User login
Things We Do for No Reason: Prescribing Docusate for Constipation in Hospitalized Adults
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
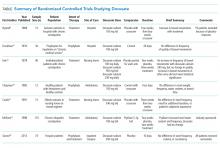
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
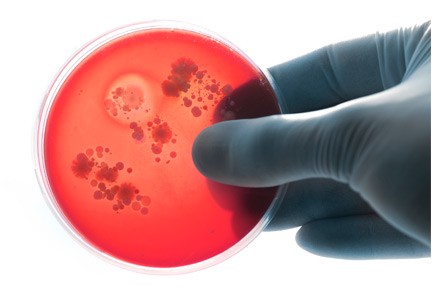
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
© 2019 Society of Hospital Medicine
Managing malignant pleural effusion
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
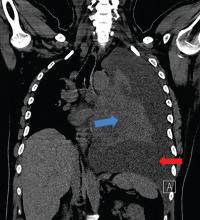
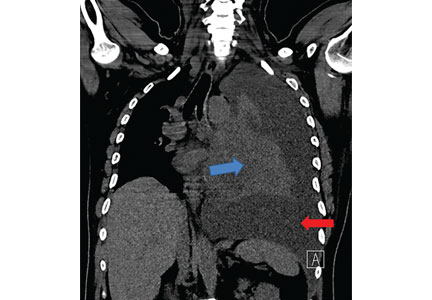
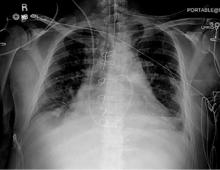
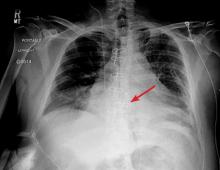
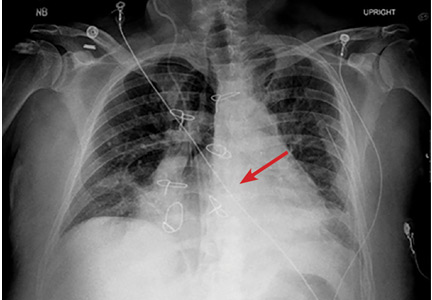
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
KEY POINTS
- Asymptomatic pleural effusion in patients currently on chemotherapy does not require treatment but should be monitored for progression.
- Indwelling pleural catheters are best used to treat effusion with lung collapse and are increasingly used as first-line therapy in other settings.
- Chemical or mechanical pleurodesis results in filling the pleural space to prevent further fluid accumulation and can be accomplished by one of several methods.
- For patients near the end of life, simple thoracentesis, repeated as needed, is a reasonable strategy.
Heart failure guidelines: What you need to know about the 2017 focused update
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
KEY POINTS
- Despite advances in treatment, heart failure remains highly morbid, common, and costly. Prevention is key.
- Strategies to prevent progression to clinical heart failure in high-risk patients include new blood pressure targets (< 130/80 mm Hg) and B-type natriuretic peptide screening to prompt referral to a cardiovascular specialist.
- An aldosterone receptor antagonist might be considered to decrease hospitalizations in appropriately selected stage C HFpEF patients. Routine use of nitrates or phosphodiesterase-5 inhibitors in such patients is not recommended.
- Outpatient intravenous iron infusions are reasonable in persistently symptomatic New York Heart Association stage II to III heart failure with reduced ejection fraction (HFrEF) to improve functional capacity and quality of life.
- The new systolic blood pressure target is less than 130 mm Hg for stage A heart failure, stage C HFrEF, and stage C HFpEF.
Dancing sternal wires: A radiologic sign of sternal dehiscence
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
The next day, routine radiography showed widely separated sternal wires (Figure 3), indicating significant progression of sternal dehiscence. The patient subsequently underwent open reduction and internal fixation of the sternum.
STERNAL DEHISCENCE
Physical examination may reveal tenderness to palpation, but findings that are more characteristic are an audible click and rocking of the sternum with coughing or forced chest movements.3
Plain chest radiography can clearly show early signs of sternal dehiscence; however, physicians rarely scrutinize the films for wire placement. Subtle signs include loss of sternal alignment with shifting of the segments and central sternal lucency. Gross signs start to appear when 2 or more wires are displaced; these signs are dramatic and rarely missed.
Loss of alignment and central sternal lucency are the earliest radiographic signs of dehiscence. Awareness of early subtle signs can lead to prompt diagnosis and treatment to prevent progression to gross sternal dehiscence.
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
- Olbrecht VA, Barreiro CJ, Bonde PN, et al. Clinical outcomes of noninfectious sternal dehiscence after median sternotomy. Ann Thorac Surg 2006; 82(3):902–907. doi:10.1016/j.athoracsur.2006.04.058
- Efthymiou CA, Kay PH, Nair UR. Repair of spontaneous right ventricular rupture following sternal dehiscence. A novel technique. Interact Cardiovasc Thorac Surg 2010; 10(1):12–13. doi:10.1510/icvts.2009.217810
- Santarpino G, Pfeiffer S, Concistré G, Fischlein T. Sternal wound dehiscence from intense coughing in a cardiac surgery patient: could it be prevented? G Chir 2013; 34(4):112-113. pmid:23660161
Repeating blood cultures after initial bacteremia: When and how often?
