User login
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
Expert calls for paradigm shift in lab monitoring of some dermatology drugs
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
FROM ODAC 2021
Is COVID-19 accelerating progress toward high-value care?
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
As Rachna Rawal, MD, was donning her personal protective equipment (PPE), a process that has become deeply ingrained into her muscle memory, a nurse approached her to ask, “Hey, for Mr. Smith, any chance we can time these labs to be done together with his medication administration? We’ve been in and out of that room a few times already.”
As someone who embraces high-value care, this simple suggestion surprised her. What an easy strategy to minimize room entry with full PPE, lab testing, and patient interruptions. That same day, someone else asked, “Do we need overnight vitals?”
COVID-19 has forced hospitalists to reconsider almost every aspect of care. It feels like every decision we make including things we do routinely – labs, vital signs, imaging – needs to be reassessed to determine the actual benefit to the patient balanced against concerns about staff safety, dwindling PPE supplies, and medication reserves. We are all faced with frequently answering the question, “How will this intervention help the patient?” This question lies at the heart of delivering high-value care.
High-value care is providing the best care possible through efficient use of resources, achieving optimal results for each patient. While high-value care has become a prominent focus over the past decade, COVID-19’s high transmissibility without a cure – and associated scarcity of health care resources – have sparked additional discussions on the front lines about promoting patient outcomes while avoiding waste. Clinicians may not have realized that these were high-value care conversations.
The United States’ health care quality and cost crises, worsened in the face of the current pandemic, have been glaringly apparent for years. Our country is spending more money on health care than anywhere else in the world without desired improvements in patient outcomes. A 2019 JAMA study found that 25% of all health care spending, an estimated $760 to $935 billion, is considered waste, and a significant proportion of this waste is due to repetitive care, overuse and unnecessary care in the U.S.1
Examples of low-value care tests include ordering daily labs in stable medicine inpatients, routine urine electrolytes in acute kidney injury, and folate testing in anemia. The Choosing Wisely® national campaign, Journal of Hospital Medicine’s “Things We Do For No Reason,” and JAMA Internal Medicine’s “Teachable Moment” series have provided guidance on areas where common testing or interventions may not benefit patient outcomes.
The COVID-19 pandemic has raised questions related to other widely-utilized practices: Can medication times be readjusted to allow only one entry into the room? Will these labs or imaging studies actually change management? Are vital checks every 4 hours needed?
Why did it take the COVID-19 threat to our medical system to force many of us to have these discussions? Despite prior efforts to integrate high-value care into hospital practices, long-standing habits and deep-seeded culture are challenging to overcome. Once clinicians develop practice habits, these behaviors tend to persist throughout their careers.2 In many ways, COVID-19 was like hitting a “reset button” as health care professionals were forced to rapidly confront their deeply-ingrained hospital practices and habits. From new protocols for patient rounding to universal masking and social distancing to ground-breaking strategies like awake proning, the response to COVID-19 has represented an unprecedented rapid shift in practice. Previously, consequences of overuse were too downstream or too abstract for clinicians to see in real-time. However, now the ramifications of these choices hit closer to home with obvious potential consequences – like spreading a terrifying virus.
There are three interventions that hospitalists should consider implementing immediately in the COVID-19 era that accelerate us toward high-value care. Routine lab tests, imaging, and overnight vitals represent opportunities to provide patient-centered care while also remaining cognizant of resource utilization.
One area in hospital medicine that has proven challenging to significantly change practice has been routine daily labs. Patients on a general medical inpatient service who are clinically stable generally do not benefit from routine lab work.3 Avoiding these tests does not increase mortality or length of stay in clinically stable patients.3 However, despite this evidence, many patients with COVID-19 and other conditions experience lab draws that are not timed together and are done each morning out of “routine.” Choosing Wisely® recommendations from the Society of Hospital Medicine encourage clinicians to question routine lab work for COVID-19 patients and to consider batching them, if possible.3,4 In COVID-19 patients, the risks of not batching tests are magnified, both in terms of the patient-centered experience and for clinician safety. In essence, COVID-19 has pushed us to consider the elements of safety, PPE conservation and other factors, rather than making decisions based solely on their own comfort, convenience, or historical practice.
Clinicians are also reconsidering the necessity of imaging during the pandemic. The “Things We Do For No Reason” article on “Choosing Wisely® in the COVID-19 era” highlights this well.4 It is more important now than ever to decide whether the timing and type of imaging will change management for your patient. Questions to ask include: Can a portable x-ray be used to avoid patient travel and will that CT scan help your patient? A posterior-anterior/lateral x-ray can potentially provide more information depending on the clinical scenario. However, we now need to assess if that extra information is going to impact patient management. Downstream consequences of these decisions include not only risks to the patient but also infectious exposures for staff and others during patient travel.
Lastly, overnight vital sign checks are another intervention we should analyze through this high-value care lens. The Journal of Hospital Medicine released a “Things We Do For No Reason” article about minimizing overnight vitals to promote uninterrupted sleep at night.5 Deleterious effects of interrupting the sleep of our patients include delirium and patient dissatisfaction.5 Studies have shown the benefits of this approach, yet the shift away from routine overnight vitals has not yet widely occurred.
COVID-19 has pressed us to save PPE and minimize exposure risk; hence, some centers are coordinating the timing of vitals with medication administration times, when feasible. In the stable patient recovering from COVID-19, overnight vitals may not be necessary, particularly if remote monitoring is available. This accomplishes multiple goals: Providing high quality patient care, reducing resource utilization, and minimizing patient nighttime interruptions – all culminating in high-value care.
Even though the COVID-19 pandemic has brought unforeseen emotional, physical, and financial challenges for the health care system and its workers, there may be a silver lining. The pandemic has sparked high-value care discussions, and the urgency of the crisis may be instilling new practices in our daily work. This virus has indeed left a terrible wake of destruction, but may also be a nudge to permanently change our culture of overuse to help us shape the habits of all trainees during this tumultuous time. This experience will hopefully culminate in a culture in which clinicians routinely ask, “How will this intervention help the patient?”
