User login
A shocking diagnosis
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
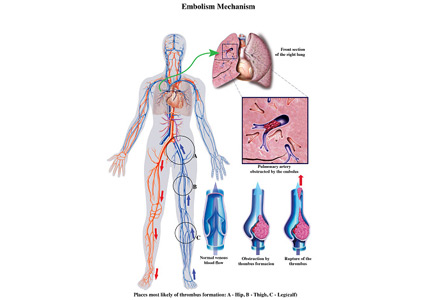
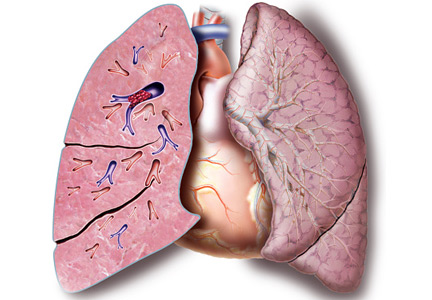
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
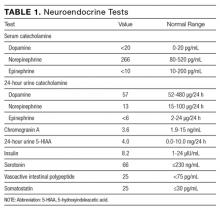
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
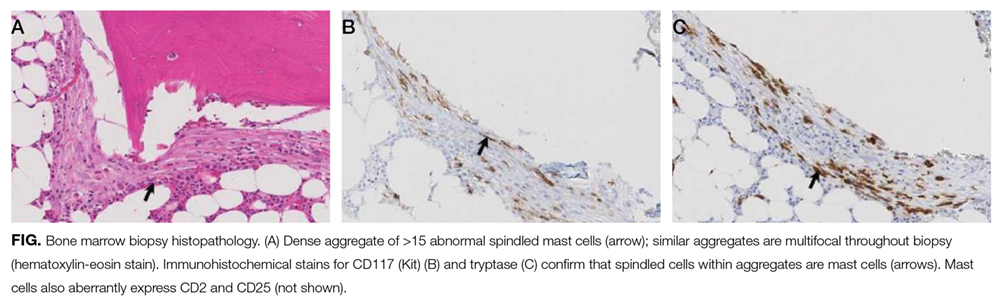
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.
DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
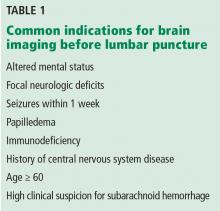
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.

DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.

DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
© 2017 Society of Hospital Medicine
Impact of patient-centered discharge tools: A systematic review
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
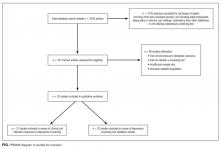
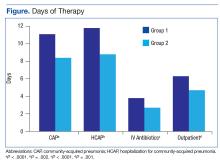
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
Screening for depression in hospitalized medical patients
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.
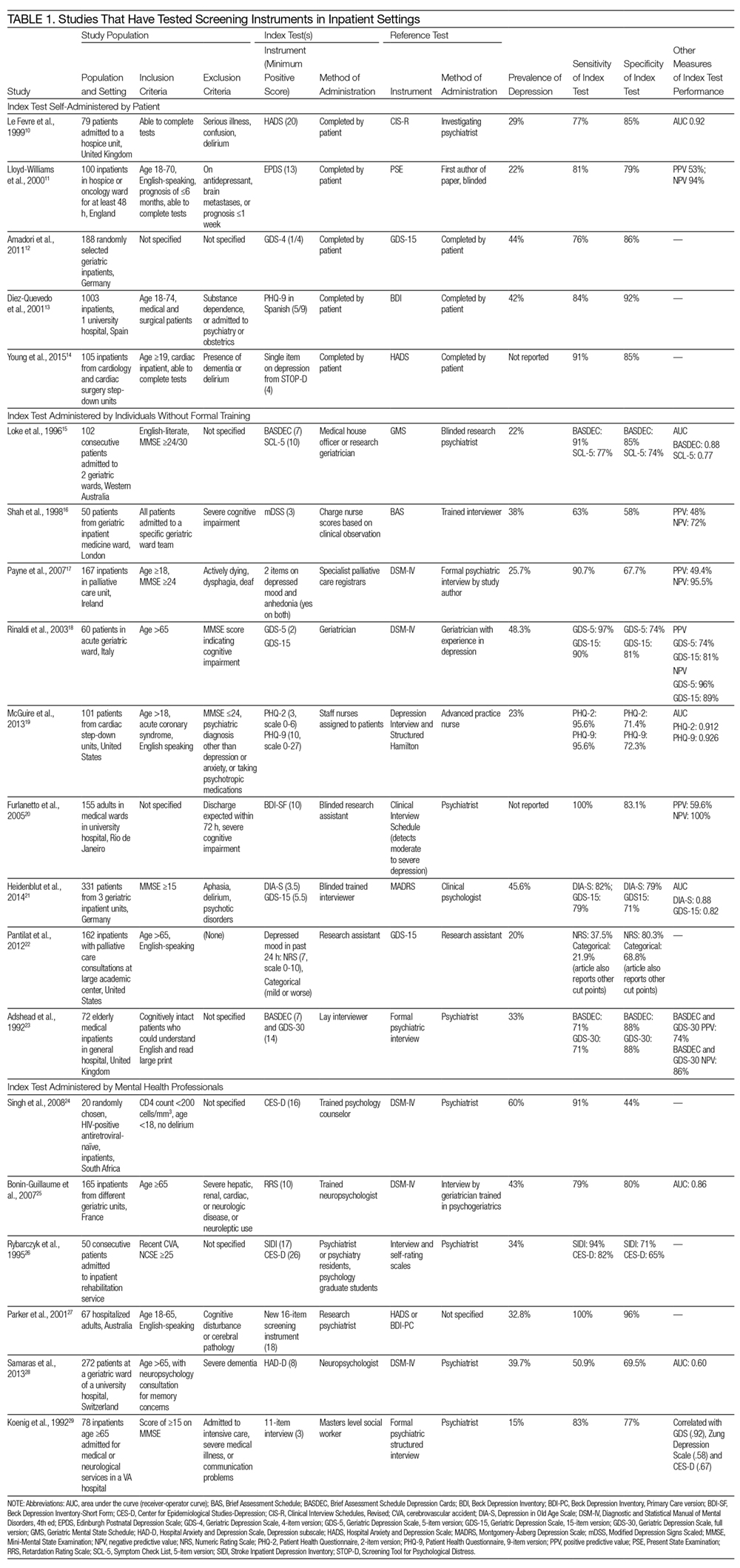
The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.
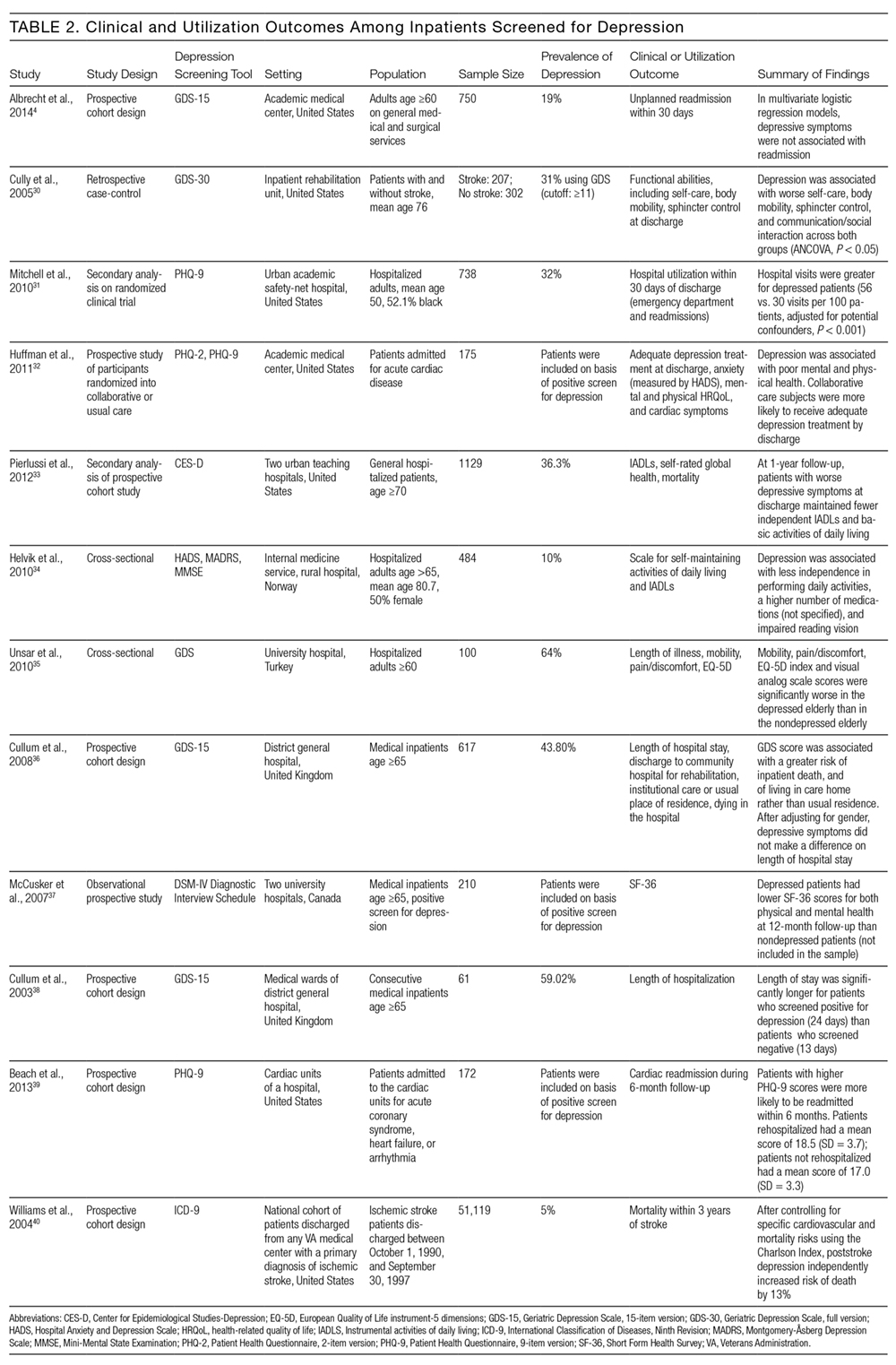
Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
© 2017 Society of Hospital Medicine
Acute kidney injury is important in the hospital and afterward
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
Acute kidney injury (AKI) is a major contributor to morbidity and mortality in hospitalized patients across the world.1 Affecting up to 20% of all admissions (depending on which definition of AKI is used),2 AKI is the most common reason for new-inpatient nephrology consultation. Recent data suggest that AKI incidence has risen rapidly, by up to 10% per year.3,4
