User login
Beyond Reporting Early Warning Score Sensitivity: The Temporal Relationship and Clinical Relevance of “True Positive” Alerts that Precede Critical Deterioration
Patients at risk for clinical deterioration in the inpatient setting may not be identified efficiently or effectively by health care providers. Early warning systems that link clinical observations to rapid response mechanisms (such as medical emergency teams) have the potential to improve outcomes, but rigorous studies are lacking.1 The pediatric Rothman Index (pRI) is an automated early warning system sold by the company PeraHealth that is integrated with the electronic health record. The system incorporates vital signs, labs, and nursing assessments from existing electronic health record data to provide a single numeric score that generates alerts based on low absolute scores and acute decreases in score (low scores indicate high mortality risk).2 Automated alerts or rules based on the pRI score are meant to bring important changes in clinical status to the attention of clinicians.
Adverse outcomes (eg, unplanned intensive care unit [ICU] transfers and mortality) are associated with low pRI scores, and scores appear to decline prior to such events.2 However, the limitation of this and other studies evaluating the sensitivity of early warning systems3-6 is that the generated alerts are assigned “true positive” status if they precede clinical deterioration, regardless of whether or not they provide meaningful information to the clinicians caring for the patients. There are two potential critiques of this approach. First, the alert may have preceded a deterioration event but may not have been clinically relevant (eg, an alert triggered by a finding unrelated to the patient’s acute health status, such as a scar that was newly documented as an abnormal skin finding and as a result led to a worsening in the pRI). Second, even if the preceding alert demonstrated clinical relevance to a deterioration event, the clinicians at the bedside may have been aware of the patient’s deterioration for hours and have already escalated care. In this situation, the alert would simply confirm what the clinician already knew.
To better understand the relationship between early warning system acuity alerts and clinical practice, we examined a cohort of hospitalized patients who experienced a critical deterioration event (CDE)7 and who would have triggered a preceding pRI alert. We evaluated the clinical relationship of the alert to the CDE (ie, whether the alert reflected physiologic changes related to a CDE or was instead an artifact of documentation) and identified whether the alert would have preceded evidence that clinicians recognized deterioration or escalated care.
METHODS
Patients and Setting
This retrospective cross-sectional study was performed at Children’s Hospital of Philadelphia (CHOP), a freestanding children’s hospital with 546 beds. Eligible patients were hospitalized on nonintensive care, noncardiology, surgical wards between January 1, 2013, and December 31, 2013. The CHOP Institutional Review Board (IRB) approved the study with waivers of consent and assent. A HIPAA Business Associate Agreement and an IRB Reliance Agreement were in place with PeraHealth to permit data transfer.
Definition of Critical Deterioration Events
Critical deterioration events (CDEs) were defined according to an existing, validated measure7 as unplanned transfers to the ICU with continuous or bilevel positive airway pressure, tracheal intubation, and/or vasopressor infusion in the 12 hours after transfer. At CHOP, all unplanned ICU transfers are routed through the hospital’s rapid response or code blue teams, so these patients were identified using an existing database managed by the CHOP Resuscitation Committee. In the database, the elements of CDEs are entered as part of ongoing quality improvement activities. The time of CDE was defined as the time of the rapid response call precipitating unplanned transfer to the ICU.
The Pediatric Rothman Index
The pRI is an automated acuity score that has been validated in hospitalized pediatric patients.2 The pRI is calculated using existing variables from the electronic health record, including manually entered vital signs, laboratory values, cardiac rhythm, and nursing assessments of organ systems. The weights assigned to continuous variables are a function of deviation from the norm.2,8 (See Supplement 1 for a complete list of variables.)
The pRI is integrated with the electronic health record and automatically generates a score each time a new data observation becomes available. Changes in score over time and low absolute scores generate a graduated series of alerts ranging from medium to very high acuity. This analysis used PeraHealth’s standard pRI alerts. Medium acuity alerts occurred when the pRI score decreased by ≥30% in 24 hours. A high acuity alert occurred when the pRI score decreased by ≥40% in 6 hours. A very high acuity alert occurred when the pRI absolute score was ≤ 30.
Development of the Source Dataset
In 2014, CHOP shared one year of clinical data with PeraHealth as part of the process of deciding whether or not to implement the pRI. The pRI algorithm retrospectively generated scores and acuity alerts for all CHOP patients who experienced CDEs between January 1, 2013, and December 31, 2013. The pRI algorithm was not active in the hospital environment during this time period; the scores and acuity alerts were not visible to clinicians. This dataset was provided to the investigators at CHOP to conduct this project.
Data Collection
Pediatric intensive care nurses trained in clinical research data abstraction from the CHOP Critical Care Center for Evidence and Outcomes performed the chart review for this study. Chart abstraction comparisons were completed on the first 15 charts to ensure interrater reliability, and additional quality assurance checks were performed on intermittent charts to ensure consistency and definition adherence. We managed all data using Research Electronic Data Capture.9
To study the value of alerts labeled as “true positives,” we restricted the dataset to CDEs in which acuity alert(s) within the prior 72 hours would have been triggered if the pRI had been in clinical use at the time.
To identify the clinical relationship between pRI and CDE, we reviewed each chart with the goal of determining whether the preceding acuity alerts were clinically associated with the etiology of the CDE. We determined the etiology of the CDE by reviewing the cause(s) identified in the note written by rapid response or code blue team responders or by the admitting clinical team after transfer to the ICU. We then used a tool provided by PeraHealth to identify the specific score components that led to worsening pRI. If the score components that worsened were (a) consistent with a clinical change as opposed to a documentation artifact and (b) an organ system change that was plausibly related to the CDE etiology, we concluded that the alert was clinically related to the etiology of the CDE.
We defined documentation artifacts as instances in nursing documentation in which a finding unrelated to the patient’s acute health status, such as a scar, was newly documented as abnormal and led to worsening pRI. Any cases in which the clinical relevance was unclear underwent review by additional members of the team
To determine the temporal relationship among pRI, CDE, and clinician awareness or action, we then sought to systematically determine whether the preceding acuity alerts preceded documented evidence of clinicians recognizing deterioration or escalation of care. We made the a priori decision that acuity alerts that occurred more than 24 hours prior to a deterioration event had questionable clinical actionability. Therefore, we restricted this next analysis to CDEs with acuity alerts during the 24 hours prior to a CDE. We reviewed time-stamped progress notes written by clinicians in the 24 hours period prior to the time of the CDE and identified whether the notes reflected an adverse change in patient status or a clinical intervention. We then compared the times of these notes with the times of the alerts and CDEs. Given that documentation of change in clinical status often occurs after clinical intervention, we also reviewed new orders placed in the 24 hours prior to each CDE to determine escalation of care. We identified the following orders as reflective of escalation of care independent of specific disease process: administration of intravenous fluid bolus, blood product, steroid, or antibiotic, increased respiratory support, new imaging studies, and new laboratory studies. We then compared the time of each order with the time of the alert and CDE.
RESULTS
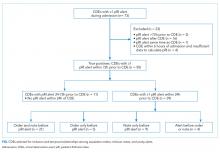
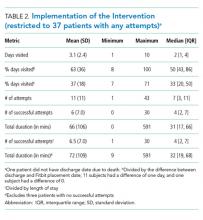
During the study period, 73 events met the CDE criteria and had a pRI alert during admission. Of the 73 events, 50 would have triggered at least one pRI alert in the 72-hour period leading up to the CDE (sensitivity 68%). Of the 50 events, 39 generated pRI alerts in the 24 hours leading up to the event, and 11 others generated pRI alerts between 24 and 72 hours prior to the event but did not generate any alerts during the 24 hours leading up to the event (Figure).
Patient Characteristics
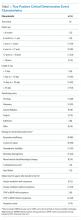
The 50 CDEs labeled as true positives occurred in 46 unique patients. Table 1 displays the event characteristics.
Acuity Alerts
A total of 79 pRI alerts preceded the 50 CDEs. Of these acuity alerts, 44 (56%) were medium acuity alerts, 17 (22%) were high acuity alerts, and 18 (23%) were very high acuity alerts. Of the 50 CDEs that would have triggered pRI alerts, 33 (66%) would have triggered a single acuity alert and 17 (34%) would have triggered multiple acuity alerts.
Of the 50 CDEs, 39 (78%) had a preceding acuity alert within 24 hours prior to the CDE. In these cases, the alert preceded the CDE by a median of 3.1 hours (interquartile range of 0.7 to 10.3 hours).
We assessed the score components that caused each alert to trigger. All of the vital sign and laboratory components were assessed as clinically related to the CDE’s etiology. By contrast, about half of nursing assessment components were assessed as clinically related to the etiology of the CDE (Table 2). Abnormal cardiac, respiratory, and neurologic assessments were most frequently assessed as clinically relevant.
Escalation Orders
To determine whether the pRI alert would have preceded the earliest documented treatment efforts, we restricted evaluation to the 39 CDEs that had at least one alert in the 24-hour window prior to the CDE. When we reviewed escalation orders placed by clinicians, we found that in 26 cases (67%), the first clinician order reflecting escalation of care would have preceded the first pRI alert within the 24-hour period prior to the CDE. In 13 cases (33%), the first pRI alert would have preceded the first escalation order placed by the clinician. The first pRI alert and the first escalation order would have occurred within the same 1-hour period in 6 of these cases.
Provider Notes
Temporal Relationships
In Supplement 2, we present the proportion of CDEs in which the order or note preceded the pRI alert for each abnormal organ system.
The Figure shows the temporal relationships among escalation orders, clinician notes, and acuity alerts for the 39 CDEs with one or more alerts in the 24 hours leading up to the event. In 21 cases (54%), both an escalation order and a note preceded the first acuity alert. In 14 cases (36%), either an escalation order or a note preceded the first acuity alert. In four cases (10%), the alert preceded any documented evidence that clinicians had recognized deterioration or escalating care.
DISCUSSION
The main finding of this study is that 90% of CDE events that generated “true positive” pRI alerts had evidence suggesting that clinicians had already recognized deterioration and/or were already escalating care before most pRI alerts would have been triggered.
The impacts of early warning scores on patient safety outcomes are not well established. In a recent 21-hospital cluster randomized trial of the BedsidePEWS, a pediatric early warning score system, investigators found that implementing the system does not significantly decrease all-cause mortality in hospitalized children, although hospitals using the BedsidePEWS have low rates of significant CDEs.10 In other studies, early warning scores were often coimplemented with rapid response teams, and separating the incremental benefit of the scoring tool from the availability of a rapid response team is usually not possible.11
Therefore, the benefits of early warning scores are often inferred based on their test characteristics (eg, sensitivity and positive predictive value).12 Sensitivity, which is the proportion of patients who deteriorated and also triggered the early warning score within a reasonable time window preceding the event, is an important consideration when deciding whether an early warning score is worth implementing. A challenging follow-up question that goes beyond sensitivity is how often an early warning score adds new knowledge by identifying patients on a path toward deterioration who were not yet recognized. This study is the first to address that follow-up question. Our results revealed that the score appeared to precede evidence of clinician recognition of deterioration in 10% of CDEs. In some patients, the alert could have contributed to a detection of deterioration that was not previously evident. In the portion of CDEs in which the alert and escalation order or note occurred within the same one-hour window, the alert could have been used as confirmation of clinical suspicion. Notably, we did not evaluate the 16 cases in which a CDE preceded any pRI alert because we chose to focus on “true positive” cases in which pRI alerts preceded CDEs. These events could have had timely recognition by clinicians that we did not capture, so these results may provide an overestimation of CDEs in which the pRI preceded clinician recognition.
Prior work has described a range of mechanisms by which early warning scores can impact patient safety.13 The results of this study suggest limited incremental benefit for the pRI to alert physicians and nurses to new concerning changes at this hospital, although the benefits to low-resourced community hospitals that care for children may be great. The pRI score may also serve as evidence that empowers nurses to overcome barriers to further escalate care, even if the process of escalation has already begun. In addition to empowering nurses, the score may support trainees and clinicians with varying levels of pediatric expertise in the decision to escalate care. Evaluating these potential benefits would require prospective study.
We used the pRI alerts as they were already defined by PeraHealth for CHOP, and different alert thresholds may change score performance. Our study did not identify additional variables to improve score performance, but they can be investigated in future research.
This study had several limitations. First, this work is a single-center study with highly skilled pediatric providers, a mature rapid response system, and low rates of cardiopulmonary arrest outside ICUs. Therefore, the results that we obtained were not immediately generalizable. In a community environment with nurses and physicians who are less experienced in caring for ill children, an early warning score with high sensitivity may be beneficial in ensuring patient safety.
Second, by using escalation orders and notes from the patient chart, we did not capture all the undocumented ways in which clinicians demonstrate awareness of deterioration. For example, a resident may alert the attending on service or a team may informally request consultation with a specialist. We also gave equal weight to escalation orders and clinician notes as evidence of recognition of deterioration. It could be that either orders or notes more closely correlated with clinician awareness.
Finally, the data were from 2013. Although the score components have not changed, efforts to standardize nursing assessments may have altered the performance of the score in the intervening years.
CONCLUSIONS
In most patients who had a CDE at a large freestanding children’s hospital, escalation orders or documented changes in patient status would have occurred before a pRI alert. However, in a minority of patients, the alert could have contributed to the detection of deterioration that was not previously evident.
Disclosures
The authors have nothing to disclose
Funding
The study was supported by funds from the Department of Biomedical and Health Informatics at Children’s Hospital of Philadelphia. PeraHealth, the company that sells the Rothman Index software, provided a service to the investigators but no funding. They applied their proprietary scoring algorithm to the data from Children’s Hospital of Philadelphia to generate alerts retrospectively. This service was provided free of charge in 2014 during the time period when Children’s Hospital of Philadelphia was considering purchasing and implementing PeraHealth software, which it subsequently did. We did not receive any funding for the study from PeraHealth. PeraHealth personnel did not influence the study design, the interpretation of data, the writing of the report, or the decision to submit the article for publication.
1. Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. doi: 10.1016/j.resuscitation.2014.01.013. PubMed
2. Rothman MJ, Tepas JJ, Nowalk AJ, et al. Development and validation of a continuously age-adjusted measure of patient condition for hospitalized children using the electronic medical record. J Biomed Inform. 2017;66 (Supplement C):180-193. doi: 10.1016/j.jbi.2016.12.013. PubMed
3. Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4):e763-e769. doi: 10.1542/peds.2009-0338. PubMed
4. Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013;132(4):e841-e850. doi: 10.1542/peds.2012-3594. PubMed
5. Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care Lond Engl. 2009;13(4):R135. doi: 10.1186/cc7998. PubMed
6. Hollis RH, Graham LA, Lazenby JP, et al. A role for the early warning score in early identification of critical postoperative complications. Ann Surg. 2016;263(5):918-923. doi: 10.1097/SLA.0000000000001514. PubMed
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. doi: 10.1542/peds.2011-2784. PubMed
8. Rothman MJ, Rothman SI, Beals J. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. doi: 10.1016/j.jbi.2013.06.011. PubMed
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
10. Parshuram CS, Dryden-Palmer K, Farrell C, et al. Effect of a pediatric early warning system on all-cause mortality in hospitalized pediatric patients: the EPOCH randomized clinical trial. JAMA. 2018;319(10):1002-1012. doi: 10.1001/jama.2018.0948. PubMed
11. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. doi: 10.1001/jamapediatrics.2013.3266. PubMed
12. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285. doi: 10.1186/s13054-015-0999-1. PubMed
13. Bonafide CP, Roberts KE, Weirich CM, et al. Beyond statistical prediction: qualitative evaluation of the mechanisms by which pediatric early warning scores impact patient safety. J Hosp Med. 2013;8(5):248-253. doi: 10.1002/jhm.2026. PubMed
Patients at risk for clinical deterioration in the inpatient setting may not be identified efficiently or effectively by health care providers. Early warning systems that link clinical observations to rapid response mechanisms (such as medical emergency teams) have the potential to improve outcomes, but rigorous studies are lacking.1 The pediatric Rothman Index (pRI) is an automated early warning system sold by the company PeraHealth that is integrated with the electronic health record. The system incorporates vital signs, labs, and nursing assessments from existing electronic health record data to provide a single numeric score that generates alerts based on low absolute scores and acute decreases in score (low scores indicate high mortality risk).2 Automated alerts or rules based on the pRI score are meant to bring important changes in clinical status to the attention of clinicians.
Adverse outcomes (eg, unplanned intensive care unit [ICU] transfers and mortality) are associated with low pRI scores, and scores appear to decline prior to such events.2 However, the limitation of this and other studies evaluating the sensitivity of early warning systems3-6 is that the generated alerts are assigned “true positive” status if they precede clinical deterioration, regardless of whether or not they provide meaningful information to the clinicians caring for the patients. There are two potential critiques of this approach. First, the alert may have preceded a deterioration event but may not have been clinically relevant (eg, an alert triggered by a finding unrelated to the patient’s acute health status, such as a scar that was newly documented as an abnormal skin finding and as a result led to a worsening in the pRI). Second, even if the preceding alert demonstrated clinical relevance to a deterioration event, the clinicians at the bedside may have been aware of the patient’s deterioration for hours and have already escalated care. In this situation, the alert would simply confirm what the clinician already knew.
To better understand the relationship between early warning system acuity alerts and clinical practice, we examined a cohort of hospitalized patients who experienced a critical deterioration event (CDE)7 and who would have triggered a preceding pRI alert. We evaluated the clinical relationship of the alert to the CDE (ie, whether the alert reflected physiologic changes related to a CDE or was instead an artifact of documentation) and identified whether the alert would have preceded evidence that clinicians recognized deterioration or escalated care.
METHODS
Patients and Setting
This retrospective cross-sectional study was performed at Children’s Hospital of Philadelphia (CHOP), a freestanding children’s hospital with 546 beds. Eligible patients were hospitalized on nonintensive care, noncardiology, surgical wards between January 1, 2013, and December 31, 2013. The CHOP Institutional Review Board (IRB) approved the study with waivers of consent and assent. A HIPAA Business Associate Agreement and an IRB Reliance Agreement were in place with PeraHealth to permit data transfer.
Definition of Critical Deterioration Events
Critical deterioration events (CDEs) were defined according to an existing, validated measure7 as unplanned transfers to the ICU with continuous or bilevel positive airway pressure, tracheal intubation, and/or vasopressor infusion in the 12 hours after transfer. At CHOP, all unplanned ICU transfers are routed through the hospital’s rapid response or code blue teams, so these patients were identified using an existing database managed by the CHOP Resuscitation Committee. In the database, the elements of CDEs are entered as part of ongoing quality improvement activities. The time of CDE was defined as the time of the rapid response call precipitating unplanned transfer to the ICU.
The Pediatric Rothman Index
The pRI is an automated acuity score that has been validated in hospitalized pediatric patients.2 The pRI is calculated using existing variables from the electronic health record, including manually entered vital signs, laboratory values, cardiac rhythm, and nursing assessments of organ systems. The weights assigned to continuous variables are a function of deviation from the norm.2,8 (See Supplement 1 for a complete list of variables.)
The pRI is integrated with the electronic health record and automatically generates a score each time a new data observation becomes available. Changes in score over time and low absolute scores generate a graduated series of alerts ranging from medium to very high acuity. This analysis used PeraHealth’s standard pRI alerts. Medium acuity alerts occurred when the pRI score decreased by ≥30% in 24 hours. A high acuity alert occurred when the pRI score decreased by ≥40% in 6 hours. A very high acuity alert occurred when the pRI absolute score was ≤ 30.
Development of the Source Dataset
In 2014, CHOP shared one year of clinical data with PeraHealth as part of the process of deciding whether or not to implement the pRI. The pRI algorithm retrospectively generated scores and acuity alerts for all CHOP patients who experienced CDEs between January 1, 2013, and December 31, 2013. The pRI algorithm was not active in the hospital environment during this time period; the scores and acuity alerts were not visible to clinicians. This dataset was provided to the investigators at CHOP to conduct this project.
Data Collection
Pediatric intensive care nurses trained in clinical research data abstraction from the CHOP Critical Care Center for Evidence and Outcomes performed the chart review for this study. Chart abstraction comparisons were completed on the first 15 charts to ensure interrater reliability, and additional quality assurance checks were performed on intermittent charts to ensure consistency and definition adherence. We managed all data using Research Electronic Data Capture.9
To study the value of alerts labeled as “true positives,” we restricted the dataset to CDEs in which acuity alert(s) within the prior 72 hours would have been triggered if the pRI had been in clinical use at the time.
To identify the clinical relationship between pRI and CDE, we reviewed each chart with the goal of determining whether the preceding acuity alerts were clinically associated with the etiology of the CDE. We determined the etiology of the CDE by reviewing the cause(s) identified in the note written by rapid response or code blue team responders or by the admitting clinical team after transfer to the ICU. We then used a tool provided by PeraHealth to identify the specific score components that led to worsening pRI. If the score components that worsened were (a) consistent with a clinical change as opposed to a documentation artifact and (b) an organ system change that was plausibly related to the CDE etiology, we concluded that the alert was clinically related to the etiology of the CDE.
We defined documentation artifacts as instances in nursing documentation in which a finding unrelated to the patient’s acute health status, such as a scar, was newly documented as abnormal and led to worsening pRI. Any cases in which the clinical relevance was unclear underwent review by additional members of the team
To determine the temporal relationship among pRI, CDE, and clinician awareness or action, we then sought to systematically determine whether the preceding acuity alerts preceded documented evidence of clinicians recognizing deterioration or escalation of care. We made the a priori decision that acuity alerts that occurred more than 24 hours prior to a deterioration event had questionable clinical actionability. Therefore, we restricted this next analysis to CDEs with acuity alerts during the 24 hours prior to a CDE. We reviewed time-stamped progress notes written by clinicians in the 24 hours period prior to the time of the CDE and identified whether the notes reflected an adverse change in patient status or a clinical intervention. We then compared the times of these notes with the times of the alerts and CDEs. Given that documentation of change in clinical status often occurs after clinical intervention, we also reviewed new orders placed in the 24 hours prior to each CDE to determine escalation of care. We identified the following orders as reflective of escalation of care independent of specific disease process: administration of intravenous fluid bolus, blood product, steroid, or antibiotic, increased respiratory support, new imaging studies, and new laboratory studies. We then compared the time of each order with the time of the alert and CDE.
RESULTS
During the study period, 73 events met the CDE criteria and had a pRI alert during admission. Of the 73 events, 50 would have triggered at least one pRI alert in the 72-hour period leading up to the CDE (sensitivity 68%). Of the 50 events, 39 generated pRI alerts in the 24 hours leading up to the event, and 11 others generated pRI alerts between 24 and 72 hours prior to the event but did not generate any alerts during the 24 hours leading up to the event (Figure).
Patient Characteristics
The 50 CDEs labeled as true positives occurred in 46 unique patients. Table 1 displays the event characteristics.
Acuity Alerts
A total of 79 pRI alerts preceded the 50 CDEs. Of these acuity alerts, 44 (56%) were medium acuity alerts, 17 (22%) were high acuity alerts, and 18 (23%) were very high acuity alerts. Of the 50 CDEs that would have triggered pRI alerts, 33 (66%) would have triggered a single acuity alert and 17 (34%) would have triggered multiple acuity alerts.
Of the 50 CDEs, 39 (78%) had a preceding acuity alert within 24 hours prior to the CDE. In these cases, the alert preceded the CDE by a median of 3.1 hours (interquartile range of 0.7 to 10.3 hours).
We assessed the score components that caused each alert to trigger. All of the vital sign and laboratory components were assessed as clinically related to the CDE’s etiology. By contrast, about half of nursing assessment components were assessed as clinically related to the etiology of the CDE (Table 2). Abnormal cardiac, respiratory, and neurologic assessments were most frequently assessed as clinically relevant.
Escalation Orders
To determine whether the pRI alert would have preceded the earliest documented treatment efforts, we restricted evaluation to the 39 CDEs that had at least one alert in the 24-hour window prior to the CDE. When we reviewed escalation orders placed by clinicians, we found that in 26 cases (67%), the first clinician order reflecting escalation of care would have preceded the first pRI alert within the 24-hour period prior to the CDE. In 13 cases (33%), the first pRI alert would have preceded the first escalation order placed by the clinician. The first pRI alert and the first escalation order would have occurred within the same 1-hour period in 6 of these cases.
Provider Notes
Temporal Relationships
In Supplement 2, we present the proportion of CDEs in which the order or note preceded the pRI alert for each abnormal organ system.
The Figure shows the temporal relationships among escalation orders, clinician notes, and acuity alerts for the 39 CDEs with one or more alerts in the 24 hours leading up to the event. In 21 cases (54%), both an escalation order and a note preceded the first acuity alert. In 14 cases (36%), either an escalation order or a note preceded the first acuity alert. In four cases (10%), the alert preceded any documented evidence that clinicians had recognized deterioration or escalating care.
DISCUSSION
The main finding of this study is that 90% of CDE events that generated “true positive” pRI alerts had evidence suggesting that clinicians had already recognized deterioration and/or were already escalating care before most pRI alerts would have been triggered.
The impacts of early warning scores on patient safety outcomes are not well established. In a recent 21-hospital cluster randomized trial of the BedsidePEWS, a pediatric early warning score system, investigators found that implementing the system does not significantly decrease all-cause mortality in hospitalized children, although hospitals using the BedsidePEWS have low rates of significant CDEs.10 In other studies, early warning scores were often coimplemented with rapid response teams, and separating the incremental benefit of the scoring tool from the availability of a rapid response team is usually not possible.11
Therefore, the benefits of early warning scores are often inferred based on their test characteristics (eg, sensitivity and positive predictive value).12 Sensitivity, which is the proportion of patients who deteriorated and also triggered the early warning score within a reasonable time window preceding the event, is an important consideration when deciding whether an early warning score is worth implementing. A challenging follow-up question that goes beyond sensitivity is how often an early warning score adds new knowledge by identifying patients on a path toward deterioration who were not yet recognized. This study is the first to address that follow-up question. Our results revealed that the score appeared to precede evidence of clinician recognition of deterioration in 10% of CDEs. In some patients, the alert could have contributed to a detection of deterioration that was not previously evident. In the portion of CDEs in which the alert and escalation order or note occurred within the same one-hour window, the alert could have been used as confirmation of clinical suspicion. Notably, we did not evaluate the 16 cases in which a CDE preceded any pRI alert because we chose to focus on “true positive” cases in which pRI alerts preceded CDEs. These events could have had timely recognition by clinicians that we did not capture, so these results may provide an overestimation of CDEs in which the pRI preceded clinician recognition.
Prior work has described a range of mechanisms by which early warning scores can impact patient safety.13 The results of this study suggest limited incremental benefit for the pRI to alert physicians and nurses to new concerning changes at this hospital, although the benefits to low-resourced community hospitals that care for children may be great. The pRI score may also serve as evidence that empowers nurses to overcome barriers to further escalate care, even if the process of escalation has already begun. In addition to empowering nurses, the score may support trainees and clinicians with varying levels of pediatric expertise in the decision to escalate care. Evaluating these potential benefits would require prospective study.
We used the pRI alerts as they were already defined by PeraHealth for CHOP, and different alert thresholds may change score performance. Our study did not identify additional variables to improve score performance, but they can be investigated in future research.
This study had several limitations. First, this work is a single-center study with highly skilled pediatric providers, a mature rapid response system, and low rates of cardiopulmonary arrest outside ICUs. Therefore, the results that we obtained were not immediately generalizable. In a community environment with nurses and physicians who are less experienced in caring for ill children, an early warning score with high sensitivity may be beneficial in ensuring patient safety.
Second, by using escalation orders and notes from the patient chart, we did not capture all the undocumented ways in which clinicians demonstrate awareness of deterioration. For example, a resident may alert the attending on service or a team may informally request consultation with a specialist. We also gave equal weight to escalation orders and clinician notes as evidence of recognition of deterioration. It could be that either orders or notes more closely correlated with clinician awareness.
Finally, the data were from 2013. Although the score components have not changed, efforts to standardize nursing assessments may have altered the performance of the score in the intervening years.
CONCLUSIONS
In most patients who had a CDE at a large freestanding children’s hospital, escalation orders or documented changes in patient status would have occurred before a pRI alert. However, in a minority of patients, the alert could have contributed to the detection of deterioration that was not previously evident.
Disclosures
The authors have nothing to disclose
Funding
The study was supported by funds from the Department of Biomedical and Health Informatics at Children’s Hospital of Philadelphia. PeraHealth, the company that sells the Rothman Index software, provided a service to the investigators but no funding. They applied their proprietary scoring algorithm to the data from Children’s Hospital of Philadelphia to generate alerts retrospectively. This service was provided free of charge in 2014 during the time period when Children’s Hospital of Philadelphia was considering purchasing and implementing PeraHealth software, which it subsequently did. We did not receive any funding for the study from PeraHealth. PeraHealth personnel did not influence the study design, the interpretation of data, the writing of the report, or the decision to submit the article for publication.
Patients at risk for clinical deterioration in the inpatient setting may not be identified efficiently or effectively by health care providers. Early warning systems that link clinical observations to rapid response mechanisms (such as medical emergency teams) have the potential to improve outcomes, but rigorous studies are lacking.1 The pediatric Rothman Index (pRI) is an automated early warning system sold by the company PeraHealth that is integrated with the electronic health record. The system incorporates vital signs, labs, and nursing assessments from existing electronic health record data to provide a single numeric score that generates alerts based on low absolute scores and acute decreases in score (low scores indicate high mortality risk).2 Automated alerts or rules based on the pRI score are meant to bring important changes in clinical status to the attention of clinicians.
Adverse outcomes (eg, unplanned intensive care unit [ICU] transfers and mortality) are associated with low pRI scores, and scores appear to decline prior to such events.2 However, the limitation of this and other studies evaluating the sensitivity of early warning systems3-6 is that the generated alerts are assigned “true positive” status if they precede clinical deterioration, regardless of whether or not they provide meaningful information to the clinicians caring for the patients. There are two potential critiques of this approach. First, the alert may have preceded a deterioration event but may not have been clinically relevant (eg, an alert triggered by a finding unrelated to the patient’s acute health status, such as a scar that was newly documented as an abnormal skin finding and as a result led to a worsening in the pRI). Second, even if the preceding alert demonstrated clinical relevance to a deterioration event, the clinicians at the bedside may have been aware of the patient’s deterioration for hours and have already escalated care. In this situation, the alert would simply confirm what the clinician already knew.
To better understand the relationship between early warning system acuity alerts and clinical practice, we examined a cohort of hospitalized patients who experienced a critical deterioration event (CDE)7 and who would have triggered a preceding pRI alert. We evaluated the clinical relationship of the alert to the CDE (ie, whether the alert reflected physiologic changes related to a CDE or was instead an artifact of documentation) and identified whether the alert would have preceded evidence that clinicians recognized deterioration or escalated care.
METHODS
Patients and Setting
This retrospective cross-sectional study was performed at Children’s Hospital of Philadelphia (CHOP), a freestanding children’s hospital with 546 beds. Eligible patients were hospitalized on nonintensive care, noncardiology, surgical wards between January 1, 2013, and December 31, 2013. The CHOP Institutional Review Board (IRB) approved the study with waivers of consent and assent. A HIPAA Business Associate Agreement and an IRB Reliance Agreement were in place with PeraHealth to permit data transfer.
Definition of Critical Deterioration Events
Critical deterioration events (CDEs) were defined according to an existing, validated measure7 as unplanned transfers to the ICU with continuous or bilevel positive airway pressure, tracheal intubation, and/or vasopressor infusion in the 12 hours after transfer. At CHOP, all unplanned ICU transfers are routed through the hospital’s rapid response or code blue teams, so these patients were identified using an existing database managed by the CHOP Resuscitation Committee. In the database, the elements of CDEs are entered as part of ongoing quality improvement activities. The time of CDE was defined as the time of the rapid response call precipitating unplanned transfer to the ICU.
The Pediatric Rothman Index
The pRI is an automated acuity score that has been validated in hospitalized pediatric patients.2 The pRI is calculated using existing variables from the electronic health record, including manually entered vital signs, laboratory values, cardiac rhythm, and nursing assessments of organ systems. The weights assigned to continuous variables are a function of deviation from the norm.2,8 (See Supplement 1 for a complete list of variables.)
The pRI is integrated with the electronic health record and automatically generates a score each time a new data observation becomes available. Changes in score over time and low absolute scores generate a graduated series of alerts ranging from medium to very high acuity. This analysis used PeraHealth’s standard pRI alerts. Medium acuity alerts occurred when the pRI score decreased by ≥30% in 24 hours. A high acuity alert occurred when the pRI score decreased by ≥40% in 6 hours. A very high acuity alert occurred when the pRI absolute score was ≤ 30.
Development of the Source Dataset
In 2014, CHOP shared one year of clinical data with PeraHealth as part of the process of deciding whether or not to implement the pRI. The pRI algorithm retrospectively generated scores and acuity alerts for all CHOP patients who experienced CDEs between January 1, 2013, and December 31, 2013. The pRI algorithm was not active in the hospital environment during this time period; the scores and acuity alerts were not visible to clinicians. This dataset was provided to the investigators at CHOP to conduct this project.
Data Collection
Pediatric intensive care nurses trained in clinical research data abstraction from the CHOP Critical Care Center for Evidence and Outcomes performed the chart review for this study. Chart abstraction comparisons were completed on the first 15 charts to ensure interrater reliability, and additional quality assurance checks were performed on intermittent charts to ensure consistency and definition adherence. We managed all data using Research Electronic Data Capture.9
To study the value of alerts labeled as “true positives,” we restricted the dataset to CDEs in which acuity alert(s) within the prior 72 hours would have been triggered if the pRI had been in clinical use at the time.
To identify the clinical relationship between pRI and CDE, we reviewed each chart with the goal of determining whether the preceding acuity alerts were clinically associated with the etiology of the CDE. We determined the etiology of the CDE by reviewing the cause(s) identified in the note written by rapid response or code blue team responders or by the admitting clinical team after transfer to the ICU. We then used a tool provided by PeraHealth to identify the specific score components that led to worsening pRI. If the score components that worsened were (a) consistent with a clinical change as opposed to a documentation artifact and (b) an organ system change that was plausibly related to the CDE etiology, we concluded that the alert was clinically related to the etiology of the CDE.
We defined documentation artifacts as instances in nursing documentation in which a finding unrelated to the patient’s acute health status, such as a scar, was newly documented as abnormal and led to worsening pRI. Any cases in which the clinical relevance was unclear underwent review by additional members of the team
To determine the temporal relationship among pRI, CDE, and clinician awareness or action, we then sought to systematically determine whether the preceding acuity alerts preceded documented evidence of clinicians recognizing deterioration or escalation of care. We made the a priori decision that acuity alerts that occurred more than 24 hours prior to a deterioration event had questionable clinical actionability. Therefore, we restricted this next analysis to CDEs with acuity alerts during the 24 hours prior to a CDE. We reviewed time-stamped progress notes written by clinicians in the 24 hours period prior to the time of the CDE and identified whether the notes reflected an adverse change in patient status or a clinical intervention. We then compared the times of these notes with the times of the alerts and CDEs. Given that documentation of change in clinical status often occurs after clinical intervention, we also reviewed new orders placed in the 24 hours prior to each CDE to determine escalation of care. We identified the following orders as reflective of escalation of care independent of specific disease process: administration of intravenous fluid bolus, blood product, steroid, or antibiotic, increased respiratory support, new imaging studies, and new laboratory studies. We then compared the time of each order with the time of the alert and CDE.