Dr. Rawal is clinical assistant professor of medicine, University of Pittsburgh. Dr. Linker is assistant professor of medicine, Mount Sinai Hospital, Icahn School of Medicine at Mount Sinai, New York. Dr. Moriates is associate professor of internal medicine, Dell Medical School at the University of Texas at Austin.
References
1. Shrank W et al. Waste in The US healthcare system. JAMA. 2019;322(15):1501-9.
2. Chen C et al. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-93.
3. Eaton KP et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-9.
4. Cho H et al. Choosing Wisely in the COVID-19 Era: Preventing harm to healthcare workers. J Hosp Med. 2020;15(6):360-2.
5. Orlov N and Arora V. Things we do for no reason: Routine overnight vital sign checks. J Hosp Med. 2020;15(5):272-27.
COVID-19 in children: New cases down for third straight week
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
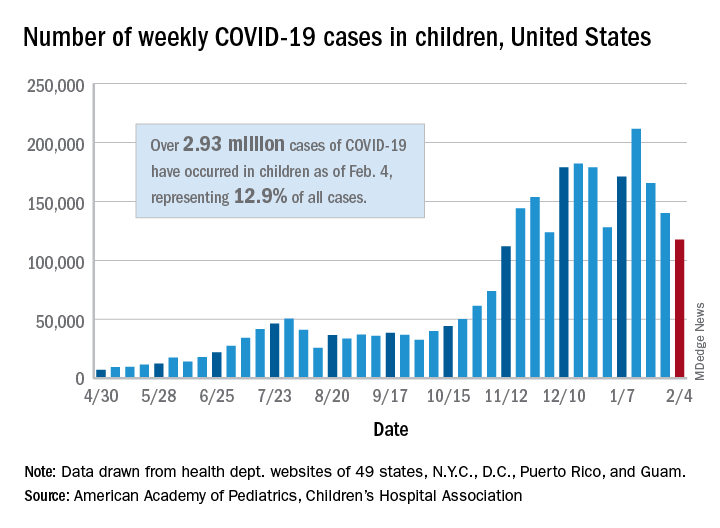
New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
New COVID-19 cases in children dropped for the third consecutive week, even as children continue to make up a larger share of all cases, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

New child cases totaled almost 118,000 for the week of Jan. 29-Feb. 4, continuing the decline that began right after the United States topped 200,000 cases for the only time Jan. 8-14, the AAP and the CHA said in their weekly COVID-19 report.
For the latest week, however, children represented 16.0% of all new COVID-19 cases, continuing a 5-week increase that began in early December 2020, after the proportion had dropped to 12.6%, based on data collected from the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. During the week of Sept. 11-17, children made up 16.9% of all cases, the highest level seen during the pandemic.
The 2.93 million cases that have been reported in children make up 12.9% of all cases since the pandemic began, and the overall rate of pediatric coronavirus infection is 3,899 cases per 100,000 children in the population. Taking a step down from the national level, 30 states are above that rate and 18 are below it, along with D.C., New York City, Puerto Rico, and Guam (New York and Texas are excluded), the AAP and CHA reported.
There were 12 new COVID-19–related child deaths in the 43 states, along with New York City and Guam, that are reporting such data, bringing the total to 227. Nationally, 0.06% of all deaths have occurred in children, with rates ranging from 0.00% (11 states) to 0.26% (Nebraska) in the 45 jurisdictions, the AAP/CHA report shows.
Child hospitalizations rose to 1.9% of all hospitalizations after holding at 1.8% since mid-November in 25 reporting jurisdictions (24 states and New York City), but the hospitalization rate among children with COVID held at 0.8%, where it has been for the last 4 weeks. Hospitalization rates as high as 3.8% were recorded early in the pandemic, the AAP and CHA noted.
U.K. COVID-19 variant doubling every 10 days in the U.S.: Study
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
The SARS-CoV-2 variant first detected in the United Kingdom is rapidly becoming the dominant strain in several countries and is doubling every 10 days in the United States, according to new data.
The findings by Nicole L. Washington, PhD, associate director of research at the genomics company Helix, and colleagues were posted Feb. 7, 2021, on the preprint server medRxiv. The paper has not been peer-reviewed in a scientific journal.
The researchers also found that the transmission rate in the United States of the variant, labeled B.1.1.7, is 30%-40% higher than that of more common lineages.
While clinical outcomes initially were thought to be similar to those of other SARS-CoV-2 variants, early reports suggest that infection with the B.1.1.7 variant may increase death risk by about 30%.
A coauthor of the current study, Kristian Andersen, PhD, told the New York Times , “Nothing in this paper is surprising, but people need to see it.”
Dr. Andersen, a virologist at the Scripps Research Institute in La Jolla, Calif., added that “we should probably prepare for this being the predominant lineage in most places in the United States by March.”
The study of the B.1.1.7 variant adds support for the Centers for Disease Control and Prevention prediction in January that it would dominate by March.
“Our study shows that the U.S. is on a similar trajectory as other countries where B.1.1.7 rapidly became the dominant SARS-CoV-2 variant, requiring immediate and decisive action to minimize COVID-19 morbidity and mortality,” the researchers wrote.
The authors pointed out that the B.1.1.7 variant became the dominant SARS-CoV-2 strain in the United Kingdom within a couple of months of its detection.
“Since then, the variant has been increasingly observed across many European countries, including Portugal and Ireland, which, like the U.K., observed devastating waves of COVID-19 after B.1.1.7 became dominant,” the authors wrote.
“Category 5” storm
The B.1.1.7 variant has likely been spreading between U.S. states since at least December, they wrote.
This news organization reported on Jan. 15 that, as of Jan. 13, the B.1.1.7 variant was seen in 76 cases across 12 U.S. states, according to an early release of the CDC’s Morbidity and Mortality Weekly Report.