AKI is associated with a variety of serious short- and long-term complications. Approximately 33% to 60% of critically ill patients who develop dialysis-requiring AKI do not survive to hospital discharge, and mortality associated with dialysis-requiring AKI is greater than that associated with other serious conditions such as myocardial infarction or acute respiratory distress syndrome.5 Even relatively mild AKI in the acute inpatient setting appears to be an independent risk factor for mortality.6
For several decades, many physicians believed that AKI was a self-limited process followed by complete recovery of renal function to pre-AKI levels among survivors. (Numerous trainees have been taught some variant of the old adage: “If the patients survive, so will their kidneys.”) But studies linking AKI with the development of new-onset chronic kidney disease (CKD) or the accelerated progression of pre-existing CKD have changed this view.7 One important reason the long-term impact of AKI hasn’t been appreciated is that, traditionally, clinical studies of AKI examined inhospital outcomes such as short-term mortality and resource usage and did not consider what transpired months to years after discharge. More recently, epidemiologic studies linking inpatient events with outpatient outcomes have filled this knowledge gap.8 Contemporary animal models of AKI have shed light on potential mechanisms of maladaptive repair after AKI, characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis, and chronic interstitial inflammation, all of which result in renal function decline. So over the last decade there has been a paradigm shift in how we think about AKI and CKD. Rather than distinct entities, AKI and CKD are now viewed as interconnected syndromes since AKI is a risk factor for CKD progression and CKD is a risk factor for new episodes of AKI.9
Two studies published in this issue of the Journal of Hospital Medicine augment our understanding of AKI and its clinical impact in hospitalized patients. Analyzing data from the National Inpatient Sample, Silver et al.10 found that hospitalizations that include AKI are substantially costlier and associated with longer lengths of stay than