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
Repeat cultures are indicated in specific scenarios, but for most patients, frequent and indiscriminate repetition after an initial positive culture is unnecessary and may be associated with excessive use of resources. Prospective studies and practice guidelines are needed to help further define the indications.
THE TENDENCY TO REPEAT CULTURES
Current literature lacks strong evidence for repeating previously positive blood cultures collected appropriately—ie, 10 mL of blood for aerobic culture and 10 mL for anaerobic culture from 2 different sites, and a positive result from both sets. However, because of the risk of serious complications of bacteremia, particularly in critically ill patients, many clinicians order multiple, repeated sets of blood cultures.
Tabriz et al1 found that one-third of hospitalized patients got repeat cultures after an initial set, regardless of the result of the first set. Most (83.4%) of those cultures yielded no growth, 9.1% grew the same pathogen, and 5.0% were contaminated. Finding a new pathogen was rare, occurring in only 2.5% of repeated cultures.
Wiggers et al2 reported an even higher number of repeat cultures ordered for patients who had an initially positive culture: 38.9%.2 And in another study,3 half of the patients received more than 2 consecutive cultures.
Drawbacks
Unrestrained ordering of repeat blood cultures can increase the risk of a false-positive result, leading to more cultures, echocardiography, other imaging tests, and unnecessary antimicrobial therapy, all of which puts patients at risk of adverse effects of treatment and missed alternative diagnoses and increases the length and cost of hospitalization.4
Advantages
On the other hand, repeat blood cultures may increase the diagnostic yield for conditions such as infective endocarditis and may have implications for the duration of antibiotic therapy.1 The duration of therapy for bacteremia is usually determined from the last negative culture; hence, documenting clearance of bacteremia can determine a precise end-date for antibiotic therapy.
Bacteremia due to Staphylococcus aureus and to endovascular and epidural sources has been found to be independently associated with persistent bacteremia, detected in 6.6% of 1,801 index cases of bacteremia in a retrospective cohort study.2 An endovascular source (adjusted odds ratio [OR] 7.66, 95% confidence interval [CI] 2.30–25.48), an epidural source (adjusted OR 26.99, 95% CI, 1.91–391.08), and S aureus bacteremia (adjusted OR 4.49, 95% CI 1.88–10.73) were independently associated with persistent bacteremia. Escherichia coli (5.1%, P = .006), viridans group streptococci (1.7%, P = .035), and beta-hemolytic streptococci (0%, P = .028) were associated with a lower likelihood of persistent bacteremia. Patients with persistent bacteremia were less likely to have achieved source control within 48 hours of the index event (29.7% vs 52.5%, P < .001).2
WHEN REPEATING CULTURES IS APPROPRIATE
Repeating blood cultures after an initial positive result is superfluous, except in certain situations.
Suspected endovascular infection
Patients with endocarditis, thrombophlebitis, an indwelling device for epidural access, or a cardiovascular implantable electronic device should have repeat cultures after an initial positive culture. Implantable electronic device infection is suspected in the following cases: sustained positive blood culture (> 24 hours); relapsing bacteremia despite a course of appropriate antibiotic therapy; presence of an implantable cardioverter defibrillator; presence of a prosthetic cardiac valve; and an episode of bacteremia within 3 months of device placement.5
S aureus bacteremia
Repeat blood culture is warranted for S aureus bacteremia regardless of methicillin susceptibility.1 But persistent methicillin-resistant S aureus (MRSA) bacteremia changes the management of these patients.6 For example, the source of infection should be identified, followed by debridement or drainage, and then either high-dose or combination antimicrobial therapy.6 Infective endocarditis from persistent MRSA bacteremia is an indication for surgery.6
Persistent S aureus bacteremia may change the duration of therapy, as the common practice is to continue treating uncomplicated gram-positive bacteremia for 14 days from the date of the first negative culture. Infection leading to infective endocarditis increases the duration of antibiotic therapy to at least 4 weeks.
Candidemia
Candidemia is an absolute indication for repeat blood culture.7 Patients with persistent candidemia should undergo imaging of the genitourinary tract, liver, and spleen as part of the evaluation for a deep-tissue source of infection.7 Also, if the patient is initially treated with an echinocandin, therapy can be transitioned to fluconazole if the isolate is azole-susceptible, the patient’s condition is clinically stable, and repeat cultures are negative.7 Therefore, repeating cultures has therapeutic implications.
Confirming response to therapy
In patients with infective endocarditis or other endovascular infection caused by S aureus, Enterococcus species, or gram-negative bacilli,1 repeat blood culture should be done to confirm therapeutic response. Patients with infective endocarditis whose condition is stable can be discharged to receive outpatient parenteral antibiotic therapy. However, patients with uncontrolled heart failure, systemic emboli, abscess, persistent fever, or persistently positive cultures are not candidates for outpatient therapy and require repeat cultures.8
Multidrug-resistant gram-negative bacilli
Bacteremia due to multidrug-resistant gram-negative bacilli requires repeat blood cultures to document clearance of bacteremia and to ensure the efficacy of antibiotics, as these organisms pose a higher risk of treatment failure, and combination synergistic regimens may be needed if bacteremia does not clear.
