User login
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
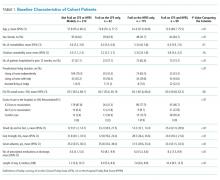
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
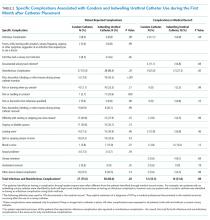
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine
Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Failing to Question a Penicillin Allergy History
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
©2019 Society of Hospital Medicine
Trends in VA Telerehabilitation Patients and Encounters Over Time and by Rurality
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
Historically, the Veterans Health Administration (VHA) has excelled at improving veterans’ access to health care and enhancing foundational services, such as prosthetics and other veteran-centric services, and this continues to be the VHA’s top priority.1 Travel distance and time are often barriers to accessing health care for many veterans.2-11 For veterans with disabilities who must overcome additional physical, cognitive, and emotional obstacles to access vital rehabilitation services, these geographic obstacles are magnified. Further compounding the challenge is that rehabilitation therapies frequently require multiple encounters. Telerehabilitation is a promising solution for veterans in need of rehabilitation to regain optimal functioning. This alternative mode of service delivery can help veterans overcome geographic access barriers by delivering health care directly to veterans in their homes or nearby community-based outpatient clinics.12,13
A growing body of evidence supports telerehabilitation. In a 2017 systematic review and meta-analysis, Cottrell and colleagues reviewed and analyzed data from 13 studies that met their inclusion criteria; specifically, their meta-analytic sample comprised adults aged ≥ 18 years presenting with any diagnosed primary musculoskeletal condition; treatment interventions via a real-time telerehabilitation medium, trials that had a comparison group with the same condition; provided clinical outcomes data, and included published randomized and nonrandomized controlled trials.14 Based on their aggregated results, they concluded that real-time telerehabilitation was effective in improving physical function (standardized mean difference [SMD], 0.63; 95% CI, 0.92-2.33; I2, 93%), and reducing pain (SMD, 0.66; 95% CI, −0.27- .60; I2, 96%) in patients with any diagnosed primary musculoskeletal condition.14
Two other systematic reviews conducted by Pietrzak and colleagues and Agostini and colleagues also demonstrated the clinical effectiveness of telerehabilitation.15,16 Clinical effectiveness was defined as changes in health, functional status, and satisfaction with the telerehabilitation services delivered. The studies examined in the review included those that provided online self-management and education in addition to exercise via teleconferencing in real time.
Pietrzak and colleagues found that Internet-based osteoarthritis self-management interventions significantly improved 4 of 6 health status measures reviewed (ie, pain, fatigue, activity limitation, health distress, disability, and self‐reported global health).15 User acceptance and satisfaction were high (≥ 70% satisfied) in all studies meeting the inclusion criteria.
Agostini and colleagues found that telerehabilitation was more effective than other modes of delivering rehabilitation to regain motor function in cardiac (SMD, 0.24; 95% CI, 0.04-0.43) and total knee arthroplasty (Timed Up and Go test: SMD, −5.17; 95% CI, −9.79- −0.55) patients.16 Some evidence from VHA and non-VHA studies also support the use of telerehabilitation to reduce health care costs,17-19 improve treatment adherence,12,20 and enhance patient physical, cognitive and mobility function, as well as patient satisfaction and health-related quality of life.13,21-24
Since the first recorded use of telehealth in 1959, the application of technology to deliver health care, including rehabilitation services, has increased exponentially.14 In fiscal year (FY) 2017 alone, the VA provided > 2 million episodes of care for > 700,000 veterans using telehealth services.2
Although the process for accessing telerehabilitation may vary throughout the VA, typically a few common factors make a veteran eligible for this mode of rehabilitation care delivery: Veterans must meet criteria for a specific program (eg, amputation, occupational therapy, and physical therapy) and receive VA care from a VA medical facility or clinic that offers telehealth services. Care providers must believe that the veteran would benefit from telerehabilitation (eg, limited mobility and long-distance travel to the facility) and that they would be able to receive an appropriate consult. The veteran must meet the following requirements: (1) willingness to consent to a visit via telehealth; (2) access to required equipment/e-mail; and (3) a caregiver to assist if they are unable to complete a visit independently.
In this article, we provide an overview of the growth of telerehabilitation in the VHA. Data are presented for specific telerehabilitation programs over time and by rurality.
