User login
In reply: Not all joint pain is arthritis
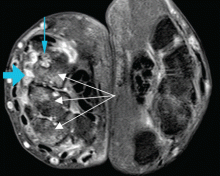
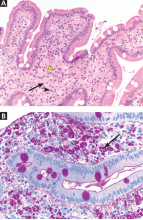
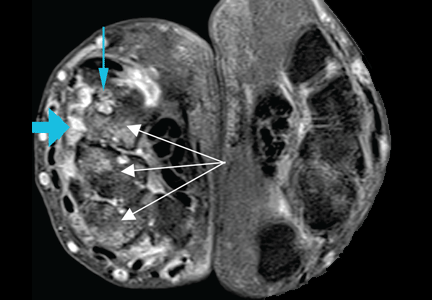
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
When people with diabetes go to surgery
Over the past decade, recommendations about the ideal glucose target in hospitalized diabetic patients have fluctuated. The controversy has extended to diabetic patients in various types of intensive care units and to those headed to the operating room. Although proposals exist on how to manage diabetes in the operating room, including intraoperative insulin infusions, outcomes probably depend more on how glucose is managed during the patient’s postoperative stay in the hospital. For patients who are less critically ill and less medically complex, continuous insulin infusions are used infrequently, and insulin is often prescribed by algorithm or, archaically, by some form of “catch-up” sliding scale. Studies indicate that even the fairly loose glucose target of 70 to 180 mg/dL is achieved consistently in only a few patients.1
In view of a number of observations, including the link between hyperglycemia and postoperative wound infections, studies were designed to test the hypothesis that aggressively keeping glucose levels quite low in critically ill and postoperative diabetic patients would be beneficial. Instead, most of these studies found that overly tight glucose control in these settings led to untoward outcomes—and not only as the result of hypoglycemic episodes. Aiming for a modest serum glucose target of 150 to 200 mg/dL can significantly reduce the postoperative death rate, but the beneficial reduction is no greater if the target is less than 150 mg/dL.
With a looser glucose target, pre- and perioperative management of insulin-dependent diabetic patients can be simplified. Dobri and Lansang discuss the key practical principles of managing insulin before the patient goes to the operating suite. They emphasize relevant pearls of insulin physiology and discuss several scenarios we often encounter.
In fact, the principles they review are equally useful to remember when we admit diabetic patients to the hospital with orders to keep them “npo” while planning and awaiting tests or other procedures. A key take-home point is that severely insulinopenic patients require some exogenous basal insulin, even when not eating.
- Lopes R, Albrecht A, Williams J, et al. Postoperative glucose control following coronary artery bypass graft surgery: predictors and clinical outcomes. J Am Coll Cardiol 2013; 61:e1601.
Over the past decade, recommendations about the ideal glucose target in hospitalized diabetic patients have fluctuated. The controversy has extended to diabetic patients in various types of intensive care units and to those headed to the operating room. Although proposals exist on how to manage diabetes in the operating room, including intraoperative insulin infusions, outcomes probably depend more on how glucose is managed during the patient’s postoperative stay in the hospital. For patients who are less critically ill and less medically complex, continuous insulin infusions are used infrequently, and insulin is often prescribed by algorithm or, archaically, by some form of “catch-up” sliding scale. Studies indicate that even the fairly loose glucose target of 70 to 180 mg/dL is achieved consistently in only a few patients.1
In view of a number of observations, including the link between hyperglycemia and postoperative wound infections, studies were designed to test the hypothesis that aggressively keeping glucose levels quite low in critically ill and postoperative diabetic patients would be beneficial. Instead, most of these studies found that overly tight glucose control in these settings led to untoward outcomes—and not only as the result of hypoglycemic episodes. Aiming for a modest serum glucose target of 150 to 200 mg/dL can significantly reduce the postoperative death rate, but the beneficial reduction is no greater if the target is less than 150 mg/dL.
With a looser glucose target, pre- and perioperative management of insulin-dependent diabetic patients can be simplified. Dobri and Lansang discuss the key practical principles of managing insulin before the patient goes to the operating suite. They emphasize relevant pearls of insulin physiology and discuss several scenarios we often encounter.
In fact, the principles they review are equally useful to remember when we admit diabetic patients to the hospital with orders to keep them “npo” while planning and awaiting tests or other procedures. A key take-home point is that severely insulinopenic patients require some exogenous basal insulin, even when not eating.
Over the past decade, recommendations about the ideal glucose target in hospitalized diabetic patients have fluctuated. The controversy has extended to diabetic patients in various types of intensive care units and to those headed to the operating room. Although proposals exist on how to manage diabetes in the operating room, including intraoperative insulin infusions, outcomes probably depend more on how glucose is managed during the patient’s postoperative stay in the hospital. For patients who are less critically ill and less medically complex, continuous insulin infusions are used infrequently, and insulin is often prescribed by algorithm or, archaically, by some form of “catch-up” sliding scale. Studies indicate that even the fairly loose glucose target of 70 to 180 mg/dL is achieved consistently in only a few patients.1
In view of a number of observations, including the link between hyperglycemia and postoperative wound infections, studies were designed to test the hypothesis that aggressively keeping glucose levels quite low in critically ill and postoperative diabetic patients would be beneficial. Instead, most of these studies found that overly tight glucose control in these settings led to untoward outcomes—and not only as the result of hypoglycemic episodes. Aiming for a modest serum glucose target of 150 to 200 mg/dL can significantly reduce the postoperative death rate, but the beneficial reduction is no greater if the target is less than 150 mg/dL.
With a looser glucose target, pre- and perioperative management of insulin-dependent diabetic patients can be simplified. Dobri and Lansang discuss the key practical principles of managing insulin before the patient goes to the operating suite. They emphasize relevant pearls of insulin physiology and discuss several scenarios we often encounter.
In fact, the principles they review are equally useful to remember when we admit diabetic patients to the hospital with orders to keep them “npo” while planning and awaiting tests or other procedures. A key take-home point is that severely insulinopenic patients require some exogenous basal insulin, even when not eating.
- Lopes R, Albrecht A, Williams J, et al. Postoperative glucose control following coronary artery bypass graft surgery: predictors and clinical outcomes. J Am Coll Cardiol 2013; 61:e1601.
- Lopes R, Albrecht A, Williams J, et al. Postoperative glucose control following coronary artery bypass graft surgery: predictors and clinical outcomes. J Am Coll Cardiol 2013; 61:e1601.
How should we manage insulin therapy before surgery?
Continuing at least part of the basal insulin is the reasonable, physiologic approach to controlling glucose levels before surgery in patients with diabetes. The process involves three basic steps:
- Ascertaining the type of diabetes
- Adjusting the basal insulin dosage
- Stopping the prandial insulin.
The steps are the same whether the surgery is major or minor. These recommendations are based on general principles of insulin action, data from large databases of surgical inpatients, and expert clinical experience translated into standardized protocols.1,2
WHY CONTINUE THE INSULIN?
Stopping or decreasing insulin because of a fear of hypoglycemia is not appropriate, as the resulting hyperglycemia can lead to delayed wound healing, wound infection, fluid and electrolyte shifts, diabetic ketoacidosis, and hyperosmolar states.
Insulin inhibits both gluconeogenesis and conversion of glycogen to glucose, processes that occur regardless of food intake. It also inhibits degradation of fats to fatty acids and of fatty acids to ketones. This is why inadequate insulin dosing can lead to uncontrolled hyperglycemia and even ketoacidosis, and thus why long-acting insulin is needed in a fasting state.
STEP 1: ASCERTAIN THE TYPE OF DIABETES
Does the patient have type 1 or type 2 diabetes, and does that even matter?
The type of diabetes should not matter, since ideally the insulin should be dosed the same for both types. However, the consequences of inappropriate insulin management may be different.
Usually, the type of diabetes can be ascertained by the history. If the patient was diagnosed at age 40 or later and was on oral medication for years before insulin was started, then he or she most likely has type 2. If the patient was younger than 40 at the time of diagnosis, was lean, and was started on insulin within a year of diagnosis, then he or she likely has type 1.
If this information is not available or is unreliable and the patient has been on insulin for many years, we recommend viewing the patient as being insulinopenic, ie, not producing enough insulin endogenously and thus requiring insulin at all times.