RESULTS
During the study period, 73 events met the CDE criteria and had a pRI alert during admission. Of the 73 events, 50 would have triggered at least one pRI alert in the 72-hour period leading up to the CDE (sensitivity 68%). Of the 50 events, 39 generated pRI alerts in the 24 hours leading up to the event, and 11 others generated pRI alerts between 24 and 72 hours prior to the event but did not generate any alerts during the 24 hours leading up to the event (Figure).
Patient Characteristics
The 50 CDEs labeled as true positives occurred in 46 unique patients. Table 1 displays the event characteristics.
Acuity Alerts
A total of 79 pRI alerts preceded the 50 CDEs. Of these acuity alerts, 44 (56%) were medium acuity alerts, 17 (22%) were high acuity alerts, and 18 (23%) were very high acuity alerts. Of the 50 CDEs that would have triggered pRI alerts, 33 (66%) would have triggered a single acuity alert and 17 (34%) would have triggered multiple acuity alerts.
Of the 50 CDEs, 39 (78%) had a preceding acuity alert within 24 hours prior to the CDE. In these cases, the alert preceded the CDE by a median of 3.1 hours (interquartile range of 0.7 to 10.3 hours).
We assessed the score components that caused each alert to trigger. All of the vital sign and laboratory components were assessed as clinically related to the CDE’s etiology. By contrast, about half of nursing assessment components were assessed as clinically related to the etiology of the CDE (Table 2). Abnormal cardiac, respiratory, and neurologic assessments were most frequently assessed as clinically relevant.
Escalation Orders
To determine whether the pRI alert would have preceded the earliest documented treatment efforts, we restricted evaluation to the 39 CDEs that had at least one alert in the 24-hour window prior to the CDE. When we reviewed escalation orders placed by clinicians, we found that in 26 cases (67%), the first clinician order reflecting escalation of care would have preceded the first pRI alert within the 24-hour period prior to the CDE. In 13 cases (33%), the first pRI alert would have preceded the first escalation order placed by the clinician. The first pRI alert and the first escalation order would have occurred within the same 1-hour period in 6 of these cases.
Provider Notes
Temporal Relationships
In Supplement 2, we present the proportion of CDEs in which the order or note preceded the pRI alert for each abnormal organ system.
The Figure shows the temporal relationships among escalation orders, clinician notes, and acuity alerts for the 39 CDEs with one or more alerts in the 24 hours leading up to the event. In 21 cases (54%), both an escalation order and a note preceded the first acuity alert. In 14 cases (36%), either an escalation order or a note preceded the first acuity alert. In four cases (10%), the alert preceded any documented evidence that clinicians had recognized deterioration or escalating care.
DISCUSSION
The main finding of this study is that 90% of CDE events that generated “true positive” pRI alerts had evidence suggesting that clinicians had already recognized deterioration and/or were already escalating care before most pRI alerts would have been triggered.
The impacts of early warning scores on patient safety outcomes are not well established. In a recent 21-hospital cluster randomized trial of the BedsidePEWS, a pediatric early warning score system, investigators found that implementing the system does not significantly decrease all-cause mortality in hospitalized children, although hospitals using the BedsidePEWS have low rates of significant CDEs.10 In other studies, early warning scores were often coimplemented with rapid response teams, and separating the incremental benefit of the scoring tool from the availability of a rapid response team is usually not possible.11
Therefore, the benefits of early warning scores are often inferred based on their test characteristics (eg, sensitivity and positive predictive value).12 Sensitivity, which is the proportion of patients who deteriorated and also triggered the early warning score within a reasonable time window preceding the event, is an important consideration when deciding whether an early warning score is worth implementing. A challenging follow-up question that goes beyond sensitivity is how often an early warning score adds new knowledge by identifying patients on a path toward deterioration who were not yet recognized. This study is the first to address that follow-up question. Our results revealed that the score appeared to precede evidence of clinician recognition of deterioration in 10% of CDEs. In some patients, the alert could have contributed to a detection of deterioration that was not previously evident. In the portion of CDEs in which the alert and escalation order or note occurred within the same one-hour window, the alert could have been used as confirmation of clinical suspicion. Notably, we did not evaluate the 16 cases in which a CDE preceded any pRI alert because we chose to focus on “true positive” cases in which pRI alerts preceded CDEs. These events could have had timely recognition by clinicians that we did not capture, so these results may provide an overestimation of CDEs in which the pRI preceded clinician recognition.
Prior work has described a range of mechanisms by which early warning scores can impact patient safety.13 The results of this study suggest limited incremental benefit for the pRI to alert physicians and nurses to new concerning changes at this hospital, although the benefits to low-resourced community hospitals that care for children may be great. The pRI score may also serve as evidence that empowers nurses to overcome barriers to further escalate care, even if the process of escalation has already begun. In addition to empowering nurses, the score may support trainees and clinicians with varying levels of pediatric expertise in the decision to escalate care. Evaluating these potential benefits would require prospective study.
We used the pRI alerts as they were already defined by PeraHealth for CHOP, and different alert thresholds may change score performance. Our study did not identify additional variables to improve score performance, but they can be investigated in future research.
This study had several limitations. First, this work is a single-center study with highly skilled pediatric providers, a mature rapid response system, and low rates of cardiopulmonary arrest outside ICUs. Therefore, the results that we obtained were not immediately generalizable. In a community environment with nurses and physicians who are less experienced in caring for ill children, an early warning score with high sensitivity may be beneficial in ensuring patient safety.
Second, by using escalation orders and notes from the patient chart, we did not capture all the undocumented ways in which clinicians demonstrate awareness of deterioration. For example, a resident may alert the attending on service or a team may informally request consultation with a specialist. We also gave equal weight to escalation orders and clinician notes as evidence of recognition of deterioration. It could be that either orders or notes more closely correlated with clinician awareness.
Finally, the data were from 2013. Although the score components have not changed, efforts to standardize nursing assessments may have altered the performance of the score in the intervening years.
CONCLUSIONS
In most patients who had a CDE at a large freestanding children’s hospital, escalation orders or documented changes in patient status would have occurred before a pRI alert. However, in a minority of patients, the alert could have contributed to the detection of deterioration that was not previously evident.
Disclosures
The authors have nothing to disclose
Funding
The study was supported by funds from the Department of Biomedical and Health Informatics at Children’s Hospital of Philadelphia. PeraHealth, the company that sells the Rothman Index software, provided a service to the investigators but no funding. They applied their proprietary scoring algorithm to the data from Children’s Hospital of Philadelphia to generate alerts retrospectively. This service was provided free of charge in 2014 during the time period when Children’s Hospital of Philadelphia was considering purchasing and implementing PeraHealth software, which it subsequently did. We did not receive any funding for the study from PeraHealth. PeraHealth personnel did not influence the study design, the interpretation of data, the writing of the report, or the decision to submit the article for publication.
1. Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. doi: 10.1016/j.resuscitation.2014.01.013. PubMed
2. Rothman MJ, Tepas JJ, Nowalk AJ, et al. Development and validation of a continuously age-adjusted measure of patient condition for hospitalized children using the electronic medical record. J Biomed Inform. 2017;66 (Supplement C):180-193. doi: 10.1016/j.jbi.2016.12.013. PubMed
3. Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4):e763-e769. doi: 10.1542/peds.2009-0338. PubMed
4. Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013;132(4):e841-e850. doi: 10.1542/peds.2012-3594. PubMed
5. Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care Lond Engl. 2009;13(4):R135. doi: 10.1186/cc7998. PubMed
6. Hollis RH, Graham LA, Lazenby JP, et al. A role for the early warning score in early identification of critical postoperative complications. Ann Surg. 2016;263(5):918-923. doi: 10.1097/SLA.0000000000001514. PubMed
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. doi: 10.1542/peds.2011-2784. PubMed
8. Rothman MJ, Rothman SI, Beals J. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. doi: 10.1016/j.jbi.2013.06.011. PubMed
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
10. Parshuram CS, Dryden-Palmer K, Farrell C, et al. Effect of a pediatric early warning system on all-cause mortality in hospitalized pediatric patients: the EPOCH randomized clinical trial. JAMA. 2018;319(10):1002-1012. doi: 10.1001/jama.2018.0948. PubMed
11. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. doi: 10.1001/jamapediatrics.2013.3266. PubMed
12. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285. doi: 10.1186/s13054-015-0999-1. PubMed
13. Bonafide CP, Roberts KE, Weirich CM, et al. Beyond statistical prediction: qualitative evaluation of the mechanisms by which pediatric early warning scores impact patient safety. J Hosp Med. 2013;8(5):248-253. doi: 10.1002/jhm.2026. PubMed
1. Alam N, Hobbelink EL, van Tienhoven AJ, van de Ven PM, Jansma EP, Nanayakkara PWB. The impact of the use of the Early Warning Score (EWS) on patient outcomes: a systematic review. Resuscitation. 2014;85(5):587-594. doi: 10.1016/j.resuscitation.2014.01.013. PubMed
2. Rothman MJ, Tepas JJ, Nowalk AJ, et al. Development and validation of a continuously age-adjusted measure of patient condition for hospitalized children using the electronic medical record. J Biomed Inform. 2017;66 (Supplement C):180-193. doi: 10.1016/j.jbi.2016.12.013. PubMed
3. Akre M, Finkelstein M, Erickson M, Liu M, Vanderbilt L, Billman G. Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4):e763-e769. doi: 10.1542/peds.2009-0338. PubMed
4. Seiger N, Maconochie I, Oostenbrink R, Moll HA. Validity of different pediatric early warning scores in the emergency department. Pediatrics. 2013;132(4):e841-e850. doi: 10.1542/peds.2012-3594. PubMed
5. Parshuram CS, Hutchison J, Middaugh K. Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care Lond Engl. 2009;13(4):R135. doi: 10.1186/cc7998. PubMed
6. Hollis RH, Graham LA, Lazenby JP, et al. A role for the early warning score in early identification of critical postoperative complications. Ann Surg. 2016;263(5):918-923. doi: 10.1097/SLA.0000000000001514. PubMed
7. Bonafide CP, Roberts KE, Priestley MA, et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874-e881. doi: 10.1542/peds.2011-2784. PubMed
8. Rothman MJ, Rothman SI, Beals J. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848. doi: 10.1016/j.jbi.2013.06.011. PubMed
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010. PubMed
10. Parshuram CS, Dryden-Palmer K, Farrell C, et al. Effect of a pediatric early warning system on all-cause mortality in hospitalized pediatric patients: the EPOCH randomized clinical trial. JAMA. 2018;319(10):1002-1012. doi: 10.1001/jama.2018.0948. PubMed
11. Bonafide CP, Localio AR, Roberts KE, Nadkarni VM, Weirich CM, Keren R. Impact of rapid response system implementation on critical deterioration events in children. JAMA Pediatr. 2014;168(1):25-33. doi: 10.1001/jamapediatrics.2013.3266. PubMed
12. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19:285. doi: 10.1186/s13054-015-0999-1. PubMed
13. Bonafide CP, Roberts KE, Weirich CM, et al. Beyond statistical prediction: qualitative evaluation of the mechanisms by which pediatric early warning scores impact patient safety. J Hosp Med. 2013;8(5):248-253. doi: 10.1002/jhm.2026. PubMed
© 2018 Society of Hospital Medicine.
Assess Before Rx: Reducing the Overtreatment of Asymptomatic Blood Pressure Elevation in the Inpatient Setting
With the presence of hypertension in 25% of patients admitted to the hospital,1 its proper management is imperative. A hypertensive crisis is a severe elevation of blood pressure, defined as systolic ≥180 mm Hg and/or diastolic ≥120 mm Hg. It is further classified as either a hypertensive emergency which includes the presence of end-organ damage,2 or hypertensive urgency, defined as asymptomatic blood pressure elevation.3 Although hypertensive emergencies account for only 1%-2% of patients with hypertension,4 they are associated with a high one-year mortality rate (>79%).5 Hypertensive emergency requires immediate reduction of blood pressure with IV antihypertensive drugs to limit organ damage. In contrast, as per national guidelines, inpatient management of hypertensive urgency requires gradual reductions of blood pressure over hours to days using oral antihypertensives.2 It is also recommended that alternative etiologies, such as anxiety or pain, be considered before treatment is initiated.1
Clinicians often inappropriately treat asymptomatic hypertension in the inpatient setting,6,7 using intravenous (IV) antihypertensive medications despite evidence showing potential harm.5,8 This can lead to unpredictable reductions in blood pressure.7,9 A recent retrospective analysis demonstrated that 32.6% of patients had a blood pressure reduction greater than 25% after the use of an IV antihypertensive.7 Reductions greater than 25% lead to shifts in autoregulation, which may result in patient harm, such as hypotension, decreased renal perfusion, and stroke.9 IV medications are also more expensive than oral agents, due to the additional cost of administration.
Although overtreatment of asymptomatic hypertension with IV antihypertensive medications is common,7 initiatives to address this in inpatient settings are lacking in the literature. The aim of this quality improvement initiative was to reduce unnecessary IV antihypertensive treatment for hypertensive urgency in the inpatient setting.
METHODS
Setting
An interdisciplinary quality improvement intervention was initiated on two inpatient medicine units at an urban, 1,134-bed tertiary medical center affiliated with the Icahn School of Medicine at Mount Sinai. Members of the Mount Sinai High Value Care Committee and the Student High Value Care Initiative10 developed this project. The intervention was implemented in stages from March 2017 to February 2018. It targeted nurses, housestaff, nurse practitioners, and attendings on general medical teaching and nonteaching services. The components of the intervention included education, a treatment algorithm, audit and feedback, and electronic medical record (EMR) change. This project was submitted to the Quality Committee in the Department of Medicine and determined to be a quality improvement project rather than research and thus, an IRB submission was not required.
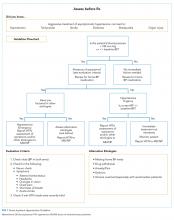
Treatment Algorithm and Education
A clinical algorithm was designed with nursing and cardiology representatives to provide guidance for nurses regarding the best practice for evaluation of inpatient hypertension, focusing on assessing patients before recommending treatment (“Assess Before Rx”; Figure 1). Educational sessions reinforcing the clinical algorithm were held monthly at nursing huddles. These involved an introduction session providing the background and purpose of the project, with follow-up sessions including interactive mock cases on the assessment of hypertensive urgency.
A second treatment algorithm was designed, with housestaff and cardiology input, to provide guidance for the internal medicine housestaff and nurse practitioners. It utilized a similar approach regarding identification, evaluation, and assessment of alternate etiologies but included more detailed treatment recommendations with a table outlining the oral medications used for hypertensive urgency (Figure 2). The flowchart and table were uploaded to an existing mobile application used by housestaff and nurse practitioners for quick access. The mobile application is frequently used by housestaff and contains many clinical resources. Additionally, e-mails including the purpose of the project and the treatment algorithm were sent to rotating housestaff at the start of each new medicine rotation.
Audit and Feedback
Monthly feedback was e-mailed to the nurses, which reinforced the goals and provided positive feedback on outcomes with an announcement of the “Nurse of the Month.” The winners were selected based on the most accurate and appropriate documentation of their assessments determined through retrospective chart review.
Targeted e-mail feedback was also sent to providers who ordered IV antihypertensives without the appropriate indication. The e-mails included the medical record number, date and time of the order, any alternate etiologies that were documented, and any adverse events that occurred as a result of the medication.
Systems Change: Electronic Medical Record Orders
EMR advisory warnings were placed on IV antihypertensive orders of labetalol and hydralazine. The alerts served to nonintrusively remind providers to assess for symptoms before placing the order to ensure that the order was appropriate.
Data Collection and Assessment
Seven-month preintervention (January-July 2016) and 12-month postintervention (March 2017-February 2018) data were compared. The months prior to intervention were excluded to account for project development and educational lag. Data were obtained from EMR utilization reports of one-time orders of IV labetalol and hydralazine, and retrospective chart review. Patients who were pregnant, less than 18 years of age, or postoperative were excluded. Orders were designated as inappropriate if there was no evidence of hypertensive emergency through documentation in progress notes, or if the patient was able to take oral medication (not NPO). Adverse events were defined as a blood pressure drop of more than 25%, a change in the heart rate by more than 20 beats per minute, or the need for IV fluids, based on previous studies.7 Although decreased blood pressure is not necessarily dangerous in and of itself, adverse events arising from blood pressure decreasing too rapidly from IV antihypertensives are well documented.9,11 The presence of alternate etiologies of high blood pressure that were documented in progress notes, including pain, anxiety, agitation, and holding of home blood pressure medications, were recorded. The numbers of inappropriate orders pre- and postintervention were compared. Confounding factors of patient age and length of stay (LOS) were compared pre- and postintervention in order to rule out other factors to which the intervention’s effect could be attributed.
For this study, orders were reported on the standardized form of orders per 1,000 patient days. This was calculated as the number of orders divided by the total number of patient days from the two medicine units. For the univariate analysis, pre- and postintervention orders were compared for the different order categories using a t-test. Results were considered statistically significant at P < .05. Data analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, North Carolina).
Additionally, a cost analysis was performed to estimate the hospital-wide annual cost of inappropriate orders. The analysis used the cost per dose12 and included nurse-time derived from the median salary of those on our units. The hospital-wide cost was extrapolated to estimate the potential annual savings for the institution.
RESULTS
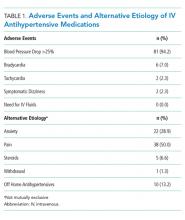
A total of 260 one-time orders of IV antihypertensives were analyzed in this study, 127 in the seven-month preintervention period and 133 in the 12-month postintervention period. The majority, 67.3% (n = 175), were labetalol orders. Inappropriate orders (ie, neither NPO nor hypertensive emergency) decreased from 8.3 to 3.3 orders per 1,000 patient days (P = .0099; Figure 3).
In total, there were 86 adverse events (33.1%), the majority of which (94.2%, n = 81) were a >25% decrease in blood pressure (Table 1). The number of adverse events per 1,000 patient days decreased from 4.4 in the preintervention period to 1.9 postintervention, P = .0112. Of the inappropriate orders, adverse events decreased from 3.7 to 0.8 per 1,000 patient days, P = .0072. Overall, there were 76 orders (29.2%) with documented alternate etiologies. The number of orders per 1,000 patient days with an alternate etiology decreased from 4.7 in the preintervention period to 1.2 postintervention, P =.0044 (Table 2). Descriptive analysis of patient characteristics pre-
Cost analysis estimated a $17,890 annual hospital-wide cost for unnecessary IV antihypertensive medications before the intervention. The estimate was calculated using the number of orders on the two medical units observed during the seven-month preintervention period, extrapolated to a 12-month period and to the total number of 15 medical units in the hospital. The intervention on the two studied medical units themselves led to an estimated $1,421 cost reduction (59.6%). Had the intervention been implemented hospital-wide with similar results, the resulting cost reduction would have amounted to $10,662.
DISCUSSION
Our initiative successfully demonstrated a significant reduction of 60% in inappropriate one-time orders of IV antihypertensives per 1,000 patient days. Accordingly, the number of adverse events per 1,000 patient days decreased by 57%. There was also a decrease in the number and percentage of IV orders with documented alternate etiologies. We hypothesize that this was due to nurses and physicians assessing and treating these conditions prior to treating hypertension in the intervention period, consequently avoiding an IV order.
The goal of the intervention was to have nurses assess for end-organ damage and alternate etiologies and include this information on their assessment provided to the physician, which would result in appropriate treatment of elevated blood pressure. By performing an interdisciplinary intervention, we addressed the knowledge deficit of both nurses and physicians, improved the triage of elevated blood pressure, and likely decreased the number of pages to providers.
To our knowledge, this is the first intervention addressing the inpatient overuse of IV antihypertensive medications for the treatment of asymptomatic hypertension. Additionally, this study bolsters prior evidence that the use of IV antihypertensives in asymptomatic patients leads to a large number of adverse events.7 A third of patients in the preintervention period had documented alternate etiologies of their blood pressure elevation, highlighting the need to assess and potentially treat these causes prior to treating blood pressure itself.
Reducing unnecessary treatment of asymptomatic blood pressure elevation is challenging. Evidence shows that both clinicians and patients overestimate the benefits and underestimate the harms of medical interventions.13,14 This unfortunately leads to unjustified enthusiasm for medical treatments, which can worsen outcomes.15 Additionally, there may be a lack of knowledge of the guidelines, as well as the amount of time required in the full assessment of hypertensive urgency, that creates a culture of “treating the number.”
Changing physician behavior is difficult.16 However, active forms of continuing education and multifaceted interventions, such as ours, are most effective.17 Our message focused on patient safety and harm reduction, addressed clinicians’ safety concerns, and included stories of real cases where this overuse led to adverse events—all of which are encouraged in order to facilitate clinician engagement.18
There were limitations to this study. Only blood pressure elevations associated with an IV antihypertensive order and not all blood pressure elevations meeting the criteria for hypertensive urgency in general were examined. Additionally, our documentation of symptoms of hypertensive emergency and alternate etiologies was based only on documentation in the medical record. Ideally, we would have liked to conduct an interrupted time series analysis to assess the effect of the intervention over time; however, there were not enough orders of IV antihypertensives to perform such an analysis.
CONCLUSION
Treatment of asymptomatic blood pressure with IV antihypertensive medications can lead to patient harm. To reduce inappropriate treatment, our Student High Value Care team set out to challenge this common practice. Our interdisciplinary intervention successfully reduced unnecessary IV antihypertensive treatment. This may serve as a model for other institutions.
Disclosures
There are no relevant conflicts of interest to disclose for any authors.
1. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065. PubMed
3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151. PubMed
4. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2011. 3.
5. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
7. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
9. Ipek E, Oktay AA, Krim SR. Hypertensive crisis: an update on clinical approach and management. Curr Opin Cardiol. 2017;32(4):397-406. doi: 10.1097/HCO.0000000000000398. PubMed
10. Cho HC, Dunn A, Di Capua J, Lee IT, Makhni S, Korenstein DR. Student high value care committee: a model for student-led implementation [abstract 286]. J Hosp Med. 2017. PubMed
11. Yang JY, Chiu S, Krouss M. Overtreatment of asymptomatic hypertension-urgency is not an emergency: a teachable moment. JAMA Intern Med. 2018;178(5):704-705. doi: 10.1001/jamainternmed.2018.0126. PubMed
12. Malesker MA, Hilleman DE. Intravenous labetalol compared with intravenous nicardipine in the management of hypertension in critically ill patients. J Crit Care. 2012;27(5):528 e527-514. doi: 10.1016/j.jcrc.2011.12.005. PubMed
13. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. doi: 10.1001/jamainternmed.2016.8254. PubMed
14. Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274-286. doi: 10.1001/jamainternmed.2014.6016. PubMed
15. Casarett D. The science of choosing wisely--overcoming the therapeutic illusion. N Engl J Med. 2016;374(13):1203-1205. doi: 10.1056/NEJMp1516803. PubMed
16. Wilensky G. Changing physician behavior is harder than we thought. JAMA. 2016;316(1):21-22. doi: 10.1001/jama.2016.8019. PubMed
17. Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015;21(1):75-84.
18. Pasik S, Korenstein D, Israilov S, Cho HJ. Engagement in eliminating overuse: the argument for safety and beyond. J Patient Saf. 2018. doi: 10.1097/PTS.0000000000000487. PubMed
With the presence of hypertension in 25% of patients admitted to the hospital,1 its proper management is imperative. A hypertensive crisis is a severe elevation of blood pressure, defined as systolic ≥180 mm Hg and/or diastolic ≥120 mm Hg. It is further classified as either a hypertensive emergency which includes the presence of end-organ damage,2 or hypertensive urgency, defined as asymptomatic blood pressure elevation.3 Although hypertensive emergencies account for only 1%-2% of patients with hypertension,4 they are associated with a high one-year mortality rate (>79%).5 Hypertensive emergency requires immediate reduction of blood pressure with IV antihypertensive drugs to limit organ damage. In contrast, as per national guidelines, inpatient management of hypertensive urgency requires gradual reductions of blood pressure over hours to days using oral antihypertensives.2 It is also recommended that alternative etiologies, such as anxiety or pain, be considered before treatment is initiated.1
Clinicians often inappropriately treat asymptomatic hypertension in the inpatient setting,6,7 using intravenous (IV) antihypertensive medications despite evidence showing potential harm.5,8 This can lead to unpredictable reductions in blood pressure.7,9 A recent retrospective analysis demonstrated that 32.6% of patients had a blood pressure reduction greater than 25% after the use of an IV antihypertensive.7 Reductions greater than 25% lead to shifts in autoregulation, which may result in patient harm, such as hypotension, decreased renal perfusion, and stroke.9 IV medications are also more expensive than oral agents, due to the additional cost of administration.
Although overtreatment of asymptomatic hypertension with IV antihypertensive medications is common,7 initiatives to address this in inpatient settings are lacking in the literature. The aim of this quality improvement initiative was to reduce unnecessary IV antihypertensive treatment for hypertensive urgency in the inpatient setting.
METHODS
Setting
An interdisciplinary quality improvement intervention was initiated on two inpatient medicine units at an urban, 1,134-bed tertiary medical center affiliated with the Icahn School of Medicine at Mount Sinai. Members of the Mount Sinai High Value Care Committee and the Student High Value Care Initiative10 developed this project. The intervention was implemented in stages from March 2017 to February 2018. It targeted nurses, housestaff, nurse practitioners, and attendings on general medical teaching and nonteaching services. The components of the intervention included education, a treatment algorithm, audit and feedback, and electronic medical record (EMR) change. This project was submitted to the Quality Committee in the Department of Medicine and determined to be a quality improvement project rather than research and thus, an IRB submission was not required.
Treatment Algorithm and Education
A clinical algorithm was designed with nursing and cardiology representatives to provide guidance for nurses regarding the best practice for evaluation of inpatient hypertension, focusing on assessing patients before recommending treatment (“Assess Before Rx”; Figure 1). Educational sessions reinforcing the clinical algorithm were held monthly at nursing huddles. These involved an introduction session providing the background and purpose of the project, with follow-up sessions including interactive mock cases on the assessment of hypertensive urgency.
A second treatment algorithm was designed, with housestaff and cardiology input, to provide guidance for the internal medicine housestaff and nurse practitioners. It utilized a similar approach regarding identification, evaluation, and assessment of alternate etiologies but included more detailed treatment recommendations with a table outlining the oral medications used for hypertensive urgency (Figure 2). The flowchart and table were uploaded to an existing mobile application used by housestaff and nurse practitioners for quick access. The mobile application is frequently used by housestaff and contains many clinical resources. Additionally, e-mails including the purpose of the project and the treatment algorithm were sent to rotating housestaff at the start of each new medicine rotation.
Audit and Feedback
Monthly feedback was e-mailed to the nurses, which reinforced the goals and provided positive feedback on outcomes with an announcement of the “Nurse of the Month.” The winners were selected based on the most accurate and appropriate documentation of their assessments determined through retrospective chart review.
Targeted e-mail feedback was also sent to providers who ordered IV antihypertensives without the appropriate indication. The e-mails included the medical record number, date and time of the order, any alternate etiologies that were documented, and any adverse events that occurred as a result of the medication.
Systems Change: Electronic Medical Record Orders
EMR advisory warnings were placed on IV antihypertensive orders of labetalol and hydralazine. The alerts served to nonintrusively remind providers to assess for symptoms before placing the order to ensure that the order was appropriate.
Data Collection and Assessment
Seven-month preintervention (January-July 2016) and 12-month postintervention (March 2017-February 2018) data were compared. The months prior to intervention were excluded to account for project development and educational lag. Data were obtained from EMR utilization reports of one-time orders of IV labetalol and hydralazine, and retrospective chart review. Patients who were pregnant, less than 18 years of age, or postoperative were excluded. Orders were designated as inappropriate if there was no evidence of hypertensive emergency through documentation in progress notes, or if the patient was able to take oral medication (not NPO). Adverse events were defined as a blood pressure drop of more than 25%, a change in the heart rate by more than 20 beats per minute, or the need for IV fluids, based on previous studies.7 Although decreased blood pressure is not necessarily dangerous in and of itself, adverse events arising from blood pressure decreasing too rapidly from IV antihypertensives are well documented.9,11 The presence of alternate etiologies of high blood pressure that were documented in progress notes, including pain, anxiety, agitation, and holding of home blood pressure medications, were recorded. The numbers of inappropriate orders pre- and postintervention were compared. Confounding factors of patient age and length of stay (LOS) were compared pre- and postintervention in order to rule out other factors to which the intervention’s effect could be attributed.
For this study, orders were reported on the standardized form of orders per 1,000 patient days. This was calculated as the number of orders divided by the total number of patient days from the two medicine units. For the univariate analysis, pre- and postintervention orders were compared for the different order categories using a t-test. Results were considered statistically significant at P < .05. Data analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, North Carolina).
Additionally, a cost analysis was performed to estimate the hospital-wide annual cost of inappropriate orders. The analysis used the cost per dose12 and included nurse-time derived from the median salary of those on our units. The hospital-wide cost was extrapolated to estimate the potential annual savings for the institution.
RESULTS
A total of 260 one-time orders of IV antihypertensives were analyzed in this study, 127 in the seven-month preintervention period and 133 in the 12-month postintervention period. The majority, 67.3% (n = 175), were labetalol orders. Inappropriate orders (ie, neither NPO nor hypertensive emergency) decreased from 8.3 to 3.3 orders per 1,000 patient days (P = .0099; Figure 3).
In total, there were 86 adverse events (33.1%), the majority of which (94.2%, n = 81) were a >25% decrease in blood pressure (Table 1). The number of adverse events per 1,000 patient days decreased from 4.4 in the preintervention period to 1.9 postintervention, P = .0112. Of the inappropriate orders, adverse events decreased from 3.7 to 0.8 per 1,000 patient days, P = .0072. Overall, there were 76 orders (29.2%) with documented alternate etiologies. The number of orders per 1,000 patient days with an alternate etiology decreased from 4.7 in the preintervention period to 1.2 postintervention, P =.0044 (Table 2). Descriptive analysis of patient characteristics pre-
Cost analysis estimated a $17,890 annual hospital-wide cost for unnecessary IV antihypertensive medications before the intervention. The estimate was calculated using the number of orders on the two medical units observed during the seven-month preintervention period, extrapolated to a 12-month period and to the total number of 15 medical units in the hospital. The intervention on the two studied medical units themselves led to an estimated $1,421 cost reduction (59.6%). Had the intervention been implemented hospital-wide with similar results, the resulting cost reduction would have amounted to $10,662.
DISCUSSION
Our initiative successfully demonstrated a significant reduction of 60% in inappropriate one-time orders of IV antihypertensives per 1,000 patient days. Accordingly, the number of adverse events per 1,000 patient days decreased by 57%. There was also a decrease in the number and percentage of IV orders with documented alternate etiologies. We hypothesize that this was due to nurses and physicians assessing and treating these conditions prior to treating hypertension in the intervention period, consequently avoiding an IV order.
The goal of the intervention was to have nurses assess for end-organ damage and alternate etiologies and include this information on their assessment provided to the physician, which would result in appropriate treatment of elevated blood pressure. By performing an interdisciplinary intervention, we addressed the knowledge deficit of both nurses and physicians, improved the triage of elevated blood pressure, and likely decreased the number of pages to providers.
To our knowledge, this is the first intervention addressing the inpatient overuse of IV antihypertensive medications for the treatment of asymptomatic hypertension. Additionally, this study bolsters prior evidence that the use of IV antihypertensives in asymptomatic patients leads to a large number of adverse events.7 A third of patients in the preintervention period had documented alternate etiologies of their blood pressure elevation, highlighting the need to assess and potentially treat these causes prior to treating blood pressure itself.
Reducing unnecessary treatment of asymptomatic blood pressure elevation is challenging. Evidence shows that both clinicians and patients overestimate the benefits and underestimate the harms of medical interventions.13,14 This unfortunately leads to unjustified enthusiasm for medical treatments, which can worsen outcomes.15 Additionally, there may be a lack of knowledge of the guidelines, as well as the amount of time required in the full assessment of hypertensive urgency, that creates a culture of “treating the number.”
Changing physician behavior is difficult.16 However, active forms of continuing education and multifaceted interventions, such as ours, are most effective.17 Our message focused on patient safety and harm reduction, addressed clinicians’ safety concerns, and included stories of real cases where this overuse led to adverse events—all of which are encouraged in order to facilitate clinician engagement.18
There were limitations to this study. Only blood pressure elevations associated with an IV antihypertensive order and not all blood pressure elevations meeting the criteria for hypertensive urgency in general were examined. Additionally, our documentation of symptoms of hypertensive emergency and alternate etiologies was based only on documentation in the medical record. Ideally, we would have liked to conduct an interrupted time series analysis to assess the effect of the intervention over time; however, there were not enough orders of IV antihypertensives to perform such an analysis.
CONCLUSION
Treatment of asymptomatic blood pressure with IV antihypertensive medications can lead to patient harm. To reduce inappropriate treatment, our Student High Value Care team set out to challenge this common practice. Our interdisciplinary intervention successfully reduced unnecessary IV antihypertensive treatment. This may serve as a model for other institutions.
Disclosures
There are no relevant conflicts of interest to disclose for any authors.
With the presence of hypertension in 25% of patients admitted to the hospital,1 its proper management is imperative. A hypertensive crisis is a severe elevation of blood pressure, defined as systolic ≥180 mm Hg and/or diastolic ≥120 mm Hg. It is further classified as either a hypertensive emergency which includes the presence of end-organ damage,2 or hypertensive urgency, defined as asymptomatic blood pressure elevation.3 Although hypertensive emergencies account for only 1%-2% of patients with hypertension,4 they are associated with a high one-year mortality rate (>79%).5 Hypertensive emergency requires immediate reduction of blood pressure with IV antihypertensive drugs to limit organ damage. In contrast, as per national guidelines, inpatient management of hypertensive urgency requires gradual reductions of blood pressure over hours to days using oral antihypertensives.2 It is also recommended that alternative etiologies, such as anxiety or pain, be considered before treatment is initiated.1
Clinicians often inappropriately treat asymptomatic hypertension in the inpatient setting,6,7 using intravenous (IV) antihypertensive medications despite evidence showing potential harm.5,8 This can lead to unpredictable reductions in blood pressure.7,9 A recent retrospective analysis demonstrated that 32.6% of patients had a blood pressure reduction greater than 25% after the use of an IV antihypertensive.7 Reductions greater than 25% lead to shifts in autoregulation, which may result in patient harm, such as hypotension, decreased renal perfusion, and stroke.9 IV medications are also more expensive than oral agents, due to the additional cost of administration.