Methods
The VHA Support Service Center works with VHA program offices and field users to provide field-focused business, clinical, and special topic reports. An online portal provides access to these customizable reports organized as data cubes, which represent data dimensions (ie, clinic type) and measures (ie, number of unique patients). For this study, we used the Connected Care, Telehealth, Call Centers Clinical Video Telehealth/Store and Forward Telehealth data cube clinical stop codes to identify the numbers of telerehabilitation veteran users and encounters across time. The following telerehabilitation clinic-stop codes were selected: 197 (polytrauma/traumatic brain injury [TBI]–individuals), 201 (Physical Medicine and Rehabilitation [PM&R] Service), 205 (physical therapy), 206 (occupational therapy), 211 (PM&R amputation clinic), 418 (amputation clinic), 214 (kinesiotherapy), and 240 (PM&R assistive technology clinic). Data for total unique patients served and the total number of encounters were extracted at the national level and by rurality from FY 2012 to FY 2017, providing the past 5 years of VHA telerehabilitation data.
It is important to note that in FY 2015, the VHA changed its definition of rurality to a rural-urban commuting areas (RUCA)-based system (www.ruralhealth.va.gov/rural-definition.asp). Prior to FY 2015, the VHA used the US Census Bureau (CB) urbanized area definitions. According to CB, an urbanized area contains a central city and surrounding area that totals > 50,000 in population. It also includes places outside of urbanized areas with populations > 2,500. Rural areas are defined as all other areas. VHA added a third category, highly rural, which is defined as areas that had < 7 people per square mile. In the RUCA system, each census tract defined by the CB is given a score. The VHA definitions are as follows:
- Urban (U)—census tracts with RUCA scores of 1.0 or 1.1. These tracts are determined by the CB as being in an urban core and having the majority of their workers commute within that same core (1.0). If 30% to 49% commute to an even larger urban core, then the code is 1.1;
- Rural (R)—all tracts not receiving scores in the urban or highly rural tiers; and
- Highly rural (H)—tracts with a RUCA score of 10.0. These are the most remote occupied land areas. Less than 10% of workers travel to CB-defined urbanized areas or urban clusters.
In addition, VHA recently added an “I” category to complement “U,” “R,” and “H.” The “I” value is assigned to veterans living on the US insular islands (ie, territories): Guam, American Samoa, Northern Marianas, and US Virgin Islands. For the analysis by rurality in this study, we excluded veterans living in the insular islands and those of unknown rurality (< 1.0% of patients and encounters). Further, because the numbers of highly rural veterans were relatively small (< 2% of patients and encounters), the rural and highly rural categories were combined and compared with urban-dwelling veterans.
Results
Overall, the workload for telerehabilitation nearly quadrupled over the 5-year period (Table 1 and Figure 1).
Interesting trends were seen by clinic type. Some clinics increased substantially, whereas others showed only moderate increases, and in 1 case (PM&R Service), a decrease. For example, there is significant growth in the number of patients and encounters involving physical therapy through telerehabilitation. This telerehabilitation clinic increased its workload from 1,676 patients with 3,016 encounters in FY 2012 to 9,136 patients with 11,834 encounters in FY 2017, accounting for 62.6% of total growth in patients and 56.8% of total growth in encounters.
Other clinics showing substantial growth over time included occupational therapy and polytrauma/TBI-individual secondary evaluation. Kinesiotherapy telerehabilitation was almost nonexistent in the VHA during FY 2012, with only 23 patients having 23 encounters. By FY 2017, there were 563 patients with 624 kinesiotherapy telerehabilitation encounters, equating to staggering increases in 5 years: 2,348% for patients and 2,613% for encounters. Similarly, the Physical Medicine and Rehabilitation Assistive Technology clinics had very low numbers in FY 2012 (patients, 2; encounters, 3) and increased over time; albeit, at a slow rate.
Trends by Rurality
Trends by rural location of patients and encounters must be interpreted with caution because of the changing rural definition between FY 2014 and FY 2015 (Tables 2 and 3; Figures 3 and 4).
The increased total number of patients seen between FY 2012 and FY 2014 (old definition) was 225% for rural veterans vs 134% for urban veterans. Between FY 2015 and FY 2017 (new definition), the increase was lower for both groups (rural, 13.4%; urban, 7.3%), but rural veterans still increased at a higher rate than did urban dwellers.
Discussion
Our primary aim was to provide data on the growth of telerehabilitation in the VHA over the past 5 years. Our secondary aim was to examine growth in the use of telerehabilitation by rurality. Specifically, we provided an overview of telerehabilitation growth in terms of unique patients and overall encounters in the VHA by rurality from FY 2012 to FY 2014 and FY 2015 to FY 2017 using the following programs: Polytrauma/TBI, PM&R Service, physical therapy, occupational therapy, PM&R amputation clinic, amputation clinic, kinesiotherapy, and PM&R assistive technology clinic. Our findings demonstrated a noteworthy increase in telerehabilitation encounters and unique patients over time for these programs. These findings were consistent with the overall trend of continued growth and expansion of telehealth within the VHA.
