User login
Buried Deep
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
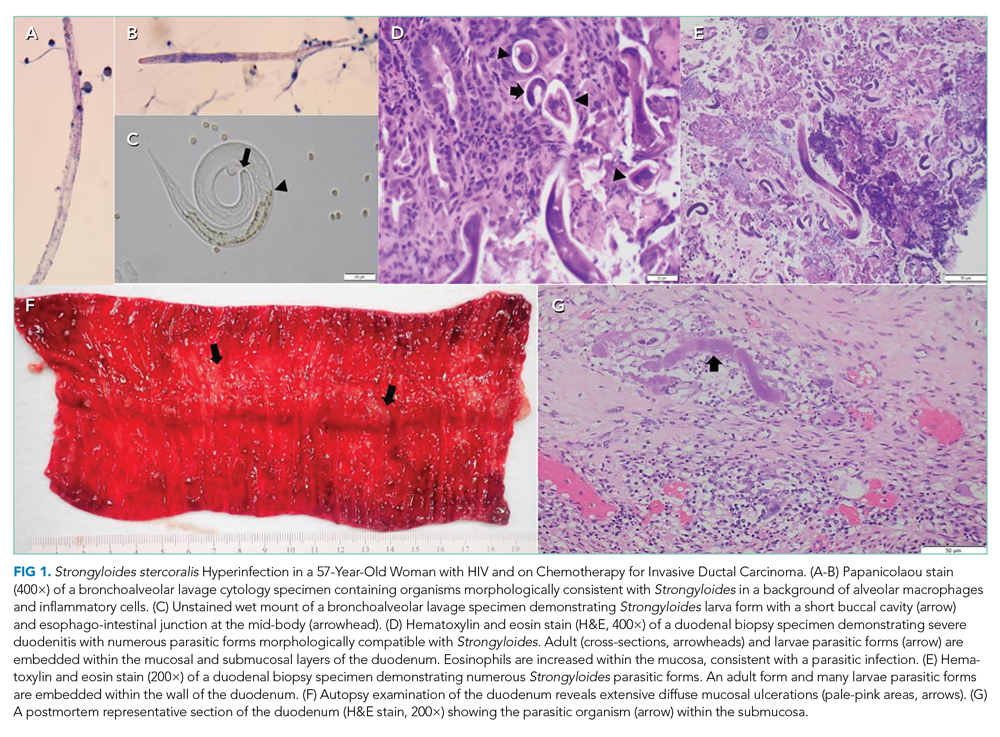
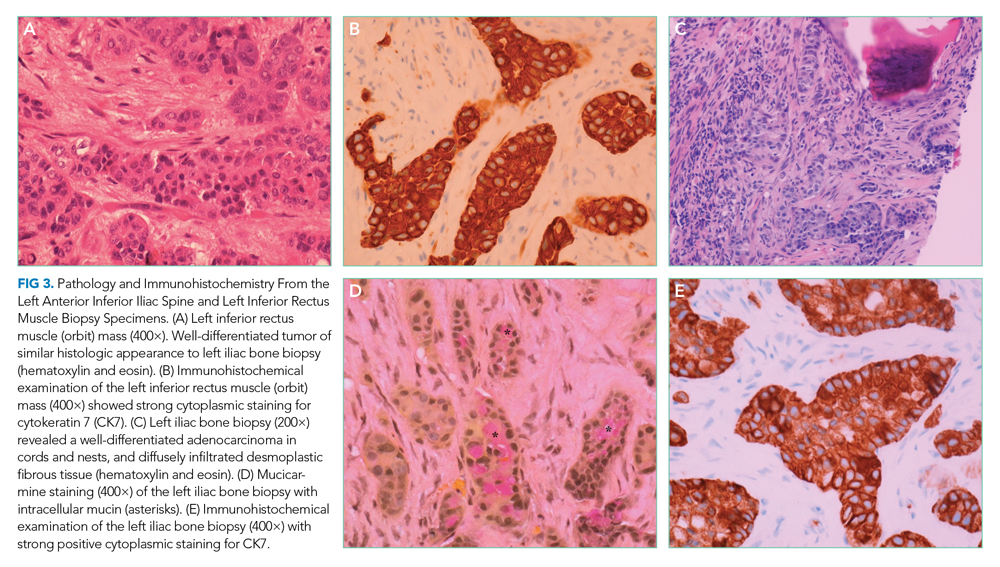
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
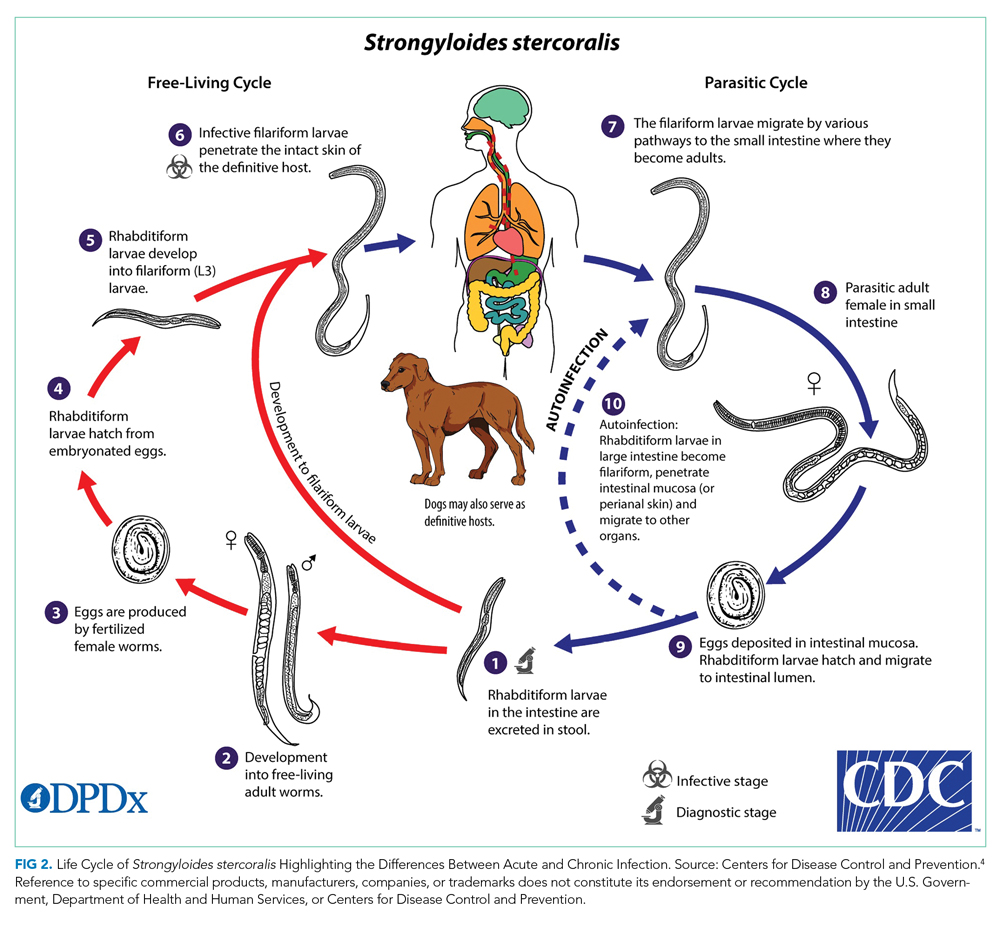
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 56-year-old-woman with a history of HIV and locally invasive ductal carcinoma recently treated with mastectomy and adjuvant doxorubicin and cyclophosphamide, now on paclitaxel, was transferred from another hospital with worsening nausea, epigastric pain, and dyspnea. She had been admitted multiple times to both this hospital and another hospital and had extensive workup over the previous 2 months for gastrointestinal (GI) bleeding and progressive dyspnea with orthopnea and paroxysmal nocturnal dyspnea in the setting of a documented 43-lb weight loss.
Her past medical history was otherwise significant only for the events of the previous few months. Eight months earlier, she was diagnosed with grade 3 triple-negative (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2) invasive ductal carcinoma and underwent mastectomy with negative sentinel lymph node biopsy. She completed four cycles of adjuvant doxorubicin and cyclophosphamide and most recently completed cycle three of paclitaxel chemotherapy.
Her HIV disease was controlled with an antiretroviral regimen of dolutegravir/rilpivirine. She had an undetectable viral load for 20 years (CD4, 239 cells/μL 2 weeks prior to transfer).
Her social history included a 1-pack-year smoking history. She denied alcohol or illicit drug use. Family history included pancreatic cancer in her father and endometrial cancer in her paternal grandmother. She was originally from Mexico but moved to Illinois 27 years earlier.
Work-up for her dyspnea was initiated 7 weeks earlier: noncontrast CT of the chest showed extensive diffuse interstitial thickening and ground-glass opacities bilaterally. Bronchoscopy showed no gross abnormalities, and bronchial washings were negative for bacteria, fungi, Pneumocystis jirovecii , acid-fast bacilli, and cancer. She also had a TTE, which showed an ejection fraction of 65% to 70% and was only significant for a pulmonary artery systolic pressure of 45 mm Hg . She was diagnosed with paclitaxel-induced pneumonitis and was discharged home with prednisone 50 mg daily, dapsone, pantoprazole, and 2 L oxygen via nasal cannula.
Two weeks later, she was admitted for coffee-ground emesis and epigastric pain. Her hemoglobin was 5.9 g/dL, for which she was transfused 3 units of packed red blood cells. EGD showed bleeding from diffuse duodenitis, which was treated with argon plasma coagulation. She was also found to have bilateral pulmonary emboli and lower-extremity deep venous thromboses. An inferior vena cava filter was placed, and she was discharged. One week later, she was readmitted with melena, and repeat EGD showed multiple duodenal ulcers with no active bleeding. Colonoscopy was normal. She was continued on prednisone 40 mg daily, as any attempts at tapering the dose resulted in hypotension.
At the time of transfer, she had presented to the outside hospital with worsening nausea and epigastric pain, increasing postprandial abdominal pain, ongoing weight loss, worsening dyspnea on exertion, paroxysmal nocturnal dyspnea, and orthopnea. She denied symptoms of GI bleeding at that time.
Her imaging is consistent with, albeit not specific for, paclitaxel-induced acute pneumonitis. Her persistent dyspnea may be due to worsening of this pneumonitis.
Upon arrival on physical exam, her temperature was 35.4° C, heart rate 112 beats per minute, blood pressure 135/96 mm Hg, respiratory rate 34 breaths per minute, and oxygen saturation 97% on room air. She was ill- appearing and in mild respiratory distress with severe muscle wasting. Cervical and supraclavicular lymphadenopathy were not detected. Heart sounds were normal without murmurs. Her jugular venous pressure was approximately 7 cm H2O. She had no lower-extremity edema. On lung exam, diffuse rhonchi were audible bilaterally with no crackles or wheezing. There was no accessory muscle use. No clubbing was present. Her abdomen was soft and mildly tender in the epigastrium with normal bowel sounds.
Her labs revealed a white blood cell (WBC) count of 5,050/μL (neutrophils, 3,600/μL; lymphocytes, 560/μL; eosinophils, 560/μL; hemoglobin, 8.7 g/dL; mean corpuscular volume, 89.3 fL; and platelets, 402,000/μL). Her CD4 count was 235 cells/μL. Her comprehensive metabolic panel demonstrated a sodium of 127 mmol/L; potassium, 4.0 mmol/L; albumin, 2.0 g/dL; calcium, 8.6 mg/dL; creatinine, 0.41 mg/dL; aspartate aminotransferase (AST), 11 U/L; alanine aminotransferase (ALT), 17 U/L; and serum osmolarity, 258 mOs/kg. Her lipase was 30 U/L, and lactate was 0.8 mmol/L. Urine studies showed creatinine 41 mg/dL, osmolality 503 mOs/kg, and sodium 53 mmol/L.
At this point, the patient has been diagnosed with multiple pulmonary emboli and recurrent GI bleeding from duodenal ulcers with chest imaging suggestive of taxane-induced pulmonary toxicity. She now presents with worsening dyspnea and upper-GI symptoms.
Her dyspnea may represent worsening of her taxane-induced lung disease. However, she may have developed a superimposed infection, heart failure, or further pulmonary emboli
On exam, she is in respiratory distress, almost mildly hypothermic and tachycardic with rhonchi on auscultation. This combination of findings could reflect worsening of her pulmonary disease and/or infection on the background of her cachectic state. Her epigastric tenderness, upper-GI symptoms, and anemia have continued to cause concern for persistent duodenal ulcers
Her anemia could represent ongoing blood loss since her last EGD or an inflammatory state due to infection. Also of concern is her use of dapsone, which can lead to hemolysis with or without glucose-6-phosphate dehydrogenase deficiency (G6PD), and this should be excluded.
She has hypotonic hyponatremia and apparent euvolemia with a high urine sodium and osmolality; this suggests syndrome of inappropriate antidiuretic hormone secretion, which may be due to her ongoing pulmonary disease process.
On day 3 of her hospitalization, her abdominal pain became more diffuse and colicky, with two episodes of associated nonbloody bilious vomiting. During the next 48 hours, her abdominal pain and tenderness worsened diffusely but without rigidity or peritoneal signs. She developed mild abdominal distention. An abdominal X-ray showed moderate to large stool burden and increased bowel dilation concerning for small bowel obstruction. A nasogastric tube was placed, with initial improvement of her abdominal pain and distention. On the morning of day six of hospitalization, she had approximately 100 mL of hematemesis. She immediately became hypotensive to the 50s/20s, and roughly 400 mL of sanguineous fluid was suctioned from her nasogastric tube. She was promptly given intravenous (IV) fluids and 2 units of cross-matched packed red blood cells with normalization of her blood pressure and was transferred to the medical intensive care unit (MICU).
Later that day, she had an EGD that showed copious clots and a severely friable duodenum with duodenal narrowing. Duodenal biopsies were taken.
The duodenal ulcers have led to a complication of stricture formation and obstruction resulting in some degree of small bowel obstruction. EGD with biopsies can shed light on the etiology of these ulcers and can specifically exclude viral, fungal, protozoal, or mycobacterial infection; infiltrative diseases (lymphoma, sarcoidosis, amyloidosis); cancer; and inflammatory noninfectious diseases such as vasculitis/connective tissue disorder. Biopsy specimens should undergo light and electron microscopy (for protozoa-like Cryptosporidium); stains for fungal infections such as histoplasmosis, Candida, and Cryptococcus; and stains for mycobacterium. Immunohistochemistry and polymerase chain reaction (PCR) testing can identify CMV, HIV, HSV, and EBV within the duodenal tissue.
She remained on methylprednisolone 30 mg IV because of her known history of pneumonitis and concern for adrenal insufficiency in the setting of acute illness. Over the next 3 days, she remained normotensive with a stable hemoglobin and had no further episodes of hematemesis. She was transferred to the general medical floor.
One day later, she required an additional unit of cross-matched red blood cells because of a hemoglobin decrease to 6.4 g/dL. The next day, she developed acute-onset respiratory distress and was intubated for hypoxemic respiratory failure and readmitted to the MICU.
Her drop in hemoglobin may reflect ongoing bleeding from the duodenum or may be due to diffuse alveolar hemorrhage (DAH) complicating her pneumonitis. The deterioration in the patient’s respiratory status could represent worsening of her taxane pneumonitis (possibly complicated by DAH or acute respiratory distress syndrome), as fatalities have been reported despite steroid treatment. However, as stated earlier, it is prudent to exclude superimposed pulmonary infection or recurrent pulmonary embolism. Broad-spectrum antibiotics should be provided to cover hospital-acquired pneumonia. Transfusion-related acute lung injury (TRALI) as a cause of her respiratory distress is much less likely given onset after 24 hours from transfusion. Symptoms of TRALI almost always develop within 1 to 2 hours of starting a transfusion, with most starting within minutes. The timing of respiratory distress after 24 hours of transfusion also makes transfusion-associated circulatory overload unlikely, as this presents within 6 to 12 hours of a transfusion being completed and generally in patients receiving large transfusion volumes who have underlying cardiac or renal disease.
Her duodenal pathology revealed Strongyloides stercoralis infection (Figure 1), and she was placed on ivermectin. Steroids were continued due to concern for adrenal insufficiency in the setting of critical illness and later septic shock. Bronchoscopy was also performed, and a specimen grew S stercoralis. She developed septic shock from disseminated S stercoralis infection that required vasopressors. Her sanguineous orogastric output increased, and her abdominal distension worsened, concerning for an intra-abdominal bleed or possible duodenal perforation. As attempts were made to stabilize the patient, ultimately, she experienced cardiac arrest and died.
The patient succumbed to hyperinfection/dissemination of strongyloidiasis. Her risk factors for superinfection included chemotherapy and high-dose steroids, which led to an unchecked autoinfection.
A high index of suspicion remains the most effective overall diagnostic tool for superinfection, which carries a mortality rate of up to 85% even with treatment. Therefore, prevention is the best treatment. Asymptomatic patients with epidemiological exposure or from endemic areas should be evaluated for empiric treatment of S stercoralis prior to initiation of immunosuppressive treatment.
COMMENTARY
Strongyloides stercoralis is a helminth responsible for one of the most overlooked tropical diseases worldwide.1 It is estimated that 370 million individuals are infected with S stercoralis globally, and prevalence in the endemic tropics and subtropics is 10% to 40%.2,3Strongyloides stercoralis infection is characterized by typically nonspecific cutaneous, pulmonary, and GI symptoms, and chronic infection can often be asymptomatic. Once the infection is established, the entirety of the S stercoralis unique life cycle can occur inside the human host, forming a cycle of endogenous autoinfection that can keep the host chronically infected and infectious for decades (Figure 24). While our patient was likely chronically infected for 27 years, cases of patients being infected for up to 75 years have been reported.5 Though mostly identified in societies where fecal contamination of soil and poor sanitation are common, S stercoralis should be considered among populations who have traveled to endemic areas and are immunocompromised.
Most chronic S stercoralis infections are asymptomatic, but infection can progress to the life-threatening hyperinfection phase, which has a mortality rate of approximately 85%.6 Hyperinfection and disseminated disease occur when there is a rapid proliferation of larvae within the pulmonary and GI tracts, but in the case of disseminated disease, may travel to the liver, brain, and kidneys.7,8 Typically, this is caused by decreased cellular immunity, often due to preexisting conditions such as human T-cell leukemia virus type 1 (HTLV-1) or medications that allow larvae proliferation to go unchecked.6,7 One common class of medications known to increase risk of progression to hyperinfection is corticosteroids, which are thought to both depress immunity and directly increase larvae population growth.6,9 Our patient had been on a prolonged course of steroids for her pulmonary symptoms, with increased doses during her acute illness because of concern for adrenal insufficiency; this likely further contributed to her progression to hyperinfection syndrome. Furthermore, the patient was also immunocompromised from chemotherapy. In addition, she had HIV, which has a controversial association with S stercoralis infection. While previously an AIDS-defining illness, prevalence data indicate a significant co-infection rate between S stercoralis and HIV, but it is unlikely that HIV increases progression to hyperinfection.3
Diagnosing chronic S stercoralis infection is difficult given the lack of a widely accepted gold standard for diagnosis. Traditionally, diagnosis relied on direct visualization of larvae with stool microscopy studies. However, to obtain adequate sensitivity from this method, up to seven serial stool samples must be examined, which is impractical from patient, cost, and efficiency standpoints.10 While other stool-based techniques, such as enriching the stool sample, stool agar plate culture, or PCR-based stool analysis, improve sensitivity, all stool-based studies are limited by intermittent larvae shedding and low worm burden associated with chronic infection.11 Conversely, serologic studies have higher sensitivity, but concerns exist about lower specificity due to potential cross-reactions with other helminths and the persistence of antibodies even after larvae eradication.11,12 Patients with suspected S stercoralis infection and pulmonary infiltrates on imaging may have larvae visible on sputum cultures. A final diagnostic method is direct visualization via biopsy during endoscopy or bronchoscopy, which is typically recommended in cases where suspicion is high yet stool studies have been negative.13 Our patient’s diagnosis was made by duodenal biopsy after her stool study was negative for S stercoralis.
Deciding who to test is difficult given the nonspecific nature of the symptoms but critically important because of the potential for mortality if the disease progresses to hyperinfection. Diagnosis should be suspected in a patient who has spent time in an endemic area and presents with any combination of pulmonary, dermatologic, or GI symptoms. If suspicion for infection is high in a patient being assessed for solid organ transplant or high-dose steroids, prophylactic treatment with ivermectin should be considered. Given the difficulty in diagnosis, some have suggested using eosinophilia as a key diagnostic element, but this has poor predictive value, particularly if the patient is on corticosteroids.7 This patient did not manifest with significant eosinophilia throughout her hospitalization.
This case highlights the difficulties of S stercoralis diagnosis given the nonspecific and variable symptoms, limitations in testing, and potential for remote travel history to endemic regions. It further underscores the need for provider vigilance when starting patients on immunosuppression, even with steroids, given the potential to accelerate chronic infections that were previously buried deep in the mucosa into a lethal hyperinfectious state.
TEACHING POINTS
- The cycle of autoinfection by S stercoralis allows it to persist for decades even while asymptomatic. This means patients can present with infection years after travel to endemic regions.
- Because progression to hyperinfection syndrome carries a high mortality rate and is associated with immunosuppressants, particularly corticosteroids, screening patients from or who have spent time in endemic regions for chronic S stercoralis infection is recommended prior to beginning immunosuppression.
- Diagnosing chronic S stercoralis infection is difficult given the lack of a highly accurate, gold-standard test. Therefore, if suspicion for infection is high yet low-sensitivity stool studies have been negative, direct visualization with a biopsy is a diagnostic option.
Acknowledgment
The authors thank Dr Nicholas Moore, microbiologist at Rush University Medical Center, for his assistance in obtaining and preparing the histology images.
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
1. Olsen A, van Lieshout L, Marti H, et al. Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103(10):967-972. https://doi.org/10.1016/j.trstmh.2009.02.013
2. Bisoffi Z, Buonfrate D, Montresor A, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7(5):e2214. https://doi.org/10.1371/journal.pntd.0002214
3. Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7(7):e2288. https://doi.org/10.1371/journal.pntd.0002288
4. Silva AJ, Moser M. Life cycle of Strongyloides stercoralis. Accessed June 5, 2020. https://www.cdc.gov/parasites/strongyloides/biology.html
5. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis. 2011;17(5):931-932. https://doi.org/10.3201/eid1705.100490
6. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273. https://doi.org/10.1017/S0031182016000834
7. Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24(2):93-96. https://doi.org/10.1155/2013/290814
8. Kassalik M, Mönkemüller K. Strongyloides stercoralis hyperinfection syndrome and disseminated disease. Gastroenterol Hepatol (N Y). 2011;7(11):766-768.
9. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5(4):345-355. https://doi.org/10.1128/cmr.5.4.345
10. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33(7):1040-1047. https://doi.org/10.1086/322707
11. Buonfrate D, Requena-Mendez A, Angheben A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(2):e0006229. dohttps://doi.org/10.1371/journal.pntd.0006229
12. Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52(3):371-378. https://doi.org/10.1016/j.jmii.2018.10.001
13. Lowe RC, Chu JN, Pierce TT, Weil AA, Branda JA. Case 3-2020: a 44-year-old man with weight loss, diarrhea, and abdominal pain. N Engl J Med. 2020;382(4):365-374. https://doi.org/10.1056/NEJMcpc1913473
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Obtaining Urine Testing in Older Adults With Delirium Without Signs or Symptoms of Urinary Tract Infection
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 78-year-old female nursing home resident presents to the emergency department for evaluation of a several-hour history of confusion and restlessness. The patient is accompanied by one of her caregivers from the nursing home. Initial evaluation reveals an awake but inattentive, disoriented, and agitated woman who can answer basic questions appropriately. The caregiver denies the patient having had any antecedent concerns, such as pain with urination, abdominal pain, subjective fevers, chills, or night sweats. Vital signs include a temperature of 37.5 °C (99.5 °F), heart rate of 90 beats per minute, blood pressure of 110/60 mm Hg, respiratory rate of 14 breaths per minute, and oxygen saturation of 98% on room air. The patient has a normal lung and abdominal exam without any suprapubic or flank tenderness. There is no Foley catheter in place.
BACKGROUND
Delirium, defined by the World Health Organization’s 10th revision of the International Classification of Diseases as “an etiologically nonspecific organic cerebral syndrome characterized by concurrent disturbances of consciousness and attention, perception, thinking, memory, psychomotor behavior, emotion, and the sleep-wake schedule,” is associated with poor clinical outcomes in older patients.1,2 Mental status changes, which can arise rapidly over the course of hours to days, often fluctuate, with most cases resolving within days of onset.3 In the United States, more than 2.6 million adults aged 65 years and older develop delirium each year, accounting for an estimated $38 to $152 billion in annual healthcare expenditures.4
WHY YOU MIGHT THINK URINE TESTING IS HELPFUL IN OLDER ADULTS WITH DELIRIUM WHO HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
Some clinicians believe that the evaluation for delirium should include an empiric urinary infectious workup with urinalysis and/or urine cultures, even in the absence of local genitourinary symptoms or other signs of infection. In fact, altered mental status is the most common indication for ordering a urine culture in older adult patients.5
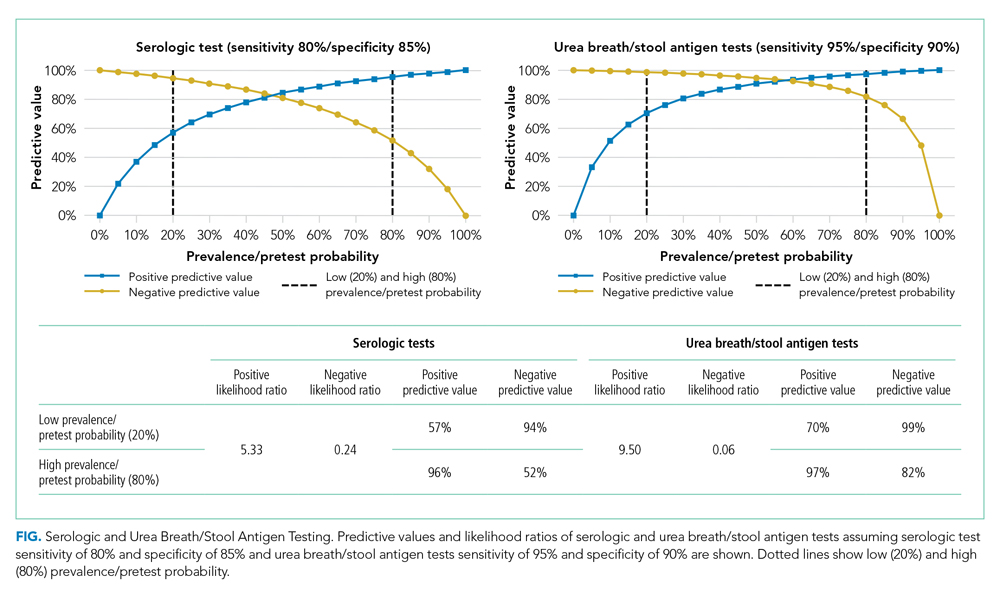
Urinary tract infections (UTIs) account for almost 25% of all reported infections in older patients, with delirium occurring in up to 30% of this patient population.6 As one study demonstrated, given this population’s very high prevalence of asymptomatic bacteriuria (ASB), urine studies sent during a delirium work-up often yield positive findings (defined as ≥105 colony-forming units [CFU]/mL [≥108 CFU/L]) in older patients with no signs or symptoms attributable to UTI.7 The incidence of ASB increases significantly with age, with prevalence estimated to be between 6% and 10% in women older than 60 years and approximately 5% in men older than 65 years.5 Among older patients residing in long-term care facilities, up to 50% of female residents and up to 40% of male residents have ASB.8 These findings, in part, created the common perception of causation between UTI and delirium.
WHY YOU SHOULD NOT OBTAIN URINE TESTING IN OLDER ADULTS WITH DELIRIUM IF THEY HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
A recent systematic review demonstrated that there is insufficient evidence to associate UTI with acute confusion in older patients.9 The Centers for Disease Control and Prevention’s National Health Safety Network notes that at least one of the following criteria must be present for the diagnosis of UTI in noncatheterized patients: fever (>38 °C), suprapubic tenderness, costovertebral angle tenderness, urinary frequency, urinary urgency, or dysuria.10 Recent studies have identified that ASB—by definition, without dysuria, frequency, bladder discomfort, or fever—is an unlikely cause of delirium.6,11
The 2019 Infectious Diseases Society of America (IDSA) practice guidelines suggest that clinicians not screen for ASB in older functionally or cognitively impaired patients with no local genitourinary symptoms or other signs of infection. The IDSA acknowledges that the potential adverse outcomes of antimicrobial therapy, including Clostridioides difficile infection, increased antimicrobial resistance, or adverse drug effects, outweigh the potential benefit of treatment given the absence of evidence that such treatment improves outcomes for this vulnerable patient population (strong recommendation, very low-quality evidence).12 Per the IDSA guidelines, recommendations are strong when there is “moderate- or high-quality evidence that the desirable consequences outweigh the undesirable consequences for a course of action” and “may also be strong when there is high-quality evidence of harm and benefits are uncertain (ie, low or very low quality),” as in this case scenario. Studies of older institutionalized and hospitalized patients have found that ASB often results in inappropriate antimicrobial use with limited benefit.7,13,14 In addition to noting the lack of benefit from treatment, these studies have found that these patients treated with antimicrobials have worse outcomes when compared to untreated patients with ASB. One study of hospitalized patients treated for ASB concluded that participants given antimicrobial agents experienced longer durations of hospitalization, with no benefits from treatment.13 Moreover, another study identified poor long-term functional recovery in patients treated for ASB.14
Overtreatment also has public health implications given that it may increase the prevalence of multidrug-resistant bacteria in long-term care facilities.15 One recent study of nursing home residents demonstrated an association between bacteriuria, increased antibiotic use, and subsequent isolation of multidrug-resistant gram-negative organisms.16 The increased prevalence of these organisms limits options for oral antibiotic therapies in the outpatient setting, potentially leading to increased healthcare utilization and further harms relating to institutionalization in this vulnerable patient population. In light of the ethical concept of nonmaleficence, recognizing the potential harms of treating ASB without clear benefit is important for clinicians to take into account when considering urinalysis in this patient population.
In addition, obtaining a urine culture in an older patient with no signs or symptoms of UTI may lead to premature closure from a diagnostic perspective, resulting in missed diagnoses during clinical evaluation. A missed alternative diagnosis could then cause additional, ongoing harm to the patient if left untreated. Subsequent harms from delayed treatment can thus compound the direct harms and added costs incurred by inappropriate testing and treatment of patients with delirium.