Febrile neutropenia
Blood cultures are important in the management of febrile neutropenia. In a study by Rosenblum et al,9 repeat cultures were positive in 10.9% of patients with febrile neutropenia after an initial negative culture, but many of those organisms were of low pathogenicity, and a significant proportion were coagulase-negative staphylococci.10 Another study showed that the frequency of detecting new pathogens by repeat culture in recurrent febrile neutropenia was higher than that in persistent febrile neutropenia (8% vs 2%) (P = .0491); a history of recent bacteremia was identified as a significant predictor of positive culture in recurrent febrile neutropenia.11
Persistent or new infection
Persistence of fever, leukocytosis, or other signs of infection 72 hours after appropriate antibiotic therapy is started requires follow-up blood cultures.
New episode of sepsis. A new episode of sepsis should be confirmed12 using the systemic inflammatory response syndrome criteria, the newer definition of Sepsis-related Organ Failure Assessment (SOFA) in the intensive-care unit, or the quick SOFA in general units. If the patient develops new signs of sepsis after response to treatment for initial bacteremia, repeat blood cultures should be considered.
Central line-associated bloodstream infection requires repeat cultures.13 Persistence of bacteremia in this type of infection extends the duration of therapy, as most clinicians determine treatment duration from the last negative culture. Persistent bacteremia also influences the decision to salvage or remove the catheter. Microbiologic clearance of bacteremia on blood culture can also guide the time of reinsertion if the catheter was removed.
Concern for an unresolved focus of infection such as abscess, joint infection, or retained catheter is an indication for repeat blood cultures.
Bacteremia of unknown source. In clinical practice, we encounter scenarios in which blood cultures are positive but no source can be identified. In those situations, it is important to repeat blood cultures to document clearance. If bacteremia persists, we need to continue searching for the source.
WHEN ROUTINELY REPEATING CULTURES IS NOT INDICATED
Repeat blood cultures are not routinely indicated in patients with streptococcal bacteremia, uncomplicated gram-negative bacteremia, and bacteremia associated with localized infection such as cellulitis, community-acquired pneumonia, or pyelonephritis.2,4 A study of patients with gram-negative bacteremia found that 17 repeated cultures needed to be drawn to yield 1 positive culture.14
Isolated fever or leukocytosis does not accurately predict bacteremia.4 A study that excluded neutropenic and intensive-care patients reported none of the initially negative cultures to be positive when repeated.15
Ordering repeat cultures in response to persistent fever is a common practice, even though fever is typical in the first 72 hours of antibiotic therapy. Such cultures rarely if ever reveal new pathogens, and results can be predicted based on cultures before the start of antibiotics.15 For patients on antibiotics, physicians should therefore wait for results of the preantibiotic cultures rather than order new cultures in response to persistent fever.15
WOULD WE MISS PERSISTENT BACTEREMIA?
In theory, not repeating blood cultures could miss persistent bacteremia, but this is unlikely if the concerns discussed above are considered. Further, persistent bacteremia would result in clinical signs and symptoms that should prompt repeat cultures.
FREQUENCY OF REPEAT BLOOD CULTURES
There are no evidence-based guidelines for the frequency of repeating cultures. The Infectious Diseases Society of America recommends repeating blood cultures 2 to 4 days after the index positive culture in the case of multidrug-resistant S aureus bacteremia, and every day or every other day for candidemia.6,7,9
A study evaluating the practice patterns of repeating cultures after an initial bacteremia showed that 34.7% were done within 24 hours and 44.7% were done in 2 to 4 days.1 There is no evidence that repeating blood cultures daily is necessary in these patients. As a general rule, it should be done 48 to 72 hours after a positive culture.
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
- Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect 2004; 10(7):624–627. doi:10.1111/j.1469-0691.2004.00893.x
- Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016; 16:286. doi:10.1186/s12879-016-1622-z
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case–control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Coburn B, Morris AM, Tomlinson G, Detsky AS. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012; 308(5):502–511. doi:10.1001/jama.2012.8262
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Interdisciplinary Council on Quality of Care; American Heart Association. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121(3):458–477. doi:10.1161/CIRCULATIONAHA.109.192665
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52(3):e18–e55. doi:10.1093/cid/ciq146
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62(4):e1–e50. doi:10.1093/cid/civ933
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Rosenblum J, Lin J, Kim M, Levy AS. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2013; 60(6):923–927. doi:10.1002/pbc.24358
- Thomas MW, Chauvenet AR, O'Suoji C. Repeating blood cultures in neutropenic children with persistent fevers when the initial blood culture is negative. Pediatr Blood Cancer 2014; 61(2):194. doi:10.1002/pbc.24834
- Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49(10):748–757. doi:10.1080/23744235.2017.1340665
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315(8):801–810. doi:10.1001/jama.2016.0287
- Shah H, Bosch W, Thompson KM, Hellinger WC. Intravascular catheter-related bloodstream infection. Neurohospitalist 2013; 3(3):144–151. doi:10.1177/1941874413476043
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32(11):1651–1655. doi:10.1086/320527
Follow-up blood cultures are often needed after bacteremia
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
Bacteremia is common and associated with significant morbidity and mortality. Bloodstream infections rank among the leading causes of death in North America and Europe.1
In this issue, Mushtaq et al2 contend that follow-up blood cultures after initial bacteremia are not needed for most hospitalized patients. Not repeating blood cultures after initial bacteremia has been proposed to decrease hospitalization length, consultations, and healthcare costs in some clinical settings. However, without follow-up cultures, it can be difficult to assess the adequacy of treatment of bacteremia and associated underlying infections.
