User login
Risk of Intestinal Necrosis With Sodium Polystyrene Sulfonate: A Systematic Review and Meta-analysis
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
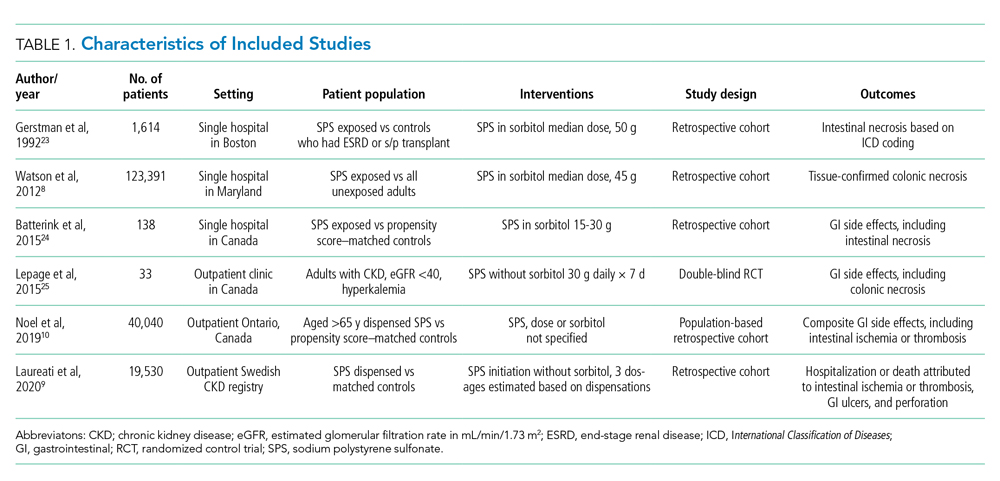
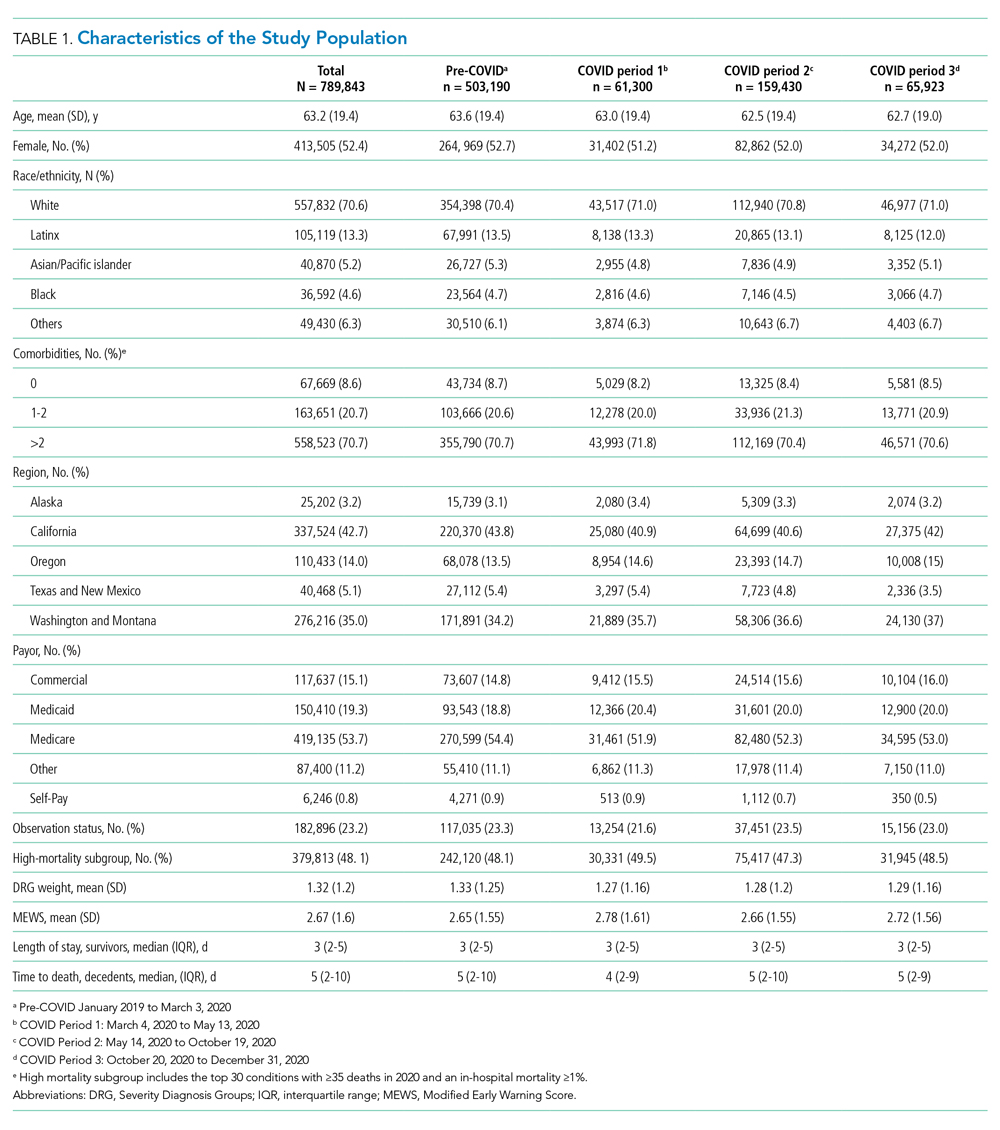
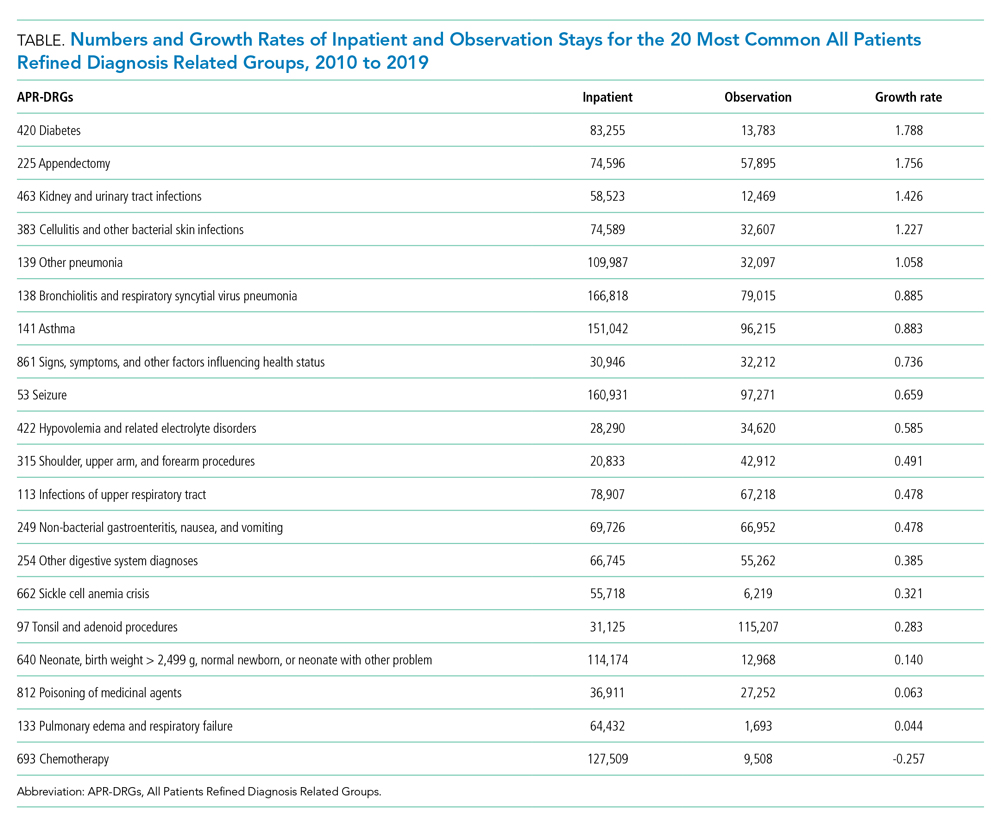
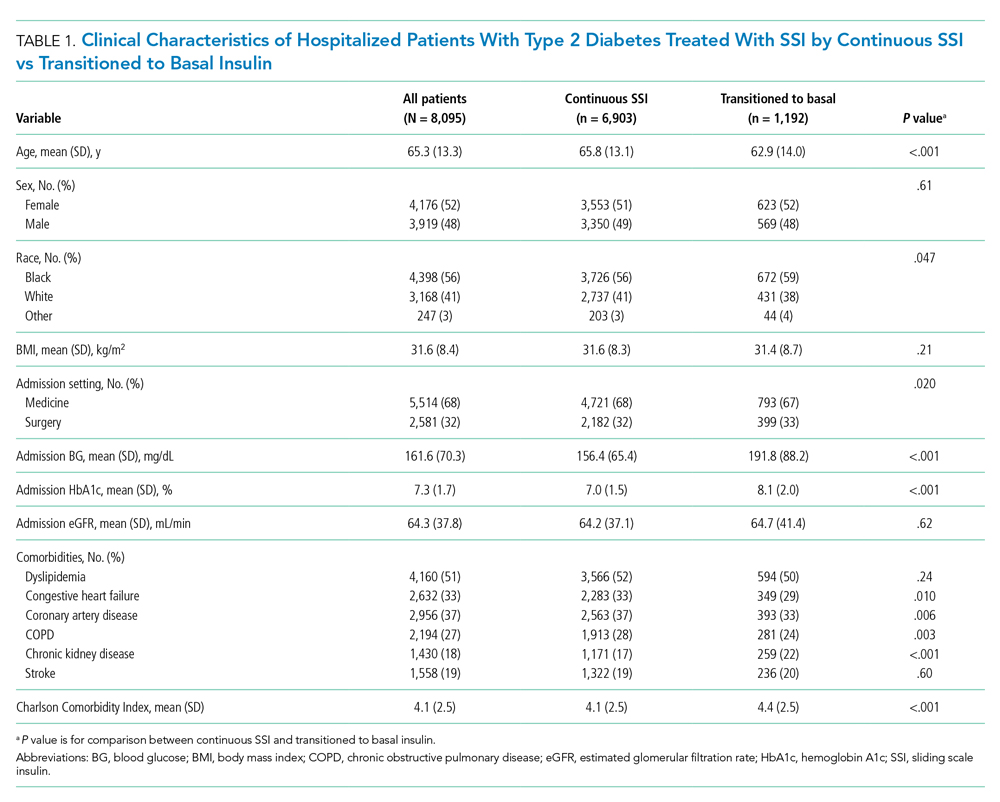
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25
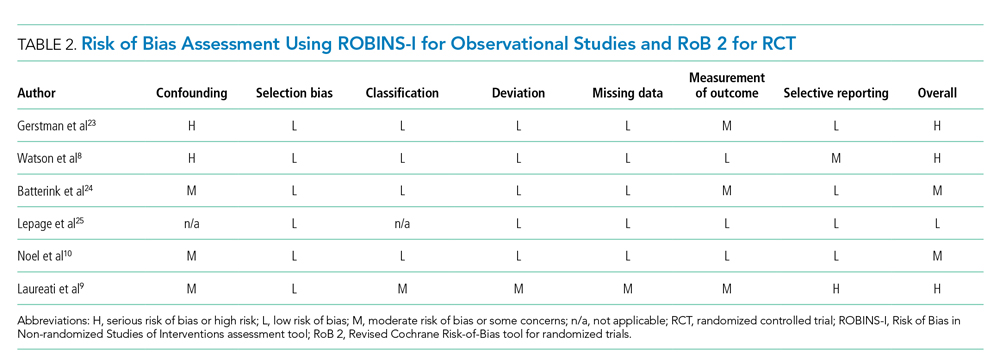
Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10
Quantitative Outcomes
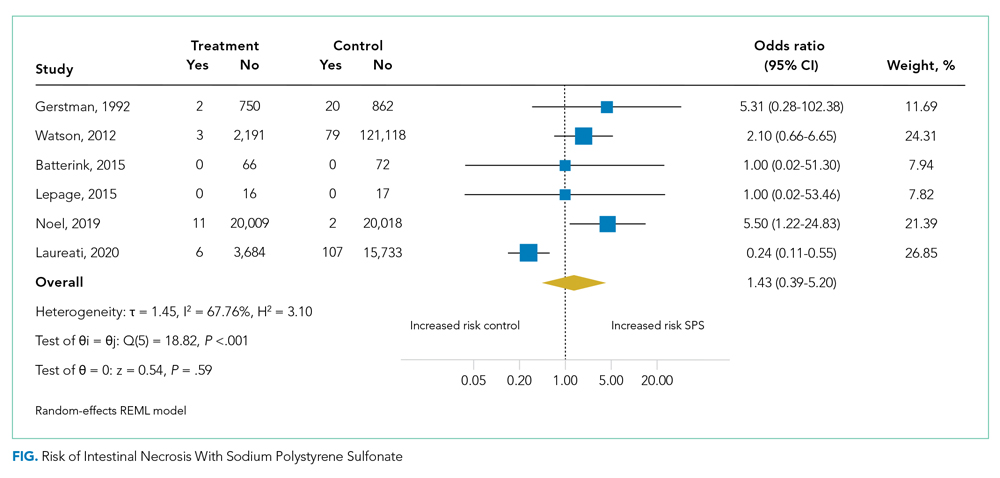
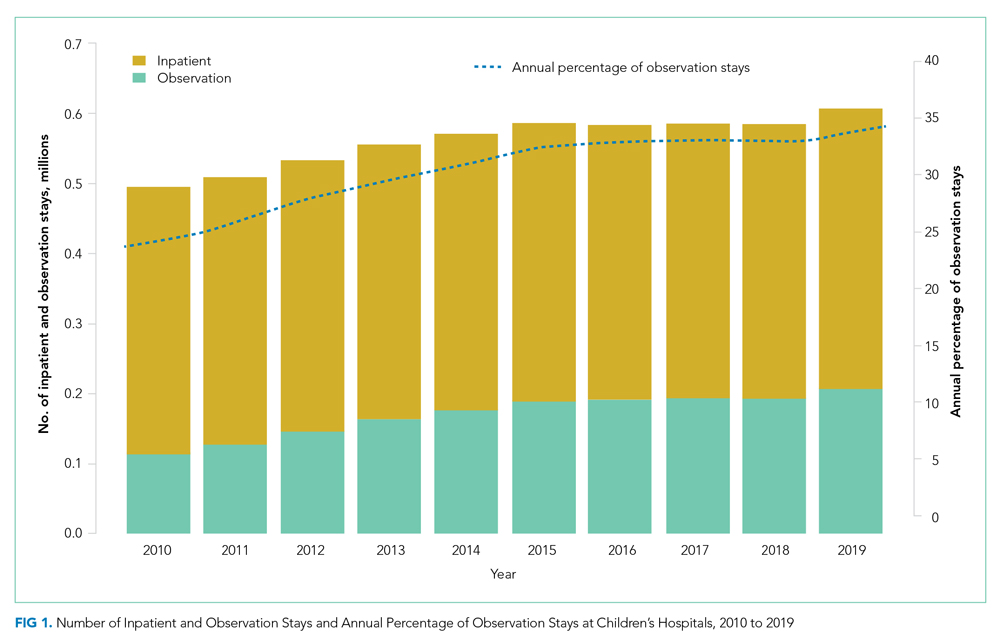
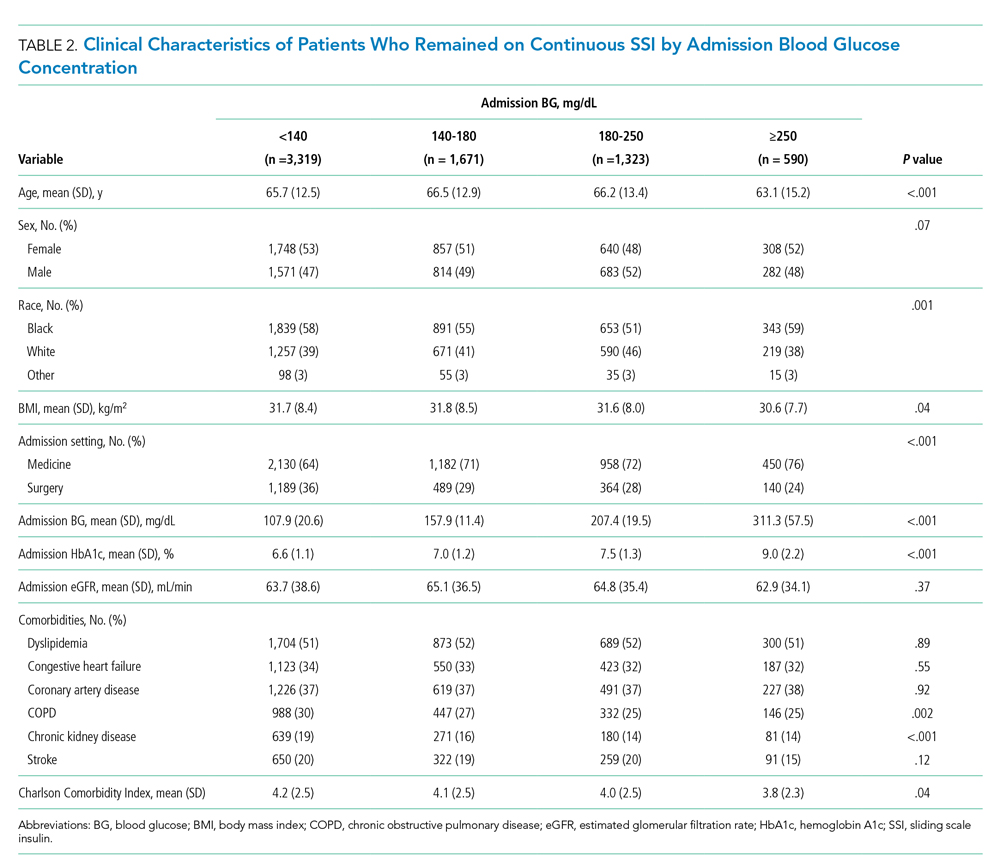
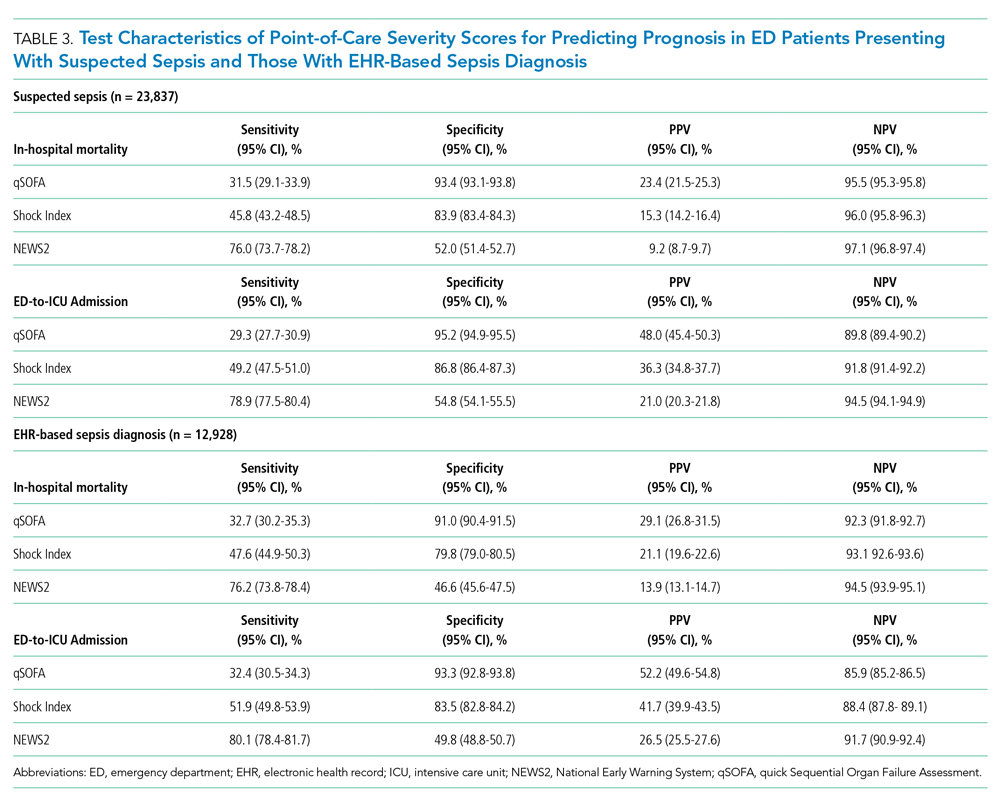
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).
For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.
DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25
Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10
Quantitative Outcomes
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).
For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.
DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
Sodium polystyrene sulfonate (SPS) was first approved in the United States in 1958 and is a commonly prescribed medication for hyperkalemia.1 SPS works by exchanging potassium for sodium in the colonic lumen, thereby promoting potassium loss in the stool. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s,2 leading some authors to recommend against the use of SPS.3,4 In 2009, the US Food and Drug Administration (FDA) warned against concomitant sorbitol administration, which was implicated in some studies.4,5 The concern about gastrointestinal side effects has also led to the development and FDA approval of two new cation-exchange resins for treatment of hyperkalemia.6 A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects.7 Because the included studies were all case reports or case series and therefore did not include comparison groups, it could not be determined whether SPS had a causal role in gastrointestinal side effects, and the authors could only conclude that there was a “possible” association. In contrast to case reports, several large cohort studies have been published more recently and report the risk of severe gastrointestinal adverse events associated with SPS compared with controls.8-10 While some studies found an increased risk, others have not. Given this uncertainty, we undertook a systematic review of studies that report the incidence of severe gastrointestinal side effects with SPS compared with controls.
METHODS
Data Sources and Search Strategy
A systematic search of the literature was conducted by a medical librarian using the Cochrane Library, Embase, Medline, Google Scholar, PubMed, Scopus, and Web of Science Core Collection databases to find relevant articles published from database inception to October 4, 2020. The search was peer reviewed by a second medical librarian using Peer Review of Electronic Search Strategies (PRESS).11 Databases were searched using a combination of controlled vocabulary and free-text terms for “SPS” and “bowel necrosis.” Details of the full search strategy are listed in Appendix A. References from all databases were imported into an EndNote X9 library, duplicates removed, and then uploaded into Coviden
Data Extraction and Quality Assessment
We used a standardized form to extract data, which included author, year, country, study design, setting, number of patients, SPS formulation, dosing, exposure, sorbitol content, outcomes of intestinal necrosis and the composite severe gastrointestinal adverse events, and the duration of time from SPS exposure to outcome occurrence. Two reviewers (JLH and AER) independently assessed the methodological quality of included studies using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool for observational studies13 and the Revised Cochrane risk of bias (RoB 2) tool for randomized controlled trials (RCTs).14 Additionally, two reviewers (JLH and CGG) graded overall strength of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.15 Disagreement was resolved by consensus.
Data Synthesis and Analysis
The proportion of patients with intestinal necrosis was compared using random effects meta-analysis using the restricted maximum likelihood method.16 For the two studies that reported hazard ratios (HRs), meta-analysis was performed after log transformation of the HRs and CIs. One study that performed survival analysis presented data for both the duration of the study (up to 11 years) and up to 1 year after exposure.9 We used the data up to 1 year after exposure because we believed later events were more likely to be due to chance than exposure to SPS. For studies with zero events, we used the treat ment-arm continuity correction, which has been reported to be preferable to the standard fixed-correction factor.17 We also performed two sensitivity analyses, including omitting the studies with zero events and performing meta-analysis using risk difference. The prevalence of intestinal ischemia was pooled using the DerSimonian and Laird18 random effects model with Freeman-Tukey19 double arcsine transformation. Heterogeneity was estimated using the I² statistic. I² values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.20 Meta-regression and tests for small-study effects were not performed because of the small number of included studies.21 In addition to random effects meta-analysis, we calculated the 90% predicted interval for future studies for the pooled effect of intestinal ischemia.22 Statistical analysis was performed using meta and metaprop commands in Stata/IC, version 16.1 (StataCorp).
RESULTS
Selected Studies
The electronic search yielded 806 unique articles, of which 791 were excluded based on title and abstract, leaving 15 articles for full-text review (Appendix B). Appendix C describes the nine studies that were excluded, including the reason for exclusion. Table 1 describes the characteristics of the six studies that met study inclusion criteria. Studies were published between 1992 and 2020. Three studies were from Canada,10,24,25 two from the United States,8,23 and one from Sweden.9 Three studies occurred in an outpatient setting,9,10,25 and three were described as inpatient studies.8,23,24 SPS preparations included sorbitol in three studies,8,23,24 were not specified in one study,10 and were not included in two studies.9,25 SPS dosing varied widely, with median doses of 15 to 30 g in three studies,9,24,25 45 to 50 g in two studies,8,23 and unspecified in one study.10 Duration of exposure typically ranged from 1 to 7 days but was not consistently described. For example, two of the studies did not report duration of exposure,8,10 and a third study reported a single dispensation of 450 g in 41% of patients, with the remaining 59% averaging three dispensations within the first year.9 Sample size ranged from 33 to 123,391 patients. Most patients were male, and mean ages ranged from 44 to 78 years. Two studies limited participation to those with chronic kidney disease (CKD) with glomerular filtration rate (GFR) <4024 or CKD stage 4 or 5 or dialysis.9 Two studies specifically limited participation to patients with potassium levels of 5.0 to 5.9 mmol/L.24,25 All six studies reported outcomes for intestinal necrosis, and four reported composite outcomes for major adverse gastrointestinal events.9,10,24,25
Table 2 describes the assessment of risk of bias using the ROBINS-I tool for the five retrospective observational studies and the RoB 2 tool for the one RCT.13,14 Three studies were rated as having serious risk of bias, with the remainder having a moderate risk of bias or some concerns. Two studies were judged as having a serious risk of bias because of potential confounding.8,23 To be judged low or moderate risk, studies needed to measure and control for potential risk factors for intestinal ischemia, such as age, diabetes, vascular disease, and heart failure.26,27 One study also had serious risk of bias for selective reporting because the published abstract of the study used a different analysis and had contradictory results from the published study.9,28 An additional area of risk of bias that did not fit into the ROBINS-I tool is that the two studies that used survival analysis chose durations for the outcome that were longer than would be expected for adverse events from SPS to be evident. One study chose 30 days and the other up to a maximum of 11 years from the time of exposure.9,10
Quantitative Outcomes
Six studies including 26,716 patients treated with SPS and controls reported the proportion of patients who developed intestinal necrosis. The Figure shows the individual study and pooled results for intestinal necrosis. The prevalence of intestinal ischemia in patients treated with SPS was 0.1% (95% CI, 0.03%-0.17%). The pooled odds ratio (OR) of intestinal necrosis was 1.43 (95% CI, 0.39-5.20). The 90% predicted interval for future studies was 0.08 to 26.6. Two studies reported rates of intestinal necrosis using survival analysis. The pooled HR from these studies was 2.00 (95% CI, 0.45-8.78). Two studies performed survival analysis for a composite outcome of severe gastrointestinal adverse events. The pooled HR for these two studies was 1.46 (95% CI, 1.01-2.11).
For the meta-analysis of intestinal necrosis, we found moderate-high statistical significance (Q = 18.82; P < .01; I² = 67.8%). Sensitivity analysis removing each study did not affect heterogeneity, with the exception of removing the study by Laureati et al,9 which resolved the heterogeneity (Q = 1.7, P = .8, I² = 0%). The pooled effect for intestinal necrosis also became statistically significant after removing Laureati et al (OR, 2.87; 95% CI, 1.24-6.63).9 We also performed two subgroup analyses, including studies that involved the concomitant use of sorbitol8,23,24 compared with studies that did not9,25 and subgroup analysis removing studies with zero events. Studies that included sorbitol found higher rates of intestinal necrosis (OR, 2.26; 95% CI, 0.80-6.38; I² = 0%) compared with studies that did not include sorbitol (OR, 0.25; 95% CI, 0.11-0.57; I² = 0%; test of group difference, P < .01). Removing the three studies with zero events resulted in a similar overall effect (OR, 1.30; 95% CI, 0.21-8.19). Finally, a meta-analysis using risk difference instead of ORs found a non–statistically significant difference in rate of intestinal necrosis favoring the control group (risk difference, −0.00033; 95% CI, −0.0022 to 0.0015; I² = 84.6%).