hospitalizations without AKI. The authors also highlight that the additional economic costs of AKI exceeded those of many other higher-profile yet less-common acute medical conditions, such as myocardial infarction and gastrointestinal bleeding. These results re-emphasize the important economic burden of AKI at a national level and expand on prior literature by confirming findings previously limited to single-center and regional studies. Better defining the impact AKI has on our healthcare system could help ensure that adequate resources are invested to combat AKI.
The second study, by Rutter et al.,11 found that among hospitalized patients with normal baseline renal function, use of vancomycin in combination with piperacillin-tazobactam is associated with a higher incidence of AKI after antibiotic exposure than use of either agent as monotherapy. This association persisted even after adjusting for potential confounders such as underlying comorbidities, exposure to nephrotoxic agents, documented hypotension, and baseline renal impairment. This study adds to a growing body of literature that suggests synergistic nephrotoxicity between vancomycin and piperacillin-tazobactam. It underscores that any medical intervention—even treatments typically envisioned as non-hazardous and frequently life-saving—involve inherent risks and should prompt the medical community to promote proper antimicrobial stewardship. Whether such exposures to vancomycin or beta-lactam derivatives cause AKI via direct tubular damage, interstitial nephritis, or some other novel mechanism remains to be elucidated. Better delineation of the contemporary causes of AKI, including increased antibiotic exposure, is the first step toward identifying ways to reduce AKI incidence.
Both of these papers serve to highlight the clinical importance of AKI among hospitalized patients. Their findings re-emphasize the need for vigilance in detecting AKI and intervening early to achieve the best clinical outcomes.
