User login
Excess Mortality Among Patients Hospitalized During the COVID-19 Pandemic
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.
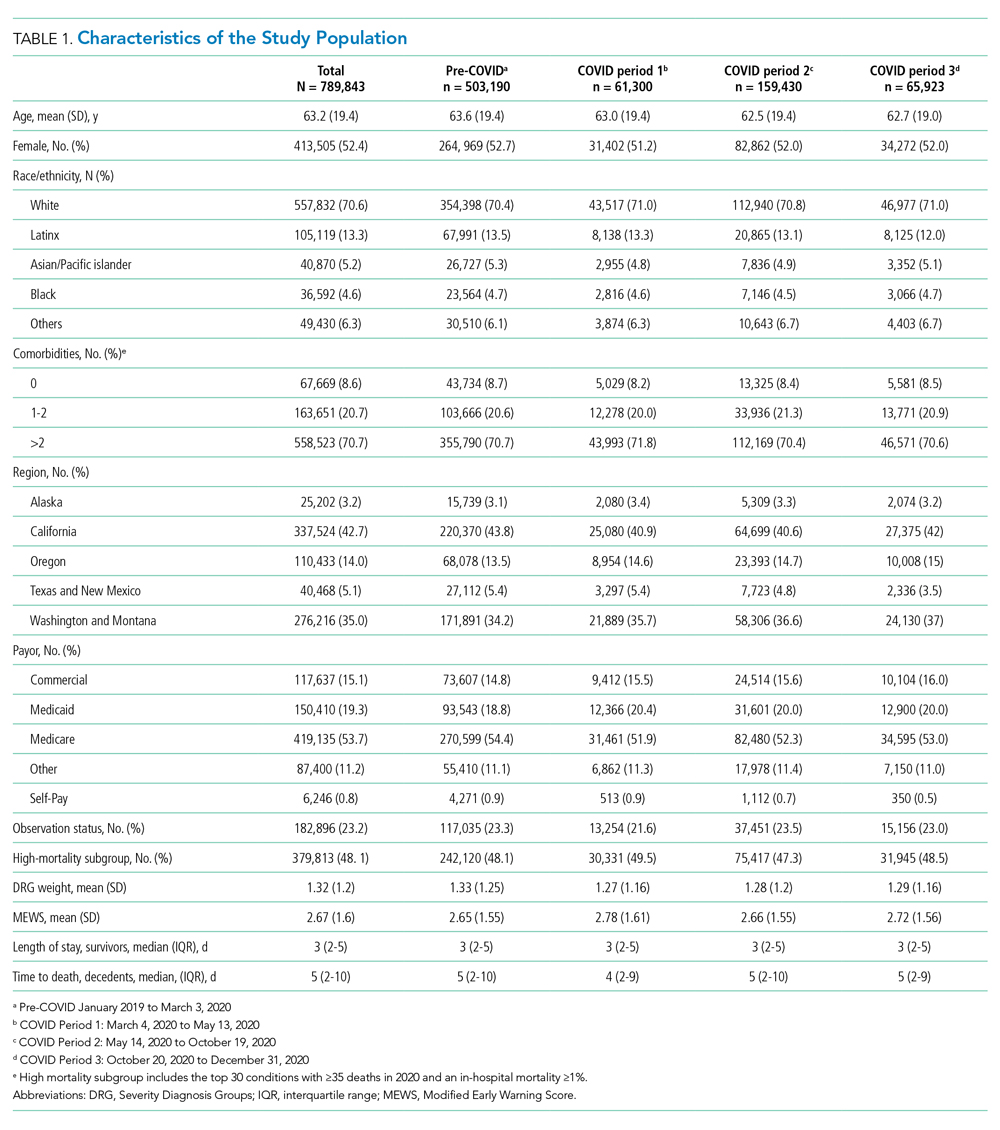
Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.