As of Feb. 7, there were 690 cases of the B.1.1.7 variant in the US in 33 states, according to the CDC.
Dr. Washington and colleagues examined more than 500,000 coronavirus test samples from cases across the United States that were tested at San Mateo, Calif.–based Helix facilities since July.
In the study, they found inconsistent prevalence of the variant across states. By the last week in January, the researchers estimated the proportion of B.1.1.7 in the U.S. population to be about 2.1% of all COVID-19 cases, though they found it made up about 2% of all COVID-19 cases in California and about 4.5% of cases in Florida. The authors acknowledged that their data is less robust outside of those two states.
Though that seems a relatively low frequency, “our estimates show that its growth rate is at least 35%-45% increased and doubling every week and a half,” the authors wrote.
“Because laboratories in the U.S. are only sequencing a small subset of SARS-CoV-2 samples, the true sequence diversity of SARS-CoV-2 in this country is still unknown,” they noted.
Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, said last week that the United States is facing a “Category 5” storm with the spread of the B.1.1.7 variant as well as the variants first identified in South Africa and Brazil.
“We are going to see something like we have not seen yet in this country,” Dr. Osterholm said recently on NBC’s Meet the Press.
Lead author Nicole L. Washington and many of the coauthors are employees of Helix. Other coauthors are employees of Illumina. Three coauthors own stock in ILMN. The work was funded by Illumina, Helix, the Innovative Genomics Institute, and the New Frontiers in Research Fund provided by the Canadian Institutes of Health Research.
A version of this article first appeared on Medscape.com.
Are diagnosticians chasing COVID-linked zebras and missing horses?
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The emergence of multiple inflammatory syndrome in children (MIS-C) in association with COVID-19 may be complicating the investigation and diagnosis of more common viral and bacterial infections, potentially delaying treatment and prolonging hospital stays.
Two recent articles published online in Hospital Pediatrics provide evidence of this phenomenon. The articles outlined case studies of children who underwent extensive investigation for MIS-C when in fact they had less severe and more common infections. MIS-C is a severe but rare syndrome that involves systemic hyperinflammation with fever and multisystem organ dysfunction similar to that of Kawasaki disease (KD).
In one of the articles, Matthew Molloy, MD, MPH, of the division of pediatric hospital medicine at Cincinnati Children’s Hospital Medical Center, and colleagues aptly asked: “What are we missing in our search for MIS-C?”
E. coli, not SARS-CoV-2
That question arose from a case involving a 3-year-old boy who had a 6-day history of fever and fatigue. Three days earlier, he had tested negative for strep antigen and COVID-19. He had a persistent, high fever, reduced appetite, and reduced urine output and was taken to the ED. On physical examination, there was no rash, skin peeling, redness of the eye or oral mucosa, congestion, rhinorrhea, cough, shortness of breath, chest pain, abdominal pain, nausea, vomiting, or diarrhea.
Urinalysis results and exam findings were suspicious for pyelonephritis. Other findings from an extensive laboratory workup raised the alarm that the boy was suffering from MIS-C as opposed to incomplete KD. After admission to hospital medicine, the cardiology, rheumatology, and infectious disease teams were called in to consult.
Repeat labs were planned for the following day before initiating therapy. On day 2, the child’s urine culture was positive for gram-negative rods, later identified as Escherichia coli. The boy was started on ceftriaxone. Left renal scarring was apparent on ultrasound. The patient’s condition resolved after 36 hours, and he was discharged home with antibiotics.
‘Diagnosis derailed’
Calling this a case of “diagnosis derailed,” the authors noted that, in the pre-COVID era, this child’s signs and symptoms would likely have triggered a more targeted and less costly evaluation for more common infectious and noninfectious causes, including pyelonephritis, absent any physical exam findings consistent with KD.
“However, the patient presented in the midst of the COVID-19 pandemic with growing awareness of a new clinical entity,” Dr. Molloy and colleagues wrote. “Anchored to the patient’s persistent fever, the medical team initiated an extensive, costly, and ultimately unnecessary workup to avoid missing the diagnosis of MIS-C; a not yet well-described diagnosis with potentially severe morbidity.”
Confirmation bias and diagnostic momentum likely contributed to the early focus on MIS-C rather than more common alternatives, the authors acknowledged. The addition of mildly abnormal laboratory data not typically obtained in the evaluation of fever led the team astray. “The diagnosis and definitive treatment may have been made earlier had the focus on concern for MIS-C not been present,” Dr. Molloy said in an interview.
Keeping value in care
The authors recognized that their initial approach to evaluating for MIS-C provided low-value care. “In our desire to not ‘miss’ MIS-C, we were performing costly evaluations that at times produced mildly abnormal, nonspecific results,” they wrote. That triggered a cascade of specialty consultations, follow-up testing, and an unwarranted diagnostic preoccupation with MIS-C.
Determining the extra price tag for the child’s workup would be complex and difficult because there is a difference in the cost to the hospital and the cost to the family, Dr. Molloy said. “However, there are potential cost savings that would be related to making a correct diagnosis in a timely manner in terms of preventing downstream effects from delayed diagnoses.”
Even as clinicians struggle with the challenging SARS-CoV-2 learning curve, Dr. Molloy and associates urged them to continue to strive for high-value care, with an unwavering focus on using only necessary resources, a stewardship the pandemic has shown to be critical.
“The COVID-19 pandemic has been an incredibly stressful time for physicians and for families,” Dr. Molloy said. “COVID-19 and related conditions like MIS-C are new, and we are learning more and more about them every week. These diagnoses are understandably on the minds of physicians and families when children present with fever.” Notwithstanding, the boy’s case underscores the need for clinicians to consider alternate diagnoses and the value of the care provided.
Impact of bias
Dr. Molloy’s group brings home the cognitive biases practitioners often suffer from, including anchoring and confirmation bias and diagnostic momentum, according to J. Howard Smart, MD, chief of pediatrics at Sharp Mary Birch Hospital for Women and Newborns, San Diego, and an assistant clinical professor of pediatrics at University of California, San Diego.
