User login
MRI Is an Important Tool in Identifying Silent JIA
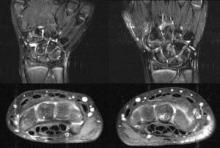
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Coronary CT Angiography Increases Interventions, Doesn't Improve Outcomes
Asymptomatic, low-risk patients with abnormal results on screening coronary computed tomographic angiography tend to undergo further invasive procedures and to initiate medical therapy, but don’t show better outcomes at 18 months than do those who aren’t screened, according to a report published online May 23 in Archives of Internal Medicine.
In addition, patients who receive normal results on CCTA screening tend to discontinue aspirin or statin use. "Whether this will prove to be cost effective or potentially harmful owing to a false sense of reassurance is currently unknown," according to Dr. John W. McEvoy of Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, and his associates.
Both findings demonstrate that physicians and patients sometimes "dramatically change practice based on CCTA findings," so rigorous randomized controlled trials that closely examine the usefulness of this imaging technique are called for, they added.
The investigators "sought to evaluate the downstream implications of CCTA testing" in what they described as the first study to examine the issue in a large matched cohort. They used data from a group of 1,000 asymptomatic, low-risk South Korean adults who had already undergone CCTA as part of a 6-month health-promotion effort at Seoul National University Bundang Hospital, as well as 1,000 matched subjects who declined to have CCTA in that promotion.
The mean age of the study population was 50 years, and 63% of the subjects were men.
The majority, 79%, had normal CCTA results; 21% had abnormal results. This included 7% who were found to have significant coronary artery stenosis.
Significantly more (5.5%) of the subjects who underwent CCTA were referred for further testing, including myocardial single-photon emission CT (SPECT) and coronary angiography, compared with subjects who did not have CCTA (2.2%). Yet the percentage of abnormal SPECT results was the same in both groups.
Revascularization procedures also were more common in the CCTA group (13 subjects) than in the control group (1 subject), "despite their asymptomatic status and low Framingham Risk Score." Twelve patients underwent PCI and 1 underwent CABG in the CCTA group.
However, the rate of major coronary events was extremely low (0.1%) and identical between the two study groups, regardless of CCTA screening status.
"This raises great concern regarding the use of CCTA imaging in low-risk groups," Dr. McEvoy and his associates said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.204]).
"Our data concur with the prevailing notion that screening CCTA does not have a role in low-risk patients."
Subjects who underwent CCTA had increased rates of statin use compared with those who did not, both at 90 days (34% vs. 8%) and 18 months (20% vs. 6%). The odds ratio for statin use among subjects who had abnormal CCTA results was 4.6, compared with subjects who did not undergo CCTA.
Abnormal CCTA results also were associated with an increased use of aspirin. The odds ratio for aspirin use among subjects who had abnormal CCTA findings was 6.8 at 90 days and 4.2 at 18 months, compared with subjects who did not undergo CCTA.
It was interesting to note that the use of both medications decreased markedly over time in both groups. This "serves as a reminder that medication compliance is a complex phenomenon determined only in part by a single health care intervention (such as an imaging test like CCTA)," the investigators noted.
Overall, the study findings "highlight the need to consider the pretest probability of disease before performing imaging tests in patients who may be subsequently exposed to potentially harmful downstream procedures with questionable prognostic benefit," they said.
In an accompanying editorial, Dr. Michael S. Lauer wrote: "The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening."
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes, said Dr. Lauer of the National Heart, Lung, and Blood Institute.
Dr. McEvoy and Dr. Lauer reported no relevant financial disclosures.
"The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening," said Dr. Michael S. Lauer.
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said.
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes.
Dr. Lauer is at the National Heart, Lung, and Blood Institute, Bethesda, Md. He reported no relevant financial disclosures. These remarks were taken from an editorial accompanying the study (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
"The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening," said Dr. Michael S. Lauer.
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said.
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes.
Dr. Lauer is at the National Heart, Lung, and Blood Institute, Bethesda, Md. He reported no relevant financial disclosures. These remarks were taken from an editorial accompanying the study (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
"The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening," said Dr. Michael S. Lauer.
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said.
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes.
Dr. Lauer is at the National Heart, Lung, and Blood Institute, Bethesda, Md. He reported no relevant financial disclosures. These remarks were taken from an editorial accompanying the study (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
Asymptomatic, low-risk patients with abnormal results on screening coronary computed tomographic angiography tend to undergo further invasive procedures and to initiate medical therapy, but don’t show better outcomes at 18 months than do those who aren’t screened, according to a report published online May 23 in Archives of Internal Medicine.
In addition, patients who receive normal results on CCTA screening tend to discontinue aspirin or statin use. "Whether this will prove to be cost effective or potentially harmful owing to a false sense of reassurance is currently unknown," according to Dr. John W. McEvoy of Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, and his associates.
Both findings demonstrate that physicians and patients sometimes "dramatically change practice based on CCTA findings," so rigorous randomized controlled trials that closely examine the usefulness of this imaging technique are called for, they added.
