User login
Worst TB outbreak in 20 years reported in Washington state
Tuberculosis cases are increasing in Washington, which has put public health officials on “heightened alert,” according to a recent announcement from the Washington State Department of Health.
Widespread disruptions in health care and missed tuberculosis diagnoses during the COVID-19 pandemic have likely added to the increase – both locally and globally.
“It’s been 20 years since we saw a cluster of TB cases like this,” Tao Sheng Kwan-Gett, MD, the state’s chief science officer, said in the announcement.
“The pandemic has likely contributed to the rise in cases and the outbreak in at least one correctional facility,” he said. “Increased access to TB testing and treatment in the community is going to be key to getting TB under control.”
Case numbers appeared to fall in Washington during the first year of the pandemic, possibly because of less reporting and missed diagnoses. But in 2021, cases rose quickly. The state reported 199 cases, marking a 22% increase from 2020.
So far this year, 70 cases have been reported, including 17 new cases that all have connections with each other and several state prisons.
The state’s Department of Corrections, Department of Health, and the Centers for Disease Control and Prevention are working together on testing and decreasing spread, MaryAnn Curl, MD, the chief medical officer for the Department of Corrections, said in the statement.
Tuberculosis cases are increasing worldwide. For the first time in more than a decade, TB deaths increased to about 1.5 million, according to the World Health Organization’s 2021 Global Tuberculosis Report.
Across the U.S., the number of reported TB cases significantly declined at the beginning of the pandemic in 2020 but increased again in 2021, according to a recent CDC study.
The Kansas Department of Health also reported an outbreak of TB cases in March, according to USA Today.
At the beginning of the pandemic, some people with TB may have been diagnosed with COVID-19 because both are infectious diseases that attack the lungs and have similar symptoms, the Washington Health Department said.
Like COVID-19, tuberculosis can spread through the air when an infected person coughs or sneezes. But unlike COVID-19, TB typically requires that you have prolonged exposure to become infected.
Symptoms of tuberculosis can include chest pain and coughing, with or without blood, as well as fever, night sweats, weight loss, and fatigue.
Tuberculosis is preventable, treatable, and curable, the Washington Health Department said. Those who travel to countries where TB is more common face higher risks for exposure, as well as those who live or work in settings where TB may spread, such as homeless shelters, prisons, jails, and nursing homes.
People can develop inactive TB, also called latent TB, which doesn’t have any symptoms and isn’t contagious. If people with inactive TB don’t get quick diagnosis or treatment, the infection can become active TB and cause symptoms. State health officials estimated that about 200,000 people in Washington have inactive TB.
Tuberculosis treatment can take a minimum of 6 months, and if it’s not followed carefully, symptoms can become more severe, the Health Department said. Incomplete treatment can also contribute to the spread of antibiotic-resistant strains of tuberculosis.
A version of this article first appeared on WebMD.com.
Tuberculosis cases are increasing in Washington, which has put public health officials on “heightened alert,” according to a recent announcement from the Washington State Department of Health.
Widespread disruptions in health care and missed tuberculosis diagnoses during the COVID-19 pandemic have likely added to the increase – both locally and globally.
“It’s been 20 years since we saw a cluster of TB cases like this,” Tao Sheng Kwan-Gett, MD, the state’s chief science officer, said in the announcement.
“The pandemic has likely contributed to the rise in cases and the outbreak in at least one correctional facility,” he said. “Increased access to TB testing and treatment in the community is going to be key to getting TB under control.”
Case numbers appeared to fall in Washington during the first year of the pandemic, possibly because of less reporting and missed diagnoses. But in 2021, cases rose quickly. The state reported 199 cases, marking a 22% increase from 2020.
So far this year, 70 cases have been reported, including 17 new cases that all have connections with each other and several state prisons.
The state’s Department of Corrections, Department of Health, and the Centers for Disease Control and Prevention are working together on testing and decreasing spread, MaryAnn Curl, MD, the chief medical officer for the Department of Corrections, said in the statement.
Tuberculosis cases are increasing worldwide. For the first time in more than a decade, TB deaths increased to about 1.5 million, according to the World Health Organization’s 2021 Global Tuberculosis Report.
Across the U.S., the number of reported TB cases significantly declined at the beginning of the pandemic in 2020 but increased again in 2021, according to a recent CDC study.
The Kansas Department of Health also reported an outbreak of TB cases in March, according to USA Today.
At the beginning of the pandemic, some people with TB may have been diagnosed with COVID-19 because both are infectious diseases that attack the lungs and have similar symptoms, the Washington Health Department said.
Like COVID-19, tuberculosis can spread through the air when an infected person coughs or sneezes. But unlike COVID-19, TB typically requires that you have prolonged exposure to become infected.
Symptoms of tuberculosis can include chest pain and coughing, with or without blood, as well as fever, night sweats, weight loss, and fatigue.
Tuberculosis is preventable, treatable, and curable, the Washington Health Department said. Those who travel to countries where TB is more common face higher risks for exposure, as well as those who live or work in settings where TB may spread, such as homeless shelters, prisons, jails, and nursing homes.
People can develop inactive TB, also called latent TB, which doesn’t have any symptoms and isn’t contagious. If people with inactive TB don’t get quick diagnosis or treatment, the infection can become active TB and cause symptoms. State health officials estimated that about 200,000 people in Washington have inactive TB.
Tuberculosis treatment can take a minimum of 6 months, and if it’s not followed carefully, symptoms can become more severe, the Health Department said. Incomplete treatment can also contribute to the spread of antibiotic-resistant strains of tuberculosis.
A version of this article first appeared on WebMD.com.
Tuberculosis cases are increasing in Washington, which has put public health officials on “heightened alert,” according to a recent announcement from the Washington State Department of Health.
Widespread disruptions in health care and missed tuberculosis diagnoses during the COVID-19 pandemic have likely added to the increase – both locally and globally.
“It’s been 20 years since we saw a cluster of TB cases like this,” Tao Sheng Kwan-Gett, MD, the state’s chief science officer, said in the announcement.
“The pandemic has likely contributed to the rise in cases and the outbreak in at least one correctional facility,” he said. “Increased access to TB testing and treatment in the community is going to be key to getting TB under control.”
Case numbers appeared to fall in Washington during the first year of the pandemic, possibly because of less reporting and missed diagnoses. But in 2021, cases rose quickly. The state reported 199 cases, marking a 22% increase from 2020.
So far this year, 70 cases have been reported, including 17 new cases that all have connections with each other and several state prisons.
The state’s Department of Corrections, Department of Health, and the Centers for Disease Control and Prevention are working together on testing and decreasing spread, MaryAnn Curl, MD, the chief medical officer for the Department of Corrections, said in the statement.
Tuberculosis cases are increasing worldwide. For the first time in more than a decade, TB deaths increased to about 1.5 million, according to the World Health Organization’s 2021 Global Tuberculosis Report.
Across the U.S., the number of reported TB cases significantly declined at the beginning of the pandemic in 2020 but increased again in 2021, according to a recent CDC study.
The Kansas Department of Health also reported an outbreak of TB cases in March, according to USA Today.
At the beginning of the pandemic, some people with TB may have been diagnosed with COVID-19 because both are infectious diseases that attack the lungs and have similar symptoms, the Washington Health Department said.
Like COVID-19, tuberculosis can spread through the air when an infected person coughs or sneezes. But unlike COVID-19, TB typically requires that you have prolonged exposure to become infected.
Symptoms of tuberculosis can include chest pain and coughing, with or without blood, as well as fever, night sweats, weight loss, and fatigue.
Tuberculosis is preventable, treatable, and curable, the Washington Health Department said. Those who travel to countries where TB is more common face higher risks for exposure, as well as those who live or work in settings where TB may spread, such as homeless shelters, prisons, jails, and nursing homes.
People can develop inactive TB, also called latent TB, which doesn’t have any symptoms and isn’t contagious. If people with inactive TB don’t get quick diagnosis or treatment, the infection can become active TB and cause symptoms. State health officials estimated that about 200,000 people in Washington have inactive TB.