“But it is one thing to recognize these in retrospect and quite another to consider whether they may be happening to you yourself in real time,” he said in an interview. “It is almost as if we need to have a ‘time out,’ where we stop and ask ourselves whether there is something else that could be explaining our patient’s presentation, something that would be more common and more likely to be occurring.”
According to Dr. Smart, who was not involved in Dr. Molloy’s study, the team’s premature diagnostic focus on MIS-C was almost the inverse of what typically happens with KD. “It is usually the case that Kawasaki disease does not enter the differential diagnosis until late in the course of the fever, typically on day 5 or later, when it may have been better to think of it earlier,” he said.
In the second article, Andrea Dean, MD, of the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, and colleagues outlined the cases of five patients aged 8-17 years who were hospitalized in May 2020 for suspected MIS-C. They exhibited inflammatory and other concerning indicators but were eventually discharged with a diagnosis of murine typhus.
This flea-borne infection, most commonly reported in the United States in the southeastern Gulf Coast region, Hawaii, and California, is often associated with a triad of fever, rash, and headache.
Cases have been rising in southern Texas, and Dr. Dean and colleagues postulated that school closures and social distancing may have increased exposure as a result of children spending more time outdoors or with pets. “Alternatively, parental concern for SARS-CoV-2 infection could mean children with symptoms are presenting to care and being referred or admitted to the hospital more frequently due to provider concern for MIS-C,” they wrote.
Cardiac involvement
The most concerning of the five cases in terms of possible MIS-C, Dr. Dean said in an interview, was that of a 12-year-old boy who had fever for 6 days in association with headache, eczematous rash, dry lips, and conjunctivitis. Laboratory tests showed a mildly elevated C-reactive protein level, hyponatremia, and thrombocytopenia, as well as sterile pyuria and mildly elevated prothrombin time. He was treated empirically with doxycycline, and his fever resolved over the next 24 hours.
An echocardiogram at initial evaluation, however, revealed mild dilation of the left anterior descending and right coronary arteries, which led to the administration of intravenous immunoglobulin and aspirin for atypical KD, in contrast to MIS-C. The authors postulated that mild cardiac involvement in disorders other than MIS-C and KD may be underrecognized.
The lesson from these cases, Dr. Dean and associates concluded, is that hospitalists must maintain a wide differential diagnosis when assessing a child with prolonged fever and evidence of systemic inflammation. The CDC stipulates that a diagnosis of MIS-C requires the absence of a plausible alternative diagnosis.
In addition to common viral, bacterial, and noninfectious disorders, a range of regional endemic rickettsial and parasitic infections must be considered as alternative diagnoses to MIS-C. “Many of these diseases cannot be reliably differentiated from MIS-C on presentation, and as community exposure to SARS-CoV-2 grows, hospitalists should be prepared to admit febrile children with evidence of systemic inflammation for brief observation periods to evaluate for MIS-C,” Dr. Dean’s group wrote. In this context, however, empiric treatment for common or even uncommon infectious diseases may avoid overdiagnosis and overtreatment of MIS-C as well as improve patient outcomes.
“We do have specific MIS-C guidelines at our institution,” Dr. Dean said, “but like all institutions, we are dealing with the broad definition of MIS-C according to the World Health Organization and the CDC, which is really the takeaway from this paper.”
More difficult differentiation
Both groups of authors pointed out that, as SARS-CoV-2 spreads throughout a community, a higher percentage of the population will have positive results on antibody testing, and such results will become less useful for differentiating between MIS-C and other conditions.
Despite these series’ cautionary lessons, other experts point to the critical importance of including MIS-C early on in the interest of efficient diagnosis and therapy. “In the cases cited, other pathologies were evaluated for and treated accordingly,” said Kara Gross Margolis, MD, AGAF, an associate professor of pediatrics in the division of pediatric gastroenterology, hepatology, and nutrition at Morgan Stanley Children’s Hospital,New York. “These papers stress the need for a balance that is important, and all potential diagnoses need to be considered, but MIS-C, due to its potential severe consequences, also needs to be on our differential now.”
In her view, as this new high-morbidity entity becomes more widespread during the pandemic, it will be increasingly important to keep this condition on the diagnostic radar.
Interestingly, in a converse example of diagnostic clouding, Dr. Gross Margolis’s group reported (Gastroenterology. 2020 Oct;159[4]:1571-4.e2) last year on a pediatric case series in which the presence of gastrointestinal symptoms in children with COVID-19–related MIS-C muddied the diagnosis by confusing this potentially severe syndrome with more common and less toxic gastrointestinal infections.
According to Dr. Smart, although the two reports don’t offer evidence for a particular diagnostic practice, they can inform the decision-making process. “It may be that we will have enough evidence shortly to say what the best practice is regarding diagnostic evaluation of possible MIS-C cases,” he said. “Until then, we must remember that common things occur commonly, even during a global pandemic.”
Neither of the two reports received any specific funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 in hospitalized children and youth
Clinical syndromes and predictors of disease severity
Clinical questions: What are the demographics and clinical features of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) syndromes, and which admitting demographics and clinical features are predictive of disease severity?
Background: In children, SARS-CoV-2 causes respiratory disease and multisystem inflammatory syndrome in children (MIS-C) as well as other clinical manifestations. The authors of this study chose to address the gap of identifying characteristics for severe disease caused by SARS-CoV-2, including respiratory disease, MIS-C and other manifestations.
Study design: Retrospective and prospective cohort analysis of hospitalized children
Setting: Participating hospitals in Tri-State Pediatric COVID-19 Consortium, including hospitals in New York, New Jersey, and Connecticut.
Synopsis: The authors identified hospitalized patients 22 years old or younger who had a positive SARS-CoV-2 test or met the U.S. Centers for Disease Control and Preventions’ MIS-C case definition. For comparative analysis, patients were divided into a respiratory disease group (based on the World Health Organization’s criteria for COVID-19), MIS-C group or other group (based on the primary reason for hospitalization).