Our findings reveal an upward trend in the total number of rural encounters and rural unique patients despite the change in the VA’s definition of rurality in FY 2015. To our knowledge, urban and rural use of telerehabilitation has not been examined previously. Under both definitions of rurality, encounters and unique patients show an important increase over time, and by year-end 2017, more than half of all patients and encounters were attributed to rural patients (53.7% and 53.9%, respectively). Indeed, the upward trend may have been more pronounced if the rural definition had not changed in FY 2015. Our early VHA stroke patients study on the difference between rural-urban patients and taxonomies showed that the RUCA definition was more likely to reduce the number of rural patients by 8.5% than the early definition used by the VHA.26
It is notable that although the use of tele-delivery of rehabilitation has continually increased, the rate of this increase was steeper from FY 2012 to FY 2014 than FY 2015 to FY 2017. For the programs under consideration in this study, the total number of rural patients/encounters increased throughout the observed periods. However, urban patients and encounters increased through FY 2016 and experienced a slight decrease in FY 2017.
The appearance of a slower rate of increase may be due to a rapid initial rate of increase through early adopters and “crossing the diffusion chasm,” a well-documented process of slower diffusion between the time of invention to penetration that often characterizes the spread of successful telehealth innovations
With an emphasis on increasing access to rehabilitation services, the VHA can expect to see a continuing increase in both the number and the percentage of telerehabilitation rural patients and encounters. The VHA has several telerehabilitation initiatives underway through the VHA’s Physical Medicine and Rehabilitation Telerehabilitation Enterprise Wide Initiative (TREWI) and Rural Veterans Telerehabilitation Initiative. These projects demonstrate the feasibility of this delivery approach and facilitate integration of this modality in clinical workflows. However, to sustain these efforts, facilities will need more infrastructure and personnel resources dedicated to the delivery of services.
In an ongoing evaluation of the TREWI, several factors seem to influence the uptake of the VHA Office of Rural Health TREWI programs. These factors are the presence or absence of a local site champion; the quality of hospital leadership support; the quality of past relationships between telerehabilitation sending sites and receiving sites; barriers to getting a telehealth service agreement in place; the availability of space; administrative know-how on setting up clinics appropriately; time involved to bring on staff; contracting issues; equipment availability and installation; cultural issues in embracing technologic innovation; training burden; hassle factors; and limited funds. Although early adopters may be able to negotiate and push through many of the barriers associated with the diffusion of telerehabilitation, the numerous barriers may slow its larger systemwide diffusion.
Telerehabilitation is a promising mode to deliver care to rural veterans who otherwise may not have access to this type of specialty care. Therefore, the identification of elements that foster telerehabilitation growth in future investigations can assist policy makers and key stakeholders in optimally leveraging program resources for maximal productivity. Future studies investigating the drivers of increases in telerehabilitation growth by rurality are warranted. Furthermore, more research is needed to examine telerehabilitation growth quality of care outcomes (eg, patient and provider satisfaction) to ensure that care is not only timely and accessible, but of high quality.
Conclusion
Disparities between rural and urban veterans compel a mode of expanding delivery of care. The VHA has embraced the use of telehealth modalities to extend its reach of rehabilitation services to veterans with disability and rehabilitation needs. Growth in telerehabilitation rural patient encounters increases access to rehabilitative care, reduces patient and caregiver travel burden, and helps ensure treatment adherence. Telerehabilitation utilization (unique patients and total encounters) is growing more rapidly for rural veterans than for their urban counterparts. Overall, telerehabilitation is filling a gap for rural veterans, as well as veterans in general with challenges in accessibility to health care. In order to make full use of the telerehabilitation services across its health care system, VA health care facilities may need to expand their effort in telerehabilitation dissemination and education among providers and veterans, particularly among providers who are less familiar with telerehabilitation services and among veterans who live in rural or highly rural areas and need special rehabilitation care.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.
1. Shane L. What’s in the VA secretary’s 10-point plan to reform his department? https://rebootcamp.militarytimes.com/news/pentagon-congress/2017/02/28/what-s-in-the-va-secretary-s-10-point-plan-to-reform-his-department. Published February 28, 2017. Accessed November 21, 2018.
2. Burgess JF, DeFiore DA. The effect of distance to a VA facility on the choice and level of utilization of VA outpatient services. Soc Science Med. 1994;39(1):95-104.
3. LaVela SL, Smith B, Weaver FM, Miskevics SA. Geographical proximity and health care utilization in veterans with SCI&D in the USA. Soc Science Med. 2004;59:2387-2399.
4. Piette JD, Moos RH. The influence of distance on ambulatory care use, death, and readmission following a myocardial infarction. Health Serv Res. 1996;31(5):573-591.
5. Schmitt SK, Phibbs CS, Piette JD. The influence of distance on utilization of outpatient mental health aftercare following inpatient substance abuse treatment. Addictive Behav. 2003;28(6):1183-1192.
