User login
Inpatient Glycemic Control With Sliding Scale Insulin in Noncritical Patients With Type 2 Diabetes: Who Can Slide?
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.
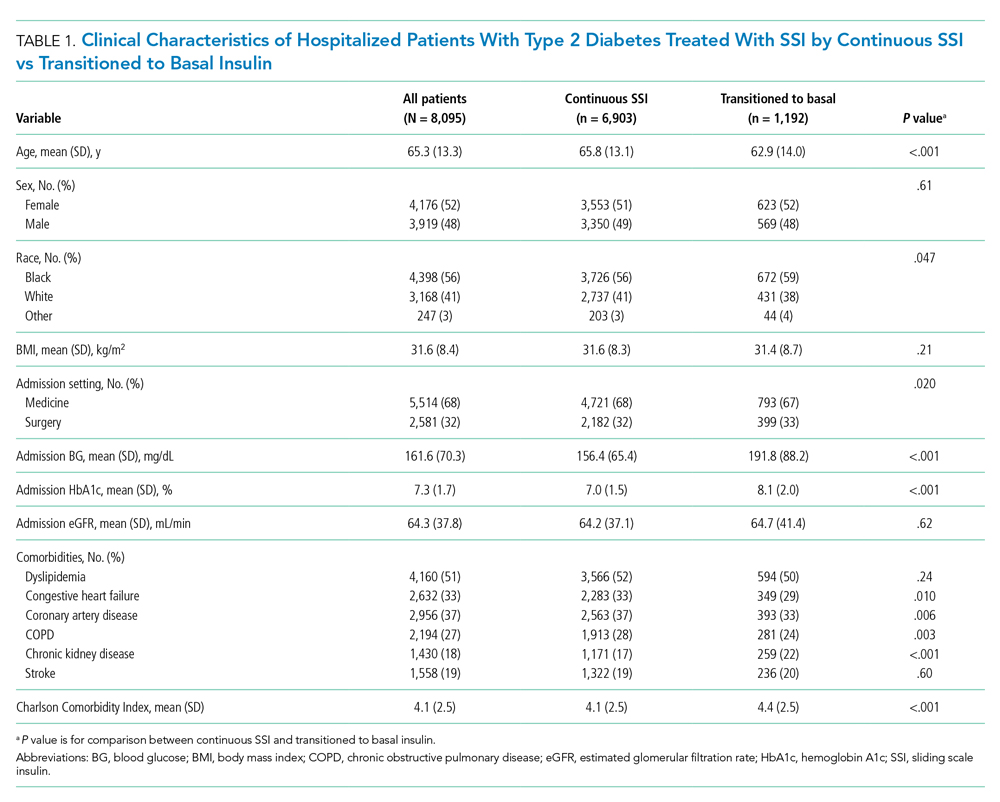
Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).
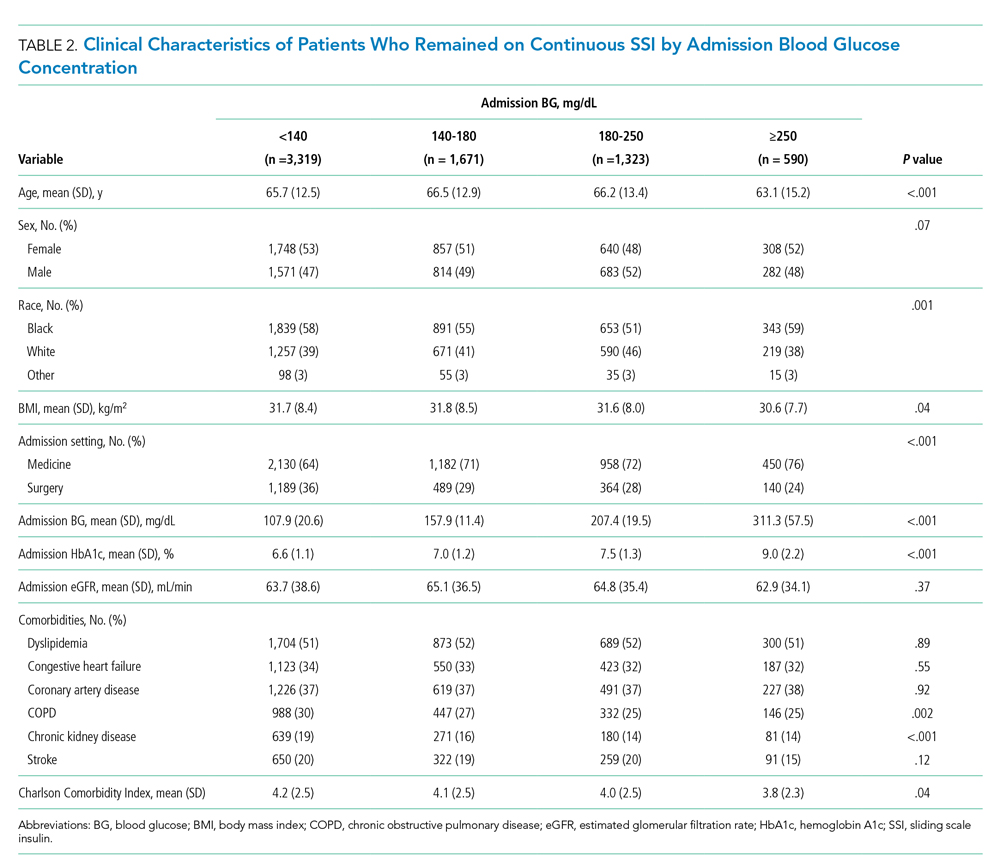
Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.

HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).

DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.

Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).

Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.

HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).

DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
Sliding scale insulin (SSI) for inpatient glycemic control was first proposed by Elliott P Joslin in 1934 when he recommended titration of insulin based on urine glucose levels.1 As bedside glucose meters became widely available, physicians transitioned to dosing SSI based on capillary blood glucose (BG) levels,2,3 and SSI became widely used for the management of inpatient hyperglycemia.1 However, during the past decade, there has been strong opposition to the use of SSI in hospitals. Many authors oppose its use, highlighting the retrospective rather than prospective nature of SSI therapy and concerns about inadequate glycemic control.4-6 In 2004, the American College of Endocrinology first released a position statement discouraging the use of SSI alone and recommended basal-bolus insulin as the preferred method of glycemic control for inpatients with type 2 diabetes (T2D).7 The American Diabetes Association (ADA) inpatient guidelines in 20058 and the Endocrine Society guidelines in 20129 also opposed SSI monotherapy and reaffirmed that a basal-bolus insulin regimen should be used for most non–critically ill patients with diabetes. Those guidelines remain in place currently.
Several randomized controlled trials (RCTs) and meta-analyses have shown that basal-bolus insulin regimens provide superior glycemic control in non–critical inpatients when compared with SSI alone.10-14 In addition, the RABBIT 2 (Randomized Study of Basal-Bolus Insulin Therapy in the Inpatient Management of Patients With Type 2 Diabetes) trial showed a significant reduction in perioperative complications10 among surgical patients when treated with basal-bolus insulin therapy. Despite these studies and strong recommendations against its use, SSI continues to be widely used in the United States. According to a 2007 survey of 44 US hospitals, 41% of noncritical patients with hyperglycemia were treated with SSI alone.15 In addition, SSI remains one of the most commonly prescribed insulin regimens in many countries around the world.16-19 The persistence of SSI use raises questions as to why clinicians continue to use a therapy that has been strongly criticized. Some authors point to convenience and fear of hypoglycemia with a basal-bolus insulin regimen.20,21 Alternatively, it is possible that SSI usage remains so pervasive because it is effective in a subset of patients. In fact, a 2018 Cochrane review concluded that existing evidence is not sufficiently robust to definitively recommend basal-bolus insulin over SSI for inpatient diabetes management of non–critically ill patients despite existing guidelines.22
Owing to the ongoing controversy and widespread use of SSI, we designed an exploratory analysis to understand the rationale for such therapy by investigating whether a certain subpopulation of hospitalized patients with T2D may achieve target glycemic control with SSI alone. We hypothesized that noncritical patients with mild hyperglycemia and admission BG <180 mg/dL would do well with SSI alone and may not require intensive treatment with basal-bolus insulin regimens. To address this question, we used electronic health records with individual-level patient data to assess inpatient glycemic control of non–critically ill patients with T2D treated with SSI alone.
METHODS
Participants
Data from 25,813 adult noncritical inpatients with T2D, with an index admission between June 1, 2010, and June 30, 2018, were obtained through the Emory Healthcare Clinical Data Warehouse infrastructure program. All patients were admitted to Emory Healthcare hospitals, including Emory University Hospital, Emory University Hospital Midtown, and Emory Saint Joseph’s Hospital, in Atlanta, Georgia. Data were extracted for each patient during the index hospitalization, including demographics, anthropometrics, and admission and inpatient laboratory values. Information was collected on daily point-of-care glucose values, hemoglobin A1c (HbA1c), hypoglycemic events, insulin doses, hospital complications, comorbidities, and hospital setting (medical vs surgical admission). International Classification of Diseases, 9th and 10th Revisions (ICD-9/10) codes were used to determine diagnosis of T2D, comorbidities, and complications.
From our initial dataset, we identified 16,366 patients who were treated with SSI during hospitalization. We excluded patients who were admitted to the intensive care unit (ICU) or placed on intravenous insulin, patients with missing admission BG values, and patients with a length of stay less than 1 day. To prevent inclusion of patients presenting in diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome, we excluded patients with an admission BG >500 mg/dL. We then excluded 6,739 patients who received basal insulin within the first 2 days of hospitalization, as well as 943 patients who were treated with noninsulin (oral or injectable) antidiabetic agents. Our final dataset included 8,095 patients (Appendix Figure).
Patients in the SSI cohort included all patients who were treated with short-acting insulin only (regular insulin or rapid-acting [lispro, aspart, glulisine] insulin analogs) during the first 2 days of hospitalization. Patients who remained on only short-acting insulin during the entire hospitalization were defined as continuous SSI patients. Patients who subsequently received basal insulin after day 2 of hospitalization were defined as patients who transitioned to basal. Patients were stratified according to admission BG levels (first BG available on day of admission) and HbA1c (when available during index admission). We compared the baseline characteristics and clinical outcomes of patients who remained on SSI alone throughout the entirety of hospitalization with those of patients who required transition to basal insulin. The mean hospital BG was calculated by taking the average of all BG measurements during the hospital stay. We defined hypoglycemia as a BG <70 mg/dL and severe hypoglycemia as BG <40 mg/dL. Repeated hypoglycemia values were excluded if they occurred within a period of 2 hours.
Outcome Measures
The primary outcome was the percentage of patients with T2D achieving target glycemic control with SSI therapy, defined as mean hospital BG between 70 and 180 mg/dL without hypoglycemia <70 mg/dL during hospital stay. This threshold was determined based on 2019 ADA recommendations targeting hospital BG <180 mg/dL and avoidance of hypoglycemia.23
Statistical Analysis
Patients were stratified according to continuous SSI versus transitioned to basal treatment. Patients who remained on continuous SSI were further categorized into four categories based on admission BG: <140 mg/dL, 140 to 180 mg/dL, 180 to 250 mg/dL, and ≥250 mg/dL. Clinical characteristics were compared using Wilcoxon rank-sum tests (if continuous) and chi-square tests or Fisher exact tests (if categorical). We then compared the clinical outcomes among continuous SSI patients with different admission BG levels (<140 mg/dL, 140-180 mg/dL, 180-250 mg/dL, and ≥250 mg/dL) and with different HbA1c levels (<7%, 7%-8%, 8%-9%, ≥9%). Within each scenario, logistic regression for the outcome of poor glycemic control, defined as mean hospital BG >180 mg/dL, was performed to evaluate the HbA1c levels and admission BG levels controlling for other factors (age, gender, body mass index [BMI], race, setting [medicine versus surgery] and Charlson Comorbidity Index score). A P value < .05 was regarded as statistically significant. All analyses were performed based on available cases and conducted in SAS version 9.4 (SAS Institute Inc.).
RESULTS
Among 25,813 adult patients with T2D, 8,095 patients (31.4%) were treated with SSI alone during the first 2 days of hospitalization. Of those patients treated with SSI, 6,903 (85%) remained on continuous SSI alone during the entire hospitalization, and 1,192 (15%) were transitioned to basal insulin. The clinical characteristics of these patients on continuous SSI and those who transitioned to basal insulin are shown in Table 1. Patients who transitioned to basal insulin had significantly higher mean (SD) admission BG (191.8 [88.2] mg/dL vs 156.4 [65.4] mg/dL, P < .001) and higher mean (SD) HbA1c (8.1% [2.0%] vs 7.01% [1.5%], P < .001), compared with those who remained on continuous SSI. Patients who transitioned to basal insulin were also younger and more likely to have chronic kidney disease (CKD), but less likely to have congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease (COPD). The Charlson Comorbidity Index score was significantly higher for patients who transitioned to basal (4.4 [2.5]) than for those who remained on continuous SSI (4.1 [2.5], P < .001). There were no significant differences among sex, BMI, or glomerular filtration rate (GFR) on admission. Of those transitioned to basal insulin, 53% achieved a mean hospitalization BG <180 mg/dL, compared with 82% of those on continuous SSI. The overall rate of hypoglycemia in the continuous SSI group was 8% compared with 18% in those transitioned to basal insulin.

Of the patients who remained on continuous SSI throughout the hospitalization, 3,319 patients (48%) had admission BG <140 mg/dL, 1,671 patients (24%) had admission BG 140 to 180 mg/dL, and 1,913 patients (28%) had admission BG >180 mg/dL. Only 9% of patients who remained on continuous SSI had admission BG ≥250 mg/dL. Patients with admission BG <140 mg/dL were older, had lower BMI and HbA1c, had higher rates of COPD and CKD, and were more likely to be admitted to a surgical service compared with patients with admission BG >140 mg/dL (P < .05 for all; Table 2).

Hospital glycemic control for patients on continuous SSI according to admission BG is displayed in Table 3. Among patients who remained on continuous SSI, 96% of patients with admission BG <140 mg/dL had a mean hospital BG <180 mg/dL; of them, 86% achieved target control without hypoglycemia. Similar rates of target control were achieved in patients with admission BG 140 to 180 mg/dL (83%), in contrast to patients with admission BG ≥250 mg/dL, of whom only 18% achieved target control (P < .001). These findings parallel those seen in patients transitioned to basal insulin. Of patients in the transition group admitted with BG <140 mg/dL and <180 mg/dL, 88.5% and 84.6% had mean hospital BG <180 mg/dL, respectively, while 69.1% and 68.9% had mean BG between 70 and 180 mg/dL without hypoglycemia. The overall frequency of hypoglycemia <70 mg/dL among patients on continuous SSI was 8% and was more common in patients with admission BG <140 mg/dL (10%) compared with patients with higher admission glucose levels (BG 140-180 mg/dL [4%], 180-250 mg/dL [4%], or ≥250 mg/dL [6%], P < .001). There was no difference in rates of severe hypoglycemia <40 mg/dL among groups.

HbA1c data were available for 2,560 of the patients on continuous SSI (Table 3). Mean hospital BG increased significantly with increasing HbA1c values. Patients admitted with HbA1c <7% had lower mean (SD) hospital BG (132.2 [28.2] mg/dL) and were more likely to achieve target glucose control during hospitalization (85%) compared with those with HbA1c 7% to 8% (mean BG, 148.7 [30.8] mg/dL; 80% target control), HbA1c 8% to 9% (mean BG, 169.1 [37.9] mg/dL; 61% target control), or HbA1c ≥9% (mean BG, 194.9 [53.4] mg/dL; 38% target control) (P < .001).
In a logistic regression analysis adjusted for age, gender, BMI, race, setting (medicine vs surgery), and Charlson Comorbidity Index score, the odds of poor glycemic control increased with higher admission BG (admission BG 140-180 mg/dL: odds ratio [OR], 1.8; 95% CI, 1.5-2.2; admission BG 180-250 mg/dL: OR, 3.7; 95% CI, 3.1-4.4; admission BG ≥250 mg/dL: OR, 7.2; 95% CI, 5.8-9.0; reference admission BG <140 mg/dL; Figure). Similarly, the logistic regression analysis showed greater odds of poor in-hospital glycemic control with increasing HbA1c (OR, 6.1; 95% CI, 4.3-8.8 for HbA1c >9% compared with HbA1c <7%).

DISCUSSION
This large retrospective cohort study examined the effectiveness of SSI for glycemic control in noncritical inpatients with T2D. Our results indicate that SSI is still widely used in our hospital system, with 31.4% of our initial cohort managed with SSI alone. We found that 86% of patients with BG <140 mg/dL and 83% of patients with BG 140 to 180 mg/dL achieved glycemic control without hypoglycemia when managed with SSI alone, compared with 53% of those admitted with BG 180 to 250 mg/dL and only 18% of those with admission BG ≥250 mg/dL. This high success rate of achieving optimal BG control with SSI alone is comparable to that seen with transition to basal insulin and may explain the prevalent use of SSI for the management of patients with T2D and mild to moderate hyperglycemia.
Published clinical guideline recommendations promoting the use of basal-bolus insulin treatment algorithms are based on the results of a few RCTs that compared the efficacy of SSI vs a basal-bolus insulin regimen. These studies reported significantly lower mean daily BG concentration with basal or basal-bolus insulin therapy compared with SSI.10,11,24 However, it is interesting to note that the mean admission BG of patients treated with SSI in these RCTs ranged from 184 to 225 mg/dL. Patients in these trials were excluded if admission BG was <140 mg/dL.10,11,24 This is in contrast to our study evaluating real-world data in non–critically ill settings in which we found that 48% of patients treated with SSI had admission BG <140 mg/dL, and nearly 75% had admission BG <180 mg/dL. This suggests that by nature of study design, most RCTs excluded the population of patients who do achieve good glycemic control with SSI and may have contributed to the perception that basal insulin is preferable in all populations.
Our analysis indicates that healthcare professionals should consider admission BG when selecting the type of insulin regimen to manage patients with T2D in the hospital. Our results suggest that SSI may be appropriate for many patients with admission BG <180 mg/dL and should be avoided as monotherapy in patients with admission BG ≥180 mg/dL, as the proportion of patients achieving target control decreased with increasing admission BG. More importantly, if a patient is not controlled with SSI alone, intensification of therapy with the addition of basal insulin is indicated to achieve glycemic control. In addition, we found that the admission HbA1c is an appropriate marker to consider as well, with hospital glycemic control deteriorating with increasing HbA1c values, paralleling the admission BG. The main limitation to widespread use of HbA1c for therapeutic decision-making is access to values at time of patient admission; in our population, only 37% of patients had an HbA1c value available during the index hospitalization.
