User login
In the Hospital: Laura Shea
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
We spoke with medical social worker Laura Shea, MSW, LICSW on her role at our tertiary care hospital. Laura’s reflections on the struggles and rewards of her job may resonate with those of us who search for balance and meaning in work.
Laura, tell us about yourself. What made you want to be a social worker?
I couldn’t really picture doing anything else. I got a degree in psychology and loved counseling. Social work was a natural fit because of the social justice component and the look into larger systems. I knew I had the skill set for this, and for those most marginalized, to be a supportive person for someone who doesn’t have that.
I also have a family member with major mental illness and chronic suicidality who I supported for a very long time. In many ways, I was a personal social worker advocating on their behalf while growing up. I remember being in high school when they overdosed, and going to the ER in the middle of the night. The next morning, I was back at school. I was a total do-gooder—President of the student council and on top of my grades. I tried dealing with this while keeping up the appearance that everything was ok, even though it wasn’t.
As I got older, there were middle-of-the-night phone calls professing suicidality which were so painful. I learned a lot about compartmentalizing and resiliency. It has given me an incredible amount of empathy for family members of patients. I have learned that it’s not always simple, and decisions aren’t easy, and solutions are complicated and can feel incomplete. We often hear, “Why hasn’t the family stepped in?” Well these issues are hard for families too, I know from firsthand experience.
At the end of the day, as challenging as the work is, I get something from it. I feel honored to bear witness to some of people’s darkest moments and also some of the most beautiful moments—the joys of coming out the other side of their process and journey.
How much of your personal story do you reveal to your patients?
I rarely do. However, to some families that are particularly devastated, I do share some of my family story. I try to affirm their challenge and acknowledge that family and friends can’t always “solve this.”
We have a culture that reveres going above and beyond, however I really honor those family members who can set boundaries. Sometimes caregivers need space, that doesn’t make you a bad person. It’s actually brave and really hard to do. You can’t give from an empty well.
Laura, tell us about your typical day.
Well, it begins with responding to e-mails. Then I meet with patients and obtain collateral to prep for multidisciplinary rounds (with physicians, RNs, case managers). I usually consult on 20-30 patients a day. In the afternoon, it varies -- maybe three patients are leaving that may need my help with things like providing substance use information or shelter resources. Typically, I’ll have a few complicated long-term patients, who may have challenging family dynamics, ongoing goals of care discussions, or behavioral difficulties. These patients keep me just as busy, it’s not quite as time sensitive but I have to keep chipping away at the work.
Seems like a busy day. Do you get a break at all?
When possible, I take a walk in the woods behind the hospital on my lunch break. There’s a beautiful path, it’s an important part of my day -- getting outside and taking a step back. I bring my pager, so I am still connected.
I used to feel like I didn’t have time to take a break, and I would work through lunch. But now I find if I take a break, I am more productive the rest of the day because it makes me more mindful. It quiets me a little, gives me perspective on the stress and stressors of working in the hospital and allows me to better connect to my job and others around me.
What does a successful day look like?
Well, one involved a homeless gentleman and a search for his family. He was in his 40s, though he looked much older, and recently had been assaulted at a shelter. He presented to either the ER or was admitted to various hospitals 14 times over the past month – typically for intoxication and hypothermia. He kept saying “I just need to find my brother” though no one was taking this request too seriously. We spent a lot of time looking for his brother with the Office of Public Guardian’s help, and we actually found him! The patient hadn’t seen his brother in four years and as it turns out was searching for him too. The brother thought the patient had passed away. With his brother’s support, the patient is now housed, going to alcohol treatment, reunited with his family, and taking his medications. His whole life changed. So that was amazing, and a reminder of how rewarding this job can be.
What is most challenging about your work?
The biggest challenge is grappling with the limitations of the system, and discharging someone to the community when the community has limited resources for these patients.
Though it’s not just the limitation of resources, some patients have been through the system so many times that as a coping mechanism and to protect themselves they do everything possible to push you away. They have walls firmly up, because of prior negative experiences with providers. I am not fazed by being yelled at, but it’s hard trying to connect with someone who has learned not to let you in. These are often the patients that need the support the most, and yet I want to respect their ability to have control or to say no. It is a tough balance.
What’s fun about your job?
I love meeting new people. I met a woman a few weeks ago who was talking about being a hippie in the ‘60s in San Francisco, and how great it was and how soft millennials are. She actually put meth in her coffee because she needed a pick-me-up to clean her house. You can’t make this stuff up! It’s just really fascinating how people live their lives, and to have a window into their world and perspective is a privilege.
Do you take work home with you or do you disconnect?
I try to disconnect, however there are days when something sticks with you and you really worry and wonder about a patient. As I mentioned, you can’t give from an empty well—so I try to acknowledge this. I find that trying to have a rich life outside of work is an important part of self-care as well. Social work is a big part of my identity but it’s not entirely who I am. I focus on friends, family, travel, yoga, and things that sustain me. I can’t do my job effectively if I am not taking a step back regularly.
What advice do you have for other providers and for patients?
The hospital is so overwhelming for our patients, more so than some providers realize. I could be in the room with a patient for 45 minutes and six different providers may come in. I try to maintain that this is the patient’s bedroom I’m walking into. It’s a private, and a sacred space for them. That’s where they sleep. This is where they are trying to recover and grapple with what brought them into the hospital.
Laura, thank you so much for telling us about your work. Anything else you’d like to share with us?
Some days I’ll go home completely exhausted and wiped out, and at first, I don’t feel like I did a single solitary thing. Some of the things that I’m trying to help people work through ...it never occurred to me that someone could, for whatever reason, find themselves in such challenging situations. I don’t have a magic wand to provide someone with housing or sobriety, but maybe in that moment I can begin to make a connection. When I just listen, I am beginning to build relationships – which for some patients is something they haven’t had in a long time. It’s in these moments of being present, without an agenda, walking with them in their challenges, that I feel most connected to the work.
Thanks, Laura.
© 2019 Society of Hospital Medicine
Care Transitions Program for High-Risk Frail Older Adults is Most Beneficial for Patients with Cognitive Impairment
Unplanned hospital admissions and readmissions have become a major focus of efforts to improve the value of healthcare given that these potentially preventable events exert substantial burden on patients, caregivers, health systems, and the economy.1 The percentage of patients who are rehospitalized within 30 days have decreased from 20%-21% at the start of the Accountable Care Act and readmission penalties to approximately 18%.2-5 Rehospitalization rates are 33% at 90 days and approach 40% at six months.6,7 Readmissions cost Medicare more than $26 billion annually,4 with one in five Medicare beneficiaries readmitted within 30 days of hospital discharge.8 Centers for Medicare and Medicaid Services and other payers use condition-specific and all-cause 30-day unplanned readmission rates and potentially preventable admissions among patients with complex or multiple comorbidities for public reporting, value-based purchasing, and performance-based reimbursement.9,10 Consequently, medical groups and hospitals have begun to place an increasing emphasis on improving the transitions of care following hospitalization with the goal of reducing unplanned readmissions.11 Care transitions programs have been shown to decrease readmission rates, mortality, and emergency department (ED) visits.12
Care transitions programs vary greatly in their scope of intervention and target groups, as well as in their efficacy in reducing readmissions.13,14 The Mayo Clinic Care Transition Program, hereafter referred to as CTP, was launched in 2011. This program was modeled after other successful programs and involves home visits by a nurse practitioner (NP) and telephonic support and triage provided by a registered nurse (RN). It is offered to high-risk community-dwelling patients during their hospitalization and begins within a week of hospital discharge.
Although the CTP reduces 30-day readmissions from 20% to 17%,7 it is a highly resource-intensive, multimodal, multidisciplinary program. Moreover, whether some components of the CTP are more critical than others remains unknown. Prior studies that examined the individual components of successful CTPs have suggested that a multipronged approach that includes close patient and caregiver support is most predictive of program efficacy.13 Long-term program sustainability would benefit from optimization of the most critical components of the program while reducing or eliminating resource-intensive factors that have negligible effects on program success. We therefore examined our CTP to identify whether and which program components are most critical for preventing 30-day readmissions and whether any patient characteristics contribute risk within this complex population.
METHODS
Study Design and Setting
This study is a retrospective cohort study of patients who were enrolled in the care transitions program of Mayo Clinic Rochester during the period January 1, 2010 to June 30, 2013. Patient demographic and clinical data were obtained from electronic health records (EHR), and information regarding CTP processes and interventions was obtained from a prospectively maintained program database. The study complied with the principles of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board.
Objectives
The study aimed to describe the performance and utilization of a multidisciplinary care transitions program that has been successful in reducing readmissions for high-risk patients. The study also sought to identify patient and/or program factors associated with failure to prevent readmission within 30 days of program enrollment.
Population
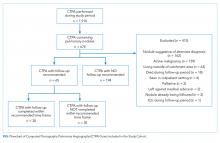
Patients who were enrolled in the CTP following hospital discharge and seen for a posthospital in-home visit prior to hospital readmission (for those readmitted) were included. Patients discharged to a skilled nursing facility were excluded. Patients were eligible for CTP enrollment if they were hospitalized for any cause, community dwelling (including assisted living) prior to hospitalization, and ≥60 years old with an Elder Risk Assessment (ERA) score ≥16.7 The ERA incorporates information regarding previous hospital days, age, and comorbid health burden and has been shown to predict 30-day readmissions, mortality, and critical illness (
Intervention
Detailed descriptions of the CTP have been previously published.7,17 Patients meeting enrollment criteria are enrolled into the CTP by a RN prior to or immediately after hospital discharge. The patient is then seen at home within one to five business days of discharge and again the following week by a NP who performs medication reconciliation; chronic illness management; and acute illness, mobility, safety, and cognition assessments. The NP also provides patient education on self-care and advance care planning. Patient and caregiver support and liaisons with community resources are provided. Home visits by an NP or MD are continued as needed for at least one month. A RN case manager performs weekly phone calls to assess changes in the patient’s clinical status and is available for phone triage of acute health issues. An interdisciplinary team composed of MDs, NPs, RNs, and pharmacists review patient management at weekly meetings. Although after-hours or weekend coverage for home visits are unavailable, an on-call primary care physician is available by phone at all times.
Primary Outcome
The primary outcome was all-cause hospital readmission within 30 days of the first CTP home visit, indicating successful program enrollment. Hospitalization was determined on the basis of billing codes from Mayo Clinic hospitals; this approach is 99% reliable in detecting readmissions for this population.18
Secondary Outcome Measures
Secondary outcome measures included six-month mortality and hospitalizations, as well as the number of hospital and ICU days and home, ED, primary care, and specialty office visits within 180 days after index hospitalizations as per the EHR. ED visits were counted only when they did not result in a hospital admission.
Independent Variables
Patient characteristics and clinical variables were retrieved from the EHR and included patient age, sex, and marital status. Comorbidities, ERA score,19 and Charlson comorbidity index (CCI)20 within two years of program enrollment were determined by using ICD-9 billing codes. The frequencies of primary care and specialty visits within six months of the index hospitalization were also ascertained using the EHR. Mobility limitations and cognitive impairment were categorized as binary variables (yes/no) and were assessed at the first home visit by the NP. The presence of mobility limitations was defined as a Barthel’s score of <7521,22 or Timed up and Go time of >20 seconds.23 Cognitive impairment was established as Kokmen below the normal cutoff for patient’s age group,24 Mini-Cog ≤2,25or AD8 ≥2.26 If these measures were not specifically documented during the first visit, clinical notes were queried for the description of pertinent cognitive and/or mobility limitations. Dementia diagnosis billing codes (ICD9 Code 290.*) were also included. High medication use was defined as >14 given the reported average medication number ranges from 8-13 in this population.27
As previously published, fidelity measures were abstracted from clinical notes by a trained nurse abstractor within 30 days of program enrollment and prior to a readmission.7 The five program fidelity measures included medication reconciliation, home service evaluation, advanced directives discussion, action plan for acute and chronic disease, safety plan, and discussion of community resources. The presence of advanced care planning was determined on the basis of visit medical notes and/or change of code status within the EHR, the identification or scanning of written advanced directives or “provider order for life-sustaining treatment,” and documentation of the discussion of resuscitation status. It was abstracted in duplicate by a nurse abstractor with physician adjudication for disagreement. Moreover, whether the initial visit met the goal of being within five days of discharge was determined by using billing data.
Analysis
The contribution of each independent variable to 30-day readmission was first directly assessed by using a univariate logistic regression model. Five patients died within 30 days without being admitted. These deaths, however, were not censored given that home death (as opposed to hospital death) was considered a positive outcome of the CTP. Multivariable modeling was performed through log rank test with backwards elimination and included all independent variables with P < .05. Variables with P values between .05 and >.1 were tested for interaction with age and sex. Age was categorized as <80 or ≥80 years. The length of hospital stay was categorized as <3 days (not qualifying for a Medicare skilled nursing facility), 3-13 days, or ≥14 days.
This study had 30% power to detect a reduction of 5% in the rates of hospital admissions; 5% is the median absolute risk reduction reported by previous randomized studies on care transitions programs previously reported.10 All analyses were performed using SAS 6.01 (SAS Inc., Cary, North Carolina).