6. Fortney JC, Booth BM, Blow FC, Bunn JY. The effects of travel barriers and age on the utilization of alcoholism treatment aftercare. Am J Drug Alcohol Abuse. 1995;21(3):391-406.
7. McCarthy JF, Blow FC, Valenstein M, et al. Veterans Affairs Health System and mental health treatment retention among patients with serious mental illness: evaluating accessibility and availability barriers. Health Serv Res. 2007;42(3):1042-1060.
8. Mooney C, Zwanziger J, Phibbs CS, Schmitt S. Is travel distance a barrier to veterans’ use of VA hospitals for medical surgical care? Soc Sci Med. 2000;50(12):1743-1755.
9. Friedman SA, Frayne SM, Berg E, et al. Travel time and attrition from VHA care among women veterans: how far is too far? Med Care. 2015;53(4)(suppl 1):S15-S22.
10. Buzza C, Ono SS, Turvey C, et al. Distance is relative: unpacking a principal barrier in rural healthcare. J Gen Intern Med. 2011;26(suppl 2):648-654.
11. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21(3):206-213.
12. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil. 2009;31(6):427-447.
13. McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Phys Med Rehabil Clin N Am. 2010;21(1):195-205.
14. Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638.
15. Pietrzak E, Cotea C, Pullman S, Nasveld P. Self-management and rehabilitation in osteoarthritis: is there a place for internet-based interventions? Telemed J E Health. 2013;19(10):800-805.
16. Agostini M, Moja L, Banzi R, et al. Telerehabilitation and recovery of motor function: a systematic review and meta-analysis. J Telemed Telecare. 2015;21(4):202-213.
17. Kortke H, Stromeyer H, Zittermann A, et al. New East-Westfalian Postoperative Therapy Concept: A telemedicine guide for the study of ambulatory rehabilitation of patients after cardiac surgery. Telemed J E-Health. 2006;12(4):475-483.
18. Tousignant M, Boissy P, Corriveau H, Moffet H. In home telerehabilitation for older adults after discharge from an acute hospital or rehabilitation unit: A proof-of- concept study and costs estimation. Disabil Rehabil Assist Technol. 2006;1(4):209-216.
19. Sanford JA, Griffiths PC, Richardson P, et al. The effects of in-home rehabilitation on task self-efficacy in mobility-impaired adults: a randomized clinical trial. J Am Geriatr Soc. 2006;54(11):1641-1648.
20. Nakamura K, Takano T, Akao C. The effectiveness of videophones in home healthcare for the elderly. Med Care. 1999;37(2):117-125.
21. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361-370.
22. Guilfoyle C, Wootton R, Hassall S, et al. User satisfaction with allied health services delivered to residential facilities via videoconferencing. J Telemed Telecare. 2003;9(1):S52-S54.23. Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320(7248):1517-1520.
24. Williams T L, May C R, Esmail A. Limitations of patient satisfaction studies in telehealthcare: a systematic review of the literature. Telemed J E-Health. 2001;7(4):293-316.
25. US Department of Veterans Affairs, Office of Telehealth Services. http://vaww.telehealth.va.gov/quality/data/index.asp. Accessed June 1, 2018. [Nonpublic document; source not verified.]
26. Jia H, Cowper D, Tang Y, et al. Post-acute stroke rehabilitation utilization: Are there difference between rural-urban patients and taxonomies? J Rural Health. 2012;28(3):242-247.
27. Cho S, Mathiassen L, Gallivan M. Crossing the chasm: from adoption to diffusion of a telehealth innovation. In: León G, Bernardos AM, Casar JR, Kautz K, De Gross JI, eds. Open IT-Based Innovation: Moving Towards Cooperative IT Transfer and Knowledge Diffusion. Boston, MA: Springer; 2008.
28. Broderick A, Lindeman D. Scaling telehealth programs: lessons from early adopters. https://www.commonwealthfund.org/publications/case-study/2013/jan/scaling-telehealth-programs-lessons-early-adopters. Published January 2013. Accessed June 1, 2018.