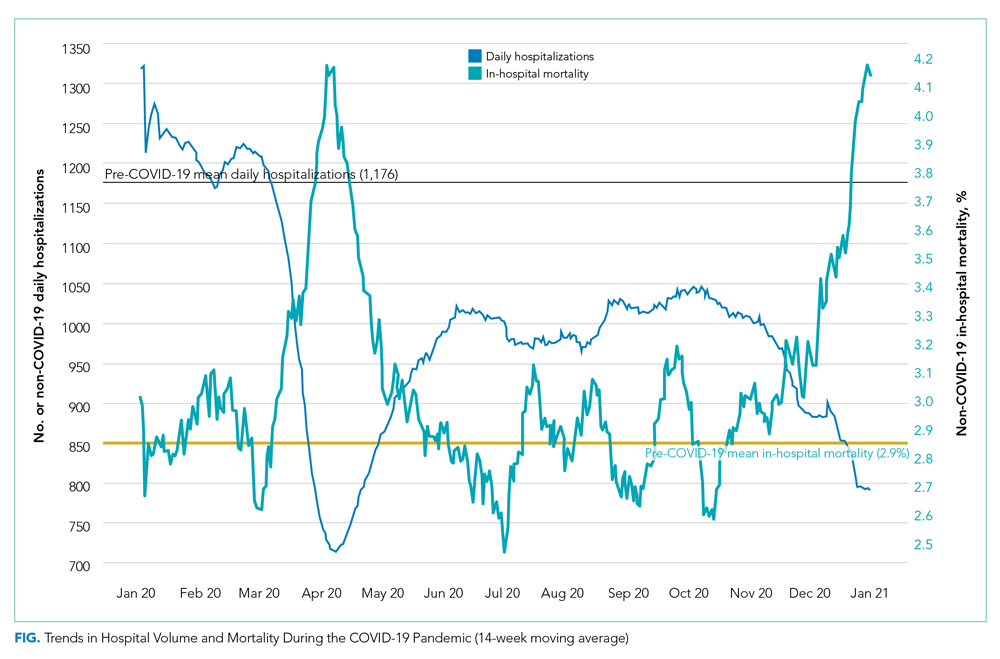
Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.
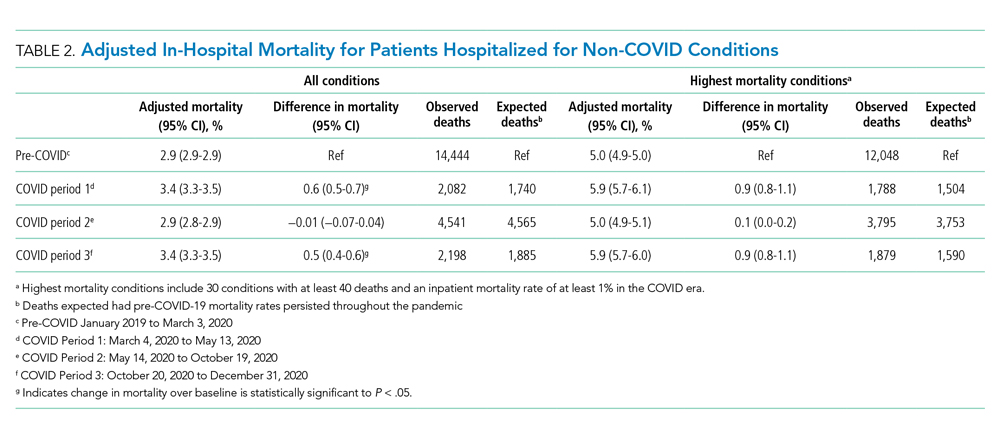
Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.
DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.

Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.

Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.

Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.

DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
One of the most striking features of the early COVID-19 pandemic was the sudden and sharp reductions in emergency department (ED) visits and hospitalizations throughout the United States.1-4 Several studies have documented lower rates of hospitalization for many emergent, time-sensitive conditions, such as acute myocardial infarction, stroke, and hyperglycemic crises, starting shortly after community transmission of COVID-19 was recognized and social distancing guidelines were implemented.5-8 In most cases, hospital volumes rebounded after an initial drop, stabilizing at somewhat lower levels than those expected from historic trends.9
The observed shifts in hospital use largely have been attributed to patients’ forgoing or delaying necessary care,10 which underscores the indirect effects of the pandemic on patients without COVID-19.11 To date, the extent to which outcomes for patients without COVID-19 have been adversely affected is less well understood. Evidence suggests patients with acute and chronic illnesses have experienced increased morbidity and mortality since the onset of the pandemic. For example, in northern California, abrupt declines in ED visits for cardiac symptoms were coupled with higher rates of out-of-hospital cardiac arrest.12 Moreover, states with higher rates of COVID-19 also reported increased deaths attributed to heart disease, diabetes, and other conditions.13
To better understand these potential indirect effects, this study used data from a large, multistate health care system to examine changes in hospital volume and its relationship to in-hospital mortality for patients without COVID-19 during the first 10 months of the pandemic.