The authors included 281 patients in the study. 51% of the patients presented with respiratory disease, 25% with MIS-C and 25% with other symptoms, including gastrointestinal, or fever. 51% of all patients were Hispanic and 23% were non-Black Hispanic. The most common pre-existing comorbidities amongst all groups were obesity (34%) and asthma (14%).
Patients with respiratory disease had a median age of 14 years while those with MIS-C had a median age of 7 years. Patients more commonly identified as non-Hispanic Black in the MIS-C group vs the respiratory group (35% vs. 18%). Obesity and medical complexity were more prevalent in the respiratory group relative to the MIS-C group. 75% of patients with MIS-C had gastrointestinal symptoms. 44% of respiratory patients had a chest radiograph with bilateral infiltrates on admission, and 18% or respiratory patients required invasive mechanical ventilation. The most common complications in the respiratory group were acute respiratory distress syndrome (17%) and acute kidney injury (11%), whereas shock (35%) and cardiac dysfunction (25%) were the most common complications in the MIS-C group. The median length of stay for all patients was 4 days (IQR 2-8 days).
Patients with MIS-C were more likely to be admitted to the intensive care unit (ICU) but all deaths (7 patients) occurred in the respiratory group. 40% of patients with respiratory disease, 56% of patients with MIS-C, and 6% of other patients met the authors’ definition of severe disease (ICU admission > 48 hours). For the respiratory group, younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph were independent predictors of severe disease based on multivariate analyses. For the MIS-C group, lower absolute lymphocyte count and increasing CRP at admission were independent predictors of severity.
Bottom line: Mortality in pediatric patients is low. Ethnicity and race were not predictive of disease severity in this model, even though 51% of the patients studied were Hispanic and 23% were non-Hispanic Black. Severity of illness for patients with respiratory disease was found to be associated with younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph. Severity of illness in patients with MIS-C was associated with lower absolute lymphocyte count and increasing CRP.
Citation: Fernandes DM, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2020 Nov 14;S0022-3476(20):31393-7. DOI: 10.1016/j.jpeds.2020.11.016.
Dr. Kumar is an assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
Clinical syndromes and predictors of disease severity
Clinical syndromes and predictors of disease severity
Clinical questions: What are the demographics and clinical features of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) syndromes, and which admitting demographics and clinical features are predictive of disease severity?
Background: In children, SARS-CoV-2 causes respiratory disease and multisystem inflammatory syndrome in children (MIS-C) as well as other clinical manifestations. The authors of this study chose to address the gap of identifying characteristics for severe disease caused by SARS-CoV-2, including respiratory disease, MIS-C and other manifestations.
Study design: Retrospective and prospective cohort analysis of hospitalized children
Setting: Participating hospitals in Tri-State Pediatric COVID-19 Consortium, including hospitals in New York, New Jersey, and Connecticut.
Synopsis: The authors identified hospitalized patients 22 years old or younger who had a positive SARS-CoV-2 test or met the U.S. Centers for Disease Control and Preventions’ MIS-C case definition. For comparative analysis, patients were divided into a respiratory disease group (based on the World Health Organization’s criteria for COVID-19), MIS-C group or other group (based on the primary reason for hospitalization).
The authors included 281 patients in the study. 51% of the patients presented with respiratory disease, 25% with MIS-C and 25% with other symptoms, including gastrointestinal, or fever. 51% of all patients were Hispanic and 23% were non-Black Hispanic. The most common pre-existing comorbidities amongst all groups were obesity (34%) and asthma (14%).
Patients with respiratory disease had a median age of 14 years while those with MIS-C had a median age of 7 years. Patients more commonly identified as non-Hispanic Black in the MIS-C group vs the respiratory group (35% vs. 18%). Obesity and medical complexity were more prevalent in the respiratory group relative to the MIS-C group. 75% of patients with MIS-C had gastrointestinal symptoms. 44% of respiratory patients had a chest radiograph with bilateral infiltrates on admission, and 18% or respiratory patients required invasive mechanical ventilation. The most common complications in the respiratory group were acute respiratory distress syndrome (17%) and acute kidney injury (11%), whereas shock (35%) and cardiac dysfunction (25%) were the most common complications in the MIS-C group. The median length of stay for all patients was 4 days (IQR 2-8 days).
Patients with MIS-C were more likely to be admitted to the intensive care unit (ICU) but all deaths (7 patients) occurred in the respiratory group. 40% of patients with respiratory disease, 56% of patients with MIS-C, and 6% of other patients met the authors’ definition of severe disease (ICU admission > 48 hours). For the respiratory group, younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph were independent predictors of severe disease based on multivariate analyses. For the MIS-C group, lower absolute lymphocyte count and increasing CRP at admission were independent predictors of severity.
Bottom line: Mortality in pediatric patients is low. Ethnicity and race were not predictive of disease severity in this model, even though 51% of the patients studied were Hispanic and 23% were non-Hispanic Black. Severity of illness for patients with respiratory disease was found to be associated with younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph. Severity of illness in patients with MIS-C was associated with lower absolute lymphocyte count and increasing CRP.
Citation: Fernandes DM, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2020 Nov 14;S0022-3476(20):31393-7. DOI: 10.1016/j.jpeds.2020.11.016.
Dr. Kumar is an assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
Clinical questions: What are the demographics and clinical features of pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) syndromes, and which admitting demographics and clinical features are predictive of disease severity?
Background: In children, SARS-CoV-2 causes respiratory disease and multisystem inflammatory syndrome in children (MIS-C) as well as other clinical manifestations. The authors of this study chose to address the gap of identifying characteristics for severe disease caused by SARS-CoV-2, including respiratory disease, MIS-C and other manifestations.
Study design: Retrospective and prospective cohort analysis of hospitalized children
Setting: Participating hospitals in Tri-State Pediatric COVID-19 Consortium, including hospitals in New York, New Jersey, and Connecticut.