Tuberculosis treatment can take a minimum of 6 months, and if it’s not followed carefully, symptoms can become more severe, the Health Department said. Incomplete treatment can also contribute to the spread of antibiotic-resistant strains of tuberculosis.
A version of this article first appeared on WebMD.com.
New HIV care guidelines from the European AIDS Clinical Society
Version 11.0 of the 2021 revised European AIDS Clinical Society (EACS) Guidelines updates all aspects of HIV care and adds recommendations on COVID-19 and antiretroviral treatment (ART) in children and adolescents, the guidelines authors reported in HIV Medicine.
“Conducting a systematic and timely annual revision of all guidelines recommendations is an EACS cornerstone,” EACS Guidelines coordinator Lene Ryom, MD, PhD, DMSc, a researcher at the University of Copenhagen, said in an interview. “These revisions ensure that the EACS Guidelines remain clinically relevant, are updated with the latest scientific evidence, and that they cover all key aspects related to HIV management.”
Key revisions in this update include:
Antiretroviral therapy (ART)
- Six recommended treatment options for first-line regimens for ART-naive adults include triple-drug regimens consisting of tenofovir (either tenofovir disoproxil fumarate or tenofovir alafenamide) with either lamivudine or emtricitabine plus dolutegravir, raltegravir, bictegravir, or doravirine; abacavir/lamivudine plus dolutegravir; or dual therapy with emtricitabine plus dolutegravir. These drug combinations are recommended in single-tablet form if available.
- Alternatives consisting of triple-drug tenofovir-based regimens along with efavirenz, rilpivirine, or boosted darunavir, are advised when no recommended regimens are feasible.
- Bimonthly injections with long-acting cabotegravir plus rilpivirine are now advised as a switch option for people who are virologically suppressed.
- Pre-exposure prophylaxis on demand is advised for cisgender men, and PrEP may be continued during pregnancy and breastfeeding for people at risk of acquiring HIV.
Drug-drug interactions (DDIs) and other prescribing issues
- Four new DDI tables cover antituberculosis drugs, anxiolytics, hormone therapy, and COVID-19 therapies.
Comorbidities
- This update acknowledged the impact of the COVID-19 pandemic on routine health care, provides recommendations, and highlights the role of shared care and consultation for anxiety and other mental health disorders.
- Treatments involving diabetes, hypertension, cardiovascular disease, heart failure, chronic kidney disease, hypercholesterolemia, obesity, cancer, and sexual health have been updated, with new information about elderly and frail patients, women’s sexual health, and special considerations for transgender people.
Viral hepatitis coinfection
Immediate treatment of recently acquired hepatitis C is recommended for people living with HIV and ongoing risk behavior. Bulevirtide is added as a treatment option for hepatitis Delta virus.
Opportunistic infections and COVID-19
- The revision adds new guidance on management of HIV and COVID-19, covering epidemiology, risk factors for severe COVID-19, COVID-19 management, HIV care during a pandemic, HIV management during COVID-19 treatment, and management of long-term COVID-19 symptoms and prophylaxis.
- It includes guidance on management of tuberculosis meningitis, cryptococcosis, Pneumocystis jirovecii pneumonia, and drug-resistant tuberculosis.
Pediatric HIV infection treatments
- This new section, developed with the European pediatric research organization Penta, updates guidance for the use of preferred and alternative first-line drugs from birth to adolescence. Combinations include new child-friendly formulations of dolutegravir as early as 4 weeks of age and 3 kg (6.6 lb) of weight as well as an increased emphasis on dolutegravir as first-line preferred agent for all children except newborns. Abacavir is recommended for children younger than 3 months.
- ART regimens for children with infectious hepatitis or tuberculosis are also provided.
Laura Jane Waters, MD, a genitourinary consultant and HIV and hepatitis lead at Central and North West London National Health Service Mortimer Market Centre, and chair of the British HIV Association (BHIVA), shared her perspective on the revision. She was not involved with the EACS Guidelines revision.
“The addition of a section on COVID-19 in people with HIV, including management, drug interactions, and vaccination, is welcomed, as is the inclusion of key references and, for selected references, the key findings,” Dr. Waters said in an interview.
“Finally, for the first time, EACS covers pediatric HIV treatment by integrating with the Penta guidelines,” she added. “This is an important evolution, considering there are still cases of vertical HIV transmission in Europe, not to mention children living with HIV who have immigrated. Ensuring high and equitable standards of HIV treatment for young people is crucial.”
“This update to the always-pragmatic EACS guidelines further diverges from the United States Department of Health & Human Services guidelines,” Dr. Waters explained. “For 6 months, both guidelines preferred the same ... regimens for first-line therapy, but since DHSS removed raltegravir-based ART in June 2021 and EACS added doravirine-based regimens in October 2021, we’re back in the more familiar territory of EACS offering a broader range of preferred choices.”
Dr. Ryom noted that modern HIV care needs to consider managing coinfections, opportunistic diseases, comorbidities, aging, addictions, and mental health.
“Ensuring an integrated and personalized approach to HIV management is becoming increasingly important in an aging population living with HIV with the potential for complex needs,” she said.
The guidelines are available in several formats: as a free smartphone app, an interactive web version, and an online PDF.
Funding information was not provided. Dr. Ryom and several coauthors disclosed no relevant financial relationships. Most of the guideline coauthors declared financial relationships with pharmaceutical companies “outside the submitted work.” Dr. Waters provided no information on conflicts of interest.
A version of this article first appeared on Medscape.com.
Version 11.0 of the 2021 revised European AIDS Clinical Society (EACS) Guidelines updates all aspects of HIV care and adds recommendations on COVID-19 and antiretroviral treatment (ART) in children and adolescents, the guidelines authors reported in HIV Medicine.
“Conducting a systematic and timely annual revision of all guidelines recommendations is an EACS cornerstone,” EACS Guidelines coordinator Lene Ryom, MD, PhD, DMSc, a researcher at the University of Copenhagen, said in an interview. “These revisions ensure that the EACS Guidelines remain clinically relevant, are updated with the latest scientific evidence, and that they cover all key aspects related to HIV management.”
Key revisions in this update include:
Antiretroviral therapy (ART)
- Six recommended treatment options for first-line regimens for ART-naive adults include triple-drug regimens consisting of tenofovir (either tenofovir disoproxil fumarate or tenofovir alafenamide) with either lamivudine or emtricitabine plus dolutegravir, raltegravir, bictegravir, or doravirine; abacavir/lamivudine plus dolutegravir; or dual therapy with emtricitabine plus dolutegravir. These drug combinations are recommended in single-tablet form if available.
- Alternatives consisting of triple-drug tenofovir-based regimens along with efavirenz, rilpivirine, or boosted darunavir, are advised when no recommended regimens are feasible.
- Bimonthly injections with long-acting cabotegravir plus rilpivirine are now advised as a switch option for people who are virologically suppressed.
- Pre-exposure prophylaxis on demand is advised for cisgender men, and PrEP may be continued during pregnancy and breastfeeding for people at risk of acquiring HIV.
Drug-drug interactions (DDIs) and other prescribing issues
- Four new DDI tables cover antituberculosis drugs, anxiolytics, hormone therapy, and COVID-19 therapies.
Comorbidities
- This update acknowledged the impact of the COVID-19 pandemic on routine health care, provides recommendations, and highlights the role of shared care and consultation for anxiety and other mental health disorders.
- Treatments involving diabetes, hypertension, cardiovascular disease, heart failure, chronic kidney disease, hypercholesterolemia, obesity, cancer, and sexual health have been updated, with new information about elderly and frail patients, women’s sexual health, and special considerations for transgender people.
Viral hepatitis coinfection
Immediate treatment of recently acquired hepatitis C is recommended for people living with HIV and ongoing risk behavior. Bulevirtide is added as a treatment option for hepatitis Delta virus.
Opportunistic infections and COVID-19
- The revision adds new guidance on management of HIV and COVID-19, covering epidemiology, risk factors for severe COVID-19, COVID-19 management, HIV care during a pandemic, HIV management during COVID-19 treatment, and management of long-term COVID-19 symptoms and prophylaxis.
