User login
Dermatology and Vaccines: We Must Do Better
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
Vaccines work. They are powerful tools that have saved millions of lives worldwide; however, a robust antivaccine movement has taken hold in the United States and worldwide despite overwhelming data in support of vaccination. In fact, vaccine hesitancy—the reluctance or refusal to vaccinate despite the availability of vaccines—was listed by the World Health Organization as one of the top 10 global health threats in 2019.1
Several vaccines have a role in dermatology, including the human papillomavirus (HPV) vaccine (Gardasil 9 [Merck Sharp & Dohme Corp]), the herpes zoster vaccines (Zostavax [Merck Sharp & Dohme Corp] and Shingrix [GlaxoSmithKline Biologicals]), and the measles-mumps-rubella vaccine, among others. These vaccinations are necessary for children and many adults alike, and they play a critical role in protecting both healthy and immunosuppressed patients.
Vaccine hesitancy is a growing threat to individual and public health that requires a response from all physicians. In our experience, dermatologists have been somewhat passive in advocating for vaccinations, possibly due to knowledge barriers or time constraints; however, this stance must change. Dermatologists must join the front lines in advocating for vaccinations, which are a proven and effective modality in promoting public health.
Dermatologists can employ the following practical tips to improve vaccination compliance among patients:
• Familiarize yourself with the Centers for Disease Control and Prevention immunization schedules and vaccination information sheets (https://www.cdc.gov/vaccines/hcp/vis/current-vis.html). Printed copies of informational handouts should be readily available to provide to patients in the office. The Centers for Disease Control and Prevention also offers tip sheets to guide conversations with patients (https://www.cdc.gov/vaccines/hcp/conversations/index.html).
• Prior to starting an immunosuppressive medication, confirm the patient’s immunization status. You should know which vaccines are live (containing an attenuated pathogen) and which are inactivated. Live vaccines typically are not administered to immunosuppressed patients.
• Use electronic medical records to help provide reminders to prompt administration of any necessary vaccines.
• Know the facts, especially regarding purported vaccine controversies, and be able to cite data on vaccine safety and efficacy. For example, when having a conversation with a patient you could state that vaccination against HPV, which can cause genital warts and certain cancers, has decreased the number of HPV infections by more than 70% in young women and 80% in teenaged girls.2 Cervical precancers were reduced by 40% in women vaccinated against HPV. Twelve years of monitoring data validates the safety and efficacy of the HPV vaccine—it is safe and effective, with benefits that outweigh any potential risks.2
• Tailor counseling based on the patient’s age and focus on benefits that directly impact the patient. For example, consider showing young adults photographs of genital warts while educating them that the HPV vaccine can help prevent this kind of infection in the future.
• Emphasize that vaccines are a routine part of comprehensive patient care and support this point by providing data and specific reasons for recommending vaccines.3 Avoid phrases such as, “Do you want the vaccine?” or “You could consider receiving the vaccine today,” which can imply that the vaccine is not necessary.
• Offer vaccines in your office or provide clear printed informational sheets directing patients to nearby primary care clinics, infectious disease clinics, or pharmacies where vaccinations are offered.
• Consider using social media to promote the benefits of vaccination among patients.
The recent coronavirus disease 2019 pandemic has brought the topic of vaccination into the limelight while highlighting that rampant misinformation can lead to distrust of health care workers. Dermatologists, along with all physicians, should be trusted advisors and advocates for public health. In addition to being knowledgeable, dermatologists must remain open-minded in having conversations with skeptical patients. Physicians must take the time and effort to promote vaccinations—the health of patients and the general public depends on it.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
- Akbar R. Ten threats to global health in 2019. World Health Organization website. https://www.who.int/emergencies/ten-threats-to-global-health-in-2019. Published March 21, 2019. Accessed November 11, 2020.
- HPV vaccination is safe and effective. Centers for Disease Control and Prevention website. https://www.cdc.gov/hpv/parents/vaccinesafety.html. Updated April 29, 2019. Accessed November 11, 2020.