Norwegian scabies
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
Clinical Guideline Highlights for the Hospitalist: The Use of Intravenous Fluids in the Hospitalized Adult
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
Hospitalized patients often receive intravenous fluids (IVF) when they cannot meet physiologic needs through oral intake in the setting of medical or surgical illness. Prescribing the optimal IVF solution to the appropriate patient is a complex decision and often occurs without the same degree of institutionalized restrictions or guidance developed for other inpatient pharmacologic agents. There is wide variation in clinical utilization of IVF due to the lack of data to guide decision making.1 When data do exist, they typically focus on a limited number of clinical situations.2 Thus, even though IVF are often considered low-risk, the frequency and lack of consistency with which they are used can result in errors, complications, and over-use of medical resources.3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
(Evidence quality: not described in the guideline, recommendation strength: not described in the guideline)
Recommendation 1
To aid in fluid management and avoid complications, the guidelines recommend that patients on IVF require careful assessment of volume status, including a detailed history, physical exam, clinical monitoring, and daily labs.2
Clinical history should focus on understanding fluid losses and intake; physical exam should include vital signs, evidence of orthostatic hypotension, capillary refill, jugular venous pulsation, and assessment for pulmonary edema. Subsequent clinical monitoring should include fluid balance (Ins and Outs) and daily weights. All patients starting or continuing IVF should have a basic metabolic panel at least daily according to the guidelines, though the authors note this frequency may be too high for some patients and needs further study.2
Recommendation 2
The guidelines describe four types of IV fluids that can be administered: crystalloids, balanced crystalloids, glucose solutions, and non blood-product colloids.2
Crystalloids include isotonic saline with 154 millimoles (mmol) of sodium and chloride. Balanced crystalloids, such as lactated Ringer’s solution, are more physiologic, with less sodium and chloride, and the addition of magnesium, potassium, and calcium. Glucose solutions are quickly metabolized and, thus, are an effective way to deliver free water. Non blood-product colloids include particles that are retained within the circulation, including proteins such as human albumin.
Recommendation 3
For each indication to administer IVF, the guidelines recommend the following formulations and considerations:2
For general resuscitation, use crystalloids with sodium content of 130-154 mmol, delivered in a bolus of at least 500 milliliters (mL) over 15 minutes or less. For sepsis, infuse at least 30 mL/kg.4 For routine maintenance, restrict the volume to 25-30 mL/kg/day of water, and include 1 mmol/kg/day of potassium, sodium, and chloride along with 50-100 g/day of glucose to prevent starvation ketosis, though glucose should be avoided in most diabetic patients. With obesity, adjust the IVF to ideal body weight, and for patients who are older, frail, or admitted with renal or cardiac impairment, consider prescribing a lower range of fluid (20-25 mL/kg/day). For redistribution or replacement, use sodium chloride or balanced crystalloids or consider colloids, which have a theoretical advantage in expanding intravascular volume while limiting interstitial edema. Note that colloids are more expensive, and definitive evidence supporting increased efficacy is lacking. Clinicians should monitor closely for hypovolemia, hypervolemia, and electrolyte abnormalities, particularly hypo- and hypernatremia that carry associated mental status implications and risk of central pontine myelinolysis. The inadvertent overuse of IVF is common in hospital settings, particularly when maintenance fluids are not discontinued upon patient improvement or when patients move between care areas. Thus, regular clinical reassessment of volume status is important.
Recommendation 4
In both noncritically ill and critically ill hospitalized patients, there is a benefit to using balanced crystalloids compared to isotonic saline in preventing major adverse kidney events and death.5,6
Two important studies in 2018 added new information to the existing NICE guidelines, addressing the previously unanswered question of the benefits of balanced crystalloids versus isotonic saline, one among non-critically ill patients and the other among critically ill patients.5,6 Prior data suggested that the use of isotonic saline is associated with multiple complications, including hyperchloremic metabolic acidosis, acute kidney injury, and death. In the non-critically ill population, the use of balanced crystalloids resulted in lower incidence of major adverse kidney events (absolute difference of 0.9%), but did not change the number of hospital days (the primary outcome).5 In the critically ill population the use of balanced crystalloids resulted in lower rates of death, new renal replacement therapy, or persistent renal dysfunction,6 and the authors found preferential use of balanced crystalloids could prevent one out of every 94 patients admitted to the ICU from experiencing these adverse outcomes. Given the similar cost associated with isotonic saline and balanced crystalloids, these new findings suggest hospitalists should select balanced crystalloids if there is no compelling clinical reason to use isotonic saline.
CRITIQUE
While conflicts of interest are often a concern in clinical guidelines due to influence by pharmaceutical, device, and specialty interests, the United Kingdom’s National Clinical Guideline Centre (NGC), which developed the NICE guidelines, is hosted by the Royal College of Physicians and has governance partnerships with the Royal College of Surgeons of England, Royal College of General Practitioners, and Royal College of Nursing. Each guideline produced by the NGC is overseen by an independent guideline committee comprised of healthcare professionals and patient representatives, and as a result, concern for conflicts of interest is low.
The NICE guidelines were created by a multidisciplinary team from multiple clinical specialties, and reviewed evidence addressing both clinical and health economic outcomes. Importantly, data from randomized controlled studies was relatively limited. The data excluded patients under 16 years of age, pregnant women, and those with severe liver or renal disease, diabetes or burns, as well as those in intensive care settings. Unfortunately, many medical patients cared for by hospitalists fall into one or more of these categories, limiting applicability of the guidelines.