- It includes guidance on management of tuberculosis meningitis, cryptococcosis, Pneumocystis jirovecii pneumonia, and drug-resistant tuberculosis.
Pediatric HIV infection treatments
- This new section, developed with the European pediatric research organization Penta, updates guidance for the use of preferred and alternative first-line drugs from birth to adolescence. Combinations include new child-friendly formulations of dolutegravir as early as 4 weeks of age and 3 kg (6.6 lb) of weight as well as an increased emphasis on dolutegravir as first-line preferred agent for all children except newborns. Abacavir is recommended for children younger than 3 months.
- ART regimens for children with infectious hepatitis or tuberculosis are also provided.
Laura Jane Waters, MD, a genitourinary consultant and HIV and hepatitis lead at Central and North West London National Health Service Mortimer Market Centre, and chair of the British HIV Association (BHIVA), shared her perspective on the revision. She was not involved with the EACS Guidelines revision.
“The addition of a section on COVID-19 in people with HIV, including management, drug interactions, and vaccination, is welcomed, as is the inclusion of key references and, for selected references, the key findings,” Dr. Waters said in an interview.
“Finally, for the first time, EACS covers pediatric HIV treatment by integrating with the Penta guidelines,” she added. “This is an important evolution, considering there are still cases of vertical HIV transmission in Europe, not to mention children living with HIV who have immigrated. Ensuring high and equitable standards of HIV treatment for young people is crucial.”
“This update to the always-pragmatic EACS guidelines further diverges from the United States Department of Health & Human Services guidelines,” Dr. Waters explained. “For 6 months, both guidelines preferred the same ... regimens for first-line therapy, but since DHSS removed raltegravir-based ART in June 2021 and EACS added doravirine-based regimens in October 2021, we’re back in the more familiar territory of EACS offering a broader range of preferred choices.”
Dr. Ryom noted that modern HIV care needs to consider managing coinfections, opportunistic diseases, comorbidities, aging, addictions, and mental health.
“Ensuring an integrated and personalized approach to HIV management is becoming increasingly important in an aging population living with HIV with the potential for complex needs,” she said.
The guidelines are available in several formats: as a free smartphone app, an interactive web version, and an online PDF.
Funding information was not provided. Dr. Ryom and several coauthors disclosed no relevant financial relationships. Most of the guideline coauthors declared financial relationships with pharmaceutical companies “outside the submitted work.” Dr. Waters provided no information on conflicts of interest.
A version of this article first appeared on Medscape.com.
Version 11.0 of the 2021 revised European AIDS Clinical Society (EACS) Guidelines updates all aspects of HIV care and adds recommendations on COVID-19 and antiretroviral treatment (ART) in children and adolescents, the guidelines authors reported in HIV Medicine.
“Conducting a systematic and timely annual revision of all guidelines recommendations is an EACS cornerstone,” EACS Guidelines coordinator Lene Ryom, MD, PhD, DMSc, a researcher at the University of Copenhagen, said in an interview. “These revisions ensure that the EACS Guidelines remain clinically relevant, are updated with the latest scientific evidence, and that they cover all key aspects related to HIV management.”
Key revisions in this update include:
Antiretroviral therapy (ART)
- Six recommended treatment options for first-line regimens for ART-naive adults include triple-drug regimens consisting of tenofovir (either tenofovir disoproxil fumarate or tenofovir alafenamide) with either lamivudine or emtricitabine plus dolutegravir, raltegravir, bictegravir, or doravirine; abacavir/lamivudine plus dolutegravir; or dual therapy with emtricitabine plus dolutegravir. These drug combinations are recommended in single-tablet form if available.
- Alternatives consisting of triple-drug tenofovir-based regimens along with efavirenz, rilpivirine, or boosted darunavir, are advised when no recommended regimens are feasible.
- Bimonthly injections with long-acting cabotegravir plus rilpivirine are now advised as a switch option for people who are virologically suppressed.
- Pre-exposure prophylaxis on demand is advised for cisgender men, and PrEP may be continued during pregnancy and breastfeeding for people at risk of acquiring HIV.
Drug-drug interactions (DDIs) and other prescribing issues
- Four new DDI tables cover antituberculosis drugs, anxiolytics, hormone therapy, and COVID-19 therapies.
Comorbidities
- This update acknowledged the impact of the COVID-19 pandemic on routine health care, provides recommendations, and highlights the role of shared care and consultation for anxiety and other mental health disorders.
- Treatments involving diabetes, hypertension, cardiovascular disease, heart failure, chronic kidney disease, hypercholesterolemia, obesity, cancer, and sexual health have been updated, with new information about elderly and frail patients, women’s sexual health, and special considerations for transgender people.
Viral hepatitis coinfection
Immediate treatment of recently acquired hepatitis C is recommended for people living with HIV and ongoing risk behavior. Bulevirtide is added as a treatment option for hepatitis Delta virus.
Opportunistic infections and COVID-19
- The revision adds new guidance on management of HIV and COVID-19, covering epidemiology, risk factors for severe COVID-19, COVID-19 management, HIV care during a pandemic, HIV management during COVID-19 treatment, and management of long-term COVID-19 symptoms and prophylaxis.
- It includes guidance on management of tuberculosis meningitis, cryptococcosis, Pneumocystis jirovecii pneumonia, and drug-resistant tuberculosis.
Pediatric HIV infection treatments
- This new section, developed with the European pediatric research organization Penta, updates guidance for the use of preferred and alternative first-line drugs from birth to adolescence. Combinations include new child-friendly formulations of dolutegravir as early as 4 weeks of age and 3 kg (6.6 lb) of weight as well as an increased emphasis on dolutegravir as first-line preferred agent for all children except newborns. Abacavir is recommended for children younger than 3 months.
- ART regimens for children with infectious hepatitis or tuberculosis are also provided.
Laura Jane Waters, MD, a genitourinary consultant and HIV and hepatitis lead at Central and North West London National Health Service Mortimer Market Centre, and chair of the British HIV Association (BHIVA), shared her perspective on the revision. She was not involved with the EACS Guidelines revision.
“The addition of a section on COVID-19 in people with HIV, including management, drug interactions, and vaccination, is welcomed, as is the inclusion of key references and, for selected references, the key findings,” Dr. Waters said in an interview.
“Finally, for the first time, EACS covers pediatric HIV treatment by integrating with the Penta guidelines,” she added. “This is an important evolution, considering there are still cases of vertical HIV transmission in Europe, not to mention children living with HIV who have immigrated. Ensuring high and equitable standards of HIV treatment for young people is crucial.”
“This update to the always-pragmatic EACS guidelines further diverges from the United States Department of Health & Human Services guidelines,” Dr. Waters explained. “For 6 months, both guidelines preferred the same ... regimens for first-line therapy, but since DHSS removed raltegravir-based ART in June 2021 and EACS added doravirine-based regimens in October 2021, we’re back in the more familiar territory of EACS offering a broader range of preferred choices.”
Dr. Ryom noted that modern HIV care needs to consider managing coinfections, opportunistic diseases, comorbidities, aging, addictions, and mental health.
“Ensuring an integrated and personalized approach to HIV management is becoming increasingly important in an aging population living with HIV with the potential for complex needs,” she said.
The guidelines are available in several formats: as a free smartphone app, an interactive web version, and an online PDF.
Funding information was not provided. Dr. Ryom and several coauthors disclosed no relevant financial relationships. Most of the guideline coauthors declared financial relationships with pharmaceutical companies “outside the submitted work.” Dr. Waters provided no information on conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
Most at-home STI testing kits fail to meet young people’s needs
The wide majority of at-home sexually transmitted infection testing kits in the United States appear to be limited to use by adults, a new study finds, and many have limitations that make them less than ideal for young people to use.
While at-home kits do allow more access to STI testing, “we need to create programs that are specific for youth because they have extra needs,” said lead author Saumya Sao, a research assistant at the department of gynecology & obstetrics at Johns Hopkins University, Baltimore, in an interview. “The only platform that did meet our needs was the program that we developed specifically.”
