User login
Spinal MRI does not enhance spondyloarthropathy diagnosis
MADRID – There is no added benefit of performing spinal MRI in the diagnosis of spondyloarthritis, the results of an international, multicenter study suggest.
Around one-quarter of patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had a negative MRI scan of the sacroiliac joints (SIJs) were reclassified as being positive for SpA by a combined evaluation of SIJ MRI and spinal MRI scans.
However, 17.5% of healthy volunteers and up to 26.8% of patients with mechanical back pain who had a negative SIJ MRI were also reclassified as having SpA. This false-positive result balances out the value of combined spinal and SIJ MRI.
"Combined MRI added little incremental value compared to SIJ MRI alone for diagnosis of nr-axSpA," said Dr. Ulrich Weber, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72:145).
"Although you get about 20% more patients – which is the good news – we found about the same magnitude of false-positive controls," he said in an interview. He added that he "was very disappointed with this result, and these data need confirming." Dr. Weber collected data for the study while at the Balgrist University Clinic in Zurich, and also as a visiting professor in the rheumatology department at the University of Alberta, Edmonton.
SIJ MRI is a major criterion of the Assessment of SpondyloArthritis classification criteria for ankylosing spondylitis (AS). If a patient is strongly suspected of having early SpA, but this is not yet visible on radiographs, then an SIJ MRI is often the next step. If this is negative, however, it may be unclear what to do next.
"Our question was, ‘Would it help to order an additional spinal MRI in this situation?’ " Dr. Weber explained. These data suggest that it is not.
The collaborative study included 130 individuals with newly diagnosed back pain and aged 50 years or younger who were recruited from clinics based in Canada, China, Denmark, and Switzerland, as well as 20 healthy control individuals in whom MRI scans of the SIJ and spine were available.
The investigators used a clinical examination and pelvic radiography to stratify patients into groups, with 50 found to have nr-axSpA, 33 with AS, and 47 with mechanical back pain.
Three separate researchers blinded to the initial stratification read the MRI of the SIJ. An MRI of the spine was then performed 6 months later, with a combined SIJ/spinal scan done 1-12 months later. The presence or absence of SpA was determined in these scans, and comparisons were made between the results for MRI of the SIJ alone versus spinal MRI alone, as well as for the SIJ alone versus a combined read of both the spinal and SIJ MRI scans.
Dr. Weber noted that he would not recommend changing current practice as a result of this study. Further data are eagerly awaited from an ongoing Danish initiative that hopes to scan and assess around 2,000 whole-body MRIs in patients with suspected spondyloarthropathy by the end of the year. "This study will be very informative and very important for us because this is a large sample size. Preliminary data on about 1,000 MRIs point in the same direction," he observed.
Other data from the study, which Dr. Weber presented separately at the meeting, looked at the frequency and possible reasons for false-positive results with spinal MRI in the control groups (Ann. Rheum. Dis. 2013;72:125).
"Patients with mechanical back pain and healthy volunteers may show spinal MRI lesions suggestive of spondyloarthritis, such as corner inflammatory lesions or corner fat lesions," he explained. "We found that about 30% of those controls were misclassified as having spondyloarthritis by evaluation of the spinal MRI alone, so without SIJ MRI," said Dr. Weber. Bone marrow edema and fat infiltration were the MRI lesions largely responsible for this misclassification.
Dr. Weber said that, at least in Switzerland, general practitioners were more likely to order a spinal MRI than an SIJ MRI to assess a young patient with nonspecific back pain. "We think that caution is needed if a classification of SpA is based on MRI of the spine alone," he concluded.
Dr. Weber had no disclosures.
MADRID – There is no added benefit of performing spinal MRI in the diagnosis of spondyloarthritis, the results of an international, multicenter study suggest.
Around one-quarter of patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had a negative MRI scan of the sacroiliac joints (SIJs) were reclassified as being positive for SpA by a combined evaluation of SIJ MRI and spinal MRI scans.
