User login
FDA finalizes medical device cybersecurity guidance
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.
The Food and Drug Administration is calling on device manufacturers that have network-connected devices to consider cybersecurity risks as part of product design.
The agency finalized guidance recommending that manufacturers submit documentation to the FDA about identified risks and the controls in place to mitigate the risks, as well as about how manufacturers plan to update software as risks are discovered.
“There is no such thing as a threat-proof medical device,” Suzanne Schwartz, director of emergency preparedness/operations and medical countermeasures at the FDA’s Center for Devices and Radiological Health, said in a statement. “It is important for medical device manufacturers to remain vigilant about cybersecurity and to appropriately protect patients from those risks.”
While directed at manufacturers, the guidance notes that medical device security “is a shared responsibility” across all stakeholders, including health care facilities, physicians, patients, and manufacturers.
FDA will host a 2-day workshop on Oct. 21-22, 2014, to discuss the guidance and collaborative approaches to medical device cybersecurity.
Emergency Ultrasound: Bedside Ultrasound for Ocular Emergencies
Ocular Ultrasound
Since the eye is predominantly composed of fluid, it is the perfect organ for ultrasound evaluation. As shown in a prospective observational study by Blaivas et al in 2002,1 the use of bedside ultrasound in the ED can assist in the rapid diagnosis of time-sensitive conditions such as retinal detachment, vitreous hemorrhage, and lens dislocation.
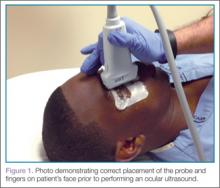
Using a high-frequency transducer, the clinician should place a tegaderm barrier over the closed eyelid of the reclined patient before next applying copious amounts of sterile gel to the top of the barrier (ultrasound gel is not bacteriostatic). Then he or she should brace a hand on the patient’s forehead or cheek to control the downward pressure exerted on the transducer (Figure 1). The transducer will sit on top of the gel and make indirect contact with the eyelid.
It is important to note that while ocular ultrasound is an excellent imaging modality, it is contraindicated in patients in whom globe rupture is suspected or obvious due to the risk of pressing on the globe with resulting increase in intraocular pressure.
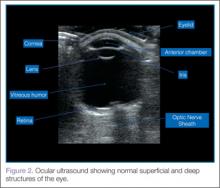
When performing ocular ultrasound, it is recommended that the clinician should scan/sweep the normal eye first and then the abnormal eye. Figure 2 demonstrates the ocular structures easily visualized by ultrasound.. Both eyes should be imaged in at least 2 planes. In addition, the patient should be instructed to look through all of the cardinal positions of gaze to maximize the ocular structures visualized.
Over-gaining the image is also helpful when looking at the posterior chamber for hemorrhage or retinal detachment. However, the time spent scanning the eye should be limited, since ultrasound could theoretically cause heating and cavitation over a prolonged period of time.
Retinal Detachment
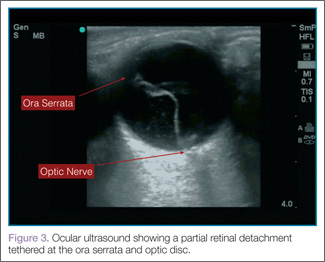
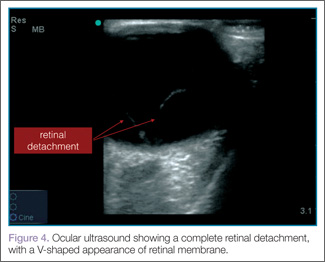
When evaluating for retinal detachment, one should focus on the posterior chamber of the eye and turn-up the far-field gain. The retina is firmly attached at two sites: the optic nerve posteriorly and the ora serrata anteriorly. Even with a complete retinal detachment, the retina is tethered at these points. Therefore, during examination, look for a hyperechoic membrane floating and moving within the vitreous body, but tethered anteriorly and posteriorly when the patient moves his or her eyes. Figures 3 and 4 are examples of ultrasound demonstrating partial and complete retinal detachment.
| 
|
Vitreous Hemorrhage
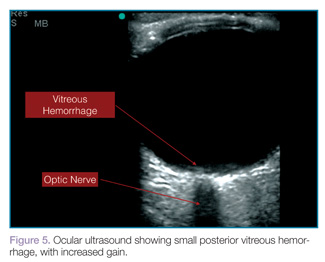
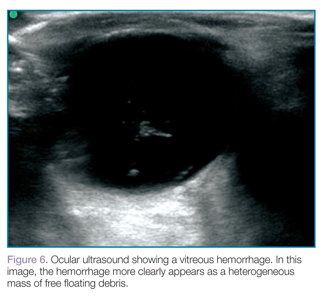
Vitreous hemorrhages will appear as hyperechoic particles or opacities within the posterior chamber (Figures 5 and 6). The appearance of hemorrhage will vary depending on age and degree, and can have the appearance of swirling or rotating particles during eye movement, resembling the movement of clothes in a washing machine.
| 
|
Lens Dislocation
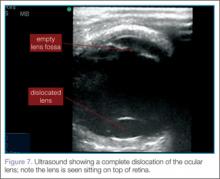
Lens dislocations are quite easy to visualize on ultrasound and are divided into partial or full dislocations. Complete dislocations are diagnosed when the lens is out of the expected position, dislodged into the anterior chamber or vitreous (Figure 7). Partial dislocations are harder to diagnose and require careful attention during active range of movement of the eye—the lens will appear to move independent of the rest of the iris/ciliary body complex.2
Conclusion
Bedside ultrasound performed in the ED can rapidly and accurately evaluate and diagnose ocular emergencies, facilitating prompt treatment.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
- Blaivas M, Theodoro D, Sierzenski PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002;9(8):791-799.
- Roque PJ, Hatch N, Barr L, Wu TS. Bedside ocular ultrasound. Crit Care Clin. 2014;30(2):227-241.
For a video clip showing a retinal detachment with acute bleeding behind the retina, visit the authors’ Web site at http://www.em.emory.edu/ultrasound/ImageWeek/lens_dislocation.
For a video clip showing the appearance of a vitreous hemorrhage as a swirling action with eye movement, visit http://youtu.be/psE1znjBF2o.
Ocular Ultrasound
Since the eye is predominantly composed of fluid, it is the perfect organ for ultrasound evaluation. As shown in a prospective observational study by Blaivas et al in 2002,1 the use of bedside ultrasound in the ED can assist in the rapid diagnosis of time-sensitive conditions such as retinal detachment, vitreous hemorrhage, and lens dislocation.
Using a high-frequency transducer, the clinician should place a tegaderm barrier over the closed eyelid of the reclined patient before next applying copious amounts of sterile gel to the top of the barrier (ultrasound gel is not bacteriostatic). Then he or she should brace a hand on the patient’s forehead or cheek to control the downward pressure exerted on the transducer (Figure 1). The transducer will sit on top of the gel and make indirect contact with the eyelid.
It is important to note that while ocular ultrasound is an excellent imaging modality, it is contraindicated in patients in whom globe rupture is suspected or obvious due to the risk of pressing on the globe with resulting increase in intraocular pressure.
When performing ocular ultrasound, it is recommended that the clinician should scan/sweep the normal eye first and then the abnormal eye. Figure 2 demonstrates the ocular structures easily visualized by ultrasound.. Both eyes should be imaged in at least 2 planes. In addition, the patient should be instructed to look through all of the cardinal positions of gaze to maximize the ocular structures visualized.
Over-gaining the image is also helpful when looking at the posterior chamber for hemorrhage or retinal detachment. However, the time spent scanning the eye should be limited, since ultrasound could theoretically cause heating and cavitation over a prolonged period of time.
Retinal Detachment
When evaluating for retinal detachment, one should focus on the posterior chamber of the eye and turn-up the far-field gain. The retina is firmly attached at two sites: the optic nerve posteriorly and the ora serrata anteriorly. Even with a complete retinal detachment, the retina is tethered at these points. Therefore, during examination, look for a hyperechoic membrane floating and moving within the vitreous body, but tethered anteriorly and posteriorly when the patient moves his or her eyes. Figures 3 and 4 are examples of ultrasound demonstrating partial and complete retinal detachment.

| 
|
Vitreous Hemorrhage
Vitreous hemorrhages will appear as hyperechoic particles or opacities within the posterior chamber (Figures 5 and 6). The appearance of hemorrhage will vary depending on age and degree, and can have the appearance of swirling or rotating particles during eye movement, resembling the movement of clothes in a washing machine.

| 
|
Lens Dislocation
Lens dislocations are quite easy to visualize on ultrasound and are divided into partial or full dislocations. Complete dislocations are diagnosed when the lens is out of the expected position, dislodged into the anterior chamber or vitreous (Figure 7). Partial dislocations are harder to diagnose and require careful attention during active range of movement of the eye—the lens will appear to move independent of the rest of the iris/ciliary body complex.2
Conclusion
Bedside ultrasound performed in the ED can rapidly and accurately evaluate and diagnose ocular emergencies, facilitating prompt treatment.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
Ocular Ultrasound
Since the eye is predominantly composed of fluid, it is the perfect organ for ultrasound evaluation. As shown in a prospective observational study by Blaivas et al in 2002,1 the use of bedside ultrasound in the ED can assist in the rapid diagnosis of time-sensitive conditions such as retinal detachment, vitreous hemorrhage, and lens dislocation.
Using a high-frequency transducer, the clinician should place a tegaderm barrier over the closed eyelid of the reclined patient before next applying copious amounts of sterile gel to the top of the barrier (ultrasound gel is not bacteriostatic). Then he or she should brace a hand on the patient’s forehead or cheek to control the downward pressure exerted on the transducer (Figure 1). The transducer will sit on top of the gel and make indirect contact with the eyelid.
It is important to note that while ocular ultrasound is an excellent imaging modality, it is contraindicated in patients in whom globe rupture is suspected or obvious due to the risk of pressing on the globe with resulting increase in intraocular pressure.
When performing ocular ultrasound, it is recommended that the clinician should scan/sweep the normal eye first and then the abnormal eye. Figure 2 demonstrates the ocular structures easily visualized by ultrasound.. Both eyes should be imaged in at least 2 planes. In addition, the patient should be instructed to look through all of the cardinal positions of gaze to maximize the ocular structures visualized.
Over-gaining the image is also helpful when looking at the posterior chamber for hemorrhage or retinal detachment. However, the time spent scanning the eye should be limited, since ultrasound could theoretically cause heating and cavitation over a prolonged period of time.
Retinal Detachment
When evaluating for retinal detachment, one should focus on the posterior chamber of the eye and turn-up the far-field gain. The retina is firmly attached at two sites: the optic nerve posteriorly and the ora serrata anteriorly. Even with a complete retinal detachment, the retina is tethered at these points. Therefore, during examination, look for a hyperechoic membrane floating and moving within the vitreous body, but tethered anteriorly and posteriorly when the patient moves his or her eyes. Figures 3 and 4 are examples of ultrasound demonstrating partial and complete retinal detachment.

| 
|
Vitreous Hemorrhage
Vitreous hemorrhages will appear as hyperechoic particles or opacities within the posterior chamber (Figures 5 and 6). The appearance of hemorrhage will vary depending on age and degree, and can have the appearance of swirling or rotating particles during eye movement, resembling the movement of clothes in a washing machine.

| 
|
Lens Dislocation
Lens dislocations are quite easy to visualize on ultrasound and are divided into partial or full dislocations. Complete dislocations are diagnosed when the lens is out of the expected position, dislodged into the anterior chamber or vitreous (Figure 7). Partial dislocations are harder to diagnose and require careful attention during active range of movement of the eye—the lens will appear to move independent of the rest of the iris/ciliary body complex.2
Conclusion
Bedside ultrasound performed in the ED can rapidly and accurately evaluate and diagnose ocular emergencies, facilitating prompt treatment.