METHODS
Setting and Participants
We examined unplanned hospitalizations from January 2019 to December 2020 at 51 community hospitals across 6 states (Alaska, Washington, Montana, Oregon, California, and Texas) in the Providence St. Joseph Health system. Hospitals within the Providence system share a common standard dataset for each encounter with a centralized cloud data warehouse from which we extracted clinical and demographic data. No hospitals entered or left the system during the study period. Hospitalizations were considered unplanned if they had an “urgent” or “emergency” service type in the record; most originated in the ED. Hospitalizations for children younger than 18 years and those with evidence of COVID-19 (International Classification of Disease, Tenth Revision, Clinical Modification U07.1, a positive COVID-19 polymerase chain reaction test during the encounter, or an infection control-assigned label of COVID-19) were excluded. The Providence St. Joseph Health Institutional Review Board approved this study.
Measures
Trends in daily hospitalizations and their relationship to adjusted in-hospital mortality (percentage of patients who died during their hospital admission) were examined over time. In preliminary models using segmented regression, we identified three distinct pandemic periods with different trends in daily hospitalizations: (1) a 10-week period corresponding to the spring COVID-19 surge (March 4 to May 13, 2020; Period 1), (2) an intervening period extending over the summer and early fall (May 14 to October 19, 2020; Period 2), and (3) a second 10-week period corresponding to the fall COVID-19 surge (October 20 to December 31, 2020; Period 3). In-hospital mortality for these periods was compared with a baseline period (pre-COVID-19) from January 1, 2019 to March 3, 2020. To further assess differences in mortality by clinical condition, hospitalizations were first grouped by primary diagnosis using Clinical Classifications Software Refined (CCSR) categories from the Agency for Healthcare Research and Quality14 and ranked by the number of observed deaths and the percentage of patients who died while hospitalized in 2020. We selected common conditions that had >35 total deaths and an in-hospital mortality rate ≥1% for condition-specific analyses, of which 30 met these criteria.
Analysis
Multivariate logistic regression was used to evaluate changes in mortality for each of the pandemic periods compared with baseline for the overall cohort and selected diagnosis groups. Our main model adjusted for age, sex, race/ethnicity (White, Black, Latinx, Asian or Pacific Islander, and other), primary payor (commercial, Medicaid, Medicare, other, and self-pay), the presence or absence of 31 chronic comorbidities in the medical record, primary admitting diagnosis grouped by CCSR category (456 total diagnostic groups), and hospital fixed-effects to account for clustering. Results are expressed as the average marginal effects of each pandemic period on in-hospital mortality (eg, adjusted percentage point change in mortality over baseline). The number of excess deaths in each period was calculated by multiplying the estimated percentage point change in mortality for each period by the total number of hospitalizations. These excess deaths were subtracted from the number of observed deaths to derive the number of deaths that would be expected if pre-pandemic mortality rates persisted.
