non-White populations and have respiratory and renal problems, they seem to have a worse survival and that makes sense – patients with comorbidities – but it feeds into the issue of, ‘Are we treating our population similarly in terms of their baseline race and ethnicity as a gap in care?’ ”
Better survival at hospitals with high volumes likely reflects more experience in handling these events, a rapid response and personnel to help with resuscitation, and overall better critical care and cath lab environment, said Dr. Naidu, director of the cardiac cath lab at Westchester Medical Center and professor of medicine at New York Medical College, both in Valhalla, N.Y.
“So that leads into two things,” he said. “One is that probably we should be working on having all high-risk patients go to centers of excellence. So, for example, [for] patients in shock, patients with STEMI, regionalization of care to the high-volume cath labs that are experienced in cardiac arrest and critical care management may be a way to go.”
“Second, if experience counts, can that experience be simulated through drills and simulations in the cath lab?” Dr. Naidu said. “Should all cath labs have drills where we have a cardiac arrest patient, and how would we respond to that? Who’s going to do the compressions? Where’s the mechanical support device? What are the things we need to have a seamless cardiac arrest protocol for arrests during the cath lab?”
Dr. Bhatt and colleagues acknowledge that despite adjustment for many key variables, the study lacked procedural details that may affect survival and information related to resuscitation efforts.
“We really do need to focus more research efforts, potentially more in the way of quality-improvement efforts, to try and help patients get these sorts of patients who are in dire straits to the cath lab but hopefully also through the hospital discharge and back home,” Dr. Bhatt said.
In an editorial accompanying the study, Matthew L. Tomey, MD, Icahn School of Medicine at Mount Sinai, New York, writes that the “findings and limitations of this study together sound a call to action.”
He also signaled the need for more research and for registries and reporting instruments to capture variables particular to in-laboratory cardiac arrest and resuscitation in the cardiac cath lab. “A necessary first step is the development of consensus data elements for supplemental reporting in cases of ILCA,” such as indication for cath lab presentation, timing of arrest relative to procedure, and cause of arrest.
Dr. Bhatt reported numerous relationships with industry. Dr. Naidu and Dr. Tomey report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The chance of surviving a cardiac arrest varies widely across hospitals in the United States, even when the arrest occurs in the highly controlled setting of a cardiac catheterization lab, a new study indicates.
Among 4,787 patients who arrested in the cath lab at 231 hospitals in the Get With The Guidelines (GWTG) Resuscitation registry, only about one-third survived to discharge. The median risk-adjusted survival rate (RASR) for all hospitals was 36%.
When stratified by RASR tertiles, however, median survival rates were 20%, 36%, and 52% for hospitals in the lowest, middle, and highest tertiles.
The odds of survival differed by 71% in similar patients presenting at two randomly selected hospitals (median odds ratio, 1.71; 95% confidence interval, 1.52-1.87).
“The good news is that cardiac arrests in the cath lab are relatively infrequent, but the bad news is that they still occur and the outcomes are, in general, pretty dismal,” senior author Deepak L. Bhatt, MD, MPH, said in an interview. “So anything that we can do as hospitals [and] health care systems to improve the care of these patients could go a long way.”
He noted that data are sparse on cardiac arrests in the cath lab but that recent studies examining in-hospital arrests in the same registry report lower hospital-wide survival rates, between 17% and 24%.
Nevertheless, the current study included only those hospitals motivated and with the resources to participate in the American Heart Association’s voluntary GWTG Resuscitation registry between January 2003 and December 2017.
“It probably does provide the best case scenario of what’s going on and, if we included every hospital in the United States or the world, probably the outcomes would be substantially worse,” said Dr. Bhatt, who was recently named director of Mount Sinai Heart and the first Dr. Valentin Fuster Professor of Cardiovascular Medicine, New York.
The results were published in JACC Cardiovascular Interventions.
Hospital and patient factors
Possible explanations for the wide disparity in survival are the small number of cardiac arrests in the cath lab, the increasing complexity of cases, and the fact that patients are often very sick and may experience a problem during a procedure, or both, Dr. Bhatt suggested. Cath labs also vary in how they handle resuscitative efforts and access to advanced mechanical support devices, such as extracorporeal membrane oxygenation (ECMO).
“It’s not available in every cath lab and, even in hospitals that have it, they may not have a given ECMO circuit available at the exact time the patient’s having a cardiac arrest,” he said. “That’s one example of something that can make, in my opinion, a big difference in whether a patient lives or dies if they’re having a cardiac arrest but may not always be easily deployed.”