- How to give a strong recommendation to adult patients who require vaccination. Medscape website. https://www.medscape.com/viewarticle/842874. Published April 16, 2015. Accessed November 11, 2020.
Infant’s COVID-19–related myocardial injury reversed
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FROM JACC CASE REPORTS
Key clinical point: Children presenting with COVID-19 should be tested for heart failure.
Major finding: A 2-month-old infant with COVID-19 had acute but reversible myocardial injury.
Study details: Single case report.
Disclosures: Dr. Sharma, MD, has no relevant financial relationships to disclose.
Source: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Two consecutive negative FUBC results clear S. aureus bacteremia
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.
In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
FROM PEDIATRICS
Challenges in the Management of Peptic Ulcer Disease
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3
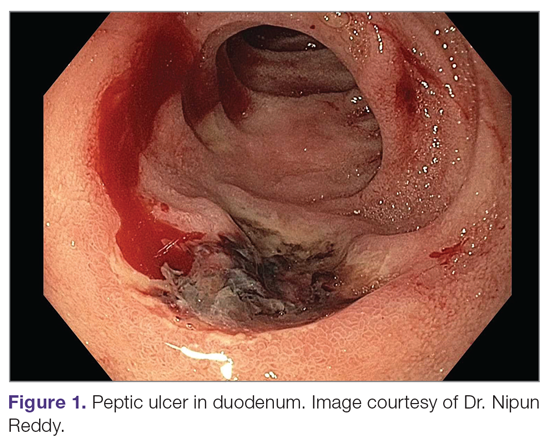
Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15
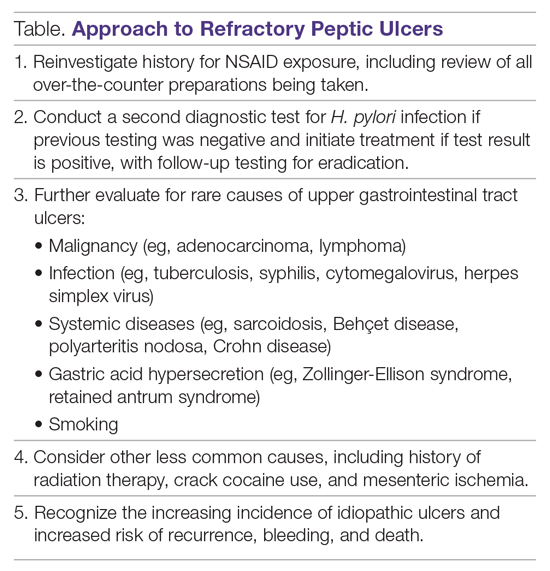
Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35
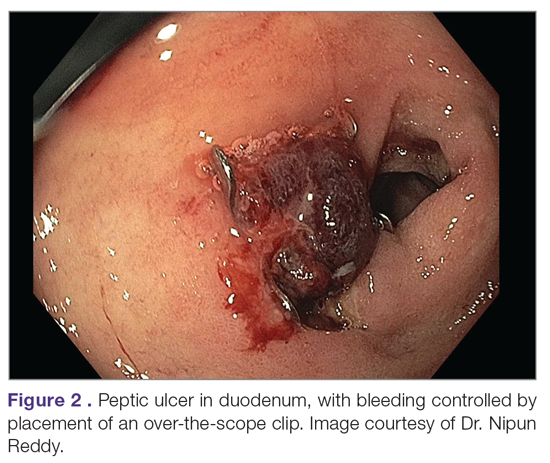
Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3

Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15

Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35

Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3

Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15

Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35

Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.
Three pillars of a successful coronavirus vaccine program in minorities
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
As COVID-19 cases soared to new daily highs across the United States, November 2020 brought some exciting and promising vaccine efficacy results. Currently, the United States has four COVID-19 vaccines in phase 3 trials: the Moderna vaccine (mRNA-1273), the Oxford/AstraZeneca vaccine (AZD1222), Pfizer/BioNTech’s (BNT162), and the Johnson & Johnson vaccine (JNJ-78436735).