The findings were released ahead of the study’s scheduled presentation at the 2022 annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (Session A117).
According to Ms. Sao, companies began to offer more at-home testing kits during the pandemic as in-person STI clinics shut down. Still, “the fact that we only found 13 self-collect mail-in STI programs shows you that this is pretty new,” she said. “There are not too many companies that do it. We found a lot more platforms that allow users to place orders for testing online, but you’re still required to go into a lab and actually do the testing.”
The researchers gathered information about 13 programs, including the one that they developed at Johns Hopkins known as Violet. Of those, seven limited testing to adults aged 18 and up, and one didn’t list an age requirement. The rest had some age requirements (such as 14 and up) or no age requirements.
The lack of full access for teens is problematic, Ms. Sao said. According to the study, “access to testing among young people is especially important because youth (ages 13-24) bear a disproportionate burden of sexually transmitted infection, accounting for 50% of cases but only 25% of the sexually active population.”
Research has suggested that young people are often wary of visiting STI clinics because they fear stigma from medical professionals or worry about being seen there, Ms. Sao said.
Tests are free in only three of the programs analyzed in the new study. Among the other programs, tests for Chlamydia trachomatis and Neisseria gonorrhoeae cost $45-$179; only two accepted insurance. “These out-of-pocket costs are really high in regard to what a young person might be able to afford for testing, especially if they would need to do repeat testing between partners, or 3 months after testing positive,” Ms. Sao said.
Most of the programs will link users to medical professionals if they test positive. This is a key feature, Ms. Sao said, in order to make sure young people have support.
As for location, most of the programs – including all those that offer free testing – are limited to certain states. Planned Parenthood, for example, only offers at-home STI testing in Maine, New Hampshire, and Vermont. The program charges patients on a sliding scale, accepts insurance, and is available for ages 14 and up. It connects users who test positive to physicians.
Another free program, TakeMeHome, is restricted to 16 states. It includes an HIV panel for ages 17+ (although it doesn’t have vaginal swab testing). It recommends that patients who are positive consult a doctor.
The researchers also found that some, but not all, of the programs send testing material in discreet packaging. This is important to young people because they may not want their parents to know that they’re getting tested.
Some of the testing programs analyzed don’t make it clear on their web sites whether their packaging is discreet, Ms. Sao said.
At Johns Hopkins, Ms. Sao has helped develop the Violet Project, which is designed to meet the needs of young people and offers free STI testing to residents of Maryland of any age for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Mailing packages are discreet, and physicians reach out to those who test positive. Fees are covered.
“We don’t have money yet to expand beyond Maryland, but we’re hopeful,” she said.
In an interview, Loma Linda (Calif.) University Health maternal-fetal medicine specialist Sarah Smithson, DO, MS, praised the study and said she supports optimizing at-home testing for young people. It may be useful for youths who first get tested in a clinic but then need follow-up testing or testing of their partners, she said.
Dr. Smithson added that transportation is often a challenge for young people. At her pregnancy clinic in California’s Inland Empire, she said, some patients live in remote areas and make virtual doctor visits because of the distance. STI testing is crucial for pregnant women, she said, “and this could be a game changer for them.”
The wide majority of at-home sexually transmitted infection testing kits in the United States appear to be limited to use by adults, a new study finds, and many have limitations that make them less than ideal for young people to use.
While at-home kits do allow more access to STI testing, “we need to create programs that are specific for youth because they have extra needs,” said lead author Saumya Sao, a research assistant at the department of gynecology & obstetrics at Johns Hopkins University, Baltimore, in an interview. “The only platform that did meet our needs was the program that we developed specifically.”
The findings were released ahead of the study’s scheduled presentation at the 2022 annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (Session A117).
According to Ms. Sao, companies began to offer more at-home testing kits during the pandemic as in-person STI clinics shut down. Still, “the fact that we only found 13 self-collect mail-in STI programs shows you that this is pretty new,” she said. “There are not too many companies that do it. We found a lot more platforms that allow users to place orders for testing online, but you’re still required to go into a lab and actually do the testing.”
The researchers gathered information about 13 programs, including the one that they developed at Johns Hopkins known as Violet. Of those, seven limited testing to adults aged 18 and up, and one didn’t list an age requirement. The rest had some age requirements (such as 14 and up) or no age requirements.
The lack of full access for teens is problematic, Ms. Sao said. According to the study, “access to testing among young people is especially important because youth (ages 13-24) bear a disproportionate burden of sexually transmitted infection, accounting for 50% of cases but only 25% of the sexually active population.”
Research has suggested that young people are often wary of visiting STI clinics because they fear stigma from medical professionals or worry about being seen there, Ms. Sao said.
Tests are free in only three of the programs analyzed in the new study. Among the other programs, tests for Chlamydia trachomatis and Neisseria gonorrhoeae cost $45-$179; only two accepted insurance. “These out-of-pocket costs are really high in regard to what a young person might be able to afford for testing, especially if they would need to do repeat testing between partners, or 3 months after testing positive,” Ms. Sao said.
Most of the programs will link users to medical professionals if they test positive. This is a key feature, Ms. Sao said, in order to make sure young people have support.
As for location, most of the programs – including all those that offer free testing – are limited to certain states. Planned Parenthood, for example, only offers at-home STI testing in Maine, New Hampshire, and Vermont. The program charges patients on a sliding scale, accepts insurance, and is available for ages 14 and up. It connects users who test positive to physicians.
Another free program, TakeMeHome, is restricted to 16 states. It includes an HIV panel for ages 17+ (although it doesn’t have vaginal swab testing). It recommends that patients who are positive consult a doctor.
The researchers also found that some, but not all, of the programs send testing material in discreet packaging. This is important to young people because they may not want their parents to know that they’re getting tested.
Some of the testing programs analyzed don’t make it clear on their web sites whether their packaging is discreet, Ms. Sao said.
At Johns Hopkins, Ms. Sao has helped develop the Violet Project, which is designed to meet the needs of young people and offers free STI testing to residents of Maryland of any age for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Mailing packages are discreet, and physicians reach out to those who test positive. Fees are covered.
“We don’t have money yet to expand beyond Maryland, but we’re hopeful,” she said.
In an interview, Loma Linda (Calif.) University Health maternal-fetal medicine specialist Sarah Smithson, DO, MS, praised the study and said she supports optimizing at-home testing for young people. It may be useful for youths who first get tested in a clinic but then need follow-up testing or testing of their partners, she said.
Dr. Smithson added that transportation is often a challenge for young people. At her pregnancy clinic in California’s Inland Empire, she said, some patients live in remote areas and make virtual doctor visits because of the distance. STI testing is crucial for pregnant women, she said, “and this could be a game changer for them.”
The wide majority of at-home sexually transmitted infection testing kits in the United States appear to be limited to use by adults, a new study finds, and many have limitations that make them less than ideal for young people to use.
While at-home kits do allow more access to STI testing, “we need to create programs that are specific for youth because they have extra needs,” said lead author Saumya Sao, a research assistant at the department of gynecology & obstetrics at Johns Hopkins University, Baltimore, in an interview. “The only platform that did meet our needs was the program that we developed specifically.”
The findings were released ahead of the study’s scheduled presentation at the 2022 annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (Session A117).
According to Ms. Sao, companies began to offer more at-home testing kits during the pandemic as in-person STI clinics shut down. Still, “the fact that we only found 13 self-collect mail-in STI programs shows you that this is pretty new,” she said. “There are not too many companies that do it. We found a lot more platforms that allow users to place orders for testing online, but you’re still required to go into a lab and actually do the testing.”
The researchers gathered information about 13 programs, including the one that they developed at Johns Hopkins known as Violet. Of those, seven limited testing to adults aged 18 and up, and one didn’t list an age requirement. The rest had some age requirements (such as 14 and up) or no age requirements.