Given recent understanding that survivors of AKI are at greater risk for more rapid loss of renal function long after hospital discharge, one goal the US Department of Health and Human Services put forth for Healthy People 2020 is to “increase the proportion of hospital patients who incurred AKI who have follow-up renal evaluation in 6 months post-discharge” (10% improvement targeted).12 Transitions of care after hospitalizations complicated by AKI require special attention to ensure that patients’ needs are optimally monitored and managed during the critical post-discharge period. One recent study analyzing discharge documentation for hospitalizations including AKI found that fewer than half of the discharge summaries and patient instructions commented on the presence, cause, or course of AKI, indicating clear room for improvement.13 And currently, it appears that only a minority of patients with AKI—even AKI severe enough to require dialysis—are seen by a nephrologist within 90 days of discharge.14
Hospitalists play a crucial role in coordinating care as vulnerable patients transition from the inpatient to outpatient setting. We suggest that AKI should be properly documented in the discharge summary. In addition, patients should be informed that they experienced AKI so they can discuss with future caregivers potential strategies to avoid additional renal insults. Discharge referrals to nephrology should be arranged for high-risk patients, including those whose renal function remains decreased at discharge or those who had recurrent AKI episodes during prior hospitalizations. For patients with pre-hospitalization baseline CKD, nephrology should be consulted before indefinitely discontinuing medications like angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. These medications are indispensable in retarding the progression of proteinuric CKD, even though they may predispose patients to AKI under certain circumstances (eg, in states of decreased renal perfusion). Adopting these simple steps may substantially improve the long-term outcomes of patients who experience AKI during hospitalization.
Acknowledgments
The authors are supported by NIH-NIDDK Grants T32DK007219 (BJL) and K24DK92291 (CYH).
Disclosure
Nothing to report.
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
1. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170-179. PubMed
2. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20. PubMed
3. Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37-42. PubMed
4. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46-61. PubMed
5. Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10(10):1859-1867. PubMed
6. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370. PubMed
7. Hsu CY. Yes, AKI truly leads to CKD. J Am Soc Nephrol. 2012;23(6):967-969. PubMed
8. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442-448. PubMed
9. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. New Engl J Med. 2014;371(1):58-66. PubMed
10. Silver SA, Long J, Zheng Y, Chertow GM. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. Full Text
11. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med. 2017;12(2):77-82. Full Text
12. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Available at: https://www.healthypeople.gov/node/4093/data_details. Accessed September 2, 2016.
13. Greer RC, Liu Y, Crews DC, Jaar BG, Rabb H, Boulware LE. Hospital discharge communications during care transitions for patients with acute kidney injury: a cross-sectional study. BMC Health Serv Res. 2016;16:449. PubMed
14. Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305-312. PubMed
© 2017 Society of Hospital Medicine
A Primary Hospital Antimicrobial Stewardship Intervention on Pneumonia Treatment Duration
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
When should brain imaging precede lumbar puncture in cases of suspected bacterial meningitis?
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
Brain imaging should precede lumbar puncture in patients with focal neurologic deficits or immunodeficiency, or with altered mental status or seizures during the previous week. However, lumbar puncture can be safely done in most patients without first obtaining brain imaging. Empiric antibiotic and corticosteroid therapy must not be delayed; they should be started immediately after the lumber puncture is done, without waiting for the results. If the lumbar puncture is going to be delayed, these treatments should be started immediately after obtaining blood samples for culture.
A MEDICAL EMERGENCY
Bacterial meningitis is a medical emergency and requires prompt recognition and treatment. It is associated with a nearly 15% death rate as well as neurologic effects such as deafness, seizures, and cognitive decline in about the same percentage of patients.1 Microbiologic information from lumbar puncture and cerebrospinal fluid analysis is an essential part of the initial workup, whenever possible. Lumbar puncture can be done safely at the bedside in most patients and so should not be delayed unless certain contraindications exist, as discussed below.2
INDICATIONS FOR BRAIN IMAGING BEFORE LUMBAR PUNCTURE
Table 1 lists common indications for brain imaging before lumbar puncture. However, there is a lack of good evidence to support them.
Current guidelines on acute bacterial meningitis from the Infectious Diseases Society of America recommend computed tomography (CT) of the brain before lumbar puncture in patients presenting with:
- Altered mental status
- A new focal neurologic deficit (eg, cranial nerve palsy, extremity weakness or drift, dysarthria, aphasia)
- Papilledema
- Seizure within the past week
- History of central nervous system disease (eg, stroke, tumor)
- Age 60 or older (likely because of the association with previous central nervous system disease)
- Immunocompromised state (due to human immunodeficiency virus infection, chemotherapy, or immunosuppressive drugs for transplant or rheumatologic disease)
- A high clinical suspicion for subarachnoid hemorrhage.3–5
However, a normal result on head CT does not rule out the possibility of increased intracranial pressure and the risk of brain herniation. Actually, patients with acute bacterial meningitis are inherently at higher risk of spontaneous brain herniation even without lumbar puncture, and some cases of brain herniation after lumbar puncture could have represented the natural course of disease. Importantly, lumbar puncture may not be independently associated with the risk of brain herniation in patients with altered mental status (Glasgow Coma Scale score ≤ 8).6 A prospective randomized study is needed to better understand when to order brain imaging before lumbar puncture and when it is safe to proceed directly to lumbar puncture.
CONTRAINDICATIONS TO LUMBAR PUNCTURE
General contraindications to lumbar puncture are listed in Table 2.