Table 3 summarizes our review findings and presents overall strength of evidence. Overall strength of evidence was found to be very low. Per GRADE criteria,15,29 strength of evidence for observational studies starts at low and may then be modified by the presence of bias, inconsistency, indirectness, imprecision, effect size, and direction of confounding. In the case of the three meta-analyses in the present study, risk of bias was serious for more than half of the study weights. Strength of evidence was also downrated for imprecision because of the low number of events and resultant wide CIs.
DISCUSSION
In total, we found six studies that reported rates of intestinal necrosis or severe gastrointestinal adverse events with SPS use compared with controls. The pooled rate of intestinal necrosis was not significantly higher for patients exposed to SPS when analyzed either as the proportion of patients with events or as HRs. The pooled rate for a composite outcome of severe gastrointestinal side effects was significantly higher (HR, 1.46; 95% CI, 1.01-2.11). The overall strength of evidence for the association of SPS with either intestinal necrosis or the composite outcome was found to be very low because of risk of bias and imprecision.
In some ways, our results emphasize the difficulty of showing a causal link between a medication and a possible rare adverse event. The first included study to assess the risk of intestinal necrosis after exposure to SPS compared with controls found only two events in the SPS group and no events in the control arm.23 Two additional studies that we found were small and did not report any events in either arm.24,25 The first large study to assess the risk of intestinal ischemia included more than 2,000 patients treated with SPS and more than 100,000 controls but found no difference in risk.8 The next large study did find increased risk of both intestinal necrosis (incidence rate, 6.82 per 1,000 person-years compared with 1.22 per 1,000 person-years for controls) and a composite outcome (incidence rate, 22.97 per 1,000 person-years compared with 11.01 per 1000 person-years for controls), but in the time to event analysis included events up to 30 days after treatment with SPS.10 A prior review of case reports of SPS and intestinal necrosis found a median of 2 days between SPS treatment and symptom onset.7 It is unlikely the authors would have had sufficient events to meaningfully compare rates if they limited the analysis to events within 7 days of SPS treatment, but events after a week of exposure are unlikely to be due to SPS. The final study to assess the association of SPS with intestinal necrosis actually found higher rates of intestinal necrosis in the control group when analyzed as proportions with events but reported a higher rate of a composite outcome of severe gastrointestinal adverse events that included nine separate International Classification of Diseases codes occurring up to 11 years after SPS exposure.9 This study was limited by evidence of selective reporting and was funded by the manufacturers of an alternative cation-exchange medication.
Based on our review of the literature, it is unclear if SPS does cause intestinal ischemia. The pooled results for intestinal ischemia analyzed as a proportion with events or with survival analysis did not find a statistically significantly increased risk. Because most o
A cost analysis of SPS vs potential alternatives such as patiromer for patients on chronic RAAS-I with a history of hyperkalemia or CKD published by Little et al26 concluded that SPS remained the cost-effective option when colonic necrosis incidence is 19.9% or less, and our systematic review reveals an incidence of 0.1% (95% CI, 0.03-0.17%). The incremental cost-effectiveness ratio was an astronomical $26,088,369 per quality-adjusted life-year gained, per Little’s analysis.
Limitations of our review are the heterogeneity of studies, which varied regarding inpatient or outpatient setting, formulations such as dosing, frequency, whether sorbitol was used, and interval from exposure to outcome measurement, which ranged from 7 days to 1 year. On sensitivity analysis, statistical heterogeneity was resolved by removing the study by Laureati et al.9 This study was notably different from the others because it included events occurring up to 1 year after exposure to SPS, which may have resulted in any true effect being diluted by later events unrelated to SPS. We did not exclude this study post hoc because this would result in bias; however, because the overall result becomes statistically significant without this study, our overall conclusion should be interpreted with caution.30 It is possible that future well-conducted studies may still find an effect of SPS on intestinal necrosis. Similarly, the finding that studies with SPS coformulated with sorbitol had statistically significantly increased risk of intestinal necrosis compared with studies without sorbitol should be interpreted with caution because the study by Laureati et al9 was included in the studies without sorbitol.
CONCLUSIONS
Based on our r
This work was presented at the Society of General Internal Medicine and Society of Hospital Medicine 2021 annual conferences.
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
1. Labriola L, Jadoul M. Sodium polystyrene sulfonate: still news after 60 years on the market. Nephrol Dial Transplant. 2020;35(9):1455-1458. https://doi.org/10.1093/ndt/gfaa004
2. Arvanitakis C, Malek G, Uehling D, Morrissey JF. Colonic complications after renal transplantation. Gastroenterology. 1973;64(4):533-538.
3. Parks M, Grady D. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med. 2019;179(8):1023-1024. https://doi.org/10.1001/jamainternmed.2019.1291
4. Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21(5):733-735. https://doi.org/10.1681/ASN.2010010079
5. Lillemoe KD, Romolo JL, Hamilton SR, Pennington LR, Burdick JF, Williams GM. Intestinal necrosis due to sodium polystyrene (Kayexalate) in sorbitol enemas: clinical and experimental support for the hypothesis. Surgery. 1987;101(3):267-272.
6. Sterns RH, Grieff M, Bernstein PL. Treatment of hyperkalemia: something old, something new. Kidney Int. 2016;89(3):546-554. https://doi.org/10.1016/j.kint.2015.11.018
7. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e269-24. https://doi.org/10.1016/j.amjmed.2012.08.016
8. Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60(3):409-416. https://doi.org/10.1053/j.ajkd.2012.04.023
9. Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518-1526. https://doi.org/10.1093/ndt/gfz150
10. Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025-1033. https://doi.org/10.1001/jamainternmed.2019.0631
11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40-46. https://doi.org/10.1016/j.jclinepi.2016.01.021
12. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. https://doi.org/10.7326/0003-4819-151-4-200908180-00136
13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919
14. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. https://doi.org/10.1016/j.jclinepi.2010.04.026
16. Raudenbush SW. Analyzing effect sizes: random-effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russel Sage Foundation; 2009:295-316.
17. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. https://doi.org/10.1002/sim.1761
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. https://doi.org/10.1016/0197-2456(86)90046-2
19. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21(4):607-611. https://doi.org/10.1214/aoms/1177729756
20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. https://doi.org/10.1136/bmj.327.7414.557
21. Higgins JPT, Chandler TJ, Cumptson M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook
22. Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. Jan 2009;172(1):137-159. https://doi.org/10.1111/j.1467-985X.2008.00552.x
23. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159-161. https://doi.org/10.1016/s0272-6386(12)80544-0
24. Batterink J, Lin J, Au-Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short-term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68(4):296-303. https://doi.org/10.4212/cjhp.v68i4.1469
25. Lepage L, Dufour AC, Doiron J, et al. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10(12):2136-2142. https://doi.org/10.2215/CJN.03640415
26. Little DJ, Nee R, Abbott KC, Watson MA, Yuan CM. Cost-utility analysis of sodium polystyrene sulfonate vs. potential alternatives for chronic hyperkalemia. Clin Nephrol. 2014;81(4):259-268. https://doi.org/10.5414/cn108103
27. Cubiella Fernández J, Núñez Calvo L, González Vázquez E, et al. Risk factors associated with the development of ischemic colitis. World J Gastroenterol. 2010;16(36):4564-4569. https://doi.org/10.3748/wjg.v16.i36.4564
28. Laureati P, Evans M, Trevisan M, et al. Sodium polystyrene sulfonate, practice patterns and associated adverse event risk; a nationwide analysis from the Swedish Renal Register [abstract]. Nephroly Dial Transplant. 2019;34(suppl 1):i94. https://doi.org/10.1093/ndt/gfz106.FP151
29. Santesso N, Carrasco-Labra A, Langendam M, et al. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. J Clin Epidemiol. 2016;74:28-39. https://doi.org/10.1016/j.jclinepi.2015.12.006
30. Deeks JJ HJ, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane, 2020. www.training.cochrane.org/handbook
© 2021 Society of Hospital Medicine
Clinical Progress Note: E-cigarette, or Vaping, Product Use-Associated Lung Injury
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
© 2021 Society of Hospital Medicine
Incidentally Detected SARS-COV-2 Among Hospitalized Patients in Los Angeles County, August to October 2020
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
Many of the 85 hospitals in Los Angeles County (LAC) routinely test patients for SARS-CoV-2, the virus that causes COVID-19, upon admission to the hospital.1 However, not all SARS-CoV-2 detections represent acute COVID-19 for at least two reasons. First, the SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) assay can report a false-positive result.2 Second, approximately 40% to 45% of persons with SARS-CoV-2 infection are asymptomatic, and RT-PCR tests can remain positive more than 2 months after an individual recovers from COVID-19; thus, SARS-CoV-2 detected on admission might represent shedding of nonviable virus from a prior unrecognized or undiagnosed infection.1,3
Public health policymakers closely monitor the rate of COVID-19 hospitalizations because it informs decisions to impose or relax COVID-19 control measures. However, the percentage of hospitalizations misclassified as COVID-19–associated because of incidentally detected SARS-CoV-2 (ie, COVID-19 was not a primary or contributing cause of hospitalization) is unknown. Therefore, we sought to determine the percentage of hospitalizations in LAC classified as having COVID-19 that might have had incidental SARS-CoV-2 detection.
METHODS
The state of California requires healthcare providers to report all COVID-19 cases and clinical laboratories to report all SARS-CoV-2 diagnostic test results. Hospitals in LAC are mandated to report daily lists of all persons hospitalized with suspected or confirmed COVID-19 to the LAC Department of Public Health (DPH) COVID-19 Hospital Electronic Surveillance System (CHESS).4 Hospitals provide daily data to CHESS containing information about patients in their facilities with COVID-19. We conducted a cross-sectional retrospective study by selecting a random set of medical records from CHESS for review.
We began regularly and systematically reviewing medical records of patients in CHESS discharged after August 1, 2020, as part of LAC DPH surveillance to characterize persons experiencing severe COVID-19, defined as illness requiring hospitalization. For severe COVID-19 surveillance, we randomly selected 45 discharged patients per week from CHESS in August 2020 and 50 discharged patients per week between September and October 2020. To ensure that the sample represented the overall age distribution of patients in CHESS, we ordered patients by birth date and selected every k record, where k represented the interval between patients needed to meet the target for the week. Before random sample selection, several free text fields from the CHESS dataset were queried to identify and remove patients who were not LAC residents, were seen in the emergency department but not admitted, were hospitalized for <1 day, were discharged from a non-acute care hospital, or if the hospital-reported patient did not have a positive SARS-CoV-2 test. We then requested full medical records for these patients from the respective hospitals. After we received the medical records, a team of four nurses independently reviewed the medical charts and excluded patients who did not meet the above listed exclusion criteria; patients were excluded at two points—during the automated query and again by manual review.
In addition, severe COVID-19 surveillance was intended to characterize primary admissions for COVID-19, defined as having a documented positive SARS-CoV-2 result within 10 days of symptom onset or hospital admission and no prior hospitalization for COVID-19. The date of the first positive result was validated by locating the positive SARS-COV-2 result in the patient’s medical record and/or the LAC COVID surveillance database; the patient was excluded from analysis if a positive SARS-CoV-2 result could not be found. Excluded discharges were not replaced by a new randomly selected patient. Instead, we oversampled the number of weekly charts to request with a goal of having 40 to 45 charts per week that met inclusion criteria for abstraction.
For this analysis, we examined medical records abstracted for discharges occurring between August 1 and October 31, 2020. We categorized hospitalizations into one of the following: (1) “likely COVID-19–associated” if the patient had
Descriptive statistics and all analyses were conducted using SAS version 9.4 (SAS Institute). Confidence limits (CL) were calculated using the proc freq CL option in SAS. Chi-square analysis was conducted to determine whether trends in hospitalization categories changed over time. Statistical significance was set at P < .05.
RESULTS
Of the 13,813 hospital discharges reported to CHESS from August to October 2020, 3,182 (23%) records were not eligible for inclusion in the random selection sample for the following reasons: 1,765 (13%) patients reported by hospitals did not have a positive COVID-19 test, 734 (5%) discharges were for non-LAC residents, 636 (5%) patients had a length of hospital stay <1 day, and 47 (<1%) discharges were from a non-acute care hospital. From the 10,631 discharges in CHESS meeting preliminary inclusion criteria from August 1 to October 31, 2020, we randomly selected 618 discharges for medical record review. Of the 618 discharges, 504 (85%) medical records were available for review as of November 30, 2020. After review of the 504 medical records, an additional 158 were excluded because 83 (13%) had a first documented positive SARS-CoV-2 test that was >10 days from hospital admission or symptom onset, 34 (6%) were previously hospitalized for COVID-19, 29 (5%) had an emergency department visit only, 6 (1%) were discharged from a non-acute care hospital, and 6 (1%) were non-LAC residents. We reviewed medical records for 346 (56%) of the 618 hospitalizations that met our inclusion criteria.
The demographic characteristics of patients included in our sample were similar to those of the overall patient population in CHESS (Table 1). Most patients in our final study population were male (54%), older than 50 years (66%), and Hispanic (60%); the median length of hospital stay for survivors was 5 days (first quartile–third quartile: 3 to 8 days).
Our analysis indicates that 71% (95% CL, 66%-75%) of hospital discharges were “likely COVID-19-associated”; 12% (CL, 9%-16%) were “not COVID-19–associated” and, therefore, had incidentally detected SARS-CoV-2; and 17% were “potentially COVID-19–associated” (CL, 13%-21%). The percentage of hospitalizations classified as “likely,” “potentially,” and “not COVID-19–associated” did not change from month-to-month during the study period (P = .81). Full-term delivery was the most common reason for hospitalization among patients with incidentally detected SARS-CoV-2 (Table 2).
DISCUSSION
The primary public health objective of the COVID-19 pandemic response has been to prevent overwhelming the healthcare system by slowing disease transmission. LAC DPH closely monitors the daily number of hospitalized COVID-19 patients, defined as hospitalization of a person with an associated positive SARS-CoV-2 result. However, increasing community transmission of SARS-CoV-2 can complicate interpretation of hospitalization data because it is likely that some patients with incidentally detected, nonviable virus will be misclassified as having COVID-19. Overestimating the burden of COVID-19–associated hospitalizations may lead public health policymakers to impose more restrictive control measures or remove restrictions more slowly. Results from this study can inform policymakers about the potential magnitude of overestimating COVID-19–associated hospitalizations.
Our results indicate that SARS-CoV-2 detection might be incidental (ie, “not COVID-19–associated”) in approximately one of eight persons hospitalized with COVID-19 in LAC. We likely underestimated the percentage of hospitalizations with incidental SARS-CoV-2 detection because our definition of “not COVID-19–associated” hospitalizations was intended to be specific for identifying patients who had no clear reason for SARS-CoV-2 testing except a presumed hospital policy of testing on admission or preoperatively. In addition, several patients classified as having a “potentially COVID-19–associated” hospitalization also had a primary reason for admission that currently does not have a clear link to COVID-19 (eg, Bell’s palsy and pelvic inflammatory disease). Although our sample size was relatively small, it was representative of all potential COVID-19 hospitalizations in LAC over a 3-month period.
CONCLUSION
Detection of SARS-CoV-2 in a person with a clinical presentation that is not compatible with COVID-19 can complicate initial clinical management because it is unclear if the result represents presymptomatic or asymptomatic infection, prolonged shedding of nonviable virus, or a false-positive result. Considering the consequences of missing a true infection, such as transmission to other staff or patients, healthcare providers are obligated to treat the test result as a real infection. Therefore, our results are not applicable to patient-level clinical management decisions, but highlight the need for policymakers and emergency preparedness personnel to consider that hospital-reported data might overestimate the burden of COVID-19 hospitalizations when making decisions that rely on hospitalization data as a metric. Additional research is needed to develop methods for correcting hospitalization data to account for patients in whom incidentally detected SARS-CoV-2 was not a direct or contributing cause of hospitalization. Adjusting COVID-19–associated hospitalization rates to account for incidental SARS-CoV-2 detection could allow for optimal resource planning by public health policymakers.
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
1. Liotti, FM, Menchinelli, G, Marchetti, S, et al. Assessment of SARS-CoV-2 RNA test results among patients who recovered from COVID-19 with prior negative results. JAMA Intern Med. 2021;181(5):702-704. https://doi.org/10.1001/jamainternmed.2020.7570
2. Centers for Disease Control and Prevention and Infectious Disease Society of America. RT-PCR Testing. Accessed April 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/diagnostics/RT-pcr-testing
3. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362-367. https://doi.org/10.7326/M20-3012
4 Los Angeles County Department of Public Health. Daily reporting of hospitalized COVID-19 positive inpatients: updated data submission requirements and guide for acute care facilities in Los Angeles County. Accessed on December 10, 2020. http://publichealth.lacounty.gov/acd/docs/HospCOVIDReportingGuide.pdf
© 2021 Society of Hospital Medicine
Excess Mortality Among Patients Hospitalized During the COVID-19 Pandemic
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.
Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.
Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.
Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.
DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.
Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.
Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.
Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.
DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.
Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.
Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.
Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.
DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
© 2021 Society of Hospital Medicine
Trends and Variation in the Use of Observation Stays at Children’s Hospitals
Payors have been refining reimbursement policies for observation and inpatient stays over the past decade, and the effects on the healthcare payment system are significant.1-4 Advocates claim that observation status could improve efficiency in the use of healthcare resources by reducing emergency department (ED) crowding and lowering costs for inpatient care.5,6 Critics consider observation status to be a cost-shifting strategy that could lead to financial burdens for patients and hospitals.7,8
Although reimbursement policies for observation stays traditionally have been set by the Centers for Medicare and Medicaid Services (CMS) in a uniform manner,4,8 state Medicaid programs and commercial health insurers have developed a variety of policies for using observation status in broader populations and hospitals.9-15 Coverage criteria and implementation timelines of these policies vary by states and commercial insurers.11-15 For example, the California Department of Health Care Services did not have a specific reimbursement rate for observation stays in 2020, while some state Medicaid programs have had reimbursement policies for observation services in place since 2010.11-15 These inconsistencies likely result in greater variation in use of observation stays across children’s hospitals than general hospitals.
Previous studies have shown rising trends in use of observation stays among adult patient populations and related implications for patients and general hospitals,16-19 but few studies have reported the trends for pediatric populations. In this study, we sought to (1) describe recent trends of observation stays for pediatric populations at children’s hospitals from 2010 through 2019 and (2) investigate features of this shifting pattern for pediatric populations and hospital-level use of observation stays.
METHODS
Study Design, Data, and Populations
We performed a retrospective analysis of the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, observation, ambulatory, and ED encounter-level data from 50 not-for-profit, tertiary care children’s hospitals affiliated with the Children’s Hospital Association (CHA).20 PHIS has an indicator to classify patient types (inpatient, observation, ED visits, ambulatory surgery, clinic visit, and others). The data are de-identified at the time of submission and subjected to validity and reliability checks by CHA and Truven Health Analytics (Ann Arbor, MI) before being included in PHIS. Each encounter in PHIS has only one patient type; therefore, encounters that transition to a higher level of care are assigned to their highest level of care (eg, a patient transitions from observation to inpatient status is classified as an inpatient encounter) to avoid duplicate counting.
To ensure consistent evaluations over time, we included 29 children’s hospitals that consistently reported both inpatient and observation data to PHIS across all quarters from 2010 through 2019. We identified the 20 most common clinical conditions using the All Patients Refined Diagnosis Related Groups (APR-DRGs; 3M Corporation) based upon their total frequencies of observation and inpatient stays over the study period. Regression analyses were conducted using all encounters within the 20 most common APR-DRGs.
Because all data have been de-identified in the PHIS database, the institutional review board at Ann and Robert H. Lurie Children’s Hospital of Chicago granted this study institutional review board–exempt status.
Main Outcome and Measures
We first presented longitudinal trends of observation stays for children’s hospitals using annual percentage of observation stays defined as:
To determine whether different pediatric populations have different trends of observation stays, we measured the growth rates of observation stays for each APR-DRG. Specifically, we first calculated the percentage of observation stays by APR-DRGs and years as described, and then calculated the growth rate of observation stays for each APR-DRG:
Next, we employed prolonged length of stay (LOS) and hospitalization resource-intensity scores for kids (H-RISK) to further investigate the shifting pattern of observation stays. Because most state Medicaid and commercial policies dictate that observation stays should not last longer than 48 hours, we defined prolonged LOS as >2 days.11-15 We defined the annual percentage of observation stays with prolonged LOS for each year as:
Numerators and denominators of the three measures were obtained by pooling all children’s hospitals included in this study. H-RISK is a continuous variable developed by CHA to measure use of intensive care for children, which is comparable across various APR-DRGs.21 Changes in the empirical distribution of H-RISK from observation stays were presented over years using percentiles.
Other measures included sex, age, race, payor, and LOS. To investigate the use of observation stays among payors, we categorized payors into five groups: private, in-state Medicaid (managed care), in-state Medicaid (Children’s Health Insurance Program [CHIP]/others), other government, and all others, according to the data availability. The “private” group consisted of commercial preferred provider organizations, commercial health maintenance organizations, and commercial others. We combined both CHIP and in-state Medicaid (others), including Medicaid fee-for-service or unspecified Medicaid together as “in-state Medicaid (CHIP/others).” Detailed categorization information is summarized in Appendix Table 1. LOS was classified into four groups: 1 day (24 hours), 2 days (48 hours), 3 to 4 days, and >4 days.
Statistical Analysis
Descriptive statistics were stratified by inpatient and observation status and were summarized using frequency, percent, median, and interquartile range (IQR). Chi-square or Wilcoxon rank-sum tests were performed to examine differences between observation and inpatient status. Trends in annual percentage of observation stays and annual percentage of observation stays with prolonged LOS were estimated using first-order autoregressive models, in which year was considered a continuous variable. A nonparametric measure of rank correlation (Spearman’s rank correlation coefficient) was employed to evaluate the correlation between year and H-RISK from observation stays.
The risk-adjusted probability of being admitted as an observation stay was estimated using generalized linear mixed models by adjusting for year, age, sex, race, payor, LOS, H-RISK, and a random intercept for each hospital to control for patient clustering within a hospital (Appendix Model). Hospital-level use of observation stays was measured by risk-adjusted percent use of observation stays for each hospital using the predicted values from generalized linear mixed models. All analyses were performed using SAS software, version 9.4 (SAS Institute) and R (R Core Team, 2019), and P < .05 was considered statistically significant.
RESULTS
Increasing Trend of Observation Stays
Over the study period, there were 5,611,001 encounters, including 3,901,873 (69.5%) inpatient and 1,709,128 (30.5%) observation stays (Appendix Table 1). The number of observation stays increased from 117,246 in 2010 to 207,842 in 2019, and the number of inpatient stays slightly increased from 378,433 to 397,994 over the 10 years (Appendix Table 1). Because of different growth rates between observation and inpatient status, the annual percentage of observation stays increased from 23.7% in 2010 to 34.3% in 2019, while the annual percentage of inpatient stays decreased from 76.3% in 2010 to 65.7% in 2019 (Appendix Table 1; Figure 1, P < .001).
Different Growth Rates of Observation Stays for Various Pediatric Populations
As shown in the Table, growth rates of observation stays increased for 19 of the 20 most common APR-DRGs. The four APR-DRGs having the highest growth rates in observation stays were appendectomy, diabetes mellitus, kidney and urinary tract infections, and cellulitis and other bacterial skin infections (Appendix Figure). In particular, the annual percentage of observation stays for appendectomy increased from 19.8% in 2010 to 54.7% in 2019, with the number of observation stays growing from 2,321 to 7,876, while the number of inpatient stays decreased from 9,384 to 6,535 (Appendix Figure). The annual percentage of observation stays for diabetes mellitus increased from 8.16% in 2010 to 22.74% in 2019. Tonsil and adenoid procedures consistently held the largest numbers of observation stays across the 10 years among all the APR-DRGs, with 115,207 and 31,125 total observation and inpatient stays, respectively (Table).
Characteristics of Observation and Inpatient Stays
Patient characteristics are summarized in Appendix Table 1. There were 542,344 (32.9%) observation stays among patients with in-state Medicaid (managed care), and 241,157 (27.4%) observation stays among in-state Medicaid (CHIP/others). The percentages of observation and inpatient stays were 29.8% and 70.2% for private payor, as well as 29.6% and 70.4% for other government payor. Overall, the median (IQR) of H-RISK among observation stays was 0.79 (0.57-1.19) vs 1.23 (0.72-2.43) for inpatient stays. There were 1,410,694 (82.5%) observation stays discharged within 1 day and 243,972 (14.3%) observation stays discharged within 2 days. However, there were 47,413 (2.8%) and 7,049 (0.4%) observation stays with LOS 3 to 4 days or >4 days, respectively.
Shifting Pattern in Observation Stays
The annual percentage of observation stays with prolonged LOS (>2 days) rose from 1.1% in 2010 to 4.6% in 2019 (P < .001; Figure 2). The empirical distribution of H-RISK from observation stays by years further suggests a slightly increasing trend in intensity of care under observation stays. As shown in Appendix Table 2, although the 1st, 5th, 10th, 25th, and 99th percentiles of H-RISK were relatively stable, the 50th, 75th, 90th, and 95th percentiles of H-RISK were increasing over time. The correlation between year and intensity of care used under observation stays (H-RISK from observation stays) was found to be weak but significantly positive (Spearman correlation coefficients = 0.04; P < .001).
Interaction coefficients from our regression model demonstrate that the existing inverse association between H-RISK and odds of admission as an observation stay became less negative over the years. In 2010, the adjusted odds ratio (OR) of H-RISK was 0.57 (95% CI, 0.55-0.59). By 2017, the adjusted OR had increased to 0.65 (95% CI, 0.64-0.66). Compared with 2010, the seven adjusted ORs of H-RISK at years 2012 through 2018 were observed to be higher and statistically significant (P < .001, Appendix Table 3).
Hospitals-Level Use of Observation Stays
After adjusting for all covariates and hospital random effects, hospital-level use of observation stays increased between 2010 and 2019 for 26 out of 29 children’s hospitals. Although observation status essentially was not used at two children’s hospitals over the study period, the median hospital-level use of observation stays was 26% in 2010 (IQR, 3%-36%) and increased to 46% (IQR: 39%; 55%) in 2019. As shown in Figure 3, the number of hospitals with a low percentage of observation stays (<26%) decreased from 15 in 2010 to 4 in 2019. The number of hospitals with a high percentage of observation stays (≥51%) increased from 5 in 2010 to 10 in 2019. Nevertheless, there remained significant variation in the use of observation stays, and the hospital-level use ranged from 0% to 67% in 2019.
DISCUSSION
By 2020, observation status has become a key component of healthcare for pediatric patients, and its relevance for children’s hospitals recently has been described.22,23 However, trends in observation stays for pediatric populations are not known. This represents the first study showing temporal trends of observation stays at children’s hospitals after 2010. Our results confirm that the increase in observation stays for pediatric populations is not attributable to decreasing patient acuity at children’s hospitals. We found a weak but significantly positive correlation between year and intensity of care used under observation stays. Although this correlation might not be clinically important, it demonstrates that patient acuity in observation stays is not decreasing. Regression results suggest that observation stays now encompass patients who need relatively higher intensity of care compared with those admitted under observation status in 2010.
This study also identifies a unique pattern in the use of observation stays among pediatric populations. Earlier studies exclusively focused on observation stays that were admitted from EDs.24 Our results indicate that observation status has been used beyond a bridge from ED care to inpatient admission. In particular, observation status has expanded to include pediatric populations with more diverse clinical conditions (eg, appendicitis and diabetes mellitus), and has become a substantial component of postprocedural admissions (Appendix Figure). Looking forward, it is likely that the use of observation stays might surpass inpatient admissions for more conditions that primarily involve short-term stays.