Dr Taylor is an assistant professor and director of postgraduate medical education, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Meer is an assistant professor and director of emergency ultrasound, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia. Dr Beck is an assistant professor, department of emergency medicine, Emory University School of Medicine, Atlanta, Georgia.
- Blaivas M, Theodoro D, Sierzenski PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002;9(8):791-799.
- Roque PJ, Hatch N, Barr L, Wu TS. Bedside ocular ultrasound. Crit Care Clin. 2014;30(2):227-241.
For a video clip showing a retinal detachment with acute bleeding behind the retina, visit the authors’ Web site at http://www.em.emory.edu/ultrasound/ImageWeek/lens_dislocation.
For a video clip showing the appearance of a vitreous hemorrhage as a swirling action with eye movement, visit http://youtu.be/psE1znjBF2o.
- Blaivas M, Theodoro D, Sierzenski PR. A study of bedside ocular ultrasonography in the emergency department. Acad Emerg Med. 2002;9(8):791-799.
- Roque PJ, Hatch N, Barr L, Wu TS. Bedside ocular ultrasound. Crit Care Clin. 2014;30(2):227-241.
For a video clip showing a retinal detachment with acute bleeding behind the retina, visit the authors’ Web site at http://www.em.emory.edu/ultrasound/ImageWeek/lens_dislocation.
For a video clip showing the appearance of a vitreous hemorrhage as a swirling action with eye movement, visit http://youtu.be/psE1znjBF2o.
My Most Unusual Case: Cesarean Scar Ectopic Pregnancy
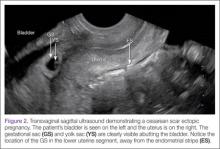
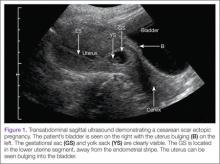
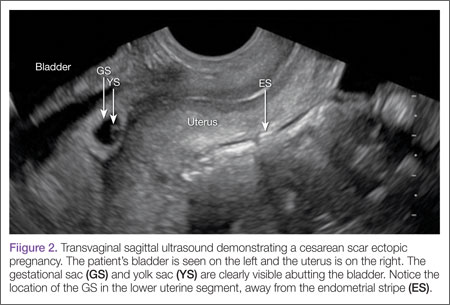
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
Cesarean scar ectopic pregnancy (CSEP) is a challenging diagnosis that warrants consideration when performing ultrasound on a pregnant patient with a previous history of cesarean delivery. It is suspected when ballooning of the lower uterine segment is noted on ultrasound,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2
Ectopic Pregnancy
Ectopic pregnancy affects approximately 2% of all pregnancies and is the leading cause of first-trimester maternal mortality.3 As front-line care providers, it is imperative that emergency physicians (EPs) recognize cases of ectopic implantation to avoid devastating outcomes.
The majority of ectopic pregnancies (97%) are located in the fallopian tubes; however, many other locations are possible, including implantation in the scar from a previous cesarean delivery.1,4 The frequency of such ectopic pregnancies is on the rise, consistent with the increasing number of cesarean deliveries performed worldwide.5 These cases present a special diagnostic challenge because patients often present asymptomatically or with painless vaginal bleeding; moreover, visualization via bedside ultrasound can be deceiving,5 and it is easy to mistake a CSEP for a viable intrauterine pregnancy.
Case
A 22-year-old woman with type 1 diabetes mellitus (DM) presented to the ED complaining of 3 days of worsening nausea and elevated blood glucose levels. She stated that although she had been taking her insulin regimen as prescribed, her symptoms progressively worsened. On the day of presentation, she developed moderate diffuse nonradiating dull abdominal pain and had several episodes of nonbloody, nonbilious emesis. She denied being pregnant and stated that her last menstrual period was 14 days ago; she further denied any vaginal discharge or bleeding. A review of her systems was otherwise benign.
In addition to type 1 DM, the patient also had a history of migraine headaches and an obstetric history of gravida 3, para 3, aborta 0. Each birth was via cesarean delivery and without complication. Her current medications included insulin glargine (Lantus) 25 units subcutaneously every night at bedtime; insulin aspart (Novolog) 7 units subcutaneously three times a day; zolpidem (Ambien) 10 mg orally every night at bedtime. A chart review was notable for several presentations of diabetic ketoacidosis (DKA) secondary to noncompliance with her diabetes regimen.
Physical examination was notable for a well-developed, well-nourished 22 year old that appeared uncomfortable but in no acute distress. Her abdomen was soft and nondistended, with diffuse moderate tenderness to palpation but no rebound or guarding. The remainder of the physical
The initial workup revealed DKA and pregnancy. Significant laboratory values included: finger-stick blood glucose, 441 mg/dL; serum ketones, 2.1 mmol/L (normal range, 0.0-0.5); anion gap, 15; and urinalysis 4+ glucose, 2+ ketones; and quantitative β-human chorionic gonadotropin (β-HCG), 5,282 IU/L (normal range, 0-5.0 IU/L ).examination was otherwise benign.
After receiving insulin, intravenous (IV) fluids, pain medication, and antiemetics, the patient stated she felt much better. She was then admitted to the inpatient floor for management of DKA and discharged uneventfully several days later. Emergency bedside transabdominal and transvaginal ultrasounds were performed by the emergency staff and identified an intrauterine gestational sac and yolk sac. The EP ordered a consultation with an obstetrician-gynecologist (OB-GYN), who saw the patient in the ED and agreed with the findings, and noted the gestational sac was consistent with a date of 5 weeks, 1 day.
Six days after discharge, however, she returned to the ED complaining of several days of weakness, vomiting, and lower abdominal pain. Significant laboratory values included: urinalysis with 4+ ketones, 1+ bacteria, + nitrites; and quantitative β-HCG 25,925 IU/L (expected range, 0-5.0); serum glucose 206 mg/dL; serum ketones 0.8 (expected range, 0-0.5); and anion gap, 12.
An emergency ultrasound identified a gestational sac, yolk sac, fetal pole, and fetal heart tones; an OB-GYN ultrasound had consistent findings, with an estimated gestational age of 6 weeks, 6 days. The patient responded well to IV fluids and antiemetics, and was asymptomatic when she was admitted to the ED observation unit for continued monitoring, fluids, and antiemetics as needed. Several hours later she again began to complain of nausea, vomiting, and poorly localized abdominal discomfort. As these symptoms persisted, the OB-GYN team returned to reevaluate the patient.
Discussion
Cesarean scar ectopic pregnancy was first described in the obstetric literature in 1978 and originally thought to be an exceedingly rare occurrence.5,6 With both the increasing number of cesarean deliveries performed and improvement in imaging technology, it is now believed that uterine scar ectopic pregnancy makes up as much as 6.1% of ectopic pregnancies in patients with a prior cesarean delivery.7 This diagnosis is well-documented in the obstetric and radiology literature, yet has never been discussed in an emergency medicine publication. Searching through both Pubmed and EMBase using the terms “cesarean” and “ectopic” yields no EM literature on the topic of CSEP. This is concerning because ultrasound of the pregnant patient is now a routine function of EPs.
Clinical history can be helpful in differentiating CSEP from alternative diagnoses. Patients undergoing spontaneous abortion are more likely to have lower abdominal cramping and experience greater loss of blood. While there is no correlation between the number of cesarean deliveries a woman has had and the likelihood of developing a CSEP, factors that impede myometrial healing (eg, preterm cesarean, cesarean after arrest of first stage of labor, chorioamnionitis) do, however, increase a patient’s risk of developing CSEP.5
Similar to tubal ectopic pregnancy, CSEP oftentimes presents early with mild, nonspecific symptoms. Thirty-nine percent of cases present with light, painless vaginal bleeding while only 25% present with abdominal pain. Moreover, 37% of cases are asymptomatic at the time of diagnosis.5
One study by found the mean gestational age at diagnosis to be 7.5 weeks.5 Delayed diagnosis places the patient at risk for uterine rupture, hemorrhage, and maternal death, making suspicion and prompt diagnosis by bedside ED ultrasound essential.7,8
Regardless of one’s clinical suspicion, the diagnosis is made (or ruled out) through ultrasound. Uterine scar ectopic pregnancy is suspected when ballooning of the lower uterine segment is noted,1 when a trophoblast is seen at a presumed cesarean scar beneath the utero-vesicular fold, and when myometrium between the gestational sac and bladder wall is thin (<8 mm).2 As seen with this patient, the diagnosis is challenging as a uterine scar ectopic pregnancy can easily be mistaken for an intrauterine pregnancy. The clinician must make every effort to ensure that the pregnancy is surrounded by appropriate myometrium. It is much easier to diagnose an ectopic pregnancy far removed from the uterus, where the uterus and pregnancy are easily visualized and independent.
Management of patients with CSEP remains outside of the scope of EM, and there is no consensus among our colleagues in OB-GYN on optimal management of these patients. Options include systemic or local injection of methotrexate and potassium chloride, or minimally invasive surgery for removal.5
As bedside ultrasound by EPs becomes standard of care for first-trimester pregnancies, a greater awareness of emergent obstetric pathologies becomes necessary. Vigilance and proper ultrasound technique will enable the EP to make the diagnosis of CSEP, minimizing maternal morbidity and mortality.
Drs Haight and Watkins are residents in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri. Dr Kane is a clinical instructor in the division of emergency medicine, Washington University School of Medicine, Saint Louis, Missouri.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
- Moschos E, Sreenarasimhaiah S, Twickler DM. First-trimester diagnosis of cesarean scar ectopic pregnancy. J Clin Ultrasound. 2008;36(8):504-511.
- Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16(6): 592-593.
- Goldner TE, Lawson HW, Xia Z, Atrash HK. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42(6):73-85.
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25(2):123-130.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol. 2006;107(6):1373-1381.
- Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus—an unusual cause of postabortal haemorrhage. A case report. S Afr Med J. 1978;53(4):142-143.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Caesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004;23(3):247-253.
- Einenkel J, Stumpp P, Kösling S, Horn LC, Höckel M. A misdiagnosed case of caesarean scar pregnancy. Arch Gynecol Obstet. 2005;271(2):178-181.
First appropriate use criteria for transthoracic echocardiography released for suspected pediatric heart disease
Appropriate use criteria of transthoracic echocardiography for suspected pediatric heart disease in outpatient settings have been issued for the first time.
The criteria, a collaboration of nine societies, will appear on the websites of the American College of Cardiology, American Society of Echocardiography, and Society for Cardiovascular Angiography and Interventions.
“Of the various diagnostic modalities, echocardiography remains the most readily available, noninvasive, and highly diagnostic tool for assessing cardiac structure, function and hemodynamics in those with suspected cardiac disease,” said Dr. Robert Campbell of Emory University, Atlanta, and his associates. Of the 113 potential indications for first-time use of outpatient transthoracic echocardiography in children and adolescents that they considered, 53 were considered appropriate, 28 possibly appropriate, and 32 rarely appropriate (J. Am. Coll. Cardiol. 2014 Sept. 30 [doi:10.1016/j.jacc.2014.08.003]). The report addressed conditions such as arrhythmias and palpitations, syncope, murmurs, and systemic disorders.
The group collaborated with an Appropriate Use Criteria (AUC) task force and an independent rating panel to produce the report. The criteria do not address posttest follow-up, inpatient pediatric echocardiography, or the assessment of children with known cardiac abnormalities, the authors said.
The writing group reported no funding sources or conflicts of interest. Three members of the rating panel and one member of the AUC task force reported financial relationships with Siemens Medical Systems or Excellus BCBS.
Appropriate use criteria of transthoracic echocardiography for suspected pediatric heart disease in outpatient settings have been issued for the first time.