Two important studies in 2018 added new information to the existing NICE guidelines, as outlined in Recommendation 4.5,6 Both of these studies occurred at a single institution, limiting their generalizability, though each study included a diverse patient population. In the ICU study, treating clinicians were aware of the composition of the assigned crystalloid so the decision to initiate renal-replacement therapy may have been susceptible to treatment bias. In addition, censoring of data collection at hospital discharge may have underestimated the true incidence of death at 30 days and overestimated persistent renal dysfunction at 30 days. Importantly, the trial design did not allow comparison of lactated Ringer’s solution versus Plasma-Lyte. The non-ICU study evaluated patients who began treatment in the emergency department and were subsequently admitted to non-ICU inpatient units—a population that mirrors much of hospitalist practice, however the un-blinded design makes bias a concern. Finally, lactated Ringer’s solution represented more than 95% of the balanced crystalloids used in the trial, so additional study is required to compare Plasma-Lyte with both saline and lactated Ringer’s solution.
AREAS IN NEED OF FUTURE STUDY
More evidence is needed to better understand the appropriate use of IVF in specific clinical scenarios, including to determine if balanced solutions, as compared with isotonic saline, are superior across a spectrum of clinical conditions. For patients with an indication for maintenance fluid administration, determining if a higher sodium content reduces the risk of hyponatremia without increasing the risk of volume overload will help guide practice. Finally, more comprehensive study of the incidence of overuse and complications as a consequence of IVF, as well as the optimal frequency of lab monitoring, is needed to guide understanding of how practicing hospitalists and health systems can help reduce harm and waste
Disclosures
The authors have nothing to disclose.
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
1. Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–221. doi: 10.1093/bja/aev067. PubMed
2. National Clinical Guideline Centre. Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital, London: Royal College of Physicians (UK); 2013 Dec. Updated May 3, 2017. https://www.nice.org.uk/guidance/cg174. Accessed January 25, 2019.
3. Hall A, Ayus J, Moritz M. Things we do for no reason: the default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9):637-640. doi: 10.12788/jhm.3040. PubMed
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2016. Intensive Care Med. 2017;43(3):304-377. doi: 10.1007/s00134-017-4683-6. PubMed
5. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. PubMed
6. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. PubMed
© 2019 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Maintenance Intravenous Fluids in Infants and Children
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
Hospitalized children with inadequate fluid intake are often administered maintenance intravenous fluids (IVFs) to support metabolic needs and sensible losses. Historically, hypotonic IVFs have been the standard, based on theoretical water and electrolyte requirements for estimated energy expenditure.1 However, when combined with increased levels of arginine vasopressin (AVP) seen in acutely ill children which impairs free-water excretion,2 hypotonic IVF can resu
KEY RECOMMENDATION FOR HOSPITALISTS
Patients between the ages of 28 days and 18 years should receive isotonic solutions with appropriate potassium chloride and dextrose for maintenance IVFs (evidence quality: high; recommendation strength: strong)
Isotonic fluids, such as 0.9% NaCl (normal saline), Hartmann solution and PlasmaLyte, contain a sodium concentration similar to that of plasma (135-144 mEq/L). Lactated Ringer solution (LR) is near-isotonic (sodium 130 mEq/L), but was not used in any of the reviewed studies and therefore not included in the recommendation. Excluded are patients with neurosurgical disorders, congenital or acquired cardiac disease, hepatic disease, cancer, renal dysfunction, diabetes insipidus, voluminous watery diarrhea, severe burns, or patients in the neonatal intensive care unit.
The primary benefit of the AAP recommendation is the reduced risk of iatrogenic hyponatremia and its associated sequelae, including complications or impact on cost of care. The number needed to treat with isotonic fluids was 7.5 to prevent any hyponatremia and 27.8 to prevent moderate hyponatremia (<130 mEq/L). Increases in readmission rates, length of stay, and cost of hospitalization have been reported in a recent meta-analysis reviewing the economic burden of hyponatremia in both adults and children.4
Potential harms from the use of isotonic fluids include hypernatremia, hyperchloremic metabolic acidosis, and fluid overload, although available data have not demonstrated an increased risk of these complications. In light of a recent normal saline (NS) shortage in the United States, limited availability is also a consideration. Plasmalyte is more costly than NS and is currently incompatible with the addition of dextrose.
CRITIQUE
Methods in Preparing Guideline
The guideline development committee included broad representation by pediatric experts in primary care, hospital medicine, emergency medicine, critical care medicine, nephrology, anesthesiology, surgery and quality improvement, as well as a guideline methodologist/informatician and epidemiologist.