Though checking for antibody markers of type 1 diabetes might give a more definitive answer, it is not practical before surgery.
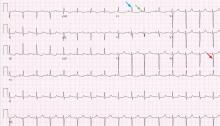
In the setting of surgical stress, withholding the basal insulin preoperatively and just giving a small dose of fast-acting (see Table 1 for the different classes of insulin) or shortacting insulin as part of a sliding scale (ie, insulin given only when the blood glucose reaches a certain high level) can send a patient with type 1 diabetes into diabetic ketoacidosis by the end of the day. This is less likely to occur in a patient with type 2 diabetes with some endogenous insulin secretion.
STEP 2: ADJUST THE BASAL INSULIN
Basal insulin is the insulin that the healthy person’s body produces when fasting. For a diabetic patient already on insulin, basal insulin is insulin injected to prevent ketogenesis, glycogenolysis, and gluconeogenesis in the fasting state.
If the basal insulin is long-acting
Long-acting insulins have a relatively peakless profile and, when properly dosed, should not result in hypoglycemia when a patient is fasting.
Preoperatively, the patient should take it as close as possible to the usual time of injection. This could be at home either at bedtime the night before surgery or the morning of surgery. If there is concern for hypoglycemia, the injection can be given when the patient is at the hospital.
- If the patient does not tend to have hypoglycemic episodes and the total daily basal insulin dose is roughly the same as the total daily mealtime (prandial) dose (eg, 50% basal, 50% prandial ratio), the full dose of basal insulin can be given.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin lispro 10 U with each meal and does not have hypoglycemic episodes, then insulin glargine 30 U should be taken at bedtime.
- If the patient has hypoglycemic episodes at home, then the basal insulin can be reduced by 25%.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin glulisine 10 U with each meal (appropriate proportion of doses, similar to the example above) but has hypoglycemic episodes at home on this regimen, then only 22 U of insulin glargine should be taken at bedtime.
- If the patient’s regimen has disproportionately more basal insulin than mealtime insulin, then the total daily doses can be added and half can be given as the basal insulin.
Example: If the patient is on insulin detemir 30 U every morning at 6 am and insulin aspart 6 U with each meal and has no hypoglycemic episodes, then 24 U of insulin detemir should be taken in the morning (ie, half of the total of 30 + 6 + 6 + 6).
- In the less common scenario of diabetes managed only with basal insulin (no other diabetes injections or oral agents), then half of the dose can be given.
- If the patient is on twice-daily long-acting basal insulin, then both the dose the night before surgery and the dose the morning of surgery should be adjusted.
If the basal insulin is intermediate-acting
The intermediate-acting insulin neutral protamine Hagedorn (NPH) is usually given twice a day because of its profile (Table 1).
- On the night before surgery, the full dose of NPH insulin should be taken, unless the patient will now skip a nighttime meal because of taking nothing by mouth, in which case the dose can be decreased by 25%.1
- On the morning of surgery, since the patient will be skipping breakfast and probably also lunch, the dose should be reduced by 50%.3,4
Special situation: Premixed insulins
Premixed insulins (70/30, 75/25) are a combination of intermediate-acting insulin and either fast-acting or short-acting insulin. In other words, they are combinations of basal and prandial insulin. Their use is thus not ideal in the preoperative period. There are two options in these situations.
One option is to switch to a regimen that includes long-acting insulin. If the patient is admitted for surgery, then the hospital staff can change the insulin regimen to long-acting basal insulin. A quick formula for conversion is to add all the premixed insulin doses and give half as basal insulin on the morning of surgery, similar to the scenario above for the patient with long-acting basal insulin that was out of proportion to the prandial insulin injections.
For example, if the usual regimen is insulin 70/30 NPH/Regular, 60 U with breakfast, 30 U with dinner, then the patient can take 45 U of insulin glargine (which is half of 60 + 30) in the morning or evening before surgery.
Another option is to adjust the dose of pre-mixed insulin. Sometimes it is not feasible or economical to change the patient’s premixed insulin just before surgery. In these situations, the patient can take half of the morning dose, followed by dextrose-containing intravenous fluids and blood glucose checks.
We recommend preoperatively giving at least part of the patient’s previous basal insulin, regardless of the type of diabetes, the type of surgery, or the fasting period.
STEP 3: STOP THE PRANDIAL INSULIN
Prandial insulin—given before each meal to cover the carbohydrates to be consumed—should be stopped the morning of surgery.3,4
WHAT ABOUT SLIDING SCALE INSULIN?
Using a sliding scale alone has no known benefit. Although it can be a quick fix to correct a high glucose level, it should be added to the basal insulin and not used as the sole insulin therapy. If a sliding scale is used, fast-acting insulin (aspart, glulisine, lispro) is preferred over regular insulin because of the more rapid onset and shorter duration of action.
Patients already using a supplemental insulin scale can apply it to correct a blood glucose above 200 mg/dL on the morning of surgery.
MAINTENANCE FLUIDS
As long as glucose levels are not very elevated (ie, > 200 mg/dL), after 12 hours on a nothing-by-mouth regimen, provide dextrose in the IV fluid to prevent hypoglycemia (eg, the patient received long-acting insulin and the glucose levels are running low) or to prevent starvation ketosis, which may result in ketones in the blood or urine. We recommend 5% dextrose in half-normal (0.45%) saline at 50 to 75 mL/hour as maintenance fluid; the infusion rate should be lower if fluid overload is a concern.
POSTOPERATIVE INSULIN MANAGEMENT
Once patients are discharged and can go back to their previous routine, they can restart their usual insulin regimen the same evening. The prandial insulin will be resumed when the regular diet is reintroduced, and the doses will be adjusted according to food intake.
- Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg 2010; 111:1378–1387.
- DiNardo M, Donihi AC, Forte P, Gieraltowski L, Korytkowski M. Standardized glycemic management and perioperative glycemic outcomes in patients with diabetes mellitus who undergo same-day surgery. Endocr Pract 2011; 17:404–411.
- Vann MA. Perioperative management of ambulatory surgical patients with diabetes mellitus. Curr Opin Anaesthesiol 2009; 22:718–724.
- Meneghini LF. Perioperative management of diabetes: translating evidence into practice. Cleve Clin J Med 2009; 76(suppl 4):S53–S59.
Continuing at least part of the basal insulin is the reasonable, physiologic approach to controlling glucose levels before surgery in patients with diabetes. The process involves three basic steps:
- Ascertaining the type of diabetes
- Adjusting the basal insulin dosage
- Stopping the prandial insulin.
The steps are the same whether the surgery is major or minor. These recommendations are based on general principles of insulin action, data from large databases of surgical inpatients, and expert clinical experience translated into standardized protocols.1,2
WHY CONTINUE THE INSULIN?
Stopping or decreasing insulin because of a fear of hypoglycemia is not appropriate, as the resulting hyperglycemia can lead to delayed wound healing, wound infection, fluid and electrolyte shifts, diabetic ketoacidosis, and hyperosmolar states.
Insulin inhibits both gluconeogenesis and conversion of glycogen to glucose, processes that occur regardless of food intake. It also inhibits degradation of fats to fatty acids and of fatty acids to ketones. This is why inadequate insulin dosing can lead to uncontrolled hyperglycemia and even ketoacidosis, and thus why long-acting insulin is needed in a fasting state.
STEP 1: ASCERTAIN THE TYPE OF DIABETES
Does the patient have type 1 or type 2 diabetes, and does that even matter?
The type of diabetes should not matter, since ideally the insulin should be dosed the same for both types. However, the consequences of inappropriate insulin management may be different.
Usually, the type of diabetes can be ascertained by the history. If the patient was diagnosed at age 40 or later and was on oral medication for years before insulin was started, then he or she most likely has type 2. If the patient was younger than 40 at the time of diagnosis, was lean, and was started on insulin within a year of diagnosis, then he or she likely has type 1.
If this information is not available or is unreliable and the patient has been on insulin for many years, we recommend viewing the patient as being insulinopenic, ie, not producing enough insulin endogenously and thus requiring insulin at all times.
Though checking for antibody markers of type 1 diabetes might give a more definitive answer, it is not practical before surgery.