Since 2013, the American Geriatrics Society (AGS) has recommended against the use of antimicrobials in older patients with no urinary tract symptoms, stating that “Antimicrobial treatment studies for asymptomatic bacteriuria in older adults demonstrate no benefits and show increased adverse antimicrobial effects.”17 The IDSA practice guidelines state the following: “In older patients with functional and/or cognitive impairment with bacteriuria and delirium (acute mental status change, confusion) and without local genitourinary symptoms or other systemic signs of infection (eg, fever or hemodynamic instability), we recommend assessment for other causes and careful observation rather than antimicrobial treatment (strong recommendation, very low-quality evidence).”12
WHEN YOU SHOULD OBTAIN URINALYSIS FOR OLDER ADULTS WITH DELIRIUM
Older patients presenting with confusion in the setting of recognized symptoms of UTI (eg, acute dysuria, urinary urgency or frequency) warrant urinalysis and urine culture. Additionally, urinalysis and urine cultures may be warranted to assess for UTI—even in the absence of a localizing source—in older patients with signs and symptoms of delirium who also exhibit systemic signs of infection (eg, fever, leukocytosis, hemodynamic instability).12
WHAT YOU SHOULD DO INSTEAD
Initial evaluation of an older patient with delirium should include a thorough review of their recent history and baseline mental status with a knowledgeable informant, a careful physical and neurologic examination, and laboratory studies to determine the presence of electrolyte or metabolic derangements as well as infection and organ failure.4 Clinicians should take into account nonmodifiable risk factors for delirium and conduct a careful review of the time course of changes in mental status and modifiable risk factors, including environment, sleep deprivation, medications, immobilization, and sensory impairments.18
To manage delirium in older patients, clinicians should identify reversible causes of the delirium and minimize modifiable exacerbating factors (eg, sensory impairment, sleep deprivation) in the immediate environment of the patient. They should also carefully review medications that may contribute to delirium, using tools such as the AGS Beers Criteria to identify high-risk medications and concerning medication combinations.19 Patients who develop local or systemic signs of infection (ie, fevers, chills, dysuria) should undergo appropriate testing, including urinalysis if there is clinical suspicion for urinary etiology.
RECOMMENDATIONS
- For older patients presenting with delirium without localized urinary symptoms or systemic signs of a serious infection, forgo routine ordering of urinalysis and urine culture.
- For older patients presenting with delirium and localized or systemic signs of infection, routine urine studies and antimicrobial therapy may be appropriate.
- For older patients presenting with delirium without localized symptoms or systemic signs of serious infection, attempt to first identify the cause of the change in mental status by obtaining history from a reliable informant, performing a thorough physical and neurologic examination, and evaluating for metabolic and electrolyte derangements.
CONCLUSION
Returning to the clinical scenario, older patients presenting with signs and symptoms of delirium should undergo further work-up to determine underlying causes for their altered mental status. The patient’s history, ideally obtained from a knowledgeable informant, should offer insight into her baseline mental status and risk factors for delirium. This should be followed by a careful physical and neurologic examination, and evaluation for electrolyte, metabolic, and other derangements. In patients without localized or systemic signs of infection, routine urine testing and treatment of bacteriuria should be avoided.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™" topics by emailing [email protected]
1. World Health Organization. 2018 International Classification of Diseases for Mortality and Morbidity Statistics. 11th Rev. Published September 20, 2020. Accessed April 12, 2021. https://icd.who.int/browse10/2019/en#/F04
2. Witlox J, Eurelings LS, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. https://doi.org/10.1001/jama.2010.1013
3. Young J, Inouye SK. Delirium in older people. BMJ. 2007;334(7598):842-846. https://doi.org/10.1136/bmj.39169.706574.ad
4. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. https://doi.org/10.1001/jama.2017.12067
5. R McKenzie, M Stewart, M. Bellantoni, TE Finucane. Bacteriuria in Individuals who become delirious. Am J Med. 2014;127(4):255-257. https://doi.org/10.1016/j.amjmed.2013.10.016
6. Balogun S, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J. 2013;17(1):22-26. https://doi.org/10.5770/cgj.17.90
7. Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med. 1987;83(1):27-33. https://doi.org/10.1016/0002-9343(87)90493-1
8. Zalmanovici Trestioreanu A, Lador A, Sauerbrun-Cutler MT, Leibovici L. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev. 2015;4:CD009534. https://doi.org/10.1002/14651858.cd009534.pub2
9. Mayne S, Bowden A, Sundvall PD, Gunnarsson R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing – a systematic literature review. BMC Geriatr. 2019;19(1):32. https://doi.org/10.1186/s12877-019-1049-7
10. Centers for Disease Control and Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. In: National Health Safety Network (NHSN) Patient Safety Component Manual. 2021:7-5. Published January 2021. Accessed April 12, 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf
11. Gupta K, Grigoryan L, Trautner B. 2017. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49-ITC64. https://doi.org/10.7326/aitc201710030
12. Nicolle LE, Gupta K, Bradley SF, et al. 2019. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. https://doi.org/10.1093/cid/ciz021
13. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019;179(11):1519-1527. https://doi.org/10.1001/jamainternmed.2019.2871
14. Dasgupta M, Brymer C, Elsayed S. 2017. Treatment of asymptomatic UTI in older delirious medical in-patients: a prospective cohort study. Arch Gerontol Geriatr. 2017;72:127-134. https://doi.org/10.1016/j.archger.2017.05.010
15. Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EMC. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56(7):1276-1280. https://doi.org/10.1111/j.1532-5415.2008.01787.x
16. Das R, Towle V, Van Ness PH, Juthani-Mehta M. 2011. Adverse outcomes in nursing home residents with increased episodes of observed bacteriuria. Infect Control Hosp Epidemiol. 2011;32(1):84-86. https://doi.org/10.1086/657664
17. American Board of Internal Medicine. Choosing Wisely. American Geriatrics Society. Antimicrobials to treat bacteriuria in older adults.” Published February 21, 2013. Accessed April 12, 2021. www.choosingwisely.org/clinician-lists/american-geriatrics-society-antimicrobials-to-treat-bacteriuria-in-older-adults/
18. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
19. 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 update AGS Beers Criteria for potential inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 78-year-old female nursing home resident presents to the emergency department for evaluation of a several-hour history of confusion and restlessness. The patient is accompanied by one of her caregivers from the nursing home. Initial evaluation reveals an awake but inattentive, disoriented, and agitated woman who can answer basic questions appropriately. The caregiver denies the patient having had any antecedent concerns, such as pain with urination, abdominal pain, subjective fevers, chills, or night sweats. Vital signs include a temperature of 37.5 °C (99.5 °F), heart rate of 90 beats per minute, blood pressure of 110/60 mm Hg, respiratory rate of 14 breaths per minute, and oxygen saturation of 98% on room air. The patient has a normal lung and abdominal exam without any suprapubic or flank tenderness. There is no Foley catheter in place.
BACKGROUND
Delirium, defined by the World Health Organization’s 10th revision of the International Classification of Diseases as “an etiologically nonspecific organic cerebral syndrome characterized by concurrent disturbances of consciousness and attention, perception, thinking, memory, psychomotor behavior, emotion, and the sleep-wake schedule,” is associated with poor clinical outcomes in older patients.1,2 Mental status changes, which can arise rapidly over the course of hours to days, often fluctuate, with most cases resolving within days of onset.3 In the United States, more than 2.6 million adults aged 65 years and older develop delirium each year, accounting for an estimated $38 to $152 billion in annual healthcare expenditures.4
WHY YOU MIGHT THINK URINE TESTING IS HELPFUL IN OLDER ADULTS WITH DELIRIUM WHO HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
Some clinicians believe that the evaluation for delirium should include an empiric urinary infectious workup with urinalysis and/or urine cultures, even in the absence of local genitourinary symptoms or other signs of infection. In fact, altered mental status is the most common indication for ordering a urine culture in older adult patients.5
Urinary tract infections (UTIs) account for almost 25% of all reported infections in older patients, with delirium occurring in up to 30% of this patient population.6 As one study demonstrated, given this population’s very high prevalence of asymptomatic bacteriuria (ASB), urine studies sent during a delirium work-up often yield positive findings (defined as ≥105 colony-forming units [CFU]/mL [≥108 CFU/L]) in older patients with no signs or symptoms attributable to UTI.7 The incidence of ASB increases significantly with age, with prevalence estimated to be between 6% and 10% in women older than 60 years and approximately 5% in men older than 65 years.5 Among older patients residing in long-term care facilities, up to 50% of female residents and up to 40% of male residents have ASB.8 These findings, in part, created the common perception of causation between UTI and delirium.
WHY YOU SHOULD NOT OBTAIN URINE TESTING IN OLDER ADULTS WITH DELIRIUM IF THEY HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
A recent systematic review demonstrated that there is insufficient evidence to associate UTI with acute confusion in older patients.9 The Centers for Disease Control and Prevention’s National Health Safety Network notes that at least one of the following criteria must be present for the diagnosis of UTI in noncatheterized patients: fever (>38 °C), suprapubic tenderness, costovertebral angle tenderness, urinary frequency, urinary urgency, or dysuria.10 Recent studies have identified that ASB—by definition, without dysuria, frequency, bladder discomfort, or fever—is an unlikely cause of delirium.6,11
The 2019 Infectious Diseases Society of America (IDSA) practice guidelines suggest that clinicians not screen for ASB in older functionally or cognitively impaired patients with no local genitourinary symptoms or other signs of infection. The IDSA acknowledges that the potential adverse outcomes of antimicrobial therapy, including Clostridioides difficile infection, increased antimicrobial resistance, or adverse drug effects, outweigh the potential benefit of treatment given the absence of evidence that such treatment improves outcomes for this vulnerable patient population (strong recommendation, very low-quality evidence).12 Per the IDSA guidelines, recommendations are strong when there is “moderate- or high-quality evidence that the desirable consequences outweigh the undesirable consequences for a course of action” and “may also be strong when there is high-quality evidence of harm and benefits are uncertain (ie, low or very low quality),” as in this case scenario. Studies of older institutionalized and hospitalized patients have found that ASB often results in inappropriate antimicrobial use with limited benefit.7,13,14 In addition to noting the lack of benefit from treatment, these studies have found that these patients treated with antimicrobials have worse outcomes when compared to untreated patients with ASB. One study of hospitalized patients treated for ASB concluded that participants given antimicrobial agents experienced longer durations of hospitalization, with no benefits from treatment.13 Moreover, another study identified poor long-term functional recovery in patients treated for ASB.14
Overtreatment also has public health implications given that it may increase the prevalence of multidrug-resistant bacteria in long-term care facilities.15 One recent study of nursing home residents demonstrated an association between bacteriuria, increased antibiotic use, and subsequent isolation of multidrug-resistant gram-negative organisms.16 The increased prevalence of these organisms limits options for oral antibiotic therapies in the outpatient setting, potentially leading to increased healthcare utilization and further harms relating to institutionalization in this vulnerable patient population. In light of the ethical concept of nonmaleficence, recognizing the potential harms of treating ASB without clear benefit is important for clinicians to take into account when considering urinalysis in this patient population.
In addition, obtaining a urine culture in an older patient with no signs or symptoms of UTI may lead to premature closure from a diagnostic perspective, resulting in missed diagnoses during clinical evaluation. A missed alternative diagnosis could then cause additional, ongoing harm to the patient if left untreated. Subsequent harms from delayed treatment can thus compound the direct harms and added costs incurred by inappropriate testing and treatment of patients with delirium.
Since 2013, the American Geriatrics Society (AGS) has recommended against the use of antimicrobials in older patients with no urinary tract symptoms, stating that “Antimicrobial treatment studies for asymptomatic bacteriuria in older adults demonstrate no benefits and show increased adverse antimicrobial effects.”17 The IDSA practice guidelines state the following: “In older patients with functional and/or cognitive impairment with bacteriuria and delirium (acute mental status change, confusion) and without local genitourinary symptoms or other systemic signs of infection (eg, fever or hemodynamic instability), we recommend assessment for other causes and careful observation rather than antimicrobial treatment (strong recommendation, very low-quality evidence).”12
WHEN YOU SHOULD OBTAIN URINALYSIS FOR OLDER ADULTS WITH DELIRIUM
Older patients presenting with confusion in the setting of recognized symptoms of UTI (eg, acute dysuria, urinary urgency or frequency) warrant urinalysis and urine culture. Additionally, urinalysis and urine cultures may be warranted to assess for UTI—even in the absence of a localizing source—in older patients with signs and symptoms of delirium who also exhibit systemic signs of infection (eg, fever, leukocytosis, hemodynamic instability).12
WHAT YOU SHOULD DO INSTEAD
Initial evaluation of an older patient with delirium should include a thorough review of their recent history and baseline mental status with a knowledgeable informant, a careful physical and neurologic examination, and laboratory studies to determine the presence of electrolyte or metabolic derangements as well as infection and organ failure.4 Clinicians should take into account nonmodifiable risk factors for delirium and conduct a careful review of the time course of changes in mental status and modifiable risk factors, including environment, sleep deprivation, medications, immobilization, and sensory impairments.18
To manage delirium in older patients, clinicians should identify reversible causes of the delirium and minimize modifiable exacerbating factors (eg, sensory impairment, sleep deprivation) in the immediate environment of the patient. They should also carefully review medications that may contribute to delirium, using tools such as the AGS Beers Criteria to identify high-risk medications and concerning medication combinations.19 Patients who develop local or systemic signs of infection (ie, fevers, chills, dysuria) should undergo appropriate testing, including urinalysis if there is clinical suspicion for urinary etiology.
RECOMMENDATIONS
- For older patients presenting with delirium without localized urinary symptoms or systemic signs of a serious infection, forgo routine ordering of urinalysis and urine culture.
- For older patients presenting with delirium and localized or systemic signs of infection, routine urine studies and antimicrobial therapy may be appropriate.
- For older patients presenting with delirium without localized symptoms or systemic signs of serious infection, attempt to first identify the cause of the change in mental status by obtaining history from a reliable informant, performing a thorough physical and neurologic examination, and evaluating for metabolic and electrolyte derangements.
CONCLUSION
Returning to the clinical scenario, older patients presenting with signs and symptoms of delirium should undergo further work-up to determine underlying causes for their altered mental status. The patient’s history, ideally obtained from a knowledgeable informant, should offer insight into her baseline mental status and risk factors for delirium. This should be followed by a careful physical and neurologic examination, and evaluation for electrolyte, metabolic, and other derangements. In patients without localized or systemic signs of infection, routine urine testing and treatment of bacteriuria should be avoided.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™" topics by emailing [email protected]
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 78-year-old female nursing home resident presents to the emergency department for evaluation of a several-hour history of confusion and restlessness. The patient is accompanied by one of her caregivers from the nursing home. Initial evaluation reveals an awake but inattentive, disoriented, and agitated woman who can answer basic questions appropriately. The caregiver denies the patient having had any antecedent concerns, such as pain with urination, abdominal pain, subjective fevers, chills, or night sweats. Vital signs include a temperature of 37.5 °C (99.5 °F), heart rate of 90 beats per minute, blood pressure of 110/60 mm Hg, respiratory rate of 14 breaths per minute, and oxygen saturation of 98% on room air. The patient has a normal lung and abdominal exam without any suprapubic or flank tenderness. There is no Foley catheter in place.
BACKGROUND
Delirium, defined by the World Health Organization’s 10th revision of the International Classification of Diseases as “an etiologically nonspecific organic cerebral syndrome characterized by concurrent disturbances of consciousness and attention, perception, thinking, memory, psychomotor behavior, emotion, and the sleep-wake schedule,” is associated with poor clinical outcomes in older patients.1,2 Mental status changes, which can arise rapidly over the course of hours to days, often fluctuate, with most cases resolving within days of onset.3 In the United States, more than 2.6 million adults aged 65 years and older develop delirium each year, accounting for an estimated $38 to $152 billion in annual healthcare expenditures.4
WHY YOU MIGHT THINK URINE TESTING IS HELPFUL IN OLDER ADULTS WITH DELIRIUM WHO HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
Some clinicians believe that the evaluation for delirium should include an empiric urinary infectious workup with urinalysis and/or urine cultures, even in the absence of local genitourinary symptoms or other signs of infection. In fact, altered mental status is the most common indication for ordering a urine culture in older adult patients.5
Urinary tract infections (UTIs) account for almost 25% of all reported infections in older patients, with delirium occurring in up to 30% of this patient population.6 As one study demonstrated, given this population’s very high prevalence of asymptomatic bacteriuria (ASB), urine studies sent during a delirium work-up often yield positive findings (defined as ≥105 colony-forming units [CFU]/mL [≥108 CFU/L]) in older patients with no signs or symptoms attributable to UTI.7 The incidence of ASB increases significantly with age, with prevalence estimated to be between 6% and 10% in women older than 60 years and approximately 5% in men older than 65 years.5 Among older patients residing in long-term care facilities, up to 50% of female residents and up to 40% of male residents have ASB.8 These findings, in part, created the common perception of causation between UTI and delirium.
WHY YOU SHOULD NOT OBTAIN URINE TESTING IN OLDER ADULTS WITH DELIRIUM IF THEY HAVE NO SIGNS OR SYMPTOMS OF URINARY TRACT INFECTION
A recent systematic review demonstrated that there is insufficient evidence to associate UTI with acute confusion in older patients.9 The Centers for Disease Control and Prevention’s National Health Safety Network notes that at least one of the following criteria must be present for the diagnosis of UTI in noncatheterized patients: fever (>38 °C), suprapubic tenderness, costovertebral angle tenderness, urinary frequency, urinary urgency, or dysuria.10 Recent studies have identified that ASB—by definition, without dysuria, frequency, bladder discomfort, or fever—is an unlikely cause of delirium.6,11
The 2019 Infectious Diseases Society of America (IDSA) practice guidelines suggest that clinicians not screen for ASB in older functionally or cognitively impaired patients with no local genitourinary symptoms or other signs of infection. The IDSA acknowledges that the potential adverse outcomes of antimicrobial therapy, including Clostridioides difficile infection, increased antimicrobial resistance, or adverse drug effects, outweigh the potential benefit of treatment given the absence of evidence that such treatment improves outcomes for this vulnerable patient population (strong recommendation, very low-quality evidence).12 Per the IDSA guidelines, recommendations are strong when there is “moderate- or high-quality evidence that the desirable consequences outweigh the undesirable consequences for a course of action” and “may also be strong when there is high-quality evidence of harm and benefits are uncertain (ie, low or very low quality),” as in this case scenario. Studies of older institutionalized and hospitalized patients have found that ASB often results in inappropriate antimicrobial use with limited benefit.7,13,14 In addition to noting the lack of benefit from treatment, these studies have found that these patients treated with antimicrobials have worse outcomes when compared to untreated patients with ASB. One study of hospitalized patients treated for ASB concluded that participants given antimicrobial agents experienced longer durations of hospitalization, with no benefits from treatment.13 Moreover, another study identified poor long-term functional recovery in patients treated for ASB.14
Overtreatment also has public health implications given that it may increase the prevalence of multidrug-resistant bacteria in long-term care facilities.15 One recent study of nursing home residents demonstrated an association between bacteriuria, increased antibiotic use, and subsequent isolation of multidrug-resistant gram-negative organisms.16 The increased prevalence of these organisms limits options for oral antibiotic therapies in the outpatient setting, potentially leading to increased healthcare utilization and further harms relating to institutionalization in this vulnerable patient population. In light of the ethical concept of nonmaleficence, recognizing the potential harms of treating ASB without clear benefit is important for clinicians to take into account when considering urinalysis in this patient population.
In addition, obtaining a urine culture in an older patient with no signs or symptoms of UTI may lead to premature closure from a diagnostic perspective, resulting in missed diagnoses during clinical evaluation. A missed alternative diagnosis could then cause additional, ongoing harm to the patient if left untreated. Subsequent harms from delayed treatment can thus compound the direct harms and added costs incurred by inappropriate testing and treatment of patients with delirium.
Since 2013, the American Geriatrics Society (AGS) has recommended against the use of antimicrobials in older patients with no urinary tract symptoms, stating that “Antimicrobial treatment studies for asymptomatic bacteriuria in older adults demonstrate no benefits and show increased adverse antimicrobial effects.”17 The IDSA practice guidelines state the following: “In older patients with functional and/or cognitive impairment with bacteriuria and delirium (acute mental status change, confusion) and without local genitourinary symptoms or other systemic signs of infection (eg, fever or hemodynamic instability), we recommend assessment for other causes and careful observation rather than antimicrobial treatment (strong recommendation, very low-quality evidence).”12
WHEN YOU SHOULD OBTAIN URINALYSIS FOR OLDER ADULTS WITH DELIRIUM
Older patients presenting with confusion in the setting of recognized symptoms of UTI (eg, acute dysuria, urinary urgency or frequency) warrant urinalysis and urine culture. Additionally, urinalysis and urine cultures may be warranted to assess for UTI—even in the absence of a localizing source—in older patients with signs and symptoms of delirium who also exhibit systemic signs of infection (eg, fever, leukocytosis, hemodynamic instability).12
WHAT YOU SHOULD DO INSTEAD
Initial evaluation of an older patient with delirium should include a thorough review of their recent history and baseline mental status with a knowledgeable informant, a careful physical and neurologic examination, and laboratory studies to determine the presence of electrolyte or metabolic derangements as well as infection and organ failure.4 Clinicians should take into account nonmodifiable risk factors for delirium and conduct a careful review of the time course of changes in mental status and modifiable risk factors, including environment, sleep deprivation, medications, immobilization, and sensory impairments.18
To manage delirium in older patients, clinicians should identify reversible causes of the delirium and minimize modifiable exacerbating factors (eg, sensory impairment, sleep deprivation) in the immediate environment of the patient. They should also carefully review medications that may contribute to delirium, using tools such as the AGS Beers Criteria to identify high-risk medications and concerning medication combinations.19 Patients who develop local or systemic signs of infection (ie, fevers, chills, dysuria) should undergo appropriate testing, including urinalysis if there is clinical suspicion for urinary etiology.
RECOMMENDATIONS
- For older patients presenting with delirium without localized urinary symptoms or systemic signs of a serious infection, forgo routine ordering of urinalysis and urine culture.
- For older patients presenting with delirium and localized or systemic signs of infection, routine urine studies and antimicrobial therapy may be appropriate.
- For older patients presenting with delirium without localized symptoms or systemic signs of serious infection, attempt to first identify the cause of the change in mental status by obtaining history from a reliable informant, performing a thorough physical and neurologic examination, and evaluating for metabolic and electrolyte derangements.
CONCLUSION
Returning to the clinical scenario, older patients presenting with signs and symptoms of delirium should undergo further work-up to determine underlying causes for their altered mental status. The patient’s history, ideally obtained from a knowledgeable informant, should offer insight into her baseline mental status and risk factors for delirium. This should be followed by a careful physical and neurologic examination, and evaluation for electrolyte, metabolic, and other derangements. In patients without localized or systemic signs of infection, routine urine testing and treatment of bacteriuria should be avoided.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™" topics by emailing [email protected]
1. World Health Organization. 2018 International Classification of Diseases for Mortality and Morbidity Statistics. 11th Rev. Published September 20, 2020. Accessed April 12, 2021. https://icd.who.int/browse10/2019/en#/F04
2. Witlox J, Eurelings LS, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. https://doi.org/10.1001/jama.2010.1013
3. Young J, Inouye SK. Delirium in older people. BMJ. 2007;334(7598):842-846. https://doi.org/10.1136/bmj.39169.706574.ad
4. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. https://doi.org/10.1001/jama.2017.12067
5. R McKenzie, M Stewart, M. Bellantoni, TE Finucane. Bacteriuria in Individuals who become delirious. Am J Med. 2014;127(4):255-257. https://doi.org/10.1016/j.amjmed.2013.10.016
6. Balogun S, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J. 2013;17(1):22-26. https://doi.org/10.5770/cgj.17.90
7. Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med. 1987;83(1):27-33. https://doi.org/10.1016/0002-9343(87)90493-1
8. Zalmanovici Trestioreanu A, Lador A, Sauerbrun-Cutler MT, Leibovici L. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev. 2015;4:CD009534. https://doi.org/10.1002/14651858.cd009534.pub2
9. Mayne S, Bowden A, Sundvall PD, Gunnarsson R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing – a systematic literature review. BMC Geriatr. 2019;19(1):32. https://doi.org/10.1186/s12877-019-1049-7
10. Centers for Disease Control and Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. In: National Health Safety Network (NHSN) Patient Safety Component Manual. 2021:7-5. Published January 2021. Accessed April 12, 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf
11. Gupta K, Grigoryan L, Trautner B. 2017. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49-ITC64. https://doi.org/10.7326/aitc201710030
12. Nicolle LE, Gupta K, Bradley SF, et al. 2019. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. https://doi.org/10.1093/cid/ciz021
13. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019;179(11):1519-1527. https://doi.org/10.1001/jamainternmed.2019.2871
14. Dasgupta M, Brymer C, Elsayed S. 2017. Treatment of asymptomatic UTI in older delirious medical in-patients: a prospective cohort study. Arch Gerontol Geriatr. 2017;72:127-134. https://doi.org/10.1016/j.archger.2017.05.010
15. Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EMC. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56(7):1276-1280. https://doi.org/10.1111/j.1532-5415.2008.01787.x
16. Das R, Towle V, Van Ness PH, Juthani-Mehta M. 2011. Adverse outcomes in nursing home residents with increased episodes of observed bacteriuria. Infect Control Hosp Epidemiol. 2011;32(1):84-86. https://doi.org/10.1086/657664
17. American Board of Internal Medicine. Choosing Wisely. American Geriatrics Society. Antimicrobials to treat bacteriuria in older adults.” Published February 21, 2013. Accessed April 12, 2021. www.choosingwisely.org/clinician-lists/american-geriatrics-society-antimicrobials-to-treat-bacteriuria-in-older-adults/
18. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
19. 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 update AGS Beers Criteria for potential inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
1. World Health Organization. 2018 International Classification of Diseases for Mortality and Morbidity Statistics. 11th Rev. Published September 20, 2020. Accessed April 12, 2021. https://icd.who.int/browse10/2019/en#/F04
2. Witlox J, Eurelings LS, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia: a meta-analysis. JAMA. 2010;304(4):443-451. https://doi.org/10.1001/jama.2010.1013
3. Young J, Inouye SK. Delirium in older people. BMJ. 2007;334(7598):842-846. https://doi.org/10.1136/bmj.39169.706574.ad
4. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161-1174. https://doi.org/10.1001/jama.2017.12067
5. R McKenzie, M Stewart, M. Bellantoni, TE Finucane. Bacteriuria in Individuals who become delirious. Am J Med. 2014;127(4):255-257. https://doi.org/10.1016/j.amjmed.2013.10.016
6. Balogun S, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. Can Geriatr J. 2013;17(1):22-26. https://doi.org/10.5770/cgj.17.90
7. Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med. 1987;83(1):27-33. https://doi.org/10.1016/0002-9343(87)90493-1
8. Zalmanovici Trestioreanu A, Lador A, Sauerbrun-Cutler MT, Leibovici L. Antibiotics for asymptomatic bacteriuria. Cochrane Database Syst Rev. 2015;4:CD009534. https://doi.org/10.1002/14651858.cd009534.pub2
9. Mayne S, Bowden A, Sundvall PD, Gunnarsson R. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing – a systematic literature review. BMC Geriatr. 2019;19(1):32. https://doi.org/10.1186/s12877-019-1049-7
10. Centers for Disease Control and Prevention. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and non-catheter-associated urinary tract infection [UTI]) events. In: National Health Safety Network (NHSN) Patient Safety Component Manual. 2021:7-5. Published January 2021. Accessed April 12, 2021. https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf
11. Gupta K, Grigoryan L, Trautner B. 2017. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49-ITC64. https://doi.org/10.7326/aitc201710030
12. Nicolle LE, Gupta K, Bradley SF, et al. 2019. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. https://doi.org/10.1093/cid/ciz021
13. Petty LA, Vaughn VM, Flanders SA, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019;179(11):1519-1527. https://doi.org/10.1001/jamainternmed.2019.2871
14. Dasgupta M, Brymer C, Elsayed S. 2017. Treatment of asymptomatic UTI in older delirious medical in-patients: a prospective cohort study. Arch Gerontol Geriatr. 2017;72:127-134. https://doi.org/10.1016/j.archger.2017.05.010
15. Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EMC. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56(7):1276-1280. https://doi.org/10.1111/j.1532-5415.2008.01787.x
16. Das R, Towle V, Van Ness PH, Juthani-Mehta M. 2011. Adverse outcomes in nursing home residents with increased episodes of observed bacteriuria. Infect Control Hosp Epidemiol. 2011;32(1):84-86. https://doi.org/10.1086/657664
17. American Board of Internal Medicine. Choosing Wisely. American Geriatrics Society. Antimicrobials to treat bacteriuria in older adults.” Published February 21, 2013. Accessed April 12, 2021. www.choosingwisely.org/clinician-lists/american-geriatrics-society-antimicrobials-to-treat-bacteriuria-in-older-adults/
18. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220. https://doi.org/10.1038/nrneurol.2009.24
19. 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 update AGS Beers Criteria for potential inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. https://doi.org/10.1111/jgs.15767
© 2021 Society of Hospital Medicine
A Longitudinal Analysis of Functional Disability, Recovery, and Nursing Home Utilization After Hospitalization for Ambulatory Care Sensitive Conditions Among Community-Living Older Persons
Acute illnesses requiring hospitalization serve as a sentinel event, with many older adults requiring assistance with activities of daily living (ADLs) upon discharge.1-3 Older adults who are frail experience even higher rates of hospital-associated disability, and rates of recovery to baseline functional status have varied.4,5 Loss of independence in ADLs has been associated with nursing home (NH) utilization, caregiver burden, and mortality.6
To date, studies have characterized functional trajectories before and after hospitalization in older persons for broad medical conditions, noting persistence of disability and incomplete recovery to baseline functional status.7 Prior evaluations have also noted the long-term disabling impact of critical conditions such as acute myocardial infarction, stroke, and sepsis,8,9 but a knowledge gap exists regarding the subsequent functional disability, recovery, and incident NH admission among older persons who are hospitalized for ambulatory care sensitive conditions (ACSCs). Often considered potentially preventable with optimal ambulatory care,10,11 ACSCs represent acute, chronic, and vaccine-preventable conditions, including urinary tract infection, congestive heart failure, diabetes mellitus, and pneumonia. Investigating the aforementioned patient-centered measures post hospitalization could provide valuable supporting evidence for the continued recognition of ACSC-related hospitalizations in national quality payment programs set forth by the Centers for Medicare & Medicaid Services (CMS).12 Demonstrating adverse outcomes after ACSC-related hospitalizations may help support interventions that target potentially preventable ACSC-related hospitalizations, such as home-based care or telehealth, with the goal of improving functional outcomes and reducing NH admission in older persons.