RESULTS
Study Population
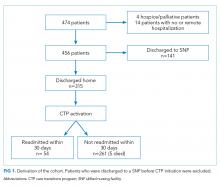
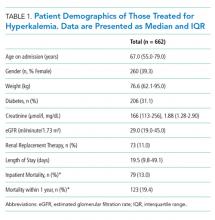
The study cohort included 315 patients who met the inclusion criteria (Fig 1). The demographic and clinical characteristics of the participants were ascertained at the time of CTP enrollment and are shown in Table 1. Patients were, on average, 82.5 (SD, 8.2) years old and had multiple comorbidities with a mean CCI score of 6.2 and ERA score of 18.5. Almost half of the patients (43.2%) exhibited cognitive impairment and more than half (51.7%) had mobility limitations. Among the patients, 42.9% had been hospitalized at least once in the 180 days prior to their CTP-qualifying hospitalization and 14.2% had ≥2 hospitalizations prior to their CTP-qualifying hospitalization. Similarly, 32.4% had at least one emergency department (ED) visit, and 3.5% had ≥3 ED visits. The majority of patients had frequent outpatient visits, with 30.8% having ≥4 office visits in primary care and 32.4% having ≥4 specialty office visits in the preceding six months.
Readmissions, Mortality, ED, and Outpatient Visits
Of the 315 patients, 54 (17.1%) had a readmission within 30 days and seven (2%) had >1 readmission. Among the patients, 126 (40.0%) were readmitted at least once within 180 days with 55 (17.5%) having more than one readmission. A total of 41 patients (13.1%) died during the six-month follow-up period. The need for both office and ED visits was reduced compared to the 180 days prior to admission with the biggest difference in ED visits: 72 (22.9%) of patients needed visits within 180 days of enrollment, as opposed to 102 (32.4%) before enrollment.
Impact of Patient Clinical Variables on Readmission Risk
Readmitted patients were less likely to exhibit cognitive impairment (29.6% vs 46.0%; P = .03) and were more likely to have high medication use (59.3% vs 44.4%; P = .047) than patients without readmission (Table 1). Readmitted patients had a higher frequency of visits to primary care (4.0 vs 3.0; P =.02) in the six months prior to admission and more hospital days in the prior year (4.6 vs 2.5; P = .04) than those without readmission.
Multivariable analysis, which included the cognitive status of the patient; the high use of medication; and the number of ED visits, primary care visits, and hospital days in the previous six months, provided a C statistic of 0.665. After backwards elimination, only the cognitive status of the patient and number of ED visits remained predictive of readmission risk.
Impact of Program Interventions on Readmission Risk
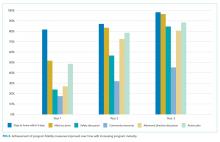
The completion of the CTP fidelity measures drastically varied with completion rates between 29.5% (community resource evaluation) and 87.0% (home visit within five days of hospital discharge; Table 2). Only 12.1% of patients received all components of the CTP at the first home visit. Readmission rates among patients who received all program components (13.2%) were lower than those among patients who did not receive all program components. This difference, however, failed to reach statistical significance. No single program component significantly reduced readmission risk. The completion rate of program fidelity measures increased with time (Figure 2). The present findings did not change even after performing sensitivity analysis that excluded the first program year. The overall agreement between chart abstractors on determining whether advance care planning occurred was 69.5% but the Cohens Kappa was only 18.4. This result was largely ascribed to the following: One abstractor counted the presence of a shorthand template used to document the delivery of an advance care planning document as discussion, whereas the other abstractor required further documentation or corroborating evidence (ie, change of code status). The majority of patients required multiple home visits to address ongoing medical needs (mean 2.7; SD = 1.3) over the first 30 days. Among these patients, only 17.1% received one visit, and 54.6% of patients received ≥3 visits. Eleven (3.5%) patients transitioned to a palliative homebound program that we began offering toward the end of this study to meet patient needs.28
DISCUSSION
The present study met our objective of identifying individual patient factors that are predictive of the success of our CTP. Cognitively impaired patients were less likely to be readmitted than cognitively intact patients. This finding is particularly important because patients with dementia constitute a subgroup that is at an increased risk of readmission after hospitalization29 and often suffer burdensome transitions at the end of life.30,31 High medication use and high number of visits to primary care and number of hospital days in the six months leading up to enrollment increase the likelihood of readmission and are plausible measures of disease severity or multi-morbidity that have been identified in previous studies.32,33 No one program intervention was found to be significantly associated with readmission. This result is consistent with prior works that demonstrated the need for multifaceted and intensive interventions to reduce readmission risk among highly complex and multimorbid patients.13,14
Our findings suggest that the provision of an alternative to stressful hospitalization to cognitively impaired patients and their caregivers may be an important benefit of care transitions programs. Having a trusted team to consult in acute situations may have enabled early intervention and crisis avoidance. Avoiding hospitalizations and ED visits may also have been in line with their goals of care.34,35 Given that program intensity varied on the basis of the discretion of the clinical team, patients with cognitive impairment and their caregivers may also have received more intensive support than cognitively intact patients.
In contrast to recent systematic reviews, our study did not find that advance directive discussion had significant effects on reductions in readmission.36,37 The lack of discussion surrounding the goals of care for patients with serious illnesses was also listed as one of four factors that are strongly associated with preventability in a national cohort of readmitted general medicine patients.38 The lack of power and incomplete documentation may have contributed to our null findings. Trust building must also occur before any meaningful discussion of the goals of care could be achieved, and follow-up time may have to be extended. Toward the end of this study, we developed an extension of our program for patients with limited life expectancy and conservative goals of care. In this extension, reductions in hospitalizations were observed among patients who had multiple goals of care discussions.28
Previous studies have shown that readmissions reduced with timely follow up among patients with heart failure.39 Our results showed no difference in readmission rate based on whether or not our patients were visited within five days from discharge, but we may have been underpowered to detect this difference. In addition, we may have missed readmissions that occurred before the enrollment visit.
The elements of the CTP were evidence based. Fidelity to program goals improved over time and reached high levels with program maturity. Only 12% of the patients received all program components at the first home visit. Patients that had all pillars addressed and documented showed a nonsignificant trend toward reduced readmission rates. NPs were given discretion as to how many visits were required to stabilize a patient and achieve program objectives. Heart failure management was driven by protocol with input from cardiology. Medication reconciliation and clinical assessment with action plan were prioritized at the first visit and thus allowed for the completion of other goals at a subsequent visit if time was insufficient. These decisions were deliberated at weekly physician-led multidisciplinary meetings. This variability allowed the team to meet chronic and urgent needs but further confounded the interpretation of our results. One possible way to interpret the lack of significant predictors of success is that through clinical assessment and flexibility, we were able to tailor our program to meet the needs of this complex multi-morbid population.
This study has important limitations. Given that it is a retrospective cohort study, we were unable to include patients who were enrolled but were either readmitted or dropped out before the first program visit. In addition, because of our study’s limited sample size and readmission rate, we had limited power to detect other potential predictor variables and test for confounding and interaction. While we included numerous variables in our analyses, we lacked information on mental health and the social determinants of health, which are known to influence readmission risk.40,41 Similarly, we lacked patient self-reported measures of health and information regarding caregiver support, which are important.42,43 Several of our predictive measures (cognitive impairment, mobility limitations, and program objective completion) were dependent on supplementing billing codes with heterogeneous data abstracted from usual clinical care as opposed to standardized research protocols. Neither method is completely accurate, nor can the combination of the two be assumed to be without inaccuracies. Failure to adequately document the clinical interventions performed by the clinical team is possibly a major confounder as evidenced by the considerable lack of agreement by our trained abstractors in determining whether advance care planning took place. The generalizability of our results is also a concern because the local population is largely white and highly educated, although our experience tells us that many of our program patients have limited means and thus may more closely resemble the general US population.44 The strength of our study is that it uses real, practice-based data that can be directly translated to practice.
CONCLUSION
This study focused on a successful high-intensity CTP. Results showed that compared with patients without dementia, patients with dementia were more likely to avoid hospitalizations as a result of enrollment in the investigated CTP. This study, however, failed to identify specific programmatic components critical for the success of the CTP. These findings support the current hypothesis that multidisciplinary, multimodal, and highly intensive interventions are necessary to care for complex and multi-morbid patients. They also suggest that compared with cognitively functional patients, cognitively impaired patients with conservative goals of care may be more likely to avoid burdensome hospitalizations when provided with early intervention in their home.
Acknowledgments
B.T. conceived and designed the study, interpreted the data, drafted and provided final revisions to the manuscript. P.Y.T, N.D.S., and J.M.N obtained funding, contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. P.A.R., R.G.M, and G.J.H., contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. S.M.P. Assisted with data acquisition and interpretation, performed the data analysis, and drafted parts of the manuscript. C.Y.Y.C, L.J.H., A.L, A.C., L.B., and R.H. helped with methodologic questions and data interpretation, and provided critical revisions to the manuscript.
All authors read and approved the final manuscript and the decision to submit the manuscript for publication.
We thank Donna Lawson, RN for her help with data abstraction and Annika Beck and Anna Jones in Mayo Clinic Biomedical Ethics Research Program for her help in preparing this manuscript for publication.
Disclosures
The authors declare no conflicts of interest.
Funding
This publication was supported by the Mayo Clinic, Robert D and Patricia E. Center for the Science of Health Care Delivery (B.T., R.H., R.G.M, L.J.H), by the Extramural Grant Program by Satellite Healthcare, a not-for-profit renal care provider (L.J.H., B.T.), and by the National Institute of Health (NIH) National Institute Of Diabetes And Digestive And Kidney Diseases grant K23 DK109134 (L.J.H.) K23DK114497 (RGM) and National Institute on Aging grant K23 AG051679 (B.T.). Additional support was provided by the National Center for Advancing Translational Sciences grant UL1 TR000135. Study contents are the sole responsibility of the authors and do not necessarily represent the official views of NIH.
The sponsors had no role in the design, execution, or reporting of this study.
Prior Presentations
Part of this data was presented in poster format at the American Geriatrics Society meeting in Washington DC 2015.
1. Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175-1177. doi: 10.1056/NEJMp1300122. PubMed
2. Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287-2295. doi: 10.1056/NEJMsa1101942. PubMed
3. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563. PubMed
5. Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N. Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2). doi: 10.5600/mmrr.003.02.b01. PubMed
6. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
7. Takahashi PY, Naessens JM, Peterson SM, et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4(1):30-35. doi: 10.1016/j.hjdsi.2015.06.006. PubMed
8. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
9. CMS. U.S. Centers for Medicare & Medicaid Services (CMS) measure methodology. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html. Accessed December 1, 2017; 2017.
10. National Committee for Quality Assurance. All-Cause Readmissions: the Number of Acute Inpatient Stays during the Measurement Year That Were Followed by an Acute Readmission for Any Diagnosis within 30 Days and the Predicted Probability of an Acute Readmission, for Patients 18 Years of Age and Older. Accessed May 18, 2017; 2014.