The criteria, a collaboration of nine societies, will appear on the websites of the American College of Cardiology, American Society of Echocardiography, and Society for Cardiovascular Angiography and Interventions.
“Of the various diagnostic modalities, echocardiography remains the most readily available, noninvasive, and highly diagnostic tool for assessing cardiac structure, function and hemodynamics in those with suspected cardiac disease,” said Dr. Robert Campbell of Emory University, Atlanta, and his associates. Of the 113 potential indications for first-time use of outpatient transthoracic echocardiography in children and adolescents that they considered, 53 were considered appropriate, 28 possibly appropriate, and 32 rarely appropriate (J. Am. Coll. Cardiol. 2014 Sept. 30 [doi:10.1016/j.jacc.2014.08.003]). The report addressed conditions such as arrhythmias and palpitations, syncope, murmurs, and systemic disorders.
The group collaborated with an Appropriate Use Criteria (AUC) task force and an independent rating panel to produce the report. The criteria do not address posttest follow-up, inpatient pediatric echocardiography, or the assessment of children with known cardiac abnormalities, the authors said.
The writing group reported no funding sources or conflicts of interest. Three members of the rating panel and one member of the AUC task force reported financial relationships with Siemens Medical Systems or Excellus BCBS.
Appropriate use criteria of transthoracic echocardiography for suspected pediatric heart disease in outpatient settings have been issued for the first time.
The criteria, a collaboration of nine societies, will appear on the websites of the American College of Cardiology, American Society of Echocardiography, and Society for Cardiovascular Angiography and Interventions.
“Of the various diagnostic modalities, echocardiography remains the most readily available, noninvasive, and highly diagnostic tool for assessing cardiac structure, function and hemodynamics in those with suspected cardiac disease,” said Dr. Robert Campbell of Emory University, Atlanta, and his associates. Of the 113 potential indications for first-time use of outpatient transthoracic echocardiography in children and adolescents that they considered, 53 were considered appropriate, 28 possibly appropriate, and 32 rarely appropriate (J. Am. Coll. Cardiol. 2014 Sept. 30 [doi:10.1016/j.jacc.2014.08.003]). The report addressed conditions such as arrhythmias and palpitations, syncope, murmurs, and systemic disorders.
The group collaborated with an Appropriate Use Criteria (AUC) task force and an independent rating panel to produce the report. The criteria do not address posttest follow-up, inpatient pediatric echocardiography, or the assessment of children with known cardiac abnormalities, the authors said.
The writing group reported no funding sources or conflicts of interest. Three members of the rating panel and one member of the AUC task force reported financial relationships with Siemens Medical Systems or Excellus BCBS.
Improved Coordination of Care for Patients with Abnormalities on Chest Imaging: The Rapid Access Chest and Lung Assessment Program
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
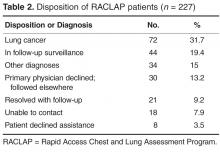
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
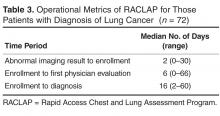
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
From the DeCesaris Cancer Institute, Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Objective: To describe the development and outcomes of a centralized evaluation service for patients with abnormalities on thoracic imaging to allow prompt and standardized review by an experienced multidisciplinary team.
- Methods: Patients with abnormal thoracic imaging studies, whether symptom-related or incidental, were referred to a specialized multidisciplinary team by radiologists, primary care physicians, or other providers. Recommendations for immediate or delayed follow-up were made based on professional society guidelines and patient characteristics. Follow-up was maintained within the program with close communication with primary care physicians.
- Results: 238 patients were referred over a 27-month period, 227 with abnormal findings on chest imaging. 171 patients (75%) accepted participation in the program. Radiologists were the most frequent referrers. Pulmonary symptoms were present in 74% of cases but were often unrelated to the findings. Patients and primary care physicians were contacted within a median of 2 days after imaging. Lung cancer was eventually diagnosed in 72 patients (32%), 51% with stage IA-IIB, at a median time of 16 days from first imaging. Physician satisfaction with the program was high.
- Conclusion: The program provided rapid and evidence-based evaluation and management of patients with thoracic imaging abnormalities, resulting in short time to diagnosis and high referring physician satisfaction.
Nonspecific abnormalities after chest imaging are a clinical dilemma for physicians and a source of anxiety for patients concerned about the possibility of malignancy. The range of abnormal findings most often involve the parenchyma but also can include nodal tissue, mediastinum, and the bony thorax. Often these findings are incidental to the symptoms that prompted the evaluation. For example, one study of over 12,000 abdominal computed tomography (CT) scans detected pulmonary nodules in 3% [1], and a study of 586 CT angiograms ordered in an emergency room found nodules in 13% and new adenopathy in 9% [2]. Furthermore, CT imaging in various lung cancer screening trials demonstrate that the prevalence of 1 or more pulmonary nodules is 8% to 51%, but the risk of malignancy is much less: 1.1% to 12% [3]. Indeed, it is estimated that due to a high prevalence of imaging, over 150,000 Americans are diagnosed with solitary pulmonary nodules (SPN) annually [2]. Although nodule characteristics such as size, shape, and stability over time can predict the likelihood of malignancy, the risk that any of these imaging abnormalities are related to a malignancy depends upon characteristics of both the lesion and the patient.
Given the nonspecific nature of many radiographic findings, management strategies and guidelines have been developed for several different types of imaging abnormalities [4–7]. However, gaps in the guidelines exist, and they often are not followed [8,9]. Radiologists are not adherent to any set of guidelines in as many as 64% of cases, despite a high level of awareness of such guidelines [10–13]. Recommendations that are not concordant with guidelines are more likely to involve excessively frequent imaging rather than inappropriately infrequent follow-up [13].
Actual cases of under- and over-imaging in surveillance and a single case of delayed diagnosis despite a radiology report highlighting a high-risk nodule prompted us to developed a centralized program to gather all patients with pulmonary imaging abnormalities into the hands of physicians most familiar with these abnormalities and the proper use of available diagnostic tools. The goals were to rely on existing guidelines tempered with clinical experience to advise patients and their primary care physicians, and to direct the most efficient diagnostic evaluation and management.
Methods
Setting
Anne Arundel Medical Center is a 385-bed acute care hospital in Annapolis, Maryland, with a medical staff of nearly 1000 physicians and mid-level providers. There are nearly 30,000 admissions and 95,000 emergency department visits annually. The medical center operates 5 regional diagnostic imaging sites that collectively perform 159,000 imaging studies annually, including 3995 chest CT scans and 5243 abdominal CT scans in 2013. The images are interpreted by 20 radiologists from a single private practice contracted to provide services at these locations. Specialist readers are deployed in nuclear medicine, musculoskeletal, neuroradiology, and breast imaging, but not in thoracic imaging.
Program Description
The goal of the Rapid Access Chest and Lung Assessment Program (RACLAP) is to perform a rapid multidisciplinary assessment of pulmonary findings related to patient symptoms or presenting as incidental findings. First contact with the primary care provider was made by either the interpreting radiologist or the nurse navigator to obtain approval for entrance into the program. At that point, the patients were contacted and offered evaluation. Once evaluated, patients provided informed consent to have their data and outcomes collected and analyzed. The assessment team included a nurse navigator to gather elements of the history, and thoracic surgeons, pulmonologists, and radiologists to make recommendations about further follow-up based on the guidelines of the Fleishner Society [5] and American College of Radiology [6] and knowledge of patient characteristics and risk factors. Patients who were judged to have lower-risk abnormalities were followed within the program for at least 2 years to document stability.
Keeping in close contact with the patient’s primary care physician, the team designed a plan for additional evaluation as necessary. If multidisciplinary consultation was required, the nurse navigator coordinated and facilitated visits to avoid duplication and delays. The RACLAP established a dedicated phone number to receive calls and messages from radiologists at any of the 5 diagnostic facilities and from emergency department or other physicians who encounter patients with abnormal chest imaging findings. Institutional review board approval was obtained for this project.
Analysis
The percentage of RACLAP patients presenting with early stage (IA–IIB) lung cancer diagnosed in the RACLAP was compared with both concurrent controls (those diagnosed during the same time period through traditional referral patterns) and with historic controls (those diagnosed in the 24 months prior to the institution of the RACLAP). A 2-sample test for binomial proportions was used for both of these comparisons.
Physician satisfaction with the program was assessed with an online survey tool sent to the 63 individual referring physicians. The survey tool consisted of 11 questions asking respondents to rate their satisfaction with various aspects of the program on a 1–10 scale where 10 was excellent.
Results
Operational metrics of the program were evaluated for the entire group. All patients were contacted within 2 business days, but data on time to evaluation is confounded by patients who had no need for urgent evaluation. However, we did quantify time to evaluation for the 72 patients who had more worrisome findings and were eventually shown to have newly diagnosed lung cancer (Table 3).
Seventy-two patients were diagnosed with lung cancer after referral (31.7%). Table 4 shows their stage at presentation and compares them to the 379 concurrent control patients diagnosed with lung cancer during these same months via traditional practice patterns and the 458 historic
The online survey was sent to 63 referring physicians and 30 responded (47% response rate). Average overall satisfaction was 8.5 on a 1 to 10 scale with 10 being the highest level of satisfaction. Likelihood of referring another patient averaged 9.1 on the same scale. Individual comments cited ease of access, the comprehensive nature of the evaluation, and the communication to the primary care physicians as the best aspects of the program.
Discussion
The discovery of suspicious findings on imaging can have a dramatic impact on patients’ quality of life and emotional well-being, with nearly all patients fearing that they have cancer [15]. Clinical uncertainty about next steps heightens their concerns. The value of data-derived guidelines in shaping the recommendations of radiologists and primary care physicians has recently been expressed [16]. We know of no data quantifying primary care or emergency department physicians’ awareness of surveillance guidelines, but experience indicates that surveillance strategies are highly idiosyncratic, with at least a few patients getting lost to follow-up altogether. Many primary care physicians rely upon recommendations in radiologists’ reports. Unfortunately, there is ample evidence that radiologists’ recommendations are not consistently concordant with guidelines [10–13], with a tendency to over-recommend follow-up tests [13].
The RACLAP program was developed to centralize the follow-up of clinically significant pulmonary imaging abnormalities in order to standardize the approach, increase adherence to professional society guidelines, and to avoid the rare but real situation of having a patient lost to follow-up. Unlike other “nodule clinics,” it was pro-active, reaching out to primary care physicians and to patients once a radiologist notified a nurse navigator of a finding. Our findings document a high acceptance of the program with 171/227 (75%) of patients and primary care physicians opting for evaluation within the program. The fact that in only 30 of 227 (13%) of potential referrals did a primary care physician decline the service indicates that the RACLAP successfully addressed an existing need among physicians. Referring physician satisfaction with the service was high reflecting the fact that the program assisted them in making difficult management decisions and discussing clinical uncertainty with patients.
Our program bears superficial similarities to the one described by Lo et al at Toronto East General Hospital [17], where a re-design of operations lead to an increase in access to thoracic oncology specialists and resulted in a reduction of wait time to evaluation by a median of 27 days. However, the goals of the 2 programs were different and the problems being addressed were dissimilar. The Canadian program was designed to shorten time from clinical suspicion to diagnosis of lung cancer and involved improving access to specialists with the creation of “shadow” slots for CT scan and bronchoscopy to facilitate prompt consultation requests, something that was not necessary in our system. Our program was focused on inserting maximum experience into the clinical decision making about imaging abnormalities to assure guideline adherence and consistency in approach.
The short interval to patient contact and evaluation described in this report compares favorably to published data on time to evaluation in referral patterns from around the world when no special efforts are made [18–21]. Alsamari et al have shown the benefit of special efforts to coordinate care of patients with apparent lung cancer with regard to timeliness of evaluation and improved stage compared to historic controls [19]. It should be noted, that even though guidelines have been promulgated for the timeliness of evaluation of symptomatic patients, it is unclear if reducing time to evaluation improves lung cancer survival [18] though it can reduce anxiety.