Previous publications have reported that hypoglycemia carries significant safety concerns, especially among a hospitalized population.25-27 As such, we included hypoglycemia as an important metric in our definition of target glycemic control rather than simply using mean hospital BG or number of hyperglycemic events to define treatment effectiveness. We did find a higher rate of hypoglycemia in patients with moderate admission BG treated with SSI compared with those with higher admission BG; however, few patients overall experienced clinically significant (<54 mg/dL) or severe (<40 mg/dL) hypoglycemia.
In our population, only 15% of patients started on SSI received additional basal insulin during hospitalization. This finding is similar to data reported in the Rabbit 2 trial, in which 14% of patients failed SSI alone, with a higher failure rate among those with higher BG on admission.10 Given the observational nature of this study, we cannot definitively state why certain patients in our population required additional basal insulin, but we can hypothesize that these patients admitted with BG ≥180 mg/dL had higher treatment failure rates and greater rates of hyperglycemia, therefore receiving intensified insulin therapy as clinically indicated at the discretion of the treating physician. Patients who transitioned from SSI to basal insulin had significantly higher admission BG and HbA1c compared with patients who remained on SSI alone. We noted that the rates of hypoglycemia were higher in the group that transitioned to basal (18% vs 8%) and similar to rates reported in previous RCTs.11,24
This observational study takes advantage of a large, diverse study population and a combination of medicine and surgery patients in a real-world setting. We acknowledge several limitations in our study. Our primary data were observational in nature, and as such, some baseline patient characteristics were notably different between groups, suggesting selection bias for treatment allocation to SSI. We do not know which patients were managed by primary teams compared with specialized diabetes consult services, which may also influence treatment regimens. We did not have access to information about patients’ at-home diabetes medication regimens or duration of diabetes, both of which have been shown in prior publications to affect an individual’s overall hospital glycemic control. Data on HbA1c values were available for only approximately one-third of patients. In addition, our study did not include patients without a history of diabetes who developed stress-induced hyperglycemia, a population that may benefit from conservative therapy such as SSI.28 A diagnosis of CKD was defined based on ICD 9/10 codes and not on admission estimated GFR. More specific data regarding stage of CKD or changes in renal function over the duration of hospitalization are not available, which could influence insulin prescribing practice. In addition, we defined the basal group as patients prescribed any form of basal insulin (NPH, glargine, detemir or degludec), and we do not have information on the use of prandial versus correction doses of rapid-acting insulin in the basal insulin–treated group.
CONCLUSION
In conclusion, our observational study indicates that the use of SSI results in appropriate target glycemic control for most noncritical medicine and surgery patients with admission BG <180 mg/dL. In agreement with previous RCTs, our study confirms that SSI as monotherapy is frequently inadequate in patients with significant hyperglycemia >180 mg/dL.10,11,24,29 We propose that an individualized approach to inpatient glycemic management is imperative, and cautious use of SSI may be a viable option for certain patients with mild hyperglycemia and admission BG <180 mg/dL. Further observational and randomized studies are needed to confirm the efficacy of SSI therapy in T2D patients with mild hyperglycemia. By identifying which subset of patients can be safely managed with SSI alone, we can better understand which patients will require escalation of therapy with intensive glucose management.
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
1. Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563-567. https://doi.org/10.1016/j.amjmed.2006.05.070
2. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633-638. https://doi.org/10.7326/0003-4819-84-6-633
3. Skyler JS, Skyler DL, Seigler DE, O’Sullivan MJ. Algorithms for adjustment of insulin dosage by patients who monitor blood glucose. Diabetes Care. 1981;4(2):311-318. https://doi.org/10.2337/diacare.4.2.311
4. Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322.
5. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552.
6. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. https://doi.org/10.2337/diacare.27.2.553
7. Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;10(1):78-82. https://doi.org/10.4158/EP.10.1.77
8. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4-S36.
9. Umpierrez GE, Hellman R, Korytkowski MT, , et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16-38. https://doi.org/10.1210/jc.2011-2098
10. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes. Diabetes Care. 2007;30(9):2181-2186. https://doi.org/10.2337/dc07-0295
11. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. https://doi.org/10.2337/dc10-1407
12. Schroeder JE, Liebergall M, Raz I, Egleston R, Ben Sussan G, Peyser A. Benefits of a simple glycaemic protocol in an orthopaedic surgery ward: a randomized prospective study. Diabetes Metab Res Rev. 2012;28:71-75. https://doi.org/10.1002/dmrr.1217
13. Lee YY, Lin YM, Leu WJ, et al. Sliding-scale insulin used for blood glucose control: a meta-analysis of randomized controlled trials. Metabolism. 2015;64(9):1183-1192. https://doi.org/10.1016/j.metabol.2015.05.011
14. Christensen MB, Gotfredsen A, Nørgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5):e2885. https://doi.org/10.1002/dmrr.2885
15. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. https://doi.org/10.2337/dc06-1715
16. Moreira ED Jr, Silveira PCB, Neves RCS, Souza C Jr, Nunes ZO, Almeida MdCC. Glycemic control and diabetes management in hospitalized patients in Brazil. Diabetol Metab Syndr. 2013;5(1):62. https://doi.org/10.1186/1758-5996-5-62
17. Akhtar ST, Mahmood K, Naqvi IH, Vaswani AS. Inpatient management of type 2 diabetes mellitus: does choice of insulin regimen really matter? Pakistan J Med Sci. 2014;30(4):895-898.
18. Gómez Cuervo C, Sánchez Morla A, Pérez-Jacoiste Asín MA, Bisbal Pardo O, Pérez Ordoño L, Vila Santos J. Effective adverse event reduction with bolus-basal versus sliding scale insulin therapy in patients with diabetes during conventional hospitalization: systematic review and meta-analysis. Endocrinol Nutr. 2016;63(4):145-156. https://doi.org/10.1016/j.endonu.2015.11.008
19. Bain A, Hasan SS, Babar ZUD. Interventions to improve insulin prescribing practice for people with diabetes in hospital: a systematic review. Diabet Med. 2019;36(8):948-960. https://doi.org/10.1111/dme.13982
20. Ambrus DB, O’Connor MJ. Things We Do For No Reason: sliding-scale insulin as monotherapy for glycemic control in hospitalized patients. J Hosp Med. 2019;14(2):114-116. https://doi.org/10.12788/jhm.3109
21. Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130-1135.
22. Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11(11):CD011296. https://doi.org/10.1002/14651858.CD011296.pub2
23. American Diabetes Association. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S173-S181. https://doi.org/10.2337/dc19-S015
24. Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174. https://doi.org/10.2337/dc12-1988
25. Turchin A, Matheny ME, Shubina M, Scanlon SV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153-1157. https://doi.org/10.2337/dc08-2127
26. Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care. 2013;36(5):1107-1110. https://doi.org/10.2337/dc12-1296
27. Zapatero A, Gómez-Huelgas R, González N, et al. Frequency of hypoglycemia and its impact on length of stay, mortality, and short-term readmission in patients with diabetes hospitalized in internal medicine wards. Endocr Pract. 2014;20(9):870-875. https://doi.org/10.4158/EP14006.OR
28. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. https://doi.org/10.1210/jcem.87.3.8341
29. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. https://doi.org/10.1370/afm.2
© 2021 Society of Hospital Medicine
Medical ICU Insulin Infusion Protocols
Observational studies in hospitalized patients with and without diabetes indicate that hyperglycemia is a predictor of poor clinical outcome and mortality.14 Early randomized controlled trials of intensified insulin therapy in patients with surgical and medical acute critical illness reported a reduction on the risk of multiorgan failure and systemic infections,35 as well as short‐ and long‐term mortality.1, 4 Recent randomized controlled trials, however, have failed to confirm the previously suggested benefits of intensive glucose control,6 and the large multicenter normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation (NICE‐SUGAR) study reported an absolute increase in mortality rate with intensive glucose control.7 In addition, intensified insulin therapy in critically‐ill patients has been shown to be associated with a higher rate of severe hypoglycemic events than less aggressive glycemic control protocols.710 These results have led to a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.
The use of intravenous continuous insulin infusion (CII) is the preferred route of insulin administration for the management of hyperglycemia in the critical care setting.1, 11 Numerous examples of successful CII algorithms in achieving glycemic control are reported in the literature.4, 5, 12 Traditionally, order forms to titrate drip to achieve a target blood glucose (BG) range using an established algorithm or by the application of mathematical rules have been used in clinical practice. Recently, computer‐based algorithms aiming to direct the nursing staff adjusting insulin infusion rate have become commercially available.13, 14 It is not known, however, if computer‐based algorithms are superior to standard paper form‐based protocols in achieving glucose control and in reducing hypoglycemic events in critically‐ill patients. Accordingly, this multicenter randomized study aimed to determine differences in glycemic control and hypoglycemic events between treatment with a computer‐guided CII device and a standard column‐based paper algorithm in critically‐ill patients in the medical intensive care unit (ICU).
Research Design and Methods
In this multicenter, prospective, open‐label randomized study, 160 adult patients admitted to a medical ICU with new hyperglycemia or with a known history of diabetes treated with diet, insulin therapy or with any combination of oral antidiabetic agents were enrolled after written informed consent had been obtained from the patient or closest family member (Figure 1). Patients with known history of diabetes had 2 BG readings >120 mg/dL while subjects without a history of diabetes had 2 BG readings >140 mg/dL prior to enrollment. We excluded patients with acute hyperglycemic crises such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state,15 patients with severely impaired renal function (serum creatinine 3.5 mg/dL), dementia, and pregnancy. This study was conducted at 4 hospital centers including Grady Memorial Hospital, Emory University Hospital, and Piedmont Hospital in Atlanta, Georgia and the Regional Medical Center in Memphis, Tennessee.

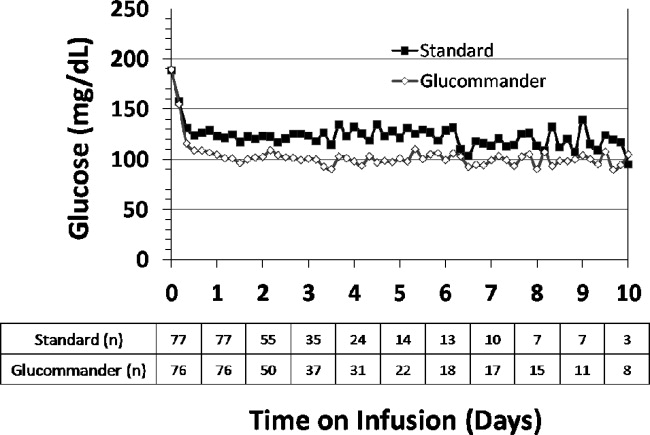
Patients were randomized using a computer randomization table to receive CII following a computer‐guided algorithm (Glucommander) or CII following a standard paper form insulin infusion algorithm. Both protocols used glulisine (Apidra) insulin and targeted a BG between 80 mg/dL and 120 mg/dL. Insulin management was directed by the specific assigned protocol and was carried out daily by the nursing staff and by members of the internal medicine residency program. The ICU physician and primary care team decided on the treatment for all other medical problem(s) for which patients were admitted. Data were collected during CII up to the first 10 days of ICU stay.
Standard and Computer‐Based CII Algorithms
The standard paper algorithm was adapted from a protocol initially published by Markovitz et al.16 (Supporting Information Appendix). The algorithm is divided into four columns based on empirically determined insulin sensitivity. The first algorithm column was for the most insulin‐sensitive patients, and the fourth algorithm column was for the most insulin resistant patients. The majority of patients started in the algorithm 1 column. Insulin‐resistant patients, such as those receiving glucocorticoids or receiving >80 units of insulin per day as outpatients, started in the algorithm 2 column. The insulin infusion rate was determined by the patient's BG level and was measured hourly until the patient was stable and within the target range. If BG targets were not achieved and the BG had not decreased by at least 60 mg/dL in the preceding hour, the patient was moved to the next column.
The characteristics and use of the Glucommander algorithm have been reported previously.13 In brief, this computer‐guided insulin algorithm directs the administration of intravenous insulin in response to BG measurement at the patient's bedside. In this study, the Glucommander program was loaded into a PalmOne (Zire 31, Tungsten E2 by Palm Inc.) handheld personal digital assistant (PDA) device. During the infusion, the nurse entered BG levels into the system and the computer recommended the insulin infusion rate and a variable time to check the next glucose testing. An alarm prompted the scheduled glucose check. The insulin infusion followed the formula: Insulin/Hour = Multiplier (BG 60). The initial multiplier or insulin sensitivity factor was 0.02. The Glucommander was programmed to adjust the multiplier to achieve and maintain target glucose.
Prior to the beginning of the study, the nursing staff at all institutions was instructed on the use of the Glucommander and paper form protocol. The insulin drip adjustment was carried out by ICU nurses in each hospital. Study investigators and coordinators rounded daily on study patients and were available for consultation and collecting data but were not involved in insulin adjustment based on the protocol.
Clinical Outcome Measures
The primary outcome of the study was to determine differences in glycemic control as measured by mean daily BG concentration between treatment groups. Secondary outcomes include differences between groups in number of hypoglycemic events (BG <60 mg/dL and <40 mg/dL), time to first glucose in target range, amount of insulin treatment (units/kg/hour), number and frequency of glucose measurements, length of stay (LOS) in the ICU and hospital, number of hyperglycemic episodes (BG >200 mg/dL), and mortality rate.
BG Monitoring
Capillary BG measurement in the standard paper protocol was performed hourly until it was within goal range for 4 hours and then every 2 hours for the duration of the infusion. Glucose measurements in the Glucommander arm were requested by the device at intervals that ranged from 20 minutes to 2 hours. The Glucommander software determined the interval between measurements based on the stability of the BG levels of the patient. The insulin infusion rate adjustment was based on the current glucose value and the slope of the glucose curve. The Glucommander alarmed at the appropriate interval to remind the nurse to check and enter the new BG value. If the BG was decreasing faster than expected, the program called for repeat BG measurements more frequently for insulin drip adjustment. If the BG was within target range for 4 consecutive readings, the Glucommander alarmed for repeat BG every 2 hours.
Laboratory Assays
Plasma glucose and glycosylated hemoglobin (HbA1c) were measured on admission. Complete blood count and complete metabolic profile were measured on admission and as otherwise determined by the treating physician.
Statistical Analysis
All data in the text, table and figures are expressed as mean standard deviation. Comparison between groups was carried out by nonparametric two‐sample Wilcoxon tests for continuous variables and chi‐square tests (or Fisher's exact tests) for categorical variables. Cochran‐Mantel‐Haenszel (CMH) or CMH exact tests were further used to adjust for site difference. Repeated measures analyses were conducted to model the probability of BG <60 mg/dL or BG<40 mg/dL based on generalized linear model with AR(1) within‐subject correlation structure. A P value <0.05 is considered as significant. We expected differences in mean BG concentration 30 mg/dL between groups. Assuming 2‐tailed alpha of 0.05, a standard deviation of approximately 40, and a one‐to‐one allocation and no subject attrition, 80 patients per treatment group were thought to be sufficient to achieve 80% power for group mean comparisons. Statistical significance was defined as a type 1 error of 0.05. Statistical analysis was performed using the SAS 9.2.
Results
The admission characteristics and clinical outcomes of interest of the study patients are shown in Table 1. A total of 160 adult patients admitted to a medical ICU with new hyperglycemia (47%) or with a known history of diabetes (53%) were randomized into the study. Of them, 7 patients were excluded due to withdrawal of consent, treatment with subcutaneous basal or supplemental short‐acting insulin during CII, or receiving less than 4 hours of CII. There were no differences in the mean age, gender, race, history of diabetes, or primary admitting diagnosis between treatment groups. The most common admitting diagnosis categories included pulmonary (22.1%), cardiovascular (21.4%), infectious (20.0%), and central nervous system (16.6%) disorders.
| Glucommander (# patients = 77) | Standard (# patients = 76) | P Value | |
|---|---|---|---|
| |||
| Age (years) | 57.8 11.0 | 58.5 13.4 | NS |
| Gender (M/F), % | 57.1/42.9 | 51.3/46.7 | NS |
| Race (W/B/H), % | 25.0/69.6/1.8 | 28.9/67.3/3.9 | NS |
| BMI (Kg/m2) | 31.6 10.4 | 30.5 8.1 | NS |
| Primary admitting diagnosis: | |||
| Cardiovascular, % | 24.7 | 18.1 | NS |
| Pulmonary, % | 24.7 | 19.4 | NS |
| Infection, % | 16.4 | 23.6 | NS |
| Cerebro‐vascular, % | 4.1 | 4.2 | NS |
| Renal, % | 1.4 | 1.4 | NS |
| Apache score | 13.4 6.1 | 16.0 8.3 | NS |
| History of diabetes, % | 53.3 | 54.3 | NS |
| Hemoglobin A1c (%) | 7.2 1.9 | 6.8 1.4 | NS |
| DM patients | 7.9 2.2 | 7.3 1.6 | NS |
| Non‐DM patients | 6.2 0.7 | 6.0 0.7 | NS |
The mean admission glucose concentration for study patients was 190.6 58.2 mg/dL and the mean A1C was 7.0 1.7%. Glycemic control parameters achieved with the CII protocols are listed in Table 2. At the start of CII, the mean BG value was similar for the Glucommander and paper protocols (189.7 64.8 mg/dL and 188.4 54.8 mg/dL, P = 0.419). The mean time to reach the BG target was shorter in the Glucommander group (4.8 2.8 vs. 7.8 9.1 hours, P < 0.001). The Glucommander group had a lower mean glucose value during insulin infusion (115.5 20.7 vs. 131.0 24.6 mg/dL, P < 0.001) and once at target goal, in a lower mean BG values (103.3 8.8 vs. 117.3 16.5 mg/dL, P < 0.001) than the standard algorithm (Figure 2). The mean inpatient BG difference between treatment groups was 15.5 mg/dL (P < 0.001), with a mean daily BG difference ranging from 17.4 mg/dL to 24.4 mg/dL less for the Glucommander group during days 2 to 6 of therapy (P < 0.01).
| Glucommander (# patients = 77) | Standard (# patients = 76) | Mean Difference (CI) or P Value | |
|---|---|---|---|
| |||
| Initial glucose (mg/dL) | 189.7 64.8 | 188.3 54.8 | 1.333 (17.701, 20.367) |
| Median (range) duration of CII (hours) | 46 (12‐240) | 47 (5‐240) | 12.939 (34.630, 8,752) |
| Insulin infusion rate (units/Kg/hour) | 0.035 0.024 | 0.028 0.021 | 0.006 (0.002, 0.014) |
| Time to achieve target BG of 80‐120 mg/dL (hours) | 4.8 2.8 | 7.8 9.1 | 3.0 (5.2, 0.9) |
| Mean BG maintained once target achieved (mg/dL) | 103.3 8.8 | 117.3 16.5 | 14.0 (18.210, 9.774) |
| % of BG tests within target range | 71.0 17.0% | 51.3 19.7% | 19.6 (13.7, 25.5) |
| Mild hypoglycemia, <60 mg/dL, n (% patients) | 33 (42.9) | 23 (31.9) | NS |
| Severe hypoglycemia, <40 mg/dL, n (% patients) | 3 (3.9) | 4 (5.6) | NS |
| Hyperglycemia, >200 mg/dL, n (% patients) | 9 (11.7) | 18 (25.0) | 0.054 |
The Glucommander algorithm was associated with tighter glycemic control and less glucose variability than the standard paper form protocol. Once patients achieved BG target, on average 71.1% of BG readings in the Glucommander and 51.3% in the standard group remained within the 80 mg/dL to 120 mg/dL target range (P < 0.001). In addition, the Glucommander was associated with a significantly lower rate of severe hyperglycemia during insulin infusion. The number of patients with 1 or more episodes of BG >200 mg/dL (11.7% vs. 25%, P = 0.057 before adjusting for potential site difference and P = 0.034 after adjusting for site difference) were less in the Glucommander group than in the standard paper regimen. In addition, 4 of these patients in spite of being on the highest insulin delivery column failed to achieve glucoses <180 and had an average in‐hospital glucose level of 204.5 32.2 mg/dL. These patients were transitioned to the Glucommander arm and withdrawn from the study. All episodes of hypoglycemia occurred after the patients achieved 1 glucose measurement within the target range. The number of patients who experienced one or more BG <40 mg/dL and <60 mg/dL was 3.9% and 42.9% in the Glucommander and 5.6% and 31.9% in the standard regimen, respectively (both, P = not significant [NS]). Similar results were obtained when site effect was accommodated (both, P = NS). Based on repeated measures analyses, the probabilities of BG reading <40 mg/dL or <60 mg/dL were not significantly different between groups (P = 0.969, P = 0.084) after accounting for within‐patient correlations with or without adjusting for time effect. None of these episodes resulted in seizures or were otherwise judged to be associated with deterioration of clinical status.