The lack of full access for teens is problematic, Ms. Sao said. According to the study, “access to testing among young people is especially important because youth (ages 13-24) bear a disproportionate burden of sexually transmitted infection, accounting for 50% of cases but only 25% of the sexually active population.”
Research has suggested that young people are often wary of visiting STI clinics because they fear stigma from medical professionals or worry about being seen there, Ms. Sao said.
Tests are free in only three of the programs analyzed in the new study. Among the other programs, tests for Chlamydia trachomatis and Neisseria gonorrhoeae cost $45-$179; only two accepted insurance. “These out-of-pocket costs are really high in regard to what a young person might be able to afford for testing, especially if they would need to do repeat testing between partners, or 3 months after testing positive,” Ms. Sao said.
Most of the programs will link users to medical professionals if they test positive. This is a key feature, Ms. Sao said, in order to make sure young people have support.
As for location, most of the programs – including all those that offer free testing – are limited to certain states. Planned Parenthood, for example, only offers at-home STI testing in Maine, New Hampshire, and Vermont. The program charges patients on a sliding scale, accepts insurance, and is available for ages 14 and up. It connects users who test positive to physicians.
Another free program, TakeMeHome, is restricted to 16 states. It includes an HIV panel for ages 17+ (although it doesn’t have vaginal swab testing). It recommends that patients who are positive consult a doctor.
The researchers also found that some, but not all, of the programs send testing material in discreet packaging. This is important to young people because they may not want their parents to know that they’re getting tested.
Some of the testing programs analyzed don’t make it clear on their web sites whether their packaging is discreet, Ms. Sao said.
At Johns Hopkins, Ms. Sao has helped develop the Violet Project, which is designed to meet the needs of young people and offers free STI testing to residents of Maryland of any age for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Mailing packages are discreet, and physicians reach out to those who test positive. Fees are covered.
“We don’t have money yet to expand beyond Maryland, but we’re hopeful,” she said.
In an interview, Loma Linda (Calif.) University Health maternal-fetal medicine specialist Sarah Smithson, DO, MS, praised the study and said she supports optimizing at-home testing for young people. It may be useful for youths who first get tested in a clinic but then need follow-up testing or testing of their partners, she said.
Dr. Smithson added that transportation is often a challenge for young people. At her pregnancy clinic in California’s Inland Empire, she said, some patients live in remote areas and make virtual doctor visits because of the distance. STI testing is crucial for pregnant women, she said, “and this could be a game changer for them.”
FROM ACOG 2022
Porcine virus a suspect in man’s death after pig heart transplant
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
A porcine cytomegalovirus (PCMV) in the heart had gone undetected before the operation and may or may not have been instrumental in David Bennett’s death 2 months later, according to a report published in MIT Technology Review.
“The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Mr. Bennett’s death and a possible reason why the heart did not last longer,” states the article, written by staff journalist Antonio Regalado.
As described in the story, the xenotransplant saga’s new twist comes from the surgeon who performed the operation, Bartley P. Griffith, MD, University of Maryland, Baltimore, who related the PCMV finding in an April 20 online presentation hosted by the American Society of Transplantation.
Mr. Bennett’s initially promising but later turbulent clinical course, described by his surgeons and widely reported upon his death, included repeated skirmishes with infection and retaliatory adjustments to his immunosuppressant regimen. Those episodes were thought to have contributed to his death, the actual cause of which is undetermined or at least not yet reported.
“We are beginning to learn why he passed on,” Dr. Griffith said in Mr. Regalado’s article, acknowledging further that the porcine virus “maybe was the actor, or could be the actor,” that set off the events leading to Bennett’s death.
Xenotransplant specialists know that PCMV is a potential problem with pig organs and know to test for it before attempting the procedure in animal models, notes the article. It refers to a published series of pig-heart transplants to baboons in Germany. The hearts “lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.”
The heart Mr. Bennett received had been extensively screened for bacteria, viruses, and other issues that could have threatened the organ and Mr. Bennett, but the effort apparently fell short. In the MIT Technology Review story, the first author of the German baboon series speculates on how the University of Maryland team might have missed PCMV.
“The U.S. team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues,” Joachim Denner, PhD, Institute of Virology, Free University of Berlin, said in the article. The virus, he contended, “can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus.”
That PCMV escaped detection before the operation “could now factor into some people’s questions over whether the experiment should have taken place at all,” the MIT Technology Review article proposes. “It’s a big red flag,” bioethicist Arthur Caplan, PhD, New York University, said in a quote, adding: “If doctors can’t prevent or control infection, ‘then such experiments are tough to justify.’ ”
A version of this article first appeared on Medscape.com.
FROM MIT TECHNOLOGY REVIEW
Don’t let FOMI lead to antibiotic overuse
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Neonatal sepsis morbidity and mortality high across rich and poor countries
LISBON – A shift toward broader-spectrum antibiotics and increasing antibiotic resistance has led to high levels of mortality and neurodevelopmental impacts in surviving babies, according to a large international study conducted on four continents.
Results of the 3-year study were presented at this week’s European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
The observational study, NeoOBS, conducted by the Global Antibiotic Research and Development Partnership (GARDP) and key partners from 2018 to 2020, explored the outcomes of more than 3,200 newborns, finding an overall mortality of 11% in those with suspected neonatal sepsis. The mortality rate increased to 18% in newborns in whom a pathogen was detected in blood culture.
More than half of infection-related deaths (59%) were due to hospital-acquired infections. Klebsiella pneumoniae was the most common pathogen isolated and is usually associated with hospital-acquired infections, which are increasingly resistant to existing antibiotic treatments, said a report produced by GARDP to accompany the results.
The study also identified a worrying trend: Hospitals are frequently using last-line agents such as carbapenems because of the high degree of antibiotic resistance in their facilities. Of note, 15% of babies with neonatal sepsis were given last-line antibiotics.
Pediatrician Julia Bielicki, MD, PhD, senior lecturer, Paediatric Infectious Diseases Research Group, St. George’s University of London, and clinician at the University of Basel Children’s Hospital, Switzerland, was a coinvestigator on the NeoOBS study.
In an interview, she explained that, as well as reducing mortality, the research is about managing infections better to prevent long-term events and improve the quality of life for survivors of neonatal sepsis. “It can have life-changing impacts for so many babies,” Dr. Bielicki said. “Improving care is much more than just making sure the baby survives the episode of sepsis – it’s about ensuring these babies can become children and adults and go on to lead productive lives.”
Also, only a minority of patients (13%) received the World Health Organization guidelines for standard of care use of ampicillin and gentamicin, and there was increasing use of last-line agents such as carbapenems and even polymyxins in some settings in low- and middle-income countries. “This is alarming and foretells the impending crisis of a lack of antibiotics to treat sepsis caused by multidrug-resistant organisms,” according to the GARDP report.
There was wide variability in antibiotic combinations used across sites in Bangladesh, Brazil, China, Greece, India, Italy, Kenya, South Africa, Thailand, Uganda, and Vietnam, and often such use was not supported by underlying data.
Dr. Bielicki remarked that there was a shift toward broad-spectrum antibiotic use. “In a high-income country, you have more restrictive patterns of antibiotic use, but it isn’t necessarily less antibiotic exposure of neonates to antibiotics, but on the whole, usually narrow-spectrum agents are used.”
In Africa and Asia, on the other hand, clinicians often have to use a broader-spectrum antibiotic empirically and may need to switch to another antibiotic very quickly. “Sometimes alternatives are not available,” she pointed out.
“Local physicians are very perceptive of this problem of antibiotic resistance in their daily practice, especially in centers with high mortality,” said Dr. Bielicki, emphasizing that it is not their fault, but is “due to the limitations in terms of the weapons available to treat these babies, which strongly demonstrates the growing problem of antimicrobial resistance affecting these babies on a global scale.”
Tim Jinks, PhD, Head of Drug Resistant Infections Priority Program at Wellcome Trust, commented on the study in a series of text messages to this news organization. “This research provides further demonstration of the urgent need for improved treatment of newborns suffering with sepsis and particularly the requirement for new antibiotics that overcome the burden of drug-resistant infections caused by [antimicrobial resistance].”
