User login
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
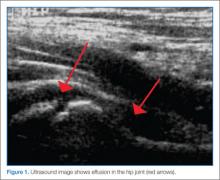
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
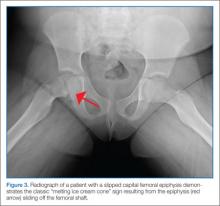
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
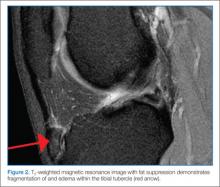
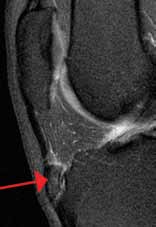
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
The child with limp represents a common scenario in the pediatric ED. Evaluation of such patients may be difficult due to vague clinical histories and nonspecific physical examination findings. The differential diagnosis is broad and includes mild self-limited processes (eg, toxic synovitis), as well as potentially limb and life-threatening etiologies (eg, osteomyelitis, malignancy). Careful attention to historical clues and a focused physical examination are often suggestive of a diagnosis, but laboratory and radiographic studies are necessary in most cases.
While trauma is the most common source of limp in children, infectious, inflammatory, and anatomic causes are also frequently encountered. This review focuses on several of the most important etiologies of limp in children: toxic synovitis, septic arthritis, osteomyelitis, Perthes disease, slipped capital femoral epiphysis (SCFE), and Osgood-Schlatter disease.
Case Presentation
Kailey, a 28-month-old girl, was brought to the ED by her parents, who stated that their child “isn’t walking right.” They noted that their daughter’s right leg had been bothering her for about a week, but that the limp had become more noticeable. Aside from a cold a week before presentation, both parents stated that the child has been healthy; they also denied any trauma or systemic symptoms such as rash, weight loss, vomiting, or diarrhea. The mother believed Kailey may have had a low-grade fever earlier in the week. There were no sick contacts at home, but the child did attend daycare.
On examination, Kailey was well-appearing on her mother’s lap. Her vital signs were unremarkable and she was afebrile. She was able to bear weight on the right leg but walked with a slightly antalgic gait, which became more apparent when she was asked to run across the room to her mother. Her right leg was normal appearing on examination with normal reflexes; however, the child appeared to wince when passively ranging her right hip and right knee joint. The emergency physician (EP) was not able to determine any particular areas of point tenderness. The remainder of the examination, including neurological and musculoskeletal examinations, was normal.
Regarding further history, the child’s parents informed the EP that they had taken their daughter to an urgent care clinic earlier in the week. X-rays taken of the girl’s right knee and hip at this visit were both normal; however, the girl’s limp had been getting worse.
Overview
Limp is a common presenting complaint in the pediatric ED, and its evaluation is often challenging as the clinical course of limp in a child varies from benign and self-limited to serious and limb-threatening. However, with careful attention to the history and physical examination, appropriate laboratory evaluation, and focused imaging studies, a diagnosis can be made in most cases and treatment initiated. Of paramount importance for clinicians is to remember that limp in a child nearly always represents an organic disease.1,2
While trauma is the most common cause of limp in children, infectious, inflammatory, and anatomic processes are other potential etiologies. A clue to the diagnosis may also be inferred from the patient’s age, as certain conditions such as fracture, Perthes disease, and transient synovitis are seen in younger children, while SCFE and Osgood-Schlatter are more common in children older than 10 years of age. Other serious conditions such as septic hip or osteomyelitis may be encountered at any age.
Initial Management
A thorough history and physical examination provide the basis for subsequent laboratory and radiographic testing of children presenting with a limp. The duration and localization of symptoms should be elicited; traumatic or infectious causes are more common among those presenting with acute (<2 weeks) complaints. The presence of systemic symptoms (eg, fever, weight loss, chills, rashes, recurrent arthralgias) increases the likelihood of underlying oncologic or rheumatologic process. Examination of the child begins with a full physical examination to uncover other possible etiologic clues such as other involved joints (juvenile idiopathic arthritis), signs of old bruising (nonaccidental trauma), firm lymph nodes (malignancy), abdominal pain (eg, appendicitis, psoas abscess, constipation), or limb-length discrepancy (developmental dysplasia of the hip).
Focused assessment of the limp itself involves watching the child walk or run; different variations of limp may also offer a clue to the diagnosis. An antalgic gait simply refers to one in which the affected leg spends less time in the weight-bearing stage, and it is most commonly seen with infection and trauma. Trendelenburg gait, frequently seen with SCFE and Perthes disease, is characterized by a downward tilt of the pelvis away from the affected side while the affected leg is bearing weight.
In many cases, it may be difficult to accurately characterize a limp due to a patient’s pain or lack of compliance. Evaluation of any limp should also focus on the joint above and below the child’s apparent main source of pain. This is particularly true of knee complaints as referred pain from the hip may often present as isolated thigh or knee pain. Areas of point tenderness, erythema, joint effusion, and warmth strongly point to an infectious source but are frequently absent early in disease presentation. While swelling and severe pain with passive movement of a joint indicate septic arthritis, limitation of joint movement at the hip can be seen with SCFE and Perthes disease.
Laboratory Studies
In most children presenting with limp, extensive laboratory testing is not needed for the diagnosis but is helpful when infectious, oncologic, and rheumatologic causes are considered. Inflammatory markers such as the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are elevated in most acute infectious processes. A complete blood count (CBC) should also be obtained in cases of suspected infection to evaluate the white blood cell count (WBC). Due to the high prevalence of joint infections and postinfectious processes caused by group A streptococcus (GAS), an antistreptolysin titer (ASO), throat culture, and rapid streptococcal antigen swab should be considered. If infection is suspected and a joint effusion is present, cell counts, gram stain, and culture from the synovial fluid should be sent to assist with diagnosis and antibiotic management. If a child appears septic, a blood culture should be obtained as well. With suspected osteomyelitis, cultures of the bone should be taken in the operating room in addition to a peripheral blood culture. Ideally, antibiotics should be withheld until cultures are sent unless the child appears acutely ill.
Diagnostic Imaging
Toxic Synovitis and Septic Arthritis
Clinicians may have difficulty differentiating between toxic synovitis (also known as transient synovitis) and septic arthritis of the hip in pediatric patients. In both toxic synovitis and septic arthritis, the child is unable to bear weight on the affected side, and ultrasound may demonstrate effusion.3,4
Toxic synovitis is thought to be a viral or postviral phenomenon, though the exact agent responsible for initiating the inflammatory cascade is not known. It has a relatively benign course and generally responds over 3 to 10 days to rest and nonsteroidal anti-inflammatory drugs (NSAIDs).5,6
Septic arthritis, however, is a serious infection with the capacity to cause permanent joint damage as well as spread into the blood or bone. Staphylococcal and streptococcal bacteria, including GAS and methicillin-resistant staphylococcus aureus (MRSA) are the most common causes of septic arthritis in the pediatric population.7 However, Kingella kingae, a gram-negative organism, is an increasingly recognized cause of septic arthritis in children younger than 3 years of age. Commonly found in the posterior pharynx, Kingella is very difficult to culture but may be detected by polymerase chain reaction (PCR) assays.8 The spectrum of GAS-related joint infections ranges from a postinfectious type that may be indistinguishable from toxic synovitis (but with evidence of recent GAS pharyngitis) to typical bacterial septic arthritis.9
Multiple clinical prediction rules and algorithms have been developed to assist with the management of these cases. The Kocher criteria, which include history of fever >101.3˚F, nonweight-bearing status, ESR >40 mm/hour, and WBC >12,000 cells/mm3 were found to be independent clinical predictors for the differentiation between transient arthritis and septic arthritis. Children with none of the risk factors had a 0.2% chance of septic arthritis while those with two risk factors had a 40% chance of septic arthritis. Subsequent validation studies among pediatric populations with a lower prevalence of septic arthritis found the positive predictive values to be lower; nevertheless, the Kocher criteria remain useful in guiding management.3,4
If a diagnosis of septic arthritis remains a consideration after the history, physical examination, and plain radiography, laboratory studies should be obtained, including a CBC, ESR, CRP, blood culture, rapid streptococcal assay, throat culture, and ASO titer. An ultrasound should also be performed to assess for the presence of a hip effusion.
Elevated inflammatory markers (ESR >40 mm/hour, CRP >20 mg/L, WBC >12,000 cells/mm3) and an effusion should prompt orthopedic consultation and arthrocentesis for synovial fluid-cell counts, gram stain, and culture. Synovial fluid WBC counts >50,000 cells/mm3 or a positive gram stain or culture is diagnostic of septic arthritis and requires treatment with intravenous (IV) antibiotics and likely operative intervention. The absence of an effusion on ultrasound, synovial fluid WBC counts <50,000 cells/mm3, and inflammatory markers that are not significantly elevated indicate an alternative diagnosis such as toxic synovitis. However, there may be cases in which the diagnosis is not clear due to ambiguous laboratory values from the blood or synovial fluid; in these cases the decision to proceed with IV antibiotics must be made in consultation with an orthopedist.10
Empiric antibiotic therapy in patients with septic arthritis should take into account the prevalence of MRSA in the community. Clindamycin is a popular choice due to its high oral bioavailability, which facilitates transition to a home regimen. Vancomycin is another option and can be reserved for more severely ill children. Empiric coverage for suspected septic arthritis in children younger than 3 years of age should also include an anti-gram negative agent, such as a cephalosporin, to treat Kingella.7
Osteomyelitis
Acute osteomyelitis is a common cause of limp among children and is usually caused by the hematogenous spread of bacteria. Staphylococcus aureus, including MRSA, is responsible for up to 90% of cases of osteomyelitis, though recent studies have identified Kingella as an etiologic agent among children younger age 3 years.7 The highly vascular metaphysis of the tibia and femur in children can become infected during times of otherwise asymptomatic bacteremia in healthy patients.8 Among younger children with less well-developed anatomic separation between the bone and joint space, it is possible for infection to spread into the joint space. Younger children with osteomyelitis may present with poorly localized pain with or without systemic symptoms; older children and those with more advanced disease may describe point tenderness on the bone. In many cases it may be difficult clinically to distinguish osteomyelitis from septic arthritis; excessive pain with passive range of motion of the joint is more indicative of septic arthritis.
Ideally, antibiotic therapy should be deferred until cultures from the site of infection are obtained operatively or via aspiration. The local staphylococcus aureus resistance pattern should guide empiric antibiotic therapy. With the rise of MRSA, clindamycin has become first-line therapy with vancomycin as an alternative for severely-ill or clindamycin-allergic patients. For children younger than 3 years of age with a more subacute presentation, the possibility of Kingella infection should be considered and treated with a cephalosporin. Some children with osteomyelitis will require operative debridement, though
many can be treated with antibiotics alone. The standard duration of antibiotic treatment is 4 to 6 weeks, with transition to an oral regimen once the patient is afebrile with downtrending inflammatory markers.7,8,11
Perthes Disease
Perthes disease (also called Legg-Calve-Perthes disease) is an idiopathic process that involves avascular necrosis and revascularization of the blood supply of the femoral head. The condition is most common in children ages 3 to 12 years and has a 4:1 male to female predominance. The remodeling takes place over the course of 2 to 4 years. During this process, the epiphysis of the femoral head is weakened and undergoes irreversible deformation that, if uncorrected, will persist throughout life. Severe degenerative arthritis may result and ultimately require hip replacement.12
Perthes disease typically presents as a subacute limp, sometimes with referred pain to the groin, thigh, or knee of the affected side. Range of motion of the hip may be limited, particularly in abduction and internal rotation. Plain films demonstrate necrotic avascular areas of the distal femoral head during active disease; after the remodeling process is complete, the femoral head often shows residual deformities. Magnetic resonance imaging is useful in cases in which plain film findings are subtle, particularly early in the course of the disease.12,13
Management of Perthes disease depends on the age of the child and clinical factors such as radiographic progression and range of motion of the hip. The goal of therapy is to limit damage to the femoral head during the revascularization process. In children younger than 5 years of age, nonsurgical management with an abduction splint to keep the femoral head contained and protected within the acetabulum may be used. Surgical osteotomies are used in older and more severely affected children to artificially contain the femoral head during the healing process.13
Slipped Capital Femoral Epiphysis
A common cause of limp among older children, the average age of presentation for SCFE is 13.5 years for boys and 12 years for girls. This condition occurs when the proximal femoral epiphysis slides posteriorly and inferiorly relative to the metaphysis. Epidemiological studies have established a connection between SCFE and obesity. Although the precise pathogenesis remains unknown, it is hypothesized that increased mechanical forces during a time of rapid pubertal growth lead to weakness at the physis. Among children who develop SCFE outside of the usual age distribution, endocrinopathies such as hypothyroidism, hypogonadism, and panhypopituitarism are often discovered.14
As with Perthes disease, SCFE may present as a chronic, subacute, or acute limp with referred pain to the groin, thigh, or knee. The patient with SCFE often has severe pain with internal rotation of the affected hip and will hold the hip in obligatory external rotation if it is flexed. Patients unable to bear weight on the affected side have unstable SCFE.
All patients with SCFE should be made nonweight bearing on the affected hip to prevent further slippage of the epiphysis. Definitive treatment involves in-situ fixation of the femoral neck with the proximal femoral epiphysis. Close orthopedic follow up is essential as roughly 50% of children with unilateral SCFE will go on to develop SCFE in the contralateral hip. Long-term complications of SCFE include osteonecrosis, joint space narrowing, and osteoarthritis.14,15
Osgood-Schlatter Disease
Osgood-Schlatter disease is a relatively benign cause of limp that is thought to occur due to repetitive trauma to the secondary ossification center tibial tubercle. Over time, the strong pull of the quadriceps muscle group on the patellar tendon causes a chronic avulsion at the site of the patellar tendon insertion on the tibial tuberosity. It most commonly develops in early puberty from ages 9 to 14 years. Affected children develop tenderness over the tibial tuberosity that is made worse with activity. On examination, pain can be elicited by having the child extend the knee against resistance or kneel. Lateral radiographs of the knee may be normal or demonstrate swelling, irregularity, or elevation of the tibial tubercle. Treatment consists of NSAIDs, ice, and physical therapy; activity restriction is generally unnecessary. Most cases resolve over 6 to 18 months as the growth plate at the secondary ossification center ossifies.16
Case Conclusion
Initial examination confirmed the presence of a limp; the differential in this age group includes toxic synovitis, septic arthritis, and osteomyelitis. Although Kailey appeared relatively well on examination, her history of recent fever and the worsening symptoms over the past week were concerning. The workup began with plain films of the pelvis and the right knee because of the possibility of referred pain and the lack of localizing signs on examination. In addition, laboratory evaluation was performed, including CBC, CRP, and ESR. Due to her young age, a rapid streptococcal assay, throat culture, or an ASO titer was not necessary.
Plain films of the pelvis and the right knee were normal. The patient’s WBC was unremarkable, but her ESR was 50 mm/hour and CRP was 25 mg/L. Given these elevated inflammatory markers, ultrasound of the right hip was ordered, which revealed a small effusion. An orthopedic specialist was consulted, who performed a sedated joint aspiration. Cell counts from the joint aspirate were sent for evaluation, as well as culture, gram stain, and a PCR for Kingella.
After joint aspiration, Kailey was admitted to the hospital overnight and was started on empiric treatment with IV clindamycin and ceftriaxone. The synovial fluid gram stain was negative, but the WBC was 65,000 cells/mm3. Over the next several days, her inflammatory markers trended downward, she remained afebrile, and her gait slowly improved. The synovial fluid culture remained negative, but the PCR was positive for Kingella. Kailey was discharged on hospital day 3 with a 21-day course of oral cephalexin.
Dr Kane is a fellow in the department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee. Dr McMorrow is an assistant professor of emergency medicine and assistant professor of pediatrics, department of pediatrics, division of pediatric emergency medicine, Vanderbilt Children’s Hospital, Nashville, Tennessee.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.
- Smith E, Anderson M, Foster H. The child with a limp: a symptom and not a diagnosis. Arch Dis Child Educ Pract Ed. 2012;97(5):185-193.
- Leung AK, Lemay JF. The limping child. J Pediatr Health Care. 2004;18(5):219-223.
- Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. Distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94(2):167,168.
- Luhmann SJ, Jones A, Schootman M, Gordon JE, Schoenecker PL, Luhmann JD. Differentiation between septic arthritis and transient synovitis of the hip in children with clinical prediction algorithms. J Bone Joint Surg Am. 2004;86-A(5):956-962.
- Do TT. Transient synovitis as a cause of painful limps in children. Curr Opin Pediatr. 2000;12(1):48-51.
- Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23(1):32-36.
- Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13(5):451-460.
- Dodwell ER. Osteomyelitis and septic arthritis in children: current concepts. Curr Opin Pediatr. 2013;25(1):58-63.
- Mignemi ME, Martus JE, Bracikowski AC, Lovejoy SA, Mencio GA, Schoenecker JG. The spectrum of group A streptococcal joint pathology in the acute care setting. Pediatr Emerg Care. 2012;28(11):
1185-1189. - Rutz E, Spoerri M. Septic arthritis of the paediatric hip - A review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg. 2013;79(2):123-134.
- Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther. 2010;8(2):175-181.
- Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45(1):87-97.
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: current principles of diagnosis
and treatment. Dtsch Arztebl Int. 2009;106(31-32):517-523. - Novais EN, Millis MB. Slipped capital femoral epiphysis: prevalence, pathogenesis, and natural history. Clin Orthop Relat Res. 2012;470(12):3432-3438.
- Peck D. Slipped capital femoral epiphysis: diagnosis and management. Am Fam Physician. 2010;82(3):258-262.
- Gholve PA, Scher DM, Khakharia S, Widmann RF, Green DW. Osgood Schlatter syndrome. Curr Opin Pediatr. 2007;19(1):44-50.