GRAM-NEGATIVE ORGANISMS
Results of retrospective studies indicate that follow-up cultures may not be routinely needed for gram-negative bacteremia. In a review by Canzoneri et al of 383 cases with subsequent follow-up cultures,3 55 (14%) were positive. The mean duration of bacteremia was 2.8 days (range 1 to 15 days). Of the 55 persistently positive blood cultures, only 8 (15%) were caused by gram-negative organisms. Limitations to this study included the lack of patient outcome data, a low event rate, and the retrospective design.4
In a retrospective case-control study of follow-up cultures for 862 episodes of Klebsiella pneumoniae bacteremia,5 independent risk factors for persistent bacteremia were intra-abdominal infection, higher Charlson comorbidity index score, solid-organ transplant, and unfavorable treatment response.
These studies confirm that persistent bacteremia is uncommon with gram-negative organisms. They also support using comorbidities and treatment response to guide the ordering of follow-up blood cultures.
WHEN IS FOLLOW-UP CULTURE USEFUL?
Although follow-up blood cultures may not be needed routinely in patients with gram- negative bacteremia, it would be difficult to extrapolate this to gram-positive organisms, especially Staphylococcus aureus.
In Canzoneri et al,3 43 (78%) of the 55 positive follow-up cultures were due to gram-positive organisms. Factors associated with positive follow-up cultures were concurrent fever, presence of a central intravenous line, end-stage renal disease on hemodialysis, and diabetes mellitus. In addition, infectious disease consultation to decide the need for follow-up cultures for S aureus bacteremia has been associated with fewer deaths, fewer relapses, and lower readmission rates.6,7
In certain clinical scenarios, follow-up blood cultures can provide useful information, such as when the source of bacteremia is endocarditis or cardiac device infection, a vascular graft, or an intravascular line. In the Infectious Diseases Society of America guidelines for diagnosis and management of catheter-related bloodstream infections, persistent or relapsing bacteremia for some organisms is a criterion for removal of a long-term central venous catheter.8
Follow-up cultures are especially useful when the focus of infection is protected from antibiotic penetration, such as in the central nervous system, joints, and abdominal or other abscess. These foci may require drainage for cure. In these cases or in the setting of unfavorable clinical treatment response, follow-up blood cultures showing persistent bacteremia can prompt a search for unaddressed or incompletely addressed foci of infection and allow for source control.
The timing of follow-up cultures is generally 1 to 2 days after the initial culture. Although Mushtaq et al propose a different approach, traditional teaching has been that the last blood culture should not be positive, and this leads to ordering follow-up blood cultures until clearance of bacteremia is documented.
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19(6):501–509. doi:10.1111/1469-0691.12195
- Mushtaq A, Bredell B, Soubani A. Repeating blood cultures after an initial bacteremia: when and how often? Cleve Clin J Med 2019; 86(2):89–92. doi:10.3949/ccjm.86a.18001
- Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65(11):1776–1779. doi:10.1093/cid/cix648
- Jones RB, Paruchuri A, Shah SS. Prospective trials are required to alter practice for follow-up blood cultures for gram-negative bacilli bacteremia. Clin Infect Dis 2018; 67(2):315–316. doi:10.1093/cid/ciy070
- Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis 2013; 13:365. doi:10.1186/1471-2334-13-365
- Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123(7):631–637. doi:10.1016/j.amjmed.2010.01.015
- Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27(3):478–486. pmid:9770144
- Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49(1):1–45. doi:10.1086/599376
Patient, Caregiver, and Clinician Perspectives on Expectations for Home Healthcare after Discharge: A Qualitative Case Study
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
Patients who are discharged from the hospital with home healthcare (HHC) are older, sicker, and more likely to be readmitted to the hospital than patients discharged home without HHC.1-3 Communication between clinicians in different settings is a key factor in successful transitions. In prior work, we focused on communication between primary care providers, hospitalists, and HHC nurses to inform efforts to improve care transitions.4,5 In one study, HHC nurses described that patients frequently have expectations beyond the scope of what skilled HHC provides,5 which prompted us to also question experiences of patients and caregivers after discharge with skilled HHC (eg, nursing and physical therapy).