Gopal et al3 analyzed clinical and radiographic data for 113 adults requiring urgent lumbar puncture and reported that altered mental status (likelihood ratio [LR] 2.2), focal neurologic deficit (LR 4.3), papilledema (LR 11.1), and clinical impression (LR 18.8) were associated with abnormalities on CT.
Hasbun et al4 prospectively analyzed whether clinical variables correlated with abnormal results of head CT that would preclude lumbar puncture in 301 patients requiring urgent lumbar puncture. They found that age 60 and older, immunodeficiency, a history of central nervous system disease, recent seizure (within 1 week), and neurologic deficits were associated with abnormal findings on head CT (eg, lesion with mass effect, midline shift). Importantly, absence of these characteristics had a 97% negative predictive value for abnormal findings on head CT. However, neither a normal head CT nor a normal clinical neurologic examination rules out increased intracranial pressure.4,7
CHIEF CONCERNS ABOUT LUMBAR PUNCTURE
Lumbar puncture is generally well tolerated. Major complications are rare2 and can be prevented by checking for contraindications and by using appropriate procedural hygiene and technique. Complications include pain at the puncture site, postprocedural headache, epidural hematoma, meningitis, osteomyelitis or discitis, bleeding, epidermoid tumor, and, most worrisome, brain herniation.
Brain herniation
Concern about causing brain herniation is the reason imaging may be ordered before lumbar puncture. Cerebral edema and increased intracranial pressure are common in patients with bacterial meningitis, as well as in other conditions such as bleeding, tumor, and abscess.1 If intracranial pressure is elevated, lumbar puncture can cause cerebral herniation with further neurologic compromise and possibly death. Herniation is believed to be due to a sudden decrease in pressure in the spinal cord caused by removal of cerebrospinal fluid. However, the only information we have about this complication comes from case reports and case series, so we don’t really know how often it happens.
On the other hand, ordering ancillary tests before lumbar puncture and starting empiric antibiotics in patients with suspected bacterial meningitis may delay treatment and lead to worse clinical outcomes and thus should be discouraged.8
Also important to note is the lack of good data regarding the safety of lumbar puncture in patients with potential hemostatic problems (thrombocytopenia, coagulopathy). The recommendation not to do lumbar puncture in these situations (Table 1) is taken from neuraxial anesthesia guidelines.9 Further, a small retrospective study of thrombocytopenic oncology patients requiring lumbar puncture did not demonstrate an increased risk of complications.10
ADDITIONAL CONSIDERATIONS
In a retrospective study in 2015, Glimåker et al6 demonstrated that lumbar puncture without prior brain CT was safe in patients with suspected acute bacterial meningitis with moderate to severe impairment of mental status, and that it led to a shorter “door-to-antibiotic time.” Lumbar puncture before imaging was also associated with a concomitant decrease in the risk of death, with no increase in the rate of complications.6
If brain imaging is to be done before lumbar puncture, then blood cultures (and cultures of other fluids, whenever appropriate) should be collected and the patient should be started on empiric management for central nervous system infection first. CT evidence of diffuse cerebral edema, focal lesions with mass effect, and ventriculomegaly should be viewed as further contraindications to lumbar puncture.1
Antibiotic therapy
When contraindications to lumbar puncture exist, the choice of antibiotic and the duration of therapy should be based on the patient’s history, demographics, risk factors, and microbiologic data from blood culture, urine culture, sputum culture, and detection of microbiological antigens.1 The choice of antibiotic is beyond the scope of this article. However, empiric antibiotic therapy with a third-generation cephalosporin (eg, ceftriaxone) and vancomycin and anti-inflammatory therapy (dexamethasone) should in most cases be started immediately after collecting samples for blood culture and must not be delayed by neuroimaging and lumbar puncture with cerebrospinal fluid sampling, given the high rates of mortality and morbidity if treatment is delayed.5,8
Consultation with the neurosurgery service regarding alternative brain ventricular fluid sampling should be considered.11
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
- Thigpen MC, Whitney CG, Messonnier NE, et al; Emerging Infections Programs Network. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011; 364:2016–2025.
- Ellenby MS, Tegtmeyer K, Lai S, Braner DA. Videos in clinical medicine. Lumbar puncture. N Engl J Med 2006; 355: e12.
- Gopal AK, Whitehouse JD, Simel DL, Corey GR. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med 1999; 159:2681–2685.
- Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med 2001; 345:1727–1733.
- Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004; 39:1267–1284.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Baraff LJ, Byyny RL, Probst MA, Salamon N, Linetsky M, Mower WR. Prevalence of herniation and intracranial shift on cranial tomography in patients with subarachnoid hemorrhage and a normal neurologic examination. Acad Emerg Med 2010; 17:423–428.
- Proulx N, Fréchette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 2005; 98:291–298.
- Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med 2010; 35:64–101.
- Ning S, Kerbel B, Callum J, Lin Y. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016; 110:393–400.
- Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med 2007; 22:194–207.