In the setting of surgical stress, withholding the basal insulin preoperatively and just giving a small dose of fast-acting (see Table 1 for the different classes of insulin) or shortacting insulin as part of a sliding scale (ie, insulin given only when the blood glucose reaches a certain high level) can send a patient with type 1 diabetes into diabetic ketoacidosis by the end of the day. This is less likely to occur in a patient with type 2 diabetes with some endogenous insulin secretion.
STEP 2: ADJUST THE BASAL INSULIN
Basal insulin is the insulin that the healthy person’s body produces when fasting. For a diabetic patient already on insulin, basal insulin is insulin injected to prevent ketogenesis, glycogenolysis, and gluconeogenesis in the fasting state.
If the basal insulin is long-acting
Long-acting insulins have a relatively peakless profile and, when properly dosed, should not result in hypoglycemia when a patient is fasting.
Preoperatively, the patient should take it as close as possible to the usual time of injection. This could be at home either at bedtime the night before surgery or the morning of surgery. If there is concern for hypoglycemia, the injection can be given when the patient is at the hospital.
- If the patient does not tend to have hypoglycemic episodes and the total daily basal insulin dose is roughly the same as the total daily mealtime (prandial) dose (eg, 50% basal, 50% prandial ratio), the full dose of basal insulin can be given.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin lispro 10 U with each meal and does not have hypoglycemic episodes, then insulin glargine 30 U should be taken at bedtime.
- If the patient has hypoglycemic episodes at home, then the basal insulin can be reduced by 25%.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin glulisine 10 U with each meal (appropriate proportion of doses, similar to the example above) but has hypoglycemic episodes at home on this regimen, then only 22 U of insulin glargine should be taken at bedtime.
- If the patient’s regimen has disproportionately more basal insulin than mealtime insulin, then the total daily doses can be added and half can be given as the basal insulin.
Example: If the patient is on insulin detemir 30 U every morning at 6 am and insulin aspart 6 U with each meal and has no hypoglycemic episodes, then 24 U of insulin detemir should be taken in the morning (ie, half of the total of 30 + 6 + 6 + 6).
- In the less common scenario of diabetes managed only with basal insulin (no other diabetes injections or oral agents), then half of the dose can be given.
- If the patient is on twice-daily long-acting basal insulin, then both the dose the night before surgery and the dose the morning of surgery should be adjusted.
If the basal insulin is intermediate-acting
The intermediate-acting insulin neutral protamine Hagedorn (NPH) is usually given twice a day because of its profile (Table 1).
- On the night before surgery, the full dose of NPH insulin should be taken, unless the patient will now skip a nighttime meal because of taking nothing by mouth, in which case the dose can be decreased by 25%.1
- On the morning of surgery, since the patient will be skipping breakfast and probably also lunch, the dose should be reduced by 50%.3,4
Special situation: Premixed insulins
Premixed insulins (70/30, 75/25) are a combination of intermediate-acting insulin and either fast-acting or short-acting insulin. In other words, they are combinations of basal and prandial insulin. Their use is thus not ideal in the preoperative period. There are two options in these situations.
One option is to switch to a regimen that includes long-acting insulin. If the patient is admitted for surgery, then the hospital staff can change the insulin regimen to long-acting basal insulin. A quick formula for conversion is to add all the premixed insulin doses and give half as basal insulin on the morning of surgery, similar to the scenario above for the patient with long-acting basal insulin that was out of proportion to the prandial insulin injections.
For example, if the usual regimen is insulin 70/30 NPH/Regular, 60 U with breakfast, 30 U with dinner, then the patient can take 45 U of insulin glargine (which is half of 60 + 30) in the morning or evening before surgery.
Another option is to adjust the dose of pre-mixed insulin. Sometimes it is not feasible or economical to change the patient’s premixed insulin just before surgery. In these situations, the patient can take half of the morning dose, followed by dextrose-containing intravenous fluids and blood glucose checks.
We recommend preoperatively giving at least part of the patient’s previous basal insulin, regardless of the type of diabetes, the type of surgery, or the fasting period.
STEP 3: STOP THE PRANDIAL INSULIN
Prandial insulin—given before each meal to cover the carbohydrates to be consumed—should be stopped the morning of surgery.3,4
WHAT ABOUT SLIDING SCALE INSULIN?
Using a sliding scale alone has no known benefit. Although it can be a quick fix to correct a high glucose level, it should be added to the basal insulin and not used as the sole insulin therapy. If a sliding scale is used, fast-acting insulin (aspart, glulisine, lispro) is preferred over regular insulin because of the more rapid onset and shorter duration of action.
Patients already using a supplemental insulin scale can apply it to correct a blood glucose above 200 mg/dL on the morning of surgery.
MAINTENANCE FLUIDS
As long as glucose levels are not very elevated (ie, > 200 mg/dL), after 12 hours on a nothing-by-mouth regimen, provide dextrose in the IV fluid to prevent hypoglycemia (eg, the patient received long-acting insulin and the glucose levels are running low) or to prevent starvation ketosis, which may result in ketones in the blood or urine. We recommend 5% dextrose in half-normal (0.45%) saline at 50 to 75 mL/hour as maintenance fluid; the infusion rate should be lower if fluid overload is a concern.
POSTOPERATIVE INSULIN MANAGEMENT
Once patients are discharged and can go back to their previous routine, they can restart their usual insulin regimen the same evening. The prandial insulin will be resumed when the regular diet is reintroduced, and the doses will be adjusted according to food intake.
Continuing at least part of the basal insulin is the reasonable, physiologic approach to controlling glucose levels before surgery in patients with diabetes. The process involves three basic steps:
- Ascertaining the type of diabetes
- Adjusting the basal insulin dosage
- Stopping the prandial insulin.
The steps are the same whether the surgery is major or minor. These recommendations are based on general principles of insulin action, data from large databases of surgical inpatients, and expert clinical experience translated into standardized protocols.1,2
WHY CONTINUE THE INSULIN?
Stopping or decreasing insulin because of a fear of hypoglycemia is not appropriate, as the resulting hyperglycemia can lead to delayed wound healing, wound infection, fluid and electrolyte shifts, diabetic ketoacidosis, and hyperosmolar states.
Insulin inhibits both gluconeogenesis and conversion of glycogen to glucose, processes that occur regardless of food intake. It also inhibits degradation of fats to fatty acids and of fatty acids to ketones. This is why inadequate insulin dosing can lead to uncontrolled hyperglycemia and even ketoacidosis, and thus why long-acting insulin is needed in a fasting state.
STEP 1: ASCERTAIN THE TYPE OF DIABETES
Does the patient have type 1 or type 2 diabetes, and does that even matter?
The type of diabetes should not matter, since ideally the insulin should be dosed the same for both types. However, the consequences of inappropriate insulin management may be different.
Usually, the type of diabetes can be ascertained by the history. If the patient was diagnosed at age 40 or later and was on oral medication for years before insulin was started, then he or she most likely has type 2. If the patient was younger than 40 at the time of diagnosis, was lean, and was started on insulin within a year of diagnosis, then he or she likely has type 1.
If this information is not available or is unreliable and the patient has been on insulin for many years, we recommend viewing the patient as being insulinopenic, ie, not producing enough insulin endogenously and thus requiring insulin at all times.
Though checking for antibody markers of type 1 diabetes might give a more definitive answer, it is not practical before surgery.
In the setting of surgical stress, withholding the basal insulin preoperatively and just giving a small dose of fast-acting (see Table 1 for the different classes of insulin) or shortacting insulin as part of a sliding scale (ie, insulin given only when the blood glucose reaches a certain high level) can send a patient with type 1 diabetes into diabetic ketoacidosis by the end of the day. This is less likely to occur in a patient with type 2 diabetes with some endogenous insulin secretion.
STEP 2: ADJUST THE BASAL INSULIN
Basal insulin is the insulin that the healthy person’s body produces when fasting. For a diabetic patient already on insulin, basal insulin is insulin injected to prevent ketogenesis, glycogenolysis, and gluconeogenesis in the fasting state.
If the basal insulin is long-acting
Long-acting insulins have a relatively peakless profile and, when properly dosed, should not result in hypoglycemia when a patient is fasting.
Preoperatively, the patient should take it as close as possible to the usual time of injection. This could be at home either at bedtime the night before surgery or the morning of surgery. If there is concern for hypoglycemia, the injection can be given when the patient is at the hospital.