While Pfizer/ BioNTech and Moderna received fast-track designation by the Food and Drug Administration, AZD1222 and JNJ-78436735 trials were resumed after a temporary hold. Pfizer/BioNTech and Moderna have also submitted an emergency-use authorization application to the FDA after favorable results from a completed phase 3 clinical trial. The results so far seem promising, with Oxford/AstraZeneca’s combined analysis from different dosing regimens resulting in an average efficacy of 70%. Pfizer/ BioNTech and Moderna have each reported vaccines that are 90% and 95% effective respectively in trials.
However, even with a safe and effective vaccine, there must be an equal emphasis on a successful coronavirus vaccine program’s three pillars in the communities that are the hardest hit: participation in the vaccine trials by minority populations, equitable allocation and distribution of vaccine for minority populations, and immunization uptake by minority populations.
1. Participation in the vaccine trials by minority populations
With a great emphasis on the inclusion of diverse populations, the Moderna vaccine clinical trials gained participation by racial and ethnic minorities. As of Oct. 21, 2020, the Moderna vaccine trial participants were 10% African American, 20% Hispanic, 4% Asian, 63% White, and 3% other.1 Pharmaceutical giant Pfizer also had approximately 42% of overall – and 45% of U.S. – participants from diverse backgrounds. The proportional registration of racially and ethnically diverse participants in other vaccine trials is also anticipated to be challenging.
Though there has been an improvement in minority participation in COVID-19 vaccine trials, it is still below the ideal representation when compared with U.S. census data.2 Ideally, participants in a clinical trial should represent the U.S. population to get a full picture of a medical product’s risks and benefits. However, recruitment rates in clinical trials have remained low among minorities for various reasons. Historically, African Americans make up only 5% of participants in U.S. clinical trials, while they represent 13% of the country’s general population; likewise, Hispanics are also underrepresented.3
The legacy of distrust in the medical system is deep-rooted and is one of the most substantial barriers to clinical trial participation. A plethora of unethical trials and experiments on the African American population have left a lasting impact. The most infamous and widely known was the “Tuskegee Study,” conducted by the United States Public Health Service to “observe the natural history of untreated syphilis” in Black populations. In the study, performed without informed consent, Black men with latent or late syphilis received no treatment, even after penicillin was discovered as a safe and reliable cure for syphilis. This human experimentation lasted for 40 years, resulting in 128 male patients who died from syphilis or its complications, 40 of their spouses infected, and 19 of their children with acquired congenital syphilis.
In another case, the father of modern gynecology, J. Marion Sims, allegedly performed experimental surgeries on enslaved Black women without consent. For more than 4 decades, North Carolina’s statewide eugenics program forcibly sterilized almost 7,600 people, many of whom were Black. Another story of exploitation involves Henrietta Lacks, whose cancer cells are the source of the HeLa cell line, responsible for some of the most important medical advances of all time. Though her cells were commercialized and generated millions for medical researchers, neither Ms. Lacks nor her family knew the cell cultures existed until more than 20 years after her death from cervical cancer. Many years later, victims and families of the Tuskegee experiment, individuals sterilized by the Eugenics Board of North Carolina, and the family of Henrietta Lacks received compensation, and Sims’s statue was taken down in 2018. Not too long ago, many criticized the FDA’s “Exception from Informed Consent policy” for compromising patients’ exercise of autonomy, and concern for overrepresenting African Americans in the U.S. EFIC trials.
Racial disparities in medical treatment and unconscious biases among providers are among the reasons for mistrust and lack of trial participation by minority populations today. Francis Collins, director of the National Institutes of Health, said that recent social upheaval sparked by the death of George Floyd has likely added to feelings of mistrust between minority groups and government or pharmaceutical companies. “Yet we need their participation if this is going to have a meaningful outcome,” he said.
While “Operation Warp Speed” is committed to developing and delivering a COVID-19 vaccine rapidly while adhering to safety and efficacy standards, the challenges to enrolling people from racial and ethnic minorities in trials have been a concern. The political partisanship and ever-shifting stances on widespread COVID-19 testing, use of facemasks, endorsement of unproven drugs for the disease, and accusations against the FDA for delaying human trials for the vaccine have contributed to the skepticism as well. Tremendous pressure for a rushed vaccine with unrealistic timelines, recent holds on AZD1222 and JNJ-78436735 as well as the AZD1222 dosage error during trials have also raised skepticism of the safety and efficacy of vaccine trials.