Synopsis: The authors identified hospitalized patients 22 years old or younger who had a positive SARS-CoV-2 test or met the U.S. Centers for Disease Control and Preventions’ MIS-C case definition. For comparative analysis, patients were divided into a respiratory disease group (based on the World Health Organization’s criteria for COVID-19), MIS-C group or other group (based on the primary reason for hospitalization).
The authors included 281 patients in the study. 51% of the patients presented with respiratory disease, 25% with MIS-C and 25% with other symptoms, including gastrointestinal, or fever. 51% of all patients were Hispanic and 23% were non-Black Hispanic. The most common pre-existing comorbidities amongst all groups were obesity (34%) and asthma (14%).
Patients with respiratory disease had a median age of 14 years while those with MIS-C had a median age of 7 years. Patients more commonly identified as non-Hispanic Black in the MIS-C group vs the respiratory group (35% vs. 18%). Obesity and medical complexity were more prevalent in the respiratory group relative to the MIS-C group. 75% of patients with MIS-C had gastrointestinal symptoms. 44% of respiratory patients had a chest radiograph with bilateral infiltrates on admission, and 18% or respiratory patients required invasive mechanical ventilation. The most common complications in the respiratory group were acute respiratory distress syndrome (17%) and acute kidney injury (11%), whereas shock (35%) and cardiac dysfunction (25%) were the most common complications in the MIS-C group. The median length of stay for all patients was 4 days (IQR 2-8 days).
Patients with MIS-C were more likely to be admitted to the intensive care unit (ICU) but all deaths (7 patients) occurred in the respiratory group. 40% of patients with respiratory disease, 56% of patients with MIS-C, and 6% of other patients met the authors’ definition of severe disease (ICU admission > 48 hours). For the respiratory group, younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph were independent predictors of severe disease based on multivariate analyses. For the MIS-C group, lower absolute lymphocyte count and increasing CRP at admission were independent predictors of severity.
Bottom line: Mortality in pediatric patients is low. Ethnicity and race were not predictive of disease severity in this model, even though 51% of the patients studied were Hispanic and 23% were non-Hispanic Black. Severity of illness for patients with respiratory disease was found to be associated with younger age, obesity, increasing white blood cell count, hypoxia, and bilateral infiltrates on chest radiograph. Severity of illness in patients with MIS-C was associated with lower absolute lymphocyte count and increasing CRP.
Citation: Fernandes DM, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2020 Nov 14;S0022-3476(20):31393-7. DOI: 10.1016/j.jpeds.2020.11.016.
Dr. Kumar is an assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of The Hospitalist.
FROM THE JOURNAL OF PEDIATRICS
CLL, MBL had lower response rates to flu vaccination, compared with healthy adults
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
Immunogenicity of the high-dose influenza vaccine (HD IIV3) in patients with chronic lymphocytic leukemia (CLL) and monoclonal B-cell lymphocytosis (MBL, the precursor state to CLL) was found lower than reported in healthy adults according to a report in Vaccine.
In addition, immunogenicity to influenza B was found to be greater in those patients with MBL, compared with those with CLL.
“Acute and chronic leukemia patients hospitalized with influenza infection document a case fatality rate of 25%-37%,” according to Jennifer A. Whitaker, MD, of the Mayo Clinic, Rochester, Minn., and colleagues in pointing out the importance of their study.
The prospective pilot study assessed the humoral immune responses of patients to the 2013-2014 and 2014-2015 HD IIV3 (Fluzone High-Dose; Sanofi Pasteur), which was administered as part of routine clinical care in 30 patients (17 with previously untreated CLL and 13 with MBL). The median patient age was 69.5 years.
The primary outcomes were seroconversion and seroprotection, as measured by hemagglutination inhibition assay (HAI).
Lower response rate
At day 28 post vaccination, the seroprotection rates for the overall cohort were 19/30 (63.3%) for A/H1N1, 21/23 (91.3%) for A/H3N2, and 13/30 (43.3%) for influenza B. Patients with MBL achieved significantly higher day 28 HAI geometric mean titers (GMT), compared with CLL patients (54.1 vs. 12.1]; P = .01), In addition, MBL patients achieved higher day 28 seroprotection rates against the influenza B vaccine strain virus than did those with CLL (76.9% vs. 17.6%; P = .002). Seroconversion rates for the overall cohort were 3/30 (10%) for A/H1N1; 5/23 (21.7%) for A/H3N2; and 3/30 (10%) for influenza B. No individual with CLL demonstrated seroconversion for influenza B, according to the researchers.
“Our studies reinforce rigorous adherence to vaccination strategies in patients with hematologic malignancy, including those with CLL, given the increased risk of serious complications among those experiencing influenza infection,” the authors stated.
“Even suboptimal responses to influenza vaccination can provide partial protection, reduce hospitalization rates, and/or prevent serious disease complications. Given the recent major issue with novel and aggressive viruses such COVID-19, we absolutely must continue with larger prospective studies to confirm these findings and evaluate vaccine effectiveness in preventing influenza or other novel viruses in these populations,” the researchers concluded.
This study was funded by the National Institutes of Health. Dr. Whitaker reported having no disclosures. Several of the coauthors reported financial relationships with a variety of pharmaceutical and biotechnology companies.
FROM VACCINE
Poor sensitivity for blood cultures drawn after antibiotics
Background: Early antibiotic administration reduces mortality in patients with severe sepsis. Administering antibiotics before blood cultures could potentially decrease time to treatment and improve outcomes, but the diagnostic yield of blood cultures drawn shortly after antibiotics is unknown.
Study design: Prospective, patient-level, pre- and post-study.
Setting: Multicenter study in USA & Canada.