- If the patient does not tend to have hypoglycemic episodes and the total daily basal insulin dose is roughly the same as the total daily mealtime (prandial) dose (eg, 50% basal, 50% prandial ratio), the full dose of basal insulin can be given.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin lispro 10 U with each meal and does not have hypoglycemic episodes, then insulin glargine 30 U should be taken at bedtime.
- If the patient has hypoglycemic episodes at home, then the basal insulin can be reduced by 25%.3
Example: If the patient is on insulin glargine 30 U at bedtime and insulin glulisine 10 U with each meal (appropriate proportion of doses, similar to the example above) but has hypoglycemic episodes at home on this regimen, then only 22 U of insulin glargine should be taken at bedtime.
- If the patient’s regimen has disproportionately more basal insulin than mealtime insulin, then the total daily doses can be added and half can be given as the basal insulin.
Example: If the patient is on insulin detemir 30 U every morning at 6 am and insulin aspart 6 U with each meal and has no hypoglycemic episodes, then 24 U of insulin detemir should be taken in the morning (ie, half of the total of 30 + 6 + 6 + 6).
- In the less common scenario of diabetes managed only with basal insulin (no other diabetes injections or oral agents), then half of the dose can be given.
- If the patient is on twice-daily long-acting basal insulin, then both the dose the night before surgery and the dose the morning of surgery should be adjusted.
If the basal insulin is intermediate-acting
The intermediate-acting insulin neutral protamine Hagedorn (NPH) is usually given twice a day because of its profile (Table 1).
- On the night before surgery, the full dose of NPH insulin should be taken, unless the patient will now skip a nighttime meal because of taking nothing by mouth, in which case the dose can be decreased by 25%.1
- On the morning of surgery, since the patient will be skipping breakfast and probably also lunch, the dose should be reduced by 50%.3,4
Special situation: Premixed insulins
Premixed insulins (70/30, 75/25) are a combination of intermediate-acting insulin and either fast-acting or short-acting insulin. In other words, they are combinations of basal and prandial insulin. Their use is thus not ideal in the preoperative period. There are two options in these situations.
One option is to switch to a regimen that includes long-acting insulin. If the patient is admitted for surgery, then the hospital staff can change the insulin regimen to long-acting basal insulin. A quick formula for conversion is to add all the premixed insulin doses and give half as basal insulin on the morning of surgery, similar to the scenario above for the patient with long-acting basal insulin that was out of proportion to the prandial insulin injections.
For example, if the usual regimen is insulin 70/30 NPH/Regular, 60 U with breakfast, 30 U with dinner, then the patient can take 45 U of insulin glargine (which is half of 60 + 30) in the morning or evening before surgery.
Another option is to adjust the dose of pre-mixed insulin. Sometimes it is not feasible or economical to change the patient’s premixed insulin just before surgery. In these situations, the patient can take half of the morning dose, followed by dextrose-containing intravenous fluids and blood glucose checks.
We recommend preoperatively giving at least part of the patient’s previous basal insulin, regardless of the type of diabetes, the type of surgery, or the fasting period.
STEP 3: STOP THE PRANDIAL INSULIN
Prandial insulin—given before each meal to cover the carbohydrates to be consumed—should be stopped the morning of surgery.3,4
WHAT ABOUT SLIDING SCALE INSULIN?
Using a sliding scale alone has no known benefit. Although it can be a quick fix to correct a high glucose level, it should be added to the basal insulin and not used as the sole insulin therapy. If a sliding scale is used, fast-acting insulin (aspart, glulisine, lispro) is preferred over regular insulin because of the more rapid onset and shorter duration of action.
Patients already using a supplemental insulin scale can apply it to correct a blood glucose above 200 mg/dL on the morning of surgery.
MAINTENANCE FLUIDS
As long as glucose levels are not very elevated (ie, > 200 mg/dL), after 12 hours on a nothing-by-mouth regimen, provide dextrose in the IV fluid to prevent hypoglycemia (eg, the patient received long-acting insulin and the glucose levels are running low) or to prevent starvation ketosis, which may result in ketones in the blood or urine. We recommend 5% dextrose in half-normal (0.45%) saline at 50 to 75 mL/hour as maintenance fluid; the infusion rate should be lower if fluid overload is a concern.
POSTOPERATIVE INSULIN MANAGEMENT
Once patients are discharged and can go back to their previous routine, they can restart their usual insulin regimen the same evening. The prandial insulin will be resumed when the regular diet is reintroduced, and the doses will be adjusted according to food intake.
- Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg 2010; 111:1378–1387.
- DiNardo M, Donihi AC, Forte P, Gieraltowski L, Korytkowski M. Standardized glycemic management and perioperative glycemic outcomes in patients with diabetes mellitus who undergo same-day surgery. Endocr Pract 2011; 17:404–411.
- Vann MA. Perioperative management of ambulatory surgical patients with diabetes mellitus. Curr Opin Anaesthesiol 2009; 22:718–724.
- Meneghini LF. Perioperative management of diabetes: translating evidence into practice. Cleve Clin J Med 2009; 76(suppl 4):S53–S59.
- Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg 2010; 111:1378–1387.
- DiNardo M, Donihi AC, Forte P, Gieraltowski L, Korytkowski M. Standardized glycemic management and perioperative glycemic outcomes in patients with diabetes mellitus who undergo same-day surgery. Endocr Pract 2011; 17:404–411.
- Vann MA. Perioperative management of ambulatory surgical patients with diabetes mellitus. Curr Opin Anaesthesiol 2009; 22:718–724.
- Meneghini LF. Perioperative management of diabetes: translating evidence into practice. Cleve Clin J Med 2009; 76(suppl 4):S53–S59.
Is anticoagulation appropriate for all patients with portal vein thrombosis?
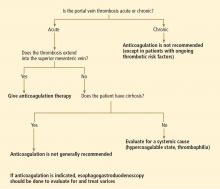
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
No. in general, the decision to treat portal vein thrombosis with anticoagulant drugs is complex and depends on whether the thrombosis is acute or chronic, and whether the cause is a local factor, cirrhosis of the liver, or a systemic condition (Table 1). A “one-size-fits-all” approach should be avoided (Figure 1).
ACUTE PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
No randomized controlled trial has yet evaluated anticoagulation in acute portal vein thrombosis. But a prospective study published in 2010 showed that the portal vein and its left or right branch were patent in 39% of anticoagulated patients (vs 13% initially), the splenic vein in 80% (vs 57% initially), and the superior mesenteric vein in 73% (vs 42% initially).1 Further, there appears to be a 20% reduction in the overall mortality rate associated with anticoagulation for acute portal vein thrombosis in retrospective studies.2
In the absence of contraindications, anticoagulation with heparin or low-molecular-weight heparin is recommended, with complete bridging to oral anticoagulation with a vitamin K antagonist. Anticoagulation should be continued for at least 3 months, and indefinitely in patients with permanent hypercoaguable risk factors.3
CHRONIC PORTAL VEIN THROMBOSIS WITHOUT CIRRHOSIS
All patients with chronic portal vein thrombosis should undergo esophagogastroduodenoscopy to evaluate for varices. Patients with large varices should be treated orally with a nonselective beta-adrenergic blocker or endoscopically. Though no prospective study has validated this practice, a retrospective analysis showed a decreased risk of first or recurrent bleeding.4
In 2007, a retrospective study showed a lower rate of death in patients with portomesenteric venous thrombosis treated with an oral vitamin K antagonist.5 Patients with chronic portal vein thrombosis with ongoing thrombotic risk factors should be treated with long-term anticoagulation after screening for varices, and if varices are present, primary prophylaxis should be started.3 With this approach, less than 5% of patients died from classic complications of portal vein thrombosis at 5 years of follow-up.4
ACUTE OR CHRONIC PORTAL VEIN THROMBOSIS WITH CIRRHOSIS
Portal vein thrombosis is common in patients with underlying cirrhosis. The risk in patients with cirrhosis significantly increases as liver function worsens. In patients with well-compensated cirrhosis, the risk is less than 1% vs 8% to 25% in those with advanced cirrhosis.6
In patients awaiting liver transplantation, a large retrospective study7 showed that the rate of partial or complete recanalization of the splanchnic veins was significantly higher in those who received anticoagulation (8 of 19) than in those who did not (0 of 10, P = .002). The rate of survival was significantly lower in those who had complete thrombotic obstruction of the portal vein at the time of surgery (P = .04). However, there was no difference in survival rates between those with partial obstruction who received anticoagulation and those with a patent portal vein.7
A later retrospective study8 showed no significant benefit in the rate of transplantation-free survival or survival after liver transplantation in patients with or without chronic portal vein thrombosis.8
Unfortunately, we have no data from prospective controlled trials and only limited data from retrospective studies to make a strong recommendation for or against anticoagulation in either acute and chronic portal vein thrombosis associated with cirrhosis. As such, each case must be evaluated on an individual basis in association with expert consultation.