To further assess whether changes in adjusted mortality could be attributed to a smaller, sicker population of patients presenting to the hospital during the pandemic (meaning that less acutely ill patients stayed home), we conducted two sensitivity analyses. First, we tested whether substituting indicators for Medicare Severity Diagnosis Groups (MS-DRG) in lieu of CCSR categories had any impact on our results. MS-DRGs are designed to account for a patient’s illness severity and expected costs, whereas CCSR categories do not.15 MS-DRGs also better distinguish between surgical versus medical conditions. We re-ran our main model using indicators for CCSR to control for diagnostic mix, but further adjusted for severity using the DRG weight for the primary diagnosis and Modified Early Warning Score (MEWS) as continuous covariates. MEWS is a physiologic scoring system that incorporates abnormal vital signs and data related to mental status during the first 24 hours of a patient’s hospitalization into a risk-based score that has been shown to predict hospital mortality and need for intensive care.16,17 These sensitivity analyses were performed on a subset of inpatient admissions because DRG data are not available for hospitalizations billed as an observation stay, and only approximately 70% of hospitals in the sample contributed vital sign data to the Providence data warehouse. All statistical analyses were conducted with R, version 3.6.3 (R Foundation for Statistical Computing) and SAS Enterprise Guide 7.1 (SAS Institute Inc).
RESULTS
The characteristics of our sample are described in Table 1. A total of 61,300, 159,430, and 65,923 hospitalizations occurred in each of the three pandemic periods, respectively, compared with 503,190 hospitalizations in the pre-pandemic period. The mean (SD) age of patients in the study was 63.2 (19.4) years; most were women (52.4%), White (70.6%), and had Medicare as their primary payor (53.7%). Less than half (42.7%) of hospitalizations occurred in California, and just under one-quarter were observation stays (23.2%). Patient characteristics were similar in the pre-COVID-19 and COVID-19 pandemic periods.

Figure 1 shows trends in hospital volume and mortality. Overall daily hospitalizations declined abruptly from a mean of 1176 per day in the pre-pandemic period to 617 per day (47.5% relative decrease) during the first 3 weeks of Period 1. Mean daily hospitalizations began to rise over the next 2 months (Period 1), reaching steady state at <1000 hospitalizations per day (15% relative decrease from baseline) during Period 2. During Period 3, we observed a decline in mean daily hospitalizations, with a low point of 882 per day on December 31, 2020 (25% relative decrease from baseline), corresponding to the end of our study period. Although hospital volumes declined during both COVID-19 surge periods, the percentage of patients who died during their hospitalization increased. There was an initial spike in in-hospital mortality that peaked approximately 1 month into the pandemic (middle of Period 1), a return to levels at or slightly below that before the pandemic by the beginning of Period 2, and then a rise throughout the autumn COVID-19 surge in Period 3, not yet peaking by the end of the study.

Adjusted in-hospital mortality for the three COVID-19 periods compared with the pre-pandemic period is presented in Table 2. The percentage of patients who died during their hospitalization rose from 2.9% in the pre-pandemic period to 3.4% during Period 1 (absolute difference, 0.6 percentage points; 95% CI, 0.5-0.7), corresponding to a 19.3% relative increase during the spring COVID-19 surge. Among the subset of patients hospitalized with 1 of the 30 conditions selected for individual analysis, mortality increased from 5.0% to 5.9% during the same time period (absolute difference, 0.9 percentage points; 95% CI, 0.8-1.1), corresponding to an 18.9% relative increase. In Period 2, in-hospital mortality was similar to that noted pre-pandemic for the overall cohort and the 30 selected conditions. During Period 3, in-hospital mortality increased by a magnitude similar to that observed in Period 1 for all hospitalizations combined (absolute difference, 0.5 percentage points; 95% CI, 0.0-0.6; corresponding to a 16.5% relative increase) as well as the subgroup with 1 of the 30 selected conditions (0.9 percentage points; 95% CI, 0.8-1.0; corresponding to an 18% relative increase). Further adjustment for severity by swapping CCSR categories with MS-DRG indicators or inclusion of DRG weight and MEWS score as covariates in our sensitivity analyses did not change our results.