However, 17.5% of healthy volunteers and up to 26.8% of patients with mechanical back pain who had a negative SIJ MRI were also reclassified as having SpA. This false-positive result balances out the value of combined spinal and SIJ MRI.
"Combined MRI added little incremental value compared to SIJ MRI alone for diagnosis of nr-axSpA," said Dr. Ulrich Weber, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72:145).
"Although you get about 20% more patients – which is the good news – we found about the same magnitude of false-positive controls," he said in an interview. He added that he "was very disappointed with this result, and these data need confirming." Dr. Weber collected data for the study while at the Balgrist University Clinic in Zurich, and also as a visiting professor in the rheumatology department at the University of Alberta, Edmonton.
SIJ MRI is a major criterion of the Assessment of SpondyloArthritis classification criteria for ankylosing spondylitis (AS). If a patient is strongly suspected of having early SpA, but this is not yet visible on radiographs, then an SIJ MRI is often the next step. If this is negative, however, it may be unclear what to do next.
"Our question was, ‘Would it help to order an additional spinal MRI in this situation?’ " Dr. Weber explained. These data suggest that it is not.
The collaborative study included 130 individuals with newly diagnosed back pain and aged 50 years or younger who were recruited from clinics based in Canada, China, Denmark, and Switzerland, as well as 20 healthy control individuals in whom MRI scans of the SIJ and spine were available.
The investigators used a clinical examination and pelvic radiography to stratify patients into groups, with 50 found to have nr-axSpA, 33 with AS, and 47 with mechanical back pain.
Three separate researchers blinded to the initial stratification read the MRI of the SIJ. An MRI of the spine was then performed 6 months later, with a combined SIJ/spinal scan done 1-12 months later. The presence or absence of SpA was determined in these scans, and comparisons were made between the results for MRI of the SIJ alone versus spinal MRI alone, as well as for the SIJ alone versus a combined read of both the spinal and SIJ MRI scans.
Dr. Weber noted that he would not recommend changing current practice as a result of this study. Further data are eagerly awaited from an ongoing Danish initiative that hopes to scan and assess around 2,000 whole-body MRIs in patients with suspected spondyloarthropathy by the end of the year. "This study will be very informative and very important for us because this is a large sample size. Preliminary data on about 1,000 MRIs point in the same direction," he observed.
Other data from the study, which Dr. Weber presented separately at the meeting, looked at the frequency and possible reasons for false-positive results with spinal MRI in the control groups (Ann. Rheum. Dis. 2013;72:125).
"Patients with mechanical back pain and healthy volunteers may show spinal MRI lesions suggestive of spondyloarthritis, such as corner inflammatory lesions or corner fat lesions," he explained. "We found that about 30% of those controls were misclassified as having spondyloarthritis by evaluation of the spinal MRI alone, so without SIJ MRI," said Dr. Weber. Bone marrow edema and fat infiltration were the MRI lesions largely responsible for this misclassification.
Dr. Weber said that, at least in Switzerland, general practitioners were more likely to order a spinal MRI than an SIJ MRI to assess a young patient with nonspecific back pain. "We think that caution is needed if a classification of SpA is based on MRI of the spine alone," he concluded.
Dr. Weber had no disclosures.
MADRID – There is no added benefit of performing spinal MRI in the diagnosis of spondyloarthritis, the results of an international, multicenter study suggest.
Around one-quarter of patients with nonradiographic axial spondyloarthritis (nr-axSpA) who had a negative MRI scan of the sacroiliac joints (SIJs) were reclassified as being positive for SpA by a combined evaluation of SIJ MRI and spinal MRI scans.
However, 17.5% of healthy volunteers and up to 26.8% of patients with mechanical back pain who had a negative SIJ MRI were also reclassified as having SpA. This false-positive result balances out the value of combined spinal and SIJ MRI.
"Combined MRI added little incremental value compared to SIJ MRI alone for diagnosis of nr-axSpA," said Dr. Ulrich Weber, who presented the findings at the annual European Congress of Rheumatology (Ann. Rheum. Dis. 2013;72:145).