“The study is a hugely important contribution to our understanding of the burden of neonatal sepsis in low- and middle- income countries,” he added, “and points toward ways that patient treatment can be improved to save more lives.”
High-, middle-, and low-income countries
The NeoOBS study gathered data from 19 hospitals in 11 high-, middle-, and low-income countries and assessed which antibiotics are currently being used to treat neonatal sepsis, as well as the degree of drug resistance associated with them. Sites included some in Italy and Greece, where most of the neonatal sepsis data currently originate, and this helped to anchor the data, Dr. Bielicki said.
The study identified babies with clinical sepsis over a 4-week period and observed how these patients were managed, particularly with respect to antibiotics, as well as outcomes including whether they recovered, remained in hospital, or died. Investigators obtained bacterial cultures from the patients and grew them to identify which organisms were causing the sepsis.
Of note, mortality varied widely between hospitals, ranging from 1% to 27%. Dr. Bielicki explained that the investigators were currently exploring the reasons behind this wide range of mortality. “There are lots of possible reasons for this, including structural factors such as how care is delivered, which is complex to measure,” she said. “It isn’t trivial to measure why, in a certain setting, mortality is low and why in another setting of comparable income range, mortality is much higher.”
Aside from the mortality results, Dr. Bielicki also emphasized that the survivors of neonatal sepsis frequently experience neurodevelopmental impacts. “A hospital may have low mortality, but many of these babies may have neurodevelopment problems, and this has a long-term impact.”
“Even though mortality might be low in a certain hospital, it might not be low in terms of morbidity,” she added.
The researchers also collected isolates from the cohort of neonates to determine which antibiotic combinations work against the pathogens. “This will help us define what sort of antibiotic regimen warrants further investigation,” Dr. Bielicki said.
Principal Investigator, Mike Sharland, MD, also from St. George’s, University of London, who is also the Antimicrobial Resistance Program Lead at Penta Child Health Research, said, in a press release, that the study had shown that antibiotic resistance is now one of the major threats to neonatal health globally. “There are virtually no studies underway on developing novel antibiotic treatments for babies with sepsis caused by multidrug-resistant infections.”
“This is a major problem for babies in all countries, both rich and poor,” he stressed.
NeoSep-1 trial to compare multiple different treatments
The results have paved the way for a major new global trial of multiple established and new antibiotics with the goal of reducing mortality from neonatal sepsis – the NeoSep1 trial.
“This is a randomized trial with a specific design that allows us to rank different treatments against each other in terms of effectiveness, safety, and costs,” Dr. Bielicki explained.
Among the antibiotics in the study are amikacin, flomoxef and amikacin, or fosfomycin and flomoxef in babies with sepsis 28 days old or younger. Similar to the NeoOBS study, patients will be recruited from all over the world, and in particular from low- and middle-income countries such as Kenya, South Africa, and other countries in Africa and Southeast Asia.
Ultimately, the researchers want to identify modifiable risk factors and enact change in practice. But Dr. Bielicki was quick to point out that it was difficult to disentangle those factors that can easily be changed. “Some can be changed in theory, but in practice it is actually difficult to change them. One modifiable risk factor that can be changed is probably infection control, so when resistant bacteria appear in a unit, we need to ensure that there is no or minimal transmission between babies.”
Luregn Schlapbach, MD, PhD, Head, department of intensive care and neonatology, University Children’s Hospital Zurich, Switzerland, welcomed the study, saying recent recognition of pediatric and neonatal sepsis was an urgent problem worldwide.
She referred to the 2017 WHO resolution recognizing that sepsis represents a leading cause of mortality and morbidity worldwide, affecting patients of all ages, across all continents and health care systems but that many were pediatric. “At that time, our understanding of the true burden of sepsis was limited, as was our knowledge of current epidemiology,” she said in an email interview. “The Global Burden of Disease study in 2020 revealed that about half of the approximatively 50 million global sepsis cases affect pediatric age groups, many of those during neonatal age.”
The formal acknowledgment of this extensive need emphasizes the “urgency to design preventive and therapeutic interventions to reduce this devastating burden,” Dr. Schlapbach said. “In this context, the work led by GARDP is of great importance – it is designed to improve our understanding of current practice, risk factors, and burden of neonatal sepsis across low- to middle-income settings and is essential to design adequately powered trials testing interventions such as antimicrobials to improve patient outcomes and reduce the further emergence of antimicrobial resistance.”
Dr. Bielicki and Dr. Schlapbach have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LISBON – A shift toward broader-spectrum antibiotics and increasing antibiotic resistance has led to high levels of mortality and neurodevelopmental impacts in surviving babies, according to a large international study conducted on four continents.
Results of the 3-year study were presented at this week’s European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
The observational study, NeoOBS, conducted by the Global Antibiotic Research and Development Partnership (GARDP) and key partners from 2018 to 2020, explored the outcomes of more than 3,200 newborns, finding an overall mortality of 11% in those with suspected neonatal sepsis. The mortality rate increased to 18% in newborns in whom a pathogen was detected in blood culture.
More than half of infection-related deaths (59%) were due to hospital-acquired infections. Klebsiella pneumoniae was the most common pathogen isolated and is usually associated with hospital-acquired infections, which are increasingly resistant to existing antibiotic treatments, said a report produced by GARDP to accompany the results.
The study also identified a worrying trend: Hospitals are frequently using last-line agents such as carbapenems because of the high degree of antibiotic resistance in their facilities. Of note, 15% of babies with neonatal sepsis were given last-line antibiotics.
Pediatrician Julia Bielicki, MD, PhD, senior lecturer, Paediatric Infectious Diseases Research Group, St. George’s University of London, and clinician at the University of Basel Children’s Hospital, Switzerland, was a coinvestigator on the NeoOBS study.
In an interview, she explained that, as well as reducing mortality, the research is about managing infections better to prevent long-term events and improve the quality of life for survivors of neonatal sepsis. “It can have life-changing impacts for so many babies,” Dr. Bielicki said. “Improving care is much more than just making sure the baby survives the episode of sepsis – it’s about ensuring these babies can become children and adults and go on to lead productive lives.”
Also, only a minority of patients (13%) received the World Health Organization guidelines for standard of care use of ampicillin and gentamicin, and there was increasing use of last-line agents such as carbapenems and even polymyxins in some settings in low- and middle-income countries. “This is alarming and foretells the impending crisis of a lack of antibiotics to treat sepsis caused by multidrug-resistant organisms,” according to the GARDP report.
There was wide variability in antibiotic combinations used across sites in Bangladesh, Brazil, China, Greece, India, Italy, Kenya, South Africa, Thailand, Uganda, and Vietnam, and often such use was not supported by underlying data.
Dr. Bielicki remarked that there was a shift toward broad-spectrum antibiotic use. “In a high-income country, you have more restrictive patterns of antibiotic use, but it isn’t necessarily less antibiotic exposure of neonates to antibiotics, but on the whole, usually narrow-spectrum agents are used.”
In Africa and Asia, on the other hand, clinicians often have to use a broader-spectrum antibiotic empirically and may need to switch to another antibiotic very quickly. “Sometimes alternatives are not available,” she pointed out.
“Local physicians are very perceptive of this problem of antibiotic resistance in their daily practice, especially in centers with high mortality,” said Dr. Bielicki, emphasizing that it is not their fault, but is “due to the limitations in terms of the weapons available to treat these babies, which strongly demonstrates the growing problem of antimicrobial resistance affecting these babies on a global scale.”
Tim Jinks, PhD, Head of Drug Resistant Infections Priority Program at Wellcome Trust, commented on the study in a series of text messages to this news organization. “This research provides further demonstration of the urgent need for improved treatment of newborns suffering with sepsis and particularly the requirement for new antibiotics that overcome the burden of drug-resistant infections caused by [antimicrobial resistance].”
“The study is a hugely important contribution to our understanding of the burden of neonatal sepsis in low- and middle- income countries,” he added, “and points toward ways that patient treatment can be improved to save more lives.”