In our experience, the risk of bleeding in patients with liver cirrhosis is substantial because of the decreased synthesis of coagulation factors and the presence of varices, whereas the efficacy and the benefits of recanalizing the portal vein in asymptomatic patients with liver cirrhosis and portal vein thrombosis are unknown. Therefore, unless the thrombosis extends into the mesenteric vein, thus posing a risk of mesenteric ischemia, we do not generally recommend anticoagulation in asymptomatic portal vein thrombosis in patients with cirrhosis.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51:210–218.
- Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med 2001; 345:1683–1688.
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005; 43:167–176.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54:691–697.
- John BV, Konjeti VR, Aggarwal A, et al. The impact of portal vein thrombosis (PVT) on cirrhotics awaiting liver transplantation (abstract). Hepatology 2010; 52(suppl1):888A–889A.
Which lower-extremity DVTs should be removed early?
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
Early thrombus removal for lower-extremity deep venous thrombosis (DVT) is at present only modestly supported by evidence and so remains controversial. It is largely aimed at preventing postthrombotic syndrome.
The decision to pursue early thrombus removal demands weighing the patient’s risk of postthrombotic syndrome against the risks and costs associated with thrombolysis and thrombectomy, such as bleeding complications. In the final analysis, this remains a subjective decision.
With these caveats in mind, the best candidate for early thrombus removal is a young patient with iliofemoral DVT with symptoms lasting fewer than 14 days.
POSTTHROMBOTIC SYNDROME IS COMMON
Anticoagulation with heparin and warfarin is the mainstay of DVT therapy. Indeed, the safety of this therapy and its effectiveness in reducing thrombus propagation and DVT recurrence are well established. Neither heparin nor warfarin, however, actively reduces the thrombus burden. Rather, both prevent the clot from propagating while it is, hopefully, gradually reabsorbed through endogenous mechanisms.
Up to 50% of DVT patients develop postthrombotic syndrome. A variety of mechanisms are involved, including persistent obstructive thrombosis and valvular injury.1 But much remains unknown about the etiology, and some patients develop the condition in the absence of abnormalities on objective testing.
Symptoms of postthrombotic syndrome can range from mild heaviness, edema, erythema, and cramping in the affected limb to debilitating pain with classic signs of venous hypertension (eg, venous ectasia and ulcers). It accounts for significant health care costs and has a detrimental effect on quality of life.1 Thus, there has been interest in early thrombus removal as initial therapy for DVT.
THROMBUS REMOVAL
Venous clots can be removed with open surgery or, more typically, with percutaneous catheter-based thrombolysis and thrombectomy devices that use high-velocity saline jets, ultrasonic energy, or wire oscillation to mechanically fragment the venous clot. All of these mechanisms help with drug delivery and pose a minimal risk of pulmonary embolism.
Evidence is weak
Patients with DVT of the iliac venous system or common femoral vein are at highest risk of postthrombotic syndrome. Therefore, the Society for Vascular Surgery and the American Venous Forum have issued a grade 2C (ie, weak) recommendation in favor of early thrombus removal in patients with a first-time episode of iliofemoral DVT with fewer than 14 days of symptoms.2 Moreover, patients must have a low risk of bleeding complications, be ambulatory, and have reasonable life expectancy.
The recommendation is buttressed by a Cochrane meta-analysis that included 101 patients.3 It concluded that there was a significant decrement in the development of postthrombotic syndrome with thrombolysis (but without mechanical thrombectomy) compared with standard therapy: the rate was 48% (29/61) with thrombolysis, and 65% (26/40) with standard therapy.3
More recently, the Catheter-Directed Thrombolysis Versus Standard Treatment for Acute Iliofemoral Deep Vein Thrombosis (CaVenT) study, a randomized prospective trial in 189 patients, demonstrated a lower rate of postthrombotic syndrome at 24 months and increased iliofemoral patency at 6 months with catheter-directed thrombolysis with alteplase (41.1% and 65.9%) vs anticoagulation with heparin and warfarin alone (55.6% and 47.4%).4
The Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-directed Thrombolysis (ATTRACT) trial is an ongoing prospective randomized multicenter trial of the effect of thrombolysis on postthrombotic syndrome that also hopes to clarify the relative benefits of different methods of pharmacomechanical clot removal.
While CaVenT has not been criticized extensively in the literature, other studies supporting early intervention for iliofemoral venous thrombosis generally have been noted to have a number of shortcomings, including a lack of randomization, and consequent bias, and the use of surrogate end points instead of a direct assessment of postthrombotic syndrome.
Reflecting the weakness of the evidence, the American College of Chest Physicians has issued a grade 2C recommendation against catheter-directed thrombolysis and against thrombectomy in favor of anticoagulant therapy.5
A subjective, case-by-case decision
The decision on standard vs interventional therapy must be made case by case. For example, thrombus removal may be more appropriate for a physically active young patient who is more likely to be impaired by postthrombotic syndrome, whereas standard warfarin therapy may be preferable for a sedentary patient. We are also more inclined to offer thrombus removal to patients who have worse symptoms.
Complicating the issue, many patients present with a mix of variables that support and oppose intervention—eg, a moderately active elderly patient with an unclear life expectancy and a history of gastrointestinal bleeding. At present, there is no way to quantitatively evaluate the risks and rewards of thrombus removal, and the final decision is essentially subjective.
Additional facts warranting consideration include the possibility that thrombolysis may require several days of therapy with daily venography for evaluation. Monitoring in the intensive care unit is normally required during the period of thrombolysis. Patients should be apprised of these elements of therapy beforehand; obviously, those who are unwilling to comply are not candidates.
Not a substitute for anticoagulation
It is important to recognize that thrombus removal is not a substitute for standard heparin-warfarin anticoagulation, which must also be prescribed.5 Thus, patients who cannot tolerate standard post-DVT anticoagulation should not undergo thrombus removal. Furthermore, the current evidence supports the use of standard anticoagulation over early thrombus removal of DVTs that are more distal in the lower extremity, such as those in the popliteal vein.5
PHLEGMASIA CERULEA DOLENS IS A SPECIAL CASE
Phlegmasia cerulea dolens—acute venous outflow obstruction associated with edema, cyanosis, and pain that in the worst cases may lead to shock, limb loss, and death—constitutes a special case. Although we lack robust supporting evidence, phlegmasia is a commonly accepted indication for early thrombus removal as a means of limb salvage.2,6
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
- Kahn SR. The post thrombotic syndrome. Thromb Res 2011; 127 (suppl 3):S89–S92.
- Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery; American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012; 55:1449–1462.
- Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev 2004; 4:CD002783.
- Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012; 379:31–38.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (suppl 2):e419S–e494S.
- Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJ. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010; 30:669–674.
Postoperative pain: Meeting new expectations
One of the most common questions patients ask when they hear that they need surgery is, “How much pain will I have, and how will you manage it?”
Pain is a common human experience that provokes both fear and anxiety, which in some cases can last a lifetime. The medical community has been slow to meet the challenge of managing it. The US National Institutes of Health states that more than 80% of patients suffer postoperative pain, with fewer than 50% receiving adequate relief.1 Patients have spoken out loudly through the Hospital Consumer Assessment of Healthcare Providers and Systems scores, demonstrating that the issue of inadequate postoperative pain management is real.
Clearly, as the push to tie reimbursement to patient satisfaction grows, clinicians have both a moral and a financial imperative to address postoperative pain.
The management of acute postoperative pain is evolving, and recognition of acute pain has progressed from considering it an afterthought or nuisance to realizing that improperly or inadequately treated postoperative pain can have a number of adverse effects, including debilitating chronic pain syndromes.2 Inadequately treated pain is also contributing to the calamitous rise in addiction to illegal substances and prescription medications.3 The time has come to take responsibility and meet the expectations of our patients.