Our program relied most heavily upon radiologists to make the referral to the RACLAP. We find that the ability to inform and organize a smaller number of radiologists is more effective than attempting to inform a much larger number of primary care physicians about the existence of the program. Even with the success of the program we noted that not all radiologists made referrals at the same frequency, suggesting variability in interest and/or awareness. The system could therefore be improved by making it easier for radiologists to participate by implementing electronic tools that allow radiologists to activate the RACLAP navigator via an inbox message in the electronic medical record as was described at the a program at the Veterans Affairs Connecticut Health Care System [19]. In addition, tools such as natural language processing and clinical decision support which “read” radiology reports and allow standardized templated recommendations, similar to breast imaging reports would improve standardization of recommendation.
The limitations of this study are chiefly related to questions regarding its generalizability, as this was a highly centralized, hospital-based program. The nurse navigator was a hospital employee and the involved physicians were all hospital-based, although only the surgeons were employed by the medical center. In addition, all 5 radiology centers and physicians in the program had access to the electronically stored images. Whether such a program could be recreated and thrive in communities without this degree of centralization, system collaboration, and leadership is unclear. Another feature of this program that raises questions of generalizability is that all the radiologists, the chief source of referrals, were employed in a single professional practice which facilitated communication and uniformity of practice. We are in the process of expanding the program to engage a larger number of radiology practices without the close relationships described above.
The high rate of new lung cancer diagnoses (32%) was surprising. Though most patients had some symptoms that provoked the imaging, many of these symptoms seemed to be unrelated to the findings, even among those subsequently found to have cancer. Our population did have a higher percentage of active smokers (19.7% compared with 14% of adults in our home county [22]), indicating perhaps a bias toward ordering imaging in those who smoke. It is possible that referring physicians, including radiologists, referred patients who had more worrisome characteristics more often. The program was intended to be universal, but we cannot exclude referral bias as a cause of the high rate of malignant diagnoses. Even so, the increased frequency of “early”- stage cancers stands.
Conclusion
Our study showed that in a community hospital–based practice, the care of patients with pulmonary imaging abnormalities can be coordinated and facilitated so that professional society guidelines for surveillance are utilized. The program required no capital and was only modestly labor intensive, requiring the deployment of a navigator who may be shared with other cancer programs. Referring physician satisfaction was high. As high resolution CT scans for lung cancer screening and other indications becomes more common, imaging abnormalities will be found increasingly. Health systems are increasingly focused on both costs of care and quality of care. In this setting, directing the evaluation of patient with abnormal lung imaging to those most experienced can be a means to achieve both higher quality and lower cost.
Acknowledgments: We acknowledge Professor Charles Mylander for expert statistical analysis and support. We are grateful to members of the Thoracic Oncology Steering Committee at Anne Arundel Medical Center for help in creating the program described above.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, [email protected].
Financial disclosures: None.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
1. Alpert JB, Fantauzzi JP, Melamud K, et al. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol 2012;1998:793–9.
2. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169:1961–5.
3. Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? Chest 2007;132(3 Suppl):94S–107S.
4. Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535–42.
5. MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400.
6. Ray Jr CE, English B, Funaki BS, et al. ACR appropriateness criteria radiologic management of thoracic nodules and masses. J Am Coll Radiol 2012;9:13–9.
7. Kanne JP, Jensen LE, Tan-Lucien HM, et al. ACR appropriateness criteria radiographically detected solitary pulmonary nodule. J Thorac Imaging 2013;28:W1–W3.
8. Edey AJ, Hansell DM. Incidentally detected small pulmonary nodules on CT. Clin Radiol 2009;64:872–84.
9. Nair A, Baldwin DR, Field JK, et al. Measurement methods and algorithms for the management of solid nodules. J Thorac Imaging 2012;27:230–9.
10. Eisenberg RL, Bankier, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218–24.
11. Lacson, RL, Prevedello LM, Andriole KP, et al. Factors associated with radiologists’ adherence to Fleischner guidelines for management of pulmonary nodules. J Am Coll Radiol 2012; 9:468–73.
12. Esmail A, Munden RF, Muhammed TL. Small pulmonary nodule management: a survey of the members of the Society of Thoracic Radiology with comparison to the Fleischner Society guidelines. J Thorac Imaging 2011;26:27–31.
13. Masciocchi M, Wagner B, Lloyd B. Quality review: Fleischner criteria adherence by radiologists in a large community hospital. J Am Coll Radiol 2012;9:336–9.
14. National Cancer Institute Patient Information page: non-small cell lung cancer. Accessed 1 Jul 2013 at www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/Patient#Keypoint4.
15. Wiener RS, Gould MK, Woloshin S, et al. What do you mean, a spot? a qualitative analysis of patients’ reactions to discussion with their physicians about pulmonary nodules. Chest 2013;143:672–7.
16. McMahon H. Compliance with Fleischner Society guidelines for management of lung nodules: lessons and opportunities. Radiology 2010;255:14–5.
17. Lo DS, Zeldin RA, Skratsins R, et al. Time to treat: a system redesign focusing on decreasing the time from suspicion of lung cancer to diagnosis. J Thorac Oncol 2007;2:1001–6.
18. Brocken P, Loers BAB, Looijen-Salamon MG, et al. Timeliness of lung cancer diagnosis and treatment in a rapid outpatient diagnostic program with combined 18FDG-PET and contrast enhanced CT scanning. Lung Cancer 2012;75:336–41.
19. Alsamarai S, Xiaopan Y, Cain HC, et al. The effect of a lung cancer care coordination program on timeliness of care. Clin Lung Cancer 2013;14:527–34.
20. Leprieur EG, Labrune S, Giraud V, et al. Delay between the initial symptomsa, the diagnosis and the onset of specific treatment in elderly patients with lung cancer. Clin Lung Cancer 2012;13:363–8.
21. Cheung WY, Butler JR, Kliewer EV, et al. Analysis of wait times and costs during the peri-diagnostic period for non small cell lung cancer. Lung Cancer 2011;72:125–31.
22. Report card of community health indicators. Anne Arundel County Department of Health. Accessed 20 Jul 2013 at www.aahealth.org/pdf/aahealth-report-card-2011.pdf.
Teen First North American to Undergo Incisionless Surgery for Bone Tumor
On July 17, Jack Campanile, age 16 years, a patient at the Hospital for Sick Children (SickKids) in Toronto, was the first teen to undergo a specialized procedure using magnetic resonance imaging (MRI) and ultrasound technology to destroy a tumor in his leg without piercing the skin. The lesion had caused the teen excruciating pain prior to electing to have this non-invasive procedure.
“With high-intensity focused ultrasound, we are moving from minimally invasive to non-invasive therapy, significantly reducing risk to the patient and fast-tracking recovery,” said interventional radiologist Michael Temple, MD, Associate Professor of Medical Imaging at the University of Toronto, who led the team that performed the surgery.
During the 30-minute procedure, the team used the MRI to pinpoint the exact location of the osteoid osteoma and to help target the ultrasound waves to burn the whole tumor. Using an MRI also afforded the team the opportunity to monitor the temperature induced by the ultrasound to ensure that there was no unexpected increase in heat in surrounding tissue.
Hours after undergoing this procedure Jack was discharged. He initially experienced a few hours of pain, and then he was totally pain-free. Overall, he recovered quickly and smoothly; there also have been no reported complications. That night he had uninterrupted sleep for the first time in months. Most of his normal activities also were resumed 2 days after surgery.
“The osteoid osteoma tumor was chosen as our pilot study because the lesion is easily accessible and while the procedure is sophisticated, it is relatively straightforward. The success of this first case is great news for Jack, and exciting for our team as we look at developing more complex incisionless treatments in the future,” said Dr. Temple.
On July 17, Jack Campanile, age 16 years, a patient at the Hospital for Sick Children (SickKids) in Toronto, was the first teen to undergo a specialized procedure using magnetic resonance imaging (MRI) and ultrasound technology to destroy a tumor in his leg without piercing the skin. The lesion had caused the teen excruciating pain prior to electing to have this non-invasive procedure.
“With high-intensity focused ultrasound, we are moving from minimally invasive to non-invasive therapy, significantly reducing risk to the patient and fast-tracking recovery,” said interventional radiologist Michael Temple, MD, Associate Professor of Medical Imaging at the University of Toronto, who led the team that performed the surgery.
During the 30-minute procedure, the team used the MRI to pinpoint the exact location of the osteoid osteoma and to help target the ultrasound waves to burn the whole tumor. Using an MRI also afforded the team the opportunity to monitor the temperature induced by the ultrasound to ensure that there was no unexpected increase in heat in surrounding tissue.
Hours after undergoing this procedure Jack was discharged. He initially experienced a few hours of pain, and then he was totally pain-free. Overall, he recovered quickly and smoothly; there also have been no reported complications. That night he had uninterrupted sleep for the first time in months. Most of his normal activities also were resumed 2 days after surgery.
“The osteoid osteoma tumor was chosen as our pilot study because the lesion is easily accessible and while the procedure is sophisticated, it is relatively straightforward. The success of this first case is great news for Jack, and exciting for our team as we look at developing more complex incisionless treatments in the future,” said Dr. Temple.
On July 17, Jack Campanile, age 16 years, a patient at the Hospital for Sick Children (SickKids) in Toronto, was the first teen to undergo a specialized procedure using magnetic resonance imaging (MRI) and ultrasound technology to destroy a tumor in his leg without piercing the skin. The lesion had caused the teen excruciating pain prior to electing to have this non-invasive procedure.
“With high-intensity focused ultrasound, we are moving from minimally invasive to non-invasive therapy, significantly reducing risk to the patient and fast-tracking recovery,” said interventional radiologist Michael Temple, MD, Associate Professor of Medical Imaging at the University of Toronto, who led the team that performed the surgery.
During the 30-minute procedure, the team used the MRI to pinpoint the exact location of the osteoid osteoma and to help target the ultrasound waves to burn the whole tumor. Using an MRI also afforded the team the opportunity to monitor the temperature induced by the ultrasound to ensure that there was no unexpected increase in heat in surrounding tissue.
Hours after undergoing this procedure Jack was discharged. He initially experienced a few hours of pain, and then he was totally pain-free. Overall, he recovered quickly and smoothly; there also have been no reported complications. That night he had uninterrupted sleep for the first time in months. Most of his normal activities also were resumed 2 days after surgery.
“The osteoid osteoma tumor was chosen as our pilot study because the lesion is easily accessible and while the procedure is sophisticated, it is relatively straightforward. The success of this first case is great news for Jack, and exciting for our team as we look at developing more complex incisionless treatments in the future,” said Dr. Temple.
Few risks seen with initial ultrasonography in nephrolithiasis
Ultrasonography is known to be less sensitive than computed tomography for diagnosing kidney stones. But the initial use of ultrasonography, followed by CT imaging if indicated, results in less cumulative radiation exposure for patients without increasing the risk of adverse clinical outcomes or missed diagnoses, according to findings published online Sept 18 in the New England Journal of Medicine (doi:10.1056/NEJMoa1404446).
The multicenter study, led by Dr. Rebecca Smith-Bindman of the University of California, San Francisco, and colleagues, randomized 2,759 patients presenting in hospital emergency departments with symptoms of nephrolithiasis to receive initial ultrasonography performed by an emergency physician (n = 908); ultrasonography performed by a radiologist (n = 893), or abdominal CT (n = 958), with all further diagnostic and management decisions left up to the physician.