2. Equitable allocation and distribution of vaccine for minority populations
Enrollment in clinical trials is just a beginning; a more significant challenge would be the vaccine’s uptake when available to the general public. We still lack a consensus on whether it is lawful for race to be an explicit criterion for priority distribution of the COVID-19 vaccine. Recently the Centers for Disease Control and Prevention suggested that the vaccine amount allotted to jurisdictions might be based on critical populations recommended for vaccination by the Advisory Committee on Immunization Practices with input from the National Academies of Sciences, Engineering, and Medicine.
The NASEM framework lays out four-phased vaccine distribution approaches, emphasizing social equity by prioritizing vaccines for geographic areas identified through CDC’s social vulnerability index (SVI) or another more specific index. SVI has been a robust composite marker of minority status and language, household composition and transportation, and housing and disability, and predicted COVID-19 case counts in the United States in several studies. The National Academy of Medicine has also recommended racial minorities receive priority vaccination because they have been hard hit and are “worse off” socioeconomically.
3. Immunization uptake by minority populations
Though minority participation is crucial in developing the vaccine, more transparency, open discussions on ethical distribution, and awareness of side effects are required before vaccine approval or emergency-use authorization. Companies behind the four major COVID-19 vaccines in development have released their trials’ protocols, details on vaccine efficacy, and each product’s makeup to increase acceptance of the vaccine.
According to a recent Pew research study, about half of U.S. adults (51%) now say they would definitely or probably get a vaccine to prevent COVID-19 if it were available today. Nearly as many (49%) say they definitely or probably would not get vaccinated at this time. Intent to get a COVID-19 vaccine has fallen from 72% in May 2020, a 21–percentage point drop, and Black adults were much less likely to say they would get a vaccine than other Americans.3 This is concerning as previous studies have shown that race and ethnicity can influence immune responses to vaccination. There is evidence of racial and ethnic differences in immune response following rubella vaccination, Hib–tetanus toxoid conjugate vaccine, antibody responses to the influenza A virus components of IIV3 or 4, and immune responses after measles vaccination.4-9
On the other hand, significant differences in reporting rates of adverse events after human papillomavirus vaccinations were found in different race and ethnicity groups in the Vaccine Adverse Event Reporting System.10 Thus, there is ample evidence that race and ethnicity affect responsiveness to a vaccine. Inequity in participation in a clinical trial may lead to an ineffective or one with a suboptimal response or even an unsafe vaccine.
When we look at other immunization programs, according to various surveys in recent years, non-Hispanic Blacks have lower annual vaccination rates for flu, pneumonia, and human papillomavirus vaccinations nationally, compared with non-Hispanic White adults.11 It is a cause of concern as a proportion of the population must be vaccinated to reach “community immunity” or “herd immunity” from vaccination. Depending on varying biological, environmental, and sociobehavioral factors, the threshold for COVID-19 herd immunity may be between 55% and 82% of the population.12 Hence, neither a vaccine trial nor an immunization program can succeed without participation from all communities and age groups.
Role of hospitalists
Hospitalists, who give immunizations as part of the hospital inpatient quality reporting program, are uniquely placed in this pandemic. Working on the front lines, we may encounter questions, concerns, rejections, and discussions about the pros and cons of the COVID-19 vaccine from patients.
Investigators at Children’s National Hospital and George Washington University, both in Washington, recently recommended three steps physicians and others can take now to ensure more people get the COVID-19 vaccine when it is available. Engaging frontline health professionals was one of the suggested steps to encourage more people to get the vaccine.13 However, it is imperative to understand that vaccine hesitancy might be an issue for health care providers as well, if concerns for scientific standards and involvement of diverse populations are not addressed.