In a prior qualitative study by Foust and colleagues, HHC patients and caregivers described disparate experiences around preparation for hospital discharge—patients expressed knowing about the timing and plans for discharge, and the caregivers frequently felt left out of this discussion.6 In other studies, caregivers of recently discharged patients have described feeling excluded from interactions with clinicians both before and after discharge.7,8 In another recent qualitative study, caregivers described uncertainty about their role compared with the HHC role in caring for the patient.9
As of 2016, a majority of states had passed the Caregiver Advise, Record, and Enable (CARE) Act, which requires hospitals to (1) record a family caregiver in the medical record, (2) inform this caregiver about discharge, and (3) deliver instructions with education about medical tasks that they will need to complete after discharge.10
METHODS
Study Design
In this qualitative descriptive case study, we interviewed HHC patients, an involved caregiver, and the HHC clinician completing the first HHC visit within 7-14 days following hospital discharge. We chose this timeframe to allow patients to receive one or more HHC visits following hospital discharge.
Population
- >65 years old,12 eligibility was initially limited to patients
- >65 years old. Due to recruitment challenges, the age range was broadened to
- >50 years old in October 2017.
Because our goal was to better understand the experience of general medicine patients with multiple comorbidities, we recruited patients from one general medicine unit at an academic hospital in Colorado. Patients on this unit were screened for eligibility Monday-Friday (excluding weekends and holidays) based on research assistant availability.
Criteria included are as follows: HHC referral, three or more comorbidities, resides in the community prior to admission (ie, not in a facility), cognitively intact, English speaking, and able to identify a caregiver participating in their care. Eligible patients were approached for written consent prior to discharge to allow us to contact them 7-14 days after discharge for an interview by phone or in their home, per their preference. At the time of consent, patients provided contact information for their informal caregiver. Caregiver eligibility criteria included the following: age ≥18 years and provides caregiving at least one hour a week before hospital discharge. HHC clinicians approached for interviews had completed the first HHC visit for the patient following discharge. Both caregivers and HHC clinicians provided verbal consent for interviews. All participants received a $25 gift card for participation in the study.
Framework and Data Collection
Our interview guides were organized by the Agency for Healthcare Research and Quality Care Coordination Framework, an approach we have taken in prior work.4,5,13 We added questions about patient preparation and self-management support to build on findings from a prior study with HHC nurses and on prior work by Coleman and colleagues.5,14 Sample questions from the interview guides for patients, caregivers, and HHC clinicians within key analysis domains are included in Appendix 1. The patient and caregiver interviews were completed by an individual with prior experience in social work and healthcare (SS). The HHC clinician interviews were completed by either this individual (SS) or a physician-researcher with experience in qualitative methods (CJ). Patients and caregivers could choose to be interviewed individually or together. All interviews were digitally recorded and transcribed verbatim.
Analysis
Patient interviews lasted an average of 43 minutes, caregiver interviews an average of 41 minutes, and HHC clinician interviews an average of 25 minutes
We observed the two main themes of clear and unclear expectations for HHC after discharge. Clear expectations occur when the patient and/or caregiver have expectations for HHC that align with the services they receive. Unclear expectations occur when the patient and/or caregiver expectations are either uncertain or misaligned with the services they receive. Although not all interviews yielded codes about clear or unclear expectations, patients described clear expectations in five cases and unclear expectations in another five cases.
In nine cases with more than one perspective available, expectations were compared within cases and found to be clear (three cases), unclear (three cases), or discordant (three cases) across perspectives. For the discordant cases, the description of clear and unclear expectations differed between patients and either their caregiver or their HHC clinician. Patients and caregivers with clear expectations for HHC frequently described prior experiences with skilled HHC or work experience within the healthcare field. In most cases with unclear expectations, the patient and caregiver did not have prior experience with HHC. In addition, the desire for assistance with personal care for patients such as showering and housekeeping was described by caregivers with unclear expectations. The results are organized into clear, unclear, and discordant expectations from the perspectives of patients, caregivers, and HHC clinicians within cases.
Clear Expectations within Cases
Clear expectations for HHC were identified across perspectives in three cases, with sample quotes provided in Table 2. In the case of patient 1, the patient and HHC nurse had known each other for over two years because the patient had a wound requiring long-term HHC services. A caregiver did not complete an interview in this case. With patient 2, the patient, caregiver, and HHC physical therapist (PT) all describe that the patient had clear expectations for HHC. In this case, the patient and caregiver describe feeling prepared because of previously receiving HHC, prior work experience in the healthcare field, and a caregiver with experience working in HHC. In the case of patient 3, the patient had previously received HHC from the same HHC nurse.
Unclear Expectations across Cases
For the three cases in which unclear expectations were described across perspectives, two of the patients described being new to HHC, with representative quotes in Table 2. Patient 4 and her caregiver are new to HHC and describe unclear expectations for both the HHC referral and the HHC role, which was also noted by the HHC clinician. Of note, the caregiver for patient 4 further described that she was unable to be present for the first HHC visit. In the case of patient 5, although the patient had previously received HHC, the patient describes not knowing why the HHC PT needs to see her after discharge, which is also noted by the HHC PT. Finally, both patient 6 and her HHC PT describe that the patient was not sure about their expectations for HHC and that HHC was a new experience for them.