High-risk diagnoses with complications within 30 days of initial imaging occurred infrequently across the groups (0.4% for all three, n = 11), with no significant differences seen among the groups (P = .30). Within 6 months, serious adverse advents occurred in 12.4% of patients assigned initial ED ultrasonography, 10.8% in those assigned radiology ultrasonography, and 11.2% of those assigned to CT (P = .50) with no significant differences in pain scores, return emergency department visits, or hospitalizations. Cumulative radiation exposure at 6 months, however, was significantly higher for the CT arm than for the two ultrasonography arms (P < .001).
Dr. Smith-Bindman and colleagues emphasized in their analysis that their results do not imply that patients with suspected nephrolithiasis should undergo only ultrasound imaging, “but rather that ultrasonography should be used as the initial diagnostic imaging test, with further imaging studies performed at the discretion of the physician on the basis of clinical judgment.” Patients with nephrolithiasis often undergo repeat imaging over time, the researchers observed, and “replacing initial CT with ultrasonography for this often-recurring disease reduced overall radiation exposure.”
Dr. Smith-Bindman and colleagues noted as a limitation of their study the fact that treatment assignment could not be blinded. The study was funded by the Agency for Healthcare Research and Quality; none of its authors declared financial conflicts of interest.
On the basis of the study findings, it is reasonable for a physician to use ultrasonography as the initial imaging method for a patient presenting to the emergency department with suspected nephrolithiasis, remembering that additional imaging studies should be used when clinically indicated. Although CT had higher sensitivity than ultrasonography, this increased sensitivity did not lead to better clinical outcomes.
It should be emphasized that ultrasonography when used alone is not very sensitive for detecting stones. However, the approach of starting with ultrasonography and then proceeding to CT if indicated resulted in similar levels of sensitivity in the three groups. It is reassuring that high-risk diagnoses were rarely missed with this approach.
Dr. Gary Curhan is with Brigham and Women’s Hospital and Harvard Medical School, Boston. Dr. Curhan disclosed financial ties with AstraZeneca, Exponent, UpToDate, Allena, the American Society of Nephrology, and the American Urological Association.
On the basis of the study findings, it is reasonable for a physician to use ultrasonography as the initial imaging method for a patient presenting to the emergency department with suspected nephrolithiasis, remembering that additional imaging studies should be used when clinically indicated. Although CT had higher sensitivity than ultrasonography, this increased sensitivity did not lead to better clinical outcomes.
It should be emphasized that ultrasonography when used alone is not very sensitive for detecting stones. However, the approach of starting with ultrasonography and then proceeding to CT if indicated resulted in similar levels of sensitivity in the three groups. It is reassuring that high-risk diagnoses were rarely missed with this approach.
Dr. Gary Curhan is with Brigham and Women’s Hospital and Harvard Medical School, Boston. Dr. Curhan disclosed financial ties with AstraZeneca, Exponent, UpToDate, Allena, the American Society of Nephrology, and the American Urological Association.
On the basis of the study findings, it is reasonable for a physician to use ultrasonography as the initial imaging method for a patient presenting to the emergency department with suspected nephrolithiasis, remembering that additional imaging studies should be used when clinically indicated. Although CT had higher sensitivity than ultrasonography, this increased sensitivity did not lead to better clinical outcomes.
It should be emphasized that ultrasonography when used alone is not very sensitive for detecting stones. However, the approach of starting with ultrasonography and then proceeding to CT if indicated resulted in similar levels of sensitivity in the three groups. It is reassuring that high-risk diagnoses were rarely missed with this approach.
Dr. Gary Curhan is with Brigham and Women’s Hospital and Harvard Medical School, Boston. Dr. Curhan disclosed financial ties with AstraZeneca, Exponent, UpToDate, Allena, the American Society of Nephrology, and the American Urological Association.
Ultrasonography is known to be less sensitive than computed tomography for diagnosing kidney stones. But the initial use of ultrasonography, followed by CT imaging if indicated, results in less cumulative radiation exposure for patients without increasing the risk of adverse clinical outcomes or missed diagnoses, according to findings published online Sept 18 in the New England Journal of Medicine (doi:10.1056/NEJMoa1404446).
The multicenter study, led by Dr. Rebecca Smith-Bindman of the University of California, San Francisco, and colleagues, randomized 2,759 patients presenting in hospital emergency departments with symptoms of nephrolithiasis to receive initial ultrasonography performed by an emergency physician (n = 908); ultrasonography performed by a radiologist (n = 893), or abdominal CT (n = 958), with all further diagnostic and management decisions left up to the physician.
High-risk diagnoses with complications within 30 days of initial imaging occurred infrequently across the groups (0.4% for all three, n = 11), with no significant differences seen among the groups (P = .30). Within 6 months, serious adverse advents occurred in 12.4% of patients assigned initial ED ultrasonography, 10.8% in those assigned radiology ultrasonography, and 11.2% of those assigned to CT (P = .50) with no significant differences in pain scores, return emergency department visits, or hospitalizations. Cumulative radiation exposure at 6 months, however, was significantly higher for the CT arm than for the two ultrasonography arms (P < .001).
Dr. Smith-Bindman and colleagues emphasized in their analysis that their results do not imply that patients with suspected nephrolithiasis should undergo only ultrasound imaging, “but rather that ultrasonography should be used as the initial diagnostic imaging test, with further imaging studies performed at the discretion of the physician on the basis of clinical judgment.” Patients with nephrolithiasis often undergo repeat imaging over time, the researchers observed, and “replacing initial CT with ultrasonography for this often-recurring disease reduced overall radiation exposure.”
Dr. Smith-Bindman and colleagues noted as a limitation of their study the fact that treatment assignment could not be blinded. The study was funded by the Agency for Healthcare Research and Quality; none of its authors declared financial conflicts of interest.
Ultrasonography is known to be less sensitive than computed tomography for diagnosing kidney stones. But the initial use of ultrasonography, followed by CT imaging if indicated, results in less cumulative radiation exposure for patients without increasing the risk of adverse clinical outcomes or missed diagnoses, according to findings published online Sept 18 in the New England Journal of Medicine (doi:10.1056/NEJMoa1404446).
The multicenter study, led by Dr. Rebecca Smith-Bindman of the University of California, San Francisco, and colleagues, randomized 2,759 patients presenting in hospital emergency departments with symptoms of nephrolithiasis to receive initial ultrasonography performed by an emergency physician (n = 908); ultrasonography performed by a radiologist (n = 893), or abdominal CT (n = 958), with all further diagnostic and management decisions left up to the physician.
High-risk diagnoses with complications within 30 days of initial imaging occurred infrequently across the groups (0.4% for all three, n = 11), with no significant differences seen among the groups (P = .30). Within 6 months, serious adverse advents occurred in 12.4% of patients assigned initial ED ultrasonography, 10.8% in those assigned radiology ultrasonography, and 11.2% of those assigned to CT (P = .50) with no significant differences in pain scores, return emergency department visits, or hospitalizations. Cumulative radiation exposure at 6 months, however, was significantly higher for the CT arm than for the two ultrasonography arms (P < .001).
Dr. Smith-Bindman and colleagues emphasized in their analysis that their results do not imply that patients with suspected nephrolithiasis should undergo only ultrasound imaging, “but rather that ultrasonography should be used as the initial diagnostic imaging test, with further imaging studies performed at the discretion of the physician on the basis of clinical judgment.” Patients with nephrolithiasis often undergo repeat imaging over time, the researchers observed, and “replacing initial CT with ultrasonography for this often-recurring disease reduced overall radiation exposure.”
Dr. Smith-Bindman and colleagues noted as a limitation of their study the fact that treatment assignment could not be blinded. The study was funded by the Agency for Healthcare Research and Quality; none of its authors declared financial conflicts of interest.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Ultrasonography may be preferable to computed tomography in initial imaging to diagnose kidney stones.
Major finding: Initial ultrasound, followed by CT if indicated, did not increase incidence of high-risk diagnoses with complications or adverse outcomes compared with initial CT; patients also saw lower cumulative radiation compared with initial CT.
Data source: Study of 2,759 patients with suspected nephrolithiasis enrolled from 15 U.S. hospital emergency departments between October 2011 and February 2013, randomized to initial screening with CT or ultrasonography, and followed up for 6 months.
Disclosures: None of the study authors declared financial conflicts of interest.
High-Risk Musculoskeletal Injuries
Case
A 2-year-old girl was carried into the ED after falling off her bed earlier in the evening. The parents did not see the child fall, but heard her crying in her room. On physical examination, the patient was in a lot of pain, would not move her left arm, and had a left elbow effusion. The radial pulse was strong, and she was able to move all of her fingers but would not move her elbow. A lateral X-ray taken of the left elbow is shown below (Figure 1).
Supracondylar Fractures
Supracondylar fractures are the most common pediatric elbow injury and disposition can range from outpatient follow-up to urgent surgical intervention. The average age of presentation is between 3 to 10 years, and the injury typically results from a fall on an outstretched hand (FOOSH) with hyperextension of the elbow. Supracondylar fractures may also occur after a direct blow to the elbow or hyperflexion.1
The supracondylar area in children, the distal portion of the humerus, is thin and weak. The force transmitted to this region by a direct blow or FOOSH injury can fracture the humerus. The brachial artery runs along the anterior humerus and can easily sustain injury. Median, ulnar, or radial nerve injuries are also common and can result in permanent disability.2 Immediate neurovascular examination is mandatory, and diminished or absent pulses, poor perfusion, and pallor are signs of ischemia. Examination should include assessment of the radial pulse and the sensory and motor function of the median, radial, and ulnar nerves. To test the median nerve (via the anterior interosseous branch), ask the patient make an “OK” sign with his or her fingers; to test the radial nerve, instruct the child to make a “thumb’s up” sign; and to test the ulnar nerve, have the child hold his or her fingers spread-out against resistance. In addition, sensation of the palmer and dorsal surfaces and in between the fingers should be confirmed.
Plain radiographs, including anteroposterior (AP), oblique, and true lateral views, should be obtained. Interpretation of pediatric elbow films can be difficult, and the stages of ossification must be considered. The helpful acronym for remembering the order of bone ossification is CRITOE (capitellum, radial head, internal [medial] epicondyle, trochlea, olecranon, and external [lateral] epicondyle) (Table 1).