OPIOIDS HAVE MAJOR DRAWBACKS
Opioid derivatives are potent analgesics and have been the traditional first-line therapy for pain. “Judicious use of opium” for painful maladies has been a mainstay of Western medicine since the 16th century and was described in writings from Mesopotamia and China more than 2,000 years ago.
The ease of administration of these drugs coupled with their efficacy in managing a broad spectrum of pain syndromes has led to their frequent and widespread use, often, unfortunately, without consideration of the potential for negative short-term and long-term consequences. Headache, drowsiness, and pruritus are common adverse effects. Less common is a slowing of bowel motility, leading to constipation, bloating, or nausea. Additionally, in 5% to 10% of patients, narcotics may actually sensitize the nerves and make bowel-related pain worse. This narcotic bowel syndrome, as discussed by Agito and Rizk in this issue of the Journal, may make the patient uncomfortable and may lead to delays in recovery and hospital discharge.4
Opioid-related respiratory depression is especially devastating in the postoperative period, potentially causing respiratory arrest and death. The frequency of drug-induced respiratory depression and clinically significant adverse outcomes prompted the Anesthesia Patient Safety Foundation (APSF) to declare in 2011, “No patient shall be harmed by opioid-induced respiratory depression.”5 The APSF has recommended using new monitoring technology to enhance detection.
While many clinicians have been moving towards aggressive pain-management practice, hospital infrastructure has not kept pace. It is often ill-equipped to adequately monitor breathing patterns and to alert personnel to the need for rapid intervention. In the 21st century, we need to respond to this challenge with a combination of tools and technology, including improved clinical assessment and monitoring equipment that has proven to save lives in the perioperative setting.
A MULTIMODAL APPROACH IS BEST
Pain management professionals have also been moving from a predominantly opioid-based regimen to a more balanced, multimodal approach. The goal is to effectively treat acute postoperative pain while reducing the use of opioids and increasing the use of nonopioid drugs and alternative therapies for both pain management and convalescence.
Studies have shown the benefits of nonopioid drugs such as nonsteroidal anti-inflammatory drugs, paracetamol (intravenous acetaminophen), antidepressants, antiepileptics, and regional or local anesthetics combined with nontraditional treatments such as Reiki, massage therapy, and deep breathing.6
Each patient’s experience of pain is unique and responds to medications and alternative therapies in a distinctly different manner. We should not assume that one intervention is suitable for every patient. It is more beneficial to individualize treatment based on protocols that target different pain pathways. This may lead to better pain management and patient satisfaction while reducing the incidence of drug overdose and unwanted side effects.
WHAT WE NEED TO DO
Although many health care professionals have the authority to prescribe potent anesthetics and analgesics, we believe that there is a lack of adequate education, supervision, and experience, and this exposes patients to risks of prescription drug overdose.7,8 All medical professionals who provide postoperative care need specific education and training to offer the best care to this vulnerable patient population. This includes specific and more extensive training in the appropriate use of controlled medications before receiving their controlled substance registration from the Drug Enforcement Agency. We must also extend education to patients and family members regarding the dangers of drug abuse and the safe use of prescription drugs.8
Finally, we need to engage and communicate more effectively with our patients, especially when they are in acute pain. How long should a patient expect to remain in pain while waiting for an assessment and intervention? The medical community must commit to rapid and consistent coverage throughout the day for all patients experiencing a new or changing pattern of pain not responding to current therapy. Problems do not end at 5 pm or at a shift change. We need to build a process of timely intervention, perhaps by using a model similar to that of the rapid response and resuscitation team, which has been effective in many institutions. When a patient is in pain, minutes spent waiting for relief seem like an eternity. The empathy we show patients by validating, not minimizing, their pain and by following a defined yet tailored therapeutic intervention may not only improve their physical discomfort, but improve their overall patient experience.
Margo McCaffery, RN, a pioneer in pain management nursing, defined pain as “whatever the experiencing person says it is, existing whenever the experiencing person says it does.”9 We have come a long way from the days when attending staff in the post-anesthesia care unit would routinely declare, “Pain never killed anyone.” As caregivers, we need to become engaged, empathetic, and effective as we meet the challenges of managing acute postoperative pain and improving our patients’ experience and outcomes.
- Relieving Pain in America. Institute of Medicine 2011. National Academies Press (US). 2011 ISBN-13: 978-0-309-21484-1.
- Lamacraft G. The link between acute postoperative pain and chronic pain syndromes. South Afr J Anaesth Analg 2012; 18:45–50.
- Binyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11:S105–S120.
- Grunkemeier DMS, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Anesthesia Patient Safety Foundation. Proceedings of “Essential Monitoring Strategies to Detect Clinically Significant Drug-Induced Respiratory Depression in the Postoperative Period” Conference, 2011. http://www.apsf.org/newsletters/pdf/fall_2011.pdf. Accessed May 13, 2013.
- So PS, Jiang JY, Qin Y. Touch therapies for pain relief in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD006535. DOI: 10.1002/14651858.CD006535.pub2.
- Polydorou S, Gunderson EW, Levin FR. Training physicians to treat substance use disorders. Curr Psychiatry Rep 2008; 10:399–404.
- CDC Grand Rounds. Prescription Drug Overdoses – a U.S Epidemic MMWR January 13, 2012/61(01);10–13.
- McCaffery M, Pasero C. Pain: Clinical Manual. 2nd ed. St. Louis: Mosby, 1999.
One of the most common questions patients ask when they hear that they need surgery is, “How much pain will I have, and how will you manage it?”
Pain is a common human experience that provokes both fear and anxiety, which in some cases can last a lifetime. The medical community has been slow to meet the challenge of managing it. The US National Institutes of Health states that more than 80% of patients suffer postoperative pain, with fewer than 50% receiving adequate relief.1 Patients have spoken out loudly through the Hospital Consumer Assessment of Healthcare Providers and Systems scores, demonstrating that the issue of inadequate postoperative pain management is real.
Clearly, as the push to tie reimbursement to patient satisfaction grows, clinicians have both a moral and a financial imperative to address postoperative pain.
The management of acute postoperative pain is evolving, and recognition of acute pain has progressed from considering it an afterthought or nuisance to realizing that improperly or inadequately treated postoperative pain can have a number of adverse effects, including debilitating chronic pain syndromes.2 Inadequately treated pain is also contributing to the calamitous rise in addiction to illegal substances and prescription medications.3 The time has come to take responsibility and meet the expectations of our patients.
OPIOIDS HAVE MAJOR DRAWBACKS
Opioid derivatives are potent analgesics and have been the traditional first-line therapy for pain. “Judicious use of opium” for painful maladies has been a mainstay of Western medicine since the 16th century and was described in writings from Mesopotamia and China more than 2,000 years ago.
The ease of administration of these drugs coupled with their efficacy in managing a broad spectrum of pain syndromes has led to their frequent and widespread use, often, unfortunately, without consideration of the potential for negative short-term and long-term consequences. Headache, drowsiness, and pruritus are common adverse effects. Less common is a slowing of bowel motility, leading to constipation, bloating, or nausea. Additionally, in 5% to 10% of patients, narcotics may actually sensitize the nerves and make bowel-related pain worse. This narcotic bowel syndrome, as discussed by Agito and Rizk in this issue of the Journal, may make the patient uncomfortable and may lead to delays in recovery and hospital discharge.4
Opioid-related respiratory depression is especially devastating in the postoperative period, potentially causing respiratory arrest and death. The frequency of drug-induced respiratory depression and clinically significant adverse outcomes prompted the Anesthesia Patient Safety Foundation (APSF) to declare in 2011, “No patient shall be harmed by opioid-induced respiratory depression.”5 The APSF has recommended using new monitoring technology to enhance detection.
While many clinicians have been moving towards aggressive pain-management practice, hospital infrastructure has not kept pace. It is often ill-equipped to adequately monitor breathing patterns and to alert personnel to the need for rapid intervention. In the 21st century, we need to respond to this challenge with a combination of tools and technology, including improved clinical assessment and monitoring equipment that has proven to save lives in the perioperative setting.
A MULTIMODAL APPROACH IS BEST
Pain management professionals have also been moving from a predominantly opioid-based regimen to a more balanced, multimodal approach. The goal is to effectively treat acute postoperative pain while reducing the use of opioids and increasing the use of nonopioid drugs and alternative therapies for both pain management and convalescence.