Table 3 and the Appendix Figure describe changes in volume and adjusted in-hospital mortality for the 30 conditions selected for analysis. There was a decrease in the mean daily admissions for all conditions studied. Among the 30 conditions, 26 showed increased mortality during Period 1, although the increase was only statistically significant for 16 of these conditions. Among the 10 most commonly admitted conditions (by number of daily hospital admissions during the baseline period), there was a statistically significant relative increase in mortality for patients with sepsis (20.1%), heart failure (17.6%), ischemic stroke (12.5%), device/graft/surgical complications (14.0%), cardiac dysrhythmias (14.4%), pneumonia (24.5%), respiratory failure (16.1%), and gastrointestinal hemorrhage (23.3%). In general, mortality returned to baseline or improved during Period 2. Thereafter, all 30 conditions showed increased mortality in Period 3. This increase was significant for only 16 conditions, which were not the same ones noted during Period 1. Of note, although there was higher mortality for some cardiovascular conditions (heart failure cardiac dysrhythmias), mortality for myocardial infarction remained unchanged from baseline across all 3 periods. In contrast, several solid cancer–related conditions showed progressively worsening mortality throughout the study, with 7.7% higher mortality in Period 1, 10.3% higher mortality in Period 2, and 16.5% higher mortality in Period 3, respectively, compared with baseline. Although a similar pattern was observed for acute renal failure and some neurologic conditions (traumatic brain injury, seizure, other nervous system disorders), mortality for drug poisonings and gastrointestinal bleeds improved over time.

DISCUSSION
In this study of unplanned hospitalizations from 51 community hospitals across 6 states in the US West, we found a significant increase in mortality—at a rate of approximately 5 to 6 excess deaths per 1000 hospitalizations—among patients admitted during the pandemic with a variety of non-COVID-19 illnesses and injuries. Higher in-hospital mortality was observed in the spring (March to May) and fall (October to December) of 2020 when COVID-19 case counts surged and shelter-in-place mandates were implemented. With the initial surge, higher mortality rates were largely transient, and, for most conditions evaluated, returned to baseline approximately 3 months after the pandemic onset. For the fall surge, mortality rates had not peaked by the end of the study period. Changes in mortality were closely and inversely correlated with hospital volume for non-COVID-19 illnesses during both surge periods.
Higher morbidity and mortality for patients without COVID-19 appears to be an unfortunate spillover effect that has been reported in several studies. Recent work examining national surveillance data suggest that up to one-third of excess deaths (deaths higher than those expected for season) early in the pandemic have occurred among patients without known COVID-19.13,18-20 Specifically, these studies estimate that mortality rates in the United States increased by 15% to 19% in the spring of 2020; of the identified excess deaths, only 38% to 77% could be attributed to COVID-19, with the remainder attributed to cardiovascular disease, diabetes, and Alzheimer’s disease, among others. In addition, reports from several European countries and China examining population death data have found similar trends,21-25 as well as a recent study examining excess deaths in nursing homes.26 Our results are largely consistent with these earlier studies in that we describe higher mortality in a sample of patients hospitalized with a variety of common conditions that otherwise are routinely treated in US hospitals. Reporting these indirect casualties of COVID-19 is important to fully understand the pandemic’s toll on patients and healthcare systems.
Our work builds on the current body of literature, highlighting the consistent relationship between rising COVID-19 case counts, hospital volume, and excess mortality over more than one surge period. Although several studies have looked at trends in hospital admissions or population mortality rates, few have examined the two outcomes together. The close correlation between daily hospital admissions and in-hospital mortality in this study suggests that the pandemic changed how patients use healthcare resources in ways that were important for their health and outcomes. The higher mortality rate that we and others have observed likely is related to patients’ delaying care because of fear of contracting COVID-19. In one survey, more than 4 in 10 adults in the United States reported that they avoided medical care during the early pandemic.10 Importantly, even a few days delay for many conditions, such as heart failure or sepsis, can result in precipitous declines in clinical status and outcomes.
It also is possible that we found increased rates of in-hospital mortality simply because patients with more moderate illness chose to stay home, resulting in a patient population enriched with those more likely to die. We found mixed evidence in our data that the observed increases in mortality could be attributable to a smaller, sicker population. Some characteristics that might be protective, such as a slightly younger mean age and lower mean DRG weight, were more common among those hospitalized during the pandemic. However, other characteristics, such as a slightly higher MEWS score and a greater percentage of total hospitalizations in the higher mortality subgroup, also were noted during the pandemic (Table 1). We do note, however, that the differences in these severity-related characteristics were small across the study periods. Further adjusting for these characteristics in our sensitivity analyses did not appreciably change our main findings, suggesting that the mortality increase could not be explained by changes in case-mix alone.