When the investigators looked specifically at hospital-level factors, only yearly volume of cardiac arrests in the cath lab was significantly associated with risk-adjusted survival (P < .01), whereas hospital size, rural or urban setting, teaching status, and geographic location were not.
In multivariate adjusted analyses, factors associated with survival to discharge included age (OR, 0.78), Black race (OR, 0.68), respiratory insufficiency (OR, 0.75), and initial cardiac arrest rhythm (OR, 3.32).
The median hospital RASR was 27% higher for ventricular tachycardia or ventricular fibrillation arrests than for arrests with a nonshockable rhythm of asystole and pulseless electrical activity (55% vs. 28%).
Notably, hospitals in the lowest tertile of risk-adjusted survival rates had a higher prevalence of non-White patients, renal and respiratory comorbidity, and arrest with nonshockable rhythm.
“We want to make sure as we’re contemplating whether to resuscitate a patient or how aggressively to resuscitate, that we aren’t letting any of our own biases, whether they have to do with race or potentially sex and gender, interfere with more objective assessments of whether the patient can in fact be saved or not,” Dr. Bhatt said.
Reached for comment, Srihari S. Naidu, MD, who chaired the writing group for the Society for Cardiovascular Angiography and Interventions’ (SCAI) consensus statement on cardiogenic shock and co-authored its document on best practices in the cardiac cath lab, said the findings show that survival in the cath lab is higher than that seen in-hospital. “Still, there’s a lot of room for improvement,” he said.
He was particularly struck by the variability in survival. “Underprivileged individuals, so those who are non-White populations and have respiratory and renal problems, they seem to have a worse survival and that makes sense – patients with comorbidities – but it feeds into the issue of, ‘Are we treating our population similarly in terms of their baseline race and ethnicity as a gap in care?’ ”
Better survival at hospitals with high volumes likely reflects more experience in handling these events, a rapid response and personnel to help with resuscitation, and overall better critical care and cath lab environment, said Dr. Naidu, director of the cardiac cath lab at Westchester Medical Center and professor of medicine at New York Medical College, both in Valhalla, N.Y.
“So that leads into two things,” he said. “One is that probably we should be working on having all high-risk patients go to centers of excellence. So, for example, [for] patients in shock, patients with STEMI, regionalization of care to the high-volume cath labs that are experienced in cardiac arrest and critical care management may be a way to go.”
“Second, if experience counts, can that experience be simulated through drills and simulations in the cath lab?” Dr. Naidu said. “Should all cath labs have drills where we have a cardiac arrest patient, and how would we respond to that? Who’s going to do the compressions? Where’s the mechanical support device? What are the things we need to have a seamless cardiac arrest protocol for arrests during the cath lab?”
Dr. Bhatt and colleagues acknowledge that despite adjustment for many key variables, the study lacked procedural details that may affect survival and information related to resuscitation efforts.
“We really do need to focus more research efforts, potentially more in the way of quality-improvement efforts, to try and help patients get these sorts of patients who are in dire straits to the cath lab but hopefully also through the hospital discharge and back home,” Dr. Bhatt said.
In an editorial accompanying the study, Matthew L. Tomey, MD, Icahn School of Medicine at Mount Sinai, New York, writes that the “findings and limitations of this study together sound a call to action.”
He also signaled the need for more research and for registries and reporting instruments to capture variables particular to in-laboratory cardiac arrest and resuscitation in the cardiac cath lab. “A necessary first step is the development of consensus data elements for supplemental reporting in cases of ILCA,” such as indication for cath lab presentation, timing of arrest relative to procedure, and cause of arrest.
Dr. Bhatt reported numerous relationships with industry. Dr. Naidu and Dr. Tomey report having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Indiana cardiologist faces multiple malpractice complaints
alleging he performed unnecessary cardiac procedures that led to physical and emotional harm.
The medical records for one patient, 70-year-old John Pflum, of Noblesville, Ind., show that Edward Harlamert, MD, performed 44 heart catheterizations and inserted at least 41 stents between 2004 and 2013, according to an investigation by WTHR 13News in Indianapolis that was published Dec. 14.
The news outlet asked four cardiologists to review and comment on John Pflum’s medical records.
“There is not a single scenario I can think of where doing this level of stents and angiograms would be justified or make sense. I have never seen this happen in the course of my medical training or my medical career,” Payal Kohli, MD, cardiologist and medical director of Cherry Creek Heart in Denver, told 13News.
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angioplasty and Interventions, who also reviewed Mr. Pflum’s medical records for 13News, said he’s “never seen a patient who has gotten this many procedures.”
Dr. Rao said that on the basis of what he saw in the records and in the images, there were several deviations from the standard of care.