The mean insulin infusion rate was slightly higher in the Glucommander regimen but the difference was not statistically significant between groups. Patients treated with the Glucommander protocol received a mean infusion rate of 0.035 0.024 unit/kg/hour for a total of 2.85 1.93 units per hour, and those treated with the paper protocol received a 0.028 0.021 units/kg/hour for a total of 2.50 2.28 units per hour, P = 0.12 and P = 0.09, respectively.
The numbers of BG measurements were similar between the Glucommander and standard paper algorithms (44.2 39.8 and 41.2 34.5 respectively, P = NS) with the number of glucose testing per patient ranging from 6 to 175 in the Glucommander and 3 to 168 in the standard group. Similarly, when normalized to the duration of insulin infusion, the frequency of BG monitoring was not different with the protocols (0.68 0.18 and 0.62 0.22 tests/hour respectively, P = NS).
Compared to the standard paper insulin infusion algorithm, patients treated with the Glucommander device had a similar mean ICU LOS (13.4 13.8 vs. 8.5 7.6 days, P = 0.145), mean hospital LOS (17.5 15.0 days vs. 23.9 26.3 days, P = 0.704) and hospital mortality (26.0% vs. 21.9%, P = 0.561).
Discussion
This study is the first to compare the safety and efficacy of a CII via a computer‐guided algorithm and a standard paper form protocol in nonsurgical patients in the ICU. Both treatment algorithms resulted in significant improvement in glycemic control with the Glucommander achieving glycemic glucose target in a shorter time of treatment, a lower mean glucose concentration, and in greater percentage of glucose measurements maintained within target range, without an increased risk of severe hypoglycemia compared to the standard paper protocol.
Hyperglycemia in hospitalized patients is a common, serious, and costly health care problem. Evidence from observational and interventional studies indicate that hyperglycemia in critical illness is associated with an increased risk of complications and mortality.25 There is ongoing debate, however, about the optimal glucose level in hospitalized patients with critical illness. Although, several cohort studies as well as early randomized trials in ICU patients reported that intensified insulin treatment to achieve a target glucose between 80 mg/dL to 110 mg/dL reported a reduction in short‐term and long‐term mortality and rates of multiorgan failure and systemic infections compared with conventionally treated patients.3, 4, 17 More recent randomized controlled trials and meta‐analyses, however, have shown that this low BG target has been difficult to achieve without increasing the risk for severe hypoglycemia.710 In addition, recent multicenter trials have failed to show significant improvement in clinical outcome or have even shown increased mortality risk with intensive glycemic control.610 Based on these reports, the American Association of Clinical Endocrinologist (AACE) and American Diabetes Association (ADA) task force on inpatient glycemic control recommended different glycemic targets in the ICU setting. Current guidelines suggest targeting a BG level between 140 mg/dL and 180 mg/dL (7.8 and 10.0 mmol/L) for the majority of ICU patients and a lower glucose targets between 110 mg/dL and 140 mg/dL (6.1 and 7.8 mmol/L) in selected ICU patients (ie, centers with extensive experience and appropriate nursing support, cardiac surgical patients, patients with stable glycemic control without hypoglycemia). Glucose targets >180 mg/dL or <110 mg/dL are no longer recommended in ICU patients.
The rate of severe hypoglycemic events (<40 mg/dL) observed in both arms of our trial was significantly lower than those reported in recent international trials of intensive glycemic control.3, 4, 8 The overall rate of severe hypoglycemic events in international trials ranged between 5% to 28.6%.3, 4, 7, 8, 18, 19 In this trial, the number of patients with severe hypoglycemia was 3.9% in the computer‐based and 5.6% in the standard paper algorithm. Repeated measures analyses show the probabilities of BG readings <40 mg/dL were similar and not significantly different between groups (P = 0.969). We observed, however, a high rate of mild hypoglycemic events in patients treated with both insulin algorithms. The number of patients with BG <60 mg/dL was 42.9% in the Glucommander and 31.9% in the standard (P = NS). Minimizing the rate of hypoglycemia events is of major importance in hospitalized patients because it has been shown that hypoglycemia may be an independent risk factor of poor clinical outcome and mortality.20 Hypoglycemia may increase the risk of ventricular arrhythmias, in part due to the prolongation QT interval21 and can impair cerebral glucose metabolism resulting in brain metabolic dysfunction, as suggested by recent clinical studies.22 Moreover, insulin‐induced hypoglycemia is also associated with increased proinflammatory cytokines (tumor necrosis factor [TNF]‐alpha, interleukin [IL]‐1beta, IL‐6, and IL‐8) and oxidative stress23 that correlate with elevations of counterregulatory hormones (catecholamines, cortisol).
The Glucommander was associated with lower glycemic variability and with a higher percentage of BG readings within target range than patients treated with the standard paper form regimen. The clinical importance of the degree of variability and rapidity of fluctuations in glucose levels in critically ill patients is a topic of recent interest. Glycemic variability has been identified as a strong independent contributor to the risk of mortality in critically ill and surgical patients.24 Low levels of glycemic variability (standard deviation [SD] <10 mg/dL or 10‐20 mg/dL) have been shown to have a statistically significant lower risk of mortality, even after adjustment for severity of illness. Further studies are needed to determine benefits on clinical outcomes from the more consistent BG control from computer‐based titration protocols.
We acknowledge the following limitations in this multicenter open label study. First, this study was conducted in the medical ICU and excluded postsurgical patients and subjects expected to undergo a major surgical procedure during the hospital stay. Although a recent meta‐analysis9 of 26 studies involving 13,567 patients reported no benefits in the general ICU population, it found a favorable effect of intensive glycemic control on mortality in surgical ICU patients (relative risk [RR], 0.63; confidence interval [CI], 0.44‐0.91). We also excluded patients with severe renal insufficiency and patients with a history of hyperglycemic crises. In addition, our study was not powered to demonstrate differences in mortality or clinical outcome between treatment groups, and the BG targets used in this study were lower than glycemic targets recently recommended by the AACE and ADA inpatient glycemic control task force.25 Raising the BG targets is likely to reduce or prevent the rate of mild and severe hypoglycemic events in the ICU.
In conclusion, the computer‐guided algorithm resulted in a more rapid and tighter glycemic control with a similar rate of hypoglycemic events than the standard paper form protocol in medical ICU patients. Our study suggests that, both treatment algorithms are appropriate alternatives for the management of hyperglycemia in critically ill patients, and the choice depends on a physician's preferences, cost considerations, and the availability of the computer guided algorithm. Large randomized clinical trials are needed to test the impact of the new AACE/ADA recommended BG targets in reducing hypoglycemic events, hospital complications, and hospital mortality in critically ill patients in the ICU.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180:821–827.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360:1283–1297.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12:R120.
- ,.Tight glucose control and hypoglycemia.Crit Care Med.2008;36:1391; author reply 1391–1392.
- ,.ICU care for patients with diabetes.Curr Opin Endocrinol Diabetes Obes.2004;11:75–81.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit.Diabetes Technol Ther.2007;9:232–240.
- ,,, et al.Hyperglycemic crises in diabetes.Diabetes Care.2004;27Suppl 1:S94–S102.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8:10–18.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
- ,,, et al.Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients.Crit Care Med.2008;36:3190–3197.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,, et al.Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2009;94:709–728.
- ,,, et al.Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study.Crit Care Med.2008;36:3233–3238.
- ,,, et al.Proinflammatory cytokines in response to insulin‐induced hypoglycemic stress in healthy subjects.Metabolism.2009;58:443–448.
- ,,, et al.Blood glucose variability is associated with mortality in the surgical intensive care unit.Am Surg.2008;74:679–685; discussion685.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Diabetes Care.2009;32:1119–1131.
Observational studies in hospitalized patients with and without diabetes indicate that hyperglycemia is a predictor of poor clinical outcome and mortality.14 Early randomized controlled trials of intensified insulin therapy in patients with surgical and medical acute critical illness reported a reduction on the risk of multiorgan failure and systemic infections,35 as well as short‐ and long‐term mortality.1, 4 Recent randomized controlled trials, however, have failed to confirm the previously suggested benefits of intensive glucose control,6 and the large multicenter normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation (NICE‐SUGAR) study reported an absolute increase in mortality rate with intensive glucose control.7 In addition, intensified insulin therapy in critically‐ill patients has been shown to be associated with a higher rate of severe hypoglycemic events than less aggressive glycemic control protocols.710 These results have led to a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.
The use of intravenous continuous insulin infusion (CII) is the preferred route of insulin administration for the management of hyperglycemia in the critical care setting.1, 11 Numerous examples of successful CII algorithms in achieving glycemic control are reported in the literature.4, 5, 12 Traditionally, order forms to titrate drip to achieve a target blood glucose (BG) range using an established algorithm or by the application of mathematical rules have been used in clinical practice. Recently, computer‐based algorithms aiming to direct the nursing staff adjusting insulin infusion rate have become commercially available.13, 14 It is not known, however, if computer‐based algorithms are superior to standard paper form‐based protocols in achieving glucose control and in reducing hypoglycemic events in critically‐ill patients. Accordingly, this multicenter randomized study aimed to determine differences in glycemic control and hypoglycemic events between treatment with a computer‐guided CII device and a standard column‐based paper algorithm in critically‐ill patients in the medical intensive care unit (ICU).
Research Design and Methods
In this multicenter, prospective, open‐label randomized study, 160 adult patients admitted to a medical ICU with new hyperglycemia or with a known history of diabetes treated with diet, insulin therapy or with any combination of oral antidiabetic agents were enrolled after written informed consent had been obtained from the patient or closest family member (Figure 1). Patients with known history of diabetes had 2 BG readings >120 mg/dL while subjects without a history of diabetes had 2 BG readings >140 mg/dL prior to enrollment. We excluded patients with acute hyperglycemic crises such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state,15 patients with severely impaired renal function (serum creatinine 3.5 mg/dL), dementia, and pregnancy. This study was conducted at 4 hospital centers including Grady Memorial Hospital, Emory University Hospital, and Piedmont Hospital in Atlanta, Georgia and the Regional Medical Center in Memphis, Tennessee.


Patients were randomized using a computer randomization table to receive CII following a computer‐guided algorithm (Glucommander) or CII following a standard paper form insulin infusion algorithm. Both protocols used glulisine (Apidra) insulin and targeted a BG between 80 mg/dL and 120 mg/dL. Insulin management was directed by the specific assigned protocol and was carried out daily by the nursing staff and by members of the internal medicine residency program. The ICU physician and primary care team decided on the treatment for all other medical problem(s) for which patients were admitted. Data were collected during CII up to the first 10 days of ICU stay.
Standard and Computer‐Based CII Algorithms
The standard paper algorithm was adapted from a protocol initially published by Markovitz et al.16 (Supporting Information Appendix). The algorithm is divided into four columns based on empirically determined insulin sensitivity. The first algorithm column was for the most insulin‐sensitive patients, and the fourth algorithm column was for the most insulin resistant patients. The majority of patients started in the algorithm 1 column. Insulin‐resistant patients, such as those receiving glucocorticoids or receiving >80 units of insulin per day as outpatients, started in the algorithm 2 column. The insulin infusion rate was determined by the patient's BG level and was measured hourly until the patient was stable and within the target range. If BG targets were not achieved and the BG had not decreased by at least 60 mg/dL in the preceding hour, the patient was moved to the next column.
The characteristics and use of the Glucommander algorithm have been reported previously.13 In brief, this computer‐guided insulin algorithm directs the administration of intravenous insulin in response to BG measurement at the patient's bedside. In this study, the Glucommander program was loaded into a PalmOne (Zire 31, Tungsten E2 by Palm Inc.) handheld personal digital assistant (PDA) device. During the infusion, the nurse entered BG levels into the system and the computer recommended the insulin infusion rate and a variable time to check the next glucose testing. An alarm prompted the scheduled glucose check. The insulin infusion followed the formula: Insulin/Hour = Multiplier (BG 60). The initial multiplier or insulin sensitivity factor was 0.02. The Glucommander was programmed to adjust the multiplier to achieve and maintain target glucose.
Prior to the beginning of the study, the nursing staff at all institutions was instructed on the use of the Glucommander and paper form protocol. The insulin drip adjustment was carried out by ICU nurses in each hospital. Study investigators and coordinators rounded daily on study patients and were available for consultation and collecting data but were not involved in insulin adjustment based on the protocol.
Clinical Outcome Measures
The primary outcome of the study was to determine differences in glycemic control as measured by mean daily BG concentration between treatment groups. Secondary outcomes include differences between groups in number of hypoglycemic events (BG <60 mg/dL and <40 mg/dL), time to first glucose in target range, amount of insulin treatment (units/kg/hour), number and frequency of glucose measurements, length of stay (LOS) in the ICU and hospital, number of hyperglycemic episodes (BG >200 mg/dL), and mortality rate.
BG Monitoring
Capillary BG measurement in the standard paper protocol was performed hourly until it was within goal range for 4 hours and then every 2 hours for the duration of the infusion. Glucose measurements in the Glucommander arm were requested by the device at intervals that ranged from 20 minutes to 2 hours. The Glucommander software determined the interval between measurements based on the stability of the BG levels of the patient. The insulin infusion rate adjustment was based on the current glucose value and the slope of the glucose curve. The Glucommander alarmed at the appropriate interval to remind the nurse to check and enter the new BG value. If the BG was decreasing faster than expected, the program called for repeat BG measurements more frequently for insulin drip adjustment. If the BG was within target range for 4 consecutive readings, the Glucommander alarmed for repeat BG every 2 hours.
Laboratory Assays
Plasma glucose and glycosylated hemoglobin (HbA1c) were measured on admission. Complete blood count and complete metabolic profile were measured on admission and as otherwise determined by the treating physician.
Statistical Analysis
All data in the text, table and figures are expressed as mean standard deviation. Comparison between groups was carried out by nonparametric two‐sample Wilcoxon tests for continuous variables and chi‐square tests (or Fisher's exact tests) for categorical variables. Cochran‐Mantel‐Haenszel (CMH) or CMH exact tests were further used to adjust for site difference. Repeated measures analyses were conducted to model the probability of BG <60 mg/dL or BG<40 mg/dL based on generalized linear model with AR(1) within‐subject correlation structure. A P value <0.05 is considered as significant. We expected differences in mean BG concentration 30 mg/dL between groups. Assuming 2‐tailed alpha of 0.05, a standard deviation of approximately 40, and a one‐to‐one allocation and no subject attrition, 80 patients per treatment group were thought to be sufficient to achieve 80% power for group mean comparisons. Statistical significance was defined as a type 1 error of 0.05. Statistical analysis was performed using the SAS 9.2.