11. Naylor MD, Hirschman KB, Hanlon AL, et al. Comparison of evidence-based interventions on outcomes of hospitalized, cognitively impaired older adults. J Comp Eff Res. 2014;3(3):245-257. doi: 10.2217/cer.14.14. PubMed
12. Le Berre M, Maimon G, Sourial N, Guériton M, Vedel I. Impact of transitional care services for chronically ill older patients: A systematic evidence review. J Am Geriatr Soc. 2017;65(7):1597-1608. doi: 10.1111/jgs.14828. PubMed
13. Leppin AL, Gionfriddo MR, Kessler M, et al. Preevnting 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi: 10.1001/jamainternmed.2014.1608. PubMed
14. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. doi: 10.7326/0003-4819-155-8-201110180-00008. PubMed
15. Takahashi PY, Tung EE, Crane SJ, Chaudhry R, Cha S, Hanson GJ. Use of the elderly risk assessment (ERA) index to predict 2-year mortality and nursing home placement among community dwelling older adults. Arch Gerontol Geriatr. 2012;54(1):34-38. doi: 10.1016/j.archger.2011.02.012. PubMed
16. Biehl M, Takahashi PY, Cha SS, Chaudhry R, Gajic O, Thorsteinsdottir B. Prediction of critical illness in elderly outpatients using elder risk assessment: a population-based study. Clin Interv Aging. 2016;11:829-834. doi: 10.2147/CIA.S99419. PubMed
17. Takahashi PY, Haas LR, Quigg SM, et al. 30-day hospital readmission of older adults using care transitions after hospitalization: a pilot prospective cohort study. Clin Interv Aging. 2013;8:729-736. doi: 10.2147/CIA.S44390. PubMed
18. Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127(6):538-546. doi: 10.1016/j.amjmed.2014.02.008. PubMed
19. Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. doi: 10.1186/1472-6963-10-338. PubMed
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8. PubMed
21. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61-63. doi: 10.3109/09638288809164103. PubMed
22. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538-1541. doi: 10.1161/01.STR.30.8.1538. PubMed
23. Bohannon RW. Reference values for the timed up and go test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64-68. doi: 10.1519/00139143-200608000-00004. PubMed
24. Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281-288. doi: 10.1016/S0025-6196(12)61905-3. PubMed
25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6. PubMed
26. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. doi: 10.1212/01.wnl.0000172958.95282.2a. PubMed
27. Farrell B, Szeto W, Shamji S. Drug-related problems in the frail elderly. Can Fam Phys. 2011;57(2):168-169. PubMed
28. Chen CY, Thorsteinsdottir B, Cha SS, et al. Health care outcomes and advance care planning in older adults who receive home-based palliative care: a pilot cohort study. J Palliat Med. 2015;18(1):38-44. doi: 10.1089/jpm.2014.0150. PubMed
29. Rao A, Suliman A, Vuik S, Aylin P, Darzi A. Outcomes of dementia: systematic review and meta-analysis of hospital administrative database studies. Arch Gerontol Geriatr. 2016;66(Suppl C):198-204. doi: 10.1016/j.archger.2016.06.008. PubMed
30. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi: 10.1056/NEJMsa1100347. PubMed
31. Wang SY, Aldridge MD, Gross CP, Canavan M, Cherlin E, Bradley E. End-of-life care transition patterns of Medicare beneficiaries. J Am Geriatr Soc. 2017;65(7):1406-1413. doi: 10.1111/jgs.14891. PubMed
32. Pedersen MK, Meyer G, Uhrenfeldt L. Risk factors for acute care hospital readmission in older persons in Western countries: a systematic review. JBI Database System Rev Implement Rep. 2017;15(2):454-485. doi: 10.11124/JBISRIR-2016-003267. PubMed

33. Edwards ST, Saha S, Prentice JC, Pizer SD. Preventing hospitalization with Veterans Affairs home-based primary care: which individuals benefit most? J Am Geriatr Soc. 2017;65(8):1676-1683. doi: 10.1111/jgs.14843. PubMed
34. Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of care preferences Among nursing home residents With advanced dementia. J Pain Symptom Manage. 2017;54(3):340-345. doi: 10.1016/j.jpainsymman.2017.04.020. PubMed
35. D’Avolio DA, Strumpf NE, Feldman J, Mitchell P, Rebholz CM. Barriers to primary care: perceptions of older adults utilizing the ED for nonurgent visits. Clin Nurs Res. 2013;22(4):416-431. doi: 10.1177/1054773813485597. PubMed
36. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000-1025. doi: 10.1177/0269216314526272. PubMed
37. Martin RS, Hayes B, Gregorevic K, Lim WK. The effects of advance care planning interventions on nursing home residents: A systematic review. J Am Med Dir Assoc. 2016;17(4):284-293. doi: 10.1016/j.jamda.2015.12.017. PubMed
38. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. doi: 10.1001/jamainternmed.2015.7863. PubMed
39. Parrinello G, Torres D, Paterna S, et al. Early and personalized ambulatory follow-up to tailor furosemide and fluid intake according to congestion in post-discharge heart failure. Intern Emerg Med. 2013;8(3):221-228. doi: 10.1007/s11739-011-0602-y. PubMed
40. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
41. Calvillo–King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269-282. doi: 10.1007/s11606-012-2235-x. PubMed
42. Rönneikkö JK, Mäkelä M, Jämsen ER, et al. Predictors for unplanned hospitalization of New Home care clients. J Am Geriatr Soc. 2017;65(2):407-414. doi: 10.1111/jgs.14486. PubMed
43. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. doi: 10.1007/s11606-009-1196-1. PubMed
44. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. doi: 10.1016/j.mayocp.2011.11.009. PubMed
Unplanned hospital admissions and readmissions have become a major focus of efforts to improve the value of healthcare given that these potentially preventable events exert substantial burden on patients, caregivers, health systems, and the economy.1 The percentage of patients who are rehospitalized within 30 days have decreased from 20%-21% at the start of the Accountable Care Act and readmission penalties to approximately 18%.2-5 Rehospitalization rates are 33% at 90 days and approach 40% at six months.6,7 Readmissions cost Medicare more than $26 billion annually,4 with one in five Medicare beneficiaries readmitted within 30 days of hospital discharge.8 Centers for Medicare and Medicaid Services and other payers use condition-specific and all-cause 30-day unplanned readmission rates and potentially preventable admissions among patients with complex or multiple comorbidities for public reporting, value-based purchasing, and performance-based reimbursement.9,10 Consequently, medical groups and hospitals have begun to place an increasing emphasis on improving the transitions of care following hospitalization with the goal of reducing unplanned readmissions.11 Care transitions programs have been shown to decrease readmission rates, mortality, and emergency department (ED) visits.12
Care transitions programs vary greatly in their scope of intervention and target groups, as well as in their efficacy in reducing readmissions.13,14 The Mayo Clinic Care Transition Program, hereafter referred to as CTP, was launched in 2011. This program was modeled after other successful programs and involves home visits by a nurse practitioner (NP) and telephonic support and triage provided by a registered nurse (RN). It is offered to high-risk community-dwelling patients during their hospitalization and begins within a week of hospital discharge.
Although the CTP reduces 30-day readmissions from 20% to 17%,7 it is a highly resource-intensive, multimodal, multidisciplinary program. Moreover, whether some components of the CTP are more critical than others remains unknown. Prior studies that examined the individual components of successful CTPs have suggested that a multipronged approach that includes close patient and caregiver support is most predictive of program efficacy.13 Long-term program sustainability would benefit from optimization of the most critical components of the program while reducing or eliminating resource-intensive factors that have negligible effects on program success. We therefore examined our CTP to identify whether and which program components are most critical for preventing 30-day readmissions and whether any patient characteristics contribute risk within this complex population.
METHODS
Study Design and Setting
This study is a retrospective cohort study of patients who were enrolled in the care transitions program of Mayo Clinic Rochester during the period January 1, 2010 to June 30, 2013. Patient demographic and clinical data were obtained from electronic health records (EHR), and information regarding CTP processes and interventions was obtained from a prospectively maintained program database. The study complied with the principles of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board.
Objectives
The study aimed to describe the performance and utilization of a multidisciplinary care transitions program that has been successful in reducing readmissions for high-risk patients. The study also sought to identify patient and/or program factors associated with failure to prevent readmission within 30 days of program enrollment.
Population
Patients who were enrolled in the CTP following hospital discharge and seen for a posthospital in-home visit prior to hospital readmission (for those readmitted) were included. Patients discharged to a skilled nursing facility were excluded. Patients were eligible for CTP enrollment if they were hospitalized for any cause, community dwelling (including assisted living) prior to hospitalization, and ≥60 years old with an Elder Risk Assessment (ERA) score ≥16.7 The ERA incorporates information regarding previous hospital days, age, and comorbid health burden and has been shown to predict 30-day readmissions, mortality, and critical illness (
Intervention
Detailed descriptions of the CTP have been previously published.7,17 Patients meeting enrollment criteria are enrolled into the CTP by a RN prior to or immediately after hospital discharge. The patient is then seen at home within one to five business days of discharge and again the following week by a NP who performs medication reconciliation; chronic illness management; and acute illness, mobility, safety, and cognition assessments. The NP also provides patient education on self-care and advance care planning. Patient and caregiver support and liaisons with community resources are provided. Home visits by an NP or MD are continued as needed for at least one month. A RN case manager performs weekly phone calls to assess changes in the patient’s clinical status and is available for phone triage of acute health issues. An interdisciplinary team composed of MDs, NPs, RNs, and pharmacists review patient management at weekly meetings. Although after-hours or weekend coverage for home visits are unavailable, an on-call primary care physician is available by phone at all times.
Primary Outcome
The primary outcome was all-cause hospital readmission within 30 days of the first CTP home visit, indicating successful program enrollment. Hospitalization was determined on the basis of billing codes from Mayo Clinic hospitals; this approach is 99% reliable in detecting readmissions for this population.18
Secondary Outcome Measures
Secondary outcome measures included six-month mortality and hospitalizations, as well as the number of hospital and ICU days and home, ED, primary care, and specialty office visits within 180 days after index hospitalizations as per the EHR. ED visits were counted only when they did not result in a hospital admission.
Independent Variables
Patient characteristics and clinical variables were retrieved from the EHR and included patient age, sex, and marital status. Comorbidities, ERA score,19 and Charlson comorbidity index (CCI)20 within two years of program enrollment were determined by using ICD-9 billing codes. The frequencies of primary care and specialty visits within six months of the index hospitalization were also ascertained using the EHR. Mobility limitations and cognitive impairment were categorized as binary variables (yes/no) and were assessed at the first home visit by the NP. The presence of mobility limitations was defined as a Barthel’s score of <7521,22 or Timed up and Go time of >20 seconds.23 Cognitive impairment was established as Kokmen below the normal cutoff for patient’s age group,24 Mini-Cog ≤2,25or AD8 ≥2.26 If these measures were not specifically documented during the first visit, clinical notes were queried for the description of pertinent cognitive and/or mobility limitations. Dementia diagnosis billing codes (ICD9 Code 290.*) were also included. High medication use was defined as >14 given the reported average medication number ranges from 8-13 in this population.27
As previously published, fidelity measures were abstracted from clinical notes by a trained nurse abstractor within 30 days of program enrollment and prior to a readmission.7 The five program fidelity measures included medication reconciliation, home service evaluation, advanced directives discussion, action plan for acute and chronic disease, safety plan, and discussion of community resources. The presence of advanced care planning was determined on the basis of visit medical notes and/or change of code status within the EHR, the identification or scanning of written advanced directives or “provider order for life-sustaining treatment,” and documentation of the discussion of resuscitation status. It was abstracted in duplicate by a nurse abstractor with physician adjudication for disagreement. Moreover, whether the initial visit met the goal of being within five days of discharge was determined by using billing data.
Analysis
The contribution of each independent variable to 30-day readmission was first directly assessed by using a univariate logistic regression model. Five patients died within 30 days without being admitted. These deaths, however, were not censored given that home death (as opposed to hospital death) was considered a positive outcome of the CTP. Multivariable modeling was performed through log rank test with backwards elimination and included all independent variables with P < .05. Variables with P values between .05 and >.1 were tested for interaction with age and sex. Age was categorized as <80 or ≥80 years. The length of hospital stay was categorized as <3 days (not qualifying for a Medicare skilled nursing facility), 3-13 days, or ≥14 days.
This study had 30% power to detect a reduction of 5% in the rates of hospital admissions; 5% is the median absolute risk reduction reported by previous randomized studies on care transitions programs previously reported.10 All analyses were performed using SAS 6.01 (SAS Inc., Cary, North Carolina).
RESULTS
Study Population
The study cohort included 315 patients who met the inclusion criteria (Fig 1). The demographic and clinical characteristics of the participants were ascertained at the time of CTP enrollment and are shown in Table 1. Patients were, on average, 82.5 (SD, 8.2) years old and had multiple comorbidities with a mean CCI score of 6.2 and ERA score of 18.5. Almost half of the patients (43.2%) exhibited cognitive impairment and more than half (51.7%) had mobility limitations. Among the patients, 42.9% had been hospitalized at least once in the 180 days prior to their CTP-qualifying hospitalization and 14.2% had ≥2 hospitalizations prior to their CTP-qualifying hospitalization. Similarly, 32.4% had at least one emergency department (ED) visit, and 3.5% had ≥3 ED visits. The majority of patients had frequent outpatient visits, with 30.8% having ≥4 office visits in primary care and 32.4% having ≥4 specialty office visits in the preceding six months.
Readmissions, Mortality, ED, and Outpatient Visits
Of the 315 patients, 54 (17.1%) had a readmission within 30 days and seven (2%) had >1 readmission. Among the patients, 126 (40.0%) were readmitted at least once within 180 days with 55 (17.5%) having more than one readmission. A total of 41 patients (13.1%) died during the six-month follow-up period. The need for both office and ED visits was reduced compared to the 180 days prior to admission with the biggest difference in ED visits: 72 (22.9%) of patients needed visits within 180 days of enrollment, as opposed to 102 (32.4%) before enrollment.
Impact of Patient Clinical Variables on Readmission Risk
Readmitted patients were less likely to exhibit cognitive impairment (29.6% vs 46.0%; P = .03) and were more likely to have high medication use (59.3% vs 44.4%; P = .047) than patients without readmission (Table 1). Readmitted patients had a higher frequency of visits to primary care (4.0 vs 3.0; P =.02) in the six months prior to admission and more hospital days in the prior year (4.6 vs 2.5; P = .04) than those without readmission.
Multivariable analysis, which included the cognitive status of the patient; the high use of medication; and the number of ED visits, primary care visits, and hospital days in the previous six months, provided a C statistic of 0.665. After backwards elimination, only the cognitive status of the patient and number of ED visits remained predictive of readmission risk.
Impact of Program Interventions on Readmission Risk
The completion of the CTP fidelity measures drastically varied with completion rates between 29.5% (community resource evaluation) and 87.0% (home visit within five days of hospital discharge; Table 2). Only 12.1% of patients received all components of the CTP at the first home visit. Readmission rates among patients who received all program components (13.2%) were lower than those among patients who did not receive all program components. This difference, however, failed to reach statistical significance. No single program component significantly reduced readmission risk. The completion rate of program fidelity measures increased with time (Figure 2). The present findings did not change even after performing sensitivity analysis that excluded the first program year. The overall agreement between chart abstractors on determining whether advance care planning occurred was 69.5% but the Cohens Kappa was only 18.4. This result was largely ascribed to the following: One abstractor counted the presence of a shorthand template used to document the delivery of an advance care planning document as discussion, whereas the other abstractor required further documentation or corroborating evidence (ie, change of code status). The majority of patients required multiple home visits to address ongoing medical needs (mean 2.7; SD = 1.3) over the first 30 days. Among these patients, only 17.1% received one visit, and 54.6% of patients received ≥3 visits. Eleven (3.5%) patients transitioned to a palliative homebound program that we began offering toward the end of this study to meet patient needs.28
DISCUSSION
The present study met our objective of identifying individual patient factors that are predictive of the success of our CTP. Cognitively impaired patients were less likely to be readmitted than cognitively intact patients. This finding is particularly important because patients with dementia constitute a subgroup that is at an increased risk of readmission after hospitalization29 and often suffer burdensome transitions at the end of life.30,31 High medication use and high number of visits to primary care and number of hospital days in the six months leading up to enrollment increase the likelihood of readmission and are plausible measures of disease severity or multi-morbidity that have been identified in previous studies.32,33 No one program intervention was found to be significantly associated with readmission. This result is consistent with prior works that demonstrated the need for multifaceted and intensive interventions to reduce readmission risk among highly complex and multimorbid patients.13,14
Our findings suggest that the provision of an alternative to stressful hospitalization to cognitively impaired patients and their caregivers may be an important benefit of care transitions programs. Having a trusted team to consult in acute situations may have enabled early intervention and crisis avoidance. Avoiding hospitalizations and ED visits may also have been in line with their goals of care.34,35 Given that program intensity varied on the basis of the discretion of the clinical team, patients with cognitive impairment and their caregivers may also have received more intensive support than cognitively intact patients.