Two other independent cardiologists who spoke with 13News voiced similar opinions.
Mr. Pflum was “getting cathed almost every month. That’s not how it’s done,” Morton Rinder, MD, an interventional cardiologist at St. Luke’s Hospital near St. Louis, told 13News.
Dr. Rinder has been hired as a medical consultant for the attorneys who filed Mr. Pflum’s malpractice complaint against Dr. Harlamert.
Cardiologists who reviewed the catheterization films for 13News said some of Mr. Pflum’s heart blockages met the 70% threshold to warrant consideration of a stent, while others clearly did not. In-stent restenosis occurred in several of the implanted stents, requiring a second open heart surgery.
In a statement, Dr. Harlamert’s attorneys told 13News that Dr. Harlamert has “always been committed to providing quality care to patients” and that he treated his cardiology patients “based on their unique circumstances, his expertise, and the tools available.
“Because of stringent privacy laws and pending litigation, a response to a local news story is not the proper forum to present a picture of any particular treatment decision, especially when that picture may be incomplete at this time,” the statement reads.
A version of this article first appeared on Medscape.com.
alleging he performed unnecessary cardiac procedures that led to physical and emotional harm.
The medical records for one patient, 70-year-old John Pflum, of Noblesville, Ind., show that Edward Harlamert, MD, performed 44 heart catheterizations and inserted at least 41 stents between 2004 and 2013, according to an investigation by WTHR 13News in Indianapolis that was published Dec. 14.
The news outlet asked four cardiologists to review and comment on John Pflum’s medical records.
“There is not a single scenario I can think of where doing this level of stents and angiograms would be justified or make sense. I have never seen this happen in the course of my medical training or my medical career,” Payal Kohli, MD, cardiologist and medical director of Cherry Creek Heart in Denver, told 13News.
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angioplasty and Interventions, who also reviewed Mr. Pflum’s medical records for 13News, said he’s “never seen a patient who has gotten this many procedures.”
Dr. Rao said that on the basis of what he saw in the records and in the images, there were several deviations from the standard of care.
Two other independent cardiologists who spoke with 13News voiced similar opinions.
Mr. Pflum was “getting cathed almost every month. That’s not how it’s done,” Morton Rinder, MD, an interventional cardiologist at St. Luke’s Hospital near St. Louis, told 13News.
Dr. Rinder has been hired as a medical consultant for the attorneys who filed Mr. Pflum’s malpractice complaint against Dr. Harlamert.
Cardiologists who reviewed the catheterization films for 13News said some of Mr. Pflum’s heart blockages met the 70% threshold to warrant consideration of a stent, while others clearly did not. In-stent restenosis occurred in several of the implanted stents, requiring a second open heart surgery.
In a statement, Dr. Harlamert’s attorneys told 13News that Dr. Harlamert has “always been committed to providing quality care to patients” and that he treated his cardiology patients “based on their unique circumstances, his expertise, and the tools available.
“Because of stringent privacy laws and pending litigation, a response to a local news story is not the proper forum to present a picture of any particular treatment decision, especially when that picture may be incomplete at this time,” the statement reads.
A version of this article first appeared on Medscape.com.
alleging he performed unnecessary cardiac procedures that led to physical and emotional harm.
The medical records for one patient, 70-year-old John Pflum, of Noblesville, Ind., show that Edward Harlamert, MD, performed 44 heart catheterizations and inserted at least 41 stents between 2004 and 2013, according to an investigation by WTHR 13News in Indianapolis that was published Dec. 14.
The news outlet asked four cardiologists to review and comment on John Pflum’s medical records.
“There is not a single scenario I can think of where doing this level of stents and angiograms would be justified or make sense. I have never seen this happen in the course of my medical training or my medical career,” Payal Kohli, MD, cardiologist and medical director of Cherry Creek Heart in Denver, told 13News.
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angioplasty and Interventions, who also reviewed Mr. Pflum’s medical records for 13News, said he’s “never seen a patient who has gotten this many procedures.”
Dr. Rao said that on the basis of what he saw in the records and in the images, there were several deviations from the standard of care.
Two other independent cardiologists who spoke with 13News voiced similar opinions.
Mr. Pflum was “getting cathed almost every month. That’s not how it’s done,” Morton Rinder, MD, an interventional cardiologist at St. Luke’s Hospital near St. Louis, told 13News.
Dr. Rinder has been hired as a medical consultant for the attorneys who filed Mr. Pflum’s malpractice complaint against Dr. Harlamert.
Cardiologists who reviewed the catheterization films for 13News said some of Mr. Pflum’s heart blockages met the 70% threshold to warrant consideration of a stent, while others clearly did not. In-stent restenosis occurred in several of the implanted stents, requiring a second open heart surgery.