To address these gaps, we evaluated ACSC-related hospitalizations among participants of the Precipitating Events Project (PEP), a 19-year longitudinal study of community-living persons who were initially nondisabled in their basic functional activities. In the 6 months following an ACSC-related hospitalization, our objectives were to describe: (1) the 6-month course of postdischarge functional disability, (2) the cumulative monthly probability of functional recovery, and (3) the cumulative monthly probability of incident NH admission.
METHODS
Study Population
Participants were drawn from the PEP study, an ongoing, prospective, longitudinal study of 754 community-dwelling persons aged 70 years or older.13 Potential participants were members of a large health plan in greater New Haven, Connecticut, and were enrolled from March 1998 through October 1999. As previously described,14 persons were oversampled if they were physically frail, as denoted by a timed score >10 seconds on the rapid gait test. Exclusion criteria included significant cognitive impairment with no available proxy, life expectancy less than 12 months, plans to leave the area, and inability to speak English. Participants were initially required to be nondisabled in four basic activities of daily living (bathing, dressing, walking across a room, and transferring from a chair). Eligibility was determined during a screening telephone interview and was confirmed during an in-home assessment. Of the eligible members, 75.2% agreed to participate in the project, and persons who declined to participate did not significantly differ in age or sex from those who were enrolled. The Yale Human Investigation Committee approved the study protocol, and all participants provided verbal informed consent.
Data Collection
From 1998 to 2017, comprehensive home-based assessments were completed by trained research nurses at baseline and at 18-month intervals over 234 months (except at 126 months), and telephone interviews were completed monthly through June 2018, to obtain information on disability over time. For participants who had significant cognitive impairment or who were unavailable, we interviewed a proxy informant using a rigorous protocol with demonstrated reliability and validity.14 All incident NH admissions, including both short- and long-term stays, were identified using the CMS Skilled Nursing Facility claims file and Long Term Care Minimum Data Set. Deaths were ascertained by review of obituaries and/or from a proxy informant, with a completion rate of 100%. A total of 688 participants (91.2%) had died after a median follow-up of 108 months, while 43 participants (5.7%) dropped out of the study after a median follow-up of 27 months. Among all participants, data were otherwise available for 99.2% of 85,531 monthly telephone interviews.
Assembly of Analytic Sample
PEP participants were considered for inclusion in the analytic sample if they had a hospitalization with an ACSC as the primary diagnosis on linked Medicare claims data. The complete list of ACSCs was defined using specifications from the Agency for Healthcare Research and Quality,15 and was assembled using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) classification prior to October 1, 2015, and ICD Tenth Revision, Clinical Modification (ICD-10-CM) classification after October 1, 2015 (Appendix Table 1). Examples of ACSCs include congestive heart failure, dehydration, urinary tract infection, and angina without procedure. As performed previously,16,17 two ACSCs (low birthweight; asthma in younger adults 18-39 years) were not included in this analysis because they were not based on full adult populations.
ACSC-related hospitalizations were included through December 2017. Participants could contribute more than one ACSC-related hospitalization over the course of the study based on the following criteria: (1) participant did not have a prior non-ACSC-related hospitalization within an 18-month interval; (2) participant did not have a prior ACSC-related hospitalization or treat-and-release emergency department (ED) visit within an 18-month interval (to ensure independence of observations if the participant was still recovering from the prior event and because some of the characteristics within Table 1 are susceptible to change in the setting of an intervening event and, hence, would not accurately reflect the status of the participant prior to ACSC-related hospitalization); (3) participant was not admitted from a NH; (4) participant did not have an in-hospital intensive care unit (ICU) stay (because persons with critical illness are a distinct population with frequent disability and prolonged recovery, as previously described18), in-hospital death, or death before first follow-up interview (because our aim was to evaluate disability and recovery after the hospitalization7).
Assembly of the primary analytic sample is depicted in the Appendix Figure. Of the 814 patients who were identified with ACSC-related hospitalizations, 107 had a prior non-ACSC-related hospitalization and 275 had a prior ACSC-related hospitalization or a treat-and-release ED visit within an 18-month interval. Of the remaining 432 ACSC-related hospitalizations, 181 were excluded: 114 patients were admitted from a NH, 38 had an in-hospital ICU stay, 3 died in the hospital, 11 died before their first follow-up interview, and 15 had withdrawn from the study. The primary analytic sample included the remaining 251 ACSC-related hospitalizations, contributed by 196 participants. Specifically, nine participants contributed three ACSC-related hospitalizations each, 37 participants contributed two hospitalizations each, and the remaining 150 participants contributed one hospitalization each. During the 6-month follow-up period, 40 participants contributing ACSC-related hospitalizations died after a median (interquartile range [IQR]) of 4 (2-5) months, and 1 person refused continued participation.
Comprehensive Assessments
During the comprehensive in-home assessments, data were obtained on demographic characteristics. Age was measured in years at the time of the ACSC-related hospitalization. In addition, we describe factors from the comprehensive assessment immediately prior to the ACSC-related hospitalization, grouped into two additional domains related to disability19: health-related and cognitive-psychosocial. The health-related factors included nine self-reported, physician-diagnosed chronic conditions and frailty. The cognitive-psychosocial factors included social support, cognitive impairment, and depressive symptoms.
Assessment of Disability
Complete details about the assessment of disability have been previously described.13,14,19,20 Briefly, disability was assessed during the monthly telephone interviews, and included four basic activities (bathing, dressing, walking across a room, and transferring from a chair), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and three mobility activities (walking a quarter mile, climbing a flight of stairs, and lifting or carrying 10 lb). Participants were asked, “At the present time, do you need help from another person to [complete the task]?” Disability was operationalized as the need for personal assistance or an inability to perform the task. Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Those who responded no were classified as being disabled in driving.19
The number of disabilities overall and for each functional domain (basic, instrumental, and mobility) was summed. Possible disability scores ranged from 0 to 13, with a score of 0 indicating complete independence in all of the items, and a score of 13 indicating complete dependence. Worse postdischarge disability was defined as a total disability score (0-13) at the first telephone interview after an ACSC-related hospitalization that was greater than the total disability score from the telephone interview immediately preceding hospitalization.
Outcome Measures
The primary outcome was the number of disabilities in all 13 basic, instrumental, and mobility activities in each of the 6 months following discharge from an ACSC-related hospitalization. To determine whether our findings were consistent across the three functional domains, we also evaluated the number of disabilities in the four basic, five instrumental, and four mobility activities separately. As secondary outcomes, we evaluated: (1) the cumulative probability of recovery within the 6-month follow-up time frame after an ACSC-related hospitalization, with “recovery” defined as return to the participant’s pre-ACSC-related hospitalization total disability score, and (2) the cumulative probability of incident NH admission within the 6 months after an ACSC-related hospitalization. Aligned with CMS and prior literature,21,22 we defined a short-term NH stay as ≤100 days and a long-term NH stay as >100 days.
Statistical Analysis
Pre-ACSC-related hospitalization characteristics were summarized by means (SDs) and frequencies with proportions. We determined the mean number of disabilities in each of the 6 months following hospital discharge, with the prehospitalization value included as a reference point. We also determined the mean (SD) number of disabilities for the three subscales of disability (basic activities of daily living [BADLs], instrumental activities of daily living [IADLs], and mobility activities). We calculated the cumulative probability of recovery within 6 months of hospital discharge. Finally, we determined the cumulative probability of incident NH admission during the 6 months after hospital discharge.
To test the robustness of our main results, we conducted a sensitivity analysis assessing disability scores of the 150 participants that contributed only one ACSC-related hospitalization. All analyses were performed using Stata, version 16.0, statistical software (StataCorp).
RESULTS
Table 1 shows the characteristics of the 251 ACSC-related hospitalizations immediately prior to hospitalization. Participants’ mean (SD) age was 85.1 (6.0) years, and the mean total disability score was 5.4. The majority were female, non-Hispanic White, frail, and lived alone. As shown in Appendix Table 2, the three most common reasons for ACSC-related hospitalizations were congestive heart failure (n = 69), bacterial pneumonia (n = 53), and dehydration (n = 44).
The Figure shows the disability scores during the 6-month follow-up period for total, basic, instrumental, and mobility activities, in panels A, B, C, and D, respectively. The exact values are provided in Appendix Table 3. After hospitalization, disability scores for total, basic, instrumental, and mobility activities peaked at month 1 and tended to improve modestly over the next 5 months, but remained greater, on average, than pre-hospitalization scores. Of the 40 participants who died within the 6-month follow-up period, 36 (90%) had worse disability scores in their last month of life than in the month prior to their ACSC-related hospitalization.
Table 2 shows the cumulative probability of functional recovery after ACSC-related hospitalizations. Recovery was incomplete, with only 70% (95% CI, 64%-76%) of hospitalizations achieving a return to the pre-hospitalization total disability score within 6 months of hospitalization.
Table 3 shows the cumulative probability of incident NH admission after an ACSC-related hospitalization. Of the 251 ACSC-related hospitalizations, incident NH admission was experienced by 38% (95% CI, 32%-44%) within 1 month and 50% (95% CI, 43%-56%) within 6 months of discharge. Short-term NH stays accounted for 90 (75.6%) of the 119 incident NH admissions within the 6 months after ACSC-related hospitalizations. Sensitivity analyses yielded comparable disability scores, shown in Appendix Table 4.
DISCUSSION
In this longitudinal study of community-living older persons, we evaluated functional disability, recovery, and incident NH admission within 6 months of hospitalization for an ACSC. Our study has three major findings. First, disability scores for total, basic, instrumental, and mobility activities at months 1 to 6 of follow-up were greater on average than pre-hospitalization scores. Second, functional recovery was not achieved by 3 of 10 participants after an ACSC-related hospitalization. Third, half of them experienced an incident NH admission within 6 months of discharge from an ACSC-related hospitalization, although about three-quarters of these were short-term stays. Our findings provide evidence that older persons experience clinically meaningful adverse patient-reported outcomes after ACSC-related hospitalizations.
Prior research involving ACSCs has focused largely on rates of hospitalization as a measure of access to primary care and the associated factors predictive of ACSC-related hospitalizations,23-26 and has not addressed subsequent patient-reported outcomes. The findings in this analysis highlight that older persons experience worsening disability immediately after an ACSC-related hospitalization, which persists for prolonged periods and often results in incomplete recovery. Prior research has assessed pre-hospitalization functional status through retrospective recall approaches,2 included only older adults discharged with incident disability,3 and examined functional status after all-cause medical illness hospitalizations.5 Our prospective analysis extends the literature by reliably capturing pre-hospital disability scores and uniquely assessing the cohort of older persons hospitalized with ACSCs.
Our work is relevant to the continued evaluation of ACSC-related hospitalizations in national quality measurement and payment initiatives among Medicare beneficiaries. In prior evaluations of ACSC-related quality measures, stakeholders have criticized the measures for limited validity due to a lack of evidence linking each utilization outcome to other patient-centered outcomes.10,27 Our work addresses this gap by demonstrating that ACSC-related hospitalizations are linked to persistent disability, incomplete functional recovery, and incident NH admissions. Given the large body of evidence demonstrating the priority older persons place on these patient-reported outcomes,28,29 our work should reassure policymakers seeking to transform quality measurement programs into a more patient-oriented enterprise.
Our findings have several clinical practice, research, and policy implications. First, more-effective clinical strategies to minimize the level of care required for acute exacerbations of ACSC-related illnesses may include: (1) substituting home-based care30 and telehealth interventions31 for traditional inpatient hospitalization, (2) making in-ED resources (ie, case management services, geriatric-focused advanced practice providers) more accessible for older persons with ACSC-related illnesses, thereby enhancing care transitions and follow-up to avoid potential current and subsequent hospitalizations, and (3) ensuring adequate ambulatory care access to all older persons, as prior work has shown variation in ACSC hospital admission rates dependent on population factors such as high-poverty neighborhoods,16 insurance status,16,32 and race/ethnicity.33
Clinical strategies have been narrow and not holistic for ACSCs; for example, many institutions have focused on pneumonia vaccinations to reduce hospitalizations, but our work supports the need to further evaluate the impact of preventing ACSC-related hospitalizations and their associated disabling consequences. For patients admitted to the hospital, clinical strategies, such as in-hospital or post-hospital mobility and activity programs, have been shown to be protective against hospital-associated disability.34,35 Furthermore, hospital discharge planning could include preparing older persons for anticipated functional disabilities, associated recoveries, and NH admission after ACSC-related hospitalizations. Risk factors contributing to post-hospitalization functional disability and recovery have been identified,19,20,36 but future work is needed to: (1) identify target populations (including those most likely to worsen) so that interventions can be offered earlier in the course of care to those who would benefit most, and (2) identify and learn from those who are resilient and have recovered, to better understand factors contributing to their success.
Our study has several strengths. First, the study is unique due to its longitudinal design, with monthly assessments of functional status. Since functional status was assessed prospectively before the ACSC-related hospitalization, we also have avoided any potential concern for recall bias that may be present if assessed after the hospitalization. Additionally, through the use of Medicare claims and the Minimum Data Set, the ascertainment of hospitalizations and NH admissions was likely complete for the studied population.
However, the study has limitations. First, functional measures were based on self-reports rather than objective measurements. Nevertheless, the self-report function is often used to guide coverage determinations in the Medicare program, as it has been shown to be associated with poor health outcomes.37 Second, we are unable to comment on the rate of functional decline or NH admission when an older person was not hospitalized in relation to an ACSC. Future analyses may benefit from using a control group (eg, older adults without an ACSC hospitalization or older adults with a non-ACSC hospitalization). Third, we used strict exclusion criteria to identify a population of older adults without recent hospitalizations to determine the isolated impact of ACSC hospitalization on disability, incident NH admission, and functional recovery. Considering this potential selection bias, our findings are likely conservative estimates of the patient-centered outcomes evaluated. Fourth, participants were not asked about feeding and toileting. However, the incidence of disability in these ADLs is low among nondisabled, community-living older persons, and it is highly uncommon for disability to develop in these ADLs without concurrent disability in the ADLs within this analysis.14,38
Finally, because our study participants were members of a single health plan in a small urban area and included nondisabled older persons living in the community, our findings may not be generalizable to geriatric patients in other settings. Nonetheless, the demographics of our cohort reflect those of older persons in New Haven County, Connecticut, which are similar to the demographics of the US population, with the exception of race and ethnicity. In addition, the generalizability of our results are strengthened by the study’s high participation rate and minimal attrition.
CONCLUSION
Within 6 months of ACSC-related hospitalizations, community-living older persons exhibited greater total disability scores than those immediately preceding hospitalization. In the same time frame, 3 of 10 older persons did not achieve functional recovery, and half experienced incident NH admission. These results provide evidence regarding the continued recognition of ACSC-related hospitalizations in federal quality measurement and payment programs and suggests the need for preventive and comprehensive interventions to meaningfully improve longitudinal outcomes.
Acknowledgments
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director. Each of these persons were paid employees of Yale School of Medicine during the conduct of this study.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556
2. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-458. https://doi.org/10.1046/j.1532-5415.2003.51152.x
3. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. https://doi.org/10.1007/s11606-012-2226-y
4. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919-1928. https://doi.org/10.1001/jama.2010.1568
5. Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171-2179. https://doi.org/10.1111/j.1532-5415.2008.02023.x
6. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461. https://doi.org/10.1016/j.jamda.2019.09.015
7. Dharmarajan K, Han L, Gahbauer EA, Leo-Summers LS, Gill TM. Disability and recovery after hospitalization for medical illness among community-living older persons: a prospective cohort study. J Am Geriatr Soc. 2020;68(3):486-495. https://doi.org/10.1111/jgs.16350
8. Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MAM, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7(6):863-871. https://doi.org/10.1161/HCQ.0000000000000008
9. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794. https://doi.org/10.1001/jama.2010.1553
10. Hodgson K, Deeny SR, Steventon A. Ambulatory care-sensitive conditions: their potential uses and limitations. BMJ Qual Saf. 2019;28(6):429-433. https://doi.org/10.1136/bmjqs-2018-008820
11. Agency for Healthcare Research and Quality (AHRQ). Quality Indicator User Guide: Prevention Quality Indicators (PQI) Composite Measures. Version 2020. Accessed November 10, 2020. https://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx.
12. Centers for Medicare & Medicaid Services. 2016 Measure information about the hospital admissions for acute and chronic ambulatory care-sensitive condition (ACSC) composite measures, calculated for the 2018 value-based payment modified program. Accessed November 24, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2016-ACSC-MIF.pdf.
13. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321. https://doi.org/10.7326/0003-4819-135-5-200109040-00007
14. Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492-1497. https://doi.org/10.1046/j.1532-5415.2002.50403.x
15. Agency for Healthcare Research and Quality. Prevention Quality Indicators Technical Specifications Updates—Version v2018 and 2018.0.1 (ICD 10-CM/PCS), June 2018. Accessed February 4, 2020. https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD10_v2018.aspx.
16. Johnson PJ, Ghildayal N, Ward AC, Westgard BC, Boland LL, Hokanson JS. Disparities in potentially avoidable emergency department (ED) care: ED visits for ambulatory care sensitive conditions. Med Care. 2012;50(12):1020-1028. https://doi.org/10.1097/MLR.0b013e318270bad4
17. Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Acad Emerg Med. 2015;22(2):172-181. https://doi.org/10.1111/acem.12579
18. Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523-529. https://doi.org/10.1001/jamainternmed.2014.7889
19. Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131-140. https://doi.org/10.7326/0003-4819-156-2-201201170-00009
20. Hardy SE, Gill TM. Factors associated with recovery of independence among newly disabled older persons. Arch Intern Med. 2005;165(1):106-112. https://doi.org/10.1001/archinte.165.1.106
21. Centers for Medicare & Medicaid Services. Nursing Home Quality Initiative—Quality Measures. Accessed June 13, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures
22. Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. https://doi.org/10.1186/s12913-017-2318-9
23. Laditka, JN, Laditka SB, Probst JC. More may be better: evidence of a negative relationship between physician supply and hospitalization for ambulatory care sensitive conditions. Health Serv Res. 2005;40(4):1148-1166. https://doi.org/10.1111/j.1475-6773.2005.00403.x
24. Ansar Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Med Care Res Rev. 2006;63(6):719-741. https://doi.org/10.1177/1077558706293637
25. Mackinko J, de Oliveira VB, Turci MA, Guanais FC, Bonolo PF, Lima-Costa MF. The influence of primary care and hospital supply on ambulatory care-sensitive hospitalizations among adults in Brazil, 1999-2007. Am J Public Health. 2011;101(10):1963-1970. https://doi.org/10.2105/AJPH.2010.198887
26. Gibson OR, Segal L, McDermott RA. A systematic review of evidence on the association between hospitalisation for chronic disease related ambulatory care sensitive conditions and primary health care resourcing. BMC Health Serv Res. 2013;13:336. https://doi.org/10.1186/1472-6963-13-336
27. Vuik SI, Fontana G, Mayer E, Darzi A. Do hospitalisations for ambulatory care sensitive conditions reflect low access to primary care? An observational cohort study of primary care usage prior to hospitalisation. BMJ Open. 2017;7(8):e015704. https://doi.org/10.1136/bmjopen-2016-015704
28. Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83(2):278-282. https://doi.org/10.1016/j.pec.2010.04.032
29. Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777-779. https://doi.org/10.1056/NEJMp1113631
30. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. https://doi.org/10.1001/jamainternmed.2018.2562
31. Shah MN, Wasserman EB, Gillespie SM, et al. High-intensity telemedicine decreases emergency department use for ambulatory care sensitive conditions by older adult senior living community residents. J Am Med Dir Assoc. 2015;16(12):1077-1081. https://doi.org/10.1016/j.jamda.2015.07.009
32. Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003;41(2):198-207. https://doi.org/10.1097/01.MLR.0000045021.70297.9F
33. O’Neil SS, Lake T, Merrill A, Wilson A, Mann DA, Bartnyska LM. Racial disparities in hospitalizations for ambulatory care-sensitive conditions. Am J Prev Med. 2010;38(4):381-388. https://doi.org/10.1016/j.amepre.2009.12.026
34. Pavon JM, Sloane RJ, Pieper RF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68:261-265. https://doi.org/10.1111/jgs.16231
35. Sunde S, Hesseberg K, Skelton DA, et al. Effects of a multicomponent high intensity exercise program on physical function and health-related quality of life in older adults with or at risk of mobility disability after discharge from hospital: a randomised controlled trial. BMC Geriatr. 2020;20(1):464. https://doi.org/10.1186/s12877-020-01829-9
36. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596-1602. https://doi.org/10.1001/jama.291.13.1596
37. Rotenberg J, Kinosian B, Boling P, Taler G, Independence at Home Learning Collaborative Writing Group. Home-based primary care: beyond extension of the independence at home demonstration. J Am Geriatr Soc. 2018;66(4):812-817. https://doi.org/10.1111/jgs.15314
38. Rodgers W, Miller B. A comparative analysis of ADL questions in surveys of older people. J Gerontol B Psychol Sci Soc Sci. 1997;52:21-36. https://doi.org/10.1093/geronb/52b.special_issue.21
Acute illnesses requiring hospitalization serve as a sentinel event, with many older adults requiring assistance with activities of daily living (ADLs) upon discharge.1-3 Older adults who are frail experience even higher rates of hospital-associated disability, and rates of recovery to baseline functional status have varied.4,5 Loss of independence in ADLs has been associated with nursing home (NH) utilization, caregiver burden, and mortality.6
To date, studies have characterized functional trajectories before and after hospitalization in older persons for broad medical conditions, noting persistence of disability and incomplete recovery to baseline functional status.7 Prior evaluations have also noted the long-term disabling impact of critical conditions such as acute myocardial infarction, stroke, and sepsis,8,9 but a knowledge gap exists regarding the subsequent functional disability, recovery, and incident NH admission among older persons who are hospitalized for ambulatory care sensitive conditions (ACSCs). Often considered potentially preventable with optimal ambulatory care,10,11 ACSCs represent acute, chronic, and vaccine-preventable conditions, including urinary tract infection, congestive heart failure, diabetes mellitus, and pneumonia. Investigating the aforementioned patient-centered measures post hospitalization could provide valuable supporting evidence for the continued recognition of ACSC-related hospitalizations in national quality payment programs set forth by the Centers for Medicare & Medicaid Services (CMS).12 Demonstrating adverse outcomes after ACSC-related hospitalizations may help support interventions that target potentially preventable ACSC-related hospitalizations, such as home-based care or telehealth, with the goal of improving functional outcomes and reducing NH admission in older persons.
To address these gaps, we evaluated ACSC-related hospitalizations among participants of the Precipitating Events Project (PEP), a 19-year longitudinal study of community-living persons who were initially nondisabled in their basic functional activities. In the 6 months following an ACSC-related hospitalization, our objectives were to describe: (1) the 6-month course of postdischarge functional disability, (2) the cumulative monthly probability of functional recovery, and (3) the cumulative monthly probability of incident NH admission.
METHODS
Study Population
Participants were drawn from the PEP study, an ongoing, prospective, longitudinal study of 754 community-dwelling persons aged 70 years or older.13 Potential participants were members of a large health plan in greater New Haven, Connecticut, and were enrolled from March 1998 through October 1999. As previously described,14 persons were oversampled if they were physically frail, as denoted by a timed score >10 seconds on the rapid gait test. Exclusion criteria included significant cognitive impairment with no available proxy, life expectancy less than 12 months, plans to leave the area, and inability to speak English. Participants were initially required to be nondisabled in four basic activities of daily living (bathing, dressing, walking across a room, and transferring from a chair). Eligibility was determined during a screening telephone interview and was confirmed during an in-home assessment. Of the eligible members, 75.2% agreed to participate in the project, and persons who declined to participate did not significantly differ in age or sex from those who were enrolled. The Yale Human Investigation Committee approved the study protocol, and all participants provided verbal informed consent.
Data Collection
From 1998 to 2017, comprehensive home-based assessments were completed by trained research nurses at baseline and at 18-month intervals over 234 months (except at 126 months), and telephone interviews were completed monthly through June 2018, to obtain information on disability over time. For participants who had significant cognitive impairment or who were unavailable, we interviewed a proxy informant using a rigorous protocol with demonstrated reliability and validity.14 All incident NH admissions, including both short- and long-term stays, were identified using the CMS Skilled Nursing Facility claims file and Long Term Care Minimum Data Set. Deaths were ascertained by review of obituaries and/or from a proxy informant, with a completion rate of 100%. A total of 688 participants (91.2%) had died after a median follow-up of 108 months, while 43 participants (5.7%) dropped out of the study after a median follow-up of 27 months. Among all participants, data were otherwise available for 99.2% of 85,531 monthly telephone interviews.
Assembly of Analytic Sample
PEP participants were considered for inclusion in the analytic sample if they had a hospitalization with an ACSC as the primary diagnosis on linked Medicare claims data. The complete list of ACSCs was defined using specifications from the Agency for Healthcare Research and Quality,15 and was assembled using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) classification prior to October 1, 2015, and ICD Tenth Revision, Clinical Modification (ICD-10-CM) classification after October 1, 2015 (Appendix Table 1). Examples of ACSCs include congestive heart failure, dehydration, urinary tract infection, and angina without procedure. As performed previously,16,17 two ACSCs (low birthweight; asthma in younger adults 18-39 years) were not included in this analysis because they were not based on full adult populations.
ACSC-related hospitalizations were included through December 2017. Participants could contribute more than one ACSC-related hospitalization over the course of the study based on the following criteria: (1) participant did not have a prior non-ACSC-related hospitalization within an 18-month interval; (2) participant did not have a prior ACSC-related hospitalization or treat-and-release emergency department (ED) visit within an 18-month interval (to ensure independence of observations if the participant was still recovering from the prior event and because some of the characteristics within Table 1 are susceptible to change in the setting of an intervening event and, hence, would not accurately reflect the status of the participant prior to ACSC-related hospitalization); (3) participant was not admitted from a NH; (4) participant did not have an in-hospital intensive care unit (ICU) stay (because persons with critical illness are a distinct population with frequent disability and prolonged recovery, as previously described18), in-hospital death, or death before first follow-up interview (because our aim was to evaluate disability and recovery after the hospitalization7).
Assembly of the primary analytic sample is depicted in the Appendix Figure. Of the 814 patients who were identified with ACSC-related hospitalizations, 107 had a prior non-ACSC-related hospitalization and 275 had a prior ACSC-related hospitalization or a treat-and-release ED visit within an 18-month interval. Of the remaining 432 ACSC-related hospitalizations, 181 were excluded: 114 patients were admitted from a NH, 38 had an in-hospital ICU stay, 3 died in the hospital, 11 died before their first follow-up interview, and 15 had withdrawn from the study. The primary analytic sample included the remaining 251 ACSC-related hospitalizations, contributed by 196 participants. Specifically, nine participants contributed three ACSC-related hospitalizations each, 37 participants contributed two hospitalizations each, and the remaining 150 participants contributed one hospitalization each. During the 6-month follow-up period, 40 participants contributing ACSC-related hospitalizations died after a median (interquartile range [IQR]) of 4 (2-5) months, and 1 person refused continued participation.
Comprehensive Assessments
During the comprehensive in-home assessments, data were obtained on demographic characteristics. Age was measured in years at the time of the ACSC-related hospitalization. In addition, we describe factors from the comprehensive assessment immediately prior to the ACSC-related hospitalization, grouped into two additional domains related to disability19: health-related and cognitive-psychosocial. The health-related factors included nine self-reported, physician-diagnosed chronic conditions and frailty. The cognitive-psychosocial factors included social support, cognitive impairment, and depressive symptoms.
Assessment of Disability
Complete details about the assessment of disability have been previously described.13,14,19,20 Briefly, disability was assessed during the monthly telephone interviews, and included four basic activities (bathing, dressing, walking across a room, and transferring from a chair), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and three mobility activities (walking a quarter mile, climbing a flight of stairs, and lifting or carrying 10 lb). Participants were asked, “At the present time, do you need help from another person to [complete the task]?” Disability was operationalized as the need for personal assistance or an inability to perform the task. Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Those who responded no were classified as being disabled in driving.19
The number of disabilities overall and for each functional domain (basic, instrumental, and mobility) was summed. Possible disability scores ranged from 0 to 13, with a score of 0 indicating complete independence in all of the items, and a score of 13 indicating complete dependence. Worse postdischarge disability was defined as a total disability score (0-13) at the first telephone interview after an ACSC-related hospitalization that was greater than the total disability score from the telephone interview immediately preceding hospitalization.
Outcome Measures
The primary outcome was the number of disabilities in all 13 basic, instrumental, and mobility activities in each of the 6 months following discharge from an ACSC-related hospitalization. To determine whether our findings were consistent across the three functional domains, we also evaluated the number of disabilities in the four basic, five instrumental, and four mobility activities separately. As secondary outcomes, we evaluated: (1) the cumulative probability of recovery within the 6-month follow-up time frame after an ACSC-related hospitalization, with “recovery” defined as return to the participant’s pre-ACSC-related hospitalization total disability score, and (2) the cumulative probability of incident NH admission within the 6 months after an ACSC-related hospitalization. Aligned with CMS and prior literature,21,22 we defined a short-term NH stay as ≤100 days and a long-term NH stay as >100 days.
Statistical Analysis
Pre-ACSC-related hospitalization characteristics were summarized by means (SDs) and frequencies with proportions. We determined the mean number of disabilities in each of the 6 months following hospital discharge, with the prehospitalization value included as a reference point. We also determined the mean (SD) number of disabilities for the three subscales of disability (basic activities of daily living [BADLs], instrumental activities of daily living [IADLs], and mobility activities). We calculated the cumulative probability of recovery within 6 months of hospital discharge. Finally, we determined the cumulative probability of incident NH admission during the 6 months after hospital discharge.
To test the robustness of our main results, we conducted a sensitivity analysis assessing disability scores of the 150 participants that contributed only one ACSC-related hospitalization. All analyses were performed using Stata, version 16.0, statistical software (StataCorp).