Ring-enhancing cerebral lesions
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
A 39-year-old woman with a history of human immunodeficiency virus (HIV) and hepatitis B virus infection was brought to the emergency department for evaluation of seizures, which had started a few days earlier. She was born and raised in a state bordering the Ohio River, an area where Histoplasma capsulatum is endemic. She denied any recent travel.
Her vital signs and neurologic examination were normal. Computed tomography of the head showed two areas of increased attenuation anterior to the frontal horns. To better characterize those lesions, magnetic resonance imaging (MRI) with contrast was done, which showed about a dozen 1-cm ring-enhancing lesions in the right cerebellum and both cerebral hemispheres (Figure 1).
Results of a complete blood cell count, metabolic profile, and chest radiography were normal. Her CD4 count was 428/μL (reference range 533–1,674) and 20% (60%–89%); her HIV viral load was 326,000 copies/mL.
She was initially treated empirically with sulfadiazine, pyrimethamine, and leukovorin for possible toxoplasmosis, which is the most common cause of ring-enhancing brain lesions in HIV patients. In the meantime, cerebrospinal fluid, blood, and urine were sent for a detailed workup for fungi, including Histoplasma. Results of the Histoplasma antibody and antigen studies of the serum, urine, and cerebrospinal fluid were positive, while cerebrospinal fluid testing for Toxoplasma by polymerase chain reaction testing was negative. Empirical treatment for toxoplasmosis was stopped and amphotericin B was started to treat disseminated histoplasmosis.
During her hospital course, she underwent brain biopsy via right frontotemporal craniotomy with resection of right frontal lesions. Pathologic study showed partially organizing abscesses with central necrosis (Figure 2), microscopy with Grocott-Gomori methenamine silver stain was positive for budding yeast forms consistent with H capsulatum (Figure 3), and special stain for acid-fast bacilli was negative for mycobacteria. Cultures of the brain biopsy specimen, blood, and cerebrospinal fluid for fungi, acid-fast bacilli, and bacteria did not reveal any growth after 28 days.
The patient was discharged home with instructions to take amphotericin B for a total of 6 weeks and then itraconazole. About 1 year later, she remained free of symptoms, although repeat MRI did not show any significant change in the size or number of histoplasmomas.
She did not comply well with her HIV treatment, and her immune status did not improve, so we decided to continue her itraconazole treatment for more than 1 year.
CEREBRAL HISTOPLASMOMA
The term “histoplasmoma” was introduced by Shapiro et al1 in 1955, when they first described numerous focal areas of softening, up to 1 cm in diameter, scattered throughout the brain at autopsy in a 41-year-old man who had died of disseminated histoplasmosis. They coined the word to describe these discrete areas of necrosis that might resemble tumors on the basis of their size, location, and capability of causing increased intracranial pressure.
Central nervous system involvement can either be a manifestation of disseminated disease or present as an isolated illness.2 It occurs in 5% to 10% of cases of disseminated histoplasmosis.3 Histoplasmosis of the central nervous system can have different manifestations; the most common presentation is chronic meningitis.4
Laboratory diagnosis is based on detecting H capsulatum antigen and antibody in the urine, blood, and cerebrospinal fluid. Tissue biopsy (histopathology) as well as cultures of tissue samples or body fluids may also establish the diagnosis.4
Toxoplasmosis and primary central nervous system lymphoma are the most common causes of brain ring-enhancing lesions in HIV patients in developed countries, while in the developing world neurocysticercosis and tuberculomas are more common.5,6 Much less common causes include brain abscesses secondary to bacterial infections (pyogenic abscess),7 cryptococcomas,8 syphilitic cerebral gummata,9 primary brain tumors (gliomas), and metastases.10
Compared with other forms of the disease, histoplasmosis of the central nervous system has higher rates of treatment failure and relapse, so treatment should be prolonged and aggressive.2,3 The cure rate with amphotericin B ranges from 33% to 61%, and higher doses produce better response rates.3
Current treatment recommendations are based on 2007 guidelines of the Infectious Diseases Society of America.11 Liposomal amphotericin B is the drug of choice because it achieves higher concentrations in the central nervous system than other drugs and is less toxic. It is given for 4 to 6 weeks, followed by itraconazole for at least 1 year and until the cerebrospinal fluid Histoplasma antigen test is negative and other cerebrospinal fluid abnormalities are resolved.
In patients who have primary disseminated histoplasmosis that includes the central nervous system, itraconazole can be given for more than 1 year or until immune recovery is achieved—or lifelong if necessary.2,12 Long-term suppressive antifungal therapy also should be considered in patients for whom appropriate initial therapy fails.2
Nephrotoxicity (acute kidney injury, hypokalemia, and hypomagnesemia), infusion-related drug reactions, and rash are among the well-described side effects of amphotericin B. Maintenance of intravascular volume and replacement of electrolytes should be an integral part of the amphotericin B treatment regimen.13
TAKE-AWAY POINTS
- Histoplasmomas should be considered in the differential diagnosis of ring-enhancing lesions of the central nervous system, along with toxoplasmosis and primary central nervous system lymphoma. This will allow timely initiation of the diagnostic workup, avoiding unnecessary and potentially risky interventions and delays in starting targeted antifungal therapy.