In contrast to recent systematic reviews, our study did not find that advance directive discussion had significant effects on reductions in readmission.36,37 The lack of discussion surrounding the goals of care for patients with serious illnesses was also listed as one of four factors that are strongly associated with preventability in a national cohort of readmitted general medicine patients.38 The lack of power and incomplete documentation may have contributed to our null findings. Trust building must also occur before any meaningful discussion of the goals of care could be achieved, and follow-up time may have to be extended. Toward the end of this study, we developed an extension of our program for patients with limited life expectancy and conservative goals of care. In this extension, reductions in hospitalizations were observed among patients who had multiple goals of care discussions.28
Previous studies have shown that readmissions reduced with timely follow up among patients with heart failure.39 Our results showed no difference in readmission rate based on whether or not our patients were visited within five days from discharge, but we may have been underpowered to detect this difference. In addition, we may have missed readmissions that occurred before the enrollment visit.
The elements of the CTP were evidence based. Fidelity to program goals improved over time and reached high levels with program maturity. Only 12% of the patients received all program components at the first home visit. Patients that had all pillars addressed and documented showed a nonsignificant trend toward reduced readmission rates. NPs were given discretion as to how many visits were required to stabilize a patient and achieve program objectives. Heart failure management was driven by protocol with input from cardiology. Medication reconciliation and clinical assessment with action plan were prioritized at the first visit and thus allowed for the completion of other goals at a subsequent visit if time was insufficient. These decisions were deliberated at weekly physician-led multidisciplinary meetings. This variability allowed the team to meet chronic and urgent needs but further confounded the interpretation of our results. One possible way to interpret the lack of significant predictors of success is that through clinical assessment and flexibility, we were able to tailor our program to meet the needs of this complex multi-morbid population.
This study has important limitations. Given that it is a retrospective cohort study, we were unable to include patients who were enrolled but were either readmitted or dropped out before the first program visit. In addition, because of our study’s limited sample size and readmission rate, we had limited power to detect other potential predictor variables and test for confounding and interaction. While we included numerous variables in our analyses, we lacked information on mental health and the social determinants of health, which are known to influence readmission risk.40,41 Similarly, we lacked patient self-reported measures of health and information regarding caregiver support, which are important.42,43 Several of our predictive measures (cognitive impairment, mobility limitations, and program objective completion) were dependent on supplementing billing codes with heterogeneous data abstracted from usual clinical care as opposed to standardized research protocols. Neither method is completely accurate, nor can the combination of the two be assumed to be without inaccuracies. Failure to adequately document the clinical interventions performed by the clinical team is possibly a major confounder as evidenced by the considerable lack of agreement by our trained abstractors in determining whether advance care planning took place. The generalizability of our results is also a concern because the local population is largely white and highly educated, although our experience tells us that many of our program patients have limited means and thus may more closely resemble the general US population.44 The strength of our study is that it uses real, practice-based data that can be directly translated to practice.
CONCLUSION
This study focused on a successful high-intensity CTP. Results showed that compared with patients without dementia, patients with dementia were more likely to avoid hospitalizations as a result of enrollment in the investigated CTP. This study, however, failed to identify specific programmatic components critical for the success of the CTP. These findings support the current hypothesis that multidisciplinary, multimodal, and highly intensive interventions are necessary to care for complex and multi-morbid patients. They also suggest that compared with cognitively functional patients, cognitively impaired patients with conservative goals of care may be more likely to avoid burdensome hospitalizations when provided with early intervention in their home.
Acknowledgments
B.T. conceived and designed the study, interpreted the data, drafted and provided final revisions to the manuscript. P.Y.T, N.D.S., and J.M.N obtained funding, contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. P.A.R., R.G.M, and G.J.H., contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. S.M.P. Assisted with data acquisition and interpretation, performed the data analysis, and drafted parts of the manuscript. C.Y.Y.C, L.J.H., A.L, A.C., L.B., and R.H. helped with methodologic questions and data interpretation, and provided critical revisions to the manuscript.
All authors read and approved the final manuscript and the decision to submit the manuscript for publication.
We thank Donna Lawson, RN for her help with data abstraction and Annika Beck and Anna Jones in Mayo Clinic Biomedical Ethics Research Program for her help in preparing this manuscript for publication.
Disclosures
The authors declare no conflicts of interest.
Funding
This publication was supported by the Mayo Clinic, Robert D and Patricia E. Center for the Science of Health Care Delivery (B.T., R.H., R.G.M, L.J.H), by the Extramural Grant Program by Satellite Healthcare, a not-for-profit renal care provider (L.J.H., B.T.), and by the National Institute of Health (NIH) National Institute Of Diabetes And Digestive And Kidney Diseases grant K23 DK109134 (L.J.H.) K23DK114497 (RGM) and National Institute on Aging grant K23 AG051679 (B.T.). Additional support was provided by the National Center for Advancing Translational Sciences grant UL1 TR000135. Study contents are the sole responsibility of the authors and do not necessarily represent the official views of NIH.
The sponsors had no role in the design, execution, or reporting of this study.
Prior Presentations
Part of this data was presented in poster format at the American Geriatrics Society meeting in Washington DC 2015.
Unplanned hospital admissions and readmissions have become a major focus of efforts to improve the value of healthcare given that these potentially preventable events exert substantial burden on patients, caregivers, health systems, and the economy.1 The percentage of patients who are rehospitalized within 30 days have decreased from 20%-21% at the start of the Accountable Care Act and readmission penalties to approximately 18%.2-5 Rehospitalization rates are 33% at 90 days and approach 40% at six months.6,7 Readmissions cost Medicare more than $26 billion annually,4 with one in five Medicare beneficiaries readmitted within 30 days of hospital discharge.8 Centers for Medicare and Medicaid Services and other payers use condition-specific and all-cause 30-day unplanned readmission rates and potentially preventable admissions among patients with complex or multiple comorbidities for public reporting, value-based purchasing, and performance-based reimbursement.9,10 Consequently, medical groups and hospitals have begun to place an increasing emphasis on improving the transitions of care following hospitalization with the goal of reducing unplanned readmissions.11 Care transitions programs have been shown to decrease readmission rates, mortality, and emergency department (ED) visits.12
Care transitions programs vary greatly in their scope of intervention and target groups, as well as in their efficacy in reducing readmissions.13,14 The Mayo Clinic Care Transition Program, hereafter referred to as CTP, was launched in 2011. This program was modeled after other successful programs and involves home visits by a nurse practitioner (NP) and telephonic support and triage provided by a registered nurse (RN). It is offered to high-risk community-dwelling patients during their hospitalization and begins within a week of hospital discharge.
Although the CTP reduces 30-day readmissions from 20% to 17%,7 it is a highly resource-intensive, multimodal, multidisciplinary program. Moreover, whether some components of the CTP are more critical than others remains unknown. Prior studies that examined the individual components of successful CTPs have suggested that a multipronged approach that includes close patient and caregiver support is most predictive of program efficacy.13 Long-term program sustainability would benefit from optimization of the most critical components of the program while reducing or eliminating resource-intensive factors that have negligible effects on program success. We therefore examined our CTP to identify whether and which program components are most critical for preventing 30-day readmissions and whether any patient characteristics contribute risk within this complex population.
METHODS
Study Design and Setting
This study is a retrospective cohort study of patients who were enrolled in the care transitions program of Mayo Clinic Rochester during the period January 1, 2010 to June 30, 2013. Patient demographic and clinical data were obtained from electronic health records (EHR), and information regarding CTP processes and interventions was obtained from a prospectively maintained program database. The study complied with the principles of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board.
Objectives
The study aimed to describe the performance and utilization of a multidisciplinary care transitions program that has been successful in reducing readmissions for high-risk patients. The study also sought to identify patient and/or program factors associated with failure to prevent readmission within 30 days of program enrollment.
Population
Patients who were enrolled in the CTP following hospital discharge and seen for a posthospital in-home visit prior to hospital readmission (for those readmitted) were included. Patients discharged to a skilled nursing facility were excluded. Patients were eligible for CTP enrollment if they were hospitalized for any cause, community dwelling (including assisted living) prior to hospitalization, and ≥60 years old with an Elder Risk Assessment (ERA) score ≥16.7 The ERA incorporates information regarding previous hospital days, age, and comorbid health burden and has been shown to predict 30-day readmissions, mortality, and critical illness (
Intervention
Detailed descriptions of the CTP have been previously published.7,17 Patients meeting enrollment criteria are enrolled into the CTP by a RN prior to or immediately after hospital discharge. The patient is then seen at home within one to five business days of discharge and again the following week by a NP who performs medication reconciliation; chronic illness management; and acute illness, mobility, safety, and cognition assessments. The NP also provides patient education on self-care and advance care planning. Patient and caregiver support and liaisons with community resources are provided. Home visits by an NP or MD are continued as needed for at least one month. A RN case manager performs weekly phone calls to assess changes in the patient’s clinical status and is available for phone triage of acute health issues. An interdisciplinary team composed of MDs, NPs, RNs, and pharmacists review patient management at weekly meetings. Although after-hours or weekend coverage for home visits are unavailable, an on-call primary care physician is available by phone at all times.
Primary Outcome
The primary outcome was all-cause hospital readmission within 30 days of the first CTP home visit, indicating successful program enrollment. Hospitalization was determined on the basis of billing codes from Mayo Clinic hospitals; this approach is 99% reliable in detecting readmissions for this population.18
Secondary Outcome Measures
Secondary outcome measures included six-month mortality and hospitalizations, as well as the number of hospital and ICU days and home, ED, primary care, and specialty office visits within 180 days after index hospitalizations as per the EHR. ED visits were counted only when they did not result in a hospital admission.
Independent Variables
Patient characteristics and clinical variables were retrieved from the EHR and included patient age, sex, and marital status. Comorbidities, ERA score,19 and Charlson comorbidity index (CCI)20 within two years of program enrollment were determined by using ICD-9 billing codes. The frequencies of primary care and specialty visits within six months of the index hospitalization were also ascertained using the EHR. Mobility limitations and cognitive impairment were categorized as binary variables (yes/no) and were assessed at the first home visit by the NP. The presence of mobility limitations was defined as a Barthel’s score of <7521,22 or Timed up and Go time of >20 seconds.23 Cognitive impairment was established as Kokmen below the normal cutoff for patient’s age group,24 Mini-Cog ≤2,25or AD8 ≥2.26 If these measures were not specifically documented during the first visit, clinical notes were queried for the description of pertinent cognitive and/or mobility limitations. Dementia diagnosis billing codes (ICD9 Code 290.*) were also included. High medication use was defined as >14 given the reported average medication number ranges from 8-13 in this population.27
As previously published, fidelity measures were abstracted from clinical notes by a trained nurse abstractor within 30 days of program enrollment and prior to a readmission.7 The five program fidelity measures included medication reconciliation, home service evaluation, advanced directives discussion, action plan for acute and chronic disease, safety plan, and discussion of community resources. The presence of advanced care planning was determined on the basis of visit medical notes and/or change of code status within the EHR, the identification or scanning of written advanced directives or “provider order for life-sustaining treatment,” and documentation of the discussion of resuscitation status. It was abstracted in duplicate by a nurse abstractor with physician adjudication for disagreement. Moreover, whether the initial visit met the goal of being within five days of discharge was determined by using billing data.
Analysis
The contribution of each independent variable to 30-day readmission was first directly assessed by using a univariate logistic regression model. Five patients died within 30 days without being admitted. These deaths, however, were not censored given that home death (as opposed to hospital death) was considered a positive outcome of the CTP. Multivariable modeling was performed through log rank test with backwards elimination and included all independent variables with P < .05. Variables with P values between .05 and >.1 were tested for interaction with age and sex. Age was categorized as <80 or ≥80 years. The length of hospital stay was categorized as <3 days (not qualifying for a Medicare skilled nursing facility), 3-13 days, or ≥14 days.
This study had 30% power to detect a reduction of 5% in the rates of hospital admissions; 5% is the median absolute risk reduction reported by previous randomized studies on care transitions programs previously reported.10 All analyses were performed using SAS 6.01 (SAS Inc., Cary, North Carolina).
RESULTS
Study Population
The study cohort included 315 patients who met the inclusion criteria (Fig 1). The demographic and clinical characteristics of the participants were ascertained at the time of CTP enrollment and are shown in Table 1. Patients were, on average, 82.5 (SD, 8.2) years old and had multiple comorbidities with a mean CCI score of 6.2 and ERA score of 18.5. Almost half of the patients (43.2%) exhibited cognitive impairment and more than half (51.7%) had mobility limitations. Among the patients, 42.9% had been hospitalized at least once in the 180 days prior to their CTP-qualifying hospitalization and 14.2% had ≥2 hospitalizations prior to their CTP-qualifying hospitalization. Similarly, 32.4% had at least one emergency department (ED) visit, and 3.5% had ≥3 ED visits. The majority of patients had frequent outpatient visits, with 30.8% having ≥4 office visits in primary care and 32.4% having ≥4 specialty office visits in the preceding six months.
Readmissions, Mortality, ED, and Outpatient Visits
Of the 315 patients, 54 (17.1%) had a readmission within 30 days and seven (2%) had >1 readmission. Among the patients, 126 (40.0%) were readmitted at least once within 180 days with 55 (17.5%) having more than one readmission. A total of 41 patients (13.1%) died during the six-month follow-up period. The need for both office and ED visits was reduced compared to the 180 days prior to admission with the biggest difference in ED visits: 72 (22.9%) of patients needed visits within 180 days of enrollment, as opposed to 102 (32.4%) before enrollment.