Observation status originally was designed as a reimbursement strategy for patients who needed short stays in dedicated ED units or hospitals, but did not qualify for inpatient services.5,25 After several changes in reimbursement policies, CMS released the “two midnight rule” for Medicare beneficiaries in 2013, which replaced condition-based criteria with time-based criteria to determine an inpatient or observation stay.1 Some Medicaid programs and commercial payors have developed similar policies. Unlike the universal policy for Medicare populations, the regulations for pediatric populations vary by states and health insurers.11-15,26-28 This might partially explain the wide variation observed among children’s hospital-level use of observation stays. For example, the California Medicaid program did not have a reimbursement rate for observation services as of 2020, while the Texas Medicaid program has had a policy for observation stays since 2010.12,13 We found that two children’s hospitals in California had the lowest use of observation stays (almost zero), whereas the hospital-level use of observation stays was more than 50% for three out of four children’s hospitals in Texas. In addition to reimbursement policies, individual hospitals also might have different strategies for observation status designation. An earlier survey showed that there was lack of consistency in billing and payor-based designations of observation status at children’s hospitals.29 These findings suggest that children’s hospital-level use of observation stays likely is influenced by reimbursement policy and practical strategy for observation status determination.
Earlier studies reported that observation status could be a more efficient use of healthcare resources.5,6 However, there are still at least two concerns relevant to children’s hospitals during the last decade. The first is whether the use of observation stays can promote cost-saving or if it is just a cost-shifting strategy. An earlier study demonstrated that observation stays with prolonged LOS might increase risk of cost-sharing among adult patients.29 Our study reveals an increasing trend of observation stays with prolonged LOS for pediatric patients. Similar to adult patients, LOS exceeding 24 or 48 hours could lead to uncovered healthcare costs and financial burdens on families.30-32 Meanwhile, children’s hospitals also might take on a higher financial liability by implementing observation status. Earlier studies have indicated that resource use between observation and inpatient stays at children’s hospitals is similar, and increasing use of observation stays might lead to financial risk rather than cost effectiveness.33 Further, administrative costs of observation determination are considerably high.34 Medicaid is the major payor for pediatric patients in children’s hospitals. In this study, more than 50% of encounters were paid through Medicaid programs. It is well known that Medicaid reimbursement rates are lower than Medicare and commercial plans.35 Therefore, the cost-saving conclusion drawn from Medicare patients cannot be generalized to pediatric populations at children’s hospitals without cautious reevaluation.
A second concern with increasing use of observation stays is selection bias in public reporting and comparisons of hospital performance. Presently, four main categories of quality indicators established by the Agency for Healthcare Research and Quality rely heavily on inpatient encounters.36 In this study, we found that the range of hospital-level use of observation stays was large. In 2019, the risk-adjusted percent use of observation stays was less than 5% at three hospitals, while the percent use was greater than 60% in another three hospitals. Therefore, comparisons made without uniform accounting of observation stays might have significant implications for national rankings of children’s hospitals across the United States. These consequences have been investigated in several published studies.22,23,37-39
There are several limitations to our study. First, the study sample was limited to children’s hospitals that consistently reported inpatient and observation data over the entire study period. Eighteen hospitals (86%) excluded from this study did not consistently submit inpatient and observation data to PHIS from 2010 through 2019. The primary purpose of this study was to present temporal trends of observation stays for children’s hospitals, and it was important to build the hospital cohort based on valid and consistent data during the study period. Appendix Table 4 presents differences of hospital characteristics by included and excluded groups of hospitals. Excluded hospitals might have fewer resources (eg, fewer pediatric intensive care beds). Nonetheless, the selection of hospitals was optimized based on data availability. Second, this study was a retrospective review of an administrative database of children’s hospitals and units. The sample does not represent all children’s hospitals or pediatric patients in the United States, but there are no available data sources—that we know of—that can generate national estimates for both inpatient and observation stays. Third, we did not attempt to conclusively infer any causal effects, and several factors could explain the increasing trends, such as reimbursement policies, hospital-level implementation strategies, determination guidelines for observation status designation, as well as changes in clinical care. Further studies should investigate impact of these factors on the use of observation stays for pediatric patients and children’s hospitals.
CONCLUSION
Observation status has been increasingly used for pediatric patients with more diverse clinical conditions, and there is a rising trend of prolonged LOS among observation stays since 2010. Considerable variation exists in hospital-level use of observation stays across children’s hospitals. Observation status could be an opportunity to improve efficiency of healthcare resource use or could lead to a financial risk for patients with prolonged LOS. Future studies should explore appropriateness of observation care in clinical practice through leveraging efficient care and alleviating financial risk.
1. Centers for Medicare & Medicaid Services. Fact Sheet: Two-Midnight Rule. Accessed April 11, 2021. https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0
2. BlueCross BlueShield of Rhode Island. Payment Policy Outpaient Observation. Accessed April 11, 2021. https://www.bcbsri.com/sites/default/files/polices/Outpatient-Observation.pdf
3. Blue Cross Blue Shield of Illinois. Observation Services Tool for Applying MCG Care Guidelines Clinical Payment and Coding Policy. Accessed April 11, 2021. https://www.bcbsil.com/pdf/standards/observation_services_cpcp.pdf
4. Medicare.gov. Inpatient or outpatient hospital status affects your costs. Accessed April 11, 2021. https://www.medicare.gov/what-medicare-covers/what-part-a-covers/inpatient-or-outpatient-hospital-status
5. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. https://doi.org/10.1377/hlthaff.2013.0662
6. Baugh CW, Venkatesh AK, Hilton JA, Samuel PA, Schuur JD, Bohan JS. Making greater use of dedicated hospital observation units for many short-stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314-2323. https://doi.org/10.1377/hlthaff.2011.0926
7. Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991-1998. https://doi.org/10.1001/jamainternmed.2013.8185
8. Baugh CW, Schuur JD. Observation care—high-value care or a cost-shifting loophole? N Engl J Med. 2013;369(4):302-305. https://doi.org/10.1056/NEJMp1304493
9. Missouri Hospital Association. A patient’s guide to observation care. Accessed April 11, 2021. https://www.mhanet.com/mhaimages/PatientsGuideToObservationCareFlyer.pdf
10. Cigna. Employee-paid hospital care coverage- summary of benefits. Accessed April 11, 2021. https://www.cigna.com/iwov-resources/national-second-sale/docs/healthy-benefits/updated-HC-benefit-summary.pdf
11. BlueCross BlueShield of Minnesota. Reimbursement policy-observation care services. Accessed April 11, 2021. https://www.bluecrossmn.com/sites/default/files/DAM/2020-07/Evaluation%20and%20Management%20004_Observation%20Care%20Services%20_09.04.17.pdf
12. California Department of Health Care Services. Public Hospital Project Frequently Asked Questions. Accessed April 11, 2021. https://www.dhcs.ca.gov/provgovpart/Documents/Public%20Hospital%20Project/PHP_Final_FAQs_January2013ADA.pdf
13. Texas Medicaid & Healthcare Partnership. Inpatient and Outpatient Hospital Servicces Handbook. Accessed May 29, 2021. https://www.tmhp.com/sites/default/files/microsites/provider-manuals/tmppm/html/TMPPM/2_Inpatient_Outpatient_Hosp_Srvs/2_Inpatient_Outpatient_Hosp_Srvs.htm
14. Alabama Medicaid. Outpatient observation. Accessed April 11, 2021. https://medicaid.alabama.gov/news_detail.aspx?ID=5121
15. NC Medicaid. Medicaid and Health Choice Clinical Coverage Policy No: 2A-1. Accessed April 11, 2021. https://files.nc.gov/ncdma/documents/files/2A-1_0.pdf
16. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. https://doi.org/10.1377/hlthaff.2012.0129
17. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. https://doi.org/10.1377/hlthaff.2014.1474
18. Wright B, Jung HY, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. Health Serv Res. 2014;49(4):1088-1107. https://doi.org/10.1111/1475-6773.12166
19. Sabbatini AK, Wright B, Hall MK, Basu A. The cost of observation care for commercially insured patients visiting the emergency department. Am J Emerg Med. 2018;36(9):1591-1596. https://doi.org/10.1016/j.ajem.2018.01.040
20. Children’s Hospital Association. Pediatric health information system. Accessed April 11, 2021. https://www.childrenshospitals.org/phis
21. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
22. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst DC, Macy ML.Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
23. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
24. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
25. Macy ML, Kim CS, Sasson C, Lozon MM, Davis MM. Pediatric observation units in the United States: a systematic review. J Hosp Med. 2010;5(3):172-182. https://doi.org/10.1002/jhm.592
26. UnitedHealthcare. Observation services policy, facility. Accessed April 11, 2021. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/medicaid-comm-plan-reimbursement/UHCCP-Facility-Observation-Services-Policy-(F7106).pdf
27. Cal SB-1076§1253.7. General acute care hospitals: observation services – Health and Safety. Accessed April 11, 2021. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520160SB1076
28. Nebraska Total Care. 2021 Provider Billing Guide. Accessed April 11, 2021. https://www.nebraskatotalcare.com/content/dam/centene/Nebraska/PDFs/ProviderRelations/NTC_Nebraska_Total_Care_Provider_Billing_Guide_508.pdf
29. Macy ML, Hall M, Shah SS, et al. Differences in designations of observation care in US freestanding children’s hospitals: are they virtual or real? J Hosp Med. 2012;7(4):287-293. https://doi.org/10.1002/jhm.949
30. Hockenberry JM, Mutter R, Barrett M, Parlato J, Ross MA. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. https://doi.org/10.1111/1475-6773.12143
31. Anthem BlueCross BlueShield. Ohio Provider Manual. Accessed April11, 2021. https://www11.anthem.com/provider/oh/f1/s0/t0/pw_g357368.pdf?refer=ahpprovider&state=oh
32. Humana. Provider manual for physicians, hospitals and healthcare providers. Accessed April 11, 2021. https://docushare-web.apps.cf.humana.com/Marketing/docushare-app?file=3932669
33. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058 https://doi.org/10.1542/peds.2012-249
34. Tejedor-Sojo J. Observation status-a name at what cost? Hosp Pediatr. 2014;4(5):321-323. https://doi.org/10.1542/hpeds.2014-0037.
35. Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health Aff (Millwood). 2015;34(12):2147-2150. https://doi.org/10.1377/hlthaff.2015.0706
36. Agency for Healthcare Research and Quality. AHRQ Quality Indicators. Accessed April 11, 2021. https://www.qualityindicators.ahrq.gov
37. Figueroa JF, Burke LG, Zheng J, Orav EJ, Jha AK. Trends in hospitalization vs observation stay for ambulatory care-sensitive conditions. JAMA Intern Med. 2019;179(12):1714-1716. https://doi.org/10.1001/jamainternmed.2019.3177
38. Markham JL, Hall M, Gay JC, Bettenhausen JL, Berry JG. Length of stay and cost of pediatric readmissions. Pediatrics. 2018;141(4):e20172934. https://doi.org/10.1542/peds.2017-2934.
39. Overman RA, Freburger JK, Assimon MM, Li X, Brookhart, MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. https://doi.org/10.1002/pds.3647.
Payors have been refining reimbursement policies for observation and inpatient stays over the past decade, and the effects on the healthcare payment system are significant.1-4 Advocates claim that observation status could improve efficiency in the use of healthcare resources by reducing emergency department (ED) crowding and lowering costs for inpatient care.5,6 Critics consider observation status to be a cost-shifting strategy that could lead to financial burdens for patients and hospitals.7,8
Although reimbursement policies for observation stays traditionally have been set by the Centers for Medicare and Medicaid Services (CMS) in a uniform manner,4,8 state Medicaid programs and commercial health insurers have developed a variety of policies for using observation status in broader populations and hospitals.9-15 Coverage criteria and implementation timelines of these policies vary by states and commercial insurers.11-15 For example, the California Department of Health Care Services did not have a specific reimbursement rate for observation stays in 2020, while some state Medicaid programs have had reimbursement policies for observation services in place since 2010.11-15 These inconsistencies likely result in greater variation in use of observation stays across children’s hospitals than general hospitals.
Previous studies have shown rising trends in use of observation stays among adult patient populations and related implications for patients and general hospitals,16-19 but few studies have reported the trends for pediatric populations. In this study, we sought to (1) describe recent trends of observation stays for pediatric populations at children’s hospitals from 2010 through 2019 and (2) investigate features of this shifting pattern for pediatric populations and hospital-level use of observation stays.
METHODS
Study Design, Data, and Populations
We performed a retrospective analysis of the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, observation, ambulatory, and ED encounter-level data from 50 not-for-profit, tertiary care children’s hospitals affiliated with the Children’s Hospital Association (CHA).20 PHIS has an indicator to classify patient types (inpatient, observation, ED visits, ambulatory surgery, clinic visit, and others). The data are de-identified at the time of submission and subjected to validity and reliability checks by CHA and Truven Health Analytics (Ann Arbor, MI) before being included in PHIS. Each encounter in PHIS has only one patient type; therefore, encounters that transition to a higher level of care are assigned to their highest level of care (eg, a patient transitions from observation to inpatient status is classified as an inpatient encounter) to avoid duplicate counting.
To ensure consistent evaluations over time, we included 29 children’s hospitals that consistently reported both inpatient and observation data to PHIS across all quarters from 2010 through 2019. We identified the 20 most common clinical conditions using the All Patients Refined Diagnosis Related Groups (APR-DRGs; 3M Corporation) based upon their total frequencies of observation and inpatient stays over the study period. Regression analyses were conducted using all encounters within the 20 most common APR-DRGs.
Because all data have been de-identified in the PHIS database, the institutional review board at Ann and Robert H. Lurie Children’s Hospital of Chicago granted this study institutional review board–exempt status.
Main Outcome and Measures
We first presented longitudinal trends of observation stays for children’s hospitals using annual percentage of observation stays defined as:
To determine whether different pediatric populations have different trends of observation stays, we measured the growth rates of observation stays for each APR-DRG. Specifically, we first calculated the percentage of observation stays by APR-DRGs and years as described, and then calculated the growth rate of observation stays for each APR-DRG:
Next, we employed prolonged length of stay (LOS) and hospitalization resource-intensity scores for kids (H-RISK) to further investigate the shifting pattern of observation stays. Because most state Medicaid and commercial policies dictate that observation stays should not last longer than 48 hours, we defined prolonged LOS as >2 days.11-15 We defined the annual percentage of observation stays with prolonged LOS for each year as:
Numerators and denominators of the three measures were obtained by pooling all children’s hospitals included in this study. H-RISK is a continuous variable developed by CHA to measure use of intensive care for children, which is comparable across various APR-DRGs.21 Changes in the empirical distribution of H-RISK from observation stays were presented over years using percentiles.
Other measures included sex, age, race, payor, and LOS. To investigate the use of observation stays among payors, we categorized payors into five groups: private, in-state Medicaid (managed care), in-state Medicaid (Children’s Health Insurance Program [CHIP]/others), other government, and all others, according to the data availability. The “private” group consisted of commercial preferred provider organizations, commercial health maintenance organizations, and commercial others. We combined both CHIP and in-state Medicaid (others), including Medicaid fee-for-service or unspecified Medicaid together as “in-state Medicaid (CHIP/others).” Detailed categorization information is summarized in Appendix Table 1. LOS was classified into four groups: 1 day (24 hours), 2 days (48 hours), 3 to 4 days, and >4 days.
Statistical Analysis
Descriptive statistics were stratified by inpatient and observation status and were summarized using frequency, percent, median, and interquartile range (IQR). Chi-square or Wilcoxon rank-sum tests were performed to examine differences between observation and inpatient status. Trends in annual percentage of observation stays and annual percentage of observation stays with prolonged LOS were estimated using first-order autoregressive models, in which year was considered a continuous variable. A nonparametric measure of rank correlation (Spearman’s rank correlation coefficient) was employed to evaluate the correlation between year and H-RISK from observation stays.
The risk-adjusted probability of being admitted as an observation stay was estimated using generalized linear mixed models by adjusting for year, age, sex, race, payor, LOS, H-RISK, and a random intercept for each hospital to control for patient clustering within a hospital (Appendix Model). Hospital-level use of observation stays was measured by risk-adjusted percent use of observation stays for each hospital using the predicted values from generalized linear mixed models. All analyses were performed using SAS software, version 9.4 (SAS Institute) and R (R Core Team, 2019), and P < .05 was considered statistically significant.
RESULTS
Increasing Trend of Observation Stays
Over the study period, there were 5,611,001 encounters, including 3,901,873 (69.5%) inpatient and 1,709,128 (30.5%) observation stays (Appendix Table 1). The number of observation stays increased from 117,246 in 2010 to 207,842 in 2019, and the number of inpatient stays slightly increased from 378,433 to 397,994 over the 10 years (Appendix Table 1). Because of different growth rates between observation and inpatient status, the annual percentage of observation stays increased from 23.7% in 2010 to 34.3% in 2019, while the annual percentage of inpatient stays decreased from 76.3% in 2010 to 65.7% in 2019 (Appendix Table 1; Figure 1, P < .001).
Different Growth Rates of Observation Stays for Various Pediatric Populations
As shown in the Table, growth rates of observation stays increased for 19 of the 20 most common APR-DRGs. The four APR-DRGs having the highest growth rates in observation stays were appendectomy, diabetes mellitus, kidney and urinary tract infections, and cellulitis and other bacterial skin infections (Appendix Figure). In particular, the annual percentage of observation stays for appendectomy increased from 19.8% in 2010 to 54.7% in 2019, with the number of observation stays growing from 2,321 to 7,876, while the number of inpatient stays decreased from 9,384 to 6,535 (Appendix Figure). The annual percentage of observation stays for diabetes mellitus increased from 8.16% in 2010 to 22.74% in 2019. Tonsil and adenoid procedures consistently held the largest numbers of observation stays across the 10 years among all the APR-DRGs, with 115,207 and 31,125 total observation and inpatient stays, respectively (Table).
Characteristics of Observation and Inpatient Stays
Patient characteristics are summarized in Appendix Table 1. There were 542,344 (32.9%) observation stays among patients with in-state Medicaid (managed care), and 241,157 (27.4%) observation stays among in-state Medicaid (CHIP/others). The percentages of observation and inpatient stays were 29.8% and 70.2% for private payor, as well as 29.6% and 70.4% for other government payor. Overall, the median (IQR) of H-RISK among observation stays was 0.79 (0.57-1.19) vs 1.23 (0.72-2.43) for inpatient stays. There were 1,410,694 (82.5%) observation stays discharged within 1 day and 243,972 (14.3%) observation stays discharged within 2 days. However, there were 47,413 (2.8%) and 7,049 (0.4%) observation stays with LOS 3 to 4 days or >4 days, respectively.
Shifting Pattern in Observation Stays
The annual percentage of observation stays with prolonged LOS (>2 days) rose from 1.1% in 2010 to 4.6% in 2019 (P < .001; Figure 2). The empirical distribution of H-RISK from observation stays by years further suggests a slightly increasing trend in intensity of care under observation stays. As shown in Appendix Table 2, although the 1st, 5th, 10th, 25th, and 99th percentiles of H-RISK were relatively stable, the 50th, 75th, 90th, and 95th percentiles of H-RISK were increasing over time. The correlation between year and intensity of care used under observation stays (H-RISK from observation stays) was found to be weak but significantly positive (Spearman correlation coefficients = 0.04; P < .001).
Interaction coefficients from our regression model demonstrate that the existing inverse association between H-RISK and odds of admission as an observation stay became less negative over the years. In 2010, the adjusted odds ratio (OR) of H-RISK was 0.57 (95% CI, 0.55-0.59). By 2017, the adjusted OR had increased to 0.65 (95% CI, 0.64-0.66). Compared with 2010, the seven adjusted ORs of H-RISK at years 2012 through 2018 were observed to be higher and statistically significant (P < .001, Appendix Table 3).
Hospitals-Level Use of Observation Stays
After adjusting for all covariates and hospital random effects, hospital-level use of observation stays increased between 2010 and 2019 for 26 out of 29 children’s hospitals. Although observation status essentially was not used at two children’s hospitals over the study period, the median hospital-level use of observation stays was 26% in 2010 (IQR, 3%-36%) and increased to 46% (IQR: 39%; 55%) in 2019. As shown in Figure 3, the number of hospitals with a low percentage of observation stays (<26%) decreased from 15 in 2010 to 4 in 2019. The number of hospitals with a high percentage of observation stays (≥51%) increased from 5 in 2010 to 10 in 2019. Nevertheless, there remained significant variation in the use of observation stays, and the hospital-level use ranged from 0% to 67% in 2019.
DISCUSSION
By 2020, observation status has become a key component of healthcare for pediatric patients, and its relevance for children’s hospitals recently has been described.22,23 However, trends in observation stays for pediatric populations are not known. This represents the first study showing temporal trends of observation stays at children’s hospitals after 2010. Our results confirm that the increase in observation stays for pediatric populations is not attributable to decreasing patient acuity at children’s hospitals. We found a weak but significantly positive correlation between year and intensity of care used under observation stays. Although this correlation might not be clinically important, it demonstrates that patient acuity in observation stays is not decreasing. Regression results suggest that observation stays now encompass patients who need relatively higher intensity of care compared with those admitted under observation status in 2010.
This study also identifies a unique pattern in the use of observation stays among pediatric populations. Earlier studies exclusively focused on observation stays that were admitted from EDs.24 Our results indicate that observation status has been used beyond a bridge from ED care to inpatient admission. In particular, observation status has expanded to include pediatric populations with more diverse clinical conditions (eg, appendicitis and diabetes mellitus), and has become a substantial component of postprocedural admissions (Appendix Figure). Looking forward, it is likely that the use of observation stays might surpass inpatient admissions for more conditions that primarily involve short-term stays.
Observation status originally was designed as a reimbursement strategy for patients who needed short stays in dedicated ED units or hospitals, but did not qualify for inpatient services.5,25 After several changes in reimbursement policies, CMS released the “two midnight rule” for Medicare beneficiaries in 2013, which replaced condition-based criteria with time-based criteria to determine an inpatient or observation stay.1 Some Medicaid programs and commercial payors have developed similar policies. Unlike the universal policy for Medicare populations, the regulations for pediatric populations vary by states and health insurers.11-15,26-28 This might partially explain the wide variation observed among children’s hospital-level use of observation stays. For example, the California Medicaid program did not have a reimbursement rate for observation services as of 2020, while the Texas Medicaid program has had a policy for observation stays since 2010.12,13 We found that two children’s hospitals in California had the lowest use of observation stays (almost zero), whereas the hospital-level use of observation stays was more than 50% for three out of four children’s hospitals in Texas. In addition to reimbursement policies, individual hospitals also might have different strategies for observation status designation. An earlier survey showed that there was lack of consistency in billing and payor-based designations of observation status at children’s hospitals.29 These findings suggest that children’s hospital-level use of observation stays likely is influenced by reimbursement policy and practical strategy for observation status determination.
Earlier studies reported that observation status could be a more efficient use of healthcare resources.5,6 However, there are still at least two concerns relevant to children’s hospitals during the last decade. The first is whether the use of observation stays can promote cost-saving or if it is just a cost-shifting strategy. An earlier study demonstrated that observation stays with prolonged LOS might increase risk of cost-sharing among adult patients.29 Our study reveals an increasing trend of observation stays with prolonged LOS for pediatric patients. Similar to adult patients, LOS exceeding 24 or 48 hours could lead to uncovered healthcare costs and financial burdens on families.30-32 Meanwhile, children’s hospitals also might take on a higher financial liability by implementing observation status. Earlier studies have indicated that resource use between observation and inpatient stays at children’s hospitals is similar, and increasing use of observation stays might lead to financial risk rather than cost effectiveness.33 Further, administrative costs of observation determination are considerably high.34 Medicaid is the major payor for pediatric patients in children’s hospitals. In this study, more than 50% of encounters were paid through Medicaid programs. It is well known that Medicaid reimbursement rates are lower than Medicare and commercial plans.35 Therefore, the cost-saving conclusion drawn from Medicare patients cannot be generalized to pediatric populations at children’s hospitals without cautious reevaluation.
A second concern with increasing use of observation stays is selection bias in public reporting and comparisons of hospital performance. Presently, four main categories of quality indicators established by the Agency for Healthcare Research and Quality rely heavily on inpatient encounters.36 In this study, we found that the range of hospital-level use of observation stays was large. In 2019, the risk-adjusted percent use of observation stays was less than 5% at three hospitals, while the percent use was greater than 60% in another three hospitals. Therefore, comparisons made without uniform accounting of observation stays might have significant implications for national rankings of children’s hospitals across the United States. These consequences have been investigated in several published studies.22,23,37-39
There are several limitations to our study. First, the study sample was limited to children’s hospitals that consistently reported inpatient and observation data over the entire study period. Eighteen hospitals (86%) excluded from this study did not consistently submit inpatient and observation data to PHIS from 2010 through 2019. The primary purpose of this study was to present temporal trends of observation stays for children’s hospitals, and it was important to build the hospital cohort based on valid and consistent data during the study period. Appendix Table 4 presents differences of hospital characteristics by included and excluded groups of hospitals. Excluded hospitals might have fewer resources (eg, fewer pediatric intensive care beds). Nonetheless, the selection of hospitals was optimized based on data availability. Second, this study was a retrospective review of an administrative database of children’s hospitals and units. The sample does not represent all children’s hospitals or pediatric patients in the United States, but there are no available data sources—that we know of—that can generate national estimates for both inpatient and observation stays. Third, we did not attempt to conclusively infer any causal effects, and several factors could explain the increasing trends, such as reimbursement policies, hospital-level implementation strategies, determination guidelines for observation status designation, as well as changes in clinical care. Further studies should investigate impact of these factors on the use of observation stays for pediatric patients and children’s hospitals.
CONCLUSION
Observation status has been increasingly used for pediatric patients with more diverse clinical conditions, and there is a rising trend of prolonged LOS among observation stays since 2010. Considerable variation exists in hospital-level use of observation stays across children’s hospitals. Observation status could be an opportunity to improve efficiency of healthcare resource use or could lead to a financial risk for patients with prolonged LOS. Future studies should explore appropriateness of observation care in clinical practice through leveraging efficient care and alleviating financial risk.
Payors have been refining reimbursement policies for observation and inpatient stays over the past decade, and the effects on the healthcare payment system are significant.1-4 Advocates claim that observation status could improve efficiency in the use of healthcare resources by reducing emergency department (ED) crowding and lowering costs for inpatient care.5,6 Critics consider observation status to be a cost-shifting strategy that could lead to financial burdens for patients and hospitals.7,8
Although reimbursement policies for observation stays traditionally have been set by the Centers for Medicare and Medicaid Services (CMS) in a uniform manner,4,8 state Medicaid programs and commercial health insurers have developed a variety of policies for using observation status in broader populations and hospitals.9-15 Coverage criteria and implementation timelines of these policies vary by states and commercial insurers.11-15 For example, the California Department of Health Care Services did not have a specific reimbursement rate for observation stays in 2020, while some state Medicaid programs have had reimbursement policies for observation services in place since 2010.11-15 These inconsistencies likely result in greater variation in use of observation stays across children’s hospitals than general hospitals.
Previous studies have shown rising trends in use of observation stays among adult patient populations and related implications for patients and general hospitals,16-19 but few studies have reported the trends for pediatric populations. In this study, we sought to (1) describe recent trends of observation stays for pediatric populations at children’s hospitals from 2010 through 2019 and (2) investigate features of this shifting pattern for pediatric populations and hospital-level use of observation stays.
METHODS
Study Design, Data, and Populations
We performed a retrospective analysis of the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, observation, ambulatory, and ED encounter-level data from 50 not-for-profit, tertiary care children’s hospitals affiliated with the Children’s Hospital Association (CHA).20 PHIS has an indicator to classify patient types (inpatient, observation, ED visits, ambulatory surgery, clinic visit, and others). The data are de-identified at the time of submission and subjected to validity and reliability checks by CHA and Truven Health Analytics (Ann Arbor, MI) before being included in PHIS. Each encounter in PHIS has only one patient type; therefore, encounters that transition to a higher level of care are assigned to their highest level of care (eg, a patient transitions from observation to inpatient status is classified as an inpatient encounter) to avoid duplicate counting.
To ensure consistent evaluations over time, we included 29 children’s hospitals that consistently reported both inpatient and observation data to PHIS across all quarters from 2010 through 2019. We identified the 20 most common clinical conditions using the All Patients Refined Diagnosis Related Groups (APR-DRGs; 3M Corporation) based upon their total frequencies of observation and inpatient stays over the study period. Regression analyses were conducted using all encounters within the 20 most common APR-DRGs.