We are only starting to develop a safe and effective immunization program. We must bring more to unrepresented communities than just vaccine trials. Information, education, availability, and access to the vaccines will make for a successful COVID-19 immunization program.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Moderna. COVE study. 2020 Oct 21. https://www.modernatx.com/sites/default/files/content_documents/2020-COVE-Study-Enrollment-Completion-10.22.20.pdf
2. U.S. Census Bureau. Quick facts: Population estimates, July 1, 2019. https://www.census.gov/quickfacts/fact/table/US/PST045219
3. Pew Research Center. U.S. Public Now Divided Over Whether To Get COVID-19 Vaccine. 2020 Sep 17. https://www.pewresearch.org/science/2020/09/17/u-s-public-now-divided-over-whether-to-get-covid-19-vaccine/
4. Haralambieva IH et al. Associations between race sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine. 2014;32:1946-53.
5. McQuillan GM et al. Seroprevalence of measles antibody in the U.S. population 1999-2004. J Infect Dis. 2007;196:1459–64. doi: 10.1086/522866.
6. Christy C et al. Effect of gender race and parental education on immunogenicity and reported reactogenicity of acellular and whole-cell pertussis vaccines. Pediatrics. 1995;96:584-7.
7. Poland GA et al. Measles antibody seroprevalence rates among immunized Inuit Innu and Caucasian subjects. Vaccine. 1999;17:1525-31.
8. Greenberg DP et al. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76-81.
9. Kurupati R et al. Race-related differences in antibody responses to the inactivated influenza vaccine are linked to distinct prevaccination gene expression profiles in blood. Oncotarget. 2016;7(39):62898-911.
10. Huang J et al. Characterization of the differential adverse event rates by race/ethnicity groups for HPV vaccine by integrating data from different sources. Front Pharmacol. 2018;9:539.
11. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=22
12. Sanche S et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7).
13. American Medical Association. How to ready patients now so they’ll get a COVID-19 vaccine later. 2020 May 27. https://www.ama-assn.org/delivering-care/public-health/how-ready-patients-now-so-they-ll-get-covid-19-vaccine-later
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
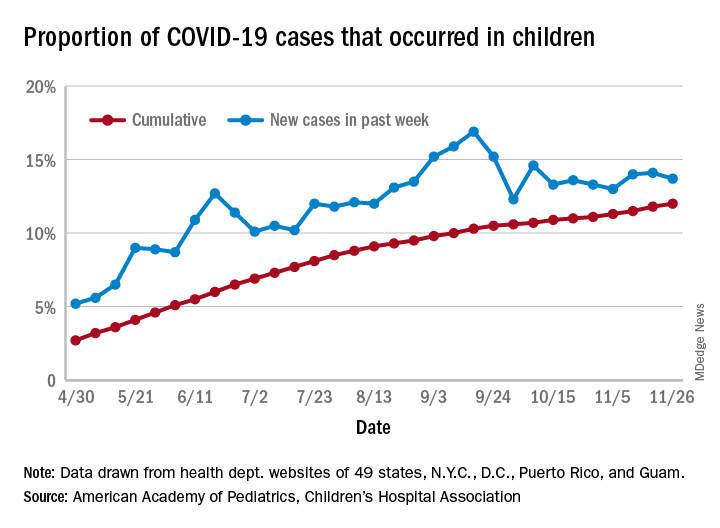
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
Prophylactic HIV treatment in female STI patients is rare
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
reported Kirk D. Henny, PhD, and colleagues of the Centers for Disease Control and Prevention.
In an effort to quantify HIV testing rates as well as the rate of pre-exposure prophylaxis (PrEP) among women with gonorrhea or syphilis, Dr. Henny and his colleagues performed a multivariate logistic regression analysis of 13,074 female patients aged 15-64 diagnosed with a STI in the absence of HIV. Data was pulled in 2017 from the IBM MarketScan commercial and Medicaid insurance databases, and the research was published in Obstetrics & Gynecology.