RESULTS
Table 1 shows the characteristics of the 251 ACSC-related hospitalizations immediately prior to hospitalization. Participants’ mean (SD) age was 85.1 (6.0) years, and the mean total disability score was 5.4. The majority were female, non-Hispanic White, frail, and lived alone. As shown in Appendix Table 2, the three most common reasons for ACSC-related hospitalizations were congestive heart failure (n = 69), bacterial pneumonia (n = 53), and dehydration (n = 44).
The Figure shows the disability scores during the 6-month follow-up period for total, basic, instrumental, and mobility activities, in panels A, B, C, and D, respectively. The exact values are provided in Appendix Table 3. After hospitalization, disability scores for total, basic, instrumental, and mobility activities peaked at month 1 and tended to improve modestly over the next 5 months, but remained greater, on average, than pre-hospitalization scores. Of the 40 participants who died within the 6-month follow-up period, 36 (90%) had worse disability scores in their last month of life than in the month prior to their ACSC-related hospitalization.
Table 2 shows the cumulative probability of functional recovery after ACSC-related hospitalizations. Recovery was incomplete, with only 70% (95% CI, 64%-76%) of hospitalizations achieving a return to the pre-hospitalization total disability score within 6 months of hospitalization.
Table 3 shows the cumulative probability of incident NH admission after an ACSC-related hospitalization. Of the 251 ACSC-related hospitalizations, incident NH admission was experienced by 38% (95% CI, 32%-44%) within 1 month and 50% (95% CI, 43%-56%) within 6 months of discharge. Short-term NH stays accounted for 90 (75.6%) of the 119 incident NH admissions within the 6 months after ACSC-related hospitalizations. Sensitivity analyses yielded comparable disability scores, shown in Appendix Table 4.
DISCUSSION
In this longitudinal study of community-living older persons, we evaluated functional disability, recovery, and incident NH admission within 6 months of hospitalization for an ACSC. Our study has three major findings. First, disability scores for total, basic, instrumental, and mobility activities at months 1 to 6 of follow-up were greater on average than pre-hospitalization scores. Second, functional recovery was not achieved by 3 of 10 participants after an ACSC-related hospitalization. Third, half of them experienced an incident NH admission within 6 months of discharge from an ACSC-related hospitalization, although about three-quarters of these were short-term stays. Our findings provide evidence that older persons experience clinically meaningful adverse patient-reported outcomes after ACSC-related hospitalizations.
Prior research involving ACSCs has focused largely on rates of hospitalization as a measure of access to primary care and the associated factors predictive of ACSC-related hospitalizations,23-26 and has not addressed subsequent patient-reported outcomes. The findings in this analysis highlight that older persons experience worsening disability immediately after an ACSC-related hospitalization, which persists for prolonged periods and often results in incomplete recovery. Prior research has assessed pre-hospitalization functional status through retrospective recall approaches,2 included only older adults discharged with incident disability,3 and examined functional status after all-cause medical illness hospitalizations.5 Our prospective analysis extends the literature by reliably capturing pre-hospital disability scores and uniquely assessing the cohort of older persons hospitalized with ACSCs.
Our work is relevant to the continued evaluation of ACSC-related hospitalizations in national quality measurement and payment initiatives among Medicare beneficiaries. In prior evaluations of ACSC-related quality measures, stakeholders have criticized the measures for limited validity due to a lack of evidence linking each utilization outcome to other patient-centered outcomes.10,27 Our work addresses this gap by demonstrating that ACSC-related hospitalizations are linked to persistent disability, incomplete functional recovery, and incident NH admissions. Given the large body of evidence demonstrating the priority older persons place on these patient-reported outcomes,28,29 our work should reassure policymakers seeking to transform quality measurement programs into a more patient-oriented enterprise.
Our findings have several clinical practice, research, and policy implications. First, more-effective clinical strategies to minimize the level of care required for acute exacerbations of ACSC-related illnesses may include: (1) substituting home-based care30 and telehealth interventions31 for traditional inpatient hospitalization, (2) making in-ED resources (ie, case management services, geriatric-focused advanced practice providers) more accessible for older persons with ACSC-related illnesses, thereby enhancing care transitions and follow-up to avoid potential current and subsequent hospitalizations, and (3) ensuring adequate ambulatory care access to all older persons, as prior work has shown variation in ACSC hospital admission rates dependent on population factors such as high-poverty neighborhoods,16 insurance status,16,32 and race/ethnicity.33
Clinical strategies have been narrow and not holistic for ACSCs; for example, many institutions have focused on pneumonia vaccinations to reduce hospitalizations, but our work supports the need to further evaluate the impact of preventing ACSC-related hospitalizations and their associated disabling consequences. For patients admitted to the hospital, clinical strategies, such as in-hospital or post-hospital mobility and activity programs, have been shown to be protective against hospital-associated disability.34,35 Furthermore, hospital discharge planning could include preparing older persons for anticipated functional disabilities, associated recoveries, and NH admission after ACSC-related hospitalizations. Risk factors contributing to post-hospitalization functional disability and recovery have been identified,19,20,36 but future work is needed to: (1) identify target populations (including those most likely to worsen) so that interventions can be offered earlier in the course of care to those who would benefit most, and (2) identify and learn from those who are resilient and have recovered, to better understand factors contributing to their success.
Our study has several strengths. First, the study is unique due to its longitudinal design, with monthly assessments of functional status. Since functional status was assessed prospectively before the ACSC-related hospitalization, we also have avoided any potential concern for recall bias that may be present if assessed after the hospitalization. Additionally, through the use of Medicare claims and the Minimum Data Set, the ascertainment of hospitalizations and NH admissions was likely complete for the studied population.
However, the study has limitations. First, functional measures were based on self-reports rather than objective measurements. Nevertheless, the self-report function is often used to guide coverage determinations in the Medicare program, as it has been shown to be associated with poor health outcomes.37 Second, we are unable to comment on the rate of functional decline or NH admission when an older person was not hospitalized in relation to an ACSC. Future analyses may benefit from using a control group (eg, older adults without an ACSC hospitalization or older adults with a non-ACSC hospitalization). Third, we used strict exclusion criteria to identify a population of older adults without recent hospitalizations to determine the isolated impact of ACSC hospitalization on disability, incident NH admission, and functional recovery. Considering this potential selection bias, our findings are likely conservative estimates of the patient-centered outcomes evaluated. Fourth, participants were not asked about feeding and toileting. However, the incidence of disability in these ADLs is low among nondisabled, community-living older persons, and it is highly uncommon for disability to develop in these ADLs without concurrent disability in the ADLs within this analysis.14,38
Finally, because our study participants were members of a single health plan in a small urban area and included nondisabled older persons living in the community, our findings may not be generalizable to geriatric patients in other settings. Nonetheless, the demographics of our cohort reflect those of older persons in New Haven County, Connecticut, which are similar to the demographics of the US population, with the exception of race and ethnicity. In addition, the generalizability of our results are strengthened by the study’s high participation rate and minimal attrition.
CONCLUSION
Within 6 months of ACSC-related hospitalizations, community-living older persons exhibited greater total disability scores than those immediately preceding hospitalization. In the same time frame, 3 of 10 older persons did not achieve functional recovery, and half experienced incident NH admission. These results provide evidence regarding the continued recognition of ACSC-related hospitalizations in federal quality measurement and payment programs and suggests the need for preventive and comprehensive interventions to meaningfully improve longitudinal outcomes.
Acknowledgments
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director. Each of these persons were paid employees of Yale School of Medicine during the conduct of this study.
Acute illnesses requiring hospitalization serve as a sentinel event, with many older adults requiring assistance with activities of daily living (ADLs) upon discharge.1-3 Older adults who are frail experience even higher rates of hospital-associated disability, and rates of recovery to baseline functional status have varied.4,5 Loss of independence in ADLs has been associated with nursing home (NH) utilization, caregiver burden, and mortality.6
To date, studies have characterized functional trajectories before and after hospitalization in older persons for broad medical conditions, noting persistence of disability and incomplete recovery to baseline functional status.7 Prior evaluations have also noted the long-term disabling impact of critical conditions such as acute myocardial infarction, stroke, and sepsis,8,9 but a knowledge gap exists regarding the subsequent functional disability, recovery, and incident NH admission among older persons who are hospitalized for ambulatory care sensitive conditions (ACSCs). Often considered potentially preventable with optimal ambulatory care,10,11 ACSCs represent acute, chronic, and vaccine-preventable conditions, including urinary tract infection, congestive heart failure, diabetes mellitus, and pneumonia. Investigating the aforementioned patient-centered measures post hospitalization could provide valuable supporting evidence for the continued recognition of ACSC-related hospitalizations in national quality payment programs set forth by the Centers for Medicare & Medicaid Services (CMS).12 Demonstrating adverse outcomes after ACSC-related hospitalizations may help support interventions that target potentially preventable ACSC-related hospitalizations, such as home-based care or telehealth, with the goal of improving functional outcomes and reducing NH admission in older persons.
To address these gaps, we evaluated ACSC-related hospitalizations among participants of the Precipitating Events Project (PEP), a 19-year longitudinal study of community-living persons who were initially nondisabled in their basic functional activities. In the 6 months following an ACSC-related hospitalization, our objectives were to describe: (1) the 6-month course of postdischarge functional disability, (2) the cumulative monthly probability of functional recovery, and (3) the cumulative monthly probability of incident NH admission.
METHODS
Study Population
Participants were drawn from the PEP study, an ongoing, prospective, longitudinal study of 754 community-dwelling persons aged 70 years or older.13 Potential participants were members of a large health plan in greater New Haven, Connecticut, and were enrolled from March 1998 through October 1999. As previously described,14 persons were oversampled if they were physically frail, as denoted by a timed score >10 seconds on the rapid gait test. Exclusion criteria included significant cognitive impairment with no available proxy, life expectancy less than 12 months, plans to leave the area, and inability to speak English. Participants were initially required to be nondisabled in four basic activities of daily living (bathing, dressing, walking across a room, and transferring from a chair). Eligibility was determined during a screening telephone interview and was confirmed during an in-home assessment. Of the eligible members, 75.2% agreed to participate in the project, and persons who declined to participate did not significantly differ in age or sex from those who were enrolled. The Yale Human Investigation Committee approved the study protocol, and all participants provided verbal informed consent.
Data Collection
From 1998 to 2017, comprehensive home-based assessments were completed by trained research nurses at baseline and at 18-month intervals over 234 months (except at 126 months), and telephone interviews were completed monthly through June 2018, to obtain information on disability over time. For participants who had significant cognitive impairment or who were unavailable, we interviewed a proxy informant using a rigorous protocol with demonstrated reliability and validity.14 All incident NH admissions, including both short- and long-term stays, were identified using the CMS Skilled Nursing Facility claims file and Long Term Care Minimum Data Set. Deaths were ascertained by review of obituaries and/or from a proxy informant, with a completion rate of 100%. A total of 688 participants (91.2%) had died after a median follow-up of 108 months, while 43 participants (5.7%) dropped out of the study after a median follow-up of 27 months. Among all participants, data were otherwise available for 99.2% of 85,531 monthly telephone interviews.
Assembly of Analytic Sample
PEP participants were considered for inclusion in the analytic sample if they had a hospitalization with an ACSC as the primary diagnosis on linked Medicare claims data. The complete list of ACSCs was defined using specifications from the Agency for Healthcare Research and Quality,15 and was assembled using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) classification prior to October 1, 2015, and ICD Tenth Revision, Clinical Modification (ICD-10-CM) classification after October 1, 2015 (Appendix Table 1). Examples of ACSCs include congestive heart failure, dehydration, urinary tract infection, and angina without procedure. As performed previously,16,17 two ACSCs (low birthweight; asthma in younger adults 18-39 years) were not included in this analysis because they were not based on full adult populations.
ACSC-related hospitalizations were included through December 2017. Participants could contribute more than one ACSC-related hospitalization over the course of the study based on the following criteria: (1) participant did not have a prior non-ACSC-related hospitalization within an 18-month interval; (2) participant did not have a prior ACSC-related hospitalization or treat-and-release emergency department (ED) visit within an 18-month interval (to ensure independence of observations if the participant was still recovering from the prior event and because some of the characteristics within Table 1 are susceptible to change in the setting of an intervening event and, hence, would not accurately reflect the status of the participant prior to ACSC-related hospitalization); (3) participant was not admitted from a NH; (4) participant did not have an in-hospital intensive care unit (ICU) stay (because persons with critical illness are a distinct population with frequent disability and prolonged recovery, as previously described18), in-hospital death, or death before first follow-up interview (because our aim was to evaluate disability and recovery after the hospitalization7).
Assembly of the primary analytic sample is depicted in the Appendix Figure. Of the 814 patients who were identified with ACSC-related hospitalizations, 107 had a prior non-ACSC-related hospitalization and 275 had a prior ACSC-related hospitalization or a treat-and-release ED visit within an 18-month interval. Of the remaining 432 ACSC-related hospitalizations, 181 were excluded: 114 patients were admitted from a NH, 38 had an in-hospital ICU stay, 3 died in the hospital, 11 died before their first follow-up interview, and 15 had withdrawn from the study. The primary analytic sample included the remaining 251 ACSC-related hospitalizations, contributed by 196 participants. Specifically, nine participants contributed three ACSC-related hospitalizations each, 37 participants contributed two hospitalizations each, and the remaining 150 participants contributed one hospitalization each. During the 6-month follow-up period, 40 participants contributing ACSC-related hospitalizations died after a median (interquartile range [IQR]) of 4 (2-5) months, and 1 person refused continued participation.
Comprehensive Assessments
During the comprehensive in-home assessments, data were obtained on demographic characteristics. Age was measured in years at the time of the ACSC-related hospitalization. In addition, we describe factors from the comprehensive assessment immediately prior to the ACSC-related hospitalization, grouped into two additional domains related to disability19: health-related and cognitive-psychosocial. The health-related factors included nine self-reported, physician-diagnosed chronic conditions and frailty. The cognitive-psychosocial factors included social support, cognitive impairment, and depressive symptoms.
Assessment of Disability
Complete details about the assessment of disability have been previously described.13,14,19,20 Briefly, disability was assessed during the monthly telephone interviews, and included four basic activities (bathing, dressing, walking across a room, and transferring from a chair), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and three mobility activities (walking a quarter mile, climbing a flight of stairs, and lifting or carrying 10 lb). Participants were asked, “At the present time, do you need help from another person to [complete the task]?” Disability was operationalized as the need for personal assistance or an inability to perform the task. Participants were also asked about a fourth mobility activity, “Have you driven a car during the past month?” Those who responded no were classified as being disabled in driving.19
The number of disabilities overall and for each functional domain (basic, instrumental, and mobility) was summed. Possible disability scores ranged from 0 to 13, with a score of 0 indicating complete independence in all of the items, and a score of 13 indicating complete dependence. Worse postdischarge disability was defined as a total disability score (0-13) at the first telephone interview after an ACSC-related hospitalization that was greater than the total disability score from the telephone interview immediately preceding hospitalization.
Outcome Measures
The primary outcome was the number of disabilities in all 13 basic, instrumental, and mobility activities in each of the 6 months following discharge from an ACSC-related hospitalization. To determine whether our findings were consistent across the three functional domains, we also evaluated the number of disabilities in the four basic, five instrumental, and four mobility activities separately. As secondary outcomes, we evaluated: (1) the cumulative probability of recovery within the 6-month follow-up time frame after an ACSC-related hospitalization, with “recovery” defined as return to the participant’s pre-ACSC-related hospitalization total disability score, and (2) the cumulative probability of incident NH admission within the 6 months after an ACSC-related hospitalization. Aligned with CMS and prior literature,21,22 we defined a short-term NH stay as ≤100 days and a long-term NH stay as >100 days.
Statistical Analysis
Pre-ACSC-related hospitalization characteristics were summarized by means (SDs) and frequencies with proportions. We determined the mean number of disabilities in each of the 6 months following hospital discharge, with the prehospitalization value included as a reference point. We also determined the mean (SD) number of disabilities for the three subscales of disability (basic activities of daily living [BADLs], instrumental activities of daily living [IADLs], and mobility activities). We calculated the cumulative probability of recovery within 6 months of hospital discharge. Finally, we determined the cumulative probability of incident NH admission during the 6 months after hospital discharge.
To test the robustness of our main results, we conducted a sensitivity analysis assessing disability scores of the 150 participants that contributed only one ACSC-related hospitalization. All analyses were performed using Stata, version 16.0, statistical software (StataCorp).
RESULTS
Table 1 shows the characteristics of the 251 ACSC-related hospitalizations immediately prior to hospitalization. Participants’ mean (SD) age was 85.1 (6.0) years, and the mean total disability score was 5.4. The majority were female, non-Hispanic White, frail, and lived alone. As shown in Appendix Table 2, the three most common reasons for ACSC-related hospitalizations were congestive heart failure (n = 69), bacterial pneumonia (n = 53), and dehydration (n = 44).
The Figure shows the disability scores during the 6-month follow-up period for total, basic, instrumental, and mobility activities, in panels A, B, C, and D, respectively. The exact values are provided in Appendix Table 3. After hospitalization, disability scores for total, basic, instrumental, and mobility activities peaked at month 1 and tended to improve modestly over the next 5 months, but remained greater, on average, than pre-hospitalization scores. Of the 40 participants who died within the 6-month follow-up period, 36 (90%) had worse disability scores in their last month of life than in the month prior to their ACSC-related hospitalization.
Table 2 shows the cumulative probability of functional recovery after ACSC-related hospitalizations. Recovery was incomplete, with only 70% (95% CI, 64%-76%) of hospitalizations achieving a return to the pre-hospitalization total disability score within 6 months of hospitalization.
Table 3 shows the cumulative probability of incident NH admission after an ACSC-related hospitalization. Of the 251 ACSC-related hospitalizations, incident NH admission was experienced by 38% (95% CI, 32%-44%) within 1 month and 50% (95% CI, 43%-56%) within 6 months of discharge. Short-term NH stays accounted for 90 (75.6%) of the 119 incident NH admissions within the 6 months after ACSC-related hospitalizations. Sensitivity analyses yielded comparable disability scores, shown in Appendix Table 4.
DISCUSSION
In this longitudinal study of community-living older persons, we evaluated functional disability, recovery, and incident NH admission within 6 months of hospitalization for an ACSC. Our study has three major findings. First, disability scores for total, basic, instrumental, and mobility activities at months 1 to 6 of follow-up were greater on average than pre-hospitalization scores. Second, functional recovery was not achieved by 3 of 10 participants after an ACSC-related hospitalization. Third, half of them experienced an incident NH admission within 6 months of discharge from an ACSC-related hospitalization, although about three-quarters of these were short-term stays. Our findings provide evidence that older persons experience clinically meaningful adverse patient-reported outcomes after ACSC-related hospitalizations.
Prior research involving ACSCs has focused largely on rates of hospitalization as a measure of access to primary care and the associated factors predictive of ACSC-related hospitalizations,23-26 and has not addressed subsequent patient-reported outcomes. The findings in this analysis highlight that older persons experience worsening disability immediately after an ACSC-related hospitalization, which persists for prolonged periods and often results in incomplete recovery. Prior research has assessed pre-hospitalization functional status through retrospective recall approaches,2 included only older adults discharged with incident disability,3 and examined functional status after all-cause medical illness hospitalizations.5 Our prospective analysis extends the literature by reliably capturing pre-hospital disability scores and uniquely assessing the cohort of older persons hospitalized with ACSCs.
Our work is relevant to the continued evaluation of ACSC-related hospitalizations in national quality measurement and payment initiatives among Medicare beneficiaries. In prior evaluations of ACSC-related quality measures, stakeholders have criticized the measures for limited validity due to a lack of evidence linking each utilization outcome to other patient-centered outcomes.10,27 Our work addresses this gap by demonstrating that ACSC-related hospitalizations are linked to persistent disability, incomplete functional recovery, and incident NH admissions. Given the large body of evidence demonstrating the priority older persons place on these patient-reported outcomes,28,29 our work should reassure policymakers seeking to transform quality measurement programs into a more patient-oriented enterprise.
Our findings have several clinical practice, research, and policy implications. First, more-effective clinical strategies to minimize the level of care required for acute exacerbations of ACSC-related illnesses may include: (1) substituting home-based care30 and telehealth interventions31 for traditional inpatient hospitalization, (2) making in-ED resources (ie, case management services, geriatric-focused advanced practice providers) more accessible for older persons with ACSC-related illnesses, thereby enhancing care transitions and follow-up to avoid potential current and subsequent hospitalizations, and (3) ensuring adequate ambulatory care access to all older persons, as prior work has shown variation in ACSC hospital admission rates dependent on population factors such as high-poverty neighborhoods,16 insurance status,16,32 and race/ethnicity.33
Clinical strategies have been narrow and not holistic for ACSCs; for example, many institutions have focused on pneumonia vaccinations to reduce hospitalizations, but our work supports the need to further evaluate the impact of preventing ACSC-related hospitalizations and their associated disabling consequences. For patients admitted to the hospital, clinical strategies, such as in-hospital or post-hospital mobility and activity programs, have been shown to be protective against hospital-associated disability.34,35 Furthermore, hospital discharge planning could include preparing older persons for anticipated functional disabilities, associated recoveries, and NH admission after ACSC-related hospitalizations. Risk factors contributing to post-hospitalization functional disability and recovery have been identified,19,20,36 but future work is needed to: (1) identify target populations (including those most likely to worsen) so that interventions can be offered earlier in the course of care to those who would benefit most, and (2) identify and learn from those who are resilient and have recovered, to better understand factors contributing to their success.
Our study has several strengths. First, the study is unique due to its longitudinal design, with monthly assessments of functional status. Since functional status was assessed prospectively before the ACSC-related hospitalization, we also have avoided any potential concern for recall bias that may be present if assessed after the hospitalization. Additionally, through the use of Medicare claims and the Minimum Data Set, the ascertainment of hospitalizations and NH admissions was likely complete for the studied population.
However, the study has limitations. First, functional measures were based on self-reports rather than objective measurements. Nevertheless, the self-report function is often used to guide coverage determinations in the Medicare program, as it has been shown to be associated with poor health outcomes.37 Second, we are unable to comment on the rate of functional decline or NH admission when an older person was not hospitalized in relation to an ACSC. Future analyses may benefit from using a control group (eg, older adults without an ACSC hospitalization or older adults with a non-ACSC hospitalization). Third, we used strict exclusion criteria to identify a population of older adults without recent hospitalizations to determine the isolated impact of ACSC hospitalization on disability, incident NH admission, and functional recovery. Considering this potential selection bias, our findings are likely conservative estimates of the patient-centered outcomes evaluated. Fourth, participants were not asked about feeding and toileting. However, the incidence of disability in these ADLs is low among nondisabled, community-living older persons, and it is highly uncommon for disability to develop in these ADLs without concurrent disability in the ADLs within this analysis.14,38
Finally, because our study participants were members of a single health plan in a small urban area and included nondisabled older persons living in the community, our findings may not be generalizable to geriatric patients in other settings. Nonetheless, the demographics of our cohort reflect those of older persons in New Haven County, Connecticut, which are similar to the demographics of the US population, with the exception of race and ethnicity. In addition, the generalizability of our results are strengthened by the study’s high participation rate and minimal attrition.
CONCLUSION
Within 6 months of ACSC-related hospitalizations, community-living older persons exhibited greater total disability scores than those immediately preceding hospitalization. In the same time frame, 3 of 10 older persons did not achieve functional recovery, and half experienced incident NH admission. These results provide evidence regarding the continued recognition of ACSC-related hospitalizations in federal quality measurement and payment programs and suggests the need for preventive and comprehensive interventions to meaningfully improve longitudinal outcomes.
Acknowledgments
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; and Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director. Each of these persons were paid employees of Yale School of Medicine during the conduct of this study.
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556
2. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-458. https://doi.org/10.1046/j.1532-5415.2003.51152.x
3. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. https://doi.org/10.1007/s11606-012-2226-y
4. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919-1928. https://doi.org/10.1001/jama.2010.1568
5. Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171-2179. https://doi.org/10.1111/j.1532-5415.2008.02023.x
6. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461. https://doi.org/10.1016/j.jamda.2019.09.015
7. Dharmarajan K, Han L, Gahbauer EA, Leo-Summers LS, Gill TM. Disability and recovery after hospitalization for medical illness among community-living older persons: a prospective cohort study. J Am Geriatr Soc. 2020;68(3):486-495. https://doi.org/10.1111/jgs.16350
8. Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MAM, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7(6):863-871. https://doi.org/10.1161/HCQ.0000000000000008
9. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794. https://doi.org/10.1001/jama.2010.1553
10. Hodgson K, Deeny SR, Steventon A. Ambulatory care-sensitive conditions: their potential uses and limitations. BMJ Qual Saf. 2019;28(6):429-433. https://doi.org/10.1136/bmjqs-2018-008820
11. Agency for Healthcare Research and Quality (AHRQ). Quality Indicator User Guide: Prevention Quality Indicators (PQI) Composite Measures. Version 2020. Accessed November 10, 2020. https://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx.
12. Centers for Medicare & Medicaid Services. 2016 Measure information about the hospital admissions for acute and chronic ambulatory care-sensitive condition (ACSC) composite measures, calculated for the 2018 value-based payment modified program. Accessed November 24, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2016-ACSC-MIF.pdf.
13. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321. https://doi.org/10.7326/0003-4819-135-5-200109040-00007
14. Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492-1497. https://doi.org/10.1046/j.1532-5415.2002.50403.x
15. Agency for Healthcare Research and Quality. Prevention Quality Indicators Technical Specifications Updates—Version v2018 and 2018.0.1 (ICD 10-CM/PCS), June 2018. Accessed February 4, 2020. https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD10_v2018.aspx.
16. Johnson PJ, Ghildayal N, Ward AC, Westgard BC, Boland LL, Hokanson JS. Disparities in potentially avoidable emergency department (ED) care: ED visits for ambulatory care sensitive conditions. Med Care. 2012;50(12):1020-1028. https://doi.org/10.1097/MLR.0b013e318270bad4
17. Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Acad Emerg Med. 2015;22(2):172-181. https://doi.org/10.1111/acem.12579
18. Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523-529. https://doi.org/10.1001/jamainternmed.2014.7889
19. Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131-140. https://doi.org/10.7326/0003-4819-156-2-201201170-00009
20. Hardy SE, Gill TM. Factors associated with recovery of independence among newly disabled older persons. Arch Intern Med. 2005;165(1):106-112. https://doi.org/10.1001/archinte.165.1.106
21. Centers for Medicare & Medicaid Services. Nursing Home Quality Initiative—Quality Measures. Accessed June 13, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures
22. Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. https://doi.org/10.1186/s12913-017-2318-9
23. Laditka, JN, Laditka SB, Probst JC. More may be better: evidence of a negative relationship between physician supply and hospitalization for ambulatory care sensitive conditions. Health Serv Res. 2005;40(4):1148-1166. https://doi.org/10.1111/j.1475-6773.2005.00403.x
24. Ansar Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Med Care Res Rev. 2006;63(6):719-741. https://doi.org/10.1177/1077558706293637
25. Mackinko J, de Oliveira VB, Turci MA, Guanais FC, Bonolo PF, Lima-Costa MF. The influence of primary care and hospital supply on ambulatory care-sensitive hospitalizations among adults in Brazil, 1999-2007. Am J Public Health. 2011;101(10):1963-1970. https://doi.org/10.2105/AJPH.2010.198887
26. Gibson OR, Segal L, McDermott RA. A systematic review of evidence on the association between hospitalisation for chronic disease related ambulatory care sensitive conditions and primary health care resourcing. BMC Health Serv Res. 2013;13:336. https://doi.org/10.1186/1472-6963-13-336
27. Vuik SI, Fontana G, Mayer E, Darzi A. Do hospitalisations for ambulatory care sensitive conditions reflect low access to primary care? An observational cohort study of primary care usage prior to hospitalisation. BMJ Open. 2017;7(8):e015704. https://doi.org/10.1136/bmjopen-2016-015704
28. Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83(2):278-282. https://doi.org/10.1016/j.pec.2010.04.032
29. Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777-779. https://doi.org/10.1056/NEJMp1113631
30. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. https://doi.org/10.1001/jamainternmed.2018.2562
31. Shah MN, Wasserman EB, Gillespie SM, et al. High-intensity telemedicine decreases emergency department use for ambulatory care sensitive conditions by older adult senior living community residents. J Am Med Dir Assoc. 2015;16(12):1077-1081. https://doi.org/10.1016/j.jamda.2015.07.009
32. Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003;41(2):198-207. https://doi.org/10.1097/01.MLR.0000045021.70297.9F
33. O’Neil SS, Lake T, Merrill A, Wilson A, Mann DA, Bartnyska LM. Racial disparities in hospitalizations for ambulatory care-sensitive conditions. Am J Prev Med. 2010;38(4):381-388. https://doi.org/10.1016/j.amepre.2009.12.026
34. Pavon JM, Sloane RJ, Pieper RF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68:261-265. https://doi.org/10.1111/jgs.16231
35. Sunde S, Hesseberg K, Skelton DA, et al. Effects of a multicomponent high intensity exercise program on physical function and health-related quality of life in older adults with or at risk of mobility disability after discharge from hospital: a randomised controlled trial. BMC Geriatr. 2020;20(1):464. https://doi.org/10.1186/s12877-020-01829-9
36. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596-1602. https://doi.org/10.1001/jama.291.13.1596
37. Rotenberg J, Kinosian B, Boling P, Taler G, Independence at Home Learning Collaborative Writing Group. Home-based primary care: beyond extension of the independence at home demonstration. J Am Geriatr Soc. 2018;66(4):812-817. https://doi.org/10.1111/jgs.15314
38. Rodgers W, Miller B. A comparative analysis of ADL questions in surveys of older people. J Gerontol B Psychol Sci Soc Sci. 1997;52:21-36. https://doi.org/10.1093/geronb/52b.special_issue.21
1. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556
2. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-458. https://doi.org/10.1046/j.1532-5415.2003.51152.x
3. Barnes DE, Mehta KM, Boscardin WJ, et al. Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28(2):261-268. https://doi.org/10.1007/s11606-012-2226-y
4. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919-1928. https://doi.org/10.1001/jama.2010.1568
5. Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171-2179. https://doi.org/10.1111/j.1532-5415.2008.02023.x
6. Loyd C, Markland AD, Zhang Y, et al. Prevalence of hospital-associated disability in older adults: a meta-analysis. J Am Med Dir Assoc. 2020;21(4):455-461. https://doi.org/10.1016/j.jamda.2019.09.015
7. Dharmarajan K, Han L, Gahbauer EA, Leo-Summers LS, Gill TM. Disability and recovery after hospitalization for medical illness among community-living older persons: a prospective cohort study. J Am Geriatr Soc. 2020;68(3):486-495. https://doi.org/10.1111/jgs.16350
8. Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MAM, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7(6):863-871. https://doi.org/10.1161/HCQ.0000000000000008
9. Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794. https://doi.org/10.1001/jama.2010.1553
10. Hodgson K, Deeny SR, Steventon A. Ambulatory care-sensitive conditions: their potential uses and limitations. BMJ Qual Saf. 2019;28(6):429-433. https://doi.org/10.1136/bmjqs-2018-008820
11. Agency for Healthcare Research and Quality (AHRQ). Quality Indicator User Guide: Prevention Quality Indicators (PQI) Composite Measures. Version 2020. Accessed November 10, 2020. https://www.qualityindicators.ahrq.gov/modules/pqi_resources.aspx.