- There is no single gold standard test for central nervous system histoplasmosis. Rather, the final diagnosis is based on the combination of clinical, laboratory, and radiologic findings.
Acknowledgment: Library research assistance provided by HSHS St. John’s Hospital Health Sciences Library staff.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
- Shapiro JL, Lux JJ, Sprofkin BE. Histoplasmosis of the central nervous system. Am J Pathol 1955; 31:319–335.
- Wheat LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central nervous system histoplasmosis. Clin Infect Dis 2005; 40:844–852.
- Wheat LJ, Batteiger BE, Sathapatayavongs B. Histoplasma capsulatum infections of the central nervous system: a clinical review. Medicine (Baltimore) 1990; 69:244–260.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM 2004; 97:413–421.
- Miller RF, Hall-Craggs MA, Costa DC, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect 1998; 74:258–264.
- Cohen WA. Intracranial bacterial infections in patients with AIDS. Neuroimaging Clin N Am 1997; 7:223–229.
- Troncoso A, Fumagalli J, Shinzato R, Gulotta H, Toller M, Bava J. CNS cryptococcoma in an HIV-positive patient. J Int Assoc Physicians AIDS Care (Chic) 2002; 1:131–133.
- Land AM, Nelson GA, Bell SG, Denby KJ, Estrada CA, Willett LL. Widening the differential for brain masses in human immunodeficiency virus-positive patients: syphilitic cerebral gummata. Am J Med Sci 2013; 346:253–255.
- Balsys R, Janousek JE, Batnitzky S, Templeton AW. Peripheral enhancement in computerized cranial tomography: a non-specific finding. Surg Neurol 1979; 11:207–216.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–825.
- Wheat J, Hafner R, Wulfsohn M, et al; National Institute of Allergy and Infectious Diseases Clinical Trials and Mycoses Study Group Collaborators. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1993; 118:610–616.
- Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol 2008; 10:161–167.
Evidence helps, but some decisions remain within the art of medicine
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
Despite advances in therapy, more than 10% of patients with acute bacterial meningitis still die of it, and more suffer significant morbidity, including cognitive dysfunction and deafness. Well-defined protocols that include empiric antibiotics and systemic corticosteroids have improved the outcomes of patients with meningitis. But, as with other closed-space infections such as septic arthritis, any delay in providing appropriate antibiotic treatment is associated with a worse prognosis. In the case of bacterial meningitis, a retrospective analysis concluded that each hour of delay in delivering antibiotics and a corticosteroid can be associated with a relative (not absolute) increase in mortality of 13%.1
The precise diagnosis of bacterial meningitis depends entirely on obtaining cerebrospinal fluid for analysis, including culture and antibiotic sensitivity testing. But that simple statement belies several current and historical complexities. From my experience, getting a prompt diagnostic lumbar puncture is not as simple as it once was.
Many hospitals have imposed patient safety initiatives, which overall have been beneficial but have had the effect that medical residents and probably even hospitalists in some medical centers are less frequently the ones doing interventional procedures. Some procedures, such as placement of pulmonary arterial catheters in the medical intensive care unit, have been shown to be less useful and to pose more risk than once believed. The tasks of placing other central lines and performing thoracenteses have been relegated to special procedure teams trained in using ultrasound guidance. Interventional radiologists now often do the visceral biopsies and lumbar punctures, and as a result, it is hoped that procedural complication rates will decline. On the other hand, these changes mean that medical residents and future staff are less experienced in performing these procedures, even though there are times that they are the only ones available to perform them. The result is a potential delay in performing a necessary lumbar puncture.
Another reason that a lumbar puncture may be delayed is concern over iatrogenic herniation if the procedure is done in a patient who has elevated intracranial pressure. We do not know precisely how often this occurs if there is an undiagnosed brain mass lesion such as an abscess, which can mimic bacterial meningitis, or a malignancy, and meningitis itself may be associated with herniation. Yet, for years physicians have hesitated to perform lumbar punctures in some patients without first ruling out a brain mass by computed tomography (CT), a diagnostic flow algorithm that often introduces at least an hour of delay in performing the procedure and in obtaining cultures before starting antibiotics.
When I was in training, we were perhaps more cavalier, appropriately or not. If the history and examination did not suggest a brain mass and the patient had retinal vein pulsations without papilledema, we did the lumbar puncture. It was a different time, and there was a different perspective on risks and benefits. More recently, the trend has been to obtain a CT scan before a lumbar puncture in several subsets of patients.