Medicaid patients were more likely to be tested for HIV
A total of 3,709 patients with commercial insurance were diagnosed with gonorrhea and 1,696 with syphilis. Among those with Medicaid, 6,172 were diagnosed with gonorrhea and 1,497 with syphilis. Medicaid patients diagnosed with either STI were more likely to be tested for HIV than the commercially insured patients. With an adjusted prevalence ratio, patients commercially insured with had either STI were more likely to be tested for HIV than patients who had no STI. Prophylactic treatment rates were similar in both insurance groups: 0.15% in the commercial insurance group and 0.26% in the Medicaid group. No patient from either group who was diagnosed with gonorrhea or syphilis and subsequently tested for HIV received pre-exposure prophylactic (PrEP) treatment.
STI diagnosis is a significant indicator of future HIV
Female patients diagnosed with either STI are more likely to contract HIV, the researchers noted. They cautioned that their findings of low HIV testing rates and the absence of prophylactic treatment means that “these missed opportunities for health care professionals to intervene with female patients diagnosed with gonorrhea or syphilis might have contributed to HIV infections that could have been averted.”
The researchers also pointed out that, in a recent analysis of pharmacy data, prophylactic prescribing for female patients with clinical indications for PrEP was 6.6%, less than one-third the coverage provided to male patients.
Future research should target understanding “individual and contextual factors associated with low HIV testing” and PrEP treatment in female patients, especially those with STIs, Dr. Henny and his colleagues advised.
In a separate interview, Constance Bohon, MD FACOG, observed: “The authors present data to document the low incidence of pre-exposure prophylaxis in women who are at substantial risk of acquiring HIV and possible causes for the low utilization of this treatment.” It is important to identify barriers to diagnosis, counseling, and treatment, she advised.
“Multicenter studies to determine the best methodologies to improve the identification, management, and treatment of these at-risk women need to be done, and the conclusions disseminated to health care providers caring for women,” Dr. Bohon said.
PrEP is an important, simple strategy for reducing HIV transmission
“Pre-exposure prophylaxis has been demonstrated to decrease HIV acquisition in those at risk by up to 90% when taken appropriately,” and yet prescribing rates are extremely low (2%-6%) in at-risk women and especially women of color. These disparities have only grown over time, with prophylactic prescriptions for women at 5% between 2012 and 2017, compared with 68% for men, Catherine S. Eppes, MD, MPH, and Jennifer McKinney, MD, MPH, said in a related editorial commenting on the Research Letter by Dr. Henny and colleagues in Obstetrics & Gynecology (2020 Dec;136[6]:1080-2).
Given the abundant research demonstrating the importance and ease of prescribing PrEP, the question remains: “why does preexposure prophylaxis uptake remain so low, especially for women and women of color? There are three important issues about preexposure prophylaxis raised by this study: the research gap, the implementation gap, and the effect of systemic racism and bias,” noted Dr. Eppes and Dr. McKinney.
Women constitute a significant portion of the population that would benefit from HIV-prevention strategies, yet they continue to be excluded from research, they noted. “Much focus on research into barriers and implementation interventions for preexposure prophylaxis have focused on men who have sex with men and transgender women,” the authors of the editorial wrote.
Most women eligible for treatment would be willing to consider it if they were aware of the option, but numerous studies have cited a lack of awareness, especially among high-risk women of color in the United States, Dr. Eppes and Dr. McKinney noted.
Clinicians also need to add it to their growing checklist of mandatory appointment discussion topics, the editorialists said. “We propose standardized inclusion of preexposure prophylaxis counseling during reproductive healthcare visits. This could be aided through an electronic medical record-based best practice advisory alert. … Standardized order sets with the medication and laboratory studies necessary for safe monitoring could facilitate ease of incorporating into routine visits,” they suggested.
“Preexposure prophylaxis is extremely effective in preventing HIV, is safe, and is the only prevention method that leaves control entirely in the hands of the female partner. As a specialty, we have a responsibility to make sure our patients know about this option,” the editorialists concluded.
The authors had no financial disclosures to report. Dr. Bohon had no conflicts of interest to report.
SOURCE: Henny KD et al. Obstet Gynecol. 2020 Dec;136(6):1083-5.
FROM OBSTETRICS & GYNECOLOGY