Other factors not dependent on patient behavior, such as barriers to accessing timely ambulatory care and impacts in the quality of care delivered, might have contributed. Shelter-in-place orders, reduced in-person access to clinicians in the ambulatory setting, slow implementation of telehealth services (with uncertainty about their equivalence to in-person exams), as well as delays in diagnostic tests and outpatient procedures could have played a role, especially during early months of the pandemic.27 Significant changes to ambulatory health care delivery might have left many patients with chronic illnesses or complex medical needs with limited care options. Importantly, these care interruptions might have had greater implications for some patients, such as those with cancer who rely on intensive, largely outpatient-based treatment.28,29 This, in part, could explain why we found persistently increased mortality among patients hospitalized with cancer after the spring surge. Later into the pandemic, however, most health systems had developed processes that allowed clinicians to resume timely care of ambulatory patients. Because of this, increases in mortality observed during the fall surge likely stem from other factors, such as patient behavior.
It is possible that care delays or changes in the quality of care delivered during the index hospitalization or pre-hospital setting might have contributed to the observed increase in mortality. This is particularly true for acute, time-sensitive conditions such as sepsis and stroke. Extra time spent donning personal protective equipment and/or new protocols instituted during the pandemic likely impacted the speed of emergency medical services transport, timeliness of ED evaluation, and delivery of definitive therapy. Although most hospitals in this study were not overwhelmed by the pandemic, the complexities associated with caring for known and suspected COVID-19 patients alongside those without the disease might have altered ideal care practices and strained healthcare teams.30 In addition, nearly all hospitalized patients during this period were deprived of in-person advocacy by family members, who were not permitted to visit.
Important limitations with this study exist. First, the data come only from hospitals in the western United States. Second, some data elements such as triage scores or vital signs were not available for the entire population, potentially limiting some risk-adjustment. Third, we were unable to determine the root cause of excess mortality based on our study design and the coded variables available. It is unknown to what extent undiagnosed COVID-19 played a role. Early in the pandemic, many community hospitals did not have access to timely COVID-19 testing, and some cases might have not been diagnosed.31 However, we do not expect this to be a significant concern in the later months of the pandemic, as testing became more widespread and hospitals implemented surveillance screening for COVID-19 for inpatients.
CONCLUSIONS
Our study indicates that the COVID-19 pandemic was associated with increased mortality among patients hospitalized for a range of clinical conditions. Although higher observed mortality rates were limited to periods of high COVID-19 activity, future studies will need to tease out the extent to which these findings relate to patient factors (ie, delayed presentation and more severe disease) or systemic factors (reduction in access or changes in quality of care). This is of key importance, and appropriate solutions will need to be developed to mitigate adverse impacts with this and future pandemics.
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
1. Baum A, Schwartz MD. Admissions to Veterans Affairs hospitals for emergency conditions during the COVID-19 pandemic. JAMA. 2020;324(1):96-99. https://doi.org/10.1001/jama.2020.9972
2. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits — United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. https://doi.org/10.15585/mmwr.mm6923e1
3. Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff. 2020;39(11):2010-2017. https://doi.org/10.1377/hlthaff.2020.00980
4. Blecker S, Jones SA, Petrilli CM, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021;181(2):269-271. https://doi.org/10.1001/jamainternmed.2020.3978
5. Bhambhvani HP, Rodrigues AJ, Yu JS, Carr JB 2nd, Hayden Gephart M. Hospital volumes of 5 medical emergencies in the COVID-19 pandemic in 2 US medical centers. JAMA Intern Med. 2021;181(2):272-274. https://doi.org/10.1001/jamainternmed.2020.3982
6. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions — United States, January–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25);795-800. https://doi.org/10.15585/mmwr.mm6925e2