Results
The admission characteristics and clinical outcomes of interest of the study patients are shown in Table 1. A total of 160 adult patients admitted to a medical ICU with new hyperglycemia (47%) or with a known history of diabetes (53%) were randomized into the study. Of them, 7 patients were excluded due to withdrawal of consent, treatment with subcutaneous basal or supplemental short‐acting insulin during CII, or receiving less than 4 hours of CII. There were no differences in the mean age, gender, race, history of diabetes, or primary admitting diagnosis between treatment groups. The most common admitting diagnosis categories included pulmonary (22.1%), cardiovascular (21.4%), infectious (20.0%), and central nervous system (16.6%) disorders.
| Glucommander (# patients = 77) | Standard (# patients = 76) | P Value | |
|---|---|---|---|
| |||
| Age (years) | 57.8 11.0 | 58.5 13.4 | NS |
| Gender (M/F), % | 57.1/42.9 | 51.3/46.7 | NS |
| Race (W/B/H), % | 25.0/69.6/1.8 | 28.9/67.3/3.9 | NS |
| BMI (Kg/m2) | 31.6 10.4 | 30.5 8.1 | NS |
| Primary admitting diagnosis: | |||
| Cardiovascular, % | 24.7 | 18.1 | NS |
| Pulmonary, % | 24.7 | 19.4 | NS |
| Infection, % | 16.4 | 23.6 | NS |
| Cerebro‐vascular, % | 4.1 | 4.2 | NS |
| Renal, % | 1.4 | 1.4 | NS |
| Apache score | 13.4 6.1 | 16.0 8.3 | NS |
| History of diabetes, % | 53.3 | 54.3 | NS |
| Hemoglobin A1c (%) | 7.2 1.9 | 6.8 1.4 | NS |
| DM patients | 7.9 2.2 | 7.3 1.6 | NS |
| Non‐DM patients | 6.2 0.7 | 6.0 0.7 | NS |
The mean admission glucose concentration for study patients was 190.6 58.2 mg/dL and the mean A1C was 7.0 1.7%. Glycemic control parameters achieved with the CII protocols are listed in Table 2. At the start of CII, the mean BG value was similar for the Glucommander and paper protocols (189.7 64.8 mg/dL and 188.4 54.8 mg/dL, P = 0.419). The mean time to reach the BG target was shorter in the Glucommander group (4.8 2.8 vs. 7.8 9.1 hours, P < 0.001). The Glucommander group had a lower mean glucose value during insulin infusion (115.5 20.7 vs. 131.0 24.6 mg/dL, P < 0.001) and once at target goal, in a lower mean BG values (103.3 8.8 vs. 117.3 16.5 mg/dL, P < 0.001) than the standard algorithm (Figure 2). The mean inpatient BG difference between treatment groups was 15.5 mg/dL (P < 0.001), with a mean daily BG difference ranging from 17.4 mg/dL to 24.4 mg/dL less for the Glucommander group during days 2 to 6 of therapy (P < 0.01).
| Glucommander (# patients = 77) | Standard (# patients = 76) | Mean Difference (CI) or P Value | |
|---|---|---|---|
| |||
| Initial glucose (mg/dL) | 189.7 64.8 | 188.3 54.8 | 1.333 (17.701, 20.367) |
| Median (range) duration of CII (hours) | 46 (12‐240) | 47 (5‐240) | 12.939 (34.630, 8,752) |
| Insulin infusion rate (units/Kg/hour) | 0.035 0.024 | 0.028 0.021 | 0.006 (0.002, 0.014) |
| Time to achieve target BG of 80‐120 mg/dL (hours) | 4.8 2.8 | 7.8 9.1 | 3.0 (5.2, 0.9) |
| Mean BG maintained once target achieved (mg/dL) | 103.3 8.8 | 117.3 16.5 | 14.0 (18.210, 9.774) |
| % of BG tests within target range | 71.0 17.0% | 51.3 19.7% | 19.6 (13.7, 25.5) |
| Mild hypoglycemia, <60 mg/dL, n (% patients) | 33 (42.9) | 23 (31.9) | NS |
| Severe hypoglycemia, <40 mg/dL, n (% patients) | 3 (3.9) | 4 (5.6) | NS |
| Hyperglycemia, >200 mg/dL, n (% patients) | 9 (11.7) | 18 (25.0) | 0.054 |
The Glucommander algorithm was associated with tighter glycemic control and less glucose variability than the standard paper form protocol. Once patients achieved BG target, on average 71.1% of BG readings in the Glucommander and 51.3% in the standard group remained within the 80 mg/dL to 120 mg/dL target range (P < 0.001). In addition, the Glucommander was associated with a significantly lower rate of severe hyperglycemia during insulin infusion. The number of patients with 1 or more episodes of BG >200 mg/dL (11.7% vs. 25%, P = 0.057 before adjusting for potential site difference and P = 0.034 after adjusting for site difference) were less in the Glucommander group than in the standard paper regimen. In addition, 4 of these patients in spite of being on the highest insulin delivery column failed to achieve glucoses <180 and had an average in‐hospital glucose level of 204.5 32.2 mg/dL. These patients were transitioned to the Glucommander arm and withdrawn from the study. All episodes of hypoglycemia occurred after the patients achieved 1 glucose measurement within the target range. The number of patients who experienced one or more BG <40 mg/dL and <60 mg/dL was 3.9% and 42.9% in the Glucommander and 5.6% and 31.9% in the standard regimen, respectively (both, P = not significant [NS]). Similar results were obtained when site effect was accommodated (both, P = NS). Based on repeated measures analyses, the probabilities of BG reading <40 mg/dL or <60 mg/dL were not significantly different between groups (P = 0.969, P = 0.084) after accounting for within‐patient correlations with or without adjusting for time effect. None of these episodes resulted in seizures or were otherwise judged to be associated with deterioration of clinical status.
The mean insulin infusion rate was slightly higher in the Glucommander regimen but the difference was not statistically significant between groups. Patients treated with the Glucommander protocol received a mean infusion rate of 0.035 0.024 unit/kg/hour for a total of 2.85 1.93 units per hour, and those treated with the paper protocol received a 0.028 0.021 units/kg/hour for a total of 2.50 2.28 units per hour, P = 0.12 and P = 0.09, respectively.
The numbers of BG measurements were similar between the Glucommander and standard paper algorithms (44.2 39.8 and 41.2 34.5 respectively, P = NS) with the number of glucose testing per patient ranging from 6 to 175 in the Glucommander and 3 to 168 in the standard group. Similarly, when normalized to the duration of insulin infusion, the frequency of BG monitoring was not different with the protocols (0.68 0.18 and 0.62 0.22 tests/hour respectively, P = NS).
Compared to the standard paper insulin infusion algorithm, patients treated with the Glucommander device had a similar mean ICU LOS (13.4 13.8 vs. 8.5 7.6 days, P = 0.145), mean hospital LOS (17.5 15.0 days vs. 23.9 26.3 days, P = 0.704) and hospital mortality (26.0% vs. 21.9%, P = 0.561).
Discussion
This study is the first to compare the safety and efficacy of a CII via a computer‐guided algorithm and a standard paper form protocol in nonsurgical patients in the ICU. Both treatment algorithms resulted in significant improvement in glycemic control with the Glucommander achieving glycemic glucose target in a shorter time of treatment, a lower mean glucose concentration, and in greater percentage of glucose measurements maintained within target range, without an increased risk of severe hypoglycemia compared to the standard paper protocol.
Hyperglycemia in hospitalized patients is a common, serious, and costly health care problem. Evidence from observational and interventional studies indicate that hyperglycemia in critical illness is associated with an increased risk of complications and mortality.25 There is ongoing debate, however, about the optimal glucose level in hospitalized patients with critical illness. Although, several cohort studies as well as early randomized trials in ICU patients reported that intensified insulin treatment to achieve a target glucose between 80 mg/dL to 110 mg/dL reported a reduction in short‐term and long‐term mortality and rates of multiorgan failure and systemic infections compared with conventionally treated patients.3, 4, 17 More recent randomized controlled trials and meta‐analyses, however, have shown that this low BG target has been difficult to achieve without increasing the risk for severe hypoglycemia.710 In addition, recent multicenter trials have failed to show significant improvement in clinical outcome or have even shown increased mortality risk with intensive glycemic control.610 Based on these reports, the American Association of Clinical Endocrinologist (AACE) and American Diabetes Association (ADA) task force on inpatient glycemic control recommended different glycemic targets in the ICU setting. Current guidelines suggest targeting a BG level between 140 mg/dL and 180 mg/dL (7.8 and 10.0 mmol/L) for the majority of ICU patients and a lower glucose targets between 110 mg/dL and 140 mg/dL (6.1 and 7.8 mmol/L) in selected ICU patients (ie, centers with extensive experience and appropriate nursing support, cardiac surgical patients, patients with stable glycemic control without hypoglycemia). Glucose targets >180 mg/dL or <110 mg/dL are no longer recommended in ICU patients.
The rate of severe hypoglycemic events (<40 mg/dL) observed in both arms of our trial was significantly lower than those reported in recent international trials of intensive glycemic control.3, 4, 8 The overall rate of severe hypoglycemic events in international trials ranged between 5% to 28.6%.3, 4, 7, 8, 18, 19 In this trial, the number of patients with severe hypoglycemia was 3.9% in the computer‐based and 5.6% in the standard paper algorithm. Repeated measures analyses show the probabilities of BG readings <40 mg/dL were similar and not significantly different between groups (P = 0.969). We observed, however, a high rate of mild hypoglycemic events in patients treated with both insulin algorithms. The number of patients with BG <60 mg/dL was 42.9% in the Glucommander and 31.9% in the standard (P = NS). Minimizing the rate of hypoglycemia events is of major importance in hospitalized patients because it has been shown that hypoglycemia may be an independent risk factor of poor clinical outcome and mortality.20 Hypoglycemia may increase the risk of ventricular arrhythmias, in part due to the prolongation QT interval21 and can impair cerebral glucose metabolism resulting in brain metabolic dysfunction, as suggested by recent clinical studies.22 Moreover, insulin‐induced hypoglycemia is also associated with increased proinflammatory cytokines (tumor necrosis factor [TNF]‐alpha, interleukin [IL]‐1beta, IL‐6, and IL‐8) and oxidative stress23 that correlate with elevations of counterregulatory hormones (catecholamines, cortisol).
The Glucommander was associated with lower glycemic variability and with a higher percentage of BG readings within target range than patients treated with the standard paper form regimen. The clinical importance of the degree of variability and rapidity of fluctuations in glucose levels in critically ill patients is a topic of recent interest. Glycemic variability has been identified as a strong independent contributor to the risk of mortality in critically ill and surgical patients.24 Low levels of glycemic variability (standard deviation [SD] <10 mg/dL or 10‐20 mg/dL) have been shown to have a statistically significant lower risk of mortality, even after adjustment for severity of illness. Further studies are needed to determine benefits on clinical outcomes from the more consistent BG control from computer‐based titration protocols.
We acknowledge the following limitations in this multicenter open label study. First, this study was conducted in the medical ICU and excluded postsurgical patients and subjects expected to undergo a major surgical procedure during the hospital stay. Although a recent meta‐analysis9 of 26 studies involving 13,567 patients reported no benefits in the general ICU population, it found a favorable effect of intensive glycemic control on mortality in surgical ICU patients (relative risk [RR], 0.63; confidence interval [CI], 0.44‐0.91). We also excluded patients with severe renal insufficiency and patients with a history of hyperglycemic crises. In addition, our study was not powered to demonstrate differences in mortality or clinical outcome between treatment groups, and the BG targets used in this study were lower than glycemic targets recently recommended by the AACE and ADA inpatient glycemic control task force.25 Raising the BG targets is likely to reduce or prevent the rate of mild and severe hypoglycemic events in the ICU.
In conclusion, the computer‐guided algorithm resulted in a more rapid and tighter glycemic control with a similar rate of hypoglycemic events than the standard paper form protocol in medical ICU patients. Our study suggests that, both treatment algorithms are appropriate alternatives for the management of hyperglycemia in critically ill patients, and the choice depends on a physician's preferences, cost considerations, and the availability of the computer guided algorithm. Large randomized clinical trials are needed to test the impact of the new AACE/ADA recommended BG targets in reducing hypoglycemic events, hospital complications, and hospital mortality in critically ill patients in the ICU.
Observational studies in hospitalized patients with and without diabetes indicate that hyperglycemia is a predictor of poor clinical outcome and mortality.14 Early randomized controlled trials of intensified insulin therapy in patients with surgical and medical acute critical illness reported a reduction on the risk of multiorgan failure and systemic infections,35 as well as short‐ and long‐term mortality.1, 4 Recent randomized controlled trials, however, have failed to confirm the previously suggested benefits of intensive glucose control,6 and the large multicenter normoglycaemia in intensive care evaluation and survival using glucose algorithm regulation (NICE‐SUGAR) study reported an absolute increase in mortality rate with intensive glucose control.7 In addition, intensified insulin therapy in critically‐ill patients has been shown to be associated with a higher rate of severe hypoglycemic events than less aggressive glycemic control protocols.710 These results have led to a heightened interest in improving the quality and safety of the management of diabetes and hyperglycemia in the hospital.
The use of intravenous continuous insulin infusion (CII) is the preferred route of insulin administration for the management of hyperglycemia in the critical care setting.1, 11 Numerous examples of successful CII algorithms in achieving glycemic control are reported in the literature.4, 5, 12 Traditionally, order forms to titrate drip to achieve a target blood glucose (BG) range using an established algorithm or by the application of mathematical rules have been used in clinical practice. Recently, computer‐based algorithms aiming to direct the nursing staff adjusting insulin infusion rate have become commercially available.13, 14 It is not known, however, if computer‐based algorithms are superior to standard paper form‐based protocols in achieving glucose control and in reducing hypoglycemic events in critically‐ill patients. Accordingly, this multicenter randomized study aimed to determine differences in glycemic control and hypoglycemic events between treatment with a computer‐guided CII device and a standard column‐based paper algorithm in critically‐ill patients in the medical intensive care unit (ICU).
Research Design and Methods
In this multicenter, prospective, open‐label randomized study, 160 adult patients admitted to a medical ICU with new hyperglycemia or with a known history of diabetes treated with diet, insulin therapy or with any combination of oral antidiabetic agents were enrolled after written informed consent had been obtained from the patient or closest family member (Figure 1). Patients with known history of diabetes had 2 BG readings >120 mg/dL while subjects without a history of diabetes had 2 BG readings >140 mg/dL prior to enrollment. We excluded patients with acute hyperglycemic crises such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state,15 patients with severely impaired renal function (serum creatinine 3.5 mg/dL), dementia, and pregnancy. This study was conducted at 4 hospital centers including Grady Memorial Hospital, Emory University Hospital, and Piedmont Hospital in Atlanta, Georgia and the Regional Medical Center in Memphis, Tennessee.


Patients were randomized using a computer randomization table to receive CII following a computer‐guided algorithm (Glucommander) or CII following a standard paper form insulin infusion algorithm. Both protocols used glulisine (Apidra) insulin and targeted a BG between 80 mg/dL and 120 mg/dL. Insulin management was directed by the specific assigned protocol and was carried out daily by the nursing staff and by members of the internal medicine residency program. The ICU physician and primary care team decided on the treatment for all other medical problem(s) for which patients were admitted. Data were collected during CII up to the first 10 days of ICU stay.
Standard and Computer‐Based CII Algorithms
The standard paper algorithm was adapted from a protocol initially published by Markovitz et al.16 (Supporting Information Appendix). The algorithm is divided into four columns based on empirically determined insulin sensitivity. The first algorithm column was for the most insulin‐sensitive patients, and the fourth algorithm column was for the most insulin resistant patients. The majority of patients started in the algorithm 1 column. Insulin‐resistant patients, such as those receiving glucocorticoids or receiving >80 units of insulin per day as outpatients, started in the algorithm 2 column. The insulin infusion rate was determined by the patient's BG level and was measured hourly until the patient was stable and within the target range. If BG targets were not achieved and the BG had not decreased by at least 60 mg/dL in the preceding hour, the patient was moved to the next column.
The characteristics and use of the Glucommander algorithm have been reported previously.13 In brief, this computer‐guided insulin algorithm directs the administration of intravenous insulin in response to BG measurement at the patient's bedside. In this study, the Glucommander program was loaded into a PalmOne (Zire 31, Tungsten E2 by Palm Inc.) handheld personal digital assistant (PDA) device. During the infusion, the nurse entered BG levels into the system and the computer recommended the insulin infusion rate and a variable time to check the next glucose testing. An alarm prompted the scheduled glucose check. The insulin infusion followed the formula: Insulin/Hour = Multiplier (BG 60). The initial multiplier or insulin sensitivity factor was 0.02. The Glucommander was programmed to adjust the multiplier to achieve and maintain target glucose.
Prior to the beginning of the study, the nursing staff at all institutions was instructed on the use of the Glucommander and paper form protocol. The insulin drip adjustment was carried out by ICU nurses in each hospital. Study investigators and coordinators rounded daily on study patients and were available for consultation and collecting data but were not involved in insulin adjustment based on the protocol.
Clinical Outcome Measures
The primary outcome of the study was to determine differences in glycemic control as measured by mean daily BG concentration between treatment groups. Secondary outcomes include differences between groups in number of hypoglycemic events (BG <60 mg/dL and <40 mg/dL), time to first glucose in target range, amount of insulin treatment (units/kg/hour), number and frequency of glucose measurements, length of stay (LOS) in the ICU and hospital, number of hyperglycemic episodes (BG >200 mg/dL), and mortality rate.
BG Monitoring
Capillary BG measurement in the standard paper protocol was performed hourly until it was within goal range for 4 hours and then every 2 hours for the duration of the infusion. Glucose measurements in the Glucommander arm were requested by the device at intervals that ranged from 20 minutes to 2 hours. The Glucommander software determined the interval between measurements based on the stability of the BG levels of the patient. The insulin infusion rate adjustment was based on the current glucose value and the slope of the glucose curve. The Glucommander alarmed at the appropriate interval to remind the nurse to check and enter the new BG value. If the BG was decreasing faster than expected, the program called for repeat BG measurements more frequently for insulin drip adjustment. If the BG was within target range for 4 consecutive readings, the Glucommander alarmed for repeat BG every 2 hours.
Laboratory Assays
Plasma glucose and glycosylated hemoglobin (HbA1c) were measured on admission. Complete blood count and complete metabolic profile were measured on admission and as otherwise determined by the treating physician.
Statistical Analysis
All data in the text, table and figures are expressed as mean standard deviation. Comparison between groups was carried out by nonparametric two‐sample Wilcoxon tests for continuous variables and chi‐square tests (or Fisher's exact tests) for categorical variables. Cochran‐Mantel‐Haenszel (CMH) or CMH exact tests were further used to adjust for site difference. Repeated measures analyses were conducted to model the probability of BG <60 mg/dL or BG<40 mg/dL based on generalized linear model with AR(1) within‐subject correlation structure. A P value <0.05 is considered as significant. We expected differences in mean BG concentration 30 mg/dL between groups. Assuming 2‐tailed alpha of 0.05, a standard deviation of approximately 40, and a one‐to‐one allocation and no subject attrition, 80 patients per treatment group were thought to be sufficient to achieve 80% power for group mean comparisons. Statistical significance was defined as a type 1 error of 0.05. Statistical analysis was performed using the SAS 9.2.