Studies have shown the benefits of nonopioid drugs such as nonsteroidal anti-inflammatory drugs, paracetamol (intravenous acetaminophen), antidepressants, antiepileptics, and regional or local anesthetics combined with nontraditional treatments such as Reiki, massage therapy, and deep breathing.6
Each patient’s experience of pain is unique and responds to medications and alternative therapies in a distinctly different manner. We should not assume that one intervention is suitable for every patient. It is more beneficial to individualize treatment based on protocols that target different pain pathways. This may lead to better pain management and patient satisfaction while reducing the incidence of drug overdose and unwanted side effects.
WHAT WE NEED TO DO
Although many health care professionals have the authority to prescribe potent anesthetics and analgesics, we believe that there is a lack of adequate education, supervision, and experience, and this exposes patients to risks of prescription drug overdose.7,8 All medical professionals who provide postoperative care need specific education and training to offer the best care to this vulnerable patient population. This includes specific and more extensive training in the appropriate use of controlled medications before receiving their controlled substance registration from the Drug Enforcement Agency. We must also extend education to patients and family members regarding the dangers of drug abuse and the safe use of prescription drugs.8
Finally, we need to engage and communicate more effectively with our patients, especially when they are in acute pain. How long should a patient expect to remain in pain while waiting for an assessment and intervention? The medical community must commit to rapid and consistent coverage throughout the day for all patients experiencing a new or changing pattern of pain not responding to current therapy. Problems do not end at 5 pm or at a shift change. We need to build a process of timely intervention, perhaps by using a model similar to that of the rapid response and resuscitation team, which has been effective in many institutions. When a patient is in pain, minutes spent waiting for relief seem like an eternity. The empathy we show patients by validating, not minimizing, their pain and by following a defined yet tailored therapeutic intervention may not only improve their physical discomfort, but improve their overall patient experience.
Margo McCaffery, RN, a pioneer in pain management nursing, defined pain as “whatever the experiencing person says it is, existing whenever the experiencing person says it does.”9 We have come a long way from the days when attending staff in the post-anesthesia care unit would routinely declare, “Pain never killed anyone.” As caregivers, we need to become engaged, empathetic, and effective as we meet the challenges of managing acute postoperative pain and improving our patients’ experience and outcomes.
One of the most common questions patients ask when they hear that they need surgery is, “How much pain will I have, and how will you manage it?”
Pain is a common human experience that provokes both fear and anxiety, which in some cases can last a lifetime. The medical community has been slow to meet the challenge of managing it. The US National Institutes of Health states that more than 80% of patients suffer postoperative pain, with fewer than 50% receiving adequate relief.1 Patients have spoken out loudly through the Hospital Consumer Assessment of Healthcare Providers and Systems scores, demonstrating that the issue of inadequate postoperative pain management is real.
Clearly, as the push to tie reimbursement to patient satisfaction grows, clinicians have both a moral and a financial imperative to address postoperative pain.
The management of acute postoperative pain is evolving, and recognition of acute pain has progressed from considering it an afterthought or nuisance to realizing that improperly or inadequately treated postoperative pain can have a number of adverse effects, including debilitating chronic pain syndromes.2 Inadequately treated pain is also contributing to the calamitous rise in addiction to illegal substances and prescription medications.3 The time has come to take responsibility and meet the expectations of our patients.
OPIOIDS HAVE MAJOR DRAWBACKS
Opioid derivatives are potent analgesics and have been the traditional first-line therapy for pain. “Judicious use of opium” for painful maladies has been a mainstay of Western medicine since the 16th century and was described in writings from Mesopotamia and China more than 2,000 years ago.
The ease of administration of these drugs coupled with their efficacy in managing a broad spectrum of pain syndromes has led to their frequent and widespread use, often, unfortunately, without consideration of the potential for negative short-term and long-term consequences. Headache, drowsiness, and pruritus are common adverse effects. Less common is a slowing of bowel motility, leading to constipation, bloating, or nausea. Additionally, in 5% to 10% of patients, narcotics may actually sensitize the nerves and make bowel-related pain worse. This narcotic bowel syndrome, as discussed by Agito and Rizk in this issue of the Journal, may make the patient uncomfortable and may lead to delays in recovery and hospital discharge.4
Opioid-related respiratory depression is especially devastating in the postoperative period, potentially causing respiratory arrest and death. The frequency of drug-induced respiratory depression and clinically significant adverse outcomes prompted the Anesthesia Patient Safety Foundation (APSF) to declare in 2011, “No patient shall be harmed by opioid-induced respiratory depression.”5 The APSF has recommended using new monitoring technology to enhance detection.
While many clinicians have been moving towards aggressive pain-management practice, hospital infrastructure has not kept pace. It is often ill-equipped to adequately monitor breathing patterns and to alert personnel to the need for rapid intervention. In the 21st century, we need to respond to this challenge with a combination of tools and technology, including improved clinical assessment and monitoring equipment that has proven to save lives in the perioperative setting.
A MULTIMODAL APPROACH IS BEST
Pain management professionals have also been moving from a predominantly opioid-based regimen to a more balanced, multimodal approach. The goal is to effectively treat acute postoperative pain while reducing the use of opioids and increasing the use of nonopioid drugs and alternative therapies for both pain management and convalescence.
Studies have shown the benefits of nonopioid drugs such as nonsteroidal anti-inflammatory drugs, paracetamol (intravenous acetaminophen), antidepressants, antiepileptics, and regional or local anesthetics combined with nontraditional treatments such as Reiki, massage therapy, and deep breathing.6
Each patient’s experience of pain is unique and responds to medications and alternative therapies in a distinctly different manner. We should not assume that one intervention is suitable for every patient. It is more beneficial to individualize treatment based on protocols that target different pain pathways. This may lead to better pain management and patient satisfaction while reducing the incidence of drug overdose and unwanted side effects.
WHAT WE NEED TO DO
Although many health care professionals have the authority to prescribe potent anesthetics and analgesics, we believe that there is a lack of adequate education, supervision, and experience, and this exposes patients to risks of prescription drug overdose.7,8 All medical professionals who provide postoperative care need specific education and training to offer the best care to this vulnerable patient population. This includes specific and more extensive training in the appropriate use of controlled medications before receiving their controlled substance registration from the Drug Enforcement Agency. We must also extend education to patients and family members regarding the dangers of drug abuse and the safe use of prescription drugs.8
Finally, we need to engage and communicate more effectively with our patients, especially when they are in acute pain. How long should a patient expect to remain in pain while waiting for an assessment and intervention? The medical community must commit to rapid and consistent coverage throughout the day for all patients experiencing a new or changing pattern of pain not responding to current therapy. Problems do not end at 5 pm or at a shift change. We need to build a process of timely intervention, perhaps by using a model similar to that of the rapid response and resuscitation team, which has been effective in many institutions. When a patient is in pain, minutes spent waiting for relief seem like an eternity. The empathy we show patients by validating, not minimizing, their pain and by following a defined yet tailored therapeutic intervention may not only improve their physical discomfort, but improve their overall patient experience.
Margo McCaffery, RN, a pioneer in pain management nursing, defined pain as “whatever the experiencing person says it is, existing whenever the experiencing person says it does.”9 We have come a long way from the days when attending staff in the post-anesthesia care unit would routinely declare, “Pain never killed anyone.” As caregivers, we need to become engaged, empathetic, and effective as we meet the challenges of managing acute postoperative pain and improving our patients’ experience and outcomes.
- Relieving Pain in America. Institute of Medicine 2011. National Academies Press (US). 2011 ISBN-13: 978-0-309-21484-1.
- Lamacraft G. The link between acute postoperative pain and chronic pain syndromes. South Afr J Anaesth Analg 2012; 18:45–50.
- Binyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11:S105–S120.
- Grunkemeier DMS, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Anesthesia Patient Safety Foundation. Proceedings of “Essential Monitoring Strategies to Detect Clinically Significant Drug-Induced Respiratory Depression in the Postoperative Period” Conference, 2011. http://www.apsf.org/newsletters/pdf/fall_2011.pdf. Accessed May 13, 2013.
- So PS, Jiang JY, Qin Y. Touch therapies for pain relief in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD006535. DOI: 10.1002/14651858.CD006535.pub2.