If the anterior humeral line—a line drawn through the anterior cortex of the humerus—fails to intersect the capitellum in its middle third, fracture of the distal humerus is present (Figure 2). The radial head should be aligned with the capitellum. Close inspection for a posterior fat pad, or “sail sign” is imperative as it indicates hemorrhage, joint effusion, or occult fracture. The presence of an anterior fat pad can be a normal variant; however, if the pad is wide and creates a “sail sign” then fracture must be assumed.1
Fracture Types
Type III supracondylar fractures are completely displaced with a fracture through the anterior and posterior cortex. Since a high-risk of injury to the upper extremity vessels and nerves is associated with these very unstable fractures, routine neurovascular checks (while awaiting operative repair) are required. Supracondylar fractures are often associated with forearm or distal radius fractures; therefore, forearm radiographs should also be obtained.3
Diskitis
Tumors
Spondylolysis
Pediatric Cervical Spine Clearance
Thankfully, cervical spine injuries are rare, occurring in approximately 1% of children after blunt trauma.9 Left unrecognized, however, these injuries may result in permanent neurological disability. Children younger than 8 years of age are more likely to injure the upper cervical spine (C1 to C3) than older children and adults. This is because children have relatively larger heads than bodies and weaker cervical muscles and ligaments, making the upper cervical spine more mobile. The Emergency X-Radiography Utilization Study (NEXUS) criteria have been validated in adults; however, criteria for clearing the pediatric cervical spine are poorly studied. Because of limited data, there are few evidence-based guidelines for the clearance of the pediatric cervical spine. A study in 2001 found that the NEXUS guidelines where helpful in reducing imaging in 20% of children, but due to the low numbers of infants in this study, caution is advised when applying the NEXUS criteria to children under 2 years of age.9
Nonaccidental Trauma: Abuse
Orthopedic injury due to nonaccidental trauma (NAT) can be difficult to distinguish from normal childhood injuries. Identification of high-risk presentations is key in diagnosing these injuries and hopefully preventing further abuse. Femur fractures in children younger than age 1 year have a high likelihood of being nonaccidental, with between 60% to 80% of femoral shaft fractures resulting from abuse. No particular pattern of fracture is pathognomonic for NAT. The American Academy of Orthopaedic Surgeons recommends that children younger than age 36 months with a diaphyseal femur fracture be evaluated for child abuse.12
Spiral fractures and transverse fractures of long bones also raise suspicion for NAT. Injury to the metaphysis of long bones, especially in nonambulatory children, is considered highly suggestive of child abuse. The classic metaphyseal lesion, called a “corner” or “bucket-handle” fracture, occurs when the extremity (usually the forearm) is pulled or twisted forcibly, or the child is shaken. X-ray will demonstrate a disruption of the metaphysis with lucency. As the developing ribs are flexible and difficult to break with minor injuries, a child with a rib fracture and no history of severe trauma, such as a motor vehicle crash or fall from a significant height, has a high likelihood of being a victim of child abuse. Skull fractures caused from accidental injury and abuse may have similar presentations. The history and mechanism are important to correlate with physical examination findings for potential inconsistencies.13
Case Conclusion
The child in this case sustained a type II supracondylar fracture. Orthopedic surgery was consulted, and the patient was taken to the operating room for closed reduction and percutaneous pinning. She was placed in a cast, and pins were removed at follow-up 4 weeks later. No residual pain or deficits remained, and she regained full function of her arm.
Dr Hewett is a pediatric emergency medicine fellow, College of Medicine, Medical University of South Carolina, Charleston.
Dr Titus is vice chair, pediatric fellowships, and fellowship director, pediatric emergency medicine; and an associate professor of pediatrics, Medical University of South Carolina, Charleston.
- Bachman D, Santora S. Orthopedic trauma. In: Fleisher GR, Ludwig S, et al, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:1538.
- Carson S, Woolridge DP, Colletti J, Kilgore K. Pediatric upper extremity injuries. Pediatr Clin North Am. 2006;53(1):41.
- Slack SE, Clancy MJ. Clearing the cervical spine of paediatric trauma patients. Emer Med J. 2004;21(2):189-193
- Brown M. Hussain K, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg. 2001;83(1):106-111.
- Cushing AH. Diskitis in children. Clin Infect Dis. 1993;17(1):1-6.
- Selbst SM, Lavelle JM, Soyupak SK, Markowitz RI. Back pain in children who present to the emergency department. Clin Pediatr (Phila). 1999;38(7):401-406.
- Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
- Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
- Viccellio P, Simon H, Pressman BD, Shah MN, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):E20.
- Hendey GW, Wolfson AB, Mower WR, Hoffman JR; National Emergency X-Radiography Utilization Study Group. Spinal cord injury without radiographic abnormality: results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J Trauma. 2002;53(1):1-4
- Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
- Offiah A, van Rijn RR, Perez-Rossello JM, Kleinman PK. Skeletal imaging of child abuse (non-accidental injury). Pediatr Radiol. 2009;39(5):461-470.
- Bonfield, CM, Naran S et al. Pediatric skull fractures: the need for surgical intervention, characteristics, complications, and outcomes. J Neurosurg Pediatr. 2014;14(2):205-211.
Case
A 2-year-old girl was carried into the ED after falling off her bed earlier in the evening. The parents did not see the child fall, but heard her crying in her room. On physical examination, the patient was in a lot of pain, would not move her left arm, and had a left elbow effusion. The radial pulse was strong, and she was able to move all of her fingers but would not move her elbow. A lateral X-ray taken of the left elbow is shown below (Figure 1).
Supracondylar Fractures
Supracondylar fractures are the most common pediatric elbow injury and disposition can range from outpatient follow-up to urgent surgical intervention. The average age of presentation is between 3 to 10 years, and the injury typically results from a fall on an outstretched hand (FOOSH) with hyperextension of the elbow. Supracondylar fractures may also occur after a direct blow to the elbow or hyperflexion.1
The supracondylar area in children, the distal portion of the humerus, is thin and weak. The force transmitted to this region by a direct blow or FOOSH injury can fracture the humerus. The brachial artery runs along the anterior humerus and can easily sustain injury. Median, ulnar, or radial nerve injuries are also common and can result in permanent disability.2 Immediate neurovascular examination is mandatory, and diminished or absent pulses, poor perfusion, and pallor are signs of ischemia. Examination should include assessment of the radial pulse and the sensory and motor function of the median, radial, and ulnar nerves. To test the median nerve (via the anterior interosseous branch), ask the patient make an “OK” sign with his or her fingers; to test the radial nerve, instruct the child to make a “thumb’s up” sign; and to test the ulnar nerve, have the child hold his or her fingers spread-out against resistance. In addition, sensation of the palmer and dorsal surfaces and in between the fingers should be confirmed.
Plain radiographs, including anteroposterior (AP), oblique, and true lateral views, should be obtained. Interpretation of pediatric elbow films can be difficult, and the stages of ossification must be considered. The helpful acronym for remembering the order of bone ossification is CRITOE (capitellum, radial head, internal [medial] epicondyle, trochlea, olecranon, and external [lateral] epicondyle) (Table 1).
If the anterior humeral line—a line drawn through the anterior cortex of the humerus—fails to intersect the capitellum in its middle third, fracture of the distal humerus is present (Figure 2). The radial head should be aligned with the capitellum. Close inspection for a posterior fat pad, or “sail sign” is imperative as it indicates hemorrhage, joint effusion, or occult fracture. The presence of an anterior fat pad can be a normal variant; however, if the pad is wide and creates a “sail sign” then fracture must be assumed.1
Fracture Types
Type III supracondylar fractures are completely displaced with a fracture through the anterior and posterior cortex. Since a high-risk of injury to the upper extremity vessels and nerves is associated with these very unstable fractures, routine neurovascular checks (while awaiting operative repair) are required. Supracondylar fractures are often associated with forearm or distal radius fractures; therefore, forearm radiographs should also be obtained.3
Diskitis
Tumors
Spondylolysis
Pediatric Cervical Spine Clearance
Thankfully, cervical spine injuries are rare, occurring in approximately 1% of children after blunt trauma.9 Left unrecognized, however, these injuries may result in permanent neurological disability. Children younger than 8 years of age are more likely to injure the upper cervical spine (C1 to C3) than older children and adults. This is because children have relatively larger heads than bodies and weaker cervical muscles and ligaments, making the upper cervical spine more mobile. The Emergency X-Radiography Utilization Study (NEXUS) criteria have been validated in adults; however, criteria for clearing the pediatric cervical spine are poorly studied. Because of limited data, there are few evidence-based guidelines for the clearance of the pediatric cervical spine. A study in 2001 found that the NEXUS guidelines where helpful in reducing imaging in 20% of children, but due to the low numbers of infants in this study, caution is advised when applying the NEXUS criteria to children under 2 years of age.9
Nonaccidental Trauma: Abuse
Orthopedic injury due to nonaccidental trauma (NAT) can be difficult to distinguish from normal childhood injuries. Identification of high-risk presentations is key in diagnosing these injuries and hopefully preventing further abuse. Femur fractures in children younger than age 1 year have a high likelihood of being nonaccidental, with between 60% to 80% of femoral shaft fractures resulting from abuse. No particular pattern of fracture is pathognomonic for NAT. The American Academy of Orthopaedic Surgeons recommends that children younger than age 36 months with a diaphyseal femur fracture be evaluated for child abuse.12
Spiral fractures and transverse fractures of long bones also raise suspicion for NAT. Injury to the metaphysis of long bones, especially in nonambulatory children, is considered highly suggestive of child abuse. The classic metaphyseal lesion, called a “corner” or “bucket-handle” fracture, occurs when the extremity (usually the forearm) is pulled or twisted forcibly, or the child is shaken. X-ray will demonstrate a disruption of the metaphysis with lucency. As the developing ribs are flexible and difficult to break with minor injuries, a child with a rib fracture and no history of severe trauma, such as a motor vehicle crash or fall from a significant height, has a high likelihood of being a victim of child abuse. Skull fractures caused from accidental injury and abuse may have similar presentations. The history and mechanism are important to correlate with physical examination findings for potential inconsistencies.13
Case Conclusion
The child in this case sustained a type II supracondylar fracture. Orthopedic surgery was consulted, and the patient was taken to the operating room for closed reduction and percutaneous pinning. She was placed in a cast, and pins were removed at follow-up 4 weeks later. No residual pain or deficits remained, and she regained full function of her arm.
Dr Hewett is a pediatric emergency medicine fellow, College of Medicine, Medical University of South Carolina, Charleston.
Dr Titus is vice chair, pediatric fellowships, and fellowship director, pediatric emergency medicine; and an associate professor of pediatrics, Medical University of South Carolina, Charleston.
Case
A 2-year-old girl was carried into the ED after falling off her bed earlier in the evening. The parents did not see the child fall, but heard her crying in her room. On physical examination, the patient was in a lot of pain, would not move her left arm, and had a left elbow effusion. The radial pulse was strong, and she was able to move all of her fingers but would not move her elbow. A lateral X-ray taken of the left elbow is shown below (Figure 1).
Supracondylar Fractures
Supracondylar fractures are the most common pediatric elbow injury and disposition can range from outpatient follow-up to urgent surgical intervention. The average age of presentation is between 3 to 10 years, and the injury typically results from a fall on an outstretched hand (FOOSH) with hyperextension of the elbow. Supracondylar fractures may also occur after a direct blow to the elbow or hyperflexion.1
The supracondylar area in children, the distal portion of the humerus, is thin and weak. The force transmitted to this region by a direct blow or FOOSH injury can fracture the humerus. The brachial artery runs along the anterior humerus and can easily sustain injury. Median, ulnar, or radial nerve injuries are also common and can result in permanent disability.2 Immediate neurovascular examination is mandatory, and diminished or absent pulses, poor perfusion, and pallor are signs of ischemia. Examination should include assessment of the radial pulse and the sensory and motor function of the median, radial, and ulnar nerves. To test the median nerve (via the anterior interosseous branch), ask the patient make an “OK” sign with his or her fingers; to test the radial nerve, instruct the child to make a “thumb’s up” sign; and to test the ulnar nerve, have the child hold his or her fingers spread-out against resistance. In addition, sensation of the palmer and dorsal surfaces and in between the fingers should be confirmed.
Plain radiographs, including anteroposterior (AP), oblique, and true lateral views, should be obtained. Interpretation of pediatric elbow films can be difficult, and the stages of ossification must be considered. The helpful acronym for remembering the order of bone ossification is CRITOE (capitellum, radial head, internal [medial] epicondyle, trochlea, olecranon, and external [lateral] epicondyle) (Table 1).