Results
The admission characteristics and clinical outcomes of interest of the study patients are shown in Table 1. A total of 160 adult patients admitted to a medical ICU with new hyperglycemia (47%) or with a known history of diabetes (53%) were randomized into the study. Of them, 7 patients were excluded due to withdrawal of consent, treatment with subcutaneous basal or supplemental short‐acting insulin during CII, or receiving less than 4 hours of CII. There were no differences in the mean age, gender, race, history of diabetes, or primary admitting diagnosis between treatment groups. The most common admitting diagnosis categories included pulmonary (22.1%), cardiovascular (21.4%), infectious (20.0%), and central nervous system (16.6%) disorders.
| Glucommander (# patients = 77) | Standard (# patients = 76) | P Value | |
|---|---|---|---|
| |||
| Age (years) | 57.8 11.0 | 58.5 13.4 | NS |
| Gender (M/F), % | 57.1/42.9 | 51.3/46.7 | NS |
| Race (W/B/H), % | 25.0/69.6/1.8 | 28.9/67.3/3.9 | NS |
| BMI (Kg/m2) | 31.6 10.4 | 30.5 8.1 | NS |
| Primary admitting diagnosis: | |||
| Cardiovascular, % | 24.7 | 18.1 | NS |
| Pulmonary, % | 24.7 | 19.4 | NS |
| Infection, % | 16.4 | 23.6 | NS |
| Cerebro‐vascular, % | 4.1 | 4.2 | NS |
| Renal, % | 1.4 | 1.4 | NS |
| Apache score | 13.4 6.1 | 16.0 8.3 | NS |
| History of diabetes, % | 53.3 | 54.3 | NS |
| Hemoglobin A1c (%) | 7.2 1.9 | 6.8 1.4 | NS |
| DM patients | 7.9 2.2 | 7.3 1.6 | NS |
| Non‐DM patients | 6.2 0.7 | 6.0 0.7 | NS |
The mean admission glucose concentration for study patients was 190.6 58.2 mg/dL and the mean A1C was 7.0 1.7%. Glycemic control parameters achieved with the CII protocols are listed in Table 2. At the start of CII, the mean BG value was similar for the Glucommander and paper protocols (189.7 64.8 mg/dL and 188.4 54.8 mg/dL, P = 0.419). The mean time to reach the BG target was shorter in the Glucommander group (4.8 2.8 vs. 7.8 9.1 hours, P < 0.001). The Glucommander group had a lower mean glucose value during insulin infusion (115.5 20.7 vs. 131.0 24.6 mg/dL, P < 0.001) and once at target goal, in a lower mean BG values (103.3 8.8 vs. 117.3 16.5 mg/dL, P < 0.001) than the standard algorithm (Figure 2). The mean inpatient BG difference between treatment groups was 15.5 mg/dL (P < 0.001), with a mean daily BG difference ranging from 17.4 mg/dL to 24.4 mg/dL less for the Glucommander group during days 2 to 6 of therapy (P < 0.01).
| Glucommander (# patients = 77) | Standard (# patients = 76) | Mean Difference (CI) or P Value | |
|---|---|---|---|
| |||
| Initial glucose (mg/dL) | 189.7 64.8 | 188.3 54.8 | 1.333 (17.701, 20.367) |
| Median (range) duration of CII (hours) | 46 (12‐240) | 47 (5‐240) | 12.939 (34.630, 8,752) |
| Insulin infusion rate (units/Kg/hour) | 0.035 0.024 | 0.028 0.021 | 0.006 (0.002, 0.014) |
| Time to achieve target BG of 80‐120 mg/dL (hours) | 4.8 2.8 | 7.8 9.1 | 3.0 (5.2, 0.9) |
| Mean BG maintained once target achieved (mg/dL) | 103.3 8.8 | 117.3 16.5 | 14.0 (18.210, 9.774) |
| % of BG tests within target range | 71.0 17.0% | 51.3 19.7% | 19.6 (13.7, 25.5) |
| Mild hypoglycemia, <60 mg/dL, n (% patients) | 33 (42.9) | 23 (31.9) | NS |
| Severe hypoglycemia, <40 mg/dL, n (% patients) | 3 (3.9) | 4 (5.6) | NS |
| Hyperglycemia, >200 mg/dL, n (% patients) | 9 (11.7) | 18 (25.0) | 0.054 |
The Glucommander algorithm was associated with tighter glycemic control and less glucose variability than the standard paper form protocol. Once patients achieved BG target, on average 71.1% of BG readings in the Glucommander and 51.3% in the standard group remained within the 80 mg/dL to 120 mg/dL target range (P < 0.001). In addition, the Glucommander was associated with a significantly lower rate of severe hyperglycemia during insulin infusion. The number of patients with 1 or more episodes of BG >200 mg/dL (11.7% vs. 25%, P = 0.057 before adjusting for potential site difference and P = 0.034 after adjusting for site difference) were less in the Glucommander group than in the standard paper regimen. In addition, 4 of these patients in spite of being on the highest insulin delivery column failed to achieve glucoses <180 and had an average in‐hospital glucose level of 204.5 32.2 mg/dL. These patients were transitioned to the Glucommander arm and withdrawn from the study. All episodes of hypoglycemia occurred after the patients achieved 1 glucose measurement within the target range. The number of patients who experienced one or more BG <40 mg/dL and <60 mg/dL was 3.9% and 42.9% in the Glucommander and 5.6% and 31.9% in the standard regimen, respectively (both, P = not significant [NS]). Similar results were obtained when site effect was accommodated (both, P = NS). Based on repeated measures analyses, the probabilities of BG reading <40 mg/dL or <60 mg/dL were not significantly different between groups (P = 0.969, P = 0.084) after accounting for within‐patient correlations with or without adjusting for time effect. None of these episodes resulted in seizures or were otherwise judged to be associated with deterioration of clinical status.
The mean insulin infusion rate was slightly higher in the Glucommander regimen but the difference was not statistically significant between groups. Patients treated with the Glucommander protocol received a mean infusion rate of 0.035 0.024 unit/kg/hour for a total of 2.85 1.93 units per hour, and those treated with the paper protocol received a 0.028 0.021 units/kg/hour for a total of 2.50 2.28 units per hour, P = 0.12 and P = 0.09, respectively.
The numbers of BG measurements were similar between the Glucommander and standard paper algorithms (44.2 39.8 and 41.2 34.5 respectively, P = NS) with the number of glucose testing per patient ranging from 6 to 175 in the Glucommander and 3 to 168 in the standard group. Similarly, when normalized to the duration of insulin infusion, the frequency of BG monitoring was not different with the protocols (0.68 0.18 and 0.62 0.22 tests/hour respectively, P = NS).
Compared to the standard paper insulin infusion algorithm, patients treated with the Glucommander device had a similar mean ICU LOS (13.4 13.8 vs. 8.5 7.6 days, P = 0.145), mean hospital LOS (17.5 15.0 days vs. 23.9 26.3 days, P = 0.704) and hospital mortality (26.0% vs. 21.9%, P = 0.561).
Discussion
This study is the first to compare the safety and efficacy of a CII via a computer‐guided algorithm and a standard paper form protocol in nonsurgical patients in the ICU. Both treatment algorithms resulted in significant improvement in glycemic control with the Glucommander achieving glycemic glucose target in a shorter time of treatment, a lower mean glucose concentration, and in greater percentage of glucose measurements maintained within target range, without an increased risk of severe hypoglycemia compared to the standard paper protocol.
Hyperglycemia in hospitalized patients is a common, serious, and costly health care problem. Evidence from observational and interventional studies indicate that hyperglycemia in critical illness is associated with an increased risk of complications and mortality.25 There is ongoing debate, however, about the optimal glucose level in hospitalized patients with critical illness. Although, several cohort studies as well as early randomized trials in ICU patients reported that intensified insulin treatment to achieve a target glucose between 80 mg/dL to 110 mg/dL reported a reduction in short‐term and long‐term mortality and rates of multiorgan failure and systemic infections compared with conventionally treated patients.3, 4, 17 More recent randomized controlled trials and meta‐analyses, however, have shown that this low BG target has been difficult to achieve without increasing the risk for severe hypoglycemia.710 In addition, recent multicenter trials have failed to show significant improvement in clinical outcome or have even shown increased mortality risk with intensive glycemic control.610 Based on these reports, the American Association of Clinical Endocrinologist (AACE) and American Diabetes Association (ADA) task force on inpatient glycemic control recommended different glycemic targets in the ICU setting. Current guidelines suggest targeting a BG level between 140 mg/dL and 180 mg/dL (7.8 and 10.0 mmol/L) for the majority of ICU patients and a lower glucose targets between 110 mg/dL and 140 mg/dL (6.1 and 7.8 mmol/L) in selected ICU patients (ie, centers with extensive experience and appropriate nursing support, cardiac surgical patients, patients with stable glycemic control without hypoglycemia). Glucose targets >180 mg/dL or <110 mg/dL are no longer recommended in ICU patients.
The rate of severe hypoglycemic events (<40 mg/dL) observed in both arms of our trial was significantly lower than those reported in recent international trials of intensive glycemic control.3, 4, 8 The overall rate of severe hypoglycemic events in international trials ranged between 5% to 28.6%.3, 4, 7, 8, 18, 19 In this trial, the number of patients with severe hypoglycemia was 3.9% in the computer‐based and 5.6% in the standard paper algorithm. Repeated measures analyses show the probabilities of BG readings <40 mg/dL were similar and not significantly different between groups (P = 0.969). We observed, however, a high rate of mild hypoglycemic events in patients treated with both insulin algorithms. The number of patients with BG <60 mg/dL was 42.9% in the Glucommander and 31.9% in the standard (P = NS). Minimizing the rate of hypoglycemia events is of major importance in hospitalized patients because it has been shown that hypoglycemia may be an independent risk factor of poor clinical outcome and mortality.20 Hypoglycemia may increase the risk of ventricular arrhythmias, in part due to the prolongation QT interval21 and can impair cerebral glucose metabolism resulting in brain metabolic dysfunction, as suggested by recent clinical studies.22 Moreover, insulin‐induced hypoglycemia is also associated with increased proinflammatory cytokines (tumor necrosis factor [TNF]‐alpha, interleukin [IL]‐1beta, IL‐6, and IL‐8) and oxidative stress23 that correlate with elevations of counterregulatory hormones (catecholamines, cortisol).
The Glucommander was associated with lower glycemic variability and with a higher percentage of BG readings within target range than patients treated with the standard paper form regimen. The clinical importance of the degree of variability and rapidity of fluctuations in glucose levels in critically ill patients is a topic of recent interest. Glycemic variability has been identified as a strong independent contributor to the risk of mortality in critically ill and surgical patients.24 Low levels of glycemic variability (standard deviation [SD] <10 mg/dL or 10‐20 mg/dL) have been shown to have a statistically significant lower risk of mortality, even after adjustment for severity of illness. Further studies are needed to determine benefits on clinical outcomes from the more consistent BG control from computer‐based titration protocols.
We acknowledge the following limitations in this multicenter open label study. First, this study was conducted in the medical ICU and excluded postsurgical patients and subjects expected to undergo a major surgical procedure during the hospital stay. Although a recent meta‐analysis9 of 26 studies involving 13,567 patients reported no benefits in the general ICU population, it found a favorable effect of intensive glycemic control on mortality in surgical ICU patients (relative risk [RR], 0.63; confidence interval [CI], 0.44‐0.91). We also excluded patients with severe renal insufficiency and patients with a history of hyperglycemic crises. In addition, our study was not powered to demonstrate differences in mortality or clinical outcome between treatment groups, and the BG targets used in this study were lower than glycemic targets recently recommended by the AACE and ADA inpatient glycemic control task force.25 Raising the BG targets is likely to reduce or prevent the rate of mild and severe hypoglycemic events in the ICU.
In conclusion, the computer‐guided algorithm resulted in a more rapid and tighter glycemic control with a similar rate of hypoglycemic events than the standard paper form protocol in medical ICU patients. Our study suggests that, both treatment algorithms are appropriate alternatives for the management of hyperglycemia in critically ill patients, and the choice depends on a physician's preferences, cost considerations, and the availability of the computer guided algorithm. Large randomized clinical trials are needed to test the impact of the new AACE/ADA recommended BG targets in reducing hypoglycemic events, hospital complications, and hospital mortality in critically ill patients in the ICU.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180:821–827.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360:1283–1297.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12:R120.
- ,.Tight glucose control and hypoglycemia.Crit Care Med.2008;36:1391; author reply 1391–1392.
- ,.ICU care for patients with diabetes.Curr Opin Endocrinol Diabetes Obes.2004;11:75–81.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit.Diabetes Technol Ther.2007;9:232–240.
- ,,, et al.Hyperglycemic crises in diabetes.Diabetes Care.2004;27Suppl 1:S94–S102.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8:10–18.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
- ,,, et al.Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients.Crit Care Med.2008;36:3190–3197.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,, et al.Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2009;94:709–728.
- ,,, et al.Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study.Crit Care Med.2008;36:3233–3238.
- ,,, et al.Proinflammatory cytokines in response to insulin‐induced hypoglycemic stress in healthy subjects.Metabolism.2009;58:443–448.
- ,,, et al.Blood glucose variability is associated with mortality in the surgical intensive care unit.Am Surg.2008;74:679–685; discussion685.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Diabetes Care.2009;32:1119–1131.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–597.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180:821–827.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360:1283–1297.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12:R120.
- ,.Tight glucose control and hypoglycemia.Crit Care Med.2008;36:1391; author reply 1391–1392.
- ,.ICU care for patients with diabetes.Curr Opin Endocrinol Diabetes Obes.2004;11:75–81.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27:461–467.
- ,,.Glucommander: a computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit.Diabetes Technol Ther.2007;9:232–240.
- ,,, et al.Hyperglycemic crises in diabetes.Diabetes Care.2004;27Suppl 1:S94–S102.
- ,,, et al.Description and evaluation of a glycemic management protocol for patients with diabetes undergoing heart surgery.Endocr Pract.2002;8:10–18.
- ,,.Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients.Metabolism.2008;57:116–120.
- ,,, et al.Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients.Crit Care Med.2008;36:3190–3197.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,, et al.Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline.J Clin Endocrinol Metab.2009;94:709–728.
- ,,, et al.Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study.Crit Care Med.2008;36:3233–3238.
- ,,, et al.Proinflammatory cytokines in response to insulin‐induced hypoglycemic stress in healthy subjects.Metabolism.2009;58:443–448.
- ,,, et al.Blood glucose variability is associated with mortality in the surgical intensive care unit.Am Surg.2008;74:679–685; discussion685.
- ,,, et al.American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control.Diabetes Care.2009;32:1119–1131.
Copyright © 2010 Society of Hospital Medicine
Insulin Infusion in the Non‐ICU Setting
Increasing evidence suggests that in hospitalized adult patients with and without diabetes, hyperglycemia is associated with increased risk of complications, prolonged length of hospitalization, and death.15 Past studies have shown that intensive glucose control in the intensive care unit (ICU) with continuous insulin infusion (CII) improves clinical outcomes by reducing the risk of multiorgan failure, systemic infection, and mortality. Effective management of hyperglycemia, an independent marker of poor outcome,1, 3, 6 is also associated with a decreased length of ICU and hospital stay79 and decreased total hospitalization cost.10 Based on several observational and interventional studies, improved control of blood glucose (BG) has been recommended for most adult patients with critical illness.2, 6, 11
Detrimental effects of hyperglycemia on outcome are not limited to patients in the ICU setting and CII has increasingly been used in non‐ICU settings. In such patients, the presence of hyperglycemia has been associated with prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 6 In general medicine and surgery services, however, hyperglycemia is frequently overlooked and inadequately addressed. Numerous reports have shown that sliding scale regular insulin (SSRI) continues to be the most common insulin prescribed regimen in the non‐ICU setting.12 This regimen is challenged by limited and variable efficacy and continued concern for hypoglycemia13; thus, a more structured, target‐driven protocol such as scheduled SC insulin or a CII protocol could facilitate glycemic control in the non‐ICU setting. Recently, we reported that a scheduled regimen using basal‐bolus insulin subcutaneously was safe, effective, and superior to SSRI in controlling BG levels in hospitalized subjects with type 2 diabetes. As in many institutions in the United States, we have used CII protocols as an alternative to subcutaneous (SC) insulin for the management of persistent hyperglycemia in non‐ICU areas during the past 10 years, particularly during the postoperative period, transplant recipients, or patients transferred from the ICU. There is, however, no clinical evidence regarding the safety, efficacy, or outcomes with the use of CII in the non‐ICU setting. Accordingly, we analyzed our experience on the efficacy and safety of CII in the management of hyperglycemia in general medicine and surgical services.
Research Design and Methods
This retrospective chart analysis was conducted in adult patients >18 years of age who were consecutively admitted to the general medical and surgical wards between July 1, 2004 and June 30, 2005 at Emory University Hospital, a 579‐bed tertiary care facility staffed exclusively by Emory University School of Medicine faculty members and residents. The CII protocol, employing regular insulin (Novolin‐R Novo Nordisk Pharmaceuticals, Princeton, NJ) with a very short half‐life, in this study is a dynamic protocol14 that has been available at all nursing stations at Emory Hospital for the past decade (Table 1). The insulin rate is calculated using the formula (BG 60) (multiplier) = units of insulin per hour. The multiplier is a value used to denote the degree of insulin sensitivity based on glucose pattern and response to insulin. The multiplier typically starts at a value of 0.02 and is adjusted by the nurse as needed to achieve target BG levels based on bedside capillary glucose measurements. Blood glucose levels were checked every 1 to 2 hours by the nursing staff (nurse:patient ratio = 1:5) according to the protocol.
| ||
| Date (mm/dd/yyyy): | Time: | Allergies: NKA |
| 1. Begin this protocol and IV fluids on ____/____/____ at __________ (time). Discontinue previous insulin orders when this protocol is started. | ||
| 2. Bedside BG monitoring q 1 h until patient is within target range two consecutive readings, and then obtain BG q 2 h. If the BG falls above or below the targeted range, resume q 1 h readings. (If using A‐line specimen, please use consistently while patient on drip). | ||
| 3. If initial BG >150 mg/dL give Regular Insulin bolus: Dose _____ units. (Dose 0.1 units/kg body weight) | ||
| 4. Insulin drip: 125 units of Regular Insulin in 250 mL 0.9% saline (1 mL of solution = 0.5 units of Insulin). | ||
| 5. Target BG Range on Insulin Drip: _____ mg/dL to _____ mg/dL (Suggested target 80‐100 for ICU patients)* | ||
| For each BG value, recalculate drip rate and disregard previous rate of infusion. | ||
| Calculate Insulin Drip rate: (BG 60) ________ (multiplier) = units of Insulin per hour ( 2 to determine cc/hour) (Typical starting multiplier 0.02 but varies by insulin sensitivity) | ||
| Adjusting Multiplier: | ||
| BG > Target Range: Increase multiplier by 0.01 | ||
| BG within Target Range: No change in multiplier | ||
| BG < Target Range: Decrease multiplier by 0.01 | ||
| 6. Treating Hypoglycemia: | ||
| 6a. BG 60‐80: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push. Adjust multiplier per protocol above | ||
| 6b. BG <60: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push | ||
| Decrease insulin drip to 50% of current infusion rate | ||
| Recheck BG in 30 minutes | ||
| BG >80: Decrease multiplier by 0.01 and then return to Step 5 formula | ||
| BG 60‐80: Repeat step 6a | ||
| BG <60: Notify MD and repeat Step 6b | ||
| 7. Continuous IV fluids ______________________ at ____________ mL/hour. (Consider changing to dextrose‐based fluids when BG <250) | ||
| 8. Additional Orders: | ||
Of 1404 patients treated with CII during the hospital stay, 1191 patients received CII in the ICU and 213 patients received CII in non‐ICU areas. The final analysis included a total of 200 non‐ICU patient records after excluding 13 patients with diabetic ketoacidosis, incomplete documentation of glycemic records, or with duration of CII for less than 3 hours. Data collected included demographics, medical history, admission diagnoses, inpatient medications, inpatient laboratory values, bedside BG measurements, insulin doses used, nutrition status during CII, length of stay, disposition at discharge, and mortality rate. Nutrition status was defined in 3 ways: (1) nil per os or nothing by mouth (NPO); (2) oral nutrition (PO‐regular or PO‐liquid); and (3) tube feeds or total parenteral nutrition (TF/TPN). Data collection was limited to the first 10 days of CII use. This study was approved by the Institutional Review Board at Emory University.