12. Centers for Medicare & Medicaid Services. 2016 Measure information about the hospital admissions for acute and chronic ambulatory care-sensitive condition (ACSC) composite measures, calculated for the 2018 value-based payment modified program. Accessed November 24, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeedbackProgram/Downloads/2016-ACSC-MIF.pdf.
13. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321. https://doi.org/10.7326/0003-4819-135-5-200109040-00007
14. Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492-1497. https://doi.org/10.1046/j.1532-5415.2002.50403.x
15. Agency for Healthcare Research and Quality. Prevention Quality Indicators Technical Specifications Updates—Version v2018 and 2018.0.1 (ICD 10-CM/PCS), June 2018. Accessed February 4, 2020. https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD10_v2018.aspx.
16. Johnson PJ, Ghildayal N, Ward AC, Westgard BC, Boland LL, Hokanson JS. Disparities in potentially avoidable emergency department (ED) care: ED visits for ambulatory care sensitive conditions. Med Care. 2012;50(12):1020-1028. https://doi.org/10.1097/MLR.0b013e318270bad4
17. Galarraga JE, Mutter R, Pines JM. Costs associated with ambulatory care sensitive conditions across hospital-based settings. Acad Emerg Med. 2015;22(2):172-181. https://doi.org/10.1111/acem.12579
18. Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523-529. https://doi.org/10.1001/jamainternmed.2014.7889
19. Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131-140. https://doi.org/10.7326/0003-4819-156-2-201201170-00009
20. Hardy SE, Gill TM. Factors associated with recovery of independence among newly disabled older persons. Arch Intern Med. 2005;165(1):106-112. https://doi.org/10.1001/archinte.165.1.106
21. Centers for Medicare & Medicaid Services. Nursing Home Quality Initiative—Quality Measures. Accessed June 13, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIQualityMeasures
22. Goodwin JS, Li S, Zhou J, Graham JE, Karmarkar A, Ottenbacher K. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res. 2017;17(1):376. https://doi.org/10.1186/s12913-017-2318-9
23. Laditka, JN, Laditka SB, Probst JC. More may be better: evidence of a negative relationship between physician supply and hospitalization for ambulatory care sensitive conditions. Health Serv Res. 2005;40(4):1148-1166. https://doi.org/10.1111/j.1475-6773.2005.00403.x
24. Ansar Z, Laditka JN, Laditka SB. Access to health care and hospitalization for ambulatory care sensitive conditions. Med Care Res Rev. 2006;63(6):719-741. https://doi.org/10.1177/1077558706293637
25. Mackinko J, de Oliveira VB, Turci MA, Guanais FC, Bonolo PF, Lima-Costa MF. The influence of primary care and hospital supply on ambulatory care-sensitive hospitalizations among adults in Brazil, 1999-2007. Am J Public Health. 2011;101(10):1963-1970. https://doi.org/10.2105/AJPH.2010.198887
26. Gibson OR, Segal L, McDermott RA. A systematic review of evidence on the association between hospitalisation for chronic disease related ambulatory care sensitive conditions and primary health care resourcing. BMC Health Serv Res. 2013;13:336. https://doi.org/10.1186/1472-6963-13-336
27. Vuik SI, Fontana G, Mayer E, Darzi A. Do hospitalisations for ambulatory care sensitive conditions reflect low access to primary care? An observational cohort study of primary care usage prior to hospitalisation. BMJ Open. 2017;7(8):e015704. https://doi.org/10.1136/bmjopen-2016-015704
28. Fried TR, Tinetti M, Agostini J, Iannone L, Towle V. Health outcome prioritization to elicit preferences of older persons with multiple health conditions. Patient Educ Couns. 2011;83(2):278-282. https://doi.org/10.1016/j.pec.2010.04.032
29. Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med. 2012;366(9):777-779. https://doi.org/10.1056/NEJMp1113631
30. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. https://doi.org/10.1001/jamainternmed.2018.2562
31. Shah MN, Wasserman EB, Gillespie SM, et al. High-intensity telemedicine decreases emergency department use for ambulatory care sensitive conditions by older adult senior living community residents. J Am Med Dir Assoc. 2015;16(12):1077-1081. https://doi.org/10.1016/j.jamda.2015.07.009
32. Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003;41(2):198-207. https://doi.org/10.1097/01.MLR.0000045021.70297.9F
33. O’Neil SS, Lake T, Merrill A, Wilson A, Mann DA, Bartnyska LM. Racial disparities in hospitalizations for ambulatory care-sensitive conditions. Am J Prev Med. 2010;38(4):381-388. https://doi.org/10.1016/j.amepre.2009.12.026
34. Pavon JM, Sloane RJ, Pieper RF, et al. Accelerometer-measured hospital physical activity and hospital-acquired disability in older adults. J Am Geriatr Soc. 2020;68:261-265. https://doi.org/10.1111/jgs.16231
35. Sunde S, Hesseberg K, Skelton DA, et al. Effects of a multicomponent high intensity exercise program on physical function and health-related quality of life in older adults with or at risk of mobility disability after discharge from hospital: a randomised controlled trial. BMC Geriatr. 2020;20(1):464. https://doi.org/10.1186/s12877-020-01829-9
36. Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596-1602. https://doi.org/10.1001/jama.291.13.1596
37. Rotenberg J, Kinosian B, Boling P, Taler G, Independence at Home Learning Collaborative Writing Group. Home-based primary care: beyond extension of the independence at home demonstration. J Am Geriatr Soc. 2018;66(4):812-817. https://doi.org/10.1111/jgs.15314
38. Rodgers W, Miller B. A comparative analysis of ADL questions in surveys of older people. J Gerontol B Psychol Sci Soc Sci. 1997;52:21-36. https://doi.org/10.1093/geronb/52b.special_issue.21
© 2021 Society of Hospital Medicine
Morning Discharges Are Also Not Associated With Emergency Department Boarding Times
We thank Dr Zorian and colleagues for their editorial1 addressing our retrospective multicenter cohort study, “Morning Discharges and Patient Length-of-Stay in Inpatient General Internal Medicine.”2 Dr Zorian and colleagues raised a question about whether morning discharges were associated with emergency department (ED) boarding times (ie, the time between the decision to admit a patient and their departure from the ED). We also received correspondence from other readers expressing interest in this metric.
We measured the association between morning discharges from general internal medicine (GIM) and ED boarding time using the same methodology and cohort as previously described in our article.2 A total of 37 admissions out of 189,781 admissions (<0.1%) did not have an ED boarding time available and were excluded. The mean (SD) boarding time for the remaining cohort (n = 189,744) was 9.63 (11.67) hours. After categorizing days in the study period into quartiles based on the number of morning discharges from GIM, we did not find a strong unadjusted association with ED boarding times (Table). After multivariable adjustment with negative binomial regression models, as previously described,2 there was a weak, statistically significant association between the number of morning discharges and ED boarding time (adjusted rate ratio, 0.995; 95% CI, 0.991-1.000), corresponding to 2.4 minutes less in ED boarding time for every additional morning discharge. Ultimately, we agree with Dr Zorian and colleagues that instead of focusing on discharge-before-noon, hospitals should consider patient flow and discharge quality more holistically.
1. Zorian A, Shine D, Mourad M. Discharge by noon: toward a better understanding of benefits and costs. J Hosp Med. 2021;16(6):384. https://doi.org/10.12788/jhm.3613
2. Kirubarajan A, Shin S, Fralick M, et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):333-338. https://10.12788/jhm.3605
We thank Dr Zorian and colleagues for their editorial1 addressing our retrospective multicenter cohort study, “Morning Discharges and Patient Length-of-Stay in Inpatient General Internal Medicine.”2 Dr Zorian and colleagues raised a question about whether morning discharges were associated with emergency department (ED) boarding times (ie, the time between the decision to admit a patient and their departure from the ED). We also received correspondence from other readers expressing interest in this metric.
We measured the association between morning discharges from general internal medicine (GIM) and ED boarding time using the same methodology and cohort as previously described in our article.2 A total of 37 admissions out of 189,781 admissions (<0.1%) did not have an ED boarding time available and were excluded. The mean (SD) boarding time for the remaining cohort (n = 189,744) was 9.63 (11.67) hours. After categorizing days in the study period into quartiles based on the number of morning discharges from GIM, we did not find a strong unadjusted association with ED boarding times (Table). After multivariable adjustment with negative binomial regression models, as previously described,2 there was a weak, statistically significant association between the number of morning discharges and ED boarding time (adjusted rate ratio, 0.995; 95% CI, 0.991-1.000), corresponding to 2.4 minutes less in ED boarding time for every additional morning discharge. Ultimately, we agree with Dr Zorian and colleagues that instead of focusing on discharge-before-noon, hospitals should consider patient flow and discharge quality more holistically.
We thank Dr Zorian and colleagues for their editorial1 addressing our retrospective multicenter cohort study, “Morning Discharges and Patient Length-of-Stay in Inpatient General Internal Medicine.”2 Dr Zorian and colleagues raised a question about whether morning discharges were associated with emergency department (ED) boarding times (ie, the time between the decision to admit a patient and their departure from the ED). We also received correspondence from other readers expressing interest in this metric.
We measured the association between morning discharges from general internal medicine (GIM) and ED boarding time using the same methodology and cohort as previously described in our article.2 A total of 37 admissions out of 189,781 admissions (<0.1%) did not have an ED boarding time available and were excluded. The mean (SD) boarding time for the remaining cohort (n = 189,744) was 9.63 (11.67) hours. After categorizing days in the study period into quartiles based on the number of morning discharges from GIM, we did not find a strong unadjusted association with ED boarding times (Table). After multivariable adjustment with negative binomial regression models, as previously described,2 there was a weak, statistically significant association between the number of morning discharges and ED boarding time (adjusted rate ratio, 0.995; 95% CI, 0.991-1.000), corresponding to 2.4 minutes less in ED boarding time for every additional morning discharge. Ultimately, we agree with Dr Zorian and colleagues that instead of focusing on discharge-before-noon, hospitals should consider patient flow and discharge quality more holistically.
1. Zorian A, Shine D, Mourad M. Discharge by noon: toward a better understanding of benefits and costs. J Hosp Med. 2021;16(6):384. https://doi.org/10.12788/jhm.3613
2. Kirubarajan A, Shin S, Fralick M, et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):333-338. https://10.12788/jhm.3605
1. Zorian A, Shine D, Mourad M. Discharge by noon: toward a better understanding of benefits and costs. J Hosp Med. 2021;16(6):384. https://doi.org/10.12788/jhm.3613
2. Kirubarajan A, Shin S, Fralick M, et al. Morning discharges and patient length of stay in inpatient general internal medicine. J Hosp Med. 2021;16(6):333-338. https://10.12788/jhm.3605
© 2021 Society of Hospital Medicine
Moment vs Movement: Mission-Based Tweeting for Physician Advocacy
“We, the members of the world community of physicians, solemnly commit ourselves to . . . advocate for social, economic, educational and political changes that ameliorate suffering and contribute to human well-being.”
— American Medical Association Oath of Professional Responsibility. 1
As individuals and groups spread misinformation on social media platforms, there is a greater need for physician health advocacy.2 We have learned through the COVID-19 pandemic that rapidly evolving information requires public-facing health experts to address misinformation and explain why healthcare providers and experts make certain recommendations.2 Physicians recognize the potential for benefit from crowdsourcing education, positive publicity, and increasing their reach to a larger platform.3
However, despite social media’s need for such expertise and these recognized benefits, many physicians are hesitant to engage on social media, citing lack of time, interest, or the proper skill set to use it effectively.3 Additional barriers may include uncertainty about employer policies, fear of saying something inaccurate or unprofessional, or inadvertently breaching patient privacy.3 While these are valid concerns, a strategic approach to curating a social media presence focuses less on the moments created by provocative tweets and more on the movement the author wishes to amplify. Here, we propose a framework for effective physician advocacy using a strategy we term Mission-Based Tweeting (MBT).
MISSION-BASED TWEETING
Physicians can use Twitter to engage large audiences.4 MBT focuses an individual’s central message by providing a framework upon which to build such engagement.5 The conceptual framework for a meaningful social media strategy through MBT is anchored on the principle that the impact of our Twitter content is more valuable than the number of followers.6 Using this framework, users begin by creating and defining their identity while engaging in meaningful online interactions. Over time, these interactions will lead to generating influence related to their established identity, which can ultimately impact the social micro-society.6 While an individual’s social media impact can be determined and reinforced through MBT, it remains important to know that MBT is not exemplified in one specific tweet, but rather in the body of work shared by an individual that continuously reinforces the mission.
TWEETING FOR THE MOMENT VS FOR THE MOVEMENT: USING MBT FOR ADVOCACY
Advocacy typically involves using one’s voice to publicly support a specific interest. With that in mind, health advocacy can be divided into two categories: (1) agency, which involves advancing the health of individual patients within a system, and (2) activism, which acts to advance the health of communities or populations or change the structure of the healthcare system.7 While many physicians accept agency as part of their day-to-day job, activism is often more difficult. For example, physicians hoping to engage in health advocacy may be unable to travel to their state or federal legislature buildings, or their employers may restrict their ability to interact with elected officials. The emergence of social media and digital technology has lowered these barriers and created more accessible opportunities for physicians to engage in advocacy efforts.
Social media can provide an opportunity for clinicians to engage with other healthcare professionals, creating movements that have far-reaching effects across the healthcare spectrum. These movements, often driven by common hashtags, have expanded greatly beyond their originators’ intent, thus demonstrating the power of social media for healthcare activism (Table).4 Physician advocacy can provide accurate information about medical conditions and treatments, dispel myths that may affect patient care, and draw attention to conditions that impact their ability to provide that care. For instance, physicians and medical students recently used Twitter during the COVID-19 pandemic to focus on the real consequences of lack of access to personal protective equipment during the pandemic (Table).8,9 In the past year, physicians have used Twitter to highlight how structural racism perpetuates racial disparities in COVID-19 and to call for action against police brutality and the killing of unarmed Black citizens. Such activism has led to media appearances and even congressional testimony—which has, in turn, provided even larger audiences for clinicians’ advocacy efforts.10 Physicians can also use MBT to advocate for the medical profession. Strategic, mission-based, social media campaigns have focused on including women; Black, Indigenous, and People of Color (BIPOC); doctors with disabilities; and LGBTQ+ physicians in the narrative of what a doctor looks like (Table).11,12
When physicians consider their personal mission statement as it applies to their social media presence, it allows them to connect to something bigger than themselves, while helping guide them away from engagements that do not align with their personal or professional values. In this manner, MBT harnesses an individual’s authenticity and helps build their personal branding, which may ultimately result in more opportunities to advance their mission. In our experience, the constant delivery of mission-based content can even accelerate one’s professional work, help amplify others’ successes and voices, and ultimately lead to more meaningful engagement and activism.
However, it is important to note that there are potential downsides to engaging on social media, particularly for women and BIPOC users. For example, in a recent online survey, almost a quarter of physicians who responded reported personal attacks on social media, with one in six female physicians reporting sexual harassment.13 This risk may increase as an individual’s visibility and reach increase.
DEVELOP YOUR MISSION STATEMENT
To aid in MBT, we have found it useful to define your personal mission statement, which should succinctly describe your core values, the specific population or cause you serve, and your overarching goals or ideals. For example, someone interested in advocating for health justice might have the following mission statement: “To create and support a healthcare workforce and graduate medical education environment that strives for excellence and values Inclusion, Diversity, Access, and Equity as not only important, but necessary, for excellence.”14 Developing a personal mission statement permits more focus in all activities, including clinical, educational, administrative, or scholarship, and allows one to succinctly communicate important values with others.15 Communicating your personal mission statement concisely can improve the quality of your interactions with others and allows you to more precisely define the qualitative and quantitative impact of your social media engagement.
ENGAGING TO AMPLIFY YOUR MISSION
There are several options for creating and delivering effective mission-driven content on Twitter.16 We propose the Five A’s of MBT (Authenticity is key, Amplify other voices, Accelerate your work, Avoid arguments, Always be professional) to provide a general guide to ensuring that your tweets honor your mission (Figure). While each factor is important, we consider authenticity the most important as it guides consistency of the message, addresses your mission, and invites discussion. In this manner, even when physicians tweet about lived experiences or scientific data that may make some individuals uncomfortable, authenticity can still lead to meaningful engagement.17
There is synergy between amplifying other voices and accelerating your own work, as both provide an opportunity to highlight your specific advocacy interest. In the earlier example, the physician advocating for health justice may create a thread highlighting inequities in COVID-19 vaccination, including their own data and that of other health justice scholars, and in doing so, provide an invaluable repository of references or speakers for a future project.
We caution that not everyone will agree with your mission, so avoiding arguments and remaining professional in these interactions is paramount. Furthermore, it is also possible that a physician’s mission and opinions may not align with those of their employer, so it is important for social media users to review and clarify their employer’s social media policies to avoid violations and related repercussions. Physicians should tweet as if they were speaking into a microphone on the record, and authenticity should ground them into projecting the same personality online as they would offline.
CONCLUSION
We believe that, by the very nature of their chosen careers, physicians should step into the tension of advocacy. We acknowledge that physicians who are otherwise vocal advocates in other areas of life may be reluctant to engage on social media. However, if the measure of “success” on Twitter is meaningful interaction, sharing knowledge, and amplifying other voices according to a specific personal mission, MBT can be a useful framework. This is a call to action for hesitant physicians to take a leap and explore this platform, and for those already using social media to reevaluate their use and reflect on their mission. Physicians have been gifted a megaphone that can be used to combat misinformation, advocate for patients and the healthcare community, and advance needed discussions to benefit those in society who cannot speak for themselves. We advocate for physicians to look beyond the moment of a tweet and consider how your voice can contribute to a movement.
Acknowledgments
The authors thank Dr Vineet Arora for her contribution to early concept development for this manuscript and the JHM editorial staff for their productive feedback and editorial comments.
1. Riddick FA Jr. The code of medical ethics of the American Medical Association. Ochsner J. 2003;5(2):6-10. https://doi.org/10.3201/eid2702.203139
2. Vraga EK, Bode L. Addressing COVID-19 misinformation on social media preemptively and responsively. Emerg Infect Dis. 2021;27(2):396-403. https://doi.org/10.3201/eid2702.203139
3. Campbell L, Evans Y, Pumper M, Moreno MA. Social media use by physicians: a qualitative study of the new frontier of medicine. BMC Med Inform Decis Mak. 2016;16:91. https://doi.org/10.1186/s12911-016-0327-y
4. Wetsman N. How Twitter is changing medical research. Nat Med. 2020;26(1):11-13. https://doi.org/10.1038/s41591-019-0697-7
5. Shapiro M. Episode 107: Vinny Arora & Charlie Wray on Social Media & CVs. Explore The Space Podcast. https://www.explorethespaceshow.com/podcasting/vinny-arora-charlie-wray-on-cvs-social-media/
6. Varghese T. i4 (i to the 4th) is a strategy for #SoMe. Accessed April 22, 2021. https://twitter.com/TomVargheseJr/status/1027181443712081920?s=20
7. Dobson S, Voyer S, Regehr G. Perspective: agency and activism: rethinking health advocacy in the medical profession. Acad Med. 2012;87(9):1161-1164. https://doi.org/10.1097/ACM.0b013e3182621c25
8. #GetMePPE. Accessed April 22, 2021. https://twitter.com/hashtag/getmeppe?f=live
9. Ouyang H. At the front lines of coronavirus, turning to social media. The New York Times. March 18, 2020. Accessed April 22, 2021. https://www.nytimes.com/2020/03/18/well/live/coronavirus-doctors-facebook-twitter-social-media-covid.html
10. Blackstock U. Combining social media advocacy with health policy advocacy. Accessed April 22, 2021. https://twitter.com/uche_blackstock/status/1270413367761666048?s=20
11. Meeks LM, Liao P, Kim N. Using Twitter to promote awareness of disabilities in medicine. Med Educ. 2019;53(5):525-526. https://doi.org/10.1111/medu.13836
12. Nolen L. To all the little brown girls out there “you can’t be what you can’t see but I hope you see me now and that you see yourself in me.” Accessed April 22, 2021. https://twitter.com/LashNolen/status/1160901502266777600?s=20.
13. Pendergrast TR, Jain S, Trueger NS, Gottlieb M, Woitowich NC, Arora VM. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552. https://doi.org/10.1001/jamainternmed.2020.7235
14. Marcelin JR. Personal mission statement. Accessed July 6, 2021. https://www.unmc.edu/intmed/residencies-fellowships/residency/diverse-taskforce/index.html.
15. Li S-TT, Frohna JG, Bostwick SB. Using your personal mission statement to INSPIRE and achieve success. Acad Pediatr. 2017;17(2):107-109. https://doi.org/10.1016/j.acap.2016.11.010
16. Alton L. 7 tips for creating engaging content every day. Accessed April 22, 2021. https://business.twitter.com/en/blog/7-tips-creating-engaging-content-every-day.html
17. Boyd R. Is everyone reading this??! Accessed April 22, 2021. https://twitter.com/RheaBoydMD/status/1273006362679578625?s=20
“We, the members of the world community of physicians, solemnly commit ourselves to . . . advocate for social, economic, educational and political changes that ameliorate suffering and contribute to human well-being.”
— American Medical Association Oath of Professional Responsibility. 1
As individuals and groups spread misinformation on social media platforms, there is a greater need for physician health advocacy.2 We have learned through the COVID-19 pandemic that rapidly evolving information requires public-facing health experts to address misinformation and explain why healthcare providers and experts make certain recommendations.2 Physicians recognize the potential for benefit from crowdsourcing education, positive publicity, and increasing their reach to a larger platform.3
However, despite social media’s need for such expertise and these recognized benefits, many physicians are hesitant to engage on social media, citing lack of time, interest, or the proper skill set to use it effectively.3 Additional barriers may include uncertainty about employer policies, fear of saying something inaccurate or unprofessional, or inadvertently breaching patient privacy.3 While these are valid concerns, a strategic approach to curating a social media presence focuses less on the moments created by provocative tweets and more on the movement the author wishes to amplify. Here, we propose a framework for effective physician advocacy using a strategy we term Mission-Based Tweeting (MBT).
MISSION-BASED TWEETING
Physicians can use Twitter to engage large audiences.4 MBT focuses an individual’s central message by providing a framework upon which to build such engagement.5 The conceptual framework for a meaningful social media strategy through MBT is anchored on the principle that the impact of our Twitter content is more valuable than the number of followers.6 Using this framework, users begin by creating and defining their identity while engaging in meaningful online interactions. Over time, these interactions will lead to generating influence related to their established identity, which can ultimately impact the social micro-society.6 While an individual’s social media impact can be determined and reinforced through MBT, it remains important to know that MBT is not exemplified in one specific tweet, but rather in the body of work shared by an individual that continuously reinforces the mission.
TWEETING FOR THE MOMENT VS FOR THE MOVEMENT: USING MBT FOR ADVOCACY
Advocacy typically involves using one’s voice to publicly support a specific interest. With that in mind, health advocacy can be divided into two categories: (1) agency, which involves advancing the health of individual patients within a system, and (2) activism, which acts to advance the health of communities or populations or change the structure of the healthcare system.7 While many physicians accept agency as part of their day-to-day job, activism is often more difficult. For example, physicians hoping to engage in health advocacy may be unable to travel to their state or federal legislature buildings, or their employers may restrict their ability to interact with elected officials. The emergence of social media and digital technology has lowered these barriers and created more accessible opportunities for physicians to engage in advocacy efforts.
Social media can provide an opportunity for clinicians to engage with other healthcare professionals, creating movements that have far-reaching effects across the healthcare spectrum. These movements, often driven by common hashtags, have expanded greatly beyond their originators’ intent, thus demonstrating the power of social media for healthcare activism (Table).4 Physician advocacy can provide accurate information about medical conditions and treatments, dispel myths that may affect patient care, and draw attention to conditions that impact their ability to provide that care. For instance, physicians and medical students recently used Twitter during the COVID-19 pandemic to focus on the real consequences of lack of access to personal protective equipment during the pandemic (Table).8,9 In the past year, physicians have used Twitter to highlight how structural racism perpetuates racial disparities in COVID-19 and to call for action against police brutality and the killing of unarmed Black citizens. Such activism has led to media appearances and even congressional testimony—which has, in turn, provided even larger audiences for clinicians’ advocacy efforts.10 Physicians can also use MBT to advocate for the medical profession. Strategic, mission-based, social media campaigns have focused on including women; Black, Indigenous, and People of Color (BIPOC); doctors with disabilities; and LGBTQ+ physicians in the narrative of what a doctor looks like (Table).11,12
When physicians consider their personal mission statement as it applies to their social media presence, it allows them to connect to something bigger than themselves, while helping guide them away from engagements that do not align with their personal or professional values. In this manner, MBT harnesses an individual’s authenticity and helps build their personal branding, which may ultimately result in more opportunities to advance their mission. In our experience, the constant delivery of mission-based content can even accelerate one’s professional work, help amplify others’ successes and voices, and ultimately lead to more meaningful engagement and activism.
However, it is important to note that there are potential downsides to engaging on social media, particularly for women and BIPOC users. For example, in a recent online survey, almost a quarter of physicians who responded reported personal attacks on social media, with one in six female physicians reporting sexual harassment.13 This risk may increase as an individual’s visibility and reach increase.
DEVELOP YOUR MISSION STATEMENT
To aid in MBT, we have found it useful to define your personal mission statement, which should succinctly describe your core values, the specific population or cause you serve, and your overarching goals or ideals. For example, someone interested in advocating for health justice might have the following mission statement: “To create and support a healthcare workforce and graduate medical education environment that strives for excellence and values Inclusion, Diversity, Access, and Equity as not only important, but necessary, for excellence.”14 Developing a personal mission statement permits more focus in all activities, including clinical, educational, administrative, or scholarship, and allows one to succinctly communicate important values with others.15 Communicating your personal mission statement concisely can improve the quality of your interactions with others and allows you to more precisely define the qualitative and quantitative impact of your social media engagement.
ENGAGING TO AMPLIFY YOUR MISSION
There are several options for creating and delivering effective mission-driven content on Twitter.16 We propose the Five A’s of MBT (Authenticity is key, Amplify other voices, Accelerate your work, Avoid arguments, Always be professional) to provide a general guide to ensuring that your tweets honor your mission (Figure). While each factor is important, we consider authenticity the most important as it guides consistency of the message, addresses your mission, and invites discussion. In this manner, even when physicians tweet about lived experiences or scientific data that may make some individuals uncomfortable, authenticity can still lead to meaningful engagement.17
There is synergy between amplifying other voices and accelerating your own work, as both provide an opportunity to highlight your specific advocacy interest. In the earlier example, the physician advocating for health justice may create a thread highlighting inequities in COVID-19 vaccination, including their own data and that of other health justice scholars, and in doing so, provide an invaluable repository of references or speakers for a future project.
We caution that not everyone will agree with your mission, so avoiding arguments and remaining professional in these interactions is paramount. Furthermore, it is also possible that a physician’s mission and opinions may not align with those of their employer, so it is important for social media users to review and clarify their employer’s social media policies to avoid violations and related repercussions. Physicians should tweet as if they were speaking into a microphone on the record, and authenticity should ground them into projecting the same personality online as they would offline.
CONCLUSION
We believe that, by the very nature of their chosen careers, physicians should step into the tension of advocacy. We acknowledge that physicians who are otherwise vocal advocates in other areas of life may be reluctant to engage on social media. However, if the measure of “success” on Twitter is meaningful interaction, sharing knowledge, and amplifying other voices according to a specific personal mission, MBT can be a useful framework. This is a call to action for hesitant physicians to take a leap and explore this platform, and for those already using social media to reevaluate their use and reflect on their mission. Physicians have been gifted a megaphone that can be used to combat misinformation, advocate for patients and the healthcare community, and advance needed discussions to benefit those in society who cannot speak for themselves. We advocate for physicians to look beyond the moment of a tweet and consider how your voice can contribute to a movement.
Acknowledgments
The authors thank Dr Vineet Arora for her contribution to early concept development for this manuscript and the JHM editorial staff for their productive feedback and editorial comments.
“We, the members of the world community of physicians, solemnly commit ourselves to . . . advocate for social, economic, educational and political changes that ameliorate suffering and contribute to human well-being.”
— American Medical Association Oath of Professional Responsibility. 1
As individuals and groups spread misinformation on social media platforms, there is a greater need for physician health advocacy.2 We have learned through the COVID-19 pandemic that rapidly evolving information requires public-facing health experts to address misinformation and explain why healthcare providers and experts make certain recommendations.2 Physicians recognize the potential for benefit from crowdsourcing education, positive publicity, and increasing their reach to a larger platform.3
However, despite social media’s need for such expertise and these recognized benefits, many physicians are hesitant to engage on social media, citing lack of time, interest, or the proper skill set to use it effectively.3 Additional barriers may include uncertainty about employer policies, fear of saying something inaccurate or unprofessional, or inadvertently breaching patient privacy.3 While these are valid concerns, a strategic approach to curating a social media presence focuses less on the moments created by provocative tweets and more on the movement the author wishes to amplify. Here, we propose a framework for effective physician advocacy using a strategy we term Mission-Based Tweeting (MBT).
MISSION-BASED TWEETING
Physicians can use Twitter to engage large audiences.4 MBT focuses an individual’s central message by providing a framework upon which to build such engagement.5 The conceptual framework for a meaningful social media strategy through MBT is anchored on the principle that the impact of our Twitter content is more valuable than the number of followers.6 Using this framework, users begin by creating and defining their identity while engaging in meaningful online interactions. Over time, these interactions will lead to generating influence related to their established identity, which can ultimately impact the social micro-society.6 While an individual’s social media impact can be determined and reinforced through MBT, it remains important to know that MBT is not exemplified in one specific tweet, but rather in the body of work shared by an individual that continuously reinforces the mission.