Although overtreatment of asymptomatic hypertension with IV antihypertensive medications is common,7 initiatives to address this in inpatient settings are lacking in the literature. The aim of this quality improvement initiative was to reduce unnecessary IV antihypertensive treatment for hypertensive urgency in the inpatient setting.
METHODS
Setting
An interdisciplinary quality improvement intervention was initiated on two inpatient medicine units at an urban, 1,134-bed tertiary medical center affiliated with the Icahn School of Medicine at Mount Sinai. Members of the Mount Sinai High Value Care Committee and the Student High Value Care Initiative10 developed this project. The intervention was implemented in stages from March 2017 to February 2018. It targeted nurses, housestaff, nurse practitioners, and attendings on general medical teaching and nonteaching services. The components of the intervention included education, a treatment algorithm, audit and feedback, and electronic medical record (EMR) change. This project was submitted to the Quality Committee in the Department of Medicine and determined to be a quality improvement project rather than research and thus, an IRB submission was not required.
Treatment Algorithm and Education
A clinical algorithm was designed with nursing and cardiology representatives to provide guidance for nurses regarding the best practice for evaluation of inpatient hypertension, focusing on assessing patients before recommending treatment (“Assess Before Rx”; Figure 1). Educational sessions reinforcing the clinical algorithm were held monthly at nursing huddles. These involved an introduction session providing the background and purpose of the project, with follow-up sessions including interactive mock cases on the assessment of hypertensive urgency.
A second treatment algorithm was designed, with housestaff and cardiology input, to provide guidance for the internal medicine housestaff and nurse practitioners. It utilized a similar approach regarding identification, evaluation, and assessment of alternate etiologies but included more detailed treatment recommendations with a table outlining the oral medications used for hypertensive urgency (Figure 2). The flowchart and table were uploaded to an existing mobile application used by housestaff and nurse practitioners for quick access. The mobile application is frequently used by housestaff and contains many clinical resources. Additionally, e-mails including the purpose of the project and the treatment algorithm were sent to rotating housestaff at the start of each new medicine rotation.
Audit and Feedback
Monthly feedback was e-mailed to the nurses, which reinforced the goals and provided positive feedback on outcomes with an announcement of the “Nurse of the Month.” The winners were selected based on the most accurate and appropriate documentation of their assessments determined through retrospective chart review.
Targeted e-mail feedback was also sent to providers who ordered IV antihypertensives without the appropriate indication. The e-mails included the medical record number, date and time of the order, any alternate etiologies that were documented, and any adverse events that occurred as a result of the medication.
Systems Change: Electronic Medical Record Orders
EMR advisory warnings were placed on IV antihypertensive orders of labetalol and hydralazine. The alerts served to nonintrusively remind providers to assess for symptoms before placing the order to ensure that the order was appropriate.
Data Collection and Assessment
Seven-month preintervention (January-July 2016) and 12-month postintervention (March 2017-February 2018) data were compared. The months prior to intervention were excluded to account for project development and educational lag. Data were obtained from EMR utilization reports of one-time orders of IV labetalol and hydralazine, and retrospective chart review. Patients who were pregnant, less than 18 years of age, or postoperative were excluded. Orders were designated as inappropriate if there was no evidence of hypertensive emergency through documentation in progress notes, or if the patient was able to take oral medication (not NPO). Adverse events were defined as a blood pressure drop of more than 25%, a change in the heart rate by more than 20 beats per minute, or the need for IV fluids, based on previous studies.7 Although decreased blood pressure is not necessarily dangerous in and of itself, adverse events arising from blood pressure decreasing too rapidly from IV antihypertensives are well documented.9,11 The presence of alternate etiologies of high blood pressure that were documented in progress notes, including pain, anxiety, agitation, and holding of home blood pressure medications, were recorded. The numbers of inappropriate orders pre- and postintervention were compared. Confounding factors of patient age and length of stay (LOS) were compared pre- and postintervention in order to rule out other factors to which the intervention’s effect could be attributed.
For this study, orders were reported on the standardized form of orders per 1,000 patient days. This was calculated as the number of orders divided by the total number of patient days from the two medicine units. For the univariate analysis, pre- and postintervention orders were compared for the different order categories using a t-test. Results were considered statistically significant at P < .05. Data analysis was conducted using SAS v. 9.4 (SAS Institute, Cary, North Carolina).
Additionally, a cost analysis was performed to estimate the hospital-wide annual cost of inappropriate orders. The analysis used the cost per dose12 and included nurse-time derived from the median salary of those on our units. The hospital-wide cost was extrapolated to estimate the potential annual savings for the institution.
RESULTS
A total of 260 one-time orders of IV antihypertensives were analyzed in this study, 127 in the seven-month preintervention period and 133 in the 12-month postintervention period. The majority, 67.3% (n = 175), were labetalol orders. Inappropriate orders (ie, neither NPO nor hypertensive emergency) decreased from 8.3 to 3.3 orders per 1,000 patient days (P = .0099; Figure 3).
In total, there were 86 adverse events (33.1%), the majority of which (94.2%, n = 81) were a >25% decrease in blood pressure (Table 1). The number of adverse events per 1,000 patient days decreased from 4.4 in the preintervention period to 1.9 postintervention, P = .0112. Of the inappropriate orders, adverse events decreased from 3.7 to 0.8 per 1,000 patient days, P = .0072. Overall, there were 76 orders (29.2%) with documented alternate etiologies. The number of orders per 1,000 patient days with an alternate etiology decreased from 4.7 in the preintervention period to 1.2 postintervention, P =.0044 (Table 2). Descriptive analysis of patient characteristics pre-
Cost analysis estimated a $17,890 annual hospital-wide cost for unnecessary IV antihypertensive medications before the intervention. The estimate was calculated using the number of orders on the two medical units observed during the seven-month preintervention period, extrapolated to a 12-month period and to the total number of 15 medical units in the hospital. The intervention on the two studied medical units themselves led to an estimated $1,421 cost reduction (59.6%). Had the intervention been implemented hospital-wide with similar results, the resulting cost reduction would have amounted to $10,662.
DISCUSSION
Our initiative successfully demonstrated a significant reduction of 60% in inappropriate one-time orders of IV antihypertensives per 1,000 patient days. Accordingly, the number of adverse events per 1,000 patient days decreased by 57%. There was also a decrease in the number and percentage of IV orders with documented alternate etiologies. We hypothesize that this was due to nurses and physicians assessing and treating these conditions prior to treating hypertension in the intervention period, consequently avoiding an IV order.
The goal of the intervention was to have nurses assess for end-organ damage and alternate etiologies and include this information on their assessment provided to the physician, which would result in appropriate treatment of elevated blood pressure. By performing an interdisciplinary intervention, we addressed the knowledge deficit of both nurses and physicians, improved the triage of elevated blood pressure, and likely decreased the number of pages to providers.
To our knowledge, this is the first intervention addressing the inpatient overuse of IV antihypertensive medications for the treatment of asymptomatic hypertension. Additionally, this study bolsters prior evidence that the use of IV antihypertensives in asymptomatic patients leads to a large number of adverse events.7 A third of patients in the preintervention period had documented alternate etiologies of their blood pressure elevation, highlighting the need to assess and potentially treat these causes prior to treating blood pressure itself.
Reducing unnecessary treatment of asymptomatic blood pressure elevation is challenging. Evidence shows that both clinicians and patients overestimate the benefits and underestimate the harms of medical interventions.13,14 This unfortunately leads to unjustified enthusiasm for medical treatments, which can worsen outcomes.15 Additionally, there may be a lack of knowledge of the guidelines, as well as the amount of time required in the full assessment of hypertensive urgency, that creates a culture of “treating the number.”
Changing physician behavior is difficult.16 However, active forms of continuing education and multifaceted interventions, such as ours, are most effective.17 Our message focused on patient safety and harm reduction, addressed clinicians’ safety concerns, and included stories of real cases where this overuse led to adverse events—all of which are encouraged in order to facilitate clinician engagement.18
There were limitations to this study. Only blood pressure elevations associated with an IV antihypertensive order and not all blood pressure elevations meeting the criteria for hypertensive urgency in general were examined. Additionally, our documentation of symptoms of hypertensive emergency and alternate etiologies was based only on documentation in the medical record. Ideally, we would have liked to conduct an interrupted time series analysis to assess the effect of the intervention over time; however, there were not enough orders of IV antihypertensives to perform such an analysis.
CONCLUSION
Treatment of asymptomatic blood pressure with IV antihypertensive medications can lead to patient harm. To reduce inappropriate treatment, our Student High Value Care team set out to challenge this common practice. Our interdisciplinary intervention successfully reduced unnecessary IV antihypertensive treatment. This may serve as a model for other institutions.
Disclosures
There are no relevant conflicts of interest to disclose for any authors.
1. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065. PubMed
3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151. PubMed
4. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2011. 3.
5. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
7. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
9. Ipek E, Oktay AA, Krim SR. Hypertensive crisis: an update on clinical approach and management. Curr Opin Cardiol. 2017;32(4):397-406. doi: 10.1097/HCO.0000000000000398. PubMed
10. Cho HC, Dunn A, Di Capua J, Lee IT, Makhni S, Korenstein DR. Student high value care committee: a model for student-led implementation [abstract 286]. J Hosp Med. 2017. PubMed
11. Yang JY, Chiu S, Krouss M. Overtreatment of asymptomatic hypertension-urgency is not an emergency: a teachable moment. JAMA Intern Med. 2018;178(5):704-705. doi: 10.1001/jamainternmed.2018.0126. PubMed
12. Malesker MA, Hilleman DE. Intravenous labetalol compared with intravenous nicardipine in the management of hypertension in critically ill patients. J Crit Care. 2012;27(5):528 e527-514. doi: 10.1016/j.jcrc.2011.12.005. PubMed
13. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. doi: 10.1001/jamainternmed.2016.8254. PubMed
14. Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274-286. doi: 10.1001/jamainternmed.2014.6016. PubMed
15. Casarett D. The science of choosing wisely--overcoming the therapeutic illusion. N Engl J Med. 2016;374(13):1203-1205. doi: 10.1056/NEJMp1516803. PubMed
16. Wilensky G. Changing physician behavior is harder than we thought. JAMA. 2016;316(1):21-22. doi: 10.1001/jama.2016.8019. PubMed
17. Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015;21(1):75-84.
18. Pasik S, Korenstein D, Israilov S, Cho HJ. Engagement in eliminating overuse: the argument for safety and beyond. J Patient Saf. 2018. doi: 10.1097/PTS.0000000000000487. PubMed
1. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065. PubMed
3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151. PubMed
4. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization; 2011. 3.
5. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
7. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
9. Ipek E, Oktay AA, Krim SR. Hypertensive crisis: an update on clinical approach and management. Curr Opin Cardiol. 2017;32(4):397-406. doi: 10.1097/HCO.0000000000000398. PubMed
10. Cho HC, Dunn A, Di Capua J, Lee IT, Makhni S, Korenstein DR. Student high value care committee: a model for student-led implementation [abstract 286]. J Hosp Med. 2017. PubMed
11. Yang JY, Chiu S, Krouss M. Overtreatment of asymptomatic hypertension-urgency is not an emergency: a teachable moment. JAMA Intern Med. 2018;178(5):704-705. doi: 10.1001/jamainternmed.2018.0126. PubMed
12. Malesker MA, Hilleman DE. Intravenous labetalol compared with intravenous nicardipine in the management of hypertension in critically ill patients. J Crit Care. 2012;27(5):528 e527-514. doi: 10.1016/j.jcrc.2011.12.005. PubMed
13. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2017;177(3):407-419. doi: 10.1001/jamainternmed.2016.8254. PubMed
14. Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274-286. doi: 10.1001/jamainternmed.2014.6016. PubMed
15. Casarett D. The science of choosing wisely--overcoming the therapeutic illusion. N Engl J Med. 2016;374(13):1203-1205. doi: 10.1056/NEJMp1516803. PubMed
16. Wilensky G. Changing physician behavior is harder than we thought. JAMA. 2016;316(1):21-22. doi: 10.1001/jama.2016.8019. PubMed
17. Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015;21(1):75-84.
18. Pasik S, Korenstein D, Israilov S, Cho HJ. Engagement in eliminating overuse: the argument for safety and beyond. J Patient Saf. 2018. doi: 10.1097/PTS.0000000000000487. PubMed
© 2019 Society of Hospital Medicine
Reducing Unnecessary Treatment of Asymptomatic Elevated Blood Pressure with Intravenous Medications on the General Internal Medicine Wards: A Quality Improvement Initiative
Elevated blood pressure (BP) is common among hospitalized adults, with prevalence estimates between 50% and 70%.1 Many factors can cause or exacerbate BP elevations in the setting of acute illness, such as pain, anxiety, medication withdrawal, and volume status, among others.2 While there are clear evidence-based recommendations for treating hypertension (HTN) in the ambulatory setting,3 guidelines for the management of elevated BP in the hospital are lacking.4,5
Hypertensive crises are generally recognized as warranting rapid reduction in BP;6-8 however, these represent the minority of cases.9,10 Far more common in the hospital are patients with asymptomatic elevated BP, a population for which there is no high-quality evidence and no guidelines supporting the use of intravenous (IV) antihypertensives.11,12 Treatment with such medications has been associated with highly variable clinical responses13-15 and may result in adverse events, such as hypotension.10
To date, only a small number of studies have investigated the treatment of asymptomatic elevated BP among hospitalized adults.10,13-15 These have suggested that IV antihypertensives are utilized frequently in this setting, often for only modestly elevated BPs; however, the studies have tended to be small, not racially diverse, and limited to noncritically ill patients. Furthermore, while it is generally accepted that reducing the use of IV antihypertensives among asymptomatic patients would have no adverse impact, to our knowledge there have been no published studies which have instituted such an initiative while measuring balancing outcomes.
The purpose of this study was to further the existing literature by defining the prevalence and effects of IV antihypertensive medication utilization among a medically complex, multiracial population of asymptomatic medical inpatients using a large electronic dataset and to evaluate the impact of a division-wide, two-tiered quality improvement (QI) initiative on the rates of IV antihypertensive utilization and patient outcomes.
METHODS
Setting
The study was conducted at the University of California, San Francisco (UCSF), an 800-bed tertiary care, academic medical center. It was approved by the UCSF Institutional Review Board. General medicine patients at UCSF are distributed between teaching and direct-care (hospitalist) services. The intensive care unit (ICU) is “open,” meaning the medicine service acts as the primary team for all nonsurgical ICU patients. This study included all adult general medicine patients admitted to UCSF Medical Center between January 1, 2017 and March 1, 2018, including those in the ICU.
Study Population and Data Collection
The UCSF Medical Center uses the electronic health record (EHR) Epic (Epic 2017, Epic Systems Corporation, Verona, Wisconsin) for all clinical care. We obtained computerized EHR data from Clarity, the relational database that stores Epic’s inpatient data in thousands of tables, including orders, medications, laboratory and radiology results, vital signs, patient demographics, and notes. We identified all adult patients hospitalized on the general medicine service with ≥1 episode of elevated BP (>160/90 mm Hg) at any point during their hospitalization who were not on a vasopressor medication at the time of the vital sign recording.
We further identified all instances in which either IV labetalol or hydralazine were administered to these patients. These two agents were chosen because they are the only IV antihypertensives used commonly at our institution for the treatment of asymptomatic elevated BP among internal medicine patients. Only those orders placed by a general medicine provider or reconciled by a general medicine provider upon transfer from another service were included. For each medication administration timestamp, we collected vital signs before and after the administration, along with the ordering provider and the clinical indication that was documented in the electronic order. To determine if a medication was administered with concern for end-organ injury, we also extracted orders that could serve as a proxy for the provider’s clinical assessment—namely electrocardiograms, serum troponins, chest x-rays, and computerized tomography scans of the head—which were placed in the one hour preceding or 15 minutes following administration of an IV antihypertensive medication.
To assess for comorbid conditions, including a preexisting diagnosis of HTN, we collected International Classification of Diseases (ICD)-9/10 diagnosis codes. Further, we also extracted All Patient Refined Diagnosis-Related Group (APR-DRG) weights, which are a standardized measure of illness severity based on relative resource consumption during hospitalization.16,17
Patients were categorized as having either “symptomatic” or “asymptomatic” elevated BP. We defined symptomatic elevated BP as having received treatment with an IV medication with provider concern for end-organ injury, as defined above. We further identified all patients in which tight BP control may be clinically indicated on the basis of the presence of any of the following ICD-9/10 diagnosis codes at the time of hospital discharge: myocardial infarction, ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, subdural hematoma, aortic dissection, hypertensive emergency, or hypertensive encephalopathy. All patients with symptomatic elevated BP or any of the above ICD-9/10 diagnoses were excluded from the analysis, since administration of IV antihypertensive medications would plausibly be warranted in these clinical scenarios.
The encounter numbers from the dataset were used to link to patient demographic data, which included age, sex, race, ethnicity, primary language, and insurance status. Finally, we identified all instances of rapid response calls, ICU transfers, and code blues (cardiopulmonary arrests) for each patient in the dataset.
Blood Pressure Measurements
BP data were collected from invasive BP (IBP) monitoring devices and noninvasive BP cuffs. For patients with BP measurements recorded concomitantly from both IBP (ie, arterial lines) in addition to noninvasive BP cuffs, the arterial line reading was favored. All systolic BP (SBP) readings >240 mm Hg from arterial lines were excluded, as this has previously been described as the upper physiologic limit for IBP readings.18
Primary Outcome
The primary outcome for the study was the proportion of patients treated with IV antihypertensive medications (labetalol or hydralazine). Using aggregate data, we calculated the number of patients who were treated at least once with an IV antihypertensive in a given month (numerator), divided by the number of patients with ≥1 episode of asymptomatic elevated BP that month (denominator). The denominator was considered to be the population of patients “at risk” of being treated with IV antihypertensive medications. For patients with multiple admissions during the study period, each admission was considered separately. These results are displayed in the upper portion of the run chart (Figure).
Secondary Outcomes
To investigate blood pressure trends over time, we analyzed BP in three ways. First, we analyzed the median SBP for the entire population. Second, to determine clinical responses to IV antihypertensive medications among patients receiving treatment, we calculated the population medians for the pretreatment SBP, the change in SBP from pretreatment baseline, and the posttreatment SBP. Third, we calculated the average median SBP on a monthly basis for the duration of the study. This was achieved by calculating the median value of all SBPs for an individual patient, then averaging across all patients in a given month. The average monthly median SBPs are displayed in the lower portion of the Figure.
To investigate whether the intervention was associated with negative patient outcomes, the proportions of several balancing outcomes were compared between pre- and postintervention periods, including ICU transfers, rapid response calls, and code blues (cardiopulmonary arrests).
Development and Implementation of an Intervention to Reduce Excessive IV Antihypertensive Use
After establishing the baseline prevalence of IV antihypertensive medication use at our institution, we developed a QI initiative with the goal of reducing IV antihypertensive medication utilization by the general medicine service for the treatment of asymptomatic patients. We hypothesized that potential contributors to overutilization might include lack of education, provider/nursing discomfort, and a system designed to mandate provider notification for even modestly elevated BPs. The QI initiative, which took place between October 2017 and December 2017, was designed to address these potential contributors and was comprised of a division-wide, two-tiered, bundled intervention. Our choice of a two-tiered approach was based on the fact that successful culture change is challenging, along with the existing evidence that multifaceted QI interventions are more often successful than single-tiered approaches.19
The first tier of the initiative included an educational campaign referred to colloquially as “NoIVForHighBP,” which targeted residents, hospitalists, and nursing staff. The campaign consisted of a series of presentations, best practice updates, handouts, and posters displayed prominently in shared workspaces. The educational content focused on alternative approaches to the management of asymptomatic elevated BP in the hospital, such as identification and treatment of pain, anxiety, volume overload, or other contributing factors (see supplemental materials). These educational outreaches occurred periodically between October 4, 2017 and November 20, 2017, with the bulk of the educational efforts taking place during November. Therefore, November 1, 2017 was designated the start date for the intervention period.
The second tier of the intervention included the liberalization of the EHR BP notification parameters on the standard inpatient admission order set from >160/90 mm Hg to >180/90 mm Hg. This change took effect on 12/6/2017. The decision to modify the BP notification parameters was based on the hypothesis that mandatory notifications for modestly elevated BPs may prompt providers to reflexively order IV antihypertensive medications, especially during times of cross-coverage or high clinical workload.
Statistical Analysis
All statistical analyses were performed using Stata software version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: StataCorp LLC). Baseline patient characteristics were compared using nonparametric tests of significance. Population median SBPs were compared between pre- and postintervention periods using Mood’s Median Test, which was selected because the data were distributed nonnormally, and variances between samples were unequal.
Among patients treated with IV antihypertensive medications, we compared the proportion of pretreatment SBPs falling into each of three specified ranges (SBP <180 mm Hg, SBP 180-199 mm Hg, and SBP >200 mm Hg) between baseline and intervention periods using chi-squared tests.
Using aggregate data, we compared the unadjusted proportion of patients treated with IV antihypertensive medications between pre- and postintervention periods using a chi-squared test. Next, using patient-level data, a logistic regression analysis was performed to examine the association between receipt of IV antihypertensive medications and time (dichotomized between pre- and postintervention periods) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
Rates of balancing outcomes were compared using chi-squared tests. A logistic regression analysis using patient-level data was also performed to investigate the association between each of these outcomes and the intervention period (pre vs post) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
RESULTS
Baseline Period
We identified 2,306 patients with ≥1 episode of asymptomatic elevated BP during the 10-month preintervention period. Patients on average experienced 9 episodes of elevated BP per hospitalization, representing 21,207 potential opportunities for treatment. Baseline characteristics are summarized in Table 1. In general, this represents an older population that was medically complex and multiracial.
Of these patients, 251 (11%) received IV hydralazine and/or labetalol at least once during their hospitalization, with a total of 597 doses administered. Among those treated, a median of 2 doses were given per patient (IQR: 1-4), 64% of which were hydralazine. The majority (380 [64%]) were ordered on an “as needed” basis, while 217 (36%) were administered as a one-time dose. Three-quarters of all doses were ordered by the teaching service (456 [76%]), with the remaining 24% ordered by the direct-care (hospitalist) service.
During the baseline period among patients receiving IV antihypertensive medications, the median SBP of the population prior to treatment was 187 mm Hg (IQR 177-199; Table 2). Treatment was initiated in 30% of patients for an SBP <180 mm Hg and in 75% for an SBP <200 mm Hg. The median time to follow-up BP check was 34 minutes (IQR 15-58). The median decrement in SBP was 20 mm Hg (IQR 5-37); however, the response to treatment was highly variable, with 2% of patients experiencing no change and 14% experiencing an increase in SBP. Seventy-nine patients (14%) had a decrement in SBP >25% following treatment.
Description of Quality Improvement Results
Following the QI initiative, a total of 934 patients experienced 9,743 episodes of asymptomatic elevated blood pressure over a 4-month period (November 1, 2017 to February 28, 2018). As shown in Table 1, patients in the postintervention period had a slightly higher median age (67 [IQR 55-80] vs 69 [IQR 57-83]; P = .01), a higher median APR-DRG weight (1.34 [IQR 0.99-1.77] vs 1.48 [1.00-1.82]; P < .001), and a longer median length of stay (4.6 [2.8-8.0] days vs 5.1 [2.9-9.2] days; P = .004). There was also a higher proportion of nonEnglish speakers, fewer Black patients, and a lower proportion of preexisting HTN, in the postintervention period.
Of the 934 patients with ≥1 episode of asymptomatic elevated BP, 70 (7%) were treated with IV antihypertensive medications, with a total of 196 doses administered. The proportion of patients treated per month during the postintervention period ranged from 6% to 8%, which was the lowest of the entire study period and below the baseline average of 10% (Figure).
In a patient-level logistic regression pre-post analysis adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight, patients admitted to the general medicine service during the postintervention period had 38% lower odds of receiving IV antihypertensive medications than those admitted during the baseline period (OR = 0.62; 95% CI 0.47-0.83; P = .001). In this adjusted model, the following factors were independently associated with increased odds of receiving treatment: APR-DRG weight (OR 1.13; 95% CI 1.07-1.20; P < .001), Black race (OR 1.81; 95% CI 1.29-2.53; P = .001), length of stay (OR 1.02; 95% CI 1.01-1.03; P < .001), and preexisting HTN (OR 4.25; 95% CI 2.75-6.56; P < .001). Older age was associated with lower odds of treatment (Table 2).
Among patients who received treatment, there were no differences between pre- and postintervention periods in the proportion of pretreatment SBP <180 mm Hg (29% vs 32%; P = .40), 180-199 mm Hg (47% vs 40%; P = .10), or >200 mm Hg (25% vs 28%; P = .31; Table 3).
Population-level median SBP was similar between pre- and postintervention periods (167 mm Hg vs 168 mm Hg, P = .78), as were unadjusted rates of rapid response calls, ICU transfers, and code blues (Table 3). After adjustment for baseline characteristics and illness severity at the patient level, the odds of rapid response calls (OR 0.84; 95% CI 0.65-1.10; P = .21) and ICU transfers (OR 1.01; 95% CI 0.75-1.38; P = .93) did not differ between pre- and postintervention periods. A regression model was not fit for cardiopulmonary arrests due to the low absolute number of events.
CONCLUSIONS
Our results suggest that treatment of asymptomatic elevated BP using IV antihypertensive medications is common practice at our institution. We found that treatment is often initiated for only modestly elevated BPs and that the clinical response to these medications is highly variable. In the baseline period, one in seven patients experienced a decrement in BP >25% following treatment, which could potentially cause harm.11 There is no evidence, neither are there any consensus guidelines, to support the rapid reduction of BP among asymptomatic patients, making this a potential valuable opportunity for reducing unnecessary treatment, minimizing waste, and avoiding harm.
While there are a few previously published studies with similar results, we add to the existing literature by studying a larger population of more than 3,000 total patients, which was uniquely multiracial, including a high proportion of non-English speakers. Furthermore, our cohort included patients in the ICU, which is reflected in the higher-than-average APR-DRG weights. Despite being critically ill, these patients arguably still do not warrant aggressive treatment of elevated BP when asymptomatic. By excluding symptomatic BP elevations using surrogate markers for end-organ damage in addition to discharge diagnosis codes indicative of conditions in which tight BP control may be warranted, we were able to study a more critically ill patient population. We were also able to describe which baseline patient characteristics convey higher adjusted odds of receiving treatment, such as preexisting HTN, younger age, illness severity, and black race.
Perhaps most significantly, our study is the first to demonstrate an effective QI intervention aimed at reducing unnecessary utilization of IV antihypertensives. We found that this can feasibly be accomplished through a combination of educational efforts and systems changes, which could easily be replicated at other institutions. While the absolute reduction in the number of patients receiving treatment was modest, if these findings were to be widely accepted and resulted in a wide-spread change in culture, there would be a potential for greater impact.
Despite the reduction in the proportion of patients receiving IV antihypertensive medications, we found no change in the median SBP compared with the baseline period, which seems to support that the intervention was well tolerated. We also found no difference in the number of ICU transfers, rapid response calls, and cardiopulmonary arrests between groups. While these findings are both reassuring, it is impossible to draw definitive conclusions about safety given the small absolute number of patients having received treatment in each group. Fortunately, current guidelines and literature support the safety of such an intervention, as there is no existing evidence to suggest that failing to rapidly lower BP among asymptomatic patients is potentially harmful.11
There are several limitations to our study. First, by utilizing a large electronic dataset, the quality of our analyses was reliant on the accuracy of the recorded EHR data. Second, in the absence of a controlled trial or control group, we cannot say definitively that our QI initiative was the direct cause of the improved rates of IV antihypertensive utilization, though the effect did persist after adjusting for baseline characteristics in patient-level models. Third, our follow-up period was relatively short, with fewer than half as many patients as in the preintervention period. This is an important limitation, since the impact of QI interventions often diminishes over time. We plan to continually monitor IV antihypertensive use, feed those data back to our group, and revitalize educational efforts should rates begin to rise. Fourth, we were unable to directly measure which patients had true end-organ injury and instead used orders placed around the time of medication administration as a surrogate marker. While this is an imperfect measure, we feel that in cases where a provider was concerned enough to even test for end-organ injury, the use of IV antihypertensives was likely justified and was therefore appropriately excluded from the analysis. Lastly, we were limited in our ability to describe associations with true clinical outcomes, such as stroke or myocardial infarction, which could theoretically be propagated by either the use or the avoidance of IV antihypertensive medications. Fortunately, based on clinical guidelines and existing evidence, there is no reason to believe that reducing IV antihypertensive use would result in increased rates of these outcomes.
Our study reaffirms the fact that overutilization of IV antihypertensive medications among asymptomatic hospitalized patients is pervasive across hospital systems. This represents a potential target for a concerted change in culture, which we have demonstrated can be feasibly accomplished through education and systems changes.
Disclosures
Dr. Auerbach has current or pending grants from the CDC, PCORI, and FDA that are unrelated to this research manuscript. He also receives royalties from UpToDate, and received an honorarium for being editor of JHM. Dr. Jacobs received a $1,000 Resident/Fellow Travel Grant from the Society of Hospital Medicine to support the cost of travel to SHM, where he presented this research as a poster in 2018. Dr. Prasad receives money from EpiExcellence, LLC for consultation, which is unrelated to this research manuscript. All other authors have nothing to disclose.
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-422. doi: 10.1002/jhm.804. PubMed
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of ypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
4. Weder AB. Treating acute hypertension in the hospital: A lacuna in the guidelines [editorial]. Hypertension. 2011;57(1):18-20. PubMed
5. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
6. Marik PE, Rivera R. Hypertensive emergencies: an update. Curr Opin Crit Care. 2011;17(6):569-580. doi:10.1097/MCC.0b013e32834cd31d. PubMed
7. Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: a systematic review of the literature. J Gen Intern Med. 2002;17(12):937-945. doi: 10.1046/j.1525-1497.2002.20389.x. PubMed
8. Padilla Ramos A, Varon J. Current and newer agents for hypertensive emergencies. Curr Hypertens Rep. 2014;16(7):450. doi: 10.1007/s11906-014-0450-z. PubMed
9. Whitworth JA, World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992. doi: 10.1097/01.hjh.0000084751.37215.d2. PubMed
10. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j.jash.2011.07.002. PubMed
11. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
12. Gauer R. Severe asymptomatic hypertension: Evaluation and treatment. Am Fam Physician. 2017;95(8):492-500. PubMed
13. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control: Antihypertensive Therapy and BP Control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
14. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
15. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
16. Averill RF, Goldfield N, Hughes, JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. Clinical Research and Documentation Departments of 3M Health Information Systems, Wallingford, Connecticut and Murray, Utah, 2003. https://www.hcup-us.ahrq.gov/. Accessed February 19, 2018.
17. Iezzoni LI, Ash AS, Shwartz M, Daley J, Hughes JS, Mackiernan YD. Predicting who dies depends on how severity is measured: Implications for evaluating patient outcomes. Ann Intern Med. 1995;123(10):763-770. PubMed
18. Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18(6):644. doi: 10.1186/s13054-014-0644-4. PubMed
19. Shojania K, Grimshaw JM. Evidence-based quality improvement: The state of the science. Health Aff (Millwood). 2005;24(1):138-150. doi: 10.1377/hlthaff.24.1.138. PubMed
Elevated blood pressure (BP) is common among hospitalized adults, with prevalence estimates between 50% and 70%.1 Many factors can cause or exacerbate BP elevations in the setting of acute illness, such as pain, anxiety, medication withdrawal, and volume status, among others.2 While there are clear evidence-based recommendations for treating hypertension (HTN) in the ambulatory setting,3 guidelines for the management of elevated BP in the hospital are lacking.4,5
Hypertensive crises are generally recognized as warranting rapid reduction in BP;6-8 however, these represent the minority of cases.9,10 Far more common in the hospital are patients with asymptomatic elevated BP, a population for which there is no high-quality evidence and no guidelines supporting the use of intravenous (IV) antihypertensives.11,12 Treatment with such medications has been associated with highly variable clinical responses13-15 and may result in adverse events, such as hypotension.10
To date, only a small number of studies have investigated the treatment of asymptomatic elevated BP among hospitalized adults.10,13-15 These have suggested that IV antihypertensives are utilized frequently in this setting, often for only modestly elevated BPs; however, the studies have tended to be small, not racially diverse, and limited to noncritically ill patients. Furthermore, while it is generally accepted that reducing the use of IV antihypertensives among asymptomatic patients would have no adverse impact, to our knowledge there have been no published studies which have instituted such an initiative while measuring balancing outcomes.
The purpose of this study was to further the existing literature by defining the prevalence and effects of IV antihypertensive medication utilization among a medically complex, multiracial population of asymptomatic medical inpatients using a large electronic dataset and to evaluate the impact of a division-wide, two-tiered quality improvement (QI) initiative on the rates of IV antihypertensive utilization and patient outcomes.
METHODS
Setting
The study was conducted at the University of California, San Francisco (UCSF), an 800-bed tertiary care, academic medical center. It was approved by the UCSF Institutional Review Board. General medicine patients at UCSF are distributed between teaching and direct-care (hospitalist) services. The intensive care unit (ICU) is “open,” meaning the medicine service acts as the primary team for all nonsurgical ICU patients. This study included all adult general medicine patients admitted to UCSF Medical Center between January 1, 2017 and March 1, 2018, including those in the ICU.
Study Population and Data Collection
The UCSF Medical Center uses the electronic health record (EHR) Epic (Epic 2017, Epic Systems Corporation, Verona, Wisconsin) for all clinical care. We obtained computerized EHR data from Clarity, the relational database that stores Epic’s inpatient data in thousands of tables, including orders, medications, laboratory and radiology results, vital signs, patient demographics, and notes. We identified all adult patients hospitalized on the general medicine service with ≥1 episode of elevated BP (>160/90 mm Hg) at any point during their hospitalization who were not on a vasopressor medication at the time of the vital sign recording.
We further identified all instances in which either IV labetalol or hydralazine were administered to these patients. These two agents were chosen because they are the only IV antihypertensives used commonly at our institution for the treatment of asymptomatic elevated BP among internal medicine patients. Only those orders placed by a general medicine provider or reconciled by a general medicine provider upon transfer from another service were included. For each medication administration timestamp, we collected vital signs before and after the administration, along with the ordering provider and the clinical indication that was documented in the electronic order. To determine if a medication was administered with concern for end-organ injury, we also extracted orders that could serve as a proxy for the provider’s clinical assessment—namely electrocardiograms, serum troponins, chest x-rays, and computerized tomography scans of the head—which were placed in the one hour preceding or 15 minutes following administration of an IV antihypertensive medication.