Impact of Patient Clinical Variables on Readmission Risk
Readmitted patients were less likely to exhibit cognitive impairment (29.6% vs 46.0%; P = .03) and were more likely to have high medication use (59.3% vs 44.4%; P = .047) than patients without readmission (Table 1). Readmitted patients had a higher frequency of visits to primary care (4.0 vs 3.0; P =.02) in the six months prior to admission and more hospital days in the prior year (4.6 vs 2.5; P = .04) than those without readmission.
Multivariable analysis, which included the cognitive status of the patient; the high use of medication; and the number of ED visits, primary care visits, and hospital days in the previous six months, provided a C statistic of 0.665. After backwards elimination, only the cognitive status of the patient and number of ED visits remained predictive of readmission risk.
Impact of Program Interventions on Readmission Risk
The completion of the CTP fidelity measures drastically varied with completion rates between 29.5% (community resource evaluation) and 87.0% (home visit within five days of hospital discharge; Table 2). Only 12.1% of patients received all components of the CTP at the first home visit. Readmission rates among patients who received all program components (13.2%) were lower than those among patients who did not receive all program components. This difference, however, failed to reach statistical significance. No single program component significantly reduced readmission risk. The completion rate of program fidelity measures increased with time (Figure 2). The present findings did not change even after performing sensitivity analysis that excluded the first program year. The overall agreement between chart abstractors on determining whether advance care planning occurred was 69.5% but the Cohens Kappa was only 18.4. This result was largely ascribed to the following: One abstractor counted the presence of a shorthand template used to document the delivery of an advance care planning document as discussion, whereas the other abstractor required further documentation or corroborating evidence (ie, change of code status). The majority of patients required multiple home visits to address ongoing medical needs (mean 2.7; SD = 1.3) over the first 30 days. Among these patients, only 17.1% received one visit, and 54.6% of patients received ≥3 visits. Eleven (3.5%) patients transitioned to a palliative homebound program that we began offering toward the end of this study to meet patient needs.28
DISCUSSION
The present study met our objective of identifying individual patient factors that are predictive of the success of our CTP. Cognitively impaired patients were less likely to be readmitted than cognitively intact patients. This finding is particularly important because patients with dementia constitute a subgroup that is at an increased risk of readmission after hospitalization29 and often suffer burdensome transitions at the end of life.30,31 High medication use and high number of visits to primary care and number of hospital days in the six months leading up to enrollment increase the likelihood of readmission and are plausible measures of disease severity or multi-morbidity that have been identified in previous studies.32,33 No one program intervention was found to be significantly associated with readmission. This result is consistent with prior works that demonstrated the need for multifaceted and intensive interventions to reduce readmission risk among highly complex and multimorbid patients.13,14
Our findings suggest that the provision of an alternative to stressful hospitalization to cognitively impaired patients and their caregivers may be an important benefit of care transitions programs. Having a trusted team to consult in acute situations may have enabled early intervention and crisis avoidance. Avoiding hospitalizations and ED visits may also have been in line with their goals of care.34,35 Given that program intensity varied on the basis of the discretion of the clinical team, patients with cognitive impairment and their caregivers may also have received more intensive support than cognitively intact patients.
In contrast to recent systematic reviews, our study did not find that advance directive discussion had significant effects on reductions in readmission.36,37 The lack of discussion surrounding the goals of care for patients with serious illnesses was also listed as one of four factors that are strongly associated with preventability in a national cohort of readmitted general medicine patients.38 The lack of power and incomplete documentation may have contributed to our null findings. Trust building must also occur before any meaningful discussion of the goals of care could be achieved, and follow-up time may have to be extended. Toward the end of this study, we developed an extension of our program for patients with limited life expectancy and conservative goals of care. In this extension, reductions in hospitalizations were observed among patients who had multiple goals of care discussions.28
Previous studies have shown that readmissions reduced with timely follow up among patients with heart failure.39 Our results showed no difference in readmission rate based on whether or not our patients were visited within five days from discharge, but we may have been underpowered to detect this difference. In addition, we may have missed readmissions that occurred before the enrollment visit.
The elements of the CTP were evidence based. Fidelity to program goals improved over time and reached high levels with program maturity. Only 12% of the patients received all program components at the first home visit. Patients that had all pillars addressed and documented showed a nonsignificant trend toward reduced readmission rates. NPs were given discretion as to how many visits were required to stabilize a patient and achieve program objectives. Heart failure management was driven by protocol with input from cardiology. Medication reconciliation and clinical assessment with action plan were prioritized at the first visit and thus allowed for the completion of other goals at a subsequent visit if time was insufficient. These decisions were deliberated at weekly physician-led multidisciplinary meetings. This variability allowed the team to meet chronic and urgent needs but further confounded the interpretation of our results. One possible way to interpret the lack of significant predictors of success is that through clinical assessment and flexibility, we were able to tailor our program to meet the needs of this complex multi-morbid population.
This study has important limitations. Given that it is a retrospective cohort study, we were unable to include patients who were enrolled but were either readmitted or dropped out before the first program visit. In addition, because of our study’s limited sample size and readmission rate, we had limited power to detect other potential predictor variables and test for confounding and interaction. While we included numerous variables in our analyses, we lacked information on mental health and the social determinants of health, which are known to influence readmission risk.40,41 Similarly, we lacked patient self-reported measures of health and information regarding caregiver support, which are important.42,43 Several of our predictive measures (cognitive impairment, mobility limitations, and program objective completion) were dependent on supplementing billing codes with heterogeneous data abstracted from usual clinical care as opposed to standardized research protocols. Neither method is completely accurate, nor can the combination of the two be assumed to be without inaccuracies. Failure to adequately document the clinical interventions performed by the clinical team is possibly a major confounder as evidenced by the considerable lack of agreement by our trained abstractors in determining whether advance care planning took place. The generalizability of our results is also a concern because the local population is largely white and highly educated, although our experience tells us that many of our program patients have limited means and thus may more closely resemble the general US population.44 The strength of our study is that it uses real, practice-based data that can be directly translated to practice.
CONCLUSION
This study focused on a successful high-intensity CTP. Results showed that compared with patients without dementia, patients with dementia were more likely to avoid hospitalizations as a result of enrollment in the investigated CTP. This study, however, failed to identify specific programmatic components critical for the success of the CTP. These findings support the current hypothesis that multidisciplinary, multimodal, and highly intensive interventions are necessary to care for complex and multi-morbid patients. They also suggest that compared with cognitively functional patients, cognitively impaired patients with conservative goals of care may be more likely to avoid burdensome hospitalizations when provided with early intervention in their home.
Acknowledgments
B.T. conceived and designed the study, interpreted the data, drafted and provided final revisions to the manuscript. P.Y.T, N.D.S., and J.M.N obtained funding, contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. P.A.R., R.G.M, and G.J.H., contributed to the conception and design of the study, analysis, and interpretation of the data, and provided critical revisions to the manuscript. S.M.P. Assisted with data acquisition and interpretation, performed the data analysis, and drafted parts of the manuscript. C.Y.Y.C, L.J.H., A.L, A.C., L.B., and R.H. helped with methodologic questions and data interpretation, and provided critical revisions to the manuscript.
All authors read and approved the final manuscript and the decision to submit the manuscript for publication.
We thank Donna Lawson, RN for her help with data abstraction and Annika Beck and Anna Jones in Mayo Clinic Biomedical Ethics Research Program for her help in preparing this manuscript for publication.
Disclosures
The authors declare no conflicts of interest.
Funding
This publication was supported by the Mayo Clinic, Robert D and Patricia E. Center for the Science of Health Care Delivery (B.T., R.H., R.G.M, L.J.H), by the Extramural Grant Program by Satellite Healthcare, a not-for-profit renal care provider (L.J.H., B.T.), and by the National Institute of Health (NIH) National Institute Of Diabetes And Digestive And Kidney Diseases grant K23 DK109134 (L.J.H.) K23DK114497 (RGM) and National Institute on Aging grant K23 AG051679 (B.T.). Additional support was provided by the National Center for Advancing Translational Sciences grant UL1 TR000135. Study contents are the sole responsibility of the authors and do not necessarily represent the official views of NIH.
The sponsors had no role in the design, execution, or reporting of this study.
Prior Presentations
Part of this data was presented in poster format at the American Geriatrics Society meeting in Washington DC 2015.
1. Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175-1177. doi: 10.1056/NEJMp1300122. PubMed
2. Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287-2295. doi: 10.1056/NEJMsa1101942. PubMed
3. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563. PubMed
5. Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N. Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2). doi: 10.5600/mmrr.003.02.b01. PubMed
6. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
7. Takahashi PY, Naessens JM, Peterson SM, et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4(1):30-35. doi: 10.1016/j.hjdsi.2015.06.006. PubMed
8. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
9. CMS. U.S. Centers for Medicare & Medicaid Services (CMS) measure methodology. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html. Accessed December 1, 2017; 2017.
10. National Committee for Quality Assurance. All-Cause Readmissions: the Number of Acute Inpatient Stays during the Measurement Year That Were Followed by an Acute Readmission for Any Diagnosis within 30 Days and the Predicted Probability of an Acute Readmission, for Patients 18 Years of Age and Older. Accessed May 18, 2017; 2014.
11. Naylor MD, Hirschman KB, Hanlon AL, et al. Comparison of evidence-based interventions on outcomes of hospitalized, cognitively impaired older adults. J Comp Eff Res. 2014;3(3):245-257. doi: 10.2217/cer.14.14. PubMed
12. Le Berre M, Maimon G, Sourial N, Guériton M, Vedel I. Impact of transitional care services for chronically ill older patients: A systematic evidence review. J Am Geriatr Soc. 2017;65(7):1597-1608. doi: 10.1111/jgs.14828. PubMed
13. Leppin AL, Gionfriddo MR, Kessler M, et al. Preevnting 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi: 10.1001/jamainternmed.2014.1608. PubMed
14. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. doi: 10.7326/0003-4819-155-8-201110180-00008. PubMed
15. Takahashi PY, Tung EE, Crane SJ, Chaudhry R, Cha S, Hanson GJ. Use of the elderly risk assessment (ERA) index to predict 2-year mortality and nursing home placement among community dwelling older adults. Arch Gerontol Geriatr. 2012;54(1):34-38. doi: 10.1016/j.archger.2011.02.012. PubMed
16. Biehl M, Takahashi PY, Cha SS, Chaudhry R, Gajic O, Thorsteinsdottir B. Prediction of critical illness in elderly outpatients using elder risk assessment: a population-based study. Clin Interv Aging. 2016;11:829-834. doi: 10.2147/CIA.S99419. PubMed
17. Takahashi PY, Haas LR, Quigg SM, et al. 30-day hospital readmission of older adults using care transitions after hospitalization: a pilot prospective cohort study. Clin Interv Aging. 2013;8:729-736. doi: 10.2147/CIA.S44390. PubMed
18. Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127(6):538-546. doi: 10.1016/j.amjmed.2014.02.008. PubMed
19. Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. doi: 10.1186/1472-6963-10-338. PubMed
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8. PubMed
21. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61-63. doi: 10.3109/09638288809164103. PubMed
22. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538-1541. doi: 10.1161/01.STR.30.8.1538. PubMed
23. Bohannon RW. Reference values for the timed up and go test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64-68. doi: 10.1519/00139143-200608000-00004. PubMed
24. Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281-288. doi: 10.1016/S0025-6196(12)61905-3. PubMed
25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6. PubMed
26. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. doi: 10.1212/01.wnl.0000172958.95282.2a. PubMed
27. Farrell B, Szeto W, Shamji S. Drug-related problems in the frail elderly. Can Fam Phys. 2011;57(2):168-169. PubMed
28. Chen CY, Thorsteinsdottir B, Cha SS, et al. Health care outcomes and advance care planning in older adults who receive home-based palliative care: a pilot cohort study. J Palliat Med. 2015;18(1):38-44. doi: 10.1089/jpm.2014.0150. PubMed
29. Rao A, Suliman A, Vuik S, Aylin P, Darzi A. Outcomes of dementia: systematic review and meta-analysis of hospital administrative database studies. Arch Gerontol Geriatr. 2016;66(Suppl C):198-204. doi: 10.1016/j.archger.2016.06.008. PubMed
30. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi: 10.1056/NEJMsa1100347. PubMed
31. Wang SY, Aldridge MD, Gross CP, Canavan M, Cherlin E, Bradley E. End-of-life care transition patterns of Medicare beneficiaries. J Am Geriatr Soc. 2017;65(7):1406-1413. doi: 10.1111/jgs.14891. PubMed
32. Pedersen MK, Meyer G, Uhrenfeldt L. Risk factors for acute care hospital readmission in older persons in Western countries: a systematic review. JBI Database System Rev Implement Rep. 2017;15(2):454-485. doi: 10.11124/JBISRIR-2016-003267. PubMed

33. Edwards ST, Saha S, Prentice JC, Pizer SD. Preventing hospitalization with Veterans Affairs home-based primary care: which individuals benefit most? J Am Geriatr Soc. 2017;65(8):1676-1683. doi: 10.1111/jgs.14843. PubMed
34. Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of care preferences Among nursing home residents With advanced dementia. J Pain Symptom Manage. 2017;54(3):340-345. doi: 10.1016/j.jpainsymman.2017.04.020. PubMed
35. D’Avolio DA, Strumpf NE, Feldman J, Mitchell P, Rebholz CM. Barriers to primary care: perceptions of older adults utilizing the ED for nonurgent visits. Clin Nurs Res. 2013;22(4):416-431. doi: 10.1177/1054773813485597. PubMed
36. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000-1025. doi: 10.1177/0269216314526272. PubMed
37. Martin RS, Hayes B, Gregorevic K, Lim WK. The effects of advance care planning interventions on nursing home residents: A systematic review. J Am Med Dir Assoc. 2016;17(4):284-293. doi: 10.1016/j.jamda.2015.12.017. PubMed
38. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. doi: 10.1001/jamainternmed.2015.7863. PubMed
39. Parrinello G, Torres D, Paterna S, et al. Early and personalized ambulatory follow-up to tailor furosemide and fluid intake according to congestion in post-discharge heart failure. Intern Emerg Med. 2013;8(3):221-228. doi: 10.1007/s11739-011-0602-y. PubMed
40. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
41. Calvillo–King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269-282. doi: 10.1007/s11606-012-2235-x. PubMed
42. Rönneikkö JK, Mäkelä M, Jämsen ER, et al. Predictors for unplanned hospitalization of New Home care clients. J Am Geriatr Soc. 2017;65(2):407-414. doi: 10.1111/jgs.14486. PubMed
43. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. doi: 10.1007/s11606-009-1196-1. PubMed
44. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. doi: 10.1016/j.mayocp.2011.11.009. PubMed
1. Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175-1177. doi: 10.1056/NEJMp1300122. PubMed
2. Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287-2295. doi: 10.1056/NEJMsa1101942. PubMed
3. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
4. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi: 10.1056/NEJMsa0803563. PubMed
5. Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N. Medicare readmission rates showed meaningful decline in 2012. Medicare Medicaid Res Rev. 2013;3(2). doi: 10.5600/mmrr.003.02.b01. PubMed
6. Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613-620. PubMed
7. Takahashi PY, Naessens JM, Peterson SM, et al. Short-term and long-term effectiveness of a post-hospital care transitions program in an older, medically complex population. Healthcare. 2016;4(1):30-35. doi: 10.1016/j.hjdsi.2015.06.006. PubMed
8. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
9. CMS. U.S. Centers for Medicare & Medicaid Services (CMS) measure methodology. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html. Accessed December 1, 2017; 2017.