High-, middle-, and low-income countries
The NeoOBS study gathered data from 19 hospitals in 11 high-, middle-, and low-income countries and assessed which antibiotics are currently being used to treat neonatal sepsis, as well as the degree of drug resistance associated with them. Sites included some in Italy and Greece, where most of the neonatal sepsis data currently originate, and this helped to anchor the data, Dr. Bielicki said.
The study identified babies with clinical sepsis over a 4-week period and observed how these patients were managed, particularly with respect to antibiotics, as well as outcomes including whether they recovered, remained in hospital, or died. Investigators obtained bacterial cultures from the patients and grew them to identify which organisms were causing the sepsis.
Of note, mortality varied widely between hospitals, ranging from 1% to 27%. Dr. Bielicki explained that the investigators were currently exploring the reasons behind this wide range of mortality. “There are lots of possible reasons for this, including structural factors such as how care is delivered, which is complex to measure,” she said. “It isn’t trivial to measure why, in a certain setting, mortality is low and why in another setting of comparable income range, mortality is much higher.”
Aside from the mortality results, Dr. Bielicki also emphasized that the survivors of neonatal sepsis frequently experience neurodevelopmental impacts. “A hospital may have low mortality, but many of these babies may have neurodevelopment problems, and this has a long-term impact.”
“Even though mortality might be low in a certain hospital, it might not be low in terms of morbidity,” she added.
The researchers also collected isolates from the cohort of neonates to determine which antibiotic combinations work against the pathogens. “This will help us define what sort of antibiotic regimen warrants further investigation,” Dr. Bielicki said.
Principal Investigator, Mike Sharland, MD, also from St. George’s, University of London, who is also the Antimicrobial Resistance Program Lead at Penta Child Health Research, said, in a press release, that the study had shown that antibiotic resistance is now one of the major threats to neonatal health globally. “There are virtually no studies underway on developing novel antibiotic treatments for babies with sepsis caused by multidrug-resistant infections.”
“This is a major problem for babies in all countries, both rich and poor,” he stressed.
NeoSep-1 trial to compare multiple different treatments
The results have paved the way for a major new global trial of multiple established and new antibiotics with the goal of reducing mortality from neonatal sepsis – the NeoSep1 trial.
“This is a randomized trial with a specific design that allows us to rank different treatments against each other in terms of effectiveness, safety, and costs,” Dr. Bielicki explained.
Among the antibiotics in the study are amikacin, flomoxef and amikacin, or fosfomycin and flomoxef in babies with sepsis 28 days old or younger. Similar to the NeoOBS study, patients will be recruited from all over the world, and in particular from low- and middle-income countries such as Kenya, South Africa, and other countries in Africa and Southeast Asia.
Ultimately, the researchers want to identify modifiable risk factors and enact change in practice. But Dr. Bielicki was quick to point out that it was difficult to disentangle those factors that can easily be changed. “Some can be changed in theory, but in practice it is actually difficult to change them. One modifiable risk factor that can be changed is probably infection control, so when resistant bacteria appear in a unit, we need to ensure that there is no or minimal transmission between babies.”
Luregn Schlapbach, MD, PhD, Head, department of intensive care and neonatology, University Children’s Hospital Zurich, Switzerland, welcomed the study, saying recent recognition of pediatric and neonatal sepsis was an urgent problem worldwide.
She referred to the 2017 WHO resolution recognizing that sepsis represents a leading cause of mortality and morbidity worldwide, affecting patients of all ages, across all continents and health care systems but that many were pediatric. “At that time, our understanding of the true burden of sepsis was limited, as was our knowledge of current epidemiology,” she said in an email interview. “The Global Burden of Disease study in 2020 revealed that about half of the approximatively 50 million global sepsis cases affect pediatric age groups, many of those during neonatal age.”
The formal acknowledgment of this extensive need emphasizes the “urgency to design preventive and therapeutic interventions to reduce this devastating burden,” Dr. Schlapbach said. “In this context, the work led by GARDP is of great importance – it is designed to improve our understanding of current practice, risk factors, and burden of neonatal sepsis across low- to middle-income settings and is essential to design adequately powered trials testing interventions such as antimicrobials to improve patient outcomes and reduce the further emergence of antimicrobial resistance.”
Dr. Bielicki and Dr. Schlapbach have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LISBON – A shift toward broader-spectrum antibiotics and increasing antibiotic resistance has led to high levels of mortality and neurodevelopmental impacts in surviving babies, according to a large international study conducted on four continents.
Results of the 3-year study were presented at this week’s European Congress of Clinical Microbiology & Infectious Diseases (ECCMID).
The observational study, NeoOBS, conducted by the Global Antibiotic Research and Development Partnership (GARDP) and key partners from 2018 to 2020, explored the outcomes of more than 3,200 newborns, finding an overall mortality of 11% in those with suspected neonatal sepsis. The mortality rate increased to 18% in newborns in whom a pathogen was detected in blood culture.
More than half of infection-related deaths (59%) were due to hospital-acquired infections. Klebsiella pneumoniae was the most common pathogen isolated and is usually associated with hospital-acquired infections, which are increasingly resistant to existing antibiotic treatments, said a report produced by GARDP to accompany the results.
The study also identified a worrying trend: Hospitals are frequently using last-line agents such as carbapenems because of the high degree of antibiotic resistance in their facilities. Of note, 15% of babies with neonatal sepsis were given last-line antibiotics.
Pediatrician Julia Bielicki, MD, PhD, senior lecturer, Paediatric Infectious Diseases Research Group, St. George’s University of London, and clinician at the University of Basel Children’s Hospital, Switzerland, was a coinvestigator on the NeoOBS study.
In an interview, she explained that, as well as reducing mortality, the research is about managing infections better to prevent long-term events and improve the quality of life for survivors of neonatal sepsis. “It can have life-changing impacts for so many babies,” Dr. Bielicki said. “Improving care is much more than just making sure the baby survives the episode of sepsis – it’s about ensuring these babies can become children and adults and go on to lead productive lives.”
Also, only a minority of patients (13%) received the World Health Organization guidelines for standard of care use of ampicillin and gentamicin, and there was increasing use of last-line agents such as carbapenems and even polymyxins in some settings in low- and middle-income countries. “This is alarming and foretells the impending crisis of a lack of antibiotics to treat sepsis caused by multidrug-resistant organisms,” according to the GARDP report.
There was wide variability in antibiotic combinations used across sites in Bangladesh, Brazil, China, Greece, India, Italy, Kenya, South Africa, Thailand, Uganda, and Vietnam, and often such use was not supported by underlying data.
Dr. Bielicki remarked that there was a shift toward broad-spectrum antibiotic use. “In a high-income country, you have more restrictive patterns of antibiotic use, but it isn’t necessarily less antibiotic exposure of neonates to antibiotics, but on the whole, usually narrow-spectrum agents are used.”
In Africa and Asia, on the other hand, clinicians often have to use a broader-spectrum antibiotic empirically and may need to switch to another antibiotic very quickly. “Sometimes alternatives are not available,” she pointed out.
“Local physicians are very perceptive of this problem of antibiotic resistance in their daily practice, especially in centers with high mortality,” said Dr. Bielicki, emphasizing that it is not their fault, but is “due to the limitations in terms of the weapons available to treat these babies, which strongly demonstrates the growing problem of antimicrobial resistance affecting these babies on a global scale.”
Tim Jinks, PhD, Head of Drug Resistant Infections Priority Program at Wellcome Trust, commented on the study in a series of text messages to this news organization. “This research provides further demonstration of the urgent need for improved treatment of newborns suffering with sepsis and particularly the requirement for new antibiotics that overcome the burden of drug-resistant infections caused by [antimicrobial resistance].”
“The study is a hugely important contribution to our understanding of the burden of neonatal sepsis in low- and middle- income countries,” he added, “and points toward ways that patient treatment can be improved to save more lives.”