Because all data have been de-identified in the PHIS database, the institutional review board at Ann and Robert H. Lurie Children’s Hospital of Chicago granted this study institutional review board–exempt status.
Main Outcome and Measures
We first presented longitudinal trends of observation stays for children’s hospitals using annual percentage of observation stays defined as:
To determine whether different pediatric populations have different trends of observation stays, we measured the growth rates of observation stays for each APR-DRG. Specifically, we first calculated the percentage of observation stays by APR-DRGs and years as described, and then calculated the growth rate of observation stays for each APR-DRG:
Next, we employed prolonged length of stay (LOS) and hospitalization resource-intensity scores for kids (H-RISK) to further investigate the shifting pattern of observation stays. Because most state Medicaid and commercial policies dictate that observation stays should not last longer than 48 hours, we defined prolonged LOS as >2 days.11-15 We defined the annual percentage of observation stays with prolonged LOS for each year as:
Numerators and denominators of the three measures were obtained by pooling all children’s hospitals included in this study. H-RISK is a continuous variable developed by CHA to measure use of intensive care for children, which is comparable across various APR-DRGs.21 Changes in the empirical distribution of H-RISK from observation stays were presented over years using percentiles.
Other measures included sex, age, race, payor, and LOS. To investigate the use of observation stays among payors, we categorized payors into five groups: private, in-state Medicaid (managed care), in-state Medicaid (Children’s Health Insurance Program [CHIP]/others), other government, and all others, according to the data availability. The “private” group consisted of commercial preferred provider organizations, commercial health maintenance organizations, and commercial others. We combined both CHIP and in-state Medicaid (others), including Medicaid fee-for-service or unspecified Medicaid together as “in-state Medicaid (CHIP/others).” Detailed categorization information is summarized in Appendix Table 1. LOS was classified into four groups: 1 day (24 hours), 2 days (48 hours), 3 to 4 days, and >4 days.
Statistical Analysis
Descriptive statistics were stratified by inpatient and observation status and were summarized using frequency, percent, median, and interquartile range (IQR). Chi-square or Wilcoxon rank-sum tests were performed to examine differences between observation and inpatient status. Trends in annual percentage of observation stays and annual percentage of observation stays with prolonged LOS were estimated using first-order autoregressive models, in which year was considered a continuous variable. A nonparametric measure of rank correlation (Spearman’s rank correlation coefficient) was employed to evaluate the correlation between year and H-RISK from observation stays.
The risk-adjusted probability of being admitted as an observation stay was estimated using generalized linear mixed models by adjusting for year, age, sex, race, payor, LOS, H-RISK, and a random intercept for each hospital to control for patient clustering within a hospital (Appendix Model). Hospital-level use of observation stays was measured by risk-adjusted percent use of observation stays for each hospital using the predicted values from generalized linear mixed models. All analyses were performed using SAS software, version 9.4 (SAS Institute) and R (R Core Team, 2019), and P < .05 was considered statistically significant.
RESULTS
Increasing Trend of Observation Stays
Over the study period, there were 5,611,001 encounters, including 3,901,873 (69.5%) inpatient and 1,709,128 (30.5%) observation stays (Appendix Table 1). The number of observation stays increased from 117,246 in 2010 to 207,842 in 2019, and the number of inpatient stays slightly increased from 378,433 to 397,994 over the 10 years (Appendix Table 1). Because of different growth rates between observation and inpatient status, the annual percentage of observation stays increased from 23.7% in 2010 to 34.3% in 2019, while the annual percentage of inpatient stays decreased from 76.3% in 2010 to 65.7% in 2019 (Appendix Table 1; Figure 1, P < .001).
Different Growth Rates of Observation Stays for Various Pediatric Populations
As shown in the Table, growth rates of observation stays increased for 19 of the 20 most common APR-DRGs. The four APR-DRGs having the highest growth rates in observation stays were appendectomy, diabetes mellitus, kidney and urinary tract infections, and cellulitis and other bacterial skin infections (Appendix Figure). In particular, the annual percentage of observation stays for appendectomy increased from 19.8% in 2010 to 54.7% in 2019, with the number of observation stays growing from 2,321 to 7,876, while the number of inpatient stays decreased from 9,384 to 6,535 (Appendix Figure). The annual percentage of observation stays for diabetes mellitus increased from 8.16% in 2010 to 22.74% in 2019. Tonsil and adenoid procedures consistently held the largest numbers of observation stays across the 10 years among all the APR-DRGs, with 115,207 and 31,125 total observation and inpatient stays, respectively (Table).
Characteristics of Observation and Inpatient Stays
Patient characteristics are summarized in Appendix Table 1. There were 542,344 (32.9%) observation stays among patients with in-state Medicaid (managed care), and 241,157 (27.4%) observation stays among in-state Medicaid (CHIP/others). The percentages of observation and inpatient stays were 29.8% and 70.2% for private payor, as well as 29.6% and 70.4% for other government payor. Overall, the median (IQR) of H-RISK among observation stays was 0.79 (0.57-1.19) vs 1.23 (0.72-2.43) for inpatient stays. There were 1,410,694 (82.5%) observation stays discharged within 1 day and 243,972 (14.3%) observation stays discharged within 2 days. However, there were 47,413 (2.8%) and 7,049 (0.4%) observation stays with LOS 3 to 4 days or >4 days, respectively.
Shifting Pattern in Observation Stays
The annual percentage of observation stays with prolonged LOS (>2 days) rose from 1.1% in 2010 to 4.6% in 2019 (P < .001; Figure 2). The empirical distribution of H-RISK from observation stays by years further suggests a slightly increasing trend in intensity of care under observation stays. As shown in Appendix Table 2, although the 1st, 5th, 10th, 25th, and 99th percentiles of H-RISK were relatively stable, the 50th, 75th, 90th, and 95th percentiles of H-RISK were increasing over time. The correlation between year and intensity of care used under observation stays (H-RISK from observation stays) was found to be weak but significantly positive (Spearman correlation coefficients = 0.04; P < .001).
Interaction coefficients from our regression model demonstrate that the existing inverse association between H-RISK and odds of admission as an observation stay became less negative over the years. In 2010, the adjusted odds ratio (OR) of H-RISK was 0.57 (95% CI, 0.55-0.59). By 2017, the adjusted OR had increased to 0.65 (95% CI, 0.64-0.66). Compared with 2010, the seven adjusted ORs of H-RISK at years 2012 through 2018 were observed to be higher and statistically significant (P < .001, Appendix Table 3).
Hospitals-Level Use of Observation Stays
After adjusting for all covariates and hospital random effects, hospital-level use of observation stays increased between 2010 and 2019 for 26 out of 29 children’s hospitals. Although observation status essentially was not used at two children’s hospitals over the study period, the median hospital-level use of observation stays was 26% in 2010 (IQR, 3%-36%) and increased to 46% (IQR: 39%; 55%) in 2019. As shown in Figure 3, the number of hospitals with a low percentage of observation stays (<26%) decreased from 15 in 2010 to 4 in 2019. The number of hospitals with a high percentage of observation stays (≥51%) increased from 5 in 2010 to 10 in 2019. Nevertheless, there remained significant variation in the use of observation stays, and the hospital-level use ranged from 0% to 67% in 2019.
DISCUSSION
By 2020, observation status has become a key component of healthcare for pediatric patients, and its relevance for children’s hospitals recently has been described.22,23 However, trends in observation stays for pediatric populations are not known. This represents the first study showing temporal trends of observation stays at children’s hospitals after 2010. Our results confirm that the increase in observation stays for pediatric populations is not attributable to decreasing patient acuity at children’s hospitals. We found a weak but significantly positive correlation between year and intensity of care used under observation stays. Although this correlation might not be clinically important, it demonstrates that patient acuity in observation stays is not decreasing. Regression results suggest that observation stays now encompass patients who need relatively higher intensity of care compared with those admitted under observation status in 2010.
This study also identifies a unique pattern in the use of observation stays among pediatric populations. Earlier studies exclusively focused on observation stays that were admitted from EDs.24 Our results indicate that observation status has been used beyond a bridge from ED care to inpatient admission. In particular, observation status has expanded to include pediatric populations with more diverse clinical conditions (eg, appendicitis and diabetes mellitus), and has become a substantial component of postprocedural admissions (Appendix Figure). Looking forward, it is likely that the use of observation stays might surpass inpatient admissions for more conditions that primarily involve short-term stays.
Observation status originally was designed as a reimbursement strategy for patients who needed short stays in dedicated ED units or hospitals, but did not qualify for inpatient services.5,25 After several changes in reimbursement policies, CMS released the “two midnight rule” for Medicare beneficiaries in 2013, which replaced condition-based criteria with time-based criteria to determine an inpatient or observation stay.1 Some Medicaid programs and commercial payors have developed similar policies. Unlike the universal policy for Medicare populations, the regulations for pediatric populations vary by states and health insurers.11-15,26-28 This might partially explain the wide variation observed among children’s hospital-level use of observation stays. For example, the California Medicaid program did not have a reimbursement rate for observation services as of 2020, while the Texas Medicaid program has had a policy for observation stays since 2010.12,13 We found that two children’s hospitals in California had the lowest use of observation stays (almost zero), whereas the hospital-level use of observation stays was more than 50% for three out of four children’s hospitals in Texas. In addition to reimbursement policies, individual hospitals also might have different strategies for observation status designation. An earlier survey showed that there was lack of consistency in billing and payor-based designations of observation status at children’s hospitals.29 These findings suggest that children’s hospital-level use of observation stays likely is influenced by reimbursement policy and practical strategy for observation status determination.
Earlier studies reported that observation status could be a more efficient use of healthcare resources.5,6 However, there are still at least two concerns relevant to children’s hospitals during the last decade. The first is whether the use of observation stays can promote cost-saving or if it is just a cost-shifting strategy. An earlier study demonstrated that observation stays with prolonged LOS might increase risk of cost-sharing among adult patients.29 Our study reveals an increasing trend of observation stays with prolonged LOS for pediatric patients. Similar to adult patients, LOS exceeding 24 or 48 hours could lead to uncovered healthcare costs and financial burdens on families.30-32 Meanwhile, children’s hospitals also might take on a higher financial liability by implementing observation status. Earlier studies have indicated that resource use between observation and inpatient stays at children’s hospitals is similar, and increasing use of observation stays might lead to financial risk rather than cost effectiveness.33 Further, administrative costs of observation determination are considerably high.34 Medicaid is the major payor for pediatric patients in children’s hospitals. In this study, more than 50% of encounters were paid through Medicaid programs. It is well known that Medicaid reimbursement rates are lower than Medicare and commercial plans.35 Therefore, the cost-saving conclusion drawn from Medicare patients cannot be generalized to pediatric populations at children’s hospitals without cautious reevaluation.
A second concern with increasing use of observation stays is selection bias in public reporting and comparisons of hospital performance. Presently, four main categories of quality indicators established by the Agency for Healthcare Research and Quality rely heavily on inpatient encounters.36 In this study, we found that the range of hospital-level use of observation stays was large. In 2019, the risk-adjusted percent use of observation stays was less than 5% at three hospitals, while the percent use was greater than 60% in another three hospitals. Therefore, comparisons made without uniform accounting of observation stays might have significant implications for national rankings of children’s hospitals across the United States. These consequences have been investigated in several published studies.22,23,37-39
There are several limitations to our study. First, the study sample was limited to children’s hospitals that consistently reported inpatient and observation data over the entire study period. Eighteen hospitals (86%) excluded from this study did not consistently submit inpatient and observation data to PHIS from 2010 through 2019. The primary purpose of this study was to present temporal trends of observation stays for children’s hospitals, and it was important to build the hospital cohort based on valid and consistent data during the study period. Appendix Table 4 presents differences of hospital characteristics by included and excluded groups of hospitals. Excluded hospitals might have fewer resources (eg, fewer pediatric intensive care beds). Nonetheless, the selection of hospitals was optimized based on data availability. Second, this study was a retrospective review of an administrative database of children’s hospitals and units. The sample does not represent all children’s hospitals or pediatric patients in the United States, but there are no available data sources—that we know of—that can generate national estimates for both inpatient and observation stays. Third, we did not attempt to conclusively infer any causal effects, and several factors could explain the increasing trends, such as reimbursement policies, hospital-level implementation strategies, determination guidelines for observation status designation, as well as changes in clinical care. Further studies should investigate impact of these factors on the use of observation stays for pediatric patients and children’s hospitals.
CONCLUSION
Observation status has been increasingly used for pediatric patients with more diverse clinical conditions, and there is a rising trend of prolonged LOS among observation stays since 2010. Considerable variation exists in hospital-level use of observation stays across children’s hospitals. Observation status could be an opportunity to improve efficiency of healthcare resource use or could lead to a financial risk for patients with prolonged LOS. Future studies should explore appropriateness of observation care in clinical practice through leveraging efficient care and alleviating financial risk.
1. Centers for Medicare & Medicaid Services. Fact Sheet: Two-Midnight Rule. Accessed April 11, 2021. https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0
2. BlueCross BlueShield of Rhode Island. Payment Policy Outpaient Observation. Accessed April 11, 2021. https://www.bcbsri.com/sites/default/files/polices/Outpatient-Observation.pdf
3. Blue Cross Blue Shield of Illinois. Observation Services Tool for Applying MCG Care Guidelines Clinical Payment and Coding Policy. Accessed April 11, 2021. https://www.bcbsil.com/pdf/standards/observation_services_cpcp.pdf
4. Medicare.gov. Inpatient or outpatient hospital status affects your costs. Accessed April 11, 2021. https://www.medicare.gov/what-medicare-covers/what-part-a-covers/inpatient-or-outpatient-hospital-status
5. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. https://doi.org/10.1377/hlthaff.2013.0662
6. Baugh CW, Venkatesh AK, Hilton JA, Samuel PA, Schuur JD, Bohan JS. Making greater use of dedicated hospital observation units for many short-stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314-2323. https://doi.org/10.1377/hlthaff.2011.0926
7. Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991-1998. https://doi.org/10.1001/jamainternmed.2013.8185
8. Baugh CW, Schuur JD. Observation care—high-value care or a cost-shifting loophole? N Engl J Med. 2013;369(4):302-305. https://doi.org/10.1056/NEJMp1304493
9. Missouri Hospital Association. A patient’s guide to observation care. Accessed April 11, 2021. https://www.mhanet.com/mhaimages/PatientsGuideToObservationCareFlyer.pdf
10. Cigna. Employee-paid hospital care coverage- summary of benefits. Accessed April 11, 2021. https://www.cigna.com/iwov-resources/national-second-sale/docs/healthy-benefits/updated-HC-benefit-summary.pdf
11. BlueCross BlueShield of Minnesota. Reimbursement policy-observation care services. Accessed April 11, 2021. https://www.bluecrossmn.com/sites/default/files/DAM/2020-07/Evaluation%20and%20Management%20004_Observation%20Care%20Services%20_09.04.17.pdf
12. California Department of Health Care Services. Public Hospital Project Frequently Asked Questions. Accessed April 11, 2021. https://www.dhcs.ca.gov/provgovpart/Documents/Public%20Hospital%20Project/PHP_Final_FAQs_January2013ADA.pdf
13. Texas Medicaid & Healthcare Partnership. Inpatient and Outpatient Hospital Servicces Handbook. Accessed May 29, 2021. https://www.tmhp.com/sites/default/files/microsites/provider-manuals/tmppm/html/TMPPM/2_Inpatient_Outpatient_Hosp_Srvs/2_Inpatient_Outpatient_Hosp_Srvs.htm
14. Alabama Medicaid. Outpatient observation. Accessed April 11, 2021. https://medicaid.alabama.gov/news_detail.aspx?ID=5121
15. NC Medicaid. Medicaid and Health Choice Clinical Coverage Policy No: 2A-1. Accessed April 11, 2021. https://files.nc.gov/ncdma/documents/files/2A-1_0.pdf
16. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. https://doi.org/10.1377/hlthaff.2012.0129
17. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. https://doi.org/10.1377/hlthaff.2014.1474
18. Wright B, Jung HY, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. Health Serv Res. 2014;49(4):1088-1107. https://doi.org/10.1111/1475-6773.12166
19. Sabbatini AK, Wright B, Hall MK, Basu A. The cost of observation care for commercially insured patients visiting the emergency department. Am J Emerg Med. 2018;36(9):1591-1596. https://doi.org/10.1016/j.ajem.2018.01.040
20. Children’s Hospital Association. Pediatric health information system. Accessed April 11, 2021. https://www.childrenshospitals.org/phis
21. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
22. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst DC, Macy ML.Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
23. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
24. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
25. Macy ML, Kim CS, Sasson C, Lozon MM, Davis MM. Pediatric observation units in the United States: a systematic review. J Hosp Med. 2010;5(3):172-182. https://doi.org/10.1002/jhm.592
26. UnitedHealthcare. Observation services policy, facility. Accessed April 11, 2021. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/medicaid-comm-plan-reimbursement/UHCCP-Facility-Observation-Services-Policy-(F7106).pdf
27. Cal SB-1076§1253.7. General acute care hospitals: observation services – Health and Safety. Accessed April 11, 2021. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520160SB1076
28. Nebraska Total Care. 2021 Provider Billing Guide. Accessed April 11, 2021. https://www.nebraskatotalcare.com/content/dam/centene/Nebraska/PDFs/ProviderRelations/NTC_Nebraska_Total_Care_Provider_Billing_Guide_508.pdf
29. Macy ML, Hall M, Shah SS, et al. Differences in designations of observation care in US freestanding children’s hospitals: are they virtual or real? J Hosp Med. 2012;7(4):287-293. https://doi.org/10.1002/jhm.949
30. Hockenberry JM, Mutter R, Barrett M, Parlato J, Ross MA. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. https://doi.org/10.1111/1475-6773.12143
31. Anthem BlueCross BlueShield. Ohio Provider Manual. Accessed April11, 2021. https://www11.anthem.com/provider/oh/f1/s0/t0/pw_g357368.pdf?refer=ahpprovider&state=oh
32. Humana. Provider manual for physicians, hospitals and healthcare providers. Accessed April 11, 2021. https://docushare-web.apps.cf.humana.com/Marketing/docushare-app?file=3932669
33. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058 https://doi.org/10.1542/peds.2012-249
34. Tejedor-Sojo J. Observation status-a name at what cost? Hosp Pediatr. 2014;4(5):321-323. https://doi.org/10.1542/hpeds.2014-0037.
35. Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health Aff (Millwood). 2015;34(12):2147-2150. https://doi.org/10.1377/hlthaff.2015.0706
36. Agency for Healthcare Research and Quality. AHRQ Quality Indicators. Accessed April 11, 2021. https://www.qualityindicators.ahrq.gov
37. Figueroa JF, Burke LG, Zheng J, Orav EJ, Jha AK. Trends in hospitalization vs observation stay for ambulatory care-sensitive conditions. JAMA Intern Med. 2019;179(12):1714-1716. https://doi.org/10.1001/jamainternmed.2019.3177
38. Markham JL, Hall M, Gay JC, Bettenhausen JL, Berry JG. Length of stay and cost of pediatric readmissions. Pediatrics. 2018;141(4):e20172934. https://doi.org/10.1542/peds.2017-2934.
39. Overman RA, Freburger JK, Assimon MM, Li X, Brookhart, MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. https://doi.org/10.1002/pds.3647.
1. Centers for Medicare & Medicaid Services. Fact Sheet: Two-Midnight Rule. Accessed April 11, 2021. https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0
2. BlueCross BlueShield of Rhode Island. Payment Policy Outpaient Observation. Accessed April 11, 2021. https://www.bcbsri.com/sites/default/files/polices/Outpatient-Observation.pdf
3. Blue Cross Blue Shield of Illinois. Observation Services Tool for Applying MCG Care Guidelines Clinical Payment and Coding Policy. Accessed April 11, 2021. https://www.bcbsil.com/pdf/standards/observation_services_cpcp.pdf
4. Medicare.gov. Inpatient or outpatient hospital status affects your costs. Accessed April 11, 2021. https://www.medicare.gov/what-medicare-covers/what-part-a-covers/inpatient-or-outpatient-hospital-status
5. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. https://doi.org/10.1377/hlthaff.2013.0662
6. Baugh CW, Venkatesh AK, Hilton JA, Samuel PA, Schuur JD, Bohan JS. Making greater use of dedicated hospital observation units for many short-stay patients could save $3.1 billion a year. Health Aff (Millwood). 2012;31(10):2314-2323. https://doi.org/10.1377/hlthaff.2011.0926
7. Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center. JAMA Intern Med. 2013;173(21):1991-1998. https://doi.org/10.1001/jamainternmed.2013.8185
8. Baugh CW, Schuur JD. Observation care—high-value care or a cost-shifting loophole? N Engl J Med. 2013;369(4):302-305. https://doi.org/10.1056/NEJMp1304493
9. Missouri Hospital Association. A patient’s guide to observation care. Accessed April 11, 2021. https://www.mhanet.com/mhaimages/PatientsGuideToObservationCareFlyer.pdf
10. Cigna. Employee-paid hospital care coverage- summary of benefits. Accessed April 11, 2021. https://www.cigna.com/iwov-resources/national-second-sale/docs/healthy-benefits/updated-HC-benefit-summary.pdf
11. BlueCross BlueShield of Minnesota. Reimbursement policy-observation care services. Accessed April 11, 2021. https://www.bluecrossmn.com/sites/default/files/DAM/2020-07/Evaluation%20and%20Management%20004_Observation%20Care%20Services%20_09.04.17.pdf
12. California Department of Health Care Services. Public Hospital Project Frequently Asked Questions. Accessed April 11, 2021. https://www.dhcs.ca.gov/provgovpart/Documents/Public%20Hospital%20Project/PHP_Final_FAQs_January2013ADA.pdf
13. Texas Medicaid & Healthcare Partnership. Inpatient and Outpatient Hospital Servicces Handbook. Accessed May 29, 2021. https://www.tmhp.com/sites/default/files/microsites/provider-manuals/tmppm/html/TMPPM/2_Inpatient_Outpatient_Hosp_Srvs/2_Inpatient_Outpatient_Hosp_Srvs.htm
14. Alabama Medicaid. Outpatient observation. Accessed April 11, 2021. https://medicaid.alabama.gov/news_detail.aspx?ID=5121
15. NC Medicaid. Medicaid and Health Choice Clinical Coverage Policy No: 2A-1. Accessed April 11, 2021. https://files.nc.gov/ncdma/documents/files/2A-1_0.pdf
16. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. https://doi.org/10.1377/hlthaff.2012.0129
17. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. https://doi.org/10.1377/hlthaff.2014.1474
18. Wright B, Jung HY, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. Health Serv Res. 2014;49(4):1088-1107. https://doi.org/10.1111/1475-6773.12166
19. Sabbatini AK, Wright B, Hall MK, Basu A. The cost of observation care for commercially insured patients visiting the emergency department. Am J Emerg Med. 2018;36(9):1591-1596. https://doi.org/10.1016/j.ajem.2018.01.040
20. Children’s Hospital Association. Pediatric health information system. Accessed April 11, 2021. https://www.childrenshospitals.org/phis
21. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9):602-608. https://doi.org/10.12788/jhm.2948
22. Gay JC, Hall M, Morse R, Fieldston ES, Synhorst DC, Macy ML.Observation encounters and length of stay benchmarking in children’s hospitals. Pediatrics. 2020;146(5):e20200120. https://doi.org/10.1542/peds.2020-0120
23. Synhorst DC, Hall M, Harris M, et al. Hospital observation status and readmission rates. Pediatrics. 2020;146(5):e2020003954. https://doi.org/10.1542/peds.2020-003954
24. Macy ML, Hall M, Shah SS, et al. Pediatric observation status: are we overlooking a growing population in children’s hospitals? J Hosp Med. 2012;7(7):530-536. https://doi.org/10.1002/jhm.1923
25. Macy ML, Kim CS, Sasson C, Lozon MM, Davis MM. Pediatric observation units in the United States: a systematic review. J Hosp Med. 2010;5(3):172-182. https://doi.org/10.1002/jhm.592
26. UnitedHealthcare. Observation services policy, facility. Accessed April 11, 2021. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/medicaid-comm-plan-reimbursement/UHCCP-Facility-Observation-Services-Policy-(F7106).pdf
27. Cal SB-1076§1253.7. General acute care hospitals: observation services – Health and Safety. Accessed April 11, 2021. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201520160SB1076
28. Nebraska Total Care. 2021 Provider Billing Guide. Accessed April 11, 2021. https://www.nebraskatotalcare.com/content/dam/centene/Nebraska/PDFs/ProviderRelations/NTC_Nebraska_Total_Care_Provider_Billing_Guide_508.pdf
29. Macy ML, Hall M, Shah SS, et al. Differences in designations of observation care in US freestanding children’s hospitals: are they virtual or real? J Hosp Med. 2012;7(4):287-293. https://doi.org/10.1002/jhm.949
30. Hockenberry JM, Mutter R, Barrett M, Parlato J, Ross MA. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. https://doi.org/10.1111/1475-6773.12143
31. Anthem BlueCross BlueShield. Ohio Provider Manual. Accessed April11, 2021. https://www11.anthem.com/provider/oh/f1/s0/t0/pw_g357368.pdf?refer=ahpprovider&state=oh
32. Humana. Provider manual for physicians, hospitals and healthcare providers. Accessed April 11, 2021. https://docushare-web.apps.cf.humana.com/Marketing/docushare-app?file=3932669
33. Fieldston ES, Shah SS, Hall M, et al. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131(6):1050-1058 https://doi.org/10.1542/peds.2012-249
34. Tejedor-Sojo J. Observation status-a name at what cost? Hosp Pediatr. 2014;4(5):321-323. https://doi.org/10.1542/hpeds.2014-0037.
35. Selden TM, Karaca Z, Keenan P, White C, Kronick R. The growing difference between public and private payment rates for inpatient hospital care. Health Aff (Millwood). 2015;34(12):2147-2150. https://doi.org/10.1377/hlthaff.2015.0706
36. Agency for Healthcare Research and Quality. AHRQ Quality Indicators. Accessed April 11, 2021. https://www.qualityindicators.ahrq.gov
37. Figueroa JF, Burke LG, Zheng J, Orav EJ, Jha AK. Trends in hospitalization vs observation stay for ambulatory care-sensitive conditions. JAMA Intern Med. 2019;179(12):1714-1716. https://doi.org/10.1001/jamainternmed.2019.3177
38. Markham JL, Hall M, Gay JC, Bettenhausen JL, Berry JG. Length of stay and cost of pediatric readmissions. Pediatrics. 2018;141(4):e20172934. https://doi.org/10.1542/peds.2017-2934.
39. Overman RA, Freburger JK, Assimon MM, Li X, Brookhart, MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. https://doi.org/10.1002/pds.3647.
© 2021 Society of Hospital Medicine
Inpatient Glycemic Control With Sliding Scale Insulin in Noncritical Patients With Type 2 Diabetes: Who Can Slide?
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.
Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).
Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.
HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).
DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.
Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).
Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.
HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).
DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.
Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).
Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.
HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).
DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
© 2021 Society of Hospital Medicine
Identifying the Sickest During Triage: Using Point-of-Care Severity Scores to Predict Prognosis in Emergency Department Patients With Suspected Sepsis
Sepsis is the leading cause of in-hospital mortality in the United States.1 Sepsis is present on admission in 85% of cases, and each hour delay in antibiotic treatment is associated with 4% to 7% increased odds of mortality.2,3 Prompt identification and treatment of sepsis is essential for reducing morbidity and mortality, but identifying sepsis during triage is challenging.2
Risk stratification scores that rely solely on data readily available at the bedside have been developed to quickly identify those at greatest risk of poor outcomes from sepsis in real time. The quick Sequential Organ Failure Assessment (qSOFA) score, the National Early Warning System (NEWS2), and the Shock Index are easy-to-calculate measures that use routinely collected clinical data that are not subject to laboratory delay. These scores can be incorporated into electronic health record (EHR)-based alerts and can be calculated longitudinally to track the risk of poor outcomes over time. qSOFA was developed to quantify patient risk at bedside in non-intensive care unit (ICU) settings, but there is no consensus about its ability to predict adverse outcomes such as mortality and ICU admission.4-6 The United Kingdom’s National Health Service uses NEWS2 to identify patients at risk for sepsis.7 NEWS has been shown to have similar or better sensitivity in identifying poorer outcomes in sepsis patients compared with systemic inflammatory response syndrome (SIRS) criteria and qSOFA.4,8-11 However, since the latest update of NEWS2 in 2017, there has been little study of its predictive ability. The Shock Index is a simple bedside score (heart rate divided by systolic blood pressure) that was developed to detect changes in cardiovascular performance before systemic shock onset. Although it was not developed for infection and has not been regularly applied in the sepsis literature, the Shock Index might be useful for identifying patients at increased risk of poor outcomes. Patients with higher and sustained Shock Index scores are more likely to experience morbidity, such as hyperlactatemia, vasopressor use, and organ failure, and also have an increased risk of mortality.12-14
Although the predictive abilities of these bedside risk stratification scores have been assessed individually using standard binary cut-points, the comparative performance of qSOFA, the Shock Index, and NEWS2 has not been evaluated in patients presenting to an emergency department (ED) with suspected sepsis.