The primary aim of the study was to determine the efficacy (mean daily BG levels) and safety (number of hyperglycemic [200 mg/dL] and hypoglycemic [60 mg/dL] events) during CII. We also determined the presence of potential risk factors associated with hypoglycemic and hyperglycemic events (age, body mass index [BMI], nutrition status, renal function, corticosteroid therapy, and use of enteral and parenteral nutrition) during CII.
Statistical Analysis
Two‐sample Wilcoxon tests and analysis of variance (ANOVA) were used to compare continuous variables. Levine's test for homogeneity of variances and log transformations were used when necessary. For categorical variables, chi square (2) analysis was used. Multivariate regression analyses controlling for age, gender, race, history of diabetes mellitus (DM), BMI, Cockcroft‐Gault estimated glomerular filtration rate (GFR), steroid use, nutrition status (via oral route vs. NPO), and number of BG tests were performed based on repeated measures linear models or linear models and were used to determine the influence of demographic and clinical characteristics on the risk of hypoglycemia, hyperglycemia, mortality, and length of stay. Model building followed the backward selection procedure. All data are expressed as mean standard deviation. Statistical significance was defined as P < 0.05.
Statistical Analysis Software (SAS), version 9.1 (SAS Institute, Inc., Cary, NC), was used to perform the statistical analysis.
Results
The cohort of 200 patients consisted of 54% males and 46% females, 53% Caucasian, 37% Black, with a mean age of 52 16 years (Table 2). Forty‐five percent of patients were admitted to the general medicine service and the remaining 55% were admitted to the surgical service for admission diagnoses that included cardiovascular disorders, trauma/surgery gastrointestinal disorders, renal disorders, and infection.
| |
| Age (years) | 52 16 |
| Gender (M/F) | 108/92 |
| Race (W/B/H/O) | 106/74//3/17 |
| Admitting service, Medical/Surgical (%/%) | 45/55 |
| BMI (kg/m2) | 28.4 7.1 |
| Known diabetes/new onset (%/%) | 90/11 |
| Admission blood glucose (mg/dL) | 325 235 |
| A1c (%) | 9.1 3 |
| CrCl (mL/minute) | 59.5 44 |
| On steroids (%) | 82 (41%) |
| Insulin drip duration (hours) | 41.6 37 |
| LOS (days) | 10 9 |
The primary indication for CII was poor glycemic control in 93.4% of patients. Forty‐one percent of subjects were receiving corticosteroids and 16% were continued on the insulin drip after transferring from an ICU. Nearly 90% of subjects had a history of diabetes and 11% were diagnosed with new‐onset diabetes. The mean admission BG concentration was 325 235 mg/dL (mean SD) and the mean A1c in 121 subjects in whom it was measured was 9.1 3%. The mean BG prior to the initiation of CII (323 184) was similar to the admission BG.
Of the 173 subjects that had well‐documented glycemic goals, the BG targeted during CII was 150 mg/dL in 85% of patients while the remaining subjects had a target BG goal that ranged from 70 to 250 mg/dL. The most commonly prescribed BG target goals were 80 to 110 mg/dL (41.6%), 80 to 120 mg/dL (13.9%), and 100 to 150 mg/dL (5.8%).
BG improved rapidly after the initiation of CII. BG on the first day of CII was 182 71 mg/dL; day 2: 142 42 mg/dL; day 3: 131 38 mg/dL; and day 4: 132 43 in response to receiving an average of 84 66 units/day, 71 61 units/day, 70 61 units/day, and 64 29 units/day, respectively (Table 3). Irrespective of the target BG goal, 67% of patients reached BG levels of 150 mg/dL by 48 hours of CII initiation. The duration of CII ranged between 4 and 240 hours, with an average of 41.6 hours and a median of 28 hours. The average insulin infusion rate during CII was 4.29 2.99 units/hour and the mean amount of insulin required to attain glycemic goals was 1.96 1.88 units/kg/day.
| Mean Daily Blood Glucose (mg/dL*) | Mean Daily IV Insulin Dose (units/day) | |
|---|---|---|
| ||
| Preinfusion | 323 184 | N/A |
| Day 1 | 182 71 | 84 66 |
| Day 2 | 142 42 | 71 61 |
| Day 3 | 131 38 | 70 61 |
| Day 4 | 132 43 | 64 29 |
During CII, 48% and 35% of patients had at least 1 episode of hyperglycemia (BG >200 mg/dL) on the second and third day of CII, respectively. Hypoglycemia (BG <60 mg/dL) was noted at least once in 22% of the cohort (day 1: 11%; day 2: 16%; and day 3: 14%); however, severe hypoglycemia (BG <40 mg/dL) only occurred in 5% of subjects. During the CII, 37% of patients experienced a BG <70 mg/dL. When BG targets were stratified (<120 mg/dL vs. 120‐180 mg/dL vs. >180 mg/dL), we found no significant association between the target BG goal and the frequency of hypoglycemic or hyperglycemic events during CII. None of the episodes of hypoglycemia were associated with significant or permanent complications.
The analysis of collected variables for influence on glycemic control (ie, BMI, age, corticosteroid use, renal function, and nutrition status) revealed that subjects with a creatinine level >1.5 mg/dL may have an increased risk of hyperglycemia (BG >200 mg/dL) (P = 0.047) but not hypoglycemia. The analysis also found that younger patients (51 16 years) were more likely to have episodes of hyperglycemia than older patients (57 13 years) (P = 0.027). Hospital length of stay and mortality rate (3%) were not associated with the rate of hyperglycemic or hypoglycemic events.
Eighty‐two percent of patients received nutrition support at some point while on the CII: 48% PO‐regular diet; 14% PO‐liquid diet; and 20% TF/TPN. Due to the titration of nutrition from NPO at CII initiation to PO, NPO status was analyzed in a time‐dependent fashion. Thus, among patients on CII on day 1, day 2, day 3, day 4, and days 510; 34.0%, 26.3%, 11.3%, 12.5%, and 10.5%, respectively, were NPO.
As compared to subjects maintained NPO, subjects that received oral nutrition while on CII had an increased rate of hyperglycemic events (BG >200 mg/dL: 86% vs. 76%, P = 0.19; >300 mg/dL: 57% vs. 53%, P = 0.69; >400 mg/dL: 32% vs. 21%, P = 0.22) and a decreased rate of hypoglycemic events (BG <70 mg/dL: 33% vs. 41%, P = 0.39; BG <60 mg/dL: 20% vs. 26%, P = 0.49; and BG <40 mg/dL: 4% vs. 6%, P = 0.65). The multivariate regression analyses, however, which considered age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests, showed that nutrition status during CII was associated with increased frequency of hyperglycemic (P = 0.042) and hypoglycemic events (P = 0.086). As compared to NPO, oral intake (PO‐regular or PO‐liquid) was associated with a significantly increased frequency of hyperglycemic (P = 0.012) and hypoglycemic events (P = 0.035). Patients treated with TPN had lower BG values than those not on TPN. Although we observed no increased number of hypoglycemic events, TPN‐treated subjects had higher mortality than non‐TPN treated subjects (P < 0.001).
Discussion
Our study aimed to determine the safety and efficacy of CII in non‐critically‐ill patients with persistent hyperglycemia in general medicine and surgical services. We observed that the use of CII was effective in controlling hyperglycemia, with two‐thirds of patients achieving their target BG 150 mg/dL by 48 hours of insulin infusion. The rate of hypoglycemic events with the use of CII in non‐ICU patients was similar to that reported in recent ICU trials with intensive glycemic control7, 8, 15, 16 and is comparable to that reported in studies using SC insulin therapy in non‐ICU settings.17, 18 The number of hypoglycemic and hyperglycemic events was significantly higher in patients allowed to eat compared to those patients kept NPO during CII. There is substantial observational evidence linking hyperglycemia in hospitalized patients (with and without diabetes) to poor outcomes. There is ongoing debate, however, about the optimal level of BG in hospitalized patients. Early cohort studies as well as randomized controlled trials (RCTs) suggest that intensive treatment of hyperglycemia reduces length of hospital and ICU stay, multiorgan failure and systemic infections, and mortality.7, 9 These positive reports led the American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) to recommend tight glycemic control (target of 80‐110 mg/dL) in critical care units. Recent multicenter controlled trials, however, have not been able to reproduce these results and in fact, have reported an increased risk of severe hypoglycemia and mortality in ICU patients in association with tight glycemic control.15, 16, 19 New glycemic targets call for more reasonable, achievable, and safer glycemic targets20, 21 in patients receiving CII in the ICU setting. The recent ADA/AACE Inpatient Task Force now recommends against aggressive BG targets of <110 mg/dL for patients in the ICU, and suggests maintaining glucose levels between 140 and 180 mg/dL during insulin therapy. However, lower targets between 110 and 140 mg/dL, while not evidence‐based, may be acceptable in a subset of patients as long as these levels can be achieved safely by a well‐trained staff.
There are no RCTs examining the effect of intensive glycemic control on outcomes or the optimal glycemic target in hospitalized patients outside the ICU setting. However, several observational studies point to a strong association between hyperglycemia and poor clinical outcomes, including prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 5 Despite the paucity of randomized controlled trials on general medical‐surgical floors, a premeal BG target of <140 mg/dL with random BG <180 mg/dL are recommended as long as this target can be safely achieved.21
Our study indicates that the use of CII in the non‐ICU setting is effective in improving glycemic control. After the first day of CII, the mean glucose level was within the recommended BG target of <180 mg/dL for patients treated with CII in the ICU. Moreover, the mean daily BG level during CII was lower than those recently reported with the use of SC basal‐bolus and insulin neutral protamine hagedorn (NPH) and regular insulin combinations in non‐ICU settings.17, 18 In the Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial, a study that compared the efficacy and safety of an SC basal‐bolus to a sliding scale insulin regimen, showed that 66% and 38% of patients, respectively, reached a target BG of <140 mg/dL.17 The Comparison of Inpatient Insulin Regimens: DEtemir plus Aspart vs. NPH plus regular in Medical Patients with Type 2 Diabetes (DEAN Trial) trial reported daily mean BG levels after the first day of 160 38 mg/dL and 158 51 mg/dL in the detemir/aspart and NPH/regular group, respectively with an achieved BG target of <140 mg/dL in 45% of patients in the detemir/aspart and in 48% in the NPH/regular18; whereas in this study we observed that most patients reached the target BG goal by 48 hours of the CII regimen.
Increasing evidence indicates that inpatient hypoglycemia is associated with short‐term and long‐term adverse outcomes.22, 23 The incidence of severe hypoglycemia (<40 mg/dL) with intensified glycemic control has ranged between 9.8% and 19%7, 15 vs. <5% in conventional treatment. In the present study, 35% of patients experienced a BG <70 mg/dL, 22% had a BG <60 mg/dL, and 5% of patients had a BG <40 mg/dL. The lower rate of hypoglycemic events with the use of CII in the non‐ICU setting observed in this study is likely the result of a more relaxed glycemic target of 80 to 150 mg/dL for the majority of subjects, as well as fewer severe comorbidities compared to patients in the ICU, where the presence of sepsis or hepatic, adrenal, or renal failure increase the risk of hypoglycemia.2224
Multivariate analyses adjusted for age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests showed that nutrition status during CII was an important factor associated with increased frequency of hyperglycemic and hypoglycemic events. Compared to subjects maintained NPO, subjects who received oral intake while on CII had a significantly increased rate of hyperglycemic and hypoglycemic events. The increased risk of hypoglycemia for those allowed to eat is expected as the protocol would mandate an increase in the CII rate in response to the prandial BG increase but does not make provisions for BG assessments or CII adjustments in relationship to the meal. These results indicate that in stable patients who are ready to start eating, CII should be stopped and transitioned to SC insulin regimen. In patients who may benefit from the continued use of CII (eg, patients requiring multistep procedures/surgeries), treatment with CII could be continued with supplemental mealtime insulin (intravenous [IV] or SC).
CII may be useful in cases of patients with persistent hyperglycemia despite scheduled SC insulin regimen; in patients where rapid glycemic control may be warranted in order to decrease the risk of increased inflammation and vascular dysfunction in acute coronary syndromes; and to enhance wound healing status post surgical procedures. Other clinical scenarios in which CII may be preferred and no ICU bed is required include cases of new‐onset diabetes with significant hyperglycemia (BG >300 mg/dL), type 1 diabetes poorly controlled with SC insulin, uncontrolled gestational diabetes, parenteral nutrition use, perioperative states, or the use of high‐dose steroids or chemotherapy.
Our findings are limited by the retrospective nature of our study and the evaluation of patients in a single university medical center. Selection bias should be considered in the interpretation of the results since each index case was selected by the attending physician to be treated with CII as opposed to another regimen for inpatient glycemic control. The selection bias, however, may be limited by the fact that the subjects in this study placed on CII seemed to be similar to those in the general hospital population. A previous pilot study from a different academic institution, however, reported that implementing CII protocols in non‐ICU patients is safe and improved glycemic control without increasing hypoglycemia.25 In addition, because most subjects in this study had a history of diabetes prior to admission, these results may not be generalizable to populations with stress‐induced hyperglycemia.
In summary, our study indicates that a CII regimen is an effective option for the management of patients with persistent hyperglycemia in the non‐critical care setting. Most patients achieved and remained within targeted BG levels during CII. The overall rate of hypoglycemic events was similar to that reported in recent randomized clinical trials in the ICU and with SC insulin therapy. The frequency of hypoglycemic and hyperglycemic events was significantly increased in patients allowed to eat during CII suggesting that CII should be stopped and patients should be transitioned to an SC insulin regimen once oral intake is initiated. Future prospective, randomized studies are needed to compare the efficacy and safety of CII protocols to SC insulin protocols in the management of patients with persistent hyperglycemia in the non‐ICU setting.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control.Crit Care Med.2003;31(2):359–366.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290(15):2041–2047.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–597.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129(3):644–650.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360(13):1283–1297.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- .Diabetes Dek Professional Edition.Eatonton, GA:American Diabetes Association;1993.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180(8):799–800.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94(2):564–569.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12(5):R120.
- ,,, et al.Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism.Circulation.2008;117(12):1610–1619.
- ,,, et al.American Association of Clinical Endocrinologists/American Diabetes Association: Consensus Statement on Inpatient Glycemic Control.Endocr Pract.2009;15(4):353–369.
- .Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction.Diab Vasc Dis Res.2008;5(4):269–275.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34(11):2714–2718.
- ,,,.New insulin infusion protocol Improves blood glucose control in hospitalized patients without increasing hypoglycemia.Jt Comm J Qual Patient Saf.2005;31(3):141–147.
Increasing evidence suggests that in hospitalized adult patients with and without diabetes, hyperglycemia is associated with increased risk of complications, prolonged length of hospitalization, and death.15 Past studies have shown that intensive glucose control in the intensive care unit (ICU) with continuous insulin infusion (CII) improves clinical outcomes by reducing the risk of multiorgan failure, systemic infection, and mortality. Effective management of hyperglycemia, an independent marker of poor outcome,1, 3, 6 is also associated with a decreased length of ICU and hospital stay79 and decreased total hospitalization cost.10 Based on several observational and interventional studies, improved control of blood glucose (BG) has been recommended for most adult patients with critical illness.2, 6, 11
Detrimental effects of hyperglycemia on outcome are not limited to patients in the ICU setting and CII has increasingly been used in non‐ICU settings. In such patients, the presence of hyperglycemia has been associated with prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 6 In general medicine and surgery services, however, hyperglycemia is frequently overlooked and inadequately addressed. Numerous reports have shown that sliding scale regular insulin (SSRI) continues to be the most common insulin prescribed regimen in the non‐ICU setting.12 This regimen is challenged by limited and variable efficacy and continued concern for hypoglycemia13; thus, a more structured, target‐driven protocol such as scheduled SC insulin or a CII protocol could facilitate glycemic control in the non‐ICU setting. Recently, we reported that a scheduled regimen using basal‐bolus insulin subcutaneously was safe, effective, and superior to SSRI in controlling BG levels in hospitalized subjects with type 2 diabetes. As in many institutions in the United States, we have used CII protocols as an alternative to subcutaneous (SC) insulin for the management of persistent hyperglycemia in non‐ICU areas during the past 10 years, particularly during the postoperative period, transplant recipients, or patients transferred from the ICU. There is, however, no clinical evidence regarding the safety, efficacy, or outcomes with the use of CII in the non‐ICU setting. Accordingly, we analyzed our experience on the efficacy and safety of CII in the management of hyperglycemia in general medicine and surgical services.
Research Design and Methods
This retrospective chart analysis was conducted in adult patients >18 years of age who were consecutively admitted to the general medical and surgical wards between July 1, 2004 and June 30, 2005 at Emory University Hospital, a 579‐bed tertiary care facility staffed exclusively by Emory University School of Medicine faculty members and residents. The CII protocol, employing regular insulin (Novolin‐R Novo Nordisk Pharmaceuticals, Princeton, NJ) with a very short half‐life, in this study is a dynamic protocol14 that has been available at all nursing stations at Emory Hospital for the past decade (Table 1). The insulin rate is calculated using the formula (BG 60) (multiplier) = units of insulin per hour. The multiplier is a value used to denote the degree of insulin sensitivity based on glucose pattern and response to insulin. The multiplier typically starts at a value of 0.02 and is adjusted by the nurse as needed to achieve target BG levels based on bedside capillary glucose measurements. Blood glucose levels were checked every 1 to 2 hours by the nursing staff (nurse:patient ratio = 1:5) according to the protocol.
| ||
| Date (mm/dd/yyyy): | Time: | Allergies: NKA |
| 1. Begin this protocol and IV fluids on ____/____/____ at __________ (time). Discontinue previous insulin orders when this protocol is started. | ||
| 2. Bedside BG monitoring q 1 h until patient is within target range two consecutive readings, and then obtain BG q 2 h. If the BG falls above or below the targeted range, resume q 1 h readings. (If using A‐line specimen, please use consistently while patient on drip). | ||
| 3. If initial BG >150 mg/dL give Regular Insulin bolus: Dose _____ units. (Dose 0.1 units/kg body weight) | ||
| 4. Insulin drip: 125 units of Regular Insulin in 250 mL 0.9% saline (1 mL of solution = 0.5 units of Insulin). | ||
| 5. Target BG Range on Insulin Drip: _____ mg/dL to _____ mg/dL (Suggested target 80‐100 for ICU patients)* | ||
| For each BG value, recalculate drip rate and disregard previous rate of infusion. | ||
| Calculate Insulin Drip rate: (BG 60) ________ (multiplier) = units of Insulin per hour ( 2 to determine cc/hour) (Typical starting multiplier 0.02 but varies by insulin sensitivity) | ||
| Adjusting Multiplier: | ||
| BG > Target Range: Increase multiplier by 0.01 | ||
| BG within Target Range: No change in multiplier | ||
| BG < Target Range: Decrease multiplier by 0.01 | ||
| 6. Treating Hypoglycemia: | ||
| 6a. BG 60‐80: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push. Adjust multiplier per protocol above | ||
| 6b. BG <60: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push | ||
| Decrease insulin drip to 50% of current infusion rate | ||
| Recheck BG in 30 minutes | ||
| BG >80: Decrease multiplier by 0.01 and then return to Step 5 formula | ||
| BG 60‐80: Repeat step 6a | ||
| BG <60: Notify MD and repeat Step 6b | ||
| 7. Continuous IV fluids ______________________ at ____________ mL/hour. (Consider changing to dextrose‐based fluids when BG <250) | ||
| 8. Additional Orders: | ||
Of 1404 patients treated with CII during the hospital stay, 1191 patients received CII in the ICU and 213 patients received CII in non‐ICU areas. The final analysis included a total of 200 non‐ICU patient records after excluding 13 patients with diabetic ketoacidosis, incomplete documentation of glycemic records, or with duration of CII for less than 3 hours. Data collected included demographics, medical history, admission diagnoses, inpatient medications, inpatient laboratory values, bedside BG measurements, insulin doses used, nutrition status during CII, length of stay, disposition at discharge, and mortality rate. Nutrition status was defined in 3 ways: (1) nil per os or nothing by mouth (NPO); (2) oral nutrition (PO‐regular or PO‐liquid); and (3) tube feeds or total parenteral nutrition (TF/TPN). Data collection was limited to the first 10 days of CII use. This study was approved by the Institutional Review Board at Emory University.