To assess for comorbid conditions, including a preexisting diagnosis of HTN, we collected International Classification of Diseases (ICD)-9/10 diagnosis codes. Further, we also extracted All Patient Refined Diagnosis-Related Group (APR-DRG) weights, which are a standardized measure of illness severity based on relative resource consumption during hospitalization.16,17
Patients were categorized as having either “symptomatic” or “asymptomatic” elevated BP. We defined symptomatic elevated BP as having received treatment with an IV medication with provider concern for end-organ injury, as defined above. We further identified all patients in which tight BP control may be clinically indicated on the basis of the presence of any of the following ICD-9/10 diagnosis codes at the time of hospital discharge: myocardial infarction, ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, subdural hematoma, aortic dissection, hypertensive emergency, or hypertensive encephalopathy. All patients with symptomatic elevated BP or any of the above ICD-9/10 diagnoses were excluded from the analysis, since administration of IV antihypertensive medications would plausibly be warranted in these clinical scenarios.
The encounter numbers from the dataset were used to link to patient demographic data, which included age, sex, race, ethnicity, primary language, and insurance status. Finally, we identified all instances of rapid response calls, ICU transfers, and code blues (cardiopulmonary arrests) for each patient in the dataset.
Blood Pressure Measurements
BP data were collected from invasive BP (IBP) monitoring devices and noninvasive BP cuffs. For patients with BP measurements recorded concomitantly from both IBP (ie, arterial lines) in addition to noninvasive BP cuffs, the arterial line reading was favored. All systolic BP (SBP) readings >240 mm Hg from arterial lines were excluded, as this has previously been described as the upper physiologic limit for IBP readings.18
Primary Outcome
The primary outcome for the study was the proportion of patients treated with IV antihypertensive medications (labetalol or hydralazine). Using aggregate data, we calculated the number of patients who were treated at least once with an IV antihypertensive in a given month (numerator), divided by the number of patients with ≥1 episode of asymptomatic elevated BP that month (denominator). The denominator was considered to be the population of patients “at risk” of being treated with IV antihypertensive medications. For patients with multiple admissions during the study period, each admission was considered separately. These results are displayed in the upper portion of the run chart (Figure).
Secondary Outcomes
To investigate blood pressure trends over time, we analyzed BP in three ways. First, we analyzed the median SBP for the entire population. Second, to determine clinical responses to IV antihypertensive medications among patients receiving treatment, we calculated the population medians for the pretreatment SBP, the change in SBP from pretreatment baseline, and the posttreatment SBP. Third, we calculated the average median SBP on a monthly basis for the duration of the study. This was achieved by calculating the median value of all SBPs for an individual patient, then averaging across all patients in a given month. The average monthly median SBPs are displayed in the lower portion of the Figure.
To investigate whether the intervention was associated with negative patient outcomes, the proportions of several balancing outcomes were compared between pre- and postintervention periods, including ICU transfers, rapid response calls, and code blues (cardiopulmonary arrests).
Development and Implementation of an Intervention to Reduce Excessive IV Antihypertensive Use
After establishing the baseline prevalence of IV antihypertensive medication use at our institution, we developed a QI initiative with the goal of reducing IV antihypertensive medication utilization by the general medicine service for the treatment of asymptomatic patients. We hypothesized that potential contributors to overutilization might include lack of education, provider/nursing discomfort, and a system designed to mandate provider notification for even modestly elevated BPs. The QI initiative, which took place between October 2017 and December 2017, was designed to address these potential contributors and was comprised of a division-wide, two-tiered, bundled intervention. Our choice of a two-tiered approach was based on the fact that successful culture change is challenging, along with the existing evidence that multifaceted QI interventions are more often successful than single-tiered approaches.19
The first tier of the initiative included an educational campaign referred to colloquially as “NoIVForHighBP,” which targeted residents, hospitalists, and nursing staff. The campaign consisted of a series of presentations, best practice updates, handouts, and posters displayed prominently in shared workspaces. The educational content focused on alternative approaches to the management of asymptomatic elevated BP in the hospital, such as identification and treatment of pain, anxiety, volume overload, or other contributing factors (see supplemental materials). These educational outreaches occurred periodically between October 4, 2017 and November 20, 2017, with the bulk of the educational efforts taking place during November. Therefore, November 1, 2017 was designated the start date for the intervention period.
The second tier of the intervention included the liberalization of the EHR BP notification parameters on the standard inpatient admission order set from >160/90 mm Hg to >180/90 mm Hg. This change took effect on 12/6/2017. The decision to modify the BP notification parameters was based on the hypothesis that mandatory notifications for modestly elevated BPs may prompt providers to reflexively order IV antihypertensive medications, especially during times of cross-coverage or high clinical workload.
Statistical Analysis
All statistical analyses were performed using Stata software version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: StataCorp LLC). Baseline patient characteristics were compared using nonparametric tests of significance. Population median SBPs were compared between pre- and postintervention periods using Mood’s Median Test, which was selected because the data were distributed nonnormally, and variances between samples were unequal.
Among patients treated with IV antihypertensive medications, we compared the proportion of pretreatment SBPs falling into each of three specified ranges (SBP <180 mm Hg, SBP 180-199 mm Hg, and SBP >200 mm Hg) between baseline and intervention periods using chi-squared tests.
Using aggregate data, we compared the unadjusted proportion of patients treated with IV antihypertensive medications between pre- and postintervention periods using a chi-squared test. Next, using patient-level data, a logistic regression analysis was performed to examine the association between receipt of IV antihypertensive medications and time (dichotomized between pre- and postintervention periods) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
Rates of balancing outcomes were compared using chi-squared tests. A logistic regression analysis using patient-level data was also performed to investigate the association between each of these outcomes and the intervention period (pre vs post) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
RESULTS
Baseline Period
We identified 2,306 patients with ≥1 episode of asymptomatic elevated BP during the 10-month preintervention period. Patients on average experienced 9 episodes of elevated BP per hospitalization, representing 21,207 potential opportunities for treatment. Baseline characteristics are summarized in Table 1. In general, this represents an older population that was medically complex and multiracial.
Of these patients, 251 (11%) received IV hydralazine and/or labetalol at least once during their hospitalization, with a total of 597 doses administered. Among those treated, a median of 2 doses were given per patient (IQR: 1-4), 64% of which were hydralazine. The majority (380 [64%]) were ordered on an “as needed” basis, while 217 (36%) were administered as a one-time dose. Three-quarters of all doses were ordered by the teaching service (456 [76%]), with the remaining 24% ordered by the direct-care (hospitalist) service.
During the baseline period among patients receiving IV antihypertensive medications, the median SBP of the population prior to treatment was 187 mm Hg (IQR 177-199; Table 2). Treatment was initiated in 30% of patients for an SBP <180 mm Hg and in 75% for an SBP <200 mm Hg. The median time to follow-up BP check was 34 minutes (IQR 15-58). The median decrement in SBP was 20 mm Hg (IQR 5-37); however, the response to treatment was highly variable, with 2% of patients experiencing no change and 14% experiencing an increase in SBP. Seventy-nine patients (14%) had a decrement in SBP >25% following treatment.
Description of Quality Improvement Results
Following the QI initiative, a total of 934 patients experienced 9,743 episodes of asymptomatic elevated blood pressure over a 4-month period (November 1, 2017 to February 28, 2018). As shown in Table 1, patients in the postintervention period had a slightly higher median age (67 [IQR 55-80] vs 69 [IQR 57-83]; P = .01), a higher median APR-DRG weight (1.34 [IQR 0.99-1.77] vs 1.48 [1.00-1.82]; P < .001), and a longer median length of stay (4.6 [2.8-8.0] days vs 5.1 [2.9-9.2] days; P = .004). There was also a higher proportion of nonEnglish speakers, fewer Black patients, and a lower proportion of preexisting HTN, in the postintervention period.
Of the 934 patients with ≥1 episode of asymptomatic elevated BP, 70 (7%) were treated with IV antihypertensive medications, with a total of 196 doses administered. The proportion of patients treated per month during the postintervention period ranged from 6% to 8%, which was the lowest of the entire study period and below the baseline average of 10% (Figure).
In a patient-level logistic regression pre-post analysis adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight, patients admitted to the general medicine service during the postintervention period had 38% lower odds of receiving IV antihypertensive medications than those admitted during the baseline period (OR = 0.62; 95% CI 0.47-0.83; P = .001). In this adjusted model, the following factors were independently associated with increased odds of receiving treatment: APR-DRG weight (OR 1.13; 95% CI 1.07-1.20; P < .001), Black race (OR 1.81; 95% CI 1.29-2.53; P = .001), length of stay (OR 1.02; 95% CI 1.01-1.03; P < .001), and preexisting HTN (OR 4.25; 95% CI 2.75-6.56; P < .001). Older age was associated with lower odds of treatment (Table 2).
Among patients who received treatment, there were no differences between pre- and postintervention periods in the proportion of pretreatment SBP <180 mm Hg (29% vs 32%; P = .40), 180-199 mm Hg (47% vs 40%; P = .10), or >200 mm Hg (25% vs 28%; P = .31; Table 3).
Population-level median SBP was similar between pre- and postintervention periods (167 mm Hg vs 168 mm Hg, P = .78), as were unadjusted rates of rapid response calls, ICU transfers, and code blues (Table 3). After adjustment for baseline characteristics and illness severity at the patient level, the odds of rapid response calls (OR 0.84; 95% CI 0.65-1.10; P = .21) and ICU transfers (OR 1.01; 95% CI 0.75-1.38; P = .93) did not differ between pre- and postintervention periods. A regression model was not fit for cardiopulmonary arrests due to the low absolute number of events.
CONCLUSIONS
Our results suggest that treatment of asymptomatic elevated BP using IV antihypertensive medications is common practice at our institution. We found that treatment is often initiated for only modestly elevated BPs and that the clinical response to these medications is highly variable. In the baseline period, one in seven patients experienced a decrement in BP >25% following treatment, which could potentially cause harm.11 There is no evidence, neither are there any consensus guidelines, to support the rapid reduction of BP among asymptomatic patients, making this a potential valuable opportunity for reducing unnecessary treatment, minimizing waste, and avoiding harm.
While there are a few previously published studies with similar results, we add to the existing literature by studying a larger population of more than 3,000 total patients, which was uniquely multiracial, including a high proportion of non-English speakers. Furthermore, our cohort included patients in the ICU, which is reflected in the higher-than-average APR-DRG weights. Despite being critically ill, these patients arguably still do not warrant aggressive treatment of elevated BP when asymptomatic. By excluding symptomatic BP elevations using surrogate markers for end-organ damage in addition to discharge diagnosis codes indicative of conditions in which tight BP control may be warranted, we were able to study a more critically ill patient population. We were also able to describe which baseline patient characteristics convey higher adjusted odds of receiving treatment, such as preexisting HTN, younger age, illness severity, and black race.
Perhaps most significantly, our study is the first to demonstrate an effective QI intervention aimed at reducing unnecessary utilization of IV antihypertensives. We found that this can feasibly be accomplished through a combination of educational efforts and systems changes, which could easily be replicated at other institutions. While the absolute reduction in the number of patients receiving treatment was modest, if these findings were to be widely accepted and resulted in a wide-spread change in culture, there would be a potential for greater impact.
Despite the reduction in the proportion of patients receiving IV antihypertensive medications, we found no change in the median SBP compared with the baseline period, which seems to support that the intervention was well tolerated. We also found no difference in the number of ICU transfers, rapid response calls, and cardiopulmonary arrests between groups. While these findings are both reassuring, it is impossible to draw definitive conclusions about safety given the small absolute number of patients having received treatment in each group. Fortunately, current guidelines and literature support the safety of such an intervention, as there is no existing evidence to suggest that failing to rapidly lower BP among asymptomatic patients is potentially harmful.11
There are several limitations to our study. First, by utilizing a large electronic dataset, the quality of our analyses was reliant on the accuracy of the recorded EHR data. Second, in the absence of a controlled trial or control group, we cannot say definitively that our QI initiative was the direct cause of the improved rates of IV antihypertensive utilization, though the effect did persist after adjusting for baseline characteristics in patient-level models. Third, our follow-up period was relatively short, with fewer than half as many patients as in the preintervention period. This is an important limitation, since the impact of QI interventions often diminishes over time. We plan to continually monitor IV antihypertensive use, feed those data back to our group, and revitalize educational efforts should rates begin to rise. Fourth, we were unable to directly measure which patients had true end-organ injury and instead used orders placed around the time of medication administration as a surrogate marker. While this is an imperfect measure, we feel that in cases where a provider was concerned enough to even test for end-organ injury, the use of IV antihypertensives was likely justified and was therefore appropriately excluded from the analysis. Lastly, we were limited in our ability to describe associations with true clinical outcomes, such as stroke or myocardial infarction, which could theoretically be propagated by either the use or the avoidance of IV antihypertensive medications. Fortunately, based on clinical guidelines and existing evidence, there is no reason to believe that reducing IV antihypertensive use would result in increased rates of these outcomes.
Our study reaffirms the fact that overutilization of IV antihypertensive medications among asymptomatic hospitalized patients is pervasive across hospital systems. This represents a potential target for a concerted change in culture, which we have demonstrated can be feasibly accomplished through education and systems changes.
Disclosures
Dr. Auerbach has current or pending grants from the CDC, PCORI, and FDA that are unrelated to this research manuscript. He also receives royalties from UpToDate, and received an honorarium for being editor of JHM. Dr. Jacobs received a $1,000 Resident/Fellow Travel Grant from the Society of Hospital Medicine to support the cost of travel to SHM, where he presented this research as a poster in 2018. Dr. Prasad receives money from EpiExcellence, LLC for consultation, which is unrelated to this research manuscript. All other authors have nothing to disclose.
Elevated blood pressure (BP) is common among hospitalized adults, with prevalence estimates between 50% and 70%.1 Many factors can cause or exacerbate BP elevations in the setting of acute illness, such as pain, anxiety, medication withdrawal, and volume status, among others.2 While there are clear evidence-based recommendations for treating hypertension (HTN) in the ambulatory setting,3 guidelines for the management of elevated BP in the hospital are lacking.4,5
Hypertensive crises are generally recognized as warranting rapid reduction in BP;6-8 however, these represent the minority of cases.9,10 Far more common in the hospital are patients with asymptomatic elevated BP, a population for which there is no high-quality evidence and no guidelines supporting the use of intravenous (IV) antihypertensives.11,12 Treatment with such medications has been associated with highly variable clinical responses13-15 and may result in adverse events, such as hypotension.10
To date, only a small number of studies have investigated the treatment of asymptomatic elevated BP among hospitalized adults.10,13-15 These have suggested that IV antihypertensives are utilized frequently in this setting, often for only modestly elevated BPs; however, the studies have tended to be small, not racially diverse, and limited to noncritically ill patients. Furthermore, while it is generally accepted that reducing the use of IV antihypertensives among asymptomatic patients would have no adverse impact, to our knowledge there have been no published studies which have instituted such an initiative while measuring balancing outcomes.
The purpose of this study was to further the existing literature by defining the prevalence and effects of IV antihypertensive medication utilization among a medically complex, multiracial population of asymptomatic medical inpatients using a large electronic dataset and to evaluate the impact of a division-wide, two-tiered quality improvement (QI) initiative on the rates of IV antihypertensive utilization and patient outcomes.
METHODS
Setting
The study was conducted at the University of California, San Francisco (UCSF), an 800-bed tertiary care, academic medical center. It was approved by the UCSF Institutional Review Board. General medicine patients at UCSF are distributed between teaching and direct-care (hospitalist) services. The intensive care unit (ICU) is “open,” meaning the medicine service acts as the primary team for all nonsurgical ICU patients. This study included all adult general medicine patients admitted to UCSF Medical Center between January 1, 2017 and March 1, 2018, including those in the ICU.
Study Population and Data Collection
The UCSF Medical Center uses the electronic health record (EHR) Epic (Epic 2017, Epic Systems Corporation, Verona, Wisconsin) for all clinical care. We obtained computerized EHR data from Clarity, the relational database that stores Epic’s inpatient data in thousands of tables, including orders, medications, laboratory and radiology results, vital signs, patient demographics, and notes. We identified all adult patients hospitalized on the general medicine service with ≥1 episode of elevated BP (>160/90 mm Hg) at any point during their hospitalization who were not on a vasopressor medication at the time of the vital sign recording.
We further identified all instances in which either IV labetalol or hydralazine were administered to these patients. These two agents were chosen because they are the only IV antihypertensives used commonly at our institution for the treatment of asymptomatic elevated BP among internal medicine patients. Only those orders placed by a general medicine provider or reconciled by a general medicine provider upon transfer from another service were included. For each medication administration timestamp, we collected vital signs before and after the administration, along with the ordering provider and the clinical indication that was documented in the electronic order. To determine if a medication was administered with concern for end-organ injury, we also extracted orders that could serve as a proxy for the provider’s clinical assessment—namely electrocardiograms, serum troponins, chest x-rays, and computerized tomography scans of the head—which were placed in the one hour preceding or 15 minutes following administration of an IV antihypertensive medication.
To assess for comorbid conditions, including a preexisting diagnosis of HTN, we collected International Classification of Diseases (ICD)-9/10 diagnosis codes. Further, we also extracted All Patient Refined Diagnosis-Related Group (APR-DRG) weights, which are a standardized measure of illness severity based on relative resource consumption during hospitalization.16,17
Patients were categorized as having either “symptomatic” or “asymptomatic” elevated BP. We defined symptomatic elevated BP as having received treatment with an IV medication with provider concern for end-organ injury, as defined above. We further identified all patients in which tight BP control may be clinically indicated on the basis of the presence of any of the following ICD-9/10 diagnosis codes at the time of hospital discharge: myocardial infarction, ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, subdural hematoma, aortic dissection, hypertensive emergency, or hypertensive encephalopathy. All patients with symptomatic elevated BP or any of the above ICD-9/10 diagnoses were excluded from the analysis, since administration of IV antihypertensive medications would plausibly be warranted in these clinical scenarios.
The encounter numbers from the dataset were used to link to patient demographic data, which included age, sex, race, ethnicity, primary language, and insurance status. Finally, we identified all instances of rapid response calls, ICU transfers, and code blues (cardiopulmonary arrests) for each patient in the dataset.
Blood Pressure Measurements
BP data were collected from invasive BP (IBP) monitoring devices and noninvasive BP cuffs. For patients with BP measurements recorded concomitantly from both IBP (ie, arterial lines) in addition to noninvasive BP cuffs, the arterial line reading was favored. All systolic BP (SBP) readings >240 mm Hg from arterial lines were excluded, as this has previously been described as the upper physiologic limit for IBP readings.18
Primary Outcome
The primary outcome for the study was the proportion of patients treated with IV antihypertensive medications (labetalol or hydralazine). Using aggregate data, we calculated the number of patients who were treated at least once with an IV antihypertensive in a given month (numerator), divided by the number of patients with ≥1 episode of asymptomatic elevated BP that month (denominator). The denominator was considered to be the population of patients “at risk” of being treated with IV antihypertensive medications. For patients with multiple admissions during the study period, each admission was considered separately. These results are displayed in the upper portion of the run chart (Figure).
Secondary Outcomes
To investigate blood pressure trends over time, we analyzed BP in three ways. First, we analyzed the median SBP for the entire population. Second, to determine clinical responses to IV antihypertensive medications among patients receiving treatment, we calculated the population medians for the pretreatment SBP, the change in SBP from pretreatment baseline, and the posttreatment SBP. Third, we calculated the average median SBP on a monthly basis for the duration of the study. This was achieved by calculating the median value of all SBPs for an individual patient, then averaging across all patients in a given month. The average monthly median SBPs are displayed in the lower portion of the Figure.
To investigate whether the intervention was associated with negative patient outcomes, the proportions of several balancing outcomes were compared between pre- and postintervention periods, including ICU transfers, rapid response calls, and code blues (cardiopulmonary arrests).
Development and Implementation of an Intervention to Reduce Excessive IV Antihypertensive Use
After establishing the baseline prevalence of IV antihypertensive medication use at our institution, we developed a QI initiative with the goal of reducing IV antihypertensive medication utilization by the general medicine service for the treatment of asymptomatic patients. We hypothesized that potential contributors to overutilization might include lack of education, provider/nursing discomfort, and a system designed to mandate provider notification for even modestly elevated BPs. The QI initiative, which took place between October 2017 and December 2017, was designed to address these potential contributors and was comprised of a division-wide, two-tiered, bundled intervention. Our choice of a two-tiered approach was based on the fact that successful culture change is challenging, along with the existing evidence that multifaceted QI interventions are more often successful than single-tiered approaches.19
The first tier of the initiative included an educational campaign referred to colloquially as “NoIVForHighBP,” which targeted residents, hospitalists, and nursing staff. The campaign consisted of a series of presentations, best practice updates, handouts, and posters displayed prominently in shared workspaces. The educational content focused on alternative approaches to the management of asymptomatic elevated BP in the hospital, such as identification and treatment of pain, anxiety, volume overload, or other contributing factors (see supplemental materials). These educational outreaches occurred periodically between October 4, 2017 and November 20, 2017, with the bulk of the educational efforts taking place during November. Therefore, November 1, 2017 was designated the start date for the intervention period.
The second tier of the intervention included the liberalization of the EHR BP notification parameters on the standard inpatient admission order set from >160/90 mm Hg to >180/90 mm Hg. This change took effect on 12/6/2017. The decision to modify the BP notification parameters was based on the hypothesis that mandatory notifications for modestly elevated BPs may prompt providers to reflexively order IV antihypertensive medications, especially during times of cross-coverage or high clinical workload.
Statistical Analysis
All statistical analyses were performed using Stata software version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, Texas: StataCorp LLC). Baseline patient characteristics were compared using nonparametric tests of significance. Population median SBPs were compared between pre- and postintervention periods using Mood’s Median Test, which was selected because the data were distributed nonnormally, and variances between samples were unequal.
Among patients treated with IV antihypertensive medications, we compared the proportion of pretreatment SBPs falling into each of three specified ranges (SBP <180 mm Hg, SBP 180-199 mm Hg, and SBP >200 mm Hg) between baseline and intervention periods using chi-squared tests.
Using aggregate data, we compared the unadjusted proportion of patients treated with IV antihypertensive medications between pre- and postintervention periods using a chi-squared test. Next, using patient-level data, a logistic regression analysis was performed to examine the association between receipt of IV antihypertensive medications and time (dichotomized between pre- and postintervention periods) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
Rates of balancing outcomes were compared using chi-squared tests. A logistic regression analysis using patient-level data was also performed to investigate the association between each of these outcomes and the intervention period (pre vs post) while adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight.
RESULTS
Baseline Period
We identified 2,306 patients with ≥1 episode of asymptomatic elevated BP during the 10-month preintervention period. Patients on average experienced 9 episodes of elevated BP per hospitalization, representing 21,207 potential opportunities for treatment. Baseline characteristics are summarized in Table 1. In general, this represents an older population that was medically complex and multiracial.
Of these patients, 251 (11%) received IV hydralazine and/or labetalol at least once during their hospitalization, with a total of 597 doses administered. Among those treated, a median of 2 doses were given per patient (IQR: 1-4), 64% of which were hydralazine. The majority (380 [64%]) were ordered on an “as needed” basis, while 217 (36%) were administered as a one-time dose. Three-quarters of all doses were ordered by the teaching service (456 [76%]), with the remaining 24% ordered by the direct-care (hospitalist) service.
During the baseline period among patients receiving IV antihypertensive medications, the median SBP of the population prior to treatment was 187 mm Hg (IQR 177-199; Table 2). Treatment was initiated in 30% of patients for an SBP <180 mm Hg and in 75% for an SBP <200 mm Hg. The median time to follow-up BP check was 34 minutes (IQR 15-58). The median decrement in SBP was 20 mm Hg (IQR 5-37); however, the response to treatment was highly variable, with 2% of patients experiencing no change and 14% experiencing an increase in SBP. Seventy-nine patients (14%) had a decrement in SBP >25% following treatment.
Description of Quality Improvement Results
Following the QI initiative, a total of 934 patients experienced 9,743 episodes of asymptomatic elevated blood pressure over a 4-month period (November 1, 2017 to February 28, 2018). As shown in Table 1, patients in the postintervention period had a slightly higher median age (67 [IQR 55-80] vs 69 [IQR 57-83]; P = .01), a higher median APR-DRG weight (1.34 [IQR 0.99-1.77] vs 1.48 [1.00-1.82]; P < .001), and a longer median length of stay (4.6 [2.8-8.0] days vs 5.1 [2.9-9.2] days; P = .004). There was also a higher proportion of nonEnglish speakers, fewer Black patients, and a lower proportion of preexisting HTN, in the postintervention period.
Of the 934 patients with ≥1 episode of asymptomatic elevated BP, 70 (7%) were treated with IV antihypertensive medications, with a total of 196 doses administered. The proportion of patients treated per month during the postintervention period ranged from 6% to 8%, which was the lowest of the entire study period and below the baseline average of 10% (Figure).
In a patient-level logistic regression pre-post analysis adjusting for age, sex, race, ethnicity, primary language, insurance status, preexisting HTN, length of stay, and APR-DRG weight, patients admitted to the general medicine service during the postintervention period had 38% lower odds of receiving IV antihypertensive medications than those admitted during the baseline period (OR = 0.62; 95% CI 0.47-0.83; P = .001). In this adjusted model, the following factors were independently associated with increased odds of receiving treatment: APR-DRG weight (OR 1.13; 95% CI 1.07-1.20; P < .001), Black race (OR 1.81; 95% CI 1.29-2.53; P = .001), length of stay (OR 1.02; 95% CI 1.01-1.03; P < .001), and preexisting HTN (OR 4.25; 95% CI 2.75-6.56; P < .001). Older age was associated with lower odds of treatment (Table 2).
Among patients who received treatment, there were no differences between pre- and postintervention periods in the proportion of pretreatment SBP <180 mm Hg (29% vs 32%; P = .40), 180-199 mm Hg (47% vs 40%; P = .10), or >200 mm Hg (25% vs 28%; P = .31; Table 3).
Population-level median SBP was similar between pre- and postintervention periods (167 mm Hg vs 168 mm Hg, P = .78), as were unadjusted rates of rapid response calls, ICU transfers, and code blues (Table 3). After adjustment for baseline characteristics and illness severity at the patient level, the odds of rapid response calls (OR 0.84; 95% CI 0.65-1.10; P = .21) and ICU transfers (OR 1.01; 95% CI 0.75-1.38; P = .93) did not differ between pre- and postintervention periods. A regression model was not fit for cardiopulmonary arrests due to the low absolute number of events.
CONCLUSIONS
Our results suggest that treatment of asymptomatic elevated BP using IV antihypertensive medications is common practice at our institution. We found that treatment is often initiated for only modestly elevated BPs and that the clinical response to these medications is highly variable. In the baseline period, one in seven patients experienced a decrement in BP >25% following treatment, which could potentially cause harm.11 There is no evidence, neither are there any consensus guidelines, to support the rapid reduction of BP among asymptomatic patients, making this a potential valuable opportunity for reducing unnecessary treatment, minimizing waste, and avoiding harm.
While there are a few previously published studies with similar results, we add to the existing literature by studying a larger population of more than 3,000 total patients, which was uniquely multiracial, including a high proportion of non-English speakers. Furthermore, our cohort included patients in the ICU, which is reflected in the higher-than-average APR-DRG weights. Despite being critically ill, these patients arguably still do not warrant aggressive treatment of elevated BP when asymptomatic. By excluding symptomatic BP elevations using surrogate markers for end-organ damage in addition to discharge diagnosis codes indicative of conditions in which tight BP control may be warranted, we were able to study a more critically ill patient population. We were also able to describe which baseline patient characteristics convey higher adjusted odds of receiving treatment, such as preexisting HTN, younger age, illness severity, and black race.
Perhaps most significantly, our study is the first to demonstrate an effective QI intervention aimed at reducing unnecessary utilization of IV antihypertensives. We found that this can feasibly be accomplished through a combination of educational efforts and systems changes, which could easily be replicated at other institutions. While the absolute reduction in the number of patients receiving treatment was modest, if these findings were to be widely accepted and resulted in a wide-spread change in culture, there would be a potential for greater impact.
Despite the reduction in the proportion of patients receiving IV antihypertensive medications, we found no change in the median SBP compared with the baseline period, which seems to support that the intervention was well tolerated. We also found no difference in the number of ICU transfers, rapid response calls, and cardiopulmonary arrests between groups. While these findings are both reassuring, it is impossible to draw definitive conclusions about safety given the small absolute number of patients having received treatment in each group. Fortunately, current guidelines and literature support the safety of such an intervention, as there is no existing evidence to suggest that failing to rapidly lower BP among asymptomatic patients is potentially harmful.11
There are several limitations to our study. First, by utilizing a large electronic dataset, the quality of our analyses was reliant on the accuracy of the recorded EHR data. Second, in the absence of a controlled trial or control group, we cannot say definitively that our QI initiative was the direct cause of the improved rates of IV antihypertensive utilization, though the effect did persist after adjusting for baseline characteristics in patient-level models. Third, our follow-up period was relatively short, with fewer than half as many patients as in the preintervention period. This is an important limitation, since the impact of QI interventions often diminishes over time. We plan to continually monitor IV antihypertensive use, feed those data back to our group, and revitalize educational efforts should rates begin to rise. Fourth, we were unable to directly measure which patients had true end-organ injury and instead used orders placed around the time of medication administration as a surrogate marker. While this is an imperfect measure, we feel that in cases where a provider was concerned enough to even test for end-organ injury, the use of IV antihypertensives was likely justified and was therefore appropriately excluded from the analysis. Lastly, we were limited in our ability to describe associations with true clinical outcomes, such as stroke or myocardial infarction, which could theoretically be propagated by either the use or the avoidance of IV antihypertensive medications. Fortunately, based on clinical guidelines and existing evidence, there is no reason to believe that reducing IV antihypertensive use would result in increased rates of these outcomes.
Our study reaffirms the fact that overutilization of IV antihypertensive medications among asymptomatic hospitalized patients is pervasive across hospital systems. This represents a potential target for a concerted change in culture, which we have demonstrated can be feasibly accomplished through education and systems changes.
Disclosures
Dr. Auerbach has current or pending grants from the CDC, PCORI, and FDA that are unrelated to this research manuscript. He also receives royalties from UpToDate, and received an honorarium for being editor of JHM. Dr. Jacobs received a $1,000 Resident/Fellow Travel Grant from the Society of Hospital Medicine to support the cost of travel to SHM, where he presented this research as a poster in 2018. Dr. Prasad receives money from EpiExcellence, LLC for consultation, which is unrelated to this research manuscript. All other authors have nothing to disclose.
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-422. doi: 10.1002/jhm.804. PubMed
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of ypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
4. Weder AB. Treating acute hypertension in the hospital: A lacuna in the guidelines [editorial]. Hypertension. 2011;57(1):18-20. PubMed
5. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
6. Marik PE, Rivera R. Hypertensive emergencies: an update. Curr Opin Crit Care. 2011;17(6):569-580. doi:10.1097/MCC.0b013e32834cd31d. PubMed
7. Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: a systematic review of the literature. J Gen Intern Med. 2002;17(12):937-945. doi: 10.1046/j.1525-1497.2002.20389.x. PubMed
8. Padilla Ramos A, Varon J. Current and newer agents for hypertensive emergencies. Curr Hypertens Rep. 2014;16(7):450. doi: 10.1007/s11906-014-0450-z. PubMed
9. Whitworth JA, World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992. doi: 10.1097/01.hjh.0000084751.37215.d2. PubMed
10. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j.jash.2011.07.002. PubMed
11. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
12. Gauer R. Severe asymptomatic hypertension: Evaluation and treatment. Am Fam Physician. 2017;95(8):492-500. PubMed
13. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control: Antihypertensive Therapy and BP Control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
14. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
15. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
16. Averill RF, Goldfield N, Hughes, JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. Clinical Research and Documentation Departments of 3M Health Information Systems, Wallingford, Connecticut and Murray, Utah, 2003. https://www.hcup-us.ahrq.gov/. Accessed February 19, 2018.
17. Iezzoni LI, Ash AS, Shwartz M, Daley J, Hughes JS, Mackiernan YD. Predicting who dies depends on how severity is measured: Implications for evaluating patient outcomes. Ann Intern Med. 1995;123(10):763-770. PubMed
18. Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18(6):644. doi: 10.1186/s13054-014-0644-4. PubMed
19. Shojania K, Grimshaw JM. Evidence-based quality improvement: The state of the science. Health Aff (Millwood). 2005;24(1):138-150. doi: 10.1377/hlthaff.24.1.138. PubMed
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417-422. doi: 10.1002/jhm.804. PubMed
2. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of ypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
4. Weder AB. Treating acute hypertension in the hospital: A lacuna in the guidelines [editorial]. Hypertension. 2011;57(1):18-20. PubMed
5. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
6. Marik PE, Rivera R. Hypertensive emergencies: an update. Curr Opin Crit Care. 2011;17(6):569-580. doi:10.1097/MCC.0b013e32834cd31d. PubMed
7. Cherney D, Straus S. Management of patients with hypertensive urgencies and emergencies: a systematic review of the literature. J Gen Intern Med. 2002;17(12):937-945. doi: 10.1046/j.1525-1497.2002.20389.x. PubMed
8. Padilla Ramos A, Varon J. Current and newer agents for hypertensive emergencies. Curr Hypertens Rep. 2014;16(7):450. doi: 10.1007/s11906-014-0450-z. PubMed
9. Whitworth JA, World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983-1992. doi: 10.1097/01.hjh.0000084751.37215.d2. PubMed
10. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j.jash.2011.07.002. PubMed
11. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
12. Gauer R. Severe asymptomatic hypertension: Evaluation and treatment. Am Fam Physician. 2017;95(8):492-500. PubMed
13. Lipari M, Moser LR, Petrovitch EA, Farber M, Flack JM. As-needed intravenous antihypertensive therapy and blood pressure control: Antihypertensive Therapy and BP Control. J Hosp Med. 2016;11(3):193-198. doi: 10.1002/jhm.2510. PubMed
14. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
15. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: Use of intravenous labetalol and hydralazine. J Clin Hypertens. 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
16. Averill RF, Goldfield N, Hughes, JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. Clinical Research and Documentation Departments of 3M Health Information Systems, Wallingford, Connecticut and Murray, Utah, 2003. https://www.hcup-us.ahrq.gov/. Accessed February 19, 2018.
17. Iezzoni LI, Ash AS, Shwartz M, Daley J, Hughes JS, Mackiernan YD. Predicting who dies depends on how severity is measured: Implications for evaluating patient outcomes. Ann Intern Med. 1995;123(10):763-770. PubMed
18. Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18(6):644. doi: 10.1186/s13054-014-0644-4. PubMed
19. Shojania K, Grimshaw JM. Evidence-based quality improvement: The state of the science. Health Aff (Millwood). 2005;24(1):138-150. doi: 10.1377/hlthaff.24.1.138. PubMed
© 2019 Society of Hospital Medicine
Treatment of Inpatient Asymptomatic Hypertension: Not a Call to Act but to Think
Your pager beeps. Your patient, Mrs. Jones, who was admitted with cellulitis and is improving, now has a blood pressure of 188/103 on routine vitals. Her nurse reports that she is comfortable and asymptomatic, but she met the “call parameters.” You review her chart and find that since admission her systolic blood pressure (SBP) has ranged from 149 to 157 mm Hg and her diastolic blood pressure (DBP) from 84 to 96 mm Hg. Her nurse asks how you would like to treat her.