High-, middle-, and low-income countries
The NeoOBS study gathered data from 19 hospitals in 11 high-, middle-, and low-income countries and assessed which antibiotics are currently being used to treat neonatal sepsis, as well as the degree of drug resistance associated with them. Sites included some in Italy and Greece, where most of the neonatal sepsis data currently originate, and this helped to anchor the data, Dr. Bielicki said.
The study identified babies with clinical sepsis over a 4-week period and observed how these patients were managed, particularly with respect to antibiotics, as well as outcomes including whether they recovered, remained in hospital, or died. Investigators obtained bacterial cultures from the patients and grew them to identify which organisms were causing the sepsis.
Of note, mortality varied widely between hospitals, ranging from 1% to 27%. Dr. Bielicki explained that the investigators were currently exploring the reasons behind this wide range of mortality. “There are lots of possible reasons for this, including structural factors such as how care is delivered, which is complex to measure,” she said. “It isn’t trivial to measure why, in a certain setting, mortality is low and why in another setting of comparable income range, mortality is much higher.”
Aside from the mortality results, Dr. Bielicki also emphasized that the survivors of neonatal sepsis frequently experience neurodevelopmental impacts. “A hospital may have low mortality, but many of these babies may have neurodevelopment problems, and this has a long-term impact.”
“Even though mortality might be low in a certain hospital, it might not be low in terms of morbidity,” she added.
The researchers also collected isolates from the cohort of neonates to determine which antibiotic combinations work against the pathogens. “This will help us define what sort of antibiotic regimen warrants further investigation,” Dr. Bielicki said.
Principal Investigator, Mike Sharland, MD, also from St. George’s, University of London, who is also the Antimicrobial Resistance Program Lead at Penta Child Health Research, said, in a press release, that the study had shown that antibiotic resistance is now one of the major threats to neonatal health globally. “There are virtually no studies underway on developing novel antibiotic treatments for babies with sepsis caused by multidrug-resistant infections.”
“This is a major problem for babies in all countries, both rich and poor,” he stressed.
NeoSep-1 trial to compare multiple different treatments
The results have paved the way for a major new global trial of multiple established and new antibiotics with the goal of reducing mortality from neonatal sepsis – the NeoSep1 trial.
“This is a randomized trial with a specific design that allows us to rank different treatments against each other in terms of effectiveness, safety, and costs,” Dr. Bielicki explained.
Among the antibiotics in the study are amikacin, flomoxef and amikacin, or fosfomycin and flomoxef in babies with sepsis 28 days old or younger. Similar to the NeoOBS study, patients will be recruited from all over the world, and in particular from low- and middle-income countries such as Kenya, South Africa, and other countries in Africa and Southeast Asia.
Ultimately, the researchers want to identify modifiable risk factors and enact change in practice. But Dr. Bielicki was quick to point out that it was difficult to disentangle those factors that can easily be changed. “Some can be changed in theory, but in practice it is actually difficult to change them. One modifiable risk factor that can be changed is probably infection control, so when resistant bacteria appear in a unit, we need to ensure that there is no or minimal transmission between babies.”
Luregn Schlapbach, MD, PhD, Head, department of intensive care and neonatology, University Children’s Hospital Zurich, Switzerland, welcomed the study, saying recent recognition of pediatric and neonatal sepsis was an urgent problem worldwide.
She referred to the 2017 WHO resolution recognizing that sepsis represents a leading cause of mortality and morbidity worldwide, affecting patients of all ages, across all continents and health care systems but that many were pediatric. “At that time, our understanding of the true burden of sepsis was limited, as was our knowledge of current epidemiology,” she said in an email interview. “The Global Burden of Disease study in 2020 revealed that about half of the approximatively 50 million global sepsis cases affect pediatric age groups, many of those during neonatal age.”
The formal acknowledgment of this extensive need emphasizes the “urgency to design preventive and therapeutic interventions to reduce this devastating burden,” Dr. Schlapbach said. “In this context, the work led by GARDP is of great importance – it is designed to improve our understanding of current practice, risk factors, and burden of neonatal sepsis across low- to middle-income settings and is essential to design adequately powered trials testing interventions such as antimicrobials to improve patient outcomes and reduce the further emergence of antimicrobial resistance.”
Dr. Bielicki and Dr. Schlapbach have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ECCMID 2022
Children and COVID: New cases up for third straight week
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.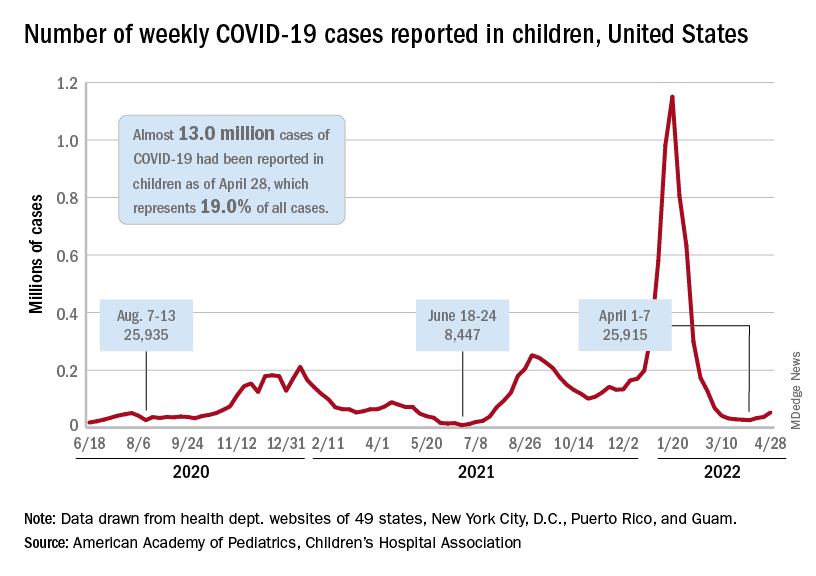
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”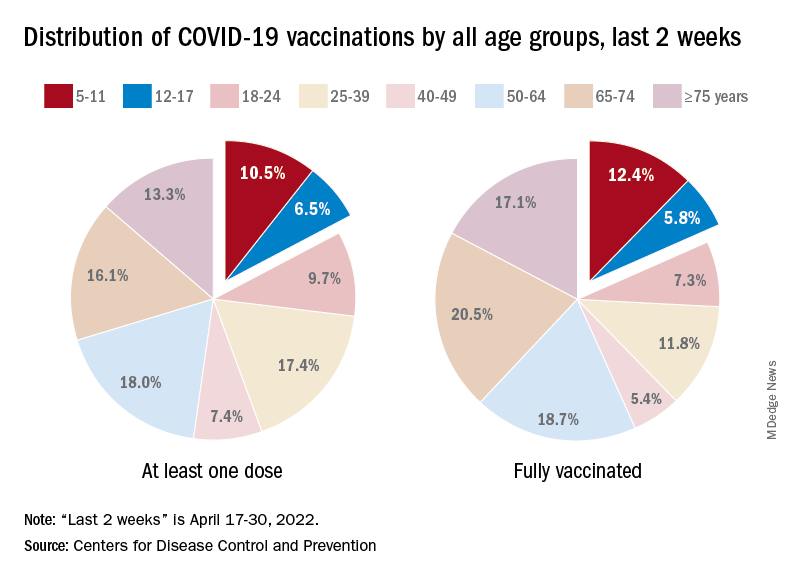
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
WHO, UNICEF warn about increased risk of measles outbreaks
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
Unexplained hepatitis cases in children reported in 10 U.S. states, more than 200 worldwide
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Painful Fungating Perianal Mass
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal bleeding in the setting of constipation. The patient’s symptoms had persisted despite multiple clinical encounters and treatment with sulfamethoxazole-trimethoprim, clotrimazole, valacyclovir, topical hydrocortisone and pramoxine, topical lidocaine, imiquimod, and psyllium seed. The patient denied engaging in receptive anal intercourse and had no notable medical or surgical history. Physical examination revealed a 6-cm hypopigmented fungating mass on the left gluteal cleft just external to the anal verge; there were no other abnormal findings. The patient denied any other systemic symptoms.