The primary aim of the study was to determine the efficacy (mean daily BG levels) and safety (number of hyperglycemic [200 mg/dL] and hypoglycemic [60 mg/dL] events) during CII. We also determined the presence of potential risk factors associated with hypoglycemic and hyperglycemic events (age, body mass index [BMI], nutrition status, renal function, corticosteroid therapy, and use of enteral and parenteral nutrition) during CII.
Statistical Analysis
Two‐sample Wilcoxon tests and analysis of variance (ANOVA) were used to compare continuous variables. Levine's test for homogeneity of variances and log transformations were used when necessary. For categorical variables, chi square (2) analysis was used. Multivariate regression analyses controlling for age, gender, race, history of diabetes mellitus (DM), BMI, Cockcroft‐Gault estimated glomerular filtration rate (GFR), steroid use, nutrition status (via oral route vs. NPO), and number of BG tests were performed based on repeated measures linear models or linear models and were used to determine the influence of demographic and clinical characteristics on the risk of hypoglycemia, hyperglycemia, mortality, and length of stay. Model building followed the backward selection procedure. All data are expressed as mean standard deviation. Statistical significance was defined as P < 0.05.
Statistical Analysis Software (SAS), version 9.1 (SAS Institute, Inc., Cary, NC), was used to perform the statistical analysis.
Results
The cohort of 200 patients consisted of 54% males and 46% females, 53% Caucasian, 37% Black, with a mean age of 52 16 years (Table 2). Forty‐five percent of patients were admitted to the general medicine service and the remaining 55% were admitted to the surgical service for admission diagnoses that included cardiovascular disorders, trauma/surgery gastrointestinal disorders, renal disorders, and infection.
| |
| Age (years) | 52 16 |
| Gender (M/F) | 108/92 |
| Race (W/B/H/O) | 106/74//3/17 |
| Admitting service, Medical/Surgical (%/%) | 45/55 |
| BMI (kg/m2) | 28.4 7.1 |
| Known diabetes/new onset (%/%) | 90/11 |
| Admission blood glucose (mg/dL) | 325 235 |
| A1c (%) | 9.1 3 |
| CrCl (mL/minute) | 59.5 44 |
| On steroids (%) | 82 (41%) |
| Insulin drip duration (hours) | 41.6 37 |
| LOS (days) | 10 9 |
The primary indication for CII was poor glycemic control in 93.4% of patients. Forty‐one percent of subjects were receiving corticosteroids and 16% were continued on the insulin drip after transferring from an ICU. Nearly 90% of subjects had a history of diabetes and 11% were diagnosed with new‐onset diabetes. The mean admission BG concentration was 325 235 mg/dL (mean SD) and the mean A1c in 121 subjects in whom it was measured was 9.1 3%. The mean BG prior to the initiation of CII (323 184) was similar to the admission BG.
Of the 173 subjects that had well‐documented glycemic goals, the BG targeted during CII was 150 mg/dL in 85% of patients while the remaining subjects had a target BG goal that ranged from 70 to 250 mg/dL. The most commonly prescribed BG target goals were 80 to 110 mg/dL (41.6%), 80 to 120 mg/dL (13.9%), and 100 to 150 mg/dL (5.8%).
BG improved rapidly after the initiation of CII. BG on the first day of CII was 182 71 mg/dL; day 2: 142 42 mg/dL; day 3: 131 38 mg/dL; and day 4: 132 43 in response to receiving an average of 84 66 units/day, 71 61 units/day, 70 61 units/day, and 64 29 units/day, respectively (Table 3). Irrespective of the target BG goal, 67% of patients reached BG levels of 150 mg/dL by 48 hours of CII initiation. The duration of CII ranged between 4 and 240 hours, with an average of 41.6 hours and a median of 28 hours. The average insulin infusion rate during CII was 4.29 2.99 units/hour and the mean amount of insulin required to attain glycemic goals was 1.96 1.88 units/kg/day.
| Mean Daily Blood Glucose (mg/dL*) | Mean Daily IV Insulin Dose (units/day) | |
|---|---|---|
| ||
| Preinfusion | 323 184 | N/A |
| Day 1 | 182 71 | 84 66 |
| Day 2 | 142 42 | 71 61 |
| Day 3 | 131 38 | 70 61 |
| Day 4 | 132 43 | 64 29 |
During CII, 48% and 35% of patients had at least 1 episode of hyperglycemia (BG >200 mg/dL) on the second and third day of CII, respectively. Hypoglycemia (BG <60 mg/dL) was noted at least once in 22% of the cohort (day 1: 11%; day 2: 16%; and day 3: 14%); however, severe hypoglycemia (BG <40 mg/dL) only occurred in 5% of subjects. During the CII, 37% of patients experienced a BG <70 mg/dL. When BG targets were stratified (<120 mg/dL vs. 120‐180 mg/dL vs. >180 mg/dL), we found no significant association between the target BG goal and the frequency of hypoglycemic or hyperglycemic events during CII. None of the episodes of hypoglycemia were associated with significant or permanent complications.
The analysis of collected variables for influence on glycemic control (ie, BMI, age, corticosteroid use, renal function, and nutrition status) revealed that subjects with a creatinine level >1.5 mg/dL may have an increased risk of hyperglycemia (BG >200 mg/dL) (P = 0.047) but not hypoglycemia. The analysis also found that younger patients (51 16 years) were more likely to have episodes of hyperglycemia than older patients (57 13 years) (P = 0.027). Hospital length of stay and mortality rate (3%) were not associated with the rate of hyperglycemic or hypoglycemic events.
Eighty‐two percent of patients received nutrition support at some point while on the CII: 48% PO‐regular diet; 14% PO‐liquid diet; and 20% TF/TPN. Due to the titration of nutrition from NPO at CII initiation to PO, NPO status was analyzed in a time‐dependent fashion. Thus, among patients on CII on day 1, day 2, day 3, day 4, and days 510; 34.0%, 26.3%, 11.3%, 12.5%, and 10.5%, respectively, were NPO.
As compared to subjects maintained NPO, subjects that received oral nutrition while on CII had an increased rate of hyperglycemic events (BG >200 mg/dL: 86% vs. 76%, P = 0.19; >300 mg/dL: 57% vs. 53%, P = 0.69; >400 mg/dL: 32% vs. 21%, P = 0.22) and a decreased rate of hypoglycemic events (BG <70 mg/dL: 33% vs. 41%, P = 0.39; BG <60 mg/dL: 20% vs. 26%, P = 0.49; and BG <40 mg/dL: 4% vs. 6%, P = 0.65). The multivariate regression analyses, however, which considered age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests, showed that nutrition status during CII was associated with increased frequency of hyperglycemic (P = 0.042) and hypoglycemic events (P = 0.086). As compared to NPO, oral intake (PO‐regular or PO‐liquid) was associated with a significantly increased frequency of hyperglycemic (P = 0.012) and hypoglycemic events (P = 0.035). Patients treated with TPN had lower BG values than those not on TPN. Although we observed no increased number of hypoglycemic events, TPN‐treated subjects had higher mortality than non‐TPN treated subjects (P < 0.001).
Discussion
Our study aimed to determine the safety and efficacy of CII in non‐critically‐ill patients with persistent hyperglycemia in general medicine and surgical services. We observed that the use of CII was effective in controlling hyperglycemia, with two‐thirds of patients achieving their target BG 150 mg/dL by 48 hours of insulin infusion. The rate of hypoglycemic events with the use of CII in non‐ICU patients was similar to that reported in recent ICU trials with intensive glycemic control7, 8, 15, 16 and is comparable to that reported in studies using SC insulin therapy in non‐ICU settings.17, 18 The number of hypoglycemic and hyperglycemic events was significantly higher in patients allowed to eat compared to those patients kept NPO during CII. There is substantial observational evidence linking hyperglycemia in hospitalized patients (with and without diabetes) to poor outcomes. There is ongoing debate, however, about the optimal level of BG in hospitalized patients. Early cohort studies as well as randomized controlled trials (RCTs) suggest that intensive treatment of hyperglycemia reduces length of hospital and ICU stay, multiorgan failure and systemic infections, and mortality.7, 9 These positive reports led the American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) to recommend tight glycemic control (target of 80‐110 mg/dL) in critical care units. Recent multicenter controlled trials, however, have not been able to reproduce these results and in fact, have reported an increased risk of severe hypoglycemia and mortality in ICU patients in association with tight glycemic control.15, 16, 19 New glycemic targets call for more reasonable, achievable, and safer glycemic targets20, 21 in patients receiving CII in the ICU setting. The recent ADA/AACE Inpatient Task Force now recommends against aggressive BG targets of <110 mg/dL for patients in the ICU, and suggests maintaining glucose levels between 140 and 180 mg/dL during insulin therapy. However, lower targets between 110 and 140 mg/dL, while not evidence‐based, may be acceptable in a subset of patients as long as these levels can be achieved safely by a well‐trained staff.
There are no RCTs examining the effect of intensive glycemic control on outcomes or the optimal glycemic target in hospitalized patients outside the ICU setting. However, several observational studies point to a strong association between hyperglycemia and poor clinical outcomes, including prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 5 Despite the paucity of randomized controlled trials on general medical‐surgical floors, a premeal BG target of <140 mg/dL with random BG <180 mg/dL are recommended as long as this target can be safely achieved.21
Our study indicates that the use of CII in the non‐ICU setting is effective in improving glycemic control. After the first day of CII, the mean glucose level was within the recommended BG target of <180 mg/dL for patients treated with CII in the ICU. Moreover, the mean daily BG level during CII was lower than those recently reported with the use of SC basal‐bolus and insulin neutral protamine hagedorn (NPH) and regular insulin combinations in non‐ICU settings.17, 18 In the Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial, a study that compared the efficacy and safety of an SC basal‐bolus to a sliding scale insulin regimen, showed that 66% and 38% of patients, respectively, reached a target BG of <140 mg/dL.17 The Comparison of Inpatient Insulin Regimens: DEtemir plus Aspart vs. NPH plus regular in Medical Patients with Type 2 Diabetes (DEAN Trial) trial reported daily mean BG levels after the first day of 160 38 mg/dL and 158 51 mg/dL in the detemir/aspart and NPH/regular group, respectively with an achieved BG target of <140 mg/dL in 45% of patients in the detemir/aspart and in 48% in the NPH/regular18; whereas in this study we observed that most patients reached the target BG goal by 48 hours of the CII regimen.
Increasing evidence indicates that inpatient hypoglycemia is associated with short‐term and long‐term adverse outcomes.22, 23 The incidence of severe hypoglycemia (<40 mg/dL) with intensified glycemic control has ranged between 9.8% and 19%7, 15 vs. <5% in conventional treatment. In the present study, 35% of patients experienced a BG <70 mg/dL, 22% had a BG <60 mg/dL, and 5% of patients had a BG <40 mg/dL. The lower rate of hypoglycemic events with the use of CII in the non‐ICU setting observed in this study is likely the result of a more relaxed glycemic target of 80 to 150 mg/dL for the majority of subjects, as well as fewer severe comorbidities compared to patients in the ICU, where the presence of sepsis or hepatic, adrenal, or renal failure increase the risk of hypoglycemia.2224
Multivariate analyses adjusted for age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests showed that nutrition status during CII was an important factor associated with increased frequency of hyperglycemic and hypoglycemic events. Compared to subjects maintained NPO, subjects who received oral intake while on CII had a significantly increased rate of hyperglycemic and hypoglycemic events. The increased risk of hypoglycemia for those allowed to eat is expected as the protocol would mandate an increase in the CII rate in response to the prandial BG increase but does not make provisions for BG assessments or CII adjustments in relationship to the meal. These results indicate that in stable patients who are ready to start eating, CII should be stopped and transitioned to SC insulin regimen. In patients who may benefit from the continued use of CII (eg, patients requiring multistep procedures/surgeries), treatment with CII could be continued with supplemental mealtime insulin (intravenous [IV] or SC).
CII may be useful in cases of patients with persistent hyperglycemia despite scheduled SC insulin regimen; in patients where rapid glycemic control may be warranted in order to decrease the risk of increased inflammation and vascular dysfunction in acute coronary syndromes; and to enhance wound healing status post surgical procedures. Other clinical scenarios in which CII may be preferred and no ICU bed is required include cases of new‐onset diabetes with significant hyperglycemia (BG >300 mg/dL), type 1 diabetes poorly controlled with SC insulin, uncontrolled gestational diabetes, parenteral nutrition use, perioperative states, or the use of high‐dose steroids or chemotherapy.
Our findings are limited by the retrospective nature of our study and the evaluation of patients in a single university medical center. Selection bias should be considered in the interpretation of the results since each index case was selected by the attending physician to be treated with CII as opposed to another regimen for inpatient glycemic control. The selection bias, however, may be limited by the fact that the subjects in this study placed on CII seemed to be similar to those in the general hospital population. A previous pilot study from a different academic institution, however, reported that implementing CII protocols in non‐ICU patients is safe and improved glycemic control without increasing hypoglycemia.25 In addition, because most subjects in this study had a history of diabetes prior to admission, these results may not be generalizable to populations with stress‐induced hyperglycemia.
In summary, our study indicates that a CII regimen is an effective option for the management of patients with persistent hyperglycemia in the non‐critical care setting. Most patients achieved and remained within targeted BG levels during CII. The overall rate of hypoglycemic events was similar to that reported in recent randomized clinical trials in the ICU and with SC insulin therapy. The frequency of hypoglycemic and hyperglycemic events was significantly increased in patients allowed to eat during CII suggesting that CII should be stopped and patients should be transitioned to an SC insulin regimen once oral intake is initiated. Future prospective, randomized studies are needed to compare the efficacy and safety of CII protocols to SC insulin protocols in the management of patients with persistent hyperglycemia in the non‐ICU setting.
Increasing evidence suggests that in hospitalized adult patients with and without diabetes, hyperglycemia is associated with increased risk of complications, prolonged length of hospitalization, and death.15 Past studies have shown that intensive glucose control in the intensive care unit (ICU) with continuous insulin infusion (CII) improves clinical outcomes by reducing the risk of multiorgan failure, systemic infection, and mortality. Effective management of hyperglycemia, an independent marker of poor outcome,1, 3, 6 is also associated with a decreased length of ICU and hospital stay79 and decreased total hospitalization cost.10 Based on several observational and interventional studies, improved control of blood glucose (BG) has been recommended for most adult patients with critical illness.2, 6, 11
Detrimental effects of hyperglycemia on outcome are not limited to patients in the ICU setting and CII has increasingly been used in non‐ICU settings. In such patients, the presence of hyperglycemia has been associated with prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 6 In general medicine and surgery services, however, hyperglycemia is frequently overlooked and inadequately addressed. Numerous reports have shown that sliding scale regular insulin (SSRI) continues to be the most common insulin prescribed regimen in the non‐ICU setting.12 This regimen is challenged by limited and variable efficacy and continued concern for hypoglycemia13; thus, a more structured, target‐driven protocol such as scheduled SC insulin or a CII protocol could facilitate glycemic control in the non‐ICU setting. Recently, we reported that a scheduled regimen using basal‐bolus insulin subcutaneously was safe, effective, and superior to SSRI in controlling BG levels in hospitalized subjects with type 2 diabetes. As in many institutions in the United States, we have used CII protocols as an alternative to subcutaneous (SC) insulin for the management of persistent hyperglycemia in non‐ICU areas during the past 10 years, particularly during the postoperative period, transplant recipients, or patients transferred from the ICU. There is, however, no clinical evidence regarding the safety, efficacy, or outcomes with the use of CII in the non‐ICU setting. Accordingly, we analyzed our experience on the efficacy and safety of CII in the management of hyperglycemia in general medicine and surgical services.