10. National Committee for Quality Assurance. All-Cause Readmissions: the Number of Acute Inpatient Stays during the Measurement Year That Were Followed by an Acute Readmission for Any Diagnosis within 30 Days and the Predicted Probability of an Acute Readmission, for Patients 18 Years of Age and Older. Accessed May 18, 2017; 2014.
11. Naylor MD, Hirschman KB, Hanlon AL, et al. Comparison of evidence-based interventions on outcomes of hospitalized, cognitively impaired older adults. J Comp Eff Res. 2014;3(3):245-257. doi: 10.2217/cer.14.14. PubMed
12. Le Berre M, Maimon G, Sourial N, Guériton M, Vedel I. Impact of transitional care services for chronically ill older patients: A systematic evidence review. J Am Geriatr Soc. 2017;65(7):1597-1608. doi: 10.1111/jgs.14828. PubMed
13. Leppin AL, Gionfriddo MR, Kessler M, et al. Preevnting 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi: 10.1001/jamainternmed.2014.1608. PubMed
14. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. doi: 10.7326/0003-4819-155-8-201110180-00008. PubMed
15. Takahashi PY, Tung EE, Crane SJ, Chaudhry R, Cha S, Hanson GJ. Use of the elderly risk assessment (ERA) index to predict 2-year mortality and nursing home placement among community dwelling older adults. Arch Gerontol Geriatr. 2012;54(1):34-38. doi: 10.1016/j.archger.2011.02.012. PubMed
16. Biehl M, Takahashi PY, Cha SS, Chaudhry R, Gajic O, Thorsteinsdottir B. Prediction of critical illness in elderly outpatients using elder risk assessment: a population-based study. Clin Interv Aging. 2016;11:829-834. doi: 10.2147/CIA.S99419. PubMed
17. Takahashi PY, Haas LR, Quigg SM, et al. 30-day hospital readmission of older adults using care transitions after hospitalization: a pilot prospective cohort study. Clin Interv Aging. 2013;8:729-736. doi: 10.2147/CIA.S44390. PubMed
18. Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127(6):538-546. doi: 10.1016/j.amjmed.2014.02.008. PubMed
19. Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. doi: 10.1186/1472-6963-10-338. PubMed
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8. PubMed
21. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61-63. doi: 10.3109/09638288809164103. PubMed
22. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538-1541. doi: 10.1161/01.STR.30.8.1538. PubMed
23. Bohannon RW. Reference values for the timed up and go test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64-68. doi: 10.1519/00139143-200608000-00004. PubMed
24. Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987;62(4):281-288. doi: 10.1016/S0025-6196(12)61905-3. PubMed
25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6. PubMed
26. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: A brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. doi: 10.1212/01.wnl.0000172958.95282.2a. PubMed
27. Farrell B, Szeto W, Shamji S. Drug-related problems in the frail elderly. Can Fam Phys. 2011;57(2):168-169. PubMed
28. Chen CY, Thorsteinsdottir B, Cha SS, et al. Health care outcomes and advance care planning in older adults who receive home-based palliative care: a pilot cohort study. J Palliat Med. 2015;18(1):38-44. doi: 10.1089/jpm.2014.0150. PubMed
29. Rao A, Suliman A, Vuik S, Aylin P, Darzi A. Outcomes of dementia: systematic review and meta-analysis of hospital administrative database studies. Arch Gerontol Geriatr. 2016;66(Suppl C):198-204. doi: 10.1016/j.archger.2016.06.008. PubMed
30. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212-1221. doi: 10.1056/NEJMsa1100347. PubMed
31. Wang SY, Aldridge MD, Gross CP, Canavan M, Cherlin E, Bradley E. End-of-life care transition patterns of Medicare beneficiaries. J Am Geriatr Soc. 2017;65(7):1406-1413. doi: 10.1111/jgs.14891. PubMed
32. Pedersen MK, Meyer G, Uhrenfeldt L. Risk factors for acute care hospital readmission in older persons in Western countries: a systematic review. JBI Database System Rev Implement Rep. 2017;15(2):454-485. doi: 10.11124/JBISRIR-2016-003267. PubMed

33. Edwards ST, Saha S, Prentice JC, Pizer SD. Preventing hospitalization with Veterans Affairs home-based primary care: which individuals benefit most? J Am Geriatr Soc. 2017;65(8):1676-1683. doi: 10.1111/jgs.14843. PubMed
34. Mitchell SL, Palmer JA, Volandes AE, Hanson LC, Habtemariam D, Shaffer ML. Level of care preferences Among nursing home residents With advanced dementia. J Pain Symptom Manage. 2017;54(3):340-345. doi: 10.1016/j.jpainsymman.2017.04.020. PubMed
35. D’Avolio DA, Strumpf NE, Feldman J, Mitchell P, Rebholz CM. Barriers to primary care: perceptions of older adults utilizing the ED for nonurgent visits. Clin Nurs Res. 2013;22(4):416-431. doi: 10.1177/1054773813485597. PubMed
36. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000-1025. doi: 10.1177/0269216314526272. PubMed
37. Martin RS, Hayes B, Gregorevic K, Lim WK. The effects of advance care planning interventions on nursing home residents: A systematic review. J Am Med Dir Assoc. 2016;17(4):284-293. doi: 10.1016/j.jamda.2015.12.017. PubMed
38. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. doi: 10.1001/jamainternmed.2015.7863. PubMed
39. Parrinello G, Torres D, Paterna S, et al. Early and personalized ambulatory follow-up to tailor furosemide and fluid intake according to congestion in post-discharge heart failure. Intern Emerg Med. 2013;8(3):221-228. doi: 10.1007/s11739-011-0602-y. PubMed
40. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
41. Calvillo–King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269-282. doi: 10.1007/s11606-012-2235-x. PubMed
42. Rönneikkö JK, Mäkelä M, Jämsen ER, et al. Predictors for unplanned hospitalization of New Home care clients. J Am Geriatr Soc. 2017;65(2):407-414. doi: 10.1111/jgs.14486. PubMed
43. Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219. doi: 10.1007/s11606-009-1196-1. PubMed
44. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-160. doi: 10.1016/j.mayocp.2011.11.009. PubMed
© 2019 Society of Hospital Medicine
AHRQ Evidence-based Practice Center Program--Applying the Knowledge to Practice to Data Cycle to Strengthen the Value of Patient Care
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
©2019 Society of Hospital Medicine
Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized Patients
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
© 2019 Society of Hospital Medicine
Follow Up of Incidental High-Risk Pulmonary Nodules on Computed Tomography Pulmonary Angiography at Care Transitions
Computed tomography pulmonary angiography (CTPA) is often used in the evaluation of suspected pulmonary embolism (PE). The detection of incidental findings that require follow-up is common; in just over 50% of cases, those incidental findings are pulmonary nodules.1 Although the majority of these nodules are benign, Fleischner Society guidelines2 recommend that patients with nodules at high risk for malignancy should undergo follow-up CT imaging within 3-12 months, with patients who smoke and have large nodules requiring closer follow up.
The failure to follow-up on abnormal test results is known to contribute to diagnostic error and can lead to patient harm.3 We sought to determine the proportion of high-risk pulmonary nodules on CTPA which did not undergo the recommended follow-up imaging.
Study Setting and Design
This retrospective cohort study included all patients who underwent CTPA in the emergency department (ED) and inpatient settings at three academic health centers (Mount Sinai Hospital, Toronto General Hospital, and Toronto Western Hospital) in Toronto, Canada between September 1, 2014, and August 31, 2015.
We examined the proportion of patients with pulmonary nodules requiring follow up who received repeat CT imaging within six weeks of the time frame recommended by the radiologist. Since we were interested in measuring the rate of an important test result that is missed (rather than accuracy of the test itself), we defined “requiring follow up” as the inclusion of explicit recommendations for follow up in the radiology report.
Montage (Philadelphia, Pennsylvania), a natural language processing software, was applied to a linked radiology information system (RIS) to identify all CTPAs that contained pulmonary nodules. We conducted manual chart review to confirm software accuracy. We initially searched the RIS for all CTPAs that were completed within the study period, resulting in the identification of 1932 imaging studies. Following a review of these 1,932 studies, we excluded 22 as they were not CTPAs. We then applied the search term, “nodule-” to 1,910 confirmed CTPAs, resulting in the identification of 836 imaging studies. Following a review of these 836 studies, we excluded 10 as they were duplicate studies. We also excluded 152 studies where the term “nodule-” did not identify a pulmonary nodule but instead referred to a radiologist reporting the absence of pulmonary nodules (eg “there were no pulmonary nodules found”) or the presence of non-lung nodules (eg thyroid nodules). This resulted in the identification of 674 CTPAs containing pulmonary nodules (Figure 1).
Thereafter, we generated a cohort with possible new lung malignancy eligible for follow-up imaging by reviewing available health records and applying the following prespecified exclusion criteria: (1) patients who died, (2) left against medical advice, (3) were critically ill during the follow-up period, (4) lived outside the hospital catchment area (Greater Toronto Area), (5) were seen in the outpatient setting, (6) identified as palliative, (7) had an active malignancy, (8) had nodules that were already being followed, or (9) had nodules with characteristics suggestive of alternate diagnoses to lung malignancy (such as infection or inflammation) with no follow up recommended as reported by the radiologist. For patients with multiple CTPAs, we included only the first study. For each eligible patient, we determined whether follow-up imaging was completed by manually reviewing the linked RIS. We reviewed available health records to determine whether the pulmonary nodule findings had been discussed with the patient and whether the patient had attended an outpatient follow-up visit. In patients for whom recommended follow-up imaging was not confirmed, we notified the ordering physician by e-mail.
Each radiology department followed the same protocol adherent to the 2005 Fleischner guidelines for identifying nodules requiring follow up.2 Virtually all CTPAs at the three study institutions are read and reported within 72 hours. The ordering physician is sometimes called at the discretion of the reading radiologist when the findings are judged to be urgent and time-sensitive in nature. For example, the ordering physician may be contacted if a CTPA is positive for segmental PE but is not typically called for incidental pulmonary nodules. It is not common practice for ordering physicians to be notified of incidental findings above and beyond the radiology report. Primary care physicians are not typically copied on radiology reports and usually do not use the same electronic health record.
Statistical Analysis
We calculated simple descriptive statistics for all results. Mean values were compared using two-tailed t-tests, categorical groups using chi-square tests, and median values using Mann-Whitney U tests. We performed all analyses using Microsoft Excel version 16.14.1 (Redmond, Washington).
Ethics Approval
This study was approved by each institution’s research ethics board.
RESULTS
Follow Up of Incidental High-Risk Pulmonary Nodules
Of the 1910 CTPAs performed over the study period (Figure), 674 (35.3%) contained pulmonary nodules. Of the 259 patients with new pulmonary nodules eligible for follow-up imaging, 194 (74.9%) did not have an explicit suggestion for follow up by the radiologist. Ninety-five percent of radiologists (184 out of 194) provided an explanation for not recommending follow up in the radiology report; the two most common reasons were small nodule size (often described as “tiny”) and no interval change compared with the prior imaging study.2 Of the 65 patients who did receive an explicit suggestion for follow up by radiology, 35 (53.8%) did not receive repeat imaging within the recommended time frame, allowing for a six-week grace period. Of these 35 patients, 10 eventually went on to receive delayed repeat imaging. The median follow-up time for the 30 patients who received timely repeat imaging was four months (IQR 2-6 months); in contrast, the median follow-up time for the 10 patients who received delayed repeat imaging was seven months (IQR 6-8 months), P = .01.
Of the 65 patients for whom follow up was recommended, the medical record showed evidence that there was a discussion between the medical team and the patient regarding patient preference for or against follow up in 55.4% (36 out of 65) of the patients. Notably, all 36 patients showed interest in receiving follow up; no patient indicated a preference for no follow up.