- Polydorou S, Gunderson EW, Levin FR. Training physicians to treat substance use disorders. Curr Psychiatry Rep 2008; 10:399–404.
- CDC Grand Rounds. Prescription Drug Overdoses – a U.S Epidemic MMWR January 13, 2012/61(01);10–13.
- McCaffery M, Pasero C. Pain: Clinical Manual. 2nd ed. St. Louis: Mosby, 1999.
- Relieving Pain in America. Institute of Medicine 2011. National Academies Press (US). 2011 ISBN-13: 978-0-309-21484-1.
- Lamacraft G. The link between acute postoperative pain and chronic pain syndromes. South Afr J Anaesth Analg 2012; 18:45–50.
- Binyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician 2008; 11:S105–S120.
- Grunkemeier DMS, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Anesthesia Patient Safety Foundation. Proceedings of “Essential Monitoring Strategies to Detect Clinically Significant Drug-Induced Respiratory Depression in the Postoperative Period” Conference, 2011. http://www.apsf.org/newsletters/pdf/fall_2011.pdf. Accessed May 13, 2013.
- So PS, Jiang JY, Qin Y. Touch therapies for pain relief in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD006535. DOI: 10.1002/14651858.CD006535.pub2.
- Polydorou S, Gunderson EW, Levin FR. Training physicians to treat substance use disorders. Curr Psychiatry Rep 2008; 10:399–404.
- CDC Grand Rounds. Prescription Drug Overdoses – a U.S Epidemic MMWR January 13, 2012/61(01);10–13.
- McCaffery M, Pasero C. Pain: Clinical Manual. 2nd ed. St. Louis: Mosby, 1999.
Stiff, numb hands
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
A 45-year-old woman with no chronic medical problems presented to the emergency room with a 1-day history of cramps and paresthesias in both hands and feet, mainly involving the fingers and toes. She said that after an argument with her daughter she began feeling anxious, and this was accompanied by shortness of breath and palpitations as well as generalized weakness, fatigue, and body aches. She also reported nausea and repeated vomiting but no abdominal pain, distention or change in bowel movements. She had had no loss of consciousness, confusion, incontinence, headache, dizziness, diplopia, or facial paresthesia.
She is a cigarette smoker, is alcohol-dependent, but does not use illicit drugs and is not on any medications.
Examination revealed a temperature of 37.1°C (98.8°F), blood pressure 150/75 mm Hg, heart rate 105 bpm, respiratory rate 24 breaths per minute, and oxygen saturation 97% on room air. She appeared very fatigued, thin, and in mild distress due to her cramps. Her mucous membranes were dry, but she had no orthostatic changes. She had noticeable carpopedal spasms (Figure 1), reproducible by inflating a blood-pressure cuff placed on her arm (Trousseau sign) (Figure 2). Also noted was the Chvostek sign—contraction of the ipsilateral facial muscles when the facial nerve is tapped just in front of the ear. The rest of the systemic evaluation was normal. Laboratory investigations were as listed in Table 1. Electrocardiography showed a prolonged QTc interval (0.5 sec). The chest radiograph was normal.
HYPERVENTILATION AND TETANY
The presumptive diagnosis was latent tetany caused by an electrolyte derangement, in this case a combination of hypocalcemia, hypomagnesemia, and hypokalemia as the result of alcohol abuse, repeated vomiting, and hyperventilation brought on by a severe attack of anxiety.
Tetany results from increased excitability of nerves and muscles, leading to painful muscle cramps.1,2 Typical symptoms include circumoral and distal paresthesias, stiffness, clumsiness, myalgia, carpopedal spasm, laryngospasm, bronchospasm, and generalized seizure. The Chvostek and Trousseau signs help to confirm the diagnosis of tetany.3,4
The differential diagnosis of carpopedal spasm includes other conditions of involuntary muscle contraction, such as myotonic disorders; myokymia from Isaac syndrome (writhing movements of the muscles under the skin visualized by continuous “rippling” movements of the muscle); stiff-man syndrome (an autoimmune-antiglutamic acid decarboxylase antibody-associated muscle rigidity that waxes and wanes with concurrent spasms); and snake envenomation.
In addition, our patient’s symptoms were probably brought on by hyperventilation. In general, patients with hyperventilation syndrome are young females who display various manifestations of underlying anxiety and can develop tetany even after a brief episode of hyperventilation. At the time of presentation, our patient was found to have mixed respiratory and metabolic alkalosis. The anxiety-induced hyperventilation likely contributed to the respiratory alkalosis. She had no other symptoms or signs to suggest an acute organic respiratory illness such as pulmonary embolism, pneumothorax, or infection. Vomiting likely caused the metabolic alkalosis and hypokalemia.
Tetany is usually triggered by acute hypocalcemia and is uncommon unless the serum ionized calcium concentration falls below 4.3 mg/dL (1.1 mmol/L), which usually corresponds to a serum total calcium concentration of 7.0 to 7.5 mg/dL (1.8 to 1.9 mmol/L). Patients with a gradual onset of hypocalcemia tend to have fewer symptoms.3,4
Although alkalosis alone can cause tetany, it also enhances tetany by reducing the level of ionized calcium in the serum. Alkalemia causes hypocalcemia by an intravascular chelative mechanism in which the decrease in concentration of hydrogen ions leaves the negatively charged binding sites on albumin available to bind ionized calcium.3
The same happens to the magnesium, a cation with the same size and valence. Significant hypomagnesemia is common in tetanic patients with hyperventilation attacks and may, by itself or in combination with hypocalcemia, cause tetany.2,5,6 Hypokalemia can develop in patients with hypomagnesemia or metabolic alkalosis and may lead to tetany.6,7 Furthermore, our patient was dependent on alcohol, and this is known to cause hypomagnesemia from the excessive urinary excretion of magnesium. This defect of alcohol-induced tubular dysfunction is reversible within 4 weeks of abstinence. Magnesium depletion can cause hypocalcemia by producing resistance to parathyroid hormone or by decreasing its secretion, and this occurs in severe hypomagnesemia, ie, when the serum magnesium concentration falls below 1.0 mg/dL (0.4 mmol/L).
IDENTIFY AND TREAT THE UNDERLYING CAUSE
The management of tetany consists of identifying and treating the underlying cause. Infusion of calcium or magnesium is effective as acute therapy for tetany, and, if appropriate, vitamin D supplementation should also be provided.3,4,7 However, if associated hyperventilation syndrome is present, patients benefit from reassurance and treatment for underlying psychological stress. The traditional treatment of rebreathing into a paper bag is no longer recommended because of the potential risk of hypoxia. Sedatives and antidepressants should be reserved for patients who have not responded to conservative treatment.
Our patient was given an explanation of the condition together with breathing exercises. She received lorazepam and was immediately treated with intravenous hydration, along with intravenous infusion of magnesium, calcium, and potassium. These interventions led to an immediate resolution of her symptoms.
Her low level of intact parathyroid hormone may also have been caused by hypomagnesemia. She was given oral magnesium, potassium, calcium, and vitamin D to continue at home. In addition, she was advised to avoid excessive alcohol consumption and to see us or her primary care doctor should the symptoms recur. As expected, all the laboratory values normalized within 1 month of abstinence from alcohol, and she has been well since.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008; 35:215–237.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Smets YF, Bokani N, de Meijer PH, Meinders AE. Tetany due to excessive use of alcohol: a possible magnesium deficiency [in Dutch]. Ned Tijdschr Geneeskd 2004; 148:641–644.
- Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 2007; 18:2649–2652.
Not all joint pain is arthritis
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
Implications of a prominent R wave in V1
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
KEY POINTS
- The utility of carbapenems is being undermined by the emergence of resistance in Enterobacteriaceae and other bacteria.
- The clinical impact of CRE falls on elderly patients exposed to these organisms in hospitals and long-term care facilities. In this vulnerable group, invasive infections with CRE exact a high death rate.
- Long-term care facilities play an important role in the transmission dynamics of CRE.
- Tigecycline and colistin are treatments of last resort against infections caused by CRE. Their use in combination with other agents, especially carbapenems, may improve outcomes and needs to be explored further.
- Early detection of CRE in the microbiology laboratory is key to guiding infection control and treatment decisions and supporting surveillance efforts.