The investigators "sought to evaluate the downstream implications of CCTA testing" in what they described as the first study to examine the issue in a large matched cohort. They used data from a group of 1,000 asymptomatic, low-risk South Korean adults who had already undergone CCTA as part of a 6-month health-promotion effort at Seoul National University Bundang Hospital, as well as 1,000 matched subjects who declined to have CCTA in that promotion.
The mean age of the study population was 50 years, and 63% of the subjects were men.
The majority, 79%, had normal CCTA results; 21% had abnormal results. This included 7% who were found to have significant coronary artery stenosis.
Significantly more (5.5%) of the subjects who underwent CCTA were referred for further testing, including myocardial single-photon emission CT (SPECT) and coronary angiography, compared with subjects who did not have CCTA (2.2%). Yet the percentage of abnormal SPECT results was the same in both groups.
Revascularization procedures also were more common in the CCTA group (13 subjects) than in the control group (1 subject), "despite their asymptomatic status and low Framingham Risk Score." Twelve patients underwent PCI and 1 underwent CABG in the CCTA group.
However, the rate of major coronary events was extremely low (0.1%) and identical between the two study groups, regardless of CCTA screening status.
"This raises great concern regarding the use of CCTA imaging in low-risk groups," Dr. McEvoy and his associates said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.204]).
"Our data concur with the prevailing notion that screening CCTA does not have a role in low-risk patients."
Subjects who underwent CCTA had increased rates of statin use compared with those who did not, both at 90 days (34% vs. 8%) and 18 months (20% vs. 6%). The odds ratio for statin use among subjects who had abnormal CCTA results was 4.6, compared with subjects who did not undergo CCTA.
Abnormal CCTA results also were associated with an increased use of aspirin. The odds ratio for aspirin use among subjects who had abnormal CCTA findings was 6.8 at 90 days and 4.2 at 18 months, compared with subjects who did not undergo CCTA.
It was interesting to note that the use of both medications decreased markedly over time in both groups. This "serves as a reminder that medication compliance is a complex phenomenon determined only in part by a single health care intervention (such as an imaging test like CCTA)," the investigators noted.
Overall, the study findings "highlight the need to consider the pretest probability of disease before performing imaging tests in patients who may be subsequently exposed to potentially harmful downstream procedures with questionable prognostic benefit," they said.
In an accompanying editorial, Dr. Michael S. Lauer wrote: "The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening."
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes, said Dr. Lauer of the National Heart, Lung, and Blood Institute.
Dr. McEvoy and Dr. Lauer reported no relevant financial disclosures.
Asymptomatic, low-risk patients with abnormal results on screening coronary computed tomographic angiography tend to undergo further invasive procedures and to initiate medical therapy, but don’t show better outcomes at 18 months than do those who aren’t screened, according to a report published online May 23 in Archives of Internal Medicine.
In addition, patients who receive normal results on CCTA screening tend to discontinue aspirin or statin use. "Whether this will prove to be cost effective or potentially harmful owing to a false sense of reassurance is currently unknown," according to Dr. John W. McEvoy of Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore, and his associates.
Both findings demonstrate that physicians and patients sometimes "dramatically change practice based on CCTA findings," so rigorous randomized controlled trials that closely examine the usefulness of this imaging technique are called for, they added.
The investigators "sought to evaluate the downstream implications of CCTA testing" in what they described as the first study to examine the issue in a large matched cohort. They used data from a group of 1,000 asymptomatic, low-risk South Korean adults who had already undergone CCTA as part of a 6-month health-promotion effort at Seoul National University Bundang Hospital, as well as 1,000 matched subjects who declined to have CCTA in that promotion.
The mean age of the study population was 50 years, and 63% of the subjects were men.
The majority, 79%, had normal CCTA results; 21% had abnormal results. This included 7% who were found to have significant coronary artery stenosis.
Significantly more (5.5%) of the subjects who underwent CCTA were referred for further testing, including myocardial single-photon emission CT (SPECT) and coronary angiography, compared with subjects who did not have CCTA (2.2%). Yet the percentage of abnormal SPECT results was the same in both groups.
Revascularization procedures also were more common in the CCTA group (13 subjects) than in the control group (1 subject), "despite their asymptomatic status and low Framingham Risk Score." Twelve patients underwent PCI and 1 underwent CABG in the CCTA group.
However, the rate of major coronary events was extremely low (0.1%) and identical between the two study groups, regardless of CCTA screening status.
"This raises great concern regarding the use of CCTA imaging in low-risk groups," Dr. McEvoy and his associates said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.204]).
"Our data concur with the prevailing notion that screening CCTA does not have a role in low-risk patients."
Subjects who underwent CCTA had increased rates of statin use compared with those who did not, both at 90 days (34% vs. 8%) and 18 months (20% vs. 6%). The odds ratio for statin use among subjects who had abnormal CCTA results was 4.6, compared with subjects who did not undergo CCTA.
Abnormal CCTA results also were associated with an increased use of aspirin. The odds ratio for aspirin use among subjects who had abnormal CCTA findings was 6.8 at 90 days and 4.2 at 18 months, compared with subjects who did not undergo CCTA.
It was interesting to note that the use of both medications decreased markedly over time in both groups. This "serves as a reminder that medication compliance is a complex phenomenon determined only in part by a single health care intervention (such as an imaging test like CCTA)," the investigators noted.
Overall, the study findings "highlight the need to consider the pretest probability of disease before performing imaging tests in patients who may be subsequently exposed to potentially harmful downstream procedures with questionable prognostic benefit," they said.
In an accompanying editorial, Dr. Michael S. Lauer wrote: "The report by McEvoy et al. serves as a powerful reminder of the two-edged effects of screening."
"Overdiagnosis is threatening to become an increasingly important public health problem because of the enthusiasm for and proliferation of unproven screening tests," he said (Arch. Intern. Med. 2011 May 23 [doi:10.1001/archinternmed.2011.205]).
"If we are going to prevent an epidemic of coronary pseudodisease, we as a profession will have to muster the courage, imagination, and discipline to design and perform the needed large-scale trials" to determine whether CCTA actually improves patient outcomes, said Dr. Lauer of the National Heart, Lung, and Blood Institute.
Dr. McEvoy and Dr. Lauer reported no relevant financial disclosures.
FROM ARCHIVES OF INTERNAL MEDICINE
Major Finding: The rate of coronary events was extremely low and identical (0.1%) between low-risk subjects who underwent CCTA screening and those who did not. The screening group was much more likely to undergo invasive procedures and initiate medical therapy.
Data Source: A cohort study of 2,000 asymptomatic, low-risk adults in South Korea who participated in a 6-month health-promotion effort that included CCTA screening in half of the subjects. All participants were followed for 18 months for the development of cardiac events.
Disclosures: No financial conflicts of interest were reported.
Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
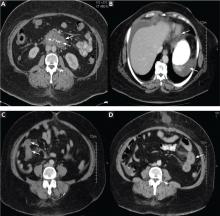
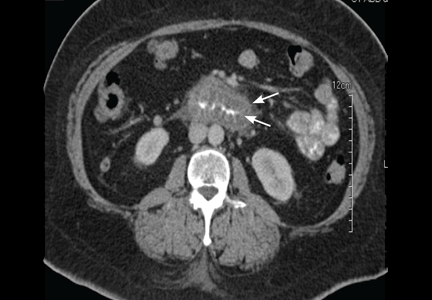
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
KEY POINTS
- Visceral angioedema due to ACE-inhibitor therapy can easily be diagnosed by clinical suspicion and abdominal computed tomography (CT).
- Many physicians are not aware of this condition and so may subject patients to unnecessary invasive procedures, including surgery and endoscopy.
- If a middle-aged woman taking an ACE inhibitor presents with abdominal pain and emesis, the differential diagnosis should include visceral angioedema, and CT should be strongly considered.
Throat Pain
Ganglion Cysts of the Posterior Cruciate Ligament
Oblique Radiographs Compared Favorably With Computed Tomography Images in Assessing Cervical Foraminal Area
MRI Greatly Improves Diagnostic Accuracy for Spondyloarthritis
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
FROM A RHEUMATOLOGY MEETING SPONSORED BY NEW YORK UNIVERSITY
MRI Greatly Improves Diagnostic Accuracy for Spondyloarthritis
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
NEW YORK – When magnetic resonance imaging is used instead of plain x-rays in patients with early inflammatory back pain, the diagnostic accuracy for spondyloarthritis jumps from 25% to 70%, according to Dr. Maxime Dougados, who spoke at a rheumatology meeting sponsored by New York University.
"Seventy-five percent of the time, you cannot make the diagnosis with plain x-rays," said Dr. Dougados, professor of rheumatology at Paris-Descartes University/Cochin Hospital in Paris and the president-elect of EULAR. He presented the Ira Goldstein Memorial Lecture at the meeting, focusing on spondyloarthritis (SpA).
Dr. Dougados presented the as yet unpublished results from the DESIR cohort, a large French national multicenter database of long-term follow-up of 708 patients presenting with early inflammatory back pain that was initiated by the French Society of Rheumatology. Patients were recruited between December 2007 and April 2010 if they had inflammatory back pain lasting more than 3 months and less than 3 years. The group will be followed for 10 years in the ongoing study. At baseline, the mean age was 35 years, 54% were female and 57% were HLA-B27 positive (Joint Bone Spine 2011 March 30 [doi: 10.1016/j.jbspin.2011.01.013]).
Looking at radiological sacroiliac changes, the diagnosis was "obvious" for 25.6% of the cohort, "doubtful" for 21.3%, and "normal" for 53.1%. "These results indicate that at the first clinical visit, the interview is very important to pick up other clinical symptoms," said Dr. Dougados. In fact, about 80% were found to have non-axial clinical manifestations, including articular peripheral involvement, enthesopathy, dactylitis, anterior chest wall pain, uveitis, or psoriasis.
Using MRI, 70% of the cohort were determined to have "obvious" sacroiliitis, about 20% had a "doubtful" diagnosis and about 10% were thought to be "normal."
"These results indicate that you can detect early abnormalities of the sacroiliac joint on MRI even if x-rays are normal," he said.
According to Dr. Dougados, these imaging findings fit well with recent results from the DECLIC study, in which 163 rheumatologists were asked to diagnose 472 patients with early inflammatory back pain, including 161 patients with spondyloarthritis, according to four different sets of criteria. The specificity of the modified New York criteria, which relies on radiographic signs of sacroiliitis (unilateral grade III or bilateral grade II), fell well below that of the modified Amor criteria, the modified ESSG (European Spondyloarthropathy Study Group) criteria, and the ASAS (Assessment of Spondyloarthritis International Society) criteria. The latter three criteria include the option of diagnosing sacroiliitis with MRI.
In the new classification criteria from the ASAS, separate criteria are listed for patients with axial SpA with and without peripheral manifestations and patients with peripheral manifestations only. For axial SpA, one diagnostic pathway requires sacroiliitis on imaging plus one or more SpA feature. Sacroiliitis on MRI is given as much weight as is sacroiliitis on radiographs (Best Pract. Res. Clin. Rheumatol. 2010;24:589-604). The other pathway requires HLA-B27 positivity plus two or more SpA features. In patients with peripheral manifestations only, the requirements include peripheral arthritis, enthesitis or dactylitis plus one or more SpA features, including sacroiliitis on imaging.
Dr. Dougados also spoke about recent findings showing that patients with SpA were more likely to have distinct noninflammatory spinal MRI lesions (known as Fatty Romanus lesions) than were patients with degenerative arthritis or spinal malignancy (Ann. Rheum. Dis. 2010; 69:891-94).
"As MRI is becoming more important, rheumatologists should be trained to interpret MRIs," he said. "You don’t need to be a specialist in radiology."
Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
FROM A RHEUMATOLOGY MEETING SPONSORED BY NEW YORK UNIVERSITY
Vitals: The diagnostic accuracy for spondyloarthritis was 70% when MRI is used.
Data source: A long-term prospective follow-up of 708 patients with early, inflammatory back pain
Disclosures: Dr. Dougados has received grants for research projects and/or honorarium fees for participation at advisory boards/symposiums from Abbott, Bristol-Myers Squibb, Merck, Pfizer, Sanofi, and UCB.
Incidental Findings Common on Post-EVAR Serial CT Scans
LAKE BUENA VISTA, FLA. – Serial computed tomography scans commonly used to monitor patients following endovascular aneurysm repair may be unnecessary after 6 years, according to the findings of a retrospective study of 2,965 scans in 608 EVAR patients.
Furthermore, such scans are more likely to detect a clinically significant incidental finding that warrants further workup than to find a problem with the endograft, Dr. Elizabeth L. Detschelt said at the annual meeting of the Society for Clinical Vascular Surgery.
The average annual rate of detection of EVAR-related findings was 4% (range 2%-5%), which remained constant over the first 6 years of follow-up, and the rate after 6 years was 0%. However, the annual detection rate for new clinically significant incidental findings on these scans was 25% (range 14%-32%), which remained constant for more than 10 years, said Dr. Detschelt of Allegheny General Hospital, Pittsburgh.
On multivariate analysis, predictors of detection of new clinically significant findings included age over 65 years, glomerular filtration rate less than 60 mL/min per 1.73 m2, and tobacco use. No predictors were identified for EVAR-related findings, she noted.
The patients underwent EVAR for infrarenal aneurysm at a single institution between Dec. 1, 1999, and Nov. 30, 2009, and were followed for a mean of 32 months. These results are particularly relevant, Dr. Detschelt said, because risks from repeated scans, which are commonly used for serial imaging in EVAR patients to monitor for endoleak and other problems, are currently a topic of intense debate.
"Recently, the literature has really been inundated with concerns about the cumulative effects of such radiation exposure by these CT scan protocols, and in addition there’s a fair amount of literature to cite a very high rate of incidental findings that we detect on this follow-up with CT protocol," said Dr. Detschelt. Although such findings require follow-up, the literature suggests that this often falls through the cracks, she added.
In this study, EVAR-related findings included endoleak, limb occlusion, and endograft migration. Clinically significant incidental findings were varied, with the most common occurring in the broad categories of genitourinary findings, hepatobiliary findings, hernias, pulmonary neoplasms, and other vascular and cardiac lesions.
Not only do the findings suggest that serial imaging is not needed for EVAR-related concerns after 6 years, but they also underscore the importance of carefully evaluating post-EVAR CT scans for clinically significant incidental findings.
"As the ordering physicians of these CT scans, it is our legal responsibility to ensure that they have appropriate workup, so this is going to mean that not only do we have to look at the scans to assess the status of our aneurysm repair, but we also have to read the radiologist’s report to make sure we’re not missing something," she said.
That’s particularly true for patients who are older, who smoke, and who have a degree of renal insufficiency, she added.
The findings also raise the question of whether post-EVAR patients should undergo monitoring using other imaging techniques such as ultrasound, or whether a less frequent CT scan protocol can be used to reduce patient exposure to radiation and reduce patient costs.
These questions – along with the bigger question of whether it is more prudent to not use CT scans in order to reduce radiation exposure or to continue with CT monitoring to pick up findings that potentially could save or improve lives – require better data to inform decision making, she concluded.