Furthermore, of the 65 patients that had follow up recommended, two patients were eventually diagnosed with lung cancer (one via lung biopsy, the other via positron emission tomography imaging); both patients did not receive timely follow-up imaging. While we did not include nodule size as an exclusion criterion, not one of the 65 patients included in the final cohort had nodules larger than 3 cm.
Physician Notification
In circumstances where we could not confirm that followed up had occurred, we notified the ordering physician by e-mail. Since 10 of the 35 patients who did not receive timely follow-up imaging went on to receive delayed repeat imaging, we notified 25 physicians. Of the 25 physicians that we e-mailed, 24 acknowledged receipt of the information. Of these 24 physicians, 14 reported conducting a detailed review of the chart, from which the following additional information was obtained: one patient expired, and five physicians notified the corresponding primary care physicians (two of whom were unaware of the nodule, and subsequently arranged further follow up with the patient).
Characteristics Associated with Timely Follow Up
Explicit mention that follow up was required in the discharge summary (P = .03), attending an outpatient follow-up visit (P < .001), and younger age (P = .03) were associated with receiving timely follow up; patient sex, smoking history, history of chronic obstructive pulmonary disease, lung nodule count, recommended follow-up time, and hospital department (defined as the discharging service) were not (Table).
DISCUSSION
In this multicenter cohort study, over 50% of patients with new high-risk pulmonary nodules detected incidentally on CTPA did not receive timely follow-up imaging. Including follow-up recommendations in the discharge summary, attending an outpatient follow-up visit, and younger age were associated with timely follow-up imaging.
Few studies have assessed the follow up of incidental nodules identified on CTPA. In a retrospective cohort study of ED patients in the United States, Blagev et al. found that only 29% received timely follow up.4 Our study contributes to the literature in several ways. First, our study included all hospitalized patients, not only those in the ED. Notably, most of our cohort were inpatients, a group of patients not previously described. Second, we examined factors associated with timely follow up, which may help to inform future quality improvement initiatives and interventions. Third, we included data from three different hospitals, which may improve generalization. Lastly, our study draws on contemporary Canadian data. Most of the studies investigating test result follow up have been conducted in the US5,6 and Europe,7 with few empirical studies describing this phenomenon within the Canadian healthcare setting. We believe that our work contributes to the existing evidence that missed test results occur across diverse healthcare systems and have yet to be solved.5-7
Our study had limitations. First, we defined follow up as repeat imaging and did not include office visits or biopsy in this definition. Second, we may have missed repeat imaging and outpatient follow-up visits that occurred outside the study hospitals. Although we were able to determine if repeat imaging and outpatient follow-up visits (eg, pulmonology or thoracic surgery clinics) had occurred within the study hospitals, we did not have access to follow-up encounters that occurred outside of the study hospitals (eg primary care clinics). We are unaware of any published regional data on the rate of outpatient follow up at the index facility following discharge. However, we know from provincial data of patients discharged from the ED with a new cardiac diagnosis that just under half are seen by a family physician, cardiologist, or internist within seven days, with just under 80% seen within 30 days.8 Third, although we attempted to capture patient preference for or against repeat imaging using chart review, the absence of documentation of patient preference did not confirm that a discussion regarding patient preferences had not occurred. Fourth, while we did exclude patients that had an active malignancy, we did not exclude patients who were younger than 35 years or were immunocompromised, which may have led to an overestimation of the percentage of patients who did not receive follow up.
Incidental findings detected on acute diagnostic tests requiring handoffs for chronic follow up are at risk of falling through the cracks. The inclusion of follow-up recommendations in discharge summaries has been shown to increase the likelihood of follow-up completion.9 Our study provides additional evidence of the urgent need for interventions aimed at closing the loop on test result follow up.5,6
Disclosures
None of the authors have any conflicts of interest to disclose in reference to this study.
Funding
JLK is supported by the Mount Sinai Hospital Department of Medicine Research Fund. PC is supported by a K24 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR062133).
1. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169(21):1961. doi: 10.1001/archinternmed.2009.360. PubMed
2. Macmahon H, Austin JHM, Gamsu G, et al. Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society. Radiology 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
3. National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC. 2015. PubMed
4. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
5. Callen J, Georgiou A, Li J, Westbrook JI. The safety implications of missed test results for hospitalized patients: a systematic review. BMJ Quality Safety 2011;20(2):194-199. doi: 10.1136/bmjqs.2010.044339.
6. Callen JL, Westbrook JI, Georgiou A, Li J. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2011;27(10):1334-1348. doi: 10.1007/s11606-011-1949-5. PubMed
7. Litchfield I, Bentham L, Lilford R, Mcmanus RJ, Hill A, Greenfield S. Test result communication in primary care: a survey of current practice. BMJ Quality Safety 2015;24(11):691-699. doi: 10.1136/bmjqs-2014-003712. PubMed
8. Atzema CL, Yu B, Ivers NM, et al. Predictors of obtaining follow-up care in the province of Ontario, Canada, following a new diagnosis of atrial fibrillation, heart failure, and hypertension in the emergency department. Cjem 2017;20(03):377-391. doi: 10.1017/cem.2017.371. PubMed
9. Moore C, McGinn T, Halm E. Tying up loose ends: Discharging patients with unresolved medical issues. Arch Intern Med 2007;167(12):1305-1311. doi: 10.1001/archinte.167.12.1305 PubMed
Computed tomography pulmonary angiography (CTPA) is often used in the evaluation of suspected pulmonary embolism (PE). The detection of incidental findings that require follow-up is common; in just over 50% of cases, those incidental findings are pulmonary nodules.1 Although the majority of these nodules are benign, Fleischner Society guidelines2 recommend that patients with nodules at high risk for malignancy should undergo follow-up CT imaging within 3-12 months, with patients who smoke and have large nodules requiring closer follow up.
The failure to follow-up on abnormal test results is known to contribute to diagnostic error and can lead to patient harm.3 We sought to determine the proportion of high-risk pulmonary nodules on CTPA which did not undergo the recommended follow-up imaging.
Study Setting and Design
This retrospective cohort study included all patients who underwent CTPA in the emergency department (ED) and inpatient settings at three academic health centers (Mount Sinai Hospital, Toronto General Hospital, and Toronto Western Hospital) in Toronto, Canada between September 1, 2014, and August 31, 2015.
We examined the proportion of patients with pulmonary nodules requiring follow up who received repeat CT imaging within six weeks of the time frame recommended by the radiologist. Since we were interested in measuring the rate of an important test result that is missed (rather than accuracy of the test itself), we defined “requiring follow up” as the inclusion of explicit recommendations for follow up in the radiology report.
Montage (Philadelphia, Pennsylvania), a natural language processing software, was applied to a linked radiology information system (RIS) to identify all CTPAs that contained pulmonary nodules. We conducted manual chart review to confirm software accuracy. We initially searched the RIS for all CTPAs that were completed within the study period, resulting in the identification of 1932 imaging studies. Following a review of these 1,932 studies, we excluded 22 as they were not CTPAs. We then applied the search term, “nodule-” to 1,910 confirmed CTPAs, resulting in the identification of 836 imaging studies. Following a review of these 836 studies, we excluded 10 as they were duplicate studies. We also excluded 152 studies where the term “nodule-” did not identify a pulmonary nodule but instead referred to a radiologist reporting the absence of pulmonary nodules (eg “there were no pulmonary nodules found”) or the presence of non-lung nodules (eg thyroid nodules). This resulted in the identification of 674 CTPAs containing pulmonary nodules (Figure 1).
Thereafter, we generated a cohort with possible new lung malignancy eligible for follow-up imaging by reviewing available health records and applying the following prespecified exclusion criteria: (1) patients who died, (2) left against medical advice, (3) were critically ill during the follow-up period, (4) lived outside the hospital catchment area (Greater Toronto Area), (5) were seen in the outpatient setting, (6) identified as palliative, (7) had an active malignancy, (8) had nodules that were already being followed, or (9) had nodules with characteristics suggestive of alternate diagnoses to lung malignancy (such as infection or inflammation) with no follow up recommended as reported by the radiologist. For patients with multiple CTPAs, we included only the first study. For each eligible patient, we determined whether follow-up imaging was completed by manually reviewing the linked RIS. We reviewed available health records to determine whether the pulmonary nodule findings had been discussed with the patient and whether the patient had attended an outpatient follow-up visit. In patients for whom recommended follow-up imaging was not confirmed, we notified the ordering physician by e-mail.
Each radiology department followed the same protocol adherent to the 2005 Fleischner guidelines for identifying nodules requiring follow up.2 Virtually all CTPAs at the three study institutions are read and reported within 72 hours. The ordering physician is sometimes called at the discretion of the reading radiologist when the findings are judged to be urgent and time-sensitive in nature. For example, the ordering physician may be contacted if a CTPA is positive for segmental PE but is not typically called for incidental pulmonary nodules. It is not common practice for ordering physicians to be notified of incidental findings above and beyond the radiology report. Primary care physicians are not typically copied on radiology reports and usually do not use the same electronic health record.
Statistical Analysis
We calculated simple descriptive statistics for all results. Mean values were compared using two-tailed t-tests, categorical groups using chi-square tests, and median values using Mann-Whitney U tests. We performed all analyses using Microsoft Excel version 16.14.1 (Redmond, Washington).
Ethics Approval
This study was approved by each institution’s research ethics board.
RESULTS
Follow Up of Incidental High-Risk Pulmonary Nodules
Of the 1910 CTPAs performed over the study period (Figure), 674 (35.3%) contained pulmonary nodules. Of the 259 patients with new pulmonary nodules eligible for follow-up imaging, 194 (74.9%) did not have an explicit suggestion for follow up by the radiologist. Ninety-five percent of radiologists (184 out of 194) provided an explanation for not recommending follow up in the radiology report; the two most common reasons were small nodule size (often described as “tiny”) and no interval change compared with the prior imaging study.2 Of the 65 patients who did receive an explicit suggestion for follow up by radiology, 35 (53.8%) did not receive repeat imaging within the recommended time frame, allowing for a six-week grace period. Of these 35 patients, 10 eventually went on to receive delayed repeat imaging. The median follow-up time for the 30 patients who received timely repeat imaging was four months (IQR 2-6 months); in contrast, the median follow-up time for the 10 patients who received delayed repeat imaging was seven months (IQR 6-8 months), P = .01.
Of the 65 patients for whom follow up was recommended, the medical record showed evidence that there was a discussion between the medical team and the patient regarding patient preference for or against follow up in 55.4% (36 out of 65) of the patients. Notably, all 36 patients showed interest in receiving follow up; no patient indicated a preference for no follow up.
Furthermore, of the 65 patients that had follow up recommended, two patients were eventually diagnosed with lung cancer (one via lung biopsy, the other via positron emission tomography imaging); both patients did not receive timely follow-up imaging. While we did not include nodule size as an exclusion criterion, not one of the 65 patients included in the final cohort had nodules larger than 3 cm.
Physician Notification
In circumstances where we could not confirm that followed up had occurred, we notified the ordering physician by e-mail. Since 10 of the 35 patients who did not receive timely follow-up imaging went on to receive delayed repeat imaging, we notified 25 physicians. Of the 25 physicians that we e-mailed, 24 acknowledged receipt of the information. Of these 24 physicians, 14 reported conducting a detailed review of the chart, from which the following additional information was obtained: one patient expired, and five physicians notified the corresponding primary care physicians (two of whom were unaware of the nodule, and subsequently arranged further follow up with the patient).
Characteristics Associated with Timely Follow Up
Explicit mention that follow up was required in the discharge summary (P = .03), attending an outpatient follow-up visit (P < .001), and younger age (P = .03) were associated with receiving timely follow up; patient sex, smoking history, history of chronic obstructive pulmonary disease, lung nodule count, recommended follow-up time, and hospital department (defined as the discharging service) were not (Table).
DISCUSSION
In this multicenter cohort study, over 50% of patients with new high-risk pulmonary nodules detected incidentally on CTPA did not receive timely follow-up imaging. Including follow-up recommendations in the discharge summary, attending an outpatient follow-up visit, and younger age were associated with timely follow-up imaging.
Few studies have assessed the follow up of incidental nodules identified on CTPA. In a retrospective cohort study of ED patients in the United States, Blagev et al. found that only 29% received timely follow up.4 Our study contributes to the literature in several ways. First, our study included all hospitalized patients, not only those in the ED. Notably, most of our cohort were inpatients, a group of patients not previously described. Second, we examined factors associated with timely follow up, which may help to inform future quality improvement initiatives and interventions. Third, we included data from three different hospitals, which may improve generalization. Lastly, our study draws on contemporary Canadian data. Most of the studies investigating test result follow up have been conducted in the US5,6 and Europe,7 with few empirical studies describing this phenomenon within the Canadian healthcare setting. We believe that our work contributes to the existing evidence that missed test results occur across diverse healthcare systems and have yet to be solved.5-7
Our study had limitations. First, we defined follow up as repeat imaging and did not include office visits or biopsy in this definition. Second, we may have missed repeat imaging and outpatient follow-up visits that occurred outside the study hospitals. Although we were able to determine if repeat imaging and outpatient follow-up visits (eg, pulmonology or thoracic surgery clinics) had occurred within the study hospitals, we did not have access to follow-up encounters that occurred outside of the study hospitals (eg primary care clinics). We are unaware of any published regional data on the rate of outpatient follow up at the index facility following discharge. However, we know from provincial data of patients discharged from the ED with a new cardiac diagnosis that just under half are seen by a family physician, cardiologist, or internist within seven days, with just under 80% seen within 30 days.8 Third, although we attempted to capture patient preference for or against repeat imaging using chart review, the absence of documentation of patient preference did not confirm that a discussion regarding patient preferences had not occurred. Fourth, while we did exclude patients that had an active malignancy, we did not exclude patients who were younger than 35 years or were immunocompromised, which may have led to an overestimation of the percentage of patients who did not receive follow up.