TWEETING FOR THE MOMENT VS FOR THE MOVEMENT: USING MBT FOR ADVOCACY
Advocacy typically involves using one’s voice to publicly support a specific interest. With that in mind, health advocacy can be divided into two categories: (1) agency, which involves advancing the health of individual patients within a system, and (2) activism, which acts to advance the health of communities or populations or change the structure of the healthcare system.7 While many physicians accept agency as part of their day-to-day job, activism is often more difficult. For example, physicians hoping to engage in health advocacy may be unable to travel to their state or federal legislature buildings, or their employers may restrict their ability to interact with elected officials. The emergence of social media and digital technology has lowered these barriers and created more accessible opportunities for physicians to engage in advocacy efforts.
Social media can provide an opportunity for clinicians to engage with other healthcare professionals, creating movements that have far-reaching effects across the healthcare spectrum. These movements, often driven by common hashtags, have expanded greatly beyond their originators’ intent, thus demonstrating the power of social media for healthcare activism (Table).4 Physician advocacy can provide accurate information about medical conditions and treatments, dispel myths that may affect patient care, and draw attention to conditions that impact their ability to provide that care. For instance, physicians and medical students recently used Twitter during the COVID-19 pandemic to focus on the real consequences of lack of access to personal protective equipment during the pandemic (Table).8,9 In the past year, physicians have used Twitter to highlight how structural racism perpetuates racial disparities in COVID-19 and to call for action against police brutality and the killing of unarmed Black citizens. Such activism has led to media appearances and even congressional testimony—which has, in turn, provided even larger audiences for clinicians’ advocacy efforts.10 Physicians can also use MBT to advocate for the medical profession. Strategic, mission-based, social media campaigns have focused on including women; Black, Indigenous, and People of Color (BIPOC); doctors with disabilities; and LGBTQ+ physicians in the narrative of what a doctor looks like (Table).11,12
When physicians consider their personal mission statement as it applies to their social media presence, it allows them to connect to something bigger than themselves, while helping guide them away from engagements that do not align with their personal or professional values. In this manner, MBT harnesses an individual’s authenticity and helps build their personal branding, which may ultimately result in more opportunities to advance their mission. In our experience, the constant delivery of mission-based content can even accelerate one’s professional work, help amplify others’ successes and voices, and ultimately lead to more meaningful engagement and activism.
However, it is important to note that there are potential downsides to engaging on social media, particularly for women and BIPOC users. For example, in a recent online survey, almost a quarter of physicians who responded reported personal attacks on social media, with one in six female physicians reporting sexual harassment.13 This risk may increase as an individual’s visibility and reach increase.
DEVELOP YOUR MISSION STATEMENT
To aid in MBT, we have found it useful to define your personal mission statement, which should succinctly describe your core values, the specific population or cause you serve, and your overarching goals or ideals. For example, someone interested in advocating for health justice might have the following mission statement: “To create and support a healthcare workforce and graduate medical education environment that strives for excellence and values Inclusion, Diversity, Access, and Equity as not only important, but necessary, for excellence.”14 Developing a personal mission statement permits more focus in all activities, including clinical, educational, administrative, or scholarship, and allows one to succinctly communicate important values with others.15 Communicating your personal mission statement concisely can improve the quality of your interactions with others and allows you to more precisely define the qualitative and quantitative impact of your social media engagement.
ENGAGING TO AMPLIFY YOUR MISSION
There are several options for creating and delivering effective mission-driven content on Twitter.16 We propose the Five A’s of MBT (Authenticity is key, Amplify other voices, Accelerate your work, Avoid arguments, Always be professional) to provide a general guide to ensuring that your tweets honor your mission (Figure). While each factor is important, we consider authenticity the most important as it guides consistency of the message, addresses your mission, and invites discussion. In this manner, even when physicians tweet about lived experiences or scientific data that may make some individuals uncomfortable, authenticity can still lead to meaningful engagement.17
There is synergy between amplifying other voices and accelerating your own work, as both provide an opportunity to highlight your specific advocacy interest. In the earlier example, the physician advocating for health justice may create a thread highlighting inequities in COVID-19 vaccination, including their own data and that of other health justice scholars, and in doing so, provide an invaluable repository of references or speakers for a future project.
We caution that not everyone will agree with your mission, so avoiding arguments and remaining professional in these interactions is paramount. Furthermore, it is also possible that a physician’s mission and opinions may not align with those of their employer, so it is important for social media users to review and clarify their employer’s social media policies to avoid violations and related repercussions. Physicians should tweet as if they were speaking into a microphone on the record, and authenticity should ground them into projecting the same personality online as they would offline.
CONCLUSION
We believe that, by the very nature of their chosen careers, physicians should step into the tension of advocacy. We acknowledge that physicians who are otherwise vocal advocates in other areas of life may be reluctant to engage on social media. However, if the measure of “success” on Twitter is meaningful interaction, sharing knowledge, and amplifying other voices according to a specific personal mission, MBT can be a useful framework. This is a call to action for hesitant physicians to take a leap and explore this platform, and for those already using social media to reevaluate their use and reflect on their mission. Physicians have been gifted a megaphone that can be used to combat misinformation, advocate for patients and the healthcare community, and advance needed discussions to benefit those in society who cannot speak for themselves. We advocate for physicians to look beyond the moment of a tweet and consider how your voice can contribute to a movement.
Acknowledgments
The authors thank Dr Vineet Arora for her contribution to early concept development for this manuscript and the JHM editorial staff for their productive feedback and editorial comments.
1. Riddick FA Jr. The code of medical ethics of the American Medical Association. Ochsner J. 2003;5(2):6-10. https://doi.org/10.3201/eid2702.203139
2. Vraga EK, Bode L. Addressing COVID-19 misinformation on social media preemptively and responsively. Emerg Infect Dis. 2021;27(2):396-403. https://doi.org/10.3201/eid2702.203139
3. Campbell L, Evans Y, Pumper M, Moreno MA. Social media use by physicians: a qualitative study of the new frontier of medicine. BMC Med Inform Decis Mak. 2016;16:91. https://doi.org/10.1186/s12911-016-0327-y
4. Wetsman N. How Twitter is changing medical research. Nat Med. 2020;26(1):11-13. https://doi.org/10.1038/s41591-019-0697-7
5. Shapiro M. Episode 107: Vinny Arora & Charlie Wray on Social Media & CVs. Explore The Space Podcast. https://www.explorethespaceshow.com/podcasting/vinny-arora-charlie-wray-on-cvs-social-media/
6. Varghese T. i4 (i to the 4th) is a strategy for #SoMe. Accessed April 22, 2021. https://twitter.com/TomVargheseJr/status/1027181443712081920?s=20
7. Dobson S, Voyer S, Regehr G. Perspective: agency and activism: rethinking health advocacy in the medical profession. Acad Med. 2012;87(9):1161-1164. https://doi.org/10.1097/ACM.0b013e3182621c25
8. #GetMePPE. Accessed April 22, 2021. https://twitter.com/hashtag/getmeppe?f=live
9. Ouyang H. At the front lines of coronavirus, turning to social media. The New York Times. March 18, 2020. Accessed April 22, 2021. https://www.nytimes.com/2020/03/18/well/live/coronavirus-doctors-facebook-twitter-social-media-covid.html
10. Blackstock U. Combining social media advocacy with health policy advocacy. Accessed April 22, 2021. https://twitter.com/uche_blackstock/status/1270413367761666048?s=20
11. Meeks LM, Liao P, Kim N. Using Twitter to promote awareness of disabilities in medicine. Med Educ. 2019;53(5):525-526. https://doi.org/10.1111/medu.13836
12. Nolen L. To all the little brown girls out there “you can’t be what you can’t see but I hope you see me now and that you see yourself in me.” Accessed April 22, 2021. https://twitter.com/LashNolen/status/1160901502266777600?s=20.
13. Pendergrast TR, Jain S, Trueger NS, Gottlieb M, Woitowich NC, Arora VM. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552. https://doi.org/10.1001/jamainternmed.2020.7235
14. Marcelin JR. Personal mission statement. Accessed July 6, 2021. https://www.unmc.edu/intmed/residencies-fellowships/residency/diverse-taskforce/index.html.
15. Li S-TT, Frohna JG, Bostwick SB. Using your personal mission statement to INSPIRE and achieve success. Acad Pediatr. 2017;17(2):107-109. https://doi.org/10.1016/j.acap.2016.11.010
16. Alton L. 7 tips for creating engaging content every day. Accessed April 22, 2021. https://business.twitter.com/en/blog/7-tips-creating-engaging-content-every-day.html
17. Boyd R. Is everyone reading this??! Accessed April 22, 2021. https://twitter.com/RheaBoydMD/status/1273006362679578625?s=20
1. Riddick FA Jr. The code of medical ethics of the American Medical Association. Ochsner J. 2003;5(2):6-10. https://doi.org/10.3201/eid2702.203139
2. Vraga EK, Bode L. Addressing COVID-19 misinformation on social media preemptively and responsively. Emerg Infect Dis. 2021;27(2):396-403. https://doi.org/10.3201/eid2702.203139
3. Campbell L, Evans Y, Pumper M, Moreno MA. Social media use by physicians: a qualitative study of the new frontier of medicine. BMC Med Inform Decis Mak. 2016;16:91. https://doi.org/10.1186/s12911-016-0327-y
4. Wetsman N. How Twitter is changing medical research. Nat Med. 2020;26(1):11-13. https://doi.org/10.1038/s41591-019-0697-7
5. Shapiro M. Episode 107: Vinny Arora & Charlie Wray on Social Media & CVs. Explore The Space Podcast. https://www.explorethespaceshow.com/podcasting/vinny-arora-charlie-wray-on-cvs-social-media/
6. Varghese T. i4 (i to the 4th) is a strategy for #SoMe. Accessed April 22, 2021. https://twitter.com/TomVargheseJr/status/1027181443712081920?s=20
7. Dobson S, Voyer S, Regehr G. Perspective: agency and activism: rethinking health advocacy in the medical profession. Acad Med. 2012;87(9):1161-1164. https://doi.org/10.1097/ACM.0b013e3182621c25
8. #GetMePPE. Accessed April 22, 2021. https://twitter.com/hashtag/getmeppe?f=live
9. Ouyang H. At the front lines of coronavirus, turning to social media. The New York Times. March 18, 2020. Accessed April 22, 2021. https://www.nytimes.com/2020/03/18/well/live/coronavirus-doctors-facebook-twitter-social-media-covid.html
10. Blackstock U. Combining social media advocacy with health policy advocacy. Accessed April 22, 2021. https://twitter.com/uche_blackstock/status/1270413367761666048?s=20
11. Meeks LM, Liao P, Kim N. Using Twitter to promote awareness of disabilities in medicine. Med Educ. 2019;53(5):525-526. https://doi.org/10.1111/medu.13836
12. Nolen L. To all the little brown girls out there “you can’t be what you can’t see but I hope you see me now and that you see yourself in me.” Accessed April 22, 2021. https://twitter.com/LashNolen/status/1160901502266777600?s=20.
13. Pendergrast TR, Jain S, Trueger NS, Gottlieb M, Woitowich NC, Arora VM. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552. https://doi.org/10.1001/jamainternmed.2020.7235
14. Marcelin JR. Personal mission statement. Accessed July 6, 2021. https://www.unmc.edu/intmed/residencies-fellowships/residency/diverse-taskforce/index.html.
15. Li S-TT, Frohna JG, Bostwick SB. Using your personal mission statement to INSPIRE and achieve success. Acad Pediatr. 2017;17(2):107-109. https://doi.org/10.1016/j.acap.2016.11.010
16. Alton L. 7 tips for creating engaging content every day. Accessed April 22, 2021. https://business.twitter.com/en/blog/7-tips-creating-engaging-content-every-day.html
17. Boyd R. Is everyone reading this??! Accessed April 22, 2021. https://twitter.com/RheaBoydMD/status/1273006362679578625?s=20
© 2021 Society of Hospital Medicine
A Short-Lived Crisis
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
A 79-year-old woman presented to the emergency department with 1 day of nausea and vomiting. On the morning of presentation, she felt mild cramping in her legs and vomited twice. She denied chest or back pain, dyspnea, diaphoresis, cough, fever, dysuria, headache, and abdominal pain. Her medical history included hypertension, osteoporosis, and a right-sided acoustic neuroma treated with radiation 12 years prior. One month before this presentation, type 2 diabetes mellitus was diagnosed (hemoglobin A1c level, 7.3%) on routine testing by her primary care physician. Her medications were losartan and alendronate. She was born in China and immigrated to the United States 50 years prior. Her husband was chronically ill with several recent hospitalizations.
Nausea and vomiting are nonspecific symptoms that can arise from systemic illness, including hyperglycemia, a drug/toxin effect, or injury/inflammation of the gastrointestinal, central nervous system, or cardiovascular systems. An acoustic neuroma recurrence or malignancy in the radiation field could trigger nausea. Muscle cramping could arise from myositis or from hypokalemia secondary to vomiting. Her husband’s recent hospitalizations add an important psychosocial dimension to her care and should prompt consideration of a shared illness depending on the nature of his illness.
The patient’s temperature was 36.7 °C; heart rate, 99 beats per minute; blood pressure, 94/58 mm Hg;respiratory rate, 16 breaths per minute; and oxygen saturation, 98% while breathing room air. Her body mass index (BMI) was 18.7 kg/m2. She appeared comfortable. The heart, lung, jugular venous, and abdominal examinations were normal. She had no lower extremity edema or muscle tenderness.
The white blood cell (WBC) count was 14,500/µL (81% neutrophils, 9% lymphocytes, 8% monocytes), hemoglobin level was 17.5 g/dL (elevated from 14.2 g/dL 8 weeks prior), and platelet count was 238,000/µL. The metabolic panel revealed the following values: sodium, 139 mmol/L; potassium, 5.1 mmol/L; chloride, 96 mmol/L; bicarbonate, 17 mmol/L; blood urea nitrogen, 40 mg/dL; creatinine, 2.2 mg/dL (elevated from 0.7 mg/dL 8 weeks prior); glucose, 564 mg/dL; aspartate transaminase, 108 U/L; alanine transaminase, 130 U/L; total bilirubin, 0.6 mg/dL; and alkaline phosphatase, 105 U/L. Creatine kinase, amylase, and lipase levels were not measured. The urinalysis showed trace ketones, protein 100 mg/dL, glucose >500 mg/dL, and <5 WBCs per high-power field. The venous blood gas demonstrated a pH of 7.20 and lactate level of 13.2 mmol/L. Serum beta-hydroxybutyrate level was 0.27 mmol/L (reference range, 0.02-0.27), serum troponin I level was 8.5 µg/L (reference range, <0.05), and
Chest x-ray showed bilateral perihilar opacities with normal heart size. Electrocardiogram (ECG) revealed new ST-segment depressions in the anterior precordial leads (Figure 1).
Her hypotension may signal septic, cardiogenic, or hypovolemic shock. The leukocytosis, anion gap acidosis, acute kidney injury, and elevated lactate are compatible with sepsis, although there is no identified source of infection. Although diabetic ketoacidosis (DKA) can explain many of these findings, the serum beta-hydroxybutyrate and urine ketones are lower than expected for that condition. Her low-normal BMI makes significant insulin resistance less likely and raises concern about pancreatic adenocarcinoma as a secondary cause of diabetes.
The nausea, ST depressions, elevated troponin and B-type natriuretic peptide levels, and bilateral infiltrates suggest acute coronary syndrome (ACS), complicated by acute heart failure leading to systemic hypoperfusion and associated lactic acidosis and kidney injury. Nonischemic causes of myocardial injury, such as sepsis, myocarditis, and stress cardiomyopathy, should also be considered. Alternatively, she could be experiencing multiorgan injury from widespread embolism (eg, endocarditis), thrombosis (eg, antiphospholipid syndrome), or inflammation (eg, vasculitis). Acute pancreatitis can cause acute hyperglycemia and multisystem disease, but she did not have abdominal pain or tenderness (and her lipase level was not measured). Treatment should include intravenous insulin, intravenous fluids (trying to balance possible sepsis or DKA with heart failure), medical management for non-ST elevation myocardial infarction (NSTEMI), and empiric antibiotics.
ACS was diagnosed, and aspirin, atorvastatin, clopidogrel, and heparin were prescribed. Insulin infusion and intravenous fluids (approximately 3 L overnight) were administered for hyperglycemia (and possible early DKA). On the night of admission, the patient became profoundly diaphoretic without fevers; the WBC count rose to 24,200/µL. Vancomycin and ertapenem were initiated for possible sepsis. Serum troponin I level increased to 11.9 µg/L; the patient did not have chest pain, and the ECG was unchanged.
The next morning, the patient reported new mild diffuse abdominal pain and had mild epigastric tenderness. The WBC count was 28,900/µL; hemoglobin, 13.2 g/dL; venous pH, 7.39; lactate, 2.9 mmol/L; lipase, 48 U/L; aspartate transaminase, 84 U/L; alanine transaminase, 72 U/L; total bilirubin, 0.7 mg/dL; alkaline phosphatase, 64 U/L; and creatinine, 1.2 mg/dL.
Her rising troponin without dynamic ECG changes makes the diagnosis of ACS less likely, although myocardial ischemia can present as abdominal pain. Other causes of myocardial injury to consider (in addition to the previously mentioned sepsis, myocarditis, and stress cardiomyopathy) are pulmonary embolism and proximal aortic dissection. The latter can lead to ischemia in multiple systems (cardiac, mesenteric, renal, and lower extremity, recalling her leg cramps on admission).
The leukocytosis and lactic acidosis in the setting of new abdominal pain raises the question of mesenteric ischemia or intra-abdominal sepsis. Her hemoglobin has decreased by 4 g, and while some of the change may be dilutional, it will be important to consider hemolysis (less likely with a normal bilirubin) or gastrointestinal bleeding (given current anticoagulant and antiplatelet therapy). An echocardiogram and computed tomography (CT) angiogram of the chest, abdomen, and pelvis are indicated to evaluate the vasculature and assess for intra-abdominal pathology.
Coronary angiography revealed a 40% stenosis in the proximal right coronary artery and no other angiographically significant disease; the left ventricular end-diastolic pressure (LVEDP) was 30 mm Hg. Transthoracic echocardiography demonstrated normal left ventricular size, left ventricular ejection fraction of 65% to 70%, impaired left ventricular relaxation, and an inferior vena cava <2 cm in diameter that collapsed with inspiration.
The angiogram shows modest coronary artery disease and points away from plaque rupture as the cause of myocardial injury. Another important consideration given her husband’s recurrent illness is stress cardiomyopathy, but she does not have the typical apical ballooning or left ventricular dysfunction. The increased LVEDP with normal left ventricular size and function with elevated filling pressures is consistent with left-sided heart failure with preserved ejection fraction. Cardiac magnetic resonance imaging could exclude an infiltrative disorder leading to diastolic dysfunction or a myocarditis that explains the troponin elevation, but both diagnoses seem unlikely.
CT of the abdomen and pelvis demonstrated a heterogeneous 3-cm mass in the left adrenal gland (Figure 2).
An adrenal mass could be a functional or nonfunctional adenoma, primary adrenal carcinoma, a metastatic malignancy, or granulomatous infection such as tuberculosis. Secretion of excess glucocorticoid, mineralocorticoid, or catecholamine should be evaluated.
Cushing syndrome could explain her hyperglycemia, leukocytosis, and heart failure (mediated by the increased risk of atherosclerosis and hypertension with hypercortisolism), although her low BMI is atypical. Primary hyperaldosteronism causes hypertension but does not cause an acute multisystem disease. Pheochromocytoma could account for the diaphoresis, hypertension, hyperglycemia, leukocytosis, and cardiac injury. A more severe form—pheochromocytoma crisis—is characterized by widespread end-organ damage, including cardiomyopathy, bowel ischemia, hepatitis, hyperglycemia with ketoacidosis, and lactic acidosis. Measurement of serum cortisol and plasma and urine fractionated metanephrines, and a dexamethasone suppression test can determine whether the adrenal mass is functional.
The intravenous insulin infusion was changed to subcutaneous dosing on hospital day 2. She had no further nausea, diaphoresis, or abdominal pain, was walking around the hospital unit unassisted, and was consuming a regular diet. By hospital day 3, insulin was discontinued. The patient remained euglycemic for the remainder of her hospitalization; hemoglobin A1c value was 7.0%. Blood cultures were sterile, and the WBC count was 12,000/µL. Thyroid-stimulating hormone level was 0.31 mIU/L (reference range, 0.45-4.12), and the free thyroxine level was 12 pmol/L (reference range, 10-18). Antibiotics were discontinued. She remained euvolemic and never required diuretic therapy. The acute myocardial injury and diastolic dysfunction were attributed to an acute stress cardiomyopathy arising from the strain of her husband’s declining health. She was discharged on hospital day 5 with aspirin, atorvastatin, metoprolol, lisinopril, and outpatient follow-up.
The rapid resolution of her multisystem process suggests a self-limited process or successful treatment of the underlying cause. Although she received antibiotics, a bacterial infection never manifested. Cardiomyopathy with a high troponin level, ECG changes, and early heart failure often requires aggressive supportive measures, which were not required here. The rapid cessation of hyperglycemia and an insulin requirement within 1 day is atypical for DKA.
Pheochromocytoma is a rare secondary cause of diabetes in which excess catecholamines cause insulin resistance and suppress insulin release. It can explain both the adrenal mass and, in the form of pheochromocytoma crisis, the severe multisystem injury. However, the patient’s hypotension (which could be explained by concomitant cardiomyopathy) and older age are not typical for pheochromocytoma.
Results of testing for adrenal biomarkers, which were sent during her hospitalization, returned several days after hospital discharge. The plasma free metanephrine level was 687 pg/mL (reference range, <57) and the plasma free normetanephrine level was 508 pg/mL (reference range, <148). Metoprolol was discontinued by her primary care physician.
Elevated plasma free metanephrine and normetanephrine levels were confirmed in the endocrinology clinic 3 weeks later. The 24-hour urine metanephrine level was 1497 µg/24 hours (reference range, 90-315), and the 24-hour urine normetanephrine level was 379 µg/24 hours (reference range, 122-676). Serum aldosterone level was 8 ng/dL (reference range, 3-16), and morning cortisol level was 8 µg/dL (reference range, 4-19). Lisinopril was discontinued, and phenoxybenzamine was prescribed.
Adrenal-protocol CT of the abdomen demonstrated that the left adrenal mass was enhanced by contrast without definite washout, which could be consistent with a pheochromocytoma.
The diagnosis of pheochromocytoma has been confirmed by biochemistry and imaging. It was appropriate to stop metoprolol, as β-blockade can lead to unopposed α-receptor agonism and hypertension. Implementation of α-blockade with phenoxybenzamine and endocrine surgery referral are indicated.
On the day she intended to fill a phenoxybenzamine prescription, the patient experienced acute generalized weakness and presented to the emergency department with hyperglycemia (glucose, 661 mg/dL), acute kidney injury (creatinine, 1.6 mg/dL), troponin I elevation (0.14 µg/L), and lactic acidosis (4.7 mmol/L). She was admitted to the hospital and rapidly improved with intravenous fluids and insulin. Phenoxybenzamine 10 mg daily was administered, and she was discharged on hospital day 2. The dosage of phenoxybenzamine was gradually increased over 2 months.
Laparoscopic left adrenalectomy was performed, with removal of a 3-cm mass. The pathologic findings confirmed the diagnosis of pheochromocytoma. Two months later she felt well. Her hypertension was controlled with lisinopril 10 mg daily. Transthoracic echocardiography 3 months after adrenalectomy demonstrated a left ventricular ejection fraction of 60% to 65%. Six months later, her hemoglobin A1c was 6.6%.
DISCUSSION
Pheochromocytoma is an abnormal growth of cells of chromaffin origin that arises in the adrenal medulla.1,2 The incidence of these often benign tumors is estimated to be 2 to 8 cases per million in the general population, and 2 to 6 per 1000 in adult patients with hypertension.1,3,4 Although clinicians commonly associate these catecholamine-secreting tumors with intermittent hypertension or diaphoresis, they have a wide spectrum of manifestations, which range from asymptomatic adrenal mass to acute multiorgan illness that mimics other life-threatening conditions. Common signs and symptoms of pheochromocytoma include hypertension (60%-70% incidence), headache (50%), diaphoresis (50%), and palpitations (50%-60%).4 The textbook triad of headache, sweating, and palpitations is seen in fewer than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is often explained by a more common condition.1,4 Approximately 5% of adrenal “incidentalomas” are pheochromocytomas that are minimally symptomatic or asymptomatic.1,3 In a study of 102 patients who underwent pheochromocytoma resection, 33% were diagnosed during evaluation of an adrenal incidentaloma.5 At the other end of the spectrum is a pheochromocytoma crisis with its mimicry of ACS and sepsis, and manifestations including severe hyperglycemia, abdominal pain, acute heart failure, and syncope.2,5-9 Aside from chronic mild hypertension and a single episode of diaphoresis during admission, our patient had none of the classic signs or symptoms of pheochromocytoma. Rather, she presented with the abrupt onset of multiorgan injury.
Diagnostic evaluation for pheochromocytoma typically includes demonstration of elevated catecholamine byproducts (metanephrines) in plasma or urine and an adrenal mass on imaging.2,10 Biopsy is contraindicated because this can lead to release of catecholamines, which can trigger a pheochromocytoma crisis.5 The Endocrine Society guidelines recommend evaluating patients for pheochromocytoma who have: (1) a known or suspected genetic syndrome linked to pheochromocytoma (eg, multiple endocrine neoplasia type 2 or Von Hippel-Lindau syndrome), (2) an adrenal mass incidentally found on imaging, regardless of a history of hypertension, or (3) signs and symptoms of pheochromocytoma.3
Patients in pheochromocytoma crisis are typically very ill, requiring intensive care unit admission for hemodynamic stabilization.1,11 Initial management is typically directed at assessing and treating for common causes of systemic illness and hemodynamic instability, such as ACS and sepsis. Although some patients with pheochromocytoma crisis may have hemodynamic collapse requiring invasive circulatory support, others improve while receiving empiric treatment for mimicking conditions. Our patient had multiorgan injury and hemodynamic instability but returned to her preadmission state within 48 to 72 hours and remained stable after the withdrawal of all therapies, including insulin and antibiotics. This rapid improvement suggested a paroxysmal condition with an “on/off” capacity mediated by endogenous mediators. Once pheochromocytoma crisis is diagnosed, hemodynamic stabilization with α-adrenergic receptor blockade and intravascular volume repletion is essential. Confirmation of the diagnosis with repeat testing after hospital discharge is important because biochemical test results are less specific in the setting of acute illness. Surgery on an elective basis is the definitive treatment. Ongoing α-adrenergic receptor blockade is essential to minimize the risk of an intraoperative pheochromocytoma crisis (because of anesthesia or tumor manipulation) and prevent cardiovascular collapse after resection of tumor.11
Although the biochemical profile of a pheochromocytoma (eg, epinephrine predominant) is not tightly linked to the phenotype, the pattern of organ injury can reflect the pleotropic effects of specific catecholamines.12 While both norepinephrine and epinephrine bind the β1-adrenergic receptor with equal affinity, epinephrine has a higher affinity for the β2-adrenergic receptor. Our patient’s initial relative hypotension was likely caused by hypovolemia from decreased oral intake, vomiting, and hyperglycemia-mediated polyuria. However, β2-adrenergic receptor agonism could have caused vasodilation, and nocardiogenic hypotension has been observed with epinephrine-predominant pheochromocytomas.13 Several of the other clinical findings in this case can be explained by widespread β-adrenergic receptor agonism. Epinephrine (whether endogenously produced or exogenously administered) can lead to cardiac injury with elevated cardiac biomarkers.1,6,14 Epinephrine administration can cause leukocytosis, which is attributed to demargination of leukocyte subsets that express β2-adrenergic receptors.15,16 Lactic acidosis in the absence of tissue hypoxia (type B lactic acidosis) occurs during epinephrine infusions in healthy volunteers.17,18 Hyperglycemia from epinephrine infusions is attributed to β-adrenergic receptor stimulation causing increased gluconeogenesis and glycogenolysis and decreased insulin secretion and tissue glucose uptake.8 Resolution of hyperglycemia and diabetes is observed in the majority of patients after resection of pheochromocytoma, and hypoglycemia immediately after surgery is common, occasionally requiring glucose infusion.19,20
Pheochromocytomas are rare tumors with a wide range of manifestations that extend well beyond the classic triad. Pheochromocytomas can present as an asymptomatic adrenal mass with normal blood pressure, as new onset diabetes, or as multiorgan injury with cardiovascular collapse. Our patient suffered from two episodes of catecholamine excess that required hospitalization, but fortunately each proved to be a short-lived crisis.
TEACHING POINTS
- The classic triad of headache, sweating, and palpitations occurs in less than 25% of patients with pheochromocytoma; among unselected general medicine patients who have this triad, each symptom is usually explained by a common medical condition.
- The presentation of pheochromocytoma varies widely, from asymptomatic adrenal incidentaloma to pheochromocytoma crisis causing multiorgan dysfunction with hemodynamic instability and mimicry of common critical illnesses like ACS, DKA, and sepsis.
- Biochemical screening for pheochromocytoma is recommended when a patient has a known or suspected genetic syndrome linked to pheochromocytoma, an adrenal mass incidentally found on imaging regardless of blood pressure, or signs and symptoms of a pheochromocytoma.