METHODS
Design and Setting
We conducted a retrospective cohort study of ED patients who presented with suspected sepsis to the University of California San Francisco (UCSF) Helen Diller Medical Center at Parnassus Heights between June 1, 2012, and December 31, 2018. Our institution is a 785-bed academic teaching hospital with approximately 30,000 ED encounters per year. The study was approved with a waiver of informed consent by the UCSF Human Research Protection Program.
Participants
We use an Epic-based EHR platform (Epic 2017, Epic Systems Corporation) for clinical care, which was implemented on June 1, 2012. All data elements were obtained from Clarity, the relational database that stores Epic’s inpatient data. The study included encounters for patients age ≥18 years who had blood cultures ordered within 24 hours of ED presentation and administration of intravenous antibiotics within 24 hours. Repeat encounters were treated independently in our analysis.
Outcomes and Measures
We compared the ability of qSOFA, the Shock Index, and NEWS2 to predict in-hospital mortality and admission to the ICU from the ED (ED-to-ICU admission). We used the
We compared demographic and clinical characteristics of patients who were positive for qSOFA, the Shock Index, and NEWS2. Demographic data were extracted from the EHR and included primary language, age, sex, and insurance status. All International Classification of Diseases (ICD)-9/10 diagnosis codes were pulled from Clarity billing tables. We used the Elixhauser comorbidity groupings19 of ICD-9/10 codes present on admission to identify preexisting comorbidities and underlying organ dysfunction. To estimate burden of comorbid illnesses, we calculated the validated van Walraven comorbidity index,20 which provides an estimated risk of in-hospital death based on documented Elixhauser comorbidities. Admission level of care (acute, stepdown, or intensive care) was collected for inpatient admissions to assess initial illness severity.21 We also evaluated discharge disposition and in-hospital mortality. Index blood culture results were collected, and dates and timestamps of mechanical ventilation, fluid, vasopressor, and antibiotic administration were obtained for the duration of the encounter.
UCSF uses an automated, real-time, algorithm-based severe sepsis alert that is triggered when a patient meets ≥2 SIRS criteria and again when the patient meets severe sepsis or septic shock criteria (ie, ≥2 SIRS criteria in addition to end-organ dysfunction and/or fluid nonresponsive hypotension). This sepsis screening alert was in use for the duration of our study.22
Statistical Analysis
We performed a subgroup analysis among those who were diagnosed with sepsis, according to the 2016 Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria.
All statistical analyses were conducted using Stata 14 (StataCorp). We summarized differences in demographic and clinical characteristics among the populations meeting each severity score but elected not to conduct hypothesis testing because patients could be positive for one or more scores. We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each score to predict in-hospital mortality and ED-to-ICU admission. To allow comparison with other studies, we also created a composite outcome of either in-hospital mortality or ED-to-ICU admission.
RESULTS
Within our sample 23,837 ED patients had blood cultures ordered within 24 hours of ED presentation and were considered to have suspected sepsis. The mean age of the cohort was 60.8 years, and 1,612 (6.8%) had positive blood cultures. A total of 12,928 patients (54.2%) were found to have sepsis. We documented 1,427 in-hospital deaths (6.0%) and 3,149 (13.2%) ED-to-ICU admissions. At ED triage 1,921 (8.1%) were qSOFA-positive, 4,273 (17.9%) were Shock Index-positive, and 11,832 (49.6%) were NEWS2-positive. At ED triage, blood pressure, heart rate, respiratory rate, and oxygen saturated were documented in >99% of patients, 93.5% had temperature documented, and 28.5% had GCS recorded. If the window of assessment was widened to 1 hour, GCS was only documented among 44.2% of those with suspected sepsis.
Demographic Characteristics and Clinical Course
qSOFA-positive patients received antibiotics more quickly than those who were Shock Index-positive or NEWS2-positive (median 1.5, 1.8, and 2.8 hours after admission, respectively). In addition, those who were qSOFA-positive were more likely to have a positive blood culture (10.9%, 9.4%, and 8.5%, respectively) and to receive an EHR-based diagnosis of sepsis (77.0%, 69.6%, and 60.9%, respectively) than those who were Shock Index- or NEWS2-positive. Those who were qSOFA-positive also were more likely to be mechanically ventilated during their hospital stay (25.4%, 19.2%, and 10.8%, respectively) and to receive vasopressors (33.5%, 22.5%, and 12.2%, respectively). In-hospital mortality also was more common among those who were qSOFA-positive at triage (23.4%, 15.3%, and 9.2%, respectively).
Because both qSOFA and NEWS2 incorporate GCS, we explored baseline characteristics of patients with GCS documented at triage (n = 6,794). These patients were older (median age 63 and 61 years, P < .0001), more likely to be male (54.9% and 53.4%, P = .0031), more likely to have renal failure (22.8% and 20.1%, P < .0001), more likely to have liver disease (14.2% and 12.8%, P = .006), had a higher van Walraven comorbidity score on presentation (median 10 and 8, P < .0001), and were more likely to go directly to the ICU from the ED (20.2% and 10.6%, P < .0001). However, among the 6,397 GCS scores documented at triage, only 1,579 (24.7%) were abnormal.
Test Characteristics of qSOFA, Shock Index, and NEWS2 for Predicting In-hospital Mortality and ED-to-ICU Admission
Among 23,837 patients with suspected sepsis, NEWS2 had the highest sensitivity for predicting in-hospital mortality (76.0%; 95% CI, 73.7%-78.2%) and ED-to-ICU admission (78.9%; 95% CI, 77.5%-80.4%) but had the lowest specificity for in-hospital mortality (52.0%; 95% CI, 51.4%-52.7%) and for ED-to-ICU admission (54.8%; 95% CI, 54.1%-55.5%) (Table 3). qSOFA had the lowest sensitivity for in-hospital mortality (31.5%; 95% CI, 29.1%-33.9%) and ED-to-ICU admission (29.3%; 95% CI, 27.7%-30.9%) but the highest specificity for in-hospital mortality (93.4%; 95% CI, 93.1%-93.8%) and ED-to-ICU admission (95.2%; 95% CI, 94.9%-95.5%). The Shock Index had a sensitivity that fell between qSOFA and NEWS2 for in-hospital mortality (45.8%; 95% CI, 43.2%-48.5%) and ED-to-ICU admission (49.2%; 95% CI, 47.5%-51.0%). The specificity of the Shock Index also was between qSOFA and NEWS2 for in-hospital mortality (83.9%; 95% CI, 83.4%-84.3%) and ED-to-ICU admission (86.8%; 95% CI, 86.4%-87.3%). All three scores exhibited relatively low PPV, ranging from 9.2% to 23.4% for in-hospital mortality and 21.0% to 48.0% for ED-to-ICU triage. Conversely, all three scores exhibited relatively high NPV, ranging from 95.5% to 97.1% for in-hospital mortality and 89.8% to 94.5% for ED-to-ICU triage.
When considering a binary cutoff, the Shock Index exhibited the highest AUROC for in-hospital mortality (0.648; 95% CI, 0.635-0.662) and had a significantly higher AUROC than qSOFA (AUROC, 0.625; 95% CI, 0.612-0.637; P = .0005), but there was no difference compared with NEWS2 (AUROC, 0.640; 95% CI, 0.628-0.652; P = .2112). NEWS2 had a significantly higher AUROC than qSOFA for predicting in-hospital mortality (P = .0227). The Shock Index also exhibited the highest AUROC for ED-to-ICU admission (0.680; 95% CI, 0.617-0.689), which was significantly higher than the AUROC for qSOFA (P < .0001) and NEWS2 (P = 0.0151). NEWS2 had a significantly higher AUROC than qSOFA for predicting ED-to-ICU admission (P < .0001). Similar findings were seen in patients found to have sepsis.
DISCUSSION
In this retrospective cohort study of 23,837 patients who presented to the ED with suspected sepsis, the standard qSOFA threshold was met least frequently, followed by the Shock Index and NEWS2. NEWS2 had the highest sensitivity but the lowest specificity for predicting in-hospital mortality and ED-to-ICU admission, making it a challenging bedside risk stratification scale for identifying patients at risk of poor clinical outcomes. When comparing predictive performance among the three scales, qSOFA had the highest specificity and the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission in this cohort of patients with suspected sepsis. These trends in sensitivity, specificity, and AUROC were consistent among those who met EHR criteria for a sepsis diagnosis. In the analysis of the three scoring systems using all available cut-points, qSOFA and NEWS2 had the highest AUROCs, followed by the Shock Index.
Considering the rapid progression from organ dysfunction to death in sepsis patients, as well as the difficulty establishing a sepsis diagnosis at triage,23 providers must quickly identify patients at increased risk of poor outcomes when they present to the ED. Sepsis alerts often are built using SIRS criteria,27 including the one used for sepsis surveillance at UCSF since 2012,22 but the white blood cell count criterion is subject to a laboratory lag and could lead to a delay in identification. Implementation of a point-of-care bedside score alert that uses readily available clinical data could allow providers to identify patients at greatest risk of poor outcomes immediately at ED presentation and triage, which motivated us to explore the predictive performance of qSOFA, the Shock Index, and NEWS2.
Our study is the first to provide a head-to-head comparison of the predictive performance of qSOFA, the Shock Index, and NEWS2, three easy-to-calculate bedside risk scores that use EHR data collected among patients with suspected sepsis. The Sepsis-3 guidelines recommend qSOFA to quickly identify non-ICU patients at greatest risk of poor outcomes because the measure exhibited predictive performance similar to the more extensive SOFA score outside the ICU.16,23 Although some studies have confirmed qSOFA’s high predictive performance,28-31 our test characteristics and AUROC findings are in line with other published analyses.4,6,10,17 The UK National Health Service is using NEWS2 to screen for patients at risk of poor outcomes from sepsis. Several analyses that assessed the predictive ability of NEWS have reported estimates in line with our findings.4,10,32 The Shock Index was introduced in 1967 and provided a metric to evaluate hemodynamic stability based on heart rate and systolic blood pressure.33 The Shock Index has been studied in several contexts, including sepsis,34 and studies show that a sustained Shock Index is associated with increased odds of vasopressor administration, higher prevalence of hyperlactatemia, and increased risk of poor outcomes in the ICU.13,14
For our study, we were particularly interested in exploring how the Shock Index would compare with more frequently used severity scores such as qSOFA and NEWS2 among patients with suspected sepsis, given the simplicity of its calculation and the easy availability of required data. In our cohort of 23,837 patients, only 159 people had missing blood pressure and only 71 had omitted heart rate. In contrast, both qSOFA and NEWS2 include an assessment of level of consciousness that can be subject to variability in assessment methods and EHR documentation across institutions.11 In our cohort, GCS within 30 minutes of ED presentation was missing in 72 patients, which could have led to incomplete calculation of qSOFA and NEWS2 if a missing value was not actually within normal limits.
Several investigations relate qSOFA to NEWS but few compare qSOFA with the newer NEWS2, and even fewer evaluate the Shock Index with any of these scores.10,11,18,29,35-37 In general, studies have shown that NEWS exhibits a higher AUROC for predicting mortality, sepsis with organ dysfunction, and ICU admission, often as a composite outcome.4,11,18,37,38 A handful of studies compare the Shock Index to SIRS; however, little has been done to compare the Shock Index to qSOFA or NEWS2, scores that have been used specifically for sepsis and might be more predictive of poor outcomes than SIRS.33 In our study, the Shock Index had a higher AUROC than either qSOFA or NEWS2 for predicting in-hospital mortality and ED-to-ICU admission measured as separate outcomes and as a composite outcome using standard cut-points for these scores.
When selecting a severity score to apply in an institution, it is important to carefully evaluate the score’s test characteristics, in addition to considering the availability of reliable data. Tests with high sensitivity and NPV for the population being studied can be useful to rule out disease or risk of poor outcome, while tests with high specificity and PPV can be useful to rule in disease or risk of poor outcome.39 When considering specificity, qSOFA’s performance was superior to the Shock Index and NEWS2 in our study, but a small percentage of the population was identified using a cut-point of qSOFA ≥2. If we used qSOFA and applied this standard cut-point at our institution, we could be confident that those identified were at increased risk, but we would miss a significant number of patients who would experience a poor outcome. When considering sensitivity, performance of NEWS2 was superior to qSOFA and the Shock Index in our study, but one-half of the population was identified using a cut-point of NEWS2 ≥5. If we were to apply this standard NEWS2 cut-point at our institution, we would assume that one-half of our population was at risk, which might drive resource use towards patients who will not experience a poor outcome. Although none of the scores exhibited a robust AUROC measure, the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission when using the standard binary cut-point, and its sensitivity and specificity is between that of qSOFA and NEWS2, potentially making it a score to use in settings where qSOFA and NEWS2 score components, such as altered mentation, are not reliably collected. Finally, our sensitivity analysis varying the binary cut-point of each score within our population demonstrated that the standard cut-points might not be as useful within a specific population and might need to be tailored for implementation, balancing sensitivity, specificity, PPV, and NPV to meet local priorities and ICU capacity.
Our study has limitations. It is a single-center, retrospective analysis, factors that could reduce generalizability. However, it does include a large and diverse patient population spanning several years. Missing GCS data could have affected the predictive ability of qSOFA and NEWS2 in our cohort. We could not reliably perform imputation of GCS because of the high missingness and therefore we assumed missing was normal, as was done in the Sepsis-3 derivation studies.16 Previous studies have attempted to impute GCS and have not observed improved performance of qSOFA to predict mortality.40 Because manually collected variables such as GCS are less reliably documented in the EHR, there might be limitations in their use for triage risk scores.
Although the current analysis focused on the predictive performance of qSOFA, the Shock Index, and NEWS2 at triage, performance of these scores could affect the ED team’s treatment decisions before handoff to the hospitalist team and the expected level of care the patient will receive after in-patient admission. These tests also have the advantage of being easy to calculate at the bedside over time, which could provide an objective assessment of longitudinal predicted prognosis.
CONCLUSION
Local priorities should drive selection of a screening tool, balancing sensitivity, specificity, PPV, and NPV to achieve the institution’s goals. qSOFA, Shock Index, and NEWS2 are risk stratification tools that can be easily implemented at ED triage using data available at the bedside. Although none of these scores performed strongly when comparing AUROCs, qSOFA was highly specific for identifying patients with poor outcomes, and NEWS2 was the most sensitive for ruling out those at high risk among patients with suspected sepsis. The Shock Index exhibited a sensitivity and specificity that fell between qSOFA and NEWS2 and also might be considered to identify those at increased risk, given its ease of implementation, particularly in settings where altered mentation is unreliably or inconsistently documented.
Acknowledgment
The authors thank the UCSF Division of Hospital Medicine Data Core for their assistance with data acquisition.
1. Jones SL, Ashton CM, Kiehne LB, et al. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54(3):303-310. https://doi.org/10.1097/MLR.0000000000000481
2. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058
3. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. https://doi.org/10.1097/01.CCM.0000217961.75225.E9
4. Churpek MM, Snyder A, Sokol S, Pettit NN, Edelson DP. Investigating the impact of different suspicion of infection criteria on the accuracy of Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores. Crit Care Med. 2017;45(11):1805-1812. https://doi.org/10.1097/CCM.0000000000002648
5. Abdullah SMOB, Sørensen RH, Dessau RBC, Sattar SMRU, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
6. Kim KS, Suh GJ, Kim K, et al. Quick Sepsis-related Organ Failure Assessment score is not sensitive enough to predict 28-day mortality in emergency department patients with sepsis: a retrospective review. Clin Exp Emerg Med. 2019;6(1):77-83. HTTPS://DOI.ORG/ 10.15441/ceem.17.294
7. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Royal College of Physicians; 2017.
8. Brink A, Alsma J, Verdonschot RJCG, et al. Predicting mortality in patients with suspected sepsis at the emergency department: a retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One. 2019;14(1):e0211133. https://doi.org/ 10.1371/journal.pone.0211133
9. Redfern OC, Smith GB, Prytherch DR, Meredith P, Inada-Kim M, Schmidt PE. A comparison of the Quick Sequential (Sepsis-Related) Organ Failure Assessment Score and the National Early Warning Score in non-ICU patients with/without infection. Crit Care Med. 2018;46(12):1923-1933. https://doi.org/10.1097/CCM.0000000000003359
10. Churpek MM, Snyder A, Han X, et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195(7):906-911. https://doi.org/10.1164/rccm.201604-0854OC
11. Goulden R, Hoyle MC, Monis J, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35(6):345-349. https://doi.org/10.1136/emermed-2017-207120
12. Biney I, Shepherd A, Thomas J, Mehari A. Shock Index and outcomes in patients admitted to the ICU with sepsis. Chest. 2015;148(suppl 4):337A. https://doi.org/https://doi.org/10.1378/chest.2281151
13. Wira CR, Francis MW, Bhat S, Ehrman R, Conner D, Siegel M. The shock index as a predictor of vasopressor use in emergency department patients with severe sepsis. West J Emerg Med. 2014;15(1):60-66. https://doi.org/10.5811/westjem.2013.7.18472
14. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14(2):168-174. https://doi.org/10.5811/westjem.2012.8.11546
15. Middleton DJ, Smith TO, Bedford R, Neilly M, Myint PK. Shock Index predicts outcome in patients with suspected sepsis or community-acquired pneumonia: a systematic review. J Clin Med. 2019;8(8):1144. https://doi.org/10.3390/jcm8081144
16. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/ 10.1001/jama.2016.0288
17. Abdullah S, Sørensen RH, Dessau RBC, Sattar S, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
18. Usman OA, Usman AA, Ward MA. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am J Emerg Med. 2018;37(8):1490-1497. https://doi.org/10.1016/j.ajem.2018.10.058
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
20. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5
21. Prin M, Wunsch H. The role of stepdown beds in hospital care. Am J Respir Crit Care Med. 2014;190(11):1210-1216. https://doi.org/10.1164/rccm.201406-1117PP
22. Narayanan N, Gross AK, Pintens M, Fee C, MacDougall C. Effect of an electronic medical record alert for severe sepsis among ED patients. Am J Emerg Med. 2016;34(2):185-188. https://doi.org/10.1016/j.ajem.2015.10.005
23. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. https://doi.org/10.1001/jama.2016.0287
24. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. https://doi.org/10.1001/jama.2017.13836
25. Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran). 2016;4(2):111-113.
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
27. Kangas C, Iverson L, Pierce D. Sepsis screening: combining Early Warning Scores and SIRS Criteria. Clin Nurs Res. 2021;30(1):42-49. https://doi.org/10.1177/1054773818823334.
28. Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of Sepsis-3 Criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301-308. https://doi.org/10.1001/jama.2016.20329
29. Finkelsztein EJ, Jones DS, Ma KC, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care. 2017;21(1):73. https://doi.org/10.1186/s13054-017-1658-5
30. Canet E, Taylor DM, Khor R, Krishnan V, Bellomo R. qSOFA as predictor of mortality and prolonged ICU admission in Emergency Department patients with suspected infection. J Crit Care. 2018;48:118-123. https://doi.org/10.1016/j.jcrc.2018.08.022
31. Anand V, Zhang Z, Kadri SS, Klompas M, Rhee C; CDC Prevention Epicenters Program. Epidemiology of Quick Sequential Organ Failure Assessment criteria in undifferentiated patients and association with suspected infection and sepsis. Chest. 2019;156(2):289-297. https://doi.org/10.1016/j.chest.2019.03.032
32. Hamilton F, Arnold D, Baird A, Albur M, Whiting P. Early Warning Scores do not accurately predict mortality in sepsis: A meta-analysis and systematic review of the literature. J Infect. 2018;76(3):241-248. https://doi.org/10.1016/j.jinf.2018.01.002
33. Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock Index in the emergency department: utility and limitations. Open Access Emerg Med. 2019;11:179-199. https://doi.org/10.2147/OAEM.S178358
34. Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012;67(4):406-411.
35. Siddiqui S, Chua M, Kumaresh V, Choo R. A comparison of pre ICU admission SIRS, EWS and q SOFA scores for predicting mortality and length of stay in ICU. J Crit Care. 2017;41:191-193. https://doi.org/10.1016/j.jcrc.2017.05.017
36. Costa RT, Nassar AP, Caruso P. Accuracy of SOFA, qSOFA, and SIRS scores for mortality in cancer patients admitted to an intensive care unit with suspected infection. J Crit Care. 2018;45:52-57. https://doi.org/10.1016/j.jcrc.2017.12.024
37. Mellhammar L, Linder A, Tverring J, et al. NEWS2 is Superior to qSOFA in detecting sepsis with organ dysfunction in the emergency department. J Clin Med. 2019;8(8):1128. https://doi.org/10.3390/jcm8081128
38. Szakmany T, Pugh R, Kopczynska M, et al. Defining sepsis on the wards: results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia. 2018;73(2):195-204. https://doi.org/10.1111/anae.14062
39. Newman TB, Kohn MA. Evidence-Based Diagnosis: An Introduction to Clinical Epidemiology. Cambridge University Press; 2009.
40. Askim Å, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med. 2017;25(1):56. https://doi.org/10.1186/s13049-017-0399-4
Sepsis is the leading cause of in-hospital mortality in the United States.1 Sepsis is present on admission in 85% of cases, and each hour delay in antibiotic treatment is associated with 4% to 7% increased odds of mortality.2,3 Prompt identification and treatment of sepsis is essential for reducing morbidity and mortality, but identifying sepsis during triage is challenging.2
Risk stratification scores that rely solely on data readily available at the bedside have been developed to quickly identify those at greatest risk of poor outcomes from sepsis in real time. The quick Sequential Organ Failure Assessment (qSOFA) score, the National Early Warning System (NEWS2), and the Shock Index are easy-to-calculate measures that use routinely collected clinical data that are not subject to laboratory delay. These scores can be incorporated into electronic health record (EHR)-based alerts and can be calculated longitudinally to track the risk of poor outcomes over time. qSOFA was developed to quantify patient risk at bedside in non-intensive care unit (ICU) settings, but there is no consensus about its ability to predict adverse outcomes such as mortality and ICU admission.4-6 The United Kingdom’s National Health Service uses NEWS2 to identify patients at risk for sepsis.7 NEWS has been shown to have similar or better sensitivity in identifying poorer outcomes in sepsis patients compared with systemic inflammatory response syndrome (SIRS) criteria and qSOFA.4,8-11 However, since the latest update of NEWS2 in 2017, there has been little study of its predictive ability. The Shock Index is a simple bedside score (heart rate divided by systolic blood pressure) that was developed to detect changes in cardiovascular performance before systemic shock onset. Although it was not developed for infection and has not been regularly applied in the sepsis literature, the Shock Index might be useful for identifying patients at increased risk of poor outcomes. Patients with higher and sustained Shock Index scores are more likely to experience morbidity, such as hyperlactatemia, vasopressor use, and organ failure, and also have an increased risk of mortality.12-14
Although the predictive abilities of these bedside risk stratification scores have been assessed individually using standard binary cut-points, the comparative performance of qSOFA, the Shock Index, and NEWS2 has not been evaluated in patients presenting to an emergency department (ED) with suspected sepsis.
METHODS
Design and Setting
We conducted a retrospective cohort study of ED patients who presented with suspected sepsis to the University of California San Francisco (UCSF) Helen Diller Medical Center at Parnassus Heights between June 1, 2012, and December 31, 2018. Our institution is a 785-bed academic teaching hospital with approximately 30,000 ED encounters per year. The study was approved with a waiver of informed consent by the UCSF Human Research Protection Program.
Participants
We use an Epic-based EHR platform (Epic 2017, Epic Systems Corporation) for clinical care, which was implemented on June 1, 2012. All data elements were obtained from Clarity, the relational database that stores Epic’s inpatient data. The study included encounters for patients age ≥18 years who had blood cultures ordered within 24 hours of ED presentation and administration of intravenous antibiotics within 24 hours. Repeat encounters were treated independently in our analysis.
Outcomes and Measures
We compared the ability of qSOFA, the Shock Index, and NEWS2 to predict in-hospital mortality and admission to the ICU from the ED (ED-to-ICU admission). We used the
We compared demographic and clinical characteristics of patients who were positive for qSOFA, the Shock Index, and NEWS2. Demographic data were extracted from the EHR and included primary language, age, sex, and insurance status. All International Classification of Diseases (ICD)-9/10 diagnosis codes were pulled from Clarity billing tables. We used the Elixhauser comorbidity groupings19 of ICD-9/10 codes present on admission to identify preexisting comorbidities and underlying organ dysfunction. To estimate burden of comorbid illnesses, we calculated the validated van Walraven comorbidity index,20 which provides an estimated risk of in-hospital death based on documented Elixhauser comorbidities. Admission level of care (acute, stepdown, or intensive care) was collected for inpatient admissions to assess initial illness severity.21 We also evaluated discharge disposition and in-hospital mortality. Index blood culture results were collected, and dates and timestamps of mechanical ventilation, fluid, vasopressor, and antibiotic administration were obtained for the duration of the encounter.
UCSF uses an automated, real-time, algorithm-based severe sepsis alert that is triggered when a patient meets ≥2 SIRS criteria and again when the patient meets severe sepsis or septic shock criteria (ie, ≥2 SIRS criteria in addition to end-organ dysfunction and/or fluid nonresponsive hypotension). This sepsis screening alert was in use for the duration of our study.22
Statistical Analysis
We performed a subgroup analysis among those who were diagnosed with sepsis, according to the 2016 Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria.
All statistical analyses were conducted using Stata 14 (StataCorp). We summarized differences in demographic and clinical characteristics among the populations meeting each severity score but elected not to conduct hypothesis testing because patients could be positive for one or more scores. We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each score to predict in-hospital mortality and ED-to-ICU admission. To allow comparison with other studies, we also created a composite outcome of either in-hospital mortality or ED-to-ICU admission.
RESULTS
Within our sample 23,837 ED patients had blood cultures ordered within 24 hours of ED presentation and were considered to have suspected sepsis. The mean age of the cohort was 60.8 years, and 1,612 (6.8%) had positive blood cultures. A total of 12,928 patients (54.2%) were found to have sepsis. We documented 1,427 in-hospital deaths (6.0%) and 3,149 (13.2%) ED-to-ICU admissions. At ED triage 1,921 (8.1%) were qSOFA-positive, 4,273 (17.9%) were Shock Index-positive, and 11,832 (49.6%) were NEWS2-positive. At ED triage, blood pressure, heart rate, respiratory rate, and oxygen saturated were documented in >99% of patients, 93.5% had temperature documented, and 28.5% had GCS recorded. If the window of assessment was widened to 1 hour, GCS was only documented among 44.2% of those with suspected sepsis.
Demographic Characteristics and Clinical Course
qSOFA-positive patients received antibiotics more quickly than those who were Shock Index-positive or NEWS2-positive (median 1.5, 1.8, and 2.8 hours after admission, respectively). In addition, those who were qSOFA-positive were more likely to have a positive blood culture (10.9%, 9.4%, and 8.5%, respectively) and to receive an EHR-based diagnosis of sepsis (77.0%, 69.6%, and 60.9%, respectively) than those who were Shock Index- or NEWS2-positive. Those who were qSOFA-positive also were more likely to be mechanically ventilated during their hospital stay (25.4%, 19.2%, and 10.8%, respectively) and to receive vasopressors (33.5%, 22.5%, and 12.2%, respectively). In-hospital mortality also was more common among those who were qSOFA-positive at triage (23.4%, 15.3%, and 9.2%, respectively).