Synopsis: During 2013-2018, 330 adult patients were recruited from seven urban EDs. Patients with severe manifestations of sepsis (spontaneous bacterial peritonitis [SBP] less than 90 mm Hg and lactic acid of 4 or more) had blood cultures drawn before and after empiric antibiotic administration. Blood cultures were positive for one or more microbial pathogens in 31.4% of patients when drawn before antibiotics and in 19.4% of patients when drawn after antibiotics (absolute difference of 12.0% (95% confidence interval, 5.4%-18.6%; P less than .001). The sensitivity of blood cultures after antibiotic administration was 52.9% (95% CI, 43%-63%).
There were several study limitations including: lack of sequential recruitment, lower than expected proportion of bacteremic patients, and variation in blood culture collection. Despite this, the magnitude of the findings are convincing and support current practice.
Bottom line: Continue to obtain blood cultures before antibiotics.
Citation: Cheng MP et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis. Ann Intern Med. 2019 Oct 15;171(8):547-54.
Dr. Waner is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Early antibiotic administration reduces mortality in patients with severe sepsis. Administering antibiotics before blood cultures could potentially decrease time to treatment and improve outcomes, but the diagnostic yield of blood cultures drawn shortly after antibiotics is unknown.
Study design: Prospective, patient-level, pre- and post-study.
Setting: Multicenter study in USA & Canada.
Synopsis: During 2013-2018, 330 adult patients were recruited from seven urban EDs. Patients with severe manifestations of sepsis (spontaneous bacterial peritonitis [SBP] less than 90 mm Hg and lactic acid of 4 or more) had blood cultures drawn before and after empiric antibiotic administration. Blood cultures were positive for one or more microbial pathogens in 31.4% of patients when drawn before antibiotics and in 19.4% of patients when drawn after antibiotics (absolute difference of 12.0% (95% confidence interval, 5.4%-18.6%; P less than .001). The sensitivity of blood cultures after antibiotic administration was 52.9% (95% CI, 43%-63%).
There were several study limitations including: lack of sequential recruitment, lower than expected proportion of bacteremic patients, and variation in blood culture collection. Despite this, the magnitude of the findings are convincing and support current practice.
Bottom line: Continue to obtain blood cultures before antibiotics.
Citation: Cheng MP et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis. Ann Intern Med. 2019 Oct 15;171(8):547-54.
Dr. Waner is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Early antibiotic administration reduces mortality in patients with severe sepsis. Administering antibiotics before blood cultures could potentially decrease time to treatment and improve outcomes, but the diagnostic yield of blood cultures drawn shortly after antibiotics is unknown.
Study design: Prospective, patient-level, pre- and post-study.
Setting: Multicenter study in USA & Canada.
Synopsis: During 2013-2018, 330 adult patients were recruited from seven urban EDs. Patients with severe manifestations of sepsis (spontaneous bacterial peritonitis [SBP] less than 90 mm Hg and lactic acid of 4 or more) had blood cultures drawn before and after empiric antibiotic administration. Blood cultures were positive for one or more microbial pathogens in 31.4% of patients when drawn before antibiotics and in 19.4% of patients when drawn after antibiotics (absolute difference of 12.0% (95% confidence interval, 5.4%-18.6%; P less than .001). The sensitivity of blood cultures after antibiotic administration was 52.9% (95% CI, 43%-63%).
There were several study limitations including: lack of sequential recruitment, lower than expected proportion of bacteremic patients, and variation in blood culture collection. Despite this, the magnitude of the findings are convincing and support current practice.
Bottom line: Continue to obtain blood cultures before antibiotics.
Citation: Cheng MP et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis. Ann Intern Med. 2019 Oct 15;171(8):547-54.
Dr. Waner is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Rheumatologic disease activity an important influencer of COVID-19 death risk
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
People with rheumatic and musculoskeletal diseases (RMDs) who contract the SARS-CoV-2 virus appear more likely to die from COVID-19 if their rheumatologic condition is not being well controlled at the time of their infection.
New data from the COVID-19 Global Rheumatology Alliance (GRA) physician registry reported in Annals of the Rheumatic Diseases have found that the odds of dying from COVID-19 were 87% higher in individuals recorded as having moderate to high disease activity versus those reported to be in remission or having low disease activity.
“I think this really highlights the importance of continuing to appropriately, and actively, treat our patients, and the importance of controlling their disease,” Pedro Machado, MD, PhD, said in an interview. Dr. Machado, an associate professor in rheumatology and muscle diseases at University College London and a consultant rheumatologist at several U.K. hospitals, has been involved in the GRA physician registry from the start, and sits on the GRA steering committee.
Alongside higher disease activity, several other important factors were found to be associated with increased odds of dying from COVID-19 – older age, male gender, and the presence of one or more comorbidities, such as hypertension combined with cardiovascular disease or chronic lung disease.
These demographic and disease-based factors have been linked to an increased risk for COVID-19–related hospitalization before, both in people with RMDs and in the general population, but the latest GRA physician registry data now take that a step further, and link them also to an increased risk for death, together with several other factors more specific to RMDs.
Logging COVID-19 rheumatologic cases
Since the start of the global pandemic, the potential effects that SARS-CoV-2 infection might have on people with RMDs in particular has concerned the rheumatology community. The main worries being that, either because of the underlying RMD itself or to its treatment, there may be immunoregulatory deficits or other risk factors that would make individuals more susceptible to not only infection but also to developing more severe COVID-19 than the general population.
These concerns led to the rapid formation of the GRA and the COVID-19 GRA physician registry in March 2020 to collect and analyze data on adults with rheumatic disease and confirmed or presumptive COVID-19. Entries into the registry are made by or under the direction of rheumatologists, and this is a voluntary process.
“This population cannot ever be entirely representative of the population of patients with rheumatic diseases,” Dr. Machado acknowledged. There will be selection and other biases that affect the reported data. That said, it’s the largest database of reported COVID-19 cases in adult rheumatology patients across the world, with more than 9,000 cases so far included from multiple registries, including those based in Europe and North and South America. Data from one of these – the French RMD cohort – have also recently been published in Annals of the Rheumatic Diseases, showing much the same findings but on a national level.