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
1. Riester A, Weismann D, Quinkler M, et al. Life-threatening events in patients with pheochromocytoma. Eur J Endocrinol. 2015;173(6):757-764. https://doi.org/10.1530/eje-15-0483
2. Whitelaw BC, Prague JK, Mustafa OG, et al. Phaeochromocytoma [corrected] crisis. Clin Endocrinol (Oxf). 2014;80(1):13-22. https://doi.org/10.1111/cen.12324
3. Lenders JW, Duh QY, Eisenhofer G, et al; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. https://doi.org/10.1210/jc.2014-1498
4. Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24(12):2331-2339. https://doi.org/10.1097/01.hjh.0000251887.01885.54
5. Shen WT, Grogan R, Vriens M, Clark OH, Duh QY. One hundred two patients with pheochromocytoma treated at a single institution since the introduction of laparoscopic adrenalectomy. Arch Surg. 2010;145(9):893-897. https://doi.org/10.1001/archsurg.2010.159
6. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013;99(14):1438-1444. https://doi.org/10.1136/heartjnl-2013-304073
7. Lee TW, Lin KH, Chang CJ, Lew WH, Lee TI. Pheochromocytoma mimicking both acute coronary syndrome and sepsis: a case report. Med Princ Pract. 2013;22(4):405-407. https://doi.org/10.1159/000343578
8. Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999-1005. https://doi.org/10.4158/ep171914.ra
9. Ueda T, Oka N, Matsumoto A, et al. Pheochromocytoma presenting as recurrent hypotension and syncope. Intern Med. 2005;44(3):222-227. https://doi.org/10.2169/internalmedicine.44.222
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552-565. https://doi.org/10.1056/nejmra1806651
11. Scholten A, Cisco RM, Vriens MR, et al. Pheochromocytoma crisis is not a surgical emergency. J Clin Endocrinol Metab. 2013;98(2):581-591. https://doi.org/10.1210/jc.2012-3020
12. Pacak K. Phaeochromocytoma: a catecholamine and oxidative stress disorder. Endocr Regul. 2011;45:65-90.
13. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf). 1992;37(3):304-306. https://doi.org/10.1111/j.1365-2265.1992.tb02326.x
14. Campbell RL, Bellolio MF, Knutson BD, et al. Epinephrine in anaphylaxis: higher risk of cardiovascular complications and overdose after administration of intravenous bolus epinephrine compared with intramuscular epinephrine. J Allergy Clin Immunol Pract. 2015;3(1):76-80. https://doi.org/10.1016/j.jaip.2014.06.007
15. Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77-91. https://doi.org/10.1006/brbi.1996.0009
16. Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2010;184(1):503-511. https://doi.org/10.4049/jimmunol.0902189
17. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-1140. https://doi.org/10.1016/j.mayocp.2013.06.012
18. Levy B. Bench-to-bedside review: is there a place for epinephrine in septic shock? Crit Care. 2005;9(6):561-565. https://doi.org/10.1186/cc3901
19. Chen Y, Hodin RA, Pandolfi C, Ruan DT, McKenzie TJ. Hypoglycemia after resection of pheochromocytoma. Surgery. 2014;156:1404-1408; discussion 1408-1409. https://doi.org/10.1016/j.surg.2014.08.020
20. Pogorzelski R, Toutounchi S, Krajewska E, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67(4):361-365. https://doi.org/10.5173/ceju.2014.04.art9
© 2021 Society of Hospital Medicine
An A-Peeling Diagnosis
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 39-year-old previously healthy man presented to the emergency department (ED) with abrupt-onset fever, headache, back pain, myalgias, chills, and photophobia. His past medical history included seasonal allergies and an episode of aseptic meningitis 8 years prior. He denied cough, dysuria, weakness, numbness, or visual changes. He denied using tobacco or injection drugs and rarely drank alcohol. His only medication was acetaminophen for fever.
The patient’s sudden fever indicates the rapid onset of an inflammatory state. While the headache and photophobia might be a result of an underlying systemic infection or an irritant like blood in the cerebral spinal fluid (CSF), one must consider meningitis. Potential sources for sudden meningitis include infectious, autoimmune (rheumatoid arthritis, systemic lupus erythematosus [SLE]), or drug-induced aseptic meningitis, and structural etiologies (ruptured cyst). Recrudescence of prior disease may also present acutely (Mollaret meningitis). Malignant etiologies, being more indolent, seem less likely. Back pain may indicate an epidural inflammatory process like epidural abscess; however, the patient denies risk factors such as injection drug use or recent procedures.
The patient’s temperature was 101.2 °F; blood pressure, 120/72 mm Hg; and heart rate, 112 bpm. He appeared comfortable, without meningismus or spinal tenderness. Pupils were reactive; eyes were without icterus, injection, or suffusion. Cardiac exam was normal. Lungs were clear to auscultation. He had no abdominal tenderness, hepatosplenomegaly, or lymphadenopathy. Cranial nerves II through XII, balance, coordination, strength, and sensation were intact. No rash was noted. Complete blood count (CBC), basic and hepatic chemistry panels, urinalysis, and serum lactate tests were within normal limits. Erythrocyte sedimentation rate (ESR) was elevated to 15 mm/h (normal range, 3-10 mm/h), C-reactive protein (CRP) to 2.4 mg/dL (normal range, <0.5 mg/dL), and procalcitonin to 0.07 ng/mL (normal range, <0.05 ng/mL). The patient was treated with intravenous (IV) fluids, ketorolac, dexamethasone, and acetaminophen, with resolution of symptoms. Given his rapid improvement, absence of meningismus, and lack of immunocompromise, lumbar puncture was deferred. A diagnosis of nonspecific viral syndrome was made. He was discharged home.
Certainly, a systemic infection (eg, influenza, adenovirus, arbovirus-related infection, HIV) could be a cause of this patient’s presentation. Notably, less than two-thirds of patients with meningitis present with the classic triad of fever, neck stiffness, and altered mental status. In this patient with fever, headache, and photophobia, aseptic meningitis should still be considered. While the negative procalcitonin and rapid clinical improvement without antibiotics make acute bacterial meningitis unlikely, nonbacterial causes of meningeal irritation can be severe and life-threatening. An assessment for jolt accentuation of the headache might have been helpful. Information about time of year, geographic exposures (vector-borne infections), and sick contacts (viral illness) can inform the clinical decision to pursue lumbar puncture. Additional history regarding his previous aseptic meningitis would be helpful, as it could suggest a recurrent inflammatory process. Causes of recurrent aseptic meningitis include infectious (herpes simplex virus [HSV], Epstein-Barr virus [EBV], syphilis), drug-related (nonsteroidal anti-inflammatory drugs [NSAIDs]), structural (epidermoid cyst with rupture), and autoimmune (lupus, Sjögren syndrome, Behçet disease) etiologies.
The mildly elevated inflammatory markers are nonspecific and reflect the patient’s known inflammatory state. The dexamethasone given for symptomatic management may have had some therapeutic effect in the setting of an autoimmune process, with additional contribution from ketorolac and acetaminophen.
He returned to the ED 3 days later with a pruritic, disseminated rash involving his palms and soles, accompanied by hand swelling and tingling. Although his headache and photophobia resolved, he reported a productive cough, nasal congestion, and sore throat. He also reported orange-pink urine without dysuria or urinary frequency. Additional questioning revealed a recent motorcycle trip to the Great Lakes region. During this trip, he did not camp, interact with animals or ticks, or swim in streams or lakes. He did not eat any raw, undercooked, or locally hunted meats. He denied new medications, soaps or detergents, or sexual contacts. He had started taking acetaminophen and ibuprofen around the clock since prior discharge.
The orange-pink urine and acute-onset palmoplantar rash with recent fever help narrow the differential. Orange-pink urine might suggest bilirubinuria from liver injury, hemolysis with hemoglobinuria, or myoglobinuria. Most concerning would be hematuria associated with glomerular injury and a systemic vasculopathy.
The rash on the palms and soles should be further characterized as blanching or nonblanching. Blanching, indicating vasodilation of intact blood vessels, is seen with many drug eruptions and viral exanthems. Nonblanching, suggesting broken capillaries (petechiae or purpura), would suggest vasculitis or vasculopathy from emboli, infection, or inflammation. A palmoplantar rash in febrile illness should first prompt evaluation for life-threatening conditions, followed by consideration of both infectious and noninfectious etiologies. Acutely fatal infections include Rocky Mountain spotted fever (RMSF), meningococcemia, toxic shock syndrome, infective endocarditis, and rat-bite fever. The rash, fever, headache, and outdoor exposure raise the possibility of a rickettsial infection, including RMSF, which can be contracted rarely around the Great Lakes. Other life-threatening infections seem unlikely, as the patient would have significantly deteriorated without proper medical care by now. Palmoplantar rash with fever can also be seen in other bacterial infections (eg, secondary syphilis, arbovirus infections, typhus) and in viral infections (eg, cytomegalovirus [CMV], EBV, human herpesvirus-6 [HHV-6], HIV, coxsackievirus, and papular-purpuric gloves and socks syndrome caused by parvovirus B19). Noninfectious considerations include drug hypersensitivity rashes, neoplasm (eg, cutaneous T-cell lymphoma), or inflammatory conditions (eg, SLE, vasculitis). Drug reaction with eosinophilia and systemic symptoms (may also present with severe illness.
The acetaminophen and ibuprofen may be masking ongoing fevers. The cough, nasal congestion, and sore throat might be part of a viral prodrome or, in tandem with fever, associated with a vasculitis such as granulomatosis with polyangiitis.
Vital signs were normal, and the patient appeared nontoxic. Physical examination demonstrated mildly cracked lips, oropharyngeal erythema with small petechiae on the soft palate, a morbilliform rash throughout his extremities and trunk (Figure 1), and confluent, brightly erythematous patches on his palms and soles with associated edema (Figure 2 and Appendix Figure). No lymphadenopathy, hepatosplenomegaly, or joint swelling was noted. CBC and basic chemistry panel remained normal; however, hepatic chemistries were notable for alanine aminotransferase (ALT) of 128 U/L, aspartate aminotransferase (AST) of 49 U/L, total bilirubin of 3.7 mg/dL, direct bilirubin of 2.4 mg/dL, total protein of 7.1 g/dL, albumin of 4.1 g/dL, and alkaline phosphatase of 197 U/L. Urinalysis detected bilirubin without blood, protein, bacteria, cells, or casts. The patient was admitted to the hospital.
The patient now has acute-onset upper respiratory symptoms with oral mucosal erythema, edema and erythema of the hands and feet with morbilliform rash of the extremities, and liver injury causing bilirubinuria. The patient’s initial symptoms may have had some response to therapy, but the current presentation suggests ongoing evolution of disease. Reactive infectious mucocutaneous eruptions include chlamydia, influenza, parainfluenza, and enteroviruses. Measles is possible given its recent resurgence; however, absence of coryza or Koplik spots and the peripheral distribution of the rash without initial truncal involvement make this less likely. Mycoplasma pneumonia–induced rash and mucositis might present with respiratory symptoms and this rash distribution, but typically involves two or more mucosal sites.
Iatrogenic causes are important to consider given the recent exposure to NSAIDs, specifically Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). In this patient, however, SJS/TEN is unlikely as it typically presents 1 to 3 weeks after exposure, with a truncal-predominant rash rarely involving the palms and soles.
Despite the absence of conjunctivitis and cervical lymphadenopathy, one additional consideration is Kawasaki disease (KD). Though more common in children, it may rarely present in adulthood. The time course of manifesting symptoms with potential steroid responsiveness raises suspicion for this diagnosis.
During a 4-day hospitalization, he developed mild bilateral conjunctivitis, peeling lips, and scleral icterus. CBC remained within normal limits. A peripheral smear demonstrated toxic neutrophilic granulation with normal erythrocytes and platelets. HIV and hepatitis A, B, and C serologies were negative. Blood cultures were negative. CRP and ESR increased to 4.3 mg/dL and 56 mm/h, respectively. Hepatic chemistries increased to ALT 155 U/L, AST 101 U/L, total bilirubin 5.1 mg/dL, direct bilirubin 3.3 mg/dL, and alkaline phosphatase 211 U/L. Right upper-quadrant ultrasound demonstrated gallbladder distention (11.3 cm × 5.0 cm; normal, 10.0 cm × 4.0 cm) without stones, wall thickening, or pericholecystic fluid; sonographic Murphy sign was negative. The liver was unremarkable with normal flow in the portal vein.
The patient’s persistent reactive neutrophilic granulation and rising CRP and ESR indicate ongoing inflammation. The largely direct hyperbilirubinemia with hepatitis, minimal findings on ultrasound imaging, and lack of Murphy sign suggest either direct infection of the liver or cholestasis. Viral serologies for EBV, HSV, and CMV should be sent, although these viruses are less commonly associated with oral rash and conjunctivitis. The marked degree of cholestasis makes adenovirus and mycoplasma less likely. Leptospirosis should be considered given the degree of liver injury with potential conjunctival suffusion. However, oral involvement would be atypical; renal injury is absent; and the patient denied pertinent exposures, vomiting, diarrhea, or persistent myalgias.
It is important to know whether the patient continued to receive
Low-grade fevers resolved without intervention. Tests were sent for tick-borne (ehrlichiosis, babesiosis, RMSF, anaplasmosis), viral (EBV, West Nile virus, parvovirus, CMV, coxsackievirus, adenovirus), other bacterial and protozoal (syphilis, Coxiella, leptospirosis, Lyme, Giardia), and autoimmune (antinuclear antibody, perinuclear antineutrophil cytoplasmic antibody, double-stranded DNA) diseases. Topical steroids and antihistamines were prescribed for a suspected viral exanthem. Empiric doxycycline was prescribed to treat possible tick-borne disease, and the patient was discharged home. At home, progressive darkening of the urine was noted. Outpatient testing demonstrated rising ALT to 377 U/L, AST to 183 U/L, total bilirubin to 5.9 mg/dL, direct bilirubin to 3.5 mg/dL, and alkaline phosphatase to 301 U/L. The patient was readmitted for further evaluation.
Despite concerns of the treating physicians, features of this case make tick-borne infections less likely. Lyme disease does not typically cause significant laboratory abnormalities and is classically associated with erythema migrans rather than a mucocutaneous rash. Relapsing fever, ehrlichioses, and rickettsial infections are associated with leukopenia and thrombocytopenia in addition to hepatocellular, rather than cholestatic, liver injury. The lack of response to doxycycline is helpful diagnostically: most tick-borne infections, in addition to leptospirosis, respond well to treatment. While babesiosis, tularemia, and Powassan or Heartland viruses transmitted by ticks are not treated with doxycycline, babesiosis often involves a hemolytic anemia (not seen in this case), and this patient’s laboratory abnormalities and rash are not characteristic of tularemia or viral tick-borne infections.
Either a new or reactivated viral infection with liver inflammation or an autoimmune etiology, specifically KD, remain the most likely etiology of the patient’s symptoms.
He remained asymptomatic during a 6-day hospitalization. His oral lesions resolved. The morbilliform rash coalesced into confluent macules with fine desquamation on the extremities and trunk. There was prominent periungual and palmar/plantar desquamation (Figure 3 and Figure 4). CBC demonstrated hemoglobin of 12.6 g/dL and platelets of 399,000/μL. CRP was undetectable at <0.5 mg/dL; however, ESR increased to 110 mm/h. Transaminases increased to ALT 551 U/L and AST 219 U/L. Serum alkaline phosphatase and bilirubin decreased without intervention. Albumin and total protein remained unchanged. All infectious and autoimmune testing sent from the prior admission returned negative.
An acute-onset viral-like prodrome with fevers potentially responsive to steroids, followed by conjunctivitis, oral erythema and cracked lips, morbilliform rash with hand and foot erythema and edema, cholestatic hepatitis, and subsequent periungual desquamation is highly suggestive of KD. It would be interesting to revisit the patient’s prior episode of aseptic meningitis to see whether any other symptoms were suggestive of KD. While intravenous immunoglobulin (IVIg) and aspirin are standard therapies for the acute febrile phase of KD, the patient is now nearly 2 weeks into his clinical course, rendering their utility uncertain. Nonetheless, screening for coronary aneurysms should be pursued, which may help confirm the diagnosis.
Upon reviewing the evolution of the findings, a diagnosis of adult-onset KD was made. IVIg 2g/kg and aspirin 325 mg were administered. Echocardiogram did not show any evidence of coronary artery aneurysm, myocarditis, pericarditis, wall motion abnormalities, or pericardial effusion. Computed tomography (CT) coronary angiogram confirmed normal coronary arteries without aneurysm. The patient was discharged home without fever on daily aspirin, and all hepatic chemistries and inflammatory markers normalized. Follow-up cardiac magnetic resonance imaging at 3 months and CT angiogram at 6 months remained normal. The patient remains well now 2 years after the original diagnosis and treatment.
DISCUSSION
KD, also known as mucocutaneous lymph node syndrome, is a vasculitis that typically affects children younger than 5 years.1 Having a sibling with KD confers a 10- to 15-fold higher risk, suggesting a genetic component to the disease.2 The highest incidence of KD is in persons of East Asian descent, but KD can affect patients of all races and ethnicities. In the United States, the majority of patients with KD are non-Hispanic White, followed by Black, Hispanic, and Asian.3 The etiology is still unknown, but it is posited that an unidentified, ubiquitous infectious agent may trigger KD in genetically susceptible individuals.4
KD can cause aneurysms and thromboses in medium-sized blood vessels throughout the body.5,6 The classic presentation involves 5 days of high fever plus four or more of the symptoms in the mnemonic CRASH: conjunctival injection, rash (polymorphous), adenopathy (cervical), strawberry tongue (or red, cracked lips and oropharyngeal edema), hand (erythema and induration of hands or feet, followed by periungual desquamation).7 Multiple organ systems may be affected, manifesting as abdominal pain, arthritis, pneumonitis, aseptic meningitis, and acalculous distention of the gallbladder (hydrops).7 The most feared consequence is coronary artery involvement, which leads to aneurysm, thrombosis, and sudden death.
Though no definitive diagnostic test exists, certain laboratory findings support the diagnosis, such as sterile pyuria, thrombocytosis, elevated CRP and ESR, transaminitis, and hypoalbuminemia.7 Diagnosis requires exclusion of illnesses with similar presentations, such as bacterial, viral, and tick-borne infections; drug hypersensitivity reactions; toxic shock syndrome; scarlet fever; juvenile rheumatoid arthritis; and other rheumatologic conditions. Some cases of KD present with fewer than four of the principal (CRASH) symptoms—these are termed “incomplete” KD. The combination of supportive laboratory findings and echocardiogram can facilitate diagnosis of incomplete KD, which carries a similar risk of coronary artery aneurysm.7
Though primarily a disease of childhood, KD can present in adults.8 Adults, compared with children, are less likely to have thrombocytosis and more likely to have cervical adenopathy, arthralgias, and hepatic test abnormalities.8 Although coronary artery aneurysms occur less frequently in adults compared with children, timely diagnosis and treatment is key to preventing this life-threatening complication.8
In children, treatment is IVIg 2 g/kg and aspirin 80 to 100 mg/kg daily until afebrile for several days.9 Some require a second dose of IVIg.9 Children are then maintained on 3 to 5 mg/kg of aspirin daily for 6 to 8 weeks.9 IVIg, given within 10 days of the onset of fever, is highly effective at preventing coronary artery aneurysms.10,11 When coronary aneurysms do occur, treatment is with aspirin or clopidogrel. Very large aneurysms require systemic anticoagulation. After the acute illness, children are monitored with serial cardiac imaging at 2 weeks and 6 to 8 weeks after diagnosis.7 In adults, the optimal imaging timing is unknown. Echocardiography often cannot visualize the coronary arteries, necessitating coronary CT angiography or cardiac MRI.
Despite the presence of classic features, this patient’s diagnosis was delayed because of the rarity of KD in adults and the need to exclude more common diseases. Furthermore, the administration of dexamethasone likely shortened his febrile period and ameliorated some symptoms,12 affecting the natural history of his illness. The diagnosis relied on three components: ruling out common diagnoses, noting two unusual findings (gallbladder hydrops, desquamating periungual rash), and broadening the differential to include adult presentations of childhood disease. Review of the literature suggests very few causes for gallbladder hydrops: impacted stones, cystic fibrosis, cystic duct narrowing due to tumor or lymph nodes, KD, and bacterial and parasitic disease (eg, salmonella, ascariasis). Gallbladder hydrops and periungual desquamation are seen together only in KD.13 Given the complexity of diagnosis in adults, the time to diagnosis is often delayed compared with that for children. While IVIg treatment is preferred within 10 days of the onset of fever, this patient received IVIg on day 14, given the relatively benign nature of IVIg and the considerable morbidity associated with coronary artery aneurysms. Dosing for aspirin is unclear in adults.8 This patient was started on 325 mg aspirin daily. He recovered fully and remains free of coronary changes at two years after initial diagnosis. This case is an excellent reminder that, after exclusion of common diagnoses, reflection on the most unusual aspects of the case and consideration of childhood diseases is particularly important in our younger patients.
TEACHING POINTS
- Extended fever should broaden the differential to include rheumatologic diagnoses.
- KD is rare in adults but can present with classic findings from childhood.
- Early treatment with IVIg and aspirin can be lifesaving in patients with KD, including adults.
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Article in Japanese. Arerugi. 1967;16(3):178-222.
2. Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9(4):185-194. https://doi.org/10.1016/j.ijid.2005.03.002
3. Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-488. https://doi.org/10.1097/INF.0b013e3181cf8705
4. Rowley A, Baker S, Arollo D, et al. A hepacivirus-like protein is targeted by the antibody response to Kawasaki disease (KD) [abstract]. Open Forum Infect Dis. 2019;6(suppl 2):S48.
5. Friedman KG, Gauvreau K, Hamaoka-Okamoto A, et al. Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. 2016;5(9):e003289. https://doi.org/10.1161/JAHA.116.003289
6. Zhao QM, Chu C, Wu L, et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics. 2019;144(6):e20192254. https://doi.org/10.1542/peds.2019-2254
7. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733. https://doi.org/10.1542/peds.2004-2182
8. Sève P, Stankovic K, Smail A, Durand DV, Marchand G, Broussolle C. Adult Kawasaki disease: report of two cases and literature review. Semin Arthritis Rheum. 2005;34(6):785-792. https://doi.org/10.1016/j.semarthrit.2005.01.012
9. Shulman ST. Intravenous immunoglobulin for the treatment of Kawasaki disease. Pediatr Ann. 2017;46(1):e25-e28. https://doi.org/10.3928/19382359-20161212-01
10. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315(6):341-347. https://doi.org/10.1056/NEJM198608073150601
11. Rowley AH, Duffy CE, Shulman ST. Prevention of giant coronary artery aneurysms in Kawasaki disease by intravenous gamma globulin therapy. J Pediatr. 1988;113(2):290-294. https://doi/org/10.1016/s0022-3476(88)80267-1
12. Lim YJ, Jung JW. Clinical outcomes of initial dexamethasone treatment combined with a single high dose of intravenous immunoglobulin for primary treatment of Kawasaki disease. Yonsei Med J. 2014;55(5):1260-1266. https://doi.org/10.3349/ymj.2014.55.5.1260
13. Sun Q, Zhang J, Yang Y. Gallbladder hydrops associated with Kawasaki disease: a case report and literature review. Clin Pediatr (Phila). 2018;57(3):341-343. https://doi.org/10.1177/0009922817696468
© 2021 Society of Hospital Medicine
Out of Sight, Not Out of Mind
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
© 2021 Society of Hospital Medicine
Things We Do For No Reason™: Serum Serologic Helicobacter pylori Testing
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 25-year-old woman for evaluation of a 2-day history of intractable vomiting. The patient reports a 6-month history of intermittent dyspepsia. Vital signs include a normal temperature, tachycardia with a heart rate of 115 beats per minute, and a blood pressure of 100/60 mm Hg. Laboratory studies, including a complete blood count, electrolyte panel, and serum lipase, are normal; a pregnancy test is negative. Computed tomography (CT) of the patient’s abdomen and pelvis shows no abnormalities. The patient rapidly improves after 2 days with fluid resuscitation and supportive care. A serologic Helicobacter pylori test ordered on admission returns positive, prompting the hospitalist to discharge the patient on a course of bismuth quadruple anti-H pylori therapy.
BACKGROUND
H pylori infection causes upper gastrointestinal symptoms and progressive gastric damage, which can lead to peptic ulcer disease and gastric cancer. When H pylori infection is diagnosed, the current American College of Gastroenterology guidelines recommend eradication of the infection.1 Even with a waning prevalence in the United States, H pylori infects approximately 17% of persons aged 20 to 29 years and 57% of persons >70 years.2 Widely available noninvasive testing options for detecting H pylori include the enzyme-linked immunosorbent assay test for immunoglobulin G antibodies (ie, serology), the stool antigen test, and the urea breath test. Invasive options include upper endoscopy with biopsy. An analysis of diagnostic testing in the United States between 2010 and 2012 showed that approximately 70% of first-time testing was serologic.3
WHY YOU MIGHT THINK SEROLOGIC
H PYLORI TESTING IS HELPFUL
Providers often select serologic testing for H pylori because of the relative ease of obtaining a blood sample compared to obtaining samples for a stool antigen or urea breath test. Stool antigen and the urea breath tests identify active infections and require a large population of H pylori in the stomach. Concurrent treatment with therapies that suppress H pylori, such as antimicrobials, bismuth, or proton pump inhibitors (PPIs), reduces the sensitivity of those tests.4 One study showed that treatment with bismuth reduced the sensitivity of urea breath and stool antigen tests to 50% and 85%, respectively, and that PPIs reduced the sensitivity of the urea breath test and stool antigen test to 60% and 75%, respectively.4 The use of antibiotics, PPIs, or bismuth, however, does not affect the test characteristics of serology.
Invasive testing with endoscopy and biopsy may also yield false-negative results. For example, providers often appropriately start PPI therapy in hospitalized patients with suspected bleeding peptic ulcers. Without concurrent treatment with a PPI, the gastric histology should show the histologic hallmarks of H pylori (ie, acute-on-chronic inflammation), as well as the organisms. However, PPI suppression of the infection and active bleeding may reduce the sensitivity of endoscopic biopsy.5,6 In one study, PPI use decreased sensitivity of histology to approximately 67% compared to polymerase chain reaction testing of the biopsy.6 Bleeding peptic ulcers do not affect the accuracy of serologic testing.
WHY SEROLOGIC TESTING FOR
H PYLORI IS NOT HELPFUL
There are three main issues with H pylori serology testing: (1) decreased sensitivity of these tests compared to other noninvasive tests, (2) inability of serology tests to distinguish between past and active infection (ie, the test is not specific for active infection), and (3) wide availability and use by commercial laboratories of serologic tests that are not approved by the US Food and Drug Administration (FDA).
A multicenter trial in the United States comparing three different serologic tests for H pylori demonstrated sensitivities ranging from 76% to 84%.7 By comparison, the main stool antigen test for H pylori available in the United States has a sensitivity of 93%.8 A recent meta-analysis showed a pooled sensitivity of 96% for urea breath tests.9 These studies demonstrate that the stool antigen and urea breath tests generally eclipse the sensitivity of the available serologic tests.
To further illustrate the issues associated with serologic testing, one may consider a population of 1,000 people with an H pylori prevalence of 35%, the estimated overall prevalence of H pylori in the United States.10 In this population, a serologic test with an 80% sensitivity would result in 70 false-negative results, whereas a urea breath or stool antigen test with a 95% sensitivity would yield only 18 false-negative results. These numbers change drastically with changing prevalence or pretest probability. In some low-prevalence or low-pretest probability scenarios, serologic tests offer little more than a “coin-flip” chance of detecting active H pylori infection (Figure).
Serologic testing offers the benefit of an immediate result but at the cost of reduced sensitivity and specificity. The superior accuracy of biopsy and urea breath and stool antigen tests is dependent upon on cessation of antimicrobials, bismuth, and PPI therapy—something that may be difficult to achieve in hospitalized patients. In the majority of cases, however, there is little evidence equating immediate diagnosis of H pylori with improved patient outcomes. The preferred strategy to reduce false-negative results is to defer stool antigen or urea breath testing until patients have been off antimicrobials, bismuth, and PPIs for 4 weeks.
Serologic tests for H pylori may remain positive for years, which decreases the specificity of these tests in confirming active or eradicated infection.11 One study evaluated three different serology tests on 82 patients 6 months after confirmed eradication by urea breath test. In this study, only seven or eight patients tested negative by serology (depending on the serology test)—a specificity of 8% to 10% for active infection.12 Another study showed that even after 1 year of confirmed eradication, 65% of patients remained seropositive, which equates to a specificity of 35%.11 These studies illustrate that serologic testing for H pylori has a very poor ability to distinguish between active and past infection.
An additional common misconception is that a positive serologic test in the absence of prior treatment for, or diagnosis of, H pylori indicates an active infection. Children and adults can spontaneously clear and become reinfected with H pylori.13,14 Therefore, serologic testing for ascertaining active H pylori infection is unreliable.
As noted, the wide availability of non-FDA-approved serologic tests offered by commercial laboratories in the United States creates another problem for serologic testing. Most immunoglobulin A (IgA) and all immunoglobulin M (IgM) tests lack FDA approval and typically have low sensitivity and specificity. One study showed that compared to stool antigen, IgA and IgM serologic tests had a sensitivity of 63% and 7%, respectively.15
WHEN MIGHT SEROLOGIC H PYLORI TESTING BE HELPFUL?
Despite its limitations, serologic testing for H pylori may have a role in some situations. Clinical scenarios associated with a high pretest probability of H pylori infection (eg, chronic peptic ulcer disease without other risk factors) increase the positive predictive value of H pylori infection. In such a situation, a positive serologic test should prompt initiation of treatment, whereas a negative serologic test does not rule out H pylori infection (Figure). In contrast, in the presence of lower pretest probability symptoms (eg, dyspepsia), positive serologic testing has such a high false-positive rate that providers must first confirm the result with a stool antigen or urea breath test before initiating treatment.
WHAT YOU SHOULD DO INSTEAD
RECOMMENDATIONS
- Use stool antigen or urea breath tests to diagnose H pylori infection noninvasively in patients without an indication for endoscopy.
- Use endoscopic biopsy with histology to diagnose H pylori infection in patients with an indication for endoscopy.
- Delay stool antigen and urea breath testing until 4 weeks after patients have ceased using medications that interfere with test results (eg, antibiotics, bismuth, PPIs); H2RAs do not interfere with testing.
- In cases of a bleeding peptic ulcer with a negative biopsy for H pylori, retest with biopsy after the bleeding resolves or retest using stool antigen or urea breath test.
- Confirm a positive serologic test via stool antigen or urea breath test before initiating treatment except in very high pretest probability clinical scenarios.
- Test to confirm eradication with biopsy, urea breath, or stool antigen test in all cases of confirmed H pylori infection.
- Do not order or try to interpret H pylori IgA and IgM tests as they have no role in the diagnosis or management of H pylori infections.