Incidental findings detected on acute diagnostic tests requiring handoffs for chronic follow up are at risk of falling through the cracks. The inclusion of follow-up recommendations in discharge summaries has been shown to increase the likelihood of follow-up completion.9 Our study provides additional evidence of the urgent need for interventions aimed at closing the loop on test result follow up.5,6
Disclosures
None of the authors have any conflicts of interest to disclose in reference to this study.
Funding
JLK is supported by the Mount Sinai Hospital Department of Medicine Research Fund. PC is supported by a K24 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR062133).
Computed tomography pulmonary angiography (CTPA) is often used in the evaluation of suspected pulmonary embolism (PE). The detection of incidental findings that require follow-up is common; in just over 50% of cases, those incidental findings are pulmonary nodules.1 Although the majority of these nodules are benign, Fleischner Society guidelines2 recommend that patients with nodules at high risk for malignancy should undergo follow-up CT imaging within 3-12 months, with patients who smoke and have large nodules requiring closer follow up.
The failure to follow-up on abnormal test results is known to contribute to diagnostic error and can lead to patient harm.3 We sought to determine the proportion of high-risk pulmonary nodules on CTPA which did not undergo the recommended follow-up imaging.
Study Setting and Design
This retrospective cohort study included all patients who underwent CTPA in the emergency department (ED) and inpatient settings at three academic health centers (Mount Sinai Hospital, Toronto General Hospital, and Toronto Western Hospital) in Toronto, Canada between September 1, 2014, and August 31, 2015.
We examined the proportion of patients with pulmonary nodules requiring follow up who received repeat CT imaging within six weeks of the time frame recommended by the radiologist. Since we were interested in measuring the rate of an important test result that is missed (rather than accuracy of the test itself), we defined “requiring follow up” as the inclusion of explicit recommendations for follow up in the radiology report.
Montage (Philadelphia, Pennsylvania), a natural language processing software, was applied to a linked radiology information system (RIS) to identify all CTPAs that contained pulmonary nodules. We conducted manual chart review to confirm software accuracy. We initially searched the RIS for all CTPAs that were completed within the study period, resulting in the identification of 1932 imaging studies. Following a review of these 1,932 studies, we excluded 22 as they were not CTPAs. We then applied the search term, “nodule-” to 1,910 confirmed CTPAs, resulting in the identification of 836 imaging studies. Following a review of these 836 studies, we excluded 10 as they were duplicate studies. We also excluded 152 studies where the term “nodule-” did not identify a pulmonary nodule but instead referred to a radiologist reporting the absence of pulmonary nodules (eg “there were no pulmonary nodules found”) or the presence of non-lung nodules (eg thyroid nodules). This resulted in the identification of 674 CTPAs containing pulmonary nodules (Figure 1).
Thereafter, we generated a cohort with possible new lung malignancy eligible for follow-up imaging by reviewing available health records and applying the following prespecified exclusion criteria: (1) patients who died, (2) left against medical advice, (3) were critically ill during the follow-up period, (4) lived outside the hospital catchment area (Greater Toronto Area), (5) were seen in the outpatient setting, (6) identified as palliative, (7) had an active malignancy, (8) had nodules that were already being followed, or (9) had nodules with characteristics suggestive of alternate diagnoses to lung malignancy (such as infection or inflammation) with no follow up recommended as reported by the radiologist. For patients with multiple CTPAs, we included only the first study. For each eligible patient, we determined whether follow-up imaging was completed by manually reviewing the linked RIS. We reviewed available health records to determine whether the pulmonary nodule findings had been discussed with the patient and whether the patient had attended an outpatient follow-up visit. In patients for whom recommended follow-up imaging was not confirmed, we notified the ordering physician by e-mail.
Each radiology department followed the same protocol adherent to the 2005 Fleischner guidelines for identifying nodules requiring follow up.2 Virtually all CTPAs at the three study institutions are read and reported within 72 hours. The ordering physician is sometimes called at the discretion of the reading radiologist when the findings are judged to be urgent and time-sensitive in nature. For example, the ordering physician may be contacted if a CTPA is positive for segmental PE but is not typically called for incidental pulmonary nodules. It is not common practice for ordering physicians to be notified of incidental findings above and beyond the radiology report. Primary care physicians are not typically copied on radiology reports and usually do not use the same electronic health record.
Statistical Analysis
We calculated simple descriptive statistics for all results. Mean values were compared using two-tailed t-tests, categorical groups using chi-square tests, and median values using Mann-Whitney U tests. We performed all analyses using Microsoft Excel version 16.14.1 (Redmond, Washington).
Ethics Approval
This study was approved by each institution’s research ethics board.
RESULTS
Follow Up of Incidental High-Risk Pulmonary Nodules
Of the 1910 CTPAs performed over the study period (Figure), 674 (35.3%) contained pulmonary nodules. Of the 259 patients with new pulmonary nodules eligible for follow-up imaging, 194 (74.9%) did not have an explicit suggestion for follow up by the radiologist. Ninety-five percent of radiologists (184 out of 194) provided an explanation for not recommending follow up in the radiology report; the two most common reasons were small nodule size (often described as “tiny”) and no interval change compared with the prior imaging study.2 Of the 65 patients who did receive an explicit suggestion for follow up by radiology, 35 (53.8%) did not receive repeat imaging within the recommended time frame, allowing for a six-week grace period. Of these 35 patients, 10 eventually went on to receive delayed repeat imaging. The median follow-up time for the 30 patients who received timely repeat imaging was four months (IQR 2-6 months); in contrast, the median follow-up time for the 10 patients who received delayed repeat imaging was seven months (IQR 6-8 months), P = .01.
Of the 65 patients for whom follow up was recommended, the medical record showed evidence that there was a discussion between the medical team and the patient regarding patient preference for or against follow up in 55.4% (36 out of 65) of the patients. Notably, all 36 patients showed interest in receiving follow up; no patient indicated a preference for no follow up.
Furthermore, of the 65 patients that had follow up recommended, two patients were eventually diagnosed with lung cancer (one via lung biopsy, the other via positron emission tomography imaging); both patients did not receive timely follow-up imaging. While we did not include nodule size as an exclusion criterion, not one of the 65 patients included in the final cohort had nodules larger than 3 cm.
Physician Notification
In circumstances where we could not confirm that followed up had occurred, we notified the ordering physician by e-mail. Since 10 of the 35 patients who did not receive timely follow-up imaging went on to receive delayed repeat imaging, we notified 25 physicians. Of the 25 physicians that we e-mailed, 24 acknowledged receipt of the information. Of these 24 physicians, 14 reported conducting a detailed review of the chart, from which the following additional information was obtained: one patient expired, and five physicians notified the corresponding primary care physicians (two of whom were unaware of the nodule, and subsequently arranged further follow up with the patient).
Characteristics Associated with Timely Follow Up
Explicit mention that follow up was required in the discharge summary (P = .03), attending an outpatient follow-up visit (P < .001), and younger age (P = .03) were associated with receiving timely follow up; patient sex, smoking history, history of chronic obstructive pulmonary disease, lung nodule count, recommended follow-up time, and hospital department (defined as the discharging service) were not (Table).
DISCUSSION
In this multicenter cohort study, over 50% of patients with new high-risk pulmonary nodules detected incidentally on CTPA did not receive timely follow-up imaging. Including follow-up recommendations in the discharge summary, attending an outpatient follow-up visit, and younger age were associated with timely follow-up imaging.
Few studies have assessed the follow up of incidental nodules identified on CTPA. In a retrospective cohort study of ED patients in the United States, Blagev et al. found that only 29% received timely follow up.4 Our study contributes to the literature in several ways. First, our study included all hospitalized patients, not only those in the ED. Notably, most of our cohort were inpatients, a group of patients not previously described. Second, we examined factors associated with timely follow up, which may help to inform future quality improvement initiatives and interventions. Third, we included data from three different hospitals, which may improve generalization. Lastly, our study draws on contemporary Canadian data. Most of the studies investigating test result follow up have been conducted in the US5,6 and Europe,7 with few empirical studies describing this phenomenon within the Canadian healthcare setting. We believe that our work contributes to the existing evidence that missed test results occur across diverse healthcare systems and have yet to be solved.5-7
Our study had limitations. First, we defined follow up as repeat imaging and did not include office visits or biopsy in this definition. Second, we may have missed repeat imaging and outpatient follow-up visits that occurred outside the study hospitals. Although we were able to determine if repeat imaging and outpatient follow-up visits (eg, pulmonology or thoracic surgery clinics) had occurred within the study hospitals, we did not have access to follow-up encounters that occurred outside of the study hospitals (eg primary care clinics). We are unaware of any published regional data on the rate of outpatient follow up at the index facility following discharge. However, we know from provincial data of patients discharged from the ED with a new cardiac diagnosis that just under half are seen by a family physician, cardiologist, or internist within seven days, with just under 80% seen within 30 days.8 Third, although we attempted to capture patient preference for or against repeat imaging using chart review, the absence of documentation of patient preference did not confirm that a discussion regarding patient preferences had not occurred. Fourth, while we did exclude patients that had an active malignancy, we did not exclude patients who were younger than 35 years or were immunocompromised, which may have led to an overestimation of the percentage of patients who did not receive follow up.
Incidental findings detected on acute diagnostic tests requiring handoffs for chronic follow up are at risk of falling through the cracks. The inclusion of follow-up recommendations in discharge summaries has been shown to increase the likelihood of follow-up completion.9 Our study provides additional evidence of the urgent need for interventions aimed at closing the loop on test result follow up.5,6
Disclosures
None of the authors have any conflicts of interest to disclose in reference to this study.
Funding
JLK is supported by the Mount Sinai Hospital Department of Medicine Research Fund. PC is supported by a K24 award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR062133).
1. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169(21):1961. doi: 10.1001/archinternmed.2009.360. PubMed
2. Macmahon H, Austin JHM, Gamsu G, et al. Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society. Radiology 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
3. National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC. 2015. PubMed
4. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
5. Callen J, Georgiou A, Li J, Westbrook JI. The safety implications of missed test results for hospitalized patients: a systematic review. BMJ Quality Safety 2011;20(2):194-199. doi: 10.1136/bmjqs.2010.044339.
6. Callen JL, Westbrook JI, Georgiou A, Li J. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2011;27(10):1334-1348. doi: 10.1007/s11606-011-1949-5. PubMed
7. Litchfield I, Bentham L, Lilford R, Mcmanus RJ, Hill A, Greenfield S. Test result communication in primary care: a survey of current practice. BMJ Quality Safety 2015;24(11):691-699. doi: 10.1136/bmjqs-2014-003712. PubMed
8. Atzema CL, Yu B, Ivers NM, et al. Predictors of obtaining follow-up care in the province of Ontario, Canada, following a new diagnosis of atrial fibrillation, heart failure, and hypertension in the emergency department. Cjem 2017;20(03):377-391. doi: 10.1017/cem.2017.371. PubMed
9. Moore C, McGinn T, Halm E. Tying up loose ends: Discharging patients with unresolved medical issues. Arch Intern Med 2007;167(12):1305-1311. doi: 10.1001/archinte.167.12.1305 PubMed
1. Hall WB, Truitt SG, Scheunemann LP, et al. The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism. Arch Intern Med 2009;169(21):1961. doi: 10.1001/archinternmed.2009.360. PubMed
2. Macmahon H, Austin JHM, Gamsu G, et al. Guidelines for Management of Small Pulmonary Nodules Detected on CT Scans: A Statement from the Fleischner Society. Radiology 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
3. National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC. 2015. PubMed
4. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
5. Callen J, Georgiou A, Li J, Westbrook JI. The safety implications of missed test results for hospitalized patients: a systematic review. BMJ Quality Safety 2011;20(2):194-199. doi: 10.1136/bmjqs.2010.044339.
6. Callen JL, Westbrook JI, Georgiou A, Li J. Failure to follow-up test results for ambulatory patients: a systematic review. J Gen Intern Med 2011;27(10):1334-1348. doi: 10.1007/s11606-011-1949-5. PubMed
7. Litchfield I, Bentham L, Lilford R, Mcmanus RJ, Hill A, Greenfield S. Test result communication in primary care: a survey of current practice. BMJ Quality Safety 2015;24(11):691-699. doi: 10.1136/bmjqs-2014-003712. PubMed
8. Atzema CL, Yu B, Ivers NM, et al. Predictors of obtaining follow-up care in the province of Ontario, Canada, following a new diagnosis of atrial fibrillation, heart failure, and hypertension in the emergency department. Cjem 2017;20(03):377-391. doi: 10.1017/cem.2017.371. PubMed
9. Moore C, McGinn T, Halm E. Tying up loose ends: Discharging patients with unresolved medical issues. Arch Intern Med 2007;167(12):1305-1311. doi: 10.1001/archinte.167.12.1305 PubMed
© 2019 Society of Hospital Medicine
National Survey of Hospitalists’ Experiences with Incidental Pulmonary Nodules
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
© 2019 Society of Hospital Medicine
Serious Choices: A Systematic Environmental Scan of Decision Aids and Their Use for Seriously Ill People Near Death
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
© 2019 Society of Hospital Medicine
Inpatient Mobility Technicians: One Step Forward?
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
©2019 Society of Hospital Medicine
Aspiration Pneumonia in Older Adults
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
© 2019 Society of Hospital Medicine
The Dyad Model for Interprofessional Academic Patient Aligned Care Teams
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.