"Although you get about 20% more patients – which is the good news – we found about the same magnitude of false-positive controls," he said in an interview. He added that he "was very disappointed with this result, and these data need confirming." Dr. Weber collected data for the study while at the Balgrist University Clinic in Zurich, and also as a visiting professor in the rheumatology department at the University of Alberta, Edmonton.
SIJ MRI is a major criterion of the Assessment of SpondyloArthritis classification criteria for ankylosing spondylitis (AS). If a patient is strongly suspected of having early SpA, but this is not yet visible on radiographs, then an SIJ MRI is often the next step. If this is negative, however, it may be unclear what to do next.
"Our question was, ‘Would it help to order an additional spinal MRI in this situation?’ " Dr. Weber explained. These data suggest that it is not.
The collaborative study included 130 individuals with newly diagnosed back pain and aged 50 years or younger who were recruited from clinics based in Canada, China, Denmark, and Switzerland, as well as 20 healthy control individuals in whom MRI scans of the SIJ and spine were available.
The investigators used a clinical examination and pelvic radiography to stratify patients into groups, with 50 found to have nr-axSpA, 33 with AS, and 47 with mechanical back pain.
Three separate researchers blinded to the initial stratification read the MRI of the SIJ. An MRI of the spine was then performed 6 months later, with a combined SIJ/spinal scan done 1-12 months later. The presence or absence of SpA was determined in these scans, and comparisons were made between the results for MRI of the SIJ alone versus spinal MRI alone, as well as for the SIJ alone versus a combined read of both the spinal and SIJ MRI scans.
Dr. Weber noted that he would not recommend changing current practice as a result of this study. Further data are eagerly awaited from an ongoing Danish initiative that hopes to scan and assess around 2,000 whole-body MRIs in patients with suspected spondyloarthropathy by the end of the year. "This study will be very informative and very important for us because this is a large sample size. Preliminary data on about 1,000 MRIs point in the same direction," he observed.
Other data from the study, which Dr. Weber presented separately at the meeting, looked at the frequency and possible reasons for false-positive results with spinal MRI in the control groups (Ann. Rheum. Dis. 2013;72:125).
"Patients with mechanical back pain and healthy volunteers may show spinal MRI lesions suggestive of spondyloarthritis, such as corner inflammatory lesions or corner fat lesions," he explained. "We found that about 30% of those controls were misclassified as having spondyloarthritis by evaluation of the spinal MRI alone, so without SIJ MRI," said Dr. Weber. Bone marrow edema and fat infiltration were the MRI lesions largely responsible for this misclassification.
Dr. Weber said that, at least in Switzerland, general practitioners were more likely to order a spinal MRI than an SIJ MRI to assess a young patient with nonspecific back pain. "We think that caution is needed if a classification of SpA is based on MRI of the spine alone," he concluded.
Dr. Weber had no disclosures.
AT THE EULAR CONGRESS 2013
Major finding: More than 25% of patients with nonradiographic axial spondyloarthritis who had a negative MRI scan of the sacroiliac joints (SIJs) were reclassified as being positive for spondyloarthritis by a combined evaluation of SIJ MRI and spinal MRI scans, but this was balanced by a similarly high rate of false-positive results.
Data source: An international, multicenter study of combined SIJ and spinal MRI in 130 patients with newly diagnosed back pain and 20 healthy controls.
Disclosures: Dr. Weber had no disclosures.
Isolated Sciatic Nerve Entrapment by Ectopic Bone After Femoral Head Fracture-Dislocation
Solitary Plasmacytoma of the Medial Clavicle
Type IIb Bony Mallet Finger: Is Anatomical Reduction of the Fracture Necessary?