If the anterior humeral line—a line drawn through the anterior cortex of the humerus—fails to intersect the capitellum in its middle third, fracture of the distal humerus is present (Figure 2). The radial head should be aligned with the capitellum. Close inspection for a posterior fat pad, or “sail sign” is imperative as it indicates hemorrhage, joint effusion, or occult fracture. The presence of an anterior fat pad can be a normal variant; however, if the pad is wide and creates a “sail sign” then fracture must be assumed.1
Fracture Types
Type III supracondylar fractures are completely displaced with a fracture through the anterior and posterior cortex. Since a high-risk of injury to the upper extremity vessels and nerves is associated with these very unstable fractures, routine neurovascular checks (while awaiting operative repair) are required. Supracondylar fractures are often associated with forearm or distal radius fractures; therefore, forearm radiographs should also be obtained.3
Diskitis
Tumors
Spondylolysis
Pediatric Cervical Spine Clearance
Thankfully, cervical spine injuries are rare, occurring in approximately 1% of children after blunt trauma.9 Left unrecognized, however, these injuries may result in permanent neurological disability. Children younger than 8 years of age are more likely to injure the upper cervical spine (C1 to C3) than older children and adults. This is because children have relatively larger heads than bodies and weaker cervical muscles and ligaments, making the upper cervical spine more mobile. The Emergency X-Radiography Utilization Study (NEXUS) criteria have been validated in adults; however, criteria for clearing the pediatric cervical spine are poorly studied. Because of limited data, there are few evidence-based guidelines for the clearance of the pediatric cervical spine. A study in 2001 found that the NEXUS guidelines where helpful in reducing imaging in 20% of children, but due to the low numbers of infants in this study, caution is advised when applying the NEXUS criteria to children under 2 years of age.9
Nonaccidental Trauma: Abuse
Orthopedic injury due to nonaccidental trauma (NAT) can be difficult to distinguish from normal childhood injuries. Identification of high-risk presentations is key in diagnosing these injuries and hopefully preventing further abuse. Femur fractures in children younger than age 1 year have a high likelihood of being nonaccidental, with between 60% to 80% of femoral shaft fractures resulting from abuse. No particular pattern of fracture is pathognomonic for NAT. The American Academy of Orthopaedic Surgeons recommends that children younger than age 36 months with a diaphyseal femur fracture be evaluated for child abuse.12
Spiral fractures and transverse fractures of long bones also raise suspicion for NAT. Injury to the metaphysis of long bones, especially in nonambulatory children, is considered highly suggestive of child abuse. The classic metaphyseal lesion, called a “corner” or “bucket-handle” fracture, occurs when the extremity (usually the forearm) is pulled or twisted forcibly, or the child is shaken. X-ray will demonstrate a disruption of the metaphysis with lucency. As the developing ribs are flexible and difficult to break with minor injuries, a child with a rib fracture and no history of severe trauma, such as a motor vehicle crash or fall from a significant height, has a high likelihood of being a victim of child abuse. Skull fractures caused from accidental injury and abuse may have similar presentations. The history and mechanism are important to correlate with physical examination findings for potential inconsistencies.13
Case Conclusion
The child in this case sustained a type II supracondylar fracture. Orthopedic surgery was consulted, and the patient was taken to the operating room for closed reduction and percutaneous pinning. She was placed in a cast, and pins were removed at follow-up 4 weeks later. No residual pain or deficits remained, and she regained full function of her arm.
Dr Hewett is a pediatric emergency medicine fellow, College of Medicine, Medical University of South Carolina, Charleston.
Dr Titus is vice chair, pediatric fellowships, and fellowship director, pediatric emergency medicine; and an associate professor of pediatrics, Medical University of South Carolina, Charleston.
- Bachman D, Santora S. Orthopedic trauma. In: Fleisher GR, Ludwig S, et al, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:1538.
- Carson S, Woolridge DP, Colletti J, Kilgore K. Pediatric upper extremity injuries. Pediatr Clin North Am. 2006;53(1):41.
- Slack SE, Clancy MJ. Clearing the cervical spine of paediatric trauma patients. Emer Med J. 2004;21(2):189-193
- Brown M. Hussain K, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg. 2001;83(1):106-111.
- Cushing AH. Diskitis in children. Clin Infect Dis. 1993;17(1):1-6.
- Selbst SM, Lavelle JM, Soyupak SK, Markowitz RI. Back pain in children who present to the emergency department. Clin Pediatr (Phila). 1999;38(7):401-406.
- Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
- Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
- Viccellio P, Simon H, Pressman BD, Shah MN, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):E20.
- Hendey GW, Wolfson AB, Mower WR, Hoffman JR; National Emergency X-Radiography Utilization Study Group. Spinal cord injury without radiographic abnormality: results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J Trauma. 2002;53(1):1-4
- Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
- Offiah A, van Rijn RR, Perez-Rossello JM, Kleinman PK. Skeletal imaging of child abuse (non-accidental injury). Pediatr Radiol. 2009;39(5):461-470.
- Bonfield, CM, Naran S et al. Pediatric skull fractures: the need for surgical intervention, characteristics, complications, and outcomes. J Neurosurg Pediatr. 2014;14(2):205-211.
- Bachman D, Santora S. Orthopedic trauma. In: Fleisher GR, Ludwig S, et al, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:1538.
- Carson S, Woolridge DP, Colletti J, Kilgore K. Pediatric upper extremity injuries. Pediatr Clin North Am. 2006;53(1):41.
- Slack SE, Clancy MJ. Clearing the cervical spine of paediatric trauma patients. Emer Med J. 2004;21(2):189-193
- Brown M. Hussain K, McHugh K, Novelli V, Jones D. Discitis in young children. J Bone Joint Surg. 2001;83(1):106-111.
- Cushing AH. Diskitis in children. Clin Infect Dis. 1993;17(1):1-6.
- Selbst SM, Lavelle JM, Soyupak SK, Markowitz RI. Back pain in children who present to the emergency department. Clin Pediatr (Phila). 1999;38(7):401-406.
- Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-674.
- Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
- Viccellio P, Simon H, Pressman BD, Shah MN, Mower WR, Hoffman JR; NEXUS Group. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):E20.
- Hendey GW, Wolfson AB, Mower WR, Hoffman JR; National Emergency X-Radiography Utilization Study Group. Spinal cord injury without radiographic abnormality: results of the National Emergency X-Radiography Utilization Study in blunt cervical trauma. J Trauma. 2002;53(1):1-4
- Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343(2):94-99.
- Offiah A, van Rijn RR, Perez-Rossello JM, Kleinman PK. Skeletal imaging of child abuse (non-accidental injury). Pediatr Radiol. 2009;39(5):461-470.
- Bonfield, CM, Naran S et al. Pediatric skull fractures: the need for surgical intervention, characteristics, complications, and outcomes. J Neurosurg Pediatr. 2014;14(2):205-211.
Approach to the Limping Child
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
Four Fracture Patterns Unique to Pediatric Patients
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Case 1
A 2-year-old girl presented to the ED with arm pain. Her mother stated that her daughter was playing with a 5-year-old sibling when she heard the child cry- out in pain and noticed she was holding her right arm by her side, not wanting to move it. Neither child gave a reliable story of the injury.
Nursemaid’s Elbow
Nursemaid’s elbow, also known as pulled elbow, subluxation of the radial head, and most recently annular ligament displacement, is a common injury in children younger than age 6 years. One study estimates that the condition represented about 1% of injury-related ED visits in 2005.1
Patients with nursemaid’s elbow typically present holding the injured arm at their side, slightly flexed and pronated. These patients appear relatively comfortable until moved actively or passively. The classic history of nursemaid’s elbow includes a traction mechanism, with the child being pulled up by one arm or being grabbed by the arm suddenly to keep him or her out of harm’s way.2 Due to the laxity of connective tissues in children of this age, the head of the radius slips out of the annular ligament causing acute pain and decreased function.
Nursemaid’s elbow is usually diagnosed by history and examination alone, with special consideration to the mechanism of injury. There is rarely swelling or bruising.3 Passive flexion and extension at the elbow may be normal, but rotational maneuvers can be painful or fully resisted.
Reduction Techniques
In 2012, Cochrane updated its earlier review on nursemaid’s elbow and in 2013 followed up with an article in Pediatrics in Review.3,4 Each covered research on reduction techniques, summarizing studies comparing supination-flexion (SF) versus hyperpronation (HP) as the initial reduction maneuver. Given that these maneuvers are difficult to camouflage, studies tend to be pseudorandomized with assessment by a nonblinded healthcare provider, decreasing the strength of the studies. In the Cochrane review, four different trials that included 379 children under age 7 years were selected for the review. In all four studies, pronation was found to have the least chance of failed first attempt, the chosen outcome for this meta-analysis. The risk ratio of failure of reduction for pronation was 0.45 (95% confidence interval [CI], 0.28-0.73).
There is some data supporting hyperpronation to be less painful as well; however, the Cochrane reviewers felt there may have been reporting bias.4 Since the time of each of these reviews, another study comprised of 150 children was conducted and also favored similar practice styles, as the hyperpronation maneuver had 95% success rate on first attempt versus 68% first-time success with supination and flexion.5
Complications and Recurrence
In a small study aimed at identifying recurrence rates for nursemaid’s elbow, Teach and Schultzman6 studied 93 children for 1 year after probable or definite diagnosis of nursemaid’s elbow. Of these children, 23.7% had recurrent radial head subluxation. Children younger than age 2 years were found to have a relative risk of 2.6 (95% CI, 1.04-6.30) for one or more recurrences when compared to children older than age 2 years.
While the great majority of children with nursemaid’s elbow do not need referral to an orthopedist, those with two or more occurrences should be considered for referral to a specialist.
Case 2
A 6-year-old boy was presented to the ED by his father, who had placed the boy’s arm in a home-made sling. The child tearfully told the provider that he fell trying to catch himself after tripping over the house pet.
FOOSH Injury
The above case depicts a very common presentation in the ED—the so-called “FOOSH” (fall onto an outstretched hand) injury. This type of injury occurs with such frequency in both adults and children that it is one of the only injury patterns with a commonly used acronym. The bony injuries seen with FOOSH in children, however, have a different pattern than those in adults.
Pediatric fractures are unique due to the difference in the structure of the bones themselves. A child’s bones are more elastic than an adult’s bones, allowing them to bow and bend before they fracture.7 Despite this malleability, pediatric bones have been noted to have a thicker periosteum. For this reason, compression or impact may interrupt the periosteal sleeve, minimally yielding an incomplete interruption of the cortex unilaterally.
One fracture pattern commonly seen in children is the torus fracture. This type of fracture is also referred to as a buckle fracture as the bone cortex on radiographic imaging appears “buckled” as a result of the compressive forces on that side of the bone (Figure 1). Since the bone itself is minimally affected, these fractures are quite stable and not at risk for complications.
In comparison, a greenstick fracture, also unique to the pediatric population, is one in which the cortex shows plastic deformity on the side of the force or impact but is interrupted on the opposite side due to the tension of the impact itself. Greenstick fractures are frequently angulated and may require reduction for anatomic alignment, but long-term complications are typically minimal. These fracture patterns are distinguished from complete fractures (as seen in adults), which are quite unstable and generally require surgical intervention.
Of note, the location of pediatric forearm fractures varies with age as well. Diaphyseal fractures are more common in prepubescent children, whereas the highest incidence of physeal injuries occurs during large growth spurts, particularly throughout adolescence.7
Management
The remodeling potential of pediatric bones also makes management unique. Pediatric orthopedic literature has well-studied acceptable angles and degrees of appropriate displacement based largely on the age of the patient and proximity to a growth plate. Knowledge of these is imperative for definitive care of such fractures but is beyond the scope of this review.
Traditional treatment of pediatric forearm fractures includes immobilization of various types and duration to minimize pain and deformity while producing the best possible outcome. Several recent studies have aimed to determine best practice for the different fracture types with the goal of producing best alignment and return to function while decreasing cost, discomfort, and number of physician visits. Another concern among healthcare providers is the risk of refracture, which in buckle fractures is estimated at approximately 2% with a median time of 8 to 16 weeks after the initial injury.7
A 2010 review by Kennedy et al8 sought to determine if the refracture rate was affected by the technique used to immobilize torus fractures. The five studies used in this review had no reports of refracture in the 443 patients included in analysis, though only one of the studies (Plint et al) followed patients for more than 6 weeks.8,9 In this study, 75 patients were randomized to either a plaster removable splint or full below-elbow cast for 3 weeks; thereafter, they were followed for 6 months, during which time none experienced refracture.9
Another outcome from the same study assessed the ability of the patient to use the affected arm in the recovery period. While those in removable splints scored better during and immediately after cast removal, no differences were present after 1 week. Not surprisingly, families preferred the soft bandages or a removable splint for treatment.