In a statement, Dr. Harlamert’s attorneys told 13News that Dr. Harlamert has “always been committed to providing quality care to patients” and that he treated his cardiology patients “based on their unique circumstances, his expertise, and the tools available.
“Because of stringent privacy laws and pending litigation, a response to a local news story is not the proper forum to present a picture of any particular treatment decision, especially when that picture may be incomplete at this time,” the statement reads.
A version of this article first appeared on Medscape.com.
New AHA statement on managing ACS in older adults
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
CRT boosts heart failure survival in extended follow-up
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
AT AHA 2022
Novel PCI screening approach detects diffuse CAD
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
A novel approach for stratifying patients into one of two phenotypes for coronary artery disease (CAD) helped differentiate those who would benefit from percutaneous coronary intervention (PCI) from those who wouldn’t, researchers in Belgium reported in a subanalysis of a single-center, randomized clinical trial.
“What this study adds is that we are actually creating a refined definition of the appropriateness criteria for PCI,” lead study author Carlos Collet, MD, PhD, of the Cardiovascular Center at OLV Hospital in Aalst, Belgium, said in an interview. “We have been too long implanting stents in diffuse disease that actually have no benefit for the patient.”
The study found that patients with diffuse CAD were almost twice as likely to have residual angina 3 months after PCI than patients with focal CAD, with respective rates of 51.9% vs. 27.5% after PCI (P = .02).
The researchers analyzed 103 patients from the TARGET-FFR (Trial of Angiography vs. pressure-Ratio-Guided Enhancement Techniques–Fractional Flow Reserve) conducted at the Golden Jubilee National Hospital in Glasgow. Study patients completed the 7-item Seattle Angina Questionnaire at baseline and at 3 months after PCI, which provided the researchers information on outcomes.
The study, published in JACC: Cardiovascular Interventions, used median pullback pressure gradient (PPG) to define focal and diffuse CAD. The operators used the PressureWire X Guidewire (Abbott Vascular) to measure fractional flow reserve (FFR).
The procedure involved administering a 200-mcg bolus of intracoronary nitrate and then positioning the pressure wire sensor at the tip of the guide catheter equalized with aortic pressure. The pressure wire was then advanced to the position sensor in the distal third of the vessel. After hyperemia was induced, coronary flow reserve was assessed using bolus thermodilution. Manual FFR pullback maneuvers were done at a constant speed for 20-30 seconds. The PPG index was calculated post hoc from the manual FFR pullback recordings obtained pre-PCI.
In this study, patients with low PPG needed longer (48 mm vs. 37 mm; P = .015) and more (1.5 vs. 1.0; P = .036) stents during PCI, Dr. Collet and colleagues reported. They concluded that patients with low PPG can be treated with medical therapy.
“The beauty of the PPG is that everything happens before you implant the stent,” Dr. Collet said. “We’re starting to understand that we cannot treat diffuse disease with a focal disease therapy.”
The challenge with differentiating diffuse from focal CAD has been that it relies on visual assessment. “It’s subject to operator variability, and that’s the reason why there are no trials with focal or diffuse disease specifically because, until now, we didn’t have any metric that quantified the diffuseness or the focality of the disease,” Dr. Collet said.
The PPG itself isn’t novel, Dr. Collet said. “The novelty is that for first time we can quantify in a reproducible way the information from the pullback,” he added.
“What this study tells us is that once you have a patient with diffuse coronary artery disease, don’t try PCI because it will not help half of them,” Patrick W. Serruys, MD, PhD, a cardiologist at the National University of Ireland, Galway, and author of the accompanying editorial, said in an interview.
He noted that one limitation of the study was that Dr. Collet and colleagues used mechanical PPG to measure the pressure gradient. “We use now a surrogate, which is angiography,” Dr. Serruys said. “It’s not exactly the same as a measurement of pressure with the pressure wire, but we know from many, many studies that it’s quite a good surrogate.” Future research should focus on use of angiography without the pressure wire to evaluate the pressure gradient.
The ongoing PPG Global registry will aim to further validate findings from the subanalysis, Dr. Collet said, and the PPG Primetime study will evaluate deferring PCI in patients with low PPG.
Dr. Collet disclosed relationships with Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, OpSens, Abbott Vascular and Philips Volcano. Dr. Serruys disclosed relationships with Sinomedical Sciences Technology, Sahajanand Medical Technological, Philips Volcano, Xeltis and HeartFlow.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
FDA tweaks Impella indications on basis of postapproval study
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.