Search strategies from recently published systematic reviews of clinical trials comparing isotonic with hypotonic maintenance IVFs were used to identify studies eligible for inclusion. A total of 17 studies with 2,455 total patients were initially identified and included. One additional study meeting inclusion criteria was found after the committee convened and excluded from the guideline.5 Three reviewers from the subcommittee performed a structured critical appraisal of each article. The methods of each trial were assessed for risk-of-bias in multiple domains, including randomization, allocation concealment, performance, detection, attrition and reporting. Forest plots were generated using random-effects models and Mantel-Haenzel statistics with the outcome of hyponatremia. The guideline underwent review by various stakeholders including AAP councils, committees, and sections, and individuals considered experts in the field.
A strength of the guideline is the high quality of the evidence and the consistent findings. All of the included studies were randomized clinical trials and the number of included patients was large. Of the 17 included studies, 16 reported a risk ratio favoring isotonic fluids over hypotonic fluids in the prevention of developing hyponatremia; the results of the study that favored hypotonic fluids were not statistically significant on their own. A sensitivity analysis was performed to exclude one study with a 20% weight, determined by multiple factors such as sample size, confidence interval, and an unusually high rate of hyponatremia in the isotonic and hypotonic fluids groups (33.3 % and 70%, respectively).6 After exclusion, there was no change in the overall estimated risk in hypotonic fluids leading to hyponatremia. Only one trial had two sources of high risk of bias (allocation concealment, attrition) and the remaining had only low or unclear risk of biases in the various domains.
The study that was excluded due to its late identification similarly shows increased risk of hyponatremia in groups administered hypotonic fluids (risk ratio 6.5-8.5), and would likely not affect the estimated risk.5
Despite differences in types of patients enrolled, rate of administered fluids, type of IVF, frequency of lab testing, and study duration, the I2 (degree of heterogeneity) of the forest plot of all included studies remained low at 14% and the increased risk of hyponatremia from hypotonic fluids remained consistent.
Due to study design differences, a limitation of the guideline is that no recommendation is made regarding the type of isotonic fluids and the rate of IVF administration. Additionally, due to the low frequency of clinically significant sequelae of hyponatremia, such as hyponatremic encephalopathy, it remains uncertain how many patients would need to be treated with isotonic fluids to prevent a rare but potentially devastating event.
Sources of Potential Conflict of Interest or Bias
The guideline was developed and funded by the AAP. A formal conflict of interest management policy was followed, and subcommittee members had no conflicts of interests or financial relationships relevant to the guideline to disclose.
Generalizability
Given the large number of patients included in the studies and heterogeneity of the population included, the recommendation applies to most patients cared for by pediatric hospitalists. Several patient exclusions relevant to the pediatric hospitalist deserve mention: neonates, kidney disease, and voluminous diarrhea. Neonates under the age of 28 days, including febrile neonates, are excluded from the guideline because of the immature concentrating abilities of neonatal kidneys. Patients with renal impairment were excluded from the guideline recommendation because several studies excluded patients with kidney disease. Hospitalists often care for children who sustain prerenal acute kidney injury from severe dehydration. In this condition, the kidney conserves water through the release of AVP. While an excluded population, these patients would be even more susceptible to develop hyponatremia if administered hypotonic fluids. Patients with “voluminous diarrhea” are excluded from the guideline because those with gastroenteritis with ongoing losses may require IVFs at rates higher than maintenance, and are particularly vulnerable to electrolyte derangements. The guideline, however, does not define voluminous diarrhea, leaving it to the discretion of the treating clinician.
Finally, it is critical to mention that IVF should be considered a therapy to be judiciously used, and discontinued when possible. While the guideline addresses the choice of fluid composition, alternatives to orally or enterally hydrate a patient are always preferred.
AREAS IN NEED OF FUTURE STUDY
While the guideline strongly recommends isotonic fluids for maintenance therapy, the choice of isotonic fluid remains with the clinician. Most included studies used NS for their isotonic groups, but Hartmann’s solution and Plasmalyte were represented in a few studies. LR, one of the more widely used balanced solutions, though slightly hypotonic (130 mEq/L), was not studied. The exclusion of LR from the included studies is unfortunate, as the benefit of balanced solutions compared to NS after significant fluid resuscitation has been shown in the setting of severe sepsis and shock.7 Hyperchloremic metabolic acidosis after fluid resuscitation with NS has raised concern about continuing NS as maintenance fluid and possibly worsening acidosis or hyperchloremia and its adverse effects.8 Further studies on the potential benefit of LR as maintenance fluid, or the potential harms of unbalanced solutions as maintenance fluids in the setting of significant resuscitation are needed.