While over half of inpatients have at least one hypertensive episode during their stay, evidence suggests that nearly all such episodes—estimates are between 98% and 99%1,2—should be treated over several days with oral antihypertensives, not acutely with intravenous medications.3-6 Current guidelines recommend that intravenous medications should be reserved for severe hypertensive episodes (SBP > 180, DBP > 120) with acute end-organ damage,7,8 but such “hypertensive emergencies” are rare on the general medicine wards. Still, hospitalists regularly face the dilemma posed by Mrs. Jones, and evidence shows they often prescribe intravenous antihypertensives.1,4,5 This unnecessary use can lead to unreliable drops in blood pressure and exposes our patients to potential harm.5,6
In this issue of the Journal of Hospital Medicine, two papers describe the frequency of inappropriate intravenous antihypertensive use in their hospitals and the subsequent quality improvement efforts implemented to reduce this practice. The first, by Jacobs et al., found that over a 10-month period, 11% of patients who experienced “asymptomatic hypertension” on an urban academic hospital medicine service were treated inappropriately with intravenous antihypertensives,9 with 14% of those experiencing an adverse event. The second paper, by Pasik et al., found that in their urban academic medical center there were 8.3 inappropriate intravenous antihypertensive orders placed per 1,000 patient days,10 with nearly half of those treated experiencing an adverse event. Based on these findings, each group then led interventions to reduce the use of intravenous antihypertensives.
While both groups engaged physicians and nurses as primary stakeholders, Pasik et al.10 worked to further expand nursing staff roles by empowering them to assess for underlying causes of hypertension, such as pain or anxiety, as well as end-organ damage via specific guided algorithms prior to contacting physicians. In doing so, they reduced intravenous antihypertensive use by 60% during the postintervention period, with a proportional reduction in adverse events. In addition to their educational initiative, Jacobs et al. aimed to limit calls by liberalizing the “ceiling” on standard nursing call parameters for blood pressure from 160/80 to 180/90. Following their intervention, intravenous antihypertensive orders were reduced by 40%, with the mean orders per patient with asymptomatic hypertension decreasing from 11% to 7% .
While these results are admirable, some caution in their interpretation is needed. For example, Jacobs et al. used electronic health record data to retrospectively identify hypertension as “symptomatic” or “asymptomatic” using laboratory, electrocardiogram, and imaging diagnostics as surrogate markers for “provider concern for end-organ damage.” Although it appropriately focused on concern for end-organ damage as justification for intravenous antihypertensives, this approach potentially underappreciated true hypertensive emergencies, thereby overestimating the amount of inappropriate use of intravenous antihypertensives. Pasik et al. utilized chart review of patients prescribed intravenous antihypertensives and therefore did not explore how often symptomatic hypertension occurred in patients who did not receive intravenous antihypertensives. Subsequently, this limited their ability to evaluate unintended harms of their initiative. To address this limitation, the authors followed a group of 111 patients who had elevated hypertension but did not receive intravenous antihypertensives and found no adverse outcomes.10 Because both studies were retrospective in nature, they were subject to biases from providers choosing intravenous antihypertensives for reasons that were neither captured by their datasets nor adjusted for. Additionally, neither study reported downstream impacts such as an increase in symptomatic hypertensive episodes or more rare events such as kidney injury, stroke, or myocardial infarction.
Given that guidelines discourage using intravenous antihypertensives, why were the efforts of Jacobs et al.9 and Pasik et al.10 needed in the first place? In a recent installment of Choosing Wisely: Things We Do For No Reason, Breu et al.11 cite two primary reasons: first, providers have unfounded fears that asymptomatic hypertension will quickly progress to cause organ damage; second, providers lack understanding of the potential harms from overtreatment. It is fitting, therefore, that both groups of authors focused on these topics in their education initiatives for physicians and nurses. Yet, as good quality improvement requires steps beyond education, it was promising to see that both authors additionally focused on intervening to change the systems and culture that existed around physician and nursing communication.
In the age of electronic health records, there has been a sustained focus on creating standardized order sets. While the value of these order sets has been widely demonstrated, there are downsides. For example, nursing call parameters in admission order sets are rarely patient-specific but account for a significant portion of nursing and physician communication. These one-size-fits-all orders limit nurses from using their clinical training and create unnecessary tensions as nurses are obligated to call covering hospitalists to address “abnormal” but clinically insignificant findings. Regular monitoring of vital signs is an integral part of caring for acutely ill inpatients but for most inpatients, the importance of vitals is to detect clinically meaningful changes, not to treat risk factors like hypertension that should be treated safely over the long term.
When inpatients become febrile, tachycardic, or hypoxic, hospitalists use critical thinking to diagnose the underlying causes. Unfortunately, high blood pressure is a vital sign that is treated differently. Many hospitalists see it as a number to fix, not a potential sign of a new underlying problem such as uncontrolled pain, anxiety, or medication side effects.8 Both groups of authors took the important first step of educating physicians to think critically when called about high blood pressure. Even more importantly, they took steps to change the system and culture in which providers make these decisions in the first place. Future work in this area would be wise to follow in these footsteps, by encouraging collaboration between hospitalist and nurses to create more logical and patient-specific call parameters that could potentially improve nursing-physician communication, and subsequently, patient care.
Changing the culture to limit the use of intravenous antihypertensives will not be easy, but it is necessary. We encourage readers to investigate intravenous antihypertensives in their own hospitals and consider how better communication between nurses and physicians could change their practice. Recalling Mrs. Jones above, the provider should engage her nurse to help confirm that her hypertension is “asymptomatic” and then consider underlying causes such as pain, anxiety, or withholding her home medications as reasons for her elevated blood pressure. After all, if nothing else, it seems clear that a call about inpatient hypertension is not a call to act, but to think.
Disclosures
The authors declare that they have no competing interests.
Funding
Dr. Lucas is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086).
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417- 422. doi: 10.1002/jhm.804. PubMed
2. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization;2011. 3.
3. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
4. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
5. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j. jash.2011.07.002. PubMed
6. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
7. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. PubMed
9. Reducing Unnecessary Treatment of Asymptomatic Elevated Blood Pressure with Intravenous Medications on the General Internal Medicine Wards: A Quality Improvement Initiative. Jacobs ZG, Najafi N, Fang MC, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3087. PubMed
10. Assess Before Rx: Reducing the Overtreatment of Asymptomatic Blood Pressure Elevation in the Inpatient Setting. Pasik SD, Chiu S, Yang J, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3125. PubMed
11. Breu AC, Axon RN. Acute treatment of hypertensive urgency. J Hosp Med. 2018;13(12):860-862. doi: 10.12788/jhm.3086. PubMed
Your pager beeps. Your patient, Mrs. Jones, who was admitted with cellulitis and is improving, now has a blood pressure of 188/103 on routine vitals. Her nurse reports that she is comfortable and asymptomatic, but she met the “call parameters.” You review her chart and find that since admission her systolic blood pressure (SBP) has ranged from 149 to 157 mm Hg and her diastolic blood pressure (DBP) from 84 to 96 mm Hg. Her nurse asks how you would like to treat her.
While over half of inpatients have at least one hypertensive episode during their stay, evidence suggests that nearly all such episodes—estimates are between 98% and 99%1,2—should be treated over several days with oral antihypertensives, not acutely with intravenous medications.3-6 Current guidelines recommend that intravenous medications should be reserved for severe hypertensive episodes (SBP > 180, DBP > 120) with acute end-organ damage,7,8 but such “hypertensive emergencies” are rare on the general medicine wards. Still, hospitalists regularly face the dilemma posed by Mrs. Jones, and evidence shows they often prescribe intravenous antihypertensives.1,4,5 This unnecessary use can lead to unreliable drops in blood pressure and exposes our patients to potential harm.5,6
In this issue of the Journal of Hospital Medicine, two papers describe the frequency of inappropriate intravenous antihypertensive use in their hospitals and the subsequent quality improvement efforts implemented to reduce this practice. The first, by Jacobs et al., found that over a 10-month period, 11% of patients who experienced “asymptomatic hypertension” on an urban academic hospital medicine service were treated inappropriately with intravenous antihypertensives,9 with 14% of those experiencing an adverse event. The second paper, by Pasik et al., found that in their urban academic medical center there were 8.3 inappropriate intravenous antihypertensive orders placed per 1,000 patient days,10 with nearly half of those treated experiencing an adverse event. Based on these findings, each group then led interventions to reduce the use of intravenous antihypertensives.
While both groups engaged physicians and nurses as primary stakeholders, Pasik et al.10 worked to further expand nursing staff roles by empowering them to assess for underlying causes of hypertension, such as pain or anxiety, as well as end-organ damage via specific guided algorithms prior to contacting physicians. In doing so, they reduced intravenous antihypertensive use by 60% during the postintervention period, with a proportional reduction in adverse events. In addition to their educational initiative, Jacobs et al. aimed to limit calls by liberalizing the “ceiling” on standard nursing call parameters for blood pressure from 160/80 to 180/90. Following their intervention, intravenous antihypertensive orders were reduced by 40%, with the mean orders per patient with asymptomatic hypertension decreasing from 11% to 7% .
While these results are admirable, some caution in their interpretation is needed. For example, Jacobs et al. used electronic health record data to retrospectively identify hypertension as “symptomatic” or “asymptomatic” using laboratory, electrocardiogram, and imaging diagnostics as surrogate markers for “provider concern for end-organ damage.” Although it appropriately focused on concern for end-organ damage as justification for intravenous antihypertensives, this approach potentially underappreciated true hypertensive emergencies, thereby overestimating the amount of inappropriate use of intravenous antihypertensives. Pasik et al. utilized chart review of patients prescribed intravenous antihypertensives and therefore did not explore how often symptomatic hypertension occurred in patients who did not receive intravenous antihypertensives. Subsequently, this limited their ability to evaluate unintended harms of their initiative. To address this limitation, the authors followed a group of 111 patients who had elevated hypertension but did not receive intravenous antihypertensives and found no adverse outcomes.10 Because both studies were retrospective in nature, they were subject to biases from providers choosing intravenous antihypertensives for reasons that were neither captured by their datasets nor adjusted for. Additionally, neither study reported downstream impacts such as an increase in symptomatic hypertensive episodes or more rare events such as kidney injury, stroke, or myocardial infarction.
Given that guidelines discourage using intravenous antihypertensives, why were the efforts of Jacobs et al.9 and Pasik et al.10 needed in the first place? In a recent installment of Choosing Wisely: Things We Do For No Reason, Breu et al.11 cite two primary reasons: first, providers have unfounded fears that asymptomatic hypertension will quickly progress to cause organ damage; second, providers lack understanding of the potential harms from overtreatment. It is fitting, therefore, that both groups of authors focused on these topics in their education initiatives for physicians and nurses. Yet, as good quality improvement requires steps beyond education, it was promising to see that both authors additionally focused on intervening to change the systems and culture that existed around physician and nursing communication.
In the age of electronic health records, there has been a sustained focus on creating standardized order sets. While the value of these order sets has been widely demonstrated, there are downsides. For example, nursing call parameters in admission order sets are rarely patient-specific but account for a significant portion of nursing and physician communication. These one-size-fits-all orders limit nurses from using their clinical training and create unnecessary tensions as nurses are obligated to call covering hospitalists to address “abnormal” but clinically insignificant findings. Regular monitoring of vital signs is an integral part of caring for acutely ill inpatients but for most inpatients, the importance of vitals is to detect clinically meaningful changes, not to treat risk factors like hypertension that should be treated safely over the long term.
When inpatients become febrile, tachycardic, or hypoxic, hospitalists use critical thinking to diagnose the underlying causes. Unfortunately, high blood pressure is a vital sign that is treated differently. Many hospitalists see it as a number to fix, not a potential sign of a new underlying problem such as uncontrolled pain, anxiety, or medication side effects.8 Both groups of authors took the important first step of educating physicians to think critically when called about high blood pressure. Even more importantly, they took steps to change the system and culture in which providers make these decisions in the first place. Future work in this area would be wise to follow in these footsteps, by encouraging collaboration between hospitalist and nurses to create more logical and patient-specific call parameters that could potentially improve nursing-physician communication, and subsequently, patient care.
Changing the culture to limit the use of intravenous antihypertensives will not be easy, but it is necessary. We encourage readers to investigate intravenous antihypertensives in their own hospitals and consider how better communication between nurses and physicians could change their practice. Recalling Mrs. Jones above, the provider should engage her nurse to help confirm that her hypertension is “asymptomatic” and then consider underlying causes such as pain, anxiety, or withholding her home medications as reasons for her elevated blood pressure. After all, if nothing else, it seems clear that a call about inpatient hypertension is not a call to act, but to think.
Disclosures
The authors declare that they have no competing interests.
Funding
Dr. Lucas is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086).
Your pager beeps. Your patient, Mrs. Jones, who was admitted with cellulitis and is improving, now has a blood pressure of 188/103 on routine vitals. Her nurse reports that she is comfortable and asymptomatic, but she met the “call parameters.” You review her chart and find that since admission her systolic blood pressure (SBP) has ranged from 149 to 157 mm Hg and her diastolic blood pressure (DBP) from 84 to 96 mm Hg. Her nurse asks how you would like to treat her.
While over half of inpatients have at least one hypertensive episode during their stay, evidence suggests that nearly all such episodes—estimates are between 98% and 99%1,2—should be treated over several days with oral antihypertensives, not acutely with intravenous medications.3-6 Current guidelines recommend that intravenous medications should be reserved for severe hypertensive episodes (SBP > 180, DBP > 120) with acute end-organ damage,7,8 but such “hypertensive emergencies” are rare on the general medicine wards. Still, hospitalists regularly face the dilemma posed by Mrs. Jones, and evidence shows they often prescribe intravenous antihypertensives.1,4,5 This unnecessary use can lead to unreliable drops in blood pressure and exposes our patients to potential harm.5,6
In this issue of the Journal of Hospital Medicine, two papers describe the frequency of inappropriate intravenous antihypertensive use in their hospitals and the subsequent quality improvement efforts implemented to reduce this practice. The first, by Jacobs et al., found that over a 10-month period, 11% of patients who experienced “asymptomatic hypertension” on an urban academic hospital medicine service were treated inappropriately with intravenous antihypertensives,9 with 14% of those experiencing an adverse event. The second paper, by Pasik et al., found that in their urban academic medical center there were 8.3 inappropriate intravenous antihypertensive orders placed per 1,000 patient days,10 with nearly half of those treated experiencing an adverse event. Based on these findings, each group then led interventions to reduce the use of intravenous antihypertensives.
While both groups engaged physicians and nurses as primary stakeholders, Pasik et al.10 worked to further expand nursing staff roles by empowering them to assess for underlying causes of hypertension, such as pain or anxiety, as well as end-organ damage via specific guided algorithms prior to contacting physicians. In doing so, they reduced intravenous antihypertensive use by 60% during the postintervention period, with a proportional reduction in adverse events. In addition to their educational initiative, Jacobs et al. aimed to limit calls by liberalizing the “ceiling” on standard nursing call parameters for blood pressure from 160/80 to 180/90. Following their intervention, intravenous antihypertensive orders were reduced by 40%, with the mean orders per patient with asymptomatic hypertension decreasing from 11% to 7% .
While these results are admirable, some caution in their interpretation is needed. For example, Jacobs et al. used electronic health record data to retrospectively identify hypertension as “symptomatic” or “asymptomatic” using laboratory, electrocardiogram, and imaging diagnostics as surrogate markers for “provider concern for end-organ damage.” Although it appropriately focused on concern for end-organ damage as justification for intravenous antihypertensives, this approach potentially underappreciated true hypertensive emergencies, thereby overestimating the amount of inappropriate use of intravenous antihypertensives. Pasik et al. utilized chart review of patients prescribed intravenous antihypertensives and therefore did not explore how often symptomatic hypertension occurred in patients who did not receive intravenous antihypertensives. Subsequently, this limited their ability to evaluate unintended harms of their initiative. To address this limitation, the authors followed a group of 111 patients who had elevated hypertension but did not receive intravenous antihypertensives and found no adverse outcomes.10 Because both studies were retrospective in nature, they were subject to biases from providers choosing intravenous antihypertensives for reasons that were neither captured by their datasets nor adjusted for. Additionally, neither study reported downstream impacts such as an increase in symptomatic hypertensive episodes or more rare events such as kidney injury, stroke, or myocardial infarction.
Given that guidelines discourage using intravenous antihypertensives, why were the efforts of Jacobs et al.9 and Pasik et al.10 needed in the first place? In a recent installment of Choosing Wisely: Things We Do For No Reason, Breu et al.11 cite two primary reasons: first, providers have unfounded fears that asymptomatic hypertension will quickly progress to cause organ damage; second, providers lack understanding of the potential harms from overtreatment. It is fitting, therefore, that both groups of authors focused on these topics in their education initiatives for physicians and nurses. Yet, as good quality improvement requires steps beyond education, it was promising to see that both authors additionally focused on intervening to change the systems and culture that existed around physician and nursing communication.
In the age of electronic health records, there has been a sustained focus on creating standardized order sets. While the value of these order sets has been widely demonstrated, there are downsides. For example, nursing call parameters in admission order sets are rarely patient-specific but account for a significant portion of nursing and physician communication. These one-size-fits-all orders limit nurses from using their clinical training and create unnecessary tensions as nurses are obligated to call covering hospitalists to address “abnormal” but clinically insignificant findings. Regular monitoring of vital signs is an integral part of caring for acutely ill inpatients but for most inpatients, the importance of vitals is to detect clinically meaningful changes, not to treat risk factors like hypertension that should be treated safely over the long term.
When inpatients become febrile, tachycardic, or hypoxic, hospitalists use critical thinking to diagnose the underlying causes. Unfortunately, high blood pressure is a vital sign that is treated differently. Many hospitalists see it as a number to fix, not a potential sign of a new underlying problem such as uncontrolled pain, anxiety, or medication side effects.8 Both groups of authors took the important first step of educating physicians to think critically when called about high blood pressure. Even more importantly, they took steps to change the system and culture in which providers make these decisions in the first place. Future work in this area would be wise to follow in these footsteps, by encouraging collaboration between hospitalist and nurses to create more logical and patient-specific call parameters that could potentially improve nursing-physician communication, and subsequently, patient care.
Changing the culture to limit the use of intravenous antihypertensives will not be easy, but it is necessary. We encourage readers to investigate intravenous antihypertensives in their own hospitals and consider how better communication between nurses and physicians could change their practice. Recalling Mrs. Jones above, the provider should engage her nurse to help confirm that her hypertension is “asymptomatic” and then consider underlying causes such as pain, anxiety, or withholding her home medications as reasons for her elevated blood pressure. After all, if nothing else, it seems clear that a call about inpatient hypertension is not a call to act, but to think.
Disclosures
The authors declare that they have no competing interests.
Funding
Dr. Lucas is supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086).
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417- 422. doi: 10.1002/jhm.804. PubMed
2. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization;2011. 3.
3. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
4. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
5. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j. jash.2011.07.002. PubMed
6. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
7. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. PubMed
9. Reducing Unnecessary Treatment of Asymptomatic Elevated Blood Pressure with Intravenous Medications on the General Internal Medicine Wards: A Quality Improvement Initiative. Jacobs ZG, Najafi N, Fang MC, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3087. PubMed
10. Assess Before Rx: Reducing the Overtreatment of Asymptomatic Blood Pressure Elevation in the Inpatient Setting. Pasik SD, Chiu S, Yang J, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3125. PubMed
11. Breu AC, Axon RN. Acute treatment of hypertensive urgency. J Hosp Med. 2018;13(12):860-862. doi: 10.12788/jhm.3086. PubMed
1. Axon RN, Cousineau L, Egan BM. Prevalence and management of hypertension in the inpatient setting: A systematic review. J Hosp Med. 2011;6(7):417- 422. doi: 10.1002/jhm.804. PubMed
2. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization;2011. 3.
3. Herzog E, Frankenberger O, Aziz E, et al. A novel pathway for the management of hypertension for hospitalized patients. Crit Pathw Cardiol. 2007;6(4):150-160. doi: 10.1097/HPC.0b013e318160c3a7. PubMed
4. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
5. Campbell P, Baker WL, Bendel SD, White WB. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473-477. doi: 10.1016/j. jash.2011.07.002. PubMed
6. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2017;12(1):7-15. doi: 10.1177/1753944717746613. PubMed
7. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. PubMed
8. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066. PubMed
9. Reducing Unnecessary Treatment of Asymptomatic Elevated Blood Pressure with Intravenous Medications on the General Internal Medicine Wards: A Quality Improvement Initiative. Jacobs ZG, Najafi N, Fang MC, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3087. PubMed
10. Assess Before Rx: Reducing the Overtreatment of Asymptomatic Blood Pressure Elevation in the Inpatient Setting. Pasik SD, Chiu S, Yang J, et al. J Hosp Med. 2019;14:XXX-XXX. doi: 10.12788/jhm.3125. PubMed
11. Breu AC, Axon RN. Acute treatment of hypertensive urgency. J Hosp Med. 2018;13(12):860-862. doi: 10.12788/jhm.3086. PubMed
© 2019 Society of Hospital Medicine
Adherence to Recommended Inpatient Hepatic Encephalopathy Workup
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
© 2019 Society of Hospital Medicine
Reviews Reenvisioned: Supporting Enhanced Practice Improvement for Hospitalists
As part of the Journal of Hospital Medicine’s® commitment to our readership, we are excited to announce innovative new review formats, designed for busy hospitalists. The state of knowledge in our field is changing rapidly, and the 21st century poses a conundrum to clinicians in the form of increasingly complex studies and guidelines amidst ever-decreasing time to digest them. As a result, it can be challenging for hospitalists to access and interpret recently published research to inform their clinical practice. Because we are committed to practical innovation for hospitalists, starting in 2019, JHM will offer focused yet informative content that places important advances into relevant clinical or methodological context and provides our readers with information that is accessible, meaningful, and actionable—all in a more concise format.
Our new Clinical Guideline Highlights for the Hospitalist is a brief, targeted review of recently published clinical guidelines, distilling the major recommendations relevant to hospital medicine and placing them in context of the available evidence. This review format also offers a critique of gaps in the literature and notes areas ripe for future study. In this issue, we debut two articles using this new approach—one aimed at adult hospitalists and the other at pediatric hospitalists—regarding recently published studies and guidelines about maintenance intravenous fluids.1-5
In 2019, we will also introduce a second new format, called Progress Notes. These reviews will be shorter than JHM’s traditional review format, and will accept two types of articles: clinical and methodological. The clinical Progress Notes will provide an update on the last several years of evidence related to diagnosis, treatment, risk stratification, and/or prevention of a clinical problem highly pertinent to hospitalists. The methodological Progress Notes will provide our readers with insight into the application of quantitative, qualitative, and quality improvement methods commonly used in work published in this journal. Our aim is to use Progress Notes as a way to enhance both clinical practice and scholarship efforts by our readers.
Finally, we will introduce “Hospital Medicine: The Year in Review,” an annual feature that concisely compiles and critiques the top articles in both adult and pediatric hospital medicine over the past year. The “Year in Review” will serve as a written corollary to the popular “Updates in Hospital Medicine” presentation at the Society of Hospital Medicine annual meeting, and will highlight important research that advanced our field or provided us a fresh perspective on hospitalist practice.
As we introduce these new review formats, it is important to note that JHM will continue to accept traditional, long-form reviews on any topic relevant to hospitalists, with a preference for rigorous systematic reviews or meta-analyses. Equally important is that JHM’s overarching commitment remains unchanged: support clinicians, leaders, and scholars in our field in their pursuit of delivering evidence-based, high-value clinical care. We hope you enjoy these new article formats and we look forward to your feedback.
Disclosures
The authors declare they have no conflicts of interest/competing interests.
1. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital. London: Royal College of Physicians (UK); 2013 Dec. PubMed
2. Selmer MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults, N Engl J Med. 2018 Mar 1;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
3. Fled LG, et. al. “Clinical Practice Guideline: Maintenance Intravenous Fluids in Children,” Pediatrics. 2018 Dec;142(6). doi: 10.1542/peds.2018-3083. PubMed
4. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. doi: 10.12788/jhm.3178. PubMed
5. Girdwood ST, Parker MW, Shaughnessy EE. Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children. J Hosp Med. 2019;14(3):170-171. doi: 10.12788/jhm.3177. PubMed
As part of the Journal of Hospital Medicine’s® commitment to our readership, we are excited to announce innovative new review formats, designed for busy hospitalists. The state of knowledge in our field is changing rapidly, and the 21st century poses a conundrum to clinicians in the form of increasingly complex studies and guidelines amidst ever-decreasing time to digest them. As a result, it can be challenging for hospitalists to access and interpret recently published research to inform their clinical practice. Because we are committed to practical innovation for hospitalists, starting in 2019, JHM will offer focused yet informative content that places important advances into relevant clinical or methodological context and provides our readers with information that is accessible, meaningful, and actionable—all in a more concise format.
Our new Clinical Guideline Highlights for the Hospitalist is a brief, targeted review of recently published clinical guidelines, distilling the major recommendations relevant to hospital medicine and placing them in context of the available evidence. This review format also offers a critique of gaps in the literature and notes areas ripe for future study. In this issue, we debut two articles using this new approach—one aimed at adult hospitalists and the other at pediatric hospitalists—regarding recently published studies and guidelines about maintenance intravenous fluids.1-5
In 2019, we will also introduce a second new format, called Progress Notes. These reviews will be shorter than JHM’s traditional review format, and will accept two types of articles: clinical and methodological. The clinical Progress Notes will provide an update on the last several years of evidence related to diagnosis, treatment, risk stratification, and/or prevention of a clinical problem highly pertinent to hospitalists. The methodological Progress Notes will provide our readers with insight into the application of quantitative, qualitative, and quality improvement methods commonly used in work published in this journal. Our aim is to use Progress Notes as a way to enhance both clinical practice and scholarship efforts by our readers.
Finally, we will introduce “Hospital Medicine: The Year in Review,” an annual feature that concisely compiles and critiques the top articles in both adult and pediatric hospital medicine over the past year. The “Year in Review” will serve as a written corollary to the popular “Updates in Hospital Medicine” presentation at the Society of Hospital Medicine annual meeting, and will highlight important research that advanced our field or provided us a fresh perspective on hospitalist practice.
As we introduce these new review formats, it is important to note that JHM will continue to accept traditional, long-form reviews on any topic relevant to hospitalists, with a preference for rigorous systematic reviews or meta-analyses. Equally important is that JHM’s overarching commitment remains unchanged: support clinicians, leaders, and scholars in our field in their pursuit of delivering evidence-based, high-value clinical care. We hope you enjoy these new article formats and we look forward to your feedback.
Disclosures
The authors declare they have no conflicts of interest/competing interests.
As part of the Journal of Hospital Medicine’s® commitment to our readership, we are excited to announce innovative new review formats, designed for busy hospitalists. The state of knowledge in our field is changing rapidly, and the 21st century poses a conundrum to clinicians in the form of increasingly complex studies and guidelines amidst ever-decreasing time to digest them. As a result, it can be challenging for hospitalists to access and interpret recently published research to inform their clinical practice. Because we are committed to practical innovation for hospitalists, starting in 2019, JHM will offer focused yet informative content that places important advances into relevant clinical or methodological context and provides our readers with information that is accessible, meaningful, and actionable—all in a more concise format.
Our new Clinical Guideline Highlights for the Hospitalist is a brief, targeted review of recently published clinical guidelines, distilling the major recommendations relevant to hospital medicine and placing them in context of the available evidence. This review format also offers a critique of gaps in the literature and notes areas ripe for future study. In this issue, we debut two articles using this new approach—one aimed at adult hospitalists and the other at pediatric hospitalists—regarding recently published studies and guidelines about maintenance intravenous fluids.1-5
In 2019, we will also introduce a second new format, called Progress Notes. These reviews will be shorter than JHM’s traditional review format, and will accept two types of articles: clinical and methodological. The clinical Progress Notes will provide an update on the last several years of evidence related to diagnosis, treatment, risk stratification, and/or prevention of a clinical problem highly pertinent to hospitalists. The methodological Progress Notes will provide our readers with insight into the application of quantitative, qualitative, and quality improvement methods commonly used in work published in this journal. Our aim is to use Progress Notes as a way to enhance both clinical practice and scholarship efforts by our readers.
Finally, we will introduce “Hospital Medicine: The Year in Review,” an annual feature that concisely compiles and critiques the top articles in both adult and pediatric hospital medicine over the past year. The “Year in Review” will serve as a written corollary to the popular “Updates in Hospital Medicine” presentation at the Society of Hospital Medicine annual meeting, and will highlight important research that advanced our field or provided us a fresh perspective on hospitalist practice.
As we introduce these new review formats, it is important to note that JHM will continue to accept traditional, long-form reviews on any topic relevant to hospitalists, with a preference for rigorous systematic reviews or meta-analyses. Equally important is that JHM’s overarching commitment remains unchanged: support clinicians, leaders, and scholars in our field in their pursuit of delivering evidence-based, high-value clinical care. We hope you enjoy these new article formats and we look forward to your feedback.
Disclosures
The authors declare they have no conflicts of interest/competing interests.
1. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital. London: Royal College of Physicians (UK); 2013 Dec. PubMed
2. Selmer MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults, N Engl J Med. 2018 Mar 1;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
3. Fled LG, et. al. “Clinical Practice Guideline: Maintenance Intravenous Fluids in Children,” Pediatrics. 2018 Dec;142(6). doi: 10.1542/peds.2018-3083. PubMed
4. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. doi: 10.12788/jhm.3178. PubMed
5. Girdwood ST, Parker MW, Shaughnessy EE. Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children. J Hosp Med. 2019;14(3):170-171. doi: 10.12788/jhm.3177. PubMed
1. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital. London: Royal College of Physicians (UK); 2013 Dec. PubMed
2. Selmer MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults, N Engl J Med. 2018 Mar 1;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
3. Fled LG, et. al. “Clinical Practice Guideline: Maintenance Intravenous Fluids in Children,” Pediatrics. 2018 Dec;142(6). doi: 10.1542/peds.2018-3083. PubMed
4. Gottenborg E, Pierce R. Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult. J Hosp Med. 2019;14(3):172-173. doi: 10.12788/jhm.3178. PubMed
5. Girdwood ST, Parker MW, Shaughnessy EE. Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children. J Hosp Med. 2019;14(3):170-171. doi: 10.12788/jhm.3177. PubMed
© 2019 Society of Hospital Medicine
Increasing Mobility via In-hospital Ambulation Protocol Delivered by Mobility Technicians: A Pilot Randomized Controlled Trial
Individuals aged 65 years and over represent 13% of the United States population and account for nearly 40% of hospital discharges.1 Bedrest hastens the functional decline of older patients2-5 and is associated with risk of serious complications, such as falls, delirium, venous thrombosis, and skin breakdown.6,7 Ambulation is widely recognized as important for improving hospital outcomes.8-10 Observational studies suggest that increases of 600 steps per day are associated with shortened length of hospital stay.9 However, randomized trials of assisted ambulation have not demonstrated consistent benefit.11-14 As a result, usual care at most hospitals in the United States does not include assisted ambulation. Even when ambulation is ordered, execution of the orders is inconsistent.15-17
Studies have demonstrated the benefits of various exercise protocols for older patients in rehabilitation facilities,18,19 medical intensive care units,20 and medical and surgical wards.13,18,21 These interventions are usually nursing centered; however, assisting patients with ambulation multiple times per day may be a burdensome addition to the myriad responsibilities of nurses.19,22,23 In fact, ambulation orders are the most frequently overlooked nursing task.24
We designed a graded protocol of assisted ambulation implemented by a dedicated patient care nursing assistant (PCNA) multiple times daily to increase patient mobility. The objective of this study was to assess the feasibility and effectiveness of such an intervention for older inpatients. We hypothesized that the intervention would prove feasible and improve hospital outcomes, including less need for inpatient rehabilitation and shorter length of stay.
METHODS
We conducted a single-blind randomized controlled trial of patients aged ≥60 years and admitted as medical inpatients to the Cleveland Clinic Main Campus, a tertiary care center with over 1,440 inpatient beds. The consent form and study protocol were approved by the Cleveland Clinic Institutional Review Board, and the study was registered with ClinicalTrials.gov (NCT02757131).
Patients
All patients who were admitted to study wards for a medical illness and evaluated by Physical Therapy (PT) were eligible for the study. PT evaluations were ordered by the medical team if deemed necessary on the basis of factors, such as age, estimated mobility, and concerns raised by the ancillary staff. All patients who were expected to be discharged to a skilled nursing facility placement or who required home PT received a PT evaluation. Assessment of mobility was documented via Activity Measure for Postacute Care Inpatient Basic Mobility “six-clicks” short form, hereafter abbreviated as “six-clicks.” Based on past experience, patients with scores <16 rarely go home (<20% of the time), and those with scores >20 usually go home regardless of ambulation. Therefore, only patients with scores of 16-20 were invited to participate in the study. Although patients who were not evaluated by PT might also benefit from the intervention, we required a six-clicks score to assess eligibility. The exclusion criteria included anticipated remaining length of stay less than three days, admission under observation status, admission to the intensive care unit (ICU,) patients receiving comfort care measures, and patients with medical conditions precluding ambulation, such as decompensated heart failure or unstable angina.
Randomization
Patients were randomized to “usual care” or “mobility technician” after baseline evaluation using a computerized system. A block randomization scheme with a size of four was used to ensure an approximately equal number of patients per group.
Intervention
Patients randomized to the intervention group were asked to participate in the ambulation protocol outlined by the PT three times daily under the supervision of the mobility technician. The protocol involved four exercise levels (sitting, standing, walking, and stairs), which were implemented depending on the patient’s physical capacity. The mobility technicians, who were PCNAs, were trained by the PT team. PCNAs have no specific degrees or certification. They are taught safe handling techniques during their job orientation, so they already had an understanding of how to transfer and assist a patient with ambulation. The mobility technician training consisted of one four-hour session run by the PT team in the physical therapy department and the nursing unit. The training included safe handling practices and basic mobility, such as transfers from bed to chair, bed to standing, walking with assistance, and walking independently with equipment such as cane, rolling walker, and walking belt. All instruction was demonstrated by the trainer, and the mobility technician was then able to practice. The mobility technician then shadowed the trainer and practiced the techniques under supervision. Competency was assessed by the trainer.
The cohort of patients randomized to “usual care” was not seen by the mobility technicians but was not otherwise restricted in nursing’s baseline ability to execute recommendations placed by the PT team. Compliance with the recommendations is highly variable and dependent on patient acuity during the shift, staffing issues, and competing duties. Cleveland Clinic promotes a “culture of mobility,” and nurses are encouraged to get patients out of bed and assist with ambulation.