Because both qSOFA and NEWS2 incorporate GCS, we explored baseline characteristics of patients with GCS documented at triage (n = 6,794). These patients were older (median age 63 and 61 years, P < .0001), more likely to be male (54.9% and 53.4%, P = .0031), more likely to have renal failure (22.8% and 20.1%, P < .0001), more likely to have liver disease (14.2% and 12.8%, P = .006), had a higher van Walraven comorbidity score on presentation (median 10 and 8, P < .0001), and were more likely to go directly to the ICU from the ED (20.2% and 10.6%, P < .0001). However, among the 6,397 GCS scores documented at triage, only 1,579 (24.7%) were abnormal.
Test Characteristics of qSOFA, Shock Index, and NEWS2 for Predicting In-hospital Mortality and ED-to-ICU Admission
Among 23,837 patients with suspected sepsis, NEWS2 had the highest sensitivity for predicting in-hospital mortality (76.0%; 95% CI, 73.7%-78.2%) and ED-to-ICU admission (78.9%; 95% CI, 77.5%-80.4%) but had the lowest specificity for in-hospital mortality (52.0%; 95% CI, 51.4%-52.7%) and for ED-to-ICU admission (54.8%; 95% CI, 54.1%-55.5%) (Table 3). qSOFA had the lowest sensitivity for in-hospital mortality (31.5%; 95% CI, 29.1%-33.9%) and ED-to-ICU admission (29.3%; 95% CI, 27.7%-30.9%) but the highest specificity for in-hospital mortality (93.4%; 95% CI, 93.1%-93.8%) and ED-to-ICU admission (95.2%; 95% CI, 94.9%-95.5%). The Shock Index had a sensitivity that fell between qSOFA and NEWS2 for in-hospital mortality (45.8%; 95% CI, 43.2%-48.5%) and ED-to-ICU admission (49.2%; 95% CI, 47.5%-51.0%). The specificity of the Shock Index also was between qSOFA and NEWS2 for in-hospital mortality (83.9%; 95% CI, 83.4%-84.3%) and ED-to-ICU admission (86.8%; 95% CI, 86.4%-87.3%). All three scores exhibited relatively low PPV, ranging from 9.2% to 23.4% for in-hospital mortality and 21.0% to 48.0% for ED-to-ICU triage. Conversely, all three scores exhibited relatively high NPV, ranging from 95.5% to 97.1% for in-hospital mortality and 89.8% to 94.5% for ED-to-ICU triage.
When considering a binary cutoff, the Shock Index exhibited the highest AUROC for in-hospital mortality (0.648; 95% CI, 0.635-0.662) and had a significantly higher AUROC than qSOFA (AUROC, 0.625; 95% CI, 0.612-0.637; P = .0005), but there was no difference compared with NEWS2 (AUROC, 0.640; 95% CI, 0.628-0.652; P = .2112). NEWS2 had a significantly higher AUROC than qSOFA for predicting in-hospital mortality (P = .0227). The Shock Index also exhibited the highest AUROC for ED-to-ICU admission (0.680; 95% CI, 0.617-0.689), which was significantly higher than the AUROC for qSOFA (P < .0001) and NEWS2 (P = 0.0151). NEWS2 had a significantly higher AUROC than qSOFA for predicting ED-to-ICU admission (P < .0001). Similar findings were seen in patients found to have sepsis.
DISCUSSION
In this retrospective cohort study of 23,837 patients who presented to the ED with suspected sepsis, the standard qSOFA threshold was met least frequently, followed by the Shock Index and NEWS2. NEWS2 had the highest sensitivity but the lowest specificity for predicting in-hospital mortality and ED-to-ICU admission, making it a challenging bedside risk stratification scale for identifying patients at risk of poor clinical outcomes. When comparing predictive performance among the three scales, qSOFA had the highest specificity and the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission in this cohort of patients with suspected sepsis. These trends in sensitivity, specificity, and AUROC were consistent among those who met EHR criteria for a sepsis diagnosis. In the analysis of the three scoring systems using all available cut-points, qSOFA and NEWS2 had the highest AUROCs, followed by the Shock Index.
Considering the rapid progression from organ dysfunction to death in sepsis patients, as well as the difficulty establishing a sepsis diagnosis at triage,23 providers must quickly identify patients at increased risk of poor outcomes when they present to the ED. Sepsis alerts often are built using SIRS criteria,27 including the one used for sepsis surveillance at UCSF since 2012,22 but the white blood cell count criterion is subject to a laboratory lag and could lead to a delay in identification. Implementation of a point-of-care bedside score alert that uses readily available clinical data could allow providers to identify patients at greatest risk of poor outcomes immediately at ED presentation and triage, which motivated us to explore the predictive performance of qSOFA, the Shock Index, and NEWS2.
Our study is the first to provide a head-to-head comparison of the predictive performance of qSOFA, the Shock Index, and NEWS2, three easy-to-calculate bedside risk scores that use EHR data collected among patients with suspected sepsis. The Sepsis-3 guidelines recommend qSOFA to quickly identify non-ICU patients at greatest risk of poor outcomes because the measure exhibited predictive performance similar to the more extensive SOFA score outside the ICU.16,23 Although some studies have confirmed qSOFA’s high predictive performance,28-31 our test characteristics and AUROC findings are in line with other published analyses.4,6,10,17 The UK National Health Service is using NEWS2 to screen for patients at risk of poor outcomes from sepsis. Several analyses that assessed the predictive ability of NEWS have reported estimates in line with our findings.4,10,32 The Shock Index was introduced in 1967 and provided a metric to evaluate hemodynamic stability based on heart rate and systolic blood pressure.33 The Shock Index has been studied in several contexts, including sepsis,34 and studies show that a sustained Shock Index is associated with increased odds of vasopressor administration, higher prevalence of hyperlactatemia, and increased risk of poor outcomes in the ICU.13,14
For our study, we were particularly interested in exploring how the Shock Index would compare with more frequently used severity scores such as qSOFA and NEWS2 among patients with suspected sepsis, given the simplicity of its calculation and the easy availability of required data. In our cohort of 23,837 patients, only 159 people had missing blood pressure and only 71 had omitted heart rate. In contrast, both qSOFA and NEWS2 include an assessment of level of consciousness that can be subject to variability in assessment methods and EHR documentation across institutions.11 In our cohort, GCS within 30 minutes of ED presentation was missing in 72 patients, which could have led to incomplete calculation of qSOFA and NEWS2 if a missing value was not actually within normal limits.
Several investigations relate qSOFA to NEWS but few compare qSOFA with the newer NEWS2, and even fewer evaluate the Shock Index with any of these scores.10,11,18,29,35-37 In general, studies have shown that NEWS exhibits a higher AUROC for predicting mortality, sepsis with organ dysfunction, and ICU admission, often as a composite outcome.4,11,18,37,38 A handful of studies compare the Shock Index to SIRS; however, little has been done to compare the Shock Index to qSOFA or NEWS2, scores that have been used specifically for sepsis and might be more predictive of poor outcomes than SIRS.33 In our study, the Shock Index had a higher AUROC than either qSOFA or NEWS2 for predicting in-hospital mortality and ED-to-ICU admission measured as separate outcomes and as a composite outcome using standard cut-points for these scores.
When selecting a severity score to apply in an institution, it is important to carefully evaluate the score’s test characteristics, in addition to considering the availability of reliable data. Tests with high sensitivity and NPV for the population being studied can be useful to rule out disease or risk of poor outcome, while tests with high specificity and PPV can be useful to rule in disease or risk of poor outcome.39 When considering specificity, qSOFA’s performance was superior to the Shock Index and NEWS2 in our study, but a small percentage of the population was identified using a cut-point of qSOFA ≥2. If we used qSOFA and applied this standard cut-point at our institution, we could be confident that those identified were at increased risk, but we would miss a significant number of patients who would experience a poor outcome. When considering sensitivity, performance of NEWS2 was superior to qSOFA and the Shock Index in our study, but one-half of the population was identified using a cut-point of NEWS2 ≥5. If we were to apply this standard NEWS2 cut-point at our institution, we would assume that one-half of our population was at risk, which might drive resource use towards patients who will not experience a poor outcome. Although none of the scores exhibited a robust AUROC measure, the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission when using the standard binary cut-point, and its sensitivity and specificity is between that of qSOFA and NEWS2, potentially making it a score to use in settings where qSOFA and NEWS2 score components, such as altered mentation, are not reliably collected. Finally, our sensitivity analysis varying the binary cut-point of each score within our population demonstrated that the standard cut-points might not be as useful within a specific population and might need to be tailored for implementation, balancing sensitivity, specificity, PPV, and NPV to meet local priorities and ICU capacity.
Our study has limitations. It is a single-center, retrospective analysis, factors that could reduce generalizability. However, it does include a large and diverse patient population spanning several years. Missing GCS data could have affected the predictive ability of qSOFA and NEWS2 in our cohort. We could not reliably perform imputation of GCS because of the high missingness and therefore we assumed missing was normal, as was done in the Sepsis-3 derivation studies.16 Previous studies have attempted to impute GCS and have not observed improved performance of qSOFA to predict mortality.40 Because manually collected variables such as GCS are less reliably documented in the EHR, there might be limitations in their use for triage risk scores.
Although the current analysis focused on the predictive performance of qSOFA, the Shock Index, and NEWS2 at triage, performance of these scores could affect the ED team’s treatment decisions before handoff to the hospitalist team and the expected level of care the patient will receive after in-patient admission. These tests also have the advantage of being easy to calculate at the bedside over time, which could provide an objective assessment of longitudinal predicted prognosis.
CONCLUSION
Local priorities should drive selection of a screening tool, balancing sensitivity, specificity, PPV, and NPV to achieve the institution’s goals. qSOFA, Shock Index, and NEWS2 are risk stratification tools that can be easily implemented at ED triage using data available at the bedside. Although none of these scores performed strongly when comparing AUROCs, qSOFA was highly specific for identifying patients with poor outcomes, and NEWS2 was the most sensitive for ruling out those at high risk among patients with suspected sepsis. The Shock Index exhibited a sensitivity and specificity that fell between qSOFA and NEWS2 and also might be considered to identify those at increased risk, given its ease of implementation, particularly in settings where altered mentation is unreliably or inconsistently documented.
Acknowledgment
The authors thank the UCSF Division of Hospital Medicine Data Core for their assistance with data acquisition.
Sepsis is the leading cause of in-hospital mortality in the United States.1 Sepsis is present on admission in 85% of cases, and each hour delay in antibiotic treatment is associated with 4% to 7% increased odds of mortality.2,3 Prompt identification and treatment of sepsis is essential for reducing morbidity and mortality, but identifying sepsis during triage is challenging.2
Risk stratification scores that rely solely on data readily available at the bedside have been developed to quickly identify those at greatest risk of poor outcomes from sepsis in real time. The quick Sequential Organ Failure Assessment (qSOFA) score, the National Early Warning System (NEWS2), and the Shock Index are easy-to-calculate measures that use routinely collected clinical data that are not subject to laboratory delay. These scores can be incorporated into electronic health record (EHR)-based alerts and can be calculated longitudinally to track the risk of poor outcomes over time. qSOFA was developed to quantify patient risk at bedside in non-intensive care unit (ICU) settings, but there is no consensus about its ability to predict adverse outcomes such as mortality and ICU admission.4-6 The United Kingdom’s National Health Service uses NEWS2 to identify patients at risk for sepsis.7 NEWS has been shown to have similar or better sensitivity in identifying poorer outcomes in sepsis patients compared with systemic inflammatory response syndrome (SIRS) criteria and qSOFA.4,8-11 However, since the latest update of NEWS2 in 2017, there has been little study of its predictive ability. The Shock Index is a simple bedside score (heart rate divided by systolic blood pressure) that was developed to detect changes in cardiovascular performance before systemic shock onset. Although it was not developed for infection and has not been regularly applied in the sepsis literature, the Shock Index might be useful for identifying patients at increased risk of poor outcomes. Patients with higher and sustained Shock Index scores are more likely to experience morbidity, such as hyperlactatemia, vasopressor use, and organ failure, and also have an increased risk of mortality.12-14
Although the predictive abilities of these bedside risk stratification scores have been assessed individually using standard binary cut-points, the comparative performance of qSOFA, the Shock Index, and NEWS2 has not been evaluated in patients presenting to an emergency department (ED) with suspected sepsis.
METHODS
Design and Setting
We conducted a retrospective cohort study of ED patients who presented with suspected sepsis to the University of California San Francisco (UCSF) Helen Diller Medical Center at Parnassus Heights between June 1, 2012, and December 31, 2018. Our institution is a 785-bed academic teaching hospital with approximately 30,000 ED encounters per year. The study was approved with a waiver of informed consent by the UCSF Human Research Protection Program.
Participants
We use an Epic-based EHR platform (Epic 2017, Epic Systems Corporation) for clinical care, which was implemented on June 1, 2012. All data elements were obtained from Clarity, the relational database that stores Epic’s inpatient data. The study included encounters for patients age ≥18 years who had blood cultures ordered within 24 hours of ED presentation and administration of intravenous antibiotics within 24 hours. Repeat encounters were treated independently in our analysis.
Outcomes and Measures
We compared the ability of qSOFA, the Shock Index, and NEWS2 to predict in-hospital mortality and admission to the ICU from the ED (ED-to-ICU admission). We used the
We compared demographic and clinical characteristics of patients who were positive for qSOFA, the Shock Index, and NEWS2. Demographic data were extracted from the EHR and included primary language, age, sex, and insurance status. All International Classification of Diseases (ICD)-9/10 diagnosis codes were pulled from Clarity billing tables. We used the Elixhauser comorbidity groupings19 of ICD-9/10 codes present on admission to identify preexisting comorbidities and underlying organ dysfunction. To estimate burden of comorbid illnesses, we calculated the validated van Walraven comorbidity index,20 which provides an estimated risk of in-hospital death based on documented Elixhauser comorbidities. Admission level of care (acute, stepdown, or intensive care) was collected for inpatient admissions to assess initial illness severity.21 We also evaluated discharge disposition and in-hospital mortality. Index blood culture results were collected, and dates and timestamps of mechanical ventilation, fluid, vasopressor, and antibiotic administration were obtained for the duration of the encounter.
UCSF uses an automated, real-time, algorithm-based severe sepsis alert that is triggered when a patient meets ≥2 SIRS criteria and again when the patient meets severe sepsis or septic shock criteria (ie, ≥2 SIRS criteria in addition to end-organ dysfunction and/or fluid nonresponsive hypotension). This sepsis screening alert was in use for the duration of our study.22
Statistical Analysis
We performed a subgroup analysis among those who were diagnosed with sepsis, according to the 2016 Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria.
All statistical analyses were conducted using Stata 14 (StataCorp). We summarized differences in demographic and clinical characteristics among the populations meeting each severity score but elected not to conduct hypothesis testing because patients could be positive for one or more scores. We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each score to predict in-hospital mortality and ED-to-ICU admission. To allow comparison with other studies, we also created a composite outcome of either in-hospital mortality or ED-to-ICU admission.
RESULTS
Within our sample 23,837 ED patients had blood cultures ordered within 24 hours of ED presentation and were considered to have suspected sepsis. The mean age of the cohort was 60.8 years, and 1,612 (6.8%) had positive blood cultures. A total of 12,928 patients (54.2%) were found to have sepsis. We documented 1,427 in-hospital deaths (6.0%) and 3,149 (13.2%) ED-to-ICU admissions. At ED triage 1,921 (8.1%) were qSOFA-positive, 4,273 (17.9%) were Shock Index-positive, and 11,832 (49.6%) were NEWS2-positive. At ED triage, blood pressure, heart rate, respiratory rate, and oxygen saturated were documented in >99% of patients, 93.5% had temperature documented, and 28.5% had GCS recorded. If the window of assessment was widened to 1 hour, GCS was only documented among 44.2% of those with suspected sepsis.
Demographic Characteristics and Clinical Course
qSOFA-positive patients received antibiotics more quickly than those who were Shock Index-positive or NEWS2-positive (median 1.5, 1.8, and 2.8 hours after admission, respectively). In addition, those who were qSOFA-positive were more likely to have a positive blood culture (10.9%, 9.4%, and 8.5%, respectively) and to receive an EHR-based diagnosis of sepsis (77.0%, 69.6%, and 60.9%, respectively) than those who were Shock Index- or NEWS2-positive. Those who were qSOFA-positive also were more likely to be mechanically ventilated during their hospital stay (25.4%, 19.2%, and 10.8%, respectively) and to receive vasopressors (33.5%, 22.5%, and 12.2%, respectively). In-hospital mortality also was more common among those who were qSOFA-positive at triage (23.4%, 15.3%, and 9.2%, respectively).
Because both qSOFA and NEWS2 incorporate GCS, we explored baseline characteristics of patients with GCS documented at triage (n = 6,794). These patients were older (median age 63 and 61 years, P < .0001), more likely to be male (54.9% and 53.4%, P = .0031), more likely to have renal failure (22.8% and 20.1%, P < .0001), more likely to have liver disease (14.2% and 12.8%, P = .006), had a higher van Walraven comorbidity score on presentation (median 10 and 8, P < .0001), and were more likely to go directly to the ICU from the ED (20.2% and 10.6%, P < .0001). However, among the 6,397 GCS scores documented at triage, only 1,579 (24.7%) were abnormal.
Test Characteristics of qSOFA, Shock Index, and NEWS2 for Predicting In-hospital Mortality and ED-to-ICU Admission
Among 23,837 patients with suspected sepsis, NEWS2 had the highest sensitivity for predicting in-hospital mortality (76.0%; 95% CI, 73.7%-78.2%) and ED-to-ICU admission (78.9%; 95% CI, 77.5%-80.4%) but had the lowest specificity for in-hospital mortality (52.0%; 95% CI, 51.4%-52.7%) and for ED-to-ICU admission (54.8%; 95% CI, 54.1%-55.5%) (Table 3). qSOFA had the lowest sensitivity for in-hospital mortality (31.5%; 95% CI, 29.1%-33.9%) and ED-to-ICU admission (29.3%; 95% CI, 27.7%-30.9%) but the highest specificity for in-hospital mortality (93.4%; 95% CI, 93.1%-93.8%) and ED-to-ICU admission (95.2%; 95% CI, 94.9%-95.5%). The Shock Index had a sensitivity that fell between qSOFA and NEWS2 for in-hospital mortality (45.8%; 95% CI, 43.2%-48.5%) and ED-to-ICU admission (49.2%; 95% CI, 47.5%-51.0%). The specificity of the Shock Index also was between qSOFA and NEWS2 for in-hospital mortality (83.9%; 95% CI, 83.4%-84.3%) and ED-to-ICU admission (86.8%; 95% CI, 86.4%-87.3%). All three scores exhibited relatively low PPV, ranging from 9.2% to 23.4% for in-hospital mortality and 21.0% to 48.0% for ED-to-ICU triage. Conversely, all three scores exhibited relatively high NPV, ranging from 95.5% to 97.1% for in-hospital mortality and 89.8% to 94.5% for ED-to-ICU triage.
When considering a binary cutoff, the Shock Index exhibited the highest AUROC for in-hospital mortality (0.648; 95% CI, 0.635-0.662) and had a significantly higher AUROC than qSOFA (AUROC, 0.625; 95% CI, 0.612-0.637; P = .0005), but there was no difference compared with NEWS2 (AUROC, 0.640; 95% CI, 0.628-0.652; P = .2112). NEWS2 had a significantly higher AUROC than qSOFA for predicting in-hospital mortality (P = .0227). The Shock Index also exhibited the highest AUROC for ED-to-ICU admission (0.680; 95% CI, 0.617-0.689), which was significantly higher than the AUROC for qSOFA (P < .0001) and NEWS2 (P = 0.0151). NEWS2 had a significantly higher AUROC than qSOFA for predicting ED-to-ICU admission (P < .0001). Similar findings were seen in patients found to have sepsis.
DISCUSSION
In this retrospective cohort study of 23,837 patients who presented to the ED with suspected sepsis, the standard qSOFA threshold was met least frequently, followed by the Shock Index and NEWS2. NEWS2 had the highest sensitivity but the lowest specificity for predicting in-hospital mortality and ED-to-ICU admission, making it a challenging bedside risk stratification scale for identifying patients at risk of poor clinical outcomes. When comparing predictive performance among the three scales, qSOFA had the highest specificity and the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission in this cohort of patients with suspected sepsis. These trends in sensitivity, specificity, and AUROC were consistent among those who met EHR criteria for a sepsis diagnosis. In the analysis of the three scoring systems using all available cut-points, qSOFA and NEWS2 had the highest AUROCs, followed by the Shock Index.
Considering the rapid progression from organ dysfunction to death in sepsis patients, as well as the difficulty establishing a sepsis diagnosis at triage,23 providers must quickly identify patients at increased risk of poor outcomes when they present to the ED. Sepsis alerts often are built using SIRS criteria,27 including the one used for sepsis surveillance at UCSF since 2012,22 but the white blood cell count criterion is subject to a laboratory lag and could lead to a delay in identification. Implementation of a point-of-care bedside score alert that uses readily available clinical data could allow providers to identify patients at greatest risk of poor outcomes immediately at ED presentation and triage, which motivated us to explore the predictive performance of qSOFA, the Shock Index, and NEWS2.
Our study is the first to provide a head-to-head comparison of the predictive performance of qSOFA, the Shock Index, and NEWS2, three easy-to-calculate bedside risk scores that use EHR data collected among patients with suspected sepsis. The Sepsis-3 guidelines recommend qSOFA to quickly identify non-ICU patients at greatest risk of poor outcomes because the measure exhibited predictive performance similar to the more extensive SOFA score outside the ICU.16,23 Although some studies have confirmed qSOFA’s high predictive performance,28-31 our test characteristics and AUROC findings are in line with other published analyses.4,6,10,17 The UK National Health Service is using NEWS2 to screen for patients at risk of poor outcomes from sepsis. Several analyses that assessed the predictive ability of NEWS have reported estimates in line with our findings.4,10,32 The Shock Index was introduced in 1967 and provided a metric to evaluate hemodynamic stability based on heart rate and systolic blood pressure.33 The Shock Index has been studied in several contexts, including sepsis,34 and studies show that a sustained Shock Index is associated with increased odds of vasopressor administration, higher prevalence of hyperlactatemia, and increased risk of poor outcomes in the ICU.13,14
For our study, we were particularly interested in exploring how the Shock Index would compare with more frequently used severity scores such as qSOFA and NEWS2 among patients with suspected sepsis, given the simplicity of its calculation and the easy availability of required data. In our cohort of 23,837 patients, only 159 people had missing blood pressure and only 71 had omitted heart rate. In contrast, both qSOFA and NEWS2 include an assessment of level of consciousness that can be subject to variability in assessment methods and EHR documentation across institutions.11 In our cohort, GCS within 30 minutes of ED presentation was missing in 72 patients, which could have led to incomplete calculation of qSOFA and NEWS2 if a missing value was not actually within normal limits.
Several investigations relate qSOFA to NEWS but few compare qSOFA with the newer NEWS2, and even fewer evaluate the Shock Index with any of these scores.10,11,18,29,35-37 In general, studies have shown that NEWS exhibits a higher AUROC for predicting mortality, sepsis with organ dysfunction, and ICU admission, often as a composite outcome.4,11,18,37,38 A handful of studies compare the Shock Index to SIRS; however, little has been done to compare the Shock Index to qSOFA or NEWS2, scores that have been used specifically for sepsis and might be more predictive of poor outcomes than SIRS.33 In our study, the Shock Index had a higher AUROC than either qSOFA or NEWS2 for predicting in-hospital mortality and ED-to-ICU admission measured as separate outcomes and as a composite outcome using standard cut-points for these scores.
When selecting a severity score to apply in an institution, it is important to carefully evaluate the score’s test characteristics, in addition to considering the availability of reliable data. Tests with high sensitivity and NPV for the population being studied can be useful to rule out disease or risk of poor outcome, while tests with high specificity and PPV can be useful to rule in disease or risk of poor outcome.39 When considering specificity, qSOFA’s performance was superior to the Shock Index and NEWS2 in our study, but a small percentage of the population was identified using a cut-point of qSOFA ≥2. If we used qSOFA and applied this standard cut-point at our institution, we could be confident that those identified were at increased risk, but we would miss a significant number of patients who would experience a poor outcome. When considering sensitivity, performance of NEWS2 was superior to qSOFA and the Shock Index in our study, but one-half of the population was identified using a cut-point of NEWS2 ≥5. If we were to apply this standard NEWS2 cut-point at our institution, we would assume that one-half of our population was at risk, which might drive resource use towards patients who will not experience a poor outcome. Although none of the scores exhibited a robust AUROC measure, the Shock Index had the highest AUROC for in-hospital mortality and ED-to-ICU admission when using the standard binary cut-point, and its sensitivity and specificity is between that of qSOFA and NEWS2, potentially making it a score to use in settings where qSOFA and NEWS2 score components, such as altered mentation, are not reliably collected. Finally, our sensitivity analysis varying the binary cut-point of each score within our population demonstrated that the standard cut-points might not be as useful within a specific population and might need to be tailored for implementation, balancing sensitivity, specificity, PPV, and NPV to meet local priorities and ICU capacity.
Our study has limitations. It is a single-center, retrospective analysis, factors that could reduce generalizability. However, it does include a large and diverse patient population spanning several years. Missing GCS data could have affected the predictive ability of qSOFA and NEWS2 in our cohort. We could not reliably perform imputation of GCS because of the high missingness and therefore we assumed missing was normal, as was done in the Sepsis-3 derivation studies.16 Previous studies have attempted to impute GCS and have not observed improved performance of qSOFA to predict mortality.40 Because manually collected variables such as GCS are less reliably documented in the EHR, there might be limitations in their use for triage risk scores.
Although the current analysis focused on the predictive performance of qSOFA, the Shock Index, and NEWS2 at triage, performance of these scores could affect the ED team’s treatment decisions before handoff to the hospitalist team and the expected level of care the patient will receive after in-patient admission. These tests also have the advantage of being easy to calculate at the bedside over time, which could provide an objective assessment of longitudinal predicted prognosis.
CONCLUSION
Local priorities should drive selection of a screening tool, balancing sensitivity, specificity, PPV, and NPV to achieve the institution’s goals. qSOFA, Shock Index, and NEWS2 are risk stratification tools that can be easily implemented at ED triage using data available at the bedside. Although none of these scores performed strongly when comparing AUROCs, qSOFA was highly specific for identifying patients with poor outcomes, and NEWS2 was the most sensitive for ruling out those at high risk among patients with suspected sepsis. The Shock Index exhibited a sensitivity and specificity that fell between qSOFA and NEWS2 and also might be considered to identify those at increased risk, given its ease of implementation, particularly in settings where altered mentation is unreliably or inconsistently documented.
Acknowledgment
The authors thank the UCSF Division of Hospital Medicine Data Core for their assistance with data acquisition.
1. Jones SL, Ashton CM, Kiehne LB, et al. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54(3):303-310. https://doi.org/10.1097/MLR.0000000000000481
2. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058
3. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. https://doi.org/10.1097/01.CCM.0000217961.75225.E9
4. Churpek MM, Snyder A, Sokol S, Pettit NN, Edelson DP. Investigating the impact of different suspicion of infection criteria on the accuracy of Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores. Crit Care Med. 2017;45(11):1805-1812. https://doi.org/10.1097/CCM.0000000000002648
5. Abdullah SMOB, Sørensen RH, Dessau RBC, Sattar SMRU, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
6. Kim KS, Suh GJ, Kim K, et al. Quick Sepsis-related Organ Failure Assessment score is not sensitive enough to predict 28-day mortality in emergency department patients with sepsis: a retrospective review. Clin Exp Emerg Med. 2019;6(1):77-83. HTTPS://DOI.ORG/ 10.15441/ceem.17.294
7. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Royal College of Physicians; 2017.