Discordant Expectation Clarity across Cases
In three of the cases, the description of clear and unclear expectations was discrepant across roles. In case 7, the caregiver and patient are new to HHC and express different perspectives about expectations for HHC. The HHC clinician, in this case, did not complete an interview. The caregiver describes not being present for the first HHC visit and no awareness that the patient was being discharged with HHC:
Caregiver: Well, we didn’t even know she had home health until she got home.
The same caregiver also expresses unclear expectations for HHC:
Caregiver: It’s pretty cloudy. They (the HHC clinicians) don’t help her with her laundry, they don’t help with the housekeeping, they don’t help… with her showers so somebody is there when she showers. They don’t do anything. The only two things like I said is the…home healthcare comes in on Wednesdays to see what she needs and then the therapy comes in one day a week.
However, the patient expresses more clear expectations that are being met by HHC.
Patient: They (HHC) have met my expectations. They come in twice a week. They do vitals, take vitals and discuss with me, you know, what my feelings are, how I’m doing and I know they have met my expectations.
In case 8, although the patient describes knowing about the HHC PT involvement in her care, she expresses some unclear expectations about an HHC nurse after discharge.
Patient: As far as home health, I didn’t have a real …plan there at the hospital… They knew about (the HHC PT) coming once a week but as far as, you know, a nurse coming by to check on me, no.
However, the HHC PT describes feeling that the patient had clear expectations for HHC after discharge:
Interviewer: Can you reflect on whether she was prepared to receive home healthcare?
HHC PT: Yeah, she was ready.
Interviewer: …do you feel like she was prepared to know what to expect from you?
HHC PT: Yeah, but I think that comes from being a previous patient also.
Finally, in case 9, the patient describes clear expectations for HHC even though they were new to HHC:
Patient: …I knew what the PT was going to do and …I still need her because I’ve lost so much weight so she’s been really good, instrumental, at giving me exercises… Occupational therapist…she’s going to teach me how to shave, she’s going to teach me how to get ready for the day.
The HHC PT describes that although the patient knew the PT role, they reflect that the patient may have been somewhat unclear about expectations for the first HHC visit:
HHC PT: He knew all that it entailed with the exception of he didn’t really know what the first day was going to be like and the first day I don’t usually do treatment because it does take a long time to get all the paperwork signed, to do the evaluation and the fact that it takes two hours to do that note.
DISCUSSION
In this qualitative case study with HHC patients, caregivers, and clinicians, the participants described varying levels of expectation clarity for HHC after discharge. We triangulated across and within cases and found three cases with clear expectations and three cases with unclear expectations for HHC across perspectives. In three additional cases, we found discordant expectations across perspectives: patients and HHC clinician expectations differed in two of the cases and a patient and caregiver differed in one case. Of interest, in all three cases of clear expectations across perspectives, the patients and/or caregivers had prior HHC or healthcare work experience. In contrast,
Prior studies in this area have included a qualitative study HHC patients, caregivers, and clinicians by Foust and colleagues in which multiple caregivers described finding out about the discharge from the patient or other caregivers, rather than being actively engaged by clinicians.6 In another recent qualitative study by Arbaje and colleagues, a majority of caregivers described “mismatched expectations” about HHC services, in which caregivers
When caregivers have unclear expectations for HHC, they could be expressing the need for more support after hospital discharge, which suggests an active role for hospital teams to assess and address additional support needs with the patients and caregivers. For example, if the patient or caregiver request additional personal care services, a home health aide could help to reduce caregiver burden and improve the support network for the patient. In a prior study in which patients were asked what would help them to make informed decisions about postacute care options, the patients described wanting to receive practical information that could describe how it would apply to their specific situation and perceived needs.18 To provide this for patients and caregivers, it would follow that hospitals could provide information about skilled HHC nursing and therapies and information about services that could meet additional needs, such as home health aides.
Limitations of this study include that it was a small qualitative case study of patients, caregivers, and HHC clinicians from one medical unit at one academic medical center. Most patients in this study had Medicare insurance, were 65 years and older, white, and female.
In conclusion, to improve care transitions for HHC patients and their caregivers, emphasizing engagement of caregivers is key to ensure that they are educated about HHC, provided with additional support as needed, and included in initial HHC visits once the patients are at home. Even though patients and caregivers with prior HHC experience often had clear expectations for HHC, a strategy to uniformly engage caregivers across a range of experience can ensure caregivers have all the information and support needed to optimize care transitions to HHC.
Disclosures
The authors have nothing to disclose.
Funding
Dr. Christine Jones is supported by grant number K08HS024569 from the Agency for Healthcare Research and Quality. Jason Falvey was supported by grant F31AG056069 from the National Institute on Aging, National Institutes of Health and is currently supported by T32AG019134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
1. Jones CD, Wald HL, Boxer RS, et al. Characteristics associated with home health care referrals at hospital discharge: results from the 2012 National Inpatient Sample. Health Serv Res. 2017;52(2):879-894. doi: 10.1111/1475-6773. PubMed
2. Avalere Health. Home Health Chartbook 2015: Prepared for the Alliance for Home Health Quality and Innovation. 2016.
3. Hospital Compare. https://www.medicare.gov/hospitalcompare/search.html. Accessed May 1, 2017.
4. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. doi: 10.1007/s11606-014-3056-x. PubMed
5. Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med. 2017;32(10):1114-1121. doi: 10.1007/s11606-017-4104-0. PubMed
6. Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34(2):194-212. doi: 10.1177/0193945911400448. PubMed
7. Blair J, Volpe M, Aggarwal B. Challenges, needs, and experiences of recently hospitalized cardiac patients and their informal caregivers. J Cardiovasc Nurs. 2014;29(1):29-37. doi: 10.1097/JCN.0b013e3182784123. PubMed
8. Coleman EA, Roman SP. Family caregivers’ experiences during transitions out of hospital. J Healthc Qual. 2015;37(1):12-21. doi: 10.1097/01.JHQ.0000460117.83437.b3. PubMed
9. Arbaje AI, Hughes A, Werner N, et al. Information management goals and process failures during home visits for middle-aged and older adults receiving skilled home healthcare services after hospital discharge: a multisite, qualitative study. BMJ Qual Saf. 2018. doi: 10.1136/bmjqs-2018-008163. PubMed
10. Coleman EA. Family caregivers as partners in care transitions: the caregiver advise record and enable act. J Hosp Med. 2016;11(12):883-885. doi: 10.1002/jhm.2637. PubMed
11. Jones AL, Harris-Kojetin L, Valverde R. Characteristics and use of home health care by men and women aged 65 and over. Natl Health Stat Report. 2012(52):1-7. PubMed
12. Tian W. An all-payer view of hospital discharge to postacute care, 2013. HCUP Statistical Brief #205. Rockville, Maryland; 2016. PubMed
13. McDonald KM, Sundaram V, Bravata DM, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, Maryland; 2007. PubMed
14. Coleman EA, Smith JD, Frank JC, Eilertsen TB, Thiare JN, Kramer AM. Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02. doi: 10.5334/ijic.60. PubMed
15. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
16. Lum HD, Jones J, Lahoff D, et al. Unique challenges of hospice for patients with heart failure: a qualitative study of hospice clinicians. Am Heart J. 2015;170(3):524-530 e523. doi: 10.1016/j.ahj.2015.06.019. PubMed
17. Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269-281. doi: 10.1586/erp.10.30. PubMed
18. Sefcik JS, Nock RH, Flores EJ, et al. Patient preferences for information on post-acute care services. Res Gerontol Nurs. 2016;9(4):175-182. doi: 10.3928/19404921-20160120-01. PubMed
19. Coleman EA, Roman SP, Hall KA, Min SJ. Enhancing the care transitions intervention protocol to better address the needs of family caregivers. J Healthc Qual. 2015;37(1):2-11. doi: 10.1097/01.JHQ.0000460118.60567.fe. PubMed
© 2019 Society of Hospital Medicine
Outpatient Parenteral Antimicrobial Therapy in Vulnerable Populations—People Who Inject Drugs and the Homeless
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Sliding-Scale Insulin as Monotherapy for Glycemic Control in Hospitalized Patients
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
© 2018 Society of Hospital Medicine
Should the Diagnosis of UTI in Young Febrile Infants Require a Positive Urinalysis?
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
Reduction of antibiotic overuse is an important goal for improving the quality of care for children and is highlighted in many of the Choosing Wisely® recommendations across disciplines.1-3 However, the evidence supporting these recommendations vary widely and many are derived from expert opinion and clinical practice guidelines rather than from original research studies.2 In this issue of the Journal of Hospital Medicine, Schroeder and colleagues identify a potential area of antibiotic overuse among young febrile infants with possible urinary tract infection (UTI).4 A wide variation in antibiotic treatment rates (0%-35%) was observed across 124 hospitals in the United States for febrile infants 7-60 days of age with uropathogen detection by urine culture but a negative urinalysis (UA). Treated infants with a negative UA were more likely to be younger (7-30 days), have respiratory symptoms, and were less likely to have abnormal inflammatory markers than infants with a positive UA.
Clinicians faced with the decision of whether or not to treat a febrile infant with uropathogen detection in the setting of a negative UA must weigh the potential benefits and harms of antibiotic use in this population. Withholding antibiotics for a young infant with UTI may increase the risk of recurrent UTI and renal scarring,5,6 while antibiotic treatment in young infants can lead to the disruption of the gut microbiome, resulting in long-term consequences that are only beginning to be understood.7-10
The American Academy of Pediatrics (AAP) UTI practice parameter requires a positive UA to establish the diagnosis of UTI in children 2-24 months of age.11 This recommendation is based primarily on studies demonstrating that uropathogen detection in the setting of a negative UA commonly represents asymptomatic bacteriuria or contamination rather than true infection.12-14 This is supported by research showing that the UA demonstrates near perfect (>99%) sensitivity for UTI in children with bacteremic UTI,12,15 and studies demonstrating lower rates of subsequent urinary infections and renal injury among infants with uropathogen detection and a negative UA compared with those with uropathogen detection and a positive UA.13,14,16
An important question is whether febrile infants within the first two months of life with uropathogen detection should be treated with antimicrobials regardless of UA findings or specifically in the setting of a negative UA. The AAP practice guideline11 deliberately omits these young infants, recognizing that evidence derived from studies of older infants and children may not be applicable to this young age group, as they may not mount as robust an inflammatory response and thus may not demonstrate pyuria in the setting of a bacterial urinary infection. Schroeder et al. demonstrate lower rates of abnormal inflammatory markers in UA negative compared with UA positive infants, a finding the authors argue supports the possibility of asymptomatic bacteriuria or contamination rather than true infection.4 The counterargument is that young infants may not mount a significant inflammatory response to true infections.