Research Design and Methods
This retrospective chart analysis was conducted in adult patients >18 years of age who were consecutively admitted to the general medical and surgical wards between July 1, 2004 and June 30, 2005 at Emory University Hospital, a 579‐bed tertiary care facility staffed exclusively by Emory University School of Medicine faculty members and residents. The CII protocol, employing regular insulin (Novolin‐R Novo Nordisk Pharmaceuticals, Princeton, NJ) with a very short half‐life, in this study is a dynamic protocol14 that has been available at all nursing stations at Emory Hospital for the past decade (Table 1). The insulin rate is calculated using the formula (BG 60) (multiplier) = units of insulin per hour. The multiplier is a value used to denote the degree of insulin sensitivity based on glucose pattern and response to insulin. The multiplier typically starts at a value of 0.02 and is adjusted by the nurse as needed to achieve target BG levels based on bedside capillary glucose measurements. Blood glucose levels were checked every 1 to 2 hours by the nursing staff (nurse:patient ratio = 1:5) according to the protocol.
| ||
| Date (mm/dd/yyyy): | Time: | Allergies: NKA |
| 1. Begin this protocol and IV fluids on ____/____/____ at __________ (time). Discontinue previous insulin orders when this protocol is started. | ||
| 2. Bedside BG monitoring q 1 h until patient is within target range two consecutive readings, and then obtain BG q 2 h. If the BG falls above or below the targeted range, resume q 1 h readings. (If using A‐line specimen, please use consistently while patient on drip). | ||
| 3. If initial BG >150 mg/dL give Regular Insulin bolus: Dose _____ units. (Dose 0.1 units/kg body weight) | ||
| 4. Insulin drip: 125 units of Regular Insulin in 250 mL 0.9% saline (1 mL of solution = 0.5 units of Insulin). | ||
| 5. Target BG Range on Insulin Drip: _____ mg/dL to _____ mg/dL (Suggested target 80‐100 for ICU patients)* | ||
| For each BG value, recalculate drip rate and disregard previous rate of infusion. | ||
| Calculate Insulin Drip rate: (BG 60) ________ (multiplier) = units of Insulin per hour ( 2 to determine cc/hour) (Typical starting multiplier 0.02 but varies by insulin sensitivity) | ||
| Adjusting Multiplier: | ||
| BG > Target Range: Increase multiplier by 0.01 | ||
| BG within Target Range: No change in multiplier | ||
| BG < Target Range: Decrease multiplier by 0.01 | ||
| 6. Treating Hypoglycemia: | ||
| 6a. BG 60‐80: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push. Adjust multiplier per protocol above | ||
| 6b. BG <60: Give D50W using formula: (100 BG) 0.3 = mL D50W IV Push | ||
| Decrease insulin drip to 50% of current infusion rate | ||
| Recheck BG in 30 minutes | ||
| BG >80: Decrease multiplier by 0.01 and then return to Step 5 formula | ||
| BG 60‐80: Repeat step 6a | ||
| BG <60: Notify MD and repeat Step 6b | ||
| 7. Continuous IV fluids ______________________ at ____________ mL/hour. (Consider changing to dextrose‐based fluids when BG <250) | ||
| 8. Additional Orders: | ||
Of 1404 patients treated with CII during the hospital stay, 1191 patients received CII in the ICU and 213 patients received CII in non‐ICU areas. The final analysis included a total of 200 non‐ICU patient records after excluding 13 patients with diabetic ketoacidosis, incomplete documentation of glycemic records, or with duration of CII for less than 3 hours. Data collected included demographics, medical history, admission diagnoses, inpatient medications, inpatient laboratory values, bedside BG measurements, insulin doses used, nutrition status during CII, length of stay, disposition at discharge, and mortality rate. Nutrition status was defined in 3 ways: (1) nil per os or nothing by mouth (NPO); (2) oral nutrition (PO‐regular or PO‐liquid); and (3) tube feeds or total parenteral nutrition (TF/TPN). Data collection was limited to the first 10 days of CII use. This study was approved by the Institutional Review Board at Emory University.
The primary aim of the study was to determine the efficacy (mean daily BG levels) and safety (number of hyperglycemic [200 mg/dL] and hypoglycemic [60 mg/dL] events) during CII. We also determined the presence of potential risk factors associated with hypoglycemic and hyperglycemic events (age, body mass index [BMI], nutrition status, renal function, corticosteroid therapy, and use of enteral and parenteral nutrition) during CII.
Statistical Analysis
Two‐sample Wilcoxon tests and analysis of variance (ANOVA) were used to compare continuous variables. Levine's test for homogeneity of variances and log transformations were used when necessary. For categorical variables, chi square (2) analysis was used. Multivariate regression analyses controlling for age, gender, race, history of diabetes mellitus (DM), BMI, Cockcroft‐Gault estimated glomerular filtration rate (GFR), steroid use, nutrition status (via oral route vs. NPO), and number of BG tests were performed based on repeated measures linear models or linear models and were used to determine the influence of demographic and clinical characteristics on the risk of hypoglycemia, hyperglycemia, mortality, and length of stay. Model building followed the backward selection procedure. All data are expressed as mean standard deviation. Statistical significance was defined as P < 0.05.
Statistical Analysis Software (SAS), version 9.1 (SAS Institute, Inc., Cary, NC), was used to perform the statistical analysis.
Results
The cohort of 200 patients consisted of 54% males and 46% females, 53% Caucasian, 37% Black, with a mean age of 52 16 years (Table 2). Forty‐five percent of patients were admitted to the general medicine service and the remaining 55% were admitted to the surgical service for admission diagnoses that included cardiovascular disorders, trauma/surgery gastrointestinal disorders, renal disorders, and infection.
| |
| Age (years) | 52 16 |
| Gender (M/F) | 108/92 |
| Race (W/B/H/O) | 106/74//3/17 |
| Admitting service, Medical/Surgical (%/%) | 45/55 |
| BMI (kg/m2) | 28.4 7.1 |
| Known diabetes/new onset (%/%) | 90/11 |
| Admission blood glucose (mg/dL) | 325 235 |
| A1c (%) | 9.1 3 |
| CrCl (mL/minute) | 59.5 44 |
| On steroids (%) | 82 (41%) |
| Insulin drip duration (hours) | 41.6 37 |
| LOS (days) | 10 9 |
The primary indication for CII was poor glycemic control in 93.4% of patients. Forty‐one percent of subjects were receiving corticosteroids and 16% were continued on the insulin drip after transferring from an ICU. Nearly 90% of subjects had a history of diabetes and 11% were diagnosed with new‐onset diabetes. The mean admission BG concentration was 325 235 mg/dL (mean SD) and the mean A1c in 121 subjects in whom it was measured was 9.1 3%. The mean BG prior to the initiation of CII (323 184) was similar to the admission BG.
Of the 173 subjects that had well‐documented glycemic goals, the BG targeted during CII was 150 mg/dL in 85% of patients while the remaining subjects had a target BG goal that ranged from 70 to 250 mg/dL. The most commonly prescribed BG target goals were 80 to 110 mg/dL (41.6%), 80 to 120 mg/dL (13.9%), and 100 to 150 mg/dL (5.8%).
BG improved rapidly after the initiation of CII. BG on the first day of CII was 182 71 mg/dL; day 2: 142 42 mg/dL; day 3: 131 38 mg/dL; and day 4: 132 43 in response to receiving an average of 84 66 units/day, 71 61 units/day, 70 61 units/day, and 64 29 units/day, respectively (Table 3). Irrespective of the target BG goal, 67% of patients reached BG levels of 150 mg/dL by 48 hours of CII initiation. The duration of CII ranged between 4 and 240 hours, with an average of 41.6 hours and a median of 28 hours. The average insulin infusion rate during CII was 4.29 2.99 units/hour and the mean amount of insulin required to attain glycemic goals was 1.96 1.88 units/kg/day.
| Mean Daily Blood Glucose (mg/dL*) | Mean Daily IV Insulin Dose (units/day) | |
|---|---|---|
| ||
| Preinfusion | 323 184 | N/A |
| Day 1 | 182 71 | 84 66 |
| Day 2 | 142 42 | 71 61 |
| Day 3 | 131 38 | 70 61 |
| Day 4 | 132 43 | 64 29 |
During CII, 48% and 35% of patients had at least 1 episode of hyperglycemia (BG >200 mg/dL) on the second and third day of CII, respectively. Hypoglycemia (BG <60 mg/dL) was noted at least once in 22% of the cohort (day 1: 11%; day 2: 16%; and day 3: 14%); however, severe hypoglycemia (BG <40 mg/dL) only occurred in 5% of subjects. During the CII, 37% of patients experienced a BG <70 mg/dL. When BG targets were stratified (<120 mg/dL vs. 120‐180 mg/dL vs. >180 mg/dL), we found no significant association between the target BG goal and the frequency of hypoglycemic or hyperglycemic events during CII. None of the episodes of hypoglycemia were associated with significant or permanent complications.
The analysis of collected variables for influence on glycemic control (ie, BMI, age, corticosteroid use, renal function, and nutrition status) revealed that subjects with a creatinine level >1.5 mg/dL may have an increased risk of hyperglycemia (BG >200 mg/dL) (P = 0.047) but not hypoglycemia. The analysis also found that younger patients (51 16 years) were more likely to have episodes of hyperglycemia than older patients (57 13 years) (P = 0.027). Hospital length of stay and mortality rate (3%) were not associated with the rate of hyperglycemic or hypoglycemic events.
Eighty‐two percent of patients received nutrition support at some point while on the CII: 48% PO‐regular diet; 14% PO‐liquid diet; and 20% TF/TPN. Due to the titration of nutrition from NPO at CII initiation to PO, NPO status was analyzed in a time‐dependent fashion. Thus, among patients on CII on day 1, day 2, day 3, day 4, and days 510; 34.0%, 26.3%, 11.3%, 12.5%, and 10.5%, respectively, were NPO.
As compared to subjects maintained NPO, subjects that received oral nutrition while on CII had an increased rate of hyperglycemic events (BG >200 mg/dL: 86% vs. 76%, P = 0.19; >300 mg/dL: 57% vs. 53%, P = 0.69; >400 mg/dL: 32% vs. 21%, P = 0.22) and a decreased rate of hypoglycemic events (BG <70 mg/dL: 33% vs. 41%, P = 0.39; BG <60 mg/dL: 20% vs. 26%, P = 0.49; and BG <40 mg/dL: 4% vs. 6%, P = 0.65). The multivariate regression analyses, however, which considered age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests, showed that nutrition status during CII was associated with increased frequency of hyperglycemic (P = 0.042) and hypoglycemic events (P = 0.086). As compared to NPO, oral intake (PO‐regular or PO‐liquid) was associated with a significantly increased frequency of hyperglycemic (P = 0.012) and hypoglycemic events (P = 0.035). Patients treated with TPN had lower BG values than those not on TPN. Although we observed no increased number of hypoglycemic events, TPN‐treated subjects had higher mortality than non‐TPN treated subjects (P < 0.001).
Discussion
Our study aimed to determine the safety and efficacy of CII in non‐critically‐ill patients with persistent hyperglycemia in general medicine and surgical services. We observed that the use of CII was effective in controlling hyperglycemia, with two‐thirds of patients achieving their target BG 150 mg/dL by 48 hours of insulin infusion. The rate of hypoglycemic events with the use of CII in non‐ICU patients was similar to that reported in recent ICU trials with intensive glycemic control7, 8, 15, 16 and is comparable to that reported in studies using SC insulin therapy in non‐ICU settings.17, 18 The number of hypoglycemic and hyperglycemic events was significantly higher in patients allowed to eat compared to those patients kept NPO during CII. There is substantial observational evidence linking hyperglycemia in hospitalized patients (with and without diabetes) to poor outcomes. There is ongoing debate, however, about the optimal level of BG in hospitalized patients. Early cohort studies as well as randomized controlled trials (RCTs) suggest that intensive treatment of hyperglycemia reduces length of hospital and ICU stay, multiorgan failure and systemic infections, and mortality.7, 9 These positive reports led the American Diabetes Association (ADA) and American Association of Clinical Endocrinologists (AACE) to recommend tight glycemic control (target of 80‐110 mg/dL) in critical care units. Recent multicenter controlled trials, however, have not been able to reproduce these results and in fact, have reported an increased risk of severe hypoglycemia and mortality in ICU patients in association with tight glycemic control.15, 16, 19 New glycemic targets call for more reasonable, achievable, and safer glycemic targets20, 21 in patients receiving CII in the ICU setting. The recent ADA/AACE Inpatient Task Force now recommends against aggressive BG targets of <110 mg/dL for patients in the ICU, and suggests maintaining glucose levels between 140 and 180 mg/dL during insulin therapy. However, lower targets between 110 and 140 mg/dL, while not evidence‐based, may be acceptable in a subset of patients as long as these levels can be achieved safely by a well‐trained staff.
There are no RCTs examining the effect of intensive glycemic control on outcomes or the optimal glycemic target in hospitalized patients outside the ICU setting. However, several observational studies point to a strong association between hyperglycemia and poor clinical outcomes, including prolonged hospital stay, infection, disability after hospital discharge, and death.1, 3, 5 Despite the paucity of randomized controlled trials on general medical‐surgical floors, a premeal BG target of <140 mg/dL with random BG <180 mg/dL are recommended as long as this target can be safely achieved.21
Our study indicates that the use of CII in the non‐ICU setting is effective in improving glycemic control. After the first day of CII, the mean glucose level was within the recommended BG target of <180 mg/dL for patients treated with CII in the ICU. Moreover, the mean daily BG level during CII was lower than those recently reported with the use of SC basal‐bolus and insulin neutral protamine hagedorn (NPH) and regular insulin combinations in non‐ICU settings.17, 18 In the Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial, a study that compared the efficacy and safety of an SC basal‐bolus to a sliding scale insulin regimen, showed that 66% and 38% of patients, respectively, reached a target BG of <140 mg/dL.17 The Comparison of Inpatient Insulin Regimens: DEtemir plus Aspart vs. NPH plus regular in Medical Patients with Type 2 Diabetes (DEAN Trial) trial reported daily mean BG levels after the first day of 160 38 mg/dL and 158 51 mg/dL in the detemir/aspart and NPH/regular group, respectively with an achieved BG target of <140 mg/dL in 45% of patients in the detemir/aspart and in 48% in the NPH/regular18; whereas in this study we observed that most patients reached the target BG goal by 48 hours of the CII regimen.
Increasing evidence indicates that inpatient hypoglycemia is associated with short‐term and long‐term adverse outcomes.22, 23 The incidence of severe hypoglycemia (<40 mg/dL) with intensified glycemic control has ranged between 9.8% and 19%7, 15 vs. <5% in conventional treatment. In the present study, 35% of patients experienced a BG <70 mg/dL, 22% had a BG <60 mg/dL, and 5% of patients had a BG <40 mg/dL. The lower rate of hypoglycemic events with the use of CII in the non‐ICU setting observed in this study is likely the result of a more relaxed glycemic target of 80 to 150 mg/dL for the majority of subjects, as well as fewer severe comorbidities compared to patients in the ICU, where the presence of sepsis or hepatic, adrenal, or renal failure increase the risk of hypoglycemia.2224
Multivariate analyses adjusted for age, gender, race, BMI, renal function, steroid use, history of diabetes, and number of BG tests showed that nutrition status during CII was an important factor associated with increased frequency of hyperglycemic and hypoglycemic events. Compared to subjects maintained NPO, subjects who received oral intake while on CII had a significantly increased rate of hyperglycemic and hypoglycemic events. The increased risk of hypoglycemia for those allowed to eat is expected as the protocol would mandate an increase in the CII rate in response to the prandial BG increase but does not make provisions for BG assessments or CII adjustments in relationship to the meal. These results indicate that in stable patients who are ready to start eating, CII should be stopped and transitioned to SC insulin regimen. In patients who may benefit from the continued use of CII (eg, patients requiring multistep procedures/surgeries), treatment with CII could be continued with supplemental mealtime insulin (intravenous [IV] or SC).
CII may be useful in cases of patients with persistent hyperglycemia despite scheduled SC insulin regimen; in patients where rapid glycemic control may be warranted in order to decrease the risk of increased inflammation and vascular dysfunction in acute coronary syndromes; and to enhance wound healing status post surgical procedures. Other clinical scenarios in which CII may be preferred and no ICU bed is required include cases of new‐onset diabetes with significant hyperglycemia (BG >300 mg/dL), type 1 diabetes poorly controlled with SC insulin, uncontrolled gestational diabetes, parenteral nutrition use, perioperative states, or the use of high‐dose steroids or chemotherapy.
Our findings are limited by the retrospective nature of our study and the evaluation of patients in a single university medical center. Selection bias should be considered in the interpretation of the results since each index case was selected by the attending physician to be treated with CII as opposed to another regimen for inpatient glycemic control. The selection bias, however, may be limited by the fact that the subjects in this study placed on CII seemed to be similar to those in the general hospital population. A previous pilot study from a different academic institution, however, reported that implementing CII protocols in non‐ICU patients is safe and improved glycemic control without increasing hypoglycemia.25 In addition, because most subjects in this study had a history of diabetes prior to admission, these results may not be generalizable to populations with stress‐induced hyperglycemia.
In summary, our study indicates that a CII regimen is an effective option for the management of patients with persistent hyperglycemia in the non‐critical care setting. Most patients achieved and remained within targeted BG levels during CII. The overall rate of hypoglycemic events was similar to that reported in recent randomized clinical trials in the ICU and with SC insulin therapy. The frequency of hypoglycemic and hyperglycemic events was significantly increased in patients allowed to eat during CII suggesting that CII should be stopped and patients should be transitioned to an SC insulin regimen once oral intake is initiated. Future prospective, randomized studies are needed to compare the efficacy and safety of CII protocols to SC insulin protocols in the management of patients with persistent hyperglycemia in the non‐ICU setting.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control.Crit Care Med.2003;31(2):359–366.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290(15):2041–2047.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–597.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129(3):644–650.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360(13):1283–1297.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- .Diabetes Dek Professional Edition.Eatonton, GA:American Diabetes Association;1993.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180(8):799–800.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94(2):564–569.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12(5):R120.
- ,,, et al.Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism.Circulation.2008;117(12):1610–1619.
- ,,, et al.American Association of Clinical Endocrinologists/American Diabetes Association: Consensus Statement on Inpatient Glycemic Control.Endocr Pract.2009;15(4):353–369.
- .Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction.Diab Vasc Dis Res.2008;5(4):269–275.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34(11):2714–2718.
- ,,,.New insulin infusion protocol Improves blood glucose control in hospitalized patients without increasing hypoglycemia.Jt Comm J Qual Patient Saf.2005;31(3):141–147.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control.Crit Care Med.2003;31(2):359–366.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26(1):57–65.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290(15):2041–2047.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27(2):553–597.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354(5):449–461.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345(19):1359–1367.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125(5):1007–1021.
- ,.Cost analysis of intensive glycemic control in critically ill adult patients.Chest.2006;129(3):644–650.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients.N Engl J Med.2009;360(13):1283–1297.
- ,,, et al.Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit.Diabetes Care.2004;27(2):461–467.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157(5):545–552.
- .Diabetes Dek Professional Edition.Eatonton, GA:American Diabetes Association;1993.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,, et al.Intensive insulin therapy and mortality among critically ill patients: a meta‐analysis including NICE‐SUGAR study data.CMAJ.2009;180(8):799–800.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes.J Clin Endocrinol Metab.2009;94(2):564–569.
- ,,, et al.Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial.Crit Care.2008;12(5):R120.
- ,,, et al.Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism.Circulation.2008;117(12):1610–1619.
- ,,, et al.American Association of Clinical Endocrinologists/American Diabetes Association: Consensus Statement on Inpatient Glycemic Control.Endocr Pract.2009;15(4):353–369.
- .Blood glucose and its prognostic implications in patients hospitalised with acute myocardial infarction.Diab Vasc Dis Res.2008;5(4):269–275.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35(10):2262–2267.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34(11):2714–2718.
- ,,,.New insulin infusion protocol Improves blood glucose control in hospitalized patients without increasing hypoglycemia.Jt Comm J Qual Patient Saf.2005;31(3):141–147.
Copyright © 2010 Society of Hospital Medicine