A 2015 analysis from Sweden1 showed that we can probably do a lumbar puncture for suspected bacterial meningitis without first doing a CT scan in most patients, even in patients with moderately impaired mentation. Perhaps some other concerns can also be assuaged if evaluated, but we don’t have data. Mirrakhimov et al, in this issue of the Journal, review the current evidence on when to do CT before a lumbar puncture, even if it may significantly delay the procedure and the timely delivery of antibiotics. A perfect algorithm that balances the risks of delaying treatment, initiating less-than-ideal empiric antibiotics potentially without definitive culture, and inducing complications from a procedure done promptly may well be impossible to develop. Evidence helps us refine the diagnostic approach, but with limited data, some important decisions unfortunately remain within the “art” rather than the science of medicine.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
- Glimåker M, Johansson B, Grindborg Ö, Bottai M, Lindquist L, Sjölin J. Adult bacterial meningitis: earlier treatment and improved outcome following guideline revision promoting prompt lumbar puncture. Clin Infect Dis 2015; 60:1162–1169.
Submassive pulmonary embolism
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
To the Editor: I read with interest the review on submassive pulmonary embolism by Ataya et al1 in the December 2016 issue. I had 3 questions or observations for the authors
First, systemic thrombolytic therapy for massive or hemodynamically unstable pulmonary embolism is given a grade 2C recommendation, similar to the level for select patients with submassive pulmonary embolism with low bleeding risk but at high risk of developing hypotension. The reference for this is the 2012 American College of Chest Physicians guidelines.2 I would like to point out that these guidelines were updated and published in February 2016,3 and systemic thrombolytic therapy for massive pulmonary embolism now carries a grade 2B recommendation. Thrombolytic therapy still has a grade 2C recommendation for select patients with submassive pulmonary embolism.
Second, the Moderate Pulmonary Embolism Treated With Thrombolysis (MOPETT) trial is described as a randomized trial in patients with moderate pulmonary hypertension and right ventricular dysfunction. I would like to point out that right ventricular dysfunction was not a criterion for enrollment in the trial.4
Finally, catheter-directed thrombolytic therapy is mentioned as an option for select patients with submassive and massive pulmonary embolism. The advantage is believed to be due to local action of the drug with fewer systemic effects. Since the protocol involves alteplase for 12 or 24 hours with a maximum dose of 24 mg, and since in most cases pulmonary embolism originates in the lower extremity, are we not exposing these patients to further clot propagation for 12 or 24 hours without the benefit of concomitant systemic anticoagulation or an inferior vena cava filter?
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
In reply: Submassive pulmonary embolism
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
In Reply: We thank Dr. Katyal for his thoughtful comments.
Dr. Katyal points out that the grade of recommendation for thrombolysis in patients with massive pulmonary embolism was upgraded from 2C to 2B in the 2016 American College of Chest Physicians (ACCP) guidelines1 compared with the 2012 guidelines2 that we cited. The upgrade in this recommendation was owing to 2 small trials and 1 large randomized controlled trial that included patients with submassive pulmonary embolism.3–5 Interestingly, these 3 studies led to an upgrade in the level of recommendation for thrombolysis in the treatment of massive pulmonary embolism, perhaps more from a safety aspect (in view of the incidence of major bleeding vs mortality). Regardless, Dr. Katyal is correct in highlighting that the new 2016 ACCP guidelines now give a grade of 2B for thrombolytic therapy in the treatment of massive pulmonary embolism. These guidelines had not been published at the time of submission of our manuscript.
Dr. Katyal is also correct that patients were not required to have right ventricular dysfunction to be enrolled in the MOPETT trial.3 As we pointed out, “Only 20% of the participants were enrolled on the basis of right ventricular dysfunction on echocardiography, whereas almost 60% had elevated cardiac biomarkers.”6
Regarding catheter-directed therapy, patients who received low-dose catheter-directed alteplase were also concurrently anticoagulated with systemic unfractionated heparin in the Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism (ULTIMA) trial.7 The ULTIMA trial authors commented that unfractionated heparin was started with an 80-U/kg bolus followed by an 18-U/kg/hour infusion to target an anti-factor Xa level of 0.3 to 0.7 μg/mL, which is considered therapeutic anticoagulation. The investigators in the SEATTLE II trial8 continued systemic unfractionated heparin but targeted a lower “intermediate” anticoagulation target (an augmented partial thromboplastin time of 40–60 seconds), so these patients weren’t completely without systemic anticoagulation either. At our institution, the current practice is to target an anti-Xa level of 0.3 to 0.7 μg/mL in patients receiving catheter-directed therapy for large-volume pulmonary embolism.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–352.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M; “MOPETT” Investigators. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013; 111:273–277.
- Meyer G, Vicaut E, Danays T, et al; PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370:1402–1411.
- Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost 2014; 12:459–468.
- Ataya A, Cope J, Shahmohammadi A, Alnuaimat H. Do patients with submassive pulmonary embolism benefit from thrombolytic therapy? Cleve Clin J Med 2016; 83:923–932.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129:479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al; SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism (The SEATTLE II Study). JACC Cardiovasc Interv 2015; 8:1382–1392.