Study Instruments—Measures of Mobility
The six-clicks instrument is a tool for measuring basic mobility. It was adapted from the Activity Measures for Post-Acute Care (AM-PAC) instrument.25 Although initially created for self-report in the post-acute care setting, six-clicks has been validated for use by PTs in the acute care setting26 and is currently in use at more than 1,000 US hospitals. Cleveland Clinic PTs have used this measure for routine evaluation since 2011. The instrument has high interrater reliability and can predict discharge disposition.27-29
Each patient was provided with a tracking device (Fitbit) attached at the wrist to record daily steps for measuring mobility. The use of Fitbit has been validated in ambulatory and inpatient settings.30 The device produces step counts within 3% of the observed step count for most patients but may undercount steps in patients with very slow gait.31 The device was provided to each enrollee and collected at discharge.
Variables
Demographic information, comorbid diagnoses, and discharge destination were extracted from the electronic medical record. Information on prehospitalization physical activity level was obtained from the initial PT assessment. Falls were tracked through the safety event reporting system.
Outcomes
The primary outcomes were discharge disposition and hospital length of stay. The secondary outcomes included average steps per day, change in six-clicks score from admission to discharge, inpatient mortality, admission to ICU, falls, deep vein thrombosis, pulmonary embolism, or pneumonia, and readmission within 30 days.
Statistical Analysis
Patient characteristics were summarized as means and standard deviations or medians and interquartile ranges for continuous variables and as frequencies and percentages for categorical variables. The t-test or Wilcoxon rank sum test was applied to compare continuous characteristics between the intervention and control groups. Chi-squared test or Fisher’s exact test was applied to compare categorical characteristics. Given its skewed distribution, the length of stay was log-transformed and compared between the two groups using Student’s t-test. Chi-squared test was used to compare categorical outcomes. The analysis of final six-clicks scores was adjusted for baseline scores, and the least-square estimates are provided. A linear mixed effects model was used to compare the number of daily steps taken because each participant had multiple steps measured. Results were adjusted for prehospital activity. In addition to comparing the total steps taken by each group, we determined the proportion of patients who exceeded a particular threshold by taking the average number of steps per day for all subjects and relating it to home discharge using the Receiver Operating Characteristics (ROC) curve. An optimal cut-off was determined to maximize the Youden index. We also compared the proportion of patients who exceeded 900 steps because this value was previously reported as an important threshold.32 All analyses were conducted using intention-to-treat principles. We also conducted a per-protocol analysis in which we limited the intervention group to those who received at least one assisted ambulation session. A dose-response analysis was also performed, in which patients were categorized as not receiving the therapy, receiving sessions on one or two days, or receiving them on more than two days.
All analyses were conducted using R-studio (Boston, MA). Statistical significance was defined as a P-value < .05. Given that this is a pilot study, the results were not adjusted for multiple comparisons.
RESULTS
Characteristics of patients in the intervention and control groups are shown in Table 1. The patients were mostly white and female, with an average age in the mid-70s (range 61-98). All measures evaluated were not significantly different between the intervention and control groups. However, more patients in the intervention group had a prehospital activity level classified as independent.
Table 2 demonstrates the feasibility of the intervention. Of patients randomized to the intervention group, 74% were ambulated at least once. Once enrolled, the patients successfully participated in assisted ambulation for about two-thirds of their hospital stay. However, the intervention was delivered for only one-third of the total length of stay because most patients were not enrolled on admission. On average, the mobility technicians made 11 attempts to ambulate each patient and 56% of these attempts were successful. The proportion of unsuccessful attempts did not change over the course of the study. The reasons for unsuccessful attempts included patient refusal (n = 102) or unavailability (n = 68), mobility technicians running out of time (n = 2), and other (n = 12).
Initially, the mobility technicians were not available on weekends. In addition, they were often reassigned to other duties by their nurse managers, who were dealing with staffing shortages. As the study progressed, we were able to secure the mobility technicians to work seven days per week and to convince the nurse managers that their role should be protected. Consequently, the median number [IQR] of successful attempts increased from 1.5 [0, 2] in the first two months to 3 [0, 5] in the next three months and finally to 5 [1.5, 13] in the final months (P < .002). The median visit duration was 10 minutes, with an interquartile range of 6-15 minutes.
In the intention-to-treat analysis, patients in the intervention group took close to 50% more steps than did the control patients. After adjustment for prehospital activity level, the difference was not statistically significant. The intervention also did not significantly affect the length of stay or discharge disposition (Table 3). In the per protocol analysis, the difference in the step count was significant, even after adjustment. The six-clicks score also significantly increased.
To assess for dose response, we compared outcomes among patients who received no intervention, those who received two or fewer days of intervention, and those who received more than two days of intervention (Table 4). The length of stay was significantly longer in patients with more than two days of intervention, likely reflecting greater opportunities for exposure to the intervention. The longer intervention time significantly increased the six-clicks score.
We examined the relationship between steps achieved and discharge disposition. Patients who achieved at least 900 steps more often went home than those who did not (79% vs. 56%, P < .05). The ROC for the model of discharge disposition using steps taken as the only predictor had an area under the curve of 0.67, with optimal discrimination at 411 steps. At a threshold of 400 steps, the model had a sensitivity of 75.9% and a specificity of 51.4%. Patients achieving 400 steps were more likely to go home than those who did not achieve that goal (71% vs. 46%, P =.01). More patients in the intervention group achieved the 900 step goal (28% vs. 19%, P = .30) and the 400 step goal (66% vs. 58%, P = .39), but neither association reached statistical significance.
DISCUSSION
In this pilot study conducted with older medical inpatients, we found that assisted ambulation provided by a dedicated mobility technician was feasible and increased the number of steps taken by patients. Not all patients in the treatment group received the intervention partly due to the fact that the program initially did not include weekends and the mobility technicians were sometimes redirected to other nursing duties. Both issues were addressed during the course of the study. In the per protocol analysis, the intervention increased the average six-clicks score and there was a nonsignificant reduction in the percentage of patients discharged to a rehabilitation facility.
A range of hospital-based mobility interventions have been described. Several of which were complex multidisciplinary interventions that included a mobility component. The compliance rates have ranged from 82% to 93.7%,12,13 although a systematic review noted that many studies do not provide this level of information.11 Interventions were carried out by nursing staff and PT with support from family members and social workers.33-35 Ambulation-specific programs have also relied on nurses and PT13,14,36 and, occasionally, on research assistants to implement assisted ambulation protocols.12,37 A recent study that employed research assistants to deliver inhospital ambulation reported achieving 51.3% of intended walks.37
In contradistinction to previous studies, we created a new role, employing PCNAs as dedicated mobility technicians. We did this for two reasons. First, the approach is less expensive than deploying registered nurses or PTs to ambulate patients and therefore more likely to be adopted by hospitals, especially if it can decrease the cost of an episode of care by avoiding subsequent inpatient rehabilitation. Mobility technicians have no degree or certification requirements and are therefore paid less than nurses or physical therapists. Second, by having a single responsibility, mobility technicians were more likely to engage in their task than nurses, who have competing responsibilities. However, when nurse staffing was short, nurse managers were tempted to recall the PCNAs for other nursing duties. It took time before PCNAs and supervisors prioritized this new responsibility. When they did, the number of attempted walks increased substantially, but the percentage of successful attempts remained constant at 56%, highlighting the difficulty of getting hospitalized patients to walk.
On average, patients who received the intervention engaged in 72 minutes of additional physical activity and averaged 990 steps per day. Observational data suggest patients accrue about 1,100 steps in the day before discharge, with older patients accruing closer to 900.21 One study found that older patients with fewer than 900 steps per day were likely to experience a functional decline.32 We also found that patients who achieved at least 900 steps were more likely to go home. However, we found that a lower threshold, namely, 400 steps, offered better discrimination between patients who go home and those who do not. Future prospective studies are needed to establish the appropriate goal for exercise interventions. A lower step goal could dramatically enhance the efficiency of the intervention.
A Cochrane review found that pooled analysis of multidisciplinary interventions that included exercise, often in the form of walking, achieved a small but significant increase in the proportion of patients discharged to home (RR 1.08, 95%CI 1.03 to 1.14).11 We found no significant change in the discharge disposition, but our study was underpowered for this endpoint. The six-clicks score showed a small but significant change in the per protocol analysis. The six-clicks score has been shown to correlate with discharge disposition,28,29 and an improvement in the score suggests that discharge disposition may be influenced.
The intervention may also not have been implemented for long enough. On average, visits were achieved for one-third of the hospital stay partly because of the delay in PT evaluation, which we required for eligibility. In practice, PT evaluation can occur just a few days before the anticipated discharge. We observed a dose dependent response among patients in the intervention group, suggesting that earlier intervention could be more effective. Earlier intervention might be achieved if the MT performed the six-clicks on potentially eligible patients.
The effects of hospitalization on mobility may be the most pronounced in the long term; one study found that 40% of hospitalized older patients manifested new ADL or IADL disability three months after discharge compared with 31% at discharge.7 Hospital-based mobility interventions may continue to affect subjects’ independence for weeks or months. In one RCT, an inpatient ambulation intervention improved mobility in the community one month after discharge.37 A hospital-based exercise program that included ambulation achieved better functional outcomes one month later.13 One RCT that combined inpatient exercise with outpatient care coordination also decreased readmission rates.34 We found that the intervention did not affect readmission.
This pilot study has several limitations. The sample size was small, and the findings need to be replicated in a larger randomized controlled trial. This is particularly important because the two study arms were not balanced in terms of their prehospital activity. After adjustment for prehospital activity, the differences in the step count in the intention-to-treat analysis were no longer significant. As we adjusted the intervention to hospital workflow, the intervention changed over time. The intention-to-treat analysis may therefore underestimate the effect of the intervention. This work provides a basis for future trial. Finally, discharge disposition depends on a complex interplay of factors, including social factors and preferences, which may not be affected by a mobility intervention.
In summary, an inhospital mobility protocol of attempting ambulation delivered by dedicated mobility technicians three times daily successfully increased the daily step counts and mobility scores of the patients. Studies with a larger sample size are needed to determine whether the proposed approach can affect length of hospital stay, discharge disposition, and overall cost of an episode of care.
Disclosures
Mary Stilphen reports consulting for CreCare and Adeo, which license and distribute AM-PAC short forms, including 6 clicks. All other authors report no conflicts of interest.
Funding
This study was supported by a Research Program Committee grant from the Cleveland Clinic.
1. National Center for Health Statistics. National Hospital Discharge Survey. 2010. PubMed
2. Corcoran PJ. Use it or lose it--the hazards of bed rest and inactivity. West J Med. 1991;154(5):536-538. PubMed
3. Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16(10):1033-1038. doi: 10.1016/0277-9536(82)90175-7. PubMed
4. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. PubMed
5. Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266-273. doi: 10.1111/j.1532-5415.2010.03276.x. PubMed
6. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160(6):809-815. doi: 10.1067/mob.2001.107919. PubMed
7. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645-652. doi: 10.1001/archinte.1996.00440060067008. PubMed
8. Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44(4):M112-M117. doi: 10.1093/geronj/44.4.M112. PubMed
9. Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170(21):1942-1943. doi: 0.1001/archinternmed.2010.422. PubMed
10. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, quiz 67-58. PubMed
11. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007(1):CD005955. doi: 10.1002/14651858.CD005955. PubMed
12. Jones CT, Lowe AJ, MacGregor L, Brand CA, Tweddle N, Russell DM. A randomised controlled trial of an exercise intervention to reduce functional decline and health service utilisation in the hospitalised elderly. Australas J Ageing. 2006;25(3):126-133. doi: 10.1111/j.1741-6612.2006.00167.x.
13. Siebens H, Aronow H, Edwards D, Ghasemi Z. A randomized controlled trial of exercise to improve outcomes of acute hospitalization in older adults. J Am Geriatr Soc. 2000;48(12):1545-1552. doi: 10.1111/j.1532-5415.2000.tb03862.x. PubMed
14. Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124(3):883-889. doi: 10.1378/chest.124.3.883. PubMed
15. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi: 10.1111/j.1532-5415.2009.02393.x. PubMed
16. Callen BL, Mahoney JE, Grieves CB, Wells TJ, Enloe M. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs. 2004;25(4):212-217. doi: 10.1016/j.gerinurse.2004.06.016. PubMed
17. Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91-95. doi: 10.1111/j.1532-5415.2010.03202.x. PubMed
18. McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients: A randomized, controlled clinical trial. Ann Intern Med. 1989;110(1):79-84. doi: PubMed
19. Said CM, Morris ME, Woodward M, Churilov L, Bernhardt J. Enhancing physical activity in older adults receiving hospital based rehabilitation: A phase II feasibility study. BMC Geriatr. 2012;12:26. doi: 10.1186/1471-2318-12-26. PubMed
20. Timmerman RA. A mobility protocol for critically ill adults. Dimens Crit Care Nurs. 2007;26(5):175-179; quiz 180-171. doi: 10.1097/01.DCC.0000286816.40570.da. PubMed
21. Sallis R, Roddy-Sturm Y, Chijioke E, et al. Stepping toward discharge: Level of ambulation in hospitalized patients. J Hosp Med. 2015;10(6):384-389. doi: 10.1002/jhm.2343. PubMed
22. Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Jr., Tinetii ME. A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: The yale geriatric care program. J Am Geriatr Soc. 1993;41(12):1353-1360. doi: 10.1111/j.1532-5415.1993.tb06487.x. PubMed
23. Kalisch BJ, Landstrom GL, Hinshaw AS. Missed nursing care: A concept analysis. J Adv Nurs. 2009;65(7):1509-1517. doi: 10.1111/j.1365-2648.2009.05027.x. PubMed
24. Kalisch BJ, Tschannen D, Lee H, Friese CR. Hospital variation in missed nursing care. Am J Med Qual. 2011;26(4):291-299. doi: 10.1177/1062860610395929. PubMed
25. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):I49-161. doi: 10.1097/01.mlr.0000103520.43902.6c. PubMed
26. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. doi: 10.2522/ptj.20130199. PubMed
27. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC 6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. doi: 10.2522/ptj.20140174. PubMed
28. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. doi: 10.2522/ptj.20130359. PubMed
29. Menendez ME, Schumacher CS, Ring D, Freiberg AA, Rubash HE, Kwon YM. Does “6-Clicks” day 1 postoperative mobility score predict discharge disposition after total hip and knee arthroplasties? J Arthroplasty. 2016;31(9):1916-1920. doi: 10.1016/j.arth.2016.02.017. PubMed
30. Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6(8):e10527-e10527. doi: 10.2196/10527. PubMed
31. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther. 2017;97(5):581-588. doi: 10.1093/ptj/pzx010. PubMed
32. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association between 900 steps a day and functional decline in older hospitalized patients. JAMA Intern Med. 2017;177(2):272-274. doi: 10.1001/jamainternmed.2016.7266. PubMed
33. Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. PubMed
34. Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc. 2009;57(3):395-402. doi: 10.1111/j.1532-5415.2009.02138.x. PubMed
35. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. doi: 10.1056/NEJM199505183322006. PubMed
36. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546. PubMed
37. Brown CJ, Foley KT, Lowman JD, Jr., et al. comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921-927. doi: 10.1001/jamainternmed.2016.1870. PubMed
Individuals aged 65 years and over represent 13% of the United States population and account for nearly 40% of hospital discharges.1 Bedrest hastens the functional decline of older patients2-5 and is associated with risk of serious complications, such as falls, delirium, venous thrombosis, and skin breakdown.6,7 Ambulation is widely recognized as important for improving hospital outcomes.8-10 Observational studies suggest that increases of 600 steps per day are associated with shortened length of hospital stay.9 However, randomized trials of assisted ambulation have not demonstrated consistent benefit.11-14 As a result, usual care at most hospitals in the United States does not include assisted ambulation. Even when ambulation is ordered, execution of the orders is inconsistent.15-17
Studies have demonstrated the benefits of various exercise protocols for older patients in rehabilitation facilities,18,19 medical intensive care units,20 and medical and surgical wards.13,18,21 These interventions are usually nursing centered; however, assisting patients with ambulation multiple times per day may be a burdensome addition to the myriad responsibilities of nurses.19,22,23 In fact, ambulation orders are the most frequently overlooked nursing task.24
We designed a graded protocol of assisted ambulation implemented by a dedicated patient care nursing assistant (PCNA) multiple times daily to increase patient mobility. The objective of this study was to assess the feasibility and effectiveness of such an intervention for older inpatients. We hypothesized that the intervention would prove feasible and improve hospital outcomes, including less need for inpatient rehabilitation and shorter length of stay.
METHODS
We conducted a single-blind randomized controlled trial of patients aged ≥60 years and admitted as medical inpatients to the Cleveland Clinic Main Campus, a tertiary care center with over 1,440 inpatient beds. The consent form and study protocol were approved by the Cleveland Clinic Institutional Review Board, and the study was registered with ClinicalTrials.gov (NCT02757131).
Patients
All patients who were admitted to study wards for a medical illness and evaluated by Physical Therapy (PT) were eligible for the study. PT evaluations were ordered by the medical team if deemed necessary on the basis of factors, such as age, estimated mobility, and concerns raised by the ancillary staff. All patients who were expected to be discharged to a skilled nursing facility placement or who required home PT received a PT evaluation. Assessment of mobility was documented via Activity Measure for Postacute Care Inpatient Basic Mobility “six-clicks” short form, hereafter abbreviated as “six-clicks.” Based on past experience, patients with scores <16 rarely go home (<20% of the time), and those with scores >20 usually go home regardless of ambulation. Therefore, only patients with scores of 16-20 were invited to participate in the study. Although patients who were not evaluated by PT might also benefit from the intervention, we required a six-clicks score to assess eligibility. The exclusion criteria included anticipated remaining length of stay less than three days, admission under observation status, admission to the intensive care unit (ICU,) patients receiving comfort care measures, and patients with medical conditions precluding ambulation, such as decompensated heart failure or unstable angina.
Randomization
Patients were randomized to “usual care” or “mobility technician” after baseline evaluation using a computerized system. A block randomization scheme with a size of four was used to ensure an approximately equal number of patients per group.
Intervention
Patients randomized to the intervention group were asked to participate in the ambulation protocol outlined by the PT three times daily under the supervision of the mobility technician. The protocol involved four exercise levels (sitting, standing, walking, and stairs), which were implemented depending on the patient’s physical capacity. The mobility technicians, who were PCNAs, were trained by the PT team. PCNAs have no specific degrees or certification. They are taught safe handling techniques during their job orientation, so they already had an understanding of how to transfer and assist a patient with ambulation. The mobility technician training consisted of one four-hour session run by the PT team in the physical therapy department and the nursing unit. The training included safe handling practices and basic mobility, such as transfers from bed to chair, bed to standing, walking with assistance, and walking independently with equipment such as cane, rolling walker, and walking belt. All instruction was demonstrated by the trainer, and the mobility technician was then able to practice. The mobility technician then shadowed the trainer and practiced the techniques under supervision. Competency was assessed by the trainer.
The cohort of patients randomized to “usual care” was not seen by the mobility technicians but was not otherwise restricted in nursing’s baseline ability to execute recommendations placed by the PT team. Compliance with the recommendations is highly variable and dependent on patient acuity during the shift, staffing issues, and competing duties. Cleveland Clinic promotes a “culture of mobility,” and nurses are encouraged to get patients out of bed and assist with ambulation.
Study Instruments—Measures of Mobility
The six-clicks instrument is a tool for measuring basic mobility. It was adapted from the Activity Measures for Post-Acute Care (AM-PAC) instrument.25 Although initially created for self-report in the post-acute care setting, six-clicks has been validated for use by PTs in the acute care setting26 and is currently in use at more than 1,000 US hospitals. Cleveland Clinic PTs have used this measure for routine evaluation since 2011. The instrument has high interrater reliability and can predict discharge disposition.27-29
Each patient was provided with a tracking device (Fitbit) attached at the wrist to record daily steps for measuring mobility. The use of Fitbit has been validated in ambulatory and inpatient settings.30 The device produces step counts within 3% of the observed step count for most patients but may undercount steps in patients with very slow gait.31 The device was provided to each enrollee and collected at discharge.
Variables
Demographic information, comorbid diagnoses, and discharge destination were extracted from the electronic medical record. Information on prehospitalization physical activity level was obtained from the initial PT assessment. Falls were tracked through the safety event reporting system.
Outcomes
The primary outcomes were discharge disposition and hospital length of stay. The secondary outcomes included average steps per day, change in six-clicks score from admission to discharge, inpatient mortality, admission to ICU, falls, deep vein thrombosis, pulmonary embolism, or pneumonia, and readmission within 30 days.
Statistical Analysis
Patient characteristics were summarized as means and standard deviations or medians and interquartile ranges for continuous variables and as frequencies and percentages for categorical variables. The t-test or Wilcoxon rank sum test was applied to compare continuous characteristics between the intervention and control groups. Chi-squared test or Fisher’s exact test was applied to compare categorical characteristics. Given its skewed distribution, the length of stay was log-transformed and compared between the two groups using Student’s t-test. Chi-squared test was used to compare categorical outcomes. The analysis of final six-clicks scores was adjusted for baseline scores, and the least-square estimates are provided. A linear mixed effects model was used to compare the number of daily steps taken because each participant had multiple steps measured. Results were adjusted for prehospital activity. In addition to comparing the total steps taken by each group, we determined the proportion of patients who exceeded a particular threshold by taking the average number of steps per day for all subjects and relating it to home discharge using the Receiver Operating Characteristics (ROC) curve. An optimal cut-off was determined to maximize the Youden index. We also compared the proportion of patients who exceeded 900 steps because this value was previously reported as an important threshold.32 All analyses were conducted using intention-to-treat principles. We also conducted a per-protocol analysis in which we limited the intervention group to those who received at least one assisted ambulation session. A dose-response analysis was also performed, in which patients were categorized as not receiving the therapy, receiving sessions on one or two days, or receiving them on more than two days.
All analyses were conducted using R-studio (Boston, MA). Statistical significance was defined as a P-value < .05. Given that this is a pilot study, the results were not adjusted for multiple comparisons.
RESULTS
Characteristics of patients in the intervention and control groups are shown in Table 1. The patients were mostly white and female, with an average age in the mid-70s (range 61-98). All measures evaluated were not significantly different between the intervention and control groups. However, more patients in the intervention group had a prehospital activity level classified as independent.
Table 2 demonstrates the feasibility of the intervention. Of patients randomized to the intervention group, 74% were ambulated at least once. Once enrolled, the patients successfully participated in assisted ambulation for about two-thirds of their hospital stay. However, the intervention was delivered for only one-third of the total length of stay because most patients were not enrolled on admission. On average, the mobility technicians made 11 attempts to ambulate each patient and 56% of these attempts were successful. The proportion of unsuccessful attempts did not change over the course of the study. The reasons for unsuccessful attempts included patient refusal (n = 102) or unavailability (n = 68), mobility technicians running out of time (n = 2), and other (n = 12).
Initially, the mobility technicians were not available on weekends. In addition, they were often reassigned to other duties by their nurse managers, who were dealing with staffing shortages. As the study progressed, we were able to secure the mobility technicians to work seven days per week and to convince the nurse managers that their role should be protected. Consequently, the median number [IQR] of successful attempts increased from 1.5 [0, 2] in the first two months to 3 [0, 5] in the next three months and finally to 5 [1.5, 13] in the final months (P < .002). The median visit duration was 10 minutes, with an interquartile range of 6-15 minutes.
In the intention-to-treat analysis, patients in the intervention group took close to 50% more steps than did the control patients. After adjustment for prehospital activity level, the difference was not statistically significant. The intervention also did not significantly affect the length of stay or discharge disposition (Table 3). In the per protocol analysis, the difference in the step count was significant, even after adjustment. The six-clicks score also significantly increased.
To assess for dose response, we compared outcomes among patients who received no intervention, those who received two or fewer days of intervention, and those who received more than two days of intervention (Table 4). The length of stay was significantly longer in patients with more than two days of intervention, likely reflecting greater opportunities for exposure to the intervention. The longer intervention time significantly increased the six-clicks score.
We examined the relationship between steps achieved and discharge disposition. Patients who achieved at least 900 steps more often went home than those who did not (79% vs. 56%, P < .05). The ROC for the model of discharge disposition using steps taken as the only predictor had an area under the curve of 0.67, with optimal discrimination at 411 steps. At a threshold of 400 steps, the model had a sensitivity of 75.9% and a specificity of 51.4%. Patients achieving 400 steps were more likely to go home than those who did not achieve that goal (71% vs. 46%, P =.01). More patients in the intervention group achieved the 900 step goal (28% vs. 19%, P = .30) and the 400 step goal (66% vs. 58%, P = .39), but neither association reached statistical significance.
DISCUSSION
In this pilot study conducted with older medical inpatients, we found that assisted ambulation provided by a dedicated mobility technician was feasible and increased the number of steps taken by patients. Not all patients in the treatment group received the intervention partly due to the fact that the program initially did not include weekends and the mobility technicians were sometimes redirected to other nursing duties. Both issues were addressed during the course of the study. In the per protocol analysis, the intervention increased the average six-clicks score and there was a nonsignificant reduction in the percentage of patients discharged to a rehabilitation facility.
A range of hospital-based mobility interventions have been described. Several of which were complex multidisciplinary interventions that included a mobility component. The compliance rates have ranged from 82% to 93.7%,12,13 although a systematic review noted that many studies do not provide this level of information.11 Interventions were carried out by nursing staff and PT with support from family members and social workers.33-35 Ambulation-specific programs have also relied on nurses and PT13,14,36 and, occasionally, on research assistants to implement assisted ambulation protocols.12,37 A recent study that employed research assistants to deliver inhospital ambulation reported achieving 51.3% of intended walks.37
In contradistinction to previous studies, we created a new role, employing PCNAs as dedicated mobility technicians. We did this for two reasons. First, the approach is less expensive than deploying registered nurses or PTs to ambulate patients and therefore more likely to be adopted by hospitals, especially if it can decrease the cost of an episode of care by avoiding subsequent inpatient rehabilitation. Mobility technicians have no degree or certification requirements and are therefore paid less than nurses or physical therapists. Second, by having a single responsibility, mobility technicians were more likely to engage in their task than nurses, who have competing responsibilities. However, when nurse staffing was short, nurse managers were tempted to recall the PCNAs for other nursing duties. It took time before PCNAs and supervisors prioritized this new responsibility. When they did, the number of attempted walks increased substantially, but the percentage of successful attempts remained constant at 56%, highlighting the difficulty of getting hospitalized patients to walk.
On average, patients who received the intervention engaged in 72 minutes of additional physical activity and averaged 990 steps per day. Observational data suggest patients accrue about 1,100 steps in the day before discharge, with older patients accruing closer to 900.21 One study found that older patients with fewer than 900 steps per day were likely to experience a functional decline.32 We also found that patients who achieved at least 900 steps were more likely to go home. However, we found that a lower threshold, namely, 400 steps, offered better discrimination between patients who go home and those who do not. Future prospective studies are needed to establish the appropriate goal for exercise interventions. A lower step goal could dramatically enhance the efficiency of the intervention.
A Cochrane review found that pooled analysis of multidisciplinary interventions that included exercise, often in the form of walking, achieved a small but significant increase in the proportion of patients discharged to home (RR 1.08, 95%CI 1.03 to 1.14).11 We found no significant change in the discharge disposition, but our study was underpowered for this endpoint. The six-clicks score showed a small but significant change in the per protocol analysis. The six-clicks score has been shown to correlate with discharge disposition,28,29 and an improvement in the score suggests that discharge disposition may be influenced.
The intervention may also not have been implemented for long enough. On average, visits were achieved for one-third of the hospital stay partly because of the delay in PT evaluation, which we required for eligibility. In practice, PT evaluation can occur just a few days before the anticipated discharge. We observed a dose dependent response among patients in the intervention group, suggesting that earlier intervention could be more effective. Earlier intervention might be achieved if the MT performed the six-clicks on potentially eligible patients.
The effects of hospitalization on mobility may be the most pronounced in the long term; one study found that 40% of hospitalized older patients manifested new ADL or IADL disability three months after discharge compared with 31% at discharge.7 Hospital-based mobility interventions may continue to affect subjects’ independence for weeks or months. In one RCT, an inpatient ambulation intervention improved mobility in the community one month after discharge.37 A hospital-based exercise program that included ambulation achieved better functional outcomes one month later.13 One RCT that combined inpatient exercise with outpatient care coordination also decreased readmission rates.34 We found that the intervention did not affect readmission.
This pilot study has several limitations. The sample size was small, and the findings need to be replicated in a larger randomized controlled trial. This is particularly important because the two study arms were not balanced in terms of their prehospital activity. After adjustment for prehospital activity, the differences in the step count in the intention-to-treat analysis were no longer significant. As we adjusted the intervention to hospital workflow, the intervention changed over time. The intention-to-treat analysis may therefore underestimate the effect of the intervention. This work provides a basis for future trial. Finally, discharge disposition depends on a complex interplay of factors, including social factors and preferences, which may not be affected by a mobility intervention.
In summary, an inhospital mobility protocol of attempting ambulation delivered by dedicated mobility technicians three times daily successfully increased the daily step counts and mobility scores of the patients. Studies with a larger sample size are needed to determine whether the proposed approach can affect length of hospital stay, discharge disposition, and overall cost of an episode of care.
Disclosures
Mary Stilphen reports consulting for CreCare and Adeo, which license and distribute AM-PAC short forms, including 6 clicks. All other authors report no conflicts of interest.
Funding
This study was supported by a Research Program Committee grant from the Cleveland Clinic.
Individuals aged 65 years and over represent 13% of the United States population and account for nearly 40% of hospital discharges.1 Bedrest hastens the functional decline of older patients2-5 and is associated with risk of serious complications, such as falls, delirium, venous thrombosis, and skin breakdown.6,7 Ambulation is widely recognized as important for improving hospital outcomes.8-10 Observational studies suggest that increases of 600 steps per day are associated with shortened length of hospital stay.9 However, randomized trials of assisted ambulation have not demonstrated consistent benefit.11-14 As a result, usual care at most hospitals in the United States does not include assisted ambulation. Even when ambulation is ordered, execution of the orders is inconsistent.15-17
Studies have demonstrated the benefits of various exercise protocols for older patients in rehabilitation facilities,18,19 medical intensive care units,20 and medical and surgical wards.13,18,21 These interventions are usually nursing centered; however, assisting patients with ambulation multiple times per day may be a burdensome addition to the myriad responsibilities of nurses.19,22,23 In fact, ambulation orders are the most frequently overlooked nursing task.24
We designed a graded protocol of assisted ambulation implemented by a dedicated patient care nursing assistant (PCNA) multiple times daily to increase patient mobility. The objective of this study was to assess the feasibility and effectiveness of such an intervention for older inpatients. We hypothesized that the intervention would prove feasible and improve hospital outcomes, including less need for inpatient rehabilitation and shorter length of stay.
METHODS
We conducted a single-blind randomized controlled trial of patients aged ≥60 years and admitted as medical inpatients to the Cleveland Clinic Main Campus, a tertiary care center with over 1,440 inpatient beds. The consent form and study protocol were approved by the Cleveland Clinic Institutional Review Board, and the study was registered with ClinicalTrials.gov (NCT02757131).
Patients
All patients who were admitted to study wards for a medical illness and evaluated by Physical Therapy (PT) were eligible for the study. PT evaluations were ordered by the medical team if deemed necessary on the basis of factors, such as age, estimated mobility, and concerns raised by the ancillary staff. All patients who were expected to be discharged to a skilled nursing facility placement or who required home PT received a PT evaluation. Assessment of mobility was documented via Activity Measure for Postacute Care Inpatient Basic Mobility “six-clicks” short form, hereafter abbreviated as “six-clicks.” Based on past experience, patients with scores <16 rarely go home (<20% of the time), and those with scores >20 usually go home regardless of ambulation. Therefore, only patients with scores of 16-20 were invited to participate in the study. Although patients who were not evaluated by PT might also benefit from the intervention, we required a six-clicks score to assess eligibility. The exclusion criteria included anticipated remaining length of stay less than three days, admission under observation status, admission to the intensive care unit (ICU,) patients receiving comfort care measures, and patients with medical conditions precluding ambulation, such as decompensated heart failure or unstable angina.
Randomization
Patients were randomized to “usual care” or “mobility technician” after baseline evaluation using a computerized system. A block randomization scheme with a size of four was used to ensure an approximately equal number of patients per group.
Intervention
Patients randomized to the intervention group were asked to participate in the ambulation protocol outlined by the PT three times daily under the supervision of the mobility technician. The protocol involved four exercise levels (sitting, standing, walking, and stairs), which were implemented depending on the patient’s physical capacity. The mobility technicians, who were PCNAs, were trained by the PT team. PCNAs have no specific degrees or certification. They are taught safe handling techniques during their job orientation, so they already had an understanding of how to transfer and assist a patient with ambulation. The mobility technician training consisted of one four-hour session run by the PT team in the physical therapy department and the nursing unit. The training included safe handling practices and basic mobility, such as transfers from bed to chair, bed to standing, walking with assistance, and walking independently with equipment such as cane, rolling walker, and walking belt. All instruction was demonstrated by the trainer, and the mobility technician was then able to practice. The mobility technician then shadowed the trainer and practiced the techniques under supervision. Competency was assessed by the trainer.
The cohort of patients randomized to “usual care” was not seen by the mobility technicians but was not otherwise restricted in nursing’s baseline ability to execute recommendations placed by the PT team. Compliance with the recommendations is highly variable and dependent on patient acuity during the shift, staffing issues, and competing duties. Cleveland Clinic promotes a “culture of mobility,” and nurses are encouraged to get patients out of bed and assist with ambulation.
Study Instruments—Measures of Mobility
The six-clicks instrument is a tool for measuring basic mobility. It was adapted from the Activity Measures for Post-Acute Care (AM-PAC) instrument.25 Although initially created for self-report in the post-acute care setting, six-clicks has been validated for use by PTs in the acute care setting26 and is currently in use at more than 1,000 US hospitals. Cleveland Clinic PTs have used this measure for routine evaluation since 2011. The instrument has high interrater reliability and can predict discharge disposition.27-29
Each patient was provided with a tracking device (Fitbit) attached at the wrist to record daily steps for measuring mobility. The use of Fitbit has been validated in ambulatory and inpatient settings.30 The device produces step counts within 3% of the observed step count for most patients but may undercount steps in patients with very slow gait.31 The device was provided to each enrollee and collected at discharge.
Variables
Demographic information, comorbid diagnoses, and discharge destination were extracted from the electronic medical record. Information on prehospitalization physical activity level was obtained from the initial PT assessment. Falls were tracked through the safety event reporting system.
Outcomes
The primary outcomes were discharge disposition and hospital length of stay. The secondary outcomes included average steps per day, change in six-clicks score from admission to discharge, inpatient mortality, admission to ICU, falls, deep vein thrombosis, pulmonary embolism, or pneumonia, and readmission within 30 days.