CONCLUSION
In the clinical scenario, the patient clinically improved with fluid resuscitation and supportive care. The history of unexplained dyspepsia is an indication to assess for H pylori infection with either urea breath test or stool antigen test. Given the positive serologic test, the provider should have retested for active infection with a stool antigen or urea breath test prior to initiating treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808-1825. https://doi.org/10.1111/j.1572-0241.2007.01393.x
2. Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181(4):1359-1363. https://doi.org/10.1086/315384
3. Theel ES, Johnson RD, Plumhoff E, Hanson CA. Use of the Optum Labs Data Warehouse to assess test ordering patterns for diagnosis of Helicobacter pylori infection in the United States. J Clin Microbiol. 2015;53(4):1358-1360. https://doi.org/10.1128/jcm.03464-14
4. Bravo LE, Realpe JL, Campo C, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroentrol. 1999;94(9):2380-2383. https://doi.org/10.1111/j.1572-0241.1999.01361.x
5. Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36(1):12-16. https://doi.org/10.1136/gut.36.1.12
6. Yakoob J, Jafri W, Abbas Z, Abid S, Islam M, Ahmed Z. The diagnostic yield of various tests for Helicobacter pylori infection in patients on acid-reducing drugs. Dig Dis Sci. 2008;53(1):95-100. https://doi.org/10.1007/s10620-007-9828-y
7. Chey WD, Murthy U, Shaw S, et al. A comparison of three fingerstick, whole blood antibody tests for Helicobacter pylori infection: a United States, multicenter trial. Am J Gastroentrol. 1999;94(6):1512-1516. https://doi.org/10.1111/j.1572-0241.1999.1135_x.x
8. Li YH, Guo H, Zhang PB, Zhao XY, Da SP. Clinical value of Helicobacter pylori stool antigen test, ImmunoCard STAT HpSA, for detecting H pylori infection. World J Gastroenterol. 2004;10(6):913-914. https://doi.org/10.3748/wjg.v10.i6.913
9. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21(4):1305-1314. https://doi.org/10.3748/wjg.v21.i4.1305
10. Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420-429. https://doi.org/10.1053/j.gastro.2017.04.022
11. Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91(1):85-88.
12. Bergey B, Marchildon P, Peacock J, Mégraud PF. What is the role of serology in assessing Helicobacter pylori eradication? Aliment Pharmacol Ther. 2003;18(6):635-639. https://doi.org/10.1046/j.1365-2036.2003.01716.x
13. Duque X, Vilchis J, Mera R, et al. Natural history of Helicobacter pylori infection in Mexican schoolchildren: incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55(2):209. https://doi.org/10.1097/mpg.0b013e318248877f
14. Luzza F, Suraci E, Larussa T, Leone I, Imeneo M. High exposure, spontaneous clearance, and low incidence of active Helicobacter pylori infection: the Sorbo San Basile study. Helicobacter. 2014;19(4):296-305. https://doi.org/10.1111/hel.12133
15. She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol. 2009;16(8):1253-1255. https://doi.org/10.1128/cvi.00149-09
16. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992-1002. Published correction appears in Clin Gastroenterol Hepatol. 2019;17(4):801. https://doi.org/10.1016/j.cgh.2019.01.006
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 25-year-old woman for evaluation of a 2-day history of intractable vomiting. The patient reports a 6-month history of intermittent dyspepsia. Vital signs include a normal temperature, tachycardia with a heart rate of 115 beats per minute, and a blood pressure of 100/60 mm Hg. Laboratory studies, including a complete blood count, electrolyte panel, and serum lipase, are normal; a pregnancy test is negative. Computed tomography (CT) of the patient’s abdomen and pelvis shows no abnormalities. The patient rapidly improves after 2 days with fluid resuscitation and supportive care. A serologic Helicobacter pylori test ordered on admission returns positive, prompting the hospitalist to discharge the patient on a course of bismuth quadruple anti-H pylori therapy.
BACKGROUND
H pylori infection causes upper gastrointestinal symptoms and progressive gastric damage, which can lead to peptic ulcer disease and gastric cancer. When H pylori infection is diagnosed, the current American College of Gastroenterology guidelines recommend eradication of the infection.1 Even with a waning prevalence in the United States, H pylori infects approximately 17% of persons aged 20 to 29 years and 57% of persons >70 years.2 Widely available noninvasive testing options for detecting H pylori include the enzyme-linked immunosorbent assay test for immunoglobulin G antibodies (ie, serology), the stool antigen test, and the urea breath test. Invasive options include upper endoscopy with biopsy. An analysis of diagnostic testing in the United States between 2010 and 2012 showed that approximately 70% of first-time testing was serologic.3
WHY YOU MIGHT THINK SEROLOGIC
H PYLORI TESTING IS HELPFUL
Providers often select serologic testing for H pylori because of the relative ease of obtaining a blood sample compared to obtaining samples for a stool antigen or urea breath test. Stool antigen and the urea breath tests identify active infections and require a large population of H pylori in the stomach. Concurrent treatment with therapies that suppress H pylori, such as antimicrobials, bismuth, or proton pump inhibitors (PPIs), reduces the sensitivity of those tests.4 One study showed that treatment with bismuth reduced the sensitivity of urea breath and stool antigen tests to 50% and 85%, respectively, and that PPIs reduced the sensitivity of the urea breath test and stool antigen test to 60% and 75%, respectively.4 The use of antibiotics, PPIs, or bismuth, however, does not affect the test characteristics of serology.
Invasive testing with endoscopy and biopsy may also yield false-negative results. For example, providers often appropriately start PPI therapy in hospitalized patients with suspected bleeding peptic ulcers. Without concurrent treatment with a PPI, the gastric histology should show the histologic hallmarks of H pylori (ie, acute-on-chronic inflammation), as well as the organisms. However, PPI suppression of the infection and active bleeding may reduce the sensitivity of endoscopic biopsy.5,6 In one study, PPI use decreased sensitivity of histology to approximately 67% compared to polymerase chain reaction testing of the biopsy.6 Bleeding peptic ulcers do not affect the accuracy of serologic testing.
WHY SEROLOGIC TESTING FOR
H PYLORI IS NOT HELPFUL
There are three main issues with H pylori serology testing: (1) decreased sensitivity of these tests compared to other noninvasive tests, (2) inability of serology tests to distinguish between past and active infection (ie, the test is not specific for active infection), and (3) wide availability and use by commercial laboratories of serologic tests that are not approved by the US Food and Drug Administration (FDA).
A multicenter trial in the United States comparing three different serologic tests for H pylori demonstrated sensitivities ranging from 76% to 84%.7 By comparison, the main stool antigen test for H pylori available in the United States has a sensitivity of 93%.8 A recent meta-analysis showed a pooled sensitivity of 96% for urea breath tests.9 These studies demonstrate that the stool antigen and urea breath tests generally eclipse the sensitivity of the available serologic tests.
To further illustrate the issues associated with serologic testing, one may consider a population of 1,000 people with an H pylori prevalence of 35%, the estimated overall prevalence of H pylori in the United States.10 In this population, a serologic test with an 80% sensitivity would result in 70 false-negative results, whereas a urea breath or stool antigen test with a 95% sensitivity would yield only 18 false-negative results. These numbers change drastically with changing prevalence or pretest probability. In some low-prevalence or low-pretest probability scenarios, serologic tests offer little more than a “coin-flip” chance of detecting active H pylori infection (Figure).
Serologic testing offers the benefit of an immediate result but at the cost of reduced sensitivity and specificity. The superior accuracy of biopsy and urea breath and stool antigen tests is dependent upon on cessation of antimicrobials, bismuth, and PPI therapy—something that may be difficult to achieve in hospitalized patients. In the majority of cases, however, there is little evidence equating immediate diagnosis of H pylori with improved patient outcomes. The preferred strategy to reduce false-negative results is to defer stool antigen or urea breath testing until patients have been off antimicrobials, bismuth, and PPIs for 4 weeks.
Serologic tests for H pylori may remain positive for years, which decreases the specificity of these tests in confirming active or eradicated infection.11 One study evaluated three different serology tests on 82 patients 6 months after confirmed eradication by urea breath test. In this study, only seven or eight patients tested negative by serology (depending on the serology test)—a specificity of 8% to 10% for active infection.12 Another study showed that even after 1 year of confirmed eradication, 65% of patients remained seropositive, which equates to a specificity of 35%.11 These studies illustrate that serologic testing for H pylori has a very poor ability to distinguish between active and past infection.
An additional common misconception is that a positive serologic test in the absence of prior treatment for, or diagnosis of, H pylori indicates an active infection. Children and adults can spontaneously clear and become reinfected with H pylori.13,14 Therefore, serologic testing for ascertaining active H pylori infection is unreliable.
As noted, the wide availability of non-FDA-approved serologic tests offered by commercial laboratories in the United States creates another problem for serologic testing. Most immunoglobulin A (IgA) and all immunoglobulin M (IgM) tests lack FDA approval and typically have low sensitivity and specificity. One study showed that compared to stool antigen, IgA and IgM serologic tests had a sensitivity of 63% and 7%, respectively.15
WHEN MIGHT SEROLOGIC H PYLORI TESTING BE HELPFUL?
Despite its limitations, serologic testing for H pylori may have a role in some situations. Clinical scenarios associated with a high pretest probability of H pylori infection (eg, chronic peptic ulcer disease without other risk factors) increase the positive predictive value of H pylori infection. In such a situation, a positive serologic test should prompt initiation of treatment, whereas a negative serologic test does not rule out H pylori infection (Figure). In contrast, in the presence of lower pretest probability symptoms (eg, dyspepsia), positive serologic testing has such a high false-positive rate that providers must first confirm the result with a stool antigen or urea breath test before initiating treatment.
WHAT YOU SHOULD DO INSTEAD
RECOMMENDATIONS
- Use stool antigen or urea breath tests to diagnose H pylori infection noninvasively in patients without an indication for endoscopy.
- Use endoscopic biopsy with histology to diagnose H pylori infection in patients with an indication for endoscopy.
- Delay stool antigen and urea breath testing until 4 weeks after patients have ceased using medications that interfere with test results (eg, antibiotics, bismuth, PPIs); H2RAs do not interfere with testing.
- In cases of a bleeding peptic ulcer with a negative biopsy for H pylori, retest with biopsy after the bleeding resolves or retest using stool antigen or urea breath test.
- Confirm a positive serologic test via stool antigen or urea breath test before initiating treatment except in very high pretest probability clinical scenarios.
- Test to confirm eradication with biopsy, urea breath, or stool antigen test in all cases of confirmed H pylori infection.
- Do not order or try to interpret H pylori IgA and IgM tests as they have no role in the diagnosis or management of H pylori infections.
CONCLUSION
In the clinical scenario, the patient clinically improved with fluid resuscitation and supportive care. The history of unexplained dyspepsia is an indication to assess for H pylori infection with either urea breath test or stool antigen test. Given the positive serologic test, the provider should have retested for active infection with a stool antigen or urea breath test prior to initiating treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 25-year-old woman for evaluation of a 2-day history of intractable vomiting. The patient reports a 6-month history of intermittent dyspepsia. Vital signs include a normal temperature, tachycardia with a heart rate of 115 beats per minute, and a blood pressure of 100/60 mm Hg. Laboratory studies, including a complete blood count, electrolyte panel, and serum lipase, are normal; a pregnancy test is negative. Computed tomography (CT) of the patient’s abdomen and pelvis shows no abnormalities. The patient rapidly improves after 2 days with fluid resuscitation and supportive care. A serologic Helicobacter pylori test ordered on admission returns positive, prompting the hospitalist to discharge the patient on a course of bismuth quadruple anti-H pylori therapy.
BACKGROUND
H pylori infection causes upper gastrointestinal symptoms and progressive gastric damage, which can lead to peptic ulcer disease and gastric cancer. When H pylori infection is diagnosed, the current American College of Gastroenterology guidelines recommend eradication of the infection.1 Even with a waning prevalence in the United States, H pylori infects approximately 17% of persons aged 20 to 29 years and 57% of persons >70 years.2 Widely available noninvasive testing options for detecting H pylori include the enzyme-linked immunosorbent assay test for immunoglobulin G antibodies (ie, serology), the stool antigen test, and the urea breath test. Invasive options include upper endoscopy with biopsy. An analysis of diagnostic testing in the United States between 2010 and 2012 showed that approximately 70% of first-time testing was serologic.3
WHY YOU MIGHT THINK SEROLOGIC
H PYLORI TESTING IS HELPFUL
Providers often select serologic testing for H pylori because of the relative ease of obtaining a blood sample compared to obtaining samples for a stool antigen or urea breath test. Stool antigen and the urea breath tests identify active infections and require a large population of H pylori in the stomach. Concurrent treatment with therapies that suppress H pylori, such as antimicrobials, bismuth, or proton pump inhibitors (PPIs), reduces the sensitivity of those tests.4 One study showed that treatment with bismuth reduced the sensitivity of urea breath and stool antigen tests to 50% and 85%, respectively, and that PPIs reduced the sensitivity of the urea breath test and stool antigen test to 60% and 75%, respectively.4 The use of antibiotics, PPIs, or bismuth, however, does not affect the test characteristics of serology.
Invasive testing with endoscopy and biopsy may also yield false-negative results. For example, providers often appropriately start PPI therapy in hospitalized patients with suspected bleeding peptic ulcers. Without concurrent treatment with a PPI, the gastric histology should show the histologic hallmarks of H pylori (ie, acute-on-chronic inflammation), as well as the organisms. However, PPI suppression of the infection and active bleeding may reduce the sensitivity of endoscopic biopsy.5,6 In one study, PPI use decreased sensitivity of histology to approximately 67% compared to polymerase chain reaction testing of the biopsy.6 Bleeding peptic ulcers do not affect the accuracy of serologic testing.
WHY SEROLOGIC TESTING FOR
H PYLORI IS NOT HELPFUL
There are three main issues with H pylori serology testing: (1) decreased sensitivity of these tests compared to other noninvasive tests, (2) inability of serology tests to distinguish between past and active infection (ie, the test is not specific for active infection), and (3) wide availability and use by commercial laboratories of serologic tests that are not approved by the US Food and Drug Administration (FDA).
A multicenter trial in the United States comparing three different serologic tests for H pylori demonstrated sensitivities ranging from 76% to 84%.7 By comparison, the main stool antigen test for H pylori available in the United States has a sensitivity of 93%.8 A recent meta-analysis showed a pooled sensitivity of 96% for urea breath tests.9 These studies demonstrate that the stool antigen and urea breath tests generally eclipse the sensitivity of the available serologic tests.
To further illustrate the issues associated with serologic testing, one may consider a population of 1,000 people with an H pylori prevalence of 35%, the estimated overall prevalence of H pylori in the United States.10 In this population, a serologic test with an 80% sensitivity would result in 70 false-negative results, whereas a urea breath or stool antigen test with a 95% sensitivity would yield only 18 false-negative results. These numbers change drastically with changing prevalence or pretest probability. In some low-prevalence or low-pretest probability scenarios, serologic tests offer little more than a “coin-flip” chance of detecting active H pylori infection (Figure).
Serologic testing offers the benefit of an immediate result but at the cost of reduced sensitivity and specificity. The superior accuracy of biopsy and urea breath and stool antigen tests is dependent upon on cessation of antimicrobials, bismuth, and PPI therapy—something that may be difficult to achieve in hospitalized patients. In the majority of cases, however, there is little evidence equating immediate diagnosis of H pylori with improved patient outcomes. The preferred strategy to reduce false-negative results is to defer stool antigen or urea breath testing until patients have been off antimicrobials, bismuth, and PPIs for 4 weeks.
Serologic tests for H pylori may remain positive for years, which decreases the specificity of these tests in confirming active or eradicated infection.11 One study evaluated three different serology tests on 82 patients 6 months after confirmed eradication by urea breath test. In this study, only seven or eight patients tested negative by serology (depending on the serology test)—a specificity of 8% to 10% for active infection.12 Another study showed that even after 1 year of confirmed eradication, 65% of patients remained seropositive, which equates to a specificity of 35%.11 These studies illustrate that serologic testing for H pylori has a very poor ability to distinguish between active and past infection.
An additional common misconception is that a positive serologic test in the absence of prior treatment for, or diagnosis of, H pylori indicates an active infection. Children and adults can spontaneously clear and become reinfected with H pylori.13,14 Therefore, serologic testing for ascertaining active H pylori infection is unreliable.
As noted, the wide availability of non-FDA-approved serologic tests offered by commercial laboratories in the United States creates another problem for serologic testing. Most immunoglobulin A (IgA) and all immunoglobulin M (IgM) tests lack FDA approval and typically have low sensitivity and specificity. One study showed that compared to stool antigen, IgA and IgM serologic tests had a sensitivity of 63% and 7%, respectively.15
WHEN MIGHT SEROLOGIC H PYLORI TESTING BE HELPFUL?
Despite its limitations, serologic testing for H pylori may have a role in some situations. Clinical scenarios associated with a high pretest probability of H pylori infection (eg, chronic peptic ulcer disease without other risk factors) increase the positive predictive value of H pylori infection. In such a situation, a positive serologic test should prompt initiation of treatment, whereas a negative serologic test does not rule out H pylori infection (Figure). In contrast, in the presence of lower pretest probability symptoms (eg, dyspepsia), positive serologic testing has such a high false-positive rate that providers must first confirm the result with a stool antigen or urea breath test before initiating treatment.
WHAT YOU SHOULD DO INSTEAD
RECOMMENDATIONS
- Use stool antigen or urea breath tests to diagnose H pylori infection noninvasively in patients without an indication for endoscopy.
- Use endoscopic biopsy with histology to diagnose H pylori infection in patients with an indication for endoscopy.
- Delay stool antigen and urea breath testing until 4 weeks after patients have ceased using medications that interfere with test results (eg, antibiotics, bismuth, PPIs); H2RAs do not interfere with testing.
- In cases of a bleeding peptic ulcer with a negative biopsy for H pylori, retest with biopsy after the bleeding resolves or retest using stool antigen or urea breath test.
- Confirm a positive serologic test via stool antigen or urea breath test before initiating treatment except in very high pretest probability clinical scenarios.
- Test to confirm eradication with biopsy, urea breath, or stool antigen test in all cases of confirmed H pylori infection.
- Do not order or try to interpret H pylori IgA and IgM tests as they have no role in the diagnosis or management of H pylori infections.
CONCLUSION
In the clinical scenario, the patient clinically improved with fluid resuscitation and supportive care. The history of unexplained dyspepsia is an indication to assess for H pylori infection with either urea breath test or stool antigen test. Given the positive serologic test, the provider should have retested for active infection with a stool antigen or urea breath test prior to initiating treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected]
1. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808-1825. https://doi.org/10.1111/j.1572-0241.2007.01393.x
2. Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181(4):1359-1363. https://doi.org/10.1086/315384
3. Theel ES, Johnson RD, Plumhoff E, Hanson CA. Use of the Optum Labs Data Warehouse to assess test ordering patterns for diagnosis of Helicobacter pylori infection in the United States. J Clin Microbiol. 2015;53(4):1358-1360. https://doi.org/10.1128/jcm.03464-14
4. Bravo LE, Realpe JL, Campo C, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroentrol. 1999;94(9):2380-2383. https://doi.org/10.1111/j.1572-0241.1999.01361.x
5. Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36(1):12-16. https://doi.org/10.1136/gut.36.1.12
6. Yakoob J, Jafri W, Abbas Z, Abid S, Islam M, Ahmed Z. The diagnostic yield of various tests for Helicobacter pylori infection in patients on acid-reducing drugs. Dig Dis Sci. 2008;53(1):95-100. https://doi.org/10.1007/s10620-007-9828-y
7. Chey WD, Murthy U, Shaw S, et al. A comparison of three fingerstick, whole blood antibody tests for Helicobacter pylori infection: a United States, multicenter trial. Am J Gastroentrol. 1999;94(6):1512-1516. https://doi.org/10.1111/j.1572-0241.1999.1135_x.x
8. Li YH, Guo H, Zhang PB, Zhao XY, Da SP. Clinical value of Helicobacter pylori stool antigen test, ImmunoCard STAT HpSA, for detecting H pylori infection. World J Gastroenterol. 2004;10(6):913-914. https://doi.org/10.3748/wjg.v10.i6.913
9. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21(4):1305-1314. https://doi.org/10.3748/wjg.v21.i4.1305
10. Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420-429. https://doi.org/10.1053/j.gastro.2017.04.022
11. Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91(1):85-88.
12. Bergey B, Marchildon P, Peacock J, Mégraud PF. What is the role of serology in assessing Helicobacter pylori eradication? Aliment Pharmacol Ther. 2003;18(6):635-639. https://doi.org/10.1046/j.1365-2036.2003.01716.x
13. Duque X, Vilchis J, Mera R, et al. Natural history of Helicobacter pylori infection in Mexican schoolchildren: incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55(2):209. https://doi.org/10.1097/mpg.0b013e318248877f
14. Luzza F, Suraci E, Larussa T, Leone I, Imeneo M. High exposure, spontaneous clearance, and low incidence of active Helicobacter pylori infection: the Sorbo San Basile study. Helicobacter. 2014;19(4):296-305. https://doi.org/10.1111/hel.12133
15. She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol. 2009;16(8):1253-1255. https://doi.org/10.1128/cvi.00149-09
16. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992-1002. Published correction appears in Clin Gastroenterol Hepatol. 2019;17(4):801. https://doi.org/10.1016/j.cgh.2019.01.006
1. Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808-1825. https://doi.org/10.1111/j.1572-0241.2007.01393.x
2. Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000;181(4):1359-1363. https://doi.org/10.1086/315384
3. Theel ES, Johnson RD, Plumhoff E, Hanson CA. Use of the Optum Labs Data Warehouse to assess test ordering patterns for diagnosis of Helicobacter pylori infection in the United States. J Clin Microbiol. 2015;53(4):1358-1360. https://doi.org/10.1128/jcm.03464-14
4. Bravo LE, Realpe JL, Campo C, Correa P. Effects of acid suppression and bismuth medications on the performance of diagnostic tests for Helicobacter pylori infection. Am J Gastroentrol. 1999;94(9):2380-2383. https://doi.org/10.1111/j.1572-0241.1999.01361.x
5. Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut. 1995;36(1):12-16. https://doi.org/10.1136/gut.36.1.12
6. Yakoob J, Jafri W, Abbas Z, Abid S, Islam M, Ahmed Z. The diagnostic yield of various tests for Helicobacter pylori infection in patients on acid-reducing drugs. Dig Dis Sci. 2008;53(1):95-100. https://doi.org/10.1007/s10620-007-9828-y
7. Chey WD, Murthy U, Shaw S, et al. A comparison of three fingerstick, whole blood antibody tests for Helicobacter pylori infection: a United States, multicenter trial. Am J Gastroentrol. 1999;94(6):1512-1516. https://doi.org/10.1111/j.1572-0241.1999.1135_x.x
8. Li YH, Guo H, Zhang PB, Zhao XY, Da SP. Clinical value of Helicobacter pylori stool antigen test, ImmunoCard STAT HpSA, for detecting H pylori infection. World J Gastroenterol. 2004;10(6):913-914. https://doi.org/10.3748/wjg.v10.i6.913
9. Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21(4):1305-1314. https://doi.org/10.3748/wjg.v21.i4.1305
10. Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420-429. https://doi.org/10.1053/j.gastro.2017.04.022
11. Cutler AF, Prasad VM. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;91(1):85-88.
12. Bergey B, Marchildon P, Peacock J, Mégraud PF. What is the role of serology in assessing Helicobacter pylori eradication? Aliment Pharmacol Ther. 2003;18(6):635-639. https://doi.org/10.1046/j.1365-2036.2003.01716.x
13. Duque X, Vilchis J, Mera R, et al. Natural history of Helicobacter pylori infection in Mexican schoolchildren: incidence and spontaneous clearance. J Pediatr Gastroenterol Nutr. 2012;55(2):209. https://doi.org/10.1097/mpg.0b013e318248877f
14. Luzza F, Suraci E, Larussa T, Leone I, Imeneo M. High exposure, spontaneous clearance, and low incidence of active Helicobacter pylori infection: the Sorbo San Basile study. Helicobacter. 2014;19(4):296-305. https://doi.org/10.1111/hel.12133
15. She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testing. Clin Vaccine Immunol. 2009;16(8):1253-1255. https://doi.org/10.1128/cvi.00149-09
16. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16(7):992-1002. Published correction appears in Clin Gastroenterol Hepatol. 2019;17(4):801. https://doi.org/10.1016/j.cgh.2019.01.006
© 2021 Society of Hospital Medicine
Improving Healthcare Value: Effectiveness of a Program to Reduce Laboratory Testing for Non-Critically-Ill Patients With COVID-19
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
The COVID-19 pandemic posed an unprecedented challenge to our current healthcare system—how to efficiently develop and standardize care for a disease process yet to be fully characterized while continuing to deliver high-value care. In the United States, many local institutions developed their own practice patterns, resulting in wide variation.
The Society of Hospital Medicine’s Choosing Wisely® recommendations include avoiding repetitive routine laboratory testing.1
In April 2020, at Dell Seton Medical Center (DSMC) at the University of Texas at Austin, we created a Therapeutics and Informatics Committee to critically review evidence-based practices, reach consensus, and guide practice patterns, with the aim of delivering high-value care. This brief report aims
METHODS
Study Design and Setting
We followed SQUIRE guidelines for reporting this quality improvement intervention.3 Using retrospective chart review, we analyzed laboratory ordering patterns for COVID-positive patients at a single safety net academic medical center in Austin, Texas. Data were abstracted using a custom SQL query of our EHR and de-identified for this analysis. Our internal review board determined that this project is a quality improvement project and did not meet the criteria of human subjects research.
Study Population
All adult (age ≥18 years), non-intensive care unit (ICU), COVID-positive patients with an observation or inpatient status discharged between
Intervention
In April 2020, we created a Therapeutics and Informatics Committee, an interprofessional group including hospitalists, infectious disease, pulmonary and critical care, pharmacy, hospital leadership, and other subspecialists, to iteratively evaluate evidence and standardize inpatient care.
On April 30, 2020, the committee met to evaluate routine laboratory tests in patients with COVID-19.
The committee revisited laboratory ordering practices on June 25, 2020, making the recommendation to further discontinue trending troponin levels and reduce the amount of baseline labs, as they were contributing little to the clinical gestalt or changing management decisions. The customized EHR order sets were updated to reflect the new recommendations, and providers were encouraged to adopt them.
Although direct feedback on ordering practices can be an effective component of a multipronged intervention for decreasing lab usage,4 in this particular case we did not provide feedback to physicians related to their lab usage for COVID-19 care. We provided education to all physicians following each local COVID management consensus guideline change through email, handbook-style updates, and occasional conferences.
Measures and Analysis
The main process measure for this study was the mean hospitalization-level proportion of calendar hospital days with at least one laboratory result for each of four separate lab types: white blood cell count (WBC, as a marker for CBC), creatinine (as a marker for chemistry panels), troponin-I, and D-dimer. First, individual hospitalization-level proportions were calculated for each patient and each lab type. For example, if a patient with a length of stay of 5 calendar days had a WBC measured 2 of those days, their WBC proportion was 0.4. Then we calculated the mean of these proportions for all patients discharged in a given week during the study period for each lab type. Using this measure allowed us to understand the cadence of lab ordering and whether labs were checked daily.
Mean daily lab proportions were plotted separately for CBC, chemistry panel, troponin I, and D-dimer on statistical process control (SPC) charts.
RESULTS
A total of 1,402 non-ICU COVID-positive patients were discharged between March 30, 2020, and March 7, 2021, from our hospital, with a median length of stay of 3.00 days (weekly discharge data are shown in the Figure). The majority of patients were Hispanic men, with a mean age of 54 years (Appendix Table).
To assess intervention fidelity of the order sets, we performed two random spot checks (on May 15, 2020, and June 2, 2020) and found that 16/18 (89%) and 21/25 (84%) of COVID admissions had used the customized order set, supporting robust uptake of the order set intervention.
Mean daily lab proportions for each of the four lab types—chemistry panels, CBCs, D-dimer, and troponin—all demonstrated special cause variation starting mid June to early July 2020 (Figure). All four charts demonstrated periods of four points below 1-sigma and eight points below the center line, with troponin and D-dimer also demonstrating periods of two points below 2-sigma and one point below the lower control limit. These periods of special cause variation were sustained through February 2021.
We evaluated the proportion of all COVID-19 patients who spent time in the ICU over the entire study period, which remained consistent at approximately 25% of our hospitalized COVID-19 population. On a SPC chart, there was no evidence of change in ICU patients following our intervention.
DISCUSSION
Whereas Choosing Wisely® recommendations have been traditionally based on well-established common areas of overuse, this example is unique in showing how these same underlying principles can be applied even in unclear situations, such as with the COVID-19 pandemic. Through multidisciplinary review of real-time evidence and accumulating local experience, the Therapeutics and Informatics Committee at our hospital was able to reach consensus and rapidly deploy an electronic order set that was widely adopted. Eventually, the order set was formally adopted into our EHR; however, the customized COVID-19 order set allowed rapid improvement and implementation of changes that could be shared among providers. As confirmed by our spot checks, this order set was widely used.
There are several limitations to this brief analysis. First, we were unable to assess patient outcomes in response to these changes, mostly due to multiple confounding variables throughout this time period with rapidly shifting census numbers, and the adoption of therapeutic interventions, such as the introduction of dexamethasone, which has shown a mortality benefit for patients with COVID-19. However, we have no reason to believe that this decrease in routine laboratory ordering was associated with adverse outcomes for our patients, and, in aggregate, the outcomes (eg, mortality, length of stay, readmissions) for COVID-19 patients at our hospital have been better than average across Vizient peer groups.6 Prior studies have shown that reduced inpatient labs do not have an adverse impact on patient outcomes.7 Furthermore, non-ICU COVID-19 is generally a single-organ disease (unlike patients with critical illness from COVID-19), making it more likely that daily labs are unnecessary in this specific patient population.
In conclusion, the principles of Choosing Wisely® can be applied even within novel and quickly evolving situations, relying on rapid and critical review of evidence, clinician consensus-building, and leveraging available interventions to drive behavior change, such as shared order sets.
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
1. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063
2. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055. https://doi.org/10.1056/NEJMsb2005114
3. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. https://doi.org/10.1136/bmjqs-2015-004411
4. Wheeler D, Marcus P, Nguyen J, et al. Evaluation of a resident-led project to decrease phlebotomy rates in the hospital: think twice, stick once. JAMA Intern Med. 2016;176(5):708-710. https://doi.org/10.1001/jamainternmed.2016.0549
5. Montgomery DC. Introduction to Statistical Quality Control. 6th ed. Wiley; 2008.
6. Nieto K, Pierce RG, Moriates C, Schulwolf E. Lessons from the pandemic: building COVID-19 Centers of Excellence. The Hospital Leader - The Official Blog of the Society of Hospital Medicine. October 13, 2020. Accessed December 11, 2020. https://thehospitalleader.org/lessons-from-the-pandemic-building-covid-19-centers-of-excellence/
7. Corson AH, Fan VS, White T, et al. A multifaceted hospitalist quality improvement intervention: decreased frequency of common labs. J Hosp Med. 2015;10(6):390-395. https://doi.org/10.1002/jhm.2354
© 2021 Society of Hospital Medicine