Hospitalization was the focus of a previous report because “you need large sample sizes” to look at endpoints that occur less frequently. When the first analysis was done, there were around 600 cases from 40 countries in the registry with sufficient data that could be used. Now, with a greater number of recorded cases, factors influencing the risk for death could be examined.
Death rate and risk factors found
Data on 3,729 COVID-19 cases in people with RMDs were included in the current analysis, all recorded in the first few months of the registry being open and up until July 1, 2020. In all, 390 (10.5%) of people died. While this is “clearly higher” than reported in the general population in most countries, the analysis was not designed to calculate a precise estimate.
“It should not be taken as an estimate of the overall death rate among patients with rheumatic diseases and COVID-19,” Dr. Machado and coauthors have been keen to point out.
“Age is always the biggest risk factor,” Dr. Machado explained. “There’s always a gradient: the older the patient, the worse the outcome.”
Indeed, there was a threefold increased risk for death among those aged 66-75 years versus those who were 65 years or younger (odds ratio, 3.00), and a sixfold increased risk for patients older than 75, compared with the younger age group (OR, 6.18).
Having both hypertension and cardiovascular disease was associated with an OR of 1.89, and coexisting chronic lung disease also significantly increased the chances of dying from COVID-19 (OR, 1.68).
Being of male sex was associated with a 46% increased risk for death from COVID-19 versus being of female sex.
The risk for COVID-19 death also rose with the use of corticosteroids. Compared with no steroid use, there was a 69% increased risk for with death at doses of 10 mg or more prednisolone equivalent per day.
“The finding about moderate to high doses of steroids being associated with a worse outcome is consistent with the first report; it was the same for hospitalization,” Dr. Machado observed.
The general consensus on steroid use in the COVID-19 setting is that they should be continued as needed, but at the lowest possible dose, as outlined in provisional recommendations set out by the recently renamed European Alliance of Associations for Rheumatology.
The GRA physician registry findings provide further support for this, suggesting that disease control should be optimized with disease-modifying antirheumatic drugs, ideally without increasing the dose of steroids.
Surprise over sulfasalazine risk
“Taking all medications into account – such as methotrexate, leflunomide, hydroxychloroquine, [tumor necrosis factor] blockers, interleukin-6 blockers, and [Janus kinase] inhibitors – it is quite reassuring because we did not see an association with worse outcome with those drugs overall,” Dr. Machado said.
However, treatment with rituximab (OR, 4.0), sulfasalazine (OR, 3.6), and immunosuppressive agents such as azathioprine, cyclophosphamide, cyclosporine, mycophenolate, or tacrolimus (OR, 2.2), were associated with higher odds of dying from COVID-19 when compared with treatment with methotrexate alone.
The findings for rituximab and immunosuppressant use were perhaps not unexpected, but the possible association between sulfasalazine and COVID-19 death was “a bit intriguing,” Dr. Machado observed. “Sulfasalazine is believed to have low immunosuppressive effect.”
This warrants further investigation, but there are likely a range of confounding factors at play. One could be that people considered to be at higher risk may have been more often prescribed sulfasalazine because it was thought to be less immunosuppressive. Another might be because people taking sulfasalazine were more likely to be smokers, and they were also not advised to protect themselves from exposure to the virus (shielding) during the first wave of the pandemic, at least not in the United Kingdom.
Rituximab caution and vaccination
“Rituximab is a concern,” Dr. Machado acknowledged. “It is a concern that rheumatologists are now aware of and they are addressing, but then it’s a concern for a very specific subgroup of patients.”
While rheumatologists are, and will continue to prescribe it, there will be even more careful consideration over when, in whom, and how to use it during, and possibly even after, the pandemic.
“COVID is here to stay, it will become endemic, and it’s going to be part of our lives like the flu virus is,” Dr. Machado predicted.
Then there is the issue on vaccinating people against COVID-19, should those on rituximab still receive it? The answer is a yes, but, as with other vaccinations it’s all about the timing of when the vaccination is given.
Societies such as the British Society for Rheumatology have already begun to include guidance on this, recommending one of the available COVID-19 vaccines is given at least a month before the next or first dose of rituximab is due. As rituximab is given every few months, with doses sometimes spaced as much as 9 months or even a year apart, this should not be too much of a problem, but it is “better to have the vaccine first,” Dr. Machado said.
Has COVID-19 care improved in RMDs?
In separate research published in The Lancet Rheumatology, April Jorge, MD, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and associates found that the risks of severe COVID-19 outcomes have improved over time, although they still “remain substantial.”
Dr. Jorge and colleagues looked at temporal trends in COVID-19 outcomes in patients with RMDs over the course of the first 6 months of the pandemic in 2020, using data from a large, multicenter, electronic health record network (TriNetX).
They formed two patient cohorts – a late (diagnosed from April 20 to July 20) and an early (diagnosed from January 20 to April 20) cohort – to see if outcomes had improved and discovered lower relative risks among patients in the late cohort for hospitalization (0.67), admission to the ICU (0.56), mechanical ventilation (0.39), acute kidney injury (0.66), renal replacement (0.53), and death (0.39).
“These results are encouraging,” but it’s difficult to match these different populations of patients, Dr. Machado said. “There are always factors that you cannot match for” and were not included in the U.S. analysis.
While there are important caveats in how the analysis was performed and thus in interpreting these data, they do “suggest that one of the reasons why outcomes have improved is because we have become better at treating these patients,” Dr. Machado added.
“Our treatment has improved, and our capacity to treat the complications has improved. We understand better how the disease behaves – we know that they can have thromboembolic complications that we can manage, and we are now able to manage ventilation issues better.”
Moreover, Dr. Machado said that, not only were clinicians more aware of what they should or should not do, there were treatments that were being used routinely or in some cases based on recent clinical trial results. “I think we are indeed treating these patients better.”
The COVID-19 GRA physician registry is financially supported by the American College of Rheumatology and EULAR. Dr. Machado had no relevant conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES











