User login
Estimated 4,870 future cancers induced by pediatric CT annually
An estimated 4,870 future cancers are induced each year because so many children are exposed to high radiation doses from CT scans, according to a report published online June 10 in JAMA Pediatrics.
Currently, the doses of radiation vary dramatically among radiologists, even for the same type of scan in children of the same age and size. Reducing the highest 25% of radiation doses to the median dose for that type of scan would prevent nearly half of these cancers from developing, said Diana L. Miglioretti, Ph.D., of the biostatistics unit at the Group Health Research Institute and the department of public health sciences at the University of Washington, Seattle, and her associates.
Noting that the ionizing radiation doses delivered by CT are 100-500 times higher than those of conventional radiography and fall within ranges that have been linked to increased cancer risk, Dr. Miglioretti and her colleagues examined time trends in CT imaging of pediatric patients from 1996 to 2010. CT exposure "is especially concerning for children because they are more sensitive to radiation-induced carcinogenesis [than are adults] and have many remaining years of life left for cancer to develop," they noted.
The researchers used data from the HMO Research Network to retrospectively assess randomly selected CT scans in children aged 15 years and younger enrolled in six health care systems covering diverse racial/ethnic and socioeconomic populations across the country. Between 152,419 and 371,095 patients were included for each year, for a total of 4,857,736 child-years of observation.
Radiation doses were calculated for a subset of 744 pediatric CTs of the head, chest, abdomen/pelvis, and spine. These regions together account for more than 95% of all pediatric CT scans. The study population was equally divided among boys and girls, and 29% of the patients were younger than 5 years at the time of their CT scans.
For children aged 5-15 years, the use of CT nearly tripled during the first decade of the study period, from 10.5 scans/1,000 in 1996 to 27.0/1,000 in 2006, then decreased somewhat to 23.9/1,000 in 2010, Dr. Miglioretti and her colleagues reported
The pattern was similar in children aged 0-5 years: CT scanning doubled from 11/1,000 in 1996 to 20/1,000 in 2006, and then dropped somewhat to 15.8/1,000 in 2010. This trend was seen across all six health care systems.
The stabilization and slight decline in pediatric CT scanning may have resulted from increased awareness about the cancer risks from pediatric imaging, particularly given the Image Gently campaign that began in 2007, they said.
Among the anatomic locations for CT scans, increases in the number of scans were greatest for abdominal and pelvic imaging, which happen to deliver the highest doses of radiation. The head was the most commonly scanned region for children of all ages, and head CTs increased by approximately 50% during the study period. Chest CTs also rose by 50%, and the number of spinal scans increased as much as ninefold, depending on the age of the patient.
Thus, the greater use of CT scans overall and the increased use of scans for regions that required higher radiation doses both contributed to the increase in radiation doses to the pediatric population, Dr. Miglioretti and her colleagues said.
However, variability in the radiation dose administered for a given type of scan also accounted for much of the increased exposure, and targeting the highest 25% of doses would yield the largest population benefits, the investigators said.
For example, radiation doses were highest for abdominal/pelvic scans, with a mean effective dose of 14.8 mSv for the oldest and largest children. But, as many as one-fourth of all the children who underwent a single abdominal/pelvic CT scan received a dose of 20 mSv or higher, Dr. Miglioretti and her associates said.
In another example, up to 14% of all head CTs delivered radiation doses of 50 mGy to the brain in a single examination and many children who require head CT undergo multiple such examinations. Reports in the literature cite 50 mGy of exposure as raising the risk of brain cancer by two- to threefold.
The investigators used the data on radiation exposure to estimate the lifetime attributable risks of various cancers nationwide.
One radiation-induced solid cancer was projected to arise from every 300-390 abdominal or pelvic scans among girls and for every 670-760 such scans among boys. For girls, one solid cancer was projected to arise from every 330-480 chest scans and from every 270-800 spinal scans, depending on the age of the child, Dr. Miglioretti and her colleagues said
The projected lifetime attributable risk of leukemia was highest among the youngest children who received head scans, and decreased with increasing age of the patient. For children younger than 5 years who underwent head CT scanning, leukemia was projected to develop in 1.9 patients/10,000 scans, while the rate was only 0.5 cases/10,000 for patients older than 10 years. Abdominal and pelvic CT scans also raised the risk of later leukemia.
"A case of leukemia was projected to result from 1 in 5,250 head scans performed for children younger than 5 years of age and from 1 in 21,160 scans for children 10-14 years of age. The risk of leukemia was 0.8-1.0 cases/10,000 abdomen and pelvic scans and 0.4-0.7 cases/10,000 chest and spine scans," the researchers said.
"Conservatively assuming that 4.25 million pediatric CT scans are performed each year in the United States, 4.0 million CT scans would be of the head, abdomen/pelvis, chest, or spine, based on our observed distribution. If radiation doses from those CT scans parallel our observed dose distributions, approximately 4,870 future cancers could be induced by pediatric CT scans each year," they wrote (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.311]).
"Cases of breast, thyroid, and lung cancers and cases of leukemia account for 68% of projected cancers in exposed girls, whereas cases of brain, lung, and colon cancers and cases of leukemia account for 51% of future cancers in boys."
The number of radiation-induced cancers could be markedly reduced if standard dose-reduction CT protocols were implemented more uniformly across the country. "Reducing the highest 25% of doses within age groups and anatomic regions to the median dose could prevent 2,090 (43%) of these cancers," Dr. Miglioretti and her colleagues said
The benefits of medically necessary CT scans far exceed the increase in cancer risk to a given patient, but CT scans that are not necessary place patients at risk for no reason. Some studies suggest that as many as one-third of pediatric CT scans are not medically necessary and eliminating them would reduce future cancers by another 33%.
"Combining these two strategies could prevent 3,020 (62%) of these cancers," Dr. Miglioretti and her colleagues said.
"It is important for both the referring physician and the radiologist to consider whether the risks of CT exceed the diagnostic value it provides over other tests," they noted.
For example, the indications for most of the abdominal and pelvic scans in this study were pain (40%), possible appendicitis (11%), or possible infection (6%). Ultrasound, which doesn’t use ionizing radiation, is a reasonable alternative for such assessments, with CT reserved for patients whose findings are equivocal or negative on ultrasonography.
Similarly, 23% of the head scans in this study were to evaluate trauma, 22% to assess upper respiratory issues, and 17% to evaluate headache. The use of CT for trauma can be reduced if highly sensitive prediction rules are used to select only the most appropriate patients, and CT has not been established as having value in the pediatric population for assessing headache or sinusitis, the researchers said.
They cautioned that their risk projections "are only estimates based on the best available evidence and are in no way definitive."
This study was supported by the National Cancer Institute. No financial conflicts of interest were reported.
"We can still do more" to decrease the use of unnecessary CT scans in children and to decrease the amount of radiation exposure in those scans that are medically necessary, said Dr. Alan R. Schroeder and Dr. Rita F. Redberg.
"This will require a shift in our culture to become more tolerant of clinical diagnoses without confirmatory imaging, more accepting of ‘watch and wait’ approaches, and less accepting of the ‘another test can’t hurt’ mentality.
"Uncertainty can be unsettling, but it is a small price to pay for protecting ourselves and our children from thousands of preventable cancers," they said.
Dr. Schroeder is in the department of pediatrics at Santa Clara Valley Medical Center, San Jose, Calif. Dr. Redberg is in the department of medicine and women’s cardiovascular services at the University of California, San Francisco. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Miglioretti’s report (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.356]).
"We can still do more" to decrease the use of unnecessary CT scans in children and to decrease the amount of radiation exposure in those scans that are medically necessary, said Dr. Alan R. Schroeder and Dr. Rita F. Redberg.
"This will require a shift in our culture to become more tolerant of clinical diagnoses without confirmatory imaging, more accepting of ‘watch and wait’ approaches, and less accepting of the ‘another test can’t hurt’ mentality.
"Uncertainty can be unsettling, but it is a small price to pay for protecting ourselves and our children from thousands of preventable cancers," they said.
Dr. Schroeder is in the department of pediatrics at Santa Clara Valley Medical Center, San Jose, Calif. Dr. Redberg is in the department of medicine and women’s cardiovascular services at the University of California, San Francisco. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Miglioretti’s report (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.356]).
"We can still do more" to decrease the use of unnecessary CT scans in children and to decrease the amount of radiation exposure in those scans that are medically necessary, said Dr. Alan R. Schroeder and Dr. Rita F. Redberg.
"This will require a shift in our culture to become more tolerant of clinical diagnoses without confirmatory imaging, more accepting of ‘watch and wait’ approaches, and less accepting of the ‘another test can’t hurt’ mentality.
"Uncertainty can be unsettling, but it is a small price to pay for protecting ourselves and our children from thousands of preventable cancers," they said.
Dr. Schroeder is in the department of pediatrics at Santa Clara Valley Medical Center, San Jose, Calif. Dr. Redberg is in the department of medicine and women’s cardiovascular services at the University of California, San Francisco. They reported no financial conflicts of interest. These remarks were taken from their editorial accompanying Dr. Miglioretti’s report (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.356]).
An estimated 4,870 future cancers are induced each year because so many children are exposed to high radiation doses from CT scans, according to a report published online June 10 in JAMA Pediatrics.
Currently, the doses of radiation vary dramatically among radiologists, even for the same type of scan in children of the same age and size. Reducing the highest 25% of radiation doses to the median dose for that type of scan would prevent nearly half of these cancers from developing, said Diana L. Miglioretti, Ph.D., of the biostatistics unit at the Group Health Research Institute and the department of public health sciences at the University of Washington, Seattle, and her associates.
Noting that the ionizing radiation doses delivered by CT are 100-500 times higher than those of conventional radiography and fall within ranges that have been linked to increased cancer risk, Dr. Miglioretti and her colleagues examined time trends in CT imaging of pediatric patients from 1996 to 2010. CT exposure "is especially concerning for children because they are more sensitive to radiation-induced carcinogenesis [than are adults] and have many remaining years of life left for cancer to develop," they noted.
The researchers used data from the HMO Research Network to retrospectively assess randomly selected CT scans in children aged 15 years and younger enrolled in six health care systems covering diverse racial/ethnic and socioeconomic populations across the country. Between 152,419 and 371,095 patients were included for each year, for a total of 4,857,736 child-years of observation.
Radiation doses were calculated for a subset of 744 pediatric CTs of the head, chest, abdomen/pelvis, and spine. These regions together account for more than 95% of all pediatric CT scans. The study population was equally divided among boys and girls, and 29% of the patients were younger than 5 years at the time of their CT scans.
For children aged 5-15 years, the use of CT nearly tripled during the first decade of the study period, from 10.5 scans/1,000 in 1996 to 27.0/1,000 in 2006, then decreased somewhat to 23.9/1,000 in 2010, Dr. Miglioretti and her colleagues reported
The pattern was similar in children aged 0-5 years: CT scanning doubled from 11/1,000 in 1996 to 20/1,000 in 2006, and then dropped somewhat to 15.8/1,000 in 2010. This trend was seen across all six health care systems.
The stabilization and slight decline in pediatric CT scanning may have resulted from increased awareness about the cancer risks from pediatric imaging, particularly given the Image Gently campaign that began in 2007, they said.
Among the anatomic locations for CT scans, increases in the number of scans were greatest for abdominal and pelvic imaging, which happen to deliver the highest doses of radiation. The head was the most commonly scanned region for children of all ages, and head CTs increased by approximately 50% during the study period. Chest CTs also rose by 50%, and the number of spinal scans increased as much as ninefold, depending on the age of the patient.
Thus, the greater use of CT scans overall and the increased use of scans for regions that required higher radiation doses both contributed to the increase in radiation doses to the pediatric population, Dr. Miglioretti and her colleagues said.
However, variability in the radiation dose administered for a given type of scan also accounted for much of the increased exposure, and targeting the highest 25% of doses would yield the largest population benefits, the investigators said.
For example, radiation doses were highest for abdominal/pelvic scans, with a mean effective dose of 14.8 mSv for the oldest and largest children. But, as many as one-fourth of all the children who underwent a single abdominal/pelvic CT scan received a dose of 20 mSv or higher, Dr. Miglioretti and her associates said.
In another example, up to 14% of all head CTs delivered radiation doses of 50 mGy to the brain in a single examination and many children who require head CT undergo multiple such examinations. Reports in the literature cite 50 mGy of exposure as raising the risk of brain cancer by two- to threefold.
The investigators used the data on radiation exposure to estimate the lifetime attributable risks of various cancers nationwide.
One radiation-induced solid cancer was projected to arise from every 300-390 abdominal or pelvic scans among girls and for every 670-760 such scans among boys. For girls, one solid cancer was projected to arise from every 330-480 chest scans and from every 270-800 spinal scans, depending on the age of the child, Dr. Miglioretti and her colleagues said
The projected lifetime attributable risk of leukemia was highest among the youngest children who received head scans, and decreased with increasing age of the patient. For children younger than 5 years who underwent head CT scanning, leukemia was projected to develop in 1.9 patients/10,000 scans, while the rate was only 0.5 cases/10,000 for patients older than 10 years. Abdominal and pelvic CT scans also raised the risk of later leukemia.
"A case of leukemia was projected to result from 1 in 5,250 head scans performed for children younger than 5 years of age and from 1 in 21,160 scans for children 10-14 years of age. The risk of leukemia was 0.8-1.0 cases/10,000 abdomen and pelvic scans and 0.4-0.7 cases/10,000 chest and spine scans," the researchers said.
"Conservatively assuming that 4.25 million pediatric CT scans are performed each year in the United States, 4.0 million CT scans would be of the head, abdomen/pelvis, chest, or spine, based on our observed distribution. If radiation doses from those CT scans parallel our observed dose distributions, approximately 4,870 future cancers could be induced by pediatric CT scans each year," they wrote (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.311]).
"Cases of breast, thyroid, and lung cancers and cases of leukemia account for 68% of projected cancers in exposed girls, whereas cases of brain, lung, and colon cancers and cases of leukemia account for 51% of future cancers in boys."
The number of radiation-induced cancers could be markedly reduced if standard dose-reduction CT protocols were implemented more uniformly across the country. "Reducing the highest 25% of doses within age groups and anatomic regions to the median dose could prevent 2,090 (43%) of these cancers," Dr. Miglioretti and her colleagues said
The benefits of medically necessary CT scans far exceed the increase in cancer risk to a given patient, but CT scans that are not necessary place patients at risk for no reason. Some studies suggest that as many as one-third of pediatric CT scans are not medically necessary and eliminating them would reduce future cancers by another 33%.
"Combining these two strategies could prevent 3,020 (62%) of these cancers," Dr. Miglioretti and her colleagues said.
"It is important for both the referring physician and the radiologist to consider whether the risks of CT exceed the diagnostic value it provides over other tests," they noted.
For example, the indications for most of the abdominal and pelvic scans in this study were pain (40%), possible appendicitis (11%), or possible infection (6%). Ultrasound, which doesn’t use ionizing radiation, is a reasonable alternative for such assessments, with CT reserved for patients whose findings are equivocal or negative on ultrasonography.
Similarly, 23% of the head scans in this study were to evaluate trauma, 22% to assess upper respiratory issues, and 17% to evaluate headache. The use of CT for trauma can be reduced if highly sensitive prediction rules are used to select only the most appropriate patients, and CT has not been established as having value in the pediatric population for assessing headache or sinusitis, the researchers said.
They cautioned that their risk projections "are only estimates based on the best available evidence and are in no way definitive."
This study was supported by the National Cancer Institute. No financial conflicts of interest were reported.
An estimated 4,870 future cancers are induced each year because so many children are exposed to high radiation doses from CT scans, according to a report published online June 10 in JAMA Pediatrics.
Currently, the doses of radiation vary dramatically among radiologists, even for the same type of scan in children of the same age and size. Reducing the highest 25% of radiation doses to the median dose for that type of scan would prevent nearly half of these cancers from developing, said Diana L. Miglioretti, Ph.D., of the biostatistics unit at the Group Health Research Institute and the department of public health sciences at the University of Washington, Seattle, and her associates.
Noting that the ionizing radiation doses delivered by CT are 100-500 times higher than those of conventional radiography and fall within ranges that have been linked to increased cancer risk, Dr. Miglioretti and her colleagues examined time trends in CT imaging of pediatric patients from 1996 to 2010. CT exposure "is especially concerning for children because they are more sensitive to radiation-induced carcinogenesis [than are adults] and have many remaining years of life left for cancer to develop," they noted.
The researchers used data from the HMO Research Network to retrospectively assess randomly selected CT scans in children aged 15 years and younger enrolled in six health care systems covering diverse racial/ethnic and socioeconomic populations across the country. Between 152,419 and 371,095 patients were included for each year, for a total of 4,857,736 child-years of observation.
Radiation doses were calculated for a subset of 744 pediatric CTs of the head, chest, abdomen/pelvis, and spine. These regions together account for more than 95% of all pediatric CT scans. The study population was equally divided among boys and girls, and 29% of the patients were younger than 5 years at the time of their CT scans.
For children aged 5-15 years, the use of CT nearly tripled during the first decade of the study period, from 10.5 scans/1,000 in 1996 to 27.0/1,000 in 2006, then decreased somewhat to 23.9/1,000 in 2010, Dr. Miglioretti and her colleagues reported
The pattern was similar in children aged 0-5 years: CT scanning doubled from 11/1,000 in 1996 to 20/1,000 in 2006, and then dropped somewhat to 15.8/1,000 in 2010. This trend was seen across all six health care systems.
The stabilization and slight decline in pediatric CT scanning may have resulted from increased awareness about the cancer risks from pediatric imaging, particularly given the Image Gently campaign that began in 2007, they said.
Among the anatomic locations for CT scans, increases in the number of scans were greatest for abdominal and pelvic imaging, which happen to deliver the highest doses of radiation. The head was the most commonly scanned region for children of all ages, and head CTs increased by approximately 50% during the study period. Chest CTs also rose by 50%, and the number of spinal scans increased as much as ninefold, depending on the age of the patient.
Thus, the greater use of CT scans overall and the increased use of scans for regions that required higher radiation doses both contributed to the increase in radiation doses to the pediatric population, Dr. Miglioretti and her colleagues said.
However, variability in the radiation dose administered for a given type of scan also accounted for much of the increased exposure, and targeting the highest 25% of doses would yield the largest population benefits, the investigators said.
For example, radiation doses were highest for abdominal/pelvic scans, with a mean effective dose of 14.8 mSv for the oldest and largest children. But, as many as one-fourth of all the children who underwent a single abdominal/pelvic CT scan received a dose of 20 mSv or higher, Dr. Miglioretti and her associates said.
In another example, up to 14% of all head CTs delivered radiation doses of 50 mGy to the brain in a single examination and many children who require head CT undergo multiple such examinations. Reports in the literature cite 50 mGy of exposure as raising the risk of brain cancer by two- to threefold.
The investigators used the data on radiation exposure to estimate the lifetime attributable risks of various cancers nationwide.
One radiation-induced solid cancer was projected to arise from every 300-390 abdominal or pelvic scans among girls and for every 670-760 such scans among boys. For girls, one solid cancer was projected to arise from every 330-480 chest scans and from every 270-800 spinal scans, depending on the age of the child, Dr. Miglioretti and her colleagues said
The projected lifetime attributable risk of leukemia was highest among the youngest children who received head scans, and decreased with increasing age of the patient. For children younger than 5 years who underwent head CT scanning, leukemia was projected to develop in 1.9 patients/10,000 scans, while the rate was only 0.5 cases/10,000 for patients older than 10 years. Abdominal and pelvic CT scans also raised the risk of later leukemia.
"A case of leukemia was projected to result from 1 in 5,250 head scans performed for children younger than 5 years of age and from 1 in 21,160 scans for children 10-14 years of age. The risk of leukemia was 0.8-1.0 cases/10,000 abdomen and pelvic scans and 0.4-0.7 cases/10,000 chest and spine scans," the researchers said.
"Conservatively assuming that 4.25 million pediatric CT scans are performed each year in the United States, 4.0 million CT scans would be of the head, abdomen/pelvis, chest, or spine, based on our observed distribution. If radiation doses from those CT scans parallel our observed dose distributions, approximately 4,870 future cancers could be induced by pediatric CT scans each year," they wrote (JAMA Pediatr. 2013 June 10 [doi:10.1001/jamapediatrics.2013.311]).
"Cases of breast, thyroid, and lung cancers and cases of leukemia account for 68% of projected cancers in exposed girls, whereas cases of brain, lung, and colon cancers and cases of leukemia account for 51% of future cancers in boys."
The number of radiation-induced cancers could be markedly reduced if standard dose-reduction CT protocols were implemented more uniformly across the country. "Reducing the highest 25% of doses within age groups and anatomic regions to the median dose could prevent 2,090 (43%) of these cancers," Dr. Miglioretti and her colleagues said
The benefits of medically necessary CT scans far exceed the increase in cancer risk to a given patient, but CT scans that are not necessary place patients at risk for no reason. Some studies suggest that as many as one-third of pediatric CT scans are not medically necessary and eliminating them would reduce future cancers by another 33%.
"Combining these two strategies could prevent 3,020 (62%) of these cancers," Dr. Miglioretti and her colleagues said.
"It is important for both the referring physician and the radiologist to consider whether the risks of CT exceed the diagnostic value it provides over other tests," they noted.
For example, the indications for most of the abdominal and pelvic scans in this study were pain (40%), possible appendicitis (11%), or possible infection (6%). Ultrasound, which doesn’t use ionizing radiation, is a reasonable alternative for such assessments, with CT reserved for patients whose findings are equivocal or negative on ultrasonography.
Similarly, 23% of the head scans in this study were to evaluate trauma, 22% to assess upper respiratory issues, and 17% to evaluate headache. The use of CT for trauma can be reduced if highly sensitive prediction rules are used to select only the most appropriate patients, and CT has not been established as having value in the pediatric population for assessing headache or sinusitis, the researchers said.
They cautioned that their risk projections "are only estimates based on the best available evidence and are in no way definitive."
This study was supported by the National Cancer Institute. No financial conflicts of interest were reported.
FROM JAMA PEDIATRICS
Major finding: Use of CT scans nearly tripled in children aged 5-15 years and doubled in those aged 0-5 years during the first decade of the study period, and then dropped somewhat from 2006 to 2010 in both age groups.
Data source: A retrospective observational study of time trends in CT scanning of up to 372,000 pediatric patients per year during 1996-2010 in six U.S. health care systems.
Disclosures: This study was supported by the National Cancer Institute. No financial conflicts of interest were reported.
Evaluation and management of premature ventricular complexes
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
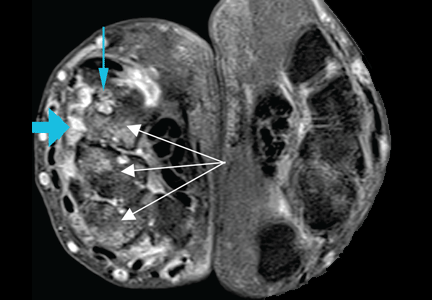
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
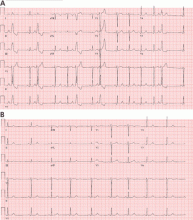
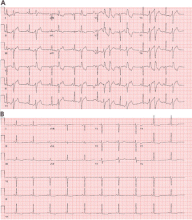
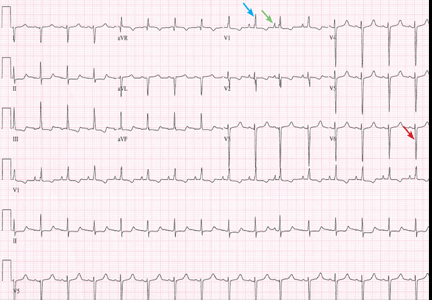
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
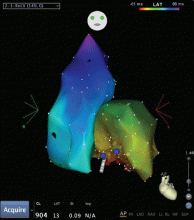
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
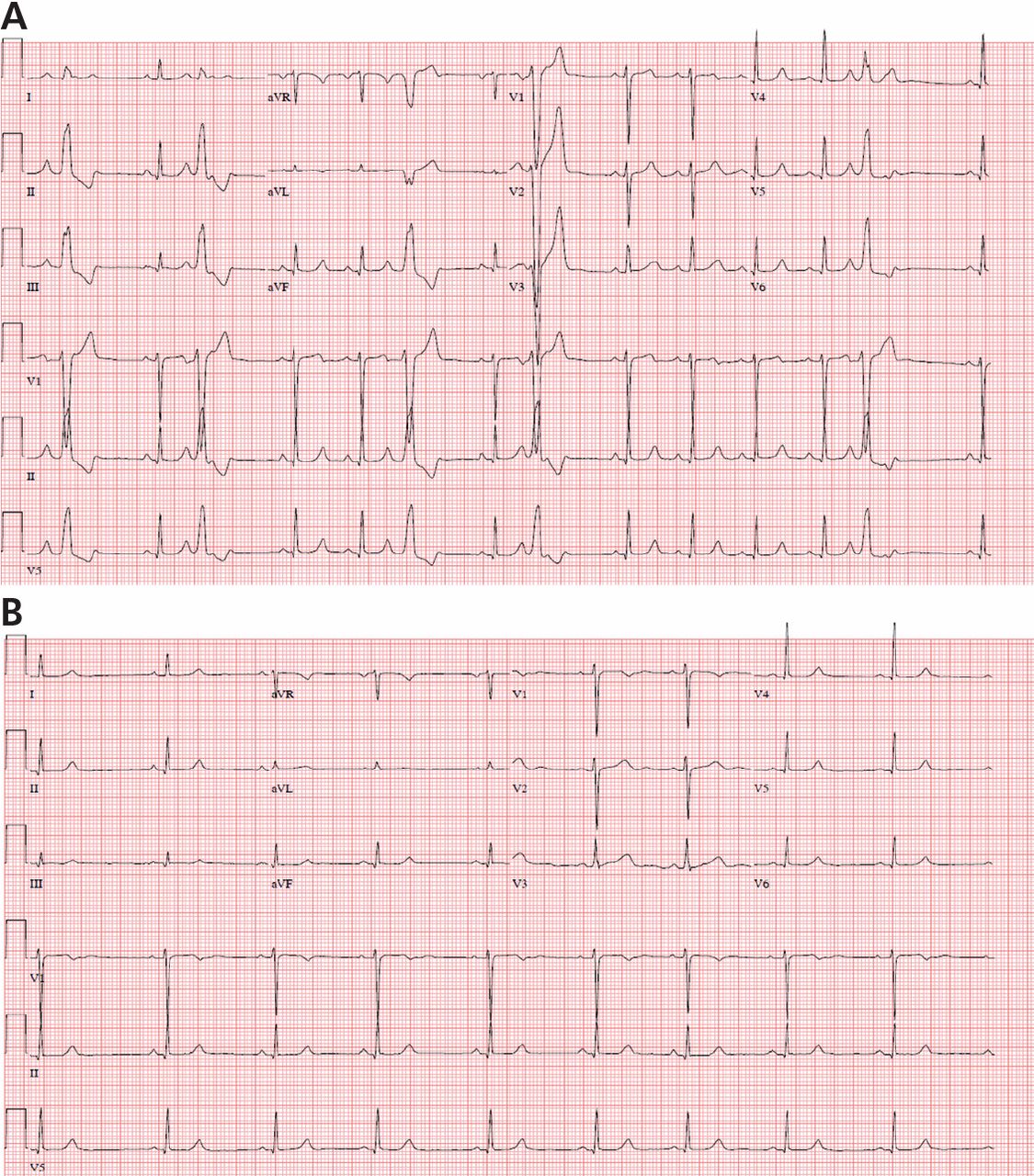
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
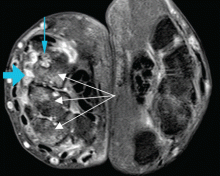
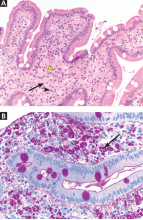
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
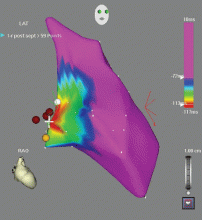
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
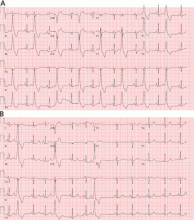
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
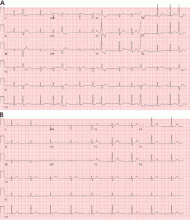
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Premature ventricular complexes (PVCs) are a common cause of palpitations, and are also often detected incidentally on electrocardiography (ECG), ambulatory monitoring, or inpatient telemetry. At the cellular level, ventricular myocytes spontaneously depolarize to create an extra systole that is “out of sync” with the cardiac cycle.
Although nearly everyone has some PVCs from time to time, people vary widely in their frequency of PVCs and their sensitivity to them.1,2 Some patients are exquisitely sensitive to even a small number of PVCs, while others are completely unaware of PVCs in a bigeminal pattern (ie, every other heartbeat). This article will review the evaluation and management of PVCs with a focus on clinical aspects.
DIAGNOSTIC EVALUATION
Personal and family history
Symptoms. The initial history should establish the presence, extent, timing, and duration of symptoms. Patients may use the word “palpitations” to describe their symptoms, but they also describe them as “hard” heartbeats, “chest-thumping,” or as a “catch” or “skipped” heartbeat. Related symptoms may include difficulty breathing, chest pain, fatigue, and dizziness.
The interview should determine whether the symptoms represent a minor nuisance or a major quality-of-life issue to the patient, and whether there are any specific associations or triggers. For example, it is very common for patients to become aware of PVCs at night, particularly in certain positions, such as lying on the left side. Patients often associate PVC symptoms with emotional stress, exercise, or caffeine or stimulant use.
Medication use. An accurate and up-to-date list of prescription medications should be screened for alpha-, beta-, or dopamine-receptor agonist drugs. Similarly, any use of over-the-counter sympathomimetic medications and nonprescription supplements should be elicited, including compounded elixirs or beverages. Many commercially available products designed to treat fatigue or increase alertness contain large doses of caffeine or other stimulants. It is also important to consider the use of illicit substances such as cocaine, amphetamine, methamphetamine, and their derivatives.
The patient’s medical and surgical history should be queried for any known structural heart disease, including coronary artery disease, myocardial infarction, congestive heart failure, valvular heart disease, congenital heart disease, and heritable conditions such as hypertrophic cardiomyopathy, prolonged QT syndromes, or other channel disorders. Pulmonary disorders such as sarcoidosis, pulmonary hypertension, or obstructive sleep apnea are also relevant. Similarly, it is important to identify endocrine disorders, including thyroid problems, sex hormone abnormalities, or adrenal gland conditions.
A careful family history should include any instance of sudden death in first-degree relatives, any heritable cardiac conditions, or coronary artery disease at an early age.
Physical examination
The physical examination should focus on findings that suggest underlying structural heart disease. Findings suggestive of congestive heart failure include elevated jugular venous pressures, abnormal cardiac sounds, pulmonary rales, abnormal arterial pulses, or peripheral edema. A murmur or a pathologic heart sound should raise suspicion of valvular or congenital heart disease when present in a young patient.
Inspection and palpation of the thyroid can reveal a related disorder. Obvious skin changes or neurologic findings can similarly reveal a systemic and possibly related clinical disorder that can have cardiac manifestations (eg, muscular dystrophy).
Electrocardiography, Holter monitoring, and other monitoring
Assessment of the cardiac rhythm includes 12-lead ECG and ambulatory Holter monitoring, typically for 24 or 48 hours.
Holter monitoring provides a continuous recording, usually in at least two or three leads. Patients are given a symptom journal or are asked to keep a diary of symptoms experienced during the monitoring period. The monitor is worn underneath clothing and is returned for download upon completion. Technicians process the data with the aid of computer software, and the final output is reviewed and interpreted by a cardiologist or cardiac electrophysiologist.
Holter monitoring for at least 24 hours is a critical step in assessing any patient with known or suspected PVCs, as it can both quantify the total burden of ventricular ectopy and identify the presence of any related ventricular tachycardia. In addition, it can detect additional supraventricular arrhythmias or bradycardia during the monitoring period. The PVC burden is an important measurement; it is expressed as the percentage of heartbeats that were ventricular extrasystoles during the monitoring period.
Both ECG and Holter monitoring are limited in that they are only snapshots of the rhythm during the period when a patient is actually hooked up. Many patients experience PVCs in clusters every very few days or weeks. Such a pattern is unlikely to be detected by a single ECG or 24- or 48-hour Holter monitoring.
A 30-day ambulatory event monitor (also known as a wearable loop recorder) is an important diagnostic tool in these scenarios. The concept is very similar to that of Holter monitoring, except that the device provides a continuous loop recording of the cardiac rhythm that is digitally stored in clips when the patient activates the device. Some wearable loop recorders also have auto-save features for heart rates falling outside of a programmed range.
Mobile outpatient cardiac telemetry is the most comprehensive form of noninvasive rhythm monitoring available. This is essentially the equivalent of continuous inpatient cardiac telemetry, but in a patient who is not hospitalized. It is a wearable ambulatory device providing continuous recordings, real-time automatic detections, and patient-activated symptom recordings. It can be used for up to 6 weeks. Advantages include detection and quantification of asymptomatic events, and real-time transmissions that the physician can act upon. The major disadvantage is cost, including coverage denial by many third-party payers.
This test is rarely indicated as part of a PVC evaluation and is typically ordered only by a cardiologist or cardiac electrophysiologist.
Noninvasive cardiac evaluation
Surface echocardiography is indicated to look for overt structural heart disease and can reliably detect abnormalities in cardiac chamber size, wall thickness, and function. Valvular heart disease is concomitantly identified by two-dimensional imaging as well as by color Doppler. The finding of significant structural heart disease in conjunction with PVCs should prompt a cardiology referral, as this carries significant prognostic implications.3–5
Exercise treadmill stress testing is appropriate for patients who experience PVCs with exercise or for whom an evaluation for coronary artery disease is indicated. The expected finding would be an increase in PVCs or ventricular tachycardia with exercise or in the subsequent recovery period. Exercise testing can be combined with either echocardiographic or nuclear perfusion imaging to evaluate the possibility of myocardial ischemia. For patients unable to exercise, pharmacologic stress testing with dobutamine or a vasodilator agent can be performed.
Advanced noninvasive cardiac imaging— such as computed tomography, magnetic resonance imaging, or positron-emission tomography—should be reserved for specific clinical indications such as congenital heart disease, suspected cardiac sarcoidosis, and infiltrative heart disease, and for specific cardiomyopathies, such as hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. For example, frequent PVCs with a left bundle branch block morphology and superior axis raise the concern for a right ventricular disorder and may prompt cardiac magnetic resonance imaging for either arrhythmogenic right ventricular cardiomyopathy or sarcoidosis.
PVCs WITHOUT STRUCTURAL HEART DISEASE
Outflow tract PVCs and ventricular tachycardia
The right or left ventricular outflow tracts, or the epicardial tissue immediately adjacent to the aortic sinuses of Valsalva are the most common sites of origin for ventricular ectopy in the absence of structural heart disease.6–9 Affected cells often demonstrate a triggered activity mechanism due to cyclic adenosine monophosphate-mediated and calcium-dependent delayed after-depolarizations.7,8
Most of these foci are in the right ventricular outflow tract, producing a left bundle branch block morphology with an inferior axis (positive R waves in limb leads II, III, and aVF) and typical precordial R-wave transition in V3 and V4 (Figure 1). A minority are in the left ventricular outflow tract, producing a right bundle branch block with an inferior axis pattern, or in the aortic sinuses with a left bundle branch block pattern but with early precordial R transition in V2 and V3.
A study in 122 patients showed that right and left outflow tract arrhythmias had similar electrophysiologic properties and pharmacologic sensitivities, providing evidence for shared mechanisms possibly due to the common embryologic origin of these structures.9
Such arrhythmias are typically catecholamine-sensitive and are sometimes inducible with burst pacing in the electrophysiology laboratory. The short ventricular coupling intervals can promote intracellular calcium overload in the affected cells, leading to triggered activity.
Therefore, outflow tract PVCs and ventricular tachycardia are commonly encountered clinically during exercise and, to an even greater extent, in the postexercise cool-down period. Similarly, they can be worse during periods of emotional stress or fatigue, when the body’s endogenous catecholamine production is elevated. However, it is worthwhile to note that there are exceptions to this principle in which faster sinus rates seem to overdrive the PVCs in some patients, causing them to become paradoxically more frequent at rest, or even during sleep.
Outflow tract PVCs can be managed medically with beta-blockers, nondihydropyridine calcium channel blockers (verapamil or diltiazem), or, less commonly, class IC drugs such as flecainide. They are also highly curable by catheter ablation (Figure 2), with procedure success rates greater than 90%.9.10
However, a subset of outflow tract PVCs nested deep in a triangle of epicardial tissue between the right and left endocardial surface and underneath the left main coronary artery can be challenging. This region has been labeled the left ventricular summit, and is shielded from ablation by an epicardial fat pad in the adjacent pericardial space.11 Ablation attempts made from the right and left endocardial surfaces as well as the epicardial surface (pericardial space) sometimes cannot adequately penetrate the tissue deep enough to reach the originating focus deep within this triangle. While ablation cannot always fully eliminate the PVC, ablation from more than one of the sites listed can generally reduce its burden, often in combination with suppressive medical therapy (Figure 3).
Fascicular PVCs
Fascicular PVCs originate from within the left ventricular His-Purkinje system12 and produce a right bundle branch block morphology with either an anterior or posterior hemiblock pattern (Figure 4). Exit from the posterior fascicle causes an anterior hemiblock pattern, and exit from the anterior fascicle a posterior hemiblock pattern. Utilization of the rapidly conducting His-Purkinje system gives these PVCs a very narrow QRS duration, sometimes approaching 120 milliseconds or shorter. This occasionally causes them to be mistaken for aberrantly conducted supraventricular beats. Such spontaneous PVCs are commonly associated with both sustained and nonsustained ventricular tachycardia and are usually sensitive to verapamil.13
Special issues relating to mapping and catheter ablation of fascicular arrhythmias involve the identification of Purkinje fiber potentials and associated procedural diagnostic maneuvers during tachycardia.14
Other sites for PVCs
Other sites of origin for PVCs in the absence of structural heart disease include ventricular tissue adjacent to the aortomitral continuity,15 the tricuspid annulus,16 the mitral valve annulus, 17 papillary muscles,18 and other Purkinje-adjacent structures such as left ventricular false tendons.19 An example of a papillary muscle PVC is shown in Figures 5 and 6.
Curable by catheter ablation
Any of these PVCs can potentially be cured by catheter ablation when present at a sufficient burden to allow for activation mapping in the electrophysiology laboratory. The threshold for offering ablation varies among operators, but is generally around 10% or greater. Pacemapping is a technique applied in the electrophysiology laboratory when medically refractory symptomatic PVCs occurring at a lower burden require ablation.
PVCs WITH AN UNDERLYING CARDIAC CONDITION
Coronary artery disease
Tissue injury and death caused by acute myocardial infarction has long been recognized as a common cause of spontaneous ventricular ectopy attributed to infarct border zones of ischemic or hibernating myocardium.20,21
Suppression has not been associated with improved outcomes, as shown for class IC drugs in the landmark Cardiac Arrhythmia Suppression Trial (CAST),22 or in the amiodarone treatment arm of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II).23 Therefore, treatment of ventricular ectopy in this patient population is usually symptom-driven unless there is hemodynamic intolerance, tachycardia-related cardiomyopathy, or a very high burden of PVCs in a patient who may be at risk of developing tachycardia-related cardiomyopathy. Antiarrhythmic drug treatment, when required, usually involves beta-blockers or class III medications such as sotalol or amiodarone.
Nonischemic dilated cardiomyopathy
This category includes patients with a wide variety of disease states including valvular heart disease, lymphocytic and other viral myocarditis, cardiac sarcoidosis, amyloidosis and other infiltrative diseases, familial conditions, and idiopathic dilated cardiomyopathy (ie, etiology unknown). Although it is a heterogeneous group, a common theme is that PVCs in this patient cohort may require epicardial mapping and ablation.24 Similarly, epicardial PVCs and ventricular tachycardia cluster at the basal posterolateral left ventricle near the mitral annulus, for unclear reasons.25
While specific criteria have been published, an epicardial focus is suggested by slowing of the initial QRS segment, pseudo-delta waves, a wider overall QRS, and Q waves in limb lead I.26
Treatment is symptom-driven unless the patient has a tachycardia-related cardiomyopathy or a high burden associated with the risk for its development. Antiarrhythmic drug therapy, when required, typically involves a beta-blocker or a class III drug such as sotalol or amiodarone. Sotalol is used in this population but has limited safety data and should be used cautiously in patients without an implantable cardioverter-defibrillator.
Arrhythmogenic right ventricular cardiomyopathy
Spontaneous ventricular ectopy and tachycardia are common, if not expected, in patients with this heritable autosomal dominant disorder. This condition is progressive and associated with the risk of sudden cardiac death. Criteria for diagnosis were established in 2010, and patients with suspected arrhythmogenic right ventricular cardiomyopathy often undergo cardiac magnetic resonance imaging.27 Diagnostic findings include fibro-fatty tissue replacement, which usually starts in the right ventricle but can progress to involve the left ventricle. PVCs and ventricular tachycardia can involve the right ventricular free wall and are often epicardial.
Catheter ablation is usually palliative, as future arrhythmias are expected. Many patients with this condition require an implantable cardioverter-defibrillator for prevention of sudden cardiac death, and some go on to cardiac transplantation as the disease progresses and ventricular arrhythmias become incessant.
Other conditions
Spontaneous ventricular ectopy is common in other heritable and acquired cardiomyopathies including hypertrophic cardiomyopathy and in infiltrative or inflammatory disorders such as cardiac amyloidosis and sarcoidosis. While technically falling under the rubric of nonischemic heart disease, the presence of spontaneous ventricular ectopy carries specific prognostic implications depending on the underlying diagnosis. Therefore, an appropriate referral for complete cardiac evaluation should be considered when a heritable disorder or other acquired structural heart disease is suspected.
TACHYCARDIA-RELATED CARDIOMYOPATHY
Tachycardia-related cardiomyopathy refers to left ventricular systolic dysfunction that is primarily caused by arrhythmias. This includes frequent PVCs or ventricular tachycardia but also atrial arrhythmias occurring at a high burden that directly weaken myocardial function over time. Although much research has been devoted to this condition, our understanding of its etiology and pathology is incomplete.
PVCs and ventricular ectopy burdens in excess of 15% to 20% have been associated with the development of this condition.28,29 However, it is important to note that cardiomyopathy can also develop at lower burdens.30 One study found that a burden greater than 24% was 79% sensitive and 78% specific for development of tachycardia-related cardiomyopathy.31 Additional studies have demonstrated specific PVC morphologic features such as slurring in the initial QRS segment and also PVCs occurring at shorter coupling intervals as being associated with cardiomyopathy.32–34
For these reasons, both quantification of the total burden and careful evaluation of available electrocardiograms and rhythm strips are important even in asymptomatic patients with frequent PVCs. Similarly, unexplained left ventricular dysfunction in patients with PVC burdens in these discussed ranges should raise suspicion for this diagnosis. Patients with tachycardia-related cardiomyopathy usually have at least partially reversible left ventricular dysfunction when identified or treated early.29,35
MEDICAL AND ABLATIVE TREATMENT
Available treatments include medical suppression and catheter ablation. One needs to exercise clinical judgment and incorporate all of the PVC-related data to make treatment decisions.
Little data for trigger avoidance and behavioral modification
Some patients report a strong association between palpitations related to PVCs and caffeine intake, other stimulants, or other dietary triggers. However, few data exist to support the role of trigger avoidance and behavioral modification in treatment. In fact, an older randomized trial in 81 men found no benefit in a program of total abstinence from caffeine and smoking, moderation of alcohol intake, and physical conditioning.36
Nonetheless, some argue in favor of advising patients to make these dietary and lifestyle changes, given the overall health benefits of aggressive risk-factor modification for cardiovascular disease.37 Certainly, a trial of trigger avoidance and behavioral modification seems reasonable for patients who have strongly associated historical triggers in the absence of structural heart disease and PVCs occurring at a low to modest burden.
Beta-blockers are the mainstay
Beta-blockers are the mainstay of medical suppression of PVCs, primarily through their effect on beta-1 adrenergic receptors to reduce intracellular cyclic adenosine monophosphate and thus decrease automaticity. Blocking beta-1 receptors also causes a negative chronotropic effect, reducing the resting sinus rate in addition to slowing atrioventricular nodal conduction.
Cardioselective beta-blockers include atenolol, betaxolol, metoprolol, and nadolol. These drugs are effective in suppressing PVCs, or at least in reducing the burden to more tolerable levels.
Beta-blockers are most strongly indicated in patients who require PVC suppression and who have concomitant coronary artery disease, prior myocardial infarction, or other cardiomyopathy, as this drug class favorably affects long-term prognosis in these conditions.
Common side effects of beta-blockers include fatigue, shortness of breath, depressed mood, and loss of libido. Side effects can present a significant challenge, particularly for younger patients. Noncardioselective beta-blockers are less commonly prescribed, with the exception of propranolol, which is an effective sympatholytic drug that blocks both beta-1 and beta-2 receptors.
Many patients with asthma or peripheral arterial disease can tolerate these drugs well despite concerns about provoked bronchospasm or claudication, respectively, and neither of these conditions is considered an absolute contraindication. Excessive bradycardia with beta-blocker therapy can lead to dizziness, lightheadedness, or overt syncope, and these drugs should be used with caution in patients with baseline sinus node dysfunction or atrioventricular nodal disease.
Nondihydropyridine calcium channel blockers
Nondihydropyridine calcium channel blockers are particularly effective for PVC suppression in patients without structural heart disease by the mechanisms previously described involving intracellular calcium channels. In particular, they are highly effective and are considered the drugs of choice in treating fascicular PVCs.
Verapamil is a potent drug in this class, but it also commonly causes constipation as a side effect. Diltiazem is less constipating but can cause fatigue, drowsiness, and headaches. Both drugs reduce the resting heart rate and slow atrioventricular nodal conduction. Patients predisposed to bradycardia or atrioventricular block can develop dizziness or overt syncope. Calcium channel blockers are also used cautiously in patients with congestive heart failure, given their potential negative inotropic effects.
Overall, calcium channel blockers are a very reasonable choice for young patients without structural heart disease who need PVC suppression.
Other antiarrhythmic drugs
Sotalol merits special consideration because it has both beta-blocker and class III antiarrhythmic properties, blocking potassium channels and prolonging cardiac repolarization. It can be very effective in PVC suppression but also creates some degree of QT prolongation. The QT-prolonging effect is accentuated in patients with baseline QT prolongation or abnormal renal function. Rarely, this can lead to torsades de pointes. As a safety precaution, some patients are admitted to the hospital when they start sotalol therapy so that they can be monitored with continuous telemetry and ECG to detect excessive QT prolongation.
Amiodarone is a versatile drug with mixed pharmacologic properties that include a predominantly potassium channel-blocking class III drug effect. However, this effect is balanced by its other pharmacologic properties that make QT prolongation less of a clinical concern. Excessive QT prolongation may still occur when used concomitantly with other QT-prolonging drugs.
Amiodarone is very effective in suppressing PVCs and ventricular arrhythmias but has considerable short-term and long-term side effects. Cumulative toxicity risks include damage to the thyroid gland, liver, skin, eyes, and lungs. Routine thyroid function testing, pulmonary function testing, and eye examinations are often considered for patients on long-term amiodarone therapy. Short-term use of this drug does not typically require such surveillance.
Catheter ablation
As mentioned in the previous sections, catheter ablation is a safe and effective treatment for PVCs. It is curative in most cases, and significantly reduces the PVC burden in others.
Procedure. Patients are brought to the electrophysiology laboratory in a fasted state and are partially sedated with an intravenous drug such as midazolam or fentanyl, or both. Steerable catheters are placed into appropriate cardiac chambers from femoral access sites, which are infiltrated with local anesthesia. Sometimes sedative or analgesic drugs must be limited if they are known to suppress PVCs.
Most operators prefer a technique called activation mapping, in which the catheter is maneuvered to home in on the precise PVC origin within the heart, which is subsequently ablated. This technique has very high success rates, but having enough spontaneous PVCs to map during the procedure is essential for the technique to succeed. Conversely, not having sufficient PVCs on the day of the procedure is a common reason that ablation fails or cannot be performed at all.
Pace-mapping is an alternate technique that does not require a continuous stream of PVCs. This involves pacing from different candidate locations inside the heart in an effort to precisely match the ECG appearance of the clinical PVC and to ablate at this site. Although activation mapping generally yields higher success rates and is preferred by most operators, pace-mapping can be successful when a perfect 12–12 match is elicited. In many cases, the two techniques are used together during the same procedure, particularly if the patient’s PVCs spontaneously wax and wane, as they often do.
Risks. Like any medical procedure, catheter ablation carries some inherent risks, including rare but potentially serious events. Unstable arrhythmias may require pace-termination from the catheter or, rarely, shock-termination externally. Even more rare is cardiac arrest requiring cardiopulmonary resuscitation. Uncommon but life-threatening complications also include pericardial effusion or cardiac tamponade requiring percutaneous drainage or, rarely, emergency surgical correction. Although such events are life-threatening, death is extremely rare.
Complications causing permanent disability are also very uncommon but include the risk of collateral injury to the conduction system requiring permanent pacemaker placement, injury to the coronary vessels requiring urgent treatment, or diaphragmatic injury affecting breathing. Left-sided cardiac ablation also carries a small risk of stroke, which is mitigated by giving intravenous heparin during the procedure.
More common but generally non-life-threatening complications include femoral vascular events such as hematomas, pseudoaneurysms, or fistulas that sometimes require subsequent treatment. These complications are generally treatable but can significantly prolong the recovery period.
Catheter ablation procedures are typically 2 to 6 hours in duration, depending on the chambers involved, PVC frequency, and other considerations. Postprocedure bed rest is required for a number of hours. A Foley catheter is sometimes used for patient comfort when a prolonged procedure is anticipated. This carries a small risk of urinary tract infection. Epicardial catheter ablation that requires access to the surface of the heart (ie, the pericardial space) is uncommon but carries some unique risks, including rare injury to coronary vessels or adjacent organs such as the liver or stomach.
Overall, both endocardial and epicardial catheter ablation can be performed safely and effectively in the overwhelming majority of patients, but understanding and explaining the potential risks remains a crucial part of the informed consent process.
TAKE-HOME POINTS
- PVCs are a common cause of palpitations but are also noted as incidental findings by ECG, Holter monitoring, and inpatient telemetry.
- The diagnostic evaluation includes an assessment for underlying structural heart disease and quantification of the total PVC burden.
- Patients without structural heart disease and with low-to-modest PVC burdens may not require specific treatment. PVCs at greater burdens, typically 15% to 20%, or with specific high-risk features carry a risk of tachycardia-related cardiomyopathy and may require treatment even if they are asymptomatic. These high-risk features include initial QRS slurring and PVCs occurring at shorter coupling intervals.
- Treatment involves medical therapy with a beta-blocker, a calcium channel blocker, or another antiarrhythmic drug, and catheter ablation in selected cases.
- Catheter ablation can be curative but is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
- Kostis JB, McCrone K, Moreyra AE, et al. Premature ventricular complexes in the absence of identifiable heart disease. Circulation 1981; 63:1351–1356.
- Sobotka PA, Mayer JH, Bauernfeind RA, Kanakis C, Rosen KM. Arrhythmias documented by 24-hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J 1981; 101:753–759.
- Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009; 95:1230–1237.
- Simpson RJ, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002; 143:535–540.
- Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985; 109:497–504.
- Gami AS, Noheria A, Lachman N, et al. Anatomical correlates relevant to ablation above the semilunar valves for the cardiac electrophysiologist: a study of 603 hearts. J Interv Card Electrophysiol 2011; 30:5–15.
- Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation 1986; 74:270–280.
- Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995; 92:421–429.
- Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006; 17:1052–1058.
- Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol 2007; 49:2035–2043.
- Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 2010; 3:616–623.
- Ouyang F, Cappato R, Ernst S, et al. Electroanatomic substrate of idiopathic left ventricular tachycardia: unidirectional block and macro-reentry within the Purkinje network. Circulation 2002; 105:462–469.
- Iwai S, Lerman BB. Management of ventricular tachycardia in patients with clinically normal hearts. Curr Cardiol Rep 2000; 2:515–521.
- Nogami A. Purkinje-related arrhythmias part I: monomorphic ventricular tachycardias. Pacing Clin Electrophysiol 2011; 34:624–650.
- Letsas KP, Efremidis M, Kollias G, Xydonas S, Sideris A. Electrocardiographic and electrophysiologic characteristics of ventricular extrasystoles arising from the aortomitral continuity. Cardiol Res Pract 2011; 2011:864964.
- Tada H, Tadokoro K, Ito S, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm 2007; 4:7–16.
- Tada H, Ito S, Naito S, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005; 45:877–886.
- Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol 2008; 1:23–29.
- Scheinman MM. Role of the His-Purkinje system in the genesis of cardiac arrhythmia. Heart Rhythm 2009; 6:1050–1058.
- Bigger JT, Dresdale FJ, Heissenbuttel RH, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease: mechanism, prevalence, significance, and management. Prog Cardiovasc Dis 1977; 19:255–300.
- Eldar M, Sievner Z, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Primary ventricular tachycardia in acute myocardial infarction: clinical characteristics and mortality. The SPRINT Study Group. Ann Intern Med 1992; 117:31–36.
- Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med 1989; 321:406–412.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol 2009; 54:799–808.
- Marchlinski FE. Perivalvular fibrosis and monomorphic ventricular tachycardia: toward a unifying hypothesis in nonischemic cardiomyopathy. Circulation 2007; 116:1998–2001.
- Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2010; 3:63–71.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010; 121:1533–1541.
- Lee GK, Klarich KW, Grogan M, Cha YM. Premature ventricular contraction-induced cardiomyopathy: a treatable condition. Circ Arrhythm Electrophysiol 2012; 5:229–236.
- Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005; 112:1092–1097.
- Kanei Y, Friedman M, Ogawa N, Hanon S, Lam P, Schweitzer P. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008; 13:81–85.
- Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010; 7:865–869.
- Moulton KP, Medcalf T, Lazzara R. Premature ventricular complex morphology. A marker for left ventricular structure and function. Circulation 1990; 81:1245–1251.
- Olgun H, Yokokawa M, Baman T, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm 2011; 8:1046–1049.
- Sun Y, Blom NA, Yu Y, et al. The influence of premature ventricular contractions on left ventricular function in asymptomatic children without structural heart disease: an echocardiographic evaluation. Int J Cardiovasc Imaging 2003; 19:295–299.
- Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm 2009; 6:1543–1549.
- DeBacker G, Jacobs D, Prineas R, et al. Ventricular premature contractions: a randomized non-drug intervention trial in normal men. Circulation 1979; 59:762–769.
- Glatter KA, Myers R, Chiamvimonvat N. Recommendations regarding dietary intake and caffeine and alcohol consumption in patients with cardiac arrhythmias: what do you tell your patients to do or not to do? Curr Treat Options Cardiovasc Med 2012; 14:529–535.
KEY POINTS
- Diagnostic evaluation should include an assessment for structural heart disease and quantification of the total PVC burden by ambulatory Holter monitoring.
- Patients without structural heart disease and low-to-modest PVC burdens do not always require treatment. PVCs at higher burdens (typically more than 15% to 20% of heartbeats) or strung together in runs of ventricular tachycardia pose a higher risk of tachycardia-related cardiomyopathy and heart failure, even if asymptomatic.
- When necessary, treatment for PVCs involves beta-blockers, calcium channel blockers, or other antiarrhythmic drugs and catheter ablation in selected cases.
- Catheter ablation can be curative, but it is typically reserved for drug-intolerant or medically refractory patients with a high PVC burden.
Managing severe acute pancreatitis
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
Severe acute pancreatitis has been known since the time of Rembrandt, with Nicolaes Tulp—the physician credited as first describing it—immortalized in the famous painting, The Anatomy Lesson. However, progress in managing this disease has been disappointing. Treatment is mainly supportive, and we lack any true disease-modifying therapy. But we are learning to recognize the disease and treat it supportively better than in the past.
The early hours of severe acute pancreatitis are critical for instituting appropriate intervention. Prompt fluid resuscitation is key to preventing immediate and later morbidity and death. This article focuses on identifying and managing the most severe form of acute pancreatitis—necrotizing disease—and its complications.
NECROTIZING DISEASE ACCOUNTS FOR MOST PANCREATITIS DEATHS
The classification and definitions of acute pancreatitis were recently revised from the 1992 Atlanta system and published early in 2013.1 In addition, the American Pancreatic Association and the International Association of Pancreatology met in 2012 to develop evidence-based guidelines on managing severe pancreatitis.
An estimated 210,000 new cases of acute pancreatitis occur each year in the United States. About 20% of cases of severe acute pancreatitis are necrotizing disease, which accounts for nearly all the morbidity and death associated with acute pancreatitis.
The clinical spectrum of acute pancreatitis ranges from mild to life-threatening, reflecting interstitial (death rate < 1%) to necrotizing histology (the latter associated with a 25% risk of death if the pancreatitis becomes infected and a 10% risk if it is sterile). When death occurs early in the disease course, it tends to be from multiorgan failure; when death occurs later in the course, it tends to be from infection. Appropriate early treatment may prevent death in both categories.
DIAGNOSING ACUTE PANCREATITIS AND PREDICTING ITS SEVERITY
The diagnosis of acute pancreatitis requires two of the following three criteria:
- Clinical presentation—epigastric pain, nausea, vomiting
- Biochemical—amylase level more than three times the upper limit of normal, or lipase more than three times the upper limit of normal
- Evidence from computed tomography (CT), ultrasonography, or magnetic resonance imaging.
Although the biochemical criteria are variably sensitive for detecting acute pancreatitis (55%–100%), the specificity is very high (93% to 99%).
Recently, urinary trypsinogen-2, measured by dipstick, has also been used to aid diagnosis. It has a reasonable sensitivity (53%–96%) and specificity (85%) if positive (> 50 ng/mL).
Speed is critical
Over the years, many clinical prediction rules have been used for predicting the severity of acute pancreatitis. The Ranson criteria,2 from 1974, and the Acute Physiology and Chronic Health Evaluation (APACHE) II system3 are cumbersome and require waiting up to 48 hours after the onset of acute pancreatitis to obtain a complete score. The Imrie-Glasgow score is another predictor.
The systemic inflammatory response syndrome (SIRS) is currently the most important indicator of prognosis.4 Originally adopted for predicting the development of organ failure with sepsis, it requires at least two of the following criteria:
- Heart rate > 90 beats/min
- Core temperature < 36°C or > 38°C
- White blood cells < 4,000 or > 12,000/mm3
- Respirations > 20/min.
The advantages of this system are that it identifies risk very early in the course of the disease and can be assessed quickly in the emergency department.
The Bedside Index for Severity of Acute Pancreatitis (BISAP) score is another simple, easy-to-perform prognostic index,5,6 calculated by assigning 1 point for each of the following if present within the first 24 hours of presentation:
- Blood urea nitrogen > 25 mg/dL
- Abnormal mental status (Glasgow coma score < 15)
- Evidence of systemic inflammatory response syndrome
- Age > 60 years
- Pleural effusion seen on imaging study.
A score of 3 points is associated with a 5.3% rate of hospital death, 4 points with 12.7%, and 5 points with 22.5%.
At its most basic, severe acute pancreatitis is defined by organ failure (at least one organ from the respiratory, renal, or cardiovascular system) lasting for more than 48 hours. Failure for each organ is defined by the Marshall scoring system.1
EARLY MANAGEMENT IS KEY TO OUTCOME
The window of opportunity to make a significant difference in outcome is within the first 12 to 24 hours of presentation. Volume resuscitation is the cornerstone of early management. By the time of presentation for severe acute pancreatitis, the pancreas is already necrotic, so the aim is to minimize the systemic inflammatory response syndrome with the goals of reducing rates of organ failure, morbidity, and death. Necrotizing pancreatitis is essentially an ischemic event, and the goal of volume resuscitation is to maintain pancreatic and intestinal microcirculation to prevent intestinal ischemia and subsequent bacterial translocation.7
Early resuscitation with lactated Ringer’s solution recommended
The evidence supporting a specific protocol for fluid resuscitation in severe acute pancreatitis is not strong, but a few studies provide guidance.
Wu et al8 randomized 40 patients with acute pancreatitis to one of four arms: “goal-directed fluid resuscitation” with either lactated Ringer’s solution or normal saline, or standard therapy (by physician discretion) with either lactated Ringer’s solution or normal saline. Goal-directed therapy involved a bolus of 20 mL/kg given over 30 to 45 minutes at presentation followed by infusion with rates dependent on an algorithm based on change in blood urea nitrogen level at set times. Patients receiving either goal-directed or standard therapy had significantly lower rates of systemic inflammatory response syndrome at 24 hours than at admission. Most striking was that treatment with lactated Ringer’s solution was associated with dramatically improved rates, whereas normal saline showed no improvement.
In a retrospective study of patients with acute pancreatitis, Warndorf et al9 identified 340 patients who received early resuscitation (more than one-third of the total 72-hour fluid volume within 24 hours of presentation) and 90 patients who received late resuscitation (less than one-third of the total 72-hour fluid volume within 24 hours of presentation). Patients who received early resuscitation developed less systemic inflammatory response syndrome and organ failure, and required fewer interventions.
Monitoring for optimum fluid resuscitation
Fluid resuscitation should be carefully managed to avoid administering either inadequate or excessive amounts of fluid. Inadequate fluid resuscitation can result in renal failure, progression of necrosis, and possibly infectious complications. Excessive resuscitation—defined as more than 4 L in the first 24 hours—is associated with respiratory failure, pancreatic fluid collections, and abdominal compartment syndrome.
Optimum resuscitation is controlled fluid expansion averaging 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
Adequate volume resuscitation can be evaluated clinically with the following goals:
- Heart rate < 120 beats per minute
- Mean arterial pressure 65–85 mm Hg
- Urinary output > 1 mL/kg per hour
- Hematocrit 35%–44%.
EARLY CT IS JUSTIFIED ONLY IF DIAGNOSIS IS UNCLEAR
The normal pancreas takes up contrast in the same way as do the liver and spleen, so its enhancement on CT is similar. If there is interstitial pancreatitis, CT shows the pancreas with normal contrast uptake, but the organ appears “boggy” with indistinct outlines. With necrotizing pancreatitis, only small areas of tissue with normal contrast may be apparent.
Peripancreatic fat necrosis may also be visible on CT. Obese patients tend to have a worse clinical course of necrotizing pancreatitis, probably because of the associated peripancreatic fat that is incorporated into the pancreatic necrosis.
For clear-cut cases of acute pancreatitis, time is wasted waiting to obtain CT images, and this could delay fluid resuscitation. Results from immediate CT almost never change the clinical management during the first week of acute pancreatitis, and obtaining CT images is usually not recommended if the diagnosis of acute pancreatitis is clear. CT’s sensitivity for detecting necrosis is only 70% in the first 48 hours of presentation, so it is easy to be fooled by a false-negative scan: frequently, a scan does not show necrotizing pancreatitis until after 72 hours. In addition, evidence from animal studies indicates that contrast agents might worsen pancreatic necrosis.
Immediate CT is justified if the diagnosis is in doubt at presentation, such as to evaluate for other intra-abdominal conditions such as intestinal ischemia or a perforated duodenal ulcer.
Contrast-enhanced CT is recommended 72 to 96 hours after presentation, or earlier if the patient is worsening despite treatment. Specific CT protocols will be included in new management guidelines, expected to be published soon.
PREVENTING INFECTIOUS COMPLICATIONS
Risk of infection is associated with the degree of pancreatic necrosis. Patients with less than 30% necrosis have a 22.5% chance of infection, whereas those with more than 50% necrosis have a 46.5% risk of infection.10
Infection can develop from a variety of sources:
Bacterial translocation from the colon and small bowel is thought to be one of the major sources of infection in necrotic pancreatitis. Volume resuscitation and maintaining gut integrity with early enteral nutrition are believed to minimize the risk of bacterial translocation.
Hematogenous spread of bacteria is another suspected source of infection into the pancreas. Again, enteral nutrition also reduces the risk by minimizing the need for central catheters.
Biliary sources may also play a role. Bile duct stones or gall bladder infection can lead to infected pancreatic necrosis.
ANTIBIOTICS NOT ROUTINELY RECOMMENDED
Treating acute pancreatitis with antibiotics has fallen in and out of favor over the past decades. From being standard practice in the 1970s, it dropped off in the 1980s and 1990s and then became more common again.
Current recommendations from the American Pancreatic Association and the International Association of Pancreatology are not to routinely use intravenous antibiotics to prevent infection in necrotizing pancreatitis because of lack of evidence that it changes overall outcome. Antibiotic usage may be associated with more bacterial resistance and the introduction of fungal infections into the pancreas.
Selective gut decontamination, involving oral and rectal administration of neomycin and other antibiotics, was shown in a single randomized trial to reduce the incidence of infection, but it is very cumbersome and is not recommended for acute pancreatitis.
Treatment with probiotics is also not recommended and was shown in one study to lead to a worse outcome.11
ENTERAL BETTER THAN TOTAL PARENTERAL NUTRITION
Enteral tube feeding with either an elemental diet or a polymeric enteral formulation is the first-line therapy for necrotizing pancreatitis. Compared with total parenteral nutrition, it reduces infection, organ failure, hospital length of stay, the need for surgical intervention, and the risk of death. Total parenteral nutrition should be considered only for patients who do not tolerate enteral feeding because of severe ileus.
Conventional thinking for many years was to provide enteral feeding with a tube passed beyond the ligament of Treitz, thinking that it reduced stimulation to the pancreas. However, recent studies indicate that nasogastric feeding is equivalent to nasojejunal feeding in terms of nutrition, maintaining gut integrity, and outcome.
INTRA-ABDOMINAL HYPERTENSION AND ABDOMINAL COMPARTMENT SYNDROME
Movement of fluid into the intracellular space (“third-spacing”) occurs in acute pancreatitis and is exacerbated by fluid resuscitation. Intra-abdominal hypertension is associated with poor outcomes in patients with severe acute pancreatitis. Especially for patients with severe pancreatitis who are on mechanical ventilation, pressure should be monitored with transvesicular bladder measurements.
Intra-abdominal hypertension is defined as a sustained intra-abdominal pressure of more than 12 mm Hg, with the following grades:
- Grade 1: 12–15 mm Hg
- Grade 2: 16–20 mm Hg
- Grade 3: 21–25 mm Hg
- Grade 4: > 25 mm Hg.
Abdominal compartment syndrome is defined as a sustained intra-abdominal pressure of more than 20 mm Hg. It is associated with new organ dysfunction or failure. It should first be managed with ultrafiltration or diuretics to try to reduce the amount of fluid in the abdomen. Lumenal decompression can be tried with nasogastric or rectal tubes for the stomach and bowels. Ascites or retroperitoneal fluid can be drained percutaneously. In addition, analgesia and sedation to reduce abdominal muscle tone can help the patient become better ventilated. Neuromuscular blockade can also relax the abdomen.
Open abdominal decompression is the treatment of last resort to relieve abdominal compartment syndrome. The abdominal wall is not closed surgically but is allowed to heal by secondary intention (it “granulates in”).12
IDENTIFYING INFECTION
Fine-needle aspiration if clinical and imaging signs are not clear
Untreated infected pancreatitis is associated with a much higher risk of death than sterile pancreatic necrosis. Unfortunately, it can be difficult to determine if a patient with necrotizing pancreatitis has an infection because fever, tachycardia, and leukocytosis are usually present regardless. It is important to determine because mechanically intervening for sterile necrosis does not improve outcome.
Fine-needle aspiration, either guided by CT or done at the bedside with ultrasonography, with evaluation with Gram stain and culture, was widely used in the 1990s in cases of necrotizing pancreatitis to determine if infection was present. There has been a shift away from this because, although it can confirm the presence of infection, the false-negative rate is 15%. Clinical and imaging signs can be relied on in most cases to determine the presence of infection, and it is now recognized that fineneedle aspiration should be used only for select cases. Clinical studies have not shown that fine-needle aspiration improves outcomes.
Clinical scenarios typical of infected pancreatic necrosis include patients who have obvious signs of infection with no identifiable source, such as those who stabilize after acute severe acute pancreatitis, and then 10 to 14 days later become worse, with a dramatically higher white blood cell count and tachycardia. Such a patient likely needs an intervention regardless of the results of fine-needle aspiration.
On the other hand, a patient with a continually up-and-down course that never stabilizes over 3 weeks, with no identifiable source of infection, and with no peripancreatic gas apparent on imaging would be a good candidate for fine-needle aspiration.
If peripancreatic gas is seen on imaging, fine-needle aspiration is unnecessary. Peripancreatic gas is traditionally attributed to gasforming bacteria within the pancreas, but in my experience, it is usually from a fistula from the necrosis to the duodenum or the colon, the fistula being caused as the necrosis erodes at the hepatic flexure, the transverse colon, or the splenic flexure.
MECHANICAL INTERVENTIONS FOR INFECTIVE NECROSIS
Late, minimally invasive procedures preferred
Conventional management has shifted away from removing the necrosis with early surgical debridement of the pancreas. Experience with myocardial infarction shows that it is not necessary to remove a sterile necrotic organ, and studies with sterile pancreatic necrosis have found that surgical intervention is associated with a higher risk of death than medical management.
Documented infection has traditionally been considered a definite indication for debridement, but even that is being called into question as more studies are emerging of infected necrosis treated successfully with antibiotics alone.
Sterile necrosis with a fulminant course is a controversial indication for surgery. It was traditionally felt that surgery was worth trying for such patients, but this is no longer common practice.
For cases in which debridement was deemed advisable, surgery was done more frequently in the past. Now, a minimally invasive approach such as with endoscopy or percutaneous catheter is also used. Waiting until at least 4 weeks after the onset of acute pancreatitis is associated with a better outcome than intervening early.
WALLED-OFF NECROSIS
Watchful waiting or minimally invasive intervention
Patients who survive multiorgan failure but are still ill more than 4 weeks after the onset of pancreatitis should be suspected of having walled-off necrosis, formerly referred to as a pancreatic phlegmon. This term was abandoned after the 1992 Atlanta symposium.13 In the mid to late 1990s, the process was referred to as organized pancreatic necrosis. It is characterized by a mature, encapsulated collection of pancreatic or peripancreatic necrosis that contains variable amounts of amylase-rich fluid from pancreatic duct disruption.
Walled-off pancreatic necrosis (WOPN) is often confused with pancreatic pseudocyst; these may appear similar on CT, and higherdensity solid debris may be visible in walled-off necrosis within an otherwise homogenous-appearing collection. Magnetic resonance imaging defines liquid and solid much better than CT.
The best way to distinguish WOPN from pseudocyst is by clinical history: a patient with a preceding history of clinically severe acute pancreatitis almost always has necrotizing pancreatitis that evolves to walled-off necrosis, usually over 3 to 4 weeks.
Endoscopic removal and other minimally invasive approaches, such as aggressive percutaneous interventions, have replaced open necrosectomy for treatment, which was associated with high morbidity and mortality rates.14–16
Intervening for sterile walled-off necrosis is still a controversial topic: although systemically ill, the patient is no longer having life-threatening consequences, and watchful waiting might be just as expedient as intervention. Evidence to support either view is lacking. Most experts believe that intervention should be done if the patient has gastric outlet obstruction and intractable pain and is unable to eat 4 to 6 weeks after the onset of pancreatitis with WOPN. Infected WOPN is considered an indication for drainage.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69–81.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–829.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874.
- Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008; 57:1698–1703.
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009; 104:966–971.
- Fisher JM, Gardner TB. The “golden hours” of management in acute pancreatitis. Am J Gastroenterol 2012; 107:1146–1150.
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:710–717.
- Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011; 9:705–709.
- Beger HG, Rau BM. Severe acute pancreatitis: clinical course and management. World J Gastroenterol 2007; 13:5043–5051.
- Besselink MG, van Santvoort HC, Buskens E, et al; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651–659.
- Fitzgerald JE, Gupta S, Masterson S, Sigurdsson HH. Laparostomy management using the ABThera open abdomen negative pressure therapy system in a grade IV open abdomen secondary to acute pancreatitis. Int Wound J 2012. doi: 1111/j.1742-481X2012.00953.x. [epub ahead of print]
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11–13, 1992. Arch Surg 1993; 128:586–590.
- Baron TH, Thaggard WG, Morgan DE, Stanley RJ. Endoscopic therapy for organized pancreatic necrosis. Gastroenterology 1996; 111:755–764.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Routine early computed tomography to evaluate patients with severe acute pancreatitis wastes time and is necessary only if the diagnosis at presentation is not clearly consistent with acute pancreatitis.
- Optimum fluid resuscitation is now recommended, using lactated Ringer’s solution at a rate of 5 to 10 mL/kg per hour, with 2,500 to 4,000 mL given in the first 24 hours.
- Enteral feeding with either an elemental diet or a polymeric enteral formulation is first-line nutritional therapy.
- Antibiotics are no longer routinely used to prevent infection.
- Relief of abdominal compartment syndrome should be attempted by multiple means before resorting to open abdominal decompression.
Ultrasound expedites pediatric emergency evaluations
LAKE BUENA VISTA, FLA. – Ultrasound expedites clinical decision making and often dictates the next step to pursue when managing children in the emergency department.
"It makes sense to use ultrasound for pediatric patients, but there’s been a delay in picking up this idea. Only now is (the use of bedside ultrasonography) becoming more prevalent in pediatric emergency medicine, Dr. Stephanie J. Doniger said at a meeting sponsored by the American College of Emergency Physicians and the American Academy of Pediatrics.
"Among the advantages is that we really never have to perform sedation, and the absolutely most important thing, we don’t need to use ionizing radiation," said Dr. Doniger, director of emergency ultrasound at Children’s Hospital and Research Center, Oakland, Calif.
Still, she said, there are no clear guidelines for ultrasonography’s use in pediatric emergencies. No one body oversees the quality in training or in outcomes. In 2009, ACEP updated its guidelines on bedside ultrasound in the emergency department. The group gave a nod to pediatric use, calling ultrasonography "an ideal diagnostic tool for children ... As in adult patients, emergency ultrasound in children can be life saving, time saving, [can] increase procedural efficiency, and [can] maximize patient safety."
The document says ultrasound is particularly useful in performing the FAST exam, and for bladder evaluations prior to instilling a catheter in infants. Dr. Doniger noted a number of other applications as well.
Ultrasonography is valuable for assessing dehydration by providing a look at the inferior vena cava. "We’re looking here for collapsibility during inspiration. Collapsibility of more than 50% correlates with dehydration."
Appendicitis is tough to image, radiographs are unreliable, and "CTs aren’t great, but we do them if we have to." But ultrasound provides a very good look into what lies beneath, and is one more way to reduce a child’s cumulative radiation dose.
A 2008 study found that a 5-minute bedside ultrasound had a sensitivity of 65% and a specificity of 90% for appendicitis. The positive predictive value was 84%, and the negative predictive value, 76%.
"That might not sound great, but it is a good result to rule in disease. If you have a high suspicion of appendicitis, then use it; if a low suspicion, then don’t."
When looking for an infected appendix, start at the point of maximal tenderness and move to the right while the child is in an oblique position. "It helps if you prop up the hip with some towels," Dr. Doniger said. "The bowel will move away and you’ll have a better view when you compress."
Look for a noncompressible tubular structure with a diameter greater than 6 mm.
The probe can also help find intussusception – a condition that x-rays identify 40%-90% of the time. The ultrasound image of intussusception is target- or doughnut-shaped – a figure formed when the bowel retracts back into itself. A study found that even beginning sonographers can identify this classic sign. Their exams had a sensitivity of 85%, a specificity of 97%, a positive predictive value of 85%, and a negative predictive value of 97%.
Even something that seems innocuous on the surface – like a splinter – will give up its secrets under the ultrasound probe. Foreign bodies may or may not show up on an x-ray, but they are obviously hypoechoic on ultrasound, she said.
Dr. Doniger presented the case of a 13-year-old who thought he got a splinter under his fingernail, but wasn’t sure. He came to the emergency department after his finger became stiff and a little painful. Ultrasound identified the culprit as splinter of wood that was more than 1 inch long.
Guiding the needle during an evaluation for painful hips for effusion is another great use for ultrasound, she said. A 2009 study determined that, compared with ultrasound alone; sonography-guided arthrocentesis for symptomatic hips had 90% sensitivity and 100% specificity, with a 100% positive predictive value and a 92% negative predictive value.
Ultrasound also provides valuable assistance in identifying the landmarks for successful needle placement when performing a spinal tap. A 2007 study equally randomized 46 children to finding the landmarks by palpation or with ultrasound. There were six failed attempts in the palpation group and one in the ultrasound group. Ultrasound was particularly helpful in obese children; four of seven palpation placements failed, compared with no failures in the ultrasound group.
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
LAKE BUENA VISTA, FLA. – Ultrasound expedites clinical decision making and often dictates the next step to pursue when managing children in the emergency department.
"It makes sense to use ultrasound for pediatric patients, but there’s been a delay in picking up this idea. Only now is (the use of bedside ultrasonography) becoming more prevalent in pediatric emergency medicine, Dr. Stephanie J. Doniger said at a meeting sponsored by the American College of Emergency Physicians and the American Academy of Pediatrics.
"Among the advantages is that we really never have to perform sedation, and the absolutely most important thing, we don’t need to use ionizing radiation," said Dr. Doniger, director of emergency ultrasound at Children’s Hospital and Research Center, Oakland, Calif.
Still, she said, there are no clear guidelines for ultrasonography’s use in pediatric emergencies. No one body oversees the quality in training or in outcomes. In 2009, ACEP updated its guidelines on bedside ultrasound in the emergency department. The group gave a nod to pediatric use, calling ultrasonography "an ideal diagnostic tool for children ... As in adult patients, emergency ultrasound in children can be life saving, time saving, [can] increase procedural efficiency, and [can] maximize patient safety."
The document says ultrasound is particularly useful in performing the FAST exam, and for bladder evaluations prior to instilling a catheter in infants. Dr. Doniger noted a number of other applications as well.
Ultrasonography is valuable for assessing dehydration by providing a look at the inferior vena cava. "We’re looking here for collapsibility during inspiration. Collapsibility of more than 50% correlates with dehydration."
Appendicitis is tough to image, radiographs are unreliable, and "CTs aren’t great, but we do them if we have to." But ultrasound provides a very good look into what lies beneath, and is one more way to reduce a child’s cumulative radiation dose.
A 2008 study found that a 5-minute bedside ultrasound had a sensitivity of 65% and a specificity of 90% for appendicitis. The positive predictive value was 84%, and the negative predictive value, 76%.
"That might not sound great, but it is a good result to rule in disease. If you have a high suspicion of appendicitis, then use it; if a low suspicion, then don’t."
When looking for an infected appendix, start at the point of maximal tenderness and move to the right while the child is in an oblique position. "It helps if you prop up the hip with some towels," Dr. Doniger said. "The bowel will move away and you’ll have a better view when you compress."
Look for a noncompressible tubular structure with a diameter greater than 6 mm.
The probe can also help find intussusception – a condition that x-rays identify 40%-90% of the time. The ultrasound image of intussusception is target- or doughnut-shaped – a figure formed when the bowel retracts back into itself. A study found that even beginning sonographers can identify this classic sign. Their exams had a sensitivity of 85%, a specificity of 97%, a positive predictive value of 85%, and a negative predictive value of 97%.
Even something that seems innocuous on the surface – like a splinter – will give up its secrets under the ultrasound probe. Foreign bodies may or may not show up on an x-ray, but they are obviously hypoechoic on ultrasound, she said.
Dr. Doniger presented the case of a 13-year-old who thought he got a splinter under his fingernail, but wasn’t sure. He came to the emergency department after his finger became stiff and a little painful. Ultrasound identified the culprit as splinter of wood that was more than 1 inch long.
Guiding the needle during an evaluation for painful hips for effusion is another great use for ultrasound, she said. A 2009 study determined that, compared with ultrasound alone; sonography-guided arthrocentesis for symptomatic hips had 90% sensitivity and 100% specificity, with a 100% positive predictive value and a 92% negative predictive value.
Ultrasound also provides valuable assistance in identifying the landmarks for successful needle placement when performing a spinal tap. A 2007 study equally randomized 46 children to finding the landmarks by palpation or with ultrasound. There were six failed attempts in the palpation group and one in the ultrasound group. Ultrasound was particularly helpful in obese children; four of seven palpation placements failed, compared with no failures in the ultrasound group.
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
LAKE BUENA VISTA, FLA. – Ultrasound expedites clinical decision making and often dictates the next step to pursue when managing children in the emergency department.
"It makes sense to use ultrasound for pediatric patients, but there’s been a delay in picking up this idea. Only now is (the use of bedside ultrasonography) becoming more prevalent in pediatric emergency medicine, Dr. Stephanie J. Doniger said at a meeting sponsored by the American College of Emergency Physicians and the American Academy of Pediatrics.
"Among the advantages is that we really never have to perform sedation, and the absolutely most important thing, we don’t need to use ionizing radiation," said Dr. Doniger, director of emergency ultrasound at Children’s Hospital and Research Center, Oakland, Calif.
Still, she said, there are no clear guidelines for ultrasonography’s use in pediatric emergencies. No one body oversees the quality in training or in outcomes. In 2009, ACEP updated its guidelines on bedside ultrasound in the emergency department. The group gave a nod to pediatric use, calling ultrasonography "an ideal diagnostic tool for children ... As in adult patients, emergency ultrasound in children can be life saving, time saving, [can] increase procedural efficiency, and [can] maximize patient safety."
The document says ultrasound is particularly useful in performing the FAST exam, and for bladder evaluations prior to instilling a catheter in infants. Dr. Doniger noted a number of other applications as well.
Ultrasonography is valuable for assessing dehydration by providing a look at the inferior vena cava. "We’re looking here for collapsibility during inspiration. Collapsibility of more than 50% correlates with dehydration."
Appendicitis is tough to image, radiographs are unreliable, and "CTs aren’t great, but we do them if we have to." But ultrasound provides a very good look into what lies beneath, and is one more way to reduce a child’s cumulative radiation dose.
A 2008 study found that a 5-minute bedside ultrasound had a sensitivity of 65% and a specificity of 90% for appendicitis. The positive predictive value was 84%, and the negative predictive value, 76%.
"That might not sound great, but it is a good result to rule in disease. If you have a high suspicion of appendicitis, then use it; if a low suspicion, then don’t."
When looking for an infected appendix, start at the point of maximal tenderness and move to the right while the child is in an oblique position. "It helps if you prop up the hip with some towels," Dr. Doniger said. "The bowel will move away and you’ll have a better view when you compress."
Look for a noncompressible tubular structure with a diameter greater than 6 mm.
The probe can also help find intussusception – a condition that x-rays identify 40%-90% of the time. The ultrasound image of intussusception is target- or doughnut-shaped – a figure formed when the bowel retracts back into itself. A study found that even beginning sonographers can identify this classic sign. Their exams had a sensitivity of 85%, a specificity of 97%, a positive predictive value of 85%, and a negative predictive value of 97%.
Even something that seems innocuous on the surface – like a splinter – will give up its secrets under the ultrasound probe. Foreign bodies may or may not show up on an x-ray, but they are obviously hypoechoic on ultrasound, she said.
Dr. Doniger presented the case of a 13-year-old who thought he got a splinter under his fingernail, but wasn’t sure. He came to the emergency department after his finger became stiff and a little painful. Ultrasound identified the culprit as splinter of wood that was more than 1 inch long.
Guiding the needle during an evaluation for painful hips for effusion is another great use for ultrasound, she said. A 2009 study determined that, compared with ultrasound alone; sonography-guided arthrocentesis for symptomatic hips had 90% sensitivity and 100% specificity, with a 100% positive predictive value and a 92% negative predictive value.
Ultrasound also provides valuable assistance in identifying the landmarks for successful needle placement when performing a spinal tap. A 2007 study equally randomized 46 children to finding the landmarks by palpation or with ultrasound. There were six failed attempts in the palpation group and one in the ultrasound group. Ultrasound was particularly helpful in obese children; four of seven palpation placements failed, compared with no failures in the ultrasound group.
None of the authors declared any financial relationships. The study was funded by the Lynn Sage Cancer Research Foundation, the Avon Foundation, and a private contribution.
EXPERT ANALYSIS AT THE ADVANCED PEDIATRIC EMERGENCY MEDICINE ASSEMBLY
In the Hips, Increasing Pain and Decreasing Range of Motion
Not all joint pain is arthritis
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
RA patients have increased aortic inflammation
NEW YORK – Patients with rheumatoid arthritis had significantly greater aortic wall inflammation than did control patients with coronary artery disease but without autoimmune disease in an 18- fluorodeoxyglucose PET imaging study. No differences in carotid artery wall inflammation were found between the groups.
"These results suggest that part of the risk of cardiovascular disease in rheumatoid arthritis patients may not only be from systemic inflammation but truly localized inflammation that is causing plaques to become more vulnerable, less stable, and more likely to rupture," said Dr. Jeffrey D. Greenberg, who presented the results of the cross-sectional comparison at the NYU Seminar in Advanced Rheumatology, sponsored by New York University.
It has been known for some time that rheumatoid arthritis (RA) patients have systemic inflammation as well as vascular inflammation and have an increased risk of cardiovascular-related death, according to Dr. Greenberg, associate director of clinical translational sciences in the division of rheumatology at New York University Langone Medical Center and the NYU Hospital for Joint Diseases. He cited multifactorial mechanisms by which RA patients may incur heightened cardiovascular risk, including traditional cardiovascular risk factors (altered lipid balance, impaired insulin sensitivity), abnormal endothelial function, and increased plaque vulnerability. He said that the results of this study demonstrate another factor – localized inflammation causing subclinical vasculitis in the aorta and perhaps coronary vessels.
Dr. Greenberg and his associates used a 10 mCi injection of 18-fluorodeoxyglucose (18-FDG) and PET imaging in two separate cohorts of patients, one consisting of 27 RA patients (aged 35-64 years) and another of 70 controls with coronary artery disease but without autoimmune disease (aged 41-76 years). The investigators determined vascular 18-FDG uptake by calculating target to background ratios (TBRs) that served as proxies for macrophage activity and inflammation in the vessel walls.
RA patients had significantly higher TBR scores than did controls for the aorta mean TBR (1.46 vs. 1.25; P less than .0001), aorta maximum TBR (2.08 vs. 1.75; P less than .0001) and the TBR of the most diseased segment of aorta (2.23 vs. 1.87; P = .0004). These values were adjusted for differences in age, gender, and body mass index between the groups in multivariate regression models. RA patients had mean disease duration of about 11 years and mean DAS28 of 4.6, with no history of coronary artery disease, myocardial infarction, or stroke.
The results of the study were presented previously at the 2012 ACR Annual Meeting.
Dr. Greenberg serves as a consultant to the Consortium of Rheumatology Researchers of North America Inc. (CORRONA) and Pfizer and holds an ownership interest in CORRONA. He also receives grant support from the American College of Rheumatology, the Arthritis National Research Foundation, the Agency for Healthcare Research and Quality, the National Institute for Arthritis, and Musculoskeletal and Skin Diseases, and the NYU Clinical and Translational Science Institute.
NEW YORK – Patients with rheumatoid arthritis had significantly greater aortic wall inflammation than did control patients with coronary artery disease but without autoimmune disease in an 18- fluorodeoxyglucose PET imaging study. No differences in carotid artery wall inflammation were found between the groups.
"These results suggest that part of the risk of cardiovascular disease in rheumatoid arthritis patients may not only be from systemic inflammation but truly localized inflammation that is causing plaques to become more vulnerable, less stable, and more likely to rupture," said Dr. Jeffrey D. Greenberg, who presented the results of the cross-sectional comparison at the NYU Seminar in Advanced Rheumatology, sponsored by New York University.
It has been known for some time that rheumatoid arthritis (RA) patients have systemic inflammation as well as vascular inflammation and have an increased risk of cardiovascular-related death, according to Dr. Greenberg, associate director of clinical translational sciences in the division of rheumatology at New York University Langone Medical Center and the NYU Hospital for Joint Diseases. He cited multifactorial mechanisms by which RA patients may incur heightened cardiovascular risk, including traditional cardiovascular risk factors (altered lipid balance, impaired insulin sensitivity), abnormal endothelial function, and increased plaque vulnerability. He said that the results of this study demonstrate another factor – localized inflammation causing subclinical vasculitis in the aorta and perhaps coronary vessels.
Dr. Greenberg and his associates used a 10 mCi injection of 18-fluorodeoxyglucose (18-FDG) and PET imaging in two separate cohorts of patients, one consisting of 27 RA patients (aged 35-64 years) and another of 70 controls with coronary artery disease but without autoimmune disease (aged 41-76 years). The investigators determined vascular 18-FDG uptake by calculating target to background ratios (TBRs) that served as proxies for macrophage activity and inflammation in the vessel walls.
RA patients had significantly higher TBR scores than did controls for the aorta mean TBR (1.46 vs. 1.25; P less than .0001), aorta maximum TBR (2.08 vs. 1.75; P less than .0001) and the TBR of the most diseased segment of aorta (2.23 vs. 1.87; P = .0004). These values were adjusted for differences in age, gender, and body mass index between the groups in multivariate regression models. RA patients had mean disease duration of about 11 years and mean DAS28 of 4.6, with no history of coronary artery disease, myocardial infarction, or stroke.
The results of the study were presented previously at the 2012 ACR Annual Meeting.
Dr. Greenberg serves as a consultant to the Consortium of Rheumatology Researchers of North America Inc. (CORRONA) and Pfizer and holds an ownership interest in CORRONA. He also receives grant support from the American College of Rheumatology, the Arthritis National Research Foundation, the Agency for Healthcare Research and Quality, the National Institute for Arthritis, and Musculoskeletal and Skin Diseases, and the NYU Clinical and Translational Science Institute.
NEW YORK – Patients with rheumatoid arthritis had significantly greater aortic wall inflammation than did control patients with coronary artery disease but without autoimmune disease in an 18- fluorodeoxyglucose PET imaging study. No differences in carotid artery wall inflammation were found between the groups.
"These results suggest that part of the risk of cardiovascular disease in rheumatoid arthritis patients may not only be from systemic inflammation but truly localized inflammation that is causing plaques to become more vulnerable, less stable, and more likely to rupture," said Dr. Jeffrey D. Greenberg, who presented the results of the cross-sectional comparison at the NYU Seminar in Advanced Rheumatology, sponsored by New York University.
It has been known for some time that rheumatoid arthritis (RA) patients have systemic inflammation as well as vascular inflammation and have an increased risk of cardiovascular-related death, according to Dr. Greenberg, associate director of clinical translational sciences in the division of rheumatology at New York University Langone Medical Center and the NYU Hospital for Joint Diseases. He cited multifactorial mechanisms by which RA patients may incur heightened cardiovascular risk, including traditional cardiovascular risk factors (altered lipid balance, impaired insulin sensitivity), abnormal endothelial function, and increased plaque vulnerability. He said that the results of this study demonstrate another factor – localized inflammation causing subclinical vasculitis in the aorta and perhaps coronary vessels.
Dr. Greenberg and his associates used a 10 mCi injection of 18-fluorodeoxyglucose (18-FDG) and PET imaging in two separate cohorts of patients, one consisting of 27 RA patients (aged 35-64 years) and another of 70 controls with coronary artery disease but without autoimmune disease (aged 41-76 years). The investigators determined vascular 18-FDG uptake by calculating target to background ratios (TBRs) that served as proxies for macrophage activity and inflammation in the vessel walls.
RA patients had significantly higher TBR scores than did controls for the aorta mean TBR (1.46 vs. 1.25; P less than .0001), aorta maximum TBR (2.08 vs. 1.75; P less than .0001) and the TBR of the most diseased segment of aorta (2.23 vs. 1.87; P = .0004). These values were adjusted for differences in age, gender, and body mass index between the groups in multivariate regression models. RA patients had mean disease duration of about 11 years and mean DAS28 of 4.6, with no history of coronary artery disease, myocardial infarction, or stroke.
The results of the study were presented previously at the 2012 ACR Annual Meeting.
Dr. Greenberg serves as a consultant to the Consortium of Rheumatology Researchers of North America Inc. (CORRONA) and Pfizer and holds an ownership interest in CORRONA. He also receives grant support from the American College of Rheumatology, the Arthritis National Research Foundation, the Agency for Healthcare Research and Quality, the National Institute for Arthritis, and Musculoskeletal and Skin Diseases, and the NYU Clinical and Translational Science Institute.
AT THE NYU SEMINAR IN ADVANCED RHEUMATOLOGY
Implications of a prominent R wave in V1
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
Epiphyseal Chondromyxoid Fibroma With Prominent Adipose Tissue: An Unusual Radiologic and Histologic Presentation
CORE320: CT angiography bests SPECT for CAD diagnosis
SAN FRANCISCO – Coronary CT angiography outperformed myocardial perfusion single-photon emission CT for the diagnosis of obstructive coronary artery disease in a prospective multicenter head-to-head comparative study.
The CORE320 (Coronary Artery Evaluation Using 320-Row Multidetector Computed Tomography Angiography and Myocardial Perfusion) study included 381 patients with suspected or known coronary artery disease (CAD) who were scheduled for invasive quantitative coronary angiography. But first they all underwent coronary CT angiography (CTA) and single-photon emission CT (SPECT), with images analyzed in blinded independent core laboratories. The time interval between the two imaging studies was a mean of 9.4 days. Invasive coronary angiography served as the diagnostic reference standard in the 16-center, 8-country study, Dr. Marcelo F. Di Carli explained at the annual meeting of the American College of Cardiology.
The primary study endpoint was test accuracy as defined by the area under the receiver operating characteristic curve for identifying the 59% of subjects with at least a 50% stenosis by invasive coronary angiography. The rate was significantly better for CTA than SPECT: 89% vs. 69%.
CTA’s superior performance was driven by its greater sensitivity in detecting stenoses of 50% or more: 91% vs. 62% for SPECT. The two imaging modalities displayed similar specificity: 74% for CTA and 67% for SPECT.
CTA had a positive predictive value of 83% and a negative predictive value of 85%, compared with 73% and 55%, respectively, for SPECT, according to Dr. Di Carli of Brigham and Women’s Hospital, Boston.
The same pattern of results was seen with regard to diagnostic accuracy in detecting patients with at least a 70% stenosis, a prespecified secondary endpoint. CTA had 94% sensitivity, 60% specificity, a positive predictive value of 66%, and a negative predictive value of 92%. SPECT showed 72% sensitivity, 67% specificity, a 64% positive predictive value, and a 73% negative predictive value.
The average radiation dose was markedly lower with CTA: 3.54 mSv compared with 10.48 mSv for SPECT.
The study was funded by Toshiba. Dr. Di Carli reported having no relevant financial conflicts.
SAN FRANCISCO – Coronary CT angiography outperformed myocardial perfusion single-photon emission CT for the diagnosis of obstructive coronary artery disease in a prospective multicenter head-to-head comparative study.
The CORE320 (Coronary Artery Evaluation Using 320-Row Multidetector Computed Tomography Angiography and Myocardial Perfusion) study included 381 patients with suspected or known coronary artery disease (CAD) who were scheduled for invasive quantitative coronary angiography. But first they all underwent coronary CT angiography (CTA) and single-photon emission CT (SPECT), with images analyzed in blinded independent core laboratories. The time interval between the two imaging studies was a mean of 9.4 days. Invasive coronary angiography served as the diagnostic reference standard in the 16-center, 8-country study, Dr. Marcelo F. Di Carli explained at the annual meeting of the American College of Cardiology.
The primary study endpoint was test accuracy as defined by the area under the receiver operating characteristic curve for identifying the 59% of subjects with at least a 50% stenosis by invasive coronary angiography. The rate was significantly better for CTA than SPECT: 89% vs. 69%.
CTA’s superior performance was driven by its greater sensitivity in detecting stenoses of 50% or more: 91% vs. 62% for SPECT. The two imaging modalities displayed similar specificity: 74% for CTA and 67% for SPECT.
CTA had a positive predictive value of 83% and a negative predictive value of 85%, compared with 73% and 55%, respectively, for SPECT, according to Dr. Di Carli of Brigham and Women’s Hospital, Boston.
The same pattern of results was seen with regard to diagnostic accuracy in detecting patients with at least a 70% stenosis, a prespecified secondary endpoint. CTA had 94% sensitivity, 60% specificity, a positive predictive value of 66%, and a negative predictive value of 92%. SPECT showed 72% sensitivity, 67% specificity, a 64% positive predictive value, and a 73% negative predictive value.
The average radiation dose was markedly lower with CTA: 3.54 mSv compared with 10.48 mSv for SPECT.
The study was funded by Toshiba. Dr. Di Carli reported having no relevant financial conflicts.
SAN FRANCISCO – Coronary CT angiography outperformed myocardial perfusion single-photon emission CT for the diagnosis of obstructive coronary artery disease in a prospective multicenter head-to-head comparative study.
The CORE320 (Coronary Artery Evaluation Using 320-Row Multidetector Computed Tomography Angiography and Myocardial Perfusion) study included 381 patients with suspected or known coronary artery disease (CAD) who were scheduled for invasive quantitative coronary angiography. But first they all underwent coronary CT angiography (CTA) and single-photon emission CT (SPECT), with images analyzed in blinded independent core laboratories. The time interval between the two imaging studies was a mean of 9.4 days. Invasive coronary angiography served as the diagnostic reference standard in the 16-center, 8-country study, Dr. Marcelo F. Di Carli explained at the annual meeting of the American College of Cardiology.
The primary study endpoint was test accuracy as defined by the area under the receiver operating characteristic curve for identifying the 59% of subjects with at least a 50% stenosis by invasive coronary angiography. The rate was significantly better for CTA than SPECT: 89% vs. 69%.
CTA’s superior performance was driven by its greater sensitivity in detecting stenoses of 50% or more: 91% vs. 62% for SPECT. The two imaging modalities displayed similar specificity: 74% for CTA and 67% for SPECT.
CTA had a positive predictive value of 83% and a negative predictive value of 85%, compared with 73% and 55%, respectively, for SPECT, according to Dr. Di Carli of Brigham and Women’s Hospital, Boston.
The same pattern of results was seen with regard to diagnostic accuracy in detecting patients with at least a 70% stenosis, a prespecified secondary endpoint. CTA had 94% sensitivity, 60% specificity, a positive predictive value of 66%, and a negative predictive value of 92%. SPECT showed 72% sensitivity, 67% specificity, a 64% positive predictive value, and a 73% negative predictive value.
The average radiation dose was markedly lower with CTA: 3.54 mSv compared with 10.48 mSv for SPECT.
The study was funded by Toshiba. Dr. Di Carli reported having no relevant financial conflicts.
AT ACC 13
Major Finding: Coronary CT angiography had a 91% sensitivity and a 74% specificity for the detection of at least 50% stenosis in 381 patients with known or suspected CAD, superior to the 62% sensitivity and 67% specificity for myocardial perfusion single-photon emission CT in the same patients.
Data Source: CORE320, an ongoing prospective 16-center study comparing the diagnostic accuracy of two widely utilized noninvasive imaging methods in detecting obstructive CAD, with invasive quantitative coronary angiography serving as the reference standard.
Disclosures: The study is funded by Toshiba. The presenter reported having no relevant financial conflicts.