Statistical Analysis
Patient characteristics were summarized as means and standard deviations or medians and interquartile ranges for continuous variables and as frequencies and percentages for categorical variables. The t-test or Wilcoxon rank sum test was applied to compare continuous characteristics between the intervention and control groups. Chi-squared test or Fisher’s exact test was applied to compare categorical characteristics. Given its skewed distribution, the length of stay was log-transformed and compared between the two groups using Student’s t-test. Chi-squared test was used to compare categorical outcomes. The analysis of final six-clicks scores was adjusted for baseline scores, and the least-square estimates are provided. A linear mixed effects model was used to compare the number of daily steps taken because each participant had multiple steps measured. Results were adjusted for prehospital activity. In addition to comparing the total steps taken by each group, we determined the proportion of patients who exceeded a particular threshold by taking the average number of steps per day for all subjects and relating it to home discharge using the Receiver Operating Characteristics (ROC) curve. An optimal cut-off was determined to maximize the Youden index. We also compared the proportion of patients who exceeded 900 steps because this value was previously reported as an important threshold.32 All analyses were conducted using intention-to-treat principles. We also conducted a per-protocol analysis in which we limited the intervention group to those who received at least one assisted ambulation session. A dose-response analysis was also performed, in which patients were categorized as not receiving the therapy, receiving sessions on one or two days, or receiving them on more than two days.
All analyses were conducted using R-studio (Boston, MA). Statistical significance was defined as a P-value < .05. Given that this is a pilot study, the results were not adjusted for multiple comparisons.
RESULTS
Characteristics of patients in the intervention and control groups are shown in Table 1. The patients were mostly white and female, with an average age in the mid-70s (range 61-98). All measures evaluated were not significantly different between the intervention and control groups. However, more patients in the intervention group had a prehospital activity level classified as independent.
Table 2 demonstrates the feasibility of the intervention. Of patients randomized to the intervention group, 74% were ambulated at least once. Once enrolled, the patients successfully participated in assisted ambulation for about two-thirds of their hospital stay. However, the intervention was delivered for only one-third of the total length of stay because most patients were not enrolled on admission. On average, the mobility technicians made 11 attempts to ambulate each patient and 56% of these attempts were successful. The proportion of unsuccessful attempts did not change over the course of the study. The reasons for unsuccessful attempts included patient refusal (n = 102) or unavailability (n = 68), mobility technicians running out of time (n = 2), and other (n = 12).
Initially, the mobility technicians were not available on weekends. In addition, they were often reassigned to other duties by their nurse managers, who were dealing with staffing shortages. As the study progressed, we were able to secure the mobility technicians to work seven days per week and to convince the nurse managers that their role should be protected. Consequently, the median number [IQR] of successful attempts increased from 1.5 [0, 2] in the first two months to 3 [0, 5] in the next three months and finally to 5 [1.5, 13] in the final months (P < .002). The median visit duration was 10 minutes, with an interquartile range of 6-15 minutes.
In the intention-to-treat analysis, patients in the intervention group took close to 50% more steps than did the control patients. After adjustment for prehospital activity level, the difference was not statistically significant. The intervention also did not significantly affect the length of stay or discharge disposition (Table 3). In the per protocol analysis, the difference in the step count was significant, even after adjustment. The six-clicks score also significantly increased.
To assess for dose response, we compared outcomes among patients who received no intervention, those who received two or fewer days of intervention, and those who received more than two days of intervention (Table 4). The length of stay was significantly longer in patients with more than two days of intervention, likely reflecting greater opportunities for exposure to the intervention. The longer intervention time significantly increased the six-clicks score.
We examined the relationship between steps achieved and discharge disposition. Patients who achieved at least 900 steps more often went home than those who did not (79% vs. 56%, P < .05). The ROC for the model of discharge disposition using steps taken as the only predictor had an area under the curve of 0.67, with optimal discrimination at 411 steps. At a threshold of 400 steps, the model had a sensitivity of 75.9% and a specificity of 51.4%. Patients achieving 400 steps were more likely to go home than those who did not achieve that goal (71% vs. 46%, P =.01). More patients in the intervention group achieved the 900 step goal (28% vs. 19%, P = .30) and the 400 step goal (66% vs. 58%, P = .39), but neither association reached statistical significance.
DISCUSSION
In this pilot study conducted with older medical inpatients, we found that assisted ambulation provided by a dedicated mobility technician was feasible and increased the number of steps taken by patients. Not all patients in the treatment group received the intervention partly due to the fact that the program initially did not include weekends and the mobility technicians were sometimes redirected to other nursing duties. Both issues were addressed during the course of the study. In the per protocol analysis, the intervention increased the average six-clicks score and there was a nonsignificant reduction in the percentage of patients discharged to a rehabilitation facility.
A range of hospital-based mobility interventions have been described. Several of which were complex multidisciplinary interventions that included a mobility component. The compliance rates have ranged from 82% to 93.7%,12,13 although a systematic review noted that many studies do not provide this level of information.11 Interventions were carried out by nursing staff and PT with support from family members and social workers.33-35 Ambulation-specific programs have also relied on nurses and PT13,14,36 and, occasionally, on research assistants to implement assisted ambulation protocols.12,37 A recent study that employed research assistants to deliver inhospital ambulation reported achieving 51.3% of intended walks.37
In contradistinction to previous studies, we created a new role, employing PCNAs as dedicated mobility technicians. We did this for two reasons. First, the approach is less expensive than deploying registered nurses or PTs to ambulate patients and therefore more likely to be adopted by hospitals, especially if it can decrease the cost of an episode of care by avoiding subsequent inpatient rehabilitation. Mobility technicians have no degree or certification requirements and are therefore paid less than nurses or physical therapists. Second, by having a single responsibility, mobility technicians were more likely to engage in their task than nurses, who have competing responsibilities. However, when nurse staffing was short, nurse managers were tempted to recall the PCNAs for other nursing duties. It took time before PCNAs and supervisors prioritized this new responsibility. When they did, the number of attempted walks increased substantially, but the percentage of successful attempts remained constant at 56%, highlighting the difficulty of getting hospitalized patients to walk.
On average, patients who received the intervention engaged in 72 minutes of additional physical activity and averaged 990 steps per day. Observational data suggest patients accrue about 1,100 steps in the day before discharge, with older patients accruing closer to 900.21 One study found that older patients with fewer than 900 steps per day were likely to experience a functional decline.32 We also found that patients who achieved at least 900 steps were more likely to go home. However, we found that a lower threshold, namely, 400 steps, offered better discrimination between patients who go home and those who do not. Future prospective studies are needed to establish the appropriate goal for exercise interventions. A lower step goal could dramatically enhance the efficiency of the intervention.
A Cochrane review found that pooled analysis of multidisciplinary interventions that included exercise, often in the form of walking, achieved a small but significant increase in the proportion of patients discharged to home (RR 1.08, 95%CI 1.03 to 1.14).11 We found no significant change in the discharge disposition, but our study was underpowered for this endpoint. The six-clicks score showed a small but significant change in the per protocol analysis. The six-clicks score has been shown to correlate with discharge disposition,28,29 and an improvement in the score suggests that discharge disposition may be influenced.
The intervention may also not have been implemented for long enough. On average, visits were achieved for one-third of the hospital stay partly because of the delay in PT evaluation, which we required for eligibility. In practice, PT evaluation can occur just a few days before the anticipated discharge. We observed a dose dependent response among patients in the intervention group, suggesting that earlier intervention could be more effective. Earlier intervention might be achieved if the MT performed the six-clicks on potentially eligible patients.
The effects of hospitalization on mobility may be the most pronounced in the long term; one study found that 40% of hospitalized older patients manifested new ADL or IADL disability three months after discharge compared with 31% at discharge.7 Hospital-based mobility interventions may continue to affect subjects’ independence for weeks or months. In one RCT, an inpatient ambulation intervention improved mobility in the community one month after discharge.37 A hospital-based exercise program that included ambulation achieved better functional outcomes one month later.13 One RCT that combined inpatient exercise with outpatient care coordination also decreased readmission rates.34 We found that the intervention did not affect readmission.
This pilot study has several limitations. The sample size was small, and the findings need to be replicated in a larger randomized controlled trial. This is particularly important because the two study arms were not balanced in terms of their prehospital activity. After adjustment for prehospital activity, the differences in the step count in the intention-to-treat analysis were no longer significant. As we adjusted the intervention to hospital workflow, the intervention changed over time. The intention-to-treat analysis may therefore underestimate the effect of the intervention. This work provides a basis for future trial. Finally, discharge disposition depends on a complex interplay of factors, including social factors and preferences, which may not be affected by a mobility intervention.
In summary, an inhospital mobility protocol of attempting ambulation delivered by dedicated mobility technicians three times daily successfully increased the daily step counts and mobility scores of the patients. Studies with a larger sample size are needed to determine whether the proposed approach can affect length of hospital stay, discharge disposition, and overall cost of an episode of care.
Disclosures
Mary Stilphen reports consulting for CreCare and Adeo, which license and distribute AM-PAC short forms, including 6 clicks. All other authors report no conflicts of interest.
Funding
This study was supported by a Research Program Committee grant from the Cleveland Clinic.
1. National Center for Health Statistics. National Hospital Discharge Survey. 2010. PubMed
2. Corcoran PJ. Use it or lose it--the hazards of bed rest and inactivity. West J Med. 1991;154(5):536-538. PubMed
3. Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16(10):1033-1038. doi: 10.1016/0277-9536(82)90175-7. PubMed
4. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. PubMed
5. Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266-273. doi: 10.1111/j.1532-5415.2010.03276.x. PubMed
6. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160(6):809-815. doi: 10.1067/mob.2001.107919. PubMed
7. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645-652. doi: 10.1001/archinte.1996.00440060067008. PubMed
8. Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44(4):M112-M117. doi: 10.1093/geronj/44.4.M112. PubMed
9. Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170(21):1942-1943. doi: 0.1001/archinternmed.2010.422. PubMed
10. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, quiz 67-58. PubMed
11. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007(1):CD005955. doi: 10.1002/14651858.CD005955. PubMed
12. Jones CT, Lowe AJ, MacGregor L, Brand CA, Tweddle N, Russell DM. A randomised controlled trial of an exercise intervention to reduce functional decline and health service utilisation in the hospitalised elderly. Australas J Ageing. 2006;25(3):126-133. doi: 10.1111/j.1741-6612.2006.00167.x.
13. Siebens H, Aronow H, Edwards D, Ghasemi Z. A randomized controlled trial of exercise to improve outcomes of acute hospitalization in older adults. J Am Geriatr Soc. 2000;48(12):1545-1552. doi: 10.1111/j.1532-5415.2000.tb03862.x. PubMed
14. Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124(3):883-889. doi: 10.1378/chest.124.3.883. PubMed
15. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi: 10.1111/j.1532-5415.2009.02393.x. PubMed
16. Callen BL, Mahoney JE, Grieves CB, Wells TJ, Enloe M. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs. 2004;25(4):212-217. doi: 10.1016/j.gerinurse.2004.06.016. PubMed
17. Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91-95. doi: 10.1111/j.1532-5415.2010.03202.x. PubMed
18. McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients: A randomized, controlled clinical trial. Ann Intern Med. 1989;110(1):79-84. doi: PubMed
19. Said CM, Morris ME, Woodward M, Churilov L, Bernhardt J. Enhancing physical activity in older adults receiving hospital based rehabilitation: A phase II feasibility study. BMC Geriatr. 2012;12:26. doi: 10.1186/1471-2318-12-26. PubMed
20. Timmerman RA. A mobility protocol for critically ill adults. Dimens Crit Care Nurs. 2007;26(5):175-179; quiz 180-171. doi: 10.1097/01.DCC.0000286816.40570.da. PubMed
21. Sallis R, Roddy-Sturm Y, Chijioke E, et al. Stepping toward discharge: Level of ambulation in hospitalized patients. J Hosp Med. 2015;10(6):384-389. doi: 10.1002/jhm.2343. PubMed
22. Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Jr., Tinetii ME. A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: The yale geriatric care program. J Am Geriatr Soc. 1993;41(12):1353-1360. doi: 10.1111/j.1532-5415.1993.tb06487.x. PubMed
23. Kalisch BJ, Landstrom GL, Hinshaw AS. Missed nursing care: A concept analysis. J Adv Nurs. 2009;65(7):1509-1517. doi: 10.1111/j.1365-2648.2009.05027.x. PubMed
24. Kalisch BJ, Tschannen D, Lee H, Friese CR. Hospital variation in missed nursing care. Am J Med Qual. 2011;26(4):291-299. doi: 10.1177/1062860610395929. PubMed
25. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):I49-161. doi: 10.1097/01.mlr.0000103520.43902.6c. PubMed
26. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. doi: 10.2522/ptj.20130199. PubMed
27. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC 6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. doi: 10.2522/ptj.20140174. PubMed
28. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. doi: 10.2522/ptj.20130359. PubMed
29. Menendez ME, Schumacher CS, Ring D, Freiberg AA, Rubash HE, Kwon YM. Does “6-Clicks” day 1 postoperative mobility score predict discharge disposition after total hip and knee arthroplasties? J Arthroplasty. 2016;31(9):1916-1920. doi: 10.1016/j.arth.2016.02.017. PubMed
30. Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6(8):e10527-e10527. doi: 10.2196/10527. PubMed
31. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther. 2017;97(5):581-588. doi: 10.1093/ptj/pzx010. PubMed
32. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association between 900 steps a day and functional decline in older hospitalized patients. JAMA Intern Med. 2017;177(2):272-274. doi: 10.1001/jamainternmed.2016.7266. PubMed
33. Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. PubMed
34. Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc. 2009;57(3):395-402. doi: 10.1111/j.1532-5415.2009.02138.x. PubMed
35. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. doi: 10.1056/NEJM199505183322006. PubMed
36. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546. PubMed
37. Brown CJ, Foley KT, Lowman JD, Jr., et al. comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921-927. doi: 10.1001/jamainternmed.2016.1870. PubMed
1. National Center for Health Statistics. National Hospital Discharge Survey. 2010. PubMed
2. Corcoran PJ. Use it or lose it--the hazards of bed rest and inactivity. West J Med. 1991;154(5):536-538. PubMed
3. Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16(10):1033-1038. doi: 10.1016/0277-9536(82)90175-7. PubMed
4. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. PubMed
5. Zisberg A, Shadmi E, Sinoff G, Gur-Yaish N, Srulovici E, Admi H. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59(2):266-273. doi: 10.1111/j.1532-5415.2010.03276.x. PubMed
6. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ, 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160(6):809-815. doi: 10.1067/mob.2001.107919. PubMed
7. Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645-652. doi: 10.1001/archinte.1996.00440060067008. PubMed
8. Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44(4):M112-M117. doi: 10.1093/geronj/44.4.M112. PubMed
9. Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170(21):1942-1943. doi: 0.1001/archinternmed.2010.422. PubMed
10. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106(1):58-67, quiz 67-58. PubMed
11. de Morton NA, Keating JL, Jeffs K. Exercise for acutely hospitalised older medical patients. Cochrane Database Syst Rev. 2007(1):CD005955. doi: 10.1002/14651858.CD005955. PubMed
12. Jones CT, Lowe AJ, MacGregor L, Brand CA, Tweddle N, Russell DM. A randomised controlled trial of an exercise intervention to reduce functional decline and health service utilisation in the hospitalised elderly. Australas J Ageing. 2006;25(3):126-133. doi: 10.1111/j.1741-6612.2006.00167.x.
13. Siebens H, Aronow H, Edwards D, Ghasemi Z. A randomized controlled trial of exercise to improve outcomes of acute hospitalization in older adults. J Am Geriatr Soc. 2000;48(12):1545-1552. doi: 10.1111/j.1532-5415.2000.tb03862.x. PubMed
14. Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124(3):883-889. doi: 10.1378/chest.124.3.883. PubMed
15. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi: 10.1111/j.1532-5415.2009.02393.x. PubMed
16. Callen BL, Mahoney JE, Grieves CB, Wells TJ, Enloe M. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs. 2004;25(4):212-217. doi: 10.1016/j.gerinurse.2004.06.016. PubMed
17. Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59(1):91-95. doi: 10.1111/j.1532-5415.2010.03202.x. PubMed
18. McVey LJ, Becker PM, Saltz CC, Feussner JR, Cohen HJ. Effect of a geriatric consultation team on functional status of elderly hospitalized patients: A randomized, controlled clinical trial. Ann Intern Med. 1989;110(1):79-84. doi: PubMed
19. Said CM, Morris ME, Woodward M, Churilov L, Bernhardt J. Enhancing physical activity in older adults receiving hospital based rehabilitation: A phase II feasibility study. BMC Geriatr. 2012;12:26. doi: 10.1186/1471-2318-12-26. PubMed
20. Timmerman RA. A mobility protocol for critically ill adults. Dimens Crit Care Nurs. 2007;26(5):175-179; quiz 180-171. doi: 10.1097/01.DCC.0000286816.40570.da. PubMed
21. Sallis R, Roddy-Sturm Y, Chijioke E, et al. Stepping toward discharge: Level of ambulation in hospitalized patients. J Hosp Med. 2015;10(6):384-389. doi: 10.1002/jhm.2343. PubMed
22. Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Jr., Tinetii ME. A controlled trial of a nursing-centered intervention in hospitalized elderly medical patients: The yale geriatric care program. J Am Geriatr Soc. 1993;41(12):1353-1360. doi: 10.1111/j.1532-5415.1993.tb06487.x. PubMed
23. Kalisch BJ, Landstrom GL, Hinshaw AS. Missed nursing care: A concept analysis. J Adv Nurs. 2009;65(7):1509-1517. doi: 10.1111/j.1365-2648.2009.05027.x. PubMed
24. Kalisch BJ, Tschannen D, Lee H, Friese CR. Hospital variation in missed nursing care. Am J Med Qual. 2011;26(4):291-299. doi: 10.1177/1062860610395929. PubMed
25. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):I49-161. doi: 10.1097/01.mlr.0000103520.43902.6c. PubMed
26. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94(3):379-391. doi: 10.2522/ptj.20130199. PubMed
27. Jette DU, Stilphen M, Ranganathan VK, Passek S, Frost FS, Jette AM. Interrater reliability of AM-PAC 6-Clicks” basic mobility and daily activity short forms. Phys Ther. 2015;95(5):758-766. doi: 10.2522/ptj.20140174. PubMed
28. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94(9):1252-1261. doi: 10.2522/ptj.20130359. PubMed
29. Menendez ME, Schumacher CS, Ring D, Freiberg AA, Rubash HE, Kwon YM. Does “6-Clicks” day 1 postoperative mobility score predict discharge disposition after total hip and knee arthroplasties? J Arthroplasty. 2016;31(9):1916-1920. doi: 10.1016/j.arth.2016.02.017. PubMed
30. Feehan LM, Geldman J, Sayre EC, et al. Accuracy of fitbit devices: Systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth. 2018;6(8):e10527-e10527. doi: 10.2196/10527. PubMed
31. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther. 2017;97(5):581-588. doi: 10.1093/ptj/pzx010. PubMed
32. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association between 900 steps a day and functional decline in older hospitalized patients. JAMA Intern Med. 2017;177(2):272-274. doi: 10.1001/jamainternmed.2016.7266. PubMed
33. Counsell SR, Holder CM, Liebenauer LL, et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. PubMed
34. Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc. 2009;57(3):395-402. doi: 10.1111/j.1532-5415.2009.02138.x. PubMed
35. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. doi: 10.1056/NEJM199505183322006. PubMed
36. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546. PubMed
37. Brown CJ, Foley KT, Lowman JD, Jr., et al. comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921-927. doi: 10.1001/jamainternmed.2016.1870. PubMed
© 2019 Society of Hospital Medicine
Examining the Utility of 30-day Readmission Rates and Hospital Profiling in the Veterans Health Administration
Using methodology created by the Centers for Medicare & Medicaid Services (CMS), the Department of Veterans Affairs (VA) calculates and reports hospital performance measures for several key conditions, including acute myocardial infarction (AMI), heart failure (HF), and pneumonia.1 These measures are designed to benchmark individual hospitals against how average hospitals perform when caring for a similar case-mix index. Because readmissions to the hospital within 30-days of discharge are common and costly, this metric has garnered extensive attention in recent years.
To summarize the 30-day readmission metric, the VA utilizes the Strategic Analytics for Improvement and Learning (SAIL) system to present internally its findings to VA practitioners and leadership.2 The VA provides these data as a means to drive quality improvement and allow for comparison of individual hospitals’ performance across measures throughout the VA healthcare system. Since 2010, the VA began using and publicly reporting the CMS-derived 30-day Risk-Stratified Readmission Rate (RSRR) on the Hospital Compare website.3 Similar to CMS, the VA uses three years of combined data so that patients, providers, and other stakeholders can compare individual hospitals’ performance across these measures.1 In response to this, hospitals and healthcare organizations have implemented quality improvement and large-scale programmatic interventions in an attempt to improve quality around readmissions.4-6 A recent assessment on how hospitals within the Medicare fee-for-service program have responded to such reporting found large degrees of variability, with more than half of the participating institutions facing penalties due to greater-than-expected readmission rates.5 Although the VA utilizes the same CMS-derived model in its assessments and reporting, the variability and distribution around this metric are not publicly reported—thus making it difficult to ascertain how individual VA hospitals compare with one another. Without such information, individual facilities may not know how to benchmark the quality of their care to others, nor would the VA recognize which interventions addressing readmissions are working, and which are not. Although previous assessments of interinstitutional variance have been performed in Medicare populations,7 a focused analysis of such variance within the VA has yet to be performed.
In this study, we performed a multiyear assessment of the CMS-derived 30-day RSRR metric for AMI, HF, and pneumonia as a useful measure to drive VA quality improvement or distinguish VA facility performance based on its ability to detect interfacility variability.
METHODS
Data Source
We used VA administrative and Medicare claims data from 2010 to 2012. After identifying index hospitalizations to VA hospitals, we obtained patients’ respective inpatient Medicare claims data from the Medicare Provider Analysis and Review (MedPAR) and Outpatient files. All Medicare records were linked to VA records via scrambled Social Security numbers and were provided by the VA Information Resource Center. This study was approved by the San Francisco VA Medical Center Institutional Review Board.
Study Sample
Our cohort consisted of hospitalized VA beneficiary and Medicare fee-for-service patients who were aged ≥65 years and admitted to and discharged from a VA acute care center with a primary discharge diagnosis of AMI, HF, or pneumonia. These comorbidities were chosen as they are publicly reported and frequently used for interfacility comparisons. Because studies have found that inclusion of secondary payer data (ie, CMS data) may affect hospital-profiling outcomes, we included Medicare data on all available patients.8 We excluded hospitalizations that resulted in a transfer to another acute care facility and those admitted to observation status at their index admission. To ensure a full year of data for risk adjustment, beneficiaries were included only if they were enrolled in Medicare for 12 months prior to and including the date of the index admission.
Index hospitalizations were first identified using VA-only inpatient data similar to methods outlined by the CMS and endorsed by the National Quality Forum for Hospital Profiling.9 An index hospitalization was defined as an acute inpatient discharge between 2010 and 2012 in which the principal diagnosis was AMI, HF, or pneumonia. We excluded in-hospital deaths, discharges against medical advice, and--for the AMI cohort only--discharges on the same day as admission. Patients may have multiple admissions per year, but only admissions after 30 days of discharge from an index admission were eligible to be included as an additional index admission.
Outcomes
A readmission was defined as any unplanned rehospitalization to either non-VA or VA acute care facilities for any cause within 30 days of discharge from the index hospitalization. Readmissions to observation status or nonacute or rehabilitation units, such as skilled nursing facilities, were not included. Planned readmissions for elective procedures, such as elective chemotherapy and revascularization following an AMI index admission, were not considered as an outcome event.
Risk Standardization for 30-day Readmission
Using approaches developed by CMS,10-12 we calculated hospital-specific 30-day RSRRs for each VA. Briefly, the RSRR is a ratio of the number of predicted readmissions within 30 days of discharge to the expected number of readmissions within 30 days of hospital discharge, multiplied by the national unadjusted 30-day readmission rate. This measure calculates hospital-specific RSRRs using hierarchical logistic regression models, which account for clustering of patients within hospitals and risk-adjusting for differences in case-mix, during the assessed time periods.13 This approach simultaneously models two levels (patient and hospital) to account for the variance in patient outcomes within and between hospitals.14 At the patient level, the model uses the log odds of readmissions as the dependent variable and age and selected comorbidities as the independent variables. The second level models the hospital-specific intercepts. According to CMS guidelines, the analysis was limited to facilities with at least 25 patient admissions annually for each condition. All readmissions were attributed to the hospital that initially discharged the patient to a nonacute setting.
Analysis
We examined and reported the distribution of patient and clinical characteristics at the hospital level. For each condition, we determined the number of hospitals that had a sufficient number of admissions (n ≥ 25) to be included in the analyses. We calculated the mean, median, and interquartile range for the observed unadjusted readmission rates across all included hospitals.
Similar to methods used by CMS, we used one year of data in the VA to assess hospital quality and variation in facility performance. First, we calculated the 30-day RSRRs using one year (2012) of data. To assess how variability changed with higher facility volume (ie, more years included in the analysis), we also calculated the 30-day RSRRs using two and three years of data. For this, we identified and quantified the number of hospitals whose RSRRs were calculated as being above or below the national VA average (mean ± 95% CI). Specifically, we calculated the number and percentage of hospitals that were classified as either above (+95% CI) or below the national average (−95% CI) using data from all three time periods. All analyses were conducted using SAS Enterprise Guide, Version 7.1. The SAS statistical packages made available by the CMS Measure Team were used to calculate RSRRs.
RESULTS
Patient Characteristics
Patients were predominantly older males (98.3%). Among those hospitalized for AMI, most of them had a history of previous coronary artery bypass graft (CABG) (69.1%), acute coronary syndrome (ACS; 66.2%), or documented coronary atherosclerosis (89.8%). Similarly, patients admitted for HF had high rates of CABG (71.3%) and HF (94.6%), in addition to cardiac arrhythmias (69.3%) and diabetes (60.8%). Patients admitted with a diagnosis of pneumonia had high rates of CABG (61.9%), chronic obstructive pulmonary disease (COPD; 58.1%), and previous diagnosis of pneumonia (78.8%; Table 1). Patient characteristics for two and three years of data are presented in Supplementary Table 1.
VA Hospitals with Sufficient Volume to Be Included in Profiling Assessments
There were 146 acute-care hospitals in the VA. In 2012, 56 (38%) VA hospitals had at least 25 admissions for AMI, 102 (70%) hospitals had at least 25 admissions for CHF, and 106 (73%) hospitals had at least 25 admissions for pneumonia (Table 1) and therefore qualified for analysis based on CMS criteria for 30-day RSRR calculation. The study sample included 3,571 patients with AMI, 10,609 patients with CHF, and 10,191 patients with pneumonia.
30-Day Readmission Rates
The mean observed readmission rates in 2012 were 20% (95% CI 19%-21%) among patients admitted for AMI, 20% (95% CI 19%-20%) for patients admitted with CHF, and 15% (95% CI 15%-16%) for patients admitted with pneumonia. No significant variation from these rates was noted following risk standardization across hospitals (Table 2). Observed and risk-standardized rates were also calculated for two and three years of data (Supplementary Table 2) but were not found to be grossly different when utilizing a single year of data.
In 2012, two hospitals (2%) exhibited HF RSRRs worse than the national average (+95% CI), whereas no hospital demonstrated worse-than-average rates (+95% CI) for AMI or pneumonia (Table 3, Figure 1). Similarly, in 2012, only three hospitals had RSRRs better than the national average (−95% CI) for HF and pneumonia.
We combined data from three years to increase the volume of admissions per hospital. Even after combining three years of data across all three conditions, only four hospitals (range: 3.5%-5.3%) had RSRRs worse than the national average (+95% CI). However, four (5.3%), eight (7.1%), and 11 (9.7%) VA hospitals had RSRRs better than the national average (−95% CI).
DISCUSSION
We found that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia showed little variation among VA hospitals. The lack of institutional 30-day readmission volume appears to be a fundamental limitation that subsequently requires multiple years of data to make this metric clinically meaningful. As the largest integrated healthcare system in the United States, the VA relies upon and makes large-scale programmatic decisions based on such performance data. The inability to detect meaningful interhospital variation in a timely manner suggests that the CMS-derived 30-day RSRR may not be a sensitive metric to distinguish facility performance or drive quality improvement initiatives within the VA.
First, we found it notable that among the 146 VA medical centers available for analysis,15 between 38% and 77% of hospitals qualified for evaluation when using CMS-based participation criteria—which excludes institutions with fewer than 25 episodes per year. Although this low degree of qualification for profiling was most dramatic when using one year of data (range: 38%-72%), we noted that it did not dramatically improve when we combined three years of data (range: 52%-77%). These findings act to highlight the population and systems differences between CMS and VA populations16 and further support the idea that CMS-derived models may not be optimized for use in the VA healthcare system.
Our findings are particularly relevant within the VA given the quarterly rate with which these data are reported within the VA SAIL scorecard.2 The VA designed SAIL for internal benchmarking to spotlight successful strategies of top performing institutions and promote high-quality, value-based care. Using one year of data, the minimum required to utilize CMS models, showed that quarterly feedback (ie, three months of data) may not be informative or useful given that few hospitals are able to differentiate themselves from the mean (±95% CI). Although the capacity to distinguish between high and low performers does improve by combining hospital admissions over three years, this is not a reasonable timeline for institutions to wait for quality comparisons. Furthermore, although the VA does present its data on CMS’s Hospital Compare website using three years of combined data, the variability and distribution of such results are not supplied.3
This lack of discriminability raises concerns about the ability to compare hospital performance between low- and high-volume institutions. Although these models function well in CMS settings with large patient volumes in which greater variability exists,5 they lose their capacity to discriminate when applied to low-volume settings such as the VA. Given that several hospitals in the US are small community hospitals with low patient volumes,17 this issue probably occurs in other non-VA settings. Although our study focuses on the VA, others have been able to compare VA and non-VA settings’ variation and distribution. For example, Nuti et al. explored the differences in 30-day RSRRs among hospitalized patients with AMI, HF, and pneumonia and similarly showed little variation, narrow distributions, and few outliers in the VA setting compared to those in the non-VA setting. For small patient volume institutions, including the VA, a focus on high-volume services, outcomes, and measures (ie, blood pressure control, medication reconciliation, etc.) may offer more discriminability between high- and low-performing facilities. For example, Patel et al. found that VA process measures in patients with HF (ie, beta-blocker and ACE-inhibitor use) can be used as valid quality measures as they exhibited consistent reliability over time and validity with adjusted mortality rates, whereas the 30-day RSRR did not.18
Our findings may have substantial financial, resource, and policy implications. Automatically developing and reporting measures created for the Medicare program in the VA may not be a good use of VA resources. In addition, facilities may react to these reported outcomes and expend local resources and finances to implement interventions to improve on a performance outcome whose measure is statistically no different than the vast majority of its comparators. Such events have been highlighted in the public media and have pointed to the fact that small changes in quality, or statistical errors themselves, can have large ramifications within the VA’s hospital rating system.19
These findings may also add to the discussion on whether public reporting of health and quality outcomes improves patient care. Since the CMS began public reporting on RSRRs in 2009, these rates have fallen for all three examined conditions (AMI, HF, and pneumonia),7,20,21 in addition to several other health outcomes.17 Although recent studies have suggested that these decreased rates have been driven by the CMS-sponsored Hospital Readmissions Reduction Program (HRRP),22 others have suggested that these findings are consistent with ongoing secular trends toward decreased readmissions and may not be completely explained by public reporting alone.23 Moreover, prior work has also found that readmissions may be strongly impacted by factors external to the hospital setting, such as patients’ social demographics (ie, household income, social isolation), that are not currently captured in risk-prediction models.24 Given the small variability we see in our data, public reporting within the VA is probably not beneficial, as only a small number of facilities are outliers based on RSRR.
Our study has several limitations. First, although we adapted the CMS model to the VA, we did not include gender in the model because >99% of all patient admissions were male. Second, we assessed only three medical conditions that were being tracked by both CMS and VA during this time period, and these outcomes may not be representative of other aspects of care and cannot be generalized to other medical conditions. Finally, more contemporary data could lead to differing results – though we note that no large-scale structural or policy changes addressing readmission rates have been implemented within the VA since our study period.
The results of this study suggest that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia may not have the capacity to properly detect interfacility variance and thus may not be an optimal quality indicator within the VA. As the VA and other healthcare systems continually strive to improve the quality of care they provide, they will require more accurate and timely metrics for which to index their performance.
Disclosures
The authors have nothing to disclose
1. Medicare C for, Baltimore MS 7500 SB, Usa M. VA Data. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Published October 19, 2016. Accessed July 15, 2018.
2. Strategic Analytics for Improvement and Learning (SAIL) - Quality of Care. https://www.va.gov/QUALITYOFCARE/measure-up/Strategic_Analytics_for_Improvement_and_Learning_SAIL.asp. Accessed July 15, 2018.
3. Snapshot. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Accessed September 10, 2018.
4. Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(4):444-450. doi: 10.1161/CIRCOUTCOMES.111.000101. PubMed
5. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
6. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. doi: 10.1161/CIRCULATIONAHA.114.010270. PubMed
7. Suter LG, Li S-X, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. doi: 10.1007/s11606-014-2862-5. PubMed
8. O’Brien WJ, Chen Q, Mull HJ, et al. What is the value of adding Medicare data in estimating VA hospital readmission rates? Health Serv Res. 2015;50(1):40-57. doi: 10.1111/1475-6773.12207. PubMed
9. NQF: All-Cause Admissions and Readmissions 2015-2017 Technical Report. https://www.qualityforum.org/Publications/2017/04/All-Cause_Admissions_and_Readmissions_2015-2017_Technical_Report.aspx. Accessed August 2, 2018.
10. Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29-37. doi: 10.1161/CIRCOUTCOMES.108.802686. PubMed
11. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. doi: 10.1161/CIRCOUTCOMES.110.957498. PubMed
12. Lindenauer PK, Normand S-LT, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142-150. doi: 10.1002/jhm.890. PubMed
13. Medicare C for, Baltimore MS 7500 SB, Usa M. OutcomeMeasures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Published October 13, 2017. Accessed July 19, 2018.
14. Nuti SV, Qin L, Rumsfeld JS, et al. Association of admission to Veterans Affairs hospitals vs non-Veterans Affairs hospitals with mortality and readmission rates among older hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2016;315(6):582-592. doi: 10.1001/jama.2016.0278. PubMed
15. Solutions VW. Veterans Health Administration - Locations. https://www.va.gov/directory/guide/division.asp?dnum=1. Accessed September 13, 2018.
16. Duan-Porter W (Denise), Martinson BC, Taylor B, et al. Evidence Review: Social Determinants of Health for Veterans. Washington (DC): Department of Veterans Affairs (US); 2017. http://www.ncbi.nlm.nih.gov/books/NBK488134/. Accessed June 13, 2018.
17. Fast Facts on U.S. Hospitals, 2018 | AHA. American Hospital Association. https://www.aha.org/statistics/fast-facts-us-hospitals. Accessed September 5, 2018.
18. Patel J, Sandhu A, Parizo J, Moayedi Y, Fonarow GC, Heidenreich PA. Validity of performance and outcome measures for heart failure. Circ Heart Fail. 2018;11(9):e005035. PubMed
19. Philipps D. Canceled Operations. Unsterile Tools. The V.A. Gave This Hospital 5 Stars. The New York Times. https://www.nytimes.com/2018/11/01/us/veterans-hospitals-rating-system-star.html. Published November 3, 2018. Accessed November 19, 2018.