During a discussion period after Dr. Detschelt’s talk, one audience member cautioned against suggesting that CT monitoring be considered for the purpose of detecting incidental nonvascular issues, saying that raises the argument of whether the general population aged 65-75 years should also undergo serial CT scans to find incidental nonvascular issues. He also noted that at his institution, the concerns about serial CT monitoring post EVAR are addressed in part by using duplex ultrasound in the immediate postoperative period, with follow-up by duplex ultrasound in those patients with no problems detected on the initial ultrasound.
He said findings from his experience and others have been published, and show that this is approach is "probably safe and effective." Dr. Detschelt responded that while duplex ultrasound is not used immediately postoperatively at her institution, there has been a move toward using it for long-term follow-up there. At many institutions, however, workforce issues come into play, because the duplex studies are more time intensive and require specially trained vascular staff, she said.
Dr. Detschelt had no disclosures.
LAKE BUENA VISTA, FLA. – Serial computed tomography scans commonly used to monitor patients following endovascular aneurysm repair may be unnecessary after 6 years, according to the findings of a retrospective study of 2,965 scans in 608 EVAR patients.
Furthermore, such scans are more likely to detect a clinically significant incidental finding that warrants further workup than to find a problem with the endograft, Dr. Elizabeth L. Detschelt said at the annual meeting of the Society for Clinical Vascular Surgery.
The average annual rate of detection of EVAR-related findings was 4% (range 2%-5%), which remained constant over the first 6 years of follow-up, and the rate after 6 years was 0%. However, the annual detection rate for new clinically significant incidental findings on these scans was 25% (range 14%-32%), which remained constant for more than 10 years, said Dr. Detschelt of Allegheny General Hospital, Pittsburgh.
On multivariate analysis, predictors of detection of new clinically significant findings included age over 65 years, glomerular filtration rate less than 60 mL/min per 1.73 m2, and tobacco use. No predictors were identified for EVAR-related findings, she noted.
The patients underwent EVAR for infrarenal aneurysm at a single institution between Dec. 1, 1999, and Nov. 30, 2009, and were followed for a mean of 32 months. These results are particularly relevant, Dr. Detschelt said, because risks from repeated scans, which are commonly used for serial imaging in EVAR patients to monitor for endoleak and other problems, are currently a topic of intense debate.
"Recently, the literature has really been inundated with concerns about the cumulative effects of such radiation exposure by these CT scan protocols, and in addition there’s a fair amount of literature to cite a very high rate of incidental findings that we detect on this follow-up with CT protocol," said Dr. Detschelt. Although such findings require follow-up, the literature suggests that this often falls through the cracks, she added.
In this study, EVAR-related findings included endoleak, limb occlusion, and endograft migration. Clinically significant incidental findings were varied, with the most common occurring in the broad categories of genitourinary findings, hepatobiliary findings, hernias, pulmonary neoplasms, and other vascular and cardiac lesions.
Not only do the findings suggest that serial imaging is not needed for EVAR-related concerns after 6 years, but they also underscore the importance of carefully evaluating post-EVAR CT scans for clinically significant incidental findings.
"As the ordering physicians of these CT scans, it is our legal responsibility to ensure that they have appropriate workup, so this is going to mean that not only do we have to look at the scans to assess the status of our aneurysm repair, but we also have to read the radiologist’s report to make sure we’re not missing something," she said.
That’s particularly true for patients who are older, who smoke, and who have a degree of renal insufficiency, she added.
The findings also raise the question of whether post-EVAR patients should undergo monitoring using other imaging techniques such as ultrasound, or whether a less frequent CT scan protocol can be used to reduce patient exposure to radiation and reduce patient costs.
These questions – along with the bigger question of whether it is more prudent to not use CT scans in order to reduce radiation exposure or to continue with CT monitoring to pick up findings that potentially could save or improve lives – require better data to inform decision making, she concluded.
During a discussion period after Dr. Detschelt’s talk, one audience member cautioned against suggesting that CT monitoring be considered for the purpose of detecting incidental nonvascular issues, saying that raises the argument of whether the general population aged 65-75 years should also undergo serial CT scans to find incidental nonvascular issues. He also noted that at his institution, the concerns about serial CT monitoring post EVAR are addressed in part by using duplex ultrasound in the immediate postoperative period, with follow-up by duplex ultrasound in those patients with no problems detected on the initial ultrasound.
He said findings from his experience and others have been published, and show that this is approach is "probably safe and effective." Dr. Detschelt responded that while duplex ultrasound is not used immediately postoperatively at her institution, there has been a move toward using it for long-term follow-up there. At many institutions, however, workforce issues come into play, because the duplex studies are more time intensive and require specially trained vascular staff, she said.
Dr. Detschelt had no disclosures.
LAKE BUENA VISTA, FLA. – Serial computed tomography scans commonly used to monitor patients following endovascular aneurysm repair may be unnecessary after 6 years, according to the findings of a retrospective study of 2,965 scans in 608 EVAR patients.
Furthermore, such scans are more likely to detect a clinically significant incidental finding that warrants further workup than to find a problem with the endograft, Dr. Elizabeth L. Detschelt said at the annual meeting of the Society for Clinical Vascular Surgery.
The average annual rate of detection of EVAR-related findings was 4% (range 2%-5%), which remained constant over the first 6 years of follow-up, and the rate after 6 years was 0%. However, the annual detection rate for new clinically significant incidental findings on these scans was 25% (range 14%-32%), which remained constant for more than 10 years, said Dr. Detschelt of Allegheny General Hospital, Pittsburgh.
On multivariate analysis, predictors of detection of new clinically significant findings included age over 65 years, glomerular filtration rate less than 60 mL/min per 1.73 m2, and tobacco use. No predictors were identified for EVAR-related findings, she noted.
The patients underwent EVAR for infrarenal aneurysm at a single institution between Dec. 1, 1999, and Nov. 30, 2009, and were followed for a mean of 32 months. These results are particularly relevant, Dr. Detschelt said, because risks from repeated scans, which are commonly used for serial imaging in EVAR patients to monitor for endoleak and other problems, are currently a topic of intense debate.
"Recently, the literature has really been inundated with concerns about the cumulative effects of such radiation exposure by these CT scan protocols, and in addition there’s a fair amount of literature to cite a very high rate of incidental findings that we detect on this follow-up with CT protocol," said Dr. Detschelt. Although such findings require follow-up, the literature suggests that this often falls through the cracks, she added.
In this study, EVAR-related findings included endoleak, limb occlusion, and endograft migration. Clinically significant incidental findings were varied, with the most common occurring in the broad categories of genitourinary findings, hepatobiliary findings, hernias, pulmonary neoplasms, and other vascular and cardiac lesions.
Not only do the findings suggest that serial imaging is not needed for EVAR-related concerns after 6 years, but they also underscore the importance of carefully evaluating post-EVAR CT scans for clinically significant incidental findings.
"As the ordering physicians of these CT scans, it is our legal responsibility to ensure that they have appropriate workup, so this is going to mean that not only do we have to look at the scans to assess the status of our aneurysm repair, but we also have to read the radiologist’s report to make sure we’re not missing something," she said.
That’s particularly true for patients who are older, who smoke, and who have a degree of renal insufficiency, she added.
The findings also raise the question of whether post-EVAR patients should undergo monitoring using other imaging techniques such as ultrasound, or whether a less frequent CT scan protocol can be used to reduce patient exposure to radiation and reduce patient costs.
These questions – along with the bigger question of whether it is more prudent to not use CT scans in order to reduce radiation exposure or to continue with CT monitoring to pick up findings that potentially could save or improve lives – require better data to inform decision making, she concluded.
During a discussion period after Dr. Detschelt’s talk, one audience member cautioned against suggesting that CT monitoring be considered for the purpose of detecting incidental nonvascular issues, saying that raises the argument of whether the general population aged 65-75 years should also undergo serial CT scans to find incidental nonvascular issues. He also noted that at his institution, the concerns about serial CT monitoring post EVAR are addressed in part by using duplex ultrasound in the immediate postoperative period, with follow-up by duplex ultrasound in those patients with no problems detected on the initial ultrasound.
He said findings from his experience and others have been published, and show that this is approach is "probably safe and effective." Dr. Detschelt responded that while duplex ultrasound is not used immediately postoperatively at her institution, there has been a move toward using it for long-term follow-up there. At many institutions, however, workforce issues come into play, because the duplex studies are more time intensive and require specially trained vascular staff, she said.
Dr. Detschelt had no disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE SOCIETY FOR CLINICAL VASCULAR SURGERY
Major Finding: The average annual rate of detection of EVAR-related findings was 4% (range 2-5%), which remained constant over the first 6 years of follow-up, and the rate after 6 years was 0%, while the annual detection rate for new clinically significant incidental findings on these scans was 25% (range 14%-32%), which remained constant for more than 10 years.
Data Source: A retrospective study of CT scans in 608 post-EVAR patients.
Disclosures: Dr. Detschelt had no disclosures.
Carotid Intima Thickness Predicts Coronary Events in Rheumatoid Arthritis
NEW YORK – Imaging seems to be the sine qua non of determining cardiovascular disease risk in patients with rheumatoid arthritis.
Dr. Jeffrey D. Greenberg noted that, over the last 10-15 years, epidemiologic studies have shown patients with rheumatoid arthritis (RA) have a twofold increase in the risk of myocardial infarction and stroke and an increase in cardiovascular-related deaths. "An important issue we face is how can we risk stratify our patients to predict who will develop cardiovascular disease? Imaging is a promising area that may help us develop biomarkers of risk or better understand pathophysiological mechanisms of RA."
The need for precise tools with which to predict risk has become more urgent with the recently published findings that carotid ultrasound measurement of carotid intima-media thickness has been found to predict coronary events in patients with RA, independent of traditional cardiovascular risk factors and manifestations of RA.
The study, conducted by Dr. Matthew R. Evans and his associates at Brooke Army Medical Center, Fort Sam Houston, Tex., found that there appears to be a dose-dependent relationship between plaque and risk, with a 2.5-fold increase with unilateral plaque and 4.3-fold increase with bilateral carotid plaque, suggesting that atherosclerosis plays a significant role in acute coronary events in patients with RA (Arthritis Rheum. 2011 [doi:10.1002/art.30265]).
In discussing Dr. Evans’s research at his presentation at a rheumatology meeting sponsored by New York University, Dr. Greenberg said that this is the first study to demonstrate the predictive value of measuring carotid intima-media thickness and plaque for cardiovascular events in RA patients.
In the Evans study, carotid ultrasounds were performed on 636 RA patients as part of the prospective ORALE (Outcome of Rheumatoid Arthritis Longitudinal Evaluation) study. These patients were followed for 3,402 person-years and, during that time, 84 patients experienced 121 new or recurrent acute coronary syndrome (ACS) events, such as myocardial infarction, unstable angina, cardiac arrest, or death from ischemic heart disease. The rate of ACS events was 3.5/100 patient-years for this group. If only those without a prior history of ACS were analyzed, this group had 66 ACS events, with an incidence of 2.1 ACS/100 person-years.
Multivariate analysis of baseline factors associated with incident or recurrent acute coronary syndromes revealed that two markers of atherosclerosis were independent predictors of a subsequent coronary event. Having a past cardiovascular event raised the risk almost threefold (hazard ratio, 2.87 [1.75, 4.73]; P = .001) and carotid intima-media thickness also raised the risk significantly (HR, 1.61 [1.24, 2.08]; P = .001). After substituting carotid plaque for intima-media thickness, the investigators found a 2.5-fold increase in risk for unilateral plaque and almost a sixfold increase in risk for bilateral plaque.
The findings confirmed that traditional demographic and cardiovascular risk factors also predict coronary events as would be expected. These include male gender (HR, 1.94 [1.11, 3.39]; P =.05), diabetes (HR, 2.24 [1.44, 3.50]; P = .001), and hypertension (HR=1.56 [1.00, 2.44]; P =.05). Measures of RA severity, such as swollen joint counts (HR, 1.03 [1.01, 1.06]; P = .01) and cumulative prednisone dose of 20 g (HR, 2.12, [1.32, 3.42]; P = .01), also had predictive value.
Dr. Greenberg, who is director of the Arthritis Translational Registry and Biorepository at NYU Hospital for Joint Diseases, is involved in ongoing studies using advanced MRI and PET techniques to visualize and quantify some of key histologic features of plaque that are most likely to rupture, which he calls "vulnerable plaque." These histologic features include macrophage content, plaque neovascularization, a lipid-rich necrotic core, and a thin fibrous cap.
Dr. Greenberg receives consulting fees from Genentech Inc.
NEW YORK – Imaging seems to be the sine qua non of determining cardiovascular disease risk in patients with rheumatoid arthritis.
Dr. Jeffrey D. Greenberg noted that, over the last 10-15 years, epidemiologic studies have shown patients with rheumatoid arthritis (RA) have a twofold increase in the risk of myocardial infarction and stroke and an increase in cardiovascular-related deaths. "An important issue we face is how can we risk stratify our patients to predict who will develop cardiovascular disease? Imaging is a promising area that may help us develop biomarkers of risk or better understand pathophysiological mechanisms of RA."
The need for precise tools with which to predict risk has become more urgent with the recently published findings that carotid ultrasound measurement of carotid intima-media thickness has been found to predict coronary events in patients with RA, independent of traditional cardiovascular risk factors and manifestations of RA.
The study, conducted by Dr. Matthew R. Evans and his associates at Brooke Army Medical Center, Fort Sam Houston, Tex., found that there appears to be a dose-dependent relationship between plaque and risk, with a 2.5-fold increase with unilateral plaque and 4.3-fold increase with bilateral carotid plaque, suggesting that atherosclerosis plays a significant role in acute coronary events in patients with RA (Arthritis Rheum. 2011 [doi:10.1002/art.30265]).
In discussing Dr. Evans’s research at his presentation at a rheumatology meeting sponsored by New York University, Dr. Greenberg said that this is the first study to demonstrate the predictive value of measuring carotid intima-media thickness and plaque for cardiovascular events in RA patients.
In the Evans study, carotid ultrasounds were performed on 636 RA patients as part of the prospective ORALE (Outcome of Rheumatoid Arthritis Longitudinal Evaluation) study. These patients were followed for 3,402 person-years and, during that time, 84 patients experienced 121 new or recurrent acute coronary syndrome (ACS) events, such as myocardial infarction, unstable angina, cardiac arrest, or death from ischemic heart disease. The rate of ACS events was 3.5/100 patient-years for this group. If only those without a prior history of ACS were analyzed, this group had 66 ACS events, with an incidence of 2.1 ACS/100 person-years.
Multivariate analysis of baseline factors associated with incident or recurrent acute coronary syndromes revealed that two markers of atherosclerosis were independent predictors of a subsequent coronary event. Having a past cardiovascular event raised the risk almost threefold (hazard ratio, 2.87 [1.75, 4.73]; P = .001) and carotid intima-media thickness also raised the risk significantly (HR, 1.61 [1.24, 2.08]; P = .001). After substituting carotid plaque for intima-media thickness, the investigators found a 2.5-fold increase in risk for unilateral plaque and almost a sixfold increase in risk for bilateral plaque.
The findings confirmed that traditional demographic and cardiovascular risk factors also predict coronary events as would be expected. These include male gender (HR, 1.94 [1.11, 3.39]; P =.05), diabetes (HR, 2.24 [1.44, 3.50]; P = .001), and hypertension (HR=1.56 [1.00, 2.44]; P =.05). Measures of RA severity, such as swollen joint counts (HR, 1.03 [1.01, 1.06]; P = .01) and cumulative prednisone dose of 20 g (HR, 2.12, [1.32, 3.42]; P = .01), also had predictive value.
Dr. Greenberg, who is director of the Arthritis Translational Registry and Biorepository at NYU Hospital for Joint Diseases, is involved in ongoing studies using advanced MRI and PET techniques to visualize and quantify some of key histologic features of plaque that are most likely to rupture, which he calls "vulnerable plaque." These histologic features include macrophage content, plaque neovascularization, a lipid-rich necrotic core, and a thin fibrous cap.
Dr. Greenberg receives consulting fees from Genentech Inc.
NEW YORK – Imaging seems to be the sine qua non of determining cardiovascular disease risk in patients with rheumatoid arthritis.
Dr. Jeffrey D. Greenberg noted that, over the last 10-15 years, epidemiologic studies have shown patients with rheumatoid arthritis (RA) have a twofold increase in the risk of myocardial infarction and stroke and an increase in cardiovascular-related deaths. "An important issue we face is how can we risk stratify our patients to predict who will develop cardiovascular disease? Imaging is a promising area that may help us develop biomarkers of risk or better understand pathophysiological mechanisms of RA."
The need for precise tools with which to predict risk has become more urgent with the recently published findings that carotid ultrasound measurement of carotid intima-media thickness has been found to predict coronary events in patients with RA, independent of traditional cardiovascular risk factors and manifestations of RA.
The study, conducted by Dr. Matthew R. Evans and his associates at Brooke Army Medical Center, Fort Sam Houston, Tex., found that there appears to be a dose-dependent relationship between plaque and risk, with a 2.5-fold increase with unilateral plaque and 4.3-fold increase with bilateral carotid plaque, suggesting that atherosclerosis plays a significant role in acute coronary events in patients with RA (Arthritis Rheum. 2011 [doi:10.1002/art.30265]).
In discussing Dr. Evans’s research at his presentation at a rheumatology meeting sponsored by New York University, Dr. Greenberg said that this is the first study to demonstrate the predictive value of measuring carotid intima-media thickness and plaque for cardiovascular events in RA patients.
In the Evans study, carotid ultrasounds were performed on 636 RA patients as part of the prospective ORALE (Outcome of Rheumatoid Arthritis Longitudinal Evaluation) study. These patients were followed for 3,402 person-years and, during that time, 84 patients experienced 121 new or recurrent acute coronary syndrome (ACS) events, such as myocardial infarction, unstable angina, cardiac arrest, or death from ischemic heart disease. The rate of ACS events was 3.5/100 patient-years for this group. If only those without a prior history of ACS were analyzed, this group had 66 ACS events, with an incidence of 2.1 ACS/100 person-years.
Multivariate analysis of baseline factors associated with incident or recurrent acute coronary syndromes revealed that two markers of atherosclerosis were independent predictors of a subsequent coronary event. Having a past cardiovascular event raised the risk almost threefold (hazard ratio, 2.87 [1.75, 4.73]; P = .001) and carotid intima-media thickness also raised the risk significantly (HR, 1.61 [1.24, 2.08]; P = .001). After substituting carotid plaque for intima-media thickness, the investigators found a 2.5-fold increase in risk for unilateral plaque and almost a sixfold increase in risk for bilateral plaque.
The findings confirmed that traditional demographic and cardiovascular risk factors also predict coronary events as would be expected. These include male gender (HR, 1.94 [1.11, 3.39]; P =.05), diabetes (HR, 2.24 [1.44, 3.50]; P = .001), and hypertension (HR=1.56 [1.00, 2.44]; P =.05). Measures of RA severity, such as swollen joint counts (HR, 1.03 [1.01, 1.06]; P = .01) and cumulative prednisone dose of 20 g (HR, 2.12, [1.32, 3.42]; P = .01), also had predictive value.
Dr. Greenberg, who is director of the Arthritis Translational Registry and Biorepository at NYU Hospital for Joint Diseases, is involved in ongoing studies using advanced MRI and PET techniques to visualize and quantify some of key histologic features of plaque that are most likely to rupture, which he calls "vulnerable plaque." These histologic features include macrophage content, plaque neovascularization, a lipid-rich necrotic core, and a thin fibrous cap.
Dr. Greenberg receives consulting fees from Genentech Inc.
FROM A RHEUMATOLOGY MEETING SPONSORED BY NEW YORK UNIVERSITY
Major Finding: Carotid intimal media thickness is an independent predictor of coronary events in patients with RA. Unilateral plaque more than doubled the risk and bilateral plaque increased the risk more than fourfold.
Data Source: Prospective study of 636 patients with RA.
Disclosures: Dr. Greenberg receives consulting fees from Genentech Inc.