8. Brink A, Alsma J, Verdonschot RJCG, et al. Predicting mortality in patients with suspected sepsis at the emergency department: a retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One. 2019;14(1):e0211133. https://doi.org/ 10.1371/journal.pone.0211133
9. Redfern OC, Smith GB, Prytherch DR, Meredith P, Inada-Kim M, Schmidt PE. A comparison of the Quick Sequential (Sepsis-Related) Organ Failure Assessment Score and the National Early Warning Score in non-ICU patients with/without infection. Crit Care Med. 2018;46(12):1923-1933. https://doi.org/10.1097/CCM.0000000000003359
10. Churpek MM, Snyder A, Han X, et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195(7):906-911. https://doi.org/10.1164/rccm.201604-0854OC
11. Goulden R, Hoyle MC, Monis J, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35(6):345-349. https://doi.org/10.1136/emermed-2017-207120
12. Biney I, Shepherd A, Thomas J, Mehari A. Shock Index and outcomes in patients admitted to the ICU with sepsis. Chest. 2015;148(suppl 4):337A. https://doi.org/https://doi.org/10.1378/chest.2281151
13. Wira CR, Francis MW, Bhat S, Ehrman R, Conner D, Siegel M. The shock index as a predictor of vasopressor use in emergency department patients with severe sepsis. West J Emerg Med. 2014;15(1):60-66. https://doi.org/10.5811/westjem.2013.7.18472
14. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14(2):168-174. https://doi.org/10.5811/westjem.2012.8.11546
15. Middleton DJ, Smith TO, Bedford R, Neilly M, Myint PK. Shock Index predicts outcome in patients with suspected sepsis or community-acquired pneumonia: a systematic review. J Clin Med. 2019;8(8):1144. https://doi.org/10.3390/jcm8081144
16. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/ 10.1001/jama.2016.0288
17. Abdullah S, Sørensen RH, Dessau RBC, Sattar S, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
18. Usman OA, Usman AA, Ward MA. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am J Emerg Med. 2018;37(8):1490-1497. https://doi.org/10.1016/j.ajem.2018.10.058
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
20. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5
21. Prin M, Wunsch H. The role of stepdown beds in hospital care. Am J Respir Crit Care Med. 2014;190(11):1210-1216. https://doi.org/10.1164/rccm.201406-1117PP
22. Narayanan N, Gross AK, Pintens M, Fee C, MacDougall C. Effect of an electronic medical record alert for severe sepsis among ED patients. Am J Emerg Med. 2016;34(2):185-188. https://doi.org/10.1016/j.ajem.2015.10.005
23. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. https://doi.org/10.1001/jama.2016.0287
24. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. https://doi.org/10.1001/jama.2017.13836
25. Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran). 2016;4(2):111-113.
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
27. Kangas C, Iverson L, Pierce D. Sepsis screening: combining Early Warning Scores and SIRS Criteria. Clin Nurs Res. 2021;30(1):42-49. https://doi.org/10.1177/1054773818823334.
28. Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of Sepsis-3 Criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301-308. https://doi.org/10.1001/jama.2016.20329
29. Finkelsztein EJ, Jones DS, Ma KC, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care. 2017;21(1):73. https://doi.org/10.1186/s13054-017-1658-5
30. Canet E, Taylor DM, Khor R, Krishnan V, Bellomo R. qSOFA as predictor of mortality and prolonged ICU admission in Emergency Department patients with suspected infection. J Crit Care. 2018;48:118-123. https://doi.org/10.1016/j.jcrc.2018.08.022
31. Anand V, Zhang Z, Kadri SS, Klompas M, Rhee C; CDC Prevention Epicenters Program. Epidemiology of Quick Sequential Organ Failure Assessment criteria in undifferentiated patients and association with suspected infection and sepsis. Chest. 2019;156(2):289-297. https://doi.org/10.1016/j.chest.2019.03.032
32. Hamilton F, Arnold D, Baird A, Albur M, Whiting P. Early Warning Scores do not accurately predict mortality in sepsis: A meta-analysis and systematic review of the literature. J Infect. 2018;76(3):241-248. https://doi.org/10.1016/j.jinf.2018.01.002
33. Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock Index in the emergency department: utility and limitations. Open Access Emerg Med. 2019;11:179-199. https://doi.org/10.2147/OAEM.S178358
34. Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012;67(4):406-411.
35. Siddiqui S, Chua M, Kumaresh V, Choo R. A comparison of pre ICU admission SIRS, EWS and q SOFA scores for predicting mortality and length of stay in ICU. J Crit Care. 2017;41:191-193. https://doi.org/10.1016/j.jcrc.2017.05.017
36. Costa RT, Nassar AP, Caruso P. Accuracy of SOFA, qSOFA, and SIRS scores for mortality in cancer patients admitted to an intensive care unit with suspected infection. J Crit Care. 2018;45:52-57. https://doi.org/10.1016/j.jcrc.2017.12.024
37. Mellhammar L, Linder A, Tverring J, et al. NEWS2 is Superior to qSOFA in detecting sepsis with organ dysfunction in the emergency department. J Clin Med. 2019;8(8):1128. https://doi.org/10.3390/jcm8081128
38. Szakmany T, Pugh R, Kopczynska M, et al. Defining sepsis on the wards: results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia. 2018;73(2):195-204. https://doi.org/10.1111/anae.14062
39. Newman TB, Kohn MA. Evidence-Based Diagnosis: An Introduction to Clinical Epidemiology. Cambridge University Press; 2009.
40. Askim Å, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med. 2017;25(1):56. https://doi.org/10.1186/s13049-017-0399-4
1. Jones SL, Ashton CM, Kiehne LB, et al. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care. 2016;54(3):303-310. https://doi.org/10.1097/MLR.0000000000000481
2. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. https://doi.org/10.1056/NEJMoa1703058
3. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596. https://doi.org/10.1097/01.CCM.0000217961.75225.E9
4. Churpek MM, Snyder A, Sokol S, Pettit NN, Edelson DP. Investigating the impact of different suspicion of infection criteria on the accuracy of Quick Sepsis-Related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores. Crit Care Med. 2017;45(11):1805-1812. https://doi.org/10.1097/CCM.0000000000002648
5. Abdullah SMOB, Sørensen RH, Dessau RBC, Sattar SMRU, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
6. Kim KS, Suh GJ, Kim K, et al. Quick Sepsis-related Organ Failure Assessment score is not sensitive enough to predict 28-day mortality in emergency department patients with sepsis: a retrospective review. Clin Exp Emerg Med. 2019;6(1):77-83. HTTPS://DOI.ORG/ 10.15441/ceem.17.294
7. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Royal College of Physicians; 2017.
8. Brink A, Alsma J, Verdonschot RJCG, et al. Predicting mortality in patients with suspected sepsis at the emergency department: a retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One. 2019;14(1):e0211133. https://doi.org/ 10.1371/journal.pone.0211133
9. Redfern OC, Smith GB, Prytherch DR, Meredith P, Inada-Kim M, Schmidt PE. A comparison of the Quick Sequential (Sepsis-Related) Organ Failure Assessment Score and the National Early Warning Score in non-ICU patients with/without infection. Crit Care Med. 2018;46(12):1923-1933. https://doi.org/10.1097/CCM.0000000000003359
10. Churpek MM, Snyder A, Han X, et al. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for detecting clinical deterioration in infected patients outside the intensive care unit. Am J Respir Crit Care Med. 2017;195(7):906-911. https://doi.org/10.1164/rccm.201604-0854OC
11. Goulden R, Hoyle MC, Monis J, et al. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35(6):345-349. https://doi.org/10.1136/emermed-2017-207120
12. Biney I, Shepherd A, Thomas J, Mehari A. Shock Index and outcomes in patients admitted to the ICU with sepsis. Chest. 2015;148(suppl 4):337A. https://doi.org/https://doi.org/10.1378/chest.2281151
13. Wira CR, Francis MW, Bhat S, Ehrman R, Conner D, Siegel M. The shock index as a predictor of vasopressor use in emergency department patients with severe sepsis. West J Emerg Med. 2014;15(1):60-66. https://doi.org/10.5811/westjem.2013.7.18472
14. Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14(2):168-174. https://doi.org/10.5811/westjem.2012.8.11546
15. Middleton DJ, Smith TO, Bedford R, Neilly M, Myint PK. Shock Index predicts outcome in patients with suspected sepsis or community-acquired pneumonia: a systematic review. J Clin Med. 2019;8(8):1144. https://doi.org/10.3390/jcm8081144
16. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774. https://doi.org/ 10.1001/jama.2016.0288
17. Abdullah S, Sørensen RH, Dessau RBC, Sattar S, Wiese L, Nielsen FE. Prognostic accuracy of qSOFA in predicting 28-day mortality among infected patients in an emergency department: a prospective validation study. Emerg Med J. 2019;36(12):722-728. https://doi.org/10.1136/emermed-2019-208456
18. Usman OA, Usman AA, Ward MA. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am J Emerg Med. 2018;37(8):1490-1497. https://doi.org/10.1016/j.ajem.2018.10.058
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004
20. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5
21. Prin M, Wunsch H. The role of stepdown beds in hospital care. Am J Respir Crit Care Med. 2014;190(11):1210-1216. https://doi.org/10.1164/rccm.201406-1117PP
22. Narayanan N, Gross AK, Pintens M, Fee C, MacDougall C. Effect of an electronic medical record alert for severe sepsis among ED patients. Am J Emerg Med. 2016;34(2):185-188. https://doi.org/10.1016/j.ajem.2015.10.005
23. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. https://doi.org/10.1001/jama.2016.0287
24. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. https://doi.org/10.1001/jama.2017.13836
25. Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg (Tehran). 2016;4(2):111-113.
26. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845.
27. Kangas C, Iverson L, Pierce D. Sepsis screening: combining Early Warning Scores and SIRS Criteria. Clin Nurs Res. 2021;30(1):42-49. https://doi.org/10.1177/1054773818823334.
28. Freund Y, Lemachatti N, Krastinova E, et al. Prognostic accuracy of Sepsis-3 Criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317(3):301-308. https://doi.org/10.1001/jama.2016.20329
29. Finkelsztein EJ, Jones DS, Ma KC, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care. 2017;21(1):73. https://doi.org/10.1186/s13054-017-1658-5
30. Canet E, Taylor DM, Khor R, Krishnan V, Bellomo R. qSOFA as predictor of mortality and prolonged ICU admission in Emergency Department patients with suspected infection. J Crit Care. 2018;48:118-123. https://doi.org/10.1016/j.jcrc.2018.08.022
31. Anand V, Zhang Z, Kadri SS, Klompas M, Rhee C; CDC Prevention Epicenters Program. Epidemiology of Quick Sequential Organ Failure Assessment criteria in undifferentiated patients and association with suspected infection and sepsis. Chest. 2019;156(2):289-297. https://doi.org/10.1016/j.chest.2019.03.032
32. Hamilton F, Arnold D, Baird A, Albur M, Whiting P. Early Warning Scores do not accurately predict mortality in sepsis: A meta-analysis and systematic review of the literature. J Infect. 2018;76(3):241-248. https://doi.org/10.1016/j.jinf.2018.01.002
33. Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock Index in the emergency department: utility and limitations. Open Access Emerg Med. 2019;11:179-199. https://doi.org/10.2147/OAEM.S178358
34. Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012;67(4):406-411.
35. Siddiqui S, Chua M, Kumaresh V, Choo R. A comparison of pre ICU admission SIRS, EWS and q SOFA scores for predicting mortality and length of stay in ICU. J Crit Care. 2017;41:191-193. https://doi.org/10.1016/j.jcrc.2017.05.017
36. Costa RT, Nassar AP, Caruso P. Accuracy of SOFA, qSOFA, and SIRS scores for mortality in cancer patients admitted to an intensive care unit with suspected infection. J Crit Care. 2018;45:52-57. https://doi.org/10.1016/j.jcrc.2017.12.024
37. Mellhammar L, Linder A, Tverring J, et al. NEWS2 is Superior to qSOFA in detecting sepsis with organ dysfunction in the emergency department. J Clin Med. 2019;8(8):1128. https://doi.org/10.3390/jcm8081128
38. Szakmany T, Pugh R, Kopczynska M, et al. Defining sepsis on the wards: results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia. 2018;73(2):195-204. https://doi.org/10.1111/anae.14062
39. Newman TB, Kohn MA. Evidence-Based Diagnosis: An Introduction to Clinical Epidemiology. Cambridge University Press; 2009.
40. Askim Å, Moser F, Gustad LT, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med. 2017;25(1):56. https://doi.org/10.1186/s13049-017-0399-4
© 2021 Society of Hospital Medicine
Drawing Down From Crisis: More Lessons From a Soldier
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
Last year, I wrote an article for the Journal of Hospital Medicine offering tips to healthcare providers in what was then an expanding COVID-19 environment.1 These lessons were drawn from my experiences during the “tough fights” and crisis situations of my military career, situations similar to what healthcare providers experienced during the pandemic.
Now, as vaccination rates rise and hospitalization rates fall, the nation and healthcare profession begin the transition to “normalcy.” What should healthcare professionals expect as they transition from a year of operating in a crisis to resumption of the habitual? What memories and lessons will linger from a long, tough fight against COVID-19, and how might physicians best approach the many post-crisis challenges they will surely face?
My military experiences inform the tips I offer to those in the medical profession. Both professions depend on adeptly leading and building a functional and effective organizational culture under trying circumstances. It may seem strange, but the challenges healthcare workers (HCWs) faced in fighting COVID-19 are comparable to what soldiers experience on a battlefield. And now, as citizens return to “normal” (however normal is defined), only naïve HCWs will believe they can simply resume their previous habits and practices. This part of the journey will present new challenges and unique opportunities.
Healthcare has changed…and so have you! Just like soldiers coming home from the battlefield face a necessarily new and different world, HCWs will also face changing circumstances, environments, and organizational requirements. Given this new landscape, I offer some of my lessons learned coming out of combat to help you adapt.
REFLECTIONS
Heading home from my last combat tour in Iraq, I found myself gazing out the aircraft window and pondering my personal experiences during a very long combat tour commanding a multinational task force. Pulling out my green soldier’s notebook, I rapidly scratched out some reflections on where I was, what I had learned, and what I needed to address personally and professionally. In talking with physicians in the healthcare organization where I now work, this emotional checklist seems to mirror some of the same thoughts they face coming out of the COVID-19 crisis.
Expect exhaustion. There’s a military axiom that “fatigue can make cowards of us all,” and while I don’t think I had succumbed to cowardice in battle, after 15 months in combat I was exhausted. Commanders in combat—or HCWs fighting a pandemic—face unrelenting demands from a variety of audiences. Leaders are asked to solve unsolvable problems, be at the right place at the right time with the right answers, have more energy than others, be upbeat, and exhibit behaviors that will motivate the “troops.” That’s true even if they’re exhausted and weary to the bone, serving on multiple teams, and attending endless meetings. There is also the common and unfortunate expectation that leaders should not take any time for themselves.
During the pandemic, most HCWs reported sleeping less, having little time to interact casually with others, and having less time for personal reflection, exercise, personal growth, or even prayer. My solution for addressing exhaustion was to develop a personal plan to address each one of these areas—mental, emotional, physical, spiritual—with a detailed rest and recovery strategy. I wrote my plan down, knowing that I would need to discuss this blueprint with both my employer and my spouse, who I suspected would have different ideas on what my schedule should look like after returning “home.” Healthcare providers have been through the same kinds of stresses and need to ask themselves: What recovery plan have I designed to help me overcome the fatigue I feel, and have I talked about this plan with the people who will be affected by it?
Take pride in what your teams accomplished. I was proud of how my teams had accomplished the impossible and how they had adapted to continually changing situations. Whenever military organizations know they’ll face the enemy in combat, they feel heightened anxiety, increased fear, and concern about the preparedness of their team. The Army, like any successful team, attempts to mitigate those emotions through training. During my reflections, I remembered the teams that came together to accomplish very tough missions. Some of those teams were those I had concerns about prior to deployment, but fortunately they often surprised me with their adaptability and successes in combat.
Leaders in healthcare can likely relate. Even in normal situations, organizational fault lines exist between physicians, nurses, and administrators. These fault lines may manifest as communication disconnects and distrust between different members who may not completely trust one another due to differences in training, culture, or role within the organization. But during a crisis, rifts dissipate and trust evolves as different cultures are forced to work together. Many healthcare organizations report that, during the COVID crisis, most personality conflicts, communication disconnects, and organizational dysfunctions receded, and organizations saw more and greater coordination and collaboration. Extensive research on leadership demonstrates that crises drive teams to communicate better and become more effective and efficient in accomplishing stated goals, resulting in team members who relish “being there” for one another like never before. These positive changes must be reinforced to ensure these newly formed high-performing teams do not revert back to work silos, which usually occurs due to distrust.
Just as important as pride in teams is the pride in the accomplishment of specific individuals during times of crisis. Diverse members of any organization deliver some of the best solutions to the toughest problems when they are included in the discussion, allowed to bring their ideas to the table, and rewarded for their actions (and their courage)! Just one example is given by Dr Sasha Shillcut as she describes the innovations and adaptations of the women physicians she observed in her organization during the COVID-19 crisis,2 and there are many examples of other organizations citing similar transformation in areas like telemedicine, emergency department procedures, and equipment design and use.3,4
Anticipate “survivor’s guilt.” During my three combat tours, 253 soldiers under my command or in my organization sacrificed their lives for the mission, and many more were wounded in action. There are times when bad dreams remind me of some of the circumstances surrounding the incidents that took the lives of those who died, and I often wake with a start and in a sweat. The first question I always ask myself in the middle of the night when this happens is, “Why did they die, and why did I survive?” That question is always followed by, “What might I have done differently to prevent those deaths?”
As we draw down from treating patients during the COVID-19 crisis, healthcare providers must also be wary of “survivor’s guilt.” Survivor’s guilt is a strong emotion for anyone who has survived a crisis, especially when their friends or loved ones have not. Healthcare providers have lost many patients, but they have also lost colleagues, friends, and family members. Because you are in the healing profession, many of you will question what more you could have done to prevent the loss of life. You likely won’t ever be completely satisfied with the answer, but I have a recommendation that may assuage your emotions.
In combat, we continually memorialized our fallen comrades in ceremonies that are attended by the entire unit. One of my commanders had an idea to keep pictures of those who had made the ultimate sacrifice, and on my desk is a box with the 253 pictures of those dedicated individuals who were killed in action under my command or in my unit. On the top of the box are the words “Make It Matter.” I look at those pictures often to remember them and their selfless service to the nation, and I often ask myself whether I am “making it matter” in my daily activities. Does your healthcare facility have plans for a memorial service for all those who died while in your care? Is there a special tribute in your hospital to those healthcare providers who paid the ultimate sacrifice in caring for patients? Most importantly, have you rededicated yourself to your profession, knowing that what you learned during the pandemic will help you be a better physician in the future, and do you have the knowledge that you are making a meaningful difference every day you serve in healthcare?
Relish being home. On that flight back to family, my excitement was palpable. But there were challenges too, as I knew I had to continue to focus on my team, my organization, and my profession. While images on the internet often show soldiers returning from war rushing into the arms of their loved ones, soldiers never leave the demands associated with wearing the cloth of the country. As a result, many marriages and families are damaged when one member who has been so singularly focused returns home and is still caught up in the demands of the job. They find it is difficult to pick up where they’ve left off, forgetting their family has also been under a different kind of intense stress.
These same challenges will face HCWs. Many of you voluntarily distanced yourself from family and friends due to a fear of transmitting the disease. Spouses and children underwent traumatic challenges in their jobs, holding together the household and piloting kids through schooling. My biggest recommendation is this: strive for a return to a healthy balance, be wary of any sharp edges that appear in your personality or in your relationships, and be open in communicating with those you love. Relying on friends, counselors, and mentors who can provide trusted advice—as well as therapy, if necessary—is not a sign of weakness, but a sign of strength and courage. The pandemic has affected our lives more than we can imagine, and “coming out” of the crisis will continue to test our humanity and civility like never before. Trust me on this one. I’ve been there.
RECOMMENDATIONS FOR POST-CRISIS ACTIONS
These reflections open us to issues physicians must address in the months after your “redeployment” from dealing with the pandemic. When soldiers redeploy from combat, every unit develops a plan to address personal and professional growth for individual members of the team. Additionally, leaders develop a plan to sustain performance and improve teams and organizational approaches. The objective? Polish the diamond from what we learned during the crisis, while preparing for those things that might detract from effectiveness in future crises. It’s an SOP (standard operating procedure) for military units to do these things. Is this approach also advisable for healthcare professionals and teams in responding to crises?
Crises increase stress on individuals and disrupt the functioning of organizations, but crises also provide phenomenal opportunities for growth.5 Adaptive organizations, be they military or healthcare, must take time to understand how the crises affected people and the organizational framework, while also preparing for potential future disruptions. While HCWs and their respective organizations are usually adept at learning from short-term emergencies (eg, limited disease outbreaks, natural disasters, mass-casualty events), they are less practiced in addressing crises that affect the profession for months. It has been a century since the medical profession has been faced with a global pandemic, but experts suggest other pandemics may be on the short-term horizon.6 We ought to use this past year of experiences to prepare for them.
Pay attention to your personal needs and the conditions of others on your team. After returning from combat, I was exhausted and stressed intellectually, physically, emotionally, and spiritually. From what I’ve seen, healthcare providers fit that same description, and the fatigue is palpable. Many of you have experienced extreme stress. I have experienced extremepost-traumatic stress, and it is important to understand that this will affect some on your team.7 In addition to addressing stress—and this is advice I give to all the physicians I know—find the time to get a physical examination. While the Army requires yearly physicals for all soldiers (especially generals!), most healthcare providers I know are shockingly deficient in taking the time to get a checkup from one of their colleagues. Commit to fixing that.
Reflect on what you have learned during this period. Take an afternoon with an adult beverage (if that’s your style) and reflect on what you learned and what others might learn from your unique experiences. Then, take some notes and shape your ideas. What did you experience? What adaptations did you or your team make during the pandemic? What worked and what didn’t? What things do you want to sustain in your practice and what things do you want to eliminate? What did you learn about the medical arts…or even about your Hippocratic Oath? If you have a mentor, share these thoughts with them; if you don’t have a mentor, find one and then share your thoughts with them. Get some outside feedback.
Assess team strengths and weaknesses. If you’re a formal physician leader (someone with a title and a position on your team), it’s your responsibility to provide feedback on both people and processes. If you’re an informal leader (someone who is a member of the team but doesn’t have specific leadership responsibilities outside your clinical role) and you don’t see this happening, volunteer to run the session for your formal leader and your organization. This session should last several hours and be held in a comfortable setting. You should prepare your team so they aren’t defensive about the points that may arise. Determine strengths and opportunities by asking for feedback on communication, behaviors, medical knowledge, emotional intelligence, and execution of tasks. Determine which processes and systems either worked or didn’t work, and either polish the approaches or drive change to improve systems as you get back to normal. Crises provide an opportunity to fix what’s broken while also reinforcing the things that worked in the crisis that might not be normal procedure. Don’t go back to old ways if those weren’t the things or the approaches you were using under critical conditions.
Encourage completion of an organization-wide after-action review (AAR). As I started writing this article, I watched CNN’s Dr Sanjay Gupta conduct a review of actions with the key physicians who contributed to the last administration’s response to the pandemic. In watching that session—and having conducted hundreds of AARs in my military career—there was discussion of obvious good and bad leadership and management procedures, process issues that needed to be addressed, and decision-making that might be applauded or questioned. Every healthcare organization ought to conduct a similar AAR, with a review of the most important aspects of actions and teamwork, the hospital’s operations, logistical preparation, and leader and organization procedures that demand to be addressed.
The successful conduct of any AAR requires asking (and getting answers to) four questions: What happened?; Why did it happen the way it did?; What needs to be fixed or “polished” in the processes, systems, or leadership approach?; and Who is responsible for ensuring the fixes or adjustments occur? The facilitator (and the key leaders of the organization) must ask the right questions, must be deeply involved in getting the right people to comment on the issues, and must “pin the rose” on someone who will be responsible for carrying through on the fixes. At the end of the AAR, after the key topics are discussed, with a plan for addressing each, the person in charge of the organization must publish an action plan with details for ensuring the fixes.
Like all citizens across our nation, my family is grateful for the skill and professionalism exhibited by clinicians and healthcare providers during this devastating pandemic. While we are all breathing a sigh of relief as we see the end in sight, true professionals must take the opportunity to learn and grow from this crisis and adapt. Hopefully, the reflections and recommendations in this article—things I learned from a different profession—will provide ideas to my new colleagues in healthcare.
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
1. Hertling M. Ten tips for a crisis: lessons from a soldier. J Hosp Med. 2020;15(5): 275-276. https://doi.org/10.12788/jhm.3424
2. Shillcut S. The inspiring women physicians of the COVID-19 pandemic. MedPage Today. April 9, 2020. Accessed July 7, 2021. https://www.kevinmd.com/blog/2020/04/the-insiring-women-physicians-of-the-covid-19-pandemic.html
3. Daley B. Three medical innovations fueled by COVID-19 that will outlast the pandemic. The Conversation. March 9, 2021. Accessed July 7, 2021. https://theconversation.com/3-medical-innovations-fueled-by-covid-19-that-will-outlast-the-pandemic-156464
4. Drees J, Dyrda L, Adams K. Ten big advancements in healthcare tech during the pandemic. Becker’s Health IT. July 6, 2020. Accessed July 7, 2021. https://www.beckershospitalreview.com/digital-transformation/10-big-advancements-in-healthcare-tech-during-the-pandemic.html
5. Wang J. Developing organizational learning capacity in crisis management. Adv Developing Hum Resources. 10(3):425-445. https://doi.org/10.1177/1523422308316464
6. Morens DM, Fauci AS. Emerging pandemic diseases: how we got COVID-19. Cell. 2020;182(5):1077-1092. https://doi.org/10.1016/j.cell.2020.08.021
7. What is posttraumatic stress disorder? American Psychiatric Association. Reviewed August 2020. Accessed July 7, 2021. https://www.psychiatry.org/patients-families/ptsd/what-is-ptsd
© 2021 Society of Hospital Medicine
Preoperative Care Assessment of Need Scores Are Associated With Postoperative Mortality and Length of Stay in Veterans Undergoing Knee Replacement
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
Risk calculators can be of great value in guiding clinical decision making, patient-centered precision medicine, and resource allocation.1 Several perioperative risk prediction models have emerged in recent decades that estimate specific hazards (eg, cardiovascular complications after noncardiac surgery) with varying accuracy and utility. In the perioperative sphere, the time windows are often limited to an index hospitalization or 30 days following surgery or discharge.2-9 Although longer periods are of interest to patients, families, and health systems, few widely used or validated models are designed to look beyond this very narrow window.10,11 In addition, perioperative risk prediction models do not routinely incorporate parameters of a wide variety of health or demographic domains, such as patterns of health care, health care utilization, or medication use.
In 2013, in response to the need for near real-time information to guide delivery of enhanced care management services, the Veterans Health Administration (VHA) Office of Informatics and Analytics developed automated risk prediction models that used detailed electronic health record (EHR) data. These models were used to report Care Assessment Need (CAN) scores each week for all VHA enrollees and include data from a wide array of health domains. These CAN scores predict the risk for hospitalization, death, or either event within 90 days and 1 year.12,13 Each score is reported as both a predicted probability (0-1) and as a percentile in relation to all other VHA enrollees (a value between 1 and 99).13 The data used to calculate CAN scores are listed in Table 1.12
Surgical procedures or admissions would not be differentiated from nonsurgical admissions or other procedural clinic visits, and as such, it is not possible to isolate the effect of undergoing a surgical procedure from another health-related event on the CAN score. At the same time though, a short-term increase in system utilization caused by an elective surgical procedure such as a total knee replacement (TKR) would presumably be reflected in a change in CAN score, but this has not been studied.
Since their introduction, CAN scores have been routinely accessed by primary care teams and used to facilitate care coordination for thousands of VHA patients. However, these CAN scores are currently not available to VHA surgeons, anesthesiologists, or other perioperative clinicians. In this study, we examine the distributions of preoperative CAN scores and explore the relationships of preoperative CAN 1-year mortality scores with 1-year survival following discharge and length of stay (LOS) during index hospitalization in a cohort of US veterans who underwent TKR, the most common elective operation performed within the VHA system.
Methods
Following approval of the Durham Veterans Affairs Medical Center Institutional Review Board, all necessary data were extracted from the VHA Corporate Data Warehouse (CDW) repository.14 Informed consent was waived due to the minimal risk nature of the study.