Case 3
A 13-year-old boy presented to the ED with right ankle pain and difficulty bearing weight. He stated that he was playing basketball when he “rolled” his right ankle coming down from a rebound.
Ankle Fractures
Ankle fractures are among the most common acute injuries of the lower extremity in children, accounting for approximately 5% of pediatric fractures and 15% of physeal injuries.10 Ankle fractures also account for up to 40% of all injuries to the skeletally immature athlete.10,11 More specifically, distal fibular physeal fractures are the most common types of pediatric ankle fracture; however, they are associated with a relatively low risk for long-term complications. In contrast, distal tibial physeal fractures are associated with a higher risk for long-term complications.12,13
Presentation and Evaluation
Typically, patients presenting with ankle fractures are too sore to bear weight, and swelling and ecchymosis can be identified anterior to the ankle. In addition, there may be diffuse tenderness throughout the ankle and point tenderness may be induced on the anterolateral aspect of the distal tibia.14 A complete evaluation of the entire lower extremity should be conducted before assuming that the injury is confined to the ankle, especially in children younger than age 5 years and/or who are nonverbal.10 When evaluating an ankle fracture, in general, orthopedic consultation should be obtained for children with neurovascular compromise, open fractures, and/or Salter-Harris III, IV, and V fractures.
The juvenile Tillaux fracture represents a Salter-Harris III physeal injury that involves the anterolateral portion of the tibia. It usually occurs in children between ages 12 and 14 years as they approach skeletal maturity and who have a partially fused tibial physis. The common mechanism of injury is inversion of the ankle with the foot pointed away from the midline (supination with external rotation). This leads to avulsion of the lateral tibial epiphysis that is attached to the anterior inferior tibiofibular ligament. The uninvolved medial portion of the epiphysis is closed.10
Radiographic Imaging
Three radiographic views should be obtained in the evaluation of pediatric ankle injuries as Tillaux fractures or other subtle injuries could be easily missed if only two views are obtained. Interpretation of the radiographs must be correlated with the physical examination.10 The fracture line is usually best seen on a mortise view (Figure 2). Computed tomography (CT) is warranted in cases in which displacement greater than 2 mm is suspected because it better defines fracture displacement and can aid in surgical planning.14 Because of its sensitivity in detecting fractures displaced more than 2 mm, CT is now the preferred imaging modality in the assessment of juvenile Tillaux fractures.15
Definitive Management
There are two important goals when treating children with ankle fractures—achieving a satisfactory reduction and avoiding physeal arrest so as to minimize the risks of angular deformity, early arthrosis, leg-length inequality, and joint stiffness.11 Juvenile Tillaux fractures with greater than 2 mm of displacement require orthopedic consultation for closed or open reduction. Closed reduction is attempted by internally rotating the foot and applying direct pressure over the anterolateral tibia. If necessary, percutaneous pins can be used for stabilization of the reduction. If closed reduction is unsuccessful, open reduction is required. Care must be taken to assure no displacement occurs after casting; this requires weekly X-ray evaluation for the first 2 weeks.12
Patients with nondisplaced Salter-Harris III fractures are treated with long-leg casting for 4 weeks with conversion to a short-leg cast or boot for an additional 4 weeks. Patients should anticipate 8 weeks of nonweight-bearing. The patient is allowed to remove the boot for range-of-motion exercises but must remain nonweight-bearing for the first 2 weeks.14
Case 4
A 3-year-old previously healthy girl presented to the ED with a limp and difficulty bearing weight. Her mother reported that the child was playing in the yard when she caught her foot on a tree root, stumbled, and fell down. Since the incident, the child has been tearful, limping, and refusing to walk.
Tibial Fractures
Tibial fractures are among the most frequent types of orthopedic injuries in young children, with only femur and forearm fractures having a higher incidence of occurrence. Tibial fractures account for up to 15% of long bone fractures in children and adolescents.16,17 The mechanism of injury varies depending on the patient’s age. In young children, the most common cause of injury is from a seemingly minor twisting around a fixed foot or from a minor fall. In older children and adults, high-energy motor vehicle accidents and sports-related injuries are more common causes.
Fractures of the tibial shaft are typically short oblique or transverse fractures of the middle or distal third of the shaft. Thirty percent of tibial shaft fractures are associated with fractures of the fibula.16
Toddler’s Fracture
The term toddler’s fracture refers to a nondisplaced oblique fracture of the tibial shaft without concomitant fibular fracture. It usually results from an indirect rotational or twisting force applied to the foot and lower leg.16-18 More specifically, the term describes a specialized case of spiral fracture of the distal tibia in patients aged 9 months to 3 years, when weight-bearing is just beginning.19,20 Such injuries commonly occur when a toddler stumbles and falls, or attempts to extricate the foot from between the bars of a crib. Often, however, the mechanism is minimal or unknown.18 Of those injuries that are witnessed, most caregivers report a minor twisting mechanism. Most children with toddler’s fracture are younger than age 6 years. Sixty-three of 76 such fractures reported by Dunbar et al17,19 occurred in children younger than 2.5 years of age. Toddler’s fractures occur more often in boys than girls, and in the right leg more often than the left. Most children will give a history of tripping or twisting their ankle.17
Evaluating the Toddler
Toddlers can be challenging patients as they can not relate history and are often uncooperative on examination. A child may present with a limp, diminished movement of the affected limb, or refuse to bear weight without a distinct history of injury. The onset of limping or refusal to bear weight after minor trauma, or without an obvious injury in a young ambulatory child, warrants a detailed examination looking for tenderness over the tibia, along with radiographic evaluation to rule out a toddler’s fracture.
The examination of the patient is rarely impressive as there is little swelling and bruising with most toddlers’ fractures. A complete clinical history is needed, including a detailed description of any observed traumatic event to exclude the existence of other injuries.
When no traumatic event is observed or an inconsistent history is provided, the physician should obtain a detailed social history, including a list of the child’s most recent caregivers and contacts.16 Because of mild clinical symptoms and frequent lack of a history of injury in this patient population, presentation for evaluation may be delayed. In such cases, by the time the extremity is examined, the fracture has begun to heal. This healing phase may be accompanied by periosteal new bone and, in the absence of a history, may erroneously suggest other, more ominous conditions such as osteomylelitis or tumor.17,18
Consideration of Abuse
Although tibial shaft fractures are rarely found in abused children, diagnosis of child abuse must be considered in cases where a tibial fracture is discovered in the nonambulatory child; his or her clinical history is inconsistent with the injury; and/or there are other physical findings suggestive of abuse. Investigation for suspected nonaccidental trauma includes a thorough physical examination, skeletal survey, and evaluation by social services personnel.16
Radiographic Imaging
Quality anteroposterior (AP) or lateral radiographs of the affected leg may show a hairline fracture, but these can easily be missed on initial plain films in almost a third of patients.21 An internal oblique view can aid in identifying nondisplaced toddler fractures.17 The AP view is the best view for observing the nondisplaced spiral fracture along the distal tibia (Figure 3).6 Occasionally, a fracture line is not identified on initial plain films and the first evidence of fracture becomes apparent on X-ray when new periosteal bone forms 7 to 10 days after the initial injury.
Definitive Treatment
Children with a classic history for a toddler’s fracture and an inability to bear weight should be immobilized with a long-leg splint or cast—even when X-rays are negative—until a definitive diagnosis can be made. Such fractures usually become visible on X-ray 7 to 10 days after injury as a result of new bone growth.22
When definitive diagnosis of a toddler’s fracture is made on plain radiographs, the child should either be immobilized in a long-leg splint with referral to an orthopedist within 5 to 7 days, or immediately casted.16
Conclusion
Fractures in both children and adults are among the most common injury-related presentations to the ED. Based on the structure and increased elasticity of bone in the pediatric patient, there are several fracture patterns unique to this population. Appropriate evaluation, diagnosis, and management in the ED helps to maximize and ensure long-term function and healing while minimizing trauma to the patient.
Dr McBride is an associate professor of pediatrics and pediatric emergency medicine, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
Dr Sutton is a pediatric resident, Wake Forest Baptist Health, Brenner Children’s Hospital, Winston-Salem, North Carolina.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.
- Brown D. Emergency department visits for nursemaid’s elbow in the United States, 2005-2006. Orthop Nurs. 2009;28(4):161,162.
- Hardy RH. Pulled elbow. J R Coll Gen Pract. 1978;28(189):224-226.
- Browner EA. Nursemaid’s elbow (annular ligament displacement). Pediatr Rev. 2013;34(8):366,367.
- Krul M, van der Wouden JC,van Suijlekom-Smit LW, Koes BM. Manipulative interventions for reducing pulled elbow in young children. Cochrane Database of Syst Rev. 2012;1:CD007759.
- Gunaydin YK, Katirci Y, Duymaz H, et al. Comparison of success and pain levels of supination-flexion and hyperpronation maneuvers in childhood nursemaid’s elbow cases. Am J Emerg Med. 2013;31(7):1078-1081.
- Teach SJ, Schutzman SA. Prospective study of recurrent radial head subluxation. Arch Pediatr Adolesc Med. 1996;150(2):164-166.
- Dolan M and Waters PM. Fractures and dislocations of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, eds. Skeletal Trauma in Children. 4th ed. Philadephia, PA: Saunders Elsevier; 2009:159-206.
- Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19(1):77-81.
- Plint AC, Perry JJ, Correll R, Gaboury I, Lawtown L. A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children. Pediatrics. 2006;117(3):691-697.
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg. 2001;9(4):268-278.
- Marsh JS, Daigneault JP. Ankle injuries in the pediatric population. Curr Opin Pediatr. 2000;12(1):52-60
- Cummings RJ. Distal tibial and fibular fractures. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1096-1104.
- Boutis K, Willan AR, Babyn P, Narayanan UG, Alman B, Schuh S. A randomized, controlled trial of a removable brace versus casting in children with low-risk ankle fractures. Pediatrics. 2007;119(6):1256-1263.
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg. 2013;21(4):234-244.
- Horn BD, Crisci K, Krug M, Pizzutillo PD, MacEwen GD. Radiologic evaluation of juvenile Tillaux fractures of the distal tibia. J Pediatr Orthop. 2001;21(2):162-164.
- Mashru RP, Herman MJ, Pizzutillo PD. Tibial shaft fractures in children and adolescents. J Am Acad Orthop Surg. 2005;139(5):345-352.
- Heinrich SD, Mooney JF. Fractures of the shaft of the tibia and fibula. In: Beaty JH, Kasser JR, eds. Rockwood and Wilkins’ Fractures in Children. 6th ed. Pennsylvania, PA: Lippincott Williams & Wilkins; 2006:1063,1064.
- John SD, Moorthy CS, Swischuk LE. Expanding the concept of the toddler’s fracture. Radiographics. 1997;17(2):367-376.
- Dunbar JS, Owen HF, Nogrady MB, McLeese R. Obscure tibial fracture of infants—the toddlers’ fracture. J Can Assoc Radiol 1964;15:136-144.
- Tenenbein M, Reed MH, Black GB. The toddler’s fracture revisited. Am J Emerg Med. 1990;8(3):208-211.
- Shravat BP, Harrop SN, Kane TP. Toddler’s fracture. J Accid Emerg Med. 1996;13(1):59-61.
- Halsey MF, Finzel KC, Carrion WV, Haralabatos SS, Gruber MA, Meinhard BP. Toddler’s fracture: presumptive diagnosis and treatment. J Pediatr Orthop. 2001;21(2):152-156.