7. Solomon MD, McNulty EJ, Rana JS, et al. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691-693. https://doi.org/10.1056/NEJMc2015630
8. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400-401. https://doi.org/10.1056/NEJMc2014816
9. Heist T, Schwartz K, Butler S. Trends in overall and non-COVID-19 hospital admissions. Kaiser Family Foundation. Accessed March 18, 2021. https://www.kff.org/health-costs/issue-brief/trends-in-overall-and-non-covid-19-hospital-admissions
10. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36);1250-1257. https://doi.org/10.15585/mmwr.mm6936a4
11. Chen J, McGeorge R. Spillover effects of the COVID-19 pandemic could drive long-term health consequences for non-COVID-19 patients. Health Affairs Blog. Accessed March 18, 2021. https://www.healthaffairs.org/do/10.1377/hblog20201020.566558/full/
12. Wong LE, Hawkins JE, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catalyst. Accessed March 18, 2021. https://catalyst.nejm.org/doi/abs/10.1056/CAT.20.0193
13. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. https://doi.org/10.1001/jama.2020.11787
14. Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. Agency for Healthcare Research and Quality, Rockville, MD. Accessed April 22, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp
15. MS-DRG Classifications and Software. Centers for Medicare & Medicaid Services. Accessed March 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
16. Jayasundera R, Neilly M, Smith TO, Myint PK. Are early warning scores useful predictors for mortality and morbidity in hospitalised acutely unwell older patients? A systematic review. J Clin Med. 2018;7(10):309. https://doi.org/10.3390/jcm7100309
17. Delgado-Hurtado JJ, Berger A, Bansal AB. Emergency department Modified Early Warning Score association with admission, admission disposition, mortality, and length of stay. J Community Hosp Intern Med Perspect. 2016;6(2):31456. https://doi.org/10.3402/jchimp.v6.31456
18. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562-1564. https://doi.org/10.1001/jama.2020.19545
19. Faust JS, Krumholz HM, Du C, et al. All-cause excess mortality and COVID-19–related mortality among US adults aged 25-44 years, March-July 2020. JAMA. 2021;325(8):785-787. https://doi.org/10.1001/jama.2020.24243
20. Weinberger DM, Chen J, Cohen T, et al. Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020. JAMA Intern Med. 2020;180(10):1336-1344. https://doi.org/10.1001/jamainternmed.2020.3391
21. Vandoros S. Excess mortality during the Covid-19 pandemic: Early evidence from England and Wales. Soc Sci Med. 2020; 258:113101. https://doi.org/10.1016/j.socscimed.2020.113101
22. Vestergaard LS, Nielsen J, Richter L, et al; ECDC Public Health Emergency Team for COVID-19. Excess all-cause mortality during the COVID-19 pandemic in Europe – preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25(26):2001214. https://doi.org/10.2807/1560-7917.ES.2020.25.26.2001214
23. Kontopantelis E, Mamas MA, Deanfield J, Asaria M, Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. J Epidemiol Community Health. 2021;75(3):213-223. https://doi.org/10.1136/jech-2020-214764
24. Liu J, Zhang L, Yan Y, et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. https://doi.org/10.1136/bmj.n415
25. Docherty KF, Butt JH, de Boer RA, et al. Excess deaths during the Covid-19 pandemic: An international comparison. Preprint. Posted online May 13, 2020. medRxiv. doi:https://doi.org/10.1101/2020.04.21.20073114
26. Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, admissions, and patient census at SNFs in 3 US cities during the COVID-19 pandemic. JAMA. 2020;324(5):507-509. https://doi.org/10.1001/jama.2020.11642
27. Rosenbaum L. The untold toll — The pandemic’s effects on patients without Covid-19. N Engl J Med. 2020; 382:2368-2371 https://doi.org/10.1056/NEJMms2009984
28. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open. 2020;10(11):e043828. https://doi.org/10.1136/bmjopen-2020-043828
29. Van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665-671. https://doi.org/10.1038/s41591-020-0874-8
30. Traylor AM, Tannenbaum SI, Thomas EJ, Salas E. Helping healthcare teams save lives during COVID-19: insights and countermeasures from team science. Am Psychol. 2020;76(1):1-13. https://doi.org/10.1037/amp0000750
31. Grimm CA. Hospital experiences responding to the COVID-19 pandemic: results of a National Pulse Survey March 23–27. U.S. Department of Health and Human Services Office of Inspector General; 2020. https://oig.hhs.gov/oei/reports/oei-06-20-00300.pdf
© 2021 Society of Hospital Medicine