Histologic Analysis of Postmeniscectomy Osteonecrosis
Hyperflexion Injury of the Thumb
Flexion Deformity of the Fifth Digit
Multiple intracardiac thrombi
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
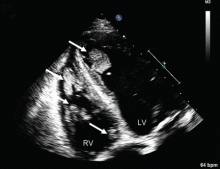
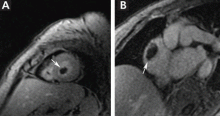
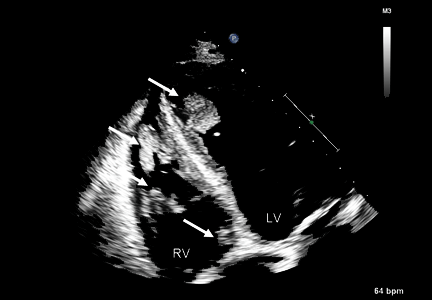
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
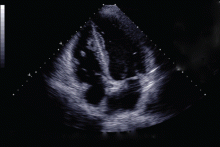
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
MRI detects high level of subclinical small joint inflammation
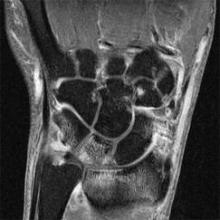
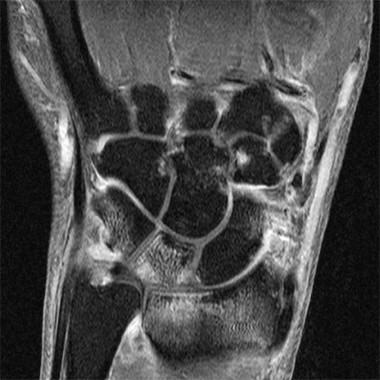
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
MADRID – A high percentage of patients with early arthritis have inflammation of the small joints that can be detected with MRI but not by physical examination.
Results of a cross-sectional study, presented by Dr. Annemarie Krabben at the annual European Congress of Rheumatology, found that 66% of wrist, 27% of metacarpophalangeal (MCP), and 13% of metatarsophalangeal (MTP) joints that were not clinically swollen showed signs of inflammation on MRI. However, inflammation on MRI was present in 92% of wrists, 86% of MCP, and 29% of MTP joints that were clinically swollen.
"You would expect that inflammation on MRI would be present in the clinically swollen joints, but we also saw inflammation in the non-swollen joints," explained Dr. Krabben of Leiden University Medical Center in the Netherlands.
Furthermore, "when you look at the joints with MRI-detected inflammation, a lot of these didn’t have clinical inflammation," she added.
Clinical joint swelling was absent but signs of bone marrow edema were detected on MRI in 60% of wrist, 53% of MCP, and 78% of MTP joints. If severe MRI-detected edema was considered, joint swelling was absent in 35%, 39%, and 58% of wrist, MCP, and MTP joints, respectively. Joints without clinical swelling showed signs of inflammation on MRI in 61% of wrist, 64% of MCP, and 77% of MTP joints.
The study involved patients with early arthritis who were part of the Leiden Early Arthritis Clinic cohort. This cohort was established in 1993 to detect and treat inflammatory disorders early in the disease state (Rheumatology [Oxford] 2011;50:93-100).
Upon entry into the cohort, patients underwent a physical examination that included 68 tender and 66 swollen joint counts and 1.5 Tesla MRI of the wrist, MCP, and MTP joints. The latter were used to determine the presence and extent of synovitis, bone marrow edema, and tenosynovitis.
In total, 1,790 small joints were examined in 179 patients who had a median duration of symptoms of 15 weeks. Overall, 30% of wrist, 15% of MCP, and 11% of MTP joints were swollen at physical examination and the majority also showed inflammation on MRI.
"There was a lot of subclinical inflammation, especially bone marrow edema, in the nonswollen joints," Dr. Krabben said. Bone marrow edema is linked to erosive disease progression, she observed and suggested that the next step is to see what happens to patients with subclinical inflammation at baseline, and whether this will eventually progress to erosive disease.