Disclosures
The authors have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832. PubMed
2. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350-1360. doi: 10.12788/jhm.3177 PubMed
3. Feld LG, Neuspiel DR, Foster BA, et al. Clinical practice guideline: maintenance intravenous fluids in children. Pediatrics. 2018;142(6). doi: 10.12788/jhm.3177 PubMed
4. Corona G, Giuliani C, Parenti G, et al. The economic burden of hyponatremia: systematic review and meta-analysis. Am J Med. 2016;129(8):823-835 e824. doi: 10.12788/jhm.3177 PubMed
5. Pemde HK, Dutta AK, Sodani R, Mishra K. Isotonic intravenous maintenance fluid reduces hospital acquired hyponatremia in young children with central nervous system infections. Indian J Pediatr. 2015;82(1):13-18. doi: 10.12788/jhm.3177 PubMed
6. Shamim A, Afzal K, Ali SM. Safety and efficacy of isotonic (0.9%) vs. hypotonic (0.18%) saline as maintenance intravenous fluids in children: a randomized controlled trial. Indian Pediatr. 2014;51(12):969-974. PubMed
7. Emrath ET, Fortenberry JD, Travers C, McCracken CE, Hebbar KB. Resuscitation with balanced fluids is associated with improved survival in pediatric severe sepsis. Crit Care Med. 2017;45(7):1177-1183. doi: 10.1097/CCM.0000000000002365 PubMed
8. Stenson EK, Cvijanovich NZ, Anas N, et al. Hyperchloremia is associated with complicated course and mortality in pediatric patients with septic shock. Pediatr Crit Care Med. 2018;19(2):155-160. doi: 10.1097/PCC.0000000000001401. PubMed
© 2019 Society of Hospital Medicine
Leadership & Professional Development: Know Your TLR
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
“Better to remain silent and be thought a fool than to speak and remove all doubt..”
—Abraham Lincoln
Have you ever been in a meeting with a supervisor wondering when you will get a chance to speak? Or have you walked away from an interview not knowing much about the candidate because you were talking all the time? If so, it might be time to consider your TLR: Talking to Listening Ratio. The TLR is a leadership pearl of great value. By keeping track of how much you talk versus how much you listen, you learn how and when to keep quiet.
As Mark Goulston wrote, “There are three stages of speaking to other people. In the first stage, you are on task, relevant and concise . . . the second stage (is) when it feels so good to talk, you don’t even notice the other person is not listening. The third stage occurs after you have lost track of what you were saying and begin to realize you might need to reel the other person back in.” Rather than finding a way to re-engage the other person by giving them a chance to talk while you listen, “. . . the usual impulse is to talk even more in an effort to regain their interest.”1
When you are talking, you are not listening—and when you are not listening, you are not learning. Executives who do all the talking at meetings do not have the opportunity to hear the ideas of others. Poor listening can make it appear as if you don’t care what others think. Worse, being a hypocompetent listener can turn you into an ineffective leader—one who does not have the trust or respect of others.
The TLR is highly relevant for hospitalists: physicians and nurses who do all the talking are not noticing what patients or families want to say or what potentially mistaken conclusions they are drawing. Similarly, quality improvement and patient safety champions who do all the talking are not discovering what frontline clinicians think about an initiative or what barriers need to be overcome for success. They are also not hearing novel approaches to the problem or different priorities that should be addressed instead.
Your goal: ensure that your TLR is less than 1. How? Make it a habit to reflect on your TLR after an encounter with a patient, colleague, or supervisor and ask yourself, “Did I listen well?” In addition to its value in monitoring your own talkativeness, use the TLR to measure others. For example, when interviewing a new hire, apply TLR to discover how much patience would be required to work with a candidate. We once interviewed a physician whose TLR was north of 20 . . . we passed on hiring them. The TLR is also helpful for managing meetings. If you find yourself in one with an over-talker (TLR >5), point to the agenda and redirect the discussion. If it’s a direct report or colleague that’s doing all the talking, remind them that you have another meeting in 30 minutes, so they will need to move things along. Better yet: share the TLR pearl with them so that they can reflect on their performance. If you’re dealing with an under-talker (eg, TLR<0.5), encourage them to voice their opinion. Who knows—you might learn a thing or two.
The most surprising aspect to us about TLR is how oblivious people tend to be about it. High TLR’ers have little idea about the effect they have on people while those with an extremely low TLR (less than 0.2) wonder why they didn’t get picked for a project or promotion. Aim for a TLR between 0.5 and 0.7. Doing so will make you a better leader and follower.
Disclosures
Drs. Saint and Chopra are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
1. Goulston M. How to Know If You Talk Too Much. Harvard Business Review. https://hbr.org/2015/06/how-to-know-if-you-talk-too-much. Accessed January 30, 2019.
Things We Do For No Reason: Contact Precautions for MRSA and VRE
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
© 2019 Society of Hospital Medicine
The Complex Problem of Women Trainees in Academic Medicine
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
© 2019 Society of Hospital Medicine