The authors appropriately highlight the paucity of literature to help differentiate true infection from asymptomatic bacteriuria or contamination in infants less than two months of age. As infants in this age group are usually treated with antibiotics for a positive urine culture regardless of UA result, robust data on short- and long-term outcomes of untreated infants are lacking. Much of the existing literature evaluates the test performance of the UA for UTI using the urine culture as the reference standard, which presents inherent limitations with incorporating the results of the UA into the definition of UTI using these data. Additionally, reported test performance of the UA for UTI varies by uropathogen type,17 fever duration,18 associated bacteremia,19 and urine concentration,20 which are important considerations when applying a strict definition of UTI that includes the UA in this age group. Conversely, more recent studies have demonstrated improved sensitivity of the dipstick and microscopic UA for the detection of UTI.15,20,21 The improved test performance may not only enhance the use of the UA as a screen for UTI in this high-risk population but also allow its potential inclusion into the definition of UTI as the authors suggest, as previous false-negative UTIs would be less frequent with improved UA testing modalities.
Ultimately, what’s missing from the equation is whether treatment of young febrile infants with uropathogen detection in the setting of a negative UA affects either short-term or long-term complications of UTI. Unfortunately, limited information exists to help inform the decision to initiate antibiotic treatment for these infants. Ideally, this question can only be answered by either an observational study evaluating outcomes of untreated infants or a randomized trial of antibiotics for infants less than two months of age with uropathogen detection in the setting of a negative UA. Until then, we may continue to observe a wide variation in antibiotic treatment rates for febrile young infants with uropathogen detection in the setting of a negative UA.
Disclosures
The authors have nothing to disclose.
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
1. Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479-485. doi: 10.1002/jhm.2064. PubMed
2. Admon AJ, Gupta A, Williams M, et al. Appraising the evidence supporting Choosing Wisely® recommendations. J Hosp Med. 2018;13(10):688-691. doi: 10.12788/jhm.2964. PubMed
3. Reyes M, Paulus E, Hronek C, et al. Choosing Wisely Campaign: Report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. doi: 10.1542/hpeds.2017-0029. PubMed
4. Schroeder AR, Lucas BP, Garber MD, McCulloh RJ, Joshi-Patel AA BE. Negative urinalyses in febrile infants 7-60 days of age treated for urinary tract infection. J Hosp Med. 2019;14(2):101-104. doi: 10.12788/jhm.3120..
5. Shaikh N, Mattoo TK, Keren R, et al. Early antibiotic treatment for pediatric febrile urinary tract infection and renal scarring. JAMA Pediatr. 2016;170(9):848-854. doi: 10.1001/jamapediatrics.2016.1181. PubMed
6. Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015;136(1):e13-e21. doi: 10.1542/peds.2015-0409. PubMed
7. Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141(4):e20172437. doi: 10.1542/peds.2017-2437. PubMed
8. Gibson MK, Crofts TS, Dantas G. Antibiotics and the developing infant gut microbiota and resistome. Curr Opin Microbiol. 2015;27:51-56. doi: 10.1016/j.mib.2015.07.007. PubMed
9. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi: 10.1016/j.jpeds.2014.09.041. PubMed
10. Dardas M, Gill SR, Grier A, et al. The impact of postnatal antibiotics on the preterm intestinal microbiome. Pediatr Res. 2014;76(2):150-158. doi: 10.1038/pr.2014.69. PubMed
11. Roberts KB, Downs SM, Finnell SM, et al. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
12. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants < 3 months of age. Pediatrics. 2015;135(6):965-971. doi: 10.1542/peds.2015-0012. PubMed
13. Wettergren B, Hellström M, Stokland E, Jodal U. Six year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
14. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatr Scand. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
15. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. doi: 10.1542/peds.2017-3068. PubMed
16. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
17. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1):e20160087. doi: 10.1542/peds.2016-0087. PubMed
18. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. PubMed
19. Roman HK, Chang PW, Schroeder AR. Diagnosis and management of bacteremic urinary tract infection in infants. Hosp Pediatr. 2015;5(1):1-8. doi: 10.1542/hpeds.2014-0051. PubMed
20. Chaudhari PP, Monuteaux MC, Bachur RG. Urine concentration and pyuria for identifying UTI in infants. Pediatrics. 2016;138(5):e20162370. PubMed
21. Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121-e1127. doi: 10.1542/peds.2013-3291. PubMed
© 2019 Society of Hospital Medicine