The study was supported by the Dutch Arthritis Foundation (Reumafonds), the Netherlands Organization for Health Research and Development, and the Center for Translational Molecular Medicine. Dr. Krabben has received research funding from Reumafonds.
AT THE EULAR CONGRESS 2013
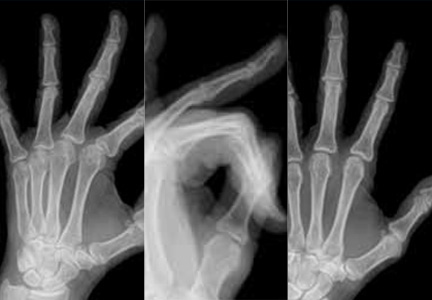
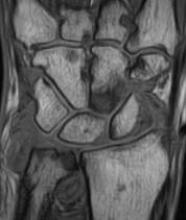
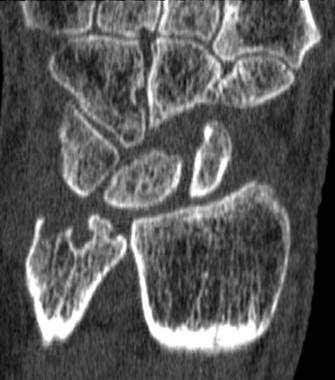
MRI score of joint narrowing has research promise
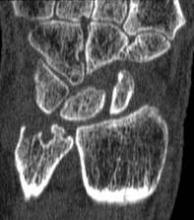
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
A magnetic resonance imaging scoring system of joint-space narrowing in rheumatoid arthritis showed "a very high" agreement with computed tomography scores and may become a useful tool in rheumatoid arthritis clinical trials after further validation, judging from data presented by Dr. Uffe Møller Døhn.
In a small study, which was conducted to validate the OMERACT-RAMRIS MRI JSN scoring system in the wrists and metacarpophalangeal (MCP) joints, there was a very high agreement between the joint-space narrowing scores on MRI and CT and moderate agreement between scores on MRI and x-ray, said Dr. Møller Døhn of Copenhagen University Hospital at Glostrup at the annual European Congress of Rheumatology. In addition, there was "high to very high" inter- and intrareader reliability, particularly for the wrist joints.
An OMERACT (Outcome Measures in Rheumatology) initiative, this scoring system is being developed to provide a more precise and sensitive method of measuring joint space damage in patients with rheumatoid arthritis (RA), but it needs to be validated through comparisons to other imaging methods.
To evaluate the degree of agreement with CT and x-ray scores, this study assessed MRI and CT images of the wrist and the second to fifth metacarpophalangeal (MCP 2-5) joints of 14 people with RA and one healthy control, who were from a clinical trial. Three readers assessed the images twice, and a single reader scored x-rays using the Sharp-Van der Heidje method, said Dr. Møller Døhn, who is in the center for rheumatology and spine diseases at the hospital.
The MRI scores of joint space narrowing "were very highly correlated" with CT scores, when comparing the wrist and MCP scores both separately and combined: Using intraclass correlation coefficients (ICCs) as a measure of agreement between scores and scorers, the MRI and CT scores for joint space narrowing were 0.94 for the MCP joints, 0.92 for the wrist, and 0.92 for the wrist and MCP joints combined. But the ICCs for the x-ray joint space narrowing scores were lower: With MRI scores, the ICCs were 0.49 for the MCP 2-5 joints and 0.55 for the wrist. With CT scores, the ICCs were 0.56 for the MCP 2-5 joints and 0.43 for the wrist.
"The most important next step is to test the scoring system in a longitudinal setting, in order to investigate the sensitivity to change," Dr. Møller Døhn said in an interview before the congress. "Before the system can be implemented as an outcome measure in clinical trials, we need to know if it is more sensitive than other methods that are already available. If it turns out that [joint space narrowing] assessment of several joints on x-ray is just as good as - or better than - MRI, then it does not add information to what we already use today."
Dr. Møller Døhn reported that he had no relevant financial disclosures.
FROM THE EULAR CONGRESS 2013