20. DeVore AD, Hammill BG, Hardy NC, Eapen ZJ, Peterson ED, Hernandez AF. Has public reporting of hospital readmission rates affected patient outcomes?: Analysis of Medicare claims data. J Am Coll Cardiol. 2016;67(8):963-972. doi: 10.1016/j.jacc.2015.12.037. PubMed
21. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017;166(5):324-331. doi: 10.7326/M16-0185. PubMed
22. Medicare C for, Baltimore MS 7500 SB, Usa M. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published March 26, 2018. Accessed July 19, 2018.
23. Radford MJ. Does public reporting improve care? J Am Coll Cardiol. 2016;67(8):973-975. doi: 10.1016/j.jacc.2015.12.038. PubMed
24. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
Using methodology created by the Centers for Medicare & Medicaid Services (CMS), the Department of Veterans Affairs (VA) calculates and reports hospital performance measures for several key conditions, including acute myocardial infarction (AMI), heart failure (HF), and pneumonia.1 These measures are designed to benchmark individual hospitals against how average hospitals perform when caring for a similar case-mix index. Because readmissions to the hospital within 30-days of discharge are common and costly, this metric has garnered extensive attention in recent years.
To summarize the 30-day readmission metric, the VA utilizes the Strategic Analytics for Improvement and Learning (SAIL) system to present internally its findings to VA practitioners and leadership.2 The VA provides these data as a means to drive quality improvement and allow for comparison of individual hospitals’ performance across measures throughout the VA healthcare system. Since 2010, the VA began using and publicly reporting the CMS-derived 30-day Risk-Stratified Readmission Rate (RSRR) on the Hospital Compare website.3 Similar to CMS, the VA uses three years of combined data so that patients, providers, and other stakeholders can compare individual hospitals’ performance across these measures.1 In response to this, hospitals and healthcare organizations have implemented quality improvement and large-scale programmatic interventions in an attempt to improve quality around readmissions.4-6 A recent assessment on how hospitals within the Medicare fee-for-service program have responded to such reporting found large degrees of variability, with more than half of the participating institutions facing penalties due to greater-than-expected readmission rates.5 Although the VA utilizes the same CMS-derived model in its assessments and reporting, the variability and distribution around this metric are not publicly reported—thus making it difficult to ascertain how individual VA hospitals compare with one another. Without such information, individual facilities may not know how to benchmark the quality of their care to others, nor would the VA recognize which interventions addressing readmissions are working, and which are not. Although previous assessments of interinstitutional variance have been performed in Medicare populations,7 a focused analysis of such variance within the VA has yet to be performed.
In this study, we performed a multiyear assessment of the CMS-derived 30-day RSRR metric for AMI, HF, and pneumonia as a useful measure to drive VA quality improvement or distinguish VA facility performance based on its ability to detect interfacility variability.
METHODS
Data Source
We used VA administrative and Medicare claims data from 2010 to 2012. After identifying index hospitalizations to VA hospitals, we obtained patients’ respective inpatient Medicare claims data from the Medicare Provider Analysis and Review (MedPAR) and Outpatient files. All Medicare records were linked to VA records via scrambled Social Security numbers and were provided by the VA Information Resource Center. This study was approved by the San Francisco VA Medical Center Institutional Review Board.
Study Sample
Our cohort consisted of hospitalized VA beneficiary and Medicare fee-for-service patients who were aged ≥65 years and admitted to and discharged from a VA acute care center with a primary discharge diagnosis of AMI, HF, or pneumonia. These comorbidities were chosen as they are publicly reported and frequently used for interfacility comparisons. Because studies have found that inclusion of secondary payer data (ie, CMS data) may affect hospital-profiling outcomes, we included Medicare data on all available patients.8 We excluded hospitalizations that resulted in a transfer to another acute care facility and those admitted to observation status at their index admission. To ensure a full year of data for risk adjustment, beneficiaries were included only if they were enrolled in Medicare for 12 months prior to and including the date of the index admission.
Index hospitalizations were first identified using VA-only inpatient data similar to methods outlined by the CMS and endorsed by the National Quality Forum for Hospital Profiling.9 An index hospitalization was defined as an acute inpatient discharge between 2010 and 2012 in which the principal diagnosis was AMI, HF, or pneumonia. We excluded in-hospital deaths, discharges against medical advice, and--for the AMI cohort only--discharges on the same day as admission. Patients may have multiple admissions per year, but only admissions after 30 days of discharge from an index admission were eligible to be included as an additional index admission.
Outcomes
A readmission was defined as any unplanned rehospitalization to either non-VA or VA acute care facilities for any cause within 30 days of discharge from the index hospitalization. Readmissions to observation status or nonacute or rehabilitation units, such as skilled nursing facilities, were not included. Planned readmissions for elective procedures, such as elective chemotherapy and revascularization following an AMI index admission, were not considered as an outcome event.
Risk Standardization for 30-day Readmission
Using approaches developed by CMS,10-12 we calculated hospital-specific 30-day RSRRs for each VA. Briefly, the RSRR is a ratio of the number of predicted readmissions within 30 days of discharge to the expected number of readmissions within 30 days of hospital discharge, multiplied by the national unadjusted 30-day readmission rate. This measure calculates hospital-specific RSRRs using hierarchical logistic regression models, which account for clustering of patients within hospitals and risk-adjusting for differences in case-mix, during the assessed time periods.13 This approach simultaneously models two levels (patient and hospital) to account for the variance in patient outcomes within and between hospitals.14 At the patient level, the model uses the log odds of readmissions as the dependent variable and age and selected comorbidities as the independent variables. The second level models the hospital-specific intercepts. According to CMS guidelines, the analysis was limited to facilities with at least 25 patient admissions annually for each condition. All readmissions were attributed to the hospital that initially discharged the patient to a nonacute setting.
Analysis
We examined and reported the distribution of patient and clinical characteristics at the hospital level. For each condition, we determined the number of hospitals that had a sufficient number of admissions (n ≥ 25) to be included in the analyses. We calculated the mean, median, and interquartile range for the observed unadjusted readmission rates across all included hospitals.
Similar to methods used by CMS, we used one year of data in the VA to assess hospital quality and variation in facility performance. First, we calculated the 30-day RSRRs using one year (2012) of data. To assess how variability changed with higher facility volume (ie, more years included in the analysis), we also calculated the 30-day RSRRs using two and three years of data. For this, we identified and quantified the number of hospitals whose RSRRs were calculated as being above or below the national VA average (mean ± 95% CI). Specifically, we calculated the number and percentage of hospitals that were classified as either above (+95% CI) or below the national average (−95% CI) using data from all three time periods. All analyses were conducted using SAS Enterprise Guide, Version 7.1. The SAS statistical packages made available by the CMS Measure Team were used to calculate RSRRs.
RESULTS
Patient Characteristics
Patients were predominantly older males (98.3%). Among those hospitalized for AMI, most of them had a history of previous coronary artery bypass graft (CABG) (69.1%), acute coronary syndrome (ACS; 66.2%), or documented coronary atherosclerosis (89.8%). Similarly, patients admitted for HF had high rates of CABG (71.3%) and HF (94.6%), in addition to cardiac arrhythmias (69.3%) and diabetes (60.8%). Patients admitted with a diagnosis of pneumonia had high rates of CABG (61.9%), chronic obstructive pulmonary disease (COPD; 58.1%), and previous diagnosis of pneumonia (78.8%; Table 1). Patient characteristics for two and three years of data are presented in Supplementary Table 1.
VA Hospitals with Sufficient Volume to Be Included in Profiling Assessments
There were 146 acute-care hospitals in the VA. In 2012, 56 (38%) VA hospitals had at least 25 admissions for AMI, 102 (70%) hospitals had at least 25 admissions for CHF, and 106 (73%) hospitals had at least 25 admissions for pneumonia (Table 1) and therefore qualified for analysis based on CMS criteria for 30-day RSRR calculation. The study sample included 3,571 patients with AMI, 10,609 patients with CHF, and 10,191 patients with pneumonia.
30-Day Readmission Rates
The mean observed readmission rates in 2012 were 20% (95% CI 19%-21%) among patients admitted for AMI, 20% (95% CI 19%-20%) for patients admitted with CHF, and 15% (95% CI 15%-16%) for patients admitted with pneumonia. No significant variation from these rates was noted following risk standardization across hospitals (Table 2). Observed and risk-standardized rates were also calculated for two and three years of data (Supplementary Table 2) but were not found to be grossly different when utilizing a single year of data.
In 2012, two hospitals (2%) exhibited HF RSRRs worse than the national average (+95% CI), whereas no hospital demonstrated worse-than-average rates (+95% CI) for AMI or pneumonia (Table 3, Figure 1). Similarly, in 2012, only three hospitals had RSRRs better than the national average (−95% CI) for HF and pneumonia.
We combined data from three years to increase the volume of admissions per hospital. Even after combining three years of data across all three conditions, only four hospitals (range: 3.5%-5.3%) had RSRRs worse than the national average (+95% CI). However, four (5.3%), eight (7.1%), and 11 (9.7%) VA hospitals had RSRRs better than the national average (−95% CI).
DISCUSSION
We found that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia showed little variation among VA hospitals. The lack of institutional 30-day readmission volume appears to be a fundamental limitation that subsequently requires multiple years of data to make this metric clinically meaningful. As the largest integrated healthcare system in the United States, the VA relies upon and makes large-scale programmatic decisions based on such performance data. The inability to detect meaningful interhospital variation in a timely manner suggests that the CMS-derived 30-day RSRR may not be a sensitive metric to distinguish facility performance or drive quality improvement initiatives within the VA.
First, we found it notable that among the 146 VA medical centers available for analysis,15 between 38% and 77% of hospitals qualified for evaluation when using CMS-based participation criteria—which excludes institutions with fewer than 25 episodes per year. Although this low degree of qualification for profiling was most dramatic when using one year of data (range: 38%-72%), we noted that it did not dramatically improve when we combined three years of data (range: 52%-77%). These findings act to highlight the population and systems differences between CMS and VA populations16 and further support the idea that CMS-derived models may not be optimized for use in the VA healthcare system.
Our findings are particularly relevant within the VA given the quarterly rate with which these data are reported within the VA SAIL scorecard.2 The VA designed SAIL for internal benchmarking to spotlight successful strategies of top performing institutions and promote high-quality, value-based care. Using one year of data, the minimum required to utilize CMS models, showed that quarterly feedback (ie, three months of data) may not be informative or useful given that few hospitals are able to differentiate themselves from the mean (±95% CI). Although the capacity to distinguish between high and low performers does improve by combining hospital admissions over three years, this is not a reasonable timeline for institutions to wait for quality comparisons. Furthermore, although the VA does present its data on CMS’s Hospital Compare website using three years of combined data, the variability and distribution of such results are not supplied.3
This lack of discriminability raises concerns about the ability to compare hospital performance between low- and high-volume institutions. Although these models function well in CMS settings with large patient volumes in which greater variability exists,5 they lose their capacity to discriminate when applied to low-volume settings such as the VA. Given that several hospitals in the US are small community hospitals with low patient volumes,17 this issue probably occurs in other non-VA settings. Although our study focuses on the VA, others have been able to compare VA and non-VA settings’ variation and distribution. For example, Nuti et al. explored the differences in 30-day RSRRs among hospitalized patients with AMI, HF, and pneumonia and similarly showed little variation, narrow distributions, and few outliers in the VA setting compared to those in the non-VA setting. For small patient volume institutions, including the VA, a focus on high-volume services, outcomes, and measures (ie, blood pressure control, medication reconciliation, etc.) may offer more discriminability between high- and low-performing facilities. For example, Patel et al. found that VA process measures in patients with HF (ie, beta-blocker and ACE-inhibitor use) can be used as valid quality measures as they exhibited consistent reliability over time and validity with adjusted mortality rates, whereas the 30-day RSRR did not.18
Our findings may have substantial financial, resource, and policy implications. Automatically developing and reporting measures created for the Medicare program in the VA may not be a good use of VA resources. In addition, facilities may react to these reported outcomes and expend local resources and finances to implement interventions to improve on a performance outcome whose measure is statistically no different than the vast majority of its comparators. Such events have been highlighted in the public media and have pointed to the fact that small changes in quality, or statistical errors themselves, can have large ramifications within the VA’s hospital rating system.19
These findings may also add to the discussion on whether public reporting of health and quality outcomes improves patient care. Since the CMS began public reporting on RSRRs in 2009, these rates have fallen for all three examined conditions (AMI, HF, and pneumonia),7,20,21 in addition to several other health outcomes.17 Although recent studies have suggested that these decreased rates have been driven by the CMS-sponsored Hospital Readmissions Reduction Program (HRRP),22 others have suggested that these findings are consistent with ongoing secular trends toward decreased readmissions and may not be completely explained by public reporting alone.23 Moreover, prior work has also found that readmissions may be strongly impacted by factors external to the hospital setting, such as patients’ social demographics (ie, household income, social isolation), that are not currently captured in risk-prediction models.24 Given the small variability we see in our data, public reporting within the VA is probably not beneficial, as only a small number of facilities are outliers based on RSRR.
Our study has several limitations. First, although we adapted the CMS model to the VA, we did not include gender in the model because >99% of all patient admissions were male. Second, we assessed only three medical conditions that were being tracked by both CMS and VA during this time period, and these outcomes may not be representative of other aspects of care and cannot be generalized to other medical conditions. Finally, more contemporary data could lead to differing results – though we note that no large-scale structural or policy changes addressing readmission rates have been implemented within the VA since our study period.
The results of this study suggest that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia may not have the capacity to properly detect interfacility variance and thus may not be an optimal quality indicator within the VA. As the VA and other healthcare systems continually strive to improve the quality of care they provide, they will require more accurate and timely metrics for which to index their performance.
Disclosures
The authors have nothing to disclose
Using methodology created by the Centers for Medicare & Medicaid Services (CMS), the Department of Veterans Affairs (VA) calculates and reports hospital performance measures for several key conditions, including acute myocardial infarction (AMI), heart failure (HF), and pneumonia.1 These measures are designed to benchmark individual hospitals against how average hospitals perform when caring for a similar case-mix index. Because readmissions to the hospital within 30-days of discharge are common and costly, this metric has garnered extensive attention in recent years.
To summarize the 30-day readmission metric, the VA utilizes the Strategic Analytics for Improvement and Learning (SAIL) system to present internally its findings to VA practitioners and leadership.2 The VA provides these data as a means to drive quality improvement and allow for comparison of individual hospitals’ performance across measures throughout the VA healthcare system. Since 2010, the VA began using and publicly reporting the CMS-derived 30-day Risk-Stratified Readmission Rate (RSRR) on the Hospital Compare website.3 Similar to CMS, the VA uses three years of combined data so that patients, providers, and other stakeholders can compare individual hospitals’ performance across these measures.1 In response to this, hospitals and healthcare organizations have implemented quality improvement and large-scale programmatic interventions in an attempt to improve quality around readmissions.4-6 A recent assessment on how hospitals within the Medicare fee-for-service program have responded to such reporting found large degrees of variability, with more than half of the participating institutions facing penalties due to greater-than-expected readmission rates.5 Although the VA utilizes the same CMS-derived model in its assessments and reporting, the variability and distribution around this metric are not publicly reported—thus making it difficult to ascertain how individual VA hospitals compare with one another. Without such information, individual facilities may not know how to benchmark the quality of their care to others, nor would the VA recognize which interventions addressing readmissions are working, and which are not. Although previous assessments of interinstitutional variance have been performed in Medicare populations,7 a focused analysis of such variance within the VA has yet to be performed.
In this study, we performed a multiyear assessment of the CMS-derived 30-day RSRR metric for AMI, HF, and pneumonia as a useful measure to drive VA quality improvement or distinguish VA facility performance based on its ability to detect interfacility variability.
METHODS
Data Source
We used VA administrative and Medicare claims data from 2010 to 2012. After identifying index hospitalizations to VA hospitals, we obtained patients’ respective inpatient Medicare claims data from the Medicare Provider Analysis and Review (MedPAR) and Outpatient files. All Medicare records were linked to VA records via scrambled Social Security numbers and were provided by the VA Information Resource Center. This study was approved by the San Francisco VA Medical Center Institutional Review Board.
Study Sample
Our cohort consisted of hospitalized VA beneficiary and Medicare fee-for-service patients who were aged ≥65 years and admitted to and discharged from a VA acute care center with a primary discharge diagnosis of AMI, HF, or pneumonia. These comorbidities were chosen as they are publicly reported and frequently used for interfacility comparisons. Because studies have found that inclusion of secondary payer data (ie, CMS data) may affect hospital-profiling outcomes, we included Medicare data on all available patients.8 We excluded hospitalizations that resulted in a transfer to another acute care facility and those admitted to observation status at their index admission. To ensure a full year of data for risk adjustment, beneficiaries were included only if they were enrolled in Medicare for 12 months prior to and including the date of the index admission.
Index hospitalizations were first identified using VA-only inpatient data similar to methods outlined by the CMS and endorsed by the National Quality Forum for Hospital Profiling.9 An index hospitalization was defined as an acute inpatient discharge between 2010 and 2012 in which the principal diagnosis was AMI, HF, or pneumonia. We excluded in-hospital deaths, discharges against medical advice, and--for the AMI cohort only--discharges on the same day as admission. Patients may have multiple admissions per year, but only admissions after 30 days of discharge from an index admission were eligible to be included as an additional index admission.
Outcomes
A readmission was defined as any unplanned rehospitalization to either non-VA or VA acute care facilities for any cause within 30 days of discharge from the index hospitalization. Readmissions to observation status or nonacute or rehabilitation units, such as skilled nursing facilities, were not included. Planned readmissions for elective procedures, such as elective chemotherapy and revascularization following an AMI index admission, were not considered as an outcome event.
Risk Standardization for 30-day Readmission
Using approaches developed by CMS,10-12 we calculated hospital-specific 30-day RSRRs for each VA. Briefly, the RSRR is a ratio of the number of predicted readmissions within 30 days of discharge to the expected number of readmissions within 30 days of hospital discharge, multiplied by the national unadjusted 30-day readmission rate. This measure calculates hospital-specific RSRRs using hierarchical logistic regression models, which account for clustering of patients within hospitals and risk-adjusting for differences in case-mix, during the assessed time periods.13 This approach simultaneously models two levels (patient and hospital) to account for the variance in patient outcomes within and between hospitals.14 At the patient level, the model uses the log odds of readmissions as the dependent variable and age and selected comorbidities as the independent variables. The second level models the hospital-specific intercepts. According to CMS guidelines, the analysis was limited to facilities with at least 25 patient admissions annually for each condition. All readmissions were attributed to the hospital that initially discharged the patient to a nonacute setting.
Analysis
We examined and reported the distribution of patient and clinical characteristics at the hospital level. For each condition, we determined the number of hospitals that had a sufficient number of admissions (n ≥ 25) to be included in the analyses. We calculated the mean, median, and interquartile range for the observed unadjusted readmission rates across all included hospitals.
Similar to methods used by CMS, we used one year of data in the VA to assess hospital quality and variation in facility performance. First, we calculated the 30-day RSRRs using one year (2012) of data. To assess how variability changed with higher facility volume (ie, more years included in the analysis), we also calculated the 30-day RSRRs using two and three years of data. For this, we identified and quantified the number of hospitals whose RSRRs were calculated as being above or below the national VA average (mean ± 95% CI). Specifically, we calculated the number and percentage of hospitals that were classified as either above (+95% CI) or below the national average (−95% CI) using data from all three time periods. All analyses were conducted using SAS Enterprise Guide, Version 7.1. The SAS statistical packages made available by the CMS Measure Team were used to calculate RSRRs.
RESULTS
Patient Characteristics
Patients were predominantly older males (98.3%). Among those hospitalized for AMI, most of them had a history of previous coronary artery bypass graft (CABG) (69.1%), acute coronary syndrome (ACS; 66.2%), or documented coronary atherosclerosis (89.8%). Similarly, patients admitted for HF had high rates of CABG (71.3%) and HF (94.6%), in addition to cardiac arrhythmias (69.3%) and diabetes (60.8%). Patients admitted with a diagnosis of pneumonia had high rates of CABG (61.9%), chronic obstructive pulmonary disease (COPD; 58.1%), and previous diagnosis of pneumonia (78.8%; Table 1). Patient characteristics for two and three years of data are presented in Supplementary Table 1.
VA Hospitals with Sufficient Volume to Be Included in Profiling Assessments
There were 146 acute-care hospitals in the VA. In 2012, 56 (38%) VA hospitals had at least 25 admissions for AMI, 102 (70%) hospitals had at least 25 admissions for CHF, and 106 (73%) hospitals had at least 25 admissions for pneumonia (Table 1) and therefore qualified for analysis based on CMS criteria for 30-day RSRR calculation. The study sample included 3,571 patients with AMI, 10,609 patients with CHF, and 10,191 patients with pneumonia.
30-Day Readmission Rates
The mean observed readmission rates in 2012 were 20% (95% CI 19%-21%) among patients admitted for AMI, 20% (95% CI 19%-20%) for patients admitted with CHF, and 15% (95% CI 15%-16%) for patients admitted with pneumonia. No significant variation from these rates was noted following risk standardization across hospitals (Table 2). Observed and risk-standardized rates were also calculated for two and three years of data (Supplementary Table 2) but were not found to be grossly different when utilizing a single year of data.
In 2012, two hospitals (2%) exhibited HF RSRRs worse than the national average (+95% CI), whereas no hospital demonstrated worse-than-average rates (+95% CI) for AMI or pneumonia (Table 3, Figure 1). Similarly, in 2012, only three hospitals had RSRRs better than the national average (−95% CI) for HF and pneumonia.
We combined data from three years to increase the volume of admissions per hospital. Even after combining three years of data across all three conditions, only four hospitals (range: 3.5%-5.3%) had RSRRs worse than the national average (+95% CI). However, four (5.3%), eight (7.1%), and 11 (9.7%) VA hospitals had RSRRs better than the national average (−95% CI).
DISCUSSION
We found that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia showed little variation among VA hospitals. The lack of institutional 30-day readmission volume appears to be a fundamental limitation that subsequently requires multiple years of data to make this metric clinically meaningful. As the largest integrated healthcare system in the United States, the VA relies upon and makes large-scale programmatic decisions based on such performance data. The inability to detect meaningful interhospital variation in a timely manner suggests that the CMS-derived 30-day RSRR may not be a sensitive metric to distinguish facility performance or drive quality improvement initiatives within the VA.
First, we found it notable that among the 146 VA medical centers available for analysis,15 between 38% and 77% of hospitals qualified for evaluation when using CMS-based participation criteria—which excludes institutions with fewer than 25 episodes per year. Although this low degree of qualification for profiling was most dramatic when using one year of data (range: 38%-72%), we noted that it did not dramatically improve when we combined three years of data (range: 52%-77%). These findings act to highlight the population and systems differences between CMS and VA populations16 and further support the idea that CMS-derived models may not be optimized for use in the VA healthcare system.
Our findings are particularly relevant within the VA given the quarterly rate with which these data are reported within the VA SAIL scorecard.2 The VA designed SAIL for internal benchmarking to spotlight successful strategies of top performing institutions and promote high-quality, value-based care. Using one year of data, the minimum required to utilize CMS models, showed that quarterly feedback (ie, three months of data) may not be informative or useful given that few hospitals are able to differentiate themselves from the mean (±95% CI). Although the capacity to distinguish between high and low performers does improve by combining hospital admissions over three years, this is not a reasonable timeline for institutions to wait for quality comparisons. Furthermore, although the VA does present its data on CMS’s Hospital Compare website using three years of combined data, the variability and distribution of such results are not supplied.3
This lack of discriminability raises concerns about the ability to compare hospital performance between low- and high-volume institutions. Although these models function well in CMS settings with large patient volumes in which greater variability exists,5 they lose their capacity to discriminate when applied to low-volume settings such as the VA. Given that several hospitals in the US are small community hospitals with low patient volumes,17 this issue probably occurs in other non-VA settings. Although our study focuses on the VA, others have been able to compare VA and non-VA settings’ variation and distribution. For example, Nuti et al. explored the differences in 30-day RSRRs among hospitalized patients with AMI, HF, and pneumonia and similarly showed little variation, narrow distributions, and few outliers in the VA setting compared to those in the non-VA setting. For small patient volume institutions, including the VA, a focus on high-volume services, outcomes, and measures (ie, blood pressure control, medication reconciliation, etc.) may offer more discriminability between high- and low-performing facilities. For example, Patel et al. found that VA process measures in patients with HF (ie, beta-blocker and ACE-inhibitor use) can be used as valid quality measures as they exhibited consistent reliability over time and validity with adjusted mortality rates, whereas the 30-day RSRR did not.18
Our findings may have substantial financial, resource, and policy implications. Automatically developing and reporting measures created for the Medicare program in the VA may not be a good use of VA resources. In addition, facilities may react to these reported outcomes and expend local resources and finances to implement interventions to improve on a performance outcome whose measure is statistically no different than the vast majority of its comparators. Such events have been highlighted in the public media and have pointed to the fact that small changes in quality, or statistical errors themselves, can have large ramifications within the VA’s hospital rating system.19
These findings may also add to the discussion on whether public reporting of health and quality outcomes improves patient care. Since the CMS began public reporting on RSRRs in 2009, these rates have fallen for all three examined conditions (AMI, HF, and pneumonia),7,20,21 in addition to several other health outcomes.17 Although recent studies have suggested that these decreased rates have been driven by the CMS-sponsored Hospital Readmissions Reduction Program (HRRP),22 others have suggested that these findings are consistent with ongoing secular trends toward decreased readmissions and may not be completely explained by public reporting alone.23 Moreover, prior work has also found that readmissions may be strongly impacted by factors external to the hospital setting, such as patients’ social demographics (ie, household income, social isolation), that are not currently captured in risk-prediction models.24 Given the small variability we see in our data, public reporting within the VA is probably not beneficial, as only a small number of facilities are outliers based on RSRR.
Our study has several limitations. First, although we adapted the CMS model to the VA, we did not include gender in the model because >99% of all patient admissions were male. Second, we assessed only three medical conditions that were being tracked by both CMS and VA during this time period, and these outcomes may not be representative of other aspects of care and cannot be generalized to other medical conditions. Finally, more contemporary data could lead to differing results – though we note that no large-scale structural or policy changes addressing readmission rates have been implemented within the VA since our study period.
The results of this study suggest that the CMS-derived 30-day risk-stratified readmission metric for AMI, HF, and pneumonia may not have the capacity to properly detect interfacility variance and thus may not be an optimal quality indicator within the VA. As the VA and other healthcare systems continually strive to improve the quality of care they provide, they will require more accurate and timely metrics for which to index their performance.
Disclosures
The authors have nothing to disclose
1. Medicare C for, Baltimore MS 7500 SB, Usa M. VA Data. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Published October 19, 2016. Accessed July 15, 2018.
2. Strategic Analytics for Improvement and Learning (SAIL) - Quality of Care. https://www.va.gov/QUALITYOFCARE/measure-up/Strategic_Analytics_for_Improvement_and_Learning_SAIL.asp. Accessed July 15, 2018.
3. Snapshot. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Accessed September 10, 2018.
4. Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(4):444-450. doi: 10.1161/CIRCOUTCOMES.111.000101. PubMed
5. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
6. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. doi: 10.1161/CIRCULATIONAHA.114.010270. PubMed
7. Suter LG, Li S-X, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. doi: 10.1007/s11606-014-2862-5. PubMed
8. O’Brien WJ, Chen Q, Mull HJ, et al. What is the value of adding Medicare data in estimating VA hospital readmission rates? Health Serv Res. 2015;50(1):40-57. doi: 10.1111/1475-6773.12207. PubMed
9. NQF: All-Cause Admissions and Readmissions 2015-2017 Technical Report. https://www.qualityforum.org/Publications/2017/04/All-Cause_Admissions_and_Readmissions_2015-2017_Technical_Report.aspx. Accessed August 2, 2018.
10. Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29-37. doi: 10.1161/CIRCOUTCOMES.108.802686. PubMed
11. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. doi: 10.1161/CIRCOUTCOMES.110.957498. PubMed
12. Lindenauer PK, Normand S-LT, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142-150. doi: 10.1002/jhm.890. PubMed
13. Medicare C for, Baltimore MS 7500 SB, Usa M. OutcomeMeasures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Published October 13, 2017. Accessed July 19, 2018.
14. Nuti SV, Qin L, Rumsfeld JS, et al. Association of admission to Veterans Affairs hospitals vs non-Veterans Affairs hospitals with mortality and readmission rates among older hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2016;315(6):582-592. doi: 10.1001/jama.2016.0278. PubMed
15. Solutions VW. Veterans Health Administration - Locations. https://www.va.gov/directory/guide/division.asp?dnum=1. Accessed September 13, 2018.
16. Duan-Porter W (Denise), Martinson BC, Taylor B, et al. Evidence Review: Social Determinants of Health for Veterans. Washington (DC): Department of Veterans Affairs (US); 2017. http://www.ncbi.nlm.nih.gov/books/NBK488134/. Accessed June 13, 2018.
17. Fast Facts on U.S. Hospitals, 2018 | AHA. American Hospital Association. https://www.aha.org/statistics/fast-facts-us-hospitals. Accessed September 5, 2018.
18. Patel J, Sandhu A, Parizo J, Moayedi Y, Fonarow GC, Heidenreich PA. Validity of performance and outcome measures for heart failure. Circ Heart Fail. 2018;11(9):e005035. PubMed
19. Philipps D. Canceled Operations. Unsterile Tools. The V.A. Gave This Hospital 5 Stars. The New York Times. https://www.nytimes.com/2018/11/01/us/veterans-hospitals-rating-system-star.html. Published November 3, 2018. Accessed November 19, 2018.
20. DeVore AD, Hammill BG, Hardy NC, Eapen ZJ, Peterson ED, Hernandez AF. Has public reporting of hospital readmission rates affected patient outcomes?: Analysis of Medicare claims data. J Am Coll Cardiol. 2016;67(8):963-972. doi: 10.1016/j.jacc.2015.12.037. PubMed
21. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017;166(5):324-331. doi: 10.7326/M16-0185. PubMed
22. Medicare C for, Baltimore MS 7500 SB, Usa M. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published March 26, 2018. Accessed July 19, 2018.
23. Radford MJ. Does public reporting improve care? J Am Coll Cardiol. 2016;67(8):973-975. doi: 10.1016/j.jacc.2015.12.038. PubMed
24. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
1. Medicare C for, Baltimore MS 7500 SB, Usa M. VA Data. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Published October 19, 2016. Accessed July 15, 2018.
2. Strategic Analytics for Improvement and Learning (SAIL) - Quality of Care. https://www.va.gov/QUALITYOFCARE/measure-up/Strategic_Analytics_for_Improvement_and_Learning_SAIL.asp. Accessed July 15, 2018.
3. Snapshot. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data.html. Accessed September 10, 2018.
4. Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6(4):444-450. doi: 10.1161/CIRCOUTCOMES.111.000101. PubMed
5. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. doi: 10.1001/jama.2016.18533. PubMed
6. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796-1803. doi: 10.1161/CIRCULATIONAHA.114.010270. PubMed
7. Suter LG, Li S-X, Grady JN, et al. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333-1340. doi: 10.1007/s11606-014-2862-5. PubMed
8. O’Brien WJ, Chen Q, Mull HJ, et al. What is the value of adding Medicare data in estimating VA hospital readmission rates? Health Serv Res. 2015;50(1):40-57. doi: 10.1111/1475-6773.12207. PubMed
9. NQF: All-Cause Admissions and Readmissions 2015-2017 Technical Report. https://www.qualityforum.org/Publications/2017/04/All-Cause_Admissions_and_Readmissions_2015-2017_Technical_Report.aspx. Accessed August 2, 2018.
10. Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29-37. doi: 10.1161/CIRCOUTCOMES.108.802686. PubMed
11. Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243-252. doi: 10.1161/CIRCOUTCOMES.110.957498. PubMed
12. Lindenauer PK, Normand S-LT, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142-150. doi: 10.1002/jhm.890. PubMed
13. Medicare C for, Baltimore MS 7500 SB, Usa M. OutcomeMeasures. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Published October 13, 2017. Accessed July 19, 2018.
14. Nuti SV, Qin L, Rumsfeld JS, et al. Association of admission to Veterans Affairs hospitals vs non-Veterans Affairs hospitals with mortality and readmission rates among older hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2016;315(6):582-592. doi: 10.1001/jama.2016.0278. PubMed
15. Solutions VW. Veterans Health Administration - Locations. https://www.va.gov/directory/guide/division.asp?dnum=1. Accessed September 13, 2018.
16. Duan-Porter W (Denise), Martinson BC, Taylor B, et al. Evidence Review: Social Determinants of Health for Veterans. Washington (DC): Department of Veterans Affairs (US); 2017. http://www.ncbi.nlm.nih.gov/books/NBK488134/. Accessed June 13, 2018.
17. Fast Facts on U.S. Hospitals, 2018 | AHA. American Hospital Association. https://www.aha.org/statistics/fast-facts-us-hospitals. Accessed September 5, 2018.
18. Patel J, Sandhu A, Parizo J, Moayedi Y, Fonarow GC, Heidenreich PA. Validity of performance and outcome measures for heart failure. Circ Heart Fail. 2018;11(9):e005035. PubMed
19. Philipps D. Canceled Operations. Unsterile Tools. The V.A. Gave This Hospital 5 Stars. The New York Times. https://www.nytimes.com/2018/11/01/us/veterans-hospitals-rating-system-star.html. Published November 3, 2018. Accessed November 19, 2018.
20. DeVore AD, Hammill BG, Hardy NC, Eapen ZJ, Peterson ED, Hernandez AF. Has public reporting of hospital readmission rates affected patient outcomes?: Analysis of Medicare claims data. J Am Coll Cardiol. 2016;67(8):963-972. doi: 10.1016/j.jacc.2015.12.037. PubMed
21. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017;166(5):324-331. doi: 10.7326/M16-0185. PubMed
22. Medicare C for, Baltimore MS 7500 SB, Usa M. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published March 26, 2018. Accessed July 19, 2018.
23. Radford MJ. Does public reporting improve care? J Am Coll Cardiol. 2016;67(8):973-975. doi: 10.1016/j.jacc.2015.12.038. PubMed
24. Barnett ML, Hsu J, McWilliams JM. Patient characteristics and differences in hospital readmission rates. JAMA Intern Med. 2015;175(11):1803-1812. doi: 10.1001/jamainternmed.2015.4660. PubMed
© 2019 Society of Hospital Medicine
Limitation of Life-Sustaining Care in the Critically Ill: A Systematic Review of the Literature
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
© 2019 Society of Hospital Medicine
Things We Do for No Reason: The Use of Thickened Liquids in Treating Hospitalized Adult Patients with Dysphagia
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
© 2019 Society of Hospital Medicine