We used Current Procedural Terminology codes (27438, 27446, 27447, 27486, 27487, 27488) and International Classification of Diseases, 9th edition clinical modification procedure codes (81.54, 81.55, 81.59, 00.80-00.84) to identify all veterans who had undergone primary or revision TKR between July 2014 and December 2015 in VHA Veterans Integrated Service Network 1 (Maine, Vermont, New Hampshire, Massachusetts, Connecticut, Rhode Island, New York, Pennsylvania, West Virginia, Virginia, North Carolina). Because we focused on outcomes following hospital discharge, patients who died before discharge were excluded from the analysis. Preoperative CAN 1-year mortality score was chosen as the measure under the assumption that long-term survival may be the most meaningful of the 4 possible CAN score measures.
Our primary objective was to determine distribution of preoperative CAN scores in the study population. Our secondary was to study relationships among the preoperative CAN 1-year mortality scores and 1-year mortality and hospital LOS.
Study Variables
For each patient, we extracted the date of index surgery. The primary exposure or independent variable was the CAN score in the week prior to this date. Because prior study has shown that CAN scores trajectories do not significantly change over time, the date-stamped CAN scores in the week before surgery represent what would have been available to clinicians in a preoperative setting.15 Since CAN scores are refreshed and overwritten every week, we extracted archived scores from the CDW.
For the 1-year survival outcome, the primary dependent variable, we queried the vital status files in the CDW for the date of death if applicable. We confirmed survival beyond 1 year by examining vital signs in the CDW for a minimum of 2 independent encounters beyond 1 year after the date of discharge. To compute the index LOS, the secondary outcome, we computed the difference between the date of admission and date of hospital discharge.
Statistical Methods
The parameters and performance of the multivariable logistic regression models developed to compute the various CAN mortality and hospitalization risk scores have been previously described.12 Briefly, Wang and colleagues created parsimonious regression models using backward selection. Model discrimination was evaluated using C (concordance)-statistic. Model calibration was assessed by comparing predicted vs observed event rates by risk deciles and performing Cox proportional hazards regression.
We plotted histograms to display preoperative CAN scores as a simple measure of distribution (Figure 1). We also examined the cumulative proportion of patients at each preoperative CAN 1-year mortality score.
Using a conventional t test, we compared means of preoperative CAN 1-year mortality scores in patients who survived vs those who died within 1 year. We also constructed a plot of the proportion of patients who had died within 1 year vs preoperative CAN 1-year mortality scores. Kaplan-Meier curves were then constructed examining 1-year survival by CAN 1-year mortality score by terciles.
Finally, we examined the relationship between preoperative CAN 1-year mortality scores and index LOS in 2 ways: We plotted LOS across CAN scores, and we constructed a
Results
We identified 8206 patients who had undergone a TKR over the 18-month study period. The overall mean (SD) for age was 65 (8.41) years; 93% were male, and 78% were White veterans. Patient demographics are well described in a previous publication.16,17
In terms of model parameters for the CAN score models, C-statistics for the 90-day outcome models were as follows: 0.833 for the model predicting hospitalization (95% CI, 0.832-0.834); 0.865 for the model predicting death (95% CI, 0.863-0.876); and 0.811 for the model predicting either event (95% CI, 0.810-0.812). C-statistics for the 1-year outcome models were 0.809 for the model predicting hospitalization (95% CI, 0.808-0.810); 0.851 for the model predicting death (95% CI, 0.849-0.852); and 0.787 for the model predicting either event (95% CI, 0.786-0.787). Models were well calibrated with α = 0 and β = 1, demonstrating strong agreement between observed and predicted event rates.
The distribution of preoperative CAN 1-year mortality scores was close to normal (median, 50; interquartile range, 40; mean [SD] 48 [25.6]) (eTable). The original CAN score models were developed having an equal number of patients in each strata and as such, are normally distributed.12 Our cohort was similar in pattern of distribution. Distributions of the remaining preoperative CAN scores (90-day mortality, 1-year hospitalization, 90-day hospitalization) are shown in Figures 2, 3, and 4. Not surprisingly, histograms for both 90-day and 1-year hospitalization were skewed toward higher scores, indicating that these patients were expected to be hospitalized in the near future.
Overall, 1.4% (110/8096) of patients died within 1 year of surgery. Comparing 1-year mortality CAN scores in survivors vs nonsurvivors, we found statistically significant differences in means (47 vs 66 respectively, P < .001) and medians (45 vs 75 respectively, P < .001) (Table 2). In the plot examining the relationship between preoperative 1-year mortality CAN scores and 1-year mortality, the percentage who died within 1 year increased initially for patients with CAN scores > 60 and again exponentially for patients with CAN scores > 80. Examining Kaplan-Meier curves, we found that survivors and nonsurvivors separated early after surgery, and the differences between the top tercile and the middle/lower terciles were statistically significant (P < .001). Mortality rates were about 0.5% in the lower and middle terciles but about 2% in the upper tercile (Figure 5).
In the plot examining the relationship between CAN scores and index LOS, the LOS rose significantly beyond a CAN score of 60 and dramatically beyond a CAN score of 80 (Figure 6). LOESS curves also showed 2 inflection points suggesting an incremental and sequential rise in the LOS with increasing CAN scores (Figure 7). Mean (SD) LOS in days for the lowest to highest terciles was 2.6 (1.7), 2.8 (2.1), and 3.6 (2.2), respectively.
Discussion
CAN scores are automatically generated each week by EHR-based multivariable risk models. These scores have excellent predictive accuracy for 90-day and 1-year mortality and hospitalization and are routinely used by VHA primary care teams to assist with clinical operations.13 We studied the distribution of CAN 1-year mortality scores in a preoperative context and examined relationships of the preoperative CAN 1-year mortality scores with postoperative mortality and LOS in 8206 veterans who underwent TKR.
There are several noteworthy findings. First, the overall 1-year mortality rate observed following TKR (1.4%) was similar to other published reports.18,19 Not surprisingly, preoperative CAN 1-year mortality scores were significantly higher in veterans who died compared with those of survivors. The majority of patients who died had a preoperative CAN 1-year mortality score > 75 while most who survived had a preoperative CAN 1-year mortality score < 45 (P < .001). Interestingly, the same scores showed a nonlinear correlation with LOS. Index LOS was about 4 days in patients in the highest tercile of CAN scores vs 2.5 days in the lowest tercile, but the initial increase in LOS was detected at a CAN score of about 55 to 60.
In addition, mortality rate varied widely in different segments of the population when grouped according to preoperative CAN scores. One-year mortality rates in the highest tercile reached 2%, about 4-fold higher than that of lower terciles (0.5%). Examination of the Kaplan-Meier curves showed that this difference in mortality between the highest tercile and the lower 2 groups appears soon after discharge and continues to increase over time, suggesting that the factors contributing to the increased mortality are present at the time of discharge and persist beyond the postoperative period. In summary, although CAN scores were not designed for use in the perioperative context, we found that preoperative CAN 1-year mortality scores are broadly predictive of mortality, but especially for increases in LOS following elective TKA, both increases in hospital LOS following elective TKA and mortality over the year after TKA.
Our findings raise several important questions. The decision to undergo elective surgery is complex. Arguably, individuals who undergo elective knee replacement should be healthy enough to undergo, recover, and reap the benefits from a procedure that does not extend life. The distribution of preoperative CAN 1-year mortality scores for our study population was similar to that of the general VHA enrollee population with similar measured mortality rates (≤ 0.5% vs ≥ 1.7% in the low and high terciles, respectively).1 Further study comparing outcomes in matched cohorts who did and did not undergo joint replacement would be of interest. In lieu of this, though, the association of high but not extreme CAN scores with increased hospital LOS may potentially be used to guide allocation of resources to this group, obviating the increased cost and risk to which this group is exposed. And the additional insight afforded by CAN scores may enhance shared decision-making models by identifying patients at the very highest risk (eg, 1-year mortality CAN score ≥ 90), patients who conceivably might not survive long enough to recover from and enjoy their reconstructed knee, who might in the long run be harmed by undergoing the procedure.
Many total joint arthroplasties are performed in older patients, a population in which frailty is increasingly recognized as a significant risk factor for poor outcomes.20,21 CAN scores reliably identify high-risk patients and have been shown to correlate with frailty in this group.22 Multiple authors have reported improved outcomes with cost reductions after implementation of programs targeting modifiable risk factors in high-risk surgical candidates.23-25 A preoperative assessment that includes the CAN score may be valuable in identifying patients who would benefit most from prehabilitation programs or other interventions designed to blunt the impact of frailty. It is true that many elements used to calculate the CAN score would not be considered modifiable, especially in the short term. However, specific contributors to frailty, such as nutritional status and polypharmacy might be potential candidates. As with all multivariable risk prediction models, there are multiple paths to a high CAN score, and further research to identify clinically relevant subgroups may help inform efforts to improve perioperative care within this population.
Hospital LOS is of intense interest for many reasons, not least its utility as a surrogate for cost and increased risk for immediate perioperative adverse events, such as multidrug-resistant hospital acquired infections, need for postacute facility-based rehabilitation, and deconditioning that increase risks of falls and fractures in the older population.26-29 In addition, its importance is magnified due to the COVID-19 pandemic context in which restarting elective surgery programs has changed traditional criteria by which patients are scheduled for surgery.
We have shown that elevated CAN scores are able to identify patients at risk for extended hospital stays and, as such, may be useful additional data in allocating scarce operating room time and other resources for optimal patient and health care provider safety.30,31 Individual surgeons and hospital systems would, of course, decide which patients should be triaged to go first, based on local priorities; however, choosing lower risk patients with minimal risk of morbidity and mortality while pursuing prehabilitation for higher risk patients is a reasonable approach.
Limitations
Our study has several limitations. Only a single surgical procedure was included, albeit the most common one performed in the VHA. In addition, no information was available concerning the precise clinical course for these patients, such as the duration of surgery, anesthetic technique, and management of acute, perioperative course. Although the assumption was made that patients received standard care in a manner such that these factors would not significantly affect either their mortality or their LOS out of proportion to their preoperative clinical status, confounding cannot be excluded. Therefore, further study is necessary to determine whether CAN scores can accurately predict mortality and/or LOS for patients undergoing other procedures. Further, a clinical trial is required to assess whether systematic provision of the CAN score at the point of surgery would impact care and, more important, impact outcomes. In addition, multivariable analyses were not performed, including and excluding various components of the CAN score models. Currently, CAN scores could be made available to the surgical/anesthesia communities at minimal or no cost and are updated automatically. Model calibration and discrimination in this particular setting were not validated.
Because our interest is in leveraging an existing resource to a current clinical and operational problem rather than in creating or validating a new tool, we chose to test the simple bivariate relationship between preoperative CAN scores and outcomes. We chose the preoperative 1-year mortality CAN score from among the 4 options under the assumption that long-term survival is the most meaningful of the 4 candidate outcomes. Finally, while the CAN scores are currently only calculated and generated for patients cared for within the VHA, few data elements are unavailable to civilian health systems. The most problematic would be documentation of actual prescription filling, but this is a topic of increasing interest to the medical and academic communities and access to such information we hope will improve.32-34
Conclusions
Although designed for use by VHA primary care teams, CAN scores also may have value for perioperative clinicians, predicting mortality and prolonged hospital LOS in those with elevated 1-year mortality scores. Advantages of CAN scores relative to other perioperative risk calculators lies in their ability to predict long-term rather than 30-day survival and that they are automatically generated on a near-real-time basis for all patients who receive care in VHA ambulatory clinics. Further study is needed to determine practical utility in shared decision making, preoperative evaluation and optimization, and perioperative resource allocation.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) National Center for Patient Safety, Field Office 10A4E, through the Patient Safety Center of Inquiry at the Durham VA Medical Center in North Carolina. The study also received support from the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13-410) at the Durham VA Health Care System.
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213
1. McNair AGK, MacKichan F, Donovan JL, et al. What surgeons tell patients and what patients want to know before major cancer surgery: a qualitative study. BMC Cancer. 2016;16:258. doi:10.1186/s12885-016-2292-3
2. Grover FL, Hammermeister KE, Burchfiel C. Initial report of the Veterans Administration Preoperative Risk Assessment Study for Cardiac Surgery. Ann Thorac Surg. 1990;50(1):12-26; discussion 27-18. doi:10.1016/0003-4975(90)90073-f
3. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
4. Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702. doi:10.1097/SLA.0b013e31824b45af
5. Keller DS, Kroll D, Papaconstantinou HT, Ellis CN. Development and validation of a methodology to reduce mortality using the veterans affairs surgical quality improvement program risk calculator. J Am Coll Surg. 2017;224(4):602-607. doi:10.1016/j.jamcollsurg.2016.12.033
6. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833-842.e831-833. doi:10.1016/j.jamcollsurg.2013.07.385
7. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152(1):26-35. doi:10.7326/0003-4819-152-1-201001050-00007
8. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi:10.1161/CIRCULATIONAHA.110.015701
9. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi:10.1161/01.cir.100.10.1043
10. Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in Veterans Health Administration surgery patients. JAMA Surg. 2016;151(5):417-422. doi:10.1001/jamasurg.2015.4882
11. Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90- day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149-1154. doi:10.1002/bjs.8813
12. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1016/j.amjcard.2012.06.038
13. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
14. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
15. Wong ES, Yoon J, Piegari RI, Rosland AM, Fihn SD, Chang ET. Identifying latent subgroups of high-risk patients using risk score trajectories. J Gen Intern Med. 2018;33(12):2120-2126. doi:10.1007/s11606-018-4653-x
16. Chen Q, Hsia HL, Overman R, et al. Impact of an opioid safety initiative on patients undergoing total knee arthroplasty: a time series analysis. Anesthesiology. 2019;131(2):369-380. doi:10.1097/ALN.0000000000002771
17. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
18. Inacio MCS, Dillon MT, Miric A, Navarro RA, Paxton EW. Mortality after total knee and total hip arthroplasty in a large integrated health care system. Perm J. 2017;21:16-171. doi:10.7812/TPP/16-171
19. Lee QJ, Mak WP, Wong YC. Mortality following primary total knee replacement in public hospitals in Hong Kong. Hong Kong Med J. 2016;22(3):237-241. doi:10.12809/hkmj154712
20. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi:10.1186/s12877-016-0329-8
21. Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2019;155(1):e194620. doi:10.1001/jamasurg.2019.4620
22. Ruiz JG, Priyadarshni S, Rahaman Z, et al. Validation of an automatically generated screening score for frailty: the care assessment need (CAN) score. BMC Geriatr. 2018;18(1):106. doi:10.1186/s12877-018-0802-7
23. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642- 3648. doi:10.1016/j.arth.2018.08.018
24. Sodhi N, Anis HK, Coste M, et al. A nationwide analysis of preoperative planning on operative times and postoperative complications in total knee arthroplasty. J Knee Surg. 2019;32(11):1040-1045. doi:10.1055/s-0039-1677790
25. Krause A, Sayeed Z, El-Othmani M, Pallekonda V, Mihalko W, Saleh KJ. Outpatient total knee arthroplasty: are we there yet? (part 1). Orthop Clin North Am. 2018;49(1):1-6. doi:10.1016/j.ocl.2017.08.002
26. Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca- Doñate R, Moliner-Lahoz J. Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis. 2017;65(4):644-652. doi:10.1093/cid/cix411
27. Nikkel LE, Kates SL, Schreck M, Maceroli M, Mahmood B, Elfar JC. Length of hospital stay after hip fracture and risk of early mortality after discharge in New York state: retrospective cohort study. BMJ. 2015;351:h6246. doi:10.1136/bmj.h6246
28. Marfil-Garza BA, Belaunzarán-Zamudio PF, Gulias-Herrero A, et al. Risk factors associated with prolonged hospital length-of-stay: 18-year retrospective study of hospitalizations in a tertiary healthcare center in Mexico. PLoS One. 2018;13(11):e0207203. doi:10.1371/journal.pone.0207203
29. Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38(12):1296-1303. doi:10.1111/j.1532-5415.1990.tb03451.x
30. Iyengar KP, Jain VK, Vaish A, Vaishya R, Maini L, Lal H. Post COVID-19: planning strategies to resume orthopaedic surgery -challenges and considerations. J Clin Orthop Trauma. 2020;11(suppl 3):S291-S295. doi:10.1016/j.jcot.2020.04.028
31. O’Connor CM, Anoushiravani AA, DiCaprio MR, Healy WL, Iorio R. Economic recovery after the COVID-19 pandemic: resuming elective orthopedic surgery and total joint arthroplasty. J Arthroplasty. 2020;35(suppl 7):S32-S36. doi:10.1016/j.arth.2020.04.038.
32. Mauseth SA, Skurtveit S, Skovlund E, Langhammer A, Spigset O. Medication use and association with urinary incontinence in women: data from the Norwegian Prescription Database and the HUNT study. Neurourol Urodyn. 2018;37(4):1448-1457. doi:10.1002/nau.23473
33. Sultan RS, Correll CU, Schoenbaum M, King M, Walkup JT, Olfson M. National patterns of commonly prescribed psychotropic medications to young people. J Child Adolesc Psychopharmacol. 2018;28(3):158-165. doi:10.1089/cap.2017.0077
34. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S.-the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21(12):702-712. doi:10.1089/dia.2019.0213
The Hospital Readmissions Reduction Program: Inconvenient Observations
Centers for Medicare and Medicaid Services (CMS)–promulgated quality metrics continue to attract critics. Physicians decry that many metrics are outside their control, while patient groups are frustrated that metrics lack meaning for beneficiaries. The Hospital Readmissions Reduction Program (HRRP) reduces payments for “excess” 30-day risk-standardized readmissions for six conditions and procedures, and may be less effective in reducing readmissions than previously reported due to intentional and increasing use of hospital observation stays.1
In this issue, Sheehy et al2 report that nearly one in five rehospitalizations were unrecognized because either the index hospitalization or the rehospitalization was an observation stay, highlighting yet another challenge with the HRRP. Limitations of their study include the use of a single year of claims data and the exclusion of Medicare Advantage claims data, as one might expect lower readmission rates in this capitated program. Opportunities for improving the HRRP could consist of updating the HRRP metric to include observation stays and, for surgical hospitalizations, extended-stay surgical recovery, wherein patients may be observed for up to 2 days following a procedure. Unfortunately, despite the HRRP missing nearly one in five readmissions, CMS would likely need additional statutory authority from Congress in order to reinterpret the definition of readmission3 to include observation stays.
Challenges with the HRRP metrics raise broader concerns about the program. For decades, administrators viewed readmissions as a utilization metric, only to have the Affordable Care Act re-designate and define all-cause readmissions as a quality metric. Yet hospitals and health systems control only some factors driving readmission. Readmissions occur for a variety of reasons, including not only poor quality of initial hospital care and inadequate care coordination, but also factors that are beyond the hospital’s purview, such as lack of access to ambulatory services, multiple and severe chronic conditions that progress or remain unresponsive to intervention,4 and demographic and social factors such as housing instability, health literacy, or residence in a food desert. These non-hospital factors reside within the domain of other market participants or local, state, and federal government agencies.
Challenges to the utility, validity, and appropriateness of HRRP metrics should remind policymakers of the dangers of over-legislating the details of healthcare policy and the statutory inflexibility that can ensue. Clinical care evolves, and artificial constructs—including payment categories such as observation status—may age poorly over time, exemplified best by the challenges of accessing post-acute care due to the 3-day rule.5 Introduced as a statutory requirement in 1967, when the average length of stay was 13.8 days and observation care did not exist as a payment category, the 3-day rule requires Medicare beneficiaries to spend 3 days admitted to the hospital in order to qualify for coverage of post-acute care, creating care gaps for observation stay patients.
Observation care itself is an artificial construct of CMS payment policy. In the Medicare program, observation care falls under Part B, exposing patients to both greater financial responsibility and billing complexity through the engagement of their supplemental insurance, even though those receiving observation care experience the same care as if hospitalized— routine monitoring, nursing care, blood draws, imaging, and diagnostic tests. While CMS requires notification of observation status and explanation of the difference in patient financial responsibility, in clinical practice, patient understanding is limited. Policymakers can support both Medicare beneficiaries and hospitals by reexamining observation care as a payment category.
Sheehy and colleagues’ work simultaneously challenges the face validity of the HRRP and the reasonableness of categorizing some inpatient stays as outpatient care in the hospital—issues that policymakers can and should address.
1. Sabbatini AK, Wright B. Excluding observation stays from readmission rates – what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
2. Sheehy AM, Kaiksow F, Powell WR, et al. The hospital readmissions reduction program’s blind spot: observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
3. The Patient Protection and Affordable Care Act, 42 USC 18001§3025 (2010).
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. https://doi.org/10.1056/NEJMp1315568
5. Patel N, Slota JM, Miller BJ. The continued conundrum of discharge to a skilled nursing facility after a medicare observation stay. JAMA Health Forum. 2020;1(5):e200577. https://doi.org/10.1001/jamahealthforum.2020.0577
Centers for Medicare and Medicaid Services (CMS)–promulgated quality metrics continue to attract critics. Physicians decry that many metrics are outside their control, while patient groups are frustrated that metrics lack meaning for beneficiaries. The Hospital Readmissions Reduction Program (HRRP) reduces payments for “excess” 30-day risk-standardized readmissions for six conditions and procedures, and may be less effective in reducing readmissions than previously reported due to intentional and increasing use of hospital observation stays.1
In this issue, Sheehy et al2 report that nearly one in five rehospitalizations were unrecognized because either the index hospitalization or the rehospitalization was an observation stay, highlighting yet another challenge with the HRRP. Limitations of their study include the use of a single year of claims data and the exclusion of Medicare Advantage claims data, as one might expect lower readmission rates in this capitated program. Opportunities for improving the HRRP could consist of updating the HRRP metric to include observation stays and, for surgical hospitalizations, extended-stay surgical recovery, wherein patients may be observed for up to 2 days following a procedure. Unfortunately, despite the HRRP missing nearly one in five readmissions, CMS would likely need additional statutory authority from Congress in order to reinterpret the definition of readmission3 to include observation stays.
Challenges with the HRRP metrics raise broader concerns about the program. For decades, administrators viewed readmissions as a utilization metric, only to have the Affordable Care Act re-designate and define all-cause readmissions as a quality metric. Yet hospitals and health systems control only some factors driving readmission. Readmissions occur for a variety of reasons, including not only poor quality of initial hospital care and inadequate care coordination, but also factors that are beyond the hospital’s purview, such as lack of access to ambulatory services, multiple and severe chronic conditions that progress or remain unresponsive to intervention,4 and demographic and social factors such as housing instability, health literacy, or residence in a food desert. These non-hospital factors reside within the domain of other market participants or local, state, and federal government agencies.
Challenges to the utility, validity, and appropriateness of HRRP metrics should remind policymakers of the dangers of over-legislating the details of healthcare policy and the statutory inflexibility that can ensue. Clinical care evolves, and artificial constructs—including payment categories such as observation status—may age poorly over time, exemplified best by the challenges of accessing post-acute care due to the 3-day rule.5 Introduced as a statutory requirement in 1967, when the average length of stay was 13.8 days and observation care did not exist as a payment category, the 3-day rule requires Medicare beneficiaries to spend 3 days admitted to the hospital in order to qualify for coverage of post-acute care, creating care gaps for observation stay patients.
Observation care itself is an artificial construct of CMS payment policy. In the Medicare program, observation care falls under Part B, exposing patients to both greater financial responsibility and billing complexity through the engagement of their supplemental insurance, even though those receiving observation care experience the same care as if hospitalized— routine monitoring, nursing care, blood draws, imaging, and diagnostic tests. While CMS requires notification of observation status and explanation of the difference in patient financial responsibility, in clinical practice, patient understanding is limited. Policymakers can support both Medicare beneficiaries and hospitals by reexamining observation care as a payment category.
Sheehy and colleagues’ work simultaneously challenges the face validity of the HRRP and the reasonableness of categorizing some inpatient stays as outpatient care in the hospital—issues that policymakers can and should address.
Centers for Medicare and Medicaid Services (CMS)–promulgated quality metrics continue to attract critics. Physicians decry that many metrics are outside their control, while patient groups are frustrated that metrics lack meaning for beneficiaries. The Hospital Readmissions Reduction Program (HRRP) reduces payments for “excess” 30-day risk-standardized readmissions for six conditions and procedures, and may be less effective in reducing readmissions than previously reported due to intentional and increasing use of hospital observation stays.1
In this issue, Sheehy et al2 report that nearly one in five rehospitalizations were unrecognized because either the index hospitalization or the rehospitalization was an observation stay, highlighting yet another challenge with the HRRP. Limitations of their study include the use of a single year of claims data and the exclusion of Medicare Advantage claims data, as one might expect lower readmission rates in this capitated program. Opportunities for improving the HRRP could consist of updating the HRRP metric to include observation stays and, for surgical hospitalizations, extended-stay surgical recovery, wherein patients may be observed for up to 2 days following a procedure. Unfortunately, despite the HRRP missing nearly one in five readmissions, CMS would likely need additional statutory authority from Congress in order to reinterpret the definition of readmission3 to include observation stays.
Challenges with the HRRP metrics raise broader concerns about the program. For decades, administrators viewed readmissions as a utilization metric, only to have the Affordable Care Act re-designate and define all-cause readmissions as a quality metric. Yet hospitals and health systems control only some factors driving readmission. Readmissions occur for a variety of reasons, including not only poor quality of initial hospital care and inadequate care coordination, but also factors that are beyond the hospital’s purview, such as lack of access to ambulatory services, multiple and severe chronic conditions that progress or remain unresponsive to intervention,4 and demographic and social factors such as housing instability, health literacy, or residence in a food desert. These non-hospital factors reside within the domain of other market participants or local, state, and federal government agencies.
Challenges to the utility, validity, and appropriateness of HRRP metrics should remind policymakers of the dangers of over-legislating the details of healthcare policy and the statutory inflexibility that can ensue. Clinical care evolves, and artificial constructs—including payment categories such as observation status—may age poorly over time, exemplified best by the challenges of accessing post-acute care due to the 3-day rule.5 Introduced as a statutory requirement in 1967, when the average length of stay was 13.8 days and observation care did not exist as a payment category, the 3-day rule requires Medicare beneficiaries to spend 3 days admitted to the hospital in order to qualify for coverage of post-acute care, creating care gaps for observation stay patients.
Observation care itself is an artificial construct of CMS payment policy. In the Medicare program, observation care falls under Part B, exposing patients to both greater financial responsibility and billing complexity through the engagement of their supplemental insurance, even though those receiving observation care experience the same care as if hospitalized— routine monitoring, nursing care, blood draws, imaging, and diagnostic tests. While CMS requires notification of observation status and explanation of the difference in patient financial responsibility, in clinical practice, patient understanding is limited. Policymakers can support both Medicare beneficiaries and hospitals by reexamining observation care as a payment category.
Sheehy and colleagues’ work simultaneously challenges the face validity of the HRRP and the reasonableness of categorizing some inpatient stays as outpatient care in the hospital—issues that policymakers can and should address.
1. Sabbatini AK, Wright B. Excluding observation stays from readmission rates – what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
2. Sheehy AM, Kaiksow F, Powell WR, et al. The hospital readmissions reduction program’s blind spot: observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
3. The Patient Protection and Affordable Care Act, 42 USC 18001§3025 (2010).
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. https://doi.org/10.1056/NEJMp1315568
5. Patel N, Slota JM, Miller BJ. The continued conundrum of discharge to a skilled nursing facility after a medicare observation stay. JAMA Health Forum. 2020;1(5):e200577. https://doi.org/10.1001/jamahealthforum.2020.0577
1. Sabbatini AK, Wright B. Excluding observation stays from readmission rates – what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
2. Sheehy AM, Kaiksow F, Powell WR, et al. The hospital readmissions reduction program’s blind spot: observation hospitalizations. J Hosp Med. 2021;16(7):409-411. https://doi.org/10.12788/jhm.3634
3. The Patient Protection and Affordable Care Act, 42 USC 18001§3025 (2010).
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. https://doi.org/10.1056/NEJMp1315568
5. Patel N, Slota JM, Miller BJ. The continued conundrum of discharge to a skilled nursing facility after a medicare observation stay. JAMA Health Forum. 2020;1(5):e200577. https://doi.org/10.1001/jamahealthforum.2020.0577